Abstract
No study examined and compared the association between intake of trans-fatty acids (TFAs) and risk of metabolic syndrome before and after significant reduction of TFA intakes in the US population. We hypothesized that the relationship might remain significant after substantial reduction of TFA intakes in the population. We used data on 1442 and 2233 adults aged ≥20 years from the National Health and Nutrition Examination Survey 1999-2000 and 2009-2010, respectively. Multivariable logistic regression analysis was used to assess the association between plasma TFA concentrations and metabolic syndrome, including each of its 5 components. The median plasma TFA concentrations were reduced from 79.8 μmol/L in 1999-2000 to 36.9 μmol/L in 2009-2010. The fully adjusted prevalence ratios comparing the highest vs the lowest quintile of plasma TFA concentrations in 1999-2000 were 3.43 (95% confidence interval, 2.39-4.92) for metabolic syndrome, 1.72 (1.38-2.14) for large waistline, 8.25 (6.34-10.74) for high triglycerides, 1.96 (1.46-2.62) for low high-density lipoprotein cholesterol, 1.14 (0.85-1.55) for high blood pressure, and 1.48 (1.19-1.85) for high fasting glucose, respectively. The corresponding prevalence ratios in 2009-2010 were 2.93 (2.41-3.54), 1.62 (1.39-1.89), 14.93 (9.28-24.02), 3.09 (2.18-4.37), 1.27 (1.11-1.46), and 1.24 (1.06-1.46), respectively. The pattern of association between TFAs and metabolic syndrome and its components did not differ by cycles. The observed associations were consistent across the subgroups examined. Despite a 54% decline in plasma TFA concentrations from 1999-2000 to 2009-2010, it was positively associated with risk of metabolic syndrome and its individual components except for blood pressure in 1999-2000. Our findings support Food and Drug Administration initiatives to remove TFAs from the industrially-produced foods.
Keywords: Trans-fatty acids, Metabolic syndrome, Prevalence ratio, Epidemiology, NHANES
1. Introduction
Individuals with metabolic syndrome, which is characterized by a constellation of hypertension, hyperglycemia, excess central body fat, high triglycerides, and low high-density lipoprotein (HDL) cholesterol concentrations, are at an increased risk for cardiovascular disease morbidity and mortality [1–3]. Metabolic syndrome affects approximately 1 in 3 US adults and is associated with increased health care use and costs, with each metabolic syndrome component resulting in an estimated 24% increase in health care costs [4,5]. Metabolic syndrome is considered largely a disease of unhealthy lifestyle factors [6], such as physical inactivity and unhealthy diet, including higher intakes of trans-fatty acids (TFAs).
TFAs are unsaturated fatty acids that contain at least 1 carbon-carbon trans double bond [7]. Studies have shown that higher dietary TFA intakes have several implications in metabolic syndrome, such as increased plasma triacylglycerols and cholesterol, and reduced HDL cholesterol levels [8–10]. Although TFAs occur naturally in meat and dairy products from ruminants, the major dietary sources of TFAs in the United States are partially hydrogenated vegetable oils [11]. Because of the adverse effects of TFAs, some countries have restricted the content of industrially produced TFAs in manufactured foods [12,13]. The US Food and Drug Administration (FDA) mandated that the quantity of TFAs must be indicated on the Nutrition Facts panels of all packaged food labels as of January 1, 2006 [14], and a recent study reported a 58% reduction of plasma TFA concentrations between 2000 and 2009 among non-Hispanic white adults in the United States [15].
Previous studies have examined the association between dietary TFAs [8–10] or TFAs in erythrocytes [16] and individual components of metabolic syndrome, but not simultaneously. In addition, no study examined and compared the association between TFAs and risk of metabolic syndrome before and after significant reduction of TFA intakes in population, and it is unknown whether the association remains detectable after the significant reduction in TFA intakes in the US population. We hypothesized that the association might remain significant after the substantial reduction in TFA intakes in the population. The purpose of this study was to examine and compare the association between plasma TFAs and metabolic syndrome before and after the FDA’s regulation on TFAs using data from the 1999-2000 and 2009-2010 National Health and Nutrition Examination Survey (NHANES).
2. Methods and materials
2.1. Study participants
NHANES uses a complex, stratified, multistage probability cluster sampling, cross-sectional design to collect health and nutritional data from a representative sample of the noninstitutionalized US population. The design and operation of NHANES have been described previously [17]. For the present study, we used data from 2 cycles of NHANES for which plasma TFAs were measured: 1999-2000 and 2009-2010. For NHANES 1999-2000, from the 1613 participants 20 years or older with morning fasting blood samples and complete TFA data, we excluded 89 pregnant women, 61 participants with missing metabolic syndrome components, and 21 participants with missing values on covariates. We recoded 47 participants with missing alcohol intake and 34 participants with missing physical activity, yielding 1442 adults for analysis (Fig. 1). For NHANES 2009-2010, of the 2462 fasting adult participants, we excluded 25 pregnant women, 154 participants with missing metabolic syndrome components, and 50 with missing values on covariates. We recoded 137 participants with missing alcohol intake, yielding 2233 adults for analysis (Fig. 1). Study protocols for NHANES were approved by the National Center for Health Statistics Research Ethics Review Board. Signed informed consent was obtained from all participants.
Fig. 1 –
Selection of study population for NHANES 1999-2000 and 2009-2010.
2.2. Metabolic syndrome
Metabolic syndrome was defined based on the National Cholesterol Education Program Adult Treatment Panel III, which was updated by the American Heart Association [6], as having 3 or more of the following: waist circumference >102 cm in men or >88 cm in women; serum triglycerides ≥150 mg/dL; HDL cholesterol <40 mg/dL in men or <50 mg/dL in women; mean systolic/diastolic blood pressure ≥130/85 mm Hg (based on the average of up to 3 readings obtained under standard conditions during a single physical examination) or self-reported use of antihypertensive medications; or fasting plasma glucose ≥100 mg/dL or self-reported use of antidiabetic medications.
2.3. Measurements of plasma TFAs and lipids profile
TFAs and all lipids analyses were conducted on morning fasting (≥8 to <24 hours) venous samples collected and analyzed according to a standardized protocol [18]. Four TFAs (elaidic acid [18:1n-9 t] OD9, vaccenic acid [18:1n-7 t] OD1, linoelaidic acid [18:2n-6 t,9 t] OTT, and palmitelaidic acid [16:1n-7 t] HDT) were measured in plasma stored at −70°C and analyzed using gas chromatography coupled with mass spectrometry (GC/MSD; Agilent Technologies, Santa Clara, CA, USA). TFA measurement details can be found in the supplemental file. The present study focused primarily on total plasma TFA concentration, which is the sum of the 4 major TFAs.
Triglyceride levels were measured using enzymatic reactions; HDL cholesterol was measured by the direct immunoassay method in 2009-2010; and in 1999-2000, the heparin manganese precipitation method was primarily used [19–21]. All lipid measurements were standardized through the Lipid Standardization Program maintained by the Centers for Disease Control and Prevention in collaboration with the National Heart, Lung, and Blood Institute [22].
2.4. Covariates
Study covariates included age, sex, race-ethnicity (non-Hispanic white, non-Hispanic black, Mexican American, or others); body mass index (BMI) (<25, 25-<30, and ≥30, calculated as weight in kilograms divided by height in meters squared); educational attainment (<12, 12, or >12 years); self-reported smoking status (current smoker, former smoker, or never smoked); alcohol consumption (0, <2, or ≥2 drinks per week or “missing” for men; 0, <1, or ≥1 drink per week or “missing” for women); diabetes status (based on participants’ self-reported history of diabetes diagnosis, or use of insulin or other diabetic medications to lower blood glucose, or fasting glucose ≥126 mg/dL); physical activity; statin use (identified using the prescription medication data files Multum Lexicon therapeutic classification “HMA-COA Reductase Inhibitors”); Healthy Eating Index–2010 (HEI-2010); and intakes of total energy, total saturated fatty acids, total polyunsaturated fatty acids, and total cholesterol from the first-day 24-hour dietary recall.
Because of changes in physical activity questions, the 2008 Physical Activity Guidelines for Americans recommendation for all adults to avoid inactivity was used as a basic assessment of activity. Physical activity status was based on whether participants reported engaging in at least 10 minutes of moderate-and/or vigorous-intensity activity per week (active, inactive, or missing) [23]. HEI-2010 scores were based on a 12-component index: total fruit, whole fruit, total vegetables, grains and beans, whole grains, dairy, total protein foods, seafood and plant protein, fatty acid, refined grains, sodium, and empty energy, with total scores ranging from 0 to 100 and a higher score indicating a healthier diet [24]. HEI-2010 scores were calculated based on the first-day 24-hour dietary recall.
2.5. Statistical analyses
Statistical analyses were performed using SUDDAN version 11 to take into account the complex sampling design. We analyzed NHANES 1999-2000 and 2009-2010 data separately. Data on characteristics were expressed as means and standard error for continuous variables or as percentages for categorical variables. We used t test to compare the differences in continuous variables between participants with and without metabolic syndrome and used χ2 test for the categorical variables. We used a series of multivariable logistic regression analysis to estimate adjusted prevalence ratios (PRs) for the risk of metabolic syndrome and each of its 5 individual components by comparing Q5, Q4, Q3, and Q2 with the lowest quintile (Q1) of plasma TFA concentrations. The first model included age, sex, race-ethnicity, and total energy. The second model added educational attainment, smoking status, alcohol consumption, statin use, physical activity level, and HEI-2010. The third model additionally adjusted for dietary intake of total saturated fatty acids, total polyunsaturated fatty acids, and total cholesterol. We did not include BMI in the model because it is highly correlated with waist circumference, which is 1 of the 5 components of metabolic syndrome. The 95% confidence intervals (CIs) for PRs were computed using the Wald method. The difference in PRs across the quintiles was tested based on Satterthwaite adjusted F test. We also performed stratified analyses to explore whether the association between plasma TFA concentrations and metabolic syndrome differed by age group (<60 vs ≥60 years), sex, race-ethnicity, educational attainment (<12 vs ≥12 years), physical activity (inactive vs activities), HEI-2010 (top 50% [score ≥44.27 or ≥47.84 for NHANES 1999-2000 and 2009-2010, respectively] vs other), BMI (normal vs overweight/obese), and statin use (yes/no). We tested collinearity among independent variables using the variance inflation factor in multivariate linear regression analysis. There was no evidence of collinearity, as the variance inflation factors were <5.0 for all independent variables [25]. We tested the interaction between plasma TFA concentrations and covariates by including the interaction terms in the multivariable logistic models based on Satterthwaite adjusted F test and used Bonferroni correction for multiple testing. We combined the 2 cycle together and tested for an interaction between plasma TFAs and cycle years on PRs.
We performed several sensitivity analyses: (1) We excluded participants with missing alcohol (n = 47) and missing physical activity (n = 34) in NHANES 1999-2000 and participants with missing alcohol (n = 137) in NHANES 2009-2010. (2) Although our methods will not be able to separate the naturally occurring and industrially produced TFAs, the vaccenic acid is the predominant trans isomer found in the fat of ruminants (naturally occurring TFA). We examined the association after excluding vaccenic acid from total TFAs. (3) We also examined the association between each of the 4 plasma TFAs (elaidic acid, vaccenic acid, linoelaidic acid, and palmitelaidic acid) and metabolic syndrome. Assuming a design effect of 1.6 (NHANES 1999-2000) and 2.0 (NHANES 2009-2010), the sample size was sufficient to detect a relative difference of 9.0% and 7.2% with 80% power at α = .05 level in NHANES 1999-2000 and 2009-2010, respectively. The power was estimated by SAS Proc Power using the adjusted sample size by the average design effect for the complex survey (release 9.3; SAS Institute Inc, Cary, NC, USA). All tests of statistical significance were 2-tailed, and a probability value <.05 was considered significant.
3. Results
The median plasma TFA concentrations were reduced from 79.8 (95% CI, 74.8-87.0) μmol/L in 1999-2000 to 36.9 (35.4-38.4) μmol/L in 2009-2010. Participants with metabolic syndrome were older and more likely to be overweight or obese, and had higher levels of total cholesterol, low-density lipoprotein cholesterol, HDL cholesterol, triglycerides, and TFAs in both 1999-2000 and 2009-2010 cycles (Table 1).
Table 1 –
Characteristics of participants aged ≥20 years by metabolic syndrome status—NHANES 1999-2000 and 2009-2010
| 1999-2000 Metabolic Syndrome |
2009-2010 Metabolic Syndrome |
|||||
|---|---|---|---|---|---|---|
| Yes (n = 558) | No (n = 884) | P value | Yes (n = 891) | No (n = 1342) | P value | |
| Age (y) | 51.7 ± 0.94 | 42.0 ± 1.01 | <.001 | 54.3 ± 0.89 | 43.5 ± 0.66 | <.001 |
| Sex (%) | ||||||
| Male | 47.5 | 50.3 | .418 | 50.0 | 48.9 | .727 |
| Female | 52.5 | 49.7 | 50.0 | 51.1 | ||
| Race/ethnicity (%) | ||||||
| Non-Hispanic white | 74.5 | 74.4 | .031 | 70.4 | 68.4 | .034 |
| Non-Hispanic black | 6.9 | 9.4 | 10.6 | 10.5 | ||
| Mexican American | 6.6 | 6.4 | 10.0 | 8.4 | ||
| Other | 12.1 | 9.9 | 9.0 | 12.7 | ||
| Educational attainment (%) | ||||||
| 0-11 y | 24.1 | 22.0 | .111 | 23.5 | 15.9 | <.001 |
| 12 y | 32.9 | 25.0 | 28.8 | 19.6 | ||
| ≥12 y | 43.1 | 53.0 | 47.7 | 64.5 | ||
| Smoking status (%) | ||||||
| Current | 24.4 | 24.9 | .131 | 18.1 | 20.2 | .001 |
| Former | 32.9 | 25.6 | 29.0 | 23.1 | ||
| Never | 42.1 | 49.6 | 53.0 | 56.7 | ||
| BMI status (%) | ||||||
| <25 | 11.3 | 52.0 | <.001 | 7.5 | 43.0 | <.001 |
| 25-<30 | 29.0 | 32.9 | 28.2 | 36.4 | ||
| ≥30 | 59.8 | 15.1 | 64.3 | 20.7 | ||
| HEI-2010 | 45.7 ± 0.89 | 45.8 ± 1.41 | .928 | 47.6 ± 0.61 | 49.5 ± 0.55 | .189 |
| Total fat (g/d) | 85.4 ± 1.78 | 83.9 ± 2.12 | .656 | 82.5 ± 2.39 | 82.1 ± 1.57 | .904 |
| Total saturated fatty acids (g/d) | 28.6 ± 0.85 | 27.7 ± 0.75 | .484 | 26.8 ± 0.94 | 26.9 ± 0.63 | .929 |
| Total polyunsaturated fatty acids (g/d) | 17.2 ± 0.35 | 18.0 ± 0.47 | .218 | 18.2 ± 0.62 | 18.2 ± 0.37 | .916 |
| Lipid profile (geometric mean) | ||||||
| Total cholesterol (mg/dL) | 207.0 ± 2.08 | 195.4 ± 1.83 | <.001 | 195.7 ± 1.68 | 189.7 ± 1.33 | .002 |
| LDL cholesterol (mg/dL) | 124.8 ± 2.02 (n = 526) | 119.7 ± 1.69 (n = 883) | .068 | 114.6 ± 1.51 (n = 860) | 110.0 ± 1.07 (n = 1339) | .005 |
| HDL cholesterol (mg/dL) | 40.8 ± 0.77 | 51.0 ± 0.64 | <.001 | 43.1 ± 0.46 | 56.8 ± 0.49 | <.001 |
| Triglycerides (mg/dL) | 179.3 ± 4.73 | 98.9 ± 1.72 | <.001 | 154.7 ± 3.16 | 89.7 ± 1.01 | <.001 |
| Total cholesterol to HDL ratio | 5.08 ± 0.10 | 3.83 ± 0.06 | <.001 | 4.53 ± 0.06 | 3.34 ± 0.03 | <.001 |
| Plasma TFA (μmol/L) (geometric mean) | 98.4 ± 3.78 | 72.7 ± 2.55 | <.001 | 46.4 ± 0.99 | 33.4 ± 0.55 | <.001 |
Values are means or geometric mean ± SE.
Abbreviation: CI, confidence intervals; HDL, high-density lipoprotein; HEI-2010, Healthy Eating Index-2010; LDL, low-density lipoprotein; NHANES, National Health and Nutrition Examination Survey; TFA, trans-fatty acid.
After adjustment for potential confounders, plasma TFAs were significantly associated with metabolic syndrome and its individual components, except for high blood pressure in the 1999-2000 cycle. The adjusted PRs comparing the highest vs the lowest quintile of plasma TFA concentrations in 1999-2000 were 3.43 (95% CI, 2.39-4.92) for metabolic syndrome, 1.72 (95% CI, 1.38-2.14) for large waistline, 8.25 (95% CI, 6.34-10.74) for high triglycerides level, 1.96 (95% CI, 1.46-2.62) for low HDL cholesterol level, 1.14 (95% CI, 0.85-1.55) for high blood pressure, and 1.48 (95% CI, 1.19-1.85) for high fasting glucose, respectively (Table 2). The corresponding adjusted PRs in 2009-2010 were 2.93 (95% CI, 2.41-3.54), 1.62 (95% CI, 1.39-1.89), 14.93 (95% CI, 9.28-24.02), 3.09 (95% CI, 2.18-4.37), 1.27 (95% CI, 1.11-1.46), and 1.24 (95% CI, 1.06-1.46), respectively (Table 3). The pattern of association between TFAs and metabolic syndrome and its components did not differ between the 1999-2000 and 2009-2010 cycles.
Table 2 –
Adjusted PRs of metabolic syndrome and its components among participants aged ≥20 years by quintiles of plasma TFA concentrations—NHANES 1999-2000
| Quintiles of plasma TFA concentrations, PRs (95% CI) |
P value for trend a | |||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | ||
| Median (range) of plasma TFA (μmol/L) | 45.79 (10.96-55.35) | 64.61 (55.36-72.28) | 79.76 (72.29-89.26) | 98.71 (89.27-115.28) | 138.24 (115.29-478.34) | |
| No. of participants | 304 | 310 | 274 | 266 | 288 | |
| Metabolic syndrome | ||||||
| M1b | 1.00 | 1.45 (1.08-1.95) | 2.01 (1.28-3.17) | 2.46 (1.58-3.83) | 3.71 (2.65-5.20) | <.001 |
| M2c | 1.00 | 1.45 (1.04-2.02) | 2.01 (1.30-3.09) | 2.20 (1.43-3.39) | 3.45 (2.39-4.97) | <.001 |
| M3d | 1.00 | 1.44 (1.04-1.98) | 1.99 (1.30-3.04) | 2.20 (1.46-3.33) | 3.43 (2.39-4.92) | <.001 |
| Large waistline | ||||||
| M1b | 1.00 | 1.15 (0.90-1.47) | 1.34 (0.96-1.89) | 1.58 (1.18-2.12) | 1.80 (1.41-2.29) | <.001 |
| M2c | 1.00 | 1.12 (0.88-1.42) | 1.34 (0.97-1.83) | 1.49 (1.15-1.93) | 1.71 (1.37-2.14) | <.001 |
| M3d | 1.00 | 1.13 (0.90-1.42) | 1.34 (0.98-1.83) | 1.51 (1.18-1.93) | 1.72 (1.38-2.14) | <.001 |
| High triglyceride level | ||||||
| M1b | 1.00 | 1.19 (0.73-1.94) | 2.89 (2.08-4.02) | 3.35 (2.53-4.43) | 6.94 (5.56-8.66) | <.001 |
| M2c | 1.00 | 1.38 (0.75-2.55) | 3.59 (2.67-4.83) | 4.00 (3.12-5.14) | 8.29 (6.39-10.75) | <.001 |
| M3d | 1.00 | 1.36 (0.74-2.49) | 3.57 (2.63-4.84) | 4.02 (3.19-5.07) | 8.25 (6.34-10.74) | <.001 |
| Low HDL cholesterol level | ||||||
| M1b | 1.00 | 1.24 (0.91-1.70) | 1.47 (1.03-2.09) | 1.80 (1.31-2.48) | 2.26 (1.77-2.89) | <.001 |
| M2c | 1.00 | 1.16 (0.82-1.63) | 1.32 (0.91-1.93) | 1.52 (1.09-2.11) | 1.93 (1.43-2.60) | <.001 |
| M3d | 1.00 | 1.17 (0.84-1.64) | 1.35 (0.93-1.95) | 1.54 (1.13-2.10) | 1.96 (1.46-2.62) | <.001 |
| High blood pressure | ||||||
| M1b | 1.00 | 1.17 (0.87-1.56) | 1.02 (0.73-1.41) | 0.97 (0.63-1.50) | 1.16 (0.87-1.56) | .740 |
| M2c | 1.00 | 1.17 (0.88-1.55) | 1.01 (0.75-1.38) | 0.94 (0.61-1.44) | 1.14 (0.84-1.54) | .911 |
| M3d | 1.00 | 1.18 (0.88-1.59) | 1.02 (0.74-1.41) | 0.95 (0.62-1.45) | 1.14 (0.85-1.55) | .894 |
| High fasting glucose | ||||||
| M1b | 1.00 | 1.31 (1.03-1.76) | 1.13 (0.73-1.76) | 1.57 (1.13-2.19) | 1.62 (1.15-2.29) | .004 |
| M2c | 1.00 | 1.31 (1.12-1.53) | 1.15 (0.78-1.69) | 1.36 (1.01-1.84) | 1.50 (1.19-1.89) | .010 |
| M3d | 1.00 | 1.29 (1.12-1.50) | 1.14 (0.78-1.66) | 1.34 (1.02-1.76) | 1.48 (1.19-1.85) | .007 |
Values are medians (ranges) for plasma TFA concentration or adjusted PR (95% CIs).
Abbreviations: HDL, high-density lipoprotein; NHANES, National Health and Nutrition Examination Survey; TFA, trans-fatty acid.
P values are presented for difference across the quintiles of TFA concentrations. All tests were 2-tailed and based on Satterthwaite adjusted F test.
M1 = adjusted for age, sex, race/ethnicity, and total energy intake.
M2 = in addition to M1, adjusted for educational attainment, smoking status, alcohol consumption, physical activity level, statin use, diabetes status, and HEI-2010.
M3 = in addition to M2, adjusted for total saturated fatty acids, total polyunsaturated fatty acids, and total cholesterol intakes.
Table 3 –
Adjusted PRs of metabolic syndrome and its components among participants aged ≥20 years by quintiles of plasma TFA concentrations—NHANES 2009-2010
| Quintiles of plasma TFA concentrations, PRs (95% CI) |
P value for trend a | |||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | ||
| Median (range) of plasma TFA (μmol/L) | 21.08 (7.33-25.46) | 29.49 (25.47-33.30) | 36.82 (33.31-41.14) | 46.62 (41.15-54.14) | 67.40 (54.15-303.20) | |
| No. of participants | 423 | 443 | 434 | 455 | 478 | |
| Metabolic syndrome | ||||||
| M1b | 1.00 | 1.23 (0.93-1.63) | 1.54 (1.19-1.99) | 1.80 (1.36-2.39) | 3.11 (2.43-3.98) | <.001 |
| M2c | 1.00 | 1.20 (0.92-1.57) | 1.52 (1.23-1.89) | 1.70 (1.36-2.13) | 2.80 (2.33-3.37) | <.001 |
| M3d | 1.00 | 1.25 (0.99-1.58) | 1.59 (1.28-1.97) | 1.80 (1.42-2.27) | 2.93 (2.41-3.54) | <.001 |
| Large waistline | ||||||
| M1b | 1.00 | 1.22 (1.08-1.38) | 1.43 (1.21-1.68) | 1.47 (1.24-1.75) | 1.71 (1.45-2.01) | <.001 |
| M2c | 1.00 | 1.18 (1.02-1.36) | 1.41 (1.19-1.67) | 1.43 (1.18-1.72) | 1.61 (1.38-1.87) | <.001 |
| M3d | 1.00 | 1.17 (1.03-1.33) | 1.42 (1.19-1.68) | 1.44 (1.19-1.74) | 1.62 (1.39-1.89) | <.001 |
| High triglyceride level | ||||||
| M1b | 1.00 | 1.43 (0.72-2.82) | 3.93 (2.32-6.66) | 6.68 (4.01-11.16) | 12.72 (7.85-20.59) | <.001 |
| M2c | 1.00 | 1.56 (0.80-3.04) | 4.66 (2.72-7.98) | 7.84 (4.79-12.84) | 14.65 (9.13-23.50) | <.001 |
| M3d | 1.00 | 1.75 (0.90-3.40) | 4.89 (2.85-8.41) | 8.18 (5.00-13.37) | 14.93 (9.28-24.02) | <.001 |
| Low HDL cholesterol level | ||||||
| M1b | 1.00 | 1.23 (0.82-1.83) | 2.01 (1.48-2.74) | 1.98 (1.42-2.76) | 3.69 (2.68-5.09) | <.001 |
| M2c | 1.00 | 1.17 (0.80-1.70) | 1.78 (1.26-2.50) | 1.71 (1.20-2.42) | 3.05 (2.18-4.27) | <.001 |
| M3d | 1.00 | 1.20 (0.83-1.74) | 1.82 (1.29-2.58) | 1.75 (1.23-2.48) | 3.09 (2.18-4.37) | <.001 |
| High blood pressure | ||||||
| M1b | 1.00 | 0.93 (0.77-1.13) | 1.07 (0.90-1.28) | 1.04 (0.86-1.24) | 1.27 (1.10-1.46) | .010 |
| M2c | 1.00 | 0.92 (0.76-1.10) | 1.09 (0.96-1.25) | 1.05 (0.89-1.23) | 1.24 (1.08-1.43) | .010 |
| M3d | 1.00 | 0.94 (0.78-1.13) | 1.12 (0.97-1.29) | 1.08 (0.92-1.27) | 1.27 (1.11-1.46) | .006 |
| High fasting glucose | ||||||
| M1b | 1.00 | 1.03 (0.81-1.31) | 1.08 (0.86-1.35) | 1.23 (0.96-1.58) | 1.34 (1.08-1.67) | <.001 |
| M2c | 1.00 | 1.02 (0.85-1.22) | 1.07 (0.91-1.26) | 1.18 (0.99-1.39) | 1.21 (1.04-1.41) | <.001 |
| M3d | 1.00 | 1.04 (0.87-1.25) | 1.10 (0.93-1.31) | 1.21 (1.02-1.44) | 1.24 (1.06-1.46) | <.001 |
Values are medians (ranges) for plasma TFA concentration or adjusted PR (95% CIs).
Abbreviations: HDL, high-density lipoprotein; NHANES, National Health and Nutrition Examination Survey; TFA, trans-fatty acid.
P values are presented for difference across the quintiles of TFA concentrations. All tests were 2-tailed and based on Satterthwaite adjusted F test.
M1 = adjusted for age, sex, race/ethnicity, and total energy intake.
M2 = in addition to M1, adjusted for educational attainment, smoking status, alcohol consumption, physical activity level, statin use, diabetes status, and HEI-2010.
M3 = in addition to M2, adjusted for total saturated fatty acids, total polyunsaturated fatty acids, and total cholesterol intakes.
The patterns of association were largely consistent across age (<60 vs ≥60 years), sex, race-ethnicity, BMI (normal vs overweight/obese), education attainment, physical activity, statin use, and HEI-2010 (≥44.27 vs <44.27 in NHANES 1999-2000 and ≥47.84 vs <47.84 in NHANES 2009-2010) subgroups (Fig. 2 and Supplemental Table S1).
Fig. 2 –
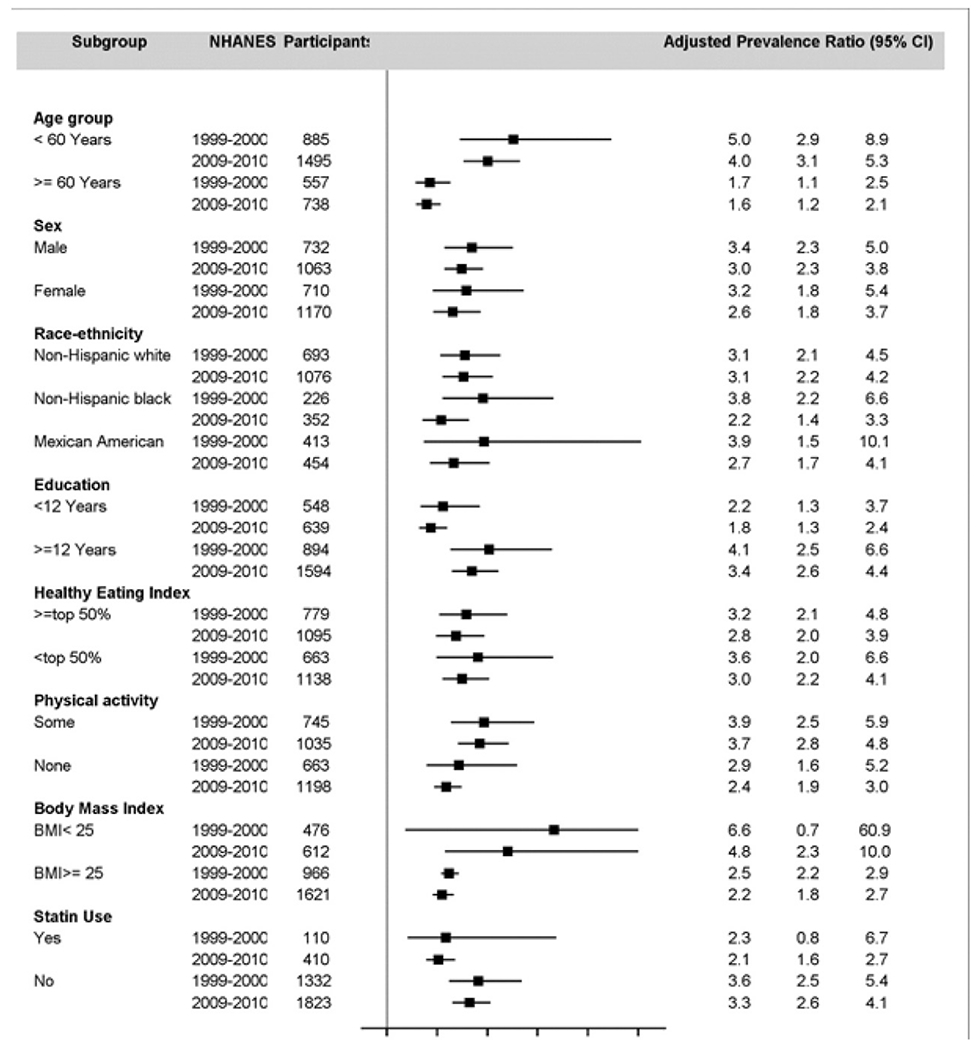
Adjusted PR of metabolic syndrome among participants aged ≥20 years comparing the highest to lowest quintiles of plasma TFA concentration—NHANES 1999-2000 and 2009-2010.
In a sensitivity analysis, we excluded participants with missing covariates and found that compared with the results when the missing data were recoded, the adjusted PRs remained consistent (Supplemental Tables S2–S3). When vaccenic acid was excluded from the total TFAs, the pattern of association was consistent, but the magnitude of association appeared to be stronger (Supplemental Tables S4–S5). The pattern of association between each of 4 TFA components and metabolic syndrome was largely consistent with that of the summarized plasma TFAs, and linoelaidic acid had the strongest association with high triglycerides (Supplemental Tables S6–S13). Analyses additionally adjusting for BMI slightly attenuated the association between TFAs and metabolic syndrome and each of its 5 components (data not shown).
4. Discussion
This analysis of a nationally representative sample of US adults suggested that plasma TFA concentrations declined dramatically from 1990-2000 to 2009-2010, which is consistent with the reduction of dietary intake of TFAs following the FDA’s food labeling regulations for TFAs in 2006 [11] as well as a previous report [15]. Plasma TFA concentrations were significantly associated with the prevalence of metabolic syndrome and its individual components before and after the significant reduction of plasma TFA concentrations in population, except for blood pressure in NHANES 1999-2000. The observed association was consistent by the subgroups examined or by NHANES 1999-2000 and 2009-2010 cycle years. It appeared that there is no threshold value of plasma TFA concentrations under which the association between plasma TFAs and risk for metabolic syndrome might become undetectable.
Studies have consistently shown that metabolic syndrome increased the risk of cardiovascular disease and diabetes mellitus, as well as cardiovascular disease and all-cause mortality [3,26]. It is estimated that 13%-44% of the excess cardiovascular disease mortality in the United States might be attributed to metabolic syndrome or metabolic syndrome–related cardiovascular disease [27]. The major underlying risk factors for metabolic syndrome include obesity, physical inactivity, unhealthy diet, cigarette smoking, and parental premature history of cardiovascular disease [28]. Identification, prevention, and treatment of metabolic syndrome to prevent cardiovascular disease, the leading cause of morbidity and mortality in United States, are imperative. However, public awareness of metabolic syndrome is limited despite its prevalence [26]. Consumption of TFAs has been identified as an important and modifiable risk factor for metabolic syndrome and the risk for cardiovascular diseases [29]. Various policies aimed at restricting industrially produced TFAs were associated with significant reductions of TFAs in foods. In addition to the 2006 food labeling regulations, FDA recently issued a regulation to further reduce TFAs in US foods by eliminating the use of partially hydrogenated oils by 2018 [30]. The 2015-2020 US Dietary Guidelines for Americans and the Institute of Medicine recommend that individuals should limit TFA intakes as much as possible to eliminate the adverse effects on health [31,32].
Although the exact mechanisms are not entirely or clearly understood, the association between dietary TFA intakes and factors contributing to metabolic syndrome have been studied and established [8–10]. Dietary TFA intakes have adverse effects on serum lipid and lipoprotein concentrations, plasma markers of inflammation (C-reactive protein and interleukin-6), weight gain, and visceral adiposity [29]. Our analyses demonstrates that higher plasma TFA concentrations are associated with an increased risk of high triglycerides, low HDL cholesterol, greater waist circumference, and high fasting glucose, results that are consistent with previous reports. Several studies showed that higher TFA intakes increased blood levels of triglycerides and reduced HDL cholesterol when compared with the intake of saturated fat and other unsaturated fats [8–10]. Two prospective cohort studies assessed the long-term effects of TFAs on weight gain and suggested that TFA might increase body weight and fat accumulation, with each 2% energy increase in TFA consumption being associated with a 0.77-cm increase in waist circumference in a 9-year follow-up study among 16 587 men [33] and a 1.6-kg weight gain in another 8-year follow-up study among 41 518 women [34]. Studies have reported inconsistent findings for the association between TFAs and insulin resistance. A meta-analysis of 7 randomized, placebo-controlled trials and 2 shortterm trials in lean, healthy individuals did not find enough evidence to support a potential benefit of reduced dietary TFA intakes on glucose homeostasis [35–37]. Other studies have reported the adverse effects of TFAs on insulin sensitivity among overweight individuals or patients with diabetes mellitus [38–40]; results were consistent with our analysis, in which about two-thirds of the study population was overweight. Mensink et al [41] measured the effect of dietary TFAs on blood pressure in 25 men and 34 women and reported that, after mixed natural diet for 3 weeks, dietary TFAs did not influence blood pressure levels in normotensive subjects relative to oleic acid. In a randomized, double-blind, and paralleled intervention trial, Dyerberg et al [42] randomly assigned 87 healthy men to dietary TFAs, 3-n polyunsaturated fatty acids, or a control group for 8 weeks. Compared with the control group, blood pressure decreased in the 3-n polyunsaturated fatty acids group, but not the TFAs group, after 12 weeks of follow-up. Our analyses indicated that higher plasma TFA concentrations were related to an increased risk of hypertension in the 2009-2010 cycle.
Our study has major strengths. To our knowledge, this is the first study to assess the association between plasma TFAs and both metabolic syndrome and its individual components in a large nationally representative sample of US adults. In addition, we used a new analytical method for measuring plasma TFA concentrations with a high analytical specificity for the plasma TFAs investigated. Moreover, the examination of plasma TFAs avoids the recall bias and measurement error resulting from dietary recalls, rendering estimates of association more objective. Finally, we were able to adjust for a wide range of potential confounding factors.
Our study has several limitations. First, the industrially produced and naturally occurring TFAs could not be differentiated. Second, HEI-2010 and intakes of total energy, total saturated fatty acids, total polyunsaturated fatty acids, and total cholesterol were from the first-day 24-hour dietary recall, which might not reflect the individuals’ usual dietary intakes. A previous validation study using 24-hour dietary recalls suggested that energy intake may be underestimated by as much as 11%. Third, we were not able to examine the correlation between dietary and plasma TFAs in our analysis because dietary TFAs were not available in NHANES. However, previous studies showed a moderate correlation between plasma and dietary TFAs assessed either by validated Food Frequency Questionnaire or erythrocyte [43,44]. Fourth, questions regarding physical activity differed between the NHANES 1999-2000 and 2009-2010 cycles. To minimize the influence of this inconsistency, we defined physically inactive as participating in less than 10 minutes of moderate and/or vigorous activity per week. Finally, our study was cross-sectional; thus, the associations between plasma TFAs and metabolic syndrome and its individual components cannot be interpreted as directly causal.
Our results suggested that despite a 54% reduction of plasma TFA concentrations in the US from 1990-2000 to 2009-2010, plasma TFA concentrations were significantly associated with the prevalence of metabolic syndrome and most of its individual components. It appeared that there is no threshold value of plasma TFA concentrations under which the association between plasma TFAs and risk for metabolic syndrome might become undetectable. Our findings support FDA initiatives to remove TFAs from industrially produced foods.
Supplementary Material
Acknowledgment
We are thankful Hubert Vesper and Samuel P. Caudill for their laboratory analyses on plasma TFAs. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The authors have no conflicts of interest to declare.
Disclaimer:
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Abbreviations:
- BMI
body mass index
- CI
confidence interval
- FDA
Food and Drug Administration
- HDL
high-density lipoprotein
- HEI-2010
Healthy Eating Index–2010
- NHANES
National Health and Nutrition Examination Survey
- PRs
prevalence ratios
- TFAs
trans-fatty acids
Footnotes
Appendix A. Supplemental materials
Supplemental materials to this article can be found online at http://dx.doi.org/10.1016/j.nutres.2017.05.008.
REFERENCES
- [1].Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA 2002;287:356–9. [DOI] [PubMed] [Google Scholar]
- [2].Gami AS, Witt BJ, Howard DE, Erwin PJ, Gami LA, Somers VK, et al. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol 2007;49:403–14. [DOI] [PubMed] [Google Scholar]
- [3].Mottillo S, Filion KB, Genest J, Joseph L, Pilote L, Poirier P, et al. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol 2010;56:1113–32. [DOI] [PubMed] [Google Scholar]
- [4].Boudreau DM, Malone DC, Raebel MA, Fishman PA, Nichols GA, Feldstein AC, et al. Health care utilization and costs by metabolic syndrome risk factors. Metab Syndr Relat Disord 2009;7:305–14. [DOI] [PubMed] [Google Scholar]
- [5].Aguilar M, Bhuket T, Torres S, Liu B, Wong RJ. Prevalence of the metabolic syndrome in the United States, 2003-2012. JAMA 2015;313:1973–4. [DOI] [PubMed] [Google Scholar]
- [6].Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. , American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation 2015;131:e29–322. [DOI] [PubMed] [Google Scholar]
- [7].Eckel RH, Borra S, Lichtenstein AH, Yin-Piazza SY, Trans Fat Conference Planning Group. Understanding the complexity of trans fatty acid reduction in the American diet: American Heart Association trans fat conference 2006: report of the trans fat conference planning group. Circulation 2007;115:2231–46. [DOI] [PubMed] [Google Scholar]
- [8].Mensink RP, Katan MB. Effect of dietary trans fatty acids on high-density and low-density lipoprotein cholesterol levels in healthy subjects. N Engl J Med 1990;323:439–45. [DOI] [PubMed] [Google Scholar]
- [9].Mensink RP, Zock PL, Kester AD, Katan MB. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am J Clin Nutr 2003;77:1146–55. [DOI] [PubMed] [Google Scholar]
- [10].Mozaffarian D, Clarke R. Quantitative effects on cardiovascular risk factors and coronary heart disease risk of replacing partially hydrogenated vegetable oils with other fats and oils. Eur J Clin Nutr 2009;63:s22–33. [DOI] [PubMed] [Google Scholar]
- [11].Doell D, Folmer D, Lee H, Honigfort M, Carberry S. Updated estimate of trans fat intake by the US population. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 2012;29:861–74. [DOI] [PubMed] [Google Scholar]
- [12].Canada H TRANSforming the food supply. http://www.hc-sc.gc.ca/fn-an/nutrition/gras-trans-fats/tf-ge/tf-gt_rep-rap-eng.php. [Accessed 25.11.16].
- [13].Leth T, Jensen HG, Mikkelsen AA, Bysted A. The effect of the regulation on trans fatty acid content in Danish food. Atherosclerosis 2006;7:s53–6. [DOI] [PubMed] [Google Scholar]
- [14].Food Drug Administration. Food labeling: trans fatty acids in nutrition labeling, nutrient content claims, and health claims. Final rule. Fed Regist 2003;68:41433–506. [PubMed] [Google Scholar]
- [15].Vesper HW, Kuiper HC, Mirel LB, Johnson CL, Pirkle JL. Levels of plasma trans-fatty acids in non-Hispanic white adults in the United States in 2000 and 2009. JAMA 2012;307:562–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Yu DX, Sun Q, Ye XW, Pan A, Zong G, Zhou YH, et al. Erythrocyte trans-fatty acids, type 2 diabetes and cardiovascular risk factors in middle-aged and older Chinese individuals. Diabetologia 2012;55:2954–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Centers for Disease Control and Prevention (CDC), National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey questionnaires, datasets, and related documentation. http://www.cdc.gov/nchs/nhanes/nhanes_questionnaires.htm. [Accessed 25.11.16].
- [18].Centers for Disease Control and Prevention (CDC). National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey laboratory protocol Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. 2009-2010. http://www.cdc.gov/nchs/nhanes/nhanes2009-2010/manuals09_10.htm. [Accessed 25.11.16]. [Google Scholar]
- [19].Allain CC, Poon LS, Chan CS, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol. Clin Chem 1974;20:470–5. [PubMed] [Google Scholar]
- [20].Carroll MD, Kit BK, Lacher DA. Total and high-density lipoprotein cholesterol in adults: National Health and Nutrition Examination Survey, 2009-2010. NCHS Data Brief 2012;92:1–8. [PubMed] [Google Scholar]
- [21].Sugiuchi H, Uji Y, Okabe H, Irie T, Uekama K, Kayahara N, et al. Direct measurement of high-density lipoprotein cholesterol in serum with polyethylene glycol-modified enzymes and sulfated alpha-cyclodextrin. Clin Chem 1995;41:717–23. [PubMed] [Google Scholar]
- [22].The Centers for Disease Control and Prevention Lipid Standardization Program. Laboratory quality assurance and standardization programs: 2012. http://www.cdc.gov/labstandards/lsp_faq.html. [Accessed 25.11.16].
- [23].United States Department of Health and Human Services. http://www.health.gov/paguidelines. [Accessed 25.11.16].
- [24].Kennedy ET, Ohls J, Carlson S, Fleming K. The healthy eating index: design and applications. J Am Diet Assoc 1995;95:1103–8. [DOI] [PubMed] [Google Scholar]
- [25].Rogerson PA. Statistical methods for geography. London: Sage; 2001. [Google Scholar]
- [26].Lewis SJ, Rodbard HW, Fox KM, Grandy S, Group SS. Self-reported prevalence and awareness of metabolic syndrome: findings from SHIELD. Int J Clin Pract 2008;62:1168–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Liu L, Miura K, Fujiyoshi A, Kadota A, Miyagawa N, Nakamura Y. Impact of metabolic syndrome on the risk of cardiovascular disease mortality in the United States and in Japan. Am J Cardiol 2014;113:84–9. [DOI] [PubMed] [Google Scholar]
- [28].Dallongeville J, Grupposo MC, Cottel D, Ferrieres J, Arveiler D, Bingham A, et al. Association between the metabolic syndrome and parental history of premature cardiovascular disease. Eur Heart J 2006;27:722–8. [DOI] [PubMed] [Google Scholar]
- [29].Micha R, Mozaffarian D. Trans fatty acids: effects on metabolic syndrome, heart disease and diabetes. Nat Rev Endocrinol 2009;5:335–44. [DOI] [PubMed] [Google Scholar]
- [30].Food Drug Administration. Final determination regarding partially hydrogenated oils. Fed Regist 2015:14883. [PubMed] [Google Scholar]
- [31].Institute of Medicine (U.S.). Panel on Macronutrients. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. Washington, D.C.: National Academies Press; 2005 [Google Scholar]
- [32].U.S. Department of Health and Human Services and U.S. Department of Agriculture. Dietary guidelines for Americans. 8th ed. Washington, DC: U.S. Government Printing Office; 2015-2020. [http://health.gov/dietaryguidelines/2015/guidelines; Accessed 25.11.16]. [Google Scholar]
- [33].Koh-Banerjee P, Chu NF, Spiegelman D, Rosner B, Colditz G, Willett W, et al. Prospective study of the association of changes in dietary intake, physical activity, alcohol consumption, and smoking with 9-y gain in waist circumference among 16 587 US men. Am J Clin Nutr 2003;78:719–27. [DOI] [PubMed] [Google Scholar]
- [34].Field AE, Willett WC, Lissner L, Colditz GA. Dietary fat and weight gain among women in the Nurses’ Health Study. Obesity 2007;15:967–76. [DOI] [PubMed] [Google Scholar]
- [35].Aronis KN, Khan SM, Mantzoros CS. Effects of trans fatty acids on glucose homeostasis: a meta-analysis of randomized, placebo-controlled clinical trials. Am J Clin Nutr 2012;96:1093–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Louheranta AM, Turpeinen AK, Vidgren HM, Schwab US, Uusitupa MI. A high-trans fatty acid diet and insulin sensitivity in young healthy women. Metabolism 1999;48:870–5. [DOI] [PubMed] [Google Scholar]
- [37].Lovejoy JC, Smith SR, Champagne CM, Most MM, Lefevre M, DeLany JP, et al. Effects of diets enriched in saturated (palmitic), monounsaturated (oleic), or trans (elaidic) fatty acids on insulin sensitivity and substrate oxidation in healthy adults. Diabetes Care 2002;25:1283–8. [DOI] [PubMed] [Google Scholar]
- [38].Christiansen E, Schnider S, Palmvig B, Tauber-Lassen E, Pedersen O. Intake of a diet high in trans monounsaturated fatty acids or saturated fatty acids. Effects on postprandial insulinemia and glycemia in obese patients with NIDDM. Diabetes Care 1997;20:881–7. [DOI] [PubMed] [Google Scholar]
- [39].Lefevre M, Lovejoy JC, Smith SR, Delany JP, Champagne C, Most MM, et al. Comparison of the acute response to meals enriched with cis- or trans-fatty acids on glucose and lipids in overweight individuals with differing FABP2 genotypes. Metabolism 2005;54:1652–8. [DOI] [PubMed] [Google Scholar]
- [40].Vega-Lopez S, Ausman LM, Jalbert SM, Erkkila AT, Lichtenstein AH. Palm and partially hydrogenated soybean oils adversely alter lipoprotein profiles compared with soybean and canola oils in moderately hyperlipidemic subjects. Am J Clin Nutr 2006;84:54–62. [DOI] [PubMed] [Google Scholar]
- [41].Mensink RP, de Louw MH, Katan MB. Effects of dietary trans fatty acids on blood pressure in normotensive subjects. Eur J Clin Nutr 1991;45:375–82. [PubMed] [Google Scholar]
- [42].Dyerberg J, Eskesen DC, Andersen PW, Astrup A, Buemann B, Christensen JH, et al. Effects of trans- and n-3 unsaturated fatty acids on cardiovascular risk markers in healthy males. An 8 weeks dietary intervention study. Eur J Clin Nutr 2004;58:1062–70. [DOI] [PubMed] [Google Scholar]
- [43].Sun Q, Ma J, Campos H, Hankinson SE, Manson JE, Stampfer MJ, et al. A prospective study of trans fatty acids in erythrocytes and risk of coronary heart disease. Circulation 2007;115:1858–65. [DOI] [PubMed] [Google Scholar]
- [44].Baylin A, Kim MK, Donovan-Palmer A, Siles X, Dougherty L, Tocco P, et al. Fasting whole blood as a biomarker of essential fatty acid intake in epidemiologic studies: comparison with adipose tissue and plasma. Am J Epidemiol 2005;162:373–81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


