Abstract
BACKGROUND AND PURPOSE:
Flow diverters with antithrombotic coatings are increasingly used to improve the safety of flow diverter treatments of intracranial aneurysms. This study aimed to investigate the safety and short-term efficacy of the new FRED X flow diverter.
MATERIALS AND METHODS:
Medical charts and procedural and imaging data of a consecutive series of patients with intracranial aneurysms who were treated with the FRED X at 9 international neurovascular centers were retrospectively analyzed.
RESULTS:
One hundred sixty-one patients (77.6% women; mean age, 55 years) with 184 aneurysms (11.2% acutely ruptured) were included in this study. Most aneurysms were located in the anterior circulation (77.0%), most frequently at the ICA (72.7%). The FRED X was successfully implanted in all procedures. Additional coiling was performed in 29.8%. In-stent balloon angioplasty was necessary in 2.5%. The rate of major adverse events was 3.1%. Thrombotic events occurred in 7 patients (4.3%) with 4 intra- and 4 postprocedural in-stent thromboses, respectively (1 patient had both peri- and postprocedural thrombosis). Of these thrombotic events, only 2 (1.2%) led to major adverse events (ischemic strokes). Postinterventional neurologic morbidity and mortality were observed in 1.9% and 1.2%, respectively. The rate of complete aneurysm occlusion after a mean follow-up of 7.0 months was 66.0%.
CONCLUSIONS:
The new FRED X is a safe and feasible device for aneurysm treatment. In this retrospective multicenter study, the rate of thrombotic complications was low, and the short-term occlusion rates are satisfactory.
The treatment of intracranial aneurysms with flow diverters (FDs) has emerged as an established treatment option for a considerable number of aneurysms.1-4 The functional principle of FDs is based on a dense mesh of stent struts, which diverts the flow within the target vessel past the aneurysm, eventually leading to the occlusion of the aneurysm. This relatively high metal coverage of the vessel wall, which is higher compared with conventional intracranial stents, can trigger thrombosis within the FD, which is a feared complication during and after FD treatments because it can lead to distal emboli and stent occlusion, eventually causing ischemic stroke.5,6 An emerging trend in the field of FD treatment of intracranial aneurysms is the use of FDs with specific antithrombotic coatings, which aim to reduce the risk of this potentially harmful complication.7,8 The Flow-Redirection Intraluminal Device (FRED; MicroVention) is one of the most frequently used FDs worldwide. Its safety and efficacy were demonstrated in numerous studies during the past years.2,9-11 After the publication of the FRED pivotal trial, it received FDA approval in the United States in 2020.12 The FRED X, which was introduced only recently, is a new version of the FRED. The novelty of this successor product is the X-technology, a specific antithrombotic surface treatment that is applied to the stent.
The aim of this multicenter study was to investigate the peri- and postprocedural safety and the short-term efficacy of the new FRED X FD for the treatment of ruptured and unruptured intracranial aneurysms. The participating centers received the FRED X as part of a limited market release.
MATERIALS AND METHODS
Study Design
The FRESH study - Treatment of Intracranial Aneurysms with the New FRED X Flow Diverter with Antithrombotic Surface Treatment Technology - is a retrospective, multicentric, observational study at 9 international high-volume neurovascular centers. A survey, which was specifically designed for this study, was completed by the physicians who performed the treatments with the FRED X. On the basis of these surveys, the clinical, radiologic, and procedural parameters of patients with ruptured and unruptured intracranial aneurysms who were treated with the FRED X between January 2020 and March 2022 were systematically analyzed. The observation period, including the assessment of postprocedural events and the degree of occlusion at the latest imaging, was from January 2020 until June 2022. A part of this patient cohort was also included in the FRED/FRED Jr/FRED X Intracranial Aneurysm Treatment Study (FRITS; not yet published, ClinicalTrials.gov Identifier: NCT03920358). The institutional ethics committees approved this study.
Patient Data
The patient data included age, sex, and the initial clinical presentation. The pre- and posttreatment clinical statuses of the patients were assessed using the mRS. For patients with an acutely ruptured aneurysm, the clinical status was assessed according to the Hunt and Hess (HH) scale.
Aneurysm Data
The assessed characteristics of the treated aneurysms included the location of the aneurysm, the aneurysm type (saccular, fusiform, blister-like, or dissecting), the size of the aneurysm (maximal diameter), and the diameter of its neck. The diameter of the parent vessel proximal and distal to the aneurysm was also assessed. Wide-neck aneurysms were defined as those with a neck diameter of ≥4 mm or aneurysms with a dome-to-neck ratio of <2.
FRED X: Device Characteristics
As mentioned in the beginning of the article, the novelty of the FRED X is a specific antithrombotic surface treatment. The X-technology, which is applied to the new FRED X device, is based on the material poly-2-methoxyethyl acrylate. The nanopolymer surface treatment is derived from the Xcoating surface treatment (Terumo), which has also been applied in other cardiovascular devices, such as oxygenators and arterial filter lines for >30 years.13,14 The surface treatment comprises an amphiphilic polymer with a hydrophobic part toward the device and a hydrophilic part toward the vessel lumen (and blood) or vessel wall, leading to a boundary layer adjacent to the stent struts, which aims to reduce protein denaturation and thus platelet adhesion. Apart from the X-technology surface treatment, the FRED X is identical to the FRED and FRED Jr with its specific dual-layer design, comprising a low-porosity inner mesh and a high-porosity outer stent. However, there was a change in the designation of the devices. For its precursor, the larger devices with a diameter of ≥3.5 mm, which featured a distal tip and had to be delivered with a 0.027-inch microcatheter, were named “FRED,” and the smaller devices with a diameter of ≤3.0 mm, which did not have a distal tip and could be delivered with a 0.021-inch microcatheter, were named “FRED Jr.” In contrast, all sizes of the new device are named FRED X, while the design of the larger (≥3.5 mm) and smaller (≤3.0) devices (distal tip, microcatheter compatibility) remained unchanged.
Procedural Parameters
The assessed procedural parameters included the peri-interventional antithrombotic medication, as well as treatment characteristics, such as the procedure duration, the number of implanted devices, additional coiling, and the need for in-stent balloon angioplasty.
Ease of deployment, vessel wall apposition, and radiopacity of the device were rated overall by the treating interventionalist for each treatment, using a 5-point scale (1, very poor; 2, poor; 3; intermediate; 4, good; 5, very good). Additionally, the general performance of the FRED X was compared with its precursor, FRED/FRED Jr, also using a 5-point scale (1, worse; 2, slightly worse; 3, equivalent; 4, slightly better; 5, better) for each treatment.
Periprocedural technical difficulties as well as peri- and postinterventional adverse events were assessed. The severity of adverse events was defined as described previously:15 A minor adverse event was defined as an event that resolved within 7 days without any clinical sequelae, while a major adverse event was defined as an ongoing clinical deficit at 7 days following the event. Clinical evaluation was performed before the procedure, immediately after the procedure, 24 (±6) hours after the procedure, at discharge, and at follow-up visits.
Follow-up
Clinical and imaging follow-up was performed according to the individual protocol of the respective centers. At the follow-up visits, the clinical condition of the patients was assessed, and imaging was performed. In follow-up imaging, the degree of in-stent stenosis was assessed and categorized as “not present,” “mild” (defined as ≤50% stenosis, compared with the immediate postinterventional diameter), “moderate” (50%–75%), “severe” (>75%), or “complete occlusion.”
Assessment of the Degree of Occlusion
The grade of aneurysm occlusion immediately after the procedure was reported according to the O’Kelly-Marotta (OKM) scale.16 Because invasive angiography was not available for all follow-ups, the grade of occlusion at the latest follow-up was reported according to the Raymond-Roy occlusion classification (RROC) for all patients.17 Adequate occlusion was defined as complete occlusion or residual neck (OKM C and D and RROC I and II).
RESULTS
One hundred sixty-one consecutive patients with 184 aneurysms treated with the FRED X device were included in this study. Patient and aneurysm characteristics are summarized in Table 1. Results subdivided into unruptured and ruptured aneurysms are summarized in the Online Supplemental Data.
Table 1:
Patient and aneurysm characteristicsa
| Characteristics | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Patient age (mean) (range) (yr) | 55 (SD, 12) (27–79) | ||||||||
| Clinical presentation | Incidental | Regrowth/persistent aneurysmb | SAH | Headache | Co-incidentalc | Diplopia | Other | ||
| 100 (62.1%) | 20 (12.4%) | 18 (11.2%) | 9 (5.6%) | 6 (3.7%) | 3 (1.9%) | 5 (3.1%) | |||
| Aneurysm location | ICA | BA | MCA | VA | Other | ||||
| 117 (72.7%) | 13 (8.1%) | 10 (6.2%) | 9 (5.6%) | 12 (7.5%) | |||||
| Aneurysm sized (mean) (range) (mm) | 7.8 (SD, 6.3) (1.0–46.0) | ||||||||
| Neck diameter (mean) (range) (mm) | 4.7 (SD, 3.8) (1.0–36.0) | ||||||||
| Dome-to-neck ratio (mean) (range) | 1.7 (SD, 0.9) (0.4–7.5) | ||||||||
| Diameter of the parent artery proximal and distal to the aneurysm mean) (range) (mm) | Proximal | Distal | |||||||
| 3.5 (SD, 0.8) (1.5–7.0) | 3.1 (SD, 0.7) (1.2–5.2) | ||||||||
| Aneurysm type | Saccular | Blister-like | Fusiform | Dissecting | |||||
| 131 (81.4%) | 14 (8.7%) | 11 (6.8%) | 5 (3.1%) | ||||||
| Sidewall or bifurcation aneurysm | Sidewall | Bifurcation | |||||||
| 136 (84.5%) | 25 (15.5%) | ||||||||
Data are as mean (minimum–maximum) or absolute number of cases (relative frequency in %).
Persistent aneurysm after previous treatment.
Aneurysm diagnosed because of a ruptured intracranial aneurysm elsewhere.
Maximal diameter.
Patient Characteristics
The mean age of the patients was 55.1 years, and 77.6% were women. The median pretreatment mRS was 0 (first quartile; third quartile, 0; 0) (mRS 0 in 126 patients [78.3%], mRS 1 in 24 [14.9%], and mRS 4 in 1 [0.6%]). The patient with mRS 4 presented with a subacute brainstem infarction caused by a large, fusiform, unruptured aneurysm of the basilar artery (BA). Of the 18 patients (11.2%) who presented with an acutely ruptured aneurysm, the HH scale was 1 (first quartile; third quartile, 1; 2.5) (HH 1 in 8 patients [53.3%], HH 2 in 3 patients [20.0%], HH 3 in 2 patients [13.3%]. and HH 5 in 2 patients [13.3%]). In 100 patients (62.1%), the aneurysm was incidental and asymptomatic.
Aneurysm Characteristics
Most patients (89.4%) had only 1 aneurysm treated with the FRED X, while in 8.1%, 2 aneurysms, and in 1.2%, 3 aneurysms were treated. The most frequent aneurysm location was the ICA (72.7%), followed by the BA (8.1%) and the MCA (6.2%). The mean aneurysm size was 7.8 mm, ranging from 1-mm blister-like to 46-mm giant aneurysms. Most aneurysms had a wide neck (90.1%), a saccular shape (81.4%), and were sidewall aneurysms (84.5%).
Antiplatelet Therapy
Pre-, intra-, and postprocedural platelet inhibition and testing of the thrombocyte aggregation response were managed according to local standards of the respective institutions. Platelet reactivity testing was performed in 91.9% of the patients. The most common antiplatelet medications were acetylsalicylic acid (ASA) plus clopidogrel (62.4%), ASA plus prasugrel (21.0%), and ASA plus ticagrelor (6.4%). Patients with acute aneurysmal SAH received periprocedural tirofiban, followed by double-antiplatelet therapy after the treatment.
Treatment
The treatment characteristics are summarized in Table 2 and the Online Supplemental Data. Two example cases are illustrated in Figs 1 and 2.
Table 2:
Treatment parametersa
| Parameters | |||||
|---|---|---|---|---|---|
| Aneurysms treated in the respective treatment session | 1 | 2 | 3 | 4 | |
| 144 (89.4%) | 13 (8.1%) | 2 (1.2%) | 2 (1.2%) | ||
| Ease of deploymentb | Very poor | Poor | Intermediate | Good | Very good |
| 0 (0%) | 1 (0.6%) | 1 (0.6%) | 12 (7.5%) | 147 (91.3%) | |
| Vessel wall appositionb | Very poor | Poor | Intermediate | Good | Very good |
| 0 (0%) | 0 (0%) | 2 (1.2%) | 8 (5.0%) | 151 (93.8%) | |
| Radiopacityb | Very poor | Poor | Intermediate | Good | Very good |
| 0 (0%) | 0 (0%) | 0 (0%) | 59 (36.7%) | 102 (63.4%) | |
| Performance compared with FRED/FRED Jrb | Worse | Slightly worse | Equivalent | Slightly better | Better |
| 0 (0%) | 0 (0%) | 154 (96.3%) | 6 (3.8%) | 0 (0%) | |
| Additional coiling | 48 (29.8%) | ||||
| In-stent balloon angioplasty | 4 (2.5%) | ||||
Data are absolute number of cases (relative frequency in %).
Rated as an overall score for each treatment (not for each implanted FD).
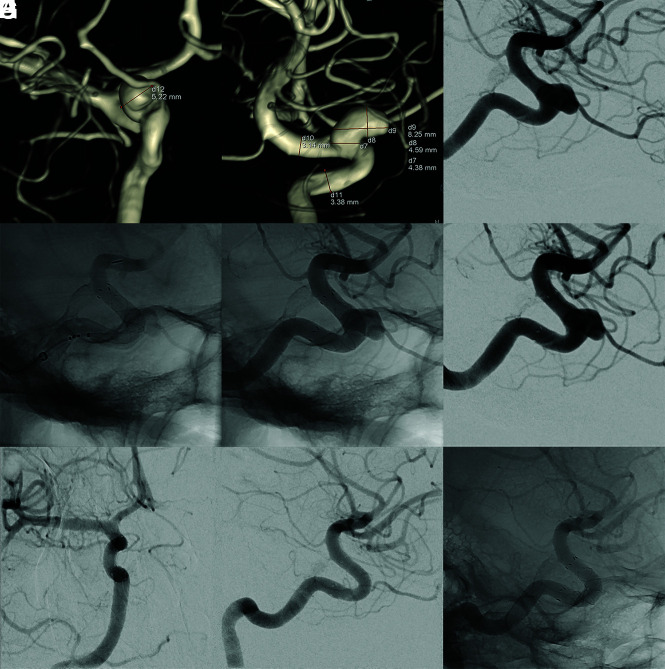
FIG 1.
Treatment of an incidental aneurysm of the ICA with the FRED X. The preinterventional angiography including 3D reconstructions (A–C) showed a saccular sidewall aneurysm of the right ICA in an asymptomatic patient. A FRED X FD was placed in the parent artery, covering the aneurysm neck (D–F). On the angiography 13 months after the treatment (G–I), the aneurysm was completely occluded.
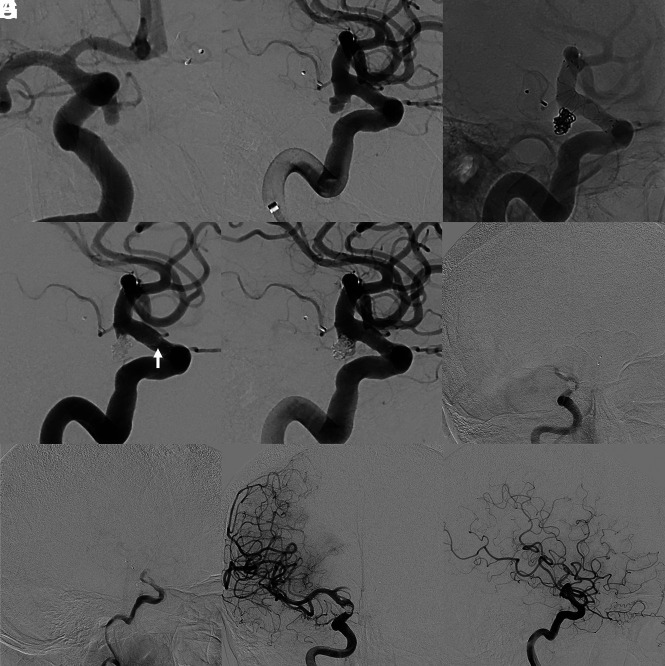
FIG 2.
Treatment of an incidental PcomA aneurysm with the FRED X and coiling with intra- and postprocedural thrombosis. This patient, who had an incidental BA aneurysm treated with a Woven EndoBridge device (WEB; MicroVention) previously, presented with an incidental, irregularly shaped aneurysm of the ICA adjacent to the PcomA (A and B). After an uneventful treatment with FRED X implantation and coiling with a jailing technique (C), intraprocedural thrombus formation at the proximal part of the flow-diverting section of the stent was observed (white arrow in D). Tirofiban was instantly administered for 30 minutes, which led to a complete resolution of the thrombosis (E). Seven days after the treatment, after discontinuation of her ticagrelor medication, the patient presented with complete occlusion of the FD (F and G) with good collateralization via the contralateral side. Recanalization of the stent was achieved by tirofiban administration for 48 hours (H and I). The patient was neurologically asymptomatic the entire time.
One hundred sixty-seven FRED X devices were implanted in 161 treatment sessions (1 FD was implanted in 155 treatments, and 2 FDs in 6 treatments) with a mean duration of 80.2 [SD, 47.5] minutes. A FRED X FD could be successfully implanted as planned in all treatments. Recapturing or repositioning of the device was required in 14.3% of the treatments. Additional coiling of the aneurysm was performed in 29.8%. The reasons for additional coiling were large aneurysm size, irregular aneurysm shape, and aneurysmal hemorrhage. In-stent balloon angioplasty to improve the vessel wall apposition was performed in 2.5%.
The ease of deployment was rated “good” or “very good” in 98.8%. It was rated “poor” in 1 case (0.6%) in which the stent kinked at a sharp curve in the vessel. Vessel wall apposition was rated good or very good in 98.8%. Radiopacity was rated good or very good in all cases (100%). When we compared the FRED X with its precursor, it was rated “equivalent” in 96.3% and “slightly better” in 3.8%.
Adverse Events and Complications
Asymptomatic and symptomatic adverse events are presented in detail in the Online Supplemental Data and summarized in Table 3.
Table 3:
Adverse events and complicationsa
| Adverse events/complications | |||||
|---|---|---|---|---|---|
| Adverse event | Technical periprocedural adverse event | Minor adverse event | Major adverse event | Neurological morbidity | Mortality |
| 5 (3.1%) | 21 (13.0%) | 5 (3.1%) | 3 (1.9%) | 2 (1.2%) | |
Data are absolute number of cases (relative frequency in %).
Intraprocedural technical adverse events occurred in 5 treatments (3.1%) and consisted of 2 cases of inadvertent stent shortening, as well as stent kinking, insufficient stent opening, and coil migration in 1 case, respectively. All of these adverse events were asymptomatic.
Most (95.8%) of recorded symptomatic adverse events occurred postinterventionally. Minor adverse events occurred in 13.0% of the patients. Nine of 21 of these events were neurologic, resulting in a minor neurologic adverse event rate of 5.6%. Major adverse events (2 ischemic strokes, 1 intracerebral hemorrhage, 1 mass effect of the aneurysm, and 1 case of vasospasms caused by a preinterventional aneurysmal SAH) were observed in 5 patients (3.1%), leading to neurologic morbidity in 3 (1.9%) and death in 2 patients (1.2%). All these major adverse events were neurologic complications. One of the major adverse events was a major ischemic stroke, which occurred 2 weeks after an uneventful treatment of an unruptured posterior communicating artery (PcomA) aneurysm, due to severe in-stent thrombosis, leading to hemodynamic cerebral infarctions. This patient had a good response to ASA and clopidogrel in the reactivity testing before treatment. Treatment in this case was performed with tirofiban, which led to a resolution of the thrombus, followed by oral medication with ASA in combination with prasugrel. The patient was discharged with mRS 3 and recovered to mRS 2 at 6 months after treatment. The second patient who had neurologic morbidity presented with a large BA aneurysm causing brainstem infarctions, which increased after the FD treatment (worsening of pre-existing stroke), without any evidence of thrombus formation. Of the patients who died, 1 patient with an enlarging giant BA aneurysm died due to increasing mass effect despite the treatment. The second case of mortality occurred in a patient with aneurysmal SAH, who developed an intracerebral hemorrhage located adjacent to the external ventricular drainage. Consequently, tirofiban had to be stopped, which led to occlusion of the stent in the ICA, eventually leading to cerebral infarction and death.
Postinterventional visual disturbances occurred in 5 patients (3.1%). In all of these patients, an aneurysm of the ICA was treated with an FD, which covered the ophthalmic artery.
A total number of 8 thrombotic events was reported in 7 patients (4.3%). In 4 of these, slight in-stent thrombosis was observed during treatment, which was immediately treated with tirofiban, leading to complete resolution of the thrombus in all cases without any clinical sequelae. Postinterventional in-stent thrombosis occurred in 1 of the 4 patients with intraprocedural thrombosis (despite complete intraprocedural thrombus resolution after tirofiban administration and after discontinuation of ticagrelor intake by the patient) and in 3 further patients with an uneventful treatment procedure. One of these patients developed an asymptomatic stent occlusion. Another stent thrombosis occurred after stopping tirofiban due to an intracerebral hemorrhage as described above. Apart from this patient, only 1 thrombotic event resulted in a major adverse event: the case of a major stroke that is described in more detail above.
Clinical Follow-up
Two patients died during the follow-up (listed under adverse events and complications), resulting in a mortality rate of 1.2%.The mean mRS at the latest follow-up, which was available for 133 patients, was 0.4 (SD, 1.0) (mRS 0 in 102 patients [76.7%], mRS 1 in 18 [13.5%], mRS 2 in 8 [6.0%], mRS 3 in 4 [3.0%], and mRS 6 in 2 [1.5%]). Deterioration of the mRS (compared with the preinterventional scale) was observed in 11 patients; 4 deteriorations were related to the procedure or the underlying disease (see adverse events and complications). For the remaining patients, mRS deterioration was caused by non-neurologic conditions.
Imaging Follow-up
Imaging follow-up was available for 145 of the 161 patients (90.1%), with a mean imaging follow-up period of 7.0 (SD, 4.3) months. The patients without follow-up imaging refused further imaging, were lost to follow-up, or died.
The imaging technique was invasive conventional angiography and MR imaging in 22.1%, conventional angiography alone in 14.5%, MR imaging and flat panel CT in 28.3%, MR imaging only in 30.3%, and flat panel CT only in 4.8%.
During follow-up, in-stent stenosis or stent occlusion was observed in 17 patients (10.6%). Most of these cases (14/17, 82.4%) were only mild stenoses. There were 2 cases of moderate in-stent stenosis and 1 case of complete stent occlusion. Of all these cases, only 1 patient was symptomatic: the above-mentioned patient with major stroke due to in-stent thrombosis. The moderate in-stent stenosis persisted in the latest follow-up imaging.
Aneurysm Occlusion
The aneurysm occlusion rates are summarized in Table 4. The immediate postinterventional occlusion rates were as follows: OKM A1 in 19.9%, A2 in 23.0%, A3 in 20.5%, B1 in 2.5%, B2 in 5.6%, B3 in 13.7%, C1 in 1.2%, C2 in 5.0%, C3 in 4.4%, and D in 4.4%.
Table 4:
Occlusion ratesa
| Occlusion rates | |||
|---|---|---|---|
| Occlusion at latest follow-upb | I: Complete occlusion | II: Residual neck | III: Residual aneurysm |
| 94 (66.2%) | 24 (16.9%) | 24 (16.9%) | |
Data are absolute number of cases (relative frequency in %).
Imaging follow-up was available for 142/161 patients with a mean follow-up period of 7.0 months, reported according to the RROC.
At the latest follow-up, aneurysm occlusion rates were as follows: RROC I in 66.2%, RROC II in 16.9% and RROC III in 16.9%, resulting in a rate of adequate aneurysm occlusion of 83.1%.
DISCUSSION
FDs have increasingly become a treatment option for cerebral aneurysms with the drawback of a higher metal density being potentially more thrombogenic than conventional stents. In this multicenter cohort, the first study reporting on the new FRED X with antithrombotic surface treatment, the FD showed a satisfactory safety profile and proved to be effective judged by short-term aneurysm occlusion.
As explained, the only modification of the FRED X toward its precursor is the new antithrombotic surface treatment. In this study, thrombotic complications occurred in 7 of 161 patients, resulting in a thrombotic complication rate of 4.3%, of which only 2 led to a major adverse event (one occurring after a necessary discontinuation of antiplatelet therapy because of an intracerebral hemorrhage). This low but still considerable number of thrombotic events indicates that even when using devices with antithrombotic coatings, high awareness is still mandatory for both peri- and postprocedural thromboses. In recent studies on the FRED and FRED Jr, the rate of thrombotic complications was slightly higher. In the Safety and Efficacy Analysis of FRED Embolic Device in Aneurysm Treatment (SAFE) study, the pivotal study for the FRED in France (published in 2019),18 thromboembolic complications occurred in 7/103 patients (6.8%). In the US pivotal trial (2022), device thrombosis was reported in 12/145 patients (8.3%).19 These thrombotic events rates are also in line with reported data for other FDs, such as the Pipeline Embolization Device (PED; Medtronic). A meta-analysis, comprising data of 1110 patients, focusing on thrombotic complications after PED treatment reported an overall rate of 7.0% thrombotic events.20 The new antithrombotic surface treatment might serve as an explanation for the slightly lower thrombotic complication rate in this study. However, the FRESH study was based on a retrospective, self-adjudicated analysis, the cohort in our study was heterogeneous, and there was no control group consisting of patients treated with the precursor devices, preventing a direct comparison of these studies and the devices regarding thrombogenicity. Prospective trials are warranted to further assess the safety of the FRED X and its potential to reduce the rate of thrombotic complications compared with conventional FDs.
The nonthrombotic complications in this study (minor adverse events in 13.0% and major adverse events in 3.1%) are similar to those reported in previous studies for the FRED and FRED Jr. In the largest study reporting on the FRED, the European Flow-Redirection Intraluminal Device Study, comprising data of 531 patients, the rate of complications and adverse events was 14%.9 In the above-mentioned US pivotal trial, the composite primary safety end point of major stroke or death within 30 days or major ipsilateral stroke or neurologic death after 30 days was met by 6.2% of the patients.
Because the FRED X is technically identical to the FRED and FRED Jr, despite the antithrombotic surface treatment, it is consistent that the occlusion rates in this study are similar to those reported for its precursor. The rate of complete occlusion at a mean follow-up of 7.0 months was 66.2% in our cohort. In the European Flow-Redirection Intraluminal Device Study, the complete occlusion rate was 82.5% at 6 months and 91.3% at 1 year. For the above-mentioned pivotal trials, the complete occlusion rate was 61.1% at 6 months and 73.3% at 1 year for the French study18 and 62.9% at 1 year for the US study.19 Occlusion rates of studies reporting on the PED are also in line with those of our study. A recent work reported a 77.9% complete occlusion rate for the new Pipeline Vantage,21 while in the Pipeline for Uncoilable or Failed Aneurysms (PUFs) trial, the early rate of complete occlusion was 73.6% at 180 days,22 reaching 95.2% at 5 years.23 The relatively high rate of additional coiling in our study (30.0%) must be considered when comparing our results with those in other studies. Future studies with long-term follow-up are needed to further assess the efficacy of the FRED X.
We acknowledge that this work has several limitations. This study was performed retrospectively and was based on a survey that was completed by the treating physicians, without data or imaging analysis by an independent core lab, indicating an inherent selection and reporting bias. Despite the relatively high number of patients in this study, the data are quite heterogeneous (eg, different aneurysm locations, high rate of additional coiling). However, the use of the FRED X in this study reflects a real-world experience, not being limited to a certain aneurysm location or rupture status. The treatments in this study were performed in the context of a limited market release to a few high-volume neurovascular centers that were selected by the manufacturer. The results may vary when the device is available freely to other centers. Furthermore, the rate of patients with follow-up conventional angiography was only 36.6%, and the follow-up period in this study was relatively short, due to the novelty of the device.
Conclusions
The FRED X is a safe and feasible FD for treatment of ruptured and unruptured intracranial aneurysms. With its new antithrombotic surface treatment, the rate of thrombotic complications was relatively low, and the short-term efficacy is promising.
ABBREVIATIONS:
- ASA
acetylsalicylic acid
- BA
basilar artery
- FD
flow diverter
- HH
Hunt and Hess
- OKM
O’Kelly-Marotta
- PcomA
posterior communicating artery
- RROC
Raymond-Roy occlusion classification
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
references
- 1.Lv X, Yang H, Liu P, et al. Flow-diverter devices in the treatment of intracranial aneurysms: a meta-analysis and systematic review. Neuroradiol J 2016;29:66–71 10.1177/1971400915621321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mohlenbruch MA, Kizilkilic O, Killer-Oberpfalzer M, et al. Multicenter experience with FRED Jr Flow Re-Direction Endoluminal Device for intracranial aneurysms in small arteries. AJNR Am J Neuroradiol 2017;38:1959–65 10.3174/ajnr.A5332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kallmes DF, Brinjikji W, Boccardi E, et al. Aneurysm Study of Pipeline in an Observational Registry (ASPIRe). Interv Neurol 2016;5:89–99 10.1159/000446503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brinjikji W, Murad MH, Lanzino G, et al. Endovascular treatment of intracranial aneurysms with flow diverters: a meta-analysis. Stroke 2013;44:442–47 10.1161/STROKEAHA.112.678151 [DOI] [PubMed] [Google Scholar]
- 5.Fiehler J, Ries T. Prevention and treatment of thromboembolism during endovascular aneurysm therapy. Klin Neuroradiol 2009;19:73–81 10.1007/s00062-009-8029-9 [DOI] [PubMed] [Google Scholar]
- 6.Ihn YK, Shin SH, Baik SK, et al. Complications of endovascular treatment for intracranial aneurysms: management and prevention. Interv Neuroradiol 2018;24:237–45 10.1177/1591019918758493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marosfoi M, Clarencon F, Langan ET, et al. Acute thrombus formation on phosphorilcholine surface modified flow diverters. J Neurointerv Surg 2018;10:406–11 10.1136/neurintsurg-2017-013175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.King RM, Langan ET, Ughi GJ, et al. Acute thrombus burden on coated flow diverters assessed by high frequency optical coherence tomography. Cardiovasc Intervent Radiol 2020;43:1218–23 10.1007/s00270-020-02482-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Killer-Oberpfalzer M, Kocer N, Griessenauer CJ, et al. European multicenter study for the evaluation of a dual-layer flow-diverting stent for treatment of wide-neck intracranial aneurysms: the European Flow-Redirection Intraluminal Device Study. AJNR Am J Neuroradiol 2018;39:841–47 10.3174/ajnr.A5592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mohlenbruch MA, Herweh C, Jestaedt L, et al. The FRED flow-diverter stent for intracranial aneurysms: clinical study to assess safety and efficacy. AJNR Am J Neuroradiol 2015;36:1155–61 10.3174/ajnr.A4251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pierot L, Spelle L, Berge J, et al. SAFE study (Safety and efficacy Analysis of FRED Embolic device in aneurysm treatment): 1-year clinical and anatomical results. J Neurointerv Surg 2019;11:184–89 10.1136/neurintsurg-2018-014261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McDougall CG, Diaz O, Boulos A, et al. Safety and efficacy results of the Flow Redirection Endoluminal Device (FRED) stent system in the treatment of intracranial aneurysms: US pivotal trial. J Neurointerv Surg 2022;14:577–84 10.1136/neurintsurg-2021-017469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gunaydin S, Farsak B, Kocakulak M, et al. Clinical performance and biocompatibility of poly(2-methoxyethylacrylate)-coated extracorporeal circuits. Ann Thorac Surg 2002;74:819–24 10.1016/s0003-4975(02)03796-7 [DOI] [PubMed] [Google Scholar]
- 14.Nutter BT, Stammers AH, Schmer RG, et al. The rheological effects of X-Coating with albumin and hetastarch on blood during cardiopulmonary bypass. J Extra Corpor Technol 2004;36:36–43 [PubMed] [Google Scholar]
- 15.Kallmes DF, Hanel R, Lopes D, et al. International retrospective study of the Pipeline embolization device: a multicenter aneurysm treatment study. AJNR Am J Neuroradiol 2015;36:108–15 10.3174/ajnr.A4111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O'Kelly CJ, Krings T, Fiorella D, et al. A novel grading scale for the angiographic assessment of intracranial aneurysms treated using flow diverting stents. Interv Neuroradiol 2010;16:133–37 10.1177/159101991001600204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mascitelli JR, Moyle H, Oermann EK, et al. An update to the Raymond-Roy occlusion classification of intracranial aneurysms treated with coil embolization. J Neurointerv Surg 2015;7:496–502 10.1136/neurintsurg-2014-011258 [DOI] [PubMed] [Google Scholar]
- 18.Pierot L, Spelle L, Berge J, et al. SAFE study (Safety and efficacy Analysis of FRED Embolic device in aneurysm treatment): 1-year clinical and anatomical results. J Neurointerv Surg 2019;11:184–189 10.1136/neurintsurg-2018-014261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McDougall CG, Diaz O, Boulos A, et al. Safety and efficacy results of the Flow Redirection Endoluminal Device (FRED) stent system in the treatment of intracranial aneurysms: US pivotal trial. J Neurointerv Surg 2022;14:577–584 10.1136/neurintsurg-2021-017469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Skukalek SL, Winkler AM, Kang J, et al. Effect of antiplatelet therapy and platelet function testing on hemorrhagic and thrombotic complications in patients with cerebral aneurysms treated with the Pipeline embolization device: a review and meta-analysis. J Neurointerv Surg 2016;8:58–65 10.1136/neurintsurg-2014-011145 [DOI] [PubMed] [Google Scholar]
- 21.Vollherbst DF, Cekirge HS, Saatci I, et al. First clinical multicenter experience with the new Pipeline Vantage flow diverter. J Neurointerv Surg 2023;15:63–69 10.1136/neurintsurg-2021-018480 [DOI] [PubMed] [Google Scholar]
- 22.Becske T, Kallmes DF, Saatci I, et al. Pipeline for uncoilable or failed aneurysms: results from a multicenter clinical trial. Radiology 2013;267:858–68 10.1148/radiol.13120099 [DOI] [PubMed] [Google Scholar]
- 23.Becske T, Brinjikji W, Potts MB, et al. Long-term clinical and angiographic outcomes following Pipeline Embolization Device treatment of complex internal carotid artery aneurysms: five-year results of the Pipeline for Uncoilable or Failed Aneurysms Trial. Neurosurgery 2017;80:40–48 10.1093/neuros/nyw014 [DOI] [PubMed] [Google Scholar]