Abstract
Introduction
Epileptogenesis has been considered one of the most prevalent diseases affecting significant numbers of individuals worldwide. Since vitamin B12 has been reported to possess antiepileptic effects, this supports that vitamin B12 deficiency is correlated to seizure occurrence. Hence, this study aimed to evaluate the neuroprotective effects of vitamin B12 injection on pentylenetetrazole (PTZ)-induced rats.
Methods
The study was performed using 40 adult female Sprague-Dawley rats (~250 g). A 45 mg/kg PTZ was intraperitoneally injected into rat models to induce seizure effects. Different groups of rat models received methyl vitamin B12 therapy at different dosages, a low dosage of 45 µg/kg and a high dosage of 85 µg/kg, at different pre-treatment periods, one day and two weeks prior to PTZ injection. A control group, which received only PTZ injection, served as a reference. The seizure latency, seizure intensity, and differences in the quality of seizures and their characteristics, from simple twitches to complete seizures, were observed after 30 minutes of PTZ injection.
Results
In general, the latency to convulsion significantly increased when vitamin B12 pre-treatment was employed. The longest latency time (LT) of 520.63±73.83 seconds was observed when a high dosage of vitamin B12 at 85 µg/kg was injected one day prior to PTZ inoculation, which was significantly higher than that of the control group at 176.88±62.67 seconds (P<0.001). Moreover, the duration of convulsion significantly decreased in which the lowest duration time (DT) of 7.00±4.68 seconds was observed when a high dosage of vitamin B12 at 85 µg/kg was injected two weeks prior to PTZ inoculation, which was significantly lower than that of the control group at 257.75±41.93 seconds (P<0.001). Lastly, the percentage of the population with PTZ-induced convulsion generally decreased after vitamin B12 pre-treatment in which majority showed more of simple less aggressive twitches rather than tonic-clonic seizures.
Conclusion
The results showed that vitamin B12 pre-treatment alleviates the seizure occurrence among PTZ-kindled rat models. These findings then suggest that vitamin B12 is a potential strategy and treatment for epilepsy and other related epileptogenesis activities.
Keywords: antiepileptic, seizure, vitamin b12, epilepsy, epileptogenesis
Introduction
Epileptogenesis is defined as molecular and cellular changes that consequently result in neuroinflammation, hypersynchrony, and hyperexcitability of cortical neurons [1,2]. Epilepsy is a chronic neurological state characterized by repetitive and unprovoked seizures [1,3-5]. Nearly 50 million people or 2% of the world population were diagnosed with epilepsy [3,5,6]. The prevalence rate of active epilepsy in most locations ranges from five to 10 cases per 1,000 population, as reported by Sander (2003) [4]. This disease can occur at all ages, and no constant age-specific incidence rate was observed; however, it was noted that this disease was less observed in younger ages compared to older age groups [4].
Since as early as the 1850s, epilepsy treatment involved different plant-based and animal-based extracts [7]. Nowadays, antiepileptic drugs (AEDs) have been extensively used as the major treatment for epilepsy; however, approximately 30%-40% of patients are resistant to these drugs [8-11]. Epilepsy is said to be drug-resistant when two AEDs given in appropriate doses fail to control seizure [5]. The ineffectiveness of AEDs and their possible side effects might contribute to some risks to the patients including neuronal damage. Although the effective drugs that can inhibit epileptogenesis are yet to be discovered, minimizing inflammatory reactions in the brain neurons can be a reasonable potential strategy against epilepsy [2]. Vitamins act as antioxidants and consequently can inhibit neuronal damage in the brain. Vitamin B12, also known as cobalamin, plays an important role in the formation of myelin sheaths in the central nervous system [12,13]. It is important in the methylation processes related to DNA and cell metabolism in which a deficiency may cause serious clinical impacts [14]. As an antioxidative and anti-inflammatory agent, vitamin B12 was observed to lessen oxidative damage and neuroinflammation, hence preventing epileptogenesis. As a matter of fact, vitamin B12 deficiency may result in a wide range of neurological, psychiatric, and hematologic consequences, including epileptic seizures [15]. Moreover, there are many studies, Erfanparast and Tamaddonfard (2015) [16] and Ikeda et al. (1997) [17], linking the antiepileptic effects of vitamin B12 to the enhancement of gamma-aminobutyric acid (GABA) activity.
In this study, the neuroprotective effect of vitamin B12 injection to pentylenetetrazol (PTZ)-induced seizure among rats has been evaluated.
Materials and methods
Adult female Sprague-Dawley rats were used. The rats were housed in cages on sawdust and had access to food and water ad libitum. Animal care and experimental ethics were applied according to the guidelines of the research and animal ethics committee of Arabian Gulf University for the use of animals in experiments, under the reference number E015-PI-10/17. Vitamin B12 and PTZ were purchased from Sigma-Aldrich Co., St. Louis, MO, USA. The solutions were prepared on each day of the experiment.
Forty adult female Sprague-Dawley rats with an approximate weight of 250 g were used. Samples were divided into five groups comprising eight rats each. Seizure was induced via intraperitoneal injection of 45 mg/kg pentylenetetrazole (PTZ). Group 1, which received only PTZ injection, served as the control group. Groups 2 and 3 received methyl vitamin B12 therapy at a low dosage of 45 µg/kg and a high dosage of 85 µg/kg, respectively, one day prior to PTZ injection. While Groups 4 and 5 received methyl vitamin B12 therapy at a low dosage of 45 µg/kg and a high dosage of 85 µg/kg, respectively, two weeks prior to PTZ injection. The seizure latency, seizure intensity, and differences in the quality of seizures and their characteristics, from simple twitches to complete seizures, were observed after 30 minutes of PTZ injection. Video monitoring of animals was employed to ensure accurate time measurement and behavioral performance. All data were presented as mean±standard error of the mean (SEM) and analyzed using a one-tailed Student’s t-test. P values of less than 0.05 were considered statistically significant. The data analyses were performed with Statistical Package for Social Sciences (SPSS) version 23 (IBM SPSS Statistics, Armonk, NY, USA).
Results
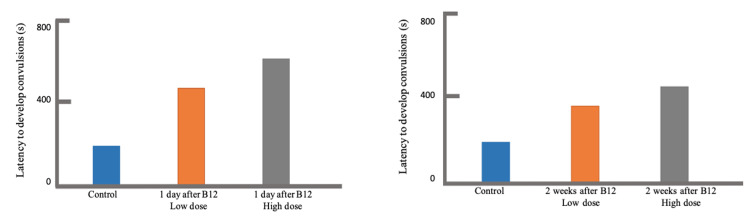
The effects of vitamin B12 in the development of PTZ-induced seizure were demonstrated in terms of latency to convulsion (Table 1) and the duration of the convulsion (Table 2). The control group, which did not receive vitamin B12 treatment, had a latency mean±SEM of 176.88±62.67 seconds. In general, the latency to convulsion significantly increased when vitamin B12 was injected into the rat specimens. The longest latency time (LT) of 520.63±73.83 seconds was observed when a high dosage of vitamin B12 at 85 µg/kg was injected one day prior to PTZ inoculation (Group 3: N=8; P<0.001), while the shortest LT of 356.13±77.22 seconds was seen when a low dosage of vitamin B12 at 45 µg/kg was injected two weeks prior to PTZ inoculation (Group 4: N=8; P=0.047), which were still both significantly higher than that of the control group. From these, the highest obtained ΔLT was 421.00 seconds, while the lowest was 179.25 seconds. As shown in Figure 1, it was observed that the latency increases, in comparison to the control, as the dosage of vitamin B12 further increases from 45 µg/kg to 85 µg/kg (Group 2 versus Group 3; Group 4 versus Group 5). In addition, latency decreases as the time gap between vitamin B12 and PTZ injection widens from one day to two weeks (Group 2 versus Group 4; Group 3 versus Group 5).
Table 1. Comparison of latency to convulsions after PTZ administration.
SEM, standard error of the mean; PTZ, pentylenetetrazole
| Specimen number | Group 1 | Group 2 | Group 3 | Group 4 | Group 5 |
| 1 | 177 | 600 | 317 | 584 | 181 |
| 2 | 67 | 600 | 600 | 120 | 1,137 |
| 3 | 147 | 560 | 587 | 163 | 244 |
| 4 | 60 | 5 | 369 | 153 | 600 |
| 5 | 82 | 600 | 1,110 | 548 | 600 |
| 6 | 104 | 600 | 600 | 500 | 103 |
| 7 | 600 | 600 | 600 | 181 | 175 |
| 8 | 178 | 600 | 600 | 600 | 600 |
| Mean, seconds | 176.88 | 520.63 | 597.88 | 356.13 | 455.00 |
| SEM, seconds | 62.67 | 73.83 | 84.04 | 77.22 | 123.21 |
| P value | --- | 0.002 | <0.001 | 0.047 | 0.032 |
Table 2. Comparison of the duration of the PTZ-induced convulsion.
SEM, standard error of the mean; PTZ, pentylenetetrazole
| Specimen number | Group 1 | Group 2 | Group 3 | Group 4 | Group 5 |
| 1 | 261 | 79 | 5 | 12 | 7 |
| 2 | 300 | 223 | 23 | 30 | 1 |
| 3 | 304 | 413 | 38 | 0 | 6 |
| 4 | 400 | 45 | 88 | 68 | 0 |
| 5 | 97 | 74 | 2 | 122 | 0 |
| 6 | 100 | 0 | 0 | 34 | 39 |
| 7 | 200 | 0 | 44 | 569 | 3 |
| 8 | 400 | 81 | 0 | 0 | 0 |
| Mean, seconds | 257.75 | 114.38 | 25.00 | 104.38 | 7.00 |
| SEM, seconds | 41.93 | 49.27 | 10.92 | 67.92 | 4.68 |
| P value | --- | 0.022 | <0.001 | 0.038 | <0.001 |
Figure 1. Comparison of latency to convulsion after vitamin B12 pre-treatment, one day (left) and two weeks (right), prior to PTZ injection.
PTZ: pentylenetetrazole
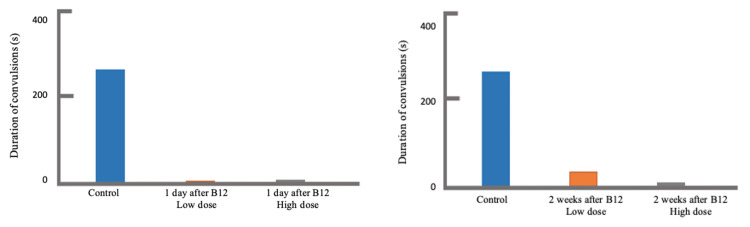
Moreover, the control group had a convulsion duration mean±SEM of 257.75±41.93 seconds. In general, the duration of convulsion significantly decreased when vitamin B12 was injected to the rat specimens. The lowest duration time (DT) of 7.00±4.68 seconds was observed when a high dosage of vitamin B12 at 85 µg/kg was injected two weeks prior to PTZ inoculation (Group 5: N=8; P<0.001), while the highest DT of 114.38±49.27 seconds was seen when a low dosage of vitamin B12 at 45 µg/kg was injected one day prior to PTZ inoculation (Group 2: N=8; P=0.022), which were still both significantly lower than that of the control group. From these, the highest obtained ΔDT was 250.75 seconds, while the lowest was 143.37 seconds. As presented in Figure 2, a shorter duration was observed as the dosage of vitamin B12 was increased from 45 µg/kg to 85 µg/kg (Group 2 versus Group 3; Group 4 versus Group 5). Consequently, the duration further shortens as the time gap between vitamin B12 and PTZ injection widens from one day to two weeks (Group 2 versus Group 4; Group 3 versus Group 5).
Figure 2. Comparison of convulsion duration after vitamin B12 pre-treatment, one day (left) and two weeks (right), prior to PTZ injection.
PTZ: pentylenetetrazole
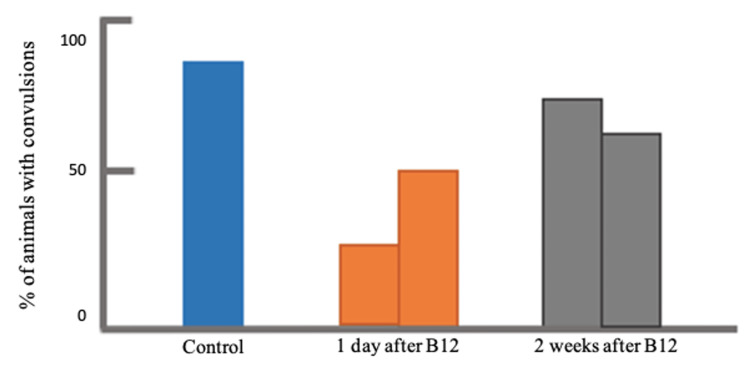
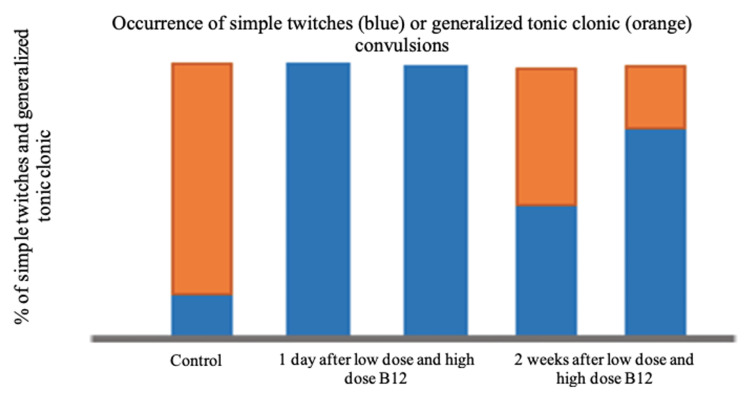
The percentage of the population that developed the incidence of PTZ-induced convulsion was also evaluated. As shown in Figure 3, the lowest percentage was observed in the group that received a low dosage of vitamin B12 at 45 µg/kg, injected one day prior to PTZ inoculation. Although there was no general trend in terms of the effect of dosage and the time of vitamin injection, the percentage of the rats that developed convulsion generally decreased after vitamin B12 pre-treatment. Furthermore, the type of convulsion was also determined and was presented in Figure 4, either simple twitches (marked in blue) or tonic-clonic (marked in orange). The results revealed that majority of the specimens in the control group experienced a tonic-clonic convulsion rather than simple twitches. On the other hand, all samples under those groups that underwent vitamin B12 pre-treatment, regardless of dosage, one day prior to PTZ injection experienced simple less aggressive twitches, which are twitches of less duration and violent contractions, while the majority of samples under those groups that underwent vitamin B12 pre-treatment, regardless of dosage, two weeks prior to PTZ injection showed more of simple less aggressive twitches rather than tonic-clonic convulsions.
Figure 3. Comparison of the percentages of PTZ-induced convulsion development after vitamin B12 pre-treatment, one day and two weeks, prior to PTZ injection.
PTZ: pentylenetetrazole
Figure 4. Comparison of the type of PTZ-induced convulsion occurring after vitamin B12 pre-treatment, one day and two weeks, prior to PTZ injection.
PTZ: pentylenetetrazole
Discussion
Neuroinflammation is widely known to occur during epileptogenesis; thus, targeting it has been considered a promising approach toward the development of treatment for epilepsy [2]. For the past decades, several studies have revealed that epileptic activities are associated with vitamin B12 deficiency, as proven by Sklar (1986) [18], which led to emerging evidence regarding the neuroprotective effects of vitamin B12, as found by Romano et al. (2014) [19] and Tamaddonfard et al. (2012) [20]. This study provided results that were nearly consistent with other literatures in which vitamin B12 pre-treatment has shown an antiepileptic effect against PTZ-induced convulsions. The main findings of this study are as follows: (1) the latency period to convulsion increased after vitamin B12 pre-treatment, (2) the duration period of convulsion decreased after vitamin B12 pre-treatment, (3) the percentage of rats experiencing convulsion decreased after vitamin B12 pre-treatment, and (4) rats with vitamin B12 pre-treatment experienced simple twitching convulsion more rather than generalized tonic-clonic convulsion. In most animal experimental models, PTZ kindling is commonly used to demonstrate the effect of antiepileptic drugs against epileptogenesis [21]. According to Morimoto et al. (2004) [21], kindling refers to the stimulation of the brain, either chemically or electrically, that leads to the lowering of the seizure threshold allowing seizure occurrence. The generally recognized mechanism for PTZ-induced seizure is the noncompetitive antagonism of the gamma-aminobutyric acid (GABA)-A receptor complex, which suppresses the function of the inhibitory synapses [22].
In this study, the latency to PTZ-induced convulsion significantly increased, while the duration significantly decreased after vitamin B12 pre-treatment, regardless of the dosage and pre-treatment period. Several previous studies have demonstrated the beneficial effects of vitamin B12 in controlling seizures attacks, such as Empanfarast et al. (2017) [23], Taskıran et al. (2018) [24], and Filiz et al. (2021) [1]. However, our experimental protocol is different than what was used in these reports. In Empanfarast et al.’s (2017) [23] experiment, vitamin B12 was administered as intracortical microinjections. Taskıran et al.’s (2018) [24] protocol consisted of multiple intraperitoneal injections of vitamin B12 for a week before testing the animals with PTZ injections, while Filiz et al.’s (2021) [1] protocol was to affect kindling procedures, which consisted of subthreshold convulsion doses of PTZ combined with vitamin B12 administration for about a month. Our experiment was aiming to confirm that a single vitamin B12 injection can modulate the convulsive effect of PTZ.
In addition, detailed information about the onset, duration, severity, and probability of seizure induction was examined. Aside from vitamin B12, some compounds with potential antiepileptic effects were also explored in PTZ-administered rat models. Karabulut et al. (2021) [2] demonstrated that thiamine or vitamin B1 has effectively suppressed the PTZ-induced epileptogenesis activities among rat models (P<0.05) in which the 50 mg/kg dosage of thiamine was found more effective in reducing the said activities (P<0.05). While Mehla et al. (2010) [25] used curcumin to delay the development of PTZ kindling among rats in which 300 mg/kg was found to show a significant increase in latency period. Focusing on the antiepileptic effects of vitamin B12, it has been documented that several epileptic activities, such as seizures, are commonly associated to vitamin B12 deficiency [15]. According to Hunt et al. (2014) [26], the main functions of vitamin B12 include the methylation processes related to DNA and cell metabolism. It affects the nervous system including its neuroprotective and neurotrophic actions, specifically in promoting growth and repair of nervous tissues during neuron injuries [1,26]. Thus, vitamin B12 deficiency might result in some serious neurological impairment. Although not clearly established, vitamin B12 level at 200 ng/L would be considered sensitive in diagnosing patients with such deficiency at 97% [26].
This study also determined the type of convulsion manifested by the rat models, either simple twitches or tonic-clonic. The results revealed that vitamin B12 pre-treatment favored the occurrence of simple less aggressive twitches rather than tonic-clonic convulsions, in which such findings were generally observed during a shorter PTZ and vitamin B12 injection gap time. In the study conducted by Filiz et al. (2021) [1], the behavioral seizure of PTZ-induced rat model was also monitored using the Racine scale in which simple twitching corresponded to low seizure effect while tonic-clonic seizures corresponded to the second highest seizure effect next to lethal seizures. The results revealed that vitamin B12 has shown protection against PTZ kindling in rats wherein oxidative stress and other neurological impairment were reduced. Similarly, Karabulut et al. (2022) [2] used the nearly equal ranking from simple twitches to clonic and lethal seizures in PTZ-induced rat models. Using thiamine as the antiepileptic component, the results revealed that seizures causing neurological and cognitive impairment have been alleviated. Relating to this, Lubana et al. (2015) [15] have demonstrated through a case report that vitamin B12 deficiency, along with high folate level, caused generalized tonic-clonic seizure in a human patient. These findings have been supported by the reduction of seizure episodes after vitamin B12 supplementation and folate normalization. As stated in their report, the deficiency in vitamin B12 hinders the methionine generation, which is relevant to the synthesis of myelin and neurotransmitters. In addition, deficiency leads to methylmalonic acid formation, a myelin destabilizer.
This study provided valuable insights into the effect of vitamin B12 on epileptogenesis in rats; however, there are still some limitations to be considered. Although PTZ is simple, it is considered laborious and time-consuming. In addition, PTZ kindling models have limitations regarding the analysis of seizure initiation and propagation patterns, which was also stated by Singh et al. (2021) [27]. Also, the trend on the effect of increasing the vitamin B12 dosage and pre-treatment period to the latency to and duration of convulsion would not be definite because this study worked at two data points each only. Lastly, the use of 100% female population may allow the results to be conclusive among female models only. Other factors affecting the seizure results, such as the device used and the expertise of the evaluators, were not reported or controlled.
Conclusions
This study provided evidence that vitamin B12 pre-treatment can reduce seizures among PTZ-induced rat models. In general, the latency to convulsion has increased after pre-treatment, while the duration of convulsion has decreased. In addition, simple twitching seizures were more likely to occur than tonic-clonic seizures after the said pre-treatment. These findings then suggest that vitamin B12 is a potential strategy and treatment for epilepsy and other related epileptogenesis activities.
Acknowledgments
Mohammed Al-Beltagi, Aysha Fakhroo, Fatima Al-Ali, Latifa Fakhroo, Marya Al-Hammadi, and Rania Snobar contributed equally to the work and should be considered co-first authors.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study
Animal Ethics
The research and animal ethics committee of Arabian Gulf University, Manama, Bahrain, Issued protocol number E015-PI-10/17
References
- 1.Protective effects of lamotrigine and vitamin B12 on pentylenetetrazole-induced epileptogenesis in rats. Filiz AK, Gumus E, Karabulut S, Tastemur Y, Taskiran AS. Epilepsy Behav. 2021;118:107915. doi: 10.1016/j.yebeh.2021.107915. [DOI] [PubMed] [Google Scholar]
- 2.Thiamine alleviates cognitive impairment and epileptogenesis by relieving brain inflammation in PTZ-induced kindling rat model. Karabulut S, Filiz AK, Akkaya R. Neurol Res. 2022;44:902–909. doi: 10.1080/01616412.2022.2066785. [DOI] [PubMed] [Google Scholar]
- 3.Neuroproteomics in epilepsy: what do we know so far? do Canto AM, Donatti A, Geraldis JC, Godoi AB, da Rosa DC, Lopes-Cendes I. Front Mol Neurosci. 2020;13:604158. doi: 10.3389/fnmol.2020.604158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The epidemiology of epilepsy revisited. Sander JW. Curr Opin Neurol. 2003;16:165–170. doi: 10.1097/01.wco.0000063766.15877.8e. [DOI] [PubMed] [Google Scholar]
- 5.Basic and clinical role of vitamins in epilepsy. Zalkhani R, Moazedi A. http://ijrabms.umsu.ac.ir/article-1-106-en.html J Res Appl Basic Med Sci. 2020;6:104–114. [Google Scholar]
- 6.Epilepsy: new advances. Moshé SL, Perucca E, Ryvlin P, Tomson T. Lancet. 2015;385:884–898. doi: 10.1016/S0140-6736(14)60456-6. [DOI] [PubMed] [Google Scholar]
- 7.Shorvon S, Perucca E, Engel J Jr. West Sussex, England: Wiley-Blackwell; 2015. The treatment of epilepsy. [Google Scholar]
- 8.Pharmacoresistant epilepsy: definition and explanation. Alexopoulos AV. Epileptology. 2013;1:38–42. [Google Scholar]
- 9.Breakthrough seizures-further analysis of the standard versus new antiepileptic drugs (SANAD) study. Bonnett LJ, Powell GA, Tudur Smith C, Marson AG. PLoS One. 2017;12:0. doi: 10.1371/journal.pone.0190035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Novel treatment options for epilepsy: focus on perampanel. Franco V, Crema F, Iudice A, Zaccara G, Grillo E. Pharmacol Res. 2013;70:35–40. doi: 10.1016/j.phrs.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 11.Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Kwan P, Arzimanoglou A, Berg AT, et al. Epilepsia. 2010;51:1069–1077. doi: 10.1111/j.1528-1167.2009.02397.x. [DOI] [PubMed] [Google Scholar]
- 12.The multi-faceted basis of vitamin B12 (cobalamin) neurotrophism in adult central nervous system: lessons learned from its deficiency. Scalabrino G. Prog Neurobiol. 2009;88:203–220. doi: 10.1016/j.pneurobio.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 13.Vitamin B12 deficiency. Killen JP, Brenninger VL. N Engl J Med. 2013;368:2040–2041. doi: 10.1056/NEJMc1304350. [DOI] [PubMed] [Google Scholar]
- 14.Green R. Encyclopedia of human nutrition. Amsterdam, Netherlands: Elsevier; 2013. Vitamin B12: physiology, dietary sources, and requirements; pp. 351–356. [Google Scholar]
- 15.Vitamin B12 deficiency and elevated folate levels: an unusual cause of generalized tonic-clonic seizure. Lubana SS, Alfishawy M, Singh N, Atkinson S. Am J Case Rep. 2015;16:386–389. doi: 10.12659/AJCR.893847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Effects of intracortical microinjection of vitamin B12 on penicillin-induced epileptiform activity in rats. Erfanparast A, Tamaddonfard E. https://www.ane.pl/pdf/7517.pdf. Acta Neurobiol Exp (Wars) 2015;75:200–207. [PubMed] [Google Scholar]
- 17.Vitamin B12 enhances GABA content but reduces glutamate content in the rat suprachiasmatic nucleus. Ikeda M, Azuma S, Inoué S. Am J Physiol. 1997;273:0–63. doi: 10.1152/ajpregu.1997.273.1.R359. [DOI] [PubMed] [Google Scholar]
- 18.Nutritional vitamin B12 deficiency in a breast-fed infant of a vegan-diet mother. Sklar R. Clin Pediatr (Phila) 1986;25:219–221. doi: 10.1177/000992288602500409. [DOI] [PubMed] [Google Scholar]
- 19.Effects of vitamin B12 on the corneal nerve regeneration in rats. Romano MR, Biagioni F, Carrizzo A, et al. Exp Eye Res. 2014;120:109–117. doi: 10.1016/j.exer.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 20.Central effect of crocin on penicillin-induced epileptiform activity in rats. Tamaddonfard E, Hamzeh Gooshchi N, Seiednejad-Yamchi S. Pharmacol Rep. 2012;64:94–101. doi: 10.1016/s1734-1140(12)70735-1. [DOI] [PubMed] [Google Scholar]
- 21.Kindling and status epilepticus models of epilepsy: rewiring the brain. Morimoto K, Fahnestock M, Racine RJ. Prog Neurobiol. 2004;73:1–60. doi: 10.1016/j.pneurobio.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 22.Pentylenetetrazole-induced kindling mouse model. Shimada T, Yamagata K. J Vis Exp. 2018:56573. doi: 10.3791/56573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Intra-hippocampal microinjection of oxytocin produced antiepileptic effect on the pentylenetetrazol-induced epilepsy in rats. Erfanparast A, Tamaddonfard E, Henareh-Chareh F. Pharmacol Rep. 2017;69:757–763. doi: 10.1016/j.pharep.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 24.The protective effects of vitamin B12 on pentylenetetrazole-induced seizures in rats. Taskıran AS, Gumus E, Gunes H, Cetindag A, Ozdemir E, Arslan G. Anat Physiol Biochem Int J. 2018;4:1–5. [Google Scholar]
- 25.Protective effect of curcumin against seizures and cognitive impairment in a pentylenetetrazole-kindled epileptic rat model. Mehla J, Reeta KH, Gupta P, Gupta YK. Life Sci. 2010;87:596–603. doi: 10.1016/j.lfs.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 26.Vitamin B12 deficiency. Hunt A, Harrington D, Robinson S. BMJ. 2014;349:0. doi: 10.1136/bmj.g5226. [DOI] [PubMed] [Google Scholar]
- 27.PTZ kindling model for epileptogenesis, refractory epilepsy, and associated comorbidities: relevance and reliability. Singh T, Mishra A, Goel RK. Metab Brain Dis. 2021;36:1573–1590. doi: 10.1007/s11011-021-00823-3. [DOI] [PubMed] [Google Scholar]