Introduction:
Blood cultures are fundamental in diagnosing and treating sepsis in the pediatric intensive care unit (PICU), but practices vary widely. Overuse can lead to false positive results and unnecessary antibiotics. Specific factors underlying decisions about blood culture use and overuse are unknown. Therefore, we aimed to identify perceived determinants of blood culture use in the PICU.
Methods:
We conducted semistructured interviews of clinicians (M.D., D.O., R.N., N.P., P.A.) from 6 PICUs who had participated in a quality improvement collaborative about blood culture practices. We developed interview questions by combining elements of the Consolidated Framework for Implementation Research and behavioral economics. We conducted telephone interviews, open-coded the transcripts, and used modified content analysis to determine key themes and mapped themes to elements of Consolidated Framework for Implementation Research and behavioral economics.
Results:
We reached thematic saturation in 24 interviews. Seven core themes emerged across 3 Consolidated Framework for Implementation Research domains: individual characteristics [personal belief in the importance of blood cultures, the perception that blood cultures are a low-risk test]; inner setting [adherence to site-specific usual practices, site-specific overall approach to PICU care (collaborative versus hierarchical), influence of non-PICU clinicians on blood culture decisions]; and outer setting [patient-specific risk factors, sepsis guidelines]. In addition, outcome bias, default bias, and loss aversion emerged as salient behavioral economics concepts.
Conclusions:
Determinants of blood culture use include individual clinician characteristics, inner setting, and outer setting, as well as default bias, outcome bias, and loss aversion. These determinants will now inform the development of candidate strategies to optimize culture practices.
INTRODUCTION
Medical overuse is a significant and poorly understood problem. Up to one-third of the United States healthcare spending represents medical overuse: care in which net benefits do not exceed the net harms and which is associated with excess cost, worse patient outcomes, and death.1–6 Examples of harm from overuse abound in a variety of specialties (cardiology, surgery, oncology, pediatrics), and overuse can occur during both diagnosis and treatment.7–10
In the pediatric intensive care unit (PICU), blood cultures are the gold standard diagnostic test for bacteremia. Clinicians typically initiate empiric broad-spectrum antibiotics concurrent with obtaining an initial set of blood cultures. Clinicians perceive blood cultures as a low-risk test compared with failing to diagnose and treat bacteremia. However, blood cultures are sometimes drawn unnecessarily in PICU patients when the pretest probability of bacteremia is low.11 False positive blood cultures cause patient harm and strain health care resources: repeat testing, unnecessary antibiotics, a longer length of stay, exposure to additional procedures and consultations, and increased costs.12–16 The accompanying antibiotic overuse is a particularly important source of harm, as it a frequent culprit in avoidable adverse drug events in children as well as a key driver of antibiotic resistance.17–21
Previous work has demonstrated a successful and safe reduction in blood culture use through quality improvement and human factors engineering methods.22–25 Survey and single-center interview data suggested significant variability in blood culture practices in the PICU and that reflexive testing and fear of missing sepsis were important factors in blood culture decisions for PICU clinicians.24,26 However, a deeper understanding of the determinants that influence blood culture decisions among PICU clinicians is a major knowledge gap preventing optimal use of blood cultures.
We conducted a multicenter qualitative study of PICU clinicians to explore blood culture practices in critically ill children. Our objectives were to identify perceived determinants of blood culture use to develop strategies to optimize testing practices in the PICU.
Methods
Design
We performed a qualitative study using semistructured interviews.
Setting, Recruitment, and Participants
We conducted interviews within an existing multicenter quality improvement collaborative called BrighT STAR.25 Briefly, BrighT STAR’s objective was to optimize blood culture practices in PICU patients. The collaborative enrolled 14 PICUs across the country, used a variety of strategies to reduce blood culture rates, and studied the effect on blood cultures, antibiotic use, and patient safety over 3 years.25
We recruited participants via emails to site leads. In addition, we conducted semistructured interviews via telephone with PICU clinicians from BrighT STAR sites after the postimplementation period. Eligible participants included bedside nurses, nurse managers, nurse practitioners, physicians’ assistants, fellows, residents, and attending physicians who worked in a direct patient-care role in a PICU. None were BrighT STAR site leads. Following the interview, we completed a verbal questionnaire to record participants’ basic demographic information.
We employed a deviance sampling approach and recruited interview participants from 6 BrighT STAR sites: 3 sites with the greatest reduction in blood culture rates and 3 with the smallest reduction in blood culture rates.27 A small financial remuneration was offered for participants’ time and participation. We obtained informed consent verbally before the start of each interview.
Interview Guide
The interview guide consisted of open-ended questions regarding protocols, practices, and the perceived risks and benefits of obtaining blood cultures in PICU patients. The researchers’ previous work on antibiotic stewardship and blood culture practices and 2 key frameworks [the Consolidated Framework for Implementation Research (CFIR) and principles from the field of behavioral economics] informed the questions.22–26,28,29
The CFIR is a pragmatic meta-theoretical framework of constructs that influence implementation. It is widely used to evaluate determinants of clinician behavior.30,31 It is a practical theory-based guide for systematically assessing potential barriers and facilitators to a new intervention or a practice change. It is a core method for first understanding context and then facilitating changes to clinician behavior.32 The CFIR has 5 domains, each with several constructs (eg, characteristics of the individual, the inner setting, the outer setting).32 Additionally, behavioral economics suggests that heuristics and cognitive biases play an important role in clinical decision-making.29 An emerging body of work suggests that combining elements of implementation science and behavioral science can facilitate a deeper understanding of important behavioral drivers of implementation and decision-making by clinicians. However, no work has yet explored this possibility in the PICU setting.33–35 We, therefore, constructed our questions by choosing certain CFIR domains identified in earlier work as important possible determinants of blood culture practices (eg, local unit culture: inner setting, and national sepsis initiatives: outer setting).24,26,28 Consistent with behavioral economics approaches, we also incorporated questions focusing on outcome bias (allowing a prior event or decision outcome to influence subsequent independent decisions) in the form of hypothetical clinical scenarios, which we hypothesized may be a driver of blood culture decisions.29 We have placed additional detail about our synthesis of CFIR and behavioral science in Supplemental Digital Content 1. (See document 1, Supplemental Digital Content 1, which shows the approach to combining elements of CFIR and behavioral science to obtain and analyze qualitative interview data. http://links.lww.com/PQ9/A476.)
Before data collection, questions were piloted with PICU clinicians and revised iteratively to create the final interview guide. (See document 2, Supplemental Digital Content 2, which shows interview questions: determinants of blood culture overuse in the pediatric intensive care unit. http://links.lww.com/PQ9/A477.)
Data Collection
Two study team members (C.W. and M.N.) conducted the interviews. Interviewer CW is a PICU physician with formal training in qualitative methods. Interviewer MN is a trained qualitative researcher from the University of Pennsylvania Mixed Methods Research Laboratory. The audio was recorded for each interview and then professionally transcribed. Data collection continued until we achieved thematic saturation and no additional new themes emerged.36–38
Data Analysis
MN and WE (another qualitative researcher from the University of Pennsylvania Mixed Methods Research Laboratory) developed a set of prespecified potential thematic codes in discussion with the study’s lead investigator (C.W.) based on preliminary data and the study’s hypotheses and organized via the CFIR and insights from behavioral economics. Subsequently, M.N. and W.E. analyzed the transcripts for content reflecting these codes and added new codes based on themes that emerged from the data. Researchers MN and WE jointly developed the emergent coding framework and revised the coding by consensus during regular meetings. Authors CW and JS reviewed the codebook. Finally, MN and WE applied the codebook to all transcripts using Nvivo 1.5 (QSR International, Burlington, Mass.), with approximately 20% of transcripts double-coded to ensure strong agreement between coders (k = 0.86).
Regulatory Oversight
This study was deemed exempt by the Institutional Review Board of the Children’s Hospital of Philadelphia.
Results
We achieved thematic saturation after completing 24 interviews with PICU clinicians (7 attending physicians, 6 trainee/supervised clinicians, and 11 nurses) from all 6 eligible sites. Interviews occurred over 4 months. Interviews lasted an average of 33 minutes (range 19–41 minutes). Table 1 displays participant demographics.
Table 1.
Characteristics of Interview Participants
| Gender (n, %) | Women 17, 71% Men 7, 29% |
|---|---|
| Age, y (median, range) | 36.5 (26–64) |
| Race (n, %) | White 22, 92%Black 1, 4%Asian 1, 4% |
| Experience as a PICU clinician (median, range) | 4.25 years (1 mo–20 y) |
| Type of clinical role in the PICU (n, %) | Nurse, 10Nurse manager, 1Physician assistant, 1Resident physician, 2Fellow physician, 3Attending physician, 7Total nurses = 11, 46%Total advanced practice providers = 1, 4%Total physicians = 12, 50%Total trainee physicians = 5, 21%Total supervising physicians = 7, 29% |
| Distribution of participants by site (n) | Site 1* = 4Site 2* = 3Site 3^ = 3Site 4^ = 7Site 5* = 3Site 6^ = 4*= low-performing site^ = high-performing site |
There were no notable differences among interview responses for any themes from the high- versus low-performing sites or when comparing nurses versus physicians. Overall, we noted 7 core emergent themes consistent with CFIR. In addition, our content analyses of responses regarding specific biases yielded the presence of outcome bias among our participants, as well as 2 additional cognitive biases not specifically hypothesized to exist a priori: default bias (the tendency to remain at the status quo because the disadvantages of leaving it loom larger than advantages), and loss aversion (the idea that losses loom larger than corresponding gains). Please see Tables 2 and 3 for illustrative quotes.
Table 2.
Representative Quotes: CFIR-guided Determinants of Blood Culture Overuse
| CFIR Domain | Theme | Representative Quote | Participant Type and Site |
|---|---|---|---|
| Individual characteristics | Personal belief in the overall importance of blood cultures | “[Without a culture] you may delay providing appropriate antibiotic coverage if needed.”“…I think there’s a sense of urgency to figure out what is going on with the patient and I think there’s a heightened awareness for the possibility of sepsis. So, I think understanding that sepsis can go south pretty quickly if it’s not under control…and that we don’t take it lightly.” | Fellow, low performing site (2)Nurse, high performing site (4) |
| Individual characteristics | Perception that blood cultures are a low-risk test | “I think there’s almost no risk of getting it besides worsening the amount of anemia that the patient can get in the ICU, but all those things are in theory reversible, right. So, I think it’s a minimal risk to get it and more benefit.”“I think that my, having been at this at this institution now, it’s made me realize that many of the blood cultures that I may have obtained in the past were unnecessary. And not only may not have provided any benefit, but may also have led to some degree of inappropriate harm, or, harm I used almost as a loose term, but inappropriate antibiotic usage certainly. And I think it’s also important that having these institutional recommendations helps remove some of that uncertainty and variability.” | Fellow, low performing site (2)Attending physician, high performing site (3) |
| Inner setting | Adherence to site-specific usual practices | “I would say my personal approach is pretty similar to the way the group practices.”“I would say that our physicians are pretty standard across the board. We are very standardized and policy driven.” | Attending physician, low performing site (2)Nurse and clinical staff leader, low performing site (5) |
| Inner setting | Site-specific overall approach to PICU care (team-based and collaborative versus hierarchical) | “So, in our unit it’s the ICU team that makes the decisions…it’s a team approach…it’s the whole team that meets and makes the decision.”“The more experienced clinicians tend to follow [the algorithm], and the less experienced ones will defer to nursing preference, so I guess that would be the way that they deviate away from it, like, ‘Oh, what do you think? Do you think that they’re meeting [the threshold for testing]? Or what do we usually do?’ Rather than actually looking at the policy. That’s usually what we’ll look at that, we have a decision tree.” | Nurse, low performing site (1)Nurse, low performing site (1) |
| Inner setting | Influence of non-PICU clinicians on blood culture decisions | “If our PICU team wants cultures or doesn’t want cultures and let’s say the heme-onc team wants cultures, I would say usually the heme-onc team wins in that perspective.” | Nurse, high performing site (4) |
| Outer setting | Patient-specific risk factors | “I think about the patient’s underlying process that’s going on to their underlying diagnosis and reason for being in the PICU in relation to the fever. That’s probably the first priority in thinking about how to or whether to order a blood culture.”“In immune compromised patients, I would be much more likely to get a blood culture in general, regardless of the other factors that are not in an immune compromised patient.”“More than a diagnosis, any patient who has a central line in place, either a tunneled line or a PICU line. It’s probably one of the biggest things” [to prompt getting a culture]. | Attending physician, high performing site (4)Attending physician, high performing site (4)Fellow, low performing site (2) |
| Outer setting | Institution-wide or national sepsis guidelines | [Discussing a 1-hour time goal for antibiotics]“The time goal puts you on the crunch to be like, okay, immediately get the culture and get the antibiotics in as soon as possible. So, I think it defaults us to sometimes get more cultures.”“Yeah obviously, an hour can go by very quickly….[] …So, I think there is definitely a sense of urgency and when there’s a sense of urgency, I think there’s always a possibility that obviously, you’re thinking quicker. So, you don’t have as much time to fully think through every possibility…” | Attending physician, high performing site (6)Resident, high performing site (6) |
Table 3.
Representative Quotes: Behavioral Economics Determinants of Blood Culture Overuse
| Cognitive bias | Representative quote | Participant type and site |
|---|---|---|
| Outcome bias(allowing a prior event or decision outcome to influence subsequent independent decisions) | “I do think that those cases, those mortalities have been crucial in my understanding that you have to be more aggressive at the beginning.” Fellow, low performing site (2)“If I recently had like a bad experience where that happened, then it’s possible that I would be more likely to get cultures sooner.” Physician’s Assistant, high performing site (4)“I think [I would have] a lot of guilt, honestly, because that was a decision on my part to just watch in six hours when it comes to bacteremia and sepsis is a big deal and I don’t think it’s something that I would just be able to roll-off, and I think it’d be something that I would take with me going forward as a clinician with other patients I encounter who become febrile. I think it would probably impact the way I go about treating all other patients and I would probably just be a little bit more fearful and not as confident.” | Fellow, low performing site (2)Physician’s Assistant, high performing site (4)Resident, high performing site (6) |
| Default bias(the tendency to continue the status quo) | “I came from an institution where at least initially, in many cases, if a patient had a fever, the immediate knee-jerk response was to get a blood culture from a central line if they had it.”“I mean, pretty much in our unit if you have a fever, you’re going to get a blood culture.”“We watch the culture for 48 hours, keep them on antibiotics…. We stop thinking about it.” | Attending physician, high performing site (3)Nurse, low performing site (1)Attending physician, low performing site (2) |
| Loss aversion(the notion that preventing a loss is more important than a corresponding potential gain) | Interviewer: How can you think about the risks versus benefits of obtaining a blood culture in the PICU patient? Interviewee: “I think there’s almost no risk of getting it besides worsening the amount of anemia that the patient can get in the ICU, but all those things are in theory reversible, right. So, I think it’s a minimal risk to get it and then there’s more benefit as long as you have a strong feeling that the patient may be bacteremic….you have to rule out that a patient may be becteremic.”“Because it’s [sepsis] just something that you definitely you don’t want to miss and it’s easy enough to draw the culture, and put them on antibiotics.” | Fellow, low performing site (2)Attending physician, low performing site (2) |
Seven Emergent Themes Linked to 3 CFIR Domains
Theme 1: personal belief in the overall importance of blood cultures (CFIR domain: individual characteristics)
A majority of participants consistently described blood cultures as very important in their practice for the evaluation of potential sepsis as well as for determining the treatment course for a PICU patient with a fever (Table 2). However, while the desire for diagnostic information from blood culture was strong, many participants simultaneously noted that they would still treat a patient with signs and symptoms strongly suggestive of sepsis (ie, hypotension, poor perfusion, elevated lactate, fever) with antibiotics even if the blood cultures were negative. Also, a minority of those interviewed described that blood cultures are very important to rule out clinical conditions similar to bacterial sepsis but have a different etiology, such as rheumatological diseases.
Theme 2: the perception that blood cultures are a low-risk test (CFIR domain: individual characteristics)
Participants commonly described their perceived risks of obtaining blood cultures (discomfort, anemia, potential to introduce bacteria) as relatively less consequential than the risk of failing to diagnose an outcome like sepsis. Only a minority felt that obtaining a false positive result could lead to unnecessary administration of antibiotics.12,13 Moreover, participants described the potential downsides of unnecessary antibiotics as less important than the potential for failing to diagnose and treat potentially dangerous bacterial infections. For most participants, the benefits of obtaining blood cultures far outweighed the risks. PICU clinicians view blood culture results as highly useful to accurately determine a patient’s treatment course, quickly identify a life-threatening pathogen in patients with blood infections, and tailor antibiotic administration to that particular bacteria. A few participants, however, openly discussed the downsides of unnecessary blood cultures (Table 2).
Theme 3: adherence to site-specific usual blood culture practices (CFIR domain: inner setting)
A particular PICU’s usual informal practice and explicit policies influenced participants’ perceptions of whether blood cultures were necessary for their patients and influenced their personal approach to ordering cultures. Many participants described specific pathways, algorithms, or thresholds used at their site to determine when to order a blood culture; most often, these outlined a certain elevated temperature, coalescence of symptoms, or timeframe, such as no cultures obtained within the last 24 hours, as justifications for ordering blood cultures. In addition, some participants described a confluence of factors that determined whether blood culture ordering was standardized or informal, particularly the perceived level of risk of bacteremia based on a patient’s clinical status. At different sites, oncology patients were singled out multiple times as having a clear, standardized approach to fever, unlike non-oncology patients.
Theme 4: a site-specific overall approach to PICU care (team-based and collaborative versus hierarchical) (CFIR domain: inner setting)
The perception of a team-based versus a more individualized model of care in their PICU influenced participants’ behavior related to obtaining blood cultures. Decisions about blood cultures could come from any PICU clinician we interviewed and were not just physician-directed. For example, in some clinical settings, bedside nurses advised their overseeing physician on the need for blood cultures, then the physician was responsible for placing the order. Some attending physicians described themselves as having “the final say” about blood culture decisions. Other participants described an explicitly collaborative, team-based approach to febrile patient care in which many clinicians participated (attending physicians, trainee physicians, nurse managers, and bedside nurses). In these arrangements, the decision to order a blood culture was made by the group based on the input of each individual.
Theme 5: influence of non-PICU clinicians on blood culture decisions (CFIR domain: inner setting)
Consulting services, such as infectious diseases or oncology, played key roles in decisions about testing or treating bacteremia in PICU patients. Participants described that non-PICU clinicians or consulting groups with longer-term knowledge of a patient’s condition or trajectory outweighed the PICU clinician’s opinion when deciding to order a blood culture; most commonly, this was the infectious diseases team. Multiple participants noted that they have deferred to non-PICU clinicians and obtained blood cultures that they would not have otherwise ordered. Participants generally described such instances as collegial and collaborative rather than adversarial.
Theme 6: patient-specific risk factors (CFIR domain: outer setting)
Nearly all participants reported that clinical factors related to individual patients were paramount in driving blood culture decisions. The primary factors noted included fever, a central venous catheter, an oncologic diagnosis, the patient’s overall immune status, and general clinical stability vs. instability.
Theme 7: external (institutional or national) sepsis guidelines (CFIR domain: outer setting)
Few participants explicitly mentioned national sepsis guidelines or initiatives (such as the Surviving Sepsis Campaign or the Children’s Hospital Association’s Improving Pediatric Sepsis Outcomes Collaborative). However, they commonly noted sepsis huddles as part of hospital-wide initiatives. During these huddles, clinicians of varying roles and expertise gather to make treatment decisions about patients suspected of sepsis. For example, participants with experience with sepsis huddles were more likely to view the decision to order a blood culture as a collaborative, team effort in which the perspectives of everyone involved in a patient’s care, from bedside nurse to infectious disease specialist, had a role. In addition, many participants noted that their hospitals had time-to-antibiotic goals that could impact how quickly they made decisions for patients with a new fever.
Cognitive Bias
We identified 3 distinct types of cognitive bias in our data: outcome bias, default bias, and loss aversion (Table 3). First, we identified outcome bias, particularly during the discussion of hypothetical cases presented during the interview, with clinicians of various roles and experience levels endorsing that a poor outcome in a previous patient would indeed play some role in how they managed a subsequent patient with similar presenting symptoms. Second, default bias was apparent more frequently than outcome bias. Many participants acknowledged that they would practice in a manner that aligns with their unit’s typical practice, even if that meant reflexive or “knee-jerk” orders for blood cultures. Finally, loss aversion emerged during discussions about the risk/benefit of blood cultures versus the risk/benefit of failing to diagnose bacteremia in a PICU patient. Numerous times, participants described missing a major diagnosis, such as bacteremia in an individual patient, as a more powerful motivator for blood culture decisions than the more abstract possibility of unnecessary testing and unnecessary antibiotics having negative consequences.
Discussion
Little work has examined and attempted to characterize PICU clinician decision-making for a common but potentially overused diagnostic test such as blood cultures. We identified 7 distinct determinants of blood culture use that align with elements of the CFIR and 3 types of cognitive biases. Combining these 2 scientific approaches yields a deep understanding of how overuse may occur by examining not only how the clinician interacts with multiple layers of their environment (ie, the CFIR domains) but also how unconscious processes within the clinician themselves may impact behavior related to diagnostic decisions (ie, heuristics and cognitive bias).30
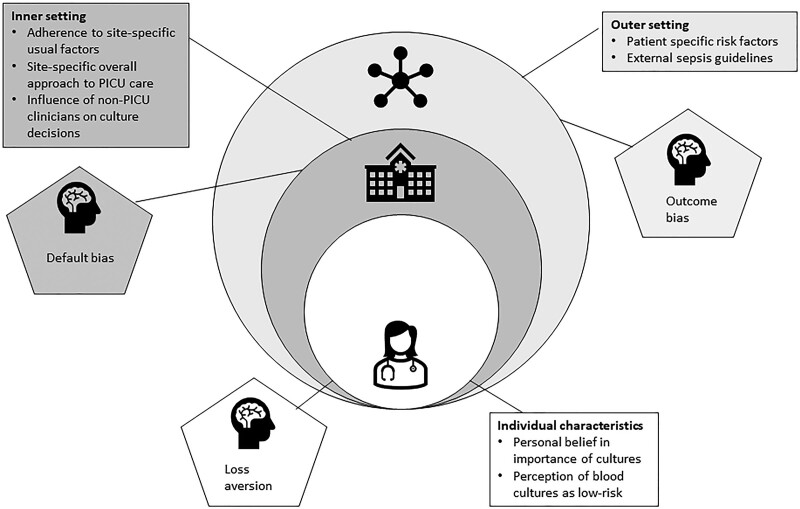
Our findings demonstrate that such decision-making is a complex process that involves multiple layers of information for clinicians to weigh in the fast-moving and high-stakes critical care setting. Potential layers include characteristics of the individual patient (such as fever, blood pressure, underlying diagnosis, and presence of a central venous catheter), characteristics of the local work environment (such as a preexisting specific PICU algorithm for evaluating fever), factors external to the local environment (such as hospital-wide or national guidelines for antibiotic timing in sepsis), and factors related to the PICU clinician themselves (such as prior experience with a poor patient outcome or personal approach to testing for bacteremia). We have constructed a conceptual model for how these layers interact and ultimately lead to decisions to test or not test for bacteremia (Fig. 1). Ultimately, this model could allow for developing strategies targeting specific drivers and improving PICU clinicians’ testing decisions.
Figure 1.
Results of CFIR and behavioral economics-guided exploration of determinants of blood culture use.
First, it appears likely that clinical factors stemming directly from the specific patient under consideration are the primary driver of blood culture decisions. In particular, participants mentioned an underlying immunocompromising condition or a central venous catheter many times. This finding is unsurprising at face value, as these are well-established risk factors for serious bacterial infection. However, this was frequently coupled with an admission of reflexive decisions to such test patients for bacteremia in the setting of any fever—the more thorough pretest clinical evaluation that PICU providers would do for a patient without these conditions was not part of their described workflow. Such reflexive behavior presents two potential harms to patients. Patients who are seriously ill or decompensating from infection could have other important treatments delayed (such as fluid boluses or the initiation of vasoactive agents) if providers fail to thoroughly evaluate the patient’s status before placing an order for blood culture and moving on cognitively to the next task. Conversely, even immunocompromised patients, and patients with CVCs, do experience noninfectious or nonbacterial causes of fever, and the unnecessary antibiotics that may be coupled with the reflexive blood culture are a source of adverse events for PICU patients.39–42
Second, for our participants, the potential benefits of testing (ie, the timely diagnosis of bacteremia) seemed to strongly outweigh the potential downsides of testing (ie, pain or anemia from unnecessary blood draws or the consequences of unnecessary antibiotics). While several participants acknowledged that unnecessary antibiotics have important negative consequences for population health, this notion appeared to carry relatively little weight in driving their decision-making about blood cultures for individual patients. This distinction raises interesting questions about how clinicians weigh the cost of their actions for society at large versus for the specific patient under their immediate care. Previous studies have demonstrated that a majority of physicians agree that their primary responsibility is to each patient rather than society at large, though PICU clinician perspectives on this concept as it relates specifically to diagnostic stewardship and antimicrobial overuse are, to date, unexplored.43 As antimicrobial resistance presents a grave threat to public health, efforts to characterize how clinicians balance specific patients’ needs versus the public’s general needs are paramount.
Third, team and institutional structure and culture have a strong role in clinical decision-making for diagnostic testing in PICU patients. This finding is notable considering the simultaneous importance of individual provider beliefs about blood cultures. Balancing an individual provider’s opinion with that of the larger PICU team, the non-PICU team (eg, consultants) or the institution (eg, antibiotic timing administration initiatives) is a complex task that likely is constantly happening without specific awareness of the cognitive workload this confers. Under ideal circumstances, this balancing act may facilitate thoughtful discussions and open dialogue between providers that benefit patient care but could also, in some environments, lead providers to make decisions that comply with typical unit practices or defer to other clinicians to minimize conflict in the name of efficiency.
Finally, our data suggest that cognitive bias impacts blood culture decision-making in the PICU. Specifically, outcome bias, default bias, and loss aversion can influence testing practices. However, the impact of such bias on patients is uncertain. While important lessons can emerge from specific patient experiences that help a clinician make better future decisions, allowing such biases to drive decisions consistently may not always lead to appropriate or ideal clinical care for patients. This concern is, to our knowledge, the first exploration of the role of cognitive bias in blood culture decisions in the PICU. More study is needed to understand the specific impact of these biases on the use of tests or treatments in critically ill children.
Our data suggest that potential targets for interventions to improve PICU decision-making for blood cultures include: the reflexive ordering of cultures without timely clinical assessment of the patient, challenges in balancing prioritization of individual patient needs versus the needs of the public as related to antibiotic resistance; potential difficulties in reconciling unit/team/institutional culture with individual clinician judgment; and recognition of the role that cognitive bias may be playing in driving diagnostic decisions. While the development of formal strategies to reduce blood culture overuse is underway, we have summarized preliminary approaches to targeting the identified determinants in Table 4.
Table 4.
Example Strategies to Target Determinants of Blood Culture Overuse
| Determinant of Culture Overuse | Potential Strategy Targeted to that Determinant |
|---|---|
| Reflexive testing as normative practice (default bias) | Timely bedside huddle to evaluate patient’s clinical status before ordering cultures |
| Perception of blood cultures as low-risk test (individual characteristics) | Education for providers about negative consequences of unnecessary testing and antibiotic resistance |
| Influence of non-PICU clinicians on culture decisions (inner setting) | Collaborative development of guideline or algorithm about blood cultures, including when cultures may be deferred, to ensure all stakeholder perspectives are represented |
| External sepsis guidelines (outer setting) | Inclusion in time-to-antibiotic algorithms of rapid specific review of patient’s risk factors for bacteremia and level of concern for bacterial infection, before orders for cultures and antibiotics |
LIMITATIONS
Our participants were clinicians from institutions that participated in BrighT STAR and, thus, have already participated in work focused on blood culture practices. This approach limits generalizability and may have introduced selection bias. In addition, our methodology inherently may involve recall bias by our participants. Finally, by using semistructured interviews constructed around prespecified hypothesized topics of interest, we also likely did not capture all possible drivers of decision-making. However, we probed during interviews for topics not included in our prespecified interview guide.
CONCLUSIONS
Multi-center PICU quality improvement work has demonstrated a safe and significant reduction in blood cultures and antibiotic use and created a novel opportunity to qualitatively study drivers of decision-making for blood cultures. We combined implementation science and behavioral economics tools to create a conceptual model for how and why PICU clinicians use blood cultures in critically ill children. This model illustrates opportunities for targeting decision-making to optimize testing practices in the PICU. Changing the use of diagnostic tests can have important downstream impacts on associated treatments. Future studies are needed to develop and then test promising strategies—work that our findings can guide.
ACKNOWLEDGMENTS
The authors wish to thank the sites enrolled in the BrighT STAR Collaborative and the interview participants for their assistance in conducting this study. Dr. Woods-Hill received support for this work from the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number K23HL151381. Dr. Milstone received support from the National Institute of Allergy and Infectious Diseases at the National Institutes of Health under Award Number from K24AI141580. The content is solely the authors’ responsibility and does not necessarily represent the official views of the National Institutes of Health.
Preliminary data of this study is presented at the 14th annual Conference for the Science of Dissemination and Implementation in Health [virtual format], December 2021.
DISCLOSURE
The authors have no financial interest to declare in relation to the content of this article.
Supplementary Material
Footnotes
Published online April 10, 2023
Supplemental digital content is available for this article. Clickable URL citations appear in the text.
To cite: Woods-Hill CZ, Nelson MN, Eriksen W, Rendle KA, Beidas RS, Bonafide CP, Brajcich MR, Milstone AM, Shea JA. Determinants of Blood Culture Use in Critically Ill Children: A Multicenter Qualitative Study. Pediatr Qual Saf 2023;8:647.
REFERENCES
- 1.Morgan DJ, Brownlee S, Leppin AL, et al. Setting a research agenda for medical overuse. BMJ. 2015;351:h4534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chassin MR, Galvin RW. Institute of medicine national roundtable on health care quality: the urgent need to improve health care quality. JAMA. 1998;280:1000–1005. [DOI] [PubMed] [Google Scholar]
- 3.Berwick DM, Hackbarth AD. Eliminating waste in US health care. JAMA. 2012;307:1513–1516. [DOI] [PubMed] [Google Scholar]
- 4.Esserman LJ, Thompson IM, Reid B. Overdiagnosis and overtreatment in cancer: an opportunity for improvement. JAMA. 2013;310:797–798. [DOI] [PubMed] [Google Scholar]
- 5.Fisher ES, Wennberg DE, Stukel TA, et al. The implications of regional variations in medicare spending. part 2: health outcomes and satisfaction with care. Ann Intern Med. 2003;138:288–298. [DOI] [PubMed] [Google Scholar]
- 6.Brownlee S, Chalkidou K, Doust J, et al. Evidence for overuse of medical services around the world. Lancet. 2017;390:156–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hannan EL, Samadashvili Z, Cozzens K, et al. Appropriateness of diagnostic catheterization for suspected coronary artery disease in New York State. Circ Cardiovasc Interv. 2014;7:19–27. [DOI] [PubMed] [Google Scholar]
- 8.Peul WC, van Houwelingen HC, van den Hout WB, et al. ; Leiden-The Hague Spine Intervention Prognostic Study Group. Surgery versus prolonged conservative treatment for sciatica. N Engl J Med. 2007;356:2245–2256. [DOI] [PubMed] [Google Scholar]
- 9.Norton WE, Chambers DA, Kramer BS. Conceptualizing de-implementation in cancer care delivery. J Clin Oncol. 2019;37:93–96. [DOI] [PubMed] [Google Scholar]
- 10.Coon ER, Quinonez RA, Morgan DJ, et al. 2018 update on pediatric medical overuse: a review. JAMA Pediatr. 2019;173:379–384. [DOI] [PubMed] [Google Scholar]
- 11.Kiragu AW, Zier J, Cornfield DN. Utility of blood cultures in postoperative pediatric intensive care unit patients. Pediatr Crit Care Med. 2009;10:364–368. [DOI] [PubMed] [Google Scholar]
- 12.Darby JM, Linden P, Pasculle W, et al. Utilization and diagnostic yield of blood cultures in a surgical intensive care unit. Crit Care Med. 1997;25:989–994. [DOI] [PubMed] [Google Scholar]
- 13.Bates DW, Goldman L, Lee TH. Contaminant blood cultures and resource utilization. The true consequences of false-positive results. JAMA. 1991;265:365–369. [PubMed] [Google Scholar]
- 14.Doern GV, Carroll KC, Diekema DJ, et al. A comprehensive update on the problem of blood culture contamination and a discussion of methods for addressing the problem. Clin Microbiol Rev. 2019;33:e00009–e00019. 10.1128/CMR.00009-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alahmadi YM, Aldeyab MA, McElnay JC, et al. Clinical and economic impact of contaminated blood cultures within the hospital setting. J Hosp Infect. 2011;77:233–236. [DOI] [PubMed] [Google Scholar]
- 16.Committee on Diagnostic Errors in Health Care; Board on Health Care Services; Institute of Medicine. In: Balogh EP, Miller BT, Ball JR, eds. Improving Diagnosis in Health Care. Washington, D.C.: National Academies Press (US); 2015. [PubMed] [Google Scholar]
- 17.Infectious Diseases Society of America (IDSA). Combating antimicrobial resistance: policy recommendations to save lives. Clin Infect Dis. 2011;52:S397–S428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shehab N, Lovegrove MC, Geller AI, et al. US emergency department visits for outpatient adverse drug events, 2013-2014. JAMA. 2016;316:2115–2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerber JS, Ross RK, Bryan M, et al. Association of broad- vs. narrow-spectrum antibiotics with treatment failure, adverse events, and quality of life in children with acute respiratory tract infections. JAMA. 2017;318:2325–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bolhuis MS, Panday PN, Pranger AD Kosterink JGW, et al. Pharmacokinetic drug interactions of antimicrobial drugs: a systematic review on oxazolidinones, rifamycines, macrolides, fluoroquinolones, and beta-lactams. Pharmaceutics. 2011;3:865–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tamma PD, Turnbull AE, Harris AD, et al. Less is more: combination antibiotic therapy for the treatment of gram-negative bacteremia in pediatric patients. JAMA Pediatr. 2013;167:903–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woods-Hill CZ, Fackler J, Nelson McMillan K, et al. Association of a clinical practice guideline with blood culture use in critically Ill children. JAMA Pediatr. 2017;171:157–164. [DOI] [PubMed] [Google Scholar]
- 23.Woods-Hill CZ, Lee L, Xie A, et al. Dissemination of a novel framework to improve blood culture use in three pediatric intensive care units. Pediatr Qual Saf 2018;3:e112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xie A, Woods-Hill CZ, King AF, et al. Work system assessment to facilitate the dissemination of a quality improvement program for optimizing blood culture use: a case study using a human factors engineering approach. J Pediatric Infect Dis Soc. 2019;8:39–45. [DOI] [PubMed] [Google Scholar]
- 25.Woods-Hill CZ, Colantuoni EA, Koontz DW, et al. ; Bright STAR Authorship Group. Association of Diagnostic Stewardship for blood cultures in critically ill children with culture rates, antibiotic use, and patient outcomes: results of the bright STAR collaborative. JAMA Pediatr. 2022;176:690–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woods-Hill CZ, Xie A, King A, et al. Practices, perceptions, and attitudes in the evaluation of critically ill children for bacteremia: a national survey. Pediatr Crit Care Med. 2020;21:e23–e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palinkas LA, Horwitz SM, Green CA, et al. Purposeful sampling for qualitative data collection and analysis in mixed method implementation research. Adm Policy Ment Health. 2015;42:533–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Damschroder LJ, Aron DC, Keith RE, et al. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blumenthal-Barby JS, Krieger H. Cognitive biases and heuristics in medical decision making: a critical review using a systematic search strategy. Med Decis Making. 2015;35:539–557. [DOI] [PubMed] [Google Scholar]
- 30.Kirk MA, Kelley C, Yankey N, et al. A systematic review of the use of the consolidated framework for implementation research. Implement Sci. 2015;11:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Skolarus TA, Lehmann T, Tabak RG, et al. Assessing citation networks for dissemination and implementation research frameworks. Implement Sci. 2017;12:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Available at: www.Cfirguide.org.
- 33.Last BS, Schriger SH, Timon C, et al. Using behavioral insights to design implementation strategies in public mental health settings: a qualitative study of clinical decision-making. Implement Sci Commun 2021;2:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang SY, Groene O. The effectiveness of behavioral economics-informed interventions on physician behavioral change: a systematic literature review. PLoS One. 2020;15:e0234149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Talat U, Schmidtke KA, Khanal S, et al. A systematic review of nudge interventions to optimize medication prescribing. Front Pharmacol. 2022;13:798916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bradley EH, Curry LA, Devers KJ. Qualitative data analysis for health services research: developing taxonomy, themes, and theory. Health Serv Res. 2007;42:1758–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hennink MM, Kaiser BN, Marconi VC. Code saturation versus meaning saturation: how many interviews are enough? Qual Health Res. 2017;27:591–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hays DG, Singh AA. Qualitative Inquiry in Clinical and Educational Settings. New York: Guilford Publications; 2012. [Google Scholar]
- 39.Alahmadi YM, Aldeyab MA, McElnay JC, et al. Clinical and economic impact of contaminated blood cultures within the hospital setting. J Hosp Infect. 2011;77:233–236. [DOI] [PubMed] [Google Scholar]
- 40.Klompas M, Calandra T, Singer M. Antibiotics for sepsis—finding the equilibrium. JAMA. 2018;320:1433–1434. [DOI] [PubMed] [Google Scholar]
- 41.Coon ER, Quinonez RA, Moyer VA, et al. Overdiagnosis: how our compulsion for diagnosis may be harming children. Pediatrics. 2014;134:1013–1023. [DOI] [PubMed] [Google Scholar]
- 42.Welch HG, Schwartz L, Woloshin S. Overdiagnosed: making people sick in the pursuit of health. Boston, Mass.: Beacon Press; 2011. [Google Scholar]
- 43.Beach MC, Meredith LS, Halpern J, et al. Physician conceptions of responsibility to individual patients and distributive justice in health care. Ann Fam Med. 2005;3:53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.