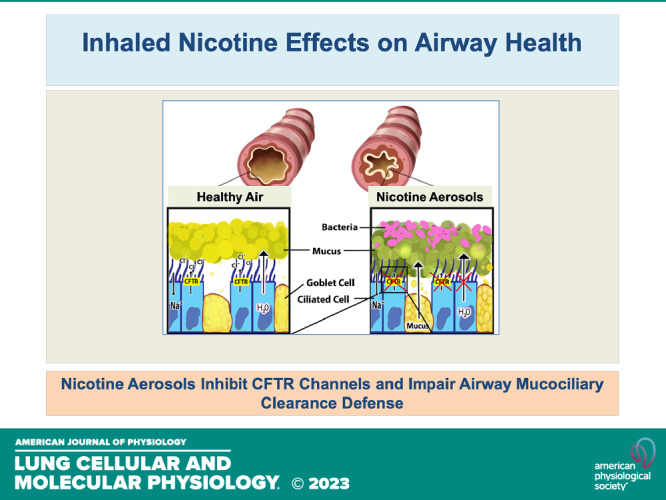
Keywords: CFTR, electronic cigarettes, mucociliary clearance, mucus physiology, nicotine
Abstract
Electronic cigarettes (e-cigs) are often promoted as safe alternatives to smoking based on the faulty perception that inhaling nicotine is safe until other harmful chemicals in cigarette smoke are absent. Previously, others and we have reported that, similar to cigarette smoke, e-cig aerosols decrease CFTR-mediated ion transport across airway epithelium. However, it is unclear whether such defective epithelial ion transport by e-cig aerosols occurs in vivo and what the singular contribution of inhaled nicotine is to impairments in mucociliary clearance (MCC), the primary physiologic defense of the airways. Here, we tested the effects of nicotine aerosols from e-cigs in primary human bronchial epithelial (HBE) cells and two animal models, rats and ferrets, known for their increasing physiologic complexity and potential for clinical translation, followed by in vitro and in vivo electrophysiologic assays for CFTR activity and micro-optical coherence tomography (μOCT) image analyses for alterations in airway mucus physiology. Data presented in this report indicate nicotine in e-cig aerosols causes 1) reduced CFTR and epithelial Na+ channel (ENaC)-mediated ion transport, 2) delayed MCC, and 3) diminished airway surface hydration, as determined by periciliary liquid depth analysis. Interestingly, the common e-cig vehicles vegetable glycerin and propylene glycol did not affect CFTR function or MCC in vivo despite their significant adverse effects in vitro. Overall, our studies contribute to an improved understanding of inhaled nicotine effects on lung health among e-cig users and inform pathologic mechanisms involved in altered host defense and increased risk for tobacco-associated lung diseases.
INTRODUCTION
The global burden of chronic obstructive pulmonary disease (COPD) is estimated to be over 251 million and continues to increase (1). COPD refers to a host of irreversible airway diseases with a progressive decline in lung function, chronic cough, and mucus hypersecretion (2). Chronic exposure to inhaled irritants such as cigarette smoke causes COPD. The continued decrease in smoking rates in the United States and other western countries represented a positive outlook regarding COPD incidence in future decades. However, the growing popularity of newer nicotine delivery devices (ENDS), such as electronic cigarettes (e-cigs), created a new challenge to the public health (3). With their variable delivery and multitude of formulations (called e-liquids), e-cigs have been challenging for research aimed at predicting their long-term harm reliably. Such knowledge regarding the safety of e-cigs is urgently needed to avoid historical mistakes committed with cigarettes. The first step in this process is a greater understanding of the pulmonary effects of inhaled nicotine aerosols since nicotine is the primary and most common ingredient among all e-cigs. With claims of reduced toxins compared with cigarette smoke (4–6), the popularity of e-cigs has rapidly increased as healthier alternatives to cigarettes. In recent years, 2.8% of American adults (7) and 5 million middle and high school students (8) were reported to be vaping.
Nicotine is present in e-cigs at levels comparable with combustible cigarettes (9, 10). Thus, nicotine must be critically evaluated in laboratory models under controlled conditions when delivered as an aerosol. The importance of testing nicotine effects is supported by the increased recognition of lung injury specifically attributable to nicotine in e-cig studies despite the reduction/absence of many other harmful agents present in cigarette smoke (11). For example, administration of nebulized nicotine to mice caused a significant reduction in the mucociliary clearance (MCC) defense (12). Similarly, MCC was reduced in sheep administered with intratracheal e-liquid containing nicotine (13). In terms of mechanisms to account for diminished MCC, acute exposure to nebulized nicotine in mice (14) and human bronchial epithelial (HBE) cells (12) led to reduced activation of CFTR, the ion channel whose dysfunction causes cystic fibrosis lung disease characterized by impaired MCC. Although these studies effectively indicate that nicotine in e-cigs can be detrimental to mucociliary clearance defense, no studies so far have conclusively established if nicotine can cause similar harm when delivered as inhaled aerosols. Relevant to this, our previous in vitro studies indicate that, similar to cigarette smoke (15), e-cig aerosols containing nicotine can also suppress CFTR activity in vitro (16). But, this finding was contradicted by an independent study conducted using human airway epithelial three-dimensional (3-D) cultures and found no adverse effects of e-cig aerosols even when the nicotine levels were several folds higher than combustible cigarette smoke (17). Regrettably, there are no conclusive in vivo studies focused on changes in epithelial ion transport and MCC defense when nicotine was administered via inhaled route as an aerosol consistent with human e-cig usage.
To better understand whether nicotine aerosols can impact human lung health, we used e-liquids prepared with scientific-grade vehicles of water, propylene glycol, vegetable glycerin, and nicotine. A nicotine level of 1.8% in the laboratory e-liquids represents human use (18) and mimics the amount encountered by lung epithelium among users of earlier generation e-cigs. Here, we present findings from in vitro and in vivo studies designed to test the hypothesis that inhaled nicotine aerosols induce airway CFTR dysfunction and impaired mucociliary clearance.
In the current study, we used differentiated human airway epithelial cells grown at the air-liquid interface and wild-type rats and ferrets proven to represent human airway anatomy and MCC defects faithfully in disease models for cystic fibrosis and COPD (19, 20). Collectively, data indicate that inhaled nicotine aerosols decrease epithelial ion transport to reduce airway surface mucus hydration and impair ciliary clearance. These data predict that inhaled nicotine aerosols administered by any ENDS will progressively impair hosts’ ability to clear inhaled irritants and pathogens, risking COPD and other lung disorders currently associated with cigarette smoking. Thus, our findings directly implicate nicotine in the increased burden of respiratory symptoms among e-cig users as determined from the analysis of more than 20,000 US adults in the Population Assessment of Tobacco and Health (PATH) study (21).
METHODS
Cell Culture and In Vitro Aerosols Exposures
Primary human bronchial epithelial (HBE) cells from consenting donors with wild-type CFTR with no history of respiratory disease and smoking/vaping were chosen. HBE cells were isolated and grown according to a protocol approved by the Institutional Review Boards at University of Alabama Birmingham, as previously discussed (22). HBE cells were expanded in submerged cultures and transferred to transwell filter inserts for culturing at air-liquid interphase until terminal differentiation to ciliated epithelial monolayers with a minimum transepithelial resistance of 500 Ω·cm2.
Aerosols were generated by a class II spinner electronic cigarette (Vision Vapors). The class II spinners had a manual refillable tank and a 1,600 mAh battery and were operated at a voltage of 4.2 V. The atomizer 2.1 Ω was primed to avoid wick burnout and changed after each exposure session. Laboratory e-liquid consisted of deionized water (vehicle control) or water with 1.8% of nicotine (Sigma Aldrich Cat. No. N3876). For in vitro experiments, HBE monolayers were placed in a custom-built chamber and exposed to aerosols generated by 8-s puffs of 35 mL/min for 10 min. Aerosols distribution was maintained by a positive clean airflow at 1 L/min into the chamber as described previously (16).
Animal Studies
Animal protocols were reviewed and approved by the Institutional Animal Care and Use Committee. Sex-matched Sprague Dawley rats (>8 wk, weighing over 250 g) and ferrets (>22 wk, weighing over >600 g in females and >1,100 g in males) with wild-type CFTR expression were randomly assigned to experimental groups of air control, vehicle aerosols from a 1:1 mixture of propylene glycol, and vegetable glycerin (Fisher Scientific), or 1.8% nicotine in vehicle mixture. During the preceding week, male and female animals were acclimatized progressively in size-appropriate tubes for nose-only inhalation. Aerosols were generated using a computer-controlled e-cigarette robot (Scireq, Montreal, Canada) set at 40 W using a 0.5-Ω atomizer. FlexiWare version 8.0 was used to control the e-cig unit (8-s puffs of 90 mL/min) and a distribution pump connected to an exposure plenum with ports for tubes with rats or ferrets. A constant negative flow of filtered air at 10 L/min was added to the plenum to ensure physiologic levels of oxygen and even the distribution of aerosols. To limit inconsistent performance and buildup of chemical residues from repeated heating, fresh batches of e-liquids and a new atomizer were used for each session. Oxygen, CO2, and CO levels were regularly measured, and animals were continuously monitored for any signs of restraint-related distress. Both rats and ferrets handled inhalation exposures of two 1-h sessions separated by 2–3 h intervals per day with no signs of distress. The resultant levels of nicotine and cotinine in blood of rats are shown in Fig. 2, F and G, respectively.
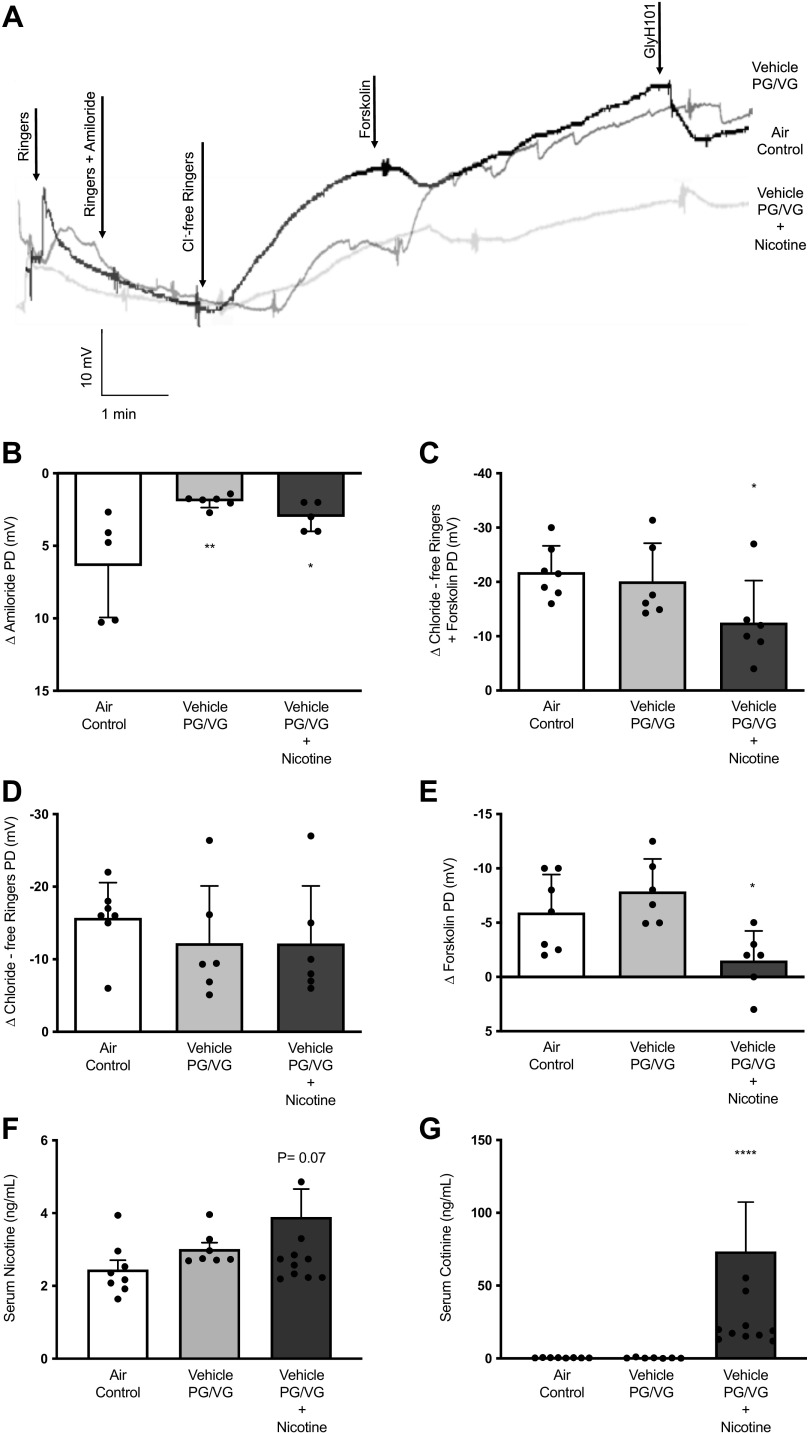
Figure 2.
Nicotine aerosol exposure diminishes rat airway CFTR function in vivo. A: overlay of representative tracings for in vivo nasal potential difference (NPD) measurements in rats following 6 wk of nose-only exposure to room air (Air control) or aerosols generated by an automated electronic cigarette from a vehicle mixture (Vehicle) containing 30% each of propylene glycol (PG) and vegetable glycerin (VG) in water, or PG/VG vehicle mixture + 1.8% nicotine (Nicotine). Summary graphs represent mean potential difference (PD) changes due to inhibition of sodium ion transport by amiloride (B) and total CFTR activity (C), as estimated by voltage changes due to chloride ion gradient + forskolin. Voltage changes are graphed separately for the portion of CFTR channels in open state at baseline and responded to chloride-free ringers and for a fraction of ion channels in closed state at baseline (D) and were opened by infusion of forskolin (E). To accurately predict the systemic burden of rats to nicotine from aerosol inhalation, serum samples were collected at euthanasia. Free nicotine and cotinine, a stable metabolite of nicotine known to circulate, were measured in rat sera using a previously reported mass spectroscopic method. Summary graphs indicate an average increase in serum nicotine (F) and cotinine (G). Data obtained from individual rats (n = 6–8/condition) were averaged, and statistical significance was interpreted by unpaired, two-tailed, nonparametric Mann–Whitney test; *P < 0.05; **P < 0.005, ****P < 0.00005.
CFTR Assay by Ussing Chamber Electrophysiology
CFTR activity in HBE cells and tracheal explants from ferrets were estimated in short-circuit current (Isc) units using modified Ussing chambers (Physiologic Instruments, San Diego, CA) under voltage clamp conditions as previously reported (23, 24). Mounted cell monolayers and tissue segments were bathed in identical ringers solution with 95% O2 and 5% CO2 followed by sequential addition of apical amiloride (100 μM), apical low chloride ringers, apical and basal forskolin (20 μM), and apical CFTR-inh172 (10 μM; HBE cells) or GlyH101 (10 μM for ferret tissues). Acquire and Analyze software (Physiologic Instruments, San Diego, CA) was used to collect and analyze epithelial ion transport changes and measure baseline transepithelial electrical resistance (TEER) before the addition of pharmacologic agents.
CFTR Assay by Nasal Potential Difference Measurement
Under conscious sedation, CFTR activity in the nasal epithelium of rats and ferrets was estimated by nasal potential difference (NPD) as described previously (25). Briefly, a nasal catheter made of PE10 tubing was inserted into a single naris of an anesthetized rat or ferret and connected to a cathode, whereas the anode via connected to a subcutaneous agar bridge. Sequential perfusion of ringers solution for baseline was followed by amiloride (100 μM), chloride-free ringers, forskolin (20 μM), and GlyH101. Chloride ion transport by nasal CFTR was estimated as the mean changes in voltage in response to infusion of chloride-free ringers and forskolin.
Micro-Optical Coherence Tomography Imaging for Mucociliary Transport
Freshly excised tracheal explants from rats and ferrets were placed on a cotton gauge soaked with RPMI-1640 at 37°C. As previously described, the mucosal surface was imaged with a high-resolution reflectance imaging modality called micro-optical coherence tomography (μOCT; 19, 26). Briefly, surface images from eight different locations were acquired with an optical beam (Photonics Superk Extreme high-power supercontinuum White Light Laser, NKT Photonics) scanning longitudinally along the ventral surface, keeping the proximal end of tracheae serving as a reference. Airway surface liquid (ASL) and periciliary liquid (PCL) depths were measured by ImageJ geometric tools. Mucociliary transport (MCT) rate was estimated by tracking native mucus particles moving along the ventral surface over multiple frames and using a previously validated algorithm (15).
Statistics
Descriptive statistics were compared using Mann–Whitney test. Error bars represent SD. All analysis parameters were set as two-sided and with α set to 0.05 to determine significance with GraphPad Prism (La Jolla, CA). SPSS ver. 25 (IBM, Armonk, NY). Pearson correlation matrix was used to examine statistical correlations between CFTR activity determined by NPD and ASL, PCL depth, and MCT rate irrespective of treatment assignment. Pearson correlation matrix was performed with a two-tailed test with confidence intervals set at 95%. GraphPad Prism was used to generate scatterplots representing relationships between dependent and independent variables after confirming the Pearson correlation matrix analysis generated by SPSS.
RESULTS
Nicotine Aerosols Diminish Airway Epithelial ENaC and CFTR Channel Functions In Vitro
The composition of commercial e-liquids varies greatly (27). Not surprisingly, nicotine is integral to most, if not all, ENDs. In pursuit of understanding e-cig safety, a larger research focus has remained on flavoring agents and other constituents in e-liquids. So far, knowledge of how inhaled nicotine aerosols may directly impact the lungs is poorly understood. We evaluated the nicotine aerosol effects on airway epithelial ion transport that maintains physiologic airway surface hydration and MCC defense to address this knowledge gap. We first exposed differentiated HBE cell monolayers to aerosols from an aqueous vehicle and 1.8% nicotine in water for 10 min and compared findings to control cells exposed to laboratory air (Air Control). After incubation for an hour, epithelial ion transport was measured by Ussing Chamber electrophysiology (Fig. 1A). When compared with air controls, HBE cells exposed to nicotine aerosols exhibited a 90% reduction in epithelial Na+ channel (ENaC)-mediated sodium absorption (ΔAmiloride-air control: −76.38 ± 13.09 μA/cm2, nicotine aerosols: −7.20 ± 4.12 μA/cm2, P < 0.05, Fig. 1B). ENaC activity in cells exposed to vehicle aerosols exhibited a downward trend that was statistically insignificant. As shown in Fig. 1C, chloride ion transport by CFTR activator, forskolin, was significantly decreased by nicotine aerosols but not, the vehicle (ΔForskolin-air control: 68.84 ± 11.85 μA/cm2, nicotine: 18.60 ± 5.66 μA/cm2, P < 0.05). In addition, chloride ion transport sensitive to CFTR inhibitor, CFTR-inh172 (ΔCFTR-inh172-air control: −58.71 ± 4.56 μA/cm2; nicotine: −40.25 ± 10.81 μA/cm2, P < 0.05, Fig. 1D) was also reduced in nicotine exposed cells. Despite reductions in ENaC and CFTR by nicotine aerosols, no discernable differences were found in transepithelial electrical resistance (TEER) of HBE cells, indicating these changes were specific to these ion channels and were not due to nonspecific cell death or loss of cell-cell contacts and monolayer integrity (Fig. 1E).
Figure 1.
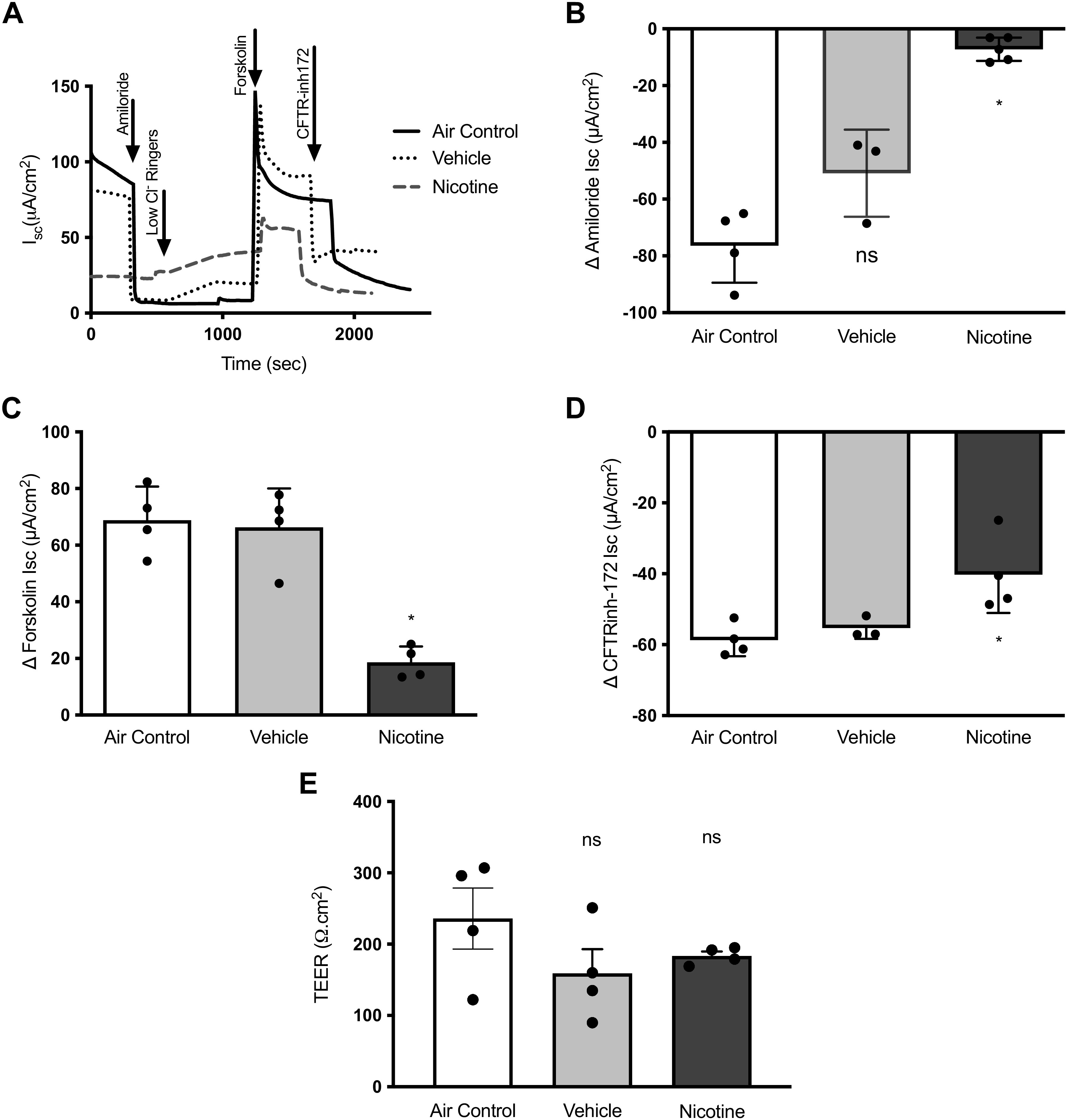
Nicotine aerosols reduce CFTR-mediated ion transport in human bronchial epithelial cells. A: a representative Ussing chamber-based electrophysiological trace depicting transepithelial ion transport measured in short circuit (Isc) units across primary human bronchial epithelial (HBE) cells exposed for 10 min to either room air (Air control), aerosolized water base (Vehicle), or aerosolized 1.8% nicotine in an aqueous vehicle (Nicotine). Summary graphs demonstrate mean changes in amiloride-sensitive sodium ion absorption (B) and CFTR-mediated anion transport by forskolin (C). D: changes in epithelial ion transport attributable to CFTR are shown by changes to the addition of a specific pharmacologic inhibitor, CFTR-inh172. E: a summary of transepithelial electrical resistance (TEER) values for HBE cell monolayers determined at baseline is shown. All Isc values are expressed as means ± SD, and statistical significance was interpreted by unpaired, two-tailed, nonparametric Mann–Whitney test; n = 5 individual HBE monolayers/condition, *P < 0.05, ns, not significant.
Inhaled Nicotine Aerosols Reduce CFTR Function in Rats
In a nose-only inhalation system, wild-type rats were administered daily with control room air, aerosols from propylene glycol (PG)/vegetable glycerin (VG) vehicle, or 1.8% nicotine in PG/VG vehicle. After 6 wk, transepithelial CFTR activity was assayed by nasal potential difference (NPD) measurement in rats under conscious sedation (Fig. 2A). NPD is a reliable tool for evaluating CFTR function in laboratory animals and patients suspected of cystic fibrosis. NPD analysis found decreased amiloride-sensitive sodium absorption by ENaC channels in rats exposed to PG/VG vehicle with and without nicotine to a similar degree (Δ Amiloride-air control: 6.38 ± 3.56 mV, vehicle PG/VG: 1.92 ± 0.44 mV, nicotine + vehicle PG/VG: 2.75 ± 0.96 mV, Fig. 2B). Interestingly, total CFTR activity was selectively reduced by nicotine aerosols and remained unaffected in animals exposed to only vehicle aerosols (Δ Chloride-free Ringers + Forskolin-air control: −21.79 ± 4.85 mV, PG/VG: −20.08 ± 7.05 mV, nicotine + PG/VG: −12.50 ± 7.77 mV, P < 0.05, Fig. 2C). Differential analysis of CFTR channels by baseline status (open/close at baseline) offered more insight. The portion of CFTR channels that were active at baseline and responded to chloride ion gradient was only modestly impacted by either aerosols (Δ Chloride-free Ringers-air control: −15.71 ± 4.86 mV, vehicle PG/VG: −12.21 ± 7.88 mV, nicotine + PG/VG: −12.17 ± 7.94 mV, P = 0.22, Fig. 2D). However, CFTR channels that were in a closed state at baseline and needed forskolin for the opening were reduced by 72% due to nicotine but not by vehicle aerosols (Δ Forskolin: air control: −5.29 ± 3.49 mV, vehicle PG/VG: −7.87 ± 3.00 mV; nicotine + PG/VG: −1.50 ± 2.74 mV; P < 0.05; Fig. 2E). Thus, CFTR was reduced explicitly by nicotine aerosols after a 6-wk exposure in rats.
Mucus Transport Is Delayed in Rats Exposed to Inhaled Nicotine Aerosols
Rat tracheal explants were imaged with micro-optical coherence tomography (μOCT) to evaluate the consequence of CFTR dysfunction by nicotine aerosols on mucus clearance. A core strength of this IR-based reflectance imaging is the ability to support integrated analyses of airway surface liquid depths and mucus transport without the use of external contrast agents or fluorescent microparticles (28). Thus, ASL and PCL depths, along with mucus transport (MCT) rates, were estimated in freshly isolated airway specimens (Fig. 3A). In rats exposed to PG/VG vehicle aerosols, there was a trend toward increased tracheal ASL height. In contrast, there was no change in ASL depth in rats administered with nicotine (ASL height-air control: 32.67 ± 25.01 μm, vehicle PG/VG: 74.21 ± 60.26 μm, nicotine + PG/VG: 24.57 ± 9.09 μm, P = ns, Fig. 3B). We next analyzed the height of PCL that extends the length of the ciliary projections and lubricates the ciliary motions. Interestingly, PCL depth was specifically affected in rats exposed to nicotine by 49% and not in vehicle-treated animals (PCL depth-air control: 5.97 ± 0.67 μm, vehicle PG/VG: 5.79 ± 0.57 μm, nicotine + PG/VG: 2.93 ± 0.33 μm, P < 0.0005, Fig. 3C). Following a similar trend, nicotine aerosols exposure caused an 85% decrease in MCT rate, whereas vehicle treatment caused no change (MCT rate-air control: 54.86 ± 10.24 μm/s, vehicle PG/VG: 49.94 ± 5.32 μm/s, nicotine + vehicle: 8.15 ± 1.66 μm/s; P < 0.005; Fig. 3D). Overall, studies in the rat model demonstrate the dramatic impact of inhaling nicotine as aerosols on airway MCC defense.
Figure 3.
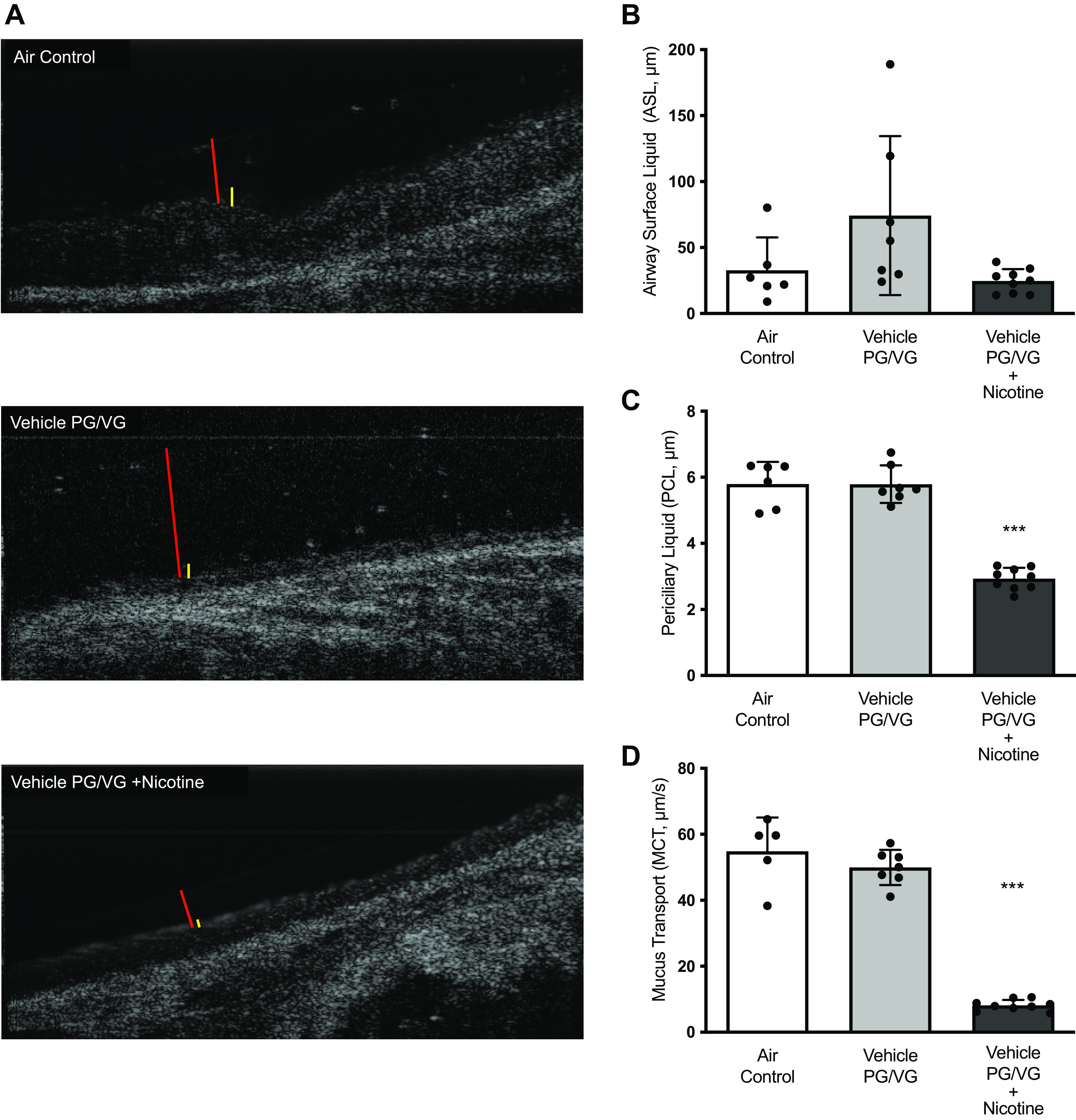
Exposure to nicotine aerosols impairs physiologic maintenance of airway surface hydration and mucus transport in rats. A: representative micro-optical coherence tomography (μOCT) images of freshly exercised trachea from rats exposed to 6 wk of nose-only control room air (Air Control), aerosols generated by an automated electronic cigarette from vehicle mixture (Vehicle) containing 30% each of propylene glycol (PG) and vegetable glycerin (VG), or PG/VG vehicle + 1.8% nicotine (Nicotine). Airway surface liquid (ASL) and periciliary liquid layer (PCL) depths are indicated by red and yellow bars. The movement of native mucus particles was tracked at multiple regions of interest on airway surface to derive the mean rate of mucociliary transport (MCT). Summary graphs illustrate the mean differences in ASL depth (B), PCL depth (C), and MCT rate (D) on rat airways. Statistical significance was interpreted by unpaired, two-tailed, nonparametric Mann–Whitney test n = 6–9/condition; ***P < 0.001.
Nicotine Aerosols Decrease CFTR-Mediated Anion Transport in Ferrets
Building on findings regarding nicotine aerosols in human cells and rats, we conducted validation studies in ferrets. Ferrets are larger than rats and closely mirror submucosal gland distribution and lung physiology in humans, and have proven invaluable in modeling muco-obstruction and chronic bronchitis seen in patients with cystic fibrosis and COPD (29–33). Wild-type ferrets were exposed to 6 wk of control room air or aerosols from nicotine in PG/VG vehicles via nose-only inhalation. We did not test PG/VG vehicle aerosols in ferrets since rat data demonstrated no adverse impacts on CFTR activity, PCL depth, or MCT rate. Moreover, these observations are in line with previous studies in mice, rats, dogs, and monkeys exposed to aerosolized PG and VG and found no evidence of significant pulmonary toxicity (34–37).
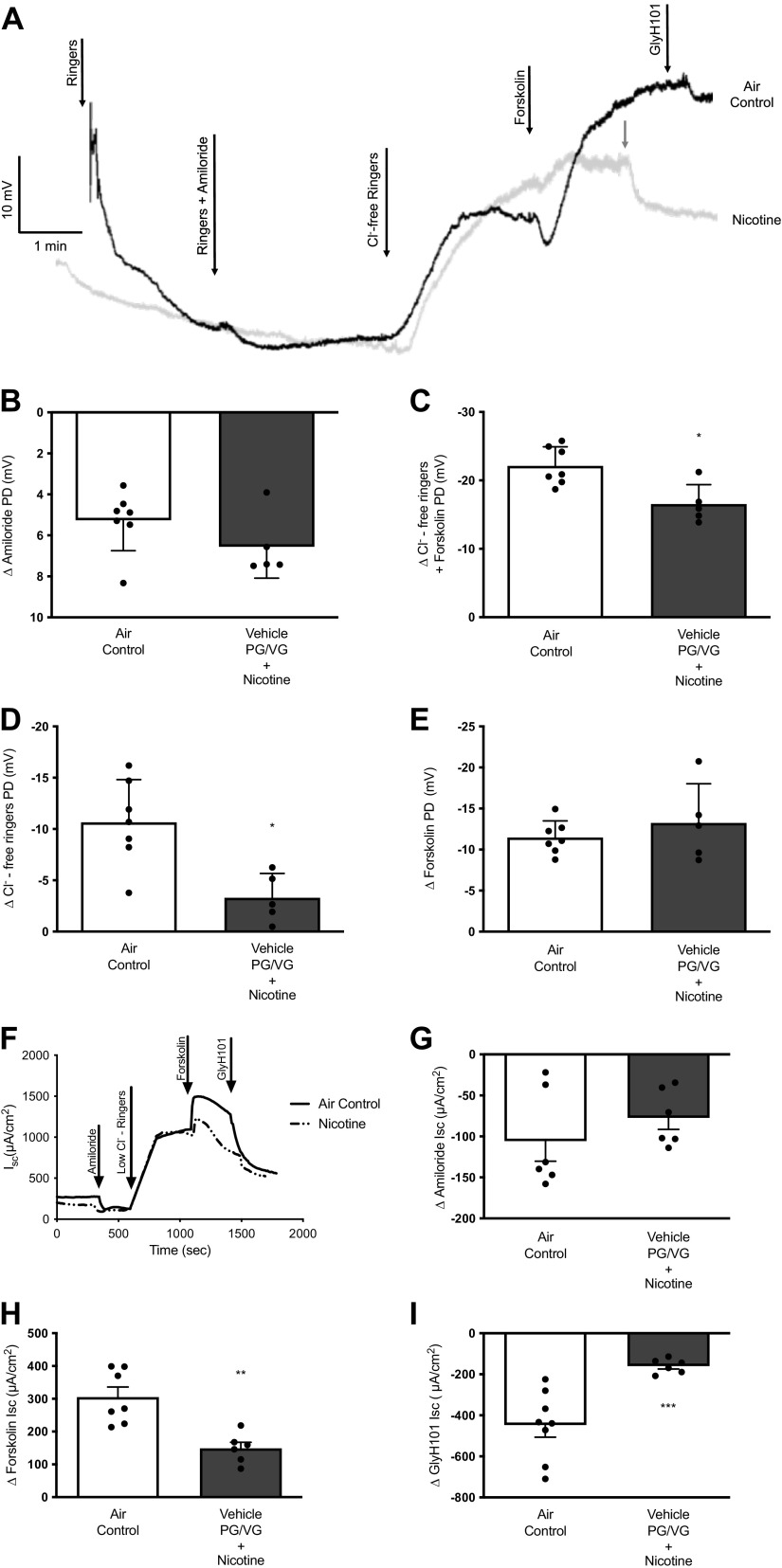
In ferrets, in vivo CFTR activity was assessed by NPD using a previously published protocol (Fig. 4A; 38). NPD analysis revealed a slight but nonsignificant increase in amiloride-sensitive ENaC function (Δ Amiloride-air control: 5.26 ± 1.49 mV, nicotine: 6.56 ± 1.53 mV, Fig. 4B). These data differ from the rat ENaC results, which demonstrated a decrease in response to both vehicle and nicotine aerosols. However, consistent with rat findings, nicotine aerosols caused a significantly lower nasal CFTR activity (Δ Chloride-free Ringers + forskolin-air control: −22.11 ± 2.80 mV, nicotine: −16.54 ± 2.85 mV, P < 0.05, Fig. 4C). When CFTR channels were differentiated based on their baseline channel status (open/close), nicotine exposure caused a reduction in CFTR channels that were open at baseline and responded to chloride ion gradient (Δ Chloride-free Ringers-air control: −10.65 ± 4.17 mV, nicotine: −3.29 ± 2.37, P < 0.05, Fig. 4D). However, the portion of CFTR channels that were in a closed state at baseline (assessed by lack of response to chloride ion gradient but demonstrating a change in voltage following the addition of forskolin) was not affected by aerosolized nicotine (Δ Forskolin-air control: −11.47 ± 2.02 mV, nicotine: −13.25 ± 4.77 mV, Fig. 4E). Next, to directly measure CFTR activity in the airway, ferret tracheal explants were analyzed by Ussing chamber electrophysiology (Fig. 4F). As compared with air controls, ferrets administered with nicotine aerosols exhibited a 36% loss in tracheal ENaC activity (Δ Amiloride-air control: −121.64 ± 44.33 μA/cm2, nicotine: −77.24 ± 38.94 μA/cm2, P < 0.05; Fig. 4G). These data differ from ENaC activity in the ferret nasal epithelium, suggesting nicotine effects are site-specific along the airways. Yet, consistent with all the previous data, nicotine aerosols exposure caused a 51% decrease in tracheal CFTR activity in ferrets (Δ Forskolin-air control: 305.10 ± 81.42 μA/cm2, nicotine: 149.00 ± 45.36 μA/cm2, P < 0.005, Fig. 4H). Mirroring this trend of diminished CFTR activation, change in tracheal Isc by CFTR inhibitor, GlyH101, was decreased by 64% in nicotine-exposed ferrets (Δ GlyH101-air control: −447.30 ± 167.20 μA/cm2, nicotine: −159.80 ± 35.22 μA/cm2, P < 0.0005, Fig. 4I). Thus, electrophysiological data from ferrets exposed to nicotine aerosols validate a sustained loss of airway CFTR function observed in rat and human airway epithelial cells.
Figure 4.
Inhalation of nicotine aerosols reduced airway CFTR function in ferrets. A: representative electrophysiologic tracings for in vivo nasal potential difference (NPD) measurements in ferrets following 6 wk of nose-only control room air (Air control) or nicotine aerosols (Nicotine). Summary graphs illustrate potential difference (PD) changes in amiloride inhibition of sodium ion transport by ENaC (B) and total CFTR function (C), as estimated by combining voltage changes following perfusion of chloride-free ringers that sets a chloride ion gradient across nasal epithelium and forskolin. CFTR-dependent voltage changes are represented by distinguishing channels in the open conformation (D) at baseline and responded to perfusion of chloride-free ringers and the proportion of channels in the closed conformation (E) at baseline and were activated by forskolin perfusion. F: representative electrophysiological tracings for tracheal explants in Ussing chambers indicating transepithelial ion transport properties. Summary graphs illustrate changes in short-circuit currents (Isc) following amiloride inhibition of ENaC (G) and forskolin-stimulated CFTR-mediated ion transport (H) under an apical chloride ion gradient. I: specific inhibitor GlyH101 distinguished epithelial ion transport that was attributable to CFTR channels. Means ± SD values represent 6–7 ferrets/condition, and statistical significance was interpreted by unpaired, two-tailed, nonparametric Mann–Whitney test; *P < 0.05; **P < 0.005; ***P < 0.0005.
Nicotine Aerosols Reduce Periciliary Liquid Depth and Mucociliary Transport in Ferrets
Ferret tracheal explants were imaged with μOCT before CFTR assessment by Ussing chamber electrophysiology, as described earlier (Fig. 5A). μOCT image analyses identified no differences in tracheal ASL depth (ASL depth-air control: 8.39 ± 4.85 μm, nicotine: 10.04 ± 9.09 μm, P = ns, Fig. 5B). However, as observed in rats, ferrets exposed to nicotine aerosols presented with a significant decrease in tracheal periciliary liquid (PCL) depth (PCL depth-air control: 5.96 ± 0.46 μm, nicotine: 3.89 ± 0.29 μm, P < 0.0005, Fig. 5C). Furthermore, this reduced PCL hydration coincided with a 49% loss in mucus transport (MCT) in nicotine-exposed ferret airways (MCT rate-air control: 136.50 ± 17.63 μm/s, nicotine: 69.37 ± 9.16 μm/s, P < 0.05, Fig. 5D). Overall, these data demonstrate that nicotine aerosols dramatically alter physiologic regulation of airway mucosal surface hydration and MCC defense, foreshadowing likely impacts on lung health in the long term.
Figure 5.
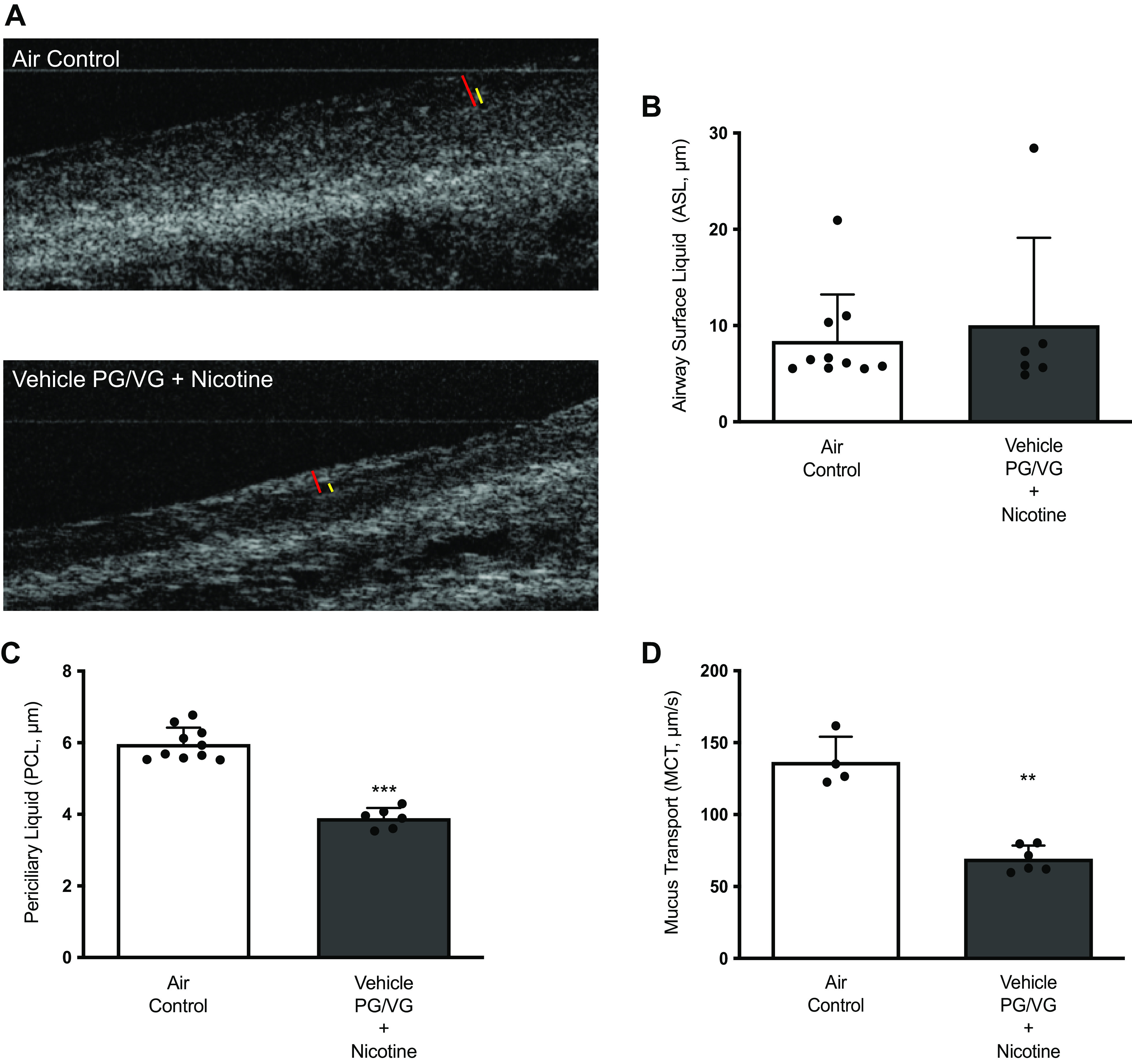
Ferret airway mucosal surface hydration and mucociliary clearance are reduced by chronic nicotine exposure. Representative micro-optical coherence tomography (μOCT) images of ferret tracheal explants following exposure to air or nicotine aerosols for 6 wk (A). Nicotine aerosol effects on airway surface hydration were assessed by measuring airway surface liquid (ASL) depth as denoted by red bars (B) and periciliary liquid layer (PCL) depth as denoted by yellow bars (C). Changes in mucus transport (MCT) rates by nicotine aerosols were estimated by tracking native mucus particle movement (D). Data obtained from individual ferrets (n = 8–10/condition) were averaged, and statistical significance was interpreted by unpaired, two-tailed, nonparametric Mann–Whitney test. **P < 0.005; ***P < 0.0005.
CFTR Dysfunction Directly Contributes to Diminished Mucociliary Defense
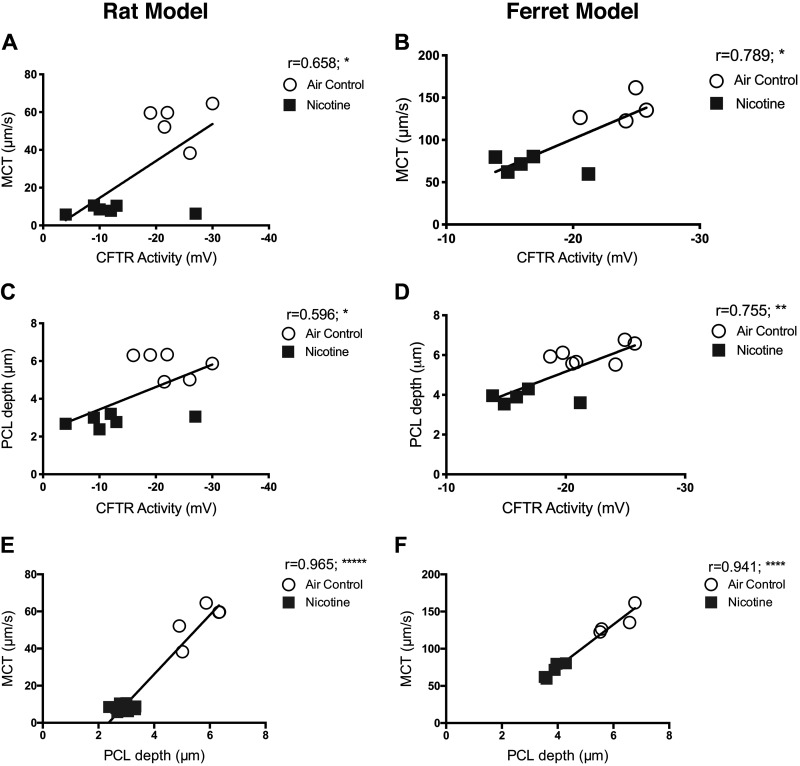
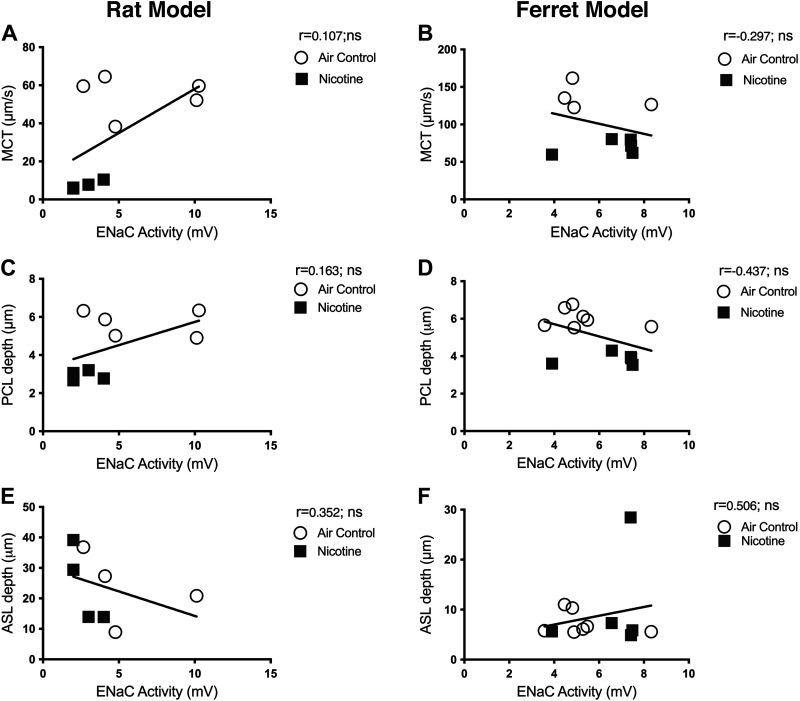
To assess any causal link between nicotine-induced CFTR dysfunction and diminished lung defense via MCC, we compared data obtained in both rat and ferret models. Specifically, CFTR activity determined in vivo by NPD and tracheal MCT in rats and ferrets exposed to air and nicotine aerosols was plotted. Pearson correlation plots reveal CFTR activity demonstrates a moderate positive association with MCT rate (rat: r = 0.658, *P < 0.05; ferret: r = 0.789, *P = <0.05, Fig. 6, A and D) and PCL depth (rat: r = 0.596, *P < 0.05; ferret: r = 0.755, **P = <0.005, Fig. 6, B and E). These trends suggested the positive correlation between nicotine-induced CFTR dysfunction and reduced MCT due to inefficient airway hydration observed as depleted PCL volume. To confirm this possibility that was previously observed in CFTR knockout rats (19), we plotted PCL depth and MCT rates in rats and ferrets. As shown in Fig. 6, C and F, PCL depth exhibits a strong positive association with MCT (rat: r = 0.965, *****P < 0.000005; ferret: r = 0.941, ****P < 0.00005). Even so, ASL height displayed no significant correlations with either CFTR function (Fig. 7, A and B) or MCT (Fig. 7, C and D). Thus, in both rats and ferrets, disruption of CFTR-mediated epithelial ion transport manifests decreased airway mucus hydration and delayed ciliary clearance. Interestingly, ENaC function was affected by nicotine aerosols in rats but not in ferrets. But, the correlative analysis found no direct association of ENaC activity on PCL depth or mucus transport rate in both animal models (Fig. 8).
Figure 6.
Reduced CFTR function by nicotine directly correlates with loss of periciliary liquid depth and mucus transport in rat and ferret airways. Functional relationships between CFTR-mediated ion transport activity by in vivo nasal potential difference (NPD) measurement and tracheal airway surface liquid (ASL) and periciliary liquid (PCL) depths and mucociliary transport (MCT) estimated by micro-optical coherence tomography (μOCT) imaging were evaluated by Pearson correlation in SPSS. Data from experiments conducted with 13–14 rats and 9–12 ferrets are used for each correlation shown, irrespective of treatment assignment. All correlations are expressed in two-tailed and at 95% confidence intervals. A and D: there was a moderately significant positive association between CFTR activity and MCT rate (rat: r = 0.658, *P < 0.05; ferret: r = 0.789, *P < 0.05). B and E: comparison of NPD and PCL depth illustrates a significantly moderate positive correlation (rat: r = 0.596, *P < 0.05; ferret: r = 0.755, **P < 0.005). C and F: PCL depth and MCT plot demonstrated a very strong positive correlation in both rat and ferret lungs (rat: r = 0.965, *****P < 0.000005; ferret: r = 0.941, ****P < 0.00005).
Figure 7.
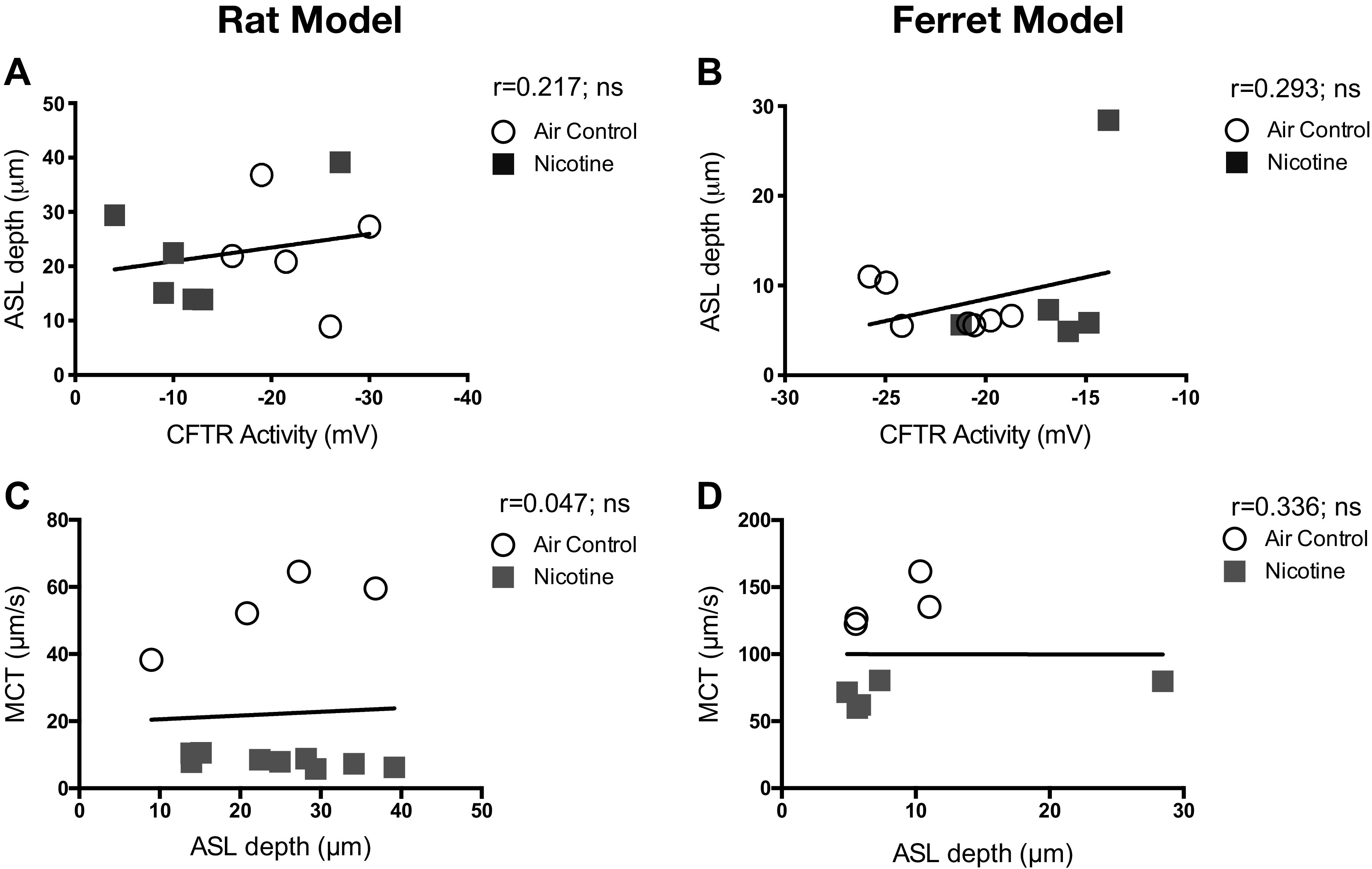
Airway surface liquid depth does not correlate with CFTR activity or determine mucus transport rate in rat and ferret airways. Pearson correlations indicate the functional relationship of airway surface liquid (ASL) depth to CFTR-mediated ion transport activity and the consequent impact on mucociliary transport (MCT) rate. Data shown represent experiments conducted with 13–14 rats and 9–12 ferrets representing all treatment groups. All correlations are expressed in two-tailed and at 95% confidence intervals. There was no significant positive association between CFTR activity and ASL height (A and B) or between ASL depth and MCT rate (C and D) in both rat and ferret lungs; ns, not significant.
Figure 8.
Airway ENaC-mediate ion transport activity does not correlate with mucus physiology parameters in rat and ferret airways. Pearson correlations indicate the functional relationship of mucociliary transport (MCT) rate, air surface liquid (ASL), and periciliary liquid (PCL) depths to ENaC-mediated ion transport activity by nasal potential difference estimates in mV. The data shown represent experiments conducted with 13–14 rats (A, C, and E) and 9–12 ferrets (B, D, and F), irrespective of treatment assignment. All correlations are expressed in two-tailed and at 95% confidence intervals. There was no significant association between ENaC activity and mucus parameters in both rat and ferret lungs. Ns, not significant.
DISCUSSION
The popularity of e-cigs is built primarily on the perception that inhaling nicotine is safe. The lack of a direct cancer link likely led to framing the conversation of tobacco safety in terms of ingredients beyond nicotine. For many addictive substances such as caffeine and alcohol, they are products available with and without the primary addictive agent. In contrast, there were no nicotine-free cigarettes to isolate nicotine-specific effects on health. Thus, it is no surprise that most tobacco products are not required to list their nicotine content but include a statement on the abuse potential. As a result, nicotine is predominantly evaluated for its effects on the brain and not extensively investigated for its potential to harm the functions of peripheral organs, including the lungs. Now, this trend of limited or lack of focus on nicotine extends to research on e-cigs. Thus, we focused on investigating nicotine-specific effects on lung defenses when administered as aerosols.
To the best of our knowledge, this is the first study that directly links inhaled nicotine aerosols to reduced mucociliary defense in vivo. We found that the adverse impact of nicotine on mucociliary clearance defense in the lungs was due to inhibitory effects on epithelial ion transport via CFTR, the defective channel in cystic fibrosis, and smoking-induced COPD (24, 39). Although these studies are consistent with in vitro effects by e-cigs reported by us (16) and others (13), we provide mechanistic data that demonstrates how decreased CFTR-mediated ion transport leads to diminished PCL depth compromising ciliary clearance of surface mucus.
The current studies on nicotine aerosols extend previous studies where it was delivered as an injectable liquid (14) or delivered via a nebulized e-liquid (13) before assessing MCC. Although many studies with commercial e-cigs are well-intentioned, they may be affected by inconsistent nicotine content and, sometimes, misleading due to inaccurate labeling (9). For many years, research on cigarettes benefited from standardized Research Cigarettes developed by the University of Kentucky. Currently, no such standardized equivalents exist for e-liquids for use by individual laboratories to arrive at reproducible findings. In our study, nicotine was administered with just PG and VG that made up the vehicle mixture and avoided using any flavoring agents. We changed atomizers after each use to maintain uniform aerosol output and minimize inconsistencies reported with commercial e-cig devices (40). However, despite the physiologic relevance of both our in vitro and in vivo delivery systems, these studies are limited by the absence of other agents typically present in e-liquids, such as flavoring agents. More importantly, the concentration of 1.8% nicotine in our e-liquids more accurately represents earlier-generation POD devices where nicotine concentration in sputum from vaping freebase nicotine was similar to that seen in cigarette (41). The newer Pod-based devices contain higher concentrations of nicotine (>60 mg/mL or >6% vol/vol) in salt form achieving transfer efficiency to inhalable aerosol at higher levels as well (>40 mg/mL) (42). Thus, the data presented here fall short in predicting the dose-dependent effects of nicotine on MCC and defining direct links to lung disease risk. Another important variable is the design of e-cig device used for cells and animal studies. The commercial e-cig devices used for in vitro studies with human cells closely approximate human e-cig use. However, using the same human e-cigs in animal models proved challenging since 1) the aerosol generation was inconsistent when the unit was operated for >20 min; and 2) larger volumes of aerosols were needed each day to simultaneously recapitulate human-level nicotine intake in groups of rats and ferrets, typically several times larger than mice. Despite the differences in devices, e-liquid composition, and nicotine delivery parameters, animal studies described here are relevant to model common human use and match recent publications from multiple laboratories in terms of laboratory e-cig devices.
Nicotine is known to directly interact with several nicotinic acetylcholine receptors (nAChRs) such as α-3, α-4, α-5, α-7, α-9, β-2, and β-4 on airway epithelial cells (43, 44) and modulates lung development (45) and function. Interestingly, when α7nAChR was deleted in mice, systemically administered nicotine failed to reduce mucus transport, whereas wild-type mice exhibited CFTR dysfunction and delayed MCC (14). Directly relevant to nicotine in e-cigs, nebulized nicotine in HBE cells decreased CFTR-mediated ion transport more than aerosolized PG/VG (12). In vivo studies in rats and ferrets similarly indicate nicotine, but not vehicle aerosols disrupt CFTR activity. Previously, mice injected with nicotine displayed a decreased CFTR function associated with reduced MCT estimated by the airway clearance of polystyrene fluorescent microspheres (14). Interestingly, when mice were exposed to nicotine aerosols, there was no change in MCT measured by radiographic clearance of 99mTc-SC compared with untreated controls (34). However, in the same study, the presence of nicotine in the aerosols blunted the increased MCT found in mice exposed to the vehicle aerosols without nicotine (34). Although mice are predictive of electrophysiological changes in CFTR function, they show no differences in mucus properties or MCC limiting their translational utility. Lack of CFTR in mice does not result in hallmark cystic fibrosis symptoms of mucus obstruction, inflammation, or chronic pathogen infections (46, 47). Thus, to determine how nicotine aerosols may impact CFTR and MCC in humans, we used ferrets and rats that are sensitive to changes in CFTR activity and are replicative of human lung mucus physiology (31, 46–49). In our study, we found no change in MCT in rats exposed to vehicle aerosols, but there was a trend toward increased ASL that was not meaningfully influencing the MCT (Fig. 7). Thus, this report clarifies the interrelationships between nicotine-induced CFTR dysfunction and loss of MCC that were not fully established in the past.
Nicotine-induced CFTR dysfunction was evident in human cells, rats, and ferrets. However, decreased ENaC activity was apparent in human cells and rats but not in ferrets. Hence in ferrets, in addition to the nasal epithelium, we also directly analyzed ENaC activity in the lower airways and still found no significant differences with nicotine aerosols. But, most importantly, the correlative analysis found no direct association of ENaC activity in both animal models on PCL depth or mucus transport rate (Fig. 8). Thus, our data assign more importance to the nicotine-induced defects in CFTR over ENaC dysfunction. Moreover, these data establish that there may be species-specific effects of nicotine that need to be carefully considered when interpreting the translational significance. These channel-specific effects are significant since CFTR and ENaC are regulated differently in the airways. In our study, we observed that nicotine inhibited both CFTR and ENaC in the airways, but only CFTR activity determined PCL depth and MCT rate. Although we do not have a precise mechanism for this effect at this time, nicotine may be acting through a common signaling pathway or by directly affecting the proteins themselves. It is relevant to note that unlike in inherited conditions such as pseudohypoaldosteronism or cystic fibrosis, smoking has been shown to disrupt both CFTR and ENaC function in smokers (17, 23) and patients with COPD (50) indiscriminately and still delays mucus clearance (15). Thus, this report is a detailed exploration of the physiologic mechanisms underlying nicotine effects on lung host defense. Ultimately, we intend our study to isolate the singular effects of nicotine and emphasize the need for focused research on it as a primary agent capable of respiratory harm.
Limitations in our study are the exclusion of PG/VG vehicle aerosol control for ferrets based on the lack of vehicle-specific effects in rats. However, the lack of changes in CFTR and MCT in rats exposed to PG/VG vehicle aerosols validate some previous studies where they had minimal impact on lung morphology (35–37) but not others that strongly establish there is enough harm from inhaling these vehicle ingredients (51). Based on our data, we feel confident that the adverse effects on MCT are more likely due to nicotine itself. Yet, our analysis does not extend to nicotine metabolites like cotinine and how their half-lives and biological effects may impact respiratory health in humans. The lack of cutoff for nicotine metabolites such as cotinine limit direct extrapolation of biological effects to e-cig users.
The short duration of e-cig aerosol exposure in our animal studies, while more than sufficient to demonstrate defects in physiologic epithelial ion transport and mucociliary clearance rate, did not cause apparent and uniform changes in airway abnormalities, remodeling, or injury. A pathologist blinded to treatment assignment did not find any significant differences in qualitative scores for mucus production, goblet cell hyperplasia, and epithelial hypertrophy. These data emphasize the need for prolonged exposure periods to enable detailed insights into lung disease processes. A statistical nuance worth noting is that we used the Mann–Whitney test since it involves a comparison of unpaired test samples (rats and ferrets were paired as groups and not on individual levels). As such, Gaussian distribution was not assumed due to the size of each cohort and the overall differences in animals at baseline. For these nonnormal distributions, we considered Mann–Whitney and Kolmogorov–Smirnov tests. The latter is generally accepted for comparing cumulative distributions. Since our data set does not meet the statistical and community-accepted standard for the Kolmogorov–Smirnov test, we elected the Mann–Whitney test to confirm statistical significance between experimental groups with an α set at 0.05. Thus, we find the Mann–Whitney test to be more appropriate than unpaired t tests that are based on the assumption that both experimental groups have the same SD.
In conclusion, nicotine inhaled as aerosols can directly reduce airway CFTR function and significantly diminish the ability of the lungs to maintain the MCC defense against inhaled pathogens and particulate matter. These data indicate that chronic use of e-cigs with nicotine might lead to increased respiratory infections and much-obstructive diseases such as COPD. These data support avoiding all nicotine use and prompt more clinical studies to ascertain the long-term health impact of all inhaled nicotine products.
DATA AVAILABILITY
Data will be made available upon reasonable request.
GRANTS
This work was supported in part by Flight Attendant Medical Research Institute Grant YFA130008 (to S. V. Raju) and by National Institutes of Health Grants R01AA027528, P30DK072482, and R35HL135816 (to S. V. Raju).
DISCLAIMERS
Sponsors had no role in study design, data interpretation, and publication.
DISCLOSURES
S. V. Raju received consulting fees or grants from Celgene, Spirovant, and Pulmotect for unrelated work. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
L.W.R., D.S., and S.V.R. conceived and designed research; L.W.R., D.S., J.L., and A.D.A. performed experiments; L.W.R., D.S., and S.V.R. analyzed data; L.W.R., D.S., and S.V.R. interpreted results of experiments; L.W.R. and S.V.R. prepared figures; L.W.R. and S.V.R. drafted manuscript; L.W.R. and S.V.R. edited and revised manuscript; L.W.R., D.S., J.L., A.D.A., and S.V.R. approved final version of manuscript.
REFERENCES
- 1.World Health Organization. Chronic Obstructive Pulmonary Disease (COPD) (Online). World Health Organization, 2017. [Google Scholar]
- 2. Haq I, Chappell S, Johnson SR, Lotya J, Daly L, Morgan K, Guetta-Baranes T, Roca J, Rabinovich R, Millar AB, Donnelly SC, Keatings V, MacNee W, Stolk J, Hiemstra PS, Miniati M, Monti S, O'Connor CM, Kalsheker N. Association of MMP-2 polymorphisms with severe and very severe COPD: a case control study of MMPs-1, 9 and 12 in a European population. BMC Med Genet 11: 7, 2010. doi: 10.1186/1471-2350-11-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dai H, Leventhal AM. Prevalence of e-cigarette use among adults in the United States, 2014-2018. JAMA 322: 1824–1827, 2019. doi: 10.1001/jama.2019.15331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Herrington JS, Myers C. Electronic cigarette solutions and resultant aerosol profiles. J Chromatogr A 1418: 192–199, 2015. doi: 10.1016/j.chroma.2015.09.034. [DOI] [PubMed] [Google Scholar]
- 5. Kucharska M, Wesołowski W, Czerczak S, Soćko R. [Testing of the composition of e-cigarette liquids—manufacturer-declared vs. true contents in a selected series of products]. Med Pr 67: 239–253, 2016. doi: 10.13075/mp.5893.00365. [DOI] [PubMed] [Google Scholar]
- 6. McNeill A, Brose LS, Calder R, Bauld L, Robson D. Evidence review of e-cigarettes and heated tobacco products 2018. A report commissioned by Public Health England. London: Public Health England, 2018. [Google Scholar]
- 7. Wang TW, Asman K, Gentzke AS, Cullen KA, Holder-Hayes E, Reyes-Guzman C, Jamal A, Neff L, King BA. Tobacco product use among adults—United States, 2017. MMWR Morb Mortal Wkly Rep 67: 1225–1232, 2018. doi: 10.15585/mmwr.mm6744a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cullen KA, Gentzke AS, Sawdey MD, Chang JT, Anic GM, Wang TW, Creamer MR, Jamal A, Ambrose BK, King BA. e-Cigarette use among youth in the United States, 2019. JAMA 322: 2095, 2019. doi: 10.1001/jama.2019.18387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Goniewicz ML, Kuma T, Gawron M, Knysak J, Kosmider L. Nicotine levels in electronic cigarettes. Nicotine Tob Res 15: 158–166, 2013. doi: 10.1093/ntr/nts103. [DOI] [PubMed] [Google Scholar]
- 10. Goniewicz ML, Knysak J, Gawron M, Kosmider L, Sobczak A, Kurek J, Prokopowicz A, Jablonska-Czapla M, Rosik-Dulewska C, Havel C, Jacob P, Benowitz N. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control 23: 133–139, 2014. doi: 10.1136/tobaccocontrol-2012-050859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Herman M, Tarran R. E-cigarettes, nicotine, the lung and the brain: multi-level cascading pathophysiology. J Physiol 598: 5063–5071, 2020. doi: 10.1113/JP278388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Garcia-Arcos I, Geraghty P, Baumlin N, Campos M, Dabo AJ, Jundi B, Cummins N, Eden E, Grosche A, Salathe M, Foronjy R. Chronic electronic cigarette exposure in mice induces features of COPD in a nicotine-dependent manner. Thorax 71: 1119–1129, 2016. doi: 10.1136/thoraxjnl-2015-208039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chung S, Baumlin N, Dennis JS, Moore R, Salathe SF, Whitney PL, Sabater J, Abraham WM, Kim MD, Salathe M. Electronic cigarette vapor with nicotine causes airway mucociliary dysfunction preferentially via TRPA1 receptors. Am J Respir Crit Care Med 200: 1134–1145, 2019. doi: 10.1164/rccm.201811-2087OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Maouche K, Medjber K, Zahm JM, Delavoie F, Terryn C, Coraux C, Pons S, Cloëz-Tayarani I, Maskos U, Birembaut P, Tournier JM. Contribution of α7 nicotinic receptor to airway epithelium dysfunction under nicotine exposure. Proc Natl Acad Sci USA 110: 4099–4104, 2013. doi: 10.1073/pnas.1216939110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Raju SV, Lin VY, Liu L, McNicholas CM, Karki S, Sloane PA, Tang L, Jackson PL, Wang W, Wilson L, Macon KJ, Mazur M, Kappes JC, DeLucas LJ, Barnes S, Kirk K, Tearney GJ, Rowe SM. The cystic fibrosis transmembrane conductance regulator potentiator ivacaftor augments mucociliary clearance abrogating cystic fibrosis transmembrane conductance regulator inhibition by cigarette smoke. Am J Respir Cell Mol Biol 56: 99–108, 2017. doi: 10.1165/rcmb.2016-0226OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lin VY, Fain MD, Jackson PL, Berryhill TF, Wilson LS, Mazur M, Barnes SJ, Blalock JE, Raju SV, Rowe SM. Vaporized E-cigarette liquids induce ion transport dysfunction in airway epithelia. Am J Respir Cell Mol Biol 61: 162–173, 2019. doi: 10.1165/rcmb.2017-0432OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rayner RE, Makena P, Prasad GL, Cormet-Boyaka E. Cigarette and ENDS preparations differentially regulate ion channels and mucociliary clearance in primary normal human bronchial 3D cultures. Am J Physiol Lung Cell Mol Physiol 317: L295–L302, 2019. doi: 10.1152/ajplung.00096.2019. [DOI] [PubMed] [Google Scholar]
- 18. Tierney PA, Karpinski CD, Brown JE, Luo W, Pankow JF. Flavour chemicals in electronic cigarette fluids. Tob Control 25: e10–e15, 2016. doi: 10.1136/tobaccocontrol-2014-052175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Birket SE, Chu KK, Liu L, Houser GH, Diephuis BJ, Wilsterman EJ, Dierksen G, Mazur M, Shastry S, Li Y, Watson JD, Smith AT, Schuster BS, Hanes J, Grizzle WE, Sorscher EJ, Tearney GJ, Rowe SM. A functional anatomic defect of the cystic fibrosis airway. Am J Respir Crit Care Med 190: 421–432, 2014. doi: 10.1164/rccm.201404-0670OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lin VY, Kaza N, Birket SE, Kim H, Edwards LJ, LaFontaine J, Liu L, Mazur M, Byzek SA, Hanes J, Tearney GJ, Raju SV, Rowe SM. Excess mucus viscosity and airway dehydration impact COPD airway clearance. Eur Respir J 55: 1900419, 2020. doi: 10.1183/13993003.00419-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xie W, Kathuria H, Galiatsatos P, Blaha MJ, Hamburg NM, Robertson RM, Bhatnagar A, Benjamin EJ, Stokes AC. Association of electronic cigarette use with incident respiratory conditions among US adults from 2013 to 2018. JAMA Netw Open 3: e2020816, 2020. doi: 10.1001/jamanetworkopen.2020.20816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pyle LC, Ehrhardt A, Mitchell LH, Fan L, Ren A, Naren AP, Li Y, Clancy JP, Bolger GB, Sorscher EJ, Rowe SM. Regulatory domain phosphorylation to distinguish the mechanistic basis underlying acute CFTR modulators. Am J Physiol Lung Cell Mol Physiol 301: L587–L597, 2011. doi: 10.1152/ajplung.00465.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sloane PA, Shastry S, Wilhelm A, Courville C, Tang LP, Backer K, Levin E, Raju SV, Li Y, Mazur M, Byan-Parker S, Grizzle W, Sorscher EJ, Dransfield MT, Rowe SM. A pharmacologic approach to acquired cystic fibrosis transmembrane conductance regulator dysfunction in smoking related lung disease. PLoS One 7: e39809, 2012. doi: 10.1371/journal.pone.0039809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Raju SV, Jackson PL, Courville CA, McNicholas CM, Sloane PA, Sabbatini G, Tidwell S, Tang LP, Liu B, Fortenberry JA, Jones CW, Boydston JA, Clancy JP, Bowen LE, Accurso FJ, Blalock JE, Dransfield MT, Rowe SM. Cigarette smoke induces systemic defects in cystic fibrosis transmembrane conductance regulator function. Am J Respir Crit Care Med 188: 1321–1330, 2013. doi: 10.1164/rccm.201304-0733OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Raju SV, Rasmussen L, Sloane PA, Tang LP, Libby EF, Rowe SM. Roflumilast reverses CFTR-mediated ion transport dysfunction in cigarette smoke-exposed mice. Respir Res 18: 173, 2017. doi: 10.1186/s12931-017-0656-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu L, Shastry S, Byan-Parker S, Houser G, K KC, Birket SE, Fernandez CM, Gardecki JA, Grizzle WE, Wilsterman EJ, Sorscher EJ, Rowe SM, Tearney GJ. An autoregulatory mechanism governing mucociliary transport is sensitive to mucus load. Am J Respir Cell Mol Biol 51: 485–493, 2014. doi: 10.1165/rcmb.2013-0499MA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhu SH, Sun JY, Bonnevie E, Cummins SE, Gamst A, Yin L, Lee M. Four hundred and sixty brands of e-cigarettes and counting: implications for product regulation. Tob Control 23: iii3–iii9, 2014. doi: 10.1136/tobaccocontrol-2014-051670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu L, Chu KK, Houser GH, Diephuis BJ, Li Y, Wilsterman EJ, Shastry S, Dierksen G, Birket SE, Mazur M, Byan-Parker S, Grizzle WE, Sorscher EJ, Rowe SM, Tearney GJ. Method for quantitative study of airway functional microanatomy using micro-optical coherence tomography. PLoS One 8: e54473, 2013. doi: 10.1371/journal.pone.0054473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jacob S, Poddar S. Mucous cells of the tracheobronchial tree in the ferret. Histochemistry 73: 599–605, 1982. doi: 10.1007/BF00493372. [DOI] [PubMed] [Google Scholar]
- 30. Vinegar A, Sinnett EE, Kosch PC, Miller ML. Pulmonary physiology of the ferret and its potential as a model for inhalation toxicology. Lab Anim Sci 35: 246–250, 1985. [PubMed] [Google Scholar]
- 31. Sehgal A, Presente A, Engelhardt JF. Developmental expression patterns of CFTR in ferret tracheal surface airway and submucosal gland epithelia. Am J Respir Cell Mol Biol 15: 122–131, 1996. doi: 10.1165/ajrcmb.15.1.8679216. [DOI] [PubMed] [Google Scholar]
- 32. Raju SV, Kim H, Byzek SA, Tang LP, Trombley JE, Jackson P, Rasmussen L, Wells JM, Libby EF, Dohm E, Winter L, Samuel SL, Zinn KR, Blalock JE, Schoeb TR, Dransfield MT, Rowe SM. A ferret model of COPD-related chronic bronchitis. JCI Insight 1: e87536, 2016. doi: 10.1172/jci.insight.87536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sun X, Sui H, Fisher JT, Yan Z, Liu X, Cho HJ, Joo NS, Zhang Y, Zhou W, Yi Y, Kinyon JM, Lei-Butters DC, Griffin MA, Naumann P, Luo M, Ascher J, Wang K, Frana T, Wine JJ, Meyerholz DK, Engelhardt JF. Disease phenotype of a ferret CFTR-knockout model of cystic fibrosis. J Clin Invest 120: 3149–3160, 2010. doi: 10.1172/JCI43052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Laube BL, Afshar-Mohajer N, Koehler K, Chen G, Lazarus P, Collaco JM, McGrath-Morrow SA. Acute and chronic in vivo effects of exposure to nicotine and propylene glycol from an E-cigarette on mucociliary clearance in a murine model. Inhal Toxicol 29: 197–205, 2017. doi: 10.1080/08958378.2017.1336585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Werley MS, McDonald P, Lilly P, Kirkpatrick D, Wallery J, Byron P, Venitz J. Non-clinical safety and pharmacokinetic evaluations of propylene glycol aerosol in Sprague-Dawley rats and Beagle dogs. Toxicology 287: 76–90, 2011. doi: 10.1016/j.tox.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 36. Renne RA, Wehner AP, Greenspan BJ, Deford HS, Ragan HA, Westerberg RB, Buschbom RL, Burger GT, Hayes AW, Suber RL, Mosberg AT. 2-Week and 13-week inhalation studies of aerosolized glycerol in rats. Inhal Toxicol 4: 95–111, 1992. doi: 10.3109/08958379209145307. [DOI] [Google Scholar]
- 37. Robertson OH, Loosli CG. Tests for the chronic toxicity of propylene glycol and triethylene glycol on monkeys and rats by vapor inhalation and oral administration. J Pharmacol Exp Ther 91: 52–76, 1947. [PubMed] [Google Scholar]
- 38. Kaza N, Raju SV, Cadillac JM, Trombley JA, Rasmussen L, Tang L, Dohm E, Harrod KS, Rowe SM. Use of ferrets for electrophysiologic monitoring of ion transport. PLoS One 12: e0186984, 2017. doi: 10.1371/journal.pone.0186984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Raju SV, Solomon GM, Dransfield MT, Rowe SM. Acquired cystic fibrosis transmembrane conductance regulator dysfunction in chronic bronchitis and other diseases of mucus clearance. Clin Chest Med 37: 147–158, 2016. doi: 10.1016/j.ccm.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. St.Helen G, Havel C, Dempsey D, Jacob P 3rd, Benowitz NL. Nicotine delivery, retention, and pharmacokinetics from various electronic cigarettes. Addiction (Abingdon, England) 111: 535–544, 2015. doi: 10.1111/add.13183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Omaiye EE, McWhirter KJ, Luo W, Pankow JF, Talbot P. High-nicotine electronic cigarette products: toxicity of JUUL fluids and aerosols correlates strongly with nicotine and some flavor chemical concentrations. Chem Res Toxicol 32: 1058–1069, 2019. doi: 10.1021/acs.chemrestox.8b00381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ghosh A, Coakley RD, Ghio AJ, Muhlebach MS, Esther CR Jr, Alexis NE, Tarran R. Chronic E-cigarette use increases neutrophil elastase and matrix metalloprotease levels in the lung. Am J Respir Crit Care Med 200: 1392–1401, 2019. doi: 10.1164/rccm.201903-0615OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zia S, Ndoye A, Nguyen VT, Grando SA. Nicotine enhances expression of the alpha 3, alpha 4, alpha 5, and alpha 7 nicotinic receptors modulating calcium metabolism and regulating adhesion and motility of respiratory epithelial cells. Res Commun Mol Pathol Pharmacol 97: 243–262, 1997. doi: 10.1186/s12931-018-0750-y. [DOI] [PubMed] [Google Scholar]
- 44. Maouche K, Polette M, Jolly T, Medjber K, Cloëz-Tayarani I, Changeux JP, Burlet H, Terryn C, Coraux C, Zahm JM, Birembaut P, Tournier JM. {alpha}7 nicotinic acetylcholine receptor regulates airway epithelium differentiation by controlling basal cell proliferation. Am J Pathol 175: 1868–1882, 2009. doi: 10.2353/ajpath.2009.090212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Noël A, Hansen S, Zaman A, Perveen Z, Pinkston R, Hossain E, Xiao R, Penn A. In utero exposures to electronic-cigarette aerosols impair the Wnt signaling during mouse lung development. Am J Physiol Lung Cell Mol Physiol 318: L705–L722, 2020. doi: 10.1152/ajplung.00408.2019. [DOI] [PubMed] [Google Scholar]
- 46. McCarron A, Donnelley M, Parsons D. Airway disease phenotypes in animal models of cystic fibrosis. Respir Res 19: 54, 2018. doi: 10.1186/s12931-018-0750-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Liu X, Luo M, Zhang L, Ding W, Yan Z, Engelhardt JF. Bioelectric properties of chloride channels in human, pig, ferret, and mouse airway epithelia. Am J Respir Cell Mol Biol 36: 313–323, 2007. doi: 10.1165/rcmb.2006-0286OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Smolich JJ, Stratford BF, Maloney JE, Ritchie BC. New features in the development of the submucosal gland of the respiratory tract. J Anat 127: 223–238, 1978. [PMC free article] [PubMed] [Google Scholar]
- 49. Engelhardt JF, Yankaskas JR, Ernst SA, Yang Y, Marino CR, Boucher RC, Cohn JA, Wilson JM. Submucosal glands are the predominant site of CFTR expression in the human bronchus. Nat Genet 2: 240–248, 1992. doi: 10.1038/ng1192-240. [DOI] [PubMed] [Google Scholar]
- 50. Dransfield MT, Wilhelm AM, Flanagan B, Courville C, Tidwell SL, Raju SV, Gaggar A, Steele C, Tang LP, Liu B, Rowe SM. Acquired cystic fibrosis transmembrane conductance regulator dysfunction in the lower airways in COPD. Chest 144: 498–506, 2013. doi: 10.1378/chest.13-0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Escobar YH, Morrison CB, Chen Y, Hickman E, Love CA, Rebuli ME, Surratt JD, Ehre C, Jaspers I. Differential responses to e-cig generated aerosols from humectants and different forms of nicotine in epithelial cells from nonsmokers and smokers. Am J Physiol Lung Cell Mol Physiol 320: L1064–L1073, 2021. doi: 10.1152/ajplung.00525.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available upon reasonable request.