Abstract
The flagellar-specific Ca2+ channel CatSper is the predominant Ca2+ entry site in mammalian sperm. CatSper-mediated Ca2+ signaling affects nearly every event that regulates sperm to acquire fertilizing capability. In this review, we summarize some of the main findings from 20 years of CatSper research and highlight recent progress and prospects.
Keywords: calcium signaling, CatSper, fertilization, ion channel, sperm
Introduction
The ultimate goal for sperm cells is to reach the eggs in time, fuse, and supply their own DNA. To adapt to the changing environments in the female reproductive tract, mammalian sperm must transduce signals during this arduous journey. Like in many other cells, calcium ions (Ca2+) are key messengers for such signaling in sperm. The 20,000-fold concentration gradient of Ca2+ across the plasma membrane and the cellular mechanisms to chelate, compartmentalize, or extrude Ca2+ enable fast, effective, and localized signal transduction (1). Ca2+ entry has been postulated as a regulator of sperm motility and fertility in mammals since the 1970s. Yet, the molecules composing the Ca2+-permeable pathways in sperm cells remained enigmatic until the discovery of CatSper (cation channel in sperm) in 2001 (2, 3). Over the past two decades, significant progress has been made in understanding the molecular and cellular mechanisms of the CatSper channel (FIGURE 1). Examples include discoveries of alkaline activation and Ca2+ selectivity of the CatSper channel by whole-sperm patch-clamp recording (4, 5), CatSper-organized linear signaling nanodomains (6, 7), Ca2+ regulation of CatSper by the Ca2+ sensor EFCAB9 (8), and the recently emerged structural data (9, 10). So far, CatSper is the only Ca2+ channel whose absence or mutations have been reported to cause male infertility in mice and humans, highlighting the importance of CatSper. In this review, we briefly walk through what was previously known about Ca2+ regulation of sperm capacitation (i.e., acquisition of fertilizing capacity) and how the key findings in CatSper ion channel research have shaped our current understanding of Ca2+ signaling in sperm cell physiology and mammalian fertilization.
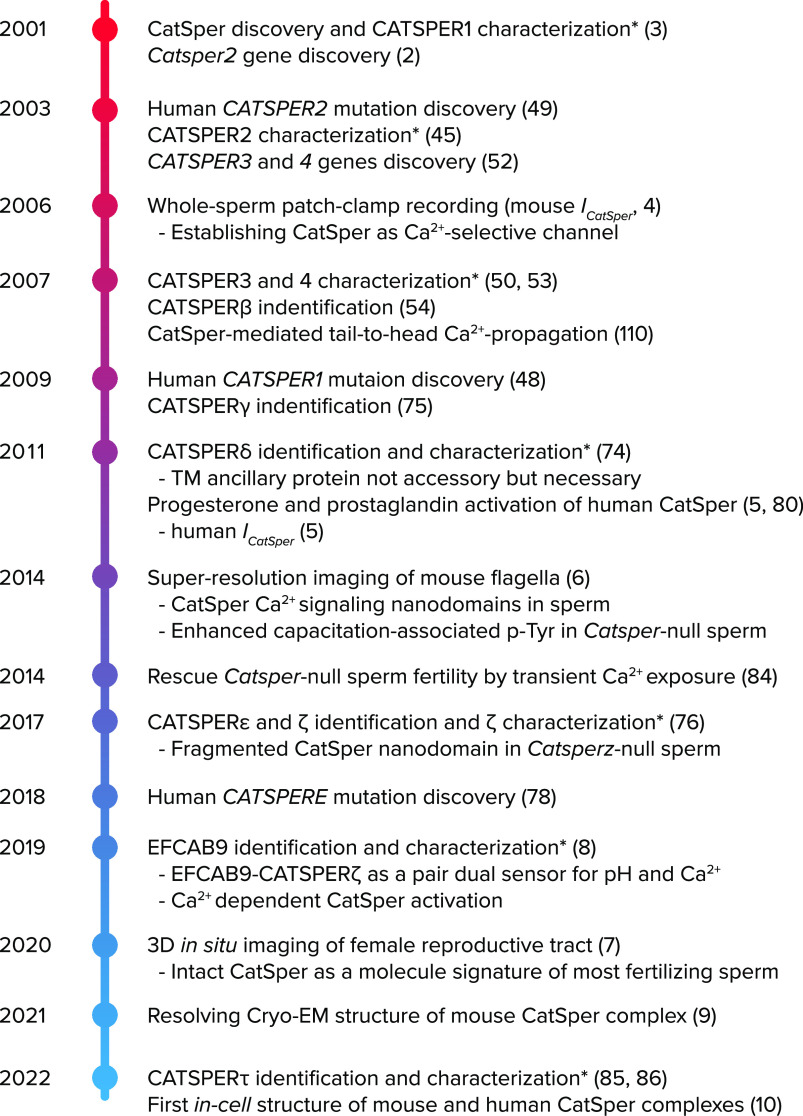
FIGURE 1.
Timeline of the key advances to understand CatSper channel Historical events in CatSper research are chronologically listed, starting with the discovery of the first CatSper component, CATSPER1 (3). Asterisks (*) indicate characterization with knockout mouse models, and numbers in parentheses indicate reference numbers.
Calcium Regulation of Sperm Capacitation—Previously Known
Ejaculated sperm undergo physiological changes in the female reproductive tract and gradually acquire the ability to fertilize an egg in a process termed capacitation (11, 12). In 1969, Yanagimachi observed (13) that the epididymal sperm flagella from golden hamsters beat asymmetrically and vigorously when incubated in vitro with follicular fluid. This flagellar beating pattern, named hyperactivated motility, was similar to that of the sperm retrieved from the ampulla of a mated female (14). Sperm hyperactivation has now been reported in capacitated sperm from various mammals including guinea pig, dog, mouse, rabbit, sheep, bull, human, pig, and horse (15). Hyperactivated sperm navigate efficiently in the mucus-filled and convoluted female tract as they work to escape the mucosal pockets and sperm reservoir in the oviduct (16, 17). Once sperm cells arrive at the ampulla, hyperactivation greatly improves the sperm’s ability to penetrate the egg’s barrier, the zona pellucida (18). All these findings highlight the importance of sperm hyperactivation in mammalian fertilization.
Concomitantly, it was noted that Ca2+ regulates the development of sperm hyperactivation. In Ca2+-free medium, no hyperactivated motility was triggered for hamster and mouse sperm (19, 20). Treating mouse sperm with a Ca2+ ionophore (A23187) induced hyperactivation-like motility but only transiently (21). Supplying Ca2+ to demembranated rat sperm also elicited flagellar curvature similar to that of hyperactivated sperm (22). Interestingly, hyperactivation of demembranated bull sperm also required an elevation of pH to 7.9–8.5 (23). All these studies strongly suggested sperm hyperactivation requires Ca2+ influx in concert with pH signaling. The timely development of ion-sensitive fluorescent probes in the 1980s prompted the measurement of intracellular Ca2+ concentration ([Ca2+]i) and intracellular pH (pHi) changes (24–26). In line with the experiments done on demembranated spermatozoa, optical methods visualizing live spermatozoa indicated a rise in [Ca2+]i and pHi when sperm are incubated in conditions mimicking those within the female reproductive tract (27, 28). In particular, Suarez et al. (29) demonstrated that [Ca2+]i is significantly higher in hyperactivated hamster sperm compared to nonhyperactivated sperm. The same study showed that the relative increase in [Ca2+]i in the tail of hyperactivating sperm is greater than that seen in the head, suggesting a Ca2+-permeable ion channel is localized in the flagellum.
Various Ca2+-permeable channels, particularly voltage-gated Ca2+ selective channels (Cav), were suggested as the Ca2+ entry site responsible for mammalian sperm hyperactivation. Early attention was drawn to fast-inactivating T-type Cav channels (30, 31) because they were electrophysiologically recorded in spermatogenic cells as the main Ca2+-conducting channel (32–34). The involvement of Cav channels was further supported by optical detection of a membrane depolarization-induced Ca2+ influx into sperm cells by elevation of extracellular K+ (35). Of note, this Ca2+ influx also required a simultaneous elevation of extracellular pH (35). Several Cav subunits detected by immunocytochemistry (35–37) were thought to be the putative Ca2+-permeable channels in sperm.
However, the development of mouse models targeting these Cav genes raised questions about their physiological significance in sperm hyperactivation. For example, knockout males for either N (Cav2.2)-type (38, 39) or T (Cav3.2)-type (40) Cav channels all had normal fertility without apparent sperm defects. Interestingly, sperm lacking another R (Cav2.3)-type Ca2+ channel swim more linearly than wild-type sperm after inducing capacitation in vitro (41), indicating a possible contribution of Cav2.3 to sperm hyperactivation. Reduction of acrosome exocytosis is also reported in Cav2.3-deficient sperm (42). Regardless, Cav2.3-deficient males are fertile; thus its physiological significance in sperm hyperactivation and acrosomal exocytosis is likely marginal. For other Cav channels for which knockout mouse models are embryonic lethal or show growth defects (43, 44), their potential involvement remains to be tested.
A Brief History of CatSper Calcium Channel
Discovery of CatSper—the Primary Ca2+-Selective Ion Channel in the Sperm Flagella
In 2001, in their search for mammalian homologs to NaChBac, the bacterial family of voltage-gated ion channels, Ren et al. (3) cloned a testis-specifically expressed gene (now known as Catsper1) that encodes an unusual, six-transmembrane-spanning protein with voltage-sensing domains (VSD) just like the topology of a voltage-dependent K+ (Kv) channel (FIGURE 2A). However, the overall homology of CATSPER1 more closely resembles a single repeat of the much larger, four-repeat (24 transmembrane domains) Cav channel and contains typical glutamate/aspartate residues conserved for Ca2+ selectivity in the predicted pore region (3). CATSPER1 localizes at the plasma membrane of the principal piece in the sperm tail. Strikingly, Catsper1-null males are completely infertile, in distinction from the knockout models of other Cav channels, despite normal sexual behavior and sperm production. The same study also showed that CATSPER1 is required for normal sperm motility, egg penetration in vitro, and cAMP-induced intracellular Ca2+ elevation.
FIGURE 2.
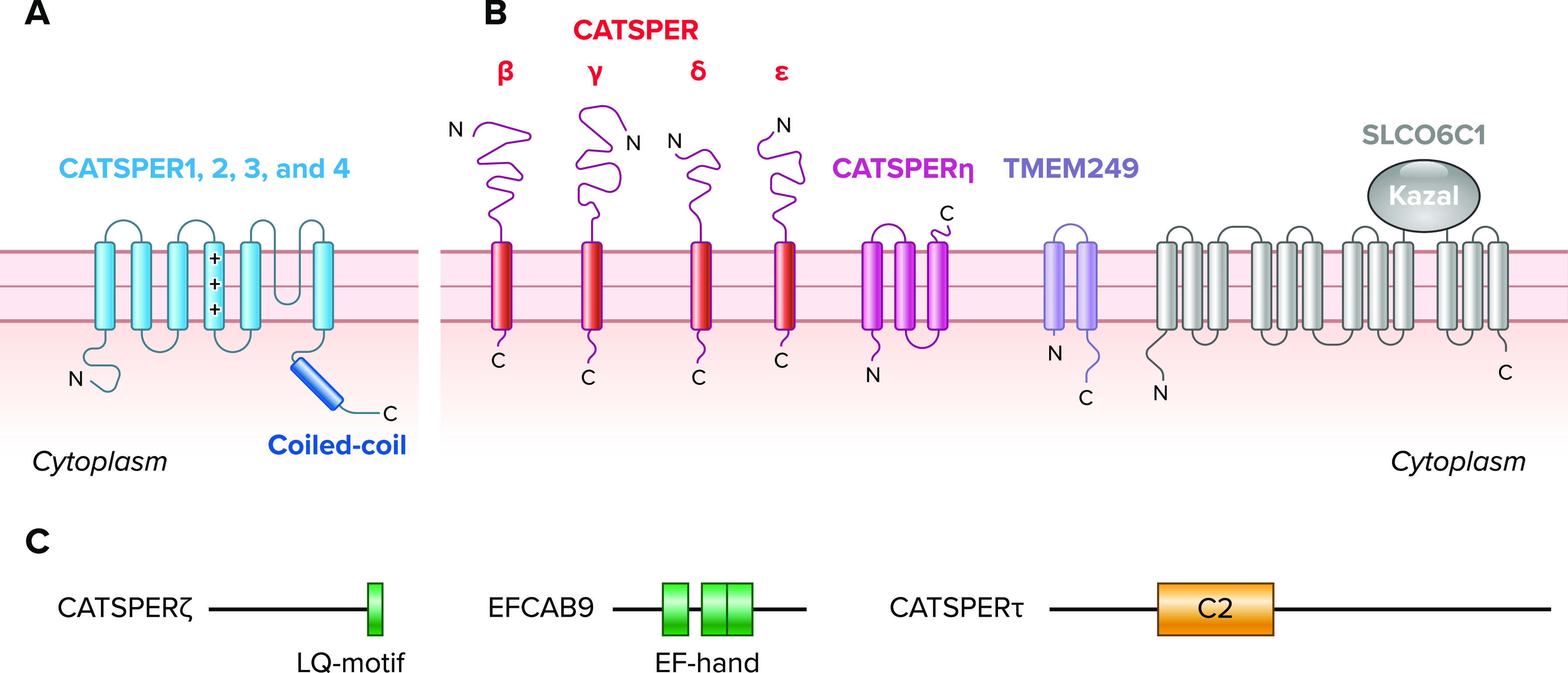
Molecular components associated with CatSper channel A: topology of pore-forming α-subunits (CATSPER1–4). The α-subunits are 6 transmembrane proteins that contain voltage-sensing domains (VSD; S1–S4), a pore-domain (S5–S6), and a coiled-coil domain at the cytoplasmic COOH termini. B: topologies of ancillary transmembrane proteins associated with CatSper pore-forming channel. Reported include single (CATSPERβ, γ, δ, and ε), 2 (TMEM249), and 3 transmembrane (CATSPERη) subunits and a 12 transmembrane anion transporter (SLCO6C1) with a Kazal domain. C: diagrams of nontransmembrane cytosolic ancillary proteins associated with CatSper channel complex. Reported proteins are shown with conserved motifs/domains in colored boxes; CATSPERζ has an IQ-like motif (LQ-motif), EFCAB9 has EF-hand Ca2+ binding domains, and CATSPERτ has a C2 domain.
Around the same time, applying signal peptide trapping from the spermatid cDNA library, Quill et al. (2) reported another CatSper gene (Catsper2) encoding a protein that shares the amino acid sequence and topology of CATSPER1. Consistent with CATSPER1, CATSPER2 is specifically expressed in the testis and is localized to the sperm principal piece (45). Moreover, both CATSPER1 and CATSPER2 are required for evoked Ca2+ entry under the above-mentioned classic high-K+, high-pH extracellular medium to develop asymmetric flagellar beating, a characteristic of hyperactivated motility (46, 47). In short, genetic depletion of either Catsper1 or Catsper2 led to identical phenotypes—male infertility due to impaired sperm hyperactivation (45, 47). These initial studies clarified that the CatSper channel is the long-sought Ca2+-permeable channel responsible for developing sperm hyperactivated motility that is crucial for male fertility. The identification of human mutations in CATSPER1 and CATSPER2 from infertile male patients with impaired sperm motility followed (48, 49), highlighting the channel’s relevance to human pathophysiology. The identical phenotypes of Catsper1- and Catsper2-null mouse models could be explained either by CATSPER1 and CATSPER2 forming the same pore of CatSper (a heteromeric pore) or by them forming two different homomeric channels that coordinate the same downstream signaling pathways.
CATSPER1–4: the Heterotetrameric Pore of the CatSper Channel
Soon after these developments, two additional CatSper pore-forming subunits (CATSPER3 and CATSPER4) were identified with exclusive gene expression in the testis and protein localization to the principal piece as with CATSPER1 and CATSPER2 (50–53). All four CatSper pore-forming subunits were predicted to contain coiled-coil domains in their COOH termini (52), suggesting protein-protein interactions help form a heterotetrameric channel pore (FIGURE 2A). This idea was further corroborated by the identical phenotypes of Catsper3 and Catsper4 knockout mice to those seen with Catsper1- and Catsper2-null mice, where null sperm are rendered incapable of hyperactivation and in which knockout males are infertile (50, 53). These inseparable phenotypes strongly suggest that the tetrameric channel pore would contain one of CATSPER1–4 each. Yet, heterologously expressing the four pore-forming subunits, either alone or all together, did not yield functional CatSper channels with detectable currents (3, 54). Instead, the proteins did not reach the cell surface in the various heterologous systems tested. This prompted the need to directly record CatSper activity in spermatozoa and search for potentially missing ancillary subunits.
Successful Application of Patch-Clamp Recording to Mouse and Human Spermatozoa
The discovery of the CATSPER1–4 and the subsequent genetic evidence made their importance in sperm physiology clear. However, CatSper’s ion selectivity (i.e., whether CatSper permeates cations nonselectively as initially presumed or conducts Ca2+ selectively) and mode of activation were unknown. The primary reasons for these unknowns were that the subunits did not form a functional channel in heterologous systems (3, 54), making the gold standard measurement by patch-clamp recording of reconstituted channels implausible. Indirect, optical methods mostly measured an averaged signal from sperm suspension (55), providing little spatial and temporal information at the single-cell level. Thus the need to directly measure native ion currents across the plasma membrane of the spermatozoa became apparent. A few years after the development of patch-clamp techniques (56, 57), the formation of high-resistance seals between the patch glass pipette and a sea urchin sperm head was reported for the first time (58). Over the next three decades, however, it remained a formidable challenge to form reliably reproducible gigaohm seals with sperm cells from any species. Such a gigaohm seal is essential to successfully break into sperm cells and to subsequently transition into the whole-cell mode of patch-clamp recording. A major culprit is the extremely low cytosolic volume of a rod-like sperm cell (e.g., ∼50 fL with ∼250 µm2 of membrane for a mouse spermatozoon, 100 times smaller than that of a typical somatic cell) in which the sperm plasma membrane is tightly attached to rigid intracellular structures, making it extremely difficult to draw sperm plasma membrane into the pipette.
To circumvent this problem, Kirichok et al. (4) recognized the cytoplasmic droplet of mouse epididymal spermatozoa as the region of the spare plasma membrane able to form gigaohm seals (FIGURE 3), which finally led to the establishment of the current “whole-sperm patch-clamp” method in 2006. For the details on developing whole-sperm patch-clamp techniques and the practical applications, more comprehensive reviews (59, 60) and a method in video format (61) are available. In this review, we only focus on how the initial landmark studies using this technique have clarified the previous conundrums in the field on the Cav responsible for sperm hyperactivation. Using whole-cell patch-clamp recording of sperm from wild-type and Catsper1-null mice, it was unambiguously demonstrated that the CatSper current (ICatSper) conducts a weakly voltage-dependent and highly Ca2+-selective current under physiological conditions (4). ICatSper, which originates from the principal piece of the sperm flagellum and is absent in Catsper1-null sperm, is potentiated by intracellular alkalinization (i.e., negative shifts in voltage sensitivity). Most of all, a monovalent cation current (a feature of Ca2+-selective channel under divalent-free conditions) was not detected in the sperm cells from all Catsper1–4 knockout mice (4, 53), leaving CatSper as the only constitutively active Ca2+-conducting channel in mouse spermatozoa. In addition, Xia and Ren (62) demonstrated T-type Ca2+ current diminishes in male germ cells during spermatogenesis and is not detected in testicular sperm and epididymal sperm, clarifying the previous speculation on the involvement of T-type Cav in sperm function. Altogether, these results indicate that intracellular alkalinization activates the sperm flagellar-specific CatSper Ca2+ channel, which is composed of a CATSPER1–4 heterotetramer, increases [Ca2+]i, and thus induces hyperactivated motility.
FIGURE 3.
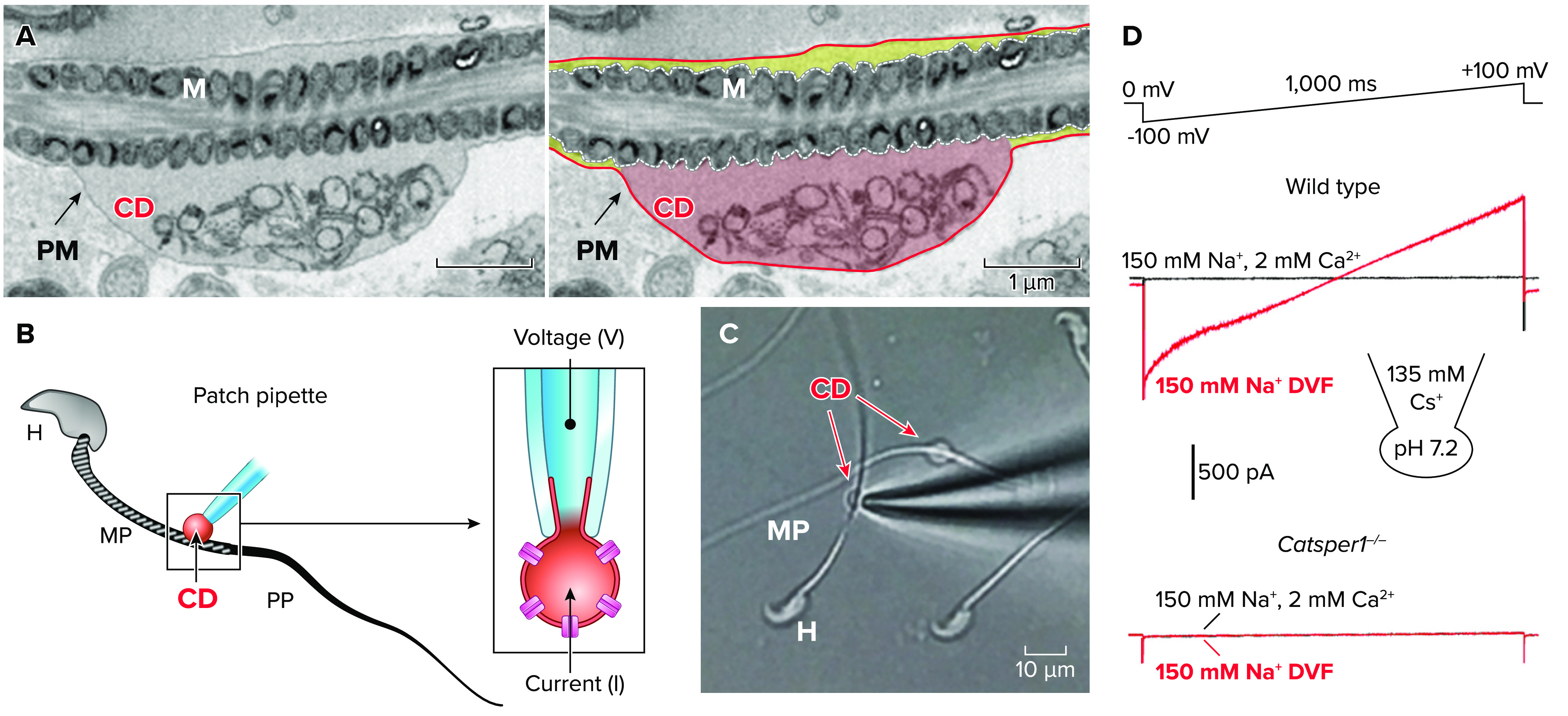
Whole-cell patch-clamping mouse sperm via a cytoplasmic droplet A: an electron microscope image of the cytoplasmic droplet of mouse sperm. Shown is the original (left) and pseudocolored (right) of the identical image. The plasma membrane in the cytoplasmic droplet region (CD; red-filled) can be drawn up into the pipette to form a gigaohm seal whereas the plasma membrane in other flagellar regions has no room as it is tightly associated with the underlying mitochondria (yellow-filled). B: a cartoon to represent the patch-clamp recording of a mouse epididymal spermatozoon in the whole cell mode. To form a gigaohm seal, the patch pipette is placed on the plasma membrane of the CD in the midpiece (MP) and then negative pressure is applied (left). The membrane drawn up into the patch pipette is broken-in to achieve a whole-cell recording mode (right). H, head; PP, principal piece. C: a bright field image showing the end of a patch pipette approaching a flagellum with the CD. D: ICatSper traces from mouse sperm by whole cell patch-clamping recording. ICatSper elicited by a voltage ramp from 0-mV holding potential in divalent-free (DVF) condition (red traces) from wild-type (top) and Catsper1-null (bottom) sperm. The traces are reproduced from Ref. 4, with permission from Nature.
In human spermatozoa, the steroid hormone progesterone has been known to induce Ca2+ influx (63, 64) and triggers Ca2+-dependent processes including sperm hyperactivation and the acrosome reaction (65). However, the mechanisms of this apparently nongenomic action of progesterone on human spermatozoa (i.e., transcriptionally silent cells) remained unknown until the development of a human sperm patch-clamp technique. Ejaculated human sperm preserves the cytoplasmic droplet (66), enabling localization of the patch pipette to the sperm’s neck region for gigaohm seal formation (5, 67). Patch clamping such ejaculated, fully mature human spermatozoa resulted in the discovery that human ICatSper is overall less (∼0.9 pF) than mouse ICatSper (∼2.5 pF). In particular, human sperm lacks ICatSper normally found at the negative membrane potential (inward current) across the sperm plasma membrane (5). Interestingly, nanomolar concentrations of progesterone and prostaglandin dramatically activate ICatSper which is further increased by intracellular alkalinization (5), indicating that the ligand-induced Ca2+ influx in human sperm is mediated by CatSper. The same study also demonstrated that the progesterone-binding site associated with CatSper is external. The progesterone-mediated CatSper activation is further verified by the absence of ICatSper in sperm from infertile patients with a deletion or a copy number variation in CATSPER2 (68, 69). In distinction from human CatSper, progesterone does not activate mouse CatSper, suggesting species-specific ligands for CatSper activation. This might be due to the species-specific and/or cell-type specific membrane receptor functionally associated with CatSper, like α/β hydrolase domain-containing protein 2 (ABHD2) suggested as the membrane progesterone receptor in humans (70). Alternatively, highly divergent CatSper components (71) might serve as binding sites for different, species-specific ligand(s). The presumable species-specific ligands may directly or indirectly activate CatSper channel concerted with alkalinization activation.
Ancillary Subunits of the CatSper Channel—Known Before the Structures
When the pore-forming α-subunits of voltage-gated ion channels are expressed alone, most can be functionally reconstituted (albeit somewhat differently from the native channels) in heterologous systems such as HEK293 or Xenopus oocytes (72). Thus ancillary subunits associated with α-subunits are often called “accessory” because they are not absolutely required to form the channel but rather are modulatory by affecting the number of channels in the plasma membrane (i.e., trafficking) and/or the biophysical properties of the channel (i.e., activation and inactivation kinetics) (73). Search for such ancillary subunits for the CatSper channel immediately followed as CatSper α-subunits failed to reconstitute functional CatSper channels in heterologous systems. Before recent structure elucidation, a separate section later in this review, four transmembrane (CATSPERβ, γ, δ, and ε) and two nontransmembrane (CATSPERζ and EFCAB9) ancillary subunits have been identified (FIGURE 2, B AND C), making the CatSper channel one of the most complex ion channels known.
A single transmembrane CATSPERβ, γ, δ, and ε are necessary for assembling the channel complex.
Four transmembrane ancillary subunits of the CatSper channel followed the discovery of the four α-subunits and helped establish the CatSper channel as a macromolecular complex. CATSPERβ, CATSPERγ, and CATSPERδ were identified as proteins in complex with CATSPER1 (54, 74, 75), followed by bioinformatics identification of CATSPERε as a protein with amino acid sequence homology to CATSPERδ (76). These four proteins share many common features. First, they are all exclusively expressed in testis. The onset of mRNA expression coincides with meiosis, followed by a significant increase in postmeiotic germ cells, as contrasted with postmeiotic gene expression of α-subunits. Second, similar to the topology of the α2δ subunit of Cav channels, the proteins were all predicted to be type I transmembrane proteins with large extracellular domains (FIGURE 2B). Third, the proteins specifically localize in the flagellar membrane of the principal piece in the same manner as the α-subunits (6, 76). Last but not the least, Catsper1-null sperm lack not only other α-(CATSPER2, 3, and 4) subunits (8, 76) but also lack all four transmembrane ancillary (CATSPERβ, γ, δ, and ε) proteins (8). All these results strongly suggested that CATSPERβ, γ, δ, and ε help form the final channel complex in mature sperm.
The generation of Catsperd-null mice provided more clues into the potential function of these transmembrane subunits (74). In the absence of CATSPERδ, the male mice are infertile and their sperm fail to hyperactivate, phenotypically similar to those lacking any one of the α-subunits. Notably, CATSPERδ-deficiency reduces CATSPER1 protein levels significantly in testis where protein-protein interactions of other CatSper subunits (β and γ) are preserved without CATSPERδ. No detectable ICatSper was recorded in Catsperd-null sperm, indicating there is no functional CatSper channel formation. As both α-subunits and other ancillary transmembrane subunits were absent in Catsperd-null sperm (similar to what was observed in Catsper1-null sperm), these results demonstrated that CATSPERδ is not an “accessory” but “necessary” subunit for CatSper channel assembly. The interdependent protein levels of these eight transmembrane subunits in mouse spermatozoa also suggested that one α-subunit pairs specifically with one of CATSPERβ, γ, δ, and ε. This idea was further supported by the copy number of CatSper subunits quantified at ∼1:1 stoichiometry in sea urchin sperm (77). The identical phenotypes of Catsperd-null sperm to those of Catsper1–4 at the molecular, cellular, and organismal levels predict that CATSPERβ, γ, and ε would all be essential for sperm hyperactivation and male fertility. Thus it is postulated that only completely assembled channels can traffic to the mouse sperm flagellum (i.e., a CatSper complex missing any transmembrane subunit would be retained in the cell body). Detailed molecular mechanisms of the CatSper channel assembly would rely on using new animal models for the genes and then further comparative studies using such models. An identified CATSPERE mutation from an infertile male patient with defective Ca2+ signal transduction also supports the physiological significance of transmembrane subunits (78).
Cytosolic EFCAB9-CATSPERζ are not necessary to form functional channels but confer pH- and Ca2+-sensitive CatSper regulation.
Patch-clamp recording of mouse and human spermatozoa (4, 67), as well as rapid-mixing fluorimetry measurement of human and sea urchin sperm (79, 80), made the universal activation mechanism of the CatSper channel by intracellular alkalinization clear. However, the functional channel could not be reconstituted in a heterologous system even when all α-subunits were expressed together with the identified transmembrane ancillary subunits. Moreover, the knockout sperm from previously existing mouse models showed identical phenotypes all lacking any detectable ICatSper current (4, 53, 74). Thus how the CatSper channel responds to the intracellular pH changes and whether Ca2+ regulates the channel activity as shown for other Cav channels have remained unexplored. Studies on two cytosolic proteins associated with the CatSper channel, CATSPERζ and the calmodulin-like EF-hand protein, EFCAB9, provided some answers to these questions (FIGURE 2C; Refs. 8, 76). Notably, a knockout of either Catsperz or Efcab9 as well as a double knockout of the two genes resulted in male subfertility with an altered sperm-beating pattern. This enables the study of the hypomorphic function of CatSper. Whereas both proteins are absent in either Catsperz- or Efcab9-null spermatozoa (i.e., these two proteins are interdependent), these null-spermatozoa contains 20–30% of the protein levels of other transmembrane α- and ancillary subunits compared to those in wild-type sperm. Most importantly, the functional CatSper channels are assembled because ICatSper was recorded in both Catsperz- and Efcab9-null spermatozoa using the whole-sperm patch-clamp technique. These results are sufficient to classify them as classical accessory subunits that regulate channel density and/or activity.
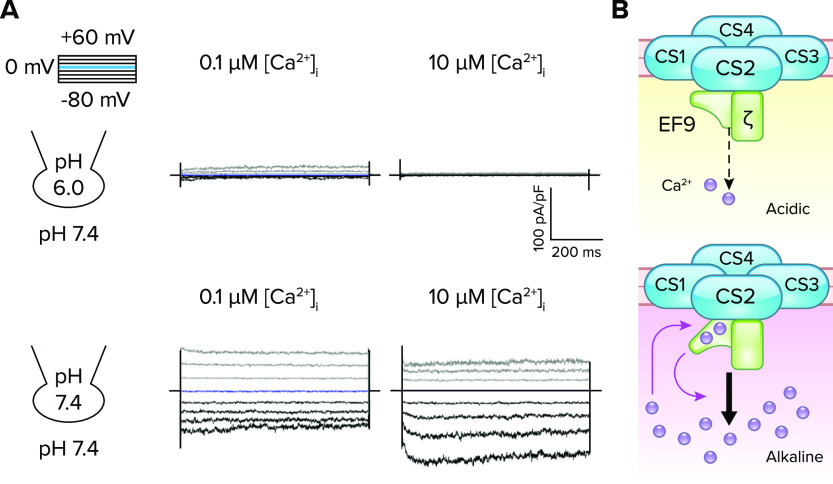
Biochemical analysis with recombinant proteins demonstrated that these two subunits form a subcomplex of CatSper via direct EFCAB9 binding with an IQ-like motif of CATSPERζ (8, 71). EFCAB9 and CATSPERζ interact in a Ca2+-dependent manner and dissociate at an elevated pH. Mutating conserved acidic residues in the EF hands of EFCAB9 or chelating Ca2+ significantly weakens their interactions, suggesting that EFCAB9 is a pH-sensitive Ca2+ sensor for the CatSper channel. Patch-clamp recording of sperm with buffered [free-Ca2+] at a fixed intracellular pH in a pipette validated this idea and revealed the full picture of the previously unappreciated, complex Ca2+ sensitivity of the CatSper channel (FIGURE 4A). At an acidic intracellular pH (pHi = 6.0), CatSper is mostly closed in both wild-type and Efcab9-null spermatozoa regardless of intracellular Ca2+ concentrations. This suggests the apparently low Ca2+ sensitivity of the CatSper channel when pHi is low. At an alkaline intracellular pH (pHi = 7.4), the basal activity of CatSper (i.e., the channel is constitutively open) and Ca2+ sensitivity is high. Under this condition, CatSper is further potentiated by increasing free [Ca2+]i. By contrast, this pH-tuned Ca2+-sensitive activation is highly diminished in sperm lacking the CATSPERζ-EFCAB9 complex (8). Conversely, when intracellular Ca2+ is buffered at a fixed concentration in the physiological range, the extent of alkaline activation of CatSper is significantly reduced in both Catsperz- and Efcab9-null spermatozoa. Thus the CATSPERζ-EFCAB9 complex serves as a dual sensor for intracellular pH and Ca2+. The complex prevents CatSper from fully activating when intracellular pH is low but releases it from this inactivation when that Ca2+ entered by alkaline-activated CatSper binds to EFCAB9, thereby altering the affinity of EFCAB9 to CATSPERζ, presumably by a conformational change (FIGURE 4B).
FIGURE 4.
pH-dependent Ca2+ sensing by EFCAB9 to modulate CatSper channel activity A: Ca2+-dependent potentiation of the CatSper current (ICatSper) in wild-type sperm under an intracellular alkaline condition. The traces are reproduced from Ref. 8, with permission from Cell. Inward ICatSper is minimally affected by increasing Ca2+ concentration ([Ca2+]i) under an acidic condition (pHi 6.0; top), but greatly potentiated in a [Ca2+]i-dependent manner under an alkaline condition (pHi 7.4; bottom). B: a schematic cartoon of pH-dependent Ca2+-sensing of EFCAB9 in regulating CatSper channel activity. In acidic intracellular pH, EFCAB9 (EF9) and its binding partner, CATSPERζ (ζ), form a tight binary complex and respond marginally to increasing Ca2+ (top). After intracellular alkalinization, EFCAB9-CATSPERζ interactions weaken, which enhances Ca2+ sensitivity of EFCAB9 (bottom). Ca2+ binding to EF-hand domains changes EFCAB9 conformation to fully activate CatSper.
CatSper-Organized Linear Ca2+ Signaling Nanodomains
Ca2+-mediated signal transduction is highly localized (81). Compartmentalized Ca2+-permeable channels are found in neuronal and immunological synapses. For example, Cav2 at presynapses and Cav1 at postsynapses form signaling complexes, which are responsible for regulating the synaptic plasticity of neurons by triggering neurotransmission and long-term potentiation, respectively (82). During T cell activation, a store-operated ORAI Ca2+ channel coalesces with STIM proteins at the immunological synapse with close localization of the mitochondria, which inhibit Ca2+ pumps to minimize Ca2+ clearance (83). However, it is not clear whether and how such localized Ca2+ signaling occurs in a spermatozoon with many tens of microns long, but submicron-wide, flagellum (e.g., 120-µm-long mouse sperm).
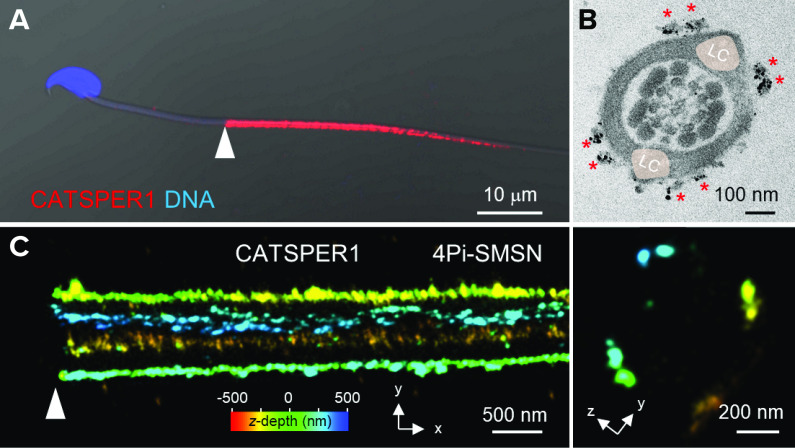
In 2014, by applying stochastic optical reconstruction microscopy (STORM) to mouse sperm, Chung et al. (6) revealed the three-dimensional (3-D) molecular distributions of various flagellar proteins including the CatSper channel. Strikingly, CatSper channels arrange in four linear “stripes” on the plasma membrane of the principal piece of the sperm tail (6). By contrast, the sperm Ca2+ pump PMCA4 is distributed evenly on the plasma membrane of the principal piece as a tapering cylinder. Other signaling molecules, such as CaMKII, calcineurin, and caveolin1, also colocalize in the unique quadrilinear domains in a CatSper-dependent manner, suggesting that CatSper specifically organizes the compartmentalized linear signaling complexes. Immunoelectron microscopy (EM) (6) and another single-molecule-based superresolution microscopy (8) suggested that the nanodomains are composed of double rows ∼100 nm apart and are localized on either side of the longitudinal columns (FIGURE 5). Quadrilinear CatSper nanodomains are also observed in human sperm (6), suggesting this spatial organization is a conserved feature of the CatSper channels in mammalian sperm. These linear Ca2+ nanodomains are predicted to be necessary for the unique long-range signaling, and consequently, effective Ca2+ transduction along the sperm tail. In Catsper1-null sperm, spatiotemporal control of downstream protein tyrosine phosphorylation is deregulated (6). The null sperm cannot reach the fertilization site in time because they are not able to pass the utero-tubal junction in the female reproductive tract (6). Yet, understanding the significance of this linear nanodomain structure is complicated by the lack of the entire channel complex, the Ca2+ signaling nanodomains, in Catsper1-null sperm due to the “all-or-none” assembly of CatSper transmembrane subunits. Navarrete et al. (84) reported treating Catsper1-null sperm transiently with a Ca2+ ionophore (A23187) enabled the sperm to hyperactivate and fertilize the eggs in vitro, bypassing the nanodomain-mediated coupling of Ca2+ entry to downstream signal transduction. Considering sperm would require dynamic regulation of Ca2+ signaling in their journey to the eggs, however, the CatSper nanodomains are likely to orchestrate Ca2+ signaling in time and space in vivo.
FIGURE 5.
CatSper-organized quadrilinear nanodomains along the principal piece of the mouse sperm tail A: a confocal image of a CATSPER1-immunostained spermatozoon. Shown is a merged image of fluorescence and the corresponding DIC images. B: a cross-section EM image of immunogold labeled CATSPER1. Shown is a reproduced image from Ref. 6, with permission from Cell. Two clusters of gold particles (red asterisks) are shown at each side of longitudinal columns (LC; pale pink). C: a 3-dimensional 4Pi-single molecule switching nanoscopy (4Pi-SMSN) image of CATSPER1 immunostained wild-type sperm tail. Images are reproduced from Ref. 8, with permission from Cell. shown in x-y (left) and y-z (right) projections. The z-axis information from the focal plane is color coded. Cross-section images from both EM and 4Pi-SMSN hint at a double row of CatSper in each quadrant. Arrowheads in A and C indicate annulus, the junctional part between midpiece and principal piece.
Accordingly, more clues on the physiological significance of the organized linear structures of CatSper signaling complexes come from multiple studies using different approaches (7, 8, 76). Motility-correlative imaging of in vitro capacitated sperm demonstrated that capacitation generates molecularly heterogeneous sperm populations in which 60% of sperm have delocalized and/or disrupted nanodomains whereas all hyperactivating sperm among the entire population maintained intact quadrilinear nanodomain structures (6). The importance of the integrity of the CatSper nanodomain is further supported by directly analyzing sperm moving to the fertilization site in time-mated females (7). 3-D in situ imaging of CLARITY-cleared female reproductive tract revealed that fertilizing sperm are characterized by intact CatSper channels, lack of tyrosine phosphorylation, and have reacted acrosomes. Of note, the CatSper nanodomain integrity shown is probed by the CATSPER1 signal in all the above-mentioned studies; however, the CATSPER1 NH2 terminus is cleaved/processed during capacitation (7). Thus it remains to be further determined if the CatSper channel containing NH2-terminally processed CATSPER1 is simply an indication of degenerating sperm or if these sperm have altered CatSper activity. Finally, loss of the EFCAB9-CATSPERζ complex reduced the two-row organization within a single CatSper quadrant to a discontinuous and irregular single row in addition to the aforementioned lower pH and Ca2+ sensitivity (8, 76). Altogether, these results highlight that CatSper nanodomain integrity is a physiological indicator of sperm motility and fertility.
Cytosolic CATSPERτ is not necessary to form a functional channel but assists channel traffic to the flagellum.
Considering the spermatozoon’s flagellar membrane accounts for ∼70–75% of the total plasma membrane (60), it is not surprising that various sperm transmembrane receptors and ion channels including CatSper are localized in the flagellar membrane. The CatSper channel is strictly located in the principal piece of the tail, and the integrity of its linearly arranged signaling nanodomains is correlated with maintaining sperm hyperactivation. Yet, the developmental and molecular mechanisms by which properly assembled CatSper channels are targeted to the flagellum and into the quadrilinear nanodomains are largely unknown. Very recently, two independent studies reported a bona fide nontransmembrane cytosolic CatSper-associated protein C2CD6 (85, 86) that is capable of trafficking to the plasma membrane on its own. It is thus now named CATSPERτ (tau for trafficking) (85).
CATSPERτ, encoded by a testis-specific gene, contains a membrane-associating C2 domain. Different from other cytosolic subunits, like EFCAB9 or CATSPERζ, mutant forms of CATSPERτ (in which the C2-domain is truncated) or its absence result in male infertility due to defective sperm hyperactivation. In CATSPERτ-deficient sperm, the trafficking of the pore-forming α-subunits and all other ancillary (either transmembrane or nontransmembrane cytosolic) subunits is severely compromised so that protein levels are ∼10% of those in wild-type sperm. However, a small, but measurable, alkalinization-activated ICatSper is detected from CATSPERτ-deficient sperm, demonstrating functional channel formation without CATSPERτ (85, 86). Thus CATSPERτ is a true accessory subunit that regulates the channel density in the plasma membrane by targeting the channel to the flagellar membrane.
Tail formation and other morphological changes occur during spermatogenesis in the testis. The nascent flagellum is elongated from the basal body of a round spermatid like a growing cilium. Hwang et al. (85) found that the assembled CatSper complex traffics to the elongating tail and arranges quadrilinearly in a CATSPERτ-dependent manner. Based on interactome analysis, it is hypothesized that CATSPERτ adapts ciliary trafficking machinery for targeting CatSper to flagella. Interestingly, heterologously expressed CATSPERτ fails to traffic to cilia. Instead, it is enriched at the basal body, suggesting channel-CATSPERτ interaction is necessary but not sufficient for flagellar trafficking and likely requires additional components in developing germ cells.
Molecular Evolution of the CatSper Channel
Comparative genomics studies suggest that CatSper channels are conserved across eukaryotes in a distinct pattern of lineage-specific gain or loss. CatSper orthologues were discovered in early metazoans [sea squirt (87) and sea anemone (88)] as well as in unicellular eukaryotes (89). Of note is the presence of CatSper orthologues in both bikont and unikont unicellular organisms, indicating that CatSper originated from their common ancestor. Yet, CatSper orthologues are absent altogether in certain animals such as frogs, mollusks, insects, and worms (8, 76, 88). Presumably, other Ca2+-permeable channels were preferentially selected in these lineages and replaced the role of CatSper in controlling flagellar movement.
In line with what we know from mouse and human studies and CatSper conservation throughout mammals, studies based on pharmacological manipulations have suggested that the CatSper channel is responsible for regulating Ca2+-mediated sperm motility in various placental mammals including the pig (90), bull (91), horse (92), and short gray-tailed opossum (71). For example, Ca2+ influx induces sperm hyperactivation in opossum, which is blocked by chelation of extracellular Ca2+ or treatment with a CatSper blocker (NNC55-0396) (71), suggesting CatSper regulation of sperm motility in ancient placental mammals like marsupials. Interestingly, one flagellum of the paired opossum sperm first begins to beat asymmetrically when incubated under capacitating conditions, indicating that sperm hyperactivation in the opossum contributes to dissociating paired sperm as well as to improving sperm’s swimming ability. In nonmammals, CatSper regulation of sperm motility has been reported in sperm from sea urchins (79) and Ciona intestinalis (93). In sea urchins, the chemoattractant resact induces intracellular alkalinization and Ca2+ entry into sperm (79). Treating the sperm with MDL12330A and mibefradil, which inhibit CatSper nonspecifically, abolishes the chemoattractant- or alkaline-induced Ca2+ influx, and it alters sperm chemotactic behavior. Markedly, genetic ablation of CatSper in C. intestinalis impairs chemotactic swimming in sperm (93). These results suggest that the CatSper channel is the conserved Ca2+-permeable path to regulate flagellar movement across metazoans.
Interestingly, EFCAB9 and CATSPERζ, which together form a dual pH and Ca2+ sensor for mammalian CatSper, have evolved completely separately. EFCAB9 has a conserved lineage (specifically like other CatSper subunits), but CATSPERζ is only conserved in mammals (8, 71, 76). The evolution of EFCAB9 and CATSPERζ suggests that Ca2+-dependent CatSper activation could be a universal mechanism like alkaline activation. Yet, mammalian spermatozoa might further fine-tune the pH-dependent Ca2+ sensitivity of CatSper by EFCAB9 interaction with a more newly evolved CATSPERζ. The mammalian invention of CATSPERζ might exist to also adapt CatSper nanodomains to the underlying fibrous sheath, which is also a mammalian-specific cytoskeletal structure.
A New Era in CATSPER Research—First Structures of the CatSper Channel
Despite the physiological importance of CatSper, structural characterization of any of the known subunits was, until recently, nonexistent. Marking 20 years since CatSper discovery, multiscale structural information of the CatSper channel complex has become available (FIGURE 6; Refs. 9, 10) due to great advances in cryogenic electron microscopy (cryo-EM). Sample processing for cryo-EM does not involve chemical fixatives or stains but freezes the sample so quickly at cryogenic temperatures that ice crystals cannot form, thus trapping it in vitreous (glass-like) ice (94). Averaging many individual particles frozen in such thin, vitreous ice, a high-resolution structure of the purified CatSper complex (i.e., single-particle cryo-EM structure) was generated (9). Attention was also drawn to directly averaging the seemingly repetitive CatSper channel complexes within the linear nanodomains by applying cryo-electron tomography (cryo-ET) to intact sperm (8, 95). Freeze fixation was combined with multiangle EM imaging to provide a 3-D volume reconstruction (i.e., tomogram), thereby revealing higher-order CatSper assembly in mouse and human sperm. The repeating CatSper units were further aligned and averaged to generate a subtomogram-averaged cryo-ET structure of the CatSper channel (i.e., in situ structure) (10). These groundbreaking works offer unprecedented details on the channel’s molecular and spatial organization, thus providing an enhanced foundational and mechanistic understanding of the CatSper channel and overall sperm motility.
FIGURE 6.
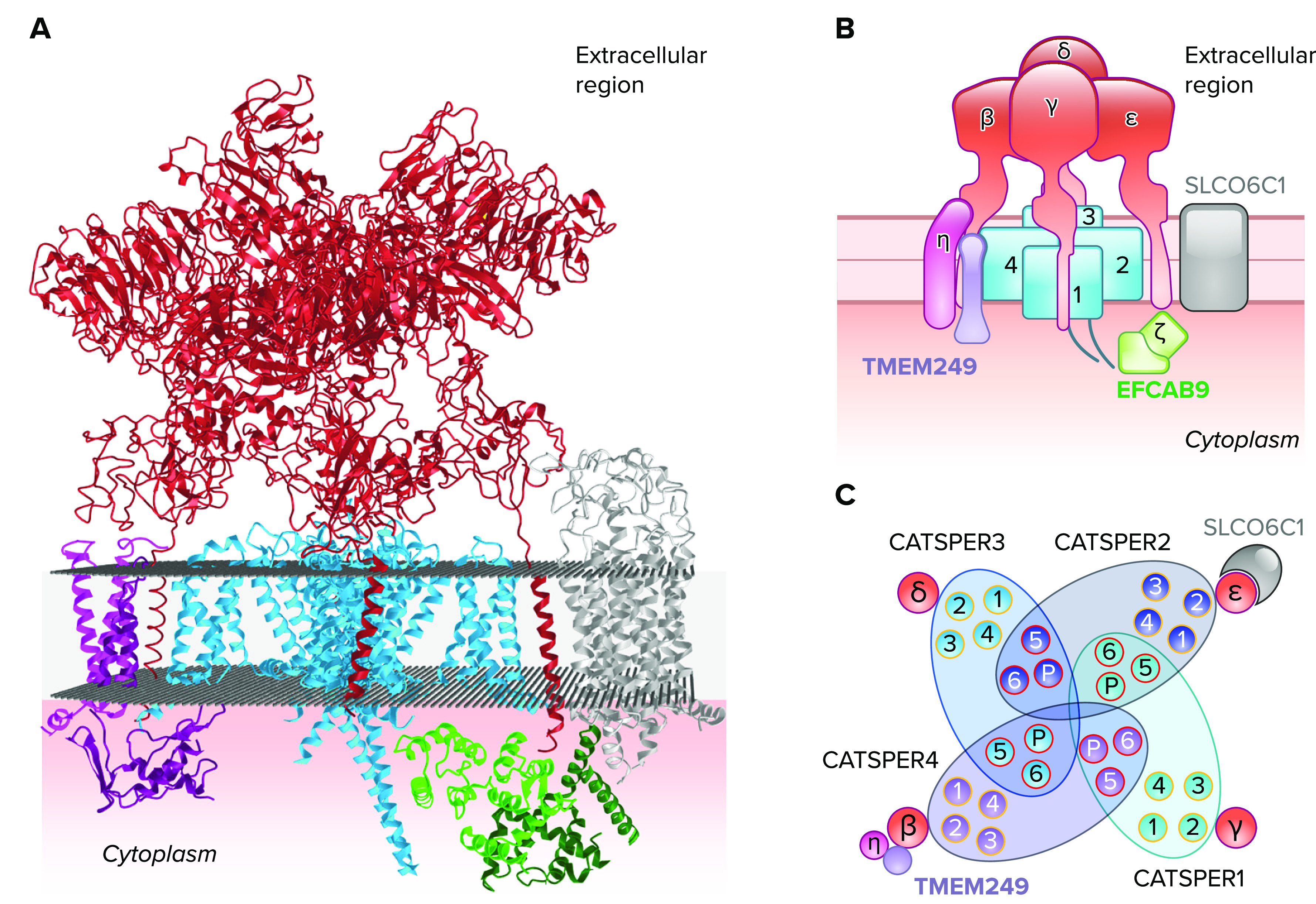
Atomic model of cryogenic electron microscopy (cryo-EM) structure of CatSper channel complex A: an atomic model of the high-resolution cryo-EM structure of a purified, monomeric mouse CatSper channel complex in a side view (PDB ID: 7EEB; Ref. 9). Pore-forming α-subunits (CATSPER1–4) are colored blue. Ancillary single transmembrane subunits (CATSPERβ, γ, δ, and ε) are marked in red. The extracellular domains of the four single transmembrane subunits form a tent-like pavilion structure over the heterotetrameric pore. TMEM249 (purple) and CATSPERη (magenta) localize close to the CATSPER4 and CATSPERβ (left side of the complex). SLCO6C1 (silver) is placed close to the CATSPER2 and CATSPERε (right side of the complex). Nontransmembrane cytosolic CATSPERζ (dark green) and EFCAB9 (green) subunits interact with each other. B: a schematic cartoon of a monomer CatSper complex. Individual subunits are shown in simplified diagrams to visualize their localization and orientation within a CatSper channel complex. C: schematic representation of α-subunit transmembrane segments in a top-down view. Domains of α-subunits swap with each other to form the channel pore. Voltage sensor domains (orange) are located close to segments 5 and 6. The pore domains (red) are centered to form a Ca2+-permeable path. Ancillary transmembrane subunits are represented in circles and SLCO6C in crescent.
Single-particle cryo-EM structure of an isolated, monomeric CatSper channel complex.
Using a knockin approach, Lin et al. (9) generated transgenic mice that expressed tandem tags at the NH2 terminus of CATSPER1. They isolated the endogenous channel complex from the testis and epididymis for single-particle cryo-EM (9). A structure of a closed CatSper channel was then visualized at an overall 2.9-Å resolution in which most previously known subunits were able to be unambiguously assigned and a few new components were also identified. As previous data predicted, a tetrameric channel is formed by CATSPER1–4, similar to the architecture of the conventional voltage-gated ion channel. Most of the extracellular domains of CATSPERβ, γ, δ, and ε sit above the channel pore like a canopy (FIGURE 6, A AND B). The overall topology and stoichiometry are surprisingly similar to the KCNQ1-KCNE2 or the Kv4-DPPX complex (96) whereas the subunit composition of the CatSper channel is much more complex.
The structure revealed that CATSPER1–4 are arranged in counterclockwise order (FIGURE 6, B AND C). Interestingly, detergent-like molecules, which could be cholesterol or steroid hormones under physiological conditions, were found in the transmembrane cavity of CATSPER1 within S3, S4, and the loop connecting S4 and S5. The molecules were not found in CATSPER2–4 (9). In this regard, it is particularly intriguing that only CATSPER1 (i.e., no other CatSper subunits) undergoes capacitation-associated cleavage and processing (6, 7). In mouse sperm, cholesterol efflux induces CatSper activation during capacitation (97) whereas cholesterol-like endogenous ligands for mouse CatSper are not known. By contrast, in human sperm, steroid derivatives and various other compounds, such as endocrine disruptors and odorants, have been reported to modulate CatSper activity (98–100), suggesting that the binding site is promiscuous as seen in mouse CATSPER1. To determine if this transmembrane binding site is a conserved feature for human CatSper, the highly anticipated human CatSper structure will first need to be mapped.
CATSPERβ, γ, δ, and ε do not share much sequence homology with each other (with an exception of 30% homology toward the COOH-terminal region between CATSPERδ and ε) nor with any known conserved domains. Yet, the cryo-EM structure now clearly demonstrates that they all show an overall similar architecture composed of multiple modular domains (NH2-terminal domain, β-propeller, Ig-like, and a stem domain before the transmembrane helix) with some variations (9). Through one-on-one pairing, the single transmembrane helix of CATSPERβ, γ, δ, and ε interacts with S2 of CATSPER4, 1, 3, and 2, respectively (FIGURE 6C). All the domains other than the transmembrane helices and the stem domains of CATSPERδ and γ do not directly interact with the channel. The stem domains of the four ancillary subunits also do not interact with each other, leaving all four sides open to allow for the free passage of ions to the selectivity filter of the channel. The Ig-like domains from the four subunits seal the top of the channel. The β-propeller domains of the four subunits form most of the canopy roof.
Identification of uncharacterized components associated with CatSper complex.
Several uncharacterized transmembrane proteins are also identified in the CatSper structure, validated by mass spectrometry (MS) analysis, increasing the complexity of the channel (8, 9). Special attention is paid to a testis-specific, organic anion transporter SLCO6C1 (solute carrier organic anion transporter family member 6c1). SLCO6C1 is located as a wing-like structure to the side of CATSPER2/CATSPERε in the native CatSper complex (FIGURES 6 AND 7; Refs. 9, 10). Finding SLCO6C1 in the native CatSper complex has made CatSper one of a growing number of channel-transporters or “chansporter” complexes (101). One of the best-known chansporter complexes and the only structurally characterized example is the KATP channel, which is formed as an obligate complex between the potassium channel Kir6.2 and the sulfonylurea receptor (SUR1; an ABC transporter), in which SUR1 is an ADP sensor modulating Kir6.2 activity (102). A closely related human homolog of the mouse SLCO6C1 is SLCO6A1, which is not conserved in rodents. If this channel-transporter interaction is a species-specific feature of mouse CatSper, or if human CatSper also forms this chansporter, will require further investigation. Deorphanizing SLCO6C1 and SLCO6A1 will address this question and potentially reveal new regulatory mechanisms of the CatSper channel affecting sperm motility and fertility.
FIGURE 7.
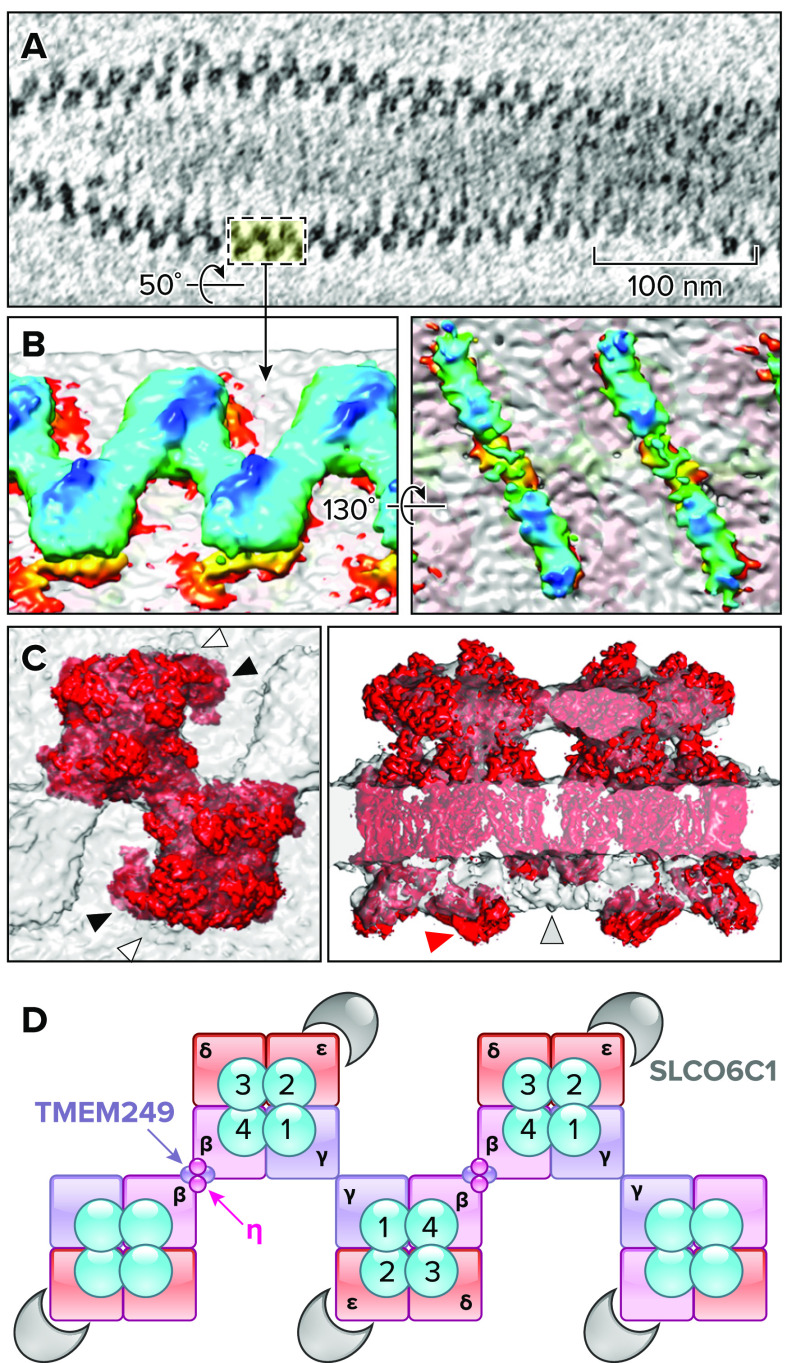
In-cell arrangement and subtomogram average structure of CatSper channel complexes A: a representative tomogram showing the CatSper arrangement in zigzag rows along mouse sperm tail from a top-down view. B: 3-dimensional isosurface rendered images of averaged, two dimer CatSper complexes. Extracellular (left) and intracellular (right) domain structures are shown. C: docking the monomeric CatSper cryogenic electron microscopy structure into the in situ map. Top view (left) shows the distinct angle of SLCO6C1 position from the cryo-EM structure (black arrowheads) and the in situ map (white arrowheads). Side view (right) is to show “cytosolic map 1” (red arrows) that protrudes from the in situ map and an intracellular region that is missing in the monomeric CatSper structure but present in the in situ CatSper structure (gray arrowhead). Images in A–C are adapted from Ref. 10, with permission from Nature Communications. D: a schematic cartoon to represent supramolecular interactions between CatSper complexes in a zigzag row. Neighboring CatSper complexes are connected by the extracellular, interdimeric interactions between two CATSPERβ (magenta) and between two CATSPERγ (purple). CATSPERη and TMEM249 are postulated to contribute to interaction within a dimer. SLCO6C1 is located at the outer corner of the channel complex as a “wing-like” structure.
In addition to SLCO6C1, three small transmembrane proteins (TMEM262, TMEM249, and an unassigned single transmembrane protein) were identified in the isolated CatSper complex (FIGURES 2B AND 6). Interestingly, all three new components are located on the side of CATSPER4/CATSPERβ in the CatSper monomer complex (FIGURE 6, B AND C). TMEM262 (now referred to as CATSPERη) is a type II membrane protein with three transmembrane domains in which transmembranes 2 and 3 form extensive hydrophobic interactions with the transmembrane domain of CATSPERβ (FIGURES 2B AND 6). TMEM249 is a putative two transmembrane protein interacting with CATSPERη and the VSD of CATSPER4 in the transmembrane and cytosolic regions, respectively. A cytosolic entity (cytosolic map 1) remains unassigned as well due to limited resolution in the intracellular regions of the cryo-EM structure (FIGURE 7). The detailed roles and physiological significance of the new CatSper subunits are waiting to be defined.
In-cell higher-order cryo-ET structures of mouse and human CatSper channel complexes.
The fragmented linear CatSper nanodomains found in Efcab9- or Catsperz-null sperm (8, 76) suggest the repeated nature of CatSper complexes within the nanodomains (8). Moreover, the CatSper nanodomain architecture was visualized in mouse flagellum with conventional scanning electron microscopy (8). Nearly 500-kDa extracellular domains are predicted for a single mouse or human CatSper complex at 1:1 stoichiometry. These data suggest that cellular cryo-ET and subtomogram averaging could aid in visualizing native CatSper channel complexes in their cellular context (95). Combined with cryo-focused ion beam (cryo-FIB) milling, which cuts 150- to 200-nm thin slices (called “lamellae”) of the sample using cryo-ET, Zhao et al. (10) resolved the 3-D in-cell higher-order organization and domain structures of the CatSper channels from intact mouse and human sperm flagella.
The 3-D reconstructed cryo-tomograms of mouse sperm flagella revealed long, continuous rows of densely packed particles in a zigzag pattern, which are well-aligned with the flagellar axis (FIGURE 7A; Ref. 10). The repeating particles are unambiguously identified as CatSper complexes because of their positions as compared to the previously reported localization for CatSper nanodomains by immuno-EM (FIGURE 5B; Ref. 6) and a wild type-mutant comparison approach. The sperm from Efcab9-null mice revealed discontinuous rows in which the repeating CatSper units form scattered and short clusters, reminiscent of the fragmented nanodomains seen with super-resolution imaging (8). New findings include structural variations in the number of zigzag rows per nanodomain in wild-type sperm flagella and in the angle of the short clusters in mutant sperm relative to the longitudinal tail axis (10). The majority consists of a single row or two rows ∼100 nm apart as previously seen by immuno-EM and super-resolution imaging (FIGURES 5 AND 7). Yet, observations also included the merging of multiple rows or the splitting of a singular row. Since capacitating sperm cells promotes heterogeneity, and since the integrity of the nanodomains is strongly correlated with the endurance of motility, hyperactivation, and fertilizing ability (6–8), it is intriguing whether endogenous structural variations of CatSper underlie such capacitation-induced, heterogenous sperm performance. Cryo-tomograms of human sperm flagella showed zigzag rows that consisted of staggered, repeating units in similar dimensions to the CatSper particles in mouse sperm, suggesting that the assembly of the higher-order CatSper complex is conserved in mammalian sperm.
Subsequent subtomogram averaging revealed several surprises (FIGURE 7B). First, the repeating, staggered CatSper units are interconnected by the extracellular domains that form a canopy with a tilted roof ridge above each tetrameric channel pore. Second, a dimer from two staggered channel units is the building block of the long zigzag rows. Intracellular domains link two neighboring channels in a diagonal array. Third, mouse CatSper contains an additional wing structure (validated as SLCO6C1 in the cryo-EM structure) connected to the outer corners of the staggered tetrameric channels rotated 180 degrees (FIGURE 7). Yet, no corresponding EM density to the wing structure was observed by cryo-ET structural analysis of human CatSper. This difference leaves the possibility of another species-specific feature of the CatSper channel between mice and humans that will require additional higher resolution structural studies for clarification. Finally, the mutant complexes affect not only the intracellular architecture, where the bulges at both ends of the diagonal arrays are missing, but also the extracellular architecture between and within dimers (10). Mutant CatSper structures in the absence of the EFCAB9-CATSPERζ complex suggest two conformations for the higher-order arrangement of CatSper complexes: the weakening of inter- or intradimer interactions. This observation leads to the question of whether the changes in the intracellular interactions of EFCAB9 and CATSPERζ during sperm capacitation (i.e., elevated pHi-induced dissociation) can similarly affect the extracellular connections in the zigzag rows. If so, it provides explanations to the plausible cooperative channel activity within each zigzag row and the efficiency of Ca2+ signal transduction along long sperm tails.
Synthesis of new knowledge based on the two complementary structures.
The two complementary structural data obtained using single particle cryo-EM and cryo-ET (9, 10), enabled fitting of the cryo-EM density and corresponding atomic model for the isolated CatSper “monomer” into the subtomogram average of the cryo-ET map (FIGURE 7C). In the averaged in situ map, two CatSper monomers fit into a dimer, especially well in the extracellular regions, revealing supramolecular interactions between the CatSper complexes and the zigzag assembly (10). The interfaces within and between dimers are formed between two CATSPERβ (intradimeric) or two CATSPERγ (interdimeric) subunits (FIGURE 7D).
There are some differences between the purified, monomeric structure and the assembled in-cell structure of CatSper complexes (FIGURE 7C). First, the positioning of SLCO6C1 with the tetrameric channel in the monomeric cryo-EM structure is rotated toward the interdimeric connection compared to the “wing” structure seen in the in situ map (10). Second, the intracellular (cytosolic) regions of the monomeric cryo-EM structure do not fit well into the in situ structure. For example, the cytosolic map 1 position in the cryo-EM structure largely protrudes from the in situ structure of the membrane. Most notably, the EM density from the cryo-EM structure that corresponds to the central part of the diagonal array linking two monomeric complexes in the in situ map is missing. Finally, there is a connecting EM density clearly visible in the in situ structure at the extracellular dimeric interface close to the membrane (10). However, a possible molecular interaction between the two cannot be explained by the present monomeric structure and corresponding atomic model because none of the newly identified transmembrane subunits (CATSPERη, TMEM249, and an unassigned single transmembrane protein) contains large extracellular domains. Thus such missing cytosolic and transmembrane components in the purified, monomeric CatSper structure are most likely due to preparation artifacts as they lack dimer interactions and native membranes. The molecular composition and stoichiometry of the central part of the intracellular region in the in situ structure remain to be determined. In this regard, it is of note that a membrane-associating C2 domain-containing C2CD6 protein, recently characterized as a CatSper-associated protein essential for fertility (85, 86), is not assigned in the cryo-EM map, but the protein was identified as one of the top five confidence hits by MS analysis of the isolated CatSper complex (9).
Linking CatSper structures to sperm motility.
Analyzing free-swimming sperm over time by 3-D high-speed digital holographic microscopy (DHM) suggests potential ways to address how the structural and conformational changes of CatSper could be linked to sperm motility (103, 104). Capacitation dramatically increases the xy-displacement of the mouse flagellum (in the out-of-plane beating direction), which is diminished in the sperm lacking CatSper components (47, 74). Using DHM, Zhao et al. (10) demonstrated that capacitation does not affect the flagellar z-displacement (the waveform amplitude). The head trajectory of free-swimming mouse sperm indicates increased spatial sampling in all directions during capacitation. By contrast, regardless of capacitation, mouse sperm swim strictly with a clockwise chirality when facing an upcoming sperm (103). In the absence of EFCAB9-CATSPERζ, the typical increase in the flagellar xy-displacement during capacitation is abolished and z-displacement becomes smaller, leading to smaller spatial sampling (10). Most dramatically, the clockwise swimming path chirality is randomized in Efcab9-null sperm (104). The connected higher-order zigzag arrangement may allow for the coordinated opening of the entire array of CatSper channels within the longitudinal nanodomains along the flagellar axis. Not only the scattered and short clusters, but also their loss of parallel angles to the longitudinal flagellar axis, likely contributes to dysregulating the domino effect of cooperative channel activity.
Future of the Field—What Is to Come After the First Structures
Over the past two decades, convincing data from the organismal to the atomic level from human and animal models have accumulated that CatSper could be a promising target for the treatment of male infertility and, conversely, for developing nonhormonal contraceptives for both men and women (105, 106). CatSper-deficient patients suffer from male infertility but are otherwise healthy except for the rare cases of deafness-infertility syndrome in which a genomic region encompassing contiguous CATSPER2 and STRC is deleted (49). Among the 13 CATSPER genes that encode either CatSper α-subunits or ancillary subunits, only 4 genes have been reported to underlie asthenozoospermia in human infertility (48, 49, 68, 69, 78, 107–109), thereby illustrating the practical difficulties of identifying damaging mutations that cause infertility in humans. Considering the large number of genes required to form the CatSper channel complex and that knockout mice of eight Catsper genes all have similar infertility phenotypes without any gross abnormality, more mutations in other CATSPER genes are expected to be discovered from infertile males in the future.
Previous studies have implicated CatSper not only in developing hyperactivated motility but also in priming other Ca2+-dependent, physiological events of sperm function such as the endurance of sperm motility (ATP homeostasis), passing the utero-tubal junction to the fertilization site, and the acrosome reaction (6, 53, 110). In all these aforementioned cases, the underlying assumption is that a loss or dysregulation of functional CatSper channels in the mature sperm results in mutant phenotypes. In this regard, mouse studies corroborate the idea that there is a critical threshold (approximately between 10% and 20–30%) of ICatSper and/or of the integrity of CatSper nanodomains to be a fertile spermatozoon (6, 8, 53, 74, 76, 85). Human sperm from subfertile and infertile patients also show significantly reduced (or no) ICatSper or Ca2+ response to progesterone activation of CatSper (111, 112). Information on the threshold for minimally sufficient CatSper function for male fertility is essential for developing drugs that enhance or inhibit CatSper function.
The CatSper structures now open a new avenue, enabling structure-based drug design. Several compounds have been identified that inhibit CatSper such as NNC55-0396, HC-056456, RU1968, and mibefradil, but they are either nonspecific or have 2–6 μM affinity (113–115). Thus molecular docking of these previously characterized compounds followed by structure optimization or rational de novo design by in silico screening coupled with automated machine learning methods will help to discover compounds specific enough for contraceptive use.
These first CatSper structures recast the following long-standing questions in the sperm biology and CatSper fields: What is the gating mechanism of CatSper channel? Where is the binding site(s) for endogenous and agonist/antagonist ligands? How does ligand binding affect the channel activity? Entirely new questions also have arisen as the present structural studies discovered the coassembly of CatSper with SLCO6C1, an uncharacterized member of the OATP6 superfamily of an organic ion transporter. For example, is SLCO6C1 a functional transporter in mouse sperm? If so, what is the substrate of this transporter, and how does transporter activity regulate CatSper activity or vice versa? Are these inventions species-specific to adapt to the female environment? What is the physiological function of the other subunits that have been newly identified?
With the ever-improving capabilities of cryo-EM, the visualization of more structures will likely help us to answer some of these questions. The current cryo-EM structure of the monomeric CatSper complex is in its closed conformation indicated by the intracellular gate that is formed by two hydrophobic rings with a radius of less than 1 Å. The CatSper structure in its open form with and without ligand binding is required to better understand the gating mechanisms for CatSper (such as the conformational change in EFCAB9). To obtain an open structure of the complex, it will be necessary to purify the CatSper complex directly from isolated sperm after inducing capacitation in vitro (i.e., not using testes and epididymis) or ideally treating them with endogenous or agonist ligands (if known). Other than the COOH-terminal regions of CATSPER2 and CATSPER3, the majority of cytosolic domains of α-subunits as well as the cytosolic map 1 are not resolved. Thus new structures with an improved resolution, especially in the intracellular regions beyond the resolution of the current cryo-EM structure, will reveal the molecular identity of the additional CatSper components. This will also provide better clues for the mechanism of CatSper dimer formation and the channel’s higher-order arrangement.
High-resolution structures of human CatSper would certainly address many of the questions posed above in this review, not to mention the contribution to developing human therapeutics. The complexity of the monomeric and the higher-order assembly of the CatSper channel explains why expressing the CatSper channel at the plasma membrane of cultured cells has remained such a long-time, outstanding challenge. Yet, high-content and/or high-throughput screening, as well as mechanism-of-action studies, would greatly benefit from making this screening strategy available. Structure-based protein engineering might finally reveal the light at the end of the tunnel.
Acknowledgments
We thank Huafeng Wang for providing a photo of whole-sperm patch-clamp recording, Tse-en Wang for preparing EM samples, Shan Xu and Song Pang for EM processing, Gillian Clouser for proofreading, and all the Chung lab members for critical reading of the draft.
This work was supported by National Institute of Child Health and Human Development Grant R01HD096745, Male Contraceptive Initiative David Sokal Innovation Award, and The Blavatnik Fund for Innovation at Yale (to J.-J.C.).
No conflicts of interest, financial or otherwise, are declared by the authors.
Author Contributions: J.Y.H. and J.-J.C. prepared figures; J.Y.H. and J.C.-C. drafted manuscript; J.Y.H. and J.C.-C. edited and revised manuscript; J.C.-C. approved final version of manuscript.
References
- 1. Clapham DE. Calcium signaling. Cell 131: 1047–1058, 2007. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 2. Quill TA, Ren D, Clapham DE, Garbers DL. A voltage-gated ion channel expressed specifically in spermatozoa. Proc Natl Acad Sci U S A 98: 12527–12531, 2001. doi: 10.1073/pnas.221454998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ren D, Navarro B, Perez G, Jackson AC, Hsu S, Shi Q, Tilly JL, Clapham DE. A sperm ion channel required for sperm motility and male fertility. Nature 413: 603–609, 2001. doi: 10.1038/35098027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kirichok Y, Navarro B, Clapham DE. Whole-cell patch-clamp measurements of spermatozoa reveal an alkaline-activated Ca2+ channel. Nature 439: 737–740, 2006. doi: 10.1038/nature04417. [DOI] [PubMed] [Google Scholar]
- 5. Lishko PV, Botchkina IL, Kirichok Y. Progesterone activates the principal Ca2+ channel of human sperm. Nature 471: 387–391, 2011. doi: 10.1038/nature09767. [DOI] [PubMed] [Google Scholar]
- 6. Chung JJ, Shim SH, Everley RA, Gygi SP, Zhuang X, Clapham DE. Structurally distinct Ca(2+) signaling domains of sperm flagella orchestrate tyrosine phosphorylation and motility. Cell 157: 808–822, 2014. doi: 10.1016/j.cell.2014.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ded L, Hwang JY, Miki K, Shi HF, Chung JJ. 3D in situ imaging of the female reproductive tract reveals molecular signatures of fertilizing spermatozoa in mice. Elife 9: e62043, 2020. doi: 10.7554/eLife.62043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hwang JY, Mannowetz N, Zhang Y, Everley RA, Gygi SP, Bewersdorf J, Lishko PV, Chung JJ. Dual sensing of physiologic pH and calcium by EFCAB9 regulates sperm motility. Cell 177: 1480–1494.e19, 2019. doi: 10.1016/j.cell.2019.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lin S, Ke M, Zhang Y, Yan Z, Wu J. Structure of a mammalian sperm cation channel complex. Nature 595: 746–750, 2021. doi: 10.1038/s41586-021-03742-6. [DOI] [PubMed] [Google Scholar]
- 10. Zhao Y, Wang H, Wiesehoefer C, Shah NB, Reetz E, Hwang JY, Huang X, Wang TE, Lishko PV, Davies KM, Wennemuth G, Nicastro D, Chung JJ. 3D structure and in situ arrangements of CatSper channel in the sperm flagellum. Nat Commun 13: 3439, 2022. doi: 10.1038/s41467-022-31050-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Austin CR. Observations on the penetration of the sperm in the mammalian egg. Aust J Sci Res B 4: 581–596, 1951. doi: 10.1071/BI9510581. [DOI] [PubMed] [Google Scholar]
- 12. Chang MC. Fertilizing capacity of spermatozoa deposited into the fallopian tubes. Nature 168: 697–698, 1951. doi: 10.1038/168697b0. [DOI] [PubMed] [Google Scholar]
- 13. Yanagimachi R. In vitro capacitation of hamster spermatozoa by follicular fluid. J Reprod Fertil 18: 275–286, 1969. doi: 10.1530/jrf.0.0180275. [DOI] [PubMed] [Google Scholar]
- 14. Yanagimachi R. The movement of golden hamster spermatozoa before and after capacitation. J Reprod Fertil 23: 193–196, 1970. doi: 10.1530/jrf.0.0230193. [DOI] [PubMed] [Google Scholar]
- 15. Kay VJ, Robertson L. Hyperactivated motility of human spermatozoa: a review of physiological function and application in assisted reproduction. Hum Reprod Update 4: 776–786, 1998. doi: 10.1093/humupd/4.6.776. [DOI] [PubMed] [Google Scholar]
- 16. Suarez SS, Osman RA. Initiation of hyperactivated flagellar bending in mouse sperm within the female reproductive tract. Biol Reprod 36: 1191–1198, 1987. doi: 10.1095/biolreprod36.5.1191. [DOI] [PubMed] [Google Scholar]
- 17. DeMott RP, Lefebvre R, Suarez SS. Carbohydrates mediate the adherence of hamster sperm to oviductal epithelium. Biol Reprod 52: 1395–1403, 1995. doi: 10.1095/biolreprod52.6.1395. [DOI] [PubMed] [Google Scholar]
- 18. Stauss CR, Votta TJ, Suarez SS. Sperm motility hyperactivation facilitates penetration of the hamster zona pellucida. Biol Reprod 53: 1280–1285, 1995. doi: 10.1095/biolreprod53.6.1280. [DOI] [PubMed] [Google Scholar]
- 19. Yanagimachi R. Requirement of extracellular calcium ions for various stages of fertilization and fertilization‐related phenomena in the hamster. Gamete Res 5: 323–344, 1982. doi: 10.1002/mrd.1120050404. [DOI] [Google Scholar]
- 20. Fraser LR. Minimum and maximum extracellular Ca2+ requirements during mouse sperm capacitation and fertilization in vitro. J Reprod Fertil 81: 77–89, 1987. doi: 10.1530/jrf.0.0810077. [DOI] [PubMed] [Google Scholar]
- 21. Suarez SS, Vincenti L, Ceglia MW. Hyperactivated motility induced in mouse sperm by calcium ionophore A23187 is reversible. J Exp Zool 244: 331–336, 1987. doi: 10.1002/jez.1402440218. [DOI] [PubMed] [Google Scholar]
- 22. Lindemann CB, Goltz JS. Calcium regulation of flagellar curvature and swimming pattern in triton X-100–extracted rat sperm. Cell Motil Cytoskeleton 10: 420–431, 1988. doi: 10.1002/cm.970100309. [DOI] [PubMed] [Google Scholar]
- 23. Ho HC, Granish KA, Suarez SS. Hyperactivated motility of bull sperm is triggered at the axoneme by Ca2+ and not cAMP. Dev Biol 250: 208–217, 2002. doi: 10.1006/dbio.2002.0797. [DOI] [PubMed] [Google Scholar]
- 24. Babcock DF, Rufo GA Jr, Lardy HA. Potassium-dependent increases in cytosolic pH stimulate metabolism and motility of mammalian sperm. Proc Natl Acad Sci U S A 80: 1327–1331, 1983. doi: 10.1073/pnas.80.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Aitken RJ, Irvine S, Kelly RW. Significance of intracellular calcium and cyclic adenosine 3',5'-monophosphate in the mechanisms by which prostaglandins influence human sperm function. J Reprod Fertil 77: 451–462, 1986. doi: 10.1530/jrf.0.0770451. [DOI] [PubMed] [Google Scholar]
- 26. Babcock DF, Pfeiffer DR. Independent elevation of cytosolic [Ca2+] and pH of mammalian sperm by voltage-dependent and pH-sensitive mechanisms. J Biol Chem 262: 15041–15047, 1987. doi: 10.1016/S0021-9258(18)48135-5. [DOI] [PubMed] [Google Scholar]
- 27. Parrish JJ, Susko-Parrish JL, First NL. Capacitation of bovine sperm by heparin: inhibitory effect of glucose and role of intracellular pH. Biol Reprod 41: 683–699, 1989. doi: 10.1095/biolreprod41.4.683. [DOI] [PubMed] [Google Scholar]
- 28. Baldi E, Casano R, Falsetti C, Krausz C, Maggi M, Forti G. Intracellular calcium accumulation and responsiveness to progesterone in capacitating human spermatozoa. J Androl 12: 323–330, 1991. [PubMed] [Google Scholar]
- 29. Suarez SS, Varosi SM, Dai X. Intracellular calcium increases with hyperactivation in intact, moving hamster sperm and oscillates with the flagellar beat cycle. Proc Natl Acad Sci U S A 90: 4660–4664, 1993. doi: 10.1073/pnas.90.10.4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Florman HM, Arnoult C, Kazam IG, Li C, O'Toole CM. A perspective on the control of mammalian fertilization by egg-activated ion channels in sperm: a tale of two channels. Biol Reprod 59: 12–16, 1998. doi: 10.1095/biolreprod59.1.12. [DOI] [PubMed] [Google Scholar]
- 31. Darszon A, Labarca P, Nishigaki T, Espinosa F. Ion channels in sperm physiology. Physiol Rev 79: 481–510, 1999. doi: 10.1152/physrev.1999.79.2.481. [DOI] [PubMed] [Google Scholar]
- 32. Arnoult C, Cardullo RA, Lemos JR, Florman HM. Activation of mouse sperm T-type Ca2+ channels by adhesion to the egg zona pellucida. Proc Natl Acad Sci U S A 93: 13004–13009, 1996. doi: 10.1073/pnas.93.23.13004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hagiwara S, Kawa K. Calcium and potassium currents in spermatogenic cells dissociated from rat seminiferous tubules. J Physiol 356: 135–149, 1984. doi: 10.1113/jphysiol.1984.sp015457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Santi CM, Darszon A, Hernandez-Cruz A. A dihydropyridine-sensitive T-type Ca2+ current is the main Ca2+ current carrier in mouse primary spermatocytes. Am J Physiol Cell Physiol 271: C1583–C1593, 1996. doi: 10.1152/ajpcell.1996.271.5.C1583. [DOI] [PubMed] [Google Scholar]
- 35. Wennemuth G, Westenbroek RE, Xu T, Hille B, Babcock DF. CaV2.2 and CaV2.3 (N- and R-type) Ca2+ channels in depolarization-evoked entry of Ca2+ into mouse sperm. J Biol Chem 275: 21210–21217, 2000. doi: 10.1074/jbc.M002068200. [DOI] [PubMed] [Google Scholar]
- 36. Trevino CL, Felix R, Castellano LE, Gutierrez C, Rodriguez D, Pacheco J, Lopez-Gonzalez I, Gomora JC, Tsutsumi V, Hernandez-Cruz A, Fiordelisio T, Scaling AL, Darszon A. Expression and differential cell distribution of low-threshold Ca(2+) channels in mammalian male germ cells and sperm. FEBS Lett 563: 87–92, 2004. doi: 10.1016/S0014-5793(04)00257-1. [DOI] [PubMed] [Google Scholar]
- 37. Westenbroek RE, Babcock DF. Discrete regional distributions suggest diverse functional roles of calcium channel alpha1 subunits in sperm. Dev Biol 207: 457–469, 1999. doi: 10.1006/dbio.1998.9172. [DOI] [PubMed] [Google Scholar]
- 38. Beuckmann CT, Sinton CM, Miyamoto N, Ino M, Yanagisawa M. N-type calcium channel alpha1B subunit (Cav2.2) knock-out mice display hyperactivity and vigilance state differences. J Neurosci 23: 6793–6797, 2003. doi: 10.1523/JNEUROSCI.23-17-06793.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kim C, Jun K, Lee T, Kim SS, McEnery MW, Chin H, Kim HL, Park JM, Kim DK, Jung SJ, Kim J, Shin HS. Altered nociceptive response in mice deficient in the alpha(1B) subunit of the voltage-dependent calcium channel. Mol Cell Neurosci 18: 235–245, 2001. doi: 10.1006/mcne.2001.1013. [DOI] [PubMed] [Google Scholar]
- 40. Chen CC, Lamping KG, Nuno DW, Barresi R, Prouty SJ, Lavoie JL, Cribbs LL, England SK, Sigmund CD, Weiss RM, Williamson RA, Hill JA, Campbell KP. Abnormal coronary function in mice deficient in alpha1H T-type Ca2+ channels. Science 302: 1416–1418, 2003. doi: 10.1126/science.1089268. [DOI] [PubMed] [Google Scholar]
- 41. Sakata Y, Saegusa H, Zong S, Osanai M, Murakoshi T, Shimizu Y, Noda T, Aso T, Tanabe T. Ca(v)2.3 (alpha1E) Ca2+ channel participates in the control of sperm function. FEBS Lett 516: 229–233, 2002. doi: 10.1016/S0014-5793(02)02529-2. [DOI] [PubMed] [Google Scholar]
- 42. Cohen R, Buttke DE, Asano A, Mukai C, Nelson JL, Ren D, Miller RJ, Cohen-Kutner M, Atlas D, Travis AJ. Lipid modulation of calcium flux through CaV2.3 regulates acrosome exocytosis and fertilization. Dev Cell 28: 310–321, 2014. doi: 10.1016/j.devcel.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jun K, Piedras-Renteria ES, Smith SM, Wheeler DB, Lee SB, Lee TG, Chin H, Adams ME, Scheller RH, Tsien RW, Shin HS. Ablation of P/Q-type Ca(2+) channel currents, altered synaptic transmission, and progressive ataxia in mice lacking the alpha(1A)-subunit. Proc Natl Acad Sci U S A 96: 15245–15250, 1999. doi: 10.1073/pnas.96.26.15245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Seisenberger C, Specht V, Welling A, Platzer J, Pfeifer A, Kuhbandner S, Striessnig J, Klugbauer N, Feil R, Hofmann F. Functional embryonic cardiomyocytes after disruption of the L-type alpha1C (Cav1.2) calcium channel gene in the mouse. J Biol Chem 275: 39193–39199, 2000. doi: 10.1074/jbc.M006467200. [DOI] [PubMed] [Google Scholar]
- 45. Quill TA, Sugden SA, Rossi KL, Doolittle LK, Hammer RE, Garbers DL. Hyperactivated sperm motility driven by CatSper2 is required for fertilization. Proc Natl Acad Sci U S A 100: 14869–14874, 2003. doi: 10.1073/pnas.2136654100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Carlson AE, Westenbroek RE, Quill T, Ren D, Clapham DE, Hille B, Garbers DL, Babcock DF. CatSper1 required for evoked Ca2+ entry and control of flagellar function in sperm. Proc Natl Acad Sci U S A 100: 14864–14868, 2003. doi: 10.1073/pnas.2536658100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Carlson AE, Quill TA, Westenbroek RE, Schuh SM, Hille B, Babcock DF. Identical phenotypes of CatSper1 and CatSper2 null sperm. J Biol Chem 280: 32238–32244, 2005. doi: 10.1074/jbc.M501430200. [DOI] [PubMed] [Google Scholar]
- 48. Avenarius MR, Hildebrand MS, Zhang Y, Meyer NC, Smith LL, Kahrizi K, Najmabadi H, Smith RJ. Human male infertility caused by mutations in the CATSPER1 channel protein. Am J Hum Genet 84: 505–510, 2009. doi: 10.1016/j.ajhg.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Avidan N, Tamary H, Dgany O, Cattan D, Pariente A, Thulliez M, Borot N, Moati L, Barthelme A, Shalmon L, Krasnov T, Ben-Asher E, Olender T, Khen M, Yaniv I, Zaizov R, Shalev H, Delaunay J, Fellous M, Lancet D, Beckmann JS. CATSPER2, a human autosomal nonsyndromic male infertility gene. Eur J Hum Genet 11: 497–502, 2003. doi: 10.1038/sj.ejhg.5200991. [DOI] [PubMed] [Google Scholar]
- 50. Jin J, Jin N, Zheng H, Ro S, Tafolla D, Sanders KM, Yan W. Catsper3 and Catsper4 are essential for sperm hyperactivated motility and male fertility in the mouse. Biol Reprod 77: 37–44, 2007. doi: 10.1095/biolreprod.107.060186. [DOI] [PubMed] [Google Scholar]
- 51. Jin JL, O’Doherty AM, Wang S, Zheng H, Sanders KM, Yan W. Catsper3 and catsper4 encode two cation channel-like proteins exclusively expressed in the testis. Biol Reprod 73: 1235–1242, 2005. doi: 10.1095/biolreprod.105.045468. [DOI] [PubMed] [Google Scholar]
- 52. Lobley A, Pierron V, Reynolds L, Allen L, Michalovich D. Identification of human and mouse CatSper3 and CatSper4 genes: characterisation of a common interaction domain and evidence for expression in testis. Reprod Biol Endocrinol 1: 53, 2003. doi: 10.1186/1477-7827-1-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Qi H, Moran MM, Navarro B, Chong JA, Krapivinsky G, Krapivinsky L, Kirichok Y, Ramsey IS, Quill TA, Clapham DE. All four CatSper ion channel proteins are required for male fertility and sperm cell hyperactivated motility. Proc Natl Acad Sci U S A 104: 1219–1223, 2007. doi: 10.1073/pnas.0610286104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Liu J, Xia J, Cho KH, Clapham DE, Ren D. CatSperbeta, a novel transmembrane protein in the CatSper channel complex. J Biol Chem 282: 18945–18952, 2007. doi: 10.1074/jbc.M701083200. [DOI] [PubMed] [Google Scholar]
- 55. Darszon A, Nishigaki T, Wood C, Trevino CL, Felix R, Beltran C. Calcium channels and Ca2+ fluctuations in sperm physiology. Int Rev Cytol 243: 79–172, 2005. doi: 10.1016/S0074-7696(05)43002-8. [DOI] [PubMed] [Google Scholar]
- 56. Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch 391: 85–100, 1981. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 57. Sakmann B, Neher E. Patch clamp techniques for studying ionic channels in excitable membranes. Annu Rev Physiol 46: 455–472, 1984. doi: 10.1146/annurev.ph.46.030184.002323. [DOI] [PubMed] [Google Scholar]
- 58. Guerrero A, Sanchez JA, Darszon A. Single-channel activity in sea urchin sperm revealed by the patch-clamp technique. FEBS Lett 220: 295–298, 1987. doi: 10.1016/0014-5793(87)80833-5. [DOI] [PubMed] [Google Scholar]
- 59. Kirichok Y, Lishko PV. Rediscovering sperm ion channels with the patch-clamp technique. Mol Hum Reprod 17: 478–499, 2011. doi: 10.1093/molehr/gar044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lishko P, Clapham DE, Navarro B, Kirichok Y. Sperm patch-clamp. Methods Enzymol 525: 59–83, 2013. doi: 10.1016/B978-0-12-397944-5.00004-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Liu B, Mundt N, Miller M, Clapham DE, Kirichok Y, Pv L. Recording electrical currents across the plasma membrane of mammalian sperm cells. J Vis Exp 168: e62049, 2021. doi: 10.3791/62049. [DOI] [PubMed] [Google Scholar]
- 62. Xia J, Ren D. Egg coat proteins activate calcium entry into mouse sperm via CATSPER channels. Biol Reprod 80: 1092–1098, 2009. doi: 10.1095/biolreprod.108.074039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Blackmore PF, Beebe SJ, Danforth DR, Alexander N. Progesterone and 17 alpha-hydroxyprogesterone. Novel stimulators of calcium influx in human sperm. J Biol Chem 265: 1376–1380, 1990. doi: 10.1016/S0021-9258(19)40024-0. [DOI] [PubMed] [Google Scholar]
- 64. Harper CV, Barratt CL, Publicover SJ. Stimulation of human spermatozoa with progesterone gradients to simulate approach to the oocyte. Induction of [Ca(2+)](i) oscillations and cyclical transitions in flagellar beating. J Biol Chem 279: 46315–46325, 2004. doi: 10.1074/jbc.M401194200. [DOI] [PubMed] [Google Scholar]
- 65. Uhler ML, Leung A, Chan SY, Wang C. Direct effects of progesterone and antiprogesterone on human sperm hyperactivated motility and acrosome reaction. Fertil Steril 58: 1191–1198, 1992. doi: 10.1016/S0015-0282(16)55568-X. [DOI] [PubMed] [Google Scholar]
- 66. Cooper TG. The epididymis, cytoplasmic droplets and male fertility. Asian J Androl 13: 130–138, 2011. doi: 10.1038/aja.2010.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lishko PV, Botchkina IL, Fedorenko A, Kirichok Y. Acid extrusion from human spermatozoa is mediated by flagellar voltage-gated proton channel. Cell 140: 327–337, 2010. doi: 10.1016/j.cell.2009.12.053. [DOI] [PubMed] [Google Scholar]
- 68. Luo T, Chen HY, Zou QX, Wang T, Cheng YM, Wang HF, Wang F, Jin ZL, Chen Y, Weng SQ, Zeng XH. A novel copy number variation in CATSPER2 causes idiopathic male infertility with normal semen parameters. Hum Reprod 34: 414–423, 2019. doi: 10.1093/humrep/dey377. [DOI] [PubMed] [Google Scholar]
- 69. Smith JF, Syritsyna O, Fellous M, Serres C, Mannowetz N, Kirichok Y, Lishko PV. Disruption of the principal, progesterone-activated sperm Ca2+ channel in a CatSper2-deficient infertile patient. Proc Natl Acad Sci U S A 110: 6823–6828, 2013. doi: 10.1073/pnas.1216588110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Miller MR, Mannowetz N, Iavarone AT, Safavi R, Gracheva EO, Smith JF, Hill RZ, Bautista DM, Kirichok Y, Lishko PV. Unconventional endocannabinoid signaling governs sperm activation via the sex hormone progesterone. Science 352: 555–559, 2016. doi: 10.1126/science.aad6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hwang JY, Maziarz J, Wagner GP, Chung JJ. Molecular evolution of CatSper in mammals and function of sperm hyperactivation in gray short-tailed opossum. Cells 10: 1047, 2021. doi: 10.3390/cells10051047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Dolphin AC. Voltage-gated calcium channels and their auxiliary subunits: physiology and pathophysiology and pharmacology. J Physiol 594: 5369–5390, 2016. doi: 10.1113/JP272262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Arikkath J, Campbell KP. Auxiliary subunits: essential components of the voltage-gated calcium channel complex. Curr Opin Neurobiol 13: 298–307, 2003. doi: 10.1016/S0959-4388(03)00066-7. [DOI] [PubMed] [Google Scholar]
- 74. Chung JJ, Navarro B, Krapivinsky G, Krapivinsky L, Clapham DE. A novel gene required for male fertility and functional CATSPER channel formation in spermatozoa. Nat Commun 2: 153, 2011. doi: 10.1038/ncomms1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wang H, Liu J, Cho KH, Ren D. A novel, single, transmembrane protein CATSPERG is associated with CATSPER1 channel protein. Biol Reprod 81: 539–544, 2009. doi: 10.1095/biolreprod.109.077107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Chung JJ, Miki K, Kim D, Shim SH, Shi HF, Hwang JY, Cai X, Iseri Y, Zhuang X, Clapham DE. CatSperzeta regulates the structural continuity of sperm Ca(2+) signaling domains and is required for normal fertility. Elife 6: e23082, 2017. doi: 10.7554/eLife.23082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Trotschel C, Hamzeh H, Alvarez L, Pascal R, Lavryk F, Bonigk W, Korschen HG, Muller A, Poetsch A, Rennhack A, Gui L, Nicastro D, Strunker T, Seifert R, Kaupp UB. Absolute proteomic quantification reveals design principles of sperm flagellar chemosensation. EMBO J 39: e102723, 2020. doi: 10.15252/embj.2019102723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Brown SG, Miller MR, Lishko PV, Lester DH, Publicover SJ, Barratt CLR, Martins Da Silva S. Homozygous in-frame deletion in CATSPERE in a man producing spermatozoa with loss of CatSper function and compromised fertilizing capacity. Hum Reprod 33: 1812–1816, 2018. doi: 10.1093/humrep/dey278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Seifert R, Flick M, Bonigk W, Alvarez L, Trotschel C, Poetsch A, Muller A, Goodwin N, Pelzer P, Kashikar ND, Kremmer E, Jikeli J, Timmermann B, Kuhl H, Fridman D, Windler F, Kaupp UB, Strunker T. The CatSper channel controls chemosensation in sea urchin sperm. EMBO J 34: 379–392, 2015. doi: 10.15252/embj.201489376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Strunker T, Goodwin N, Brenker C, Kashikar ND, Weyand I, Seifert R, Kaupp UB. The CatSper channel mediates progesterone-induced Ca2+ influx in human sperm. Nature 471: 382–386, 2011. doi: 10.1038/nature09769. [DOI] [PubMed] [Google Scholar]
- 81. Berridge MJ. Calcium microdomains: organization and function. Cell Calcium 40: 405–412, 2006. doi: 10.1016/j.ceca.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 82. Nanou E, Catterall WA. Calcium channels, synaptic plasticity, and neuropsychiatric disease. Neuron 98: 466–481, 2018. doi: 10.1016/j.neuron.2018.03.017. [DOI] [PubMed] [Google Scholar]
- 83. Trebak M, Kinet JP. Calcium signalling in T cells. Nat Rev Immunol 19: 154–169, 2019. doi: 10.1038/s41577-018-0110-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Navarrete FA, Alvau A, Lee HC, Levin LR, Buck J, Leon PM, Santi CM, Krapf D, Mager J, Fissore RA, Salicioni AM, Darszon A, Visconti PE. Transient exposure to calcium ionophore enables in vitro fertilization in sterile mouse models. Sci Rep 6: 33589, 2016. doi: 10.1038/srep33589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Hwang JY, Wang H, Lu Y, Ikawa M, Chung JJ. C2cd6-encoded CatSpertau targets sperm calcium channel to Ca(2+) signaling domains in the flagellar membrane. Cell Rep 38: 110226, 2022. doi: 10.1016/j.celrep.2021.110226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Yang F, Gracia Gervasi M, Orta G, Tourzani DA, De la Vega-Beltran JL, Ruthel G, Darszon A, Visconti PE, Wang PJ. C2CD6 regulates targeting and organization of the CatSper calcium channel complex in sperm flagella. Development 149: dev199988, 2022. doi: 10.1242/dev.199988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Okamura Y, Nishino A, Murata Y, Nakajo K, Iwasaki H, Ohtsuka Y, Tanaka-Kunishima M, Takahashi N, Hara Y, Yoshida T, Nishida M, Okado H, Watari H, Meinertzhagen IA, Satoh N, Takahashi K, Satou Y, Okada Y, Mori Y. Comprehensive analysis of the ascidian genome reveals novel insights into the molecular evolution of ion channel genes. Physiol Genomics 22: 269–282, 2005. doi: 10.1152/physiolgenomics.00229.2004. [DOI] [PubMed] [Google Scholar]
- 88. Cai X, Clapham DE. Evolutionary genomics reveals lineage-specific gene loss and rapid evolution of a sperm-specific ion channel complex: CatSpers and CatSperβ. PLoS One 3: e3569, 2008. doi: 10.1371/journal.pone.0003569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Cai X, Wang X, Clapham DE. Early evolution of the eukaryotic Ca2+ signaling machinery: conservation of the CatSper channel complex. Mol Biol Evol 31: 2735–2740, 2014. doi: 10.1093/molbev/msu218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Vicente-Carrillo A, Álvarez-Rodríguez M, Rodríguez-Martínez H. The CatSper channel modulates boar sperm motility during capacitation. Reprod Biol 17: 69–78, 2017. doi: 10.1016/j.repbio.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 91. Johnson GP, English A-M, Cronin S, Hoey DA, Meade KG, Fair S. Genomic identification, expression profiling, and functional characterization of CatSper channels in the bovine. Biol Reprod 97: 302–312, 2017. doi: 10.1093/biolre/iox082. [DOI] [PubMed] [Google Scholar]
- 92. Loux SC, Crawford KR, Ing NH, González-Fernández L, Macías-García B, Love CC, Varner DD, Velez IC, Choi YH, Hinrichs K. CatSper and the relationship of hyperactivated motility to intracellular calcium and pH kinetics in equine sperm. Biol Reprod 89: 123–115, 2013. doi: 10.1095/biolreprod.113.111708. [DOI] [PubMed] [Google Scholar]
- 93. Kijima T, Kurokawa D, Sasakura Y, Ogasawara M, Aratake S, Yoshida K, Yoshida M. CatSper mediates the chemotactic behavior and motility of the ascidian sperm. bioRxiv 2021.07.06.451356, 2021. doi: 10.1101/2021.07.06.451356. [DOI] [PMC free article] [PubMed]
- 94. Wang H, Chung JJ. Mitochondria-cytoskeleton interactions in mammalian sperm revealed by cryoelectron tomography. Proc Natl Acad Sci U S A 118: e2118020118, 2021. doi: 10.1073/pnas.2118020118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Zhao Y, Wang H, Wiesehoefer C, Shah NB, Reetz E, Hwang JY, Huang X, Lishko PV, Davies KM, Wennemuth G, Nicastro D, Chung JJ. 3D structure and in situ arrangements of CatSper channel in the sperm flagellum. Nat Commun 13: 3439, 2022. doi: 10.1038/s41467-022-31050-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Abbott GW. Kv channel ancillary subunits: where do we go from here? Physiology (Bethesda) 37: 0, 2022. doi: 10.1152/physiol.00005.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Xia J, Ren D. The BSA-induced Ca2+ influx during sperm capacitation is CATSPER channel-dependent. Reprod Biol Endocrinol 7: 119, 2009. doi: 10.1186/1477-7827-7-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Brenker C, Goodwin N, Weyand I, Kashikar ND, Naruse M, Krahling M, Muller A, Kaupp UB, Strunker T. The CatSper channel: a polymodal chemosensor in human sperm. EMBO J 31: 1654–1665, 2012. doi: 10.1038/emboj.2012.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Brenker C, Rehfeld A, Schiffer C, Kierzek M, Kaupp UB, Skakkebæk NE, Strünker T. Synergistic activation of CatSper Ca2+ channels in human sperm by oviductal ligands and endocrine disrupting chemicals. Hum Reprod 33: 1915–1923, 2018. doi: 10.1093/humrep/dey275. [DOI] [PubMed] [Google Scholar]
- 100. Schiffer C, Muller A, Egeberg DL, Alvarez L, Brenker C, Rehfeld A, Frederiksen H, Waschle B, Kaupp UB, Balbach M, Wachten D, Skakkebaek NE, Almstrup K, Strunker T. Direct action of endocrine disrupting chemicals on human sperm. EMBO Rep 15: 758–765, 2014. doi: 10.15252/embr.201438869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Neverisky DL, Abbott GW. Ion channel-transporter interactions. Crit Rev Biochem Mol Biol 51: 257–267, 2015. doi: 10.3109/10409238.2016.1172553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Vedovato N, Ashcroft FM, Puljung MC. The nucleotide-binding sites of SUR1: a mechanistic model. Biophys J 109: 2452–2460, 2015. doi: 10.1016/j.bpj.2015.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Muschol M, Wenders C, Wennemuth G. Four-dimensional analysis by high-speed holographic imaging reveals a chiral memory of sperm flagella. PLoS One 13: e0199678, 2018. doi: 10.1371/journal.pone.0199678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Wiesehofer C, Wiesehofer M, Dankert JT, Chung JJ, von Ostau NE, Singer BB, Wennemuth G. CatSper and its CaM-like Ca(2+) sensor EFCAB9 are necessary for the path chirality of sperm. FASEB J 36: e22288, 2022. doi: 10.1096/fj.202101656RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Long JE, Lee MS, Blithe DL. Male contraceptive development: update on novel hormonal and nonhormonal methods. Clin Chem 65: 153–160, 2019. doi: 10.1373/clinchem.2018.295089. [DOI] [PubMed] [Google Scholar]
- 106. Norcross NR, Georgiou I, Johnston ZC, Gruber FS, Swedlow JR, Read KD, Barratt CL, Gilbert IH. Male contraceptive development: a medicinal chemistry perspective. Eur J Med Chem 243: 114709, 2022. doi: 10.1016/j.ejmech.2022.114709. [DOI] [PubMed] [Google Scholar]
- 107. Schiffer C, Rieger S, Brenker C, Young S, Hamzeh H, Wachten D, Tuttelmann F, Ropke A, Kaupp UB, Wang T, Wagner A, Krallmann C, Kliesch S, Fallnich C, Strunker T. Rotational motion and rheotaxis of human sperm do not require functional CatSper channels and transmembrane Ca(2+) signaling. EMBO J 39: e102363, 2020. doi: 10.15252/embj.2019102363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Sinha A, Singh V, Singh S, Yadav S. Proteomic analyses reveal lower expression of TEX40 and ATP6V0A2 proteins related to calcium ion entry and acrosomal acidification in asthenozoospermic males. Life Sci 218: 81–88, 2019. doi: 10.1016/j.lfs.2018.12.016. [DOI] [PubMed] [Google Scholar]
- 109. Zhang Y, Malekpour M, Al-Madani N, Kahrizi K, Zanganeh M, Lohr NJ, Mohseni M, Mojahedi F, Daneshi A, Najmabadi H, Smith RJ. Sensorineural deafness and male infertility: a contiguous gene deletion syndrome. J Med Genet 44: 233–240, 2007. doi: 10.1136/jmg.2006.045765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Xia J, Reigada D, Mitchell CH, Ren D. CATSPER channel-mediated Ca2+ entry into mouse sperm triggers a tail-to-head propagation. Biol Reprod 77: 551–559, 2007. doi: 10.1095/biolreprod.107.061358. [DOI] [PubMed] [Google Scholar]
- 111. Kelly MC, Brown SG, Costello SM, Ramalingam M, Drew E, Publicover SJ, Barratt CL, Martins Da Silva S. Single-cell analysis of [Ca2+] i signalling in sub-fertile men: characteristics and relation to fertilization outcome. Hum Reprod 33: 1023–1033, 2018. doi: 10.1093/humrep/dey096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Williams HL, Mansell S, Alasmari W, Brown SG, Wilson SM, Sutton KA, Miller MR, Lishko PV, Barratt CLR, Publicover SJ, Martins da Silva S. Specific loss of CatSper function is sufficient to compromise fertilizing capacity of human spermatozoa. Hum Reprod 30: 2737–2746, 2015. doi: 10.1093/humrep/dev243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Alexander SP, Mathie A, Peters JA, Veale EL, Striessnig J, Kelly E, , et al. The concise guide to pharmacology 2021/22: ion channels. Br J Pharmacol 178: S157–S245, 2021. doi: 10.1111/bph.15539. [DOI] [PubMed] [Google Scholar]
- 114. Carlson AE, Burnett LA, Del Camino D, Quill TA, Hille B, Chong JA, Moran MM, Babcock DF. Pharmacological targeting of native CatSper channels reveals a required role in maintenance of sperm hyperactivation. PloS one 4: e6844, 2009. doi: 10.1371/journal.pone.0006844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Rennhack A, Schiffer C, Brenker C, Fridman D, Nitao ET, Cheng Y-M, Tamburrino L, Balbach M, Stölting G, Berger TK, Kierzek M, Alvarez L, Wachten D, Zeng X-H, Baldi E, Publicover SJ, Kaupp UB, Strünker T. A novel cross‐species inhibitor to study the function of CatSper Ca2+ channels in sperm. Br J Pharmacol 175: 3144–3161, 2018. doi: 10.1111/bph.14355. [DOI] [PMC free article] [PubMed] [Google Scholar]