Abstract
Brief (10 min) weekly exposure to low energy pulsed electromagnetic fields (PEMFs) has been shown to improve human muscle mitochondrial bioenergetics and attenuate systemic lipotoxicity following anterior cruciate ligament surgical reconstruction. Here we present data generated from 101 participants, 62% female, aged 38–91 years, recruited from the QuantumTx Demo Centre in Singapore, wherein 87% of participants (n = 88) presented with pre-existing mobility dysfunction and 13% (n = 13) were healthy volunteers. Participants were recruited if: (i) not pregnant; (ii) above 35 years of age and; (iii) without surgical implants. All participants completed mobility testing, pre- and post- PEMF intervention for 12 weeks, whereas bioelectrical impedance analysis was conducted in a subgroup of 42 and 33 participants at weeks 4 and 8, respectively. Weekly PEMF exposure was associated with significant improvements in mobility (Timed Up and Go, 5 times Sit-to-Stand, and 4m Normal Gait Speed) and body composition (increased skeletal muscle mass and reduced total and visceral fat mass), particularly in the older participants. Perception of pain was also significantly reduced. PEMF therapy may provide a manner to counteract age-associated mobility and metabolic disruptions and merits future investigation in randomized controlled trials to elucidate its clinical benefits in the frail and older adult populations.
Keywords: sarcopenia, intra-abdominal fat, frailty, muscle weakness, type 2 diabetes mellitus
INTRODUCTION
Muscle is our largest tissue mass and plays a major role in establishing human healthspan and lifespan [1–3]. Sarcopenia is an age-related disease characterized by reduced skeletal muscle mass, strength and physical function [4, 5]. In accordance with muscle’s imperative role in establishing organismal health and longevity, sarcopenia is associated with low quality of life [3, 6], falls, fractures [7] and mortality [8]. While regular exercise training and dietary protein supplementation are accepted efficacious means to retain muscle quality with age, they are difficult to implement, particularly in the frail and older adults. There is hence a growing need for the development of non-pharmacological and minimally invasive means to counteract sarcopenic muscle loss. Pulsed electromagnetic fields (PEMFs) may represent one such approach. PEMF-based therapies have been previously employed for clinical rehabilitation involving bone and other soft-tissue injuries, whether from acute trauma or degenerative conditions, in pre-clinical animal models [9] and humans [10]. For instance, rotator cuff tendinitis had been clinically treated with PEMFs as early as the 1980s [11]. In fact, in 1979, the United States Food and Drug Administration approved PEMFs therapy as safe and efficacious for treating non-union bone fractures within the categories of bone growth and osteogenic stimulation, opening the way for PEMF therapies to be used in the area of rehabilitation for orthopedic injuries [12, 13]. As such, rehabilitative PEMF studies have traditionally focussed on bone and connective tissue damage, as well as pain management [12–16]. Unfortunately, the translation of PEMF-based therapies for muscle maintenance has gone largely unexplored.
Since the 1970s a multitude of pre-clinical and clinical studies examining the effects of PEMF stimulation have appeared utilizing a broad range of repetition frequencies (Hz to MHz range), amplitudes (μT to 100s mT), signal gradients and symmetries, exposure durations (minutes to weeks), as well as cellular environments [12–17]. Not unexpectedly, the clinically related outcomes reported from these studies varied widely. As an illustrative example, an early double-blinded, randomized controlled trial (RCT) investigated the effects of PEMF treatment applied between 5–9 hours per day on patients with rotator cuff tendinopathy [11]. The administered PEMFs were set at an amplitude of ~3 mT at a repetition frequency of ~73 Hz. Patients receiving the magnetic field treatment reported less pain after 4 weeks relative to the sham treatment cohort. In another RCT also treating patients with rotator cuff tendinopathy [18], the PEMFs were administered at a frequency of 3 Hz, 80 mT for 20 minutes a day, twice a week for a total of 8 sessions. Although pain and shoulder function were significantly improved, the patients in this second study were also administered extracorporeal shockwave therapy, which may have partially obscured the beneficial contributions of the PEMF therapy per se. Systematic reviews have also recently highlighted that distinct tissue types likely respond distinctly to different PEMF exposure parameters [19–21]. Finally, many in vitro studies cannot be adequately translated to the in vivo scenario due to the common use of the aminoglycoside antibiotics during cell culturing. The aminoglycoside antibiotics, such as streptomycin, have been shown to attenuate the sensitivity of cells to magnetic field exposure, negating the relevancy of the determined in vitro field parameters to the in vivo condition [22, 23]. A need thus exists for greater standardization of the PEMF regimens used for specified clinical applications.
Recently, a muscle-specific, low energy (1.5 mT, 15 Hz, 10 minutes/week), PEMF paradigm has been developed and demonstrated effective in promoting muscle regeneration in cells [22, 23], animals [24] and humans [25] by virtue of its capacity to activate mitochondrial respiration [26]. By strategically targeting the leg musculature of humans with the coil system [25], this PEMF paradigm was capable of recapitulating some of the same mitochondrial-dependent regenerative and metabolic cascades typically invoked by exercise, particularly with regards to improving muscle mitochondrial bioenergetics and in attenuating systemic lipotoxicity [25]. The muscle mitochondrial network also exhibits heightened sensitivity to our magnetic stimulation paradigm [22] compared to that of other collateral tissues or progenitor cell classes [24, 27, 28] and hence is a favorable target with which to invoke systemic homeostasis for clinical exploitation. In this community case study, we investigated the capacity of this specific PEMF paradigm to improve physical functioning and increase lean mass in a Southeast Asian adult population. We provide evidence that weekly exposure of the quadriceps musculature to weak pulsing magnetic fields for 10 minutes each week, over the course of 8–12 weeks, improved physical performance as well as reduced both total (−3.9%) and visceral fat reserves (−3.7%), particularly in older participants. Interestingly, perceptions of pain were also reduced after 3 months of PEMF intervention.
RESULTS
Cohort characteristics are provided in Table 1. The results for the Timed up and go (TUG), 5 times Sit-to-Stand (5xSTS), and 4m normal gait speed (4mNGS) mobility tests are provided in Table 2. Statistically significant improvements in functional mobility following PEMF therapy were observed for all three tests, across all age groups. Specifically, in the TUG test, 12 weeks of PEMF therapy was associated with a significant reduction in mean time of execution for the entire cohort from a pre-PEMF value of 11.25 seconds to 9.31 seconds. The older participants showed the strongest improvements from 10.77 to 8.79 seconds and from 15.35 to 13.14 seconds for ages 66–75 and 76–91 years, respectively. Given that older adults above the age of 65 who score more than 14 seconds for TUG are associated with a higher risk of falls [29], this data assumes potential clinical importance. For 5xSTS, PEMF therapy was associated with reduced times of execution from 12.71 seconds to 10.40 seconds for the entire cohort. Again, improvements were greatest for the older participants, ranging from 12.99 to 10.32 seconds and from 15.21 to 12.74 seconds for ages 66–75 and 76–91 years, respectively, faster than the predictive 15 seconds associated with a greater risk of recurrent falls in older adults [30]. 4mNGS scores generally increased following PEMF therapy, from speeds of 0.9 ms−1 to 1.12 ms−1. Specifically, the oldest group of participants (ages 76–91 years) increased in gait speed from 0.79 to 0.92 ms−1. Importantly, a 4mNGS speed of below 0.8 ms−1 is associated with increased risks for adverse health outcomes, including disability, cognitive impairment, falls, and mortality [31]. Therefore, based on these previously published studies, the changes in functional mobility reported here following 12 weeks of PEMF therapy are clinically relevant and counteract age-related trends.
Table 1. Study demographics.
| Study demographics | Total subjects | Total mean age (±SD) | Total males | Male mean age (±SD) | Total females | Female mean age (±SD) | |
| Age (year) | (All) | 101 | 68 ± 11 | 38 | 70 ± 9 | 63 | 67 ± 12 |
| (35–55) | 14 | 50 ± 5 | 2 | 47 ± 12 | 12 | 50 ± 4 | |
| (56–65) | 24 | 61 ± 3 | 8 | 61 ± 3 | 16 | 61 ± 3 | |
| (66–75) | 41 | 71 ± 3 | 18 | 71 ± 3 | 23 | 72 ± 2 | |
| (76–91) | 22 | 82 ± 5 | 10 | 80 ± 5 | 12 | 84 ± 5 | |
Table 2. Raw values representing the mean (ave) and standard error of the mean (SEM), and mean difference (mean diff) for TUG (in secs), 5xSTS (in secs) and 4mNGS (in meters) pre- and post-PEMF therapy for 12 weeks, stratified according to age (in years).
| Mobility tests | Age (Year) | Pre (Baseline) Mean (±SD) | Post (PEMF Therapy) Mean (±SD) | Mean difference | p-value |
| TUG (sec) | [All] | 11.25 ± 7.60 | 9.31 ± 6.20 | −1.94 | <0.0001 |
| (35–55) | 8.64 ± 4.93 | 6.68 ± 1.87 | −1.96 | 0.0052 | |
| (56–65) | 9.82 ± 9.75 | 8.24 ± 7 | −1.58 | 0.0001 | |
| (66–75) | 10.77 ± 5.72 | 8.79 ± 4.74 | −1.98 | <0.0001 | |
| (76–91) | 15.35 ± 8.25 | 13.14 ± 7.87 | −2.21 | 0.0003 | |
| 5xSTS (sec) | (All) | 12.71 ± 6.54 | 10.40 ± 4.77 | −2.31 | <0.0001 |
| (35–55) | 10.18 ± 6.54 | 8.28 ± 2.59 | −1.90 | 0.0256 | |
| (56–65) | 11.43 ± 4.94 | 9.64 ± 3.92 | −1.79 | 0.0039 | |
| (66–75) | 12.99 ± 7.58 | 10.32 ± 4.82 | −2.67 | <0.0001 | |
| (76–91) | 15.21 ± 5.31 | 12.74 ± 5.79 | −2.47 | 0.0115 | |
| 4mNGS (s−1) | (All) | 0.98 ± 0.31 | 1.12 ± 0.30 | 0.14 | <0.0001 |
| (35–55) | 1.07 ± 0.29 | 1.32 ± 0.24 | 0.25 | 0.0006 | |
| (56–65) | 1.08 ± 0.34 | 1.17 ± 0.29 | 0.09 | 0.0176 | |
| (66–75) | 1.00 ± 0.26 | 1.12 ± 0.27 | 0.13 | <0.0001 | |
| (76–91) | 0.790 ± 0.31 | 0.92 ± 0.3 | 0.13 | 0.0002 |
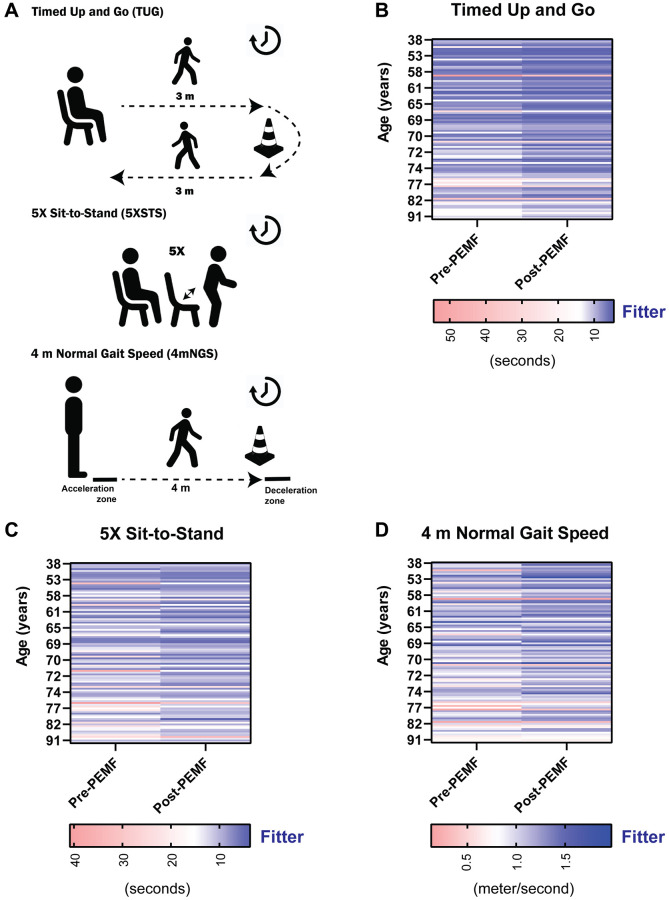
Figure 1 is a heatmap depiction of the individual responses for the entire cohort for the TUG, 5xSTS, and 4mNGS tests at baseline (pre-PEMF) and after 12 weeks of PEMF therapy. A trend was visible for each of the functional sets, whereby the pre-PEMF values worsen with greater age (increasingly redder nearer the bottom (older)), illustrating a trend of loss of function with age. In most cases, mobility and functional capacity were improved following 12 weeks of PEMF therapy (increasingly bluer on the right (post-PEMF)), irrespective of age.
Figure 1.
Individual functional assessments pre- and post-PEMF therapy. (A) Functional tests administered to study participants. Subject performance in the (B) timed-up and go (TUG; seconds), (C) 5X Sit to Stand (5xSTS; seconds), and (D) 4 m normal gait speed (4mNGS; meter/second), measured at baseline (pre-PEMF, week 1) and at week 12 (post-PEMF). Heat maps showing the responses of subjects by color gradient, with darker blue indicating functional improvement, white being the cutoffs based on known consensus for older adults, and red showing a less fit or frail characteristics. A TUG and 5xSTS score of ≥14 and ≥15 seconds, respectively are associated with increased falling risk in older adult [29, 30]. A gait speed of ≤ 0.8 m/s is correlated with an increased risk of adverse health outcomes in the older adults [31]. Statistical analysis was carried out using the Wilcoxon matched-pairs signed rank test and showed significant improvement in mobility function (p < 0.0001) with magnetic therapy for all three tests.
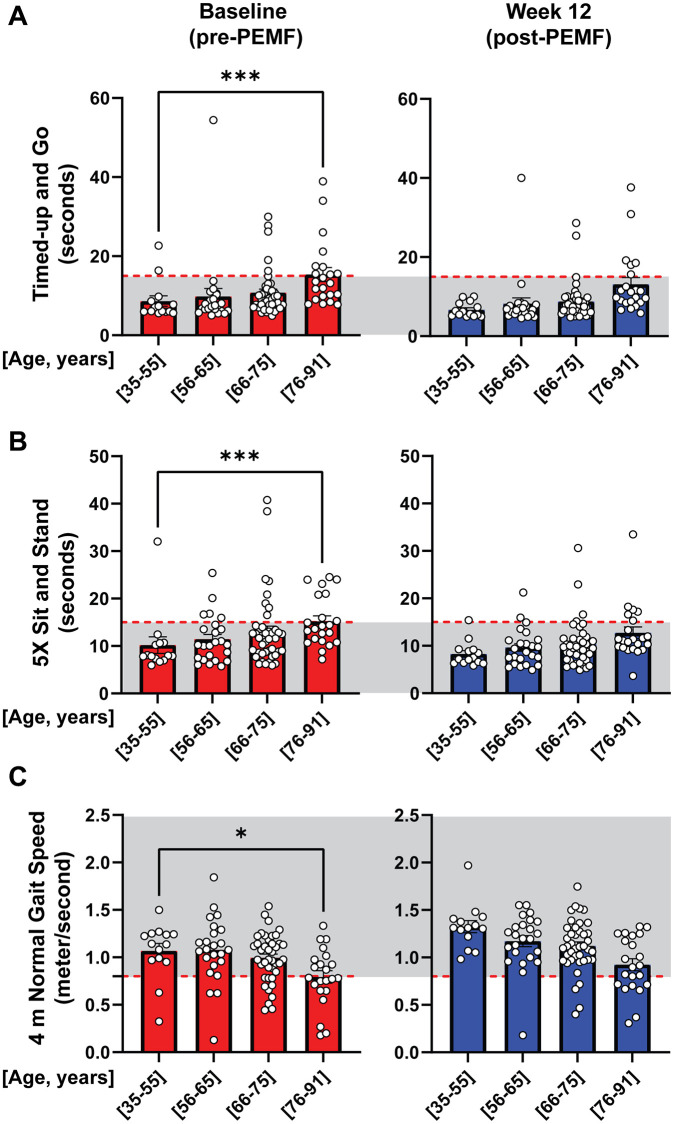
Age-associated results for the three functional tests are shown in Figure 2. Sub-group analyses of the functional tests were conducted by stratifying participants into separate age quartiles as depicted in Figure 2A–2C. The red set of histograms reveal statistically significant functional deterioration between 33–55 and 76–91 years of age at study onset (week 0). On the other hand, 12 weeks of PEMF therapy (blue histograms) was correlated with generalized functional improvements across all age groups and tests. Most notably, PEMF therapy (blue histograms) ameliorated age-dependent losses in physical capacity, as demonstrated by a loss of statistically significant differences between the “youngest” (35–55 years of age) and oldest (76–91 years of age) quartiles for all three tests (blue histograms compared with red histograms). PEMF-associated improvements are hence greatest in the older and more frail participants, which are commonly coincident phenotypes.
Figure 2.
Age-stratified changes in mobility function, pre- and post-PEMF therapy. Bar charts depicting age-stratified performance at baseline (pre-PEMF; red bars) and following 12 weeks of PEMF therapy (blue bars) in the TUG (A), 5xSTS (B), and 4mNGS (C) mobility tests. The gray shaded areas represent cutoffs of ≤14 seconds, ≤15 seconds and ≥0.8 m/s indicative of safety from physical failing reported for the TUG [29], 5xSTS [30], and 4mNGS [31], respectively. The number of subjects per age bracket are as follow: (35–55) = 14, (56–65) = 24, (66–75) = 41, and (76–91) = 22. Statistical analysis was carried out using One-Way ANOVA and Kruskal-Wallis multiple comparisons test, with *p < 0.05, **p < 0.01 and ***p < 0.001.
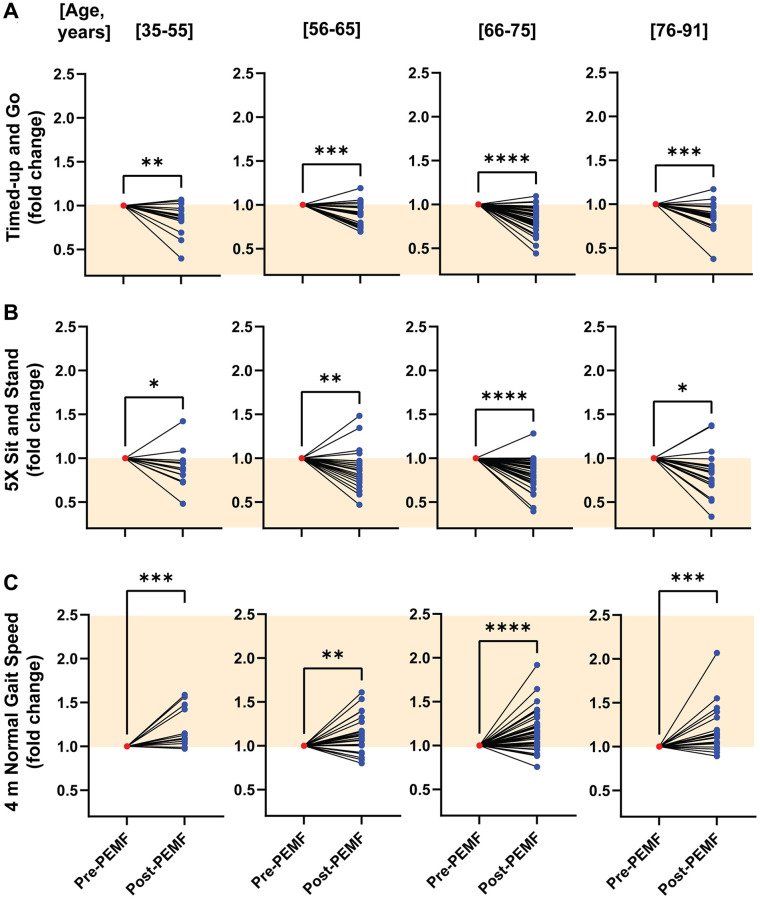
Supplementary Figure 1 shows raw data for each age group and reveals statistical significance for all three test across all age groups. To more clearly depict group trends, we normalized post- to pre-intervention values for each individual by quartile. Apparent were inter-individual trends towards improvements (orange shaded regions) associated with PEMF therapy that were most significant for the 66–75-year quartile of participants for the TUG (Figure 3A), 5xSTS (Figure 3B) and 4mNGS (Figure 3C) tests. Compared with the different age quartiles, participants in this quartile improved 1.2-fold for both TUG and 5xSTS, and 1.15-fold for 4mNGS. Supplementary Figure 2 provides the “responder” breakdown for each grouping. Notably, the percentage of responders increased after 65 years of age, indicating that the older participants experienced greater gains following PEMF therapy.
Figure 3.
Normalized age-stratified changes in mobility function before and after PEMF therapy. Fold change improvements for the (A) TUG, (B) 5xSTS and (C) 4mNGS tests after 12 weeks of PEMF therapy. Data was normalized to the respective baseline score for each subject. The orange-shaded regions depict the direction of improvement in mobility function for each test scenario. Statistical analysis was carried out using the Wilcoxon matched-pairs signed rank test, with *p < 0.05, **p < 0.01, ***p < 0.001 and ****p < 0.0001. For more information on the total number of responders and non-responders to PEMF therapy, refer to Supplementary Figure 2.
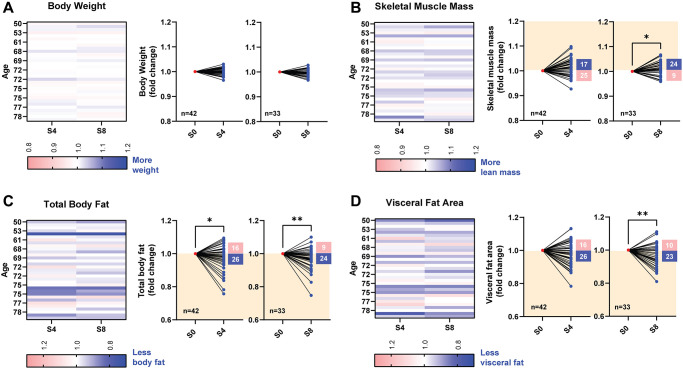
Bioelectrical impedance analyses revealed PEMF-associated changes in body composition. Whereas no significant differences in body weight were detected after 8 weeks of PEMF therapy (Figure 4A), a 1.2% (p < 0.05) increase in lean muscle mass was observed in ~72% (n = 24) of the participants (Figure 4B). By comparison, PEMF-associated changes in total body fat were more pronounced. Significant decreases in total body fat were observed in ~62% (n = 26) and ~72% (n = 24) of the participants after 4 and 8 weeks of PEMF therapy, respectively, with mean changes in total body fat of ~−3% and ~−4%, respectively (Figure 4C). Significant decreases in visceral fat were observed in ~70% (n = 23) of the participants after 8 weeks of PEMF therapy, with a mean change of ~−4% (Figure 4D). Supplementary Figure 3 shows the raw data for each grouping before normalization. Supplementary Figure 4 shows body compositional data for ages between 50–70 and 71–83 and revealed that reductions in visceral fat were greater for the older group after 8 weeks.
Figure 4.
Changes in body composition before and after 4 and 8 weeks of PEMF therapy. Body composition assessments were performed using bioelectrical impedance analysis with an InBody device at baseline (pre-PEMF), and after 4 (S4; n = 42) and 8 (S8; n = 33) sessions of weekly PEMF exposure. (A) Changes in body weight, expressed as fold change over baseline measured after 4 and 8 weekly PEMF sessions. Fold changes in skeletal muscle mass (B), total body fat (C) or visceral fat area (D) following PEMF therapy normalized to the respective baseline score for each subject (also see Supplementary Figure 3). The normalized before-after muscle and fat plots depict the fold change over baseline after 4 (S4) and 8 (S8) sessions of weekly PEMF exposure. The orange-shaded regions depict the direction for fold change improvement for each compositional assessment. The number of subjects for each trend direction is indicated in the corresponding box. Statistical analysis was carried out using the Wilcoxon matched-pairs signed rank test, with *p < 0.05 and **p < 0.01.
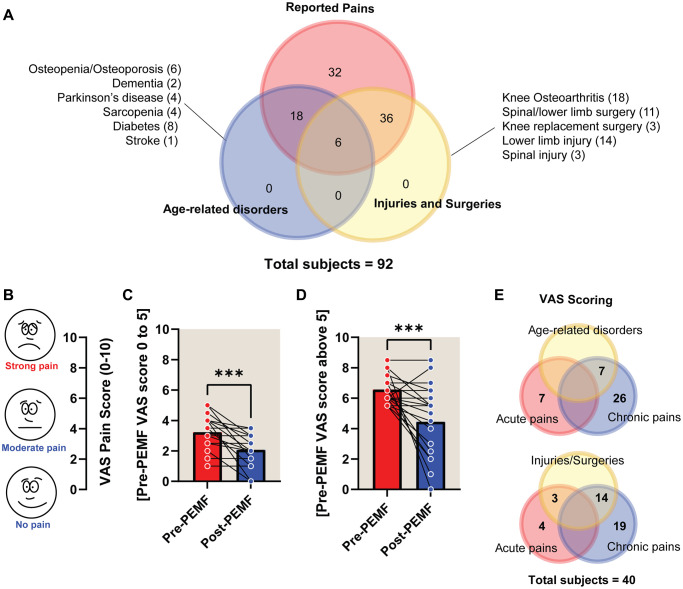
A breakdown of the health conditions of the study participants is shown in Figure 5. Of the 101 study participants (aged 38–91 years; 63 females and 38 males), 92 (60 females and 32 males) reported having pre-existing health conditions, while the remaining 9 did not. Of the 92 participants reporting health conditions, most presented with some degree of pre-existing pain; 72 (78%) were associated with age-related conditions (Figure 5A) and/or 72 (78%) with acute injuries/surgeries (Figure 5B). A Visual Analog Scale (VAS) was used to assess pain before and after PEMF treatment in a subset (40) of the participants (Figure 5B). Significant improvements in low-to-moderate pain perception (VAS:0–5) were reported after PEMF therapy (Figure 5C). Notably, severe pain perception (VAS>5) was more strongly attenuated following PEMF therapy (Figure 5D). Of these respondents, 83% (33/40) reported suffering from some form of chronic pain (Figure 5E). Only one respondent reported a severe worsening of pain following PEMF therapy (Figure 5D).
Figure 5.
Visual analogue scale (VAS) scoring of pain pre- and post-PEMF therapy for 12 weeks. (A) Venn diagram depicting the number of subjects (60 females and 32 males) reporting health conditions as either age-related (blue) or arising from injuries and/or surgeries (yellow) and associated pain (red). The number of subjects for each disorder/injury/surgical intervention are indicated within the parentheses. Pain level before and after PEMF therapy was rated using the VAS “faces” pain rating scale (B), with a rating of 10 indicating strong pain and a rating of 0 indicating no pain. VAS scores were tabulated based on pre-treatment scores between either (C) 0 and 5 (n = 19), or (D) scores above 5 (n = 21). (E) 40 of the 92 subjects completed the VAS pain questionnaire, wherein 83% (33/40) reported chronic pain, amongst whom 21% (7/33) reported age-related disorders and/or 42% (14/33) reported past or recent injuries/surgeries. The mean change in VAS score for subjects in the “0 to 5” bracket (C) was −1.23 points, from a mean of 3.23 to 2.07, before and after PEMF therapy, respectively. The “above 5” group (D) showed a −2.12-point change, from means of 6.57 to 4.45. Statistical analysis was carried out using Wilcoxon matched-pairs signed rank test and showed significant improvement in pain relief with ***p < 0.001 after magnetic therapy.
DISCUSSION
Muscle’s predominant role in establishing systemic metabolic homeostasis can be attributed to its large and dynamic pool of mitochondria [2, 32]. Succinctly, physical exercise is health- and life-extending because, to be executed, it necessitates the activation of the muscular mitochondrial pool. Mitochondrial respiratory activation and the consequent production of reactive oxygen species (ROS), in turn, serve as triggers to initiate energy-driven enzymatic and transcriptional cascades that ultimately culminate with the activation of the muscle secretome response [33, 34]. The muscle secretome is comprised of a myriad of regenerative, metabolic, anti-inflammatory, and immunity-boosting factors that are released into the systemic circulation either individually [35, 36] or vesicle-encapsulated [37–39]. For the most part, these muscle-derived secreted factors promote the maintenance and metabolism of the muscle itself, as well as that of collateral tissues, thereby accounting for the widely accepted health benefits of exercise. Sarcopenia describes the progressive loss of muscle mass, interdependent with a deterioration of mitochondria function and number, in the older adults and frail. Indeed, evidence exists that accrued mitochondrial dysfunction with advanced age contributes to age-dependent muscle loss via the activation of catabolic pathways [40]. Sarcopenia hence compromises resilience to disease and results in losses of muscle size, strength, and function. Mitochondrial/muscle usage prolongs mitochondrial/muscle functional efficiency hence, these debilitating effects of sarcopenia can be offset to a significant degree by adopting a lifestyle incorporating moderate levels of physical activity and caloric restriction [40]. The systemic metabolic attributes arising from the activation of the muscle mitochondrial pool during exercise are attributed to the enhanced expression and function of the master regulator of mitochondrial biogenesis, PGC-1α. Unfortunately, muscle has been largely overlooked in clinical strategies employing PEMF-based technologies, missing out on a valuable rehabilitative opportunity.
Sarcopenia: a pathological form of fat-muscle crosstalk
In later life, muscle loss is accompanied by increased ectopic adiposity with grave functional and metabolic consequences. Other than a strong association with age, a problem lies in identifying individuals at greatest risk for early medical intervention. Some investigators have proposed that visceral fat area, instead of Body Mass Index (BMI), should be used as a diagnostic criterion for the disorder when associated with muscle loss [41, 42]. At equivalent BMIs, Asians have been shown to generally possess higher percentages of body fat and to develop metabolic disorders such as type, 2 diabetes and cardiovascular disease, sooner than Caucasians [43, 44]. More specifically in alignment with the present study, Singaporeans have been recently shown to exhibit higher adiposity at comparable BMIs, age, and sex relative to American or European Caucasians [43, 45]. As such, BMI does not adequately reflect the amount of visceral fat or other ectopic atherogenic (inflammatory) adipose deposits, which appear to be determinant and more prominent in the South Asian population, even after adjusting for habitual levels of physical activity [44]. In accordance, the baseline BMIs of the participants of the present study (~23.5 kg/m2; Table 3) are considered overweight by the Singaporean Ministry of Health guidelines where a cut-off point of 23 kg/m2 has been assigned [43], whereas internationally it is set at 25 kg/m2. Notably, only modest, but consistent, drops in BMI were observed across all ages after 8 weeks of PEMF therapy, whereas reductions in total and visceral fat were significant and robust. The fact that once-weekly PEMF exposure (10 minutes) was associated with significant reductions in visceral fat suggests that PEMF therapy holds clinical relevance.
Table 3. Mean body mass index (BMI; kg/m2) of participants across the different age brackets, after 4-weekly or 8-weekly PEMF therapy.
| 4-weekly PEMF | (All) | (35–55) | (56–65) | (66–75) | (76–91) | |||||
| Number of subjects | 42 | 7 | 5 | 18 | 12 | |||||
| PEMF Therapy | Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post |
| Weight (kg) | 59.7 | 59.6 | 64.3 | 64.0 | 51.9 | 51.7 | 61.2 | 61.0 | 58.4 | 58.5 |
| Std. Deviation | 12.5 | 12.3 | 14.0 | 14.4 | 3.8 | 3.4 | 11.9 | 11.5 | 15.6 | 15.3 |
| BMI (kg/m2) | 23.25 | 23.21 | 23.91 | 23.80 | 20.88 | 20.81 | 23.60 | 23.53 | 23.31 | 23.39 |
| Std. Deviation | 4.03 | 3.99 | 5.02 | 5.20 | 1.66 | 1.45 | 3.76 | 3.64 | 4.55 | 4.49 |
| Minimum | 17.00 | 17.31 | 18.90 | 18.50 | 19.50 | 19.46 | 17.60 | 18.13 | 17.00 | 17.31 |
| Maximum | 33.10 | 33.21 | 32.70 | 32.48 | 23.30 | 22.84 | 33.10 | 33.21 | 32.30 | 32.34 |
| Range | 16.10 | 15.90 | 13.80 | 13.98 | 3.80 | 3.38 | 15.50 | 15.08 | 15.30 | 15.03 |
| 8-weekly PEMF | (All) | (35–55) | (56–65) | (66–75) | (76–91) | |||||
| Number of subjects | 33 | 4 | 4 | 17 | 8 | |||||
| PEMF Therapy | Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post |
| Weight (kg) | 60.5 | 60.2 | 60.5 | 60.2 | 52.9 | 52.5 | 60.8 | 60.6 | 63.6 | 63.3 |
| Std. Deviation | 11.3 | 11.2 | 7.5 | 8.6 | 3.5 | 3.6 | 12.2 | 11.7 | 13.2 | 13.3 |
| BMI (kg/m2) | 23.56 | 23.44 | 22.20 | 22.11 | 21.08 | 20.89 | 23.67 | 23.57 | 25.25 | 25.11 |
| Std. Deviation | 3.76 | 3.67 | 3.03 | 3.59 | 1.85 | 1.74 | 3.82 | 3.61 | 4.24 | 4.11 |
| Minimum | 17.60 | 17.88 | 20.20 | 19.61 | 19.50 | 19.42 | 17.60 | 17.88 | 19.60 | 19.43 |
| Maximum | 33.10 | 32.64 | 26.70 | 27.43 | 23.30 | 23.09 | 33.10 | 32.64 | 32.30 | 31.67 |
| Range | 15.50 | 14.76 | 6.50 | 7.82 | 3.80 | 3.67 | 15.50 | 14.76 | 12.70 | 12.24 |
No significant differences were observed between age brackets at any time of assessment.
Age-related increases in adipose inflammation in combination with decreases in physical activity result in the redistribution of fat to intra-abdominal (visceral fat) and intramuscular sites. The resulting accumulation of intramuscular atherogenic adipose tissue results in the production of reactive lipid species (lipotoxicity), mitochondrial dysfunction, and oxidative stress, that depress β-oxidation of fatty acids, promote insulin resistance, stimulate the secretion of pro-inflammatory cytokines, a cascade of events that generally undermine muscle viability. This shift in the muscle secretome towards pro-inflammation further exacerbates adipose inflammation, leading to a state of system-wide inflammation and the establishment of a vicious cycle of metabolic and functional decline that define the pathogenesis of sarcopenia [41, 46]. The resultant inflammatory systemic milieu is also hyper-responsive to even minor systemic perturbations, such as infections, that plays a major role in the pathophysiology of frailty [47]. Therefore, the appropriate compositional balance between muscle and fat, particularly visceral and intramuscular fat, is of utmost importance in maintaining healthspan through the modulation of body energy efficiency, myokine-adipokine crosstalk and systemic inflammatory status. Here, we report improved maintenance of skeletal muscle in conjunction with reductions of total and visceral adipose that moreover, were accompanied by improvements in indices of functional mobility, most significantly in the elderly. PEMF-based technologies may hence represent a valuable adjuvant therapy to conventional geriatric interventions intended to reduce the prevalence of frailty in the older adult population.
Muscle-targeted PEMF therapy and metabolic stabilization
In recent years the cellular responses mobilized by PEMF exposure have come into clearer focus and commonly impinge upon calcium signaling and mitochondrial respiration (ROS) to reach fruition [14, 20, 48–53]. One calcium-permeable channel in particular, the Transient Receptor Potential Cation Channel Subfamily C Member 1, or TRPC1, has received attention [20, 22, 23, 27, 28, 52, 54, 55]. A TRPC1-mitochondrial axis has been revealed that can be induced by magnetic stimulation and hence possesses the necessary functionality (calcium entry and mitochondrial interaction) to coalesce both aspects of the noted response cascade [22, 55]. Notably, exercise-dependent activation of TRPC1-mediated calcium entry promotes the development of oxidative muscle that is necessary for the execution of physical activities requiring endurance and in maintaining normal posture, and thus confers resistance to fatiguing physical activities [56–58]. Oxidative muscles are major contributors to systemic metabolic flexibility [59] and exhibit a predilection for fatty acid oxidation [60], traits that are related to their elevated mitochondrial content [61]. Accordingly, oxidative muscle was shown to be particularly responsive to magnetic exposure. For instance, 5 weeks of PEMF treatment (10 minutes/week) was sufficient to improve the running performance of mice compared to unexposed littermates [24]. The production of mitochondrial ROS and subsequent activation of PGC-1α transcriptional cascades are responses shared by both exercise [2, 32, 60–62] and our magnetic paradigm [22, 23]. The same magnetic stimulation paradigm used in the present study, consisting of brief exposures (10 minutes) to extremely low frequency (Hz-100 Hz) and low amplitude (1.5 mT) PEMFs was previously shown to stimulate both in vitro [22] and in vivo [24] myogeneses towards the oxidative phenotype, and to emulate the molecular signals characteristic of the metabolic and mitochondrial improvements associated with exercise in isolated muscle cells [22], mice [24] and humans [25]. Given this experimental backdrop, it is not entirely unexpected that this magnetic paradigm should improve functional performance and body composition in an older cohort as demonstrated in this report.
The age stratification of the standard mobility function tests reported here reveals an age-dependent decline in mobility and functional capacity at the commencement of the study (pre-PEMF intervention), trends that agree with a previous study examining the spontaneous decline in mobility function using the International Classification of Functioning, Disability and Health (ICF) framework [63]. This recent study reported a significant deterioration in the physical functioning of older adults (60–90 years) after one year of monitoring, demonstrating an increase in time taken for the TUG test from a mean of 12.8 to 14.5 seconds (P < 0.001), a decrease in the sit-stand 30-second chair stand test from a mean of 10 to 8 repetitions (P = 0.001), and an increase in the time taken for the 10-minute walk test from a mean of 12.4 to 14.4 seconds (P = 0.001) [63]. In an inverse manner, our study demonstrated improved mobility function using a similar set of functional tests (TUG, 5xSTS and 4mNGS) following PEMF therapy. With reference to the TUG test used by both studies, we demonstrated reductions in mean time scores in older subjects (>65 years), of greater absolute magnitude, but in the opposite direction (improvement, instead of worsening) and over a shorter span of time (3 months versus one year) (Table 2) associated with weekly PEMF intervention. The pre-intervention baseline values for the TUG in the present Singaporean study (15.35 seconds, 76–91 years of age) and post-assessment baseline values in the Turkish study [63] (14.5 seconds, 60-90 years of age), however, were of similar magnitude. Notably, an increase in the amount of time taken for the TUG test is associated with increased risks of falls, fractures, cardiovascular disease, dementia and Parkinson’s disease [64–68]. Longer TUG scores are also correlated with increases in fat mass, but not BMI, in an exclusively Singaporean cohort [45]. Therefore, our observation that PEMF therapy was associated with both improvements in TUG score and reduced adiposity but had less of an association with BMI (Table 3), aligns with previous studies and moreover, suggests that PEMF therapy holds potential therapeutic value for the older adult population in the areas of cognitive and physical decline, including reducing the risks of falls.
Improvements in exercise performance, adipose browning and fatty acid oxidation have been reported in small animal studies employing an analogous PEMF platform [24]. Notably, the improvements we detected in this human cohort are comparable to those previously reported for exercise intervention in the older adult population. Employing dual x-ray absorptiometry (DEXA) measurements, Aas et al. (2020) [69] detected a 1.5% increase in lean leg mass after 10 weeks of thrice weekly resistance exercise and protein supplementation in an elderly cohort (~85 years), compared to a 1.2% increase in lean body mass reported here employing bioelectrical impedance analysis after 8 weeks of PEMF therapy. For the 71–83 age group specifically, a 1.1% increase in lean body mass was detected after 8 weeks (Supplementary Figure 4). In a systematic review and meta-analysis, Lu et al. (2021) [70] reported improvements in TUG times of −0.66 seconds (standardized mean differences across studies) in the elderly (>60 years) in response to exercise training and further asserted that the TUG is a better predictor of exercise adaptations than other mobility tests. For comparison, our eldest age quartile (76–91) exhibited the greatest improvements in mean TUG time of −2.21 seconds (Table 2). Notwithstanding differences in methodologies of assessment, these studies suggest that PEMF therapy can produce comparable results to exercise in older individuals.
Magnetic mitohormesis: not a paradox, but an opportunity
Mitochondria can be considered the cell’s environmental stress sensors of a manner that is biologically adaptive [26]. Mitohormesis refers to an adaptive process whereby low levels of oxidative stress confer the installation of survival adaptations and promote regeneration, whereas greater levels of oxidative stress can stymie cell growth and survival [71]. As magnetic fields stimulate mitochondrial respiration, they can be exploited as a method with which to non-invasively produce mitohormetic responses, via a process of Magnetic Mitohormesis. Traditionally, the selection of magnetic exposure regimes has not been made with mitohormesis taken into mechanistic consideration, ultimately giving rise to disparate findings between analogous preparations. On the other hand, if taken into consideration, exposure regimes can be potentially designed to target a specific objective. For instance, we have recently shown using the same magnetic technology that stronger magnetic stimulation can be used to specifically halt breast cancer growth, employing the same molecular machinery [72]. Therefore, PEMF-based therapeutic strategies can be ultimately designed to either promote or arrest development depending on exposure intensity (duration, amplitude, and frequency) and the inflammatory status of the tissue in question. Mitohormetic principles must hence be taken into serious consideration to appropriately design efficacious PEMF-based clinical therapies.
Study limitations
The main caveat to this study was that the participant base consisted of walk-in volunteers of heterogeneous characteristics at the commencement of the trial. Notwithstanding, measured improvements were stronger in the older participants, which goes against the accepted trend of worsening with advanced age [63], as corroborated in the present study. Further suggesting an authentic therapeutic effect, improvements were consistently observed across diverse measures including the perception of pain, functional mobility, as well as body composition. Changes in body composition, such as visceral adiposity, can be considered as objective measures and less subject to perceptual or psychosomatic bias. Visceral fat content was recently shown to be less responsive to physical activity in the South Asian population [63], yet was reduced following PEMF therapy in the present Southeast Asian study, also arguing for therapeutic specificity. Given that age-related increases in visceral adiposity are a major contributor to the pathogenesis of sarcopenia [42] and its reported persistence in the Southeast Asian population [43, 44], the observation that our PEMF paradigm produced the greatest amelioration of visceral fat in our Southeast Asian older cohort (Supplementary Figure 4) indicates that our magnetic intervention holds both functional and metabolic contributions that are relevant for human aging and frailty. The possibility that the observed effects can be attributed to physical improvement with time also seems unlikely since it would be in opposition to general trends in this cohort and elsewhere [63] to naturally worsen with age. There was also no restriction on other rehabilitative measures imposed by the study team. Nonetheless, of those reported, outside rehabilitation had no effect on any of the functional assessment scored (Supplementary Figure 5), suggesting that the observed improvements were for the most part attributed to the PEMF intervention. Finally, there was no imposed restriction on pain medications during the study period which could have contributed to the reported pain reduction after PEMF therapy. However, most of the reported pain (~83%) was chronic in nature and putatively had been unresponsive to various analgesics. Although a placebo effect cannot be totally ruled out as having had an effect in the results reported for this case study, a primary placebo contribution is unlikely due to the consistency, breadth and nature of the responses observed following PEMF treatment. Moreover, the measured improvements in mobility function and body composition associated with PEMF therapy met and surpassed the cut-offs previously established in published studies [29–31, 63–68, 70] that would have needed to be exceeded in order to offset human age-related frailty and which generally worsens with age, particularly in the older adult population, where we detect greatest improvements. Therefore, the sum of these results implies an authentic therapeutic effect can be attributed to PEMF therapy. Given the potential clinical relevance of our magnetic field paradigm, further clinical investigation is certainly warranted.
CONCLUSIONS
Therapies targeting skeletal muscle hold major advantages over other tissues given muscle’s broad systemic ramifications. Here we provide initial findings that brief weekly PEMF exposure of the upper limb of humans produces clinically relevant improvements in pain, mobility, and indications of lean muscle mass, in an age-dependent manner. In accordance with previously published animal preclinical [24] and human clinical [25] studies employing analogous PEMF paradigms, adipose tissue homeostasis was particularly responsive to PEMF intervention. Most notably, the older subjects exhibited the most significant improvements in mobility and body composition. Although the results reported here are very promising, they remain to be substantiated and broadened in randomized controlled clinical trials.
METHODS
Subject recruitment
Male and female voluntary subjects without any surgical implants, who were not pregnant, without any major mobility issues, and over the age of 21 years were allowed to participate in the PEMF therapy program at the QuantumTx Demo Centre. To be included in this retrospective study, only subjects above the age of 35 years were considered and must have completed the 12-week weekly PEMF therapy with complete data points. Most study participants were word-of-mouth referrals from previous clients or joined the programme after seeing press releases of the technology. Informed consent was taken before the start of the program. This retrospective study was conducted as per the Declaration of Helsinki and approved by the NUS Institutional Review Board (NUS-IRB-2022-841).
PEMF exposure
The PEMF paradigm employed here for use in human clinical trials has previously been described [25]. The quadriceps region of subjects was exposed to PEMFs (1 mT) once a week for 10 min, over 12 weeks, on alternating legs each week. All participants were required to undertake 12 sessions of PEMF exposure. Approximately 85% of the study participants completed 12 weekly PEMF sessions without interruption; full compliance. Due to holidays, travel or illness, however, the remaining subjects completed 12 PEMF sessions within 16 weeks.
Functional and mobility assessments
Each participant completed a series of standard performance-based functional tests with modification [64–68, 73] at baseline (Week 1) and the end of the program (Week 12), to assess improvements in mobility function. These tests include (1) Timed Up and Go Test (TUG), (2) Five Times Sit to Stand Test (5xSTS), and (3) a 4-meter Normal Gait Speed (4mNGS). For TUG, seated participants were timed (in secs) from the start of rising from a chair and returning to a seated position after completing a walk and turnaround of 3 meters. For 5xSTS, seated participants were assessed for the time (in secs) required to complete a stand and sit 5 times with arms crossed against their chest. The 4mNGS test records the duration (in secs) required of the participants to complete a 4-meter walk at a comfortable pace.
Pain assessments
A Visual Analog Scale (VAS) was used for pain assessment and has been shown quite reproducible in accurately assessing chronic pain levels [74]. For the Visual Analog Scale (VAS) pain score, participants indicated their existing acute and chronic pains based on the VAS “Faces” pain rating scale from 1 (no pain) to 10 (severe pain), at week 1 and week 12.
Body compositional assessments
The measurement of the subject’s body composition was conducted using the Inbody™ 770 device (Inbody Co., Ltd.) before each weekly PEMF session and data parameters such as weight, skeletal muscle mass, body fat mass, and visceral fat area were collected at after session 4 and session 8. Body Mass Index (BMI) was calculated by dividing the weight (kg) of a person by the square of height (m2) and was an output measure of the Inbody™ 770 device.
Statistical analysis
Given that the raw data did not pass normality tests, non-parametric test was used. The Wilcoxon matched-pairs signed rank test was carried out to compare pre-and post-PEMF therapy on raw data and transformed data. Transformed data were expressed as fold change over the baseline (week 1) of each subject. The One-way ANOVA followed by Kruskal-Wallis tests was carried out to analyse the statistics for multiple comparisons. All statistical analyses were performed using GraphPad Prism version 9 for Windows.
Data availability statement
The original anonymous dataset is available on request from the corresponding authors.
Supplementary Materials
ACKNOWLEDGMENTS
The authors would like to acknowledge Loiuse Wong for her assistance in the preparation of the NUS IRB application. We would like to acknowledge the Centre of Innovation in Healthcare and the Alexandra Hospital for providing the facilities to run the study. We would also like to acknowledge funding from Enterprise Singapore and the Singapore Centre for Social Enterprise, raiSE, for supporting the development and production of devices. Manpower to oversee the study was provided by QuantumTx. Salary support for the data analysis and writing of the manuscript came from the Department of Surgery (NUS), Lee Foundation and ESR Post-doctorate Fellowship in Nanomedicine.
Abbreviations
- TUG
Time-Up and Go
- 5xSTS
5 Times sit-and-stand
- 4mNGS
4-meter normal gait speed
- PEMF
Pulsed electromagnetic fields
- VAS
Visual Analog Score
- BMI
Body Mass Index
- TRPC1
Transient Receptor Potential Canonical 1
- ROS
Reactive oxygen species
- RCT
Randomized controlled trial
- mT
milliTesla
AUTHOR CONTRIBUTIONS: The subject data were collected and compiled by SV and ST. YKT and CW compiled and analysed the data and helped in the writing of the manuscript. IG provided the PEMF device and infrastructure to conduct the study and conceptualized the study. ABM, BKK provided consultation and direction. AFO and JG wrote the manuscript. AFO helped conceptualize the study. All authors read and approved the final manuscript.
CONFLICTS OF INTEREST: AFO is an inventor on patent WO 2019/17863 A1, System, and Method for Applying Pulsed Electromagnetic Fields and is a co-founder of QuantumTx Pte. Ltd. SV, ST, IG are employees of QuantumTx Pte. Ltd. All other authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical Statement and Consent: This study was reviewed and approved for publication by the NUS Institutional Review Board (NUS-IRB-2022-841). All study participants provided informed written consent about personal and medical data collection prior to the start of the program.
FUNDING: The publication of this manuscript is supported by the Lee Foundation.
REFERENCES
- 1.Abramowitz MK, Hall CB, Amodu A, Sharma D, Androga L, Hawkins M. Muscle mass, BMI, and mortality among adults in the United States: A population-based cohort study. PLoS One. 2018; 13:e0194697. 10.1371/journal.pone.0194697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Memme JM, Erlich AT, Phukan G, Hood DA. Exercise and mitochondrial health. J Physiol. 2021; 599:803–17. 10.1113/JP278853 [DOI] [PubMed] [Google Scholar]
- 3.Hanna L, Nguo K, Furness K, Porter J, Huggins CE. Association between skeletal muscle mass and quality of life in adults with cancer: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. 2022; 13:839–57. 10.1002/jcsm.12928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AA, Schneider SM, Sieber CC, Topinkova E, et al. , and Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019; 48:16–31. 10.1093/ageing/afy169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suetta C, Haddock B, Alcazar J, Noerst T, Hansen OM, Ludvig H, Kamper RS, Schnohr P, Prescott E, Andersen LL, Frandsen U, Aagaard P, Bülow J, et al. The Copenhagen Sarcopenia Study: lean mass, strength, power, and physical function in a Danish cohort aged 20-93 years. J Cachexia Sarcopenia Muscle. 2019; 10:1316–29. 10.1002/jcsm.12477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cruz-Jentoft AJ, Sayer AA. Sarcopenia. Lancet. 2019; 393:2636–46. 10.1016/S0140-6736(19)31138-9 [DOI] [PubMed] [Google Scholar]
- 7.Yeung SSY, Reijnierse EM, Pham VK, Trappenburg MC, Lim WK, Meskers CGM, Maier AB. Sarcopenia and its association with falls and fractures in older adults: A systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. 2019; 10:485–500. 10.1002/jcsm.12411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu J, Wan CS, Ktoris K, Reijnierse EM, Maier AB. Sarcopenia Is Associated with Mortality in Adults: A Systematic Review and Meta-Analysis. Gerontology. 2022; 68:361–76. 10.1159/000517099 [DOI] [PubMed] [Google Scholar]
- 9.Wang Q, Zhou J, Wang X, Xu Y, Liang Z, Gu X, He C. Coupling induction of osteogenesis and type H vessels by pulsed electromagnetic fields in ovariectomy-induced osteoporosis in mice. Bone. 2022; 154:116211. 10.1016/j.bone.2021.116211 [DOI] [PubMed] [Google Scholar]
- 10.Krzyżańska L, Straburzyńska-Lupa A, Rąglewska P, Romanowski L. Beneficial Effects of Pulsed Electromagnetic Field during Cast Immobilization in Patients with Distal Radius Fracture. Biomed Res Int. 2020; 2020:6849352. 10.1155/2020/6849352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Binder A, Parr G, Hazleman B, Fitton-Jackson S. Pulsed electromagnetic field therapy of persistent rotator cuff tendinitis. A double-blind controlled assessment. Lancet. 1984; 1:695–8. 10.1016/s0140-6736(84)92219-0 [DOI] [PubMed] [Google Scholar]
- 12.Shupak NM, Prato FS, Thomas AW. Therapeutic uses of pulsed magnetic-field exposure: a review. URSI Radio Science Bulletin. 2003; 307:9–32. 10.23919/URSIRSB.2003.7909506 [DOI] [Google Scholar]
- 13.Waldorff EI, Zhang N, Ryaby JT. Pulsed electromagnetic field applications: A corporate perspective. J Orthop Translat. 2017; 9:60–8. 10.1016/j.jot.2017.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ehnert S, Schröter S, Aspera-Werz RH, Eisler W, Falldorf K, Ronniger M, Nussler AK. Translational Insights into Extremely Low Frequency Pulsed Electromagnetic Fields (ELF-PEMFs) for Bone Regeneration after Trauma and Orthopedic Surgery. J Clin Med. 2019; 8:2028. 10.3390/jcm8122028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Varani K, Vincenzi F, Pasquini S, Blo I, Salati S, Cadossi M, De Mattei M. Pulsed Electromagnetic Field Stimulation in Osteogenesis and Chondrogenesis: Signaling Pathways and Therapeutic Implications. Int J Mol Sci. 2021; 22:809. 10.3390/ijms22020809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Safavi AS, Sendera A, Haghighipour N, Banas-Zabczyk A. The Role of Low-Frequency Electromagnetic Fields on Mesenchymal Stem Cells Differentiation: A Systematic Review. Tissue Eng Regen Med. 2022; 19:1147–60. 10.1007/s13770-022-00473-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gualdi G, Costantini E, Reale M, Amerio P. Wound Repair and Extremely Low Frequency-Electromagnetic Field: Insight from In Vitro Study and Potential Clinical Application. Int J Mol Sci. 2021; 22:5037. 10.3390/ijms22095037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klüter T, Krath A, Stukenberg M, Gollwitzer H, Harrasser N, Knobloch K, Maffulli N, Hausdorf J, Gerdesmeyer L. Electromagnetic transduction therapy and shockwave therapy in 86 patients with rotator cuff tendinopathy: A prospective randomized controlled trial. Electromagn Biol Med. 2018; 37:175–83. 10.1080/15368378.2018.1499030 [DOI] [PubMed] [Google Scholar]
- 19.Mansourian M, Shanei A. Evaluation of Pulsed Electromagnetic Field Effects: A Systematic Review and Meta-Analysis on Highlights of Two Decades of Research In Vitro Studies. Biomed Res Int. 2021; 2021:6647497. 10.1155/2021/6647497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chansoria P, Liu H, Christiansen MG, Schürle-Finke S, Zenobi-Wong M. Untethered: using remote magnetic fields for regenerative medicine. Trends Biotechnol. 2022. [Epub ahead of print]. 10.1016/j.tibtech.2022.09.003 [DOI] [PubMed] [Google Scholar]
- 21.Markovic L, Wagner B, Crevenna R. Effects of pulsed electromagnetic field therapy on outcomes associated with osteoarthritis: A systematic review of systematic reviews. Wien Klin Wochenschr. 2022; 134:425–33. 10.1007/s00508-022-02020-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yap JLY, Tai YK, Fröhlich J, Fong CHH, Yin JN, Foo ZL, Ramanan S, Beyer C, Toh SJ, Casarosa M, Bharathy N, Kala MP, Egli M, et al. Ambient and supplemental magnetic fields promote myogenesis via a TRPC1-mitochondrial axis: evidence of a magnetic mitohormetic mechanism. FASEB J. 2019; 33:12853–72. 10.1096/fj.201900057R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong CJK, Tai YK, Yap JLY, Fong CHH, Loo LSW, Kukumberg M, Fröhlich J, Zhang S, Li JZ, Wang JW, Rufaihah AJ, Franco-Obregón A. Brief exposure to directionally-specific pulsed electromagnetic fields stimulates extracellular vesicle release and is antagonized by streptomycin: A potential regenerative medicine and food industry paradigm. Biomaterials. 2022; 287:121658. 10.1016/j.biomaterials.2022.121658 [DOI] [PubMed] [Google Scholar]
- 24.Tai YK, Ng C, Purnamawati K, Yap JLY, Yin JN, Wong C, Patel BK, Soong PL, Pelczar P, Fröhlich J, Beyer C, Fong CHH, Ramanan S, et al. Magnetic fields modulate metabolism and gut microbiome in correlation with Pgc-1α expression: Follow-up to an in vitro magnetic mitohormetic study. FASEB J. 2020; 34:11143–67. 10.1096/fj.201903005RR [DOI] [PubMed] [Google Scholar]
- 25.Stephenson MC, Krishna L, Pannir Selvan RM, Tai YK, Kit Wong CJ, Yin JN, Toh SJ, Torta F, Triebl A, Fröhlich J, Beyer C, Li JZ, Tan SS, et al. Magnetic field therapy enhances muscle mitochondrial bioenergetics and attenuates systemic ceramide levels following ACL reconstruction: Southeast Asian randomized-controlled pilot trial. J Orthop Translat. 2022; 35:99–112. 10.1016/j.jot.2022.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Franco-Obregón A. Magnetic mitohormesis: A non-invasive therapy for inflammatory disorders? Biocell. 2023; 47:239–44. 10.32604/biocell.2023.025357 [DOI] [Google Scholar]
- 27.Parate D, Franco-Obregón A, Fröhlich J, Beyer C, Abbas AA, Kamarul T, Hui JHP, Yang Z. Enhancement of mesenchymal stem cell chondrogenesis with short-term low intensity pulsed electromagnetic fields. Sci Rep. 2017; 7:9421. 10.1038/s41598-017-09892-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madanagopal TT, Tai YK, Lim SH, Fong CH, Cao T, Rosa V, Franco-Obregón A. Pulsed electromagnetic fields synergize with graphene to enhance dental pulp stem cell-derived neurogenesis by selectively targeting TRPC1 channels. Eur Cell Mater. 2021; 41:216–32. 10.22203/eCM.v041a16 [DOI] [PubMed] [Google Scholar]
- 29.Shumway-Cook A, Brauer S, Woollacott M. Predicting the probability for falls in community-dwelling older adults using the Timed Up & Go Test. Phys Ther. 2000; 80:896–903. 10.1093/ptj/80.9.896 [DOI] [PubMed] [Google Scholar]
- 30.Buatois S, Miljkovic D, Manckoundia P, Gueguen R, Miget P, Vançon G, Perrin P, Benetos A. Five times sit to stand test is a predictor of recurrent falls in healthy community-living subjects aged 65 and older. J Am Geriatr Soc. 2008; 56:1575–7. 10.1111/j.1532-5415.2008.01777.x [DOI] [PubMed] [Google Scholar]
- 31.Abellan van Kan G, Rolland Y, Andrieu S, Bauer J, Beauchet O, Bonnefoy M, Cesari M, Donini LM, Gillette Guyonnet S, Inzitari M, Nourhashemi F, Onder G, Ritz P, et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging. 2009; 13:881–9. 10.1007/s12603-009-0246-z [DOI] [PubMed] [Google Scholar]
- 32.Memme JM, Hood DA. Molecular Basis for the Therapeutic Effects of Exercise on Mitochondrial Defects. Front Physiol. 2021; 11:615038. 10.3389/fphys.2020.615038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scheele C, Nielsen S, Pedersen BK. ROS and myokines promote muscle adaptation to exercise. Trends Endocrinol Metab. 2009; 20:95–9. 10.1016/j.tem.2008.12.002 [DOI] [PubMed] [Google Scholar]
- 34.Louzada RA, Bouviere J, Matta LP, Werneck-de-Castro JP, Dupuy C, Carvalho DP, Fortunato RS. Redox Signaling in Widespread Health Benefits of Exercise. Antioxid Redox Signal. 2020. [Epub ahead of print]. 10.1089/ars.2019.7949 [DOI] [PubMed] [Google Scholar]
- 35.Karstoft K, Pedersen BK. Skeletal muscle as a gene regulatory endocrine organ. Curr Opin Clin Nutr Metab Care. 2016; 19:270–5. 10.1097/MCO.0000000000000283 [DOI] [PubMed] [Google Scholar]
- 36.Severinsen MCK, Pedersen BK. Muscle-Organ Crosstalk: The Emerging Roles of Myokines. Endocr Rev. 2020; 41:594–609. 10.1210/endrev/bnaa016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qin W, Dallas SL. Exosomes and Extracellular RNA in Muscle and Bone Aging and Crosstalk. Curr Osteoporos Rep. 2019; 17:548–59. 10.1007/s11914-019-00537-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aoi W, Tanimura Y. Roles of Skeletal Muscle-Derived Exosomes in Organ Metabolic and Immunological Communication. Front Endocrinol (Lausanne). 2021; 12:697204. 10.3389/fendo.2021.697204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vechetti IJ Jr, Peck BD, Wen Y, Walton RG, Valentino TR, Alimov AP, Dungan CM, Van Pelt DW, von Walden F, Alkner B, Peterson CA, McCarthy JJ. Mechanical overload-induced muscle-derived extracellular vesicles promote adipose tissue lipolysis. FASEB J. 2021; 35:e21644. 10.1096/fj.202100242R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leduc-Gaudet JP, Hussain SNA, Barreiro E, Gouspillou G. Mitochondrial Dynamics and Mitophagy in Skeletal Muscle Health and Aging. Int J Mol Sci. 2021; 22:8179. 10.3390/ijms22158179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zamboni M, Rubele S, Rossi AP. Sarcopenia and obesity. Curr Opin Clin Nutr Metab Care. 2019; 22:13–9. 10.1097/MCO.0000000000000519 [DOI] [PubMed] [Google Scholar]
- 42.Li CW, Yu K, Shyh-Chang N, Jiang Z, Liu T, Ma S, Luo L, Guang L, Liang K, Ma W, Miao H, Cao W, Liu R, et al. Pathogenesis of sarcopenia and the relationship with fat mass: descriptive review. J Cachexia Sarcopenia Muscle. 2022; 13:781–94. 10.1002/jcsm.12901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen KK, Wee SL, Pang BWJ, Lau LK, Jabbar KA, Seah WT, Ng TP. Relationship between BMI with percentage body fat and obesity in Singaporean adults - The Yishun Study. BMC Public Health. 2021; 21:1030. 10.1186/s12889-021-11070-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wulan SN, Raza Q, Prasmita HS, Martati E, Maligan JM, Mageshwari U, Fatima I, Plasqui G. Energy Metabolism in Relation to Diet and Physical Activity: A South Asian Perspective. Nutrients. 2021; 13:3776. 10.3390/nu13113776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tou NX, Wee SL, Pang BW, Lau LK, Jabbar KA, Seah WT, Chen KK, Ng TP. Association of fat mass index versus appendicular lean mass index with physical function–The Yishun Study. Aging and Health Research. 2022; 2:100097. 10.1016/j.ahr.2022.100097 [DOI] [Google Scholar]
- 46.Li B, Li Y, Zhang Y, Liu P, Song Y, Zhou Y, Ma L. Visceral Fat Obesity Correlates with Frailty in Middle-Aged and Older Adults. Diabetes Metab Syndr Obes. 2022; 15:2877–84. 10.2147/DMSO.S383597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013; 381:752–62. 10.1016/S0140-6736(12)62167-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang H, Zhang X. Magnetic Fields and Reactive Oxygen Species. Int J Mol Sci. 2017; 18:2175. 10.3390/ijms18102175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Uzieliene I, Bernotas P, Mobasheri A, Bernotiene E. The Role of Physical Stimuli on Calcium Channels in Chondrogenic Differentiation of Mesenchymal Stem Cells. Int J Mol Sci. 2018; 19:2998. 10.3390/ijms19102998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saliev T, Begimbetova D, Masoud AR, Matkarimov B. Biological effects of non-ionizing electromagnetic fields: Two sides of a coin. Prog Biophys Mol Biol. 2019; 141:25–36. 10.1016/j.pbiomolbio.2018.07.009 [DOI] [PubMed] [Google Scholar]
- 51.Barati M, Darvishi B, Javidi MA, Mohammadian A, Shariatpanahi SP, Eisavand MR, Madjid Ansari A. Cellular stress response to extremely low-frequency electromagnetic fields (ELF-EMF): An explanation for controversial effects of ELF-EMF on apoptosis. Cell Prolif. 2021; 54:e13154. 10.1111/cpr.13154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Özgün A, Garipcan B. Magnetic field-induced Ca2+ intake by mesenchymal stem cells is mediated by intracellular Zn2+ and accompanied by a Zn2+ influx. Biochim Biophys Acta Mol Cell Res. 2021; 1868:119062. 10.1016/j.bbamcr.2021.119062 [DOI] [PubMed] [Google Scholar]
- 53.Huang M, Li P, Chen F, Cai Z, Yang S, Zheng X, Li W. Is extremely low frequency pulsed electromagnetic fields applicable to gliomas? A literature review of the underlying mechanisms and application of extremely low frequency pulsed electromagnetic fields. Cancer Med. 2023; 12:2187–98. 10.1002/cam4.5112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ma Q, Chen C, Deng P, Zhu G, Lin M, Zhang L, Xu S, He M, Lu Y, Duan W, Pi H, Cao Z, Pei L, et al. Extremely Low-Frequency Electromagnetic Fields Promote In Vitro Neuronal Differentiation and Neurite Outgrowth of Embryonic Neural Stem Cells via Up-Regulating TRPC1. PLoS One. 2016; 11:e0150923. 10.1371/journal.pone.0150923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kurth F, Tai YK, Parate D, van Oostrum M, Schmid YRF, Toh SJ, Yap JLY, Wollscheid B, Othman A, Dittrich PS, Franco-Obregón A. Cell-Derived Vesicles as TRPC1 Channel Delivery Systems for the Recovery of Cellular Respiratory and Proliferative Capacities. Adv Biosyst. 2020; 4:e2000146. 10.1002/adbi.202000146 [DOI] [PubMed] [Google Scholar]
- 56.Zanou N, Shapovalov G, Louis M, Tajeddine N, Gallo C, Van Schoor M, Anguish I, Cao ML, Schakman O, Dietrich A, Lebacq J, Ruegg U, Roulet E, et al. Role of TRPC1 channel in skeletal muscle function. Am J Physiol Cell Physiol. 2010; 298:C149–62. 10.1152/ajpcell.00241.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zanou N, Schakman O, Louis P, Ruegg UT, Dietrich A, Birnbaumer L, Gailly P. Trpc1 ion channel modulates phosphatidylinositol 3-kinase/Akt pathway during myoblast differentiation and muscle regeneration. J Biol Chem. 2012; 287:14524–34. 10.1074/jbc.M112.341784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xia L, Cheung KK, Yeung SS, Yeung EW. The involvement of transient receptor potential canonical type 1 in skeletal muscle regrowth after unloading-induced atrophy. J Physiol. 2016; 594:3111–26. 10.1113/JP271705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hamilton MT, Hamilton DG, Zderic TW. A potent physiological method to magnify and sustain soleus oxidative metabolism improves glucose and lipid regulation. iScience. 2022; 25:104869. 10.1016/j.isci.2022.104869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cheng CF, Ku HC, Lin H. PGC-1α as a Pivotal Factor in Lipid and Metabolic Regulation. Int J Mol Sci. 2018; 19:3447. 10.3390/ijms19113447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, Michael LF, Puigserver P, Isotani E, Olson EN, Lowell BB, Bassel-Duby R, Spiegelman BM. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature. 2002; 418:797–801. 10.1038/nature00904 [DOI] [PubMed] [Google Scholar]
- 62.Aragón-Vela J, Solis-Urra P, Ruiz-Ojeda FJ, Álvarez-Mercado AI, Olivares-Arancibia J, Plaza-Diaz J. Impact of Exercise on Gut Microbiota in Obesity. Nutrients. 2021; 13:3999. 10.3390/nu13113999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kahraman T, Çekok FK, Üğüt BO, Keskinoğlu P, Genç A. One-Year Change in the Physical Functioning of Older People According to the International Classification of Functioning Domains. J Geriatr Phys Ther. 2021; 44:E9–17. 10.1519/JPT.0000000000000234 [DOI] [PubMed] [Google Scholar]
- 64.Beauchet O, Fantino B, Allali G, Muir SW, Montero-Odasso M, Annweiler C. Timed Up and Go test and risk of falls in older adults: a systematic review. J Nutr Health Aging. 2011; 15:933–8. 10.1007/s12603-011-0062-0 [DOI] [PubMed] [Google Scholar]
- 65.Lee JE, Shin DW, Jeong SM, Son KY, Cho B, Yoon JL, Park BJ, Kwon IS, Lee J, Kim S. Association Between Timed Up and Go Test and Future Dementia Onset. J Gerontol A Biol Sci Med Sci. 2018; 73:1238–43. 10.1093/gerona/glx261 [DOI] [PubMed] [Google Scholar]
- 66.Jeong SM, Shin DW, Han K, Jung JH, Chun S, Jung HW, Son KY. Timed up-and-go test is a useful predictor of fracture incidence. Bone. 2019; 127:474–81. 10.1016/j.bone.2019.07.018 [DOI] [PubMed] [Google Scholar]
- 67.Yoo JE, Jang W, Shin DW, Jeong SM, Jung HW, Youn J, Han K, Kim B. Timed Up and Go Test and the Risk of Parkinson's Disease: A Nation-wide Retrospective Cohort Study. Mov Disord. 2020; 35:1263–7. 10.1002/mds.28055 [DOI] [PubMed] [Google Scholar]
- 68.Chun S, Shin DW, Han K, Jung JH, Kim B, Jung HW, Son KY, Lee SP, Lee SC. The Timed Up and Go test and the ageing heart: Findings from a national health screening of 1,084,875 community-dwelling older adults. Eur J Prev Cardiol. 2021; 28:213–9. 10.1177/2047487319882118 [DOI] [PubMed] [Google Scholar]
- 69.Aas SN, Seynnes O, Benestad HB, Raastad T. Strength training and protein supplementation improve muscle mass, strength, and function in mobility-limited older adults: a randomized controlled trial. Aging Clin Exp Res. 2020; 32:605–16. 10.1007/s40520-019-01234-2 [DOI] [PubMed] [Google Scholar]
- 70.Lu L, Mao L, Feng Y, Ainsworth BE, Liu Y, Chen N. Effects of different exercise training modes on muscle strength and physical performance in older people with sarcopenia: a systematic review and meta-analysis. BMC Geriatr. 2021; 21:708. 10.1186/s12877-021-02642-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ristow M, Schmeisser K. Mitohormesis: Promoting Health and Lifespan by Increased Levels of Reactive Oxygen Species (ROS). Dose Response. 2014; 12:288–341. 10.2203/dose-response.13-035.Ristow [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tai YK, Chan KKW, Fong CHH, Ramanan S, Yap JLY, Yin JN, Yip YS, Tan WR, Koh APF, Tan NS, Chan CW, Huang RYJ, Li JZ, et al. Modulated TRPC1 Expression Predicts Sensitivity of Breast Cancer to Doxorubicin and Magnetic Field Therapy: Segue Towards a Precision Medicine Approach. Front Oncol. 2022; 11:783803. 10.3389/fonc.2021.783803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lusardi MM, Pellecchia GL, Schulman M. Functional performance in community living older adults. J Geriatr Phys Ther. 2003; 26:14–22. [Google Scholar]
- 74.Shafshak TS, Elnemr R. The Visual Analogue Scale Versus Numerical Rating Scale in Measuring Pain Severity and Predicting Disability in Low Back Pain. J Clin Rheumatol. 2021; 27:282–5. 10.1097/RHU.0000000000001320 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original anonymous dataset is available on request from the corresponding authors.