Abstract
Conservation of chicken germplasm is crucial in supporting commercial breeds for sustainable egg and meat production and preserving the genetic diversity of indigenous breeds for future breeding. Cryopreservation of chicken fertilized eggs or embryos is not feasible, owing to the large yolk-laden structure of the eggs. Primordial germ cells (PGCs), the embryonic precursors of gametes, are the best candidates for the cryobanking of chicken germplasm. Effective cryobanking of chicken PGCs requires an optimal cryopreservation protocol. Cryomedia containing dimethyl sulfoxide (DMSO) or DMSO combined with serum have been widely used for the cryopreservation of chicken PGCs. However, as cryoprotectants are yet to be optimized for chicken PGCs, the efficacy of cryomedia can be further improved. Here, we investigated the cryoprotective effects of propylene glycol (PG), an alternative to DMSO, on chicken PGCs. We found that the addition of non-permeable cryoprotectants, such as trehalose or chicken serum, to DMSO or PG cryomedia improved the recovery and survival rates of post-thawed PGCs. We further investigated the cryoprotective effects of trehalose and chicken serum and found that these additives have different cryoprotective actions. Based on these findings, we designed two different cryomedia: DTS, including 5% DMSO, 0.3 M trehalose, and 1% chicken serum, and PTS, including 7.5% PG, 0.1 M trehalose, and 5% chicken serum. Among the different PGC lines and freshly isolated PGCs, the cryomedia showed similar post-thaw recovery rates. Following transplantation, post-thawed male PGCs can colonize gonads and differentiate into functional sperm. We successfully revived the offspring of Kurokashiwa, a rare chicken breed in Japan, with cryopreserved PGCs. In conclusion, we developed two different cryomedia that achieved > 50% recovery of viable PGCs after thawing while maintaining germline competency.
Keywords: Dimethyl sulfoxide, Primordial germ cell, Propylene glycol, Serum, Slow-freezing, trehalose
The conservation of chicken genetic resources is critically important in the maintenance of economically valuable breeds for the sustainable production of eggs and meat. The biosecurity of chicken production is critical, especially considering that chicken populations are vulnerable to infectious diseases, such as avian influenza. Chicken genetic conservation is also important for preserving the genetic diversity of extant noncommercial breeds that can be reintroduced for future needs. However, most local chicken breeds are at risk of losing their genetic diversity because of their small population size. Thus, the issue of chicken germplasm conservation should be urgently addressed on national and global scales.
Cryopreservation techniques for fertilized eggs or embryos used in mammalian germplasm conservation are not applicable to birds because of their large yolk mass. The cryopreservation of chicken semen can be achieved using traditional methods [1, 2]. However, chicken semen cryopreservation is only effective in recovering single genes from males, omitting the female-derived W chromosome and mitochondrial DNA. In Japanese quail, the cryopreservation of immature ovaries and ovarian grafting into immunocompromised recipients can produce functional ova [3]. Although ovarian cryopreservation ensures the preservation of female germplasm, ovarian grafting involves highly technical surgery and raises welfare issues.
As an alternative, cryopreservation of primordial germ cells (PGCs), the first germ cell population established during early development, allows the conservation of the entire chicken genome. A unique biological property of avian PGCs is that they circulate temporarily in the bloodstream during early development [4], allowing their manipulation [5]. Avian PGCs can be transferred intravascularly to the recipient embryos. They settle in the gonads and then produce functional gametes after sexual maturation [6,7,8]. Partially inbred lines and rare breeds of chickens have been reconstituted from cryopreserved PGCs through intravascular transplantation [9, 10]. These recent advances in PGC-mediated technologies in chickens now facilitate the cryobanking of PGCs from various chicken breeds.
The practicality of PGC cryobanking programs is affected by the post-thaw recovery, viability, and quality of chicken PGCs [11]. Cellular membranes can be preserved through cryopreservation by adding suitable cryoprotectants (CPA), which inhibit ice formation, reducing cryogenic damage. Since the first successful cryopreservation of chicken PGCs [12], avian PGCs have commonly been cryopreserved using cryomedium containing dimethyl sulfoxide (DMSO), either alone or in conjunction with serum, using the slow-freezing method [9, 10, 13,14,15,16,17]. However, cryopreservation protocols for chicken PGCs have not been examined in detail, owing to the limited availability of PGCs. Recently, feeder cell-free cultures of chicken PGCs were established by Whyte et al. [18]. Chicken PGCs expand in vitro, allowing the use of sufficient numbers of PGCs for cryobiological studies.
We examined various types of CPA and their concentrations based on viable cell recovery after thawing using cultured PGCs. Based on these results, we designed two different cryomedia to achieve > 50% recovery rate of viable cells after thawing. Next, we determined whether male PGCs after cryopreservation in these cryomedia could settle to the gonads and differentiate into functional sperm following transplantation. Finally, we applied these cryomedia in the cryopreservation of Kurokashiwa, a rare chicken breed in Japan.
Materials and Methods
Birds and animal care
Fertilized eggs of the White Leghorn (WL) MB line were purchased from the National Livestock Breeding Center, Okazaki, Japan. Barred Plymouth Rock (BPR) and Kurokashiwa chickens were maintained at a farm at Hiroshima University, Higashi-Hiroshima, Japan. All animal care and use in this study were conducted in accordance with the animal experimentation guidelines of the Hiroshima University Animal Research Committee.
Culture of PGCs
In this study, PGC lines from two males (W4 and W19) and one female (W2), derived from WL embryos, were used. PGC lines of two males (K8 and K10) and two females (K5 and K6), derived from Kurokashiwa embryos, were also used. PGC lines were cultured as described by Whyte et al. (2015) [18] with slight modification. PGCs (5 × 104 cells) were seeded in 500 μl of the culture medium in a 24-well plate (Greiner Bio-one, Stonehouse, UK). The total volume of the medium in each well was changed every 2 day. The culture medium contained avian Knockout Dulbecco’s Modified Eagle Medium (DMEM) basal medium (250 mOsm/kg, 12.0 mM glucose, and calcium-chloride-free [18]), B-27 supplement, 2.0 mM GlutaMax, 1 × non-essential amino acids (NEAA), 0.1 mM β-mercaptoethanol, 1 × nucleosides, 1.2 mM sodium pyruvate, 2 mg/ml ovalbumin (Sigma Aldrich, St Louis, MO, USA), 100 μg/ml sodium heparin (Sigma Aldrich), 20 μg/ml ovotransferrin (Sigma Aldrich), 0.2% chicken serum, 25 ng/ml Human Activin A (PeproTech, Rocky Hill, NJ, USA), and 4 ng/ml human basic fibroblast growth factor (FGF) (R&D Biosystems, Minneapolis, MN, USA). Unless otherwise specified, all reagents were purchased from Thermo Fisher Scientific (Waltham, MA, USA).
Cryopreservation of PGCs
DMEM (330 mOsm/kg, calcium-chloride-free; 21068028, Thermo Fisher Scientific) was diluted to 24% of additional volume with distilled water to adjust to the osmolality equivalent of avian Knockout DMEM (250 mOsm/kg) and was used as a basal medium for cryopreservation.
For cryopreservation, 1 × 104 cultured PGCs were suspended in 100 μl of each cryomedium and transferred to cryovials. The cryovials were cooled at a rate of approximately −1°C/min using a commercial freezing container (Nalgene, Rochester, NY, USA) in a deep freezer at −80°C overnight. Thereafter, cryovials were transferred to liquid nitrogen (LN2: −196°C) and stored for at least one month before analysis.
To optimize the cryomedia for chicken PGCs, we characterized their cryobiological properties using a male PGC line (W4). To explore the cryoprotective effects of various permeable CPA, cultured PGCs were cryopreserved in each cryomedium at the same osmotic pressure, including 10% (v/v) glycerol (GLY), 8% (v/v) ethylene glycol (EG), 9.5% (v/v) DMSO, and 10% (v/v) propylene glycol (PG). We further explored different concentrations of DMSO (2.5%–12.5%) and PG (5.0%–15.0%) for optimization. To investigate the effect of permeable and non-permeable CPA, cultured PGCs were cryopreserved in each cryoprotectant solution, including permeable (5% DMSO or 7.5% PG) and non-permeable (0.1–0.5 M trehalose and/or 1%–10% chicken serum) CPA.
To evaluate the cryoprotective capacity of optimized cryomedia, which included 5% DMSO, 0.3 M trehalose, and 1% chicken serum (DTS) or 7.5% PG and 0.1 M trehalose, along with 5% chicken serum (PTS), a male PGC line (W4) was cryopreserved in DTS, PTS, or CELLBANKER® 1 (CB1; ZENOAQ, Koriyama, Japan), which has been used for the cryopreservation of chicken PGCs [9, 14]. To evaluate the feasibility of DTS and PTS cryomedia, two PGC lines (male: W19, female: W2) and freshly isolated PGCs from blood (naive PGCs) were cryopreserved in DTS or PTS cryomedia. Naive PGCs were harvested from the blood obtained from WL embryos at stages 14–16 [19], using Nycodenz density gradient centrifugation [20] with minor modification. Briefly, PBS(–) containing 10% chicken serum was used as a buffer, instead of KAv-1 medium. Morphologically normal naive PGCs were picked with a fine-glass micropipette under a phase-contrast microscope (IX73, OLYMPUS, Tokyo, Japan). Owing to the limited number of naive PGCs, 250 cells were suspended in 100 μl of cryomedium and used for freezing.
Thawing of PGCs and post-thaw evaluation
A basal medium containing 5% chicken serum was used as the thawing medium. Cryovials were placed in a water bath at 37°C until the icy masses disappeared, and the cell suspension (100 μl) was diluted with 900 μl of thawing media. After centrifugation at 300 × g for 4 min, 950 μl of the supernatant was carefully discarded, and the cell suspension (approximately 50 μl) was diluted with 450 μl of basal media. For the analysis of cultured PGCs, 80 μl of the total volume of cell suspension (approximately 500 μl) was used to calculate the recovery rate and viability of frozen-thawed PGCs. Cell suspension (80 μl) was added with an equal amount of 0.4% Trypan blue solution, and the mixture was incubated for 2 min at 25°C. The viability rates of frozen-thawed PGCs were determined using the Trypan blue exclusion assay. If the recovery rate of the cultured PGCs after freeze-thaw was 100%, the number of PGCs in 80 μl of cell suspension was calculated as 1600 cells, based on the total volume of cell suspension (500 μl) and total number of PGCs frozen per vial (1 × 104 cells). Therefore, the recovery rate of PGCs was determined by dividing the number of PGCs recovered after freeze-thaw by 1600. The viability of PGCs was calculated by dividing the number of surviving PGCs by the number of PGCs recovered after freeze-thaw. The recovery rate of viable PGCs was calculated by dividing the number of surviving PGCs recovered after freeze-thaw by 1600.
For the analysis of naive PGCs, the total volume of cell suspension (approximately 500 μl) was used to measure the recovery rate and viability of post-thawed PGCs. The recovery rate of PGCs was determined by dividing the number of PGCs recovered after freeze-thaw by 250. The recovery rate of viable PGCs was calculated by dividing the number of surviving PGCs recovered after freeze-thaw by 250.
Gonadal migration assay
A male PGC line (W4) was used in the gonadal migration assay. Cultured PGCs were fluorescently labeled with PKH-26 (Zynaxis, Malvern, PA, USA), as described by Yamamoto et al. (2007) [21]. A few labeled PGCs were later used for transplantation. The remaining labeled PGCs were immediately suspended in either DTS or PTS cryomedium and then cryopreserved in aliquots of approximately 1 × 105 cells per vial at −196°C for > 1 month. Approximately 1 × 103 unfrozen or frozen-thawed PGCs were intravascularly injected into the dorsal aorta of BPR embryos at stages 15–16 through a window (~20 mm diameter) in the narrow end of the eggshell. After sealing the window in the eggshell with cling film, the manipulated embryos were incubated for six days in total, until they reached stage 29. Whole gonads attached to the adjacent mesonephros were collected. Immunofluorescence of whole-mount gonads was carried out as previously reported [22], using an anti-chicken vasa homolog (CVH) monoclonal antibody (1:5; [23]) followed by Alexa 488-conjugated donkey anti-mouse IgG (1:400; Jackson ImmunoResearch, West Grove, PA, USA). The number of CVH+ PKH-26-labeled cells observed in both the left and right gonads was counted under a fluorescence microscope (M205FCA; Leica Microsystems, Wetzlar, Germany) and photographed using a CCD camera (DFC7000T; Leica Microsystems).
Molecular sexing
Blood samples collected from recipient BPR or WL embryos were suspended in 10 μl distilled water and used for PCR without DNA extraction. For molecular sexing, the conserved regions of the chromodomain helicase DNA binding protein (CHD)-W and CHD-Z genes were amplified by PCR, using primers 2550 F (5’-GTTACTGATTCGTCTACGAGA-3’) and 2718 R (5’-ATTGAAATGATCCAGTGCTTG-3’). The reaction mixture (15 μl) contained 3 μl of the diluted blood sample, 0.3 μl of MightyAmp™ DNA Polymerase Ver. 3 (Takara Bio, Kusatsu, Japan), 7.5 μl 2 × MightyAmp Buffer Ver. 3, 0.45 μl of each primer, and 3.3 μl distilled water. PCR conditions were as follows: denaturation at 95°C for 2 min, followed by 35 cycles of denaturation at 98°C for 10 sec, annealing at 60°C for 15 sec, and extension at 68°C for 40 sec. PCR products were analyzed on 1.5% agarose gel with a 1 × Tris–acetic acid–ethylenediaminetetraacetic acid running buffer. The electrophoretic bands were visualized by staining with GelRedTM (Biotium, Fremont, CA, USA) for 15 min.
Germline transmission assay
Two male PGC lines (W4 and W19) were used to demonstrate the germline competency of PGCs after storage in the cryomedia developed in this study. Cultured PGCs (1 × 105 cells/vial) were frozen in either DTS or PTS cryomedia and then cryopreserved at −196°C for > 1 month. After thawing, the PGCs were immediately used for transplantation. Approximately 1 μl of blood was collected from the dorsal aorta of BPR embryos at stage 14 through a ~20 mm diameter window at the sharp end of the eggshell. Each blood sample was diluted to 20 μl with distilled water and used for molecular sexing. Approximately 2 × 103 frozen-thawed PGCs were microinjected into the dorsal aorta of male BPR embryos at stages 15–16. The window in the eggshell was sealed with cling film and incubated until hatching occurred. Hatched recipient BPR chicks were raised to sexual maturity. Donor PGCs were obtained from WL, a breed with white feathers that is homozygous dominant white (I/I). Recipient BPR embryos possess pigmented feathers and are homozygous recessive (i/i) for dominant white. Freshly obtained semen from each BPR recipient rooster was artificially inseminated with normal BPR hens (i/i), and the feather color of the offspring was examined. White color with small patches of black pigmentation (I/i) indicates that the offspring were derived from donor WL PGCs, whereas black color (i/i) indicates that the offspring were derived from recipient BPR PGCs.
Cryopreservation of PGC lines from a rare chicken breed
Kurokashiwa, which was designated as a Japanese natural monument in 1951, was used as a model for rare chicken breeds. The PGC lines of two males (K8 and K10) and two females (K5 and K6) of Kurokashiwa were frozen in DTS or PTS cryomedia and stored for > 1 month. Post-thawed PGCs (approximately 1 × 103 cells) were immediately injected into the bloodstream of WL embryos with same-sex combinations of donor PGCs and recipient embryos. Germline transmission of mature male WL recipients was analyzed by mating them with normal female BPR chickens via artificial insemination, and the feather color of their offspring was examined. Black offspring (i/i) indicated that the offspring was derived from donor Kurokashiwa PGCs, whereas white offspring with small patches of black pigmentation (I/i) indicated that the offspring was derived from recipient WL PGCs. Artificial insemination was conducted with three pairs of male and female WL recipients to produce Kurokashiwa progeny from frozen-thawed male and female PGCs.
To determine the effects of long-term storage of cultured PGCs in LN2 on post-thaw recovery and viability, a female PGC line (K5) was cryopreserved in DTS or PTS cryomedia for > 2.5 years.
Statistical analysis
Three independent experiments were performed for each experimental condition tested, and each experiment was performed at least in triplicate unless otherwise stated. All data are presented as mean ± SEM. Data were analyzed using one-way ANOVA followed by Tukey’s multiple comparison test. To compare two datasets, an unpaired t-test was used. Significant differences between CB1 and other cryomedia and between W4 and other PGC lines were determined using the Wilcoxon test. P < 0.05 was considered statistically significant.
Results
DMSO and PG exhibited cryoprotective capacity on chicken PGCs
We first explored the optimal CPA permeability for chicken PGCs based on the post-thaw recovery and viability of cultured PGCs. When cultured PGCs were cryopreserved in a basal medium (DMEM) without CPA, most died because of low recovery and viability rates (Figs. 1A and B). The addition of various permeable CPA, that is, GLY, EG, DMSO, and PG, significantly increased the recovery rates of PGCs after freeze-thaw (Fig. 1A; P < 0.05). The post-thaw viability of recovered PGCs revealed that DMSO and PG exhibited cryoprotective effects, whereas GLY and EG exhibited negligible cryoprotective effects (Fig. 1B). Therefore, the recovery rates of surviving PGCs after freeze-thaw in DMSO (26.4% ± 2.3%) and PG (27.8% ± 0.3%) solutions were significantly higher than in GLY (6.7% ± 0.2%) and EG (7.1% ± 0.4%) solutions (Fig. 1A; P < 0.05). These results clearly demonstrate that DMSO and PG possess high cryoprotective capacity for chicken PGCs.
Fig. 1.
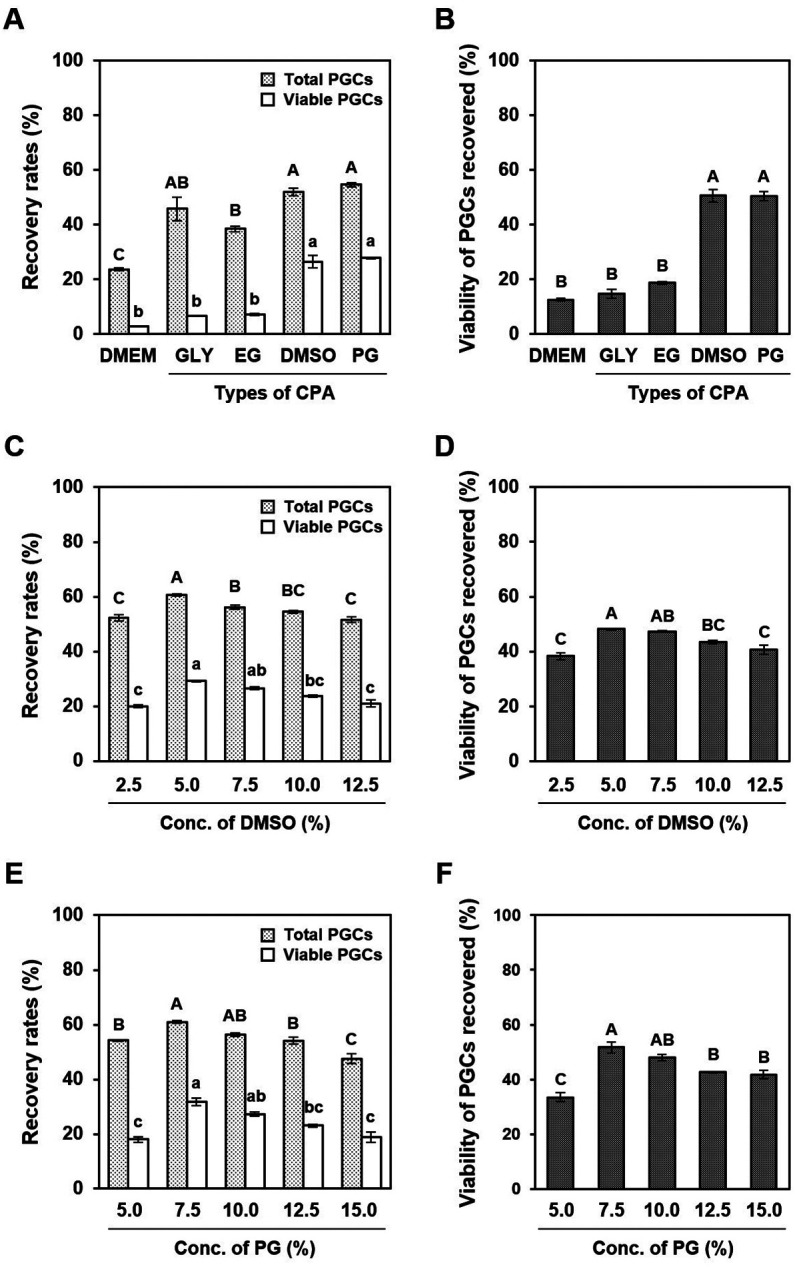
Cryoprotective effects of various permeable cryoprotectants (CPA) on the post-thaw recovery and viability of cultured primordial germ cells (PGCs). (A and B) Cultured PGCs were cryopreserved with different types of permeable CPA, namely, basal medium only (DMEM), 10% (v/v) glycerol (GLY), 8% (v/v) ethylene glycol (EG), 9.5% (v/v) dimethyl sulfoxide (DMSO), or 10% (v/v) propylene glycol (PG). (A) Recovery rates of total PGCs, including dead cells (light gray) and viable cells (white) and (B) the viability of PGCs recovered after thawing. (C–F) Cultured PGCs were cryopreserved with different concentrations of (C and D) DMSO or (E and F) PG. (C and E) Recovery rates of total PGCs, including dead (light gray) and viable cells (white) after freeze-thaw with each cryoprotectant solution. (D and F) Viability of PGCs recovered after thawing with each cryoprotectant solution. All data are presented as mean ± SEM. Data with different letters are significantly different (P < 0.05).
Cryoprotective effects of DMSO and PG
We further investigated the optimal concentrations of DMSO and PG for the cryopreservation of cultured PGCs based on the post-thaw recovery of viable cells. Both the post-thaw recovery of PGCs and viability of recovered PGCs in DMSO and PG solutions peaked at 5% and 7.5%, respectively, and then decreased in a concentration-dependent manner (Figs. 1C–F). Thus, post-thaw recovery rates of surviving PGCs in DMSO and PG solutions were highest at 5% (29.2% ± 0.2%) and 7.5% (31.8% ± 1.4%), respectively (Figs. 1C and E).
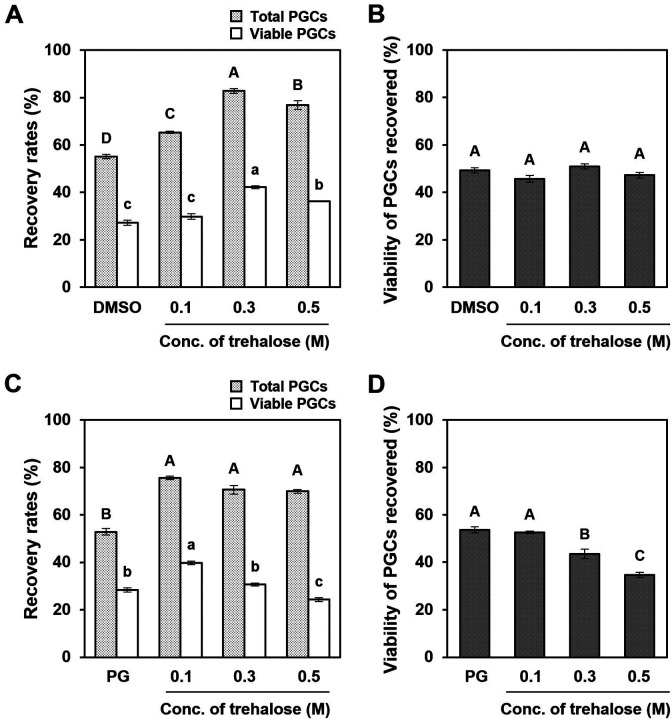
Trehalose improved post-thaw recovery of chicken PGCs
We evaluated the cryoprotective effects of trehalose, a non-permeable CPA, in combination with DMSO or PG, on cultured PGCs. We found that the addition of trehalose to 5% DMSO or 7.5% PG solutions significantly increased the recovery rates of post-thaw PGCs (Figs. 2A and C; P < 0.05). Moreover, the post-thaw viability of PGCs was constant for all concentrations of trehalose (0 to 0.5 M) in DMSO solution but decreased above 0.3 M trehalose in PG solution (Figs. 2B and D). Hence, the recovery rates of viable PGCs were highest in cryomedia, including 0.3 M trehalose and 5% DMSO (42.2% ± 0.6%) and 0.1 M trehalose and 7.5% PG (39.8% ± 0.7%). These results suggest that trehalose improves the post-thaw recovery of cultured PGCs.
Fig. 2.
Cryoprotective effects of trehalose in a combination of DMSO or PG on the post-thaw recovery and viability of cultured PGCs. Cultured PGCs were cryopreserved with different concentrations of trehalose (0–0.5 M) in combination with (A and B) 5% DMSO or (C and D) 7.5% PG. (A and C) Recovery rates of total PGCs, including dead (light gray) and viable cells (white) after cryopreservation with each cryoprotectant solution. (B and D) Viability of PGCs recovered after freeze-thaw with each cryoprotectant solution. All data are presented as mean ± SEM. Data with different letters are significantly different (P < 0.05).
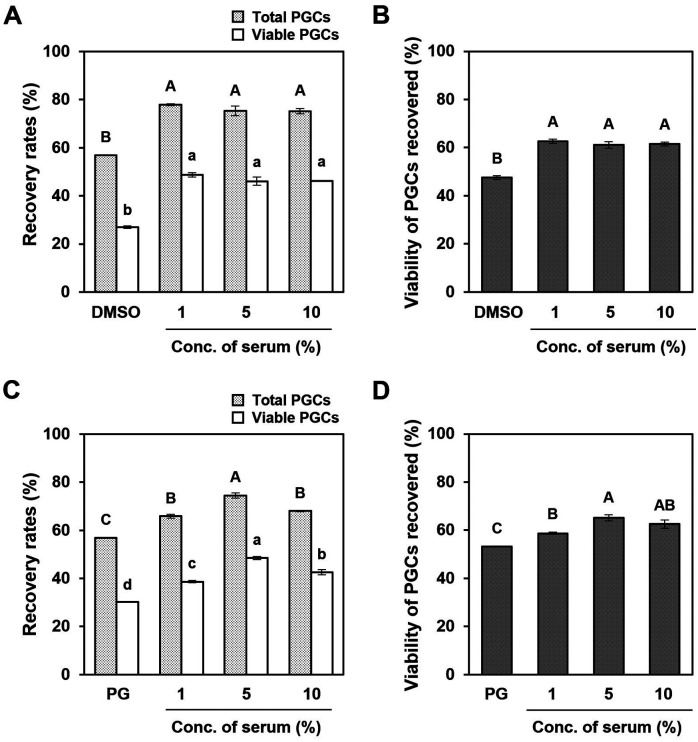
Chicken serum improved the post-thaw viability of chicken PGCs
We also evaluated the effect of chicken serum in combination with DMSO or PG on the cryoprotective capacity of cultured PGCs. When chicken serum was added along with 5% DMSO or 7.5% PG, the recovery of post-thawed PGCs was significantly increased (Figs. 3A and C; P < 0.05). Furthermore, the viability of PGCs recovered after thawing increased dramatically with the addition of chicken serum to DMSO or PG solutions (Figs. 3B and D). In the DMSO solution, the highest recovery rate of surviving PGCs after thawing was obtained by adding 1% chicken serum (Fig. 3A; 48.8% ± 0.9%). As shown in Fig. 3C, the percent recovery of viable PGCs peaked at 5% chicken serum in PG solution (48.5% ± 0.6%). These results suggest that chicken serum improves both the post-thaw recovery and viability of cultured PGCs. Therefore, we selected 1% and 5% chicken serum as additives to DMSO and PG solutions hereafter, respectively.
Fig. 3.
Cryoprotective effects of chicken serum in combination with DMSO or PG on the post-thaw recovery and viability of cultured PGCs. Cultured PGCs were cryopreserved with different concentrations of chicken serum (0%–10%) in combination with (A and B) 5% DMSO or (C and D) 7.5% PG. (A and C) Recovery rates of total PGCs, including dead (light gray) and viable cells (white) after cryopreservation with each cryoprotectant solution. (B and D) Viability of PGCs recovered after thawing with each cryoprotectant solution. All data are presented as mean ± SEM. Data with different letters are significantly different (P < 0.05).
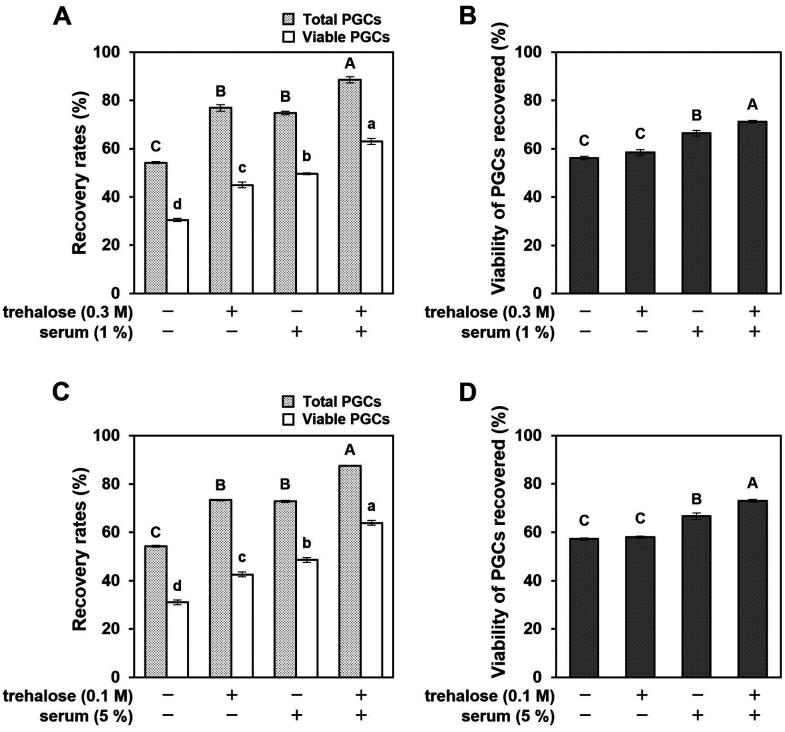
Additive cryoprotective effect of trehalose and chicken serum
Because of the differences in the cryoprotective actions of trehalose and chicken serum on cultured PGCs, we further examined whether these CPA could exhibit an additive effect. Hence, cultured PGCs were cryopreserved in DMSO or PG solutions, either alone or in combination with trehalose and/or chicken serum. Both the recovery and viability of post-thawed PGCs were additively increased by adding trehalose and chicken serum along with DMSO or PG (Figs. 4A–D). The addition of both trehalose and chicken serum drastically improved the recovery rates of viable PGCs in both DMSO (63.0% ± 1.3%) and PG (63.9% ± 0.1%) solutions (Figs. 4A and C). These results strongly suggested that trehalose and chicken serum have different cryoprotective effects.
Fig. 4.
Additive effect of trehalose and chicken serum on the post-thaw recovery and viability of cultured PGCs. Cultured PGCs were cryopreserved in 5% DMSO or 7.5% PG solutions either alone or combined with trehalose and/or chicken serum. (A and C) Recovery rates of total PGCs, including dead (light gray) and viable cells (white), thawing with each cryoprotectant solution. (B and D) Viability of PGCs recovered after freeze-thaw with each cryoprotectant solution. All data are presented as mean ± SEM. Data with different letters are significantly different (P < 0.05).
Based on these results, we confirmed the final concentrations of cocktail constituents were as follows: DTS cryomedium – 5% DMSO, 0.3 M trehalose, and 1% chicken serum; PTS cryomedium – 7.5% PG, 0.1 M trehalose, and 5% chicken serum. We examined the morphology of the cultured PGCs (W4) before and immediately after thawing (Supplementary Fig. 1A). Although some cells ruptured, the majority of frozen-thawed PGCs remained morphologically normal when compared to unfrozen PGCs, regardless of the cryomedia. We next evaluated the cryoprotective capacity of these cryomedia by comparing them with that of CB1, which is useful for slow-freezing naive PGCs [9, 14]. When cultured PGCs (W4) were cryopreserved in DTS or PTS cryomedia, the post-thaw recovery rates of viable cells were slightly higher than those in CB1; however, the differences were not significant (Supplementary Figs. 1B–D). We further determined whether DTS and PTS cryomedia could be used on various PGC lines (W2 and W19) and naive PGCs. The percent recovery of total and viable cells after freeze-thaw in W4 was statistically similar to those in W2, W19, and naive PGCs in both DTS and PTS cryomedia (Supplementary Figs. 1E, F, H, and I; P < 0.05). The percent viability of post-thawed PGCs in W4 was similar among PGC lines in DTS cryomedium but significantly higher than that of W19 in PTS cryomedia (Supplementary Figs. 1G and J; P < 0.05).
Post-thawed PGCs can settle to gonads following transplantation
We examined whether PGCs after cryopreservation with DTS or PTS cryomedia could be incorporated into the gonads of recipient embryos following transplantation. Approximately 1 × 103 fluorescently labeled cultured PGCs (W4), before or after freeze-thaw, were transferred to the bloodstream of two-day-old recipient embryos. The viability rates of PGCs decreased from 94% to approximately 70% after freeze-thawing. Gonadal migration of transplanted PGCs from both the unfrozen control and that frozen with DTS or PTS was observed in all recipient embryos (Figs. 5A and B). However, the number of CVH+ PKH-26-labeled germ cells in recipient gonads declined to less than half after freeze-thawing, regardless of the cryomedia (Fig. 5C; P < 0.05). These results suggest that cultured PGCs settled embryonic gonads less frequently than unfrozen PGCs immediately after thawing.
Fig. 5.
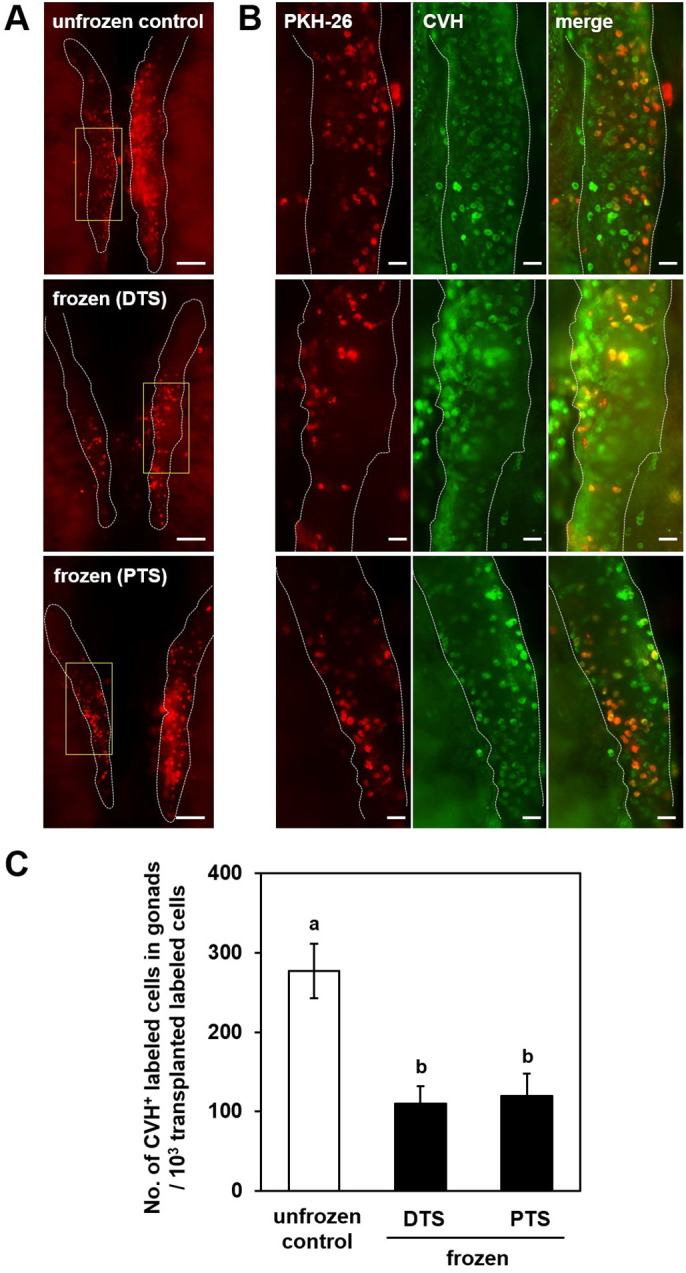
Gonadal migration of cultured PGCs after freeze-thaw. (A) Images of recipient gonads receiving PKH-26-labeled cells (about 1 × 103 cells) without cryopreservation (unfrozen control; upper) and after cryopreservation in DTS (middle) or PTS (bottom) cryomedia. Gonads are outlined with white dashed lines. (B) Higher magnification of boxed regions in (A). Left panels show PKH-26 (red), middle panels show immunofluorescence images of chicken vasa homolog (CVH) (green), and right panels show merged images of the left and middle panels. (C) Number of CVH+ PKH-26-labeled cells in recipient gonads. Scale bars: (A) 200 μm; (B) 100 μm. In (C), mean ± SEM for 15 (unfrozen control), 20 (frozen in DTS), and 20 (frozen in PTS) recipients are shown (a, b P < 0.05).
Production of functional sperm from cryopreserved PGCs
Next, we determined whether cultured PGCs frozen in DTS or PTS cryomedia maintained germline competency. In this study, two male PGC lines (W4 and W19) were cryopreserved in DTS or PTS cryomedia for > 1 month. After thawing, approximately 2 × 103 PGCs were microinjected into male recipient BPR embryos. Progeny tests with normal BPR hens revealed that all recipient BPR roosters sired white progeny with small black patches of variable frequencies (Supplementary Figs. 2A and B). These results suggest that male PGCs can differentiate into functional sperm, even after cryopreservation with DTS or PTS cryomedia.
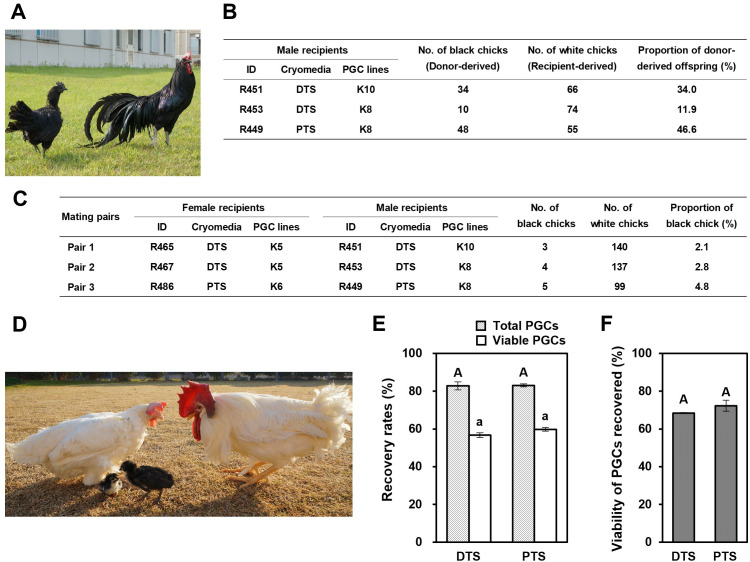
Production of offspring derived from cryopreserved PGCs from a rare chicken breed (Kurokashiwa)
Finally, we demonstrated the applicability of DTS and PTS cryomedia in the cryobanking of PGC lines from the rare chicken breed Kurokashiwa (Fig. 6A). We cryopreserved Kurokashiwa PGC lines of two males (K8 and K10) and two females (K5 and K6) in DTS or PTS cryomedia for > 1 month and transplanted them intravascularly to the WL embryos. Four WL recipients (two males and two females) in the DTS group and two WL recipients (one male and one female) in the PTS group survived to sexual maturity. Progeny tests of the three WL recipient males showed that they produced functional sperm originating from Kurokashiwa PGCs (Fig. 6B). Progeny tests were also performed with three pairs of WL recipient roosters and hens (two pairs in the DTS group and one pair in the PTS group). When 284 and 104 F1 progeny obtained by mating in the DTS and PTS groups were checked, 7 and 5 offspring exhibited black feather color, respectively (Figs. 6C and D). Therefore, we successfully produced offspring of a rare chicken breed derived from male and female PGCs after cryopreservation in DTS and PTS cryomedia.
Fig. 6.
Revival of a rare chicken breed derived from cryopreserved PGCs. (A) Female and male Kurokashiwa. This rare breed was used to verify the utility of DTS and PTS cryomedia for cryobanking of PGCs in (B)–(F). (B) Percentage of donor-derived offspring from White Leghorn (WL) recipient roosters transplanted with frozen-thawed PGCs of Kurokashiwa by mating with normal Barred Plymouth Rock (BPR) hens. (C) Percentage of donor-derived offspring by crossing female and male WL recipients. (D) Kurokashiwa offspring (black feather) revived from WL recipient parents (white feather) by the transplantation of frozen-thawed PGCs. (E) Recovery and (F) viability rates of a female PGC line (K5) of Kurokashiwa after storage in liquid nitrogen for > 2.5 years. Bars with light gray and white indicate total cells and viable cells, respectively. Values in (E) and (F) are shown as mean ± SEM. Data with different letters are significantly different (P < 0.05).
To determine the feasibility of DTS and PTS cryomedia for cryobanking PGCs, we checked whether long-term storage of cultured PGCs in LN2 affected their recovery and viability after thawing. When a female PGC line (K5) was cryopreserved in LN2 for > 2.5 years, the post-thawed recovery and viable rates of PGCs were statistically similar between the DTS and PTS cryomedia (Figs. 6E and F). The percent recovery of viable cells in DTS and PTS cryomedia after cryopreservation for > 2.5 years (56.8% ± 1.2% vs. 59.9% ± 1.0%, respectively) was similar to that after cryopreservation for > 1 month (Supplementary Figs. 1F and I). These results indicate that these cryomedia maintained the survival of cultured PGCs, even after being cryopreserved for 2.5 years.
Discussion
CPA is broadly separated into two categories: permeable and nonpermeable. Permeable CPA, such as GLY, EG, DMSO, and PG, are generally small, non-ionic molecules that can pass through cell membranes and enter a cell. Hence, permeable CPA could replace intracellular water and prevent ice crystal formation during freezing. In contrast, non-penetrating CPA include small (e.g., trehalose and sucrose) and large (e.g., albumin) molecules that do not penetrate the cell membranes. Non-permeable CPA can prevent ice crystal formation extracellularly via direct interaction with water and intracellularly via dehydration of cells. The success of cryopreservation mostly depends on both CPA type and concentration [24]. Chicken PGC cryopreservation has been regularly carried out using DMSO as a permeable CPA, either alone or in conjunction with serum as a non-permeable CPA, by the slow-freezing method. However, cryopreservation protocols for chicken PGCs have not yet been well defined. In the present study, we established two different cryomedia for slow-freezing cultured chicken PGCs by optimizing the type and concentration of CPA based on post-thaw recovery and viability.
DMSO has been the most widely used CPA since the discovery of its cryoprotective ability in 1959 [25]. The cryopreservation of chicken PGCs has been used with 4%–10% DMSO as the CPA [9, 10, 12,13,14,15,16,17, 26]. As DMSO is known to be toxic to cells and tissues depending on its concentration [27,28,29], the viability of post-thawed PGCs is affected by the concentration of DMSO in the cryomedia. We demonstrated that the recovery percentage of viable PGCs peaked at 5% DMSO treatment (Fig. 1C). Our results further showed that PG had a cryoprotective effect on chicken PGCs equivalent to that of DMSO, and the 7.5% concentration was evaluated as the most effective (Fig. 1E). Moore et al. (2006) [30] reported that EG was superior to DMSO in freezing chicken PGCs. In their study, dissociated gonadal cells, including PGCs obtained from 10 embryos, were frozen in a cryomedia containing 10% EG in straw at a cooling rate of −1°C/min; then, the viability of post-thawed PGCs was analyzed by flow cytometry. This cryopreservation protocol yielded a viable rate of 74.3% ± 3.3% of post-thawed chicken PGCs. However, we could not reproduce their results, and the percentage of viable PGCs retrieved after freeze-thaw was only 18.7% ± 0.5% (Fig. 1A). In the present study, approximately 1 × 104 cultured PGCs were cryopreserved in a cryomedia containing 8% EG in a cryotube at a cooling rate of −1°C/min; then, the viability of frozen-thawed PGCs was analyzed using the Trypan blue exclusion method. It is difficult to compare our results directly with those of Moore et al. (2006) [30]; however, differences in the number of cells frozen and types of freezing devices used might have a large effect on the post-thaw viability of chicken PGCs.
Sugars have cryoprotective properties and are less toxic than permeable CPA. The primary action of sugars is to reduce the risk of intracellular ice formation owing to dehydration [31]. Trehalose has less cytotoxicity and has been efficiently used for freezing various types of animal cells, including germ cells [32, 33]. In addition to the osmotic effect, its cryoprotective action is also related to specific interactions with cell membrane phospholipids and labile proteins by preventing their damage and denaturation owing to desiccation and oxidative stress [34,35,36]. The use of trehalose in combination with permeable CPA improved the post-thaw viability of various cell types [37,38,39]. This study revealed that trehalose, in combination with 5% DMSO or 7.5% PG as a permeable CPA, enhanced the recovery of chicken PGCs after freeze-thaw (Figs. 2A and C). A higher concentration of trehalose was required in the DMSO-based freezing solution, in which the osmolarity was lower than that in the PG-based freezing solution, until the post-thaw recovery rate reached its peak. Therefore, the primary action of trehalose in the slow-freezing of cultured PGCs is the inhibition of intracellular ice formation and resultant cell damage owing to enhanced dehydration.
Serum acts as a buffer during cryopreservation to modulate osmotic shock, protect cellular membranes, and reduce the risk of crystallization or recrystallization [40]. Despite the use of fetal bovine serum and chicken serum in conjugation with DMSO to cryopreserve chicken PGCs [9, 10, 15, 16, 41], their cryoprotective effect on chicken PGCs has not been well studied. Here, we revealed that the addition of chicken serum improved both the recovery and viability of frozen-thawed cultured PGCs when combined with 5% DMSO or 7.5% PG as a permeable CPA (Fig. 3). Furthermore, we demonstrated that trehalose and chicken serum possessed an additive cryoprotective effect on cultured PGCs (Fig. 4), suggesting that these non-permeable CPA have different mechanisms of action in slow-freezing. Albumin is the most abundant protein in serum, acting as a stabilizing cell membrane protein, and has been widely used as an effective serum replacement for the cryopreservation of various types of cells, including germ cells [42,43,44,45,46,47]. Thus, serum-free cryomedia for chicken PGCs could be improved by investigating the replacement of serum with recombinant albumin to remove the risk of vial and serum-borne pathogen contamination of cryopreserved cells.
Using the percent recovery of viable PGCs after freeze-thaw as a parameter, we optimized the composition ratios of trehalose and serum in DMSO- (DTS: 5% DMSO, 0.3 M trehalose, and 1% chicken serum) and PG-based cryomedia (PTS: 7.5% PG, 0.1 M trehalose, and 5% chicken serum). The post-thaw recovery rates of viable PGCs with these cryomedia were comparable and consistently achieved in all PGC lines, including freshly isolated naive PGCs (Supplementary Figs. 1F and I). We previously demonstrated that CB1, a commercially available serum-containing DMSO-based cryomedium, is useful for the slow-freezing of naive PGCs, with a higher percentage recovery of viable cells (range: 45.8%–47.3%) than other cryomedia [41, 48]. Therefore, we used CB1 as the standard to evaluate the cryoprotective capacity of DTS and PTS cryomedia. Here, our results showed that the average recovery rates of viable PGCs with DTS (58.8% ± 3.1%) and PTS cryomedia (63.9% ± 2.8%) were equal or slightly superior to those of CB1 (57.7% ± 1.3%; Supplementary Figs. 1B–D).
Intravascular transplantation is the most reliable method for assessing the functional activity of PGCs stored in the DTS or PTS cryomedia. Therefore, post-thawed PGCs were immediately transplanted, and the number of germ cells that settled in recipient gonads was quantified on post-transplantation day 4. The number of germ cells that settled was comparable between the two cryomedia but decreased to less than half after freeze-thaw (Fig. 5C). This result is compatible with our previous study, showing that naive PGCs settled at a low frequency after freeze-thawing [48]. Although the viability of post-thawed PGCs, as compared to that of unfrozen PGCs (approximately 70% vs. 94%) is taken into consideration, post-thawed PGCs settled recipient gonads less frequently than unfrozen PGCs. There are two possibilities for this: one is the elimination of PGCs that were damaged by freeze-thaw in the recipient and the other is a decrease in colonizing ability after freeze-thaw. Tonus et al. (2016) [26] reported that the viability of chicken PGCs decreased 1 to 2 days after post-thaw, possibly because of apoptosis, before reaching the level of unfrozen controls after one week of culture. Therefore, it is likely that some of the post-thawed PGCs are eliminated after transplantation owing to apoptosis, as observed in culture. It is recommended that post-thawed PGCs be cultured to recover from the damage caused by freeze-thawing prior to transplantation.
To further evaluate the germline competency of frozen-thawed PGCs, progeny tests of the recipients were conducted. Offspring originating from post-thawed PGCs were obtained from all recipient roosters (Supplementary Fig. 2B and Fig. 6B). We also successfully revived offspring derived from frozen-thawed PGCs of Kurokashiwa by mating recipient roosters and hens (Figs. 6C and D). These results suggest that both DTS and PTS cryomedia can maintain the germline competency of cultured PGCs after storage in LN2. Furthermore, our success with a rare chicken breed (Kurokashiwa) indicates that these cryomedia could be extended to various chicken breeds for cryobanking of PGCs. Our cryomedia maintained cultured PGCs with high recovery and viability, even after long-term storage in LN2 (Figs. 6E and F). Further studies are required to determine whether the long-term cryopreservation of PGCs affects their ability to produce gametes and offspring. In this study, the germline transmission rates of post-thawed PGCs from recipients were highly variable. This result reinforces the requirement for sterile recipients to be applied to cryobanking chicken PGCs. Future experiments will use either chemically sterilized or genetically sterilized recipient chicken embryos to increase the germline transmission rates of frozen-thawed PGCs after transplantation [49,50,51].
In conclusion, this study demonstrated that PG has a cryoprotective effect on chicken PGCs equivalent to that of DMSO, a commonly used permeable CPA. Our study further revealed that trehalose improves the post-thaw recovery of cultured PGCs and possesses an additive cryoprotective effect when used with serum. Based on these findings, we designed DMSO- and PG-based cryomedia that achieved > 50% recovery of viable PGCs after thawing while retaining their germline competency. Kurokashiwa was included in the present study as a model of a rare chicken breed, and its progeny were produced from PGCs cryopreserved in these cryomedia. The cryomedia developed in this study offers a more reliable and inexpensive protocol for the long-term cryopreservation of chicken PGCs. The prospective application of these cryomedia for cell-based cryobanking will contribute to the preservation of chicken genetic diversity for sustainability and adaptation to future poultry demands.
Conflict of interests
The authors declare no conflict of interest.
Supplementary
Acknowledgments
We are grateful to Dr. H. Horiuch for kindly providing the anti-CVH mAb. The authors thank all members of the Laboratory of Animal Breeding and Genetics, Hiroshima University for the care and breeding of chickens. This work was supported in part by the Grants-in-Aid for NBRP Fundamental Technologies Upgrading Program from the Japan Agency for Medical Research and Development (AMED) and Grants-in-Aid for Scientific Research on Innovative Areas (18H05551 to YN) from the Japan Society for the Promotion of Science (JSPS).
References
- 1.Lake PE, Ravie O, McAdam J. Preservation of fowl semen in liquid nitrogen: application to breeding programmes. Br Poult Sci 1981; 22: 71–77. [DOI] [PubMed] [Google Scholar]
- 2.Tajima A, Graham EF, Hawkins DM. Estimation of the relative fertilizing ability of frozen chicken spermatozoa using a heterospermic competition method. J Reprod Fertil 1989; 85: 1–5. [DOI] [PubMed] [Google Scholar]
- 3.Liu J, Song Y, Cheng KM, Silversides FG. Production of donor-derived offspring from cryopreserved ovarian tissue in Japanese quail (Coturnix japonica). Biol Reprod 2010; 83: 15–19. [DOI] [PubMed] [Google Scholar]
- 4.Fujimoto T, Ukeshima A, Kiyofuji R. The origin, migration and morphology of the primordial germ cells in the chick embryo. Anat Rec 1976; 185: 139–145. [DOI] [PubMed] [Google Scholar]
- 5.Yasuda Y, Tajima A, Fujimoto T, Kuwana T. A method to obtain avian germ-line chimaeras using isolated primordial germ cells. J Reprod Fertil 1992; 96: 521–528. [DOI] [PubMed] [Google Scholar]
- 6.Tajima A, Naito M, Yasuda Y, Kuwana T. Production of germ line chimera by transfer of primordial germ cells in the domestic chicken (Gallus domesticus). Theriogenology 1993; 40: 509–519. [DOI] [PubMed] [Google Scholar]
- 7.Naito M, Tajima A, Yasuda Y, Kuwana T. Production of germline chimeric chickens, with high transmission rate of donor-derived gametes, produced by transfer of primordial germ cells. Mol Reprod Dev 1994a; 39: 153–161. [DOI] [PubMed] [Google Scholar]
- 8.Nakamura Y, Usui F, Miyahara D, Mori T, Ono T, Kagami H, Takeda K, Nirasawa K, Tagami T. X-irradiation removes endogenous primordial germ cells (PGCs) and increases germline transmission of donor PGCs in chimeric chickens. J Reprod Dev 2012; 58: 432–437. [DOI] [PubMed] [Google Scholar]
- 9.Nakamura Y, Usui F, Miyahara D, Mori T, Ono T, Takeda K, Nirasawa K, Kagami H, Tagami T. Efficient system for preservation and regeneration of genetic resources in chicken: concurrent storage of primordial germ cells and live animals from early embryos of a rare indigenous fowl (Gifujidori). Reprod Fertil Dev 2010a; 22: 1237–1246. [DOI] [PubMed] [Google Scholar]
- 10.Nandi S, Whyte J, Taylor L, Sherman A, Nair V, Kaiser P, McGrew MJ. Cryopreservation of specialized chicken lines using cultured primordial germ cells. Poult Sci 2016; 95: 1905–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakamura Y. Avian Biotechnology. In: Sasanami T (ed.), Avian Reproduction: from behavior to molecules. Singapore: Springer; 2017: 187–214. [Google Scholar]
- 12.Naito M, Tajima A, Tagami T, Yasuda Y, Kuwana T. Preservation of chick primordial germ cells in liquid nitrogen and subsequent production of viable offspring. J Reprod Fertil 1994b; 102: 321–325. [DOI] [PubMed] [Google Scholar]
- 13.Tajima A, Naito M, Yasuda Y, Kuwana T. Production of germ-line chimeras by transfer of cryopreserved gonadal primordial germ cells (gPGCs) in chicken. J Exp Zool 1998; 280: 265–267. [PubMed] [Google Scholar]
- 14.Nakamura Y, Tasai M, Takeda K, Nirasawa K, Tagami T. Production of functional gametes from cryopreserved primordial germ cells of the Japanese quail. J Reprod Dev 2013; 59: 580–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woodcock ME, Gheyas AA, Mason AS, Nandi S, Taylor L, Sherman A, Smith J, Burt DW, Hawken R, McGrew MJ. Reviving rare chicken breeds using genetically engineered sterility in surrogate host birds. Proc Natl Acad Sci USA 2019; 116: 20930–20937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lázár B, Molnár M, Sztán N, Végi B, Drobnyák Á, Tóth R, Tokodyné Szabadi N, McGrew MJ, Gócza E, Patakiné Várkonyi E. Successful cryopreservation and regeneration of a partridge colored Hungarian native chicken breed using primordial germ cells. Poult Sci 2021; 100: 101207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakajima Y, Asano A, Tajima A. Developmental potential of cryopreserved gonadal germ cells from 7-day-old chick embryos recovered using the PBS(-) method. Br Poult Sci 2022; 63: 46–53. [DOI] [PubMed] [Google Scholar]
- 18.Whyte J, Glover JD, Woodcock M, Brzeszczynska J, Taylor L, Sherman A, Kaiser P, McGrew MJ. FGF, Insulin, and SMAD Signaling Cooperate for Avian Primordial Germ Cell Self-Renewal. Stem Cell Reports 2015; 5: 1171–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J Morphol 1951; 88: 49–92. [PubMed] [Google Scholar]
- 20.Zhao DF, Kuwana T. Purification of avian circulating primordial germ cells by nycodenz density gradient centrifugation. Br Poult Sci 2003; 44: 30–35. [DOI] [PubMed] [Google Scholar]
- 21.Yamamoto Y, Usui F, Nakamura Y, Ito Y, Tagami T, Nirasawa K, Matsubara Y, Ono T, Kagami H. A novel method to isolate primordial germ cells and its use for the generation of germline chimeras in chicken. Biol Reprod 2007; 77: 115–119. [DOI] [PubMed] [Google Scholar]
- 22.Nakamura Y, Usui F, Atsumi Y, Otomo A, Teshima A, Ono T, Takeda K, Nirasawa K, Kagami H, Tagami T. Effects of busulfan sustained-release emulsion on depletion and repopulation of primordial germ cells in early chicken embryos. J Poult Sci 2009; 46: 127–135. [Google Scholar]
- 23.Nakano M, Arisawa K, Yokoyama S, Nishimoto M, Yamashita Y, Sakashita M, Ezaki R, Matsuda H, Furusawa S, Horiuchi H. Characteristics of novel chicken embryonic stem cells established using chicken leukemia inhibitory factor. J Poult Sci 2011; 48: 64–72. [Google Scholar]
- 24.Fernández-Santos MR, Esteso MC, Montoro V, Soler AJ, Garde JJ. Cryopreservation of Iberian red deer (Cervus elaphus hispanicus) epididymal spermatozoa: effects of egg yolk, glycerol and cooling rate. Theriogenology 2006; 66: 1931–1942. [DOI] [PubMed] [Google Scholar]
- 25.Lovelock JE, Bishop MW. Prevention of freezing damage to living cells by dimethyl sulphoxide. Nature 1959; 183: 1394–1395. [DOI] [PubMed] [Google Scholar]
- 26.Tonus C, Connan D, Waroux O, Vandenhove B, Wayet J, Gillet L, Desmecht D, Antoine N, Ectors FJ, Grobet L. Cryopreservation of chicken primordial germ cells by vitrification and slow freezing: A comparative study. Theriogenology 2017; 88: 197–206. [DOI] [PubMed] [Google Scholar]
- 27.Rowley SD, Anderson GL. Effect of DMSO exposure without cryopreservation on hematopoietic progenitor cells. Bone Marrow Transplant 1993; 11: 389–393. [PubMed] [Google Scholar]
- 28.Katayama Y, Yano T, Bessho A, Deguchi S, Sunami K, Mahmut N, Shinagawa K, Omoto E, Makino S, Miyamoto T, Mizuno S, Fukuda T, Eto T, Fujisaki T, Ohno Y, Inaba S, Niho Y, Harada M. The effects of a simplified method for cryopreservation and thawing procedures on peripheral blood stem cells. Bone Marrow Transplant 1997; 19: 283–287. [DOI] [PubMed] [Google Scholar]
- 29.Hunt CJ, Armitage SE, Pegg DE. Cryopreservation of umbilical cord blood: 2. Tolerance of CD34(+) cells to multimolar dimethyl sulphoxide and the effect of cooling rate on recovery after freezing and thawing. Cryobiology 2003; 46: 76–87. [DOI] [PubMed] [Google Scholar]
- 30.Moore DT, Purdy PH, Blackburn HD. A method for cryopreserving chicken primordial germ cells. Poult Sci 2006; 85: 1784–1790. [DOI] [PubMed] [Google Scholar]
- 31.Swain JE, Smith GD. Cryoprotectants. In: Quinn P, Chian R-C (eds.), Fertility Cryopreservation. Cambridge: Cambridge University Press, 2010: 24–38. [Google Scholar]
- 32.Lee YA, Kim YH, Kim BJ, Kim BG, Kim KJ, Auh JH, Schmidt JA, Ryu BY. Cryopreservation in trehalose preserves functional capacity of murine spermatogonial stem cells. PLoS One 2013; 8: e54889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee YA, Kim YH, Ha SJ, Kim KJ, Kim BJ, Kim BG, Choi SH, Kim IC, Schmidt JA, Ryu BY. Cryopreservation of porcine spermatogonial stem cells by slow-freezing testis tissue in trehalose. J Anim Sci 2014; 92: 984–995. [DOI] [PubMed] [Google Scholar]
- 34.Rudolph AS, Crowe JH. Membrane stabilization during freezing: the role of two natural cryoprotectants, trehalose and proline. Cryobiology 1985; 22: 367–377. [DOI] [PubMed] [Google Scholar]
- 35.Honadel TE, Killian GJ. Cryopreservation of murine embryos with trehalose and glycerol. Cryobiology 1988; 25: 331–337. [DOI] [PubMed] [Google Scholar]
- 36.Benaroudj N, Lee DH, Goldberg AL. Trehalose accumulation during cellular stress protects cells and cellular proteins from damage by oxygen radicals. J Biol Chem 2001; 276: 24261–24267. [DOI] [PubMed] [Google Scholar]
- 37.Erdag G, Eroglu A, Morgan J, Toner M. Cryopreservation of fetal skin is improved by extracellular trehalose. Cryobiology 2002; 44: 218–228. [DOI] [PubMed] [Google Scholar]
- 38.Choi YH, Chang YJ. The influence of cooling rate, developmental stage, and the addition of sugar on the cryopreservation of larvae of the pearl oyster Pinctada fucata martensii. Cryobiology 2003; 46: 190–193. [DOI] [PubMed] [Google Scholar]
- 39.Ntai A, La Spada A, De Blasio P, Biunno I. Trehalose to cryopreserve human pluripotent stem cells. Stem Cell Res (Amst) 2018; 31: 102–112. [DOI] [PubMed] [Google Scholar]
- 40.Day JG, McLella MR. Introduction. In: Day JG, McLella MR (eds.), Cryopreservation and freeze-drying protocols. Totowa: Humana Press; 1995: 1–5. [DOI] [PubMed] [Google Scholar]
- 41.Setioko AR, Tagami T, Tase H, Nakamura Y, Takeda K, Tagami T. Cryopreservation of primordial germ cells (PGCs) from White Leghorn embryos using commercial cryoprotectants. J Poult Sci 2007; 44: 73–77. [Google Scholar]
- 42.Delbosc B, Herve P, Carbillet JP, Montard M. Corneal cryopreservation in man: a proposal for an original technic. J Fr Ophtalmol 1984; 7: 321–331. [PubMed] [Google Scholar]
- 43.Lazzari L, Lucchi S, Montemurro T, Porretti L, Lopa R, Rebulla P, Sirchia G. Evaluation of the effect of cryopreservation on ex vivo expansion of hematopoietic progenitors from cord blood. Bone Marrow Transplant 2001; 28: 693–698. [DOI] [PubMed] [Google Scholar]
- 44.Balci D, Can A. The assessment of cryopreservation conditions for human umbilical cord stroma-derived mesenchymal stem cells towards a potential use for stem cell banking. Curr Stem Cell Res Ther 2013; 8: 60–72. [DOI] [PubMed] [Google Scholar]
- 45.Briquet A, Halleux A, Lechanteur C, Beguin Y. Neuropeptides to replace serum in cryopreservation of mesenchymal stromal cells? Cytotherapy 2013; 15: 1385–1394. [DOI] [PubMed] [Google Scholar]
- 46.Jung SE, Jin JH, Ahn JS, Kim YH, Yun MH, Kim SH, Kim BJ, Ryu BY. Effect of serum replacement on murine spermatogonial stem cell cryopreservation. Theriogenology 2021; 159: 165–175. [DOI] [PubMed] [Google Scholar]
- 47.Behnamifar A, Bernal B, Torres O, Luis-Chincoya H, GGil M, García-Casado P, Rahimi S, Woelder H, Santiago-Moreno J. Evaluation of two methods for adding cryoprotectant to semen and effects of bovine serum albumin on quality characteristics of cryopreserved rooster spermatozoa. Poult Sci 2021; 100: 101093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nakamura Y, Usui F, Miyahara D, Mori T, Watanabe H, Ono T, Takeda K, Nirasawa K, Kagami H, Tagami T. Viability and functionality of primordial germ cells after freeze-thaw in chickens. J Poult Sci 2011; 48: 57–63. [Google Scholar]
- 49.Nakamura Y, Yamamoto Y, Usui F, Atsumi Y, Ito Y, Ono T, Takeda K, Nirasawa K, Kagami H, Tagami T. Increased proportion of donor primordial germ cells in chimeric gonads by sterilisation of recipient embryos using busulfan sustained-release emulsion in chickens. Reprod Fertil Dev 2008; 20: 900–907. [DOI] [PubMed] [Google Scholar]
- 50.Nakamura Y, Usui F, Ono T, Takeda K, Nirasawa K, Kagami H, Tagami T. Germline replacement by transfer of primordial germ cells into partially sterilized embryos in the chicken. Biol Reprod 2010; 83: 130–137. [DOI] [PubMed] [Google Scholar]
- 51.Ballantyne M, Taylor L, Hu T, Meunier D, Nandi S, Sherman A, Flack B, Henshall JM, Hawken RJ, McGrew MJ. Avian primordial germ cells are bipotent for male or female gametogenesis. Front Cell Dev Biol 2021; 9: 726827. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.