Abstract
Social hierarchy greatly impacts physical and mental health, but the relationship between social hierarchy and depression/anxiety and the underlying neural mechanism remain unclear. The present study used the tube test to determine the social hierarchy status of mice and then performed several behavioral tests to evaluate depression-like and anxiety-like behaviors. Electrophysiological techniques were used to record the firing activities of glutamatergic pyramidal neurons and local field potentials in the medial prefrontal cortex (mPFC). The results suggested that the mice in each cage (4 per cage) established a stable social hierarchy after 2 weeks. Subordinate mice displayed significantly fewer pushing and advancing behaviors, and more retreat behaviors compared with dominant mice. Furthermore, subordinate mice had significantly more immobility durations in the TST, but significantly fewer distances, entries, and time into the center in the OFT, as well as significantly less percent of distances, entries, and time into the open arms in the EPMT, compared with dominant mice, which indicated that subordinate mice displayed depression- and anxiety-like behaviors. In addition, chronic restraint stress (CRS) significantly induced depression- and anxiety-like behaviors in mice and altered social dominance behaviors in the tube test. CRS mice displayed significantly fewer pushing and advancing behaviors, and more retreat behaviors compared with control mice. Furthermore, low social rank and CRS significantly decreased the firing of pyramidal neurons and γ-oscillation activity in the mPFC. Taken together, the present study revealed an inverse relationship between social hierarchy and depression/anxiety, and the neural basis underlying this association might be the excitability of pyramidal neurons and γ oscillation in the mPFC. These findings established an important foundation for a depression/anxiety model based on social hierarchy and provided a new avenue for the development of therapies for stress-related mood disorders.
Keywords: Social hierarchy, Depression, mPFC, Pyramidal neurons, γ oscillation
Graphical abstract
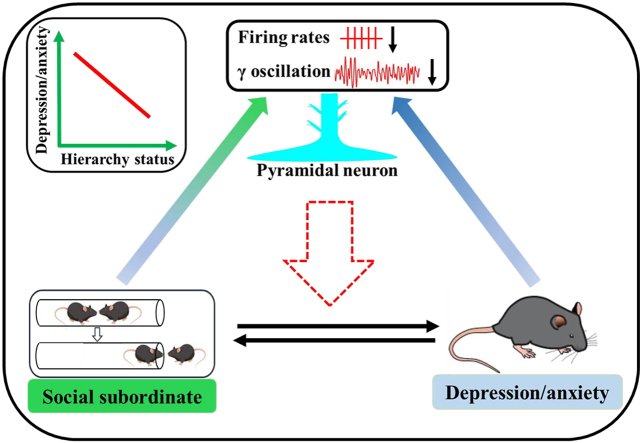
The possible neural basis underlying interactions between social hierarchy and depression/anxiety. There is an inverse relationship between social hierarchy and depression/anxiety-like behaviors (as illustrated in the top left corner). The long-term subordinate status could induce depression/anxiety-like behaviors, and depression/anxiety could alter social dominance behaviors. The subordinate status could decrease the firing rates of pyramidal neurons and γ-oscillation activities in the mPFC, and depression/anxiety could also decrease the firing rates of pyramidal neurons and γ-oscillation activities in the mPFC, which indicated that the pyramidal neurons in the mPFC might mediate the interaction between social hierarchy and depression/anxiety-like behaviors.
Highlights
-
•
There was an inverse relationship between social hierarchy and depression/anxiety-like behaviors.
-
•
Social hierarchy increased the firing activities of pyramidal neurons and γ oscillation in the mPFC.
-
•
Chronic restraint stress decreased the firing activities of pyramidal neurons and γ-oscillation activities in the mPFC.
-
•
The pyramidal neurons regulated the interaction between social hierarchy and depression/anxiety-like behaviors.
1. Introduction
Social hierarchy is a ubiquitous principle of social organization and characterizes the group structure of many species, from fish to primates (Byrne and Bates, 2010; Grosenick et al., 2007; Paz et al., 2004). The social hierarchy has extensive effects on individuals’ survival and physical and mental health (Johnson et al., 2012; Sapolsky, 2005). Specifically, recent preclinical evidence suggests that social dominance relates to autism spectrum disorder (Harris et al., 2021), but it remains unclear how social hierarchy status interacts with other psychiatric disorders, such as depression and anxiety. Depression and anxiety are highly comorbid concurrently and both related to stressful life events (Gorman, 1996). In addition, depression and anxiety-like behaviors appear simultaneously in rodent models of chronic stress (Chiba et al., 2012). Therefore, elucidating the role of social hierarchy status in the pathogenesis of depression and anxiety is very important.
However, there is little evidence of a relationship between social hierarchy and depression/anxiety due to a paucity of studies. An early epidemiological study revealed that the prevalence of depression was higher in individuals with lower socioeconomic status (SES) (Murphy et al., 1991). In addition, investigating the neural mechanisms underlying the potential relationship between social hierarchy and depression/anxiety in humans might be complicated. Fortunately, laboratory rodents are a useful model organism for investigating social hierarchies because they are social animals; thus, they can be used to investigate the neuronal basis underlying dominance behaviors (Wang et al., 2014). The tube test was first developed to investigate differences in the social hierarchy between inbred strains (Lindzey et al., 1961) and was a standard behavioral test of social hierarchy in laboratory mice (Fan et al., 2019). Social dominance is determined based on an individual's hierarchical rank in the tube test (Wang et al., 2011). However, few reports have investigated whether low social rank leads to depression-/anxiety-like behaviors in rodents.
Chronic stress might play an important role in social hierarchy and depression/anxiety. Indeed, several depression/anxiety models have been established based on chronic stress, such as chronic restraint stress (CRS). In addition, it is generally accepted that chronic stress greatly contributes to the pathogenesis of depression and anxiety (Liu et al., 2020; Seo et al., 2017), and several studies have also revealed that chronic stress has marked effects on social behaviors and dominance hierarchies (Krishnan et al., 2007; Park et al., 2018). It was previously reported that prior acute stress exposure renders rats prone to be in a long-term subordinate status in the food competition test (Cordero and Sandi, 2007). Interestingly, a recent study also suggested that CRS was associated with decreased social dominance behaviors in the tube test (Park et al., 2018), providing hints of a potential relationship among chronic stress, depression/anxiety, and social hierarchy.
However, the precise neural mechanisms regulating social hierarchy and depression/anxiety remain unclear. Previous evidence suggested that several brain regions, such as the amygdala, hippocampus, striatum, intraparietal sulcus, ventromedial prefrontal cortex, and lateral prefrontal cortex (Singer, 2012), were involved in the perception and learning of social hierarchy. Specifically, the medial prefrontal cortex (mPFC) has been implicated in many behaviors and is highly important for social dominance behaviors. Recent studies have also shown that social hierarchy regulates the neural activity of mPFC projections to the basolateral amygdala (Dulka et al., 2018), and the dominant rats display significantly greater c-fos immunoreactivity in the prelimbic and infralimbic subcortex of the mPFC, indicating that the mPFC was involved in social dominance behaviors. Many studies have demonstrated that the mPFC also played an essential role in depression (Seo et al., 2017; Xu et al., 2019, Xu et al., 2019) and anxiety (Chocyk et al., 2013; Déziel and Tasker, 2018). Importantly, synaptic activity in the mPFC might mediate core symptoms of depression and anxiety induced by chronic social stress (Covington et al., 2010). Therefore, the mPFC might be a crucial brain region for chronic stress, depression/anxiety, and social hierarchy.
Nevertheless, direct electrophysiological evidence reflecting the effects of social hierarchy and chronic stress on neural activity, such as that of single neurons and local field potentials (LFPs) in the mPFC, is scarce. In particular, excitatory glutamatergic pyramidal neurons, the predominant neurons in the mPFC (accounting for ∼80–90% of the total population in the mPFC) (Xu et al., 2019), greatly contribute to depression/anxiety (Adams, 2009; Larsen et al., 2022). In addition, γ-oscillation activity in the mPFC, the generation and regulation of which depends on the interaction between excitatory glutamatergic pyramidal neurons and inhibitory GABAergic interneurons (Sohal, 2012), might be implicated in depression (Fitzgerald and Watson, 2018). However, little is known about how the firing activities of pyramidal neurons and γ-oscillation activity in the mPFC are altered during social dominance and depression-/anxiety-like behaviors. More importantly, elucidating these issues could suggest a new avenue for developing preventions and interventions for depression/anxiety caused by social factors.
To investigate the relationship between social hierarchy and depression/anxiety, the present study used several behavioral tests to explore the effects of social hierarchy on depression- and anxiety-like behaviors in a rodent model. Furthermore, in vivo electrophysiological techniques were used to record the firing activities of single neurons and the magnitude of LFPs in the mPFC and clarify the mechanistic links among chronic stress, depression/anxiety, and social hierarchy.
2. Materials and methods
2.1. Animals
Male C57BL/6 J mice weighing 22–28 g (purchased from Beijing SPF Laboratory Animal Technology Company, Beijing, China) were used in the present study. The mice were housed in groups under standard conditions at a controlled temperature (23 ± 1 °C), humidity (50 ± 10%), and a 12-h light/dark cycle. Food and water were available ad libitum. All procedures followed the current laws of China and the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH publication No. 86–23, revised 2011) and were approved by the Institutional Animal Care and Use Committee of the Beijing Institute of Pharmacology and Toxicology. All efforts were made to minimize animal suffering and reduce the number of animals used.
2.2. Tube test
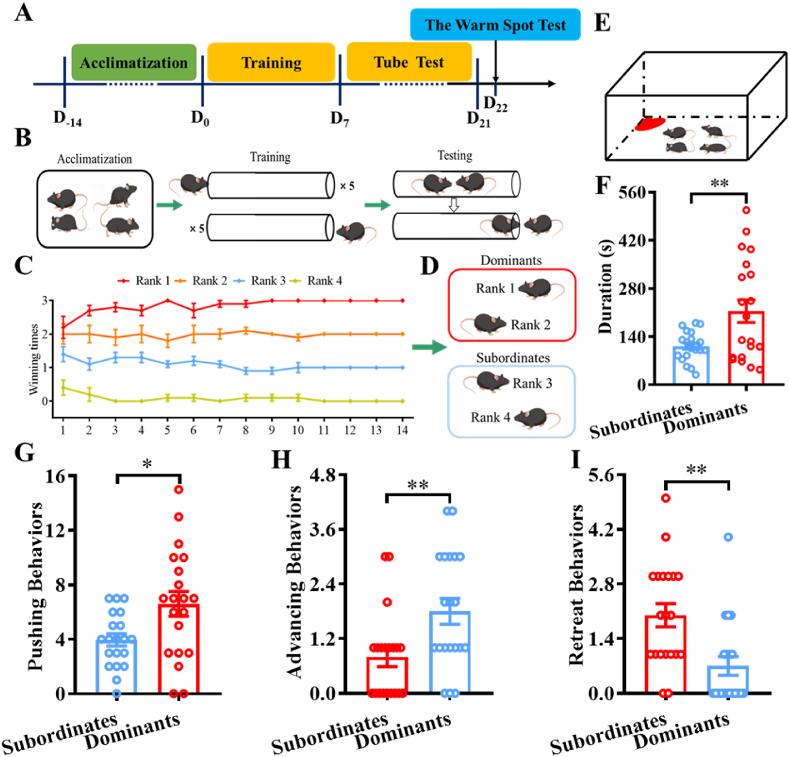
The tube test was performed as previously described (Fan et al., 2019; Zhou et al., 2017) and was applied to mice that had been living together for 2 weeks. In the training session, each mouse was trained to go through a clear plexiglass tube (diameter: 3 cm; length: 30 cm) for 5 trials from each end of the tube for 7 consecutive days, and the social hierarchy in each cage was evaluated for the following 14 consecutive days in the testing session (Fig. 1B). Six tube tests were performed in each cage of 4 mice according to a round-robin design. In the test session, a pair of mice were released (at each end of the tube) and met in the middle; the mouse that retreated outside the tube was recorded as the loser, and the other mouse was recorded as the winner. The number of winnings was calculated as an index of social dominance, and the four mice in a cage were ranked from 1 to 4. The mice ranked 1 and 2 were labeled dominant mice, and the other two (ranked 3 and 4) were labeled subordinate mice (Fig. 1D). The tube was cleaned with 70% ethanol solution to remove odor, urine, and feces before each trial. A representative confrontation between two mice in the tube test is illustrated in Video #1 (see Supplementary Materials). The pushing, advancing, and retreat behaviors in the tube test were recorded and analyzed by the VisuTube Rodent Behavior Analysis system (Shanghai XinRuan Information Technology Co., Ltd., Shanghai, China). The other behavioral tests and electrophysiological recordings were performed after that a stable social hierarchy was established in each cage of 4 mice was established, and the detailed experimental procedures are illustrated in Fig. 1A.
Fig. 1.
A social hierarchy was established in each cage of four mice. A) Schematic drawing of the experimental timeline. B) Training and testing sessions of the tube test. C) Establishment and maintenance of the social hierarchy in each cage of four mice. D) Mice were divided into dominant mice and subordinate mice according to their ranks in the tube test. E) Schematic cartoon illustrating the warm spot test. F) Duration of warm spot occupation by dominant and subordinate mice. G, H, and I) Pushing, advancing, and retreat behaviors of dominant and subordinate mice in the tube test, respectively. *p < 0.05, **p < 0.01, compared with subordinate mice, n = 20 per group.
2.3. Warm spot test
The warm spot test in the present study (Fig. 1E) was adapted from previous methods (Šabanović et al., 2020; Zhou et al., 2017). First, each group of 4 mice was habituated to the cold cage (28 cm × 20 cm), and the floor of the cold cage was cooled to 0 °C by ice (without a warm spot) for 30 min. Second, mice were placed into a cold test cage with a round warm spot (diameter: 3 cm) heated to 34 °C; mice then competed for the warm spot for 20 min. Notably, the warm spot was located in one corner and was only large enough for one adult mouse. The warm spot test for each cage was videotaped, and the time that each mouse occupied the warm spot was recorded. After each test, the test cage was cleaned with 70% ethanol solution to remove odor, urine, and feces. The details regarding the exploratory behaviors of mice in each cage are illustrated in Fig. S2 and Video #2 (see Supplementary Materials).
2.4. Chronic restraint stress (CRS)
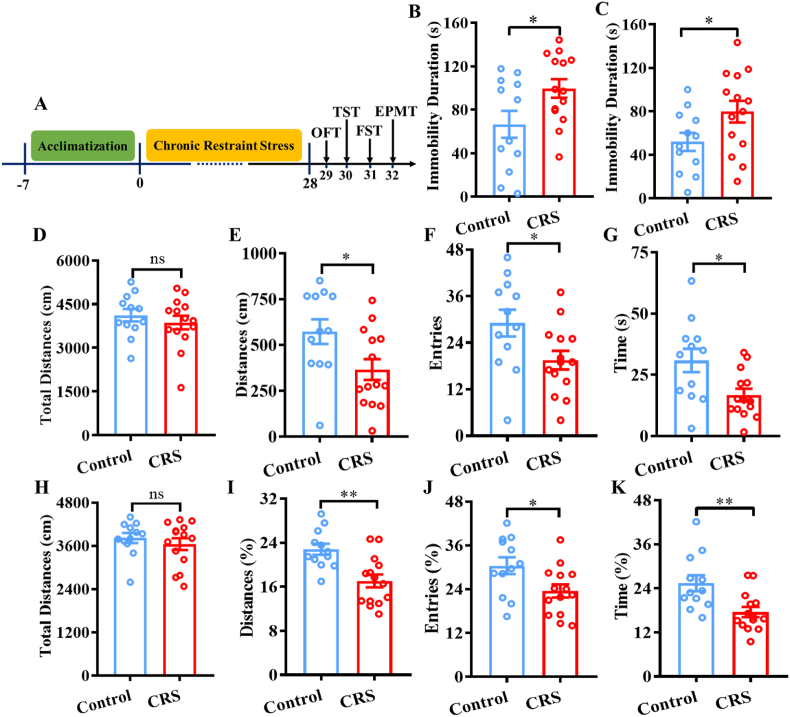
CRS was performed as previously described (Chiba et al., 2012). Mice were restrained in a 50-ml centrifuge tube (diameter: 3 cm, length: 10 cm) with some small holes in the periphery for 6 h per day (10:00–16:00) for 28 consecutive days. Mice could freely breathe in the tube but could not move forward or backward. The tail suspension test (TST), forced swim test (FST), elevated plus-maze test (EPMT), and open field test (OFT) were then carried out. The detailed timeline of behavioral tests is illustrated in Fig. 6A.
Fig. 6.
Effects of chronic restraint stress (CRS) on depression- and anxiety-like behaviors. A) Schematic drawing of the experimental timeline. B) Immobility durations in the TST of control mice and CRS mice. C) Latency to feed in the NSFT of control mice and CRS mice. D) Total distances in the OFT of control mice and CRS mice. E, F, and G) Distances, entries, and time into the center, respectively, of control mice and CRS mice. H) Total distances in the open and closed arms in the EPMT of control mice and CRS mice. I, J, and K) Percent of the distances, entries, and time into the open arms, respectively, of control mice and CRS mice. *p < 0.05, **p < 0.01, compared with control mice, n = 12–14 per group.
2.5. Open field test (OFT)
The open field apparatus consisted of a plexiglass box (60 × 60 × 16 cm), and the central area (30 × 30 cm) of the field was defined as the central zone. Mice were individually placed into a corner of the box, and their locomotor activities in the open field apparatus were videotaped for 5 min and analyzed by a video tracking system (Labmaze V3.0, Zhongshi Technology).
2.6. Elevated plus-maze test (EPMT)
The present study performed the EPMT as previously reported (Komada et al., 2008). Each mouse was placed into the center of the maze facing an open arm. The exploratory behavior of each mouse in the maze was recorded for 5 min and analyzed by a video tracking system (Labmaze V3.0, Zhongshi Technology). The maze was cleaned with 70% ethanol solution to remove odor, urine, and feces after each test.
2.7. Tail suspension test (TST)
The present study performed the TST according to previously reported methods (Cryan et al., 2005). Mice were suspended from the top of an apparatus (55 cm × 60 cm × 11.5 cm) using adhesive tape, which was placed approximately 1 cm from the tip of the tail. Mice were considered immobile only when they hung passively without moving (completely motionless). The total immobility duration in the last 4 min of a 6-min test session was recorded.
2.8. Forced swimming test (FST)
The present study performed the FST according to previously reported methods (Slattery and Cryan, 2012). Mice were placed individually into glass cylinders (height: 25 cm, diameter: 10 cm) containing 18 cm of water maintained at 24 ± 1 °C for 6 min. The total immobility duration in the last 4 min of a 6-min test session was recorded.
2.9. Novelty-suppressed feeding test (NSFT)
The present study performed the NSFT as previously described (Bodnoff et al., 1988). Each mouse was placed into one corner of an open plastic box (40 × 40 × 30 cm) with 8 pellets in the center after 24 h of food deprivation. The exploratory behavior of each mouse in the plastic box was observed for 5 min, and the latency to feed was recorded. Chewing and biting were defined as eating behaviors, and new food pellets were placed into the center after each test.
2.10. Single-unit (in vivo) recording
Mice were anesthetized with urethane (1.2 g/kg i.p.) before electrophysiological recording, and supplementary doses were given as required to maintain full general anesthesia. The mice were placed in a stereotaxic frame for implantation with a 16-channel recording electrode in the mPFC (AP: 2.43 mm; ML: 0.4–0.6 mm; DV: 1.2–1.4 mm). Spontaneous extracellular action potentials of the putative pyramidal neuron in the mPFC were recorded by using 16-channel amplifiers with 500×gain and 220–5900-Hz bandpass filters (Plexon, Dallas, TX), and the signal amplified from each electrode was digitized at a 40-kHz sampling rate. Spike sorting was performed with Offline Sorter software (Plexon, Dallas, TX) using a combination of previously described automatic and manual sorting techniques (Homayoun and Moghaddam, 2007), and all electrophysiological data were imported into NeuroExplorer (Plexon) for subsequent analysis. Spikes were identified when a minimum waveform reached an amplitude threshold of 3 standard deviations (SDs) higher than the noise amplitude (SNR >3). The valley-to-peak span of the potential waveform was calculated with MATLAB code.
2.11. Local field potential (LFP) recording
LFPs were also recorded using the data acquisition system (Plexon, Dallas, TX, USA) with a sampling rate of 1 kHz. A 16-channel silicon electrode was implanted in the mPFC (AP: 2.43 mm; ML: 0.4–0.6 mm; DV: 1.2–1.4 mm) to record the LFPs with bandpass filtering (0.1–200 Hz). All LFP data were saved and then analyzed with Neuroexplorer (Nex Technologies), and a 50-Hz notch filter was applied to remove the powerline artifact and the baseline drifts. Power spectral density (PSD) describes how the power of an LFP was distributed over frequency, the PSD was calculated using Neuroexplorer with 4096 points and Hanning (64) in the present study. The mean of all γ power recorded from 16 channels was analyzed. In addition, the γ power (30–80 Hz) was expressed as a percent of the total PSD (the sum of all powers). After in vivo recordings, electrode placement was verified by sectioning the mouse brain, and mouse data were excluded if the implantation site was incorrect.
2.12. Statistical analysis
All data in the present study were presented as the mean ± S.E.M. and were analyzed with GraphPad Prism 8.0 software. Statistical differences between the two groups were analyzed by Student's t-test (two-tailed). For all tests, p < 0.05 was considered to be statistically significant.
3. Results
3.1. Establishment and maintenance of social hierarchy status in mice
We first used the tube test to determine the social hierarchy of mice (Fig. 1B), and the results showed that a stable social hierarchy was established in each cage after 2 weeks of competition in the tube (Fig. 1C). To investigate the effects of social hierarchy status on behaviors, the present study classified the mice ranked 1 and 2 as dominant mice and the other two mice (ranked 3 and 4) as subordinate mice (Fig. 1D). In the tube test, dominant mice showed significantly more pushing (t(38) = 2.617, p < 0.05) and advancing behaviors (t(38) = 2.802, p < 0.01) than subordinate mice (Fig. 1G–H). Subordinate mice showed significantly more retreat behaviors than dominant mice (t(38) = 3.380, p < 0.01) (Fig. 1I). In the warm spot test, dominant mice spent significantly more time occupying the warm spot (t(38) = 2.961, p < 0.01) (Fig. 1F) than subordinate mice, indicating that the social hierarchy status in each cage was established and maintained after 2 weeks of tube tests.
3.2. Correlations between social rank with dominance behaviors
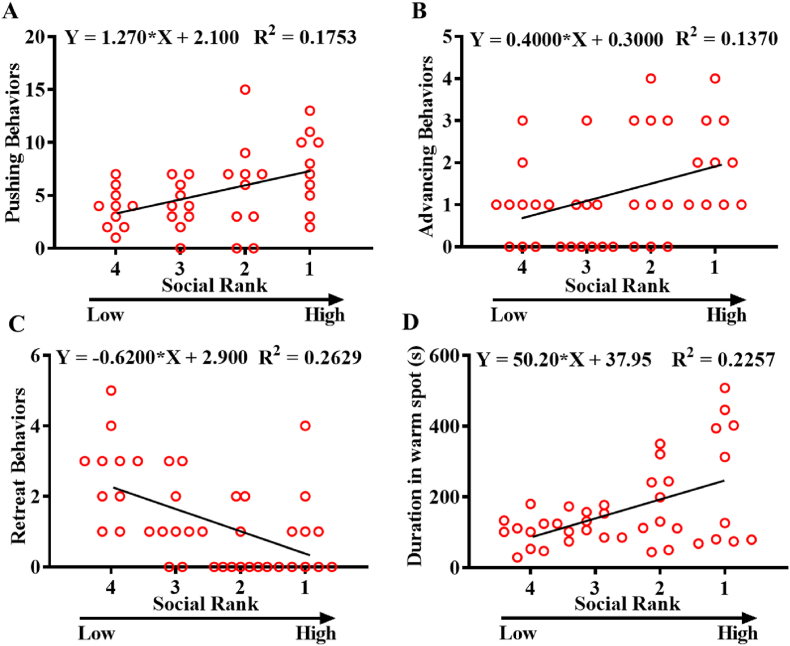
We performed a simple linear regression analysis to investigate the relationship between social rank and dominance behaviors. Pushing (F(1,38) = 8.079, p < 0.01, Fig. 2A) and advancing behaviors (F(1,38) = 6.032, p < 0.05, Fig. 2B) were positively correlated with social rank. Retreat behaviors were negatively correlated with social rank (F(1,38) = 13.56, p < 0.001, Fig. 2C). In addition, social rank was positively correlated with duration of warm spot occupation (F(1,38) = 11.08, p < 0.01, Fig. 2D). These results indicated that there was an inverse relationship between social rank and dominance behaviors.
Fig. 2.
Regression analysis between social rank with dominance behaviors. Regressions between social rank with pushing (A), advancing (B), retreat behaviors (C), and duration of warm spot occupation (D).
3.3. Involvement of firing activities of pyramidal neurons in the mPFC in social hierarchy
We then investigated the neural mechanism underlying social hierarchy in mice. Neuronal firing activity in the mPFC was recorded after a stable social hierarchy was established in each cage (Fig. 3A). The electrophysiological data showed that single units classified as putative pyramidal neurons in the mPFC had slow firing rates (0.4 Hz ∼ 3 Hz) and wide spike waveforms (Fig. S3), and the average valley-to-peak width of waveforms of the pyramidal neurons was approximately 0.5309 ± 0.01354 ms. The average value of the valleys was −4.084 ± 0.4465 mV, and the average value of the peaks was 2.829 ± 0.2128 mV (Table S1). Social hierarchy and CRS had no significant effect on the valley-to-peak width of waveforms of pyramidal neurons in the mPFC (Fig. S4). The firing rates and inter-spike intervals of action potentials described the excitability of putative pyramidal neurons in the mPFC and were analyzed in the following experiments.
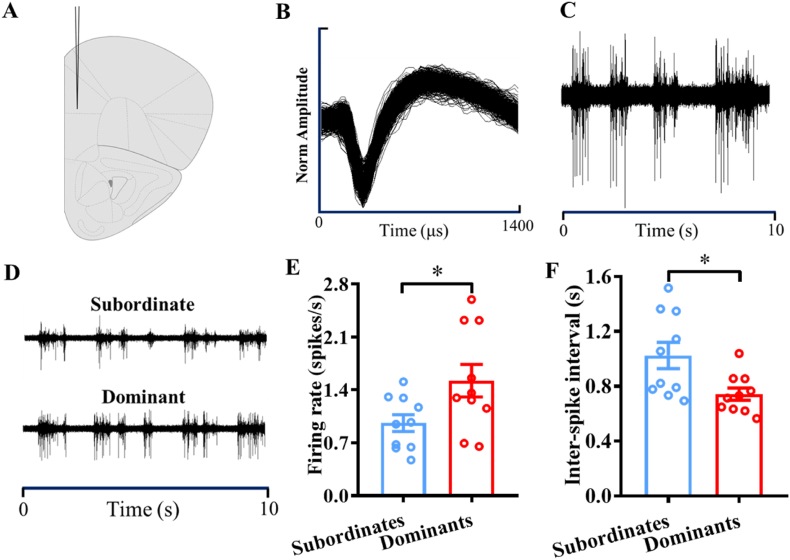
Fig. 3.
Involvement of firing activities of pyramidal neurons in the mPFC in social hierarchy. A) Diagram of electrode location in the mPFC. B) Superimposed composite of all the waveforms for the same pyramidal neuron. C) Spontaneous discharge signals of a representative pyramidal neuron. D) Comparison of the spontaneous discharge signals of representative pyramidal neurons in subordinate and dominant mice. E) Effects of social hierarchy status on the firing rates of pyramidal neurons. F) Effects of social hierarchy status on the inter-spike intervals of pyramidal neurons. *p < 0.05, compared with subordinate mice, n = 10–11 per group.
Furthermore, we investigated the changes in the firing rates and inter-spike intervals of pyramidal neurons in the mPFC. The superimposed composite of all waveforms for the same pyramidal neurons was depicted in Fig. 3B. These electrophysiological results suggested that social hierarchy significantly altered the firing activities of pyramidal neurons in the mPFC. Compared with subordinate mice, dominant mice had significantly greater firing rates of pyramidal neurons (t(18) = 2.317, p < 0.05) (Fig. 3E) and significantly lower inter-spike intervals of pyramidal neurons (t(18) = 2.663, p < 0.05) (Fig. 3F).
3.4. Associations between social hierarchy and depression-/anxiety-like behaviors
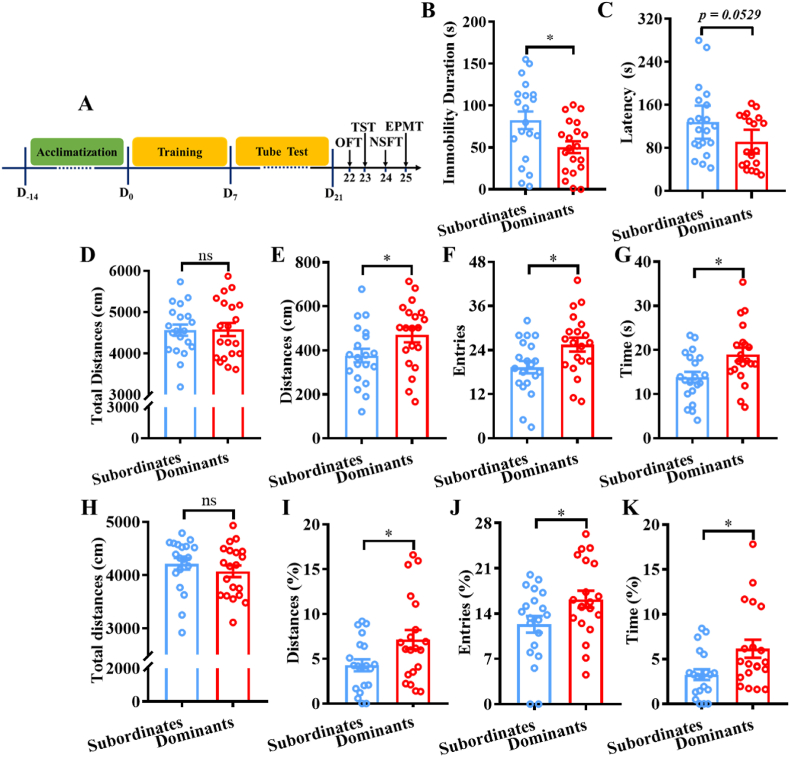
To investigate the associations between social hierarchy and depression-/anxiety-like behaviors, we performed behavioral tests after establishing a stable social hierarchy in each cage through 2 weeks of tube tests (Fig. 4A). The behavioral data suggested that social hierarchy had significant effects on depression-like behaviors in mice. Specifically, compared with dominant mice, subordinate mice had significantly greater immobility durations in the TST (t(38) = 2.517, p < 0.05) (Fig. 4B), and had slightly greater latency to feed in the NSFT (t(38) = 1.998, p = 0.0529) (Fig. 4C), which indicated that subordinate mice showed significant depression-like behaviors. In addition, the social hierarchy had significant effects on anxiety-like behaviors in mice. The OFT data showed that social hierarchy did not influence the total distances (t(38) = 0.07876, p > 0.05) (Fig. 4D). However, compared with dominant mice, subordinate mice exhibited significantly more distances into the center area (t(38) = 2.062, p < 0.05) (Fig. 4E), entries into the center area (t(38) = 2.441, p < 0.05) (Fig. 4F), and time spent in the center area (t(38) = 2.618, p < 0.05) (Fig. 4G). The EPMT data showed that social hierarchy did not influence the total distances into the open and closed arms (t(38) = 0.9133, p > 0.05) (Fig. 4H). However, compared with dominant mice, subordinate mice exhibited significantly lower percent of distances into the open arms (t(38) = 2.273, p < 0.05) (Fig. 4I), percent of entries into the open arms (t(38) = 2.094, p < 0.05) (Fig. 4J), and percent of time spent in the open arms (t(38) = 2.477, p < 0.05) (Fig. 4K). All the above results indicated that lower social hierarchy status led to depression-like and anxiety-like behaviors.
Fig. 4.
Associations between social hierarchy and depression-/anxiety-like behaviors. A) Schematic drawing of the experimental timeline. B) Immobility durations in the TST of subordinate mice and dominant mice. C) Latency to feed in the NSFT of subordinate mice and dominant mice. D) Total distances in the OFT of subordinate mice and dominant mice. E, F, and G) Distances, entries, and time into the center in the OFT, respectively, of subordinate mice and dominant mice. H) Total distances into the open and closed arms in the EPMT of subordinate mice and dominant mice. I, J, and K) Percent of the distances, entries, and time into the open arms in the EPMT, respectively, of subordinate mice and dominant mice. *p < 0.05, compared with subordinate mice, n = 20 per group.
3.5. Correlations between social dominance behaviors with depression- and anxiety-like behaviors
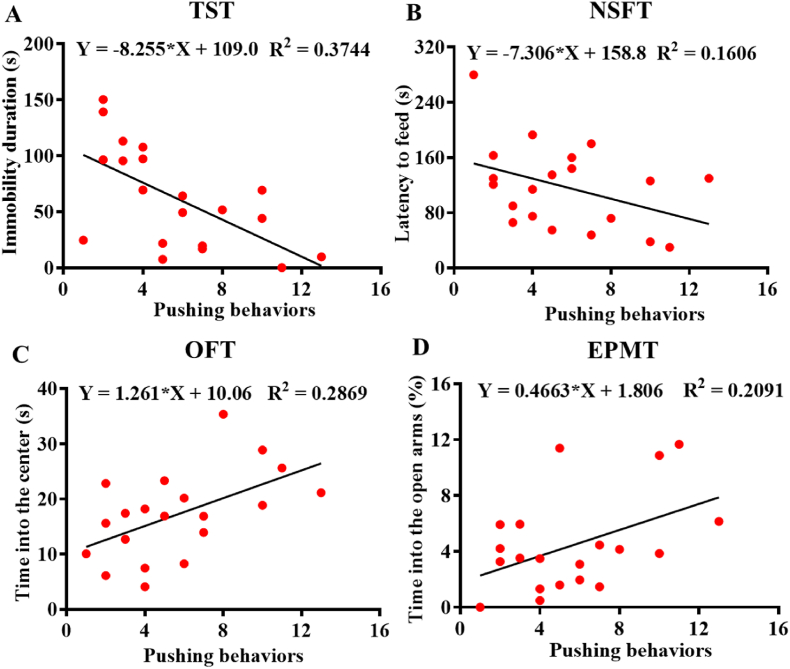
To further investigate the relationship between dominance behaviors and depression-/anxiety-like behaviors, we performed a simple linear regression analysis between pushing behaviors and depression- and anxiety-like behaviors (Fig. 5). Notably, there was a negative correlation between pushing behaviors and immobility durations in the TST (F(1,18) = 10.77, p < 0.01) (Fig. 5A). There was a weak negative correlation between pushing behaviors and latency to feed in the NSFT (F(1,18) = 3.444, p = 0.0799) (Fig. 5B). Moreover, pushing behaviors were positively correlated with the time spent in the center area in the OFT (F(1,18) = 7.244, p < 0.05) (Fig. 5C) as well as the percent of time spent in the open arms in the EPMT (F(1,18) = 4.759, p < 0.05) (Fig. 5D). These results indicated that social dominance behaviors were negatively correlated with depression- and anxiety-like behaviors. Similarly, we found that dominance behaviors were also negatively correlated with depression-/anxiety-like behaviors in the CRS model (see Fig. S7 in the Supplementary Materials).
Fig. 5.
Correlation analysis between social dominance behaviors with depression- and anxiety-like behaviors. A) Correlation between pushing behaviors and immobility durations in the TST. B) Correlation between pushing behaviors and latency to feed in the NSFT. C) Correlation between pushing behaviors and time spent in the center in the OFT. D) Correlation between pushing behaviors and the percent of time spent in the open arms in the EPMT.
3.6. Effects of chronic restraint stress on depression- and anxiety-like behaviors
We then performed a new experiment to investigate whether the social dominance behaviors were altered in the rodent model of depression. Specifically, we restrained mice for 6 h each day for 28 consecutive days for 4 weeks and then performed the tube test. Before the tube test, the depression- and anxiety-like behaviors were first evaluated in CRS mice and control mice. The behavioral data suggested that CRS had significant effects on depression-like behaviors in mice. Compared with control mice, CRS mice exhibited significantly more immobility durations in the TST (t(24) = 2.247, p < 0.05) (Fig. 6B) and in the FST (t(24) = 2.088, p < 0.05) (Fig. 6C), indicating that the CRS mice showed significant depression-like behaviors. In addition, the present results suggested that CRS also had significant effects on anxiety-like behaviors in mice. The OFT data showed that CRS did not affect the total distances (t(24) = 0.777, p > 0.05) (Fig. 6D). However, compared with control mice, CRS mice exhibited significantly more distances into the center area (t(24) = 2.379, p < 0.05) (Fig. 6E), entries into the center area (t(24) = 2.318, p < 0.05) (Fig. 6F), and time into the center area (t(24) = 2.702, p < 0.05) (Fig. 6G). The EPMT data showed that CRS did not influence the total distances into the open and closed arms (t(24) = 0.7722, p > 0.05) (Fig. 6H). However, compared with control mice, CRS mice exhibited significantly lower percent of distances into the open arms (t(24) = 3.635, p < 0.01) (Fig. 6I), percent of entries into the open arms (t(24) = 2.410, p < 0.05) (Fig. 6J), and percent of time spent in the open arms (t(24) = 3.103, p < 0.01) (Fig. 6K).
3.7. Effects of chronic restraint stress on dominance behaviors and firing activities of pyramidal neurons in the mPFC
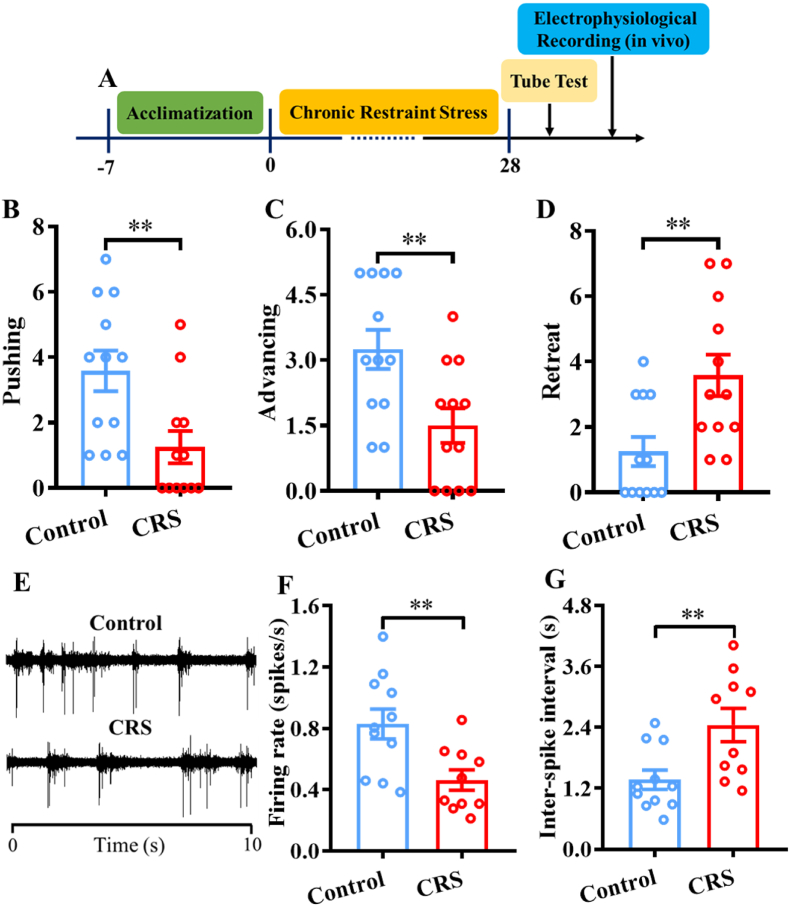
To investigate the effects of CRS on social dominant behaviors and neuronal activities, we performed the tube test and then recorded the firing activities of pyramidal neurons in the mPFC in mice exposed to 28 consecutive days of CRS (Fig. 7A). The tube test data suggested that CRS had significant effects on social dominance behaviors. Specifically, compared with control mice, CRS mice exhibited significantly fewer pushing (t(22) = 2.940, p < 0.01) and advancing behaviors (t(22) = 2.925, p < 0.01) and significantly more retreat behaviors (t(22) = 3.013, p < 0.01) (Fig. 7B–D). More importantly, the electrophysiological data suggested that CRS significantly decreased the spontaneous firing activities of pyramidal neurons in the mPFC. Specifically, compared with control mice, CRS mice exhibited significantly lower firing rates of pyramidal neurons (t(19) = 3.309, p < 0.01) (Fig. 7F) and significantly greater inter-spike intervals of pyramidal neurons (t(19) = 2.919, p < 0.01) (Fig. 7G), which indicated that CRS decreased social dominance behaviors and the excitability of pyramidal neurons in the mPFC.
Fig. 7.
Effects of chronic restraint stress (CRS) on pyramidal neurons in the mPFC. A) Schematic drawing of the experimental timeline. B, C, and D) Effects of CRS on pushing, advancing, and retreat behaviors of the control and CRS groups, respectively. E) Comparison of the spontaneous discharge signals of representative pyramidal neurons in the control and CRS groups. F) Effects of CRS on the firing rates of pyramidal neurons. G) Effects of CRS on the inter-spike intervals of pyramidal neurons. **p < 0.01, compared with control mice, n = 10–12 per group.
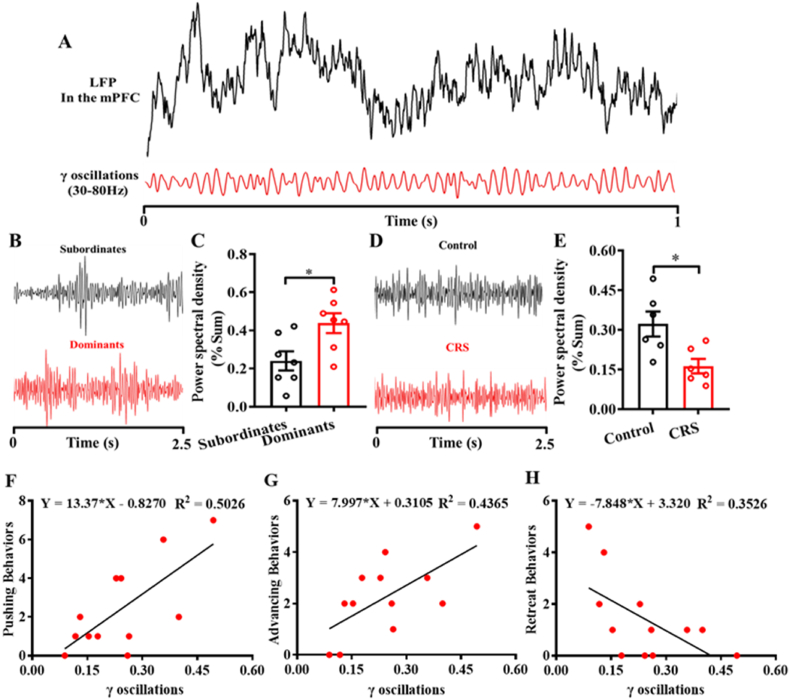
3.8. Effects of social hierarchy and chronic restraint stress on γ-oscillation activity in the mPFC
To further investigate the neural mechanism linking social hierarchy and depression/anxiety-like behaviors, we extracted the γ oscillations from the LFP in the mPFC and examined the effects of social hierarchy and CRS on γ oscillations. Surprisingly, the electrophysiological results suggested that social hierarchy significantly affected γ-oscillation activity in the mPFC, and subordinate mice had significantly lower percent of γ out of the total power spectral density (t(12) = 2.752, p < 0.05, Fig. 8C) compared with dominant mice. In addition, CRS significantly decreased γ-oscillation activity in the mPFC, and CRS mice had significantly lower percent of γ out of the total power spectral density (t(10) = 2.919, p < 0.05, Fig. 8E) compared with control mice. Moreover, to investigate the relationship between neural activities in the mPFC and dominance behaviors in the CRS study, we performed a linear regression between γ oscillations and dominance behaviors. Interestingly, pushing (F(1,10) = 10.10, p < 0.01, Fig. 8F) and advancing (F(1,10) = 7.747, p < 0.05, Fig. 8G) behaviors were positively correlated with γ oscillations, and retreat behaviors were negatively correlated with γ oscillations (F(1,10) = 5.446, p < 0.05, Fig. 8H). These results suggested that there was an inverse relationship between neural activities in the mPFC and dominance behaviors in the tube test.
Fig. 8.
Effects of social hierarchy and chronic restraint stress (CRS) on γ-oscillation activity in the mPFC. A) Raw local field potential (LFP) recorded from the mPFC and γ oscillations (30–80 Hz) extracted from the raw LFP. B) and C) Effects of social hierarchy on γ oscillations in the mPFC. D) and E) Effects of CRS on γ-oscillation activity in the mPFC. Correlation between pushing (F), advancing (G), retreat behaviors (H), and γ oscillations in the CRS study.*p < 0.05, compared with control mice or subordinate mice, n = 6–7 per group.
4. Discussion
The present study demonstrated that there were inverse relationships between social hierarchy and depression-like and anxiety-like behaviors. In addition, both low social hierarchy status and CRS decreased the firing activities of glutamatergic pyramidal neurons and γ-oscillation activity in the mPFC, which might be implicated in interactions between social hierarchy and depression/anxiety.
The present study used the tube test to evaluate the social hierarchy status in each cage; a 2-week period of inescapable confrontation in the plexiglass tube (i.e., the tube test), which might be regarded as chronic social defeat stress, seriously affected the social and mood-related behaviors of mice. Given that repeated defeat by dominant mice in the tube test might induce depression-/anxiety-like behaviors in subordinate mice, the present study performed the tube test in mice for 2 weeks. The present data suggested the time course of the tube test might play an important role in inducing depression-/anxiety-like behaviors. Intriguingly, a negative correlation was revealed between social hierarchy and depression-/anxiety-like behaviors, consistent with previous findings in humans that depression was more prevalent in individuals with lower socioeconomic status SES (Murphy et al., 1991). Thus, these findings lay an important foundation for establishing the depression/anxiety model based on the social hierarchy. In addition, the present results suggested that social hierarchy did not affect the weight of mice (see Fig. S1), which excluded the influence of weight on the social hierarchy. All the above results were of great importance for subsequent exploration of the relationship between social hierarchy and depression/anxiety as well as the underlying neural mechanisms.
On the one hand, social hierarchy status markedly affected mood-related behaviors. Social hierarchy status might be regarded as one of the most important risk factors for depression (Byrne and Bates, 2010), and social factors might be one of the most important types of factors affecting the occurrence and development of depression. In addition, a nonhuman primate study suggested that there might be a close association between social hierarchy status and huddling behaviors, a core depression-like behavior in nonhuman primate models, in macaques (Qin et al., 2013). Consistent with findings in nonhuman primates, in the present study, subordinate mice showed longer immobility durations and significantly depression-like behaviors in the TST. Interestingly, the present results were supported by a study that found subordinate mice showed significantly more depression-like behaviors in the FST (Horii et al., 2017). The present results also suggested that subordinate mice exhibited significantly more anxiety-like behaviors in the OFT and EPMT. Interestingly, a behavioral study in rodents also observed an inverse relationship between social hierarchy and anxiety-like behaviors, and subordinate mice spent significantly less time in the open arms in the EPMT and showed significantly more anxiety-like behaviors than dominant mice (Horii et al., 2017). However, the relationship between anxiety and social hierarchy seemed complicated. Another study reported that dominant mice showed significantly more anxiety-like behaviors than subordinate mice in the EPMT and OFT (Larrieu et al., 2017). As the repeated sessions involving inescapable confrontation in the plexiglass tube might have induced chronic stress, the different time courses of the tube test might account for discrepancies between the results of these two studies. Overall, we found inverse correlations between social hierarchy and depression-/anxiety-like behaviors, paving the way for future investigations of the sociobiological mechanisms underlying depression/anxiety.
On the other hand, chronic stress might be an important factor that seriously affects social behaviors. In particular, chronic stress exerts numerous pathophysiological effects on brain functions and behaviors (Sandi and Haller, 2015). In the present study, 4 weeks of CRS decreased social dominance behaviors. In addition, mice exposed to CRS for 4 weeks showed significant depression-like behaviors in the TST and FST and significant anxiety-like behaviors in the OFT and EPMT, consistent with previous studies that suggested mice exhibited depression- and anxiety-like behaviors after 4 weeks of CRS (Chiba et al., 2012; Lin et al., 2021; Liu et al., 2020). In the present study, after 4 weeks of CRS, mice showed significantly fewer pushing and advancing behaviors and more retreat behaviors than control mice, indicating that CRS significantly reduced dominance behaviors in the tube test. Consistent with these findings, another study reported that 3 weeks (3 h/day) of CRS decreased winning points in the tube test (Park et al., 2018). Similarly, chronic social defeat stress (CSDS), which was based on resident-intruder aggression, increased defensive and submissive behaviors in test mice, which behaved similarly to subordinate mice in the tube test (Krishnan et al., 2007).
Herein, the firing activities of pyramidal neurons and γ-oscillation activities in the mPFC were implicated in the mechanistic links among chronic stress, depression/anxiety, and social hierarchy. Depression and anxiety shared neural mechanisms, and the dysfunction of pyramidal neurons in the mPFC was involved in depression and anxiety (Xu et al., 2019). Many studies have reported that glutamatergic pyramidal neurons in the mPFC were involved in social and mood-related behaviors (Larsen et al., 2022; Oh et al., 2012). In the present study, the spontaneous action potentials of putative pyramidal neurons in subordinate mice significantly decreased. Similarly, another recent study found that increased firing activities of pyramidal neurons in the mPFC occurred during resistance and pushing behaviors and that activating pyramidal neurons led to more wins and reversed the social ranks of mice in the tube test (Zhou et al., 2017). Second, the spontaneous action potentials of putative pyramidal neurons significantly decreased in mice exposed to 4 weeks of CRS in the present study. Consistent with the present findings, several morphological studies suggested that chronic stress could significantly decrease the apical and basal dendrites of pyramidal neurons in the mPFC (Cook and Wellman, 2004; Radley et al., 2004). Finally, the two electrophysiological results revealed that changes in the firing of pyramidal neurons in the mPFC were similar in mice exposed to CRS and those of lower social rank, which indicated that pyramidal neurons in the mPFC might play an essential role in the social hierarchy and depression-/anxiety-like behaviors (as illustrated in Fig. 8). These findings provided promising insights into the neural mechanisms underlying social hierarchy and depression/anxiety and might pave the way for the development of pharmacological targets for addressing stress-related mood disorders.
Interestingly, neural network activity in the mPFC (e.g., γ oscillations), as found in this study, might greatly contribute to the relationship between social hierarchy and depression/anxiety. Several studies have reported that γ oscillations were closely associated with the excitatory/inhibitory (E/I) functional balance in the mPFC (Byron et al., 2021; Sohal, 2012), which is maintained by a balance of excitatory glutamatergic pyramidal neurons and inhibitory GABAergic interneurons. The rhythmic firing of γ oscillation could enhance communication between pyramidal neurons and interneurons, reinforcing the importance of the E/I balance in the effects of antipsychotic drugs, such as antidepressants (Yin et al., 2021). Consistent with these changes in the firing of pyramidal neurons in the mPFC, social hierarchy and CRS significantly affected γ-oscillation activity in the mPFC. Specifically, the γ-oscillation activity of mice with low social hierarchy status and mice exposed to CRS both significantly decreased. Consistent with these findings, Ito and his colleagues found that CRS also decreased γ power in the anterior cingulate cortex of the mPFC (Ito et al., 2020). A similar effect of chronic stress on γ power was observed in another brain region (Tomar et al., 2021). Specifically, exposure to chronic immobilization stress (CIS) for 2 h per day for 10 d decreased γ oscillation in the hippocampal CA1 subregion. Overall, the present results of altered γ oscillations and altered excitability of pyramidal neurons in the mPFC (as illustrated in the Graphical Abstract) have implications for the investigation of neural mechanisms underlying the interaction of social hierarchy and depression/anxiety.
There are a few limitations to the current work that should be considered when interpreting the results. First, to measure the order of immobility duration and social dominance, it's necessary to investigate the immobility duration in the FST before and after the tube test for the next research. Second, the direction of causation between neuronal activities in the mPFC and social hierarchy, which might be vital for the hypothesis, needs to be determined. Promisingly, optogenetic manipulation of pyramidal neurons in the mPFC and observation of whether social hierarchy will be reversed might help address this issue. Third, considering the structural similarity of the tube used for the chronic restraint stress and for the tube test, in which mice were presented with an inescapable situation and might exhibit learned helplessness (another classic model of depression), the changes in social hierarchy in the tube test after 4-week CRS could not entirely be attributed to chronic stress. To address this issue, further studies should use other rodent models, such as chronic mild unpredictable stress (CUMS) and chronic social defeated stress (CSDS), to investigate the effects of chronic stress on social hierarchy. In addition, dichotomizing in social rank in the present study might limit the explanation for the hypothesis (Altman and Royston, 2006), and mice with intermediate rank might convey a different view to investigate the neural mechanism underlying social hierarchy. Dividing mice in each cage into more ranks, and investigating neuronal firing activities in the mPFC in each rank might provide insights into understanding the neural mechanism of social hierarchy. Further electrophysiological studies are needed to collect more data to elucidate the relationship between social rank with neuronal activity in the mPFC. Finally, due to the reversible effects of CRS, further studies are needed to investigate the changes in social dominance, depression-/anxiety-like behaviors, and neuronal firing activities in the mPFC at later time points after CRS in the future.
In conclusion, the present study sheds light on the interactions between social hierarchy and depression-/anxiety-like behaviors, and these data might provide an important reference for understanding the sociobiological mechanism underlying depression/anxiety. Understanding the neural circuitry of the social hierarchy might provide new insight into the development of therapies for social stress-related clinical mood disorders.
CRediT authorship contribution statement
Yong-Yu Yin: Conceptualization, Investigation, Methodology, Validation, Formal analysis, Writing – original draft, Writing – review & editing, Visualization. Zhao-Kai Lai: Investigation, Methodology, Validation, Formal analysis, Writing – review & editing, Visualization. Jiao- Zhao Yan: Investigation, Methodology, Validation, Visualization. Qian-Qian Wei: Investigation, Methodology, Visualization. Bin Wang: Investigation, Validation, Visualization. Li-Ming Zhang: Conceptualization, Writing – review & editing, Funding acquisition. Yun-Feng Li: Conceptualization, Methodology, Writing – review & editing, Funding acquisition.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Acknowledgments
We gratefully thank Dr. Shi-Xin Lai from the School of Medicine, Sun Yat-Sen University for her advice and assistance in conducting these experiments. This work was supported by National Science and Technology Innovation 2030 Major Program (No. 2021ZD0200900) and the National Natural Science Foundation of China (No. 81773708, 82204360).
Handling Editor: Dr. Tallie Z Baram
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ynstr.2023.100536.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Adams J.D., Jr. Chemical interactions with pyramidal neurons in layer 5 of the cerebral cortex: control of pain and anxiety. Curr. Med. Chem. 2009;16(27):3476–3479. doi: 10.2174/092986709789057626. [DOI] [PubMed] [Google Scholar]
- Altman D.G., Royston P. The cost of dichotomising continuous variables. BMJ. 2006;332(7549):1080. doi: 10.1136/bmj.332.7549.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnoff S.R., Suranyi-Cadotte B., Aitken D.H., Quirion R., Meaney M.J. The effects of chronic antidepressant treatment in an animal model of anxiety. Psychopharmacology (Berl) 1988;95(3):298–302. doi: 10.1007/BF00181937. [DOI] [PubMed] [Google Scholar]
- Byrne R.W., Bates L.A. Primate social cognition: uniquely primate, uniquely social, or just unique? Neuron. 2010;65(6):815–830. doi: 10.1016/j.neuron.2010.03.010. [DOI] [PubMed] [Google Scholar]
- Byron N., Semenova A., Sakata S. Mutual interactions between brain states and alzheimer's disease pathology: a focus on gamma and slow oscillations. Biology. 2021;10(8) doi: 10.3390/biology10080707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba S., Numakawa T., Ninomiya M., Richards M.C., Wakabayashi C., Kunugi H. Chronic restraint stress causes anxiety- and depression-like behaviors, downregulates glucocorticoid receptor expression, and attenuates glutamate release induced by brain-derived neurotrophic factor in the prefrontal cortex. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2012;39(1):112–119. doi: 10.1016/j.pnpbp.2012.05.018. [DOI] [PubMed] [Google Scholar]
- Chocyk A., Majcher-Maślanka I., Dudys D., Przyborowska A., Wędzony K. Impact of early-life stress on the medial prefrontal cortex functions - a search for the pathomechanisms of anxiety and mood disorders. Pharmacol. Rep. 2013;65(6):1462–1470. doi: 10.1016/s1734-1140(13)71506-8. [DOI] [PubMed] [Google Scholar]
- Cook S.C., Wellman C.L. Chronic stress alters dendritic morphology in rat medial prefrontal cortex. J. Neurobiol. 2004;60(2):236–248. doi: 10.1002/neu.20025. [DOI] [PubMed] [Google Scholar]
- Cordero M.I., Sandi C. Stress amplifies memory for social hierarchy. Front. Neurosci. 2007;1(1):175–184. doi: 10.3389/neuro.01.1.1.013.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covington H.E., 3rd, Lobo M.K., Maze I., Vialou V., Hyman J.M., Zaman S.…Nestler E.J. Antidepressant effect of optogenetic stimulation of the medial prefrontal cortex. J. Neurosci. 2010;30(48):16082–16090. doi: 10.1523/JNEUROSCI.1731-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan J.F., Mombereau C., Vassout A. The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci. Biobehav. Rev. 2005;29(4–5):571–625. doi: 10.1016/j.neubiorev.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Déziel R.A., Tasker R.A. Bilateral ischaemic lesions of the medial prefrontal cortex are anxiogenic in the rat. Acta Neuropsychiatr. 2018;30(3):181–186. doi: 10.1017/neu.2017.32. [DOI] [PubMed] [Google Scholar]
- Dulka B.N., Bress K.S., Grizzell J.A., Cooper M.A. Social dominance modulates stress-induced neural activity in medial prefrontal cortex projections to the basolateral amygdala. Neuroscience. 2018;388:274–283. doi: 10.1016/j.neuroscience.2018.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Z., Zhu H., Zhou T., Wang S., Wu Y., Hu H. Using the tube test to measure social hierarchy in mice. Nat. Protoc. 2019;14(3):819–831. doi: 10.1038/s41596-018-0116-4. [DOI] [PubMed] [Google Scholar]
- Fitzgerald P.J., Watson B.O. Gamma oscillations as a biomarker for major depression: an emerging topic. Transl. Psychiatry. 2018;8(1):177. doi: 10.1038/s41398-018-0239-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman J.M. Comorbid depression and anxiety spectrum disorders. Depress. Anxiety. 1996;4(4):160–168. doi: 10.1002/(SICI)1520-6394(1996)4:4<160::AID-DA2>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Grosenick L., Clement T.S., Fernald R.D. Fish can infer social rank by observation alone. Nature. 2007;445(7126):429–432. doi: 10.1038/nature05511. [DOI] [PubMed] [Google Scholar]
- Harris E., Myers H., Saxena K., Mitchell-Heggs R., Kind P., Chattarji S., Morris R.G.M. Experiential modulation of social dominance in a SYNGAP1 rat model of Autism Spectrum Disorders. Eur. J. Neurosci. 2021;54(10):7733–7748. doi: 10.1111/ejn.15500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homayoun H., Moghaddam B. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J. Neurosci. 2007;27(43):11496–11500. doi: 10.1523/JNEUROSCI.2213-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horii Y., Nagasawa T., Sakakibara H., Takahashi A., Tanave A., Matsumoto Y.…Koide T. Hierarchy in the home cage affects behaviour and gene expression in group-housed C57BL/6 male mice. Sci. Rep. 2017;7(1):6991. doi: 10.1038/s41598-017-07233-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito R., Nakano T., Hojo Y., Hashizume M., Koshiba M., Murakoshi T. Chronic restraint stress affects network oscillations in the anterior cingulate cortex in mice. Neuroscience. 2020;437:172–183. doi: 10.1016/j.neuroscience.2020.04.021. [DOI] [PubMed] [Google Scholar]
- Johnson S.L., Leedom L.J., Muhtadie L. The dominance behavioral system and psychopathology: evidence from self-report, observational, and biological studies. Psychol. Bull. 2012;138(4):692–743. doi: 10.1037/a0027503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komada M., Takao K., Miyakawa T. Elevated plus maze for mice. J. Vis. Exp. 2008;22 doi: 10.3791/1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V., Han M.H., Graham D.L., Berton O., Renthal W., Russo S.J.…Nestler E.J. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131(2):391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Larrieu T., Cherix A., Duque A., Rodrigues J., Lei H., Gruetter R., Sandi C. Hierarchical status predicts behavioral vulnerability and nucleus accumbens metabolic profile following chronic social defeat stress. Curr. Biol. 2017;27(14):2202. doi: 10.1016/j.cub.2017.06.027. 2210.e2204. [DOI] [PubMed] [Google Scholar]
- Larsen N.Y., Vihrs N., Møller J., Sporring J., Tan X., Li X.…Nyengaard J.R. Layer III pyramidal cells in the prefrontal cortex reveal morphological changes in subjects with depression, schizophrenia, and suicide. Transl. Psychiatry. 2022;12(1):363. doi: 10.1038/s41398-022-02128-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S., Li Q., Jiang S., Xu Z., Jiang Y., Liu L.…Wang P. Crocetin ameliorates chronic restraint stress-induced depression-like behaviors in mice by regulating MEK/ERK pathways and gut microbiota. J. Ethnopharmacol. 2021;268 doi: 10.1016/j.jep.2020.113608. [DOI] [PubMed] [Google Scholar]
- Lindzey G., Winston H., Manosevitz M. Social dominance in inbred mouse strains. Nature. 1961;191:474–476. doi: 10.1038/191474a0. [DOI] [PubMed] [Google Scholar]
- Liu W.Z., Zhang W.H., Zheng Z.H., Zou J.X., Liu X.X., Huang S.H.…Pan B.X. Identification of a prefrontal cortex-to-amygdala pathway for chronic stress-induced anxiety. Nat. Commun. 2020;11(1):2221. doi: 10.1038/s41467-020-15920-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J.M., Olivier D.C., Monson R.R., Sobol A.M., Federman E.B., Leighton A.H. Depression and anxiety in relation to social status. A prospective epidemiologic study. Arch. Gen. Psychiatr. 1991;48(3):223–229. doi: 10.1001/archpsyc.1991.01810270035004. [DOI] [PubMed] [Google Scholar]
- Oh D.H., Son H., Hwang S., Kim S.H. Neuropathological abnormalities of astrocytes, GABAergic neurons, and pyramidal neurons in the dorsolateral prefrontal cortices of patients with major depressive disorder. Eur. Neuropsychopharmacol. 2012;22(5):330–338. doi: 10.1016/j.euroneuro.2011.09.001. [DOI] [PubMed] [Google Scholar]
- Park M.J., Seo B.A., Lee B., Shin H.S., Kang M.G. Stress-induced changes in social dominance are scaled by AMPA-type glutamate receptor phosphorylation in the medial prefrontal cortex. Sci. Rep. 2018;8(1) doi: 10.1038/s41598-018-33410-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz Y.M.C.G., Bond A.B., Kamil A.C., Balda R.P. Pinyon jays use transitive inference to predict social dominance. Nature. 2004;430(7001):778–781. doi: 10.1038/nature02723. [DOI] [PubMed] [Google Scholar]
- Qin D.D., Dominic Rizak J., Feng X.L., Chu X.X., Yang S.C., Li C.L.…Hu X.T. Social rank and cortisol among female rhesus macaques (Macaca mulatta) Dongwuxue Yanjiu. 2013;34(E2):E42–E49. doi: 10.3724/SP.J.1141.2013.E02E42. [DOI] [PubMed] [Google Scholar]
- Radley J.J., Sisti H.M., Hao J., Rocher A.B., McCall T., Hof P.R.…Morrison J.H. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience. 2004;125(1):1–6. doi: 10.1016/j.neuroscience.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Šabanović M., Liu H., Mlambo V., Aqel H., Chaudhury D. What it takes to be at the top: the interrelationship between chronic social stress and social dominance. Brain Behav. 2020;10(12) doi: 10.1002/brb3.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandi C., Haller J. Stress and the social brain: behavioural effects and neurobiological mechanisms. Nat. Rev. Neurosci. 2015;16(5):290–304. doi: 10.1038/nrn3918. [DOI] [PubMed] [Google Scholar]
- Sapolsky R.M. The influence of social hierarchy on primate health. Science. 2005;308(5722):648–652. doi: 10.1126/science.1106477. [DOI] [PubMed] [Google Scholar]
- Seo J.S., Wei J., Qin L., Kim Y., Yan Z., Greengard P. Cellular and molecular basis for stress-induced depression. Mol. Psychiatr. 2017;22(10):1440–1447. doi: 10.1038/mp.2016.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer T. The past, present and future of social neuroscience: a European perspective. Neuroimage. 2012;61(2):437–449. doi: 10.1016/j.neuroimage.2012.01.109. [DOI] [PubMed] [Google Scholar]
- Slattery D.A., Cryan J.F. Using the rat forced swim test to assess antidepressant-like activity in rodents. Nat. Protoc. 2012;7(6):1009–1014. doi: 10.1038/nprot.2012.044. [DOI] [PubMed] [Google Scholar]
- Sohal V.S. Insights into cortical oscillations arising from optogenetic studies. Biol. Psychiatr. 2012;71(12):1039–1045. doi: 10.1016/j.biopsych.2012.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomar A., Polygalov D., McHugh T.J. Differential impact of acute and chronic stress on CA1 spatial coding and gamma oscillations. Front. Behav. Neurosci. 2021;15 doi: 10.3389/fnbeh.2021.710725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Kessels H.W., Hu H. The mouse that roared: neural mechanisms of social hierarchy. Trends Neurosci. 2014;37(11):674–682. doi: 10.1016/j.tins.2014.07.005. [DOI] [PubMed] [Google Scholar]
- Wang F., Zhu J., Zhu H., Zhang Q., Lin Z., Hu H. Bidirectional control of social hierarchy by synaptic efficacy in medial prefrontal cortex. Science. 2011;334(6056):693–697. doi: 10.1126/science.1209951. [DOI] [PubMed] [Google Scholar]
- Xu H., Liu L., Tian Y., Wang J., Li J., Zheng J.…Xu H. A disinhibitory microcircuit mediates conditioned social fear in the prefrontal cortex. Neuron. 2019;102(3):668. doi: 10.1016/j.neuron.2019.02.026. -682.e665. [DOI] [PubMed] [Google Scholar]
- Xu P., Chen A., Li Y., Xing X., Lu H. Medial prefrontal cortex in neurological diseases. Physiol. Genom. 2019;51(9):432–442. doi: 10.1152/physiolgenomics.00006.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y.Y., Wang Y.H., Liu W.G., Yao J.Q., Yuan J., Li Z.H.…Li Y.F. The role of the excitation:inhibition functional balance in the mPFC in the onset of antidepressants. Neuropharmacology. 2021;191 doi: 10.1016/j.neuropharm.2021.108573. [DOI] [PubMed] [Google Scholar]
- Zhou T., Zhu H., Fan Z., Wang F., Chen Y., Liang H.…Hu H. History of winning remodels thalamo-PFC circuit to reinforce social dominance. Science. 2017;357(6347):162–168. doi: 10.1126/science.aak9726. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.