Abstract
Background
Variation in the use of treatments and hospitalization for anaphylaxis would suggest a lack of consensus in therapeutic approach.
Objective
To evaluate trends and practice variation in the emergency department (ED) care of children with anaphylaxis in a large US cohort.
Methods
We conducted a 48-site retrospective cohort study using the Pediatric Health Information System from January 2016 through September 2022. Children under 18 years with a primary diagnosis of anaphylaxis were included. Care trends were assessed using negative binomial regression modeling. Rates of medication use, hospitalizations, and revisits were reported as medians with IQRs.
Results
There were 42,909 ED visits for anaphylaxis with a 4.2% per year increase in visit incidence (95% CI 1.8, 6.7) during the study period. The median hospitalization rate was 3.5% (IQR 2.2, 6.0) and the 3-day ED revisit rate was 0.6% (IQR 0.4, 0.9). The hospital-level median use of therapies included: intramuscular epinephrine (55.3%, IQR 50.1, 59.9), systemic steroids (73.8%, IQR 63.9, 81.4), antihistamines (59.9%, IQR 53.5, 65.5), H2-receptor antagonists (56.8%, IQR 42.3, 66.2), bronchodilators (15.1%, IQR 12.5, 17.0), inhaled epinephrine (1.1%, IQR 0.6, 1.9), and fluid boluses (19.8%, IQR 11.3, 29.3). Severe reactions requiring ICU admission (1.5%, IQR 0.8, 2.2), vasopressors (0.3%, IQR 0.0, 0.6), and intubation (0.2%, IQR 0.0, 0.3) were rare.
Conclusion
ED visits for anaphylaxis increased during the study period, but hospitalization rates were low. Substantial variation exists between EDs regarding the use of anaphylaxis therapies, supporting the need for future research to evaluate the efficacy of these medications.
Keywords: anaphylaxis, antihistamines, bronchodilators, corticosteroids, emergency department, epinephrine, hospitalizations, variation, trends
Introduction
Anaphylaxis is a serious, potentially life-threatening allergic reaction with an apparent rising US and global burden.1,2 From 2008–2016 there were over 400,000 emergency department (ED) visits for anaphylaxis in the US,3 during which time visits doubled among all patient ages and tripled in children.3 These visits are associated with long ED observation periods, potential hospitalizations, and undue burdens on patients and families including missed work and school and healthcare bills.4–6
Epinephrine is the first-line treatment,7 with delayed administration identified as a risk factor for biphasic reactions.8–10 Beyond the use of epinephrine, clinicians frequently use adjunctive therapies to manage reactions.7 These include antihistamines, H2-receptor antagonists, inhaled bronchodilators, intravenous (IV) fluid boluses and systemic corticosteroids, which have a theoretical role in improving symptoms and preventing biphasic reactions.11,12 We reported that among 10,351 children presenting to pediatric EDs with anaphylaxis from 2009–2013, the use of adjunctive therapies was common: steroids − 71%, antihistamines − 60%, and H2-receptor antagonists − 53%. In 2020, a new anaphylaxis practice parameter was published by the Joint Task Force on Practice Parameters that suggested “against administering glucocorticoids or antihistamines as an intervention to prevent biphasic anaphylaxis,” while stipulating that the certainty of evidence for this recommendation was very low.13
Based on this parameter, we sought to conduct a follow-up study to evaluate more recent trends and variation in the ED care of children with anaphylaxis across US children’s hospitals.
Methods
Study design
We conducted a 48-site, retrospective cohort study of children with anaphylaxis in pediatric EDs from January 2016 through September 2022 using the Pediatric Health Information System (PHIS) database (Children’s Hospital Association, Overland Park, KS). PHIS contains clinical and resource utilization data for inpatient, ambulatory surgery, ED, and observation unit encounters from US children’s hospitals. For this study, we evaluated data from the 48 hospitals with complete data during the study period. The study was approved by the Boston Children’s Hospital institutional review board.
Eligibility criteria and case ascertainment
Children less than 18 years of age were eligible for inclusion who presented to a PHIS ED during the study period with a primary International Classification of Disease, 10th revision, Clinical Modification (ICD-10) code for anaphylaxis. Codes included T88.6XXA (anaphylactic reaction due to adverse effect of correct drug or medicament properly administered, initial encounter), T78.08XA (anaphylactic reaction due to eggs, initial encounter), T78.06XA (anaphylactic reaction due to food additives, initial encounter), T78.04XA (anaphylactic reaction due to fruits and vegetables, initial encounter), T78.07XA (anaphylactic reaction due to milk and dairy products, initial encounter), T78.03XA (anaphylactic reaction due to other fish, initial encounter), T78.09XA (anaphylactic reaction due to other food products, initial encounter), T80.59XA (anaphylactic reaction due to other serum, initial encounter), T78.01XA (anaphylactic reaction due to peanuts, initial encounter), T78.02XA (anaphylactic reaction due to shellfish (crustaceans), initial encounter), T78.05XA (anaphylactic reaction due to tree nuts and seeds, initial encounter), T78.00XA (anaphylactic reaction due to unspecified food, initial encounter), T80.52XA (anaphylactic reaction due to vaccination, initial encounter), T78.2XXA (anaphylactic shock, unspecified, initial encounter).14 Encounters with a diagnosis of mast cell activation disorder/mast cell neoplasms were excluded. For patients with multiple ED visits for anaphylaxis within 3 days, the first ED visit was classified as the index encounter and the subsequent encounter was classified as a revisit. Patients with multiple ED encounters during the study period were included in all analyses.
Data elements
Data elements included demographics (age, sex, race, ethnicity, insurance type [private vs. public]), the reaction trigger (based on primary diagnosis code which may underestimate the true incidence of reaction specific triggers and does not account for other anaphylaxis triggers and comorbidities including oral food challenges or oral immunotherapy), and ED medications/therapies: intramuscular (IM) epinephrine, systemic corticosteroids, inhaled bronchodilators, inhaled (racemic) epinephrine, antihistamines, H2-receptor antagonists, IV fluid boluses, vasopressors (IV epinephrine, dopamine, norepinephrine), and intubation. Other data elements included final ED disposition (ED discharge, admission, intensive care unit [ICU] admission) and 3-day ED revisits for anaphylaxis among patients discharged from the ED during the index encounter. Epinephrine is commonly given at home, school, or by prehospital emergency medical personnel. Because of the lack of pre-ED data, we could not assess pre-ED medication usage and thus could not report on true overall epinephrine usage in the cohort. PHIS does not account for whether reactions were initial or subsequent, whether patients follow with allergists, and what medications or referrals were placed at ED discharge. Thus, we could not determine the proportion of patients discharged with epinephrine auto-injectors or referred for allergist follow-up, both of which are standards of care.13
Risk factors for severe reactions
We also sought to evaluate risk factors for severe reactions, defined a priori as any of the following: ICU admission, receipt of vasopressors, or intubation. Candidate clinical predictors were based on biologic plausibility as well as previously identified risk factors for severe, refractory or biphasic anaphylaxis.4,5,9,15,16 However, given PHIS is an administrative database, we only included data elements that we could access and thus could not include other variables previously shown to modify the risk of severe reactions (e.g., hypotension, history of uncontrolled asthma, wheezing). Candidate predictor variables included: age (0–3 years, 4–10 years, ≥ 11 years), sex, trigger (food, non-food, unknown) and therapies (systemic steroids, inhaled bronchodilators, inhaled epinephrine, antihistamines, H2-receptor antagonists, and IV fluid boluses).
Data analysis
Medians and IQRs or counts with proportions were used to report demographic and reaction trigger details. Negative binomial regression was used to evaluate trends in ED visit counts for anaphylaxis during the study period; visit count was the dependent variable and year was the independent variable. Medians and IQRs were used to evaluate between-ED hospitalization rates, ICU admissions, and 3-day ED revisits. To evaluate extent to which increases in hospitalizations were associated with a lower risk of revisits, we measured the Pearson correlation coefficient across EDs. Medians and IQRs along with box plots were used to report the between-ED proportions of patients who received each anaphylaxis medications and intubation. We reported trends in the use of admissions and anaphylaxis treatments using logistic regression. The dependent variable was receipt of the intervention, and the independent variable was year. We adjusted confidence intervals using robust standard errors clustering on site. These trends were reported in terms of the relative change per year (e.g. by reporting an odds ratio of 0.85 as −15%). Multivariable logistic regression was used to evaluate the associations between candidate predictors and the composite outcome of severe reactions, which were reported as adjusted ORs with corresponding 95% confidence intervals (CIs).All statistics were calculated using R version 4.2.0. While we did not report p values for comparisons or trends, we used 95% confidence intervals as the measure of precision.
Results
From 2016 to 2022, there 42,909 encounters for anaphylaxis across 48 pediatric EDs. See Table 1 for demographics and reaction triggers. The median patient age was 6.9 years (IQR 2.5, 12.7), 39.8% of encounters had public insurance, and based on ICD-10 codes foods were the most common trigger (55.5%) followed by unspecific trigger (44.1%) and medications (2.8%). Figure 1 shows trends in ED anaphylaxis visits and admissions during the study period, with visits increasing by 4.2% per year (95% CI 1.8, 6.7). There was a 38.5% decline in ED visits (95% CI −42.9, −33.8) from the pre-pandemic period peak of 1,841 visits (quarter 2 of 2019) to the pandemic low of 1,132 visits (quarter 2 of 2020); however, visits have since returned to pre-pandemic levels. Over the 6 years, there were 1,793 hospitalizations, and 716 children (1.7%) were admitted to the ICU. The median hospitalization rate was 3.5 % (IQR 2.2, 6.0) and there was a 13.8% relative decrease (IQR −17.9, −9.5) in hospitalization rate per year during the study period. Severe, potentially life-threatening reactions were uncommon based on surrogate measures of severe reactions, namely 0.2% (n=77) and 0.5% (n=197) of patients were intubated or received vasopressors respectively. There were 6 fatalities reported during the study period and 0.6 % (IQR 0.4, 0.9) of patients discharged from the ED during the index encounter had a 3-day ED revisit for anaphylaxis of which 11.4% (n=31) were hospitalized. See Table 2 for a summary of the 6 fatalities. Across EDs, hospitalizations and revisit rates were not correlated (Pearson correlation coefficient −0.05, 95% CI −0.33, 0.23).
Table 1.
Demographics and reaction triggers
| Characteristics | All patients (N =42909), n (%) |
|---|---|
| Age in years, median (IQR) | 6.9 (2.5, 12.7) |
| Male | 24031 (56.0) |
| Race and ethnicity | |
| White | 17694 (41.2) |
| Black | 11033 (25.7) |
| Hispanic | 6736 (15.7) |
| Asian | 2858 (6.7) |
| Other | 3733 (8.7) |
| Insurance type | |
| Private | 23761 (56.2) |
| Public | 16831 (39.8) |
| Other | 1711 (4.0) |
| Trigger (based on ICD code) | |
| Unspecified | 18914 (44.1) |
| Foods | |
| Tree nuts and seeds | 5403 (12.6) |
| Peanuts | 5305 (12.4) |
| Other food products | 3616 (8.4) |
| Unspecified food | 2618 (6.1) |
| Eggs | 1766 (4.1) |
| Milk and dairy products | 1272 (3.0) |
| Other fish | 925 (2.2) |
| Shellfish | 943 (2.2) |
| Fruits and vegetables | 642 (1.5) |
| Food additives | 63 (0.1) |
| Drug/medication | 1190 (2.8) |
| Vaccination | 128 (0.3) |
| Other serum | 81 (0.2) |
| Blood/products | 43 (0.1) |
Figure 1:
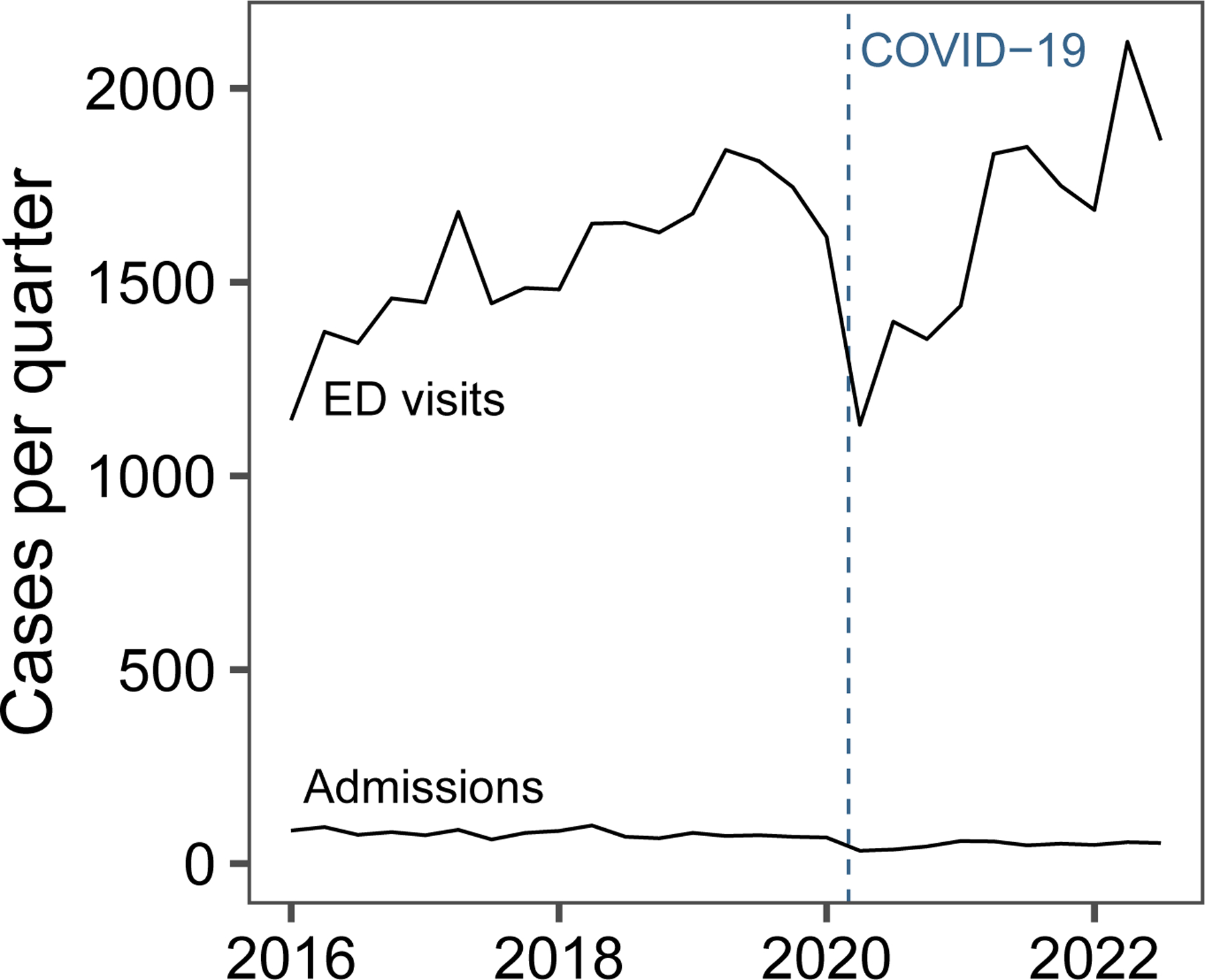
Trends in pediatric ED visits and hospitalizations for anaphylaxis from 2016–2022 across 48 hospitals.
Table 2:
Anaphylaxis fatalities
| Patient characteristics | |||||
|---|---|---|---|---|---|
| Age (years) | Sex | Race | Insurance | ICD-10 Trigger | |
| Patient | |||||
| Patient 1 | 3 | Male | White | Private | Food |
| Patient 2 | 5 | Male | White | Private | Food |
| Patient 3 | 7 | Male | Black | Public | Food |
| Patient 4 | 9 | Female | Black | Private | Unknown |
| Patient 5 | 13 | Female | White | Public | Food |
| Patient 6 | 15 | Male | Black | Public | Non-food |
Table 3 shows the proportion of patients who received each anaphylaxis therapy, the median and IQR of medication use among the included hospitals, and the per-year trend in medication use over the study period. Figure 2 is a box plot showing the qualitatively wide variability in medication usage between EDs. Figure 3 depicts changes in medication use from 2016–2022. The median rate of IM epinephrine administration in the ED was 55.3% (IQR 501, 59.9). Notably, the median rate of corticosteroid use was 73.8% (IQR 63.9, 81.4, range 28.2, 89.9) and there was a 19.6% (IQR −26.3, −12.4) yearly decline in usage over the study period. The median use of antihistamines was 59.9% (IQR 53.5, 65.5), while 56.8% (IQR 42.3, 66.2) and 19.8% (IQR 11.3, 29.3) of patients received H2-receptor antagonists and IV fluid boluses respectively.
Table 3.
Therapies, admission rates, and 3-day ED revisits
| Characteristics | n (%) N= 42909 | Median (IQR) | Per year change |
|---|---|---|---|
| Therapies | |||
| IM epinephrine | 22846 (53.2) | 55.3 (50.1, 59.9) | −0.2 (−3.8, 3.5) |
| Corticosteroids | 28125 (65.5) | 73.8 (63.9, 81.4) | −19.6 (−26.3, −12.4) |
| Antihistamines | 24527 (57.2) | 59.9 (53.5, 65.5) | −5.9 (−9.3, −2.5) |
| H2 antagonists | 21419 (49.9) | 56.8 (42.3, 66.2) | −14.3 (−20.6, −7.5) |
| Bronchodilators | 6256 (14.6) | 15.1 (12.5, 17.0) | −11.3 (−13.7, −8.9) |
| Racemic epinephrine | 640 (1.5) | 1.1 (0.6, 1.9) | −8.5 (−13.2, −3.5) |
| IV fluid bolus | 8315 (19.4) | 19.8 (11.3, 29.3) | −12.0 (−17.3, −6.4) |
| Intubation | 77 (0.2) | 0.2 (0.0, 0.3) | −4.7 (−14.3, 6.1) |
| Vasopressor | 197 (0.5) | 0.3 (0.0, 0.6) | −2.6 (−14.5, 11.0) |
| ED-disposition | |||
| Admission | 1793 (4.2) | 3.5 (2.2, 6.0) | −13.8 (−17.9, −9.5) |
| ICU admission | 716 (1.7) | 1.5 (0.8, 2.2) | −5.7 (−11.0, −0.2) |
| Fatalities | 6 (0.0) | -- | -- |
| 3-day ED revisit * | 272 (0.7) | 0.6 (0.4, 0.9) | −1.6 (−8.6, 5.9) |
The denominator for calculating ED revisits for anaphylaxis was 41,116 because revisits were only assessed for patients discharged from the ED during the index encounter and did not include patients hospitalized during the index encounter.
Figure 2:

Use of specific medications among children with anaphylaxis across 48 pediatric hospitals. Quartile coefficients of dispersion are shown for each therapy. Antihistamine (e.g., diphenhydramine, cetirizine); Glucocorticoid = systemic corticosteroids (e.g., prednisolone/prednisone, dexamethasone, methylprednisolone); H2-blocker = H2-receptor antagonists; IV fluid = IV fluid bolus; Bronchodilator = inhaled bronchodilators; inhaled epi = inhaled (racemic) epinephrine.
Figure 3:
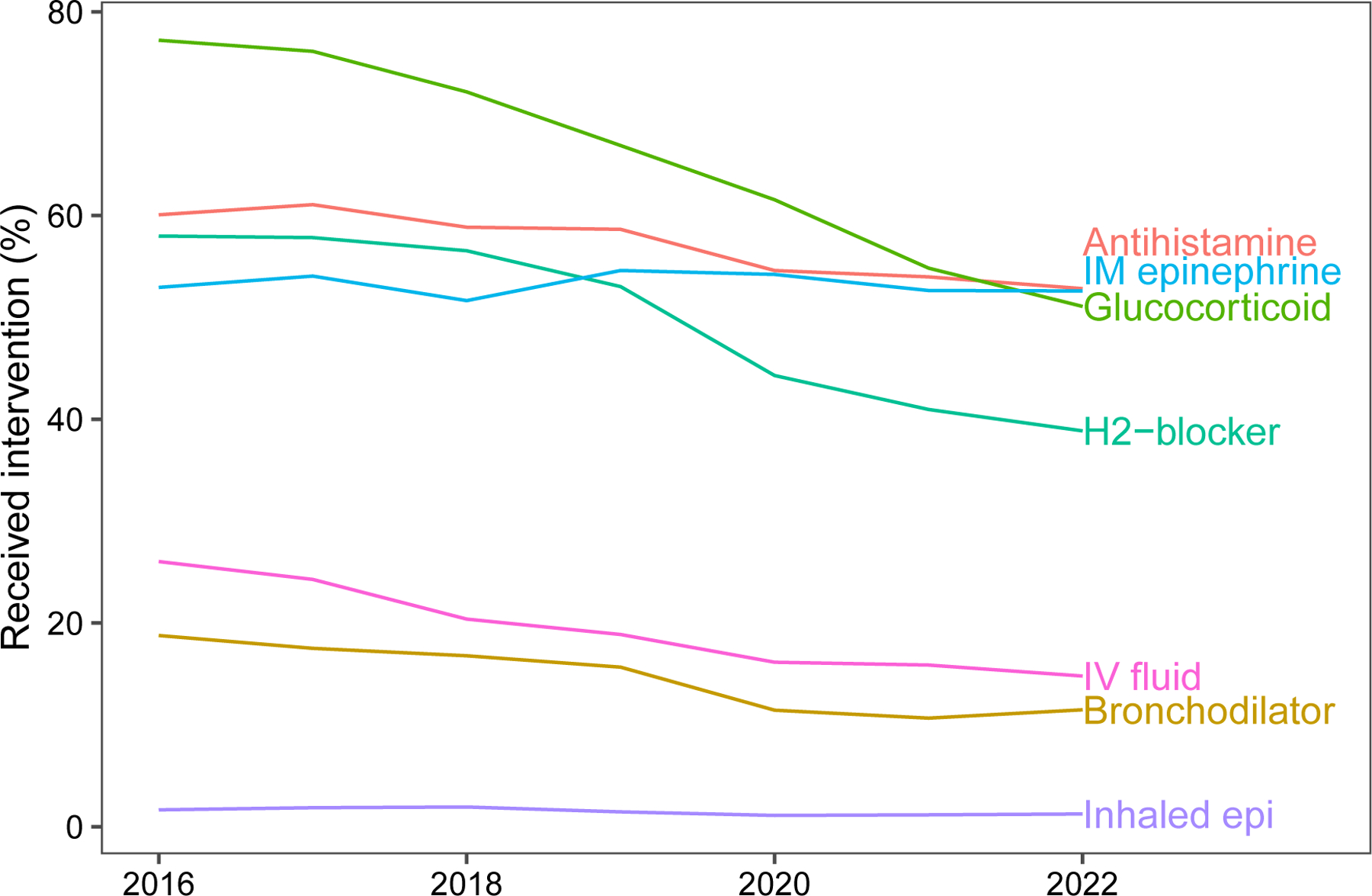
Trends in the use of anaphylaxis therapies from 2016 to 2022 across 48 pediatric hospitals. Glucocorticoid = systemic corticosteroids (e.g., prednisolone/prednisone, dexamethasone, methylprednisolone); Antihistamine (e.g., diphenhydramine, cetirizine); H2-blocker = H2-receptor antagonists; IV fluid = IV fluid bolus; Bronchodilator = inhaled bronchodilators; inhaled epi = inhaled (racemic) epinephrine; Vasopressor (e.g., dopamine, epinephrine, norepinephrine).
Risk factors for severe reactions
In the multivariable model (Table 4), risk factors for severe reactions included patients > 10 years of age (OR 1.4 [IQR 1.2, 1.8]), non-food trigger (OR 2.5 [IQR 2.0, 3.2]), and use of antihistamines (OR 1.9 [IQR 1.5, 2.4]), H2-receptor antagonists (OR 2.8 [2.2, 3.6]), inhaled bronchodilators (OR 3.4 [2.9, 3.9]), inhaled racemic epinephrine (OR 4.5 [IQR 3.5, 5.8]), glucocorticoids (OR 1.9 [1.4, 2.6]), or IV fluid boluses (OR 7.7 [6.3, 9.4]). Interestingly, unknown trigger was protective against severe reactions (OR 0.5 [0.4, 0.6]).
Table 4.
Risk factors for severe reactions, multivariate model
| Candidate risk factor | OR (95% CI) |
|---|---|
| Age ≥ 11 years | 1.4 (1.2, 1.8) |
| Trigger | |
| Non-food | 2.5 (2.0, 3.2) |
| Unknown | 0.5 (0.4, 0.6) |
| Therapies | |
| Systemic steroids | 1.9 (1.4, 2.6) |
| Inhaled bronchodilators | 3.4 (2.9, 3.9) |
| Inhaled epinephrine | 4.5 (3.5, 5.8) |
| Antihistamines | 1.9 (1.5, 2.4) |
| H2-receptor antagonists | 2.8 (2.2, 3.6) |
| IV fluid boluses | 7.7 (6.3, 9.4) |
Discussion
We conducted the largest study to date evaluating trends and variation in the care of more than 42,000 children with anaphylaxis in the US. From 2016 to 2022, ED visits for anaphylaxis increased by 4.2% per year with a median hospitalization rate of 3.5% (IQR 2.2, 6.0). We hypothesized that COVID-19 would have resulted in a reduction in ED visits for anaphylaxis secondary to a decline in accidental food exposures (the most common cause of anaphylaxis in children) in schools, restaurants, or travel because of lockdowns and other COVID-19 restrictions. Our findings support this hypothesis as ED visits declined significantly at the start of the pandemic but have since returned to pre-pandemic levels.
This study builds upon prior research showing that pediatric ED visits for anaphylaxis tripled during the past decade as well as a 2016 PHIS study that showed significant variation in the use of anaphylaxis therapies and hospitalization rates.3,17 From 2009 to 2013, hospitalization rates ranged from 12% to 95% with a median rate of 41%. In contrast, in the present study there was a significant decline in hospitalization rates, which may reflect a shift in national practice based on emerging data that anaphylaxis hospitalizations can be safely reduced without a resultant increase in ED-revisits.5,11,18,19 However, the decline in hospitalization rates must be viewed in the context of increasing anaphylaxis visits nationally, an increase that appears to be driven by a rise in non-severe cases as evident by the low admission rate or need for ICU-level care among patients in the cohort.
The 2016 PHIS study reported high rates of adjunctive anaphylaxis therapy use (systemic steroids − 75%, antihistamines − 60%, H2-receptor antagonists − 53%, IV fluids − 26%) which is consistent with findings from the present study. Interestingly, although systemic steroid use declined from 2016 to 2022, the median steroid use rate remained high at 73.8%. These findings are consistent with data from the European Anaphylaxis Registry (2007–2015; n= 1,970) in which 82% of children with anaphylaxis received corticosteroids, 76% antihistamines, and 23% β2-agonists.20
Data from the present study and the European registry are incongruous with recommendations made in the 2020 anaphylaxis practice parameter that advises “against administering glucocorticoids or antihistamines as an intervention to prevent biphasic anaphylaxis.”13 However, these recommendations are based on low quality evidence from predominantly small, retrospective studies with significant biases including confounding by severity.12,13,21 Practically, it is difficult to reduce corticosteroid usage given the perceived therapeutic benefit, because steroids are viewed as safe (despite more recent evidence showing that a single corticosteroid burst is associated with adverse events in children)22 and inexpensive, and because clinicians have considerable experience using steroids to treat other pediatric conditions including croup and asthma.23,24 Furthermore, although there is not high-quality data supporting the use of corticosteroids there is equally poor data refuting their efficacy.25 As such, there is a need for a definitive RCT to evaluate the efficacy of systemic steroids to treat anaphylaxis and prevent biphasic reactions.26 We anticipate that without such a RCT it will be challenging to reduce practice variation.
Contributors to practice variation related to the use of antihistamines, inhaled bronchodilators, and IV fluid boluses is difficult to extrapolate from PHIS given the database does not include data about symptoms, examination findings, and reaction courses. Given anaphylaxis is heterogenous condition with variable phenotypic responses and severity,27 it is expected that there will be treatment variation based on the presence/absence of symptoms that may require targeted treatments beyond the use of IM epinephrine. This includes administering antihistamines to manage pruritus, urticaria, and angioedema, inhaled bronchodilators for patients with wheezing or respiratory distress, and IV fluid boluses for those with dizziness, hypotension, or collapse. Although targeted therapeutic strategies to manage specific symptoms and examination findings is clinically appropriate, one-size-fits all management strategies in which all patients receive antihistamines, bronchodilators, steroids, and IV fluids independent of reaction characteristics is ill-advised. This “kitchen-sink” approach contributes to medication overuse for therapies that lack requisite efficacy data and may lead to epinephrine underuse for clinicians attempting to manage reactions with second-line therapies. There is opportunity to employ quality improvement methodology to improve provider adherence to practice guidelines. Such an approach has been used to reduce anaphylaxis hospitalizations and likely would be an effective strategy to standardize care specific to the use of adjunctive anaphylaxis therapies.11
Lastly, our data reinforce the fact that although anaphylaxis is potentially life-threatening, most reactions are safely managed in the ED without the need for inpatient or ICU care. This is reflected in the low hospitalization rate in the cohort and the infrequent use of high-acuity resuscitative efforts including vasopressors or intubation. Although we could not account for all previously identified risk factors for severe reactions given the limitations of the PHIS database, we found that severe reactions were more common among older children as well as those with non-food triggers. Patients with severe reactions were also more likely to receive common anaphylaxis therapies including antihistamines, steroids, bronchodilators, and fluid boluses. These therapies are likely surrogate measures of severe signs/symptoms that ultimately fulfilled our criteria for severe reactions instead of being independent risk factors for adverse outcomes.
Limitations
PHIS is an administrative database and thus does not include clinical information specific symptoms and examination findings. Thus, we cannot account for the association between reaction characteristics and treatment variation in the cohort. Additionally, because cases were identified using ICD-10 codes, we may have missed cases of anaphylaxis which were not assigned the appropriate ICD code. However, this should not impact our findings given our primary objective was to assess care trends and variation in the use of anaphylaxis therapies. Furthermore, we could not evaluate the overall rate of epinephrine use in the cohort given the database does not include medications administered prior to ED arrival.9,28 These limitations reinforce the need for a US registry akin to the European registry that includes robust pre-ED data to evaluate the epidemiology of anaphylaxis including care trends and outcomes (persistent, refractory, biphasic anaphylaxis) over time.29 We are hopeful that ongoing global adoption of ICD-11 will allow for improved epidemiologic surveillance data of anaphylaxis triggers and outcomes which may in turn result in practice and/or policy changes to mitigate the societal impact of anaphylaxis.30 Next, despite a low 3-day ED revisit rate, we cannot account for patients who returned to other EDs or care settings including urgent cares or primary care offices. Lastly, PHIS data are limited to pediatric hospitals and thus our findings may not be generalizable to the care of children with anaphylaxis managed in other EDs including patients cared for in rural settings.
Conclusions
Pediatric ED visits to children’s hospitals for anaphylaxis increased by 4.2% per year from 2016–2022, during which time there was variation in the use of adjunctive anaphylaxis therapies. 73.8% of patients received corticosteroids and 59.9% received antihistamines. RCTs are needed to evaluate the efficacy of corticosteroids and other anaphylaxis therapies and to reduce practice variation and optimize patient outcomes.
Highlights box.
1. What is already known about this topic?
Prior data suggest variation in the therapeutic approach for children with anaphylaxis in US pediatric emergency departments.
2. What does this article add to our knowledge?
ED visits for anaphylaxis increased during the study period, but hospitalization rates were low. Substantial variation exists between EDs regarding the use of anaphylaxis therapies.
3. How does this study impact current management guidelines?
Findings from this study conflict with existing practice guidelines that advise against using corticosteroids to treat anaphylaxis and/or prevent biphasic reactions. Randomized controlled trials are needed to evaluate the efficacy of adjunctive anaphylaxis therapies.
Funding:
Division of Emergency Medicine, Cincinnati Children’s Hospital Medical Center. The project described was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health, under Award Number 2UL1TR001425- 05A1. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The project described was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health, under Award Number 2KL2TR001426-05A1. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Dr. Michelson received funding through award K08HS026503 from the Agency for Healthcare Research and Quality.
Abbreviations:
- ED
Emergency Department
- ICD-10
International Classification of Disease, 10th revision, Clinical Modification
- ICU
Intensive Care Unit
- IM
intramuscular
- IV
Intravenous
- PHIS
Pediatric Health Information System
- RCT
Randomized Controlled Trial
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of potential conflict of interest: H. A. Sampson receives funding to his institution for grants from the NIH/NIAID and is employed part-time by and has received stock options from DBV Technologies. T. E. Dribin, M. I. Neuman, D. Schnadower, J. Porter, K.A. Michelson report no conflicts of interest, no other relationships, or activities that could appear to have influenced the submitted work.
References
- 1.Sampson HA, Muñoz-Furlong A, Campbell RL, Adkinson NF, Bock SA, Branum A, et al. Second symposium on the definition and management of anaphylaxis: Summary report—Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol. 2006;117(2):391–7. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0091674905027235 [DOI] [PubMed] [Google Scholar]
- 2.Turner PJ, Campbell DE, Motosue MS, Campbell RL. Global Trends in Anaphylaxis Epidemiology and Clinical Implications. J Allergy Clin Immunol Pract. 2020;8(4):1169–76. Available from: https://www.sciencedirect.com/science/article/pii/S2213219819309675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Michelson KA, Dribin TE, Vyles D, Neuman MI. Trends in emergency care for anaphylaxis. J Allergy Clin Immunol Pract. 2020;8(2):767–768.e2. [DOI] [PubMed] [Google Scholar]
- 4.Dribin TE, Michelson KA, Monuteaux MC, Schnadower D, Neuman MI. Timing and predictors of repeat epinephrine administration among children hospitalized for anaphylaxis. J Allergy Clin Immunol Pract. 2020;8:1400–1402.e2. [DOI] [PubMed] [Google Scholar]
- 5.Dribin TE, Michelson KA, Michael CM, Stack AM, Farbman KS, Schneider LC, et al. Identification of children with anaphylaxis at low risk of receiving acute inpatient therapies. PLoS One. 2019;14(2):e0211949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bilaver LA, Chadha AS, Doshi P, O’Dweyer L, Gupta RS. Economic burden of food allergy- A systematic review. Ann Allergy, Asthma Immunol. 2019;122(4):373–80. [DOI] [PubMed] [Google Scholar]
- 7.Campbell RL, Li JTC, Nicklas RA, Sadosty AT. Emergency department diagnosis and treatment of anaphylaxis: a practice parameter. Ann Allergy, Asthma Immunol. 2014;113(6):599–608. Available from: https://www.sciencedirect.com/science/article/pii/S1081120614007431 [DOI] [PubMed] [Google Scholar]
- 8.Alqurashi W, Stiell I, Chan K, Neto G, Alsadoon A, Wells G. Epidemiology and clinical predictors of biphasic reactions in children with anaphylaxis. Ann Allergy, Asthma Immunol [Internet]. 2015;115(3):217–223.e2. Available from: https://www.sciencedirect.com/science/article/pii/S1081120615003385 [DOI] [PubMed] [Google Scholar]
- 9.Lee S, Bellolio MF, Hess EP, Erwin P, Murad MH, Campbell RL. Time of Onset and Predictors of Biphasic Anaphylactic Reactions: A Systematic Review and Meta-analysis. J Allergy Clin Immunol Pract. 2015;3(3):408–416.e2. Available from: https://www.sciencedirect.com/science/article/pii/S221321981400645X [DOI] [PubMed] [Google Scholar]
- 10.Lee JM, Greenes DS. Biphasic Anaphylactic Reactions in Pediatrics. Pediatrics. 2000. Oct 1;106(4):762–6. Available from: 10.1542/peds.106.4.762 [DOI] [PubMed] [Google Scholar]
- 11.Farbman KS, Michelson KA, Neuman MI, Dribin TE, Schneider LC, Stack AM. Reducing hospitalization rates for children with anaphylaxis. Pediatrics. 2017;139(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alqurashi W, Ellis AK. Do Corticosteroids Prevent Biphasic Anaphylaxis? J Allergy Clin Immunol Pract. 2017;5(5):1194–205. Available from: https://www.sciencedirect.com/science/article/pii/S2213219817303859 [DOI] [PubMed] [Google Scholar]
- 13.Shaker MS, Wallace DV, Golden DBK, Oppenheimer J, Bernstein JA, Campbell RL, et al. Anaphylaxis--a 2020 practice parameter update, systematic review, and Grading of Recommendations, Assessment, Development and Evaluation (GRADE) analysis. J Allergy Clin Immunol. 2020. Apr;145(4):1082–123. [DOI] [PubMed] [Google Scholar]
- 14.Dribin TE, Michelson KA, Vyles D, Neuman MI, Brousseau DC, Mistry RD, et al. PEMCRC anaphylaxis study protocol: a multicentre cohort study to derive and validate clinical decision models for the emergency department management of children with anaphylaxis. BMJ Open. 2021. Jan;11(1):e037341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Motosue MS, Bellolio MF, Van Houten HK, Shah ND, Campbell RL. Risk factors for severe anaphylaxis in the United States. Ann Allergy, Asthma Immunol. 2017;119(4):356–361.e2. Available from: http://linkinghub.elsevier.com/retrieve/pii/S1081120617305550 [DOI] [PubMed] [Google Scholar]
- 16.Lee S, Peterson A, Lohse CM, Hess EP, Campbell RL. Further Evaluation of Factors That May Predict Biphasic Reactions in Emergency Department Anaphylaxis Patients. J Allergy Clin Immunol Pract. 2017;5(5):1295–301. [DOI] [PubMed] [Google Scholar]
- 17.Michelson KA, Monuteaux MC, Neuman MI. Variation and Trends in Anaphylaxis Care in United States Children’s Hospitals. Acad Emerg Med. 2016. May;23(5):623–7. [DOI] [PubMed] [Google Scholar]
- 18.Shaker M, Wallace D, Golden DBK, Oppenheimer J, Greenhawt M. Simulation of Health and Economic Benefits of Extended Observation of Resolved Anaphylaxis. JAMA Netw Open. 2019. Oct;2(10):e1913951–e1913951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaffney LK, Porter J, Gerling M, Schneider LC, Stack AM, Shah D, et al. Safely Reducing Hospitalizations for Anaphylaxis in Children Through an Evidence-Based Guideline. Pediatrics. 2022. Jan 20;149(2):e2020045831. Available from: 10.1542/peds.2020-045831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grabenhenrich LB, Dölle S, Moneret-Vautrin A, Köhli A, Lange L, Spindler T, et al. Anaphylaxis in children and adolescents: The European Anaphylaxis Registry. J Allergy Clin Immunol. 2016;137(4):1128–1137.e1. Available from: https://www.sciencedirect.com/science/article/pii/S0091674915029917 [DOI] [PubMed] [Google Scholar]
- 21.Grunau BE, Wiens MO, Rowe BH, McKay R, Li J, Yi TW, et al. Emergency Department Corticosteroid Use for Allergy or Anaphylaxis Is Not Associated With Decreased Relapses. Ann Emerg Med. 2015;66(4):381–9. Available from: https://www.sciencedirect.com/science/article/pii/S0196064415001900 [DOI] [PubMed] [Google Scholar]
- 22.Yao T-C, Wang J-Y, Chang S-M, Chang Y-C, Tsai Y-F, Wu AC, et al. Association of Oral Corticosteroid Bursts With Severe Adverse Events in Children. JAMA Pediatr. 2021. Jul 1;175(7):723–9. Available from: 10.1001/jamapediatrics.2021.0433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bjornson CL, Klassen TP, Williamson J, Brant R, Mitton C, Plint A, et al. A Randomized Trial of a Single Dose of Oral Dexamethasone for Mild Croup. N Engl J Med. 2004. Sep 23;351(13):1306–13. Available from: 10.1056/NEJMoa033534 [DOI] [PubMed] [Google Scholar]
- 24.Keeney GE, Gray MP, Morrison AK, Levas MN, Kessler EA, Hill GD, et al. Dexamethasone for Acute Asthma Exacerbations in Children: A Meta-analysis. Pediatrics. 2014. Mar 1;133(3):493–9. Available from: 10.1542/peds.2013-2273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Campbell DE. Anaphylaxis Management: Time to Re-Evaluate the Role of Corticosteroids. J Allergy Clin Immunol Pract. 2019;7(7):2239–40. Available from: https://www.sciencedirect.com/science/article/pii/S221321981930621X [DOI] [PubMed] [Google Scholar]
- 26.Dribin TE, Schnadower D, Wang J, Camargo CA Jr, Michelson KA, Shaker M, et al. Anaphylaxis knowledge gaps and future research priorities: a consensus report. J Allergy Clin Immunol. 2022. Mar 1;149(3):999–1009. Available from: 10.1016/j.jaci.2021.07.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dribin TE, Schnadower D, Spergel JM, Campbell RL, Shaker M, Neuman MI, et al. Severity grading system for acute allergic reactions: a multidisciplinary Delphi study. J Allergy Clin Immunol. 2021. Jan;148(1):173–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Campbell RL, Bashore CJ, Lee S, Bellamkonda VR, Li JTC, Hagan JB, et al. Predictors of Repeat Epinephrine Administration for Emergency Department Patients with Anaphylaxis. J Allergy Clin Immunol Pract. 2015;3(4):576–84. Available from: http://linkinghub.elsevier.com/retrieve/pii/S2213219815002275 [DOI] [PubMed] [Google Scholar]
- 29.Dribin TE, Sampson HA, Camargo CA Jr., Brousseau DC, Spergel JM, Neuman MI, et al. Persistent, refractory, and biphasic anaphylaxis: a multidisciplinary Delphi study. J Allergy Clin Immunol. 2020. Aug;146(5):1089–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tanno LK, Demoly P. Allergy in the World Health Organization’s International Classification of Diseases (ICD)-11. Pediatr Allergy Immunol. 2022. Jan 1;33(S27):5–7. Available from: 10.1111/pai.13616 [DOI] [PubMed] [Google Scholar]


