Abstract
Purpose:
The use of patient race in medicine is controversial for its potential either to exacerbate or address health disparities. Polygenic risk scores (PRS) have emerged as a tool for risk stratification models used in preventive medicine. We examined whether PRS results impact primary care physician (PCP) medical decision-making and whether that impact varies by patient race.
Methods:
Using an online survey with a randomized experimental design among PCPs in a national database, we ascertained decision-making around atherosclerotic cardiovascular disease prevention and prostate cancer screening for case scenario patients who were clinically identical except for randomized reported race.
Results:
Across 369 PCPs (email open rate 10.8%, partial completion rate 93.7%), recommendations varied with PRS results in expected directions (low-risk results, no available PRS results, and high-risk results). Still, physicians randomized to scenarios with Black patients were more likely to recommend statin therapy than those randomized to scenarios with White patients (OR 1.74, 95% CI 1.16, 2.59, p=0.007), despite otherwise identical clinical profiles and independent of PRS results. Similarly, physicians were more likely to recommend prostate cancer screening for Black patients compared with White patients (OR 1.58, 95% CI 1.06, 2.35, p=0.025), despite otherwise identical clinical and genetic profiles.
Conclusion:
Despite advances in precision risk stratification, physicians will likely continue to use patient race implicitly or explicitly in medical decision-making.
Keywords: Polygenic risk scores, prevention, prostate cancer, cardiovascular disease, health disparities
INTRODUCTION
The use of patient race in medical decision-making is controversial. Race is a social construct that correlates poorly with a complex interplay of genetic variation, socioeconomic status, and other social determinants of health.1–3 Despite the widespread understanding that race has no biological meaning, it is explicitly included as a variable or risk factor in dozens of clinical prediction rules or management guidelines in use today.4 While some have pointed out that the inclusion of race in clinical algorithms perpetuates existing health disparities,4,5 others have countered that race-blind medicine ignores these disparities and can hinder progress towards addressing them.1,2,6 For some diseases, the use of race in medical decision-making was historically but erroneously justified as a surrogate for causal genetic differences in disease susceptibility. The advent of increasingly large genetic data sets and better understanding of the genetic architecture of disease have positioned patient genotype, not the social construct of race, as a more direct measure of underlying biological differences,7 although not accounting for social determinants of health such as environmental exposures, access to health care, and prior history of discrimination.
Polygenic risk scores (PRS) have emerged as an approach to improving the precision of risk stratification models currently used in disease prevention. Research is actively examining whether PRS enable the identification of patients whose genetic susceptibility might make them more or less likely to benefit from established prevention strategies recommended for the general population. The number of commercial laboratories offering PRS tests continues to increase,8–11 and some healthcare systems are now implementing clinical PRS programs.12,13 To date, the accuracy of PRS in ancestrally diverse populations has been limited by the European bias of most genome-wide association studies from which the PRS are derived, representing a health equity concern as PRS are implemented clinically.14–16
Primary care physicians (PCPs) oversee most routine health screenings and primary prevention interventions and will thus be on the front line of any mainstreaming of PRS in preventive medicine. It remains unknown whether and how PCPs will use PRS to change their medical decision-making and whether that impact will vary by the social construct of patient race. Physicians might increasingly rely on patient genotype instead of race in explicit or implicit risk stratification. Given that PRS are a new technology whose limitations might be unfamiliar, it is unknown whether physicians might downweight their value for risk stratification in patients whose genetic ancestries they perceive to be underrepresented in genetic studies. We examined these questions through a national survey of PCPs, using a randomized experimental design to identify whether patient race impacts physician decision-making in the presence of PRS results.
METHODS
We followed the Checklist for Reporting Results of Internet E-Surveys (CHERRIES) in presenting this research (Supplement).17
Survey development
The full survey is available in the Supplement and included both novel questions and questions adapted from previous surveys of PCPs on implementation and understanding of genetic and genomic medicine technologies. The survey was piloted among eight primary care practitioners for comprehension and interpretation of questions. The survey began with a brief introduction to PRS, including a statement that they have been most extensively studied in populations of European descent; respondents were not explicitly told that PRS performance varies by genetic ancestry. Survey questions consisted of three major sections: 1) clinical case scenarios, 2) questions about PCPs’ perceived utility and barriers to the use of PRS, and 3) respondent education and demographics. This manuscript presents the results of the clinical case scenarios.
Case scenarios
We developed novel clinical scenarios, modeled after previous surveys of primary care physicians,18,19 for the use of PRS in primary care for two common diseases: atherosclerotic cardiovascular disease (ASCVD) and prostate cancer. We chose these two diseases as examples that have validated multiancestry PRS20,21 and clinical guidelines for prevention that are evidence-based but also allow for clinician judgment and patient preference.22,23 We specifically chose patient profiles illustrating common “gray areas” for these diseases, to examine how PRS and race might influence PCP decision-making in cases where they have the most discretion. All scenarios assumed shared decision-making and stated that the patient expressed no preference for or against a certain action and asked for the PCP’s recommendation. The case scenarios were reviewed by a medical education expert to ensure consistency with the presentation of patient race and genetic ancestry in medical boards questions.
Atherosclerotic cardiovascular disease
Standard of care for the primary prevention of ASCVD includes estimating a patient’s 10-year risk and tailoring statin therapy to that level of risk. Guidelines differ on whether to initiate statin therapy for patients at 5%−10% (intermediate) 10-year risk.23,24 We designed a case scenario of a generally healthy 60-year-old man with 10-year ASCVD risk of 7.5%, a value indicating intermediate risk at which guidelines recommend consideration of statin initiation if consistent with patient preferences.23 After seeing a brief summary of current clinical guidelines of the primary ASCVD prevention, respondents were shown a series of 3 patients with identical clinical characteristics (all with 10-year ASCVD risk 7.5%), except that one had no available PRS results, one had PRS results indicating high genetic risk of coronary artery disease, and one had PRS results indicating low genetic risk of coronary artery disease.25 For each of the 3 patients, respondents were asked to indicate their level of agreement with recommending statin therapy, ordering additional cardiac testing, or referring to a cardiology or genetics specialist, using a 5-item Likert response ranging from strongly disagree to strongly agree.
Prostate cancer
Because the harms of prostate cancer screening with prostate-specific antigen (PSA) testing do not significantly outweigh the risks for most men, guidelines, including those from the United States Preventive Services Task Force (USPSTF), do not recommend universal screening.22,26,27 The USPSTF considers family history and African-American race as prostate cancer risk factors but does not make separate screening recommendations for these groups. We designed a case scenario of a 45-year-old man asking his PCP for recommendation about prostate cancer screening. Similarly to the ASCVD cases, respondents were shown a series of 3 patients with identical clinical characteristics, except that one had no available PRS results, one had PRS results indicating high genetic risk of prostate cancer, and one had PRS results indicating low genetic risk.20 For each of the 3 patients, respondents were asked to indicate whether they would recommend prostate cancer screening at age 45, 50, 55, or 60, or whether they would not recommend screening, assuming shared decision-making.
Randomization
Respondents were randomly assigned to one of 4 versions of the survey, which varied the race of the patients in the clinical case scenarios. That is, respondents saw clinical cases about ASCVD prevention in men of either self-identified European-American (White) or African-American (Black) race and, independently, saw clinical cases about prostate cancer screening in men of either White or Black race. This within-disease randomization ensured that respondents were not influenced by an explicit comparison between their own management decisions for a White patient to those for a Black patient.
Respondent characteristics
The survey concluded with questions about prior genetics education28 and self-reported race and ethnicity using U.S Census categories. Other respondent characteristics were obtained by linking individual survey responses to the IQVIA ONEKEY database (see Population and sampling strategy below): age, years in practice, gender, practice specialty, practice size, and geography by state (Tables 1–2).
Table 1. Characteristics of physician survey non-invitees and invitees.
Non-invitees are defined as eligible primary care physicians in the IQVIA ONEKEY physician database who were not selected to receive the survey invitation. Non-respondents are defined as physicians who received the email invitation but did not complete at least the patient case scenario questions (Q1-Q6). Partial or full completers are defined as respondents completing at least the patient case scenario questions (Q1-Q6) of the survey. Among-group effects assessed via Cramér’s V with 95% confidence interval for nominal variables (where values ≤0.2 indicate weak association) and η2 with 95% confidence interval for continuous variables (the ratio of variance explained by invitee/respondent status, see Supplement).
| Non-invitees (n=235,226) | Non-respondents (n=26,631) | Partial or full completers (n=369) | Effect size estimates by respondent status | |
|---|---|---|---|---|
| Mean (SD) age, years | 50.1 (14.1) | 52.5 (13.7) | 55.1 (13.0) | η2 <0.01 (0.00, 0.00) |
|
| ||||
| Mean (SD) time since medical school graduation, years | 22.7 (14.2) | 24.6 (13.8) | 27.3 (13.4) | η2 <0.01 (0.00, 0.00) |
|
| ||||
| Gender, n (%) | Cramér’s V 0.01 (0.01, 0.01) |
|||
| Female | 98,091 (41.7%)a | 10,662 (40.0%) | 137 (37.1%) | |
| Male | 137,129 (58.3%)a | 15,969 (60.0%) | 232 (62.9%) | |
|
| ||||
| Specialty, n (%) | Cramér’s V 0.04 (0.03, 0.04) |
|||
| Internal medicine | 121,072 (51.5%) | 13,497 (50.7%) | 202 (54.7%) | |
| Family medicine | 108,999 (46.3%) | 12,472 (46.8%) | 159 (43.1%) | |
| General practice | 5,155 (2.2%) | 662 (2.5%) | 8 (2.2%) | |
|
| ||||
| US region, n (%) | Cramér’s V 0.01 (0.01, 0.01) |
|||
| Midwest | 52,758 (22.4%) | 5,742 (21.6%) | 82 (22.2%) | |
| Northeast | 47,201 (20.0%) | 4,976 (18.7%) | 83 (22.5%) | |
| South | 78,693 (33.5%) | 9,405 (35.3%) | 98 (26.6%) | |
| West | 56,574 (24.1%) | 6,508 (24.4%) | 106 (28.7%) | |
Six non-invitees are categorized as gender “unknown” in the database.
Table 2: Additional characteristics of survey respondents.
Data are from 369 survey respondents with at least partial (Q1-Q6) survey completion.
| Characteristic | |
|---|---|
| Self-reported race, n (%) | |
| Asian | 73 (19.8%) |
| Black of African American | 12 (3.3%) |
| Native Hawaiian or Other Pacific Islander | 5 (1.4%) |
| White | 232 (62.9%) |
| Multiracial | 7 (1.9%) |
| Prefer not to answer / Other / Missing | 40 (10.8%) |
|
| |
| Self-reported ethnicity, n (%) | |
|
| |
| Hispanic / Latinx | 15 (4.1%) |
| Not Hispanic / Latinx | 330 (89.4%) |
| Prefer not to answer / Missing | 24 (6.5%) |
|
| |
| Genetics training beyond medical schoola, n (%) | |
|
| |
| No additional training | 335 (90.8%) |
| Genetics residency/fellowship | 5 (1.4%) |
| Genetics education course | 18 (4.9%) |
| Residency rotation in genetics | 3 (0.8%) |
| Graduate degree | 2 (0.5%) |
| Other training | 12 (3.3%) |
| Missing | 4 (1.1%) |
Proportions sum to greater than 100% because multiple selections were allowed.
Population and sampling strategy
We defined the target population as PCPs who care for adult patients, including physicians practicing family medicine, general practice, or internal medicine. We worked with database licensee IQVIAⓇ to recruit respondents from the ONEKEY national physician database of more than 250,000 active physicians who have opted in to receiving email survey invitations. The database includes demographic, training, and practice-related data from the American Medical Association (AMA) Physician Masterfile and other sources.
Recruitment, consent, and enrollment
Staff at IQVIA sent email invitations to the survey to a random sample of 27,000 eligible PCPs from the database. These emails described the survey as a 8–10 minute survey about precision prevention in primary care using genetic risk scores. Respondents clicked a unique web link to access the survey, hosted by the QualtricsXM Survey Tool (Qualtrics, Provo, UT). The survey link brought physicians to a brief informed consent page, at the bottom of which respondents electronically documented consent by clicking to proceed to the first page of the survey. Neither race nor ancestry was mentioned in the invitation email or consent page. Upon accessing the survey link, respondents were randomly allocated in a 1:1:1:1 ratio to the 4 versions of the survey. The first email campaign was launched April 18, 2021 and offered respondents a $25 Amazon gift card for completing the survey. On April 27, 2021, a second invitation offering a $50 Amazon gift card was emailed to PCPs who had opened the first email but not yet accessed the survey link from the first email. The survey was closed on August 27, 2021.
Hypotheses
For ASCVD prevention, we hypothesized that, compared to a patient with no PRS results, respondents would be more likely to initiate statin for a patient with a high-risk coronary artery disease PRS and less likely to initiate statin for a patient with a low-risk PRS. Given the weaker performance of PRS among non-European ancestry groups, we hypothesized that the magnitude of these effects would be smaller for the case scenario with a Black patient than that with a White patient; such an observation would require that physicians were aware of this limitation. Similarly, for prostate cancer screening, we hypothesized that, compared to a patient with no PRS results, respondents would be more likely to screen a patient with a high-risk prostate cancer PRS and less likely to screen a patient with a low-risk prostate cancer PRS and that the magnitude of these effects would be smaller for the scenarios with a Black patient. Details about data validation and statistical analysis are described in the Supplement.
RESULTS
Participant response and characteristics
Of 25,803 physicians who received the email invitation without bounceback, 2,776 opened the email (email open rate 10.8%) and 409 participants clicked the hyperlink to view the survey website (survey view rate 409/2,776, 14.7%). Of PCPs who viewed the survey, 394 consented to study participation (participation rate 394/409, 96.3%) and 366 PCPs completed the entire survey (completion rate 366/394, 92.9%). An additional 3 participants completed the clinical scenarios (Q1-Q6) but did not complete the survey in its entirety (partial completion rate 369/394, 93.7%).
Respondents had similar demographic characteristics to physicians in the database who were not invited to participate and to physicians who were invited but did not respond (Table 1). Among the 369 respondents, 232 (62.9%), 73 (19.8%), 12 (3.3%), and 5 (1.4%) self-reported White, Asian, Black/African-American and Native Hawaiian/Other Pacific Islander race, respectively; 15 (4.1%) self-reported Hispanic/Latinx ethnicity (Table 2). The majority (335, 90.8%) reported no additional genetics training beyond medical school (Table 2).
ASCVD case scenarios
In the ASCVD case scenarios, 38%, 56%, and 89% of respondents agreed with recommending statin therapy for patients with a low-risk PRS, no PRS, and a high-risk PRS for coronary artery disease, respectively (Table S2 in Supplement). 29%, 47%, and 70% agreed with recommending additional cardiac testing for patients with a low-risk PRS, no PRS, and a high-risk PRS, respectively. 8%, 9%, and 38% agreed with specialist referral for patients with a low-risk PRS, no PRS, and a high-risk PRS, respectively. In ordinal logistic regression models, compared to patients with no available PRS results, respondents were less likely (OR 0.51, 95% CI 0.41, 0.64, p<0.001) and more likely (OR 5.26, 95% CI 3.53, 7.85, p<0.001) to recommend statin therapy to patients with low-risk and high-risk PRS, respectively (Figure 1).
Figure 1.
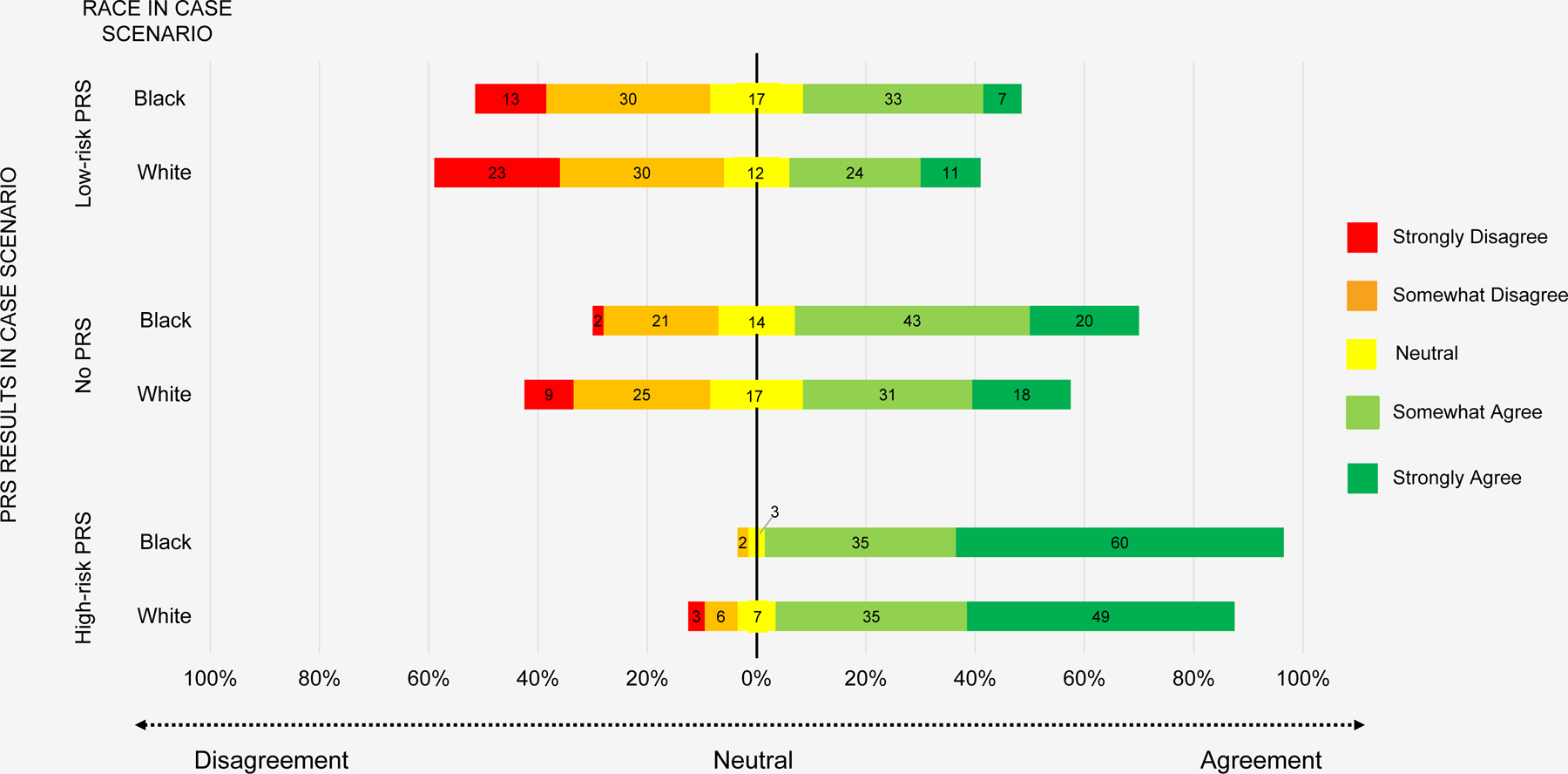
Physician agreement with initiating statin therapy for ASCVD risk reduction by PRS result and race of scenario patient
In each level of PRS result in the ASCVD scenarios, more respondents agreed with recommending statin therapy for the Black patients compared with the White patients: 41% vs. 35% (low-risk PRS), 63% vs. 49% (no PRS), and 95% vs. 84% (high-risk PRS, Table S2 in Supplement). 31% and 26% (low-risk PRS), 52% and 42%) (no PRS), and 73% and 67%) (high-risk PRS) agreed with recommending additional cardiac testing for Black and White patients, respectively. 10% and 7% (low-risk PRS), 8% and 10% (no PRS), and 37% and 39% (high-risk PRS) agreed with specialist referral for Black and White patients, respectively. In regression models accounting for within-person responses across all PRS scenarios, PCPs were more likely to agree with recommending statin therapy for Black patients compared with White patients (OR 1.74, 95% CI 1.16, 2.59, p=0.007), despite identical absolute risk estimates presented in the case scenarios. We did not observe a significant interaction between patient race and PRS results (Tables S4-S5). Physicians were not significantly more likely to recommend additional cardiac testing (OR 1.24, 95% CI 0.89, 1.73, p=0.21) or specialist referral (OR 0.98, 95% CI 0.68, 1.42, p=0.92) for Black patients compared to White patients (Figure S1-S2 in Supplement).
Prostate cancer screening case scenarios
Across all respondents, 17%, 23%, 27%, and 5% recommended prostate cancer screening for the case patient with no PRS results beginning at age 45, 50, 55, and 60 years, respectively; 28% did not recommend screening at any age (Table S2 in Supplement). For patients with a low-risk PRS, no PRS, and a high-risk PRS for prostate cancer, 59%, 72%, and 98% of respondents recommended screening at some age, respectively. More respondents recommended screening at some age for the Black patients compared with the White patients: 63% vs. 53% (low-risk PRS), 81% vs. 66% (no PRS), and 98% vs. 97% (high-risk PRS).
In logistic regression models, compared with patients with no available PRS results, respondents were less likely (OR 0.56, 95% CI 0.46, 0.68, p<0.001) and more likely (OR 16.1, 95% CI 8.46, 30.70, p<0.001) to recommend prostate cancer screening to patients with PRS indicating low and high prostate cancer risk, respectively (Figure 2). Across all PRS results, physicians were more likely to recommend PSA testing for Black patients compared with White patients (OR 1.58, 95% CI 1.06, 2.35, p=0.025). Among respondents who recommended screening, physicians were 3 times more likely (OR 2.97, 95% CI 1.71, 5.18, p<0.001) to recommend screening at age 45, ten years before USPSTF guidelines recommend screening initiation, for Black patients compared to White patients, independent of PRS results. Table S6 in the Supplement shows the likelihood of statin initiation and PSA testing by patient race, stratified by PRS category. In exploratory models, there were no significant interactions between self-reported race and ethnicity of the PCP respondents and the likelihood of recommending statins or prostate cancer screening (Table S7 in Supplement).
Figure 2.
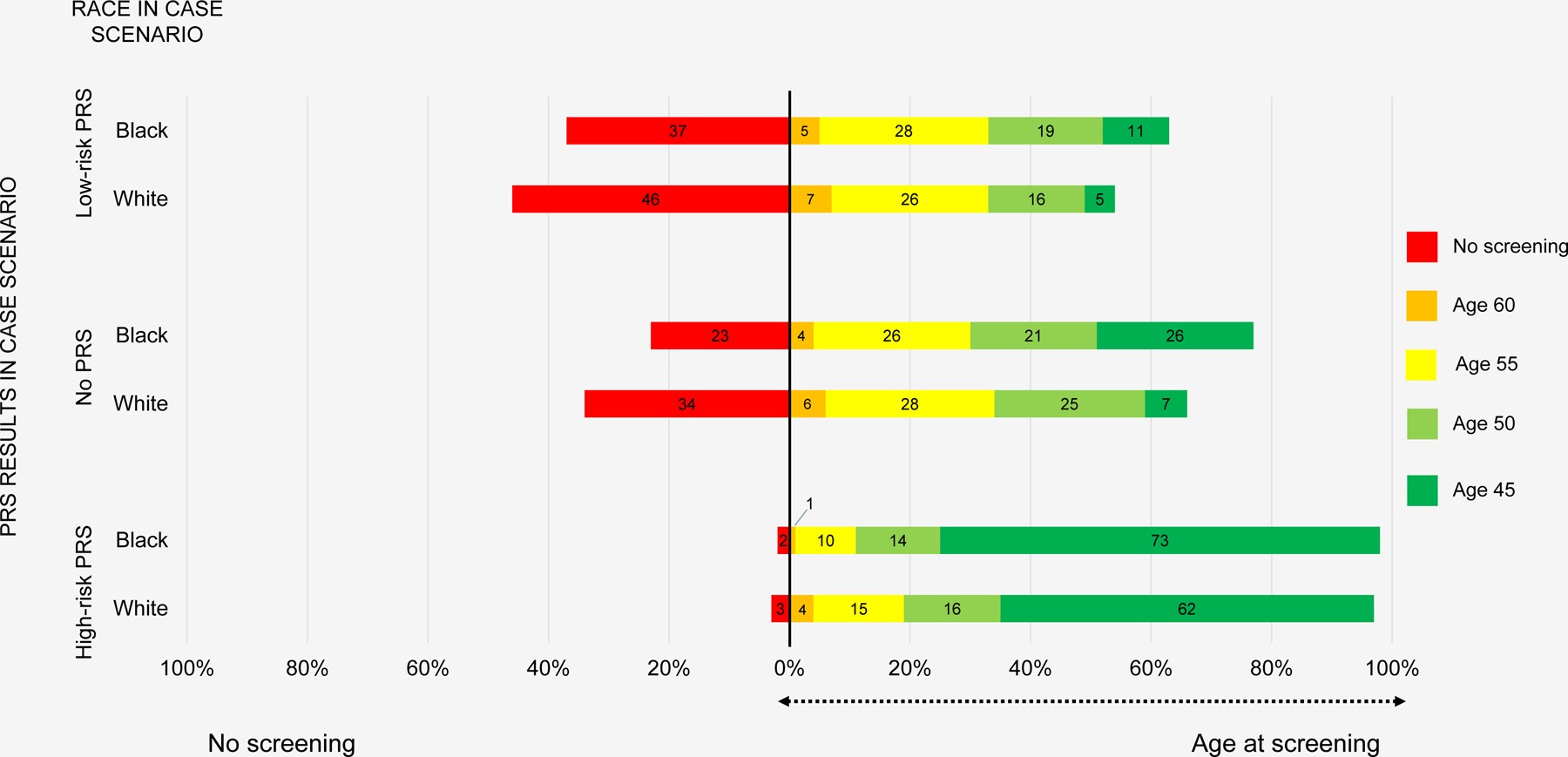
Physician recommendation for whether and at what age to recommend prostate cancer screening by PRS result and race of scenario patient
DISCUSSION
Together, the results of these randomized clinical scenarios suggest that, at least in the early days of precision prevention, both PRS and patient race influence physicians’ medical decisions in some contexts. Physicians’ recommendations varied with PRS results in the expected directions, but patient race remained a strong independent predictor. As compared with White patients, physicians were more likely to recommend statin therapy and prostate cancer screening to Black patients, despite otherwise identical clinical and genetic risk profiles. The findings suggest that physicians found clinical utility in PRS for genotype-informed risk stratification but that, independent of PRS, physicians still used patient race either implicitly or explicitly in medical decision-making.
These observations prompt two main questions: why did PCP recommendations differ by patient race, and do those differences represent racial biases that could result in patient harm? Our results cannot definitively answer these questions. For the first, the survey’s randomized design allowed the unconfounded isolation of a strong effect of patient race in PCP decision-making, independent of PRS, both in a context where Black race is explicitly mentioned in clinical guidelines (prostate cancer prevention) and another where it is assumed to be accounted for in risk calculation (ASCVD prevention). We could not observe the cognitive processes underlying these differences, which may have differed between the two disease scenarios.For the ASCVD prevention scenarios, respondents were told that all patients had identical absolute 10-year risk estimates of 7.5%, as determined by the American College of Cardiology/American Heart Association (ACC/AHA) Pooled Cohort Equations (PCE), which were developed and validated in racially diverse populations.29,30 Respondents may have implicitly brought their own additional risk perceptions and biases, or misunderstanding of the PCE, in recommending statins more often for Black patients in the scenarios. For the prostate cancer scenarios, respondents were told that guidelines list African American race as a risk factor but do not make separate screening recommendations. Respondents’ perception of increased absolute risk for Black men as compared with White men likely persisted despite otherwise identical patient characteristics and measures of relative genetic risk. What is clear from our findings, however, is that PRS results did not remove the influence of race on PCP decision-making.
A second important question then is whether the observed racial differences in PCP recommendations are appropriate in these precision prevention scenarios or whether they represent harmful racial biases. This question does not have an easy answer. It may be reassuring that physicians recommended more preventive services (statin therapy and prostate cancer screening) for Black patients compared with otherwise identical White patients. Still, all medical interventions carry some risk of harm, so racial differences in care merit scrutiny. Black American men have a 33% higher lifetime risk of prostate cancer and are twice as likely to die of the disease compared with White men.31 Although USPSTF guidelines do not include separate screening recommendations, they do mention Black race as an additional risk factor.22 Given these disparities, what would it have meant if we had observed that survey respondents’ did not recommend screening more often to Black patients? In contrast, the ACC/AHA developed the PCE specifically to address the concern that previous risk models were not well calibrated to African Americans,29,30 and yet our survey respondents were still more likely to recommend statins for the Black patients with identical ACC/AHA estimates. Significant racial disparities in cardiovascular disease persist in the US: compared with White individuals, Black individuals have higher prevalence of ASCVD risk factors such as hypertension and diabetes and are more than twice as likely to die of cardiovascular disease.32 Although we observed racial differences in PCP recommendations, it is not clear that these differences indicate harmful biases or health inequities in these two scenarios.
Nonetheless, our findings offer insights for some of the pressing questions for research and clinical implementation programs already propelling PRS into the clinic.12,13 First, if PRS are to be included in clinical risk prediction, what, if anything, is the appropriate role of the social construct of race in such models? Although much more work remains, methodological advances in PRS derivation are continually improving the validity of PRS interpretations across genetic ancestry and racial groups,16,20,33,34 and research studies such as the Genomic Medicine at VA (GenoVA) Study have begun implementing such PRS clinically.35 Other studies such as the Women Informed to Screen Depending on Measures of Risk (WISDOM) Study and the Electronic Medical Records and Genomics (eMERGE) Network are additionally incorporating PRS into existing clinical prediction models for conditions like breast cancer and ASCVD that include other risk factors such as race, family history, and smoking status.13,36 Of course, racial categories themselves belie significant within-group heterogeneity. In an ideal future, prevention guidelines would be based on risk models that include more precisely measured causal risk factors, including causal genetic variants, environmental exposures, and other social determinants of health, although such models might become too unwieldy for clinical implementation. Moreover, it is inconceivable that any clinical prediction model could accurately include all human diversity that are causally relevant for a given disease, from genetic factors to sociocultural determinants. In the meantime, race remains a problematic proxy for disease risk. Many concerns about the inclusion of race in clinical prediction models have focused on the perpetuation of existing health disparities through underdiagnosis and under-referral to specialty care.4 However, in some contexts, such as the large prostate cancer risk disparity observed for Black men, to ignore race in clinical prediction models and guidelines might inappropriately limit screening in this higher-risk group. If race is to be included in a prediction model, the downstream consequences for clinical care should be considered. In the two scenarios in our study, we did not observe that physicians’ implicit or explicit use of race in decision-making limited the care Black patients received.
Second, as prediction models grow increasingly complex through the inclusion of PRS, what support will PCPs need for their appropriate use? Consensus is emerging on how researchers and laboratories should transparently report the development and validation of a given PRS, including any limitations of performance in different population groups.35,37,38 As warranted by the evidence, clinical guidelines should be updated to specify the appropriate (and inappropriate) uses of PRS for a given disease; such guidelines should explicitly state and justify whether and how race as a social construct is relevant in the guideline.35–37 This should be done only as a means of addressing health disparities and without suggesting that race has biological or genetic meaning. Healthcare systems can play a role in designing support systems to standardize the appropriate use of genetic results, including evidence-based care pathways for the management of common PRS results, ideally integrated within electronic medical records;39–41 care teams comprised of some combination of genetic counselors, genetic care coordinators, and relevant specialists;39,40,42 and educational resources such as synchronous or asynchronous courses with continuing medical education credit.42,40,28,41 Nonetheless, even if epidemiology can develop perfect prediction models and healthcare systems can implement PCP support, our randomized study suggests physicians might still use race explicitly or implicitly in medical management. If our observations represent racial bias, there are no easy solutions to closing the gap. A recent systematic review of interventions designed to reduce biases among healthcare providers found that interventions targeting implicit bias recognition and management can promote bias awareness but have not been shown to effect sustained reduction of bias or improvement in patient care or health disparities.43 More work is needed from the individual provider to systems levels to address this entrenched problem.
Finally, patients should play a role in the equitable implementation of clinical PRS. Patient engagement efforts have already elucidated some relevant perspectives, including a preference to receive PRS results even if less accurate in certain genetic ancestry groups.38,44 For the individual patient, a clinician should transparently convey the strengths and limitations of PRS. Doing so will allow the patient to participate in shared decision-making, an approach through which patients and clinicians discuss the best available evidence, consider options, and develop informed preferences for a healthcare decision.45 Some patients may choose to eschew decision-making based on data from populations they feel do not represent them; others may prefer using imperfect data in their medical decisions over having no data.
Limitations of the present study include the invitation email open rate of 10.8% and survey view rate of 14.7%, although these compare favorably with those of other large national physician surveys.46–48 Survey respondents may not be representative of all US PCPs, but the randomized experimental design of the patient scenarios ensures the unconfounded internal validity of the between-group comparisons. Second, although we observed significant differences in PCP decision-making by patient race and PRS results, we may have had limited power to detect statistically significant race-PRS interactions. Third, physician responses to the case scenarios may not correspond to their actual practice in real-world patient care. By design, the case scenarios were narrowly described, to allow us to isolate the effects of PRS results and patient race in common decision-making contexts. As a result, respondents did not have access to other patient information they might otherwise have, including social determinants of health and other clinical risk factors. We also do not know how physician decision-making would vary, if at all, for patients of racial identities other than Black and White.
In conclusion, results of this national survey suggest that physicians will use both PRS and the social construct of race, implicitly or explicitly, in the precision prevention era. Researchers, clinicians, and policymakers must ensure that the use of PRS and race in medical decision-making, if they are used at all, promotes improved health outcomes for all patients.
Supplementary Material
Acknowledgements
The authors thank Kopika Kuhathaas for her assistance in preparing data figures. This work was funded by the National Human Genome Research Institute / National Institutes of Health (R35 HG010706, JLV).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Ethics declaration
The study was approved by the Harvard Longwood Campus Institutional Review Board (Protocol #20–2098). Informed consent was obtained from all respondents.
Conflict of interests
The authors declare no conflicts of interest. CAB, AAA, and JLV are employees of the U.S. Department of Veterans Affairs (VA). The views expressed in this manuscript do not represent those of the U.S. government or VA.
Data availability statement
The dataset supporting the current study is available from the corresponding author on request.
REFERENCES
- 1.Borrell LN, Elhawary JR, Fuentes-Afflick E, et al. Race and Genetic Ancestry in Medicine — A Time for Reckoning with Racism. N Engl J Med 2021;384(5):474–480. doi: 10.1056/NEJMms2029562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ioannidis JPA, Powe NR, Yancy C. Recalibrating the Use of Race in Medical Research. JAMA 2021;325(7):623–624. doi: 10.1001/jama.2021.0003 [DOI] [PubMed] [Google Scholar]
- 3.Flanagin A, Frey T, Christiansen SL, AMA Manual of Style Committee. Updated Guidance on the Reporting of Race and Ethnicity in Medical and Science Journals. JAMA 2021;326(7):621–627. doi: 10.1001/jama.2021.13304 [DOI] [PubMed] [Google Scholar]
- 4.Vyas DA, Eisenstein LG, Jones DS. Hidden in Plain Sight — Reconsidering the Use of Race Correction in Clinical Algorithms. N Engl J Med 2020;383(9):874–882. doi: 10.1056/NEJMms2004740 [DOI] [PubMed] [Google Scholar]
- 5.Eneanya ND, Yang W, Reese PP. Reconsidering the Consequences of Using Race to Estimate Kidney Function. JAMA 2019;322(2):113–114. doi: 10.1001/jama.2019.5774 [DOI] [PubMed] [Google Scholar]
- 6.Oni-Orisan A, Mavura Y, Banda Y, Thornton TA, Sebro R. Embracing Genetic Diversity to Improve Black Health. N Engl J Med Published online 2021. doi: 10.1056/NEJMms2031080 [DOI] [PubMed]
- 7.Bonham VL, Callier SL, Royal CD. Will Precision Medicine Move Us beyond Race? N Engl J Med 2016;374(21):2003–2005. doi: 10.1056/NEJMp1511294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hughes E, Tshiaba P, Wagner S, et al. Integrating Clinical and Polygenic Factors to Predict Breast Cancer Risk in Women Undergoing Genetic Testing. JCO Precis Oncol 2021;5:PO.20.00246. doi: 10.1200/PO.20.00246 [DOI] [PMC free article] [PubMed]
- 9.PROSTATENOW™ – GENETICSNOW: Genetic testing solutions by GoPath Diagnostics Accessed May 27, 2022. https://geneticsnow.com/prostatenow/
- 10.Genomics plc. Genomics for health systems. Genomics plc Accessed October 24, 2022. https://www.genomicsplc.com/precision-health/health-systems/
- 11.Allelica. cardioriskSCORE. cardioriskSCORE https://www.allelica.com/cardioriskscore
- 12.England NHS. Accelerating Genomic Medicine in the NHS; 2022. Accessed October 19, 2022. https://www.england.nhs.uk/long-read/accelerating-genomic-medicine-in-the-nhs/
- 13.National Human Genome Research Institute (NHGRI). Electronic Medical Records and Genomics (eMERGE) Network Published February 23, 2020. Accessed November 9, 2022. https://https://www.genome.gov/Funded-Programs-Projects/Electronic-Medical-Records-and-Genomics-Network-eMERGE
- 14.Martin AR, Kanai M, Kamatani Y, Okada Y, Neale BM, Daly MJ. Clinical use of current polygenic risk scores may exacerbate health disparities. Nat Genet 2019;51(4):584–591. doi: 10.1038/s41588-019-0379-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lewis CM, Vassos E. Polygenic risk scores: from research tools to clinical instruments. Genome Med 2020;12(1):44. doi: 10.1186/s13073-020-00742-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y, Tsuo K, Kanai M, Neale BM, Martin AR. Challenges and Opportunities for Developing More Generalizable Polygenic Risk Scores. Annu Rev Biomed Data Sci 2022;5:293–320. doi: 10.1146/annurev-biodatasci-111721-074830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eysenbach G Improving the quality of Web surveys: the Checklist for Reporting Results of Internet E-Surveys (CHERRIES). J Med Internet Res 2004;6(3):e34. doi: 10.2196/jmir.6.3.e34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yasmeen S, Romano PS, Tancredi DJ, Saito NH, Rainwater J, Kravitz RL. Screening mammography beliefs and recommendations: a web-based survey of primary care physicians. BMC Health Serv Res 2012;12(1):1–10. doi: 10.1186/1472-6963-12-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Persell SD. Potential Use of 10-Year and Lifetime Coronary Risk Information for Preventive Cardiology Prescribing Decisions: A Primary Care Physician Survey. Arch Intern Med 2010;170(5):470. doi: 10.1001/archinternmed.2009.525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Conti DV, Darst BF, Moss LC, et al. Trans-ancestry genome-wide association meta-analysis of prostate cancer identifies new susceptibility loci and informs genetic risk prediction. Nat Genet 2021;53(1):65–75. doi: 10.1038/s41588-020-00748-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dikilitas O, Schaid DJ, Kosel ML, et al. Predictive Utility of Polygenic Risk Scores for Coronary Heart Disease in Three Major Racial and Ethnic Groups. Am J Hum Genet 2020;106(5):707–716. doi: 10.1016/j.ajhg.2020.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grossman DC, Curry SJ, Owens DK, et al. Screening for Prostate Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2018;319(18):1901–1913. doi: 10.1001/jama.2018.3710 [DOI] [PubMed] [Google Scholar]
- 23.Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019;140(11):e596–e646. doi: 10.1161/CIR.0000000000000678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.US Preventive Services Task Force. Statin Use for the Primary Prevention of Cardiovascular Disease in Adults: US Preventive Services Task Force Recommendation Statement. JAMA 2016;316(19):1997–2007. doi: 10.1001/jama.2016.15450 [DOI] [PubMed] [Google Scholar]
- 25.Khera AV, Chaffin M, Aragam KG, et al. Genome-wide polygenic scores for common diseases identify individuals with risk equivalent to monogenic mutations. Nat Genet 2018;50(9):1219–1224. doi: 10.1038/s41588-018-0183-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qaseem A, Barry MJ, Denberg TD, Owens DK, Shekelle P. Screening for prostate cancer: a guidance statement from the Clinical Guidelines Committee of the American College of Physicians. Ann Intern Med 2013;158(10):761–769. doi: 10.7326/0003-4819-158-10-201305210-00633 [DOI] [PubMed] [Google Scholar]
- 27.Carter HB, Albertsen PC, Barry MJ, et al. Early detection of prostate cancer: AUA Guideline. J Urol 2013;190(2):419–426. doi: 10.1016/j.juro.2013.04.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Christensen KD, Vassy JL, Jamal L, et al. Are physicians prepared for whole genome sequencing? a qualitative analysis. Clin Genet 2016;89(2):228–234. doi: 10.1111/cge.12626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goff DC, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA Guideline on the Assessment of Cardiovascular Risk: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation Published online November 12, 2013. doi: 10.1161/01.cir.0000437741.48606.98 [DOI] [Google Scholar]
- 30.Carnethon MR, Pu J, Howard G, et al. Cardiovascular Health in African Americans: A Scientific Statement From the American Heart Association. Circulation 2017;136(21):e393–e423. doi: 10.1161/CIR.0000000000000534 [DOI] [PubMed] [Google Scholar]
- 31.Cancer of the Prostate - Cancer Stat Facts SEER. Accessed November 3, 2021. https://seer.cancer.gov/statfacts/html/prost.html
- 32.National Center for Health Statistics. Racial and Ethnic Disparities in Heart Disease; 2019. Accessed July 22, 2022. https://www.cdc.gov/nchs/hus/spotlight/HeartDiseaseSpotlight_2019_0404.pdf
- 33.Ge T, Irvin MR, Patki A, et al. Development and validation of a trans-ancestry polygenic risk score for type 2 diabetes in diverse populations. Genome Med 2022;14(1):70. doi: 10.1186/s13073-022-01074-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.National Human Genome Research Institute. Polygenic RIsk MEthods in Diverse populations (PRIMED) Consortium Accessed July 2, 2021. https://www.genome.gov/Funded-Programs-Projects/PRIMED-Consortium
- 35.Hao L, Kraft P, Berriz GF, et al. Development of a clinical polygenic risk score assay and reporting workflow. Nat Med 2022;28(5):1006–1013. doi: 10.1038/s41591-022-01767-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shieh Y, Eklund M, Madlensky L, et al. Breast Cancer Screening in the Precision Medicine Era: Risk-Based Screening in a Population-Based Trial. J Natl Cancer Inst 2017;109(5). doi: 10.1093/jnci/djw290 [DOI] [PubMed]
- 37.Wand H, Lambert SA, Tamburro C, et al. Improving reporting standards for polygenic scores in risk prediction studies. Nature 2021;591(7849):211–219. doi: 10.1038/s41586-021-03243-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lewis ACF, Perez EF, Prince AER, et al. Patient and provider perspectives on polygenic risk scores: implications for clinical reporting and utilization. Genome Med 2022;14(1):1–16. doi: 10.1186/s13073-022-01117-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wildin RS, Giummo CA, Reiter AW, Peterson TC, Leonard DGB. Primary Care Implementation of Genomic Population Health Screening Using a Large Gene Sequencing Panel. Front Genet 2022;13. Accessed October 21, 2022. 10.3389/fgene.2022.867334 [DOI] [PMC free article] [PubMed]
- 40.Lemke AA, Amendola LM, Kuchta K, et al. Primary Care Physician Experiences with Integrated Population-Scale Genetic Testing: A Mixed-Methods Assessment. J Pers Med 2020;10(4):165. doi: 10.3390/jpm10040165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hayward J, McDermott J, Qureshi N, Newman W. Pharmacogenomic testing to support prescribing in primary care: a structured review of implementation models. Pharmacogenomics 2021;22(12):761–776. doi: 10.2217/pgs-2021-0032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Slunecka JL, van der Zee MD, Beck JJ, et al. Implementation and implications for polygenic risk scores in healthcare. Hum Genomics 2021;15(1):46. doi: 10.1186/s40246-021-00339-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vela MB, Erondu AI, Smith NA, Peek ME, Woodruff JN, Chin MH. Eliminating Explicit and Implicit Biases in Health Care: Evidence and Research Needs. Annu Rev Public Health 2022;43:477–501. doi: 10.1146/annurev-publhealth-052620-103528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suckiel SA, Braganza GT, Aguiñiga KL, et al. Perspectives of diverse Spanish- and English-speaking patients on the clinical use of polygenic risk scores. Genet Med Off J Am Coll Med Genet 2022;24(6):1217–1226. doi: 10.1016/j.gim.2022.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Makoul G, Clayman ML. An integrative model of shared decision making in medical encounters. Patient Educ Couns 2006;60(3):301–312. doi: 10.1016/j.pec.2005.06.010 [DOI] [PubMed] [Google Scholar]
- 46.Ramos C, Johnston E, Ozanich G. Electronic Health Record (EHR) Reporting Program: Volutary User-Reported Criteria Urban Institute; 2020. Accessed February 23, 2022. https://www.urban.org/research/publication/electronic-health-record-reporting-program-voluntary-user-reported-criteria [Google Scholar]
- 47.Hickner J, Thompson PJ, Wilkinson T, et al. Primary care physicians’ challenges in ordering clinical laboratory tests and interpreting results. J Am Board Fam Med JABFM 2014;27(2):268–274. doi: 10.3122/jabfm.2014.02.130104 [DOI] [PubMed] [Google Scholar]
- 48.Haga SB, Kim E, Myers RA, Ginsburg GS. Primary Care Physicians’ Knowledge, Attitudes, and Experience with Personal Genetic Testing. J Pers Med 2019;9(2):29. doi: 10.3390/jpm9020029 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset supporting the current study is available from the corresponding author on request.


