Abstract
Objectives:
This study aimed to explore the impact of myocardial bridging (MB) on early development of cardiac allograft vasculopathy and long-term graft survival after heart transplantation.
Background:
MB has been reported to be associated with acceleration of proximal plaque development and endothelial dysfunction in native coronary atherosclerosis. However, its clinical significance in heart transplantation remains unclear.
Methods:
In 103 heart-transplant recipients, serial (baseline and 1-year post-transplant) volumetric intravascular ultrasound (IVUS) analyses were performed in the first 50 mm of the left anterior descending (LAD) artery. Standard IVUS indices were evaluated in 3 equally divided LAD segments (proximal, middle, and distal segments). MB was defined by IVUS as an echolucent muscular band lying on top of the artery. The primary endpoint was death or re-transplantation, assessed for up to 12.2 years (median follow-up: 4.7 years).
Results:
IVUS identified MB in 62% of the study population. At baseline, MB patients had smaller intimal volume in the distal LAD than non-MB patients (p=0.002). During the first year, vessel volume decreased diffusely irrespective of the presence of MB. Intimal growth diffusely distributed in non-MB patients, whereas MB patients demonstrated significantly augmented intimal formation in the proximal LAD. Kaplan-Meier analysis revealed significantly lower event-free survival in patients with versus without MB (log-rank p=0.02). In multivariate analysis, the presence of MB was independently associated with late adverse events [hazard ratio 5.1 (1.6–22.2)].
Conclusion:
MB appears to relate to accelerated proximal intimal growth and reduced long-term survival in heart-transplant recipients.
Keywords: myocardial bridging, heart transplantation, intravascular ultrasound, acute cellular rejection
Myocardial bridging (MB) is a common anatomic variant predominantly involving the mid-distal segment of the left anterior descending coronary artery (LAD).(1) Although MB was historically considered a benign variant, recent studies have indicated that MB can lead to significant clinical symptoms, arrhythmia, or adverse cardiac events in a certain patient subset.(1–4) In addition to the reduced blood flow reserve and decreased blood perfusion that results from delayed arterial relaxation in diastole, the accelerated proximal plaque formation(5–7) and endothelial dysfunction(8) may represent additional potential mechanisms contributing to poor clinical outcomes in patients with MB.
Cardiac allograft vasculopathy (CAV), typically characterized by diffuse coronary intimal thickening with pathological vessel remodeling, remains a leading cause of long-term mortality and morbidity after heart transplantation.(9,10) While the pathogenesis of CAV largely involves innate and adaptive immune responses, phenotypic expression of vascular lesions appears to be the consequence of cumulative endothelial injury induced not only by alloimmune responses but also by nonspecific insults in the context of impaired repair mechanisms in heart-transplant recipients. Hence, we hypothesized that presence of MB in a donor heart may add another layer of risk, identification of which may enable targeted therapy to improve long-term outcomes. This study aimed to explore the possible impact of MB on early development of CAV and long-term graft survival in patients after heart transplantation surgery.
METHODS
Study Design and Population
This is a retrospective analysis of previous work(10) in which stable heart-transplant recipients with preserved renal function (baseline serum creatinine ≤2.0 mg/dl) who survived at least 1-year post-transplantation and underwent prescheduled serial IVUS at baseline (within 8 weeks) and 1 year were screened between January 2002 and January 2013. As previously described,(10) this population included patients from 2 prospective trials performed to evaluate the role of cytomegalovirus or an angiotensin-converting enzyme inhibitor in CAV development (ClinicalTrials.gov: NCT01078363). All recipients received standard immunosuppressive therapy, including induction therapy with daclizumab or rabbit antithymocyte globulin (rATG), corticosteroids, an antiproliferative agent (sirolimus or mycophenolate mofetil), and a calcineurin inhibitor (cyclosporine or tacrolimus). Patients were monitored for acute cellular rejection (ACR) using right ventricular endomyocardial biopsies at scheduled intervals post-transplant: weekly during the first month, biweekly until the third month, monthly until the sixth months, and then at 9 and 12 months. Significant ACR was defined as the International Society for Heart and Lung Transplantation 2004 revised grade of ≥2R.(10) Patients were followed beyond the first year, and the primary endpoint was all-cause death or re-transplantation. The protocol was approved by the Stanford University Institutional Review Board, and informed consent was obtained from all patients.
Intravascular Ultrasound
Intravascular ultrasound (IVUS) was performed using a 40-MHz mechanical system (Galaxy with Atlantis SR Pro or OptiCross with iLab; Boston Scientific Corporation) in a standard manner with automated pullback at 0.5 mm/sec. Images were analyzed with a validated system (echoPlaque; Indec Systems) at an independent core laboratory blinded to clinical and angiographic information (Stanford Cardiovascular Core Analysis Laboratory). Vessel, lumen and intimal areas were manually traced at end-diastole at approximately 1-mm intervals throughout the first 50 mm of LAD with automated interpolation of the remaining frames.(10) Volumes calculated using Simpson’s method were standardized as volume index (volume / analyzed length, mm3/mm). In addition to entire vessel analysis, axial distribution of vascular change was assessed by segmental analysis performed in 3 equally divided subsegments: proximal, middle, and distal segments of the first 50 mm of LAD.(10)
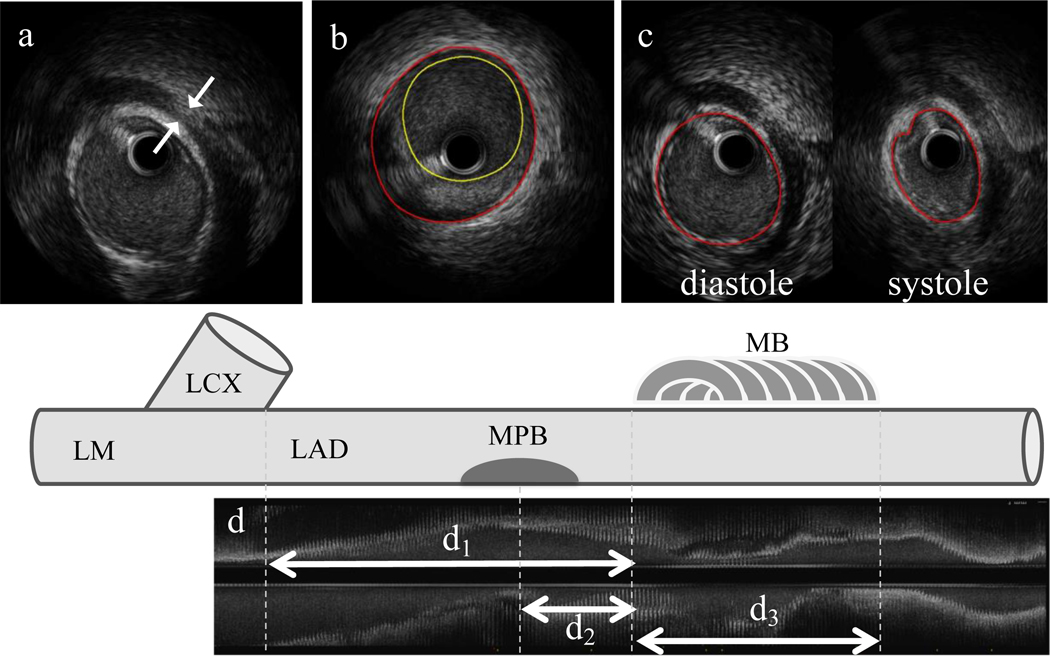
MB was defined as an echolucent band (halo) lying on top of the artery (Figure 1).(11) The reproducibility of IVUS-defined MB was evaluated by inter-observer valiance of 2 independent investigators blinded to each other’s results. Anatomical IVUS assessment of MB included length of the MB segment and thickness of the halo: MB length was measured from the first proximal appearance of the halo (MB entrance) to its distal end (MB exit), and thickness of the halo was measured at the thickest part above the artery during diastole. Location of MB was assessed as a distance from the LAD ostium to the MB entrance. Maximum plaque burden (intimal area / vessel area × 100, %) proximal to the MB was also obtained in a 20-mm non-tunneled segment immediately proximal to the MB entrance (maxPB20mm).(6) For functional MB assessment, arterial compression was calculated as the decrease in vessel area during systole standardized by vessel area at end-diastole (%). The reproducibility of IVUS-defined arterial compression at this core laboratory was reported previously.(6)
Figure 1.
IVUS Measurement of MB Properties.
MB was defined as an echolucent band (halo) partially surrounding the artery.
a. Thickness of a halo (white arrows) was measured by caliper in diastole.
b: MPB within 20-mm proximal to MB was measured by planimetry. Red circle: vessel area; yellow circle: lumen area.
c: Arterial compression was calculated as a change in vessel area during the cardiac cycle standardized by diastolic vessel area.
d: Longitudinal IVUS view showing distance and length measurements: d1 = distance from LAD ostium to MB entrance; d2 = distance from MPB to MB entrance; d3 = length of MB.
MB: myocardial bridging, MPB: maximum plaque burden, IVUS: intravascular ultrasound, LAD: left anterior descending artery, LM: left main, LCX: left circumflex.
Statistical Analysis
Statistical calculations were performed with JMP® 10 (SAS Institute Inc.). Data are expressed as frequencies and percentages for categorical variables, and as mean±SD or median with interquartile range for continuous variables. Categorical comparisons were performed using a chi-square test or Fisher exact test. Continuous values were compared using the unpaired or paired Student t test, Wilcoxon rank sum test, Mann-Whitney U test, or 1-way analysis of variance (ANOVA), as appropriate. A 2-way repeated measures ANOVA was used to test for group and time effects and their interactions. Associations between continuous variables were investigated using linear regression analysis. Survival analysis was performed by applying the Kaplan-Meier method and the log-rank test. Hazard ratios (HR) and 95% confidence intervals (CI) were analyzed with Cox proportional hazards regression models to identify factors associated with the study’s primary endpoint. A p-value <0.05 was considered statistically significant.
RESULTS
Baseline Characteristics and Clinical Outcomes
The inclusion criteria were met in 118 consecutive patients with prescheduled serial IVUS examination. Among these, 15 were excluded because of missing IVUS images (n=3), poor quality images due to nonuniform rotational distortion (n=4), air bubbles (n=1), partial scanning (n=6), or inconsistent pullback (n=1). As a result, 103 patients were included in this analysis. During the first-year post-transplant, ACR occurred in 27 patients (26.2%). After one-year post-transplant, patients were followed for up to 12.2 years (median: 4.7 years). During this period, 19 patients died (12 cardiac deaths, 1 from ACR, 1 from cancer, 2 from infection, and 3 of unknown causes) and 1 patient underwent re-transplantation.
IVUS identified MB in 64 patients (62.1%). The inter-observer valiance for the detection of MB was evaluated using randomly selected 30 patients, which verified the satisfactory agreement with Cohen’s kappa coefficient of 0.93.
Quantitative characteristics of MB were as follows: MB entrance was located at 39.7±9.4 mm distal to the LAD ostium; MB length was 19.5±10.5 mm; halo thickness was 0.56±0.33 mm and arterial compression was 19.2±11.6%.
Baseline clinical characteristics, medications, incidence of ACR, and laboratory findings during the first-year post-transplant were similar between patients with and without MB, except for higher prevalence of diabetes mellitus and more frequent use of insulin and tacrolimus in patients with MB (Table 1).
Table 1.
Clinical Characteristics
| All Patients (n=103) | Non-MB (n=39) | MB (n=64) | p | |
|---|---|---|---|---|
|
| ||||
| Recipient Profile | ||||
|
| ||||
| Age (yr) | 48 ± 16 | 46 ± 18 | 50 ± 15 | 0.25 |
| Sex (male, %) | 68.9 | 69.2 | 68.8 | 0.96 |
| Height (cm) | 171.5 ± 11.2 | 170.6 ± 14.3 | 172.0 ± 8.8 | 0.544 |
| Weight (kg) | 76.9 ± 17.7 | 76.0 ± 20.1 | 77.4 ± 16.1 | 0.67 |
| Body Mass Index (kg/m2) | 26.0 ± 5.2 | 25.9 ± 5.7 | 26.1 ± 4.8 | 0.88 |
| CMV IgG Positive (%) | 65.1 | 53.9 | 71.9 | 0.06 |
| Donor/recipient CMV Primary Mismatch (%) | 39.8 | 46.2 | 35.9 | 0.30 |
| Family History of CAD (%) | 14.5 | 14.3 | 14.8 | 0.96 |
| Diabetes (%) | 14.6 | 2.6 | 21.9 | 0.007 |
| Hypertension (%) | 36.9 | 35.9 | 37.5 | 0.87 |
| Hyperlipidemia (%) | 28.2 | 30.8 | 26.6 | 0.65 |
|
| ||||
| Donor Profile | ||||
|
| ||||
| Age (yr) | 30 ± 13 | 30 ± 15 | 30 ± 12 | 0.98 |
| Sex (male, %) | 73.8 | 71.8 | 75.0 | 0.72 |
| Donor-recipient Sex Mismatch (%) | 22.3 | 23.1 | 21.9 | 0.89 |
| Cold Ischemic Time (min) | 221.4 ± 50.0 | 212.1 ± 52.4 | 226.9 ± 48.1 | 0.17 |
| Medications | ||||
| Statins at 1 yr (%) | 94.2 | 92.3 | 95.3 | 0.53 |
| Antidiabetic Medications* at 1yr (%) | 25.2 | 18.0 | 29.7 | 0.18 |
| Insulin at 1 yr (%) | 23.3 | 12.8 | 29.7 | 0.049 |
| Immunosuppressive Regimen | ||||
| rATG (%) | 37.9 | 35.0 | 39.1 | 0.75 |
| Tacrolimus (%) | 51.5 | 38.5 | 59.4 | 0.04 |
| Sirolimus (%) | 13.6 | 12.8 | 14.1 | 0.86 |
| Laboratory and Other Test Findings | ||||
| Total Cholesterol at 1 yr, (mg/dl) | 171 ± 40 | 166 ± 40 | 175 ± 39 | 0.28 |
| Triglycerides at 1 yr (mg/dl) | 148 ± 101 | 143 ± 97 | 151 ± 104 | 0.70 |
| LVEF at 1 yr (%) | 60.3 ± 7.8 | 61.1 ± 4.5 | 59.8 ± 9.2 | 0.43 |
| Acute Cellular Rejection ≥2R (%) | 27.1 | 25.6 | 28.1 | 0.78 |
Values are expressed as % or mean ± SD.
Oral antidiabetic drugs and/or insulin.
MB = myocardial bridging; CMV = cytomegalovirus; CAD = coronary artery disease; IgG = immunoglobulin G; rATG = rabbitantithymocyte globulin; LVEF = left ventricular ejection fraction.
Serial IVUS Results in Patients With Versus Without Myocardial Bridging
At baseline, vessel volumes did not differ significantly between patients with and without MB (Table 2). In contrast, there was a significant difference in intimal volumes between the 2 groups: MB patients had smaller intimal volume in the distal LAD than non-MB patients (p=0.0002).
Table 2.
Baseline IVUS Indices
| All(n=103) | Non-MB (n=39) | MB (n=64) | p value | |
|---|---|---|---|---|
| Vessel VI (mm3/mm) | ||||
| Entire Vessel | 15.05 ± 3.50 | 15.02 ± 4.01 | 15.07 ± 3.19 | 0.95 |
| Proximal Segment | 18.70 ± 4.29 | 17.83 ± 4.54 | 19.23 ± 4.07 | 0.11 |
| Middle Segment | 15.26 ± 4.26 | 15.30 ± 4.63 | 15.24 ± 4.06 | 0.95 |
| Distal Segment | 11.20 ± 3.43 | 11.94 ± 3.84 | 10.75 ± 3.09 | 0.09 |
| Lumen VI (mm3/mm) | ||||
| Entire Vessel | 12.35 ± 2.92 | 11.98 ± 3.35 | 12.58 ± 2.63 | 0.32 |
| Proximal Segment | 15.26 ± 3.81 | 14.20 ± 3.88 | 15.92 ± 3.63 | 0.03 |
| Middle Segment | 12.46 ± 3.49 | 12.11 ± 3.82 | 12.67 ± 3.29 | 0.43 |
| Distal Segment | 9.33 ± 2.83 | 9.64 ± 3.19 | 9.15 ± 2.59 | 0.39 |
| Intimal VI (mm3/mm) | ||||
| Entire Vessel | 2.70 ± 1.38 | 3.04 ± 1.65 | 2.50 ± 1.16 | 0.05 |
| Proximal Segment | 3.43 ± 1.76 | 3.64 ± 1.90 | 3.31 ± 1.69 | 0.37 |
| Middle Segment | 2.80 ± 1.74 | 3.19 ± 2.02 | 2.57 ± 1.51 | 0.08 |
| Distal Segment | 1.86 ± 1.09 | 2.30 ± 1.43 | 1.61 ± 0.72 | 0.0002 |
Values are expressed as mean ± SD.
VI = volume index; MB = myocardial bridging; IVUS = intravascular ultrasound.
In serial analysis of the overall population, vessel volume decreased from baseline to one-year post-transplant (15.1±3.5 to 14.0±3.6 mm3/mm; p<0.0001) with an increase in intimal volume (2.7±1.4 to 3.2±1.7 mm3/mm; p<0.0001). During this follow-up period, vessel volume decreased diffusely irrespective of the presence of MB (Figure 2). Intimal growth was diffusely distributed in patients without MB (Figure 2). In contrast, patients with MB demonstrated a significantly greater increase in intimal volume at the proximal LAD segment compared with the middle and distal LAD segments (p=0.037) (Figures 2 and 3). Accordingly, lumen loss in the proximal segment tended to be greater in MB patients than in non-MB patients (p=0.17).
Figure 2.
Serial Changes in IVUS Indices
During one-year post-transplant, vessel volume decreased diffusely irrespective of the presence of myocardial bridging (MB). Intimal growth was also diffusely distributed in patients without MB, whereas patients with MB demonstrated a significantly greater increase in intimal volume at the proximal LAD segment compared with the middle and distal LAD segments. Changes (Δ) are expressed as percentages (%) standardized by baseline values.
Figure 3.
A Representative Case
Left: longitudinal IVUS images of the first 50 mm of LAD; Right: cross-sectional IVUS images at the proximal, middle and distal segments (upper: baseline, lower: one-year follow-up, respectively). MB (blue arrows) was observed primarily at the distal segment. The proximal segment showed a greater increase in intimal volume during the follow-up, compared with the middle and distal segments.
In patients with MB, the maxPB20mm was 24.1±10.2% at baseline and 34.3±14.1% at follow-up. Among the baseline MB characteristics, only arterial compression showed a correlation with the maxPB20mm at one year (Figure 4).
Figure 4.
Correlation between MB Characteristics and maxPB20mm at 1 year
Among baseline MB characteristics, only arterial compression showed a positive correlation with maxPB20mm at one-year follow-up.
Late Mortality in Patients With Versus Without Myocardial Bridging
In this study population, a total of 19 patients died during follow-up (17 patients with MB: 2 patients without MB). In the MB group, all but 4 deaths (2 infection, 1 accident and 1 undefined) were confirmed as cardiovascular events directly related to CAV (8 heart failure, 4 sudden cardiac death and 1 myocardial infarction). In the non-MB group, only one patient had sudden cardiac death and the other died of an undefined cause.
All-cause death after one-year post-transplant was significantly higher in patients with MB compared to those without MB (26.6% vs. 5.1%, p=0.0065). Kaplan-Meier analysis over a median follow-up period of 4.7 years revealed a significantly lower event-free survival in patients with MB compared with those without MB (HR=3.9, log-rank p=0.02) (Figure 5). Although there were several clinical differences between patients with and without MB, presence of MB was independently associated with late adverse events (all-cause death and re-transplantation) in the multivariate analysis (HR=5.1, p=0.003) that included all variables with a p-value ≤0.05 on univariate analysis (Table 3). This finding also persisted (HR=4.9, p=0.03) when the analysis was limited to cardiac death and re-transplantation (Table 4).
Figure 5.
Long-term Survival: Patients With Versus Without MB
Kaplan-Meier analysis over a median follow-up period of 4.7 years demonstrated a significantly lower event-free survival in patients with versus without MB. HR = hazard ratio; CI = confidence interval.
Table 3.
Factors Associated With All-Cause Death and/or Re-Transplantation
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| HR | 95% CI | p value | HR | 95% CI | p value | |
|
| ||||||
| Recipient Age, per 1 yr | 1.02 | 0.98–1.04 | 0.46 | |||
| Donor Cytomegalovirus IgG Positive (%) | 1.57 | 0.64–4.20 | 0.33 | |||
| Diabetes at Baseline | 1.82 | 0.52–5.05 | 0.32 | |||
| Hypertension at Baseline | 0.33 | 0.05–1.18 | 0.09 | |||
| Hyperlipidemia at Baseline | 0.54 | 0.16–1.50 | 0.25 | |||
| Total Cholesterol at 1 yr, per 1 mg/dl | 1.01 | 0.996–1.02 | 0.16 | |||
| Triglycerides at 1 yr, per 1 mg/dl | 1.00 | 0.997–1.00 | 0.58 | |||
| Insulin Use | 1.80 | 0.67–4.40 | 0.23 | |||
| LVEF at 1 yr, per −1% | 0.98 | 0.93–1.03 | 0.38 | |||
| Acute Cellular Rejection Grade ≥2R During the First Year | 4.36 | 1.79–10.96 | 0.002 | 3.49 | 1.30–9.80 | 0.01 |
| rATG | 0.38 | 0.02–2.25 | 0.32 | |||
| Tacrolimus | 0.24 | 0.04–0.89 | 0.03 | 0.22 | 0.03–0.82 | 0.02 |
| Sirolimus | 2.24 | 0.86–6.50 | 0.09 | |||
| Female Donor | 1.94 | 0.75–4.72 | 0.17 | |||
| Donor-recipient Sex Mismatch | 0.37 | 0.06–1.28 | 0.13 | |||
| Tansplant-period (2002 – 2007 vs. 2008 – 2013) | 1.74 | 0.44–8.53 | 0.44 | |||
| Donor Atherosclerosis (MIT at Baseline ≥ 0.5mm) | 0.65 | 0.27–1.59 | 0.34 | |||
| Baseline Vessel Volume, per 1% | 1.00 | 0.89–1.13 | 0.97 | |||
| Baseline Lumen Volume, per 1% | 1.02 | 0.89–1.17 | 0.77 | |||
| Baseline Intimal volume, per 1% | 0.92 | 0.64–1.22 | 0.58 | |||
| Increase in MIT ≥0.5mm During the First Year | 1.46 | 0.53–5.17 | 0.49 | |||
| Delta Vessel Volume During the First Year | ||||||
| Entire | 0.95 | 0.92–0.99 | 0.01 | |||
| Proximal | 0.97 | 0.94–0.998 | 0.04 | |||
| Middle | 0.97 | 0.94–1.01 | 0.11 | |||
| Distal | 0.96 | 0.93–0.99 | 0.007 | 0.96 | 0.87–1.05 | 0.38 |
| Delta Lumen Volume During the First Year | ||||||
| Entire | 0.95 | 0.92–0.99 | 0.005 | |||
| Proximal | 0.97 | 0.94–0.995 | 0.02 | |||
| Middle | 0.97 | 0.94–1.00 | 0.08 | |||
| Distal | 0.96 | 0.93–0.99 | 0.007 | 1.00 | 0.92–1.10 | 0.99 |
| Delta Intimal Volume During the First Year | ||||||
| Entire | 1.00 | 0.99–1.01 | 0.48 | |||
| Proximal | 1.00 | 1.00–1.01 | 0.32 | |||
| Middle | 1.00 | 0.99–1.01 | 0.52 | |||
| Distal | 1.00 | 0.99–1.01 | 0.89 | |||
| MB | 3.80 | 1.27–16.30 | 0.01 | 5.06 | 1.65–22.17 | 0.003 |
All variables with a p-value ≤0.05 on univariate analysis were included in multivariate analysis.
CI = confidence interval; HR = hazard ratio; MIT = maximum intimal thickness; other abbreviations as in Table 1.
Table 4.
Factors Associated With Cardiac Death and/or Re-Transplantation
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| HR | 95% CI | p value | HR | 95% CI | p value | |
|
| ||||||
| Recipient Age, per 1 yr | 0.97 | 0.95–0.998 | 0.03 | 0.97 | 0.93–1.00 | 0.051 |
| Donor Cytomegalovirus IgG Positive (%) | 1.20 | 0.42–3.64 | 0.74 | |||
| Diabetes at Baseline | 1.13 | 0.18–4.17 | 0.87 | |||
| Hypertension at Baseline | 0.24 | 0.01–1.21 | 0.09 | |||
| Hyperlipidemia at Baseline | 0.33 | 0.05–1.23 | 0.11 | |||
| Total Cholesterol at 1 yr, per 1 mg/dl | 1.01 | 0.99–1.02 | 0.22 | |||
| Triglycerides at 1 yr, per 1 mg/dl | 1.00 | 0.995–1.00 | 0.96 | |||
| Insulin Use | 1.85 | 057–5.38 | 0.28 | |||
| LVEF at 1 yr, per −1% | 0.99 | 0.94–1.06 | 0.76 | |||
| Acute Cellular Rejection Grade ≥2R During the First Year | 2.61 | 0.85–7.61 | 0.09 | |||
| rATG | 0.91 | 0.04–7.22 | 0.93 | |||
| Tacrolimus | 0.41 | 0.06–1.59 | 0.22 | |||
| Sirolimus | 3.28 | 1.11–9.68 | 0.03 | 3.68 | 0.99–14.40 | 0.052 |
| Female Donor | 1.73 | 0.53–5.01 | 0.34 | |||
| Donor-recipient Sex Mismatch | 0.53 | 0.08–1.94 | 0.37 | |||
| Transplant-period (2002 – 2007 vs. 2008 – 2013) | 1.34 | 0.26–10.21 | 0.74 | |||
| Donor Atherosclerosis (MIT at Baseline ≥ 0.5mm) | 0.87 | 0.30–2.66 | 0.80 | |||
| Baseline Vessel Volume, per 1% | 0.94 | 0.82–2.08 | 0.40 | |||
| Baseline Lumen Volume, per 1% | 0.93 | 0.66–1.35 | 0.93 | |||
| Baseline Intimal volume, per 1% | 1.01 | 0.94–1.07 | 0.73 | |||
| Increase in MIT ≥0.5mm During the First Year | 1.97 | 0.54–12.72 | 0.33 | |||
| Delta Vessel Volume During the First Year | ||||||
| Entire | 0.95 | 0.92–0.995 | 0.03 | |||
| Proximal | 0.97 | 0.93–1.00 | 0.07 | |||
| Middle | 0.98 | 0.94–1.02 | 0.34 | |||
| Distal | 0.95 | 0.91–0.99 | 0.004 | 1.06 | 0.93–1.19 | 0.38 |
| Delta Lumen Volume During the First Year | ||||||
| Entire | 0.96 | 0.92–0.99 | 0.03 | |||
| Proximal | 0.97 | 0.94–1.00 | 0.09 | |||
| Middle | 0.98 | 0.95–1.02 | 0.26 | |||
| Distal | 0.95 | 0.91–0.99 | 0.004 | 0.91 | 0.81–1.00 | 0.12 |
| Delta Intimal Volume During the First Year | ||||||
| Entire | 0.99 | 0.98–1.01 | 0.56 | |||
| Proximal | 1.00 | 0.98–1.01 | 0.41 | |||
| Middle | 1.00 | 0.99–1.01 | 0.96 | |||
| Distal | 1.00 | 0.98–1.01 | 0.60 | |||
| MB | 4.01 | 1.09–25.80 | 0.04 | 4.92 | 1.18–34.60 | 0.03 |
DISCUSSION
The main findings of this study are as follows: 1) MB was present in 62% of the study population; 2) unlike patients without MB who demonstrated diffuse intimal growth along the entire LAD during the first-year post-transplant, patients with MB had significantly augmented intimal proliferation in the proximal LAD segment; and 3) presence of MB was an independent predictor for long-term mortality and/or re-transplantation in multivariate analyses. To the best of our knowledge, this is the first study to suggest that MB may play a role in long-term outcomes in heart-transplant recipients, as is the case of native atherosclerosis.
Prevalence of Myocardial Bridging in Heart-Transplant Recipients
The prevalence of MB remains poorly defined, especially in heart-transplant recipients. In studies involving native coronary, the prevalence of MB has been reported to be 0.4–16% by angiography, 23% by IVUS, 3.5–58% by multidetector computed tomography (MDCT), and up to 85% in autopsy series.(1,12–14) Aside from the diverse study populations, the wide range of those reported rates are likely attributable to the different sensitivities of various diagnostic modalities for the detection of MB. Specifically, contrast angiography is considered one of the least sensitive diagnostic methods, as MB can be diagnosed only indirectly by detecting systolic squeezing or “milking” of the artery, rather than by directly visualizing the MB structure. Nevertheless, a previous transplant study reported the angiographic prevalence of MB as 33%,(15) which is considerably higher than that reported by angiography in native coronary studies. The present IVUS study also identified MB more frequently compared with a previous IVUS report investigating non-transplant patients with native LAD lesions.(12) Considering the heart transplantation procedure, it is unlikely that the surgical maneuver itself can contribute to the higher prevalence or detection rates of MB by angiography and IVUS. One may rather hypothesize that the higher prevalence of MB in patients with heart transplantation may be due to the fact that heart-transplant donors are different from the general population in several important ways. Specifically, stroke, head trauma, and anoxia are reported as the most frequent causes of donor brain death, accounting for 97% of cadaveric solid organ donors in the United States,(16) some of which might have been related to clinically unrecognized arrhythmia, accidents due to syncope, or other concealed adverse cardiac events possibly associated with MB. Although this hypothesis is yet to be investigated by systematic evaluation of a large transplant population, MB does not appear to be a rare finding in patients with heart transplantation, thus highlighting the importance of the current study results.
Effects of Myocardial Bridging on Development of Cardiac Allograft Vasculopathy
In patients with native coronary atherosclerosis, it has been widely recognized that the presence of MB contributes to the distribution of atherosclerosis in the LAD. Specifically, multiple studies using angiography, IVUS, MDCT, or autopsy have demonstrated that the LAD segment proximal to the MB is vulnerable to the development of atherosclerosis.(17) This preferential location of atherosclerotic plaque formation in relation to the MB likely results from the unique hemodynamic stress of this segment driven by the force of the MB muscle contraction.(6) This includes lower wall shear stress and abnormal non-laminar flow profiles (i.e., oscillatory flow reversal with or without significant collision with antegrade coronary flow) both of which are postulated to predispose to enhanced lipid transfer across the endothelium and resultant atherosclerosis. A recent clinical study with computational fluid dynamics showed that WSS in the proximal segment was lower in MB patients compared with patients without an MB, with an inverse correlation between the proximal segment WSS and the MB systolic compression.(18) As expected, there was an inverse correlation between WSS in the proximal segment and proximal plaque burden as well. The significant correlation of proximal plaque burden with arterial compression but not with other anatomical MB properties was also demonstrated in our previous IVUS study of non-transplant MB patients.(6) The current study data shown in Figure 4 are aligned with the results of these native coronary studies while the relatively rapid increase in proximal plaque burden during the one-year follow-up observed in the MB patients (Figure 2) may indicate potential acceleration of this vascular reaction owing to possible interaction with alloimmune responses after heart transplantation. Finally, a clinical physiology study also demonstrated that the intracoronary pressure within the LAD segment proximal to the MB was significantly higher than the aortic blood pressure, suggesting potential augmentation of intimal injury at this segment.(19)
Although the pathophysiology of CAV is considerably different from that of native coronary atherosclerosis, the vascular effects of MB discussed above may theoretically enhance the development of CAV by mediating endothelial injury and/or dysfunction leading to vascular inflammation. Despite CAV being traditionally referred to as “chronic rejection”, recent studies suggest that vascular lesions of transplanted hearts result from complex interactions between nonspecific insults and alloimmune responses.(20) The alloantigen-independent factors inducing endothelial dysfunction could directly or indirectly trigger alloimmune responses by activating complement and coagulation pathways, recruiting inflammatory cells, promoting trafficking of recipient dendritic cells into the allograft, and regulating T-cell differentiation. Separate from those immunologic pathways, the presence of MB, as a congenital variant existing in the donor heart prior to the transplant, might also contribute to higher prevalence of donor-transmitted atherosclerotic disease in the proximal LAD segment, potentially adding another risk for the development of vascular lesions at this important coronary segment.
Impact of Myocardial Bridging on Long-Term Mortality
The present study revealed that presence of MB was independently associated with long-term patient and/or graft failure after heart transplantation. In the general population, MB has been shown to cause myocardial ischemia, conduction disturbances,(19) and myocardial infarction,(3) possibly leading to sudden cardiac death in some cases.(4) Several different mechanisms have been proposed to account for these adverse events: 1) alteration in the effective blood perfusion of the affected myocardial territory owing to delayed arterial relaxation in diastole as often represented by decreased diastolic fractional flow reserve (dFFR); 2) the Venturi effect on the septal branches that can cause functional ischemia of the septal wall; 3) coronary vasospasm and platelet aggregation stimulated by external systolic compression and endothelial dysfunction; and 4) accelerated plaque formation in the segment proximal to the MB as investigated in the current study. While all these possible mechanisms may apply to heart-transplant recipients, cardiac denervation associated with the transplantation procedure may further affect the impaired coronary physiology in patients with MB by shortening of diastole due to increased resting heart rates (typically 80–100 bpm) that is often unresponsive to beta blockers. Sustained tachycardia in response to physical stress is commonly seen in the absence of vagal reinnervation, which may also exacerbate the abnormal coronary physiology in patients with MB during daily activity.
One intriguing question regards the stability of the vascular disease located proximally to the MB in heart-transplant patients. In native coronary atherosclerosis, a recent autopsy series of patients with LAD-related infarction (with or without MB) and normal hearts with MB suggested that plaques in the LAD proximal to the MB are prone to rupture, resulting in myocardial infarction at a younger age.(17) While the present study was unable to address this question due to the limited tissue characterization capability of grayscale IVUS, detailed assessment of plaque vulnerability using advanced imaging techniques, such as radiofrequency IVUS, optical coherence tomography, or hybrid IVUS with near-infrared diffuse reflectance spectroscopy, might be informative. In this context, long-term effects of stenting in such lesions may also be a clinically relevant question. Despite the diffuse nature of CAV overall, a recent clinical IVUS study demonstrated that the vessel change in the proximal LAD segment early after heart transplantation was the primary determinant of long-term mortality or re-transplantation.(10) In native coronary arteries, stenting the lesion proximal to MB is associated with favorable outcomes as long as inadvertent stent placement into the MB segment is avoided.(21) On the other hand, it remains unknown whether this focal treatment itself (i.e., stenting the proximal lesion without correcting the significant hemodynamic abnormalities caused by the distal MB) can serve as an effective treatment option to improve the long-term outcomes of transplant recipients. In the current study, none of the enrolled patients underwent stenting in the LAD during the follow-up, and therefore, further studies are warranted to address this important question.
Clinical Implications
Aside from its prognostic utility for risk stratification of heart-transplant recipients, one clinically important question is whether long-term clinical outcomes could be improved through treatment of MB found in the donor heart. In general, therapeutic strategies for patients with symptomatic MB include 3 proposed options: medication, stent placement in the tunneled segment, and surgical treatment. Medical management primarily consists of beta-blockers to reduce compression of the artery by the muscular band and slow the heart rate, thereby increasing the diastolic period. While this noninvasive treatment may help improve the hemodynamic abnormalities (i.e., coronary flow disturbance and intracoronary pressure augmentation), its possible protective effect against CAV development remains to be investigated. Stent placement can directly reduce the coronary compression by the MB, but controversy exists due to potential complications, such as coronary perforation, stent strut fracture, and instent restenosis, derived from mechanical interaction between the implanted device and MB. In contrast, surgical treatment of MB with supra-arterial myotomy (or “unroofing”) can directly address the pathology with durable normalization of both anatomic and hemodynamic abnormalities.(22) This procedure has been attempted in symptomatic MB patients since 1975 with excellent mid and long-term outcomes reported. Surgical treatment may be preferable particularly for pediatric patients or patients with a long MB who are unsuitable for stent placement in the bridged segment. In heart transplantation, it would be ideal if an adjunctive unroofing procedure could be performed during the transplant surgery, although the epicardial adipose tissue overlying the LAD artery usually precludes operators from precisely identifying a tunneled coronary segment. Since complete unroofing with minimum risk of surgical complications is considered crucial for successful results, adding detailed diagnostic evaluation, such as stress echocardiography, IVUS, dFFR,(19) or MDCT, early after transplant surgery would be a more realistic strategy to determine the optimal treatment for a given patient. In particular, despite its lower spatial resolution than IVUS, MDCT may offer unique advantages as a widely available clinical imaging tool for noninvasive screening of MBs. Previous clinical studies demonstrated reasonable agreements between MDCT and IVUS for the assessment of length, location, and depth of MBs with direct comparison of the two modalities in non-transplant patients.(23) (24) Finally, further investigation is required to define high-risk patients who would most benefit from invasive treatment of MB.
Study Limitations
As this was a retrospective, single-center study with a relatively small sample size, the present findings need to be confirmed in prospective trials with predefined endpoints. Indeed, in the current study, patients with MB had higher prevalence of diabetes mellitus and more frequent use of insulin. Since the prevalence of MB in donor hearts, as well as the diagnostic accuracy of IVUS, would not be affected by recipients’ baseline comorbidities, this difference is likely attributed to the small sample size of the study. While multivariate analysis with Cox proportional hazards regression models showed the independent impact of MB on long-term mortality, this bias may theoretically have affected the unadjusted IVUS results of CAV progression. Proximal plaque progression as the primary IVUS finding, however, was not significantly different between the MB patients with versus without diabetes (32.3% vs. 27.4%, respectively; p=0.52). In addition, even when confining the analysis population to non-diabetic patients (n=88, 50 MB: 38 non-MB), a significantly lower event-free survival in patient with MB remained compared with those without MB (HR: 5.9, Log-Rank p=0.008, 95% CI: 1.63–37.42).
Our standard post-transplant immunosuppressive regimen has changed over time, such that patients were treated with different regimens during the study period. Specifically, in the early phase, sirolimus was used selectively for patients with severe or recurrent rejection, significant CAV progression, and/or renal insufficiency, which most likely explains the worse outcome in patients treated with sirolimus in the current analysis, even after the adjustment with known factors by multivariate analysis. It is also worth noting that tacrolimus was more frequently used in patients with MB, although multivariate analysis suggested a possible protective effect of tacrolimus with respect to patient and graft survival (i.e., opposite to the deleterious effect of MB).
Finally, as discussed earlier, the present study was conducted with conventional grayscale IVUS technology with limited capability of tissue characterization. This study was not designed to further stratify MB patients using anatomic and/or functional IVUS findings with sufficient statistical power, which warrants future investigation in larger populations.
CONCLUSIONS
Presence of MB appeared to be associated with accelerated proximal plaque proliferation and reduced long-term survival in patients after heart transplantation. Further investigation is warranted to evaluate the possible benefits of adjunctive treatment of MB to improve long-term clinical outcomes.
HIGHLIGHTS.
Myocardial bridging in patients after heart transplantation:
might have higher prevalence than considered;
appeared to be associated with accelerated proximal plaque proliferation,
and reduced long-term survival.
ACKNOLEDGMENTS
The authors appreciate Heidi N. Bonneau, RN, MS, CCA for her expert review and editing advice.
FUNDS:
This study was funded in part by R01 HL093475 (WFF) from the National Institutes of Health, (National Heart Lung and Blood Institute), Bethesda, MD.
Footnotes
DISCLOSURE: Nothing to disclose
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Ishikawa Y, Akasaka Y, Ito K et al. Significance of anatomical properties of myocardial bridge on atherosclerosis evolution in the left anterior descending coronary artery. Atherosclerosis 2006;186:380–9. [DOI] [PubMed] [Google Scholar]
- 2.Iversen S, Hake U, Mayer E, Erbel R, Diefenbach C, Oelert H. Surgical treatment of myocardial bridging causing coronary artery obstruction. Scand J Thorac Cardiovasc Surg 1992;26:107–11. [DOI] [PubMed] [Google Scholar]
- 3.Ishikawa Y, Akasaka Y, Suzuki K et al. Anatomic properties of myocardial bridge predisposing to myocardial infarction. Circulation 2009;120:376–83. [DOI] [PubMed] [Google Scholar]
- 4.Desseigne P, Tabib A, Loire R. [Myocardial bridging on the left anterior descending coronary artery and sudden death. Apropos of 19 cases with autopsy]. Arch Mal Coeur Vaiss 1991;84:511–6. [PubMed] [Google Scholar]
- 5.Loukas M, Von Kriegenbergh K, Gilkes M et al. Myocardial bridges: A review. Clin Anat 2011;24:675–83. [DOI] [PubMed] [Google Scholar]
- 6.Yamada R, Tremmel JA, Tanaka S et al. Functional Versus Anatomic Assessment of Myocardial Bridging by Intravascular Ultrasound: Impact of Arterial Compression on Proximal Atherosclerotic Plaque. J Am Heart Assoc 2016;5:e001735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ishikawa Y, Kawawa Y, Kohda E, Shimada K, Ishii T. Significance of the anatomical properties of a myocardial bridge in coronary heart disease. Circulation journal : official journal of the Japanese Circulation Society 2011;75:1559–66. [DOI] [PubMed] [Google Scholar]
- 8.Kim JW, Seo HS, Na JO et al. Myocardial bridging is related to endothelial dysfunction but not to plaque as assessed by intracoronary ultrasound. Heart 2008;94:765–9. [DOI] [PubMed] [Google Scholar]
- 9.Stehlik J, Edwards LB, Kucheryavaya AY et al. The Registry of the International Society for Heart and Lung Transplantation: 29th official adult heart transplant report−-2012. J Heart Lung Transplant 2012;31:1052–64. [DOI] [PubMed] [Google Scholar]
- 10.Okada K, Kitahara H, Yang HM et al. Paradoxical Vessel Remodeling of the Proximal Segment of the Left Anterior Descending Artery Predicts Long-Term Mortality After Heart Transplantation. JACC Heart Fail 2015;3:942–52. [DOI] [PubMed] [Google Scholar]
- 11.Yamada R, Turcott RG, Connolly AJ et al. Histological characteristics of myocardial bridge with an ultrasonic echolucent band. Comparison between intravascular ultrasound and histology. Circulation journal : official journal of the Japanese Circulation Society 2014;78:502–4. [DOI] [PubMed] [Google Scholar]
- 12.Tsujita K, Maehara A, Mintz GS et al. Comparison of angiographic and intravascular ultrasonic detection of myocardial bridging of the left anterior descending coronary artery. Am J Cardiol 2008;102:1608–13. [DOI] [PubMed] [Google Scholar]
- 13.Kim PJ, Hur G, Kim SY et al. Frequency of myocardial bridges and dynamic compression of epicardial coronary arteries: a comparison between computed tomography and invasive coronary angiography. Circulation 2009;119:1408–16. [DOI] [PubMed] [Google Scholar]
- 14.Mohlenkamp S, Hort W, Ge J, Erbel R. Update on myocardial bridging. Circulation 2002;106:2616–22. [DOI] [PubMed] [Google Scholar]
- 15.Wymore P, Yedlicka JW, Garcia-Medina V et al. The incidence of myocardial bridges in heart transplants. Cardiovasc Intervent Radiol 1989;12:202–6. [DOI] [PubMed] [Google Scholar]
- 16.Singhal AK, Sheng X, Drakos SG, Stehlik J. Impact of donor cause of death on transplant outcomes: UNOS registry analysis. Transplant Proc 2009;41:3539–44. [DOI] [PubMed] [Google Scholar]
- 17.Ishikawa Y, Akasaka Y, Akishima-Fukasawa Y et al. Histopathologic profiles of coronary atherosclerosis by myocardial bridge underlying myocardial infarction. Atherosclerosis 2013;226:118–23. [DOI] [PubMed] [Google Scholar]
- 18.Yong ASC, Pargaonkar VS, Wong CCY et al. Abnormal shear stress and residence time are associated with proximal coronary atheroma in the presence of myocardial bridging. Int J Cardiol 2021;340:7–13. [DOI] [PubMed] [Google Scholar]
- 19.Lin S, Tremmel JA, Yamada R et al. A novel stress echocardiography pattern for myocardial bridge with invasive structural and hemodynamic correlation. J Am Heart Assoc 2013;2:e000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmauss D, Weis M. Cardiac allograft vasculopathy: recent developments. Circulation 2008;117:2131–41. [DOI] [PubMed] [Google Scholar]
- 21.Tsujita K, Maehara A, Mintz GS et al. Impact of myocardial bridge on clinical outcome after coronary stent placement. Am J Cardiol 2009;103:1344–8. [DOI] [PubMed] [Google Scholar]
- 22.Boyd JH, Pargaonkar VS, Scoville DH et al. Surgical Unroofing of Hemodynamically Significant Left Anterior Descending Myocardial Bridges. Ann Thorac Surg 2017;103:1443–1450. [DOI] [PubMed] [Google Scholar]
- 23.Forsdahl SH, Rogers IS, Schnittger I et al. Myocardial Bridges on Coronary Computed Tomography Angiography-Correlation With Intravascular Ultrasound and Fractional Flow Reserve. Circulation journal : official journal of the Japanese Circulation Society 2017;81:1894–1900. [DOI] [PubMed] [Google Scholar]
- 24.Hashikata T, Honda Y, Wang H et al. Impact of Diastolic Vessel Restriction on Quality of Life in Symptomatic Myocardial Bridging Patients Treated With Surgical Unroofing: Preoperative Assessments With Intravascular Ultrasound and Coronary Computed Tomography Angiography. Circ Cardiovasc Interv 2021;14:e011062. [DOI] [PubMed] [Google Scholar]