Abstract
Introduction:
Vaccinations against preventable respiratory infections such as Streptococcus pneumoniae and influenza are important in immunosuppressed solid organ transplant (SOT) recipients. Little is known about the role of age, race, ethnicity, sex, and sociodemographic factors including rurality, or socioeconomic status (SES) associated with vaccine uptake in this population.
Methods:
We conducted a population-based study using the Rochester Epidemiology Project, a medical records linkage system, to assess socioeconomic and demographic factors associated with influenza and pneumococcal vaccination rates among adult recipients of solid organ transplantation (aged 19–64 years) living in four counties in southeastern Minnesota. Vaccination data were obtained from the Minnesota Immunization Information Connection from June 1, 2010 to June 30, 2020. Vaccination rate was assessed with Poisson and logistic regression models.
Results:
A total of 468 SOT recipients were identified with an overall vaccination rate of 57%–63% for influenza and 56% for pneumococcal vaccines. As expected, vaccination for pneumococcal vaccine positively correlated with influenza vaccination. Rural patients had decreased vaccination in both compared to urban patients, even after adjusting for age, sex, race, ethnicity, and SES. Although the population was mostly White and non-Hispanic, neither vaccination differed by race or ethnicity, but influenza vaccination did by SES. Among organ transplant groups, liver and lung recipients were least vaccinated for influenza, and heart recipients were least up-to-date on pneumococcal vaccines.
Conclusions:
Rates of vaccination were below national goals. Rurality was associated with undervaccination. Further investigation is needed to understand and address barriers to vaccination among transplant recipients.
Keywords: flu, geographic disparities, organ transplant, pneumococcal, vaccines
1 |. INTRODUCTION
Although infection is the most common non-cardiovascular cause of death after solid organ transplant (SOT), with respiratory infec tion the highest type of infections, vaccinated SOT recipients have decreased morbidity and mortality.1,2 There are many potential and harmful infections posttransplant, with influenza the most common vaccine-preventable infection within the first 5-year posttransplant.1,2
United States (US) vaccination recommendations are well delineated for SOT recipients. Yearly influenza vaccination is recommended for all adult SOT candidates and recipients.3 Likewise, prior to 2022, all SOT candidates and recipients should have received a 13-valent pneumococcal conjugate vaccine (PCV13) followed by pneumococcal vaccination with 23-valent pneumococcal polysaccharide vaccine (PPSV23) at least 8 weeks later. These should occur before transplant or within 2–6 months after transplant,4–10 with a booster 5 years after the first dose of PPSV23. However, vaccination rates are suboptimal— influenza coverage has been reported around 50% and pneumococcus at 25% among recipients of solid organ transplantation.11 Understanding the demographic traits and characteristics that increase the risk of undervaccination therefore becomes critically important in this population.
Health-care disparities may lead to differential access to or uptake of vaccinations. Given the screening and evaluation phases pretransplant, along with the intensive health-care follow-up in the first year following transplant surgery, these disparities may be quite different among transplant patients compared to the general population. In many facets of health care, underserved communities, such as those with lower socioeconomic status (SES), of minority races and ethnicity, and living in rural areas, experience disparities in health-care access. In non-transplant populations, vaccine disparities are clearly seen by race, with up to 9% lower coverage rates observed among Black persons compared to White persons.12,11 But a recent study of SOT patients showed that White and older patients were less likely to undergo vaccination.13 Very little is known about the role individual-level SES and geographic location play in vaccine uptake in the US. Additionally, there are limited data on disparities on vaccines in transplant populations, and we sought to understand the vaccine behaviors of the high-risk adult population aged 19–64 because they are historically less well vaccinated than the older population. Individual-level SES data are not routinely collected alongside vaccination data, making it difficult to parse out how SES impacts vaccination. Associations observed at the area-level suggest potentially lower rates of vaccine coverage among populations with lower SES, along with higher rates of hospitalizations for influenza in states with a higher proportion of persons living below the poverty line.14 Although there are sparse data thus far, geographic region is now being evaluated as a social determinant of health, and those living in urban settings with less poverty are more likely receive pneumococcal vaccinations.15–17
Our primary objective was to examine influenza and pneumococcal vaccination rates for SOT recipients by age, race, ethnicity, sex, rural status, SES, and marital status in a population-based study to better understand any vaccination disparities in this population.
2 |. METHODS
We conducted a population-based study to assess associations between patient demographics and vaccination status of SOT recipients for influenza and pneumococcus. Approval was obtained from the Mayo Clinic (21–004470) and Olmsted Medical Center (024-OMC-21) Institutional Review Boards.
2.1 |. Rochester Epidemiology Project (REP)
The Rochester Epidemiology Project (REP) (R01 AG34676) provides an established and unique research infrastructure for tracking the health of the catchment population in the Midwest. The REP links the health-care records of Olmsted Medical Center, Mayo Clinic, Mayo Clinic Health System, Olmsted County Public Health Services, Zumbro Valley Health Center, and other area health-care institutions including area pharmacies to provide extensive, longitudinal, population-based health-care information for persons residing in a 27-county region of southern MN and western WI (700 000 persons included from the 1.2 million residing in the area).18–21
2.2 |. Population
We utilized the population-based research infrastructure of the REP to examine demographics of individual-level SES as measured by the HOUsing-based SocioEconomic Status (HOUSES) index, race, and geographic region (urban/suburban/rural). This was assessed for association with the vaccination of influenza and pneumococcus among high-risk vaccine-eligible adults aged 19–64-year old with an SOT (heart, kidney, liver, and lung) transplantation as determined by Current Procedure Terminology (CPT), International Classification of Diseases (ICD)-9 or ICD-10 codes living in a four-county region (Olmsted, Wabasha, Dodge, Goodhue) in southeastern Minnesota between January 1, 2010 and June 30, 2020.
2.3 |. Demographics
Demographics, such as age, race, ethnicity, sex, address, and marital status, were collected from medical records via the REP. Address and age were used at the time of index date, which occurred at the start of the study or at the time of high-risk diagnosis. Race and ethnicity data were obtained through the medical records. If a patient was classified as falling into multiple race categories, they were considered ‘Other/Mixed’ race. Similarly, highest education level, as gathered from the REP, was narrowed to three groups for modeling: high school or less, some college, and college graduate or graduate school.
2.4 |. Transplant data
Transplant information was also obtained from REP, and each patient was included into the cohort based on the date of SOT within the time of the study. We also included patients when a transplant was received before the start of the study if the individuals still met the age criteria. If a patient had more than one SOT, their first transplant was used for classification purposes. Transplantation did not have to be performed within the catchment area of the REP, but the person did have to reside within the catchment area.
2.5 |. Socioeconomic status
HOUSES is a validated method that uses address-linked real property data from the county assessor’s office and matches to the subject’s address in the medical record.22 To capture the individual-level measure of SES, HOUSES utilizes publically available property data that are maintained by the county for taxation purposes. HOUSES includes data on assessed value, square footage of the housing unit, and a number of bedrooms and bathrooms.22–25 The address used was the one each patient resided in at the time of their index date. A standardized HOUSES index score was previously created after transforming variables to z score and then categorized into quartiles. HOUSES data, at the time of the patient’s index date, were available for our four REP counties, which is what determined the counties included in this study. Higher HOUSES quartile is associated with higher SES. Quartile 4 is the highest quartile. Each standardized quartile is based on the overall population. Thus, the study population does not always have a quarter of its population in each quartile. HOUSES has previously been used in SOT populations to study the role of SES in graft failure and in pediatric populations to study pertussis and human papillomavirus vaccinations.23–26
2.6 |. Rurality
Geographic status was classified by 2010 Rural–Urban Commuting Area (RUCA) codes after geocoding subjects addresses as of June 1, 2010 (or closest one to index date).27 Urban areas are those living in a RUCA code 1–3, suburban were those living in codes 4–7 with rural coded as 8–10.
2.7 |. Vaccination status
The state immunization registry Minnesota Immunization Information Connection (MIIC) was used to obtain influenza and pneumococcus vaccination data. MIIC captures statewide community pharmacies and health system administration as well as out-of-state immunizations recorded in the patient’s area medical records.19–21 Pneumococcal vaccination data were obtained dating back to 1997 to ensure that high-risk individuals were accurately and comprehensively captured as up-to-date on appropriate recommendations. Because universal influenza vaccination was recommended beginning in 2010, we evaluated data from July 1, 2010 to June 30, 2020.3
2.8 |. Ascertainment of vaccination status and timeliness
Vaccination timeframe for influenza vaccination was an annual vaccine each year (July 1 through June 30) per current Advisory Committee on Immunization Practices (ACIP) standards with timely vaccination considered by October 31 of each year.5,6,7,9 Timeliness and time undervaccinated can only be assessed in patients who received pneumococcal vaccine(s) after 2010. On-time vaccine uptake for pneumococcus was vaccination within 1 month of high-risk diagnosis that led to the need for transplantation. If initial diagnosis data were not available, then transplant was utilized as the high-risk diagnosis. Following transplant, on-time was considered within 6 months of transplant. Posttransplant patients are recommended to receive PCV13 followed by PPSV23 within 6 months, followed by a second PPSV23 5 years later, and were considered on time if within 6 years after first PPSV23. Time undervaccinated was the number of months outside the recommended timely vaccines. If a person went the entire year without getting an influenza vaccine, it was counted as 12 months undervaccinated.
2.9 |. Statistical analysis
Patient demographics are provided with median (interquartile range [IQR]) for continuous data and number (percentage) for categorical data. For regression modeling, race was narrowed to three groups due to convergence problems with smaller individual groups: Asian, Other/Mixed (including unknown race or refusal to specify race), and White.
Influenza vaccination rates (for a given influenza season) were calculated along with corresponding 95% binomial confidence intervals (CIs). These rates were based on patients who met inclusion criteria each influenza season. A rate was calculated based on a number of influenza vaccines eligible for and a number of influenza vaccines received by patient demographics. Pneumococcal vaccination status is provided with number (percentage). The relationship between patient demographics and influenza vaccination rate was assessed with unadjusted and adjusted Poisson regression models, offset by a number of vaccines eligible for. Based on the different influenza rates for each person based on time eligible and number of vaccines received, the Poisson modeling provides estimates that represent the difference in influenza vaccination rate ratios (RR) between groups. The relationship between patient demographics and pneumococcal status was assessed with unadjusted and adjusted logistic regression models and described as odds ratios (OR). Multivariable models adjusted for age, sex, race, and covariates with p-values <.1. For all analyses, p-values <.05 were used to signify statistical significance, without correction for multiple testing. All analyses were performed in SAS Studio 3.8 (SAS Institute Inc., Cary, NC).
3 |. RESULTS
3.1 |. Cohort characteristics
There were 468 unique patients who were SOT recipients aged 19– 64 living in the four-county region in southeast Minnesota (Figure 1). The most common transplant was kidney (70%) with the least common being lung (7%). There were 133 patients with more than one organ transplant. The median age at the time of transplant was 50-year old, with more males (57%), mostly White (83%) and non-Hispanic (93%) (Table 1). These demographics reflect those of the study area where 76.3% of the population is non-Hispanic White and 6.8% is Black.28 Over a third of patients were currently or had a history of smoking (38.2%). A majority lived in an urban setting (81%) with an even mixture of SES as measured by HOUSES, although the least number of people were in the highest SES group (Q4 = 19%). The majority received at least some college education (77%). Based on the distribution of our population, age was broken down into categories of 19–34, 35–44, 45–54, and 55–64 years of age.
FIGURE 1.
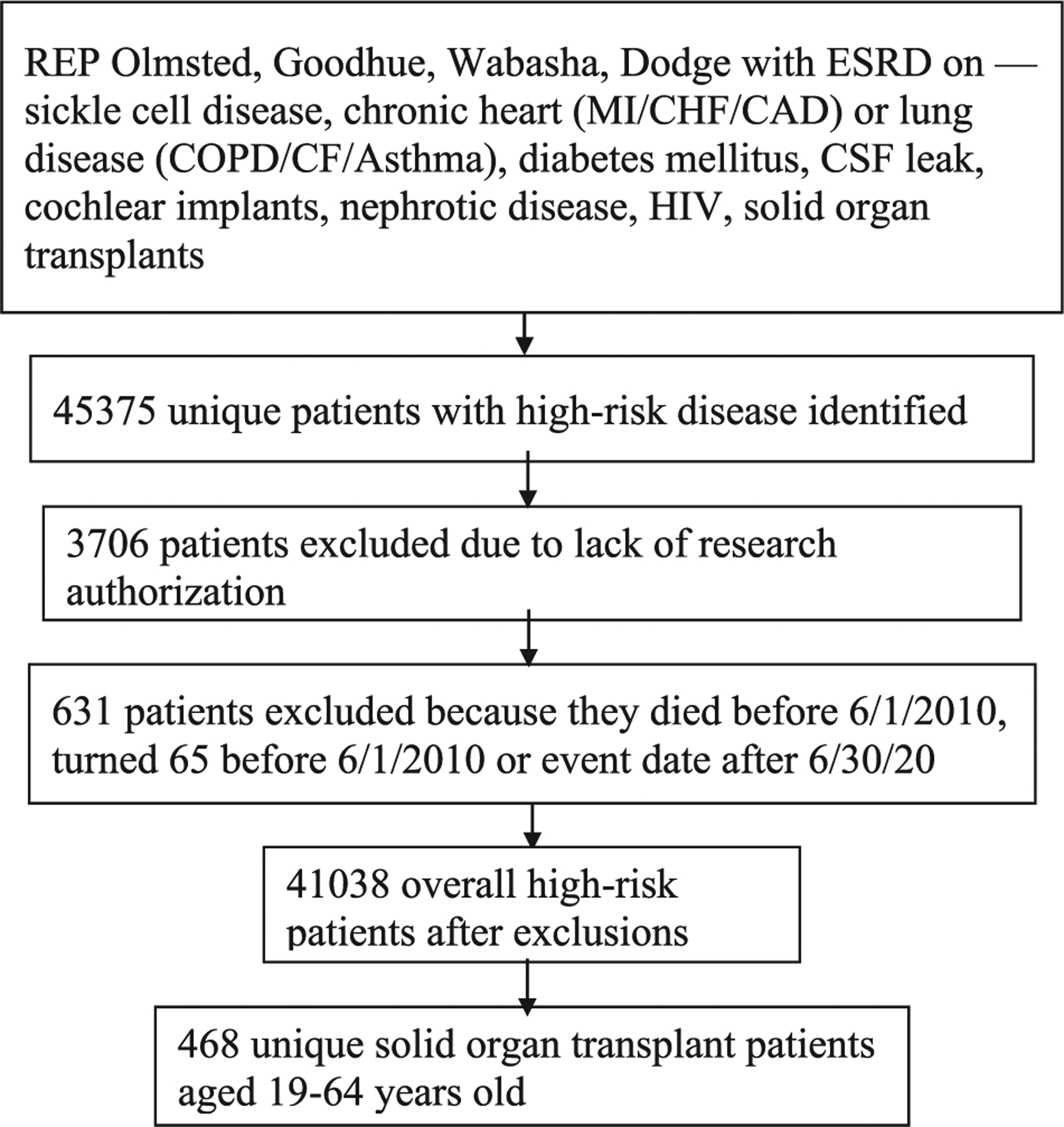
Algorithm for patient selection
TABLE 1.
Patient demographics (N = 468)
| Overall | |
|---|---|
| Age at index, median (IQR) | 50.2 (39.6, 57.2) |
| Sex, n (%) | |
| Female | 203 (43.4) |
| Male | 265 (56.6) |
| Transplant type, n (%)* | |
| Heart | 67 (14.3) |
| Kidney | 326 (69.7) |
| Liver | 136 (29.1) |
| Lung | 33 (7.1) |
| Race, n (%) | |
| Asian | 21 (4.5) |
| Black | 22 (4.7) |
| Hawaiian/Pacific Islander | 0 (0) |
| Native American | 3 (0.6) |
| Other/Mixed | 35 (7.5) |
| Refused/Unknown | 0(0) |
| White | 387 (82.7) |
| Ethnicity, n (%) | |
| Hispanic | 32 (6.8) |
| Non-Hispanic | 436 (93.2) |
| Geographic region, n (%), N = 432 | |
| Urban | 350 (81.0) |
| Suburban | 51 (11.8) |
| Rural | 31 (7.2) |
| HOUSES index, n (%), N = 429 | |
| Quartile 1 | 121 (28.2) |
| Quartile 2 | 115 (26.8) |
| Quartile 3 | 111 (25.9) |
| Quartile 4 | 82 (19.1) |
| Marital status, n (%), N = 412 | |
| Single | 111 (26.9) |
| Married | 240 (58.3) |
| Divorced | 53 (12.9) |
| Widowed | 6 (1.5) |
| Other | 2 (0.5) |
| Education level, n (%), N = 449 | |
| Eighth grade or less | 6 (1.3) |
| Some high school | 12 (2.7) |
| High school/GED | 83 (18.5) |
| Some college or 2-year degree | 172 (38.3) |
| 4-year college degree | 70 (15.6) |
| Post graduate studies | 106 (23.6) |
| Smoker, n (%) | |
| Yes | 179 (38.2) |
| No | 289 (61.8) |
Abbreviation: HOUSES, HOUsing-based SocioEconomic Status; IQR, interquartile range.
133 patients are in multiple transplant groups.
3.2 |. Influenza vaccination
Eighty-five percent (N = 397) of patients received at least one influenza vaccine, with 74% (N = 340) receiving two or more influenza vaccines. The average influenza vaccination rate over the 10-year period was 56% and ranged between 57% and 63% (Figure A1). Among all patients, the median (IQR) time undervaccinated was 26 (12, 53) months (Figure 2). Only 6% of our cohort were never undervaccinated.
FIGURE 2.
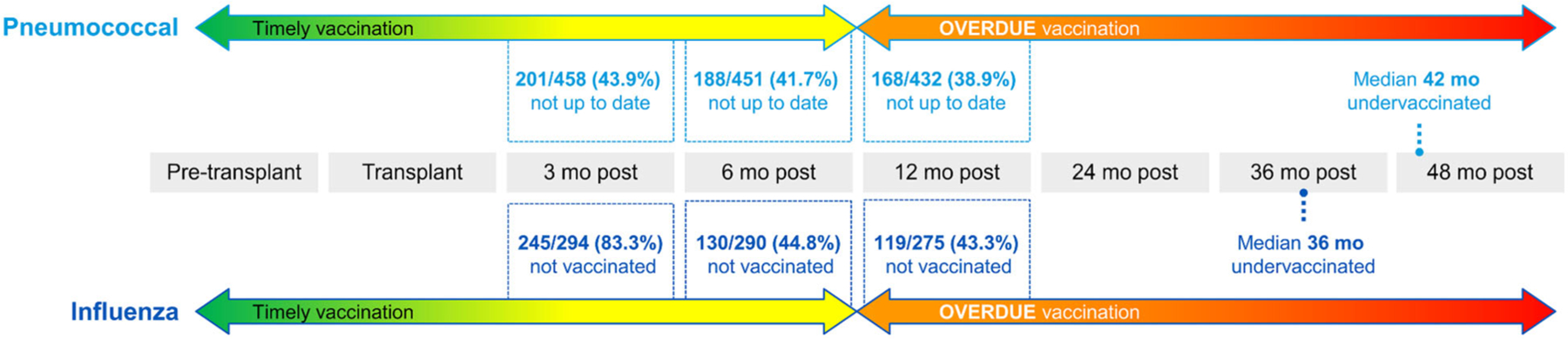
Time transplant patients spent undervaccinated. These calculations did not exclude patients who had transplants prior to 9/1/2010. About 10 patients had transplants during the first 9 months of 2010 so the flu denominators are about 160 less than the pneumonia denominators. The denominators are less than our total n of 468. That is to account for several things including date they turned 65 was within 3, 6, 9, 12 months of transplant; similar criteria applied for those who died within those time periods, excluding patients who had transplant before 9/1/2010 because we do not have flu data from 9/1/2009 to 12/31/2009.
There was no significant difference in influenza vaccination rate by the type of transplant received, ethnicity, marital status, sex, or smoking status. Higher SES was associated with higher rates of influenza vaccination when comparing Quartile 3 to Quartile 1 (RR = 1.147, 95% CI = 1.004, 1.305). Those in the second-highest SES category were the best vaccinated (Table A1). Factors that demonstrated the lowest vaccination uptake included: liver transplants, those of non-White or Asian race, or individuals living outside an urban environment. However, the increased uptake by Asian race was not significant when adjusting for age, sex, SES, and geographic region. Those living in urban settings were more likely to obtain their influenza vaccines than those living in suburban (RR = .801, 95% CI = .671, .956) or rural areas (RR = .874, 95% CI = .718, 1.065) (Table 2A) Although there was a trend toward higher vaccine coverage in those with more education, this was not significant.
TABLE 2A.
Poisson regression models of influenza vaccination rate by patient demographics
| Unadjusted | Adjusted | |||||
|---|---|---|---|---|---|---|
| N = 468 | RR | 95% CI | p-Value | RR | 95% CI | p-Value |
| First transplant | .410 | - | ||||
| Kidney | Ref | - | - | - | - | - |
| Heart | 1.010 | (.886, 1.152) | .878 | - | - | - |
| Liver | .921 | (.818,1.036) | .171 | - | - | - |
| Lung | 1.092 | (.871,1.369) | .445 | - | - | - |
| Ethnicity | 1.106 | (.916,1.334) | .295 | - | - | - |
| Marital status | .480 | - | - | - | ||
| Married | Ref | - | - | - | - | - |
| Single | .979 | (.877,1.093) | .703 | - | - | - |
| Other | .934 | (.835,1.044) | .228 | - | - | - |
| Smoker | .953 | (.870,1.045) | .306 | - | - | - |
| Age | 1.014 | (.995,1.034) | .152 | 1.003 | (.999, 1.007) | .140 |
| Sex | 1.072 | (.980,1.172) | .130 | .993 | (.898,1.092) | .890 |
| Race | .001 | .532 | ||||
| White | Ref | - | - | - | - | - |
| Asian | 1.181 | (.973,1.434) | .092 | 1.096 | (.898,1.337) | .370 |
| Other | .784 | (.675, .911) | .001 | .951 | (.808,1.119) | .542 |
| Geographic region | .002 | .021 | ||||
| Urban | Ref | - | - | - | - | - |
| Suburban | .772 | (.656, .909) | .002 | .801 | (.671, .956) | .014 |
| Rural | .837 | (.695,1.010) | .063 | .874 | (.718,1.065) | .181 |
| HOUSES | .100 | .148 | ||||
| Quartile 1 | Ref | - | - | - | - | - |
| Quartile 2 | 1.098 | (.970,1.244) | .139 | 1.053 | (.925,1.199) | .438 |
| Quartile 3 | 1.169 | (1.033,1.322) | .013 | 1.147 | (1.008,1.305) | .037 |
| Quartile 4 | 1.082 | (.944,1.240) | .260 | 1.009 | (.873,1.166) | .904 |
| Education | .077 | .162 | ||||
| High school or less | Ref | - | - | - | - | - |
| Some college | 1.116 | (.988,1.260) | .077 | 1.083 | (.954,1.230) | .218 |
| College or more | 1.143 | (1.015,1.288) | .028 | 1.131 | (.996,1.285) | .058 |
Note: The reference group for gender is male. The reference group for race is White. The reference group from ethnicity is non-Hispanic. The reference group for smoker is no. Multivariable models adjusted for age, sex, race, and covariates with p-values <.1 (geographic region, HOUSES, education). For all analyses, p-values <.05 were used to signify statistical significance.
Abbreviations: CI, confidence interval; HOUSES, HOUsing-based SocioEconomic Status; RR, rate ratio.
There was a 3.25% (95% CI: −7.23, .73; p-value = .109) lower mean difference in influenza vaccination rate when comparing the first 3 years following transplant to the remaining years following transplant, demonstrating a nonsignificant difference comparing number of influenza vaccines received initially posttransplant compared to further out from transplant (data not shown). This is representative of 296 patients who were eligible for this analysis versus the 468 total transplant patients. Patients were excluded for reasons such as diagnosis during or after the 2017–18 influenza season because we could not assess beyond the first three influenza seasons posttransplant. For influenza, there was a drastic increase in vaccination between the 3-and 6-month posttransplant time point. Those that had pneumococcal vaccines up-to-date had 26% higher rates of influenza vaccination.
3.3 |. Pneumococcal vaccination
Overall, 261 (55.8%) were up-to-date on pneumococcal vaccination. Of all the organ transplant patients, 383 (81.8%) received at least 1 pneumococcal vaccine. Of those who received at least 1 pneumococcal vaccine, 261 (68.1%) were considered up-to-date on appropriate pneumococcal vaccines. Of those who received only 1 pneumococcal vaccine, 22 received PCV13 and 59 received PPSV23. Of those who received 2 pneumococcal vaccines but were not fully up-to-date with a booster, 115 received PCV13 and 206 received PPSV23. Those with heart transplants were half as likely to be up-to-date with pneumococcal vaccines when compared to kidney transplants (OR = .509, 95% CI = .292, .889), in the unadjusted modeling (Table 2B). Pneumococcal vaccines were lowest in other/mixed race, those living in suburban and rural regions, or unmarried. There was no difference in vaccination uptake between men and women.
TABLE 2B.
Logistic regression models of pneumococcal vaccination compliance by patient variables
| N = 468 | Unadjusted | Adjusted | ||||
|---|---|---|---|---|---|---|
| RR | 95% CI | p-Value | RR | 95% CI | p-Value | |
| First transplant | .122 | - | ||||
| Kidney | Ref | - | - | - | - | - |
| Heart | .509 | (.292, .889) | .018 | - | - | - |
| Liver | .929 | (.588, 1.468) | .753 | - | - | - |
| Lung | .778 | (.565, 1.072) | .125 | - | - | - |
| Ethnicity | 1.059 | (.739,1.518) | .754 | - | - | - |
| HOUSES | .507 | - | - | - | ||
| Quartile 1 (lowest) | Ref | - | - | - | - | - |
| Quartile 2 | 1.258 | (.748, 2.115) | .388 | - | - | - |
| Quartile 3 | 1.233 | (.730, 2.082) | .434 | - | - | - |
| Quartile 4 (highest) | 1.074 | (.780, 1.479) | .660 | - | - | - |
| Education | .250 | - | - | - | ||
| High school or less | Ref | - | - | - | - | - |
| Some college | 1.429 | (.871, 2.343) | .158 | - | - | - |
| College or more | 1.285 | (.955,1.729) | .098 | - | - | - |
| Smoker | 1.255 | (.854,1.845) | .237 | - | - | - |
| Age | 1.012 | (.997,1.027) | .129 | 1.011 | (.993,1.030) | .213 |
| Sex | .996 | (.829,1.198) | .968 | 1.020 | (.836,1.246) | .844 |
| Race | .154 | .331 | ||||
| White | Ref | - | - | - | - | - |
| Asian | 1.978 | (.989, 3.959) | .054 | 1.718 | (.841, 3.507) | .137 |
| Other | .661 | (.409,1.068) | .091 | .735 | (.443,1.221) | .234 |
| Geographic region | .002 | .003 | ||||
| Urban | Ref | - | - | - | - | - |
| Suburban | .387 | (.211, .709) | .002 | .386 | (.208, .715) | .003 |
| Rural | .566 | (.405, .792) | .001 | .568 | (.404, .798) | .001 |
| Marital status | .057 | .296 | ||||
| Married | Ref | - | - | - | - | - |
| Single | .588 | (.374, .926) | .022 | .711 | (.428,1.181) | .187 |
| Other | .753 | (.590, .962) | .023 | .812 | (.624,1.055) | .119 |
Note: Up-to-date if received PCV13 followed by PPSV23 within 6 months of transplant or had already received pretransplant, followed by a second PPSV23 5 years later. The reference group for gender is male. The reference group for race is White. The reference group from ethnicity is non-Hispanic. The reference group for smoker is no. Multivariable models adjusted for age, sex, race, and covariates with p-values <.1 (geographic region, marital status). For all analyses, p-values <.05 were used to signify statistical significance.
Abbreviations: CI, confidence interval; HOUSES, HOUsing-based SocioEconomic Status; OR = odds ratio.
Geographic region was again associated with a significant difference in pneumococcal vaccines. Those in suburban areas were 62% less likely to be up-to-date compared to those in urban areas (OR = .386, 95% CI = .208, .715). Those in rural areas were 44% less likely to be upto-date compared to those in urban areas (OR = .568, 95% CI = .404, .798).
Timeliness was evaluated in patients who were vaccinated after 2010 only. Among this group, the median (IQR) time undervaccinated for pneumococcal vaccine was 18 months (0, 56.5). Overall, 52/168 (31.0%) were never undervaccinated (Figure 2).
4 |. DISCUSSION
Vaccine coverage in this high-risk population of transplant recipients is especially important because infection is the leading cause of death in transplant patients.10 The Healthy People 2020 national target for influenza coverage in the high-risk population was 90%, but nationally the baseline coverage in 2008 for noninstitutionalized high-risk adults aged 18–64 was 38.6%, with Minnesota’s highest coverage in the past 10 years at 53%.29 The Healthy People 2020 goal for high-risk adults was to achieve 60% pneumococcal vaccination; however, only 17% of eligible patients in 2008 received a pneumococcal vaccination.30 Overall, we found that SOT patients are vastly under national influenza and pneumococcal vaccine goals. This high level of undervaccination is seen most significantly in those living outside urban settings for both influenza and pneumococcal vaccines. Additionally, higher SES as measured by HOUSES demonstrated that a higher (but not the highest) level of SES was significantly associated with increased influenza vaccination, as was college education. Those who were vaccinated for pneumococcal disease were more likely to be vaccinated for influenza. Further time out from transplantation correlated with decreasing influenza vaccinations.
Influenza and pneumococcal vaccines have been documented as low in those awaiting kidney transplant at a tertiary hospital in the US, with 55% obtaining influenza and 36% completing pneumococcal vaccines between 2010 and 2014.29 Black patients are a third as likely as White patients to receive a pneumococcal vaccine, which is believed to be due to inequities in healthcare and mistrust of the medical community, not due to provider misunderstanding of recommendations or genetic differences.29 Recommendations by health-care providers are often a top reason cited for those receiving vaccination, and therefore, the lack of provider recommendations can contribute to decreased vaccination uptake.8,31–34 However, vaccine uptake has also been influenced by the anti-vaccine movement across the country, which often claims vaccines are unnatural, unnecessary, ineffective, or unsafe.35 Additional barriers to vaccination include difficult access, patient hesitancy, and concern for side effects.36
Our data showed higher influenza vaccine coverage in the organ transplant population compared to the national average, with a mean of 60% influenza vaccination over 10 years. This is slightly higher than prior studies that showed only 50% of high-risk individuals received their influenza vaccine.37 Although the vaccination rate of influenza nationally for high-risk adults is 16.6%, our cohort of organ transplant recipients demonstrated that 55.8% were up-to-date on pneumococcal vaccinations.30 Although our organ-transplant group is close to the national goal of 60%, there is still significant work that needs to be done to achieve higher levels of vaccination. Prior studies also demonstrated that those who received their pneumococcal vaccine were more likely to be up-to-date on their influenza vaccination, with a significantly higher influenza vaccination among those vaccinated for pneumococcus.10,37–39
In a prior survey of high-risk adults, only 14.8% and 18.5% of patients stated that the pneumococcal vaccine was offered to them in the last year and 5 years, respectively.33 This demonstrates that there could be an inherent problem with how we provide vaccines, especially to our high-risk populations if vaccines are not regularly being offered. Given that there was also a strong correlation between pneumococcal vaccine ascertainment and influenza vaccination, these discussions may occur by some providers regularly or because certain people are more apt to obtain recommended vaccinations. Each organ transplant practice may have different protocols and strategies for discussing vaccines; it was especially apparent that there could be a difference in practice patterns because heart transplant recipients were the only ones to have statistically significant lower numbers of pneumococcal vaccines. It is unclear why those with heart, liver, and lung transplants are less well vaccinated, but it is thought that it could have to do with the chronicity of disease, which may be more acute compared to end-stage kidney disease because dialysis centers often require vaccinations, and there is more time to obtain immunizations.
4.1 |. Sociodemographic factors
We sought to understand differences in rurality as few studies have evaluated the impact of geographic region in vaccine uptake. A longitudinal study in the US demonstrated that for all races living in rural areas, those in more rural areas experienced higher mortality rates despite other factors, and that over time the gaps in mortality between urban and rural settings has been widening.40 In the 65 and older population, a study by McLaughlin et al. found that those in rural locations were vastly undervaccinated, and that they also have higher rates of mortality related to pneumonia.38 Our research studies were similar to McLaughlin, but in our younger, high-risk population, with organ transplant recipients living in suburban or rural areas that are 20% less likely to obtain influenza vaccines (p = .009). These differences were even more pronounced in pneumococcal vaccines, where those in suburban areas were over 60% less likely to be up-to-date, and those in rural areas were only half as likely to be up-to-date. This may often be due to health-care access as there are fewer health-care facilities outside urban settings, low health literacy, or there is no appropriate stock of vaccinations.16,41,42 Other studies have shown lower levels of confidence in vaccines in rural areas.43
Although we found no statistically significant difference in SES for pneumococcal vaccine, there was a 15% higher odds of obtaining an influenza vaccine for those with higher socioeconomic status (HOUSES Q3) compared to the lowest SES (HOUSES Q1), when adjusting for all other factors. It is unexpected that in all evaluations of our data, the highest socioeconomic group (HOUSES Q4) did not have higher vaccination rates, but the second-highest group (HOUSES Q3) consistently had the highest vaccination rates. Over the past two decades, there has been a decline in vaccination coverage in children when parents had higher education and income when vaccination was previously higher in this group compared to their less educated and poorer peers.44
Due to limitations of the available financial and insurance records, we are unable to determine if patients were eligible for or received reim bursement after vaccinations, and therefore, conclusions regarding the influence of out-of-pocket costs’ on patient behaviors cannot be made.
Prior studies have shown a significant difference in vaccine coverage that correlate with the level of college education.37,38 We corrobo rated that for influenza, those with a college or greater education had 13% higher odds of receiving their annual vaccines (95% CI = .996, 1.285); however, this did not hold true for pneumococcal vaccines.
Previous vaccine studies have shown significant disparities in uptake by non-White races with the lowest uptake in Black persons.30,37 It has been shown that despite controlling for SES, education, and place of residence, minority races still demonstrated lower vaccine uptake (sometimes up to 20%–30% lower) in spite of having higher rates of pneumococcal disease.33,38,46 Because of small numbers, we had to combine multiple races, including Black and American Indian/Native Alaskan races, into one group for modeling. We found that our small but diverse group of minorities trended toward lower rates of vaccination in both influenza (RR = .951, 95% CI = .808, 1.092) and pneumococcus (OR = .735, 95% CI = .443, 1.221); however, it was not statistically significant when corrected for geographic region, age, sex, and SES. Asian persons seemed to have higher rates of vaccination than those of White and other races; however, once age, sex, geographic region, and SES were adjusted for, race and ethnicity no longer remained significant.
Transplant patients are typically very closely followed after receiving their transplant, with multiple points of health-care contact initially. It is very concerning that there is still a significant amount of time that people are still not vaccinated for pneumococcal and influenza in an appropriate manner. A median of 18 months undervaccinated for pneumococcal disease, with 31% never vaccinated comprises many missed opportunities. Guidelines from AST suggest vaccines are safe to give 3– 6-month posttransplant, but there still may be center variation in when to give vaccinations.48 Our center starts influenza and pneumococcal vaccination on the first posttransplant vaccination visit. Although we recognize that influenza vaccination may not be as effective early post-transplant, vaccination is still recommended, and in the event of an exposure, chemoprophylaxis may also be considered.
4.2 |. Implications and next steps for research and practice
Overall, vaccine refusal is a complex issue, and there are many reasons why a person is not up-to-date on recommended vaccines. Generally, the factors involved include patient, physician, and system factors.49 Studies of vaccine hesitancy have shown similar trends to our population of unvaccinated persons—with those of younger ages, Black race, and lower education more likely to be unvaccinated.43 We explored some of the drivers in vaccine uptake and found that geographic location is one of the most important factors in decreased vaccination.
New recommendations as of 2022 supplant PCV13 with PCV15 or 20 and may be simpler for people to understand. Those receiving PCV15 still get one dose of PPSV23 with the new recommendations.50 The next steps will require the determination of why coverage is low especially, outside urban settings and to find methods to increase coverage. Because access and knowledge may be key aspects, some studies have suggested ordering and administering vaccines upon hospital discharge, whereas others recommend electronic reminders to patients and providers in the outpatient setting.34 A study also found that after consultation with an infectious disease specialist, almost all patients agreed to be vaccinated.10 Prior analyses have shown that the costs of pneumococcal vaccines are significantly lower than the cost of pneumonia, so it is prudent for all insurance companies to both cover and promote vaccination.51
4.3 |. Strengths
This study captured high-risk persons across different levels of SESs and geographic regions to assess vaccine coverage over a 10-year period within multiple health-care systems, including immunization data across the entire state, and did not require self-reporting of vaccines. Utilizing individual-level SES is more accurate than area-level measures for health outcomes. This is the first study evaluating the association between SES and rurality with vaccine coverage.
4.4 |. Limitations
This study is only in four counties in southeastern Minnesota, an area that is primarily White and non-Hispanic. Although the counties outside of Olmsted have a higher population of non-White races and a variety of income levels, there is still a limitation with low numbers of other races and Hispanic ethnicity. The locality of the study does limit its external validity outside similar populations within the US. Although we did not find a disparity in vaccine uptake by race, the proportion of recipients in our study reporting Black race was low, which is similar to national findings. Only deceased kidney and heart transplant recipients have a significantly higher percentage of Black patients.52,53 Further studies in a population with a higher proportion of Black SOT recipients should be performed to validate the findings. This study only evaluated SOT patients aged 19–64, so cannot extrapolate the findings to those over 65 that could have different findings and are at even higher risk given the older age. Although we captured all statewide administered vaccines, those administered at federal sites such as the Veteran’s Administration were not captured.
5 |. CONCLUSIONS
Organ transplant patients in southeastern Minnesota are undervaccinated for influenza and pneumococcus, which is concerning given their high-risk for morbidity and mortality from infection. This was especially true in those living outside urban centers, and those of lower SES who were vaccinated less for influenza. Race was not found to be significant when adjusted for other patient demographics. Further research should focus on implementing vaccine reminders in transplant clinics with on-site vaccine administration available, as well as having remote vaccine clinic availability for those in rural settings.
ACKNOWLEDGMENTS
This study used the resources of the Rochester Epidemiology Project (REP) medical records-linkage system, which is supported by the National Institute on Aging (NIA; AG 058738), by the Mayo Clinic Research Committee, and by fees paid annually by REP users. The content of this article is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health (NIH) or the Mayo Clinic.
Funding information
National Institute on Aging, Grant/Award Number: AG 058738; Mayo Clinic Research Committee; National Cancer Institute Grant/Award: CA 217889
APPENDIX
FIGURE A1.
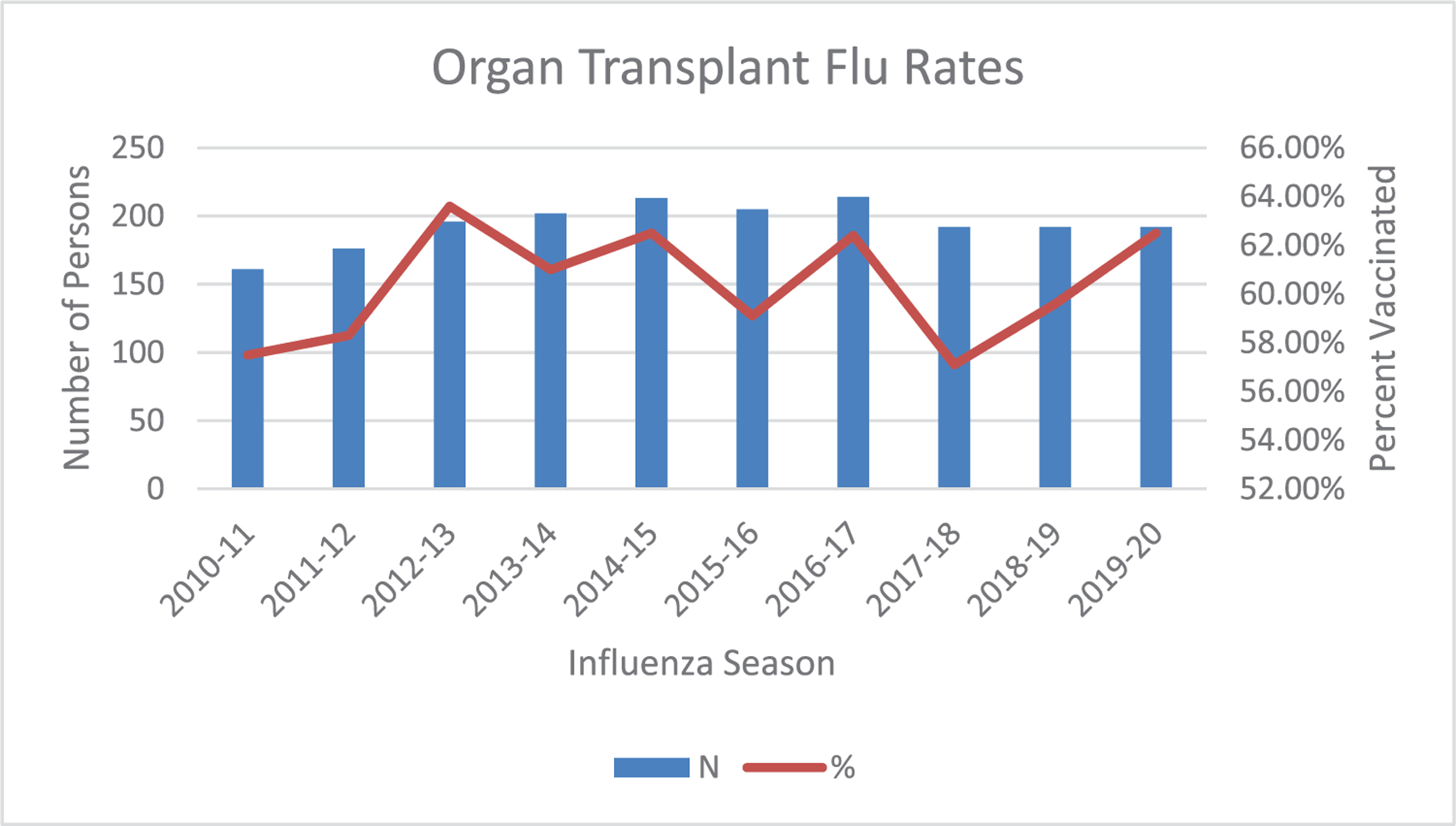
Annual influenza vaccine coverage
TABLE A1.
Solid organ transplant influenza and pneumococcal vaccination rate descriptive relationship
| N = 468 | Flu ratea | Pneumococcal up to dateb |
|---|---|---|
| First transplant | ||
| Heart | 66.7 (40.0, 90.0) | 26 (41.9%) |
| Kidney | 66.7 (33.3, 90.0) | 166 (58.7%) |
| Liver | 61.3 (22.2, 85.7) | 58 (56.9%) |
| Lung | 71.4 (50.0, 90.0) | 11 (52.4%) |
| Sex | ||
| Female | 66.7 (33.3, 90.0) | 113 (55.7%) |
| Male | 66.7 (30.0, 87.5) | 148 (55.9%) |
| Race | ||
| Asian | 83.3 (50.0, 100) | 16 (76.2%) |
| Black | 52.8 (16.7, 75.0) | 13 (59.1%) |
| Native American | 85.7 (25.0, 100) | 2 (66.7%) |
| Other/mixed | 42.9 (12.5, 75.0) | 16 (45.7%) |
| White | 66.7 (33.3, 90.0) | 214 (55.3%) |
| Ethnicity | ||
| Hispanic | 50.0 (27.5, 90.0) | 17 (53.1%) |
| Non-Hispanic | 66.7 (33.3, 90.0) | 244 (56.0%) |
| Geographic region | ||
| Urban | 70.0 (40.0, 90.0) | 212 (60.6%) |
| Suburban | 42.9 (16.7, 80.0) | 19 (37.3%) |
| Rural | 60.0 (14.3, 80.0) | 13 (41.9%) |
| HOUSES index | ||
| Quartile 1 | 60.0 (33.3, 90.0) | 68 (56.2%) |
| Quartile 2 | 70.0 (40.0, 90.0) | 71 (61.7%) |
| Quartile 3 | 75.0 (50.0, 100) | 68 (61.3%) |
| Quartile 4 | 62.5 (40.0, 87.5) | 43 (52.4%) |
| Marital status | ||
| Single | 62.5 (28.6, 90.0) | 53 (47.8%) |
| Married | 66.7 (40.0, 90.0) | 146 (60.8%) |
| Divorced | 50.0 (20.0, 83.3) | 32 (60.4%) |
| Widowed | 25.0 (0, 87.5) | 3 (50.0%) |
| Education level | ||
| Eighth grade or less | 41.7 (25.0, 55.6) | 3 (50.0%) |
| Some high school | 48.8 (7.1, 81.7) | 6 (50.0%) |
| High school/GED | 66.7 (25.0, 87.5) | 42 (50.6%) |
| Some college or 2-year degree | 70.0 (35.4, 90.0) | 102 (59.3%) |
| 4-year college degree | 71.4 (40.0, 90.0) | 41 (58.6%) |
| Post graduate studies | 66.7 (50.0, 90.0) | 65 (61.3%) |
| Smoker | ||
| Yes | 62.5 (37.5, 85.7) | 106 (59.2%) |
| No | 66.7 (30.0, 90.0) | 155 (53.6%) |
Abbreviation: HOUSES, HOUsing-based SocioEconomic Status.
Presented as median and IQR or influenza vaccination rate.
Presented as n (%). Number of vaccines eligible for is median number of years were eligible for influenza vaccine based upon development of high-risk
diagnosis, appropriate age during the 10-year time period. Number received is out of those years they were eligible, the median number of influenza vaccines received in each group. Up-to-date if received PCV13 followed by PPSV23 within 6 months of transplant or had already received pretransplant, followed by a second PPSV23 5 years later.
Footnotes
CONFLICT OF INTEREST
RMJ has shares of Johnson & Johnson through an inheritance and has served on safety review committees as well as data and safety monitoring committees for Merck for the last 3 years. All other authors have no conflicts of interest.
SUPPORTING INFORMATION
Additional supporting information can be found online in the Supporting Information section at the end of this article.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1.Kumar D, Ferreira VH, Blumberg E, et al. A 5-year prospective multi-center evaluation of influenza infection in transplant recipients. Clin Infect Dis. 2018;67(9):1322–1329. [DOI] [PubMed] [Google Scholar]
- 2.Kinnunen S, Karhapaa P, Juutilainen A, Finne P, Helantera I. Secular trends in infection-related mortality after kidney transplantation. Clin J Am Soc Nephrol. 2018;13(5):755–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fiore AE, Uyeki TM, Broder K, et al. Prevention and control of influenza with vaccines: recommendations of the Advisory Commit tee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep. 2010;59(RR-8):1–62. [PubMed] [Google Scholar]
- 4.Rubin LG, Levin MJ, Ljungman P, et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis. 2014;58(3):309–318. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention Advisory Committee on Immunization Practices. Updated recommendations for prevention of invasive pneumococcal disease among adults using the 23-valent pneumococcal polysaccharide vaccine (PPSV23). MMWR Morb Mortal Wkly Rep. 2010;59(34):1102–1106. [PubMed] [Google Scholar]
- 6.Matanock A, Lee G, Gierke R, Kobayashi M, Leidner A, Pilishvili T. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: updated recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 2019;68(46):1069–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tomczyk S, Bennett NM, Stoecker C, et al. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2014;63(37):822–825. [PMC free article] [PubMed] [Google Scholar]
- 8.Hunter P, Fryhofer SA, Szilagyi PG. Vaccination of adults in general medical practice. Mayo Clin Proc. 2020;95(1):169–183. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2012;61(40):816–819. [PubMed] [Google Scholar]
- 10.Runyo F, Matignon M, Audureau E, et al. Infectious disease consultation is effective in boosting vaccine coverage in patients awaiting kidney transplantation: a French prospective study. Transpl Infect Dis. 2021;23(4):e13607. [DOI] [PubMed] [Google Scholar]
- 11.National Center for Immunization and Respiratory Diseases. Adult Vax View. Centers for Disease Control and Prevention; 2020. [Google Scholar]
- 12.Grohskopf LA, Liburd LC, Redfield RR. Addressing influenza vaccination disparities during the COVID-19 pandemic. JAMA. 2020;324(11):1029–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Serper M, Liu CH, Blumberg EA, et al. A pragmatic outreach pilot to understand and overcome barriers to COVID-19 vaccination in abdominal organ transplant. Transpl Infect Dis. 2021;23(5):e13722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tam K, Yousey-Hindes K, Hadler JL. Influenza-related hospitalization of adults associated with low census tract socioeconomic status and female sex in New Haven County, Connecticut, 2007–2011. Influenza Other Respir Viruses. 2014;8(3):274–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gatwood J, Ramachandran S, Shuvo SA, et al. Social determinants of health and adult influenza vaccination: a nationwide claims analysis. J Manag Care Spec Pharm. 2022;28(2):196–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gatwood J, Chiu CY, Shuvo S, et al. Role of social determinants of health in pneumococcal vaccination among high-risk adults. Vaccine. 2021;39(14):1951–1962. [DOI] [PubMed] [Google Scholar]
- 17.Jain B, Paguio JA, Yao JS, et al. Rural-urban differences in influenza vaccination among adults in the United States, 2018–2019. Am J Public Health. 2022;112(2):304–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.St Sauver JL, Grossardt BR, Yawn BP, Melton LJ 3rd, Rocca WA. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester epidemiology project. Am J Epidemiol. 2011;173(9):1059–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ 3rd. His tory of the Rochester epidemiology project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87(12):1202–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.St Sauver JL, Grossardt BR, Yawn BP, et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol. 2012;41(6):1614–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rocca WA, Grossardt BR, Brue SM, et al. Data resource profile: expansion of the Rochester epidemiology project medical records-linkage system (E-REP). Int J Epidemiol. 2018;47(2):368–368j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Juhn YJ, Beebe TJ, Finnie DM, et al. Development and initial testing of a new socioeconomic status measure based on housing data. J Urban Health. 2011;88(5):933–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.MacLaughlin KL, Jacobson RM, Sauver JLS, et al. An innovative housing-related measure for individual socioeconomic status and human papillomavirus vaccination coverage: a population-based cross-sectional study. Vaccine. 2020;38(39):6112–6119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stevens MA, Beebe TJ, Wi CI, Taler SJ, St Sauver JL, Juhn YJ. HOUSES index as an innovative socioeconomic measure predicts graft failure among kidney transplant recipients. Transplantation. 2020;104(11):2383–2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson MD, Urm SH, Jung JA, et al. Housing data-based socioeconomic index and risk of invasive pneumococcal disease: an exploratory study. Epidemiol Infect. 2013;141(4):880–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hammer R, Capili C, Wi CI, Ryu E, Rand-Weaver J, Juhn YJ. A new socioeconomic status measure for vaccine research in children using individual housing data: a population-based case-control study. BMC Public Health. 2016;16(1):1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.USDA. 2010. Rural-Urban Commuting Area (RUCA) Codes. https://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes/documentation/ [Google Scholar]
- 28.US Census. Racial and Ethnic Diversity in the United States: 2010 Census and 2020 Census. US Department of Commerce; 2021. [Google Scholar]
- 29.Lee DH, Boyle SM, Malat G, Sharma A, Bias T, Doyle AM. Low rates of vaccination in listed kidney transplant candidates. Transpl Infect Dis. 2016;18(1):155–159. [DOI] [PubMed] [Google Scholar]
- 30.Immunization and Infectious Diseases and Global Health Progress Review. 2021.
- 31.Nowak GJ, Sheedy K, Bursey K, Smith TM, Basket M. Promoting influenza vaccination: insights from a qualitative meta-analysis of 14 years of influenza-related communications research by U.S. Centers for Disease Control and Prevention (CDC). Vaccine. 2015;33(24):2741–2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lau D, Hu J, Majumdar SR, Storie DA, Rees SE, Johnson JA. Interventions to improve influenza and pneumococcal vaccination rates among community-dwelling adults: a systematic review and meta-analysis. Ann Fam Med. 2012;10(6):538–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gatwood J, McKnight M, Frederick K, et al. Extent of and reasons for vaccine hesitancy in adults at high-risk for pneumococcal disease. Am J Health Promot. 2021;35(7):908–916. [DOI] [PubMed] [Google Scholar]
- 34.Rehm SJ, File TM, Metersky M, Nichol KL, Schaffner W, National foundation for infectious diseases pneumococcal disease advisory Board. Identifying barriers to adult pneumococcal vaccination: an NFID task force meeting. Postgrad Med. 2012;124(3):71–79. [DOI] [PubMed] [Google Scholar]
- 35.Jacobson RM, St Sauver JL, Finney Rutten LJ. Vaccine hesitancy. Mayo Clin Proc. 2015;90(11):1562–1568. [DOI] [PubMed] [Google Scholar]
- 36.Luz PM, Johnson RE, Brown HE. Workplace availability, risk group and perceived barriers predictive of 2016–17 influenza vaccine uptake in the United States: a cross-sectional study. Vaccine. 2017;35(43):5890–5896. [DOI] [PubMed] [Google Scholar]
- 37.Lu PJ, O’Halloran A, Ding H, Srivastav A, Williams WW. Uptake of influenza vaccination and missed opportunities among adults with high-risk conditions, United States, 2013. Am J Med. 2016;129(6):636.e631–636.e611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McLaughlin JM, Swerdlow DL, Khan F, et al. Disparities in uptake of 13-valent pneumococcal conjugate vaccine among older adults in the United States. Hum Vaccin Immunother. 2019;15(4):841–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jimenez-Garcia R, Lopez-de-Andres A, Hernandez-Barrera V, et al. Influenza vaccination in people with type 2 diabetes, coverage, predictors of uptake, and perceptions. Result of the MADIABETES cohort a 7 years follow up study. Vaccine. 2017;35(1):101–108. [DOI] [PubMed] [Google Scholar]
- 40.Singh GK, Siahpush M. Widening rural-urban disparities in all-cause mortality and mortality from major causes of death in the USA, 1969–2009. J Urban Health. 2014;91(2):272–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gai Y, Gu NY. Relationship between local family physician supply and influenza vaccination after controlling for individual and neighbor-hood effects. Am J Infect Control. 2014;42(5):500–505. [DOI] [PubMed] [Google Scholar]
- 42.Macintosh J, Luthy KE, Beckstrand RL, Eden LM, Orton J. Vaccination perceptions of school employees in a rural school district. Vaccine. 2014;32(37):4766–4771. [DOI] [PubMed] [Google Scholar]
- 43.McElfish PA, Willis DE, Shah SK, Bryant-Moore K, Rojo MO, Selig JP. Sociodemographic determinants of COVID-19 vaccine hesitancy, fear of infection, and protection self-efficacy. J Prim Care Community Health. 2021;12:21501327211040746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Silveira MF, Buffarini R, Bertoldi AD, et al. The emergence of vaccine hesitancy among upper-class Brazilians: results from four birth cohorts, 1982–2015. Vaccine. 2020;38(3):482–488. [DOI] [PubMed] [Google Scholar]
- 45.Kawai K, Kawai AT. Racial/ethnic and socioeconomic disparities in adult vaccination coverage. Am J Prev Med. 2021;61(4):465–473. [DOI] [PubMed] [Google Scholar]
- 46.Fry CA, Silverman EP, Miller S. Addressing pneumococcal vaccine uptake disparities among African-American Adults in the United States. Public Health Nurs. 2016;33(4):277–282. [DOI] [PubMed] [Google Scholar]
- 47.Kini A, Morgan R, Kuo H, et al. Differences and disparities in seasonal influenza vaccine, acceptance, adverse reactions, and coverage by age, sex, gender, and race. Vaccine. 2022;40:1643–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Danziger-Isakov L, Kumar D. Vaccination of solid organ transplant candidates and recipients: guidelines from the American society of transplantation infectious diseases community of practice. Clin Transplant. 2019;33(9):e13563. [DOI] [PubMed] [Google Scholar]
- 49.Weltermann B, Herwig A, Dehnen D, Herzer K. Vaccination status of pneumococcal and other vaccines in 444 liver transplant patients compared to a representative population sample. Ann Transplant. 2016;21:200–207. [DOI] [PubMed] [Google Scholar]
- 50.Kobayashi M, Farrar JL, Gierke R, et al. Use of 15-valent pneumococcal conjugate vaccine and 20-valent pneumococcal conjugate vaccine among U.S. adults: updated recommendations of the advisory committee on immunization practices – United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71(4):109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wateska AR, Nowalk MP, Lin CJ, et al. An intervention to improve pneumococcal vaccination uptake in high risk 50–64 year olds vs. expanded age-based recommendations: an exploratory cost-effectiveness analysis. Hum Vaccin Immunother. 2019;15(4):863–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hart A, Lentine KL, Smith JM, et al. OPTN/SRTR 2019 annual data report: kidney. Am J Transplant. 2021;21(suppl 2):21–137. [DOI] [PubMed] [Google Scholar]
- 53.Colvin M, Smith JM, Ahn Y, et al. OPTN/SRTR 2019 annual data report: heart. Am J Transplant. 2021;21(suppl 2):356–440. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


