SUMMARY ABSTRACT
In this manuscript, we describe the design and rationale of a randomized controlled trial in pediatric Fontan patients to test the hypothesis that a live-video-supervised exercise (aerobic+resistance) intervention will improve cardiac and physical capacity; muscle mass, strength, and function; and endothelial function. Survival of children with single ventricles beyond the neonatal period has increased dramatically with the staged Fontan palliation. Yet, long-term morbidity remains high. By age 40, 50% of Fontan patients will have died or undergone heart transplantation. Factors that contribute to onset and progression of heart failure in Fontan patients remain incompletely understood. However, it is established that Fontan patients have poor exercise capacity which is associated with a greater risk of morbidity and mortality. Furthermore, decreased muscle mass, abnormal muscle function, and endothelial dysfunction in this patient population is known to contribute to disease progression. In adult patients with two ventricles and heart failure, reduced exercise capacity, muscle mass, and muscle strength are powerful predictors of poor outcomes, and exercise interventions can not only improve exercise capacity and muscle mass, but also reverse endothelial dysfunction. Despite these known benefits of exercise, pediatric Fontan patients do not exercise routinely due to their chronic condition, perceived restrictions to exercise, and parental overprotection. Limited exercise interventions in children with congenital heart disease have demonstrated that exercise is safe and effective; however, these studies have been conducted in small, heterogeneous groups, and most had few Fontan patients. Critically, adherence is a major limitation in pediatric exercise interventions delivered on-site, with adherence rates as low as 10%, due to distance from site, transportation difficulties, and missed school or workdays. To overcome these challenges, we utilize live-video conferencing to deliver the supervised exercise sessions. Our multidisciplinary team of experts will assess the effectiveness of a live-video-supervised exercise intervention, rigorously designed to maximize adherence, and improve key and novel measures of health in pediatric Fontan patients associated with poor long-term outcomes. Our ultimate goal is the translation of this model to clinical application as an “exercise prescription” to intervene early in pediatric Fontan patients and decrease long-term morbidity and mortality.
Keywords: Exercise, Fontan, pediatric, intervention, muscle mass, endothelial function, VO2, randomized clinical trial
INTRODUCTION
STUDY OVERVIEW
We designed a randomized controlled trial in pediatric Fontan patients to test the hypothesis that a live-video-supervised exercise (aerobic + resistance) intervention will improve cardiac and physical capacity; muscle mass, strength and function; and endothelial function.
Figure 1 provides an overview of our milestone-driven trial: a single-center, single-blinded, randomized, controlled (delayed-entry) clinical trial, assessing the impact of a live-video-supervised exercise (aerobic + resistance) intervention in 150 pediatric Fontan patients (9–19 years) in small peer groups. After baseline testing, patients will be randomized to the 3-month exercise intervention or usual care (75 per arm). Following repeat testing at 3 months, patients in usual care will continue their routine clinical care for 6 more months while patients randomized to exercise intervention at baseline will follow a maintenance regimen for 6 months. Both groups will return for study testing at 9 months from baseline. The usual care arm will then start the exercise intervention and return for study testing at 12 months.
Figure 1.
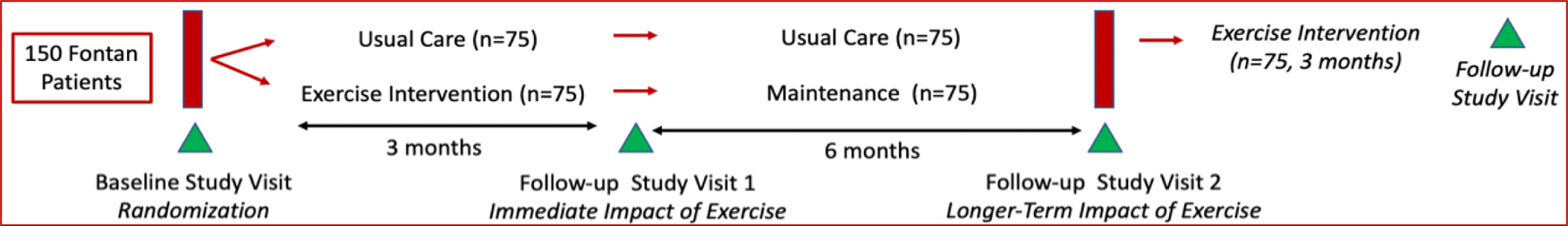
Study Design
Our trial is rigorously designed following CONSORT guidelines.1 There are several strengths to the design including the delayed entry of the usual care group into the exercise intervention and the group exercise sessions which will enhance recruitment and retention. The delated entry of the usual care group into the exercise intervention allows a longer-term comparison of the usual care group to the intervention group from baseline to 9 months at 3 time points. Furthermore, the group exercise sessions provide essential peer support. Our team of investigators have specific expertise in each of the tested measures to ensure robust and unbiased methodology, analysis, interpretation, and reporting of results, with consideration of biological variables including sex and Tanner Stage.
RATIONALE
Survival of children with single ventricles beyond the neonatal period has increased dramatically with the staged Fontan palliation. Yet, long-term morbidity remains high. By age 40, 50% of Fontan patients will have died or undergone heart transplantation. With >1,000 Fontan palliations performed in the US annually, there is a burgeoning population of Fontan patients at risk for progressive heart failure and death. Factors that contribute to onset and progression of heart failure in Fontan patients remain incompletely understood. However, it is established that Fontan patients have poor exercise capacity which is associated with a greater risk of morbidity and mortality.2–5 Furthermore, decreased muscle mass, abnormal muscle function, and endothelial dysfunction in this patient population is known to contribute to disease progression.6, 7 In adult patients with two ventricles and heart failure, reduced exercise capacity, muscle mass, and muscle strength are powerful predictors of poor outcomes, and exercise interventions can not only improve exercise capacity and muscle mass, but also reverse endothelial dysfunction.8–15
Pediatric Fontan patients do not exercise routinely due to their chronic condition, perceived restrictions to exercise, and parental overprotection (Figure 2).6, 16–18 A limited number of exercise interventions in children with congenital heart disease have demonstrated that exercise is safe and effective; however, these studies have been conducted in small, heterogeneous groups, and most had few Fontan patients. Furthermore, these interventions have not studied the impact of exercise on muscle mass or mitochondrial function, or endothelial function.
Figure 2.
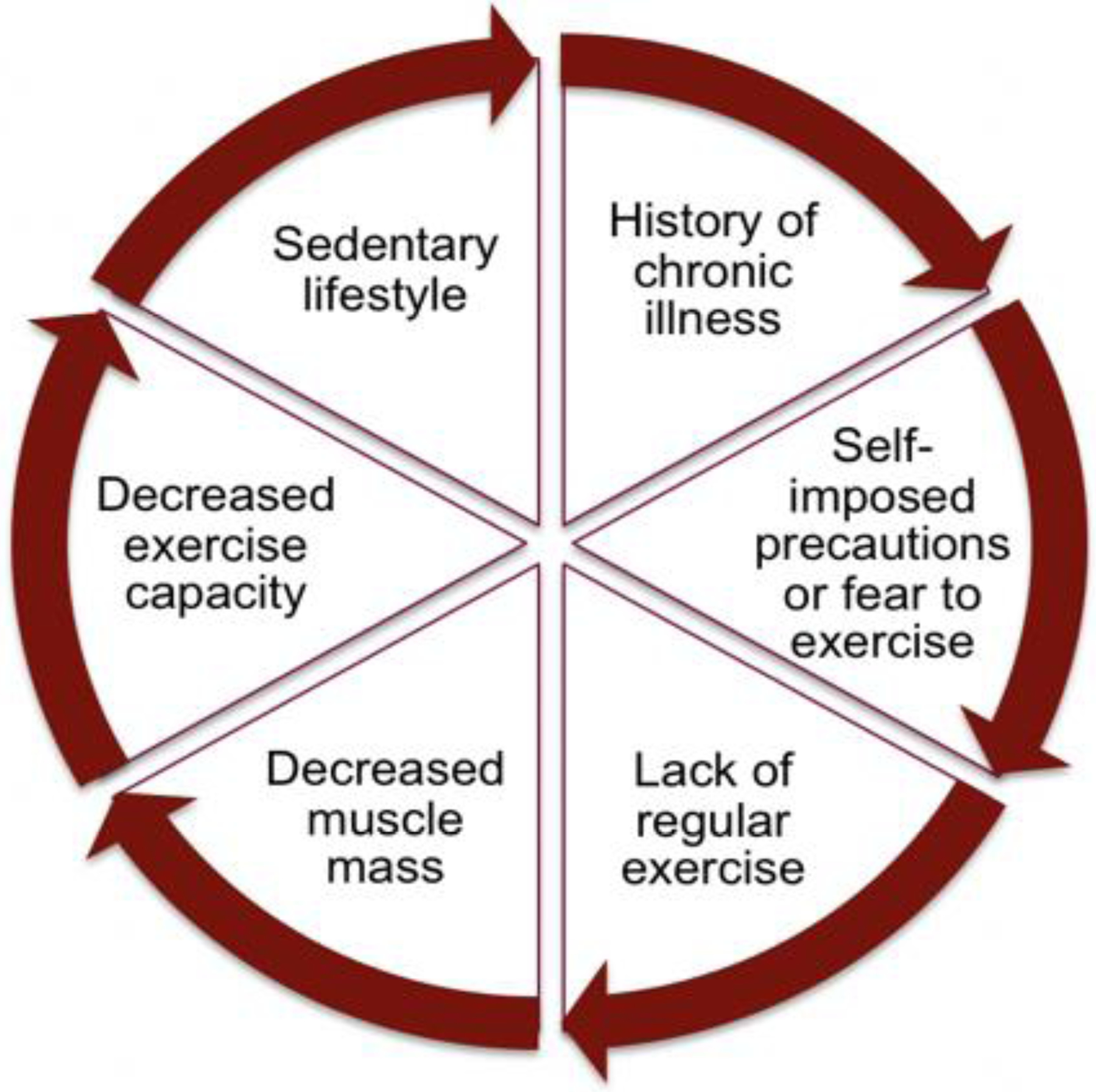
Barriers to Exercise in Pediatric Fontan Patients
Critically, adherence is a major limitation in pediatric exercise interventions delivered on-site, with adherence rates as low as 10%, due to distance from site, transportation difficulties, and missed school or work days.19 To overcome these challenges, we will utilize live-video conferencing to deliver the supervised exercise sessions (Figure 3). In our pilot exercise interventions, this approach resulted in excellent adherence (>85%) and improved exercise capacity and endothelial function.20, 21
Figure 3.
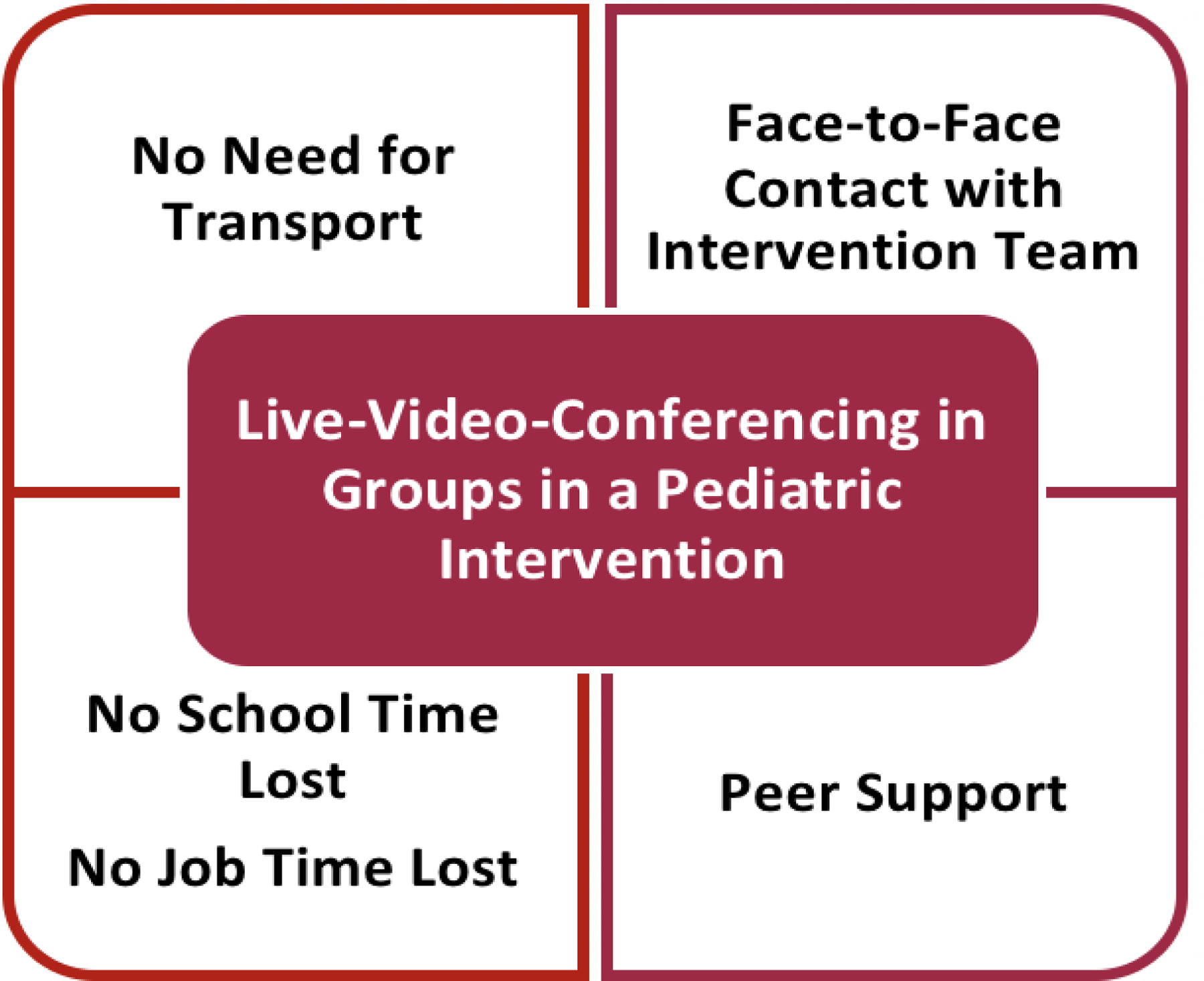
Benefits of Live-Video-Supervised Pediatric Group Intervention.
PATIENT SELECTION
Participants:
Inclusion criteria are: 1) 9–19 years of age; 2) Fontan palliation; 3) Ability to fast overnight; 4) Cardiac clearance to exercise by primary cardiologist; 5) Presence of an adult at home during exercise sessions for patients <14 years old; 6) English-speaking patient. Exclusion criteria are: 1) NYHA Class IV (severe heart failure), 2) Acute illness within the past three months; 3) Active protein losing enteropathy (albumin <2.5 mg/dL); 4) Implanted pacemaker; and 5) Cognitive delay deemed severe enough to inhibit the ability to follow the exercise program. To ensure eligibility of patients from different socioeconomic backgrounds, all enrolled patients will receive a tablet which they will keep (whether they complete the intervention or not) and internet service subscription during the intervention if they do not have Internet access at home.
Randomization
Consented patients will be randomly assigned to intervention or control (usual care) arms. Randomization will be performed using SAS Proc Plan with two stratification variables, Tanner Stage and sex, to account for potentially confounding biological variables, in a 1:1 ratio using permuted blocks of randomly selected size (e.g., 2 or 4), to ensure balanced allocations across the two groups within each stratum.
Blinding
The study is single-blinded. At the first study visit, none of the staff administering the study tests will be aware of the randomization assignment. After completion of the baseline study visit tests, the study coordinator will be aware of the participants randomization group in order to prepare the intervention equipment and schedule the training sessions. Participants and their parents/guardians will be informed which study arm they are assigned to at the end of the baseline study visit. As the study coordinators will perform the endothelial function testing, they will be the only team member who will be aware of the participant’s randomization arm when conducting a study test during follow-up visits. They will be blinded to the previous endothelial function test results in the study’s electronic data capture system. No one else conducting tests during study visits will be aware of which randomization arm the participant will be in. Participants and their parents will be asked to refrain from discussing their randomization arm with anyone but the study coordinator.
INTERVENTION
Intervention Arm (Figure 1):
Patients randomized to exercise intervention at baseline will participate in live-video-supervised exercise sessions x3/week for 3 months, followed by a maintenance regimen for 6 months. During maintenance, patients will continue live-video-supervised exercise sessions only x1/week and will be instructed to exercise on their own x2/week following an individually prescribed exercise program while using their heart rate monitor to track their activity.
Control Arm (Usual Care) (Figure 1):
Patients randomized to usual care at baseline will continue their routine clinical care for 9 months and will then start the 3-month exercise intervention.
Intervention Platform:
This intervention will use Zoom.
Supervised Exercise Intervention via Live-Video-Conferencing in Small Peer Groups:
The exercise protocol is designed to improve aerobic capacity, physical functioning, and overall muscle strength and endurance. Each participant will be provided with an exercise kit that includes a medicine ball, foam roller, sliders, exercise band, and resistance bands.
Pre-Exercise Assessment of Exercise and Functional Capacity:
Patients will only be included after clearance by their primary cardiologist. The exercise intervention is designed to accommodate each participant’s physical capabilities based on their level of conditioning, total score from the initial Functional Movement Screening (FMS) (Figure 4)22, and cardiopulmonary exercise test conducted at the baseline visit. Participants who fall into an “at risk” category by FMS (score <14), will be given specific exercise assignments to improve mobility and stability and decrease any left/right asymmetries.
Figure 4.
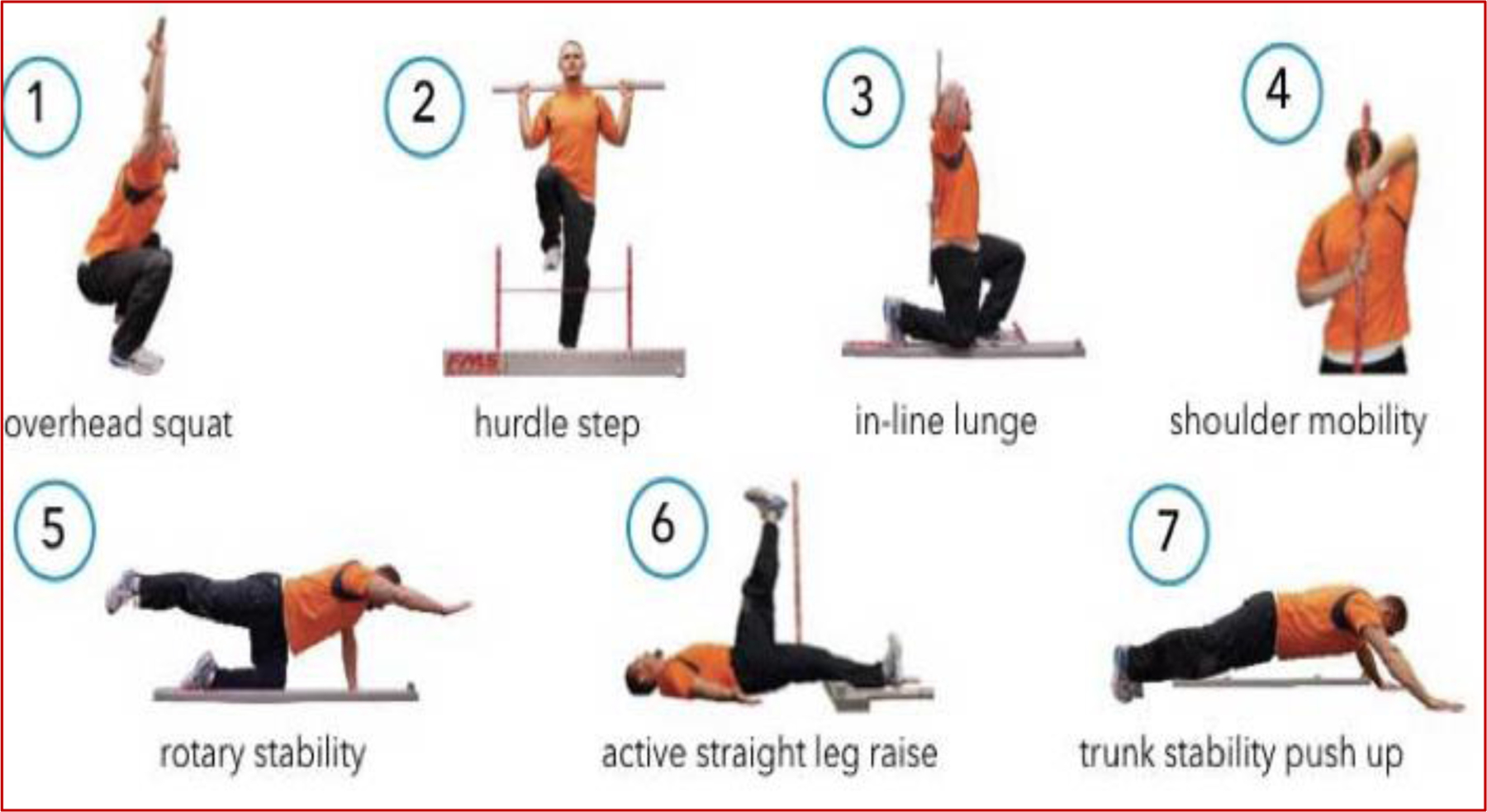
Functional Movement Screening
Safety during Exercise:
The first three exercise sessions (first week) will be one-on-one to allow the trainer to assess the participant’s baseline abilities as well as teach the participant proper exercise techniques. To maximize safety for participants, the certified trainer will ask pre-exercise check-in questions about hydration status, last snack/meal, hours slept the night before, self-perceived well-being on 1–10 scale (if <5, the session is rescheduled), and any health issues since the last exercise session. During exercise sessions patients’ heart rates are continually monitored. If there are any concerns, the patient’s cardiologist and the PI will be informed.
Exercise Intervention:
The trainer will host each supervised exercise session using live-video-conferencing (Figure 5) with small groups of patients (2–3 patients). The 3-month program will consist of 60-minute supervised exercise sessions x3/week. The specific movements and progression of intensity in the exercise protocol is based on the “overload principle”; i.e. the body’s ability to adapt to new demands gradually with progressive increase of more load or resistance than encountered previously.23
Figure 5.
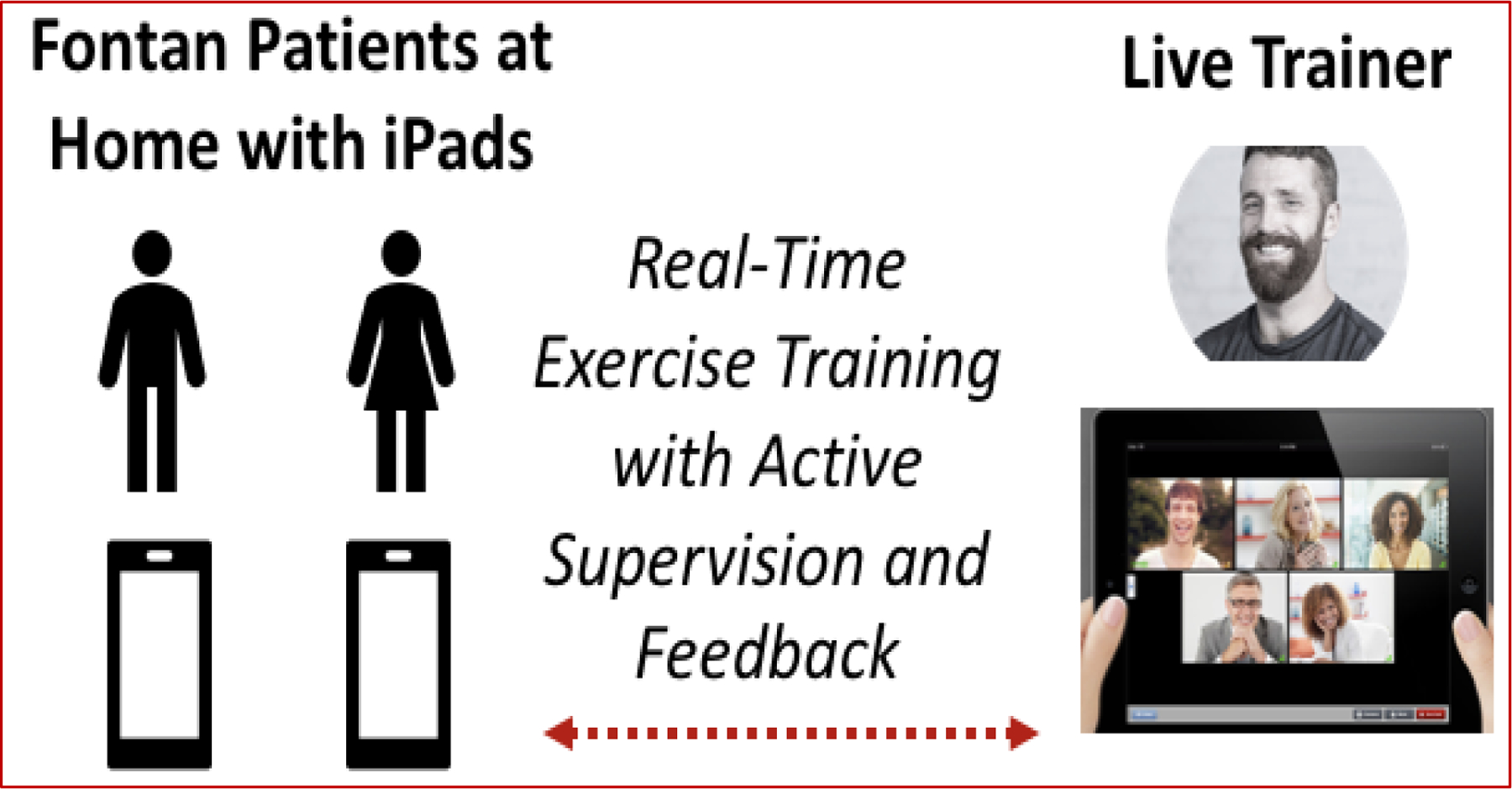
Supervised-Live-Video Exercise
Exercise sessions will primarily be in a circuit format. Patients will have ≤30-second rest periods between exercises, and ≤2-minute rest periods between circuits. The exercise protocol will have sections focused on mobility, stability, and aerobic capacity. Exercise sessions will not increase by more than 10% in total volume from session to session. Resistance exercise will involve an introduction to a low-resistance, high-repetition regimen including upper and lower body major muscle groups under individualized supervision in accordance with established guidelines.24 During the initial sessions, subjects will perform 12 to 15 repetitions at 70% of the 5-repetition maximum with 2-minute rest periods between sets. Subjects will gradually increase to two sets of these exercises, with increasing resistance as tolerated. Subjects will be given a medicine ball and resistance bands in accordance with their capabilities.
During each session, the trainer will record the number of exercises and repetitions performed and the rate of perceived exertion. Perceived exercise intensity will be targeted in the 12 to 14 range on the Borg 6–20 scale.25 Heart rate will be continuously monitored (real-time) via heart rate monitor, targeted to achieve 60% of heart rate reserve initially and gradually increasing as tolerated to 70–80% of heart rate reserve. Exercises will be rotated in intervals weekly throughout to maintain interest and motivation. If any concerns arise the trainer will stop the training session and contact the clinical team.
Maintenance:
There are limited data on strategies for maintaining health gains of lifestyle interventions over the long term; with virtually no published data in pediatric Fontan patients. We designed our intervention following recommendations by the AHA on strategies to sustain health benefits gained after an intervention and include scheduled face-to-face contact with the intervention team (via live-video-conferencing) and peer support (via peer groups). Furthermore, to promote self-efficacy and self-management based on key aspects of social cognitive theory and control theory,26–28 we have incorporated goal setting, self-monitoring, problem solving, continuous feedback, and reinforcement into our protocol (Figure 6).29
Figure 6.
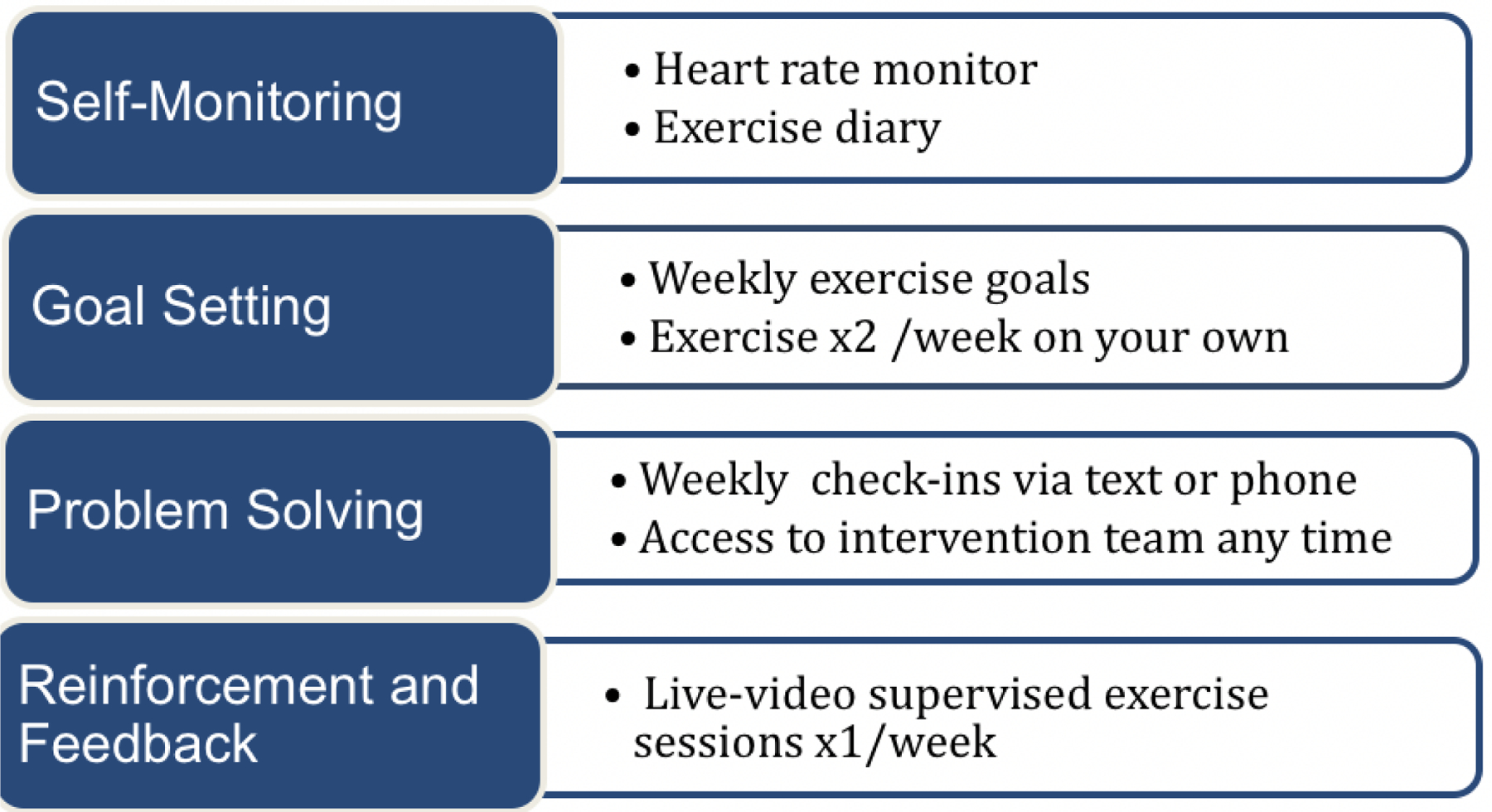
Key Concepts to Maintain Health Benefits
STUDY VISITS
There will be three study visits for both arms, at baseline, 3 months, and 9 months, and another visit at 12 months for the usual care arm. The baseline study visit will occur as soon as possible after written consent and assent are obtained. Phone call reminders and check-ins will be used to maximize attendance to follow-up visits for both arms. The estimated duration of each study visit is six hours. Participants and their parents will report a detailed medical history including medications.
Testing Protocol:
The testing protocol (Table 1) has been constructed to assess the health profile of pediatric Fontan patients specifically for cardiac and physical capacity; muscle mass, strength and function; and endothelial function. Specifics of each test in Table 1 are detailed under the corresponding Aim below. Patients will be asked to come in after an overnight fast of 12 hours except for consumption of water and prescription medications. They will also be asked to follow a nitrate-free diet for three days prior to the visit to minimize confounding for biomarkers of endothelial function. Patients will have a snack after vascular testing and blood work, prior to the cardiopulmonary exercise test. Height, weight, and blood pressure will be measured. Pubertal status (Tanner stage) will be assessed with a validated self-assessment questionnaire.30
Table 1.
Study Tests
| Study Visit | Baseline | 3 mo | 9 mo | 12* mo |
|---|---|---|---|---|
| Cardiopulmonary stress test (peak VO2) | ✓ | ✓ | ✓ | ✓ |
| Cardiac echocardiogram (longitudinal strain) | ✓ | ✓ | ✓ | ✓ |
| Functional Movement Screening (FMS) | ✓ | ✓ | ✓ | ✓ |
| Physical Functioning and Summary Scores (PedsQL) | ✓ | ✓ | ✓ | ✓ |
| Dual-energy X-Ray Absorption (lean leg mass) | ✓ | ✓ | ✓ | ✓ |
| Dynamometer (muscle strength) | ✓ | ✓ | ✓ | ✓ |
| Endothelial Function Testing (EndoPAT index) | ✓ | ✓ | ✓ | ✓ |
| Blood work (plasma nitrogen oxide, lipid profile) | ✓ | ✓ | ✓ | ✓ |
PedsQL:Pediatric Quality of Life Inventory TM; mo: month
usual care arm only
Post-Intervention and Post-Follow-Up Surveys:
Each patient and parent will receive a survey at follow-up study visits to understand their level of satisfaction with the intervention, their long-term plans to use the tools provided to exercise, and if they would like to continue this exercise routine as part of their clinical care.
OUTCOME MEASURES:
This study is designed to determine if the intervention improves: (Aim 1) cardiac and physical capacity (primary outcome: peak VO2 which is the oxygen consumption at peak exercise), (Aim 2) muscle mass strength and function; and (Aim 3) endothelial function in pediatric Fontan patients.
Peak VO2:
Peak VO2, our primary outcome, is highly reproducible and is widely accepted as the single best measure of cardiorespiratory fitness and maximal aerobic capacity.31, 32 In Fontan patients, peak VO2 is a powerful predictor of poor outcomes.33 The subjects will undergo progressive cardiopulmonary exercise testing with continuous monitoring by 12-lead EKG and gas exchange by measuring breath-to-breath ventilation. Exercise capacity will be measured by a cardiopulmonary exercise test to obtain the maximal oxygen uptake (peak VO2). The exercise tests will be performed, analyzed, and reported according to a standardized protocol and utilizeing a computerized database.34
Strain echocardiography:
Ventricular dysfunction is common in young adults with a Fontan circulation and is a risk factor for mortality.35 Assessment of ventricular function in this population is challenging due to highly variable ventricular geometry. Thus, we will assess ventricular function by strain echocardiography (longitudinal strain) which provides angle independence and decreased load dependence.36 Strain reflects global deformation of the ventricular myocardium during the cardiac cycle using 2D-speckle tracking and has been validated against tagged cardiac magnetic resonance imaging.36 Echocardiograms will be performed on a Phillips Epiq machine (Phillips, Andover) according to our standard imaging protocol.
Functional Movement Screening (FMS):
FMS is a screening instrument (Figure 4) that evaluates selected, fundamental movement patterns to determine potential injury risk. It consists of seven movements that assess movement quality with a quantitative grading system (total score of 21).22 Participants are considered “at risk” for injury if they score <14.
Physical Functioning and Summary Scores:
The PedsQL Measurement Model is a modular approach to measuring health-related quality of life in healthy children and adolescents and those with acute and chronic health conditions.37
Muscle mass and strength:
Whole body Dual-energy X-ray absorptiometry (DXA) scans will be obtained to assess muscle mass with the Horizon A Platform (Hologic Inc., Bedford, MA) with Apex software v5.5 and standard positioning. The test-retest coefficient of variation for lean mass (kg) is 1.4 to 1.7%.38 We use leg lean mass, as opposed to total body lean mass, since appendicular lean mass is the international standard for assessment of sarcopenia in adults39 and we have demonstrated that leg lean mass is associated with cardiorespiratory fitness in children with congenital heart disease while total body lean mass is not.6 Maximal handgrip strength (kg) will be measured with a handgrip dynamometer (Takei, Tokyo).40 The subject will stand upright with the shoulder adducted holding the dynamometer, with the handle adjusted to the participant’s hand size. Three maximal effort trials lasting 4–5 sec interspersed with 60-sec rests will be performed and the highest value retained for analysis. Isokinetic knee extensor and flexor strength and endurance will be evaluated using a Biodex System Pro dynamometer (Shirley, NY). Participants will be seated with their backs supported and hips positioned at 120° of flexion and secured to the seat of the dynamometer with thigh and pelvic straps. All tests will be performed on the non-dominant leg at an angular velocity of 60°/s. The best of the five maximal voluntary efforts for each of flexion and extension will be used as the measure of absolute strength and reported as peak torque (Nm) at 60°. In prior studies of retest reliability of knee strength at this velocity, the intra-class correlation coefficient was 0.95 to 0.99.41, 42 We will also test endurance (total muscular work) with 30 repetitions at maximal effort at 180°/sec.
Creatine chemical exchange saturation transfer (CrCEST) muscle function analysis (exploratory aim):
Evaluation of muscle function will be performed on a 7T whole body MRI scanner (GE Healthcare, Waukesha, WI) with a 28-channel coil (Quality Electrodynamics, Cleveland, OH) for calf imaging. Chemical Exchange Saturation Transfer Imaging of Creatine asymmetry (CrCESTasym) maps, which measures muscle free creatine concentration with high spatial resolution, will be acquired before and after exercise. This technique provides a measure of the mitochondrial oxidative phosphorylation capacity in skeletal muscle, allowing us to quantitate physiologic changes in metabolic capacity.43 CrCEST will use an in-house developed sequence as described.44 Subjects will perform two minutes of mild plantar flexion exercise within the scanner using an MR compatible pneumatical foot pedal.44 CrCESTasym maps of the calf will be computed with a 30-second temporal resolution at baseline and then eight minutes following the exercise protocol to evaluate the maximal change and the rate of recovery. Additionally, a 2D fast spin echo (FSE) sequence with iterative decomposition of water and fat with echo asymmetry and least squares estimation (IDEAL)45 will be used to acquire water and fat only images for measurement of muscle-fat fraction and muscle volume.46
Assessment of endothelial function:
Endothelial function will be assessed by non-invasive endothelial pulse amplitude testing (EndoPAT) and by blood work. EndoPAT is a noninvasive, FDA approved modality based on the principle of reactive hyperemia that assesses endothelial function (Itamar Medical). EndoPAT has been validated in adults47 and studied in several pediatric groups.48–51 This team has demonstrated excellent feasibility and reproducibility of this modality in children and adolescents which has been published.48 EndoPAT testing will be performed according to previously published laboratory protocols.48
Nitric oxide (NO) is a key modulator of endothelial function. We will measure plasma nitrogen oxide (NOx) levels by spectrophotometry with Griess reagents (Nitrate/Nitrite Colorimetric Assay Kit, Cayman). For NOx, detection limit is ~5uM; inter-assay coefficient of variation is <4%.
Statistical Considerations
Primary Outcome & Analysis:
The primary outcome of cardiac capacity is peak VO2. Peak VO2 will be measured at baseline, 3 months, 9 months, and 12 months post-randomization. Our primary analysis will evaluate change in peak VO2 at 3 months using generalized linear mixed effects models (GLMM) using intention-to-treat (ITT) principles. Sample size calculations are based on the primary outcome and analysis described above. With the assumptions including 1) a within-subject correlation of 0.80 was assumed due to the changes in a short time window54; 2) a standard deviation of 7.5 for peak VO2 in baseline Fontan patients1; 3) a 10–15% attrition at the 3 month visit, a sample size of 75 subjects per group enables to detect the mean difference of 2.5 ml/kg/min in peak VO2 between groups and reject the null hypothesis that the population means of the groups are equal with probability (power) of 85%, assuming a Type I error of 0.05.
Secondary Outcomes & Analysis:
We have numerous secondary outcomes measured at baseline, 3 months, 9 months, and 12 months post-randomization. These include the following:
Cardiac capacity: VO2 at VAT, measured at all visits,
Physical capacity as measured by the following variables: FMS and PedsQL, at all visits,
Muscle mass: leg lean mass measured by DXA scanning, at all visits,
Muscle strength and function as measured by dynamometer and muscle mitochondrial function by creatine chemical exchange saturation transfer MRI, at all visits,
Endothelial function as measured by endothelial pulse amplitude index (EndoPAT) and plasma levels of nitric oxide metabolites, at all visits.
All these secondary outcomes will leverage the same statistical framework designed for the primary analysis. If the measures are not normally distributed, an appropriate transformation will be applied before the GLMMs are performed.
Sensitivity Analyses:
We have numerous sensitivity analyses that will address key secondary objectives. These include the following: 1) per-protocol analysis in which we will estimate the treatment effect as received to account for treatment non-adherence (participation in < 80% of exercise sessions); 2) imputation analysis in which we will perform a sensitivity analysis utilizing multiple imputation to allow missingness of the primary outcome to be related to additional observed variables (other than treatment and visit time in our primary analysis); 3) longitudinal analysis in which we will perform a pre-/post-treatment study design using the only 0–12 months follow-up of the patients randomized to the usual care group. Patients in this arm will receive usual care for the first 9 months and then start the 3-month intervention.
Study Investigators and Team Structure
We have a multidisciplinary team with expertise in several fields including pediatric cardiology, vascular health and imaging, heart failure, exercise physiology, exercise interventions, biostatistics, and clinical trial design as well as vascular biology, MRI, muscle mass and strength, psychology, and telehealth.
The scientific study leadership is structured to identify barriers, make timely responses, and optimize the allocation of limited resources to meet pre-defined study objectives. Interim monitoring will consist of regular meetings with the study coordinators and intervention team, Executive Team, Study Advisory Board, and Data and Safety and Monitoring Board (DSMB) to review recruitment numbers and data collection forms, to perform quality control of study testing measures, and to receive feedback from the intervention team and for study updates. These regular meetings with the study administration will serve as control points to assess scientific performance.
Safety
Patients will be withdrawn from the study if the patient or parent wishes not to continue, or if continued participation is felt to be contraindicated by the primary cardiologist. For all withdrawn subjects, the reasons for and circumstances surrounding withdrawal will be documented. Of note, to minimize bias, all randomized subjects will be encouraged to remain in the study to complete protocol measurements, even if the subject no longer wishes to participate in the intervention.
Enrollment data will be shared on a regular basis with NHLBI. Periodic data monitoring will be ongoing throughout the trial to examine characteristics of screened ineligible patients, evaluate data completeness and conduct data cleaning. The Data and Safety Monitoring Board (DSMB) is responsible for oversight of the study data and safety considerations. If the DSMB identifies issues with recruitment or data management, it will notify the PI and an action plan will be formed to correct any issues immediately.
Potential Problems and Alternative Strategies
The greatest challenge, as with any clinical trial, is achieving the targeted enrollment. Subject availability and interest have been carefully assessed, based on the present volume in our center and patient surveys. In addition, we have built in three months extra time in case of any enrollment lags (hence leaving three months for final analyses) and incorporated strategies from physical activity interventions previously led by our team that have an excellent track record of recruitment and retention. We also have considered the possibility that the results of this follow-up period might not demonstrate maintenance of the benefits gained. We will then investigate factors contributing to this outcome. This will be achieved by two surveys sent to all patients and parents, one post-intervention and one after the post-follow-up period. These results will be important for our future studies and contribute to the body of literature on maintenance of health gains in pediatric Fontan patients.
The intervention duration may be insufficient to observe change in muscle profile of these patients. However, prior studies in other populations have demonstrated that exercise can demonstrate changes in muscle profile over 12 weeks.53, 54 Our multimodality approach and large sample size (for a pediatric cardiology study) to assess the muscle for mass, strength, as well as mitochondrial function will strengthen our ability to detect even small but meaningful changes.
We expect that not all Fontan patients will demonstrate endothelial dysfunction. Our study design is adequately powered to detect a clinically meaningful change (0.3) in EndoPAT index should there be endothelial dysfunction even in only 1/3 of the Fontan patients at baseline. Since medications, e.g. sildenafil, can affect biomarker levels, these will be adjusted for with covariate analysis.
TIMELINE
Our milestones are listed in Tables 2 and 3. As this funding mechanism is milestone driven, all milestones have to be met in Year 1 to be able to transition to Years 2–5.
Table 2.
R61 (Year 1) Milestones
| Milestones |
|---|
| Complete hiring of study coordinators |
| Register study at clinicaltrials.gov |
| Obtain IRB approval of informed consent form, assent form, data and safety monitoring plan, study flier, and recruitment documents |
| Obtain approval of study protocol, consent, assent, and data and safety monitoring plan by the DSMB |
| Finalize REDCap database |
| Finalize data completeness and quality monitoring plan |
| Finalize site performance monitoring plan |
| Finalize manual of operations |
| Conduct first meeting with the executive committee and set up subsequent meetings |
| Conduct first meeting with the study advisory board and set up subsequent meetings |
| Finalize study workflow and confirm personnel for study testing: cardiopulmonary testing, functional movement screening, endothelial testing, strain echocardiography, dynamometer, DEXA, MRI, blood work |
| Complete training for all study staff: HIPPA, study workflow, quality control, expectations, protocol, manual of operations |
| Conduct two DSMB meetings within the first 12 months |
| Finalize site performance plan |
| Finalize study website |
| Order equipment: EndoPAT probes, tablet computers, heart rate monitors |
| Finalize management and communication plan |
| Submit data and reports as applicable to NLHBI for readiness for R33 transition |
Table 3.
Milestones for Years 2–5
| Milestones by Year | Y2 | Y3 | Y4 | Y5 |
|---|---|---|---|---|
| Meet with the Data and Safety Monitoring Board annually | X | X | X | X |
| Meet with the Advisory Board regularly (every 6 months) to assess study protocol implementation performance | X | X | X | X |
| Meet with the Executive Committee regularly (every 4 months) to assess study protocol implementation performance | X | X | X | X |
| Prepare and submit annual review for NHLBI | X | X | X | X |
| Completion of 40% of projected enrollment | X | |||
| Completion of 70% of projected enrollment | X | |||
| Completion of 100% of projected enrollment | X | |||
| Prepare baseline manuscript | X | |||
| Publish baseline manuscript | X | |||
| Total patients randomized N =150 | X | |||
| Lock the database | X | |||
| Analyze data and prepare results manuscript | X | |||
| Plan to disseminate results and submit to clinicaltrial.gov within 12 months of primary completion date | X |
Funding:
This study is funded by the NIH/NHLBI (R61/R33 HL146775)
The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper and its final contents
SUMMARY AND FUTURE DIRECTIONS
In summary, we will utilize exercise, a non-pharmacologic, simple, and low-risk approach, as a novel therapy in the high-risk pediatric Fontan population (Figure 7) and utilize live-video conferencing to maximize adherence to supervised exercise using a model we have developed. Our study design will answer key questions on the impact of exercise on cardiac and physical capacity (Aim 1); muscle mass, strength, and function (Aim 2); endothelial function (Aim 3) and maintenance of the benefits post-intervention.
Figure 7.
Exercise as Therapy in Fontan Patients.
Our multidisciplinary team of experts will assess the effectiveness of a live-video-supervised exercise intervention, rigorously designed to maximize adherence, and improve key and novel measures of health in pediatric Fontan patients associated with poor long-term outcomes. There are several strengths to the design including the late entry of the usual care group into the exercise intervention and the group exercise sessions which will enhance recruitment and retention. The late entry of the usual care group into the exercise intervention allows a longer-term comparison of the usual care group to the intervention group from baseline to 9 months at 3 time points. Furthermore, the group exercise sessions provide essential peer support and also provide long term sustainability for implementation. Groups sessions ultimately require less resources and trainer time. Our team of investigators have specific expertise in each of the tested measures to ensure robust and unbiased methodology, analysis, interpretation, and reporting of results, with consideration of biological variables including sex and Tanner Stage. The Single Ventricle Program at Stanford University is structured to follow these patients in the form of a local registry that will ultimately provide data on the impact of exercise on long-term outcomes including worsening heart failure, transplant, and death.
The use of exercise as a non-pharmacologic treatment modality in pediatric Fontan patients represents a paradigm shift, where standard therapies have failed.55, 56 Our ultimate goal is the translation of this model to clinical application as an “exercise prescription” to intervene early in pediatric Fontan patients and decrease long-term morbidity and mortality, in alignment with the NIH’s mission to develop evidence-based data for new approaches to improve outcomes in youth with chronic conditions.
Highlights.
Fontan patients have poor exercise capacity associated with a greater risk of morbidity and mortality.
Fontan patients have decreased muscle mass and endothelial dysfunction contributing to disease progression.
Exercise can improve muscle mass, endothelial function, and exercise capacity.
An exercise intervention delivered via live video conferencing optimizes adherence.
We describe the design and rationale of a randomized controlled exercise intervention in pediatric Fontan patients.
Funding:
NHLBI R61/R33 HL146775
Abbreviations:
- CRCEST
Creatine chemical exchange saturation transfer
- DSMB
Data safety and monitoring board
- DXA
Dual-energy X-ray absorptiometry
- FMS
Functional movement screen
- PedsQL
Pediatric quality of life inventory scale
- EndoPAT
Endothelial pulse amplitude testing
- VAT
Ventilatory anaerobic threshold
- Peak VO2
Oxygen consumption at peak exercise
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinical Trial Number: NCT04195451
Conflict of Interest: none
References
- 1.Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. J Pharmacol Pharmacother 2010;1(2):100–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCrindle BW, Zak V, Pemberton VL, Lambert LM, Vetter VL, Lai WW, et al. Functional health status in children and adolescents after Fontan: comparison of generic and disease-specific assessments. Cardiol Young 2014;24(3):469–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uzark K, Zak V, Shrader P, McCrindle BW, Radojewski E, Varni JW, et al. Assessment of Quality of Life in Young Patients with Single Ventricle after the Fontan Operation. J Pediatr 2016;170:166–72 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manlhiot C, Knezevich S, Radojewski E, Cullen-Dean G, Williams WG, McCrindle BW. Functional health status of adolescents after the Fontan procedure -- comparison with their siblings. Can J Cardiol 2009;25(9):e294–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atz AM, Zak V, Mahony L, Uzark K, D’Agincourt N, Goldberg DJ, et al. Longitudinal Outcomes of Patients With Single Ventricle After the Fontan Procedure. J Am Coll Cardiol 2017;69(22):2735–2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Avitabile CM, Leonard MB, Zemel BS, Brodsky JL, Lee D, Dodds K, et al. Lean mass deficits, vitamin D status and exercise capacity in children and young adults after Fontan palliation. Heart 2014;100(21):1702–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Avitabile CM, Goldberg DJ, Leonard MB, Wei ZA, Tang E, Paridon SM, et al. Leg lean mass correlates with exercise systemic output in young Fontan patients. Heart 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cordina RL, O’Meagher S, Karmali A, Rae CL, Liess C, Kemp GJ, et al. Resistance training improves cardiac output, exercise capacity and tolerance to positive airway pressure in Fontan physiology. Int J Cardiol 2013;168(2):780–8. [DOI] [PubMed] [Google Scholar]
- 9.Hambrecht R, Fiehn E, Weigl C, Gielen S, Hamann C, Kaiser R, et al. Regular physical exercise corrects endothelial dysfunction and improves exercise capacity in patients with chronic heart failure. Circulation 1998;98(24):2709–15. [DOI] [PubMed] [Google Scholar]
- 10.Inai K, Saita Y, Takeda S, Nakazawa M, Kimura H. Skeletal muscle hemodynamics and endothelial function in patients after Fontan operation. Am J Cardiol 2004;93(6):792–7. [DOI] [PubMed] [Google Scholar]
- 11.Goldstein BH, Sandelin AM, Golbus JR, Warnke N, Gooding L, King KK, et al. Impact of vitamin C on endothelial function and exercise capacity in patients with a Fontan circulation. Congenit Heart Dis 2012;7(3):226–34. [DOI] [PubMed] [Google Scholar]
- 12.Gorenflo M, Zheng C, Werle E, Fiehn W, Ulmer HE. Plasma levels of asymmetrical dimethyl-L-arginine in patients with congenital heart disease and pulmonary hypertension. J Cardiovasc Pharmacol 2001;37(4):489–92. [DOI] [PubMed] [Google Scholar]
- 13.Hiramatsu T, Imai Y, Takanashi Y, Seo K, Terada M, Aoki M, et al. Time course of endothelin-1 and adrenomedullin after the Fontan procedure. Ann Thorac Surg 1999;68(1):169–72. [DOI] [PubMed] [Google Scholar]
- 14.Mahle WT, Todd K, Fyfe DA. Endothelial function following the Fontan operation. Am J Cardiol 2003;91(10):1286–8. [DOI] [PubMed] [Google Scholar]
- 15.Pearson MJ, Smart NA. Effect of exercise training on endothelial function in heart failure patients: A systematic review meta-analysis. Int J Cardiol 2017;231:234–243. [DOI] [PubMed] [Google Scholar]
- 16.Paridon SM, Mitchell PD, Colan SD, Williams RV, Blaufox A, Li JS, et al. A cross-sectional study of exercise performance during the first 2 decades of life after the Fontan operation. J Am Coll Cardiol 2008;52(2):99–107. [DOI] [PubMed] [Google Scholar]
- 17.McCrindle BW, Williams RV, Mital S, Clark BJ, Russell JL, Klein G, et al. Physical activity levels in children and adolescents are reduced after the Fontan procedure, independent of exercise capacity, and are associated with lower perceived general health. Arch Dis Child 2007;92(6):509–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Longmuir PE, Russell JL, Corey M, Faulkner G, McCrindle BW. Factors associated with the physical activity level of children who have the Fontan procedure. Am Heart J 2011;161(2):411–7. [DOI] [PubMed] [Google Scholar]
- 19.Hampl S, Paves H, Laubscher K, Eneli I. Patient engagement and attrition in pediatric obesity clinics and programs: results and recommendations. Pediatrics 2011;128 Suppl 2:S59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nourse SE, Olson I, Popat RA, Stauffer KJ, Vu CN, Berry S, et al. Live Video Diet and Exercise Intervention in Overweight and Obese Youth: Adherence and Cardiovascular Health. J Pediatr 2015;167(3):533–539 e1. [DOI] [PubMed] [Google Scholar]
- 21.Chen AC, Rosenthal DN, Couch SC, Berry S, Stauffer KJ, Brabender J, et al. Healthy hearts in pediatric heart transplant patients with an exercise and diet intervention via live video conferencing-Design and rationale. Pediatr Transplant 2019;23(1):e13316. [DOI] [PubMed] [Google Scholar]
- 22.Schneiders AG, Davidsson A, Horman E, Sullivan SJ. Functional movement screen normative values in a young, active population. Int J Sports Phys Ther 2011;6(2):75–82. [PMC free article] [PubMed] [Google Scholar]
- 23.Miller MG, Cheatham CC, Patel ND. Resistance training for adolescents. Pediatr Clin North Am 2010;57(3):671–82. [DOI] [PubMed] [Google Scholar]
- 24.Stewart K FBaRS. Resistance training in patients with coronary heart disease Champaign, IL: Human Kinetics; 2001. [Google Scholar]
- 25.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 1982;14(5):377–81. [PubMed] [Google Scholar]
- 26.Bandura A Health promotion by social cognitive means. Health education & behavior : the official publication of the Society for Public Health Education 2004;31(2):143–64. [DOI] [PubMed] [Google Scholar]
- 27.Perri MG. Improving the maintenance of weight loss in behavioral treatment of obesity in: Wadden TA, Stunkard AJ eds Handbook of Obesity Treatment; 2002. [Google Scholar]
- 28.Wilfley DE, Stein RI, Saelens BE, Mockus DS, Matt GE, Hayden-Wade HA, et al. Efficacy of maintenance treatment approaches for childhood overweight: a randomized controlled trial. JAMA 2007;298(14):1661–73. [DOI] [PubMed] [Google Scholar]
- 29.Arena R, Guazzi M, Lianov L, Whitsel L, Berra K, Lavie CJ, et al. Healthy Lifestyle Interventions to Combat Noncommunicable Disease-A Novel Nonhierarchical Connectivity Model for Key Stakeholders: A Policy Statement From the American Heart Association, European Society of Cardiology, European Association for Cardiovascular Prevention and Rehabilitation, and American College of Preventive Medicine. Mayo Clin Proc 2015;90(8):1082–103. [DOI] [PubMed] [Google Scholar]
- 30.Morris NM, Udry JR. Validation of a self-administered instrument to assess stage of adolescent development. J Youth Adolesc 1980;9(3):271–80. [DOI] [PubMed] [Google Scholar]
- 31.American Thoracic S, American College of Chest P. ATS/ACCP Statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med 2003;167(2):211–77. [DOI] [PubMed] [Google Scholar]
- 32.Bensimhon DR, Leifer ES, Ellis SJ, Fleg JL, Keteyian SJ, Pina IL, et al. Reproducibility of peak oxygen uptake and other cardiopulmonary exercise testing parameters in patients with heart failure (from the Heart Failure and A Controlled Trial Investigating Outcomes of exercise traiNing). Am J Cardiol 2008;102(6):712–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Diller GP, Giardini A, Dimopoulos K, Gargiulo G, Muller J, Derrick G, et al. Predictors of morbidity and mortality in contemporary Fontan patients: results from a multicenter study including cardiopulmonary exercise testing in 321 patients. Eur Heart J 2010;31(24):3073–83. [DOI] [PubMed] [Google Scholar]
- 34.xFVaS P. Exercise interpretation system; 1996.
- 35.Piran S, Veldtman G, Siu S, Webb GD, Liu PP. Heart failure and ventricular dysfunction in patients with single or systemic right ventricles. Circulation 2002;105(10):1189–94. [DOI] [PubMed] [Google Scholar]
- 36.Nesbitt GC, Mankad S, Oh JK. Strain imaging in echocardiography: methods and clinical applications. Int J Cardiovasc Imaging 2009;25 Suppl 1:9–22. [DOI] [PubMed] [Google Scholar]
- 37.Varni JW, Burwinkle TM, Seid M, Skarr D. The PedsQL 4.0 as a pediatric population health measure: feasibility, reliability, and validity. Ambul Pediatr 2003;3(6):329–41. [DOI] [PubMed] [Google Scholar]
- 38.Knapp KM, Welsman JR, Hopkins SJ, Shallcross A, Fogelman I, Blake GM. Obesity Increases Precision Errors in Total Body Dual-Energy X-Ray Absorptiometry Measurements. J Clin Densitom 2014. [DOI] [PubMed] [Google Scholar]
- 39.Dam TT, Peters KW, Fragala M, Cawthon PM, Harris TB, McLean R, et al. An evidence-based comparison of operational criteria for the presence of sarcopenia. J Gerontol A Biol Sci Med Sci 2014;69(5):584–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leonard MB, Zemel BS, Wrotniak BH, Klieger SB, Shults J, Stallings VA, et al. Tibia and radius bone geometry and volumetric density in obese compared to non-obese adolescents. Bone 2015;73:69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Armamento-Villareal R, Sadler C, Napoli N, Shah K, Chode S, Sinacore DR, et al. Weight loss in obese older adults increases serum sclerostin and impairs hip geometry but both are prevented by exercise training. J Bone Miner Res 2012;27(5):1215–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Feiring DC, Ellenbecker TS, Derscheid GL. Test-retest reliability of the biodex isokinetic dynamometer. J Orthop Sports Phys Ther 1990;11(7):298–300. [DOI] [PubMed] [Google Scholar]
- 43.DeBrosse C, Nanga RP, Wilson N, D’Aquilla K, Elliott M, Hariharan H, et al. Muscle oxidative phosphorylation quantitation using creatine chemical exchange saturation transfer (CrCEST) MRI in mitochondrial disorders. JCI Insight 2016;1(18):e88207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kogan F, Haris M, Singh A, Cai K, Debrosse C, Nanga RP, et al. Method for high-resolution imaging of creatine in vivo using chemical exchange saturation transfer. Magn Reson Med 2014;71(1):164–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gerdes CM, Kijowski R, Reeder SB. IDEAL imaging of the musculoskeletal system: robust water fat separation for uniform fat suppression, marrow evaluation, and cartilage imaging. AJR Am J Roentgenol 2007;189(5):W284–91. [DOI] [PubMed] [Google Scholar]
- 46.Burakiewicz J, Sinclair CDJ, Fischer D, Walter GA, Kan HE, Hollingsworth KG. Quantifying fat replacement of muscle by quantitative MRI in muscular dystrophy. J Neurol 2017;264(10):2053–2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bonetti PO, Pumper GM, Higano ST, Holmes DR Jr., Kuvin JT, Lerman A. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J Am Coll Cardiol 2004;44(11):2137–41. [DOI] [PubMed] [Google Scholar]
- 48.Selamet Tierney ES, Newburger JW, Gauvreau K, Geva J, Coogan E, Colan SD, et al. Endothelial pulse amplitude testing: feasibility and reproducibility in adolescents. J Pediatr 2009;154(6):901–5. [DOI] [PubMed] [Google Scholar]
- 49.Goldstein BH, Golbus JR, Sandelin AM, Warnke N, Gooding L, King KK, et al. Usefulness of peripheral vascular function to predict functional health status in patients with Fontan circulation. Am J Cardiol 2011;108(3):428–34. [DOI] [PubMed] [Google Scholar]
- 50.Mahmud FH, Earing MG, Lee RA, Lteif AN, Driscoll DJ, Lerman A. Altered endothelial function in asymptomatic male adolescents with type 1 diabetes. Congenit Heart Dis 2006;1(3):98–103. [DOI] [PubMed] [Google Scholar]
- 51.Sivamurthy KM, Dampier C, MacDermott M, Maureen M, Cahill M, Hsu LL. Peripheral arterial tonometry in assessing endothelial dysfunction in pediatric sickle cell disease. Pediatr Hematol Oncol 2009;26(8):589–96. [DOI] [PubMed] [Google Scholar]
- 52.Sullivan M, Genter F, Savvides M, Roberts M, Myers J, Froelicher V. The reproducibility of hemodynamic, electrocardiographic, and gas exchange data during treadmill exercise in patients with stable angina pectoris. Chest 1984;86(3):375–82. [DOI] [PubMed] [Google Scholar]
- 53.Wong PC, Chia MY, Tsou IY, Wansaicheong GK, Tan B, Wang JC, et al. Effects of a 12-week exercise training programme on aerobic fitness, body composition, blood lipids and C-reactive protein in adolescents with obesity. Ann Acad Med Singapore 2008;37(4):286–93. [PubMed] [Google Scholar]
- 54.Mosher PE, Nash MS, Perry AC, LaPerriere AR, Goldberg RB. Aerobic circuit exercise training: effect on adolescents with well-controlled insulin-dependent diabetes mellitus. Arch Phys Med Rehabil 1998;79(6):652–7. [DOI] [PubMed] [Google Scholar]
- 55.Goldberg DJ, Zak V, Goldstein BH, McCrindle BW, Menon SC, Schumacher KR, et al. Design and rationale of the Fontan Udenafil Exercise Longitudinal (FUEL) trial. Am Heart J 2018;201:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goldberg DJ, Zak V, Goldstein BH, Schumacher KR, Rhodes J, Penny DJ, et al. Results of the FUEL Trial. Circulation 2020;141(8):641–651. [DOI] [PMC free article] [PubMed] [Google Scholar]


