Abstract
Objectives
When the COVID-19 pandemic reached France early in 2020, the enforced nationwide lockdown deeply altered lifestyle as well as hospital processes and modalities of care. The aim of the study was to evaluate the impact during the lockdown of the first epidemic wave on the epidemiology of bacteremia in one French University Hospital.
Patients and Methods
Retrospective cohort study including adult patients with positive blood culture between 23rd March to 24th May 2020. The clinical-microbiological characteristics were compared with those of the period from 25th March to 26th May 2019. The data were adjusted to the number of hospitalizations (h).
Results
In 2020, 189 bacteremia were diagnosed from 1939 vials (9658 hospitalizations, 10911 emergency room consultations) compared to 143 from 1976 vials (14797 hospitalizations, 16493 emergency room consultations) recorded in 2019. The incidence of bacteremia increased up to 19.7 per 1000h in 2020 vs 9.7 in 2019 (p < 0.001). The main differences (2020 vs 2019) were: Staphylococcus aureus bacteremia (2.4 vs 1.0/1000h, p = 0.012), polymicrobial bacteremia (2.2 vs 0.9/1000h p = 0.013) and Gram-negative bacteremia (8.9 vs 4.3/1000h, p < 0.01). Conversely, Streptococcus pneumoniae incidence decreased (0 vs 0.47/1000h, p = 0.047). The standardized incidence ratio calculation confirmed these results.
Conclusion
The lockdown and the impact of the first wave of the Covid-19 pandemic on the health system resulted in increased hospital-diagnosed bacteremia and decreased pneumococcal bacteremia. Disruption and overload of ICUs, lockdown with preventive control measures, and decrease in human-to-human interaction may have been the main reasons.
Keywords: Bacteremia, Blood stream infection, Lockdown, Non-pharmaceutical interventions, SARS-CoV-2
1. Introduction
At the beginning of 2020, worldwide healthcare facilities were confronted with the severe acute respiratory syndrome coronavirus (SARS-CoV-2) pneumonia pandemic. A strict containment of populations was rapidly put in place in many countries due to the unavailability of effective tools to stop the viral spread [1]. In France, the first lockdown lasted from March 17th to May 11th, 2020.
These measures deeply impacted hospitals and their organization: deprogramming of elective surgeries; opening of a dedicated coronavirus disease 2019 (COVID-19) ward; reorganization of emergency care wards, medical departments, and intensive care units (ICU) [2], [3]. The measures taken (permission to travel for compelling reasons only, mask wearing, social distancing…) had a sudden and pronounced impact on daily life: working, teaching and travelling locally, nationally, and internationally.
Some of the microorganisms responsible for bacteremia (e.g., Streptococcus pneumoniae, Neisseria meningitidis) are related to human-to-human transmission [4], [5], while others, such as Enterobacteriaceae, often have endogenous origin [6]. In a context of reduced social interactions and reorganization of the health-care system, atypical clinical presentations of bacteremia and changed incidence of invasive infections related to human-transmitted pathogens were indirect consequences of containment. This was described by Feldman et al. [7], who found decreased incidence of S. pneumoniae bacteremia associated with social distancing measures, without any adjustment on the overall activity of the recruiting center; these results confirmed on a larger scale the findings of the international multicenter study by Brueggemann et al. [8]. A Spanish study conducted by Mormeneo Bayo et al. [9], reported a decreased number of blood cultures taken in a hospital during lockdown (second quarter 2020) compared to the same period in 2018 and 2019 (−18.8% and −22.7% p = 0.173 respectively), which remains to be confirmed.
The aim of this study is to assess the impact of a lockdown on the epidemiology of invasive infections such as bacteremia. Consequences on the incidence of bacteremia and/or its diagnosis need to be reported, as part of efforts to prepare for a future pandemic, and so s to better understand the repercussions of lockdown-associated measures on human-to-human transmitted pathogens.
2. Methods
2.1. Study design
We conducted a comparative retrospective cohort study in an adult population of patients who had a positive blood culture recorded at the microbiology laboratory of the university hospital of Nice, France (1650 beds) between 23rd March 2020 and 24th May 2020. This period covers the first containment with exclusion of transitional periods (containment beginning: 17th-23rd March 2020, and containment ending: 11th -24th May 2020). Comparative datasets were included to allow comparisons with the pre-pandemic period. We also included community-collected data to observe epidemiological trends outside the hospital center. The pre-pandemic data were extracted from our institution between 25th March and 26th May 2019, as a control period of similar duration and seasonality.
To assess the impact of non-pharmaceutical interventions (NPI) on the activity of our center over the given periods of time, the number of registered emergency department consultations and the number of hospitalizations carried out in the emergency room or in a medical unit were extracted and analyzed from the databases of the Department of Information and Medical Informatics.
To describe the trend in non-teaching health care facilities of our county, we extracted data obtained from blood culture vials of private medical laboratory network (EUROFINS LABAZUR NICE) processing bacteriological samples from community laboratories and health institutions (nine private clinics and one non-university hospital). Taken together, these facilities represent 900 hospital beds. Study periods between the different establishments were similar.
2.2. Microbiological methods
Blood cultures (aerobic FAN Plus and anaerobic FN Plus vials) from the university Hospital of Nice were incubated for five days (BactAlert 3D, bioMérieux) and identification was performed by Matrix Assisted Laser Desorption Ionization - Time of Flight (MALDI-TOF) mass spectrometry on positive broth (hereafter direct Identification) or after subculture on appropriate agar plates when the blood culture was polymicrobial or when direct identification failed.
Regarding the private medical laboratory network technical platform, the blood cultures (aerobic FA Plus and anaerobic FN Plus vials) were incubated for five days (BactAlert 3D, bioMérieux) and identification on subcultures was performed by MALDI-TOF mass spectrometry.
The methods used to perform bacterial identification did not change between the two study periods.
2.3. Patients and definition of bacteremia
The database of the Bacteriology Lab (SIRweb® software (i2a)) was used to retrieve data from all the blood culture vials registered during the periods of interest. Results from all vials collected from the same patient, regardless of the sampling site (peripheral vein, central line, PICCLINE, arterial catheter) were grouped to define a collection episode. We arbitrarily determined that a collection episode could last up to five days. After the fifth day, if other vials were registered, another collection episode was recorded. As a result, a single patient might account for several collection episodes during the same hospitalization. Data for blood culture vials used for ascitic fluid, joint fluid or pleural fluid and/or pediatric sampling cultures were excluded.
Bacteremia was defined as:
-
–
A positive peripheral blood culture for bacteria recognized as pathogenic in at least one vial (Staphylococcus aureus, Streptococcus spp, Enterococcus spp, strict anaerobic bacteria, and non-fermenting Gram-negative bacteria (GNB));
-
–
OR at least two positive vials for a commensal bacterium with the same antibiotic profile (e.g., coagulase-negative staphylococci (CoNS), Corynebacterium spp).
Monomicrobial bacteremia was defined by the presence of only one microorganism per episode. Polymicrobial bacteremia was defined by the presence of at least two microorganisms per episode. Catheter-related infection was defined as the presence of a microorganism identified on one or more vials taken from arterial catheter, PICCline, central venous line, without bacteria isolated from a simultaneous peripheral blood cultures with local or general symptoms suggestive of catheter-related infection. When the diagnosis was uncertain, a systematic review of medical computerized files was performed to assess whether the episode was catheter-related or whether it resulted from contamination.
2.4. Data analysis
Data were analyzed according to the isolated bacterial genus and species and were adjusted to hospital activity relative to the time periods studied. The number of total hospitalizations recorded, or the number of visits recorded, enabled us to obtain incidences per 1000 inpatients for medical departments, and per 1000 referral-patient for the emergency department. We then analyzed each event by calculating the standardized incidence ratio (SIR), which is the ratio between the number of events observed in 2020 and the number of events expected in 2020. The expected number of events in 2020 was calculated from the number of events observed in 2019 according to the number of hospitalizations recorded in 2020.
Private medical laboratory network data were described as raw because we were unable to subtract redundant potential vials from the same patient. In addition, due to a lack of clinical data from the patients collected, we could not define whether the positive vials corresponded to bacteremia, catheter-related infection or contamination. For these reasons we decided to report only the positivity rate and not to perform statistical analysis.
2.5. Statistical analysis
Qualitative data were analyzed with the Chi2 test or FISHER-exact tests based on headcount. The significance threshold was set at 0.05 and a P-value below 0.05 was considered as significant.
3. Results
Over the 2020 period, 9836 blood culture vials were recorded in the Bacteriology Lab, compared to 9411 in 2019. Those blood cultures were ordered in 1939 collection episodes in 2020 and 1976 collection episodes in 2019 (Fig. 1 ). In 2020, 9658 hospitalizations occurred in our center as opposed to 14797 in 2019, representing a 25% decrease between the two periods. Regarding the emergency department, in 2020 there were 10911 consultations and 4225 associated hospitalizations vs 16493 consultations and 4207 associated hospitalizations in 2019, meaning a 34% decrease of visits (normalized to hospitalization number).
Fig. 1.
Flow chart.
In the non-teaching health-care facilities, we found that a total of 1233 vials were taken in 2020 as opposed to 1554 in 2019, resulting in a 20.6% decrease. Specifically, we noted that 239 vials were positive in 2019 and 145 in 2020. The positivity rate was 15.4% (239/1554) in 2019 vs 11.8% (145/1233) in 2020.
In comparison, the positivity rate for hospital-diagnosed bacteremia was 7.2% (143/1976) in 2019 vs 9.3% (180/1939) in 2020.
3.1. Bacteremia, catheter-related infections and incidence of contamination
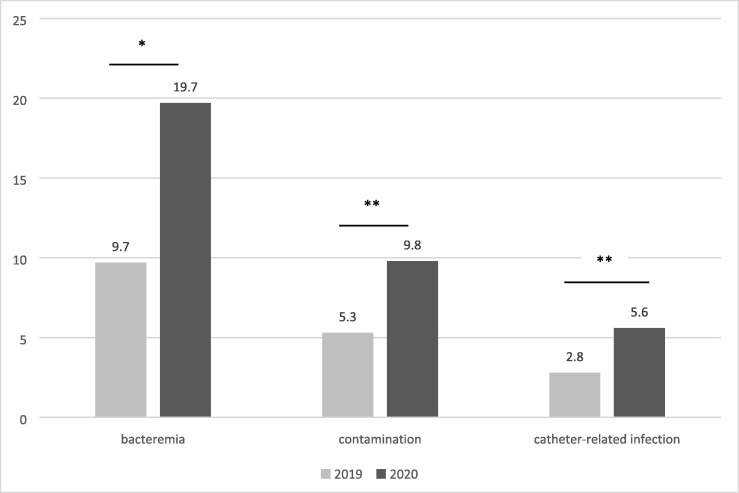
University hospital data: Crude bacteremia numbers included in the analysis are reported for the two periods (pre-pandemic 2019 vs pandemic 2020) (Fig. 1 ). Normalized to 1000 hospitalizations, we observed a significantly higher incidence of bacteremia in 2020 with 19.7 episodes /1000 hospitalizations against 9.7/1000 hospitalizations in 2019 (p < 0.001), and an increase of catheter-related infections and contamination-related episodes (Fig. 2 ).
Fig. 2.
Incidence of bacteremia, contaminations and catheter-related infections per 1000 hospitalizations. 2019 vs 2020 Note. *p<0,001 ; **p<0,01.
3.2. Standardized incidence ratio
In 2020, we observed a significant increase in bacteremia (SIR = 2.01 95% CI [1.74–2.33]), catheter-related infections (SIR = 1.88 95% CI [1.41–2.46]) and blood culture contaminations (SIR = 1.87 95% CI [1.51–2.29]) compared to 2019 (Table 1 ). In an individualized analysis at the department level (emergency, medical, and ICU departments), we observed a significant increase in bacteremia in the emergency department in 2020 with an incidence of 4.7/1000 emergency department visits vs 2.3/1000 hospitalizations in 2019 (p = 0.0007). We also noted a significantly higher incidence of bacteremia in the ICU, where 2.2/1000 hospitalizations bacteremia occurred in 2020 vs 0.6/1000 hospitalizations in 2019 (p = 0.0003) (Table 2 ). In medical departments, while incidence of bacteremia rose from 5/1000 hospitalizations in 2019 to 9.8/1000 hospitalizations, no significant difference was found (p = 0.6).
Table 1.
Standardized incidence ratio (SIR) of bacteriemia, catheter-related infections and contaminations.
| Expected events in 2020 (n) | Observed events in 2020 (n) | SIR (95% CI) | |
|---|---|---|---|
| Bacteremia | 93 | 188 | 2,01 (1.74-2.33) |
| Catheter-related infections | 29 | 54 | 1,88 (1.41-2.46) |
| Contaminations | 51 | 95 | 1,87 (1.51-2.29) |
Table 2.
Incidence of bacteremia by collection unit: emergency department, medical department, and intensive care unit.
| 2019 | 2020 | p | ||
|---|---|---|---|---|
| Emergency department | 38 | 51 | ||
| For 1000 visits | 2.3 | 4.7 | p = 0.0007 | |
| Medical department | 74 | 95 | ||
| For 1000 hospitalizations | 5 | 9.8 | p = 0.6 | |
| Intensive Care Unit | 9 | 22 | ||
| For 1000 hospitalizations | 0.6 | 2.2 | p = 0.0003 |
3.3. Microbiological data
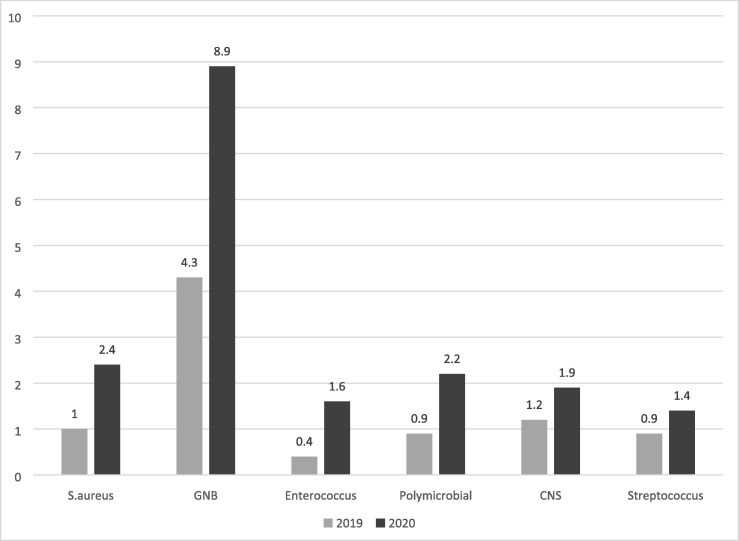
In 2020, a majority of monomicrobial bacteremia were due to GNB (8.9/1000 hospitalizations) followed by S. aureus and polymicrobial bacteremia, 2.4/1000 and 2.2/1000 hospitalizations respectively.
In 2019, the ranking of bacteremia by pathogen was roughly the same (CoNS slightly more frequent than S. aureus), but at a lower level (Fig. 3 ).
Fig. 3.
Bacteriemia incidence by germ per 1000 hospitalizations.
In 2020 (compared to 2019), we observed significantly increased incidence of S. aureus, GNB and polymicrobial bacteremia. Of note, no S. pneumoniae bacteremia was observed in 2020 compared to seven cases in 2019 (Table 3 ). Analysis of the standardized incidence ratio (SIR) for these events confirmed the significant increases described above (Table 3b ).
Table 3.
Incidence of germ bacteremia per 1000 hospitalizations (S. aureus, GRAM negative bacillus (GNB), polymicrobial, and Streptococci).
| 2019 | 2020 | p | |
|---|---|---|---|
| S. aureus | 1 | 2.4 | *p = 0.012 |
| GNB | 4.3 | 8.9 | *p < 0.01 |
| Polymicrobial | 0.9 | 2.2 | *p = 0.013 |
|
Streptococcus S.pneumoniae |
0.9 | 1.4 | p = 0.273 |
| 0.5 | 0 | *p = 0.047 |
Table 3b.
Standardized incidence ratio (SIR) of GNB, S.aureus and polymicrobial bacteriemia. 2019 vs 2020.
| Germ | Expected events in 2020 (n) | Observed events in 2020 (n) | SIR (95% CI) |
|---|---|---|---|
| GNB | 42 | 86 | 2,06 [1,65-2,55] |
| S. aureus | 10 | 22 | 2,25 [1,41-3,41] |
| Polymicrobial | 8 | 20 | 2,36 [1,44-3,64] |
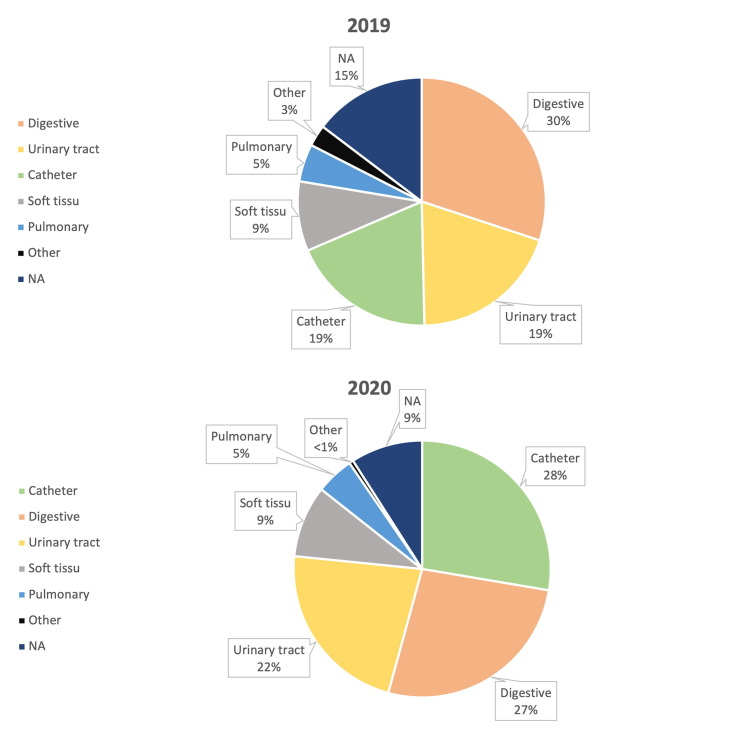
The main sources of bacteremia in 2020 vs 2019 are reported in Fig. 4 , with only slight and non-significant changes.
Fig. 4.
Sources of bacteriemia. 2019 vs 2020.
4. Discussion
The first epidemic wave of COVID-19 and the subsequent lockdown led to increased incidence and changes in the characteristics of bacteremia in our center, a university hospital referral center for a population of more than one million inhabitants.
We described higher incidence in 2020 than in 2019 not only of documented monomicrobial bacteremia due to S. aureus, but also to polymicrobial bacteremia. These results are consistent with another French study [10] which found increased incidence of E. coli, K. pneumoniae and S. aureus bacteremia in Parisian hospitals during the lockdown period compared to the same period in 2019. Conversely, a British multicenter study [11] found a decreased incidence of bacteremia related to Enterobacteriaceae, but the authors did not adjust data to level of activity, which was most certainly altered. In addition, the increase of bacteremia and catheter-related infection was observed mainly in the emergency department or intensive care units. The doubling of the incidence of catheter-related infections is linked to the increase in admissions to ICU related to COVID-19, among patients often having been hospitalized in critical care. In a context of high hospital tension and dire urgency, altered handling of catheters could explain the large number of equipment infections. These results are consistent with literature data that have reported increased catheter-related infections in relation to the COVID pandemic, particularly in critical care departments [12], [13], [14]. The increased incidence of bacteremia due to S. aureus, a microorganism very frequently associated with catheter infections, is consistent with this hypothesis.
Concomitantly with the increase in infections, in our center we observed an increase in blood culture contaminations. Conditions of practice under the pressure of an unusual influx of patients and imposed reorganizations of paramedical and medical teams may have led to degraded aseptic practices in a health emergency context. Increased incidence of CoNS contamination has also been described in patients hospitalized elsewhere for COVID-19 pneumonia [15]. The impact of these contaminations can be associated with misdiagnosis, difficult interpretation of results, and useless antibiotic treatment [16], [17], [18].
Our results on the absence of pneumococcal bacteremia during the containment period are consistent with data from the literature. Indeed, decreased incidence of viral infections due to Influenzae, a respiratory infection known as a risk factor for pneumococcal infection, was observed in France and worldwide [19], [20]. It has been shown that surgical masks, hygiene measures and compliance with other NPIs had a significant impact on the incidence of invasive infections with encapsulated germs [8]. Though they could not be thoroughly analyzed, available data from city vials highlighted decreased blood culture samples and decreased vial positivity rates. A shift of patients towards the hospital-university structure could partially explain these results. observed and described above.
Our study has some limitations. First, we have presented a French monocentric analysis, which limits generalization. The arbitrary duration of five days taken to distinguish two infectious episodes may have led us to underestimate prolonged bacteremia or early recurrence. We did not conduct any analysis on surgical wards because we did not have information on the volume of surgical procedures maintained during the 2020 lockdown compared to 2019. As regards results from the analysis of city vials, the lack of clinical data and the raw analysis without adjustment may have led us to misestimate a possible shift of patients to the university structure.
During the period analyzed, we described an increased incidence in all types of bacteremia, with the notable exception of S. pneumoniae, mainly in the emergency department and in ICUs. However, we also observed a drop of about a third in emergency room admissions. The increase could be explained by a change in the class action of emergency room admission, leading to an increased proportion of bacteremic patients. In addition, containment measures with travel restrictions, teleconsultation and deprogramming of some care activities may have led to the admission of some patients at a later stage of their pathology.
5. Conclusion
This study shows significantly increased incidence of bacteremia after adjusting for the level of activity in our center during lockdown. The exceptional situation due to the COVID-19 pandemic onset led to profound changes in patient care with a direct influence on the incidence and epidemiology of bacteremia. From bacteremia to sepsis, the deregulated response of the host may be hampered through early diagnosis and appropriate antibiotic treatment. Better understanding of the incidence in during a health emergency of bacteremia is crucial and could contribute to anticipation of diagnoses and therapeutic actions to be taken upstream from future epidemics. Among NPIs, self-isolation for febrile illnesses should always be preceded by an accurate diagnosis together with appropriate patient education, the objective being to avoid delay in the management of invasive bacterial infections.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Availability of data
Data are available upon reasonable reason.
Ethics considerations
The study was registered at ClinicalTrials.gov: NCT05640349.
CRediT authorship contribution statement
Vincent Cauhapé: Conceptualization, Formal analysis, Methodology, Writing – original draft. Brigitte Lamy: Conceptualization, Methodology, Supervision, Writing – review & editing. Romain Lotte: Writing – review & editing. Irit Touitou: Supervision, Writing – review & editing. Laurent Boyer: Writing – review & editing. Julie Contenti: Writing – review & editing. François Parisot: Writing – review & editing. Raymond Ruimy: Writing – review & editing. Michel Carles: Formal analysis, Supervision, Writing – review & editing. Johan Courjon: Conceptualization, Methodology, Supervision, Formal analysis, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
We are grateful to Cédric SEMERIA from the Department of Information and Medical Informatics.
We are grateful to Elisabeth HUYNH-VAN from the clinical research center of Nice.
References
- 1.Allocution officielle du Président de la république française du 12/03/2020. Adresse aux Français, 12 mars 2020 | Élysée (elysee.fr). 14/12/22 : Adresse aux Français, 12 mars 2020 | Élysée (elysee.fr).(Accessed on march 29, 2023)
- 2.Birkmeyer J.D., Barnato A., Birkmeyer N., Bessler R., Skinner J. The Impact Of The COVID-19 Pandemic On Hospital Admissions In The United States: Study examines trends in US hospital admissions during the COVID-19 pandemic. Health Aff (Millwood) 2020;39(11):2010–2017. doi: 10.1377/hlthaff.2020.00980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.COVIDSurg Collaborative Elective surgery cancellations due to the COVID-19 pandemic: global predictive modelling to inform surgical recovery plans: Elective surgery during the SARS-CoV-2 pandemic. Br J Surg. 2020;107(11):1440–1449. doi: 10.1002/bjs.11746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weiser J.N., Ferreira D.M., Paton J.C. Streptococcus pneumoniae: transmission, colonization and invasion. Nat Rev Microbiol. 2018;16(6):355–367. doi: 10.1038/s41579-018-0001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rouphael N.G., Stephens D.S. In: Christodoulides M., editor. vol. 799. Humana Press; Totowa, NJ: 2012. Neisseria meningitidis: Biology, Microbiology, and Epidemiology; pp. 1–20. (Neisseria Meningitidis). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oliveira J, Reygaert WC. Gram Negative Bacteria. 2022 Oct 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK538213/. [PubMed]
- 7.Feldman I., Natsheh A., Nesher G., Breuer G.S. Social distancing and bacteraemia in the time of COVID -19. Intern Med J. 2022;52(2):223–227. doi: 10.1111/imj.15560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jansen van Rensburg M.J., Shaw D., McCarthy N.D., Jolley K.A., Maiden M.C.J., van der Linden M.P.G., et al. Changes in the incidence of invasive disease due to Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria meningitidis during the COVID-19 pandemic in 26 countries and territories in the Invasive Respiratory Infection Surveillance Initiative: a prospective analysis of surveillance data. Lancet Digit. Health. 2021;3(6):e360–e370. doi: 10.1016/S2589-7500(21)00077-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mormeneo Bayo S., Palacián Ruíz M.P., Moreno Hijazo M., Villuendas Usón M.C. Bacteremia during COVID-19 pandemic in a tertiary hospital in Spain. Enfermedades Infecc Microbiol Clínica. 2022;40(4):183–186. doi: 10.1016/j.eimce.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amarsy R., Trystram D., Cambau E., Monteil C., Fournier S., Oliary J., et al. Surging bloodstream infections and antimicrobial resistance during the first wave of COVID–19: a study in a large multihospital institution in the Paris region. Int J Infect Dis. 2022;114:90–96. doi: 10.1016/j.ijid.2021.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Denny S., Rawson T.M., Hart P., Satta G., Abdulaal A., Hughes S., et al. Bacteraemia variation during the COVID-19 pandemic; a multi-centre UK secondary care ecological analysis. BMC Infect Dis. 2021;21(1):556. doi: 10.1186/s12879-021-06159-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fakih M.G., Bufalino A., Sturm L., Huang R.-H., Ottenbacher A., Saake K., et al. Coronavirus disease 2019 (COVID-19) pandemic, central-line–associated bloodstream infection (CLABSI), and catheter-associated urinary tract infection (CAUTI): The urgent need to refocus on hardwiring prevention efforts. Infect Control Hosp Epidemiol. 2022;43(1):26–31. doi: 10.1017/ice.2021.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aldawood F., El-Saed A., Zunitan M.A., Alshamrani M. Central line-associated blood stream infection during COVID-19 pandemic. J Infect Public Health. 2021;14(5):668–669. doi: 10.1016/j.jiph.2021.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kokkoris S., Papachatzakis I., Gavrielatou E., Ntaidou T., Ischaki E., Malachias S., et al. ICU-acquired bloodstream infections in critically ill patients with COVID-19. J Hosp Infect. 2021;107:95–97. doi: 10.1016/j.jhin.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sepulveda J., Westblade L.F., Whittier S., Satlin M.J., Greendyke W.G., Aaron J.G., et al. Bacteremia and Blood Culture Utilization during COVID-19 Surge in New York City. J Clin Microbiol. 2020;58(8):e00875–e920. doi: 10.1128/JCM.00875-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Hal S.J., Frostis V., Miyakis S., Marriott D., Harkness J. Prevalence and significance of coagulase-negative staphylococci isolated from blood cultures in a tertiary hospital. Scand J Infect Dis. 2008;40(6–7):551–554. doi: 10.1080/00365540701877304. [DOI] [PubMed] [Google Scholar]
- 17.Souvenir D., Anderson D.E., Palpant S., Mroch H., Askin S., Anderson J., et al. Blood Cultures Positive for Coagulase-Negative Staphylococci: Antisepsis, Pseudobacteremia, and Therapy of Patients. J Clin Microbiol. 1998;36(7):1923–1926. doi: 10.1128/JCM.36.7.1923-1926.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bates D.W., Goldman L., Lee T.H. Contaminant blood cultures and resource utilization. The true consequences of false-positive results. JAMA. 1991;265(3):365–369. PMID: 1984535. [PubMed] [Google Scholar]
- 19.Olsen S.J., Azziz-Baumgartner E., Budd A.P., Brammer L., Sullivan S., Pineda R.F., et al. Decreased influenza activity during the COVID-19 pandemic—United States, Australia, Chile, and South Africa, 2020. Am J Transplant. 2020;20(12):3681–3685. doi: 10.1111/ajt.16381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.SantéPublique France. Grippe : Bilan de la saison 2019-2020. Octobre 2020 [online]. 14/12/22: Bulletin épidémiologique grippe. Bilan de la surveillance, saison 2019-2020. (santepubliquefrance.fr).(accessed on 29 march 2023).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon reasonable reason.