Abstract
Background and Objective
In the USA, cabozantinib was approved for the treatment of patients aged ≥ 12 years with radioiodine-refractory differentiated thyroid cancer (DTC) who progressed on prior vascular endothelial growth factor (VEGFR)-targeted therapy based on the Phase 3 COSMIC-311 trial, which evaluated cabozantinib 60 mg/day versus placebo. Approved dosing is 60 mg/day for adults and for pediatric patients aged ≥ 12 years with body surface area (BSA) ≥ 1.2 m2, and 40 mg/day for pediatric patients aged ≥ 12 years with BSA < 1.2 m2. This report describes a population pharmacokinetic (PopPK) and exposure–response analysis of COSMIC-311.
Methods
A PopPK model was developed using concentration-time data from COSMIC-311 and 6 other cabozantinib studies. The final (full) PopPK model was used to simulate the effect of sex, body weight, race, and patient population. For exposure–response analysis, derived datasets from COSMIC-311 were constructed for time-to-event analyses of progression-free survival (PFS) and safety endpoints.
Results
The PopPK analysis included 4746 cabozantinib PK samples from 1745 patients and healthy volunteers. Body weight had minimal impact on cabozantinib exposure but increasing body weight was associated with increased apparent volume of distribution. Based on model-based simulation, adolescents < 40 kg had higher maximum plasma concentration at steady state of cabozantinib 60 mg/day compared to adults. Allometric scaling simulation in adolescents < 40 kg demonstrated higher exposure with 60 mg/day relative to adults receiving the same dose, while exposure with 40 mg/day in adolescents < 40 kg was similar to 60 mg/day in adults. The exposure–response analysis included 115 patients. There was no clear relationship between PFS or dose modification and cabozantinib exposure. A statistically significant relationship was demonstrated for cabozantinib exposure and hypertension (Grade ≥ 3) and fatigue/asthenia (Grade ≥ 3).
Conclusions
These results support the dosing strategy implemented in COSMIC-311 and the BSA-based label recommendations for adolescents. The cabozantinib dose should be reduced to manage adverse events as indicated.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40262-023-01210-0.
Key Points
| In this PopPK analysis of the Phase 3 COSMIC-311 study in patients with radioiodine-refractory differentiated thyroid cancer (DTC), model simulations predicted that a dose of cabozantinib 40 mg/day in adolescent patients who weigh < 40 kg would provide a similar cabozantinib exposure compared with a dose of 60 mg/day in adult patients. |
| These data support the approved cabozantinib dosing for radioiodine-refractory DTC—60 mg/day for adults and for pediatric patients aged ≥ 12 years with body surface area (BSA) ≥ 1.2 m2, and 40 mg/day for pediatric patients aged ≥ 12 years with BSA < 1.2 m2. |
| Exposure–response analyses showed that dose modification to manage adverse events was predicted to improve tolerability while maintaining efficacy. |
Introduction
Up to 15% of patients with differentiated thyroid cancer (DTC) develop radioiodine-refractory metastatic disease and have a poor prognosis [1]. Treatment options include the tyrosine kinase inhibitors (TKIs) sorafenib and lenvatinib [2, 3]. However, most patients will develop treatment resistance and disease progression [2, 3], with few second-line treatment options available.
Cabozantinib (XL184) is a TKI with multiple targets implicated in tumor growth, metastasis, and angiogenesis, including vascular endothelial growth factor (VEGFR), MET, RET, and the TAM family of receptor kinases (TYRO3, AXL, and MER) [4]. The cabozantinib tablet is approved for adults with advanced renal cell carcinoma (RCC) or hepatocellular carcinoma (HCC) previously treated with sorafenib at a dose of 60 mg once daily (QD) [5, 6]. Cabozantinib 40 mg QD in combination with nivolumab is approved for first-line treatment of adults with advanced RCC.
In September 2021, the US FDA approved cabozantinib tablets for adult and pediatric patients aged ≥ 12 years with locally advanced or metastatic DTC that has progressed after VEGFR-targeted therapy and who are ineligible for or refractory to radioiodine [7]. The recommended dose of single-agent cabozantinib is 60 mg QD for adults and for pediatric patients with a body surface area (BSA) ≥ 1.2 m2, and 40 mg QD for pediatric patients with BSA < 1.2 m2 [5]. Approval was based on results from the Phase 3 COSMIC-311 trial, in which cabozantinib 60 mg QD significantly reduced the risk of disease progression or death versus placebo in patients with previously-treated radioiodine-refractory DTC. At an interim analysis of progression-free survival (PFS) in the intention-to-treat (ITT) population, median PFS was not reached in the cabozantinib arm versus 1.9 months for placebo (hazard ratio [HR] 0.22, 96% confidence interval [CI] 0.13–0.36; p < 0.0001) [8]. The benefit was maintained after extended follow-up and in a larger ITT population with a median PFS of 11.0 months for cabozantinib versus 1.9 months for placebo (HR 0.22, 96% CI 0.15–0.32; p < 0.0001) [9].
Here we describe a cabozantinib population pharmacokinetics (PopPK) modeling and simulation analysis and an exposure–response analysis based on the interim analysis of PFS and safety data from COSMIC-311. The objectives of the PopPK analysis were to: characterize the cabozantinib concentration-time profile following cabozantinib tablet administration in healthy volunteers and in patients with various cancer types, including those with DTC from COSMIC-311; assess the effects of selected covariates on cabozantinib PK parameters; generate individual predicted cabozantinib exposure measures for subsequent analyses; and predict cabozantinib exposure in adolescent patients aged 12–17.5 years using the PopPK model developed for the adult subjects. The exposure–response analyses assessed the relationship of individual predicted cabozantinib exposure with PFS and safety endpoints in patients with DTC from COSMIC-311.
Methods
Ethics
For the cabozantinib clinical studies included in these analyses, the study protocols were approved by the institutional review board or ethics committee at each center, and all patients provided written informed consent. The studies were performed in compliance with the Declaration of Helsinki and the International Conference on Harmonization Guidelines for Good Clinical Practice.
COSMIC-311
COSMIC-311 was a randomized, double-blind, placebo-controlled study of cabozantinib in patients aged ≥ 16 years with radioiodine-refractory DTC who progressed after VEGFR-targeted therapy [8]. At the data cut-off for the analyses reported here (August 19, 2020), 187 patients were randomized 2:1 to cabozantinib 60 mg QD (n = 125) or placebo (n = 62). Patients were stratified by age (≤ 65 years vs > 65 years) and prior receipt of lenvatinib (yes vs no). Dose interruptions and dose reductions (60 mg QD to 40 mg QD, then to 20 mg QD) were permitted to manage adverse events (AEs). The dual primary endpoints were objective response rate (ORR) in the first 100 randomized patients (ORR ITT [OITT] population) after a minimum follow-up of 6 months and PFS in all randomized patients (ITT population), both assessed by a blinded independent radiology committee and Response Evaluation Criteria in Solid Tumors v1.1. An interim analysis of PFS was planned contemporaneously with the primary analysis of ORR. Meeting either endpoint would demonstrate the superiority of cabozantinib over placebo.
Tumor response and progression were assessed by magnetic resonance imaging or computed tomography at baseline, every 8 weeks after randomization for 12 months, then every 12 weeks thereafter. Safety was assessed every 2 weeks until Week 9, then every 4 weeks thereafter, with a post-treatment follow-up visit 30 days after treatment discontinuation. Adverse events were assessed with severity graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE), version 5.
At the time of the first analysis, the primary endpoint of ORR in the OITT population was not met: cabozantinib was favored versus placebo (15% vs 0%; p = 0.028) but the difference did not meet the predefined level of statistical significance (α = 0.01) [8]. However, the primary endpoint of PFS was met. The safety profile was manageable, with 70 patients (56%) having a dose reduction and 90 patients (72%) having a dose interruption of cabozantinib due to AEs.
Population Pharmacokinetic Modeling
The PopPK analysis includes plasma cabozantinib concentration-time data from COSMIC-311 and six other studies with the cabozantinib tablet formulation: a 60 mg QD dose in patients with castration-resistant prostate cancer (CRPC, XL184-306 and XL184-307, Phase 3) [10, 11], RCC (XL184-308, Phase 3) [12], and HCC (XL184-309, Phase 3) [13]; 40 mg QD in combination with nivolumab in RCC (CheckMate 9ER, Phase 3) [14]; and a single 20, 40, or 60 mg dose in healthy volunteers (XL184-020, Phase 1) [15]. Design and PK sampling schedules for each of these studies are provided in Supplementary Table S1.
A non-linear mixed effects modeling compatible dataset was constructed from source data for COSMIC-311 and added to a previously derived analysis dataset of the other six studies. Based on prior modeling experience with cabozantinib, the initial PopPK structural model was a two-compartment model with first-order absorption and first-order elimination. The initial structural model was developed with Study XL184-020 alone (healthy volunteers), as it is the only study with serial PK sampling. The other studies, which contain sparse sampling, were incorporated into the model for an assessment of the random and fixed effects. Cabozantinib concentrations were excluded from the PopPK analysis if the dose prior to the PK sample was missing, the PK sample date and time were missing, or if a cabozantinib concentration was quantifiable in PK samples prior to the first dose.
A full model was developed to explore the effect of covariates not included as structural covariates in the base model, including sex (based on previous knowledge of factors that influence cabozantinib PK) and body weight (based on consideration of cabozantinib exposure prediction in adolescent patients). See Supplementary Materials for further detail. A complete battery of diagnostic plots was generated for each model development step, and a prediction-corrected visual predictive check (pcVPC) was performed on the full model.
Potential effects of race (White vs Black vs Asian) and population (healthy volunteers vs patients with various cancer types [CRPC, HCC, RCC, and DTC]) on cabozantinib PK were evaluated using individual post hoc estimates from the final (full) model. The PK base model was used to generate individual exposure predictions for subsequent exposure–response analyses.
The final (full) PK model was used to perform stochastic simulations of steady-state exposure in adolescent patients aged 12–17.5 years with DTC taking either 40 mg (i.e., first-level dose reduction from 60 mg or starting dose for adolescents with BSA < 1.2 m2) or 60 mg QD. Guidance from the US FDA indicates that adolescent patients with a body weight ≥ 40 kg can receive the same fixed drug dose as adults based on similar drug PK or safety but that adolescents < 40 kg should switch to a body-weight or BSA-adjusted dose [16].
Two approaches were used to estimate body weight effect on cabozantinib exposures for adolescent patients aged 12 to 17.5 years: (1) using the model-based exponents on apparent clearance (CL/F) and apparent volume of distribution of the central compartment (Vc/F) parameters; and (2) replacing the model-based exponents with allometric exponents for clearance (CL) and volume of distribution of the central compartment (Vc) based on a daily dose of 40 or 60 mg (Supplementary Material). The population was simulated based on the Centers for Disease Control and Prevention (CDC) weight ranges for this age group [17]. A total of 1200 adolescents were simulated (50 for each age, in 6-month intervals, aged between 12 and 17.5 [i.e., a total of 12 groups based on age]) sampled from a normal distribution of body weight for each age and sex based on the CDC weight ranges for this age group.
Exposure–Response Analysis
Exposure–response analyses included clinical data for patients with DTC from the Phase 3 COSMIC-311 trial of cabozantinib up to the clinical data cut-off date. These analyses included cabozantinib-treated patients who received ≥ 1 dose of cabozantinib and contributed ≥ 1 measurable PK concentration to link clinical endpoints with individual predicted cabozantinib concentration. Patients missing PK information were also included and were assigned population level PK parameter estimates from the PK base model.
Derived datasets for time-to-event analyses were constructed for PFS and for the following safety endpoints: cabozantinib dose modification, palmar-plantar erythrodysesthesia (PPE, Grade ≥ 1), diarrhea (Grade ≥ 3), hypertension (Grade ≥ 3), fatigue/asthenia (Grade ≥ 3), nausea/vomiting (Grade ≥ 3), oral mucositis/stomatitis (Grade ≥ 3), and alanine aminotransferase (ALT)/aspartate aminotransferase (AST) elevation (Grade ≥ 3 per CTCAE v5). Hypertension was evaluated by two approaches: (1) blood pressure (BP) from the vital signs source data and (2) Medical Dictionary for Regulatory Activities (MedDRA) terms. Both approaches used the same cut-offs for determining events of Grade ≥ 3 severity (≥ 160 mmHg for systolic BP and ≥ 100 mmHg for diastolic BP). The safety endpoints were chosen based on common AEs associated with cabozantinib that were consistent across studies, including COSMIC-311. Empirical Bayes estimates from the PopPK model and the cabozantinib dosing history were used to generate the longitudinal individual predicted exposure metric. Exposure predictions were then merged into the derived analysis dataset.
Kaplan–Meier (KM) plots were constructed for each of the selected efficacy and safety endpoints, stratified by tertiles of average cabozantinib concentration calculated from “Time 0” until the time of event or censoring (CAVG0T), except the dose modification endpoint which was stratified by tertiles of average cabozantinib concentration over the first week of treatment (CAVG1W). The log-rank test was used to compare the cabozantinib exposure subgroups for each endpoint.
Kaplan–Meier analyses were performed using R (version 3.5.1 or higher). All data processing was performed using SAS (version 9.4) and/or R (version 3.5.1 or higher). The survdiff function in R package survival was used to perform the log-rank hypothesis test.
Results
Population Pharmacokinetic Model
The pooled PopPK analysis included 4746 quantifiable cabozantinib PK samples obtained from 1745 patients and healthy volunteers, including 205 PK samples from 101 patients in the COSMIC-311 trial who received cabozantinib and had measurable cabozantinib concentrations. Fourteen subjects were excluded from the PopPK analysis due to missing information. Demographic and covariate information for the 101 patients in COSMIC-311 receiving cabozantinib who had quantifiable PK samples are summarized in Table 1. Demographic and covariate information for all 1745 individuals (1682 patients with cancer and 63 healthy volunteers) included in the analysis are summarized in Supplementary Table S2.
Table 1.
Summary of covariate information for patients receiving cabozantinib in COSMIC-311 included in population pharmacokinetic model
| Covariate | COSMIC-311 (N = 101) |
|---|---|
| Sex | |
| Males | 45 |
| Females | 56 |
| Race | |
| Unknown | 6 |
| White | 74 |
| Black | 0 |
| Asian | 17 |
| American Indian/Native Alaskan | 2 |
| Other | 2 |
| Age (years) | |
| Median (range) | 66 (32–85) |
| Body weight (kg) | |
| Median (range) | 70.0 (41–117) |
| ALT (U/L) | |
| Median (range) | 16 (6–134) |
| AST (U/L) | |
| Median (range) | 20 (11–71) |
| Bilirubin (µmol/L) | |
| Median (range) | 7 (3–21) |
| CRCL (mL/min) | |
| Median (range) | 83.4 (26.6–182.1) |
ALT alanine aminotransferase, AST aspartate aminotransferase, CRCL, creatinine clearance
Base PopPK Model Development
Model development was initiated with PK data from a single-dose study in healthy volunteers receiving 20, 40, and 60 mg of cabozantinib (study XL184-020). The primary absorption process describing the initial absorption phase was best characterized by a model with 4 transit compartments. The secondary absorption process included a lag time, which described the increase in cabozantinib exposure at 24 h post-dose relative to the cabozantinib exposure observed at 14 h post-dose in XL184-020.
After including PK data from the other clinical trials, the structure of the residual error (RE) component of the PK model was best described by a RE term for healthy volunteers and a separate RE term for patients with various cancer types. This was considered the base model (see Supplementary Materials for base model equations).
Final Model Development
A full model was developed by incorporating potential effects of sex and body weight on CL/F and body weight on Vc/F (see Supplementary Material for model equations details). The full model was determined to be the final model upon achieving acceptable predictive performance by pcVPC evaluation. Final (full) model PK parameter estimates are reported in Table 2. Predicted mean exposure measures at steady state in adults following 60 mg cabozantinib QD based on the final PK model are summarized by study in Supplementary Table S3.
Table 2.
Pharmacokinetic parameter estimates for the final (full) PK model
| Parameter | Estimate | ASE | RSE | 95% CI | Units |
|---|---|---|---|---|---|
| CL/F | 2.05 | 0.0323 | 1.6 | (1.98–2.11) | L/h |
| Vc/F | 98.8 | 7.69 | 7.8 | (83.8–114) | L |
| Ka | 3.39 | 0.175 | 5.2 | (3.04–3.73) | h−1 |
| V3/F | 178 | 4.32 | 2.4 | (170–187) | L |
| Q/F | 15.5 | 0.976 | 6.3 | (13.6–17.4) | L/h |
| F1 | 0.735 | 0.017 | 2.3 | (0.702–0.769) | |
| ALAG4 | 19.1 | 0.0404 | 0.2 | (19.1–19.2) | h |
| Weight on CL/F | 0.144 | 0.0614 | 42.5 | (0.0241–0.265) | |
| Weight on Vc/F | 2.03 | 0.270 | 13.3 | (1.50–2.55) | |
| Female on CL/F | − 0.214 | 0.0268 | − 12.5 | (−0.266–0.161) | |
| Residual variability | |||||
| Healthy subjects | 26.6 | 0.598 | 2.3 | (25.4–27.7) | % |
| Patientsa | 36.3 | 0.615 | 1.7 | (35.1–37.5) | % |
| IIV | |||||
| CL/F | 43.1b | (41.0–45.0) | %CV | ||
| Vc/F | 100c | (88.7–111) | %CV | ||
| Ka | 39.3d | (31.0–46.1) | %CV | ||
| OFV | − 2290.11 | ||||
The final (full) model was developed based on data from 7 cabozantinib studies
ALAG absorption lag time, ASE asymptotic standard error, CI confidence interval, CL/F apparent clearance, CV coefficient of variation, F1 fraction of dose absorbed from first absorption depot, IIV interindividual variability, h hours, Ka absorption rate constant, OFV objective function value, Q/F apparent flow parameter between compartments, RSE relative standard error, V3/F apparent volume of distribution of the peripheral compartment, Vc/F apparent volume of the central compartment
aPooled subjects with various cancer types
bShrinkage is 19.6%
cShrinkage is 66.1%
dShrinkage is 82.0%
The effects of body weight and sex on the following PK parameters were assessed: steady state area under the concentration-time profile during one dosing interval (AUC0–24,ss), steady state maximum concentrations (Cmax,ss), pre-dose plasma concentrations at steady state (Cmin,ss), CL/F, and Vc/F. The reference patient was a 70-kg male with DTC receiving 60 mg cabozantinib QD. Body weight had minimal impact on cabozantinib exposure based on predicted AUC0–24,ss (< 6% change), Cmax,ss (< 14% change) and Cmin,ss (< 4% change) values, but had a notable impact on Vc/F (Supplementary Fig. S1). Lighter weight (53 kg, 5th percentile) patients had approximately 40% lower Vc/F versus a reference patient, while heavier weight (106 kg, 95th percentile) patients had an over 2-fold larger Vc/F. Females were estimated to have an approximately 20% lower CL/F than males, which corresponded to a 27% higher AUC0–24,ss, 23% higher Cmax,ss, and 29% higher Cmin,ss values. There was a lower (not clinically significant) CL/F and Vc/F in the Asian population compared to the White population. The CL/F and Vc/F range was overlapping in DTC patients and other cancer types.
Model-Based Simulations in Adolescents
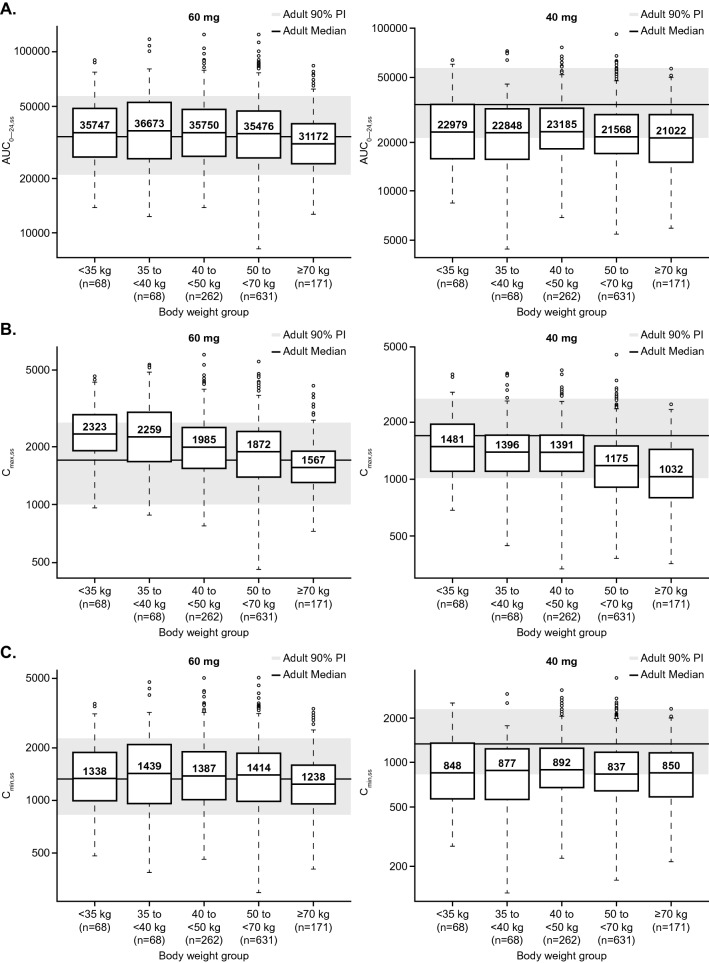
Figure 1 shows the predicted steady-state AUC0–24,ss, Cmax,ss, and Cmin,ss exposure for adolescent patients with DTC receiving 60 mg QD and 40 mg QD, stratified by weight group, based on stochastic simulations using the final PK model and the CDC weight ranges for this age group. Most of the interquartile ranges of predicted cabozantinib exposures based on these PK parameters for adolescent patients with DTC receiving 60 mg QD fell within the simulated adult exposure associated with a daily dose of 60 mg, except Cmax,ss for adolescent patients with body weight < 40 kg. The predicted Cmax,ss by weight with daily dose of 60 mg for these patients was on the high side of the range observed for adult patients receiving 60 mg QD. The predicted steady state AUC0–24,ss, Cmax,ss and Cmin,ss by weight for adolescent patients with DTC receiving 40 mg QD tended to be on the low side of the range relative to adult patients with DTC receiving 60 mg QD.
Fig. 1.
Predicted steady-state adolescent cabozantinib exposure (AUC0–24,ss [A], Cmax,ss [B], and Cmin,ss [C]) using the final (full) model (60 mg QD and 40 mg QD doses). The final (full) model was developed based on data from 7 cabozantinib studies. Lower and upper boundaries of the box represent the 1st quartile (Q1) and 3rd quartile (Q3), respectively; median is shown as a line inside the box and labelled as the value inside the box; whiskers represent minimum and maximum values that are within 1.5-times the inter-quartile range (IQR) below Q1 and above Q3, respectively; black circles represent outliers (values >1.5-times IQR below Q1 or above Q3); the gray shaded region represents the 90% prediction interval of adult reference (based on a 60 mg daily dose); solid line through the gray region is the predicted median adult reference. AUC0–24,ss steady state area under the concentration-time profile during one dosing interval (ng*h/mL), Cmax,ss steady state maximum plasma drug concentration (ng/mL), Cmin,ss pre-dose plasma drug concentrations at steady state (ng/mL), PI prediction interval
Allometric Scaling Simulations in Adolescents
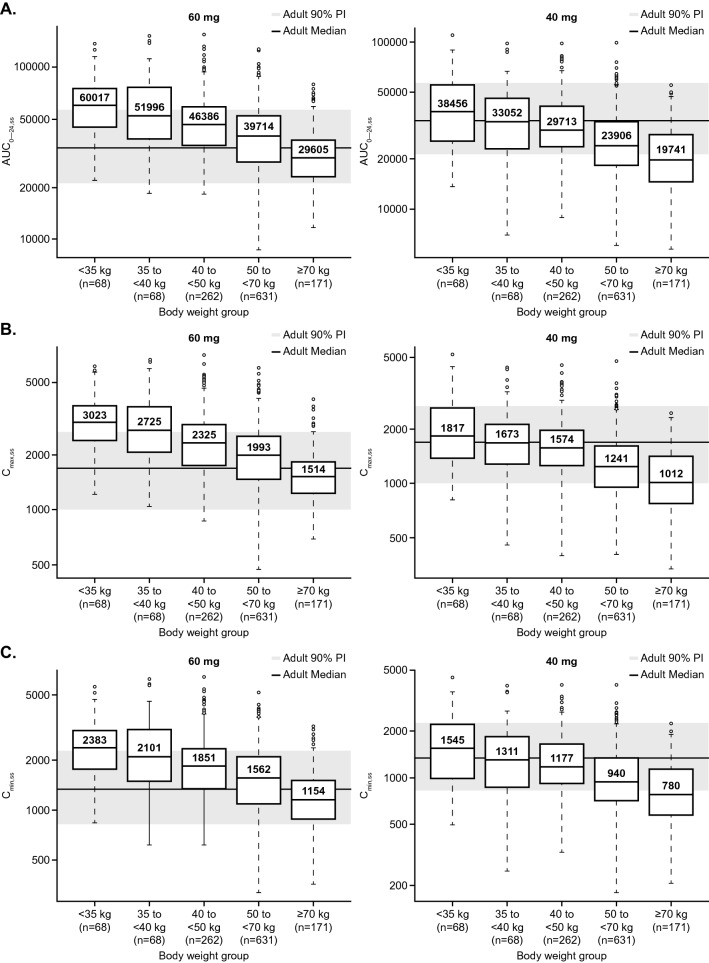
Results of the allometric scaling simulations are shown in Fig. 2. Box plots in this figure illustrate the predicted steady-state AUC0–24,ss, Cmax,ss, and Cmin,ss based on these parameter estimates and allometric exponents, stratified by weight group.
Fig. 2.
Predicted steady-state adolescent cabozantinib exposure (AUC0–24,ss [A], Cmax,ss [B], and Cmin,ss [C]) using the final (full) model and allometric scaling (60 mg QD and 40 mg QD doses). The final (full) model was developed based on data from 7 cabozantinib studies. Lower and upper boundaries of the box represent the 1st quartile (Q1) and 3rd quartile (Q3), respectively; median is shown as a line inside the box and labelled as the value inside the box; whiskers represent minimum and maximum values that are within 1.5-times the inter-quartile range (IQR) below Q1 and above Q3, respectively; black circles represent outliers (values >1.5-times IQR below Q1 or above Q3); the gray shaded region represents the 90% prediction interval of adult reference (based on a 60 mg daily dose); solid line through the gray region is the predicted median adult reference. AUC0–24,ss steady state area under the concentration-time profile during one dosing interval (ng*h/mL), Cmax,ss steady state maximum plasma drug concentration (ng/mL), Cmin,ss pre-dose plasma drug concentrations at steady state (ng/mL), PI prediction interval
Adolescents with DTC and body weight < 40 kg were predicted to have approximately 1.7-fold higher median AUC0–24,ss, Cmax,ss, and Cmin,ss for a daily 60-mg regimen relative to that of adults with DTC receiving the same dose. Exposure from 40 mg QD dosing in adolescents < 40 kg was similar (within 10% in median exposure) to that of adults receiving 60 mg QD.
For adolescents with DTC weighing ≥ 40 kg, the median exposure with daily doses of 60 mg QD was higher than in adults, but the interquartile range (IQR) was within the 90% prediction interval. As with the model-based simulation, predicted exposures with daily doses of 40 mg for adolescents weighing ≥40 kg were on the lower side of the range of exposures observed for adults receiving 60 mg QD.
Exposure Response Analyses
The number of patients with PFS or safety events and the total number of patients at risk in the COSMIC-311 trial are listed in Supplementary Table S4. In total, 125 patients had at least one documented cabozantinib dose, of whom 115 had at least one measurable concentration and were included in the exposure–response analysis (14 patients who were excluded from the PopPK analysis due to missing information were assigned typical PopPK parameters for the exposure–response analyses). The PK base model was used to generate the exposures for the 115 patients included in the exposure–response analyses.
Progression-Free Survival
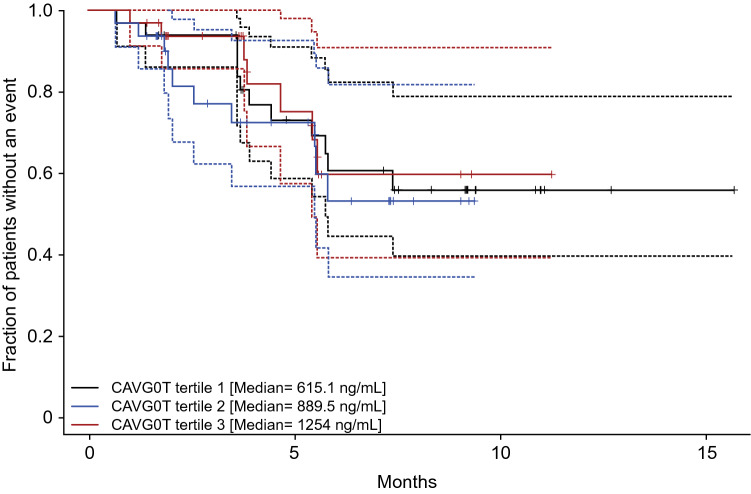
The KM plot for PFS by average cabozantinib exposure tertiles (Fig. 3) showed no clear relationship between the fraction of patients with a PFS event and the different exposure tertiles of cabozantinib. The log-rank test (p = 0.763) indicated that there was no statistically significant difference across the cabozantinib exposure tertiles (Table 3). A similar result was observed when the 14 patients missing PK information were excluded (p = 0.61).
Fig. 3.
Kaplan–Meier plot for progression-free survival by average exposure tertile. Data derived from 98 patients with at least one measurable cabozantinib concentration, a valid baseline tumor assessment, and at least one evaluable post-baseline tumor assessment in COSMIC-311. Dashed lines represent 95% confidence intervals for each exposure tertile. CAVG0T predicted average cabozantinib concentration from time zero to the event or censoring time, PFS progression-free survival
Table 3.
Log-rank tests for efficacy and safety endpoints with exposure tertiles based on data from COSMIC-311
| Tertile | N | Events observed | Events expected | Chi-square test statistic | p-value |
|---|---|---|---|---|---|
| PFS exposure tertiles | |||||
| 1 | 33 | 12 | 12.4 | 0.542 | 0.763 |
| 2 | 32 | 10 | 8.34 | ||
| 3 | 33 | 7 | 8.25 | ||
| Cabozantinib dose modification CAVG1W exposure tertiles | |||||
| 1 | 33 | 25 | 25.9 | 0.270 | 0.874 |
| 2 | 42 | 33 | 34.4 | ||
| 3 | 39 | 34 | 31.7 | ||
| PPE with exposure tertiles | |||||
| 1 | 38 | 16 | 16.9 | 0.125 | 0.939 |
| 2 | 38 | 17 | 17.3 | ||
| 3 | 39 | 22 | 20.8 | ||
| Diarrhea with exposure tertiles | |||||
| 1 | 38 | 0 | 3.12 | 5.986 | 0.0501 |
| 2 | 38 | 3 | 2.83 | ||
| 3 | 39 | 6 | 3.05 | ||
| Hypertension (BP data) with exposure tertiles | |||||
| 1 | 38 | 3 | 8.65 | 7.249 | 0.027 |
| 2 | 37 | 11 | 6.45 | ||
| 3 | 40 | 10 | 8.90 | ||
| Hypertension (MedDRA) with exposure tertiles | |||||
| 1 | 38 | 3 | 3.98 | 0.393 | 0.822 |
| 2 | 38 | 4 | 3.43 | ||
| 3 | 39 | 4 | 3.59 | ||
| Fatigue/asthenia with exposure tertiles | |||||
| 1 | 38 | 0 | 4.66 | 7.369 | 0.025 |
| 2 | 38 | 6 | 3.95 | ||
| 3 | 39 | 7 | 4.39 | ||
| Nausea/vomiting with exposure tertiles | |||||
| 1 | 38 | 2 | 1.67 | 0.324 | 0.850 |
| 2 | 38 | 1 | 1.59 | ||
| 3 | 39 | 2 | 1.74 | ||
| Oral mucositis/stomatitis with exposure tertiles | |||||
| 1 | 38 | 0 | 2.11 | 3.287 | 0.193 |
| 2 | 38 | 3 | 1.85 | ||
| 3 | 39 | 3 | 2.04 | ||
| ALT/AST elevation with exposure tertiles | |||||
| 1 | 38 | 0 | 0.365 | 2.50 | 0.287 |
| 2 | 38 | 1 | 0.286 | ||
| 3 | 39 | 0 | 0.349 | ||
PFS event = disease progression or death
ALT alanine aminotransferase, AST aspartate aminotransferase, BP blood pressure, CAVG1W average concentration for the first week of treatment; MedDRA medical dictionary for regulatory activities, N number of patients, PFS progression-free survival, PPE palmar-plantar erythrodysesthesia
Safety Endpoints
The KM plot for cabozantinib dose modification by average cabozantinib exposure based on the predicted average cabozantinib CAVG1W showed no clear relationship between cabozantinib dose modification and cabozantinib exposure (Supplementary Fig. S2). The log-rank test indicated no statistically significant difference across the cabozantinib exposure tertiles (Table 3).
For hypertension assessed by BP data (using the vital signs source data), the KM plot showed a smaller fraction of patients with hypertension of Grade ≥ 3 in the lowest cabozantinib exposure tertile compared to the highest exposure tertile (Supplementary Fig. S3), and the log-rank test (p = 0.027) indicated a statistically significant relationship between cabozantinib exposure and the incidence of hypertension Grade ≥ 3 (Table 3). For hypertension assessed by MedDRA, neither the KM plot nor the log-rank test (Supplementary Fig. S4; Table 3) indicated any clear relationship between hypertension of Grade ≥ 3 and cabozantinib exposure. It should be noted that there were more Grade ≥ 3 hypertension AEs based on the BP source data (24/115 patients) than there were using the MedDRA terms (11/115 patients). While both analyses used the same systolic and diastolic BP cut-offs to determine Grade ≥3 events, systolic and diastolic BP were counted separately in the BP vital signs data, resulting in more safety events.
The KM plot for fatigue/asthenia by average cabozantinib exposure tertiles showed no instances of Grade ≥ 3 in the lowest cabozantinib exposure tertile and similar rates among the higher two exposure tertiles (Supplementary Fig. S5). The log-rank test (p = 0.025) indicated a statistically significant relationship between cabozantinib exposure and the incidence of fatigue/asthenia Grade ≥ 3 (Table 3).
For PPE (Grade ≥ 1), diarrhea (Grade ≥ 3), nausea/vomiting (Grade ≥ 3), mucositis/stomatitis (Grade ≥ 3), and ALT/AST elevations (Grade ≥ 3), there was no statistically significant difference in rates of these AEs across the cabozantinib exposure tertiles based on the log-rank test (Table 3). The KM plot for PPE by average cabozantinib exposure tertiles indicated no significant relationship between cabozantinib exposure and the rate of PPE, although there was a trend of an increasing frequency at the higher cabozantinib exposures after the first month (Supplementary Fig. S6). The KM plot for diarrhea by average cabozantinib exposure tertiles indicated no patients with diarrhea of Grade ≥ 3 in the lowest exposure tertile and similar rates of diarrhea among patients in the two higher exposure tertiles (Supplementary Fig. S7). The KM plots for nausea/vomiting and mucositis/stomatitis (Supplementary Figs. S8 and S9) demonstrated no clear relationship between the rate of these AEs (Grade ≥ 3) and cabozantinib exposure. Likewise, the KM plot for ALT/AST elevation (Grade ≥ 3) did not indicate any relationship with cabozantinib exposure (Supplementary Fig. S10); only one instance of this AE occurred, which was in cabozantinib exposure tertile 2.
Discussion
A PopPK model was developed for the cabozantinib tablet based on data from 7 clinical trials, including COSMIC-311. Cabozantinib PK was described using a 2-compartment disposition model with dual first-order absorption and first-order elimination. The absorption process was described by two parallel processes: a slow absorption phase, which was described by 4 transit absorption compartments to capture the observed peak concentration around 3 h, and a delayed absorption process characterized by a lag time to describe the increase in cabozantinib exposure observed approximately 24 h after the first dose in the single dose study, XL184-020. The model appropriately described the observed peak concentration around 3 h and the second absorption phase around 24 h.
There was no marked difference between cabozantinib PK of patients with DTC and that of patients with other cancer types. Previous studies have presented the PopPK of cabozantinib in healthy subjects and patients with cancer, which included the tablet formulation of cabozantinib in various cancer types as well as the capsule formulation in patients with medullary thyroid cancer (MTC) [18, 19]. In these analyses, MTC was identified as a factor impacting cabozantinib PK. In the current analysis, data from the capsule formulation in patients with MTC were not included. Body weight had minimal impact on cabozantinib exposure in adult patients. Although females were predicted to have approximately 23–29% higher exposures than males, the difference was small relative to interpatient PK variability (43.1% and 100% coefficient of variation for CL/F and Vc/F, respectively [Table 2]), the exposure difference by sex for efficacy and safety endpoints was < 15%, and most safety endpoints were comparable between male and female patients (data not shown). The Asian population had lower CL/F and Vc/F compared with other races based on a post hoc analysis, which could be attributed to lower body weight in the Asian population and the small percentage (12%) of Asian subjects included in the analysis. Overall, the range of cabozantinib PK in the Asian population was overlapping with the White population. The results indicated that no intrinsic or extrinsic factors evaluated in the PopPK model had a clinically meaningful effect on cabozantinib PK. Hence, the PopPK analysis indicates there is no need for dose adjustment for cabozantinib based on body weight, sex, race, age, ALT, AST, total bilirubin, or creatinine clearance for adult patients with DTC, consistent with prior analyses [18, 19].
Although the eligibility criteria for COSMIC-311 allowed patients aged ≥ 16 years, only adults were enrolled. The PopPK model based on adult data was used to extrapolate adolescent cabozantinib exposure and provided a rationale for dose selection for the adolescent population with a body weight of < 40 kg (approximately 10% of adolescent patients estimated from the CDC growth chart [17]).
Adolescent exposure simulations based on model-estimated weight effect on cabozantinib PK predicted that adolescents with low body weight (< 40 kg) receiving cabozantinib 60 mg QD would have similar AUC0–24,ss and Cmin,ss, but higher Cmax,ss compared to adults receiving the same dose. With a conservative allometric scaling approach for extrapolating adolescent exposure, the predicted exposure (AUC0–24,ss, Cmin and Cmax) for adolescents with a bodyweight < 40 kg dosed with cabozantinib 60 mg QD was higher than adult patients receiving the same dose. When the adolescent dose was reduced to 40 mg QD, the predicted exposure for adolescents with a bodyweight < 40 kg was similar to that of adults receiving cabozantinib 60 mg QD. Although there was no relationship between PFS and cabozantinib concentration in adults with DTC, there was a relationship between safety and cabozantinib concentration; thus, the recommended dose for an adolescent with bodyweight < 40 kg is cabozantinib 40 mg QD. The adult dose of cabozantinib 60 mg QD is appropriate for adolescents with bodyweight ≥40 kg, as the predicted exposure for this body weight group is similar to that for an adult population. With respect to BSA-based dosing for adolescents in the cabozantinib label, an adolescent with a weight of 40 kg and a height of 120 cm translates to a BSA of 1.2 m2 based on the West Nomogram for BSA estimation. A previous Phase 1 dose-escalation study of cabozantinib in pediatric patients with solid tumors (age range 4–18 years) estimated a recommended dose of 40 mg/m2/day, consistent with a 60 mg/day dose in adults [20].
The current exposure–response analyses used a log-ranked test and found no relationship between PFS and average cabozantinib concentration for patients with DTC in the COSMIC-311 study, supporting a dose of 60 mg QD with the option to reduce the dose to manage AEs and improve tolerability. Earlier analyses have demonstrated a relationship between average cabozantinib concentration and PFS in METEOR (RCC) and CELESTIAL (HCC) populations using Cox proportional hazard methodology [21, 22]. However, a direct comparison between these studies is difficult given the differences in indication and sample size (METEOR [n = 329], CELESTIAL [n = 467], and COSMIC-311 [n = 115]). An additional exposure–response analysis in patients with metastatic RCC treated in routine care (n = 59) showed no clear relationship between cabozantinib exposure and PFS [23]. However, an improvement in PFS was observed in patients who had a dose reduction. Patients with a dose reduction were shown to have higher initial exposure, suggesting that high initial exposure may be beneficial while AEs could be managed by dose reduction.
The EXAMINER study, a double-blind noninferiority study, recently evaluated the efficacy of the cabozantinib tablet at 60 mg QD compared with the approved cabozantinib capsule at 140 mg QD in patients with progressive metastatic MTC [24]. The 60 mg cabozantinib tablet did not demonstrate noninferiority versus the 140 mg capsule with regard to PFS (median, 11.0 vs 13.9 months; HR 1.24 [95% CI 0.90–1.70]; p = 0.19). Mean cabozantinib exposure was higher in the 140 mg QD capsule arm compared with the 60 mg QD tablet arm in the first few weeks of treatment, but exposure became similar by 9 weeks of treatment, suggesting that high initial cabozantinib exposure improves efficacy.
In this analysis, no significant relationship was observed between dose modification and average cabozantinib concentration over the first week of treatment. Increasing cabozantinib exposure was associated with increasing rates of PPE (Grade ≥1), diarrhea (Grade ≥3), fatigue/asthenia (Grade ≥3), and hypertension (Grade ≥3), with cabozantinib exposure a significant predictor of the latter two. In contrast to this study, other exposure–response analyses in patients with advanced HCC or RCC demonstrated that cabozantinib exposure is a statistically significant predictor for both PPE and diarrhea [21, 22]. The difference in safety observations between trials may be due to a change in AE definitions and a better understanding of cabozantinib AE management. No new safety signals were identified, relative to previous experience with other cancer types.
Collectively, initiating a cabozantinib dose of 60 mg QD may optimize clinical benefit given the high interpatient variability in cabozantinib exposure and rapid disease progression of DTC. However, it should be noted that some patients who receive an initial cabozantinib dose of 60 mg QD may need a dose reduction and/or dose hold to manage AEs during treatment.
In conclusion, these results support the cabozantinib dosing strategy implemented in COSMIC-311 for adult patients with radioiodine-refractory DTC previously treated with VEGFR-targeted therapy and support the pediatric dosing recommendation provided in the label [5]. Cabozantinib should be initiated at 60 mg QD for adults and for pediatric patients aged ≥ 12 years with BSA of ≥ 1.2 m2 and at 40 mg QD for pediatric patients with BSA < 1.2 m2, and the dose should be reduced to manage AEs as indicated.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgments
The study was funded by Exelixis, Inc., Alameda, CA, USA. We thank the patients, their families, the investigators, and site staff. We thank Roman Levytskyy, PhD, MBA (Exelixis, Inc., Alameda, CA, USA), for critical review of the manuscript. Editorial and writing assistance was provided by Angela Cimmino, PharmD, and Alexus Rivas, PharmD (Fishawack Communications Inc., a part of Fishawack Health, Conshohocken, PA, USA), and was funded by Exelixis, Inc.
Author Contributions
NSL, JL, and RF drafted the manuscript. All authors contributed to the study concept and design. All authors contributed to data collection. NSL and JL performed material preparation and data analyses. All authors commented on drafts of the manuscript. All authors read and approved the final manuscript.
Declarations
Ethics Approval
For the cabozantinib clinical studies included in these analyses, the study protocols were approved by the institutional review board or ethics committee at each center.
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Consent to Publish
Not applicable.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available due to confidentiality and lack of patient consent for data sharing but may be available from the corresponding author on reasonable request.
Code Availability
Not applicable.
Funding
This study was funded by Exelixis, Inc., Alameda, CA, USA (no grant number).
Conflicts of Interest
Neang S. Ly, Jing Li, Raffaella Faggioni, and Lorin K. Roskos are employees and stockholders of Exelixis. Marcia S. Brose received honoraria from Bayer, Eisai, and Lilly, research funding from Bayer (Inst), Blueprint Medicines (Inst), Eisai (Inst), Exelixis (Inst), Lilly (Inst), and Loxo (Inst), and had a consulting or advisory role with Bayer, Blueprint Medicines, Eisai, Exelixis, Lilly, and Loxo.
References
- 1.Lirov R, Worden FP, Cohen MS. The treatment of advanced thyroid cancer in the age of novel targeted therapies. Drugs. 2017;77(7):733–745. doi: 10.1007/s40265-017-0733-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brose MS, Nutting CM, Jarzab B, Elisei R, Siena S, Bastholt L, et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, Phase 3 trial. Lancet. 2014;384(9940):319–328. doi: 10.1016/S0140-6736(14)60421-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schlumberger M, Tahara M, Wirth LJ, Robinson B, Brose MS, Elisei R, et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med. 2015;372(7):621–630. doi: 10.1056/NEJMoa1406470. [DOI] [PubMed] [Google Scholar]
- 4.Yakes FM, Chen J, Tan J, Yamaguchi K, Shi Y, Yu P, et al. Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol Cancer Ther. 2011;10(12):2298–2308. doi: 10.1158/1535-7163.MCT-11-0264. [DOI] [PubMed] [Google Scholar]
- 5.Exelixis, Inc. Cabometyx (cabozantinib) [package insert]. U.S. Food and Drug Administration. 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/208692s012lbl.pdf. Accessed 13 Jan 2022.
- 6.Exelixis, Inc. Cometriq (cabozantinib) [package insert]. U.S. Food and Drug Administration. 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/203756s009lbl.pdf. Accessed 14 Mar 2021.
- 7.U.S. Food and Drug Administration. FDA approves cabozantinib for differentiated thyroid cancer. 2021. https://www.fda.gov/drugs/resources-information-approved-drugs/Fda-approves-cabozantinib-differentiated-thyroid-cancer. Accessed 28 Sep 2021.
- 8.Brose MS, Robinson B, Sherman SI, Krajewska J, Lin CC, Vaisman F, et al. Cabozantinib for radioiodine-refractory differentiated thyroid cancer (COSMIC-311): a randomised, double-blind, placebo-controlled, Phase 3 trial. Lancet Oncol. 2021;22(8):1126–1138. doi: 10.1016/S1470-2045(21)00332-6. [DOI] [PubMed] [Google Scholar]
- 9.Capdevila J, Robinson B, Sherman S, Jarzab B, Lin C, Vaisman F. Cabozantinib versus placebo in patients with radioiodine-refractory differentiated thyroid cancer who have progressed after prior VEGFR-targeted therapy: Updated results from the phase III COSMIC-311 trial and prespecified subgroup analyses by prior therapy. Ann Oncol. 2021;32(suppl_5):Late breaking abstract 67. doi: 10.1016/annonc/annonc741. [DOI] [Google Scholar]
- 10.Basch EM, Scholz M, de Bono JS, Vogelzang N, de Souza P, Marx G, et al. Cabozantinib versus mitoxantrone-prednisone in symptomatic metastatic castration-resistant prostate cancer: a randomised Phase 3 trial with a primary pain endpoint. Eur Urol. 2019;75(6):929–937. doi: 10.1016/j.eururo.2018.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith M, De Bono J, Sternberg C, Le Moulec S, Oudard S, De Giorgi U, et al. Phase III study of cabozantinib in previously treated metastatic castration-resistant prostate cancer: COMET-1. J Clin Oncol. 2016;34(25):3005–3013. doi: 10.1200/JCO.2015.65.5597. [DOI] [PubMed] [Google Scholar]
- 12.Choueiri TK, Escudier B, Powles T, Tannir NM, Mainwaring PN, Rini BI, et al. Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): final results from a randomised, open-label, Phase 3 trial. Lancet Oncol. 2016;17(7):917–927. doi: 10.1016/S1470-2045(16)30107-3. [DOI] [PubMed] [Google Scholar]
- 13.Abou-Alfa GK, Meyer T, Cheng AL, El-Khoueiry AB, Rimassa L, Ryoo BY, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018;379(1):54–63. doi: 10.1056/NEJMoa1717002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choueiri TK, Powles T, Burotto M, Escudier B, Bourlon MT, Zurawski B, et al. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2021;384(9):829–841. doi: 10.1056/NEJMoa2026982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nguyen L, Benrimoh N, Xie Y, Offman E, Lacy S. Pharmacokinetics of cabozantinib tablet and capsule formulations in healthy adults. Anticancer Drugs. 2016;27(7):669–678. doi: 10.1097/CAD.0000000000000366. [DOI] [PubMed] [Google Scholar]
- 16.Leong R, Liu Q, Li L, Liu J, Ren YR, Lee P, et al. Recommendations for dose selection for adolescent patients in relevant adult oncology clinical trials. Clin Pharmacol Ther. 2021 doi: 10.1002/cpt.2467. [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention, National Center for Health Statistics. CDC growth charts: United States. 2000. https://www.cdc.gov/growthcharts/percentile_data_files.htm. Accessed 12 Jan 2022.
- 18.Lacy S, Yang B, Nielsen J, Miles D, Nguyen L, Hutmacher M. A population pharmacokinetic model of cabozantinib in healthy volunteers and patients with various cancer types. Cancer Chemother Pharmacol. 2018;81(6):1071–1082. doi: 10.1007/s00280-018-3581-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nguyen L, Chapel S, Tran BD, Lacy S. Updated population pharmacokinetic model of cabozantinib integrating various cancer types including hepatocellular carcinoma. J Clin Pharmacol. 2019;59(11):1551–1561. doi: 10.1002/jcph.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chuk MK, Widemann BC, Minard CG, Liu X, Kim A, Bernhardt MB, et al. A Phase 1 study of cabozantinib in children and adolescents with recurrent or refractory solid tumors, including CNS tumors: Trial ADVL1211, a report from the Children's Oncology Group. Pediatr Blood Cancer. 2018;65(8):e27077. doi: 10.1002/pbc.27077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lacy S, Nielsen J, Yang B, Miles D, Nguyen L, Hutmacher M. Population exposure-response analysis of cabozantinib efficacy and safety endpoints in patients with renal cell carcinoma. Cancer Chemother Pharmacol. 2018;81(6):1061–1070. doi: 10.1007/s00280-018-3579-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen L, Chapel S, Tran BD, Lacy S. Cabozantinib exposure-response analyses of efficacy and safety in patients with advanced hepatocellular carcinoma. J Pharmacokinet Pharmacodyn. 2019;46(6):577–589. doi: 10.1007/s10928-019-09659-y. [DOI] [PubMed] [Google Scholar]
- 23.Krens SD, van Erp NP, Groenland SL, Moes D, Mulder SF, Desar IME, et al. Exposure-response analyses of cabozantinib in patients with metastatic renal cell cancer. BMC Cancer. 2022;22(1):228. doi: 10.1186/s12885-022-09338-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Capdevila J, Klochikhin A, Leboulleux S, Isaev P, Badiu C, Robinson B, et al. A randomized, double-blind noninferiority study to evaluate the efficacy of the cabozantinib tablet at 60 mg per day compared with the cabozantinib capsule at 140 mg per day in patients with progressive, metastatic medullary thyroid cancer. Thyroid. 2022;32(5):515–524. doi: 10.1089/thy.2022.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available due to confidentiality and lack of patient consent for data sharing but may be available from the corresponding author on reasonable request.