Abstract
Vascular complications of diabetes pose a severe threat to human health. Prevention and treatment protocols based on a single vascular complication are no longer suitable for the long-term management of patients with diabetes. Diabetic panvascular disease (DPD) is a clinical syndrome in which vessels of various sizes, including macrovessels and microvessels in the cardiac, cerebral, renal, ophthalmic, and peripheral systems of patients with diabetes, develop atherosclerosis as a common pathology. Pathological manifestations of DPDs usually manifest macrovascular atherosclerosis, as well as microvascular endothelial function impairment, basement membrane thickening, and microthrombosis. Cardiac, cerebral, and peripheral microangiopathy coexist with microangiopathy, while renal and retinal are predominantly microangiopathic. The following associations exist between DPDs: numerous similar molecular mechanisms, and risk-predictive relationships between diseases. Aggressive glycemic control combined with early comprehensive vascular intervention is the key to prevention and treatment. In addition to the widely recommended metformin, glucagon-like peptide-1 agonist, and sodium-glucose cotransporter-2 inhibitors, for the latest molecular mechanisms, aldose reductase inhibitors, peroxisome proliferator-activated receptor-γ agonizts, glucokinases agonizts, mitochondrial energy modulators, etc. are under active development. DPDs are proposed for patients to obtain more systematic clinical care requires a comprehensive diabetes care center focusing on panvascular diseases. This would leverage the advantages of a cross-disciplinary approach to achieve better integration of the pathogenesis and therapeutic evidence. Such a strategy would confer more clinical benefits to patients and promote the comprehensive development of DPD as a discipline.
Subject terms: Endocrine system and metabolic diseases, Metabolic disorders, Cardiology, Molecular medicine
Introduction
Diabetes mellitus (DM) and its complications pose a serious threat to human health and have become a global public health issue.1,2 Over 90% of patients with diabetes have type 2 DM (T2DM).3,4 Diabetic complications can be classified according to the involvement of cardiopathy and encephalopathy, nephropathy, retinopathy, and peripheral vasculopathy.5–7 DM increases the risk of all these complications, and multiple vasculopathy is associated with a poorer prognosis.8 Recent intensive investigations into diabetic complications have significantly promoted the understanding of the pathogenesis of this disease. However, the increasing division of medical science into various subspecialties, has resulted in a tendency to focus on localized lesions instead of integrating overall evidence. Thus, a holistic investigation of diabetic complications involving multiple systems and different angiopathies is needed.
The pathology of diabetic complications has a high degree of commonality at the vascular level; that is, complications manifest mostly as endothelial dysfunction and atherosclerosis (AS).9 DM being a risk factor for vascular disease, the several vascular comorbidities seriously affect the prognosis and treatment of patients, leading to the concept of “panvascular disease”.10–12 Since the late 20th century, the concept of a “vessel tree”13 has been proposed and “polyvascular atherosclerotic disease”14 has been defined considering coronary and non-coronary AS, mainly peripheral arterial and cerebrovascular diseases (peripheral vascular disease and cerebrovascular disease respectively). This definition indicates that comprehensive management of the multivessel disease is clinically essential for improving outcomes and prognoses. However, this definition does not consider either microvascular disease (especially in vital organs) or multidisciplinary fusion. To improve this definition, we propose the concept of diabetic panvascular disease (DPD). This is a clinical syndrome in which AS is a common pathology between macrovessels and microvessels in the cardiac, cerebral, renal, ophthalmic, and peripheral systems in patients with diabetes. The main outcomes would be cardiovascular and cerebrovascular events, and the prognosis could be improved through aggressive intervention against metabolic abnormalities.
Diabetic complications are usually classified in two dimensions: macro/microvascular disease, or complications classified by target organs. DPDs synthesize these concepts. This article systematically reviews general pathological manifestations of vascular lesions and differences in the etiology of macro/microvascular lesions; pathological manifestations and molecular mechanisms of different target organs in DPDs; common molecular mechanisms and therapeutic targets in DPDs; time course characteristics of pathological changes in organs and mutual predictive effects among DPDs to provide clues for early diagnosis. Our findings should promote the establishment of a multidisciplinary DPD management system.
Diabetes and panvasculopathy
The vasculature comprises endothelial cells (EC), smooth muscle cells (SMC), pericytes, fibroblasts, and various other types of cells. AS, endothelial barrier damage, loss of pericytes, capillary thinning, and angiogenic disorders are common pathologies of systemic vascular disease. Blood vessels, together with nerves and lymphatic vessels, are wrapped in connective tissue membranes to form vascular nerve bundles. Differences in perivascular tissues, vascular nerve bundles, and intravascular structures result in altered vascular function. When imbalanced homeostasis is characterized by abnormal glucose and lipid metabolism, activation of the renin-angiotensin-aldosterone system (RAAS) and sympathetic nervous system (SNS) directly or indirectly causes widespread vascular damage throughout the body, leading to the development of panvascular complications of DM15 (Fig. 1). SNS dominates vasoconstriction and RAAS regulates of blood volume, vascular tone, and blood pressure.16 The vascular system in target organs is tightly regulated by surrounding tissues that regulate microvascular units through physical and signal transduction. As DM progresses, patients are more likely to develop various vascular complications and experience many pathological changes, such as endothelial dysfunction, AS, and microcirculatory disorders that interact with each other, consequently leading to the development of DPD.
Fig. 1.
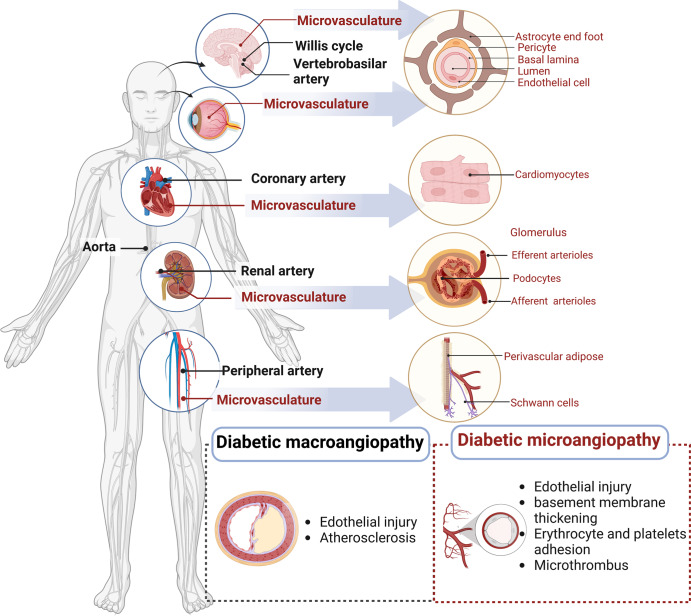
Schematic overview of panvasculopathy in diabetes mellitus. Diabetic panvasculopathy involves the cardiac, cerebral, renal, ophthalmic and peripheral systems. The macrovascular lesions are in black text. The microvascular lesions are in red. The microvascular system varies in different organs, which affects vascular function
Diabetic vasculopathy is classified as macroangiopathy and microangiopathy. Macroangiopathy includes AS of large and medium arteries (aorta, coronary, renal, basilar, and peripheral arteries), whereas microangiopathy includes endothelial damage to vessels between primary arterioles and venules, vascular basement membrane thickening, microthrombosis, platelet and red blood cell adhesion aggregation, and microcirculatory disorders. Due to differences in hemodynamics, vascular structure, and the affected cells, macro/microangiopathy present with different pathological manifestations. AS often occurs in sites of hemodynamic disturbances (preferably in elastic arteries). It manifests as macrophage foaming and EC lesions as well as mesangial SMC lesions. Microvessels are more hemodynamically stable, with a few cell layers, accompanying abundant plexus. On the other hand, differences in energy metabolic status, and organ-specific growth factors or cytokines in different target organs are also important components of the differences. Most target organs (heart, brain, peripheral vasculature) are affected by both diabetic macrovasculopathy and microvasculopathy; the retina and kidney are mainly affected by microvasculopathy (Fig. 1).
DPDs
Diabetic heart disease (DHD)
DHD includes all kinds of cardiovascular diseases secondary to DM, including coronary artery disease (CAD) and cardiomyopathy. Cardiomyopathy and CAD are often treated separately as microvascular and macrovascular diseases, DM can increase the risk of both. They are often combined in clinical settings and ultimately lead to adverse outcomes and risk of death in patients with cardiovascular disease.17
Myocardial microvascular functional changes precede structural changes in patients with diabetes. These changes finally lead to extensive macrovascular AS.18 DM complicated with coronary atherosclerotic heart disease manifests as the segmental distribution of numerous vascular branches throughout the entire process, with obvious AS. Diabetic cardiomyopathy (DCM) refers to specific myocardial structural and functional abnormalities that occur in patients without CAD and cardiac risk factors such as hypertension, with heart failure (HF) as the main manifestation of chronic cardiovascular complications of DM. The main pathological features are left ventricular hypertrophy, myocardial fibrosis, cell necrosis, and other myocardial structural changes.19,20 Myocardial involvement in vasoactive metabolite secretion or neuromodulation causes changes in the coronary artery wall pressure or endothelial shear stress. Pericoronary adipose tissue can secrete adipokines and other vasoactive mediators and/or oxidative products that can directly alter the phenotypes of perivascular adipocytes.
Investigations of biomarkers of DHD and their applications have significantly progressed. Cardiovascular events as outcomes are more beneficial for the clinical application of biomarkers from the aspect of panvascular diseases. The severity of cardiovascular disease in patients with diabetes positively correlates with the ratio of oxidized low-density lipoprotein to low-density lipoprotein-cholesterol (Ox-LDL/LDL-C). This is considered a potential biomarker for the early identification and intervention of CAD in patients with diabetes.21 Osteopontin (OPN) is a multifunctional phosphorylated glycoprotein, that functions as an inflammatory cytokine and pro-atherosclerotic factor. High levels of OPN expression in the circulation and tissues are associated with cardiovascular complications in DM, and OPN is an independent predictor of cardiovascular disease in DM.22 Serum homocysteine levels are elevated in patients with T2DM and CAD and are closely related to the severity of coronary artery lesions.23 Plasma-free fatty acids also comprise an independent risk factor for CAD in patients with DM.24 Novel biomarkers are useful for providing insights into associations between DM and cardiovascular risk and developing treatment strategies for CAD associated with DM.
Coronary AS exists in diabetic patients, and the signal transduction of AS is similar in DPDs. Chronic inflammation, abnormal lipid metabolism, and secondary autoimmunity are the main mechanisms.25 ApoB-specific CD4 T cells have been identified in humans and mice, and treg can be induced with ApoB peptides.26 Hsp60/65 is the target antigen of autoimmune T Cells.27 Hyperglycemic states can further promote autoimmune responses. In addition to glycemic control and statins, monoclonal antibodies to proprotein convertase subtilisin/kexin type 9 (PCSK9), heat shock proteins60/65 (HSP60/65), and ApoB are expected to improve AS by targeting.28
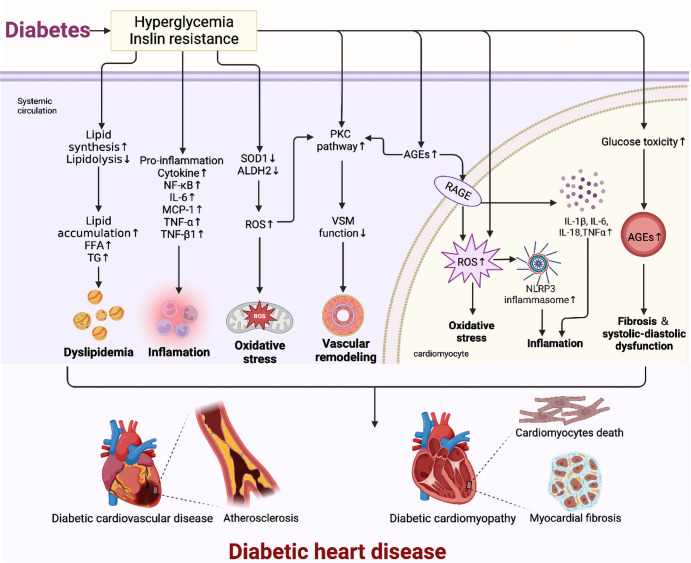
The mechanisms by which diabetes promotes cardiomyopathy have received attention, especially due to abnormal cardiac metabolism (Cardiac Metabolism), glycotoxicity and lipotoxicity, and abnormal mitochondrial function causing oxidative stress and inflammation.29 Unlike other target organs, the myocardium has high energy and oxygen requirements, and fatty acid oxidation (FAO) and aerobic oxidation of glucose are the main sources of energy for cardiac metabolism. Insulin resistance increases lipid synthesis in hepatocytes and lipolysis in adipocytes, leading to elevated circulating fatty acids and triglyceride levels. Lipid accumulation and fatty acids-induced lipotoxicity can affect myocardial FAO processes, promote endoplasmic reticulum (ER) stress, autophagy, and apoptosis, and cause ventricular remodeling.20,30 The most important metabolites of diabetic glycotoxin are advanced glycation end-products (AGEs) that are involved in the formation and evolution of DCM. These end-products bind cellular receptors of AGEs (RAGEs) that promote the production of reactive oxygen species (ROS), nuclear factor kappa-B (NF-κB), and pro-inflammatory cytokines such as interleukin (IL)-1β, IL-6, IL-18, tumor necrosis factor-alpha (TNF-α) that induce the intracellular production of abundant ROS, and initiate oxidative stress/inflammation cascade.31,32 AGEs/RAGE causing structural changes in the myocardium. Advanced glycation end-products also activate inflammatory signals through RAGEs on EC macrophages, and smooth muscle cells. This activation leads to increased ROS production and reduced nitric oxide synthesis, thus promoting the development of DCM.33,34 Hyperglycemia mediates a decrease in the expression of jund proto-oncogene subunit (JunD) and of free radical scavenger superoxide dismutase 1 and aldehyde dehydrogenase 2. It also mediates increased expression of inflammatory mediators such as NF-κB and Mcp-1, IL-6, and TNF-α that cause myocardial dysfunction and lead to the development of HF.35 Hyperglycemia promotes the increased expression of lncDACH1, which in turn promotes mitochondrial oxidative stress and apoptosis through increased ubiquitination-mediated degradation of NAD-dependent deacetylase sirtuin-3 (SIRT3), mitochondrial in mouse hearts, consequently aggravating DCM.36 Protein kinase C (PKC) is an effector in the G protein-coupled receptor system, and vascular SMC maintains vascular tone. PKC can be activated by excess ROS, AGEs, and diacylglycerol (DAG) to impair VSM function; this leads to vascular hyperresponsiveness and remodeling and accelerated development of DHD.37,38 Hyperglycemia triggers classical inflammatory pathways and oxidative stress.39,40 Hyperglycemia causes upregulates membrane cofactor protein-1 (MCP-1) and NLR family pyrin domain containing 3 inflammasome (NLPR3) expression, causing myocardial fibrosis and cardiac dysfunction, and exacerbated DCM development.41 Several molecular mechanisms synergistically act to impair the structural function of the heart and promote the development of DHD (Fig. 2).
Fig. 2.
Pathology and molecular mechanisms of DHD. The mechanisms of diabetic heart disease are complex, including oxidative stress, inflammation, and altered metabolic pathways (advanced glycosylation end product (AGE) formation, PKC pathway), which intersect and work together to ultimately lead to myocardial remodeling and dysfunction
Various interventions have conferred clinical benefits on patients with DHD (Table 1). Treatment of DHD should be comprehensive, with aggressive control of risk factors such as blood glucose, blood pressure, and lipids. Basic pharmacological therapy for CAD in patients with diabetes includes antiplatelet, cholesterol-lowering, anti-myocardial ischemia strategies, and RAAS inhibitors. Aggressive blood sugar control is necessary for treating DHD to avoid direct hyperglycemic damage. When treating T2DM complicated with cardiovascular disease, metformin should be combined with glucose-lowering drugs that have a proven cardiovascular benefit. When treating T2DM complicated with atherosclerotic cardiovascular disease, the preferred combination of metformin with either glucagon-like peptide-1 receptor agonist (GLP-1RA) or sodium/glucose cotransporter-2 inhibitors (SGLT-2i) can reduce cardiovascular events (Table 1). When treating T2DM complicated with HF, metformin should be combined with SGLT-2i, as this leads to a 39% reduction in the risk of HF hospitalization and a 46% reduction in the composite endpoint of HF hospitalization with all-cause death.42 Although GLP-1RA and dipeptidyl peptidase-4 inhibitors (DPP-4i) also confer some cardiovascular benefits, they do not offer a significant advantage for reducing the hospitalization of HF in patients with diabetes (Table 1).43,44,45
Table 1.
Cardiovascular outcomes trials (including stroke) in diabetes mellitus (2013–2022)
| Clinical trial | Clinical trials’ number | Year | Phase | Participants (n) | Intervention | Follow-up | Main outcome |
|---|---|---|---|---|---|---|---|
| Dipeptidyl peptidase-4 (DPP-4) inhibitors | |||||||
| CARMELINA475 | NCT01897532 | 2013–2016 | 3 | 6991 |
I: Iinagliptin C: placebo |
2.2 years | Cardiovascular death, nonfatal myocardial infarction, nonfatal stroke |
| NA476 | NCT01703208 | 2012–2016 | 3 | 4202 |
I: Omarigliptin C: placebo |
1.84 years | Major adverse cardiovascular event, hospitalization for heart failure |
| SAVOR-TIMI 5345 | NCT01107886 | 2010-2011 | 3 | 16492 |
I: Saxagliptin C: placebo |
2.1 years | Composite of cardiovascular death, myocardial infarction, or ischemic stroke |
| TECOS477 | NCT00790205 | 2008–2012 | 3 | 14671 |
I: Sitagliptin C: placebo |
3 years | Composite of cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, or hospitalization for unstable angina |
| Glucagon-like peptide-1 receptor (GLP-1) agonists | |||||||
| REWIND478 | NCT01394952 | 2011 | 3 | 9901 |
I: Dulaglutide C: placebo |
5.4 years | Nonfatal myocardial infarction, nonfatal stroke, and death from cardiovascular |
| SUSTAIN-644 | NCT01720446 | 2013 | 3 | 3297 |
I: Semaglutide C: placebo |
2.1 years | Cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke |
| PIONEER 6479 | NCT02692716 | 2017 | 3 | 3183 |
I: oral Semaglutide C: placebo |
1.25years | Death from cardiovascular causes, nonfatal myocardial infarction, nonfatal stroke |
| EXSCEL480 | NCT01144338 | 2010-2015 | 3 | 14752 |
I: Exenatide C: placebo |
3.2 years | Death from cardiovascular causes, nonfatal myocardial infarction, nonfatal stroke |
| LEADER481 | NCT01179048 | 2010–2012 | 3 | 9340 |
I: Liraglutide C: placebo |
3.8 years | Death from cardiovascular causes, nonfatal myocardial infarction, nonfatal stroke |
| ELIXA482 | NCT01147250 | 2010–2013 | 3 | 6991 |
I: Lixisenatide C: placebo |
2.2 years | Death from cardiovascular causes, nonfatal myocardial infarction, nonfatal stroke, hospitalization for unstable angina |
| Sodium-glucose cotransporter-2 (SGLT2) inhibitors | |||||||
| EMPA-REG OUTCOME483 | NCT01131676 | 2015 | 3 | 7020 |
I: Empagliflozin C: placebo |
3.1 years | MACE, cardiovascular, all-cause death, hospitalization for heart failure |
| CANVAS program484,485 | NCT01032629 NCT01989754 | 2017 | 3 | 10142 |
I: Canagliflozin C: placebo |
3.61 years | Cardiovascular death or hospitalized Heart failure |
| DAPA-HF486 | NCT03619213 | 2018–2022 | 3 | 3131 |
I: Dapagliflozin C: placebo |
2.3 years | Composite of worsening heart failure, cardiovascular death |
| Empa-HF100 | NCT03485092 | 2018 | 3 | 150 |
I: Empagliflozin C: placebo |
0.69 year | Left ventricular volumes |
| CREDENCE487 | NCT02065791 | 2014–2017 | 3 | 4401 |
I: Canagliflozin C: placebo |
2.62 years | Reduces the risk of kidney failure and cardiovascular events |
| DECLARE–TIMI 58488 | NCT01730534 | 2013–2018 | 3 | 17160 |
I:Dapagliflozin C: placebo |
4.2 years | MACE, composite of cardiovascular death, hospitalization for heart failure |
| EMPA-REG489 | NCT01131676 | 2010–2013 | 3 | 7020 |
I:Empagliflozin C: placebo |
3.1 years | Death from cardiovascular causes, nonfatal myocardial infarction, nonfatal stroke |
| Others | |||||||
| SAVOR-TIMI 53490 | NCT01107886 | 2010-2011 | / | 4894 |
I: Metformin C:Other antidiabetic drugs |
2.1 years | Composite of cardiovascular death, myocardial infarction, or ischemic stroke |
| TOSCA.IT491 | NCT00700856 | 2008–2014 | 3 | 3028 |
I: pioglitazone add on metformin C: sulfonylurea add on metformin |
5 years | All-cause death, nonfatal myocardial infarction, nonfatal stroke, or urgent coronary revascularization |
| PROactive492 | NCT00013208 | 2015 | / | 3606 |
I: Pioglitazone C: placebo |
7.8 years | All-cause mortality, nonfatal myocardial infarction, stroke, cardiovascular mortality, cardiac intervention, et al |
| PROFIT-J493 | UMIN000000846 | 2007–2011 | 3 | 481 |
I: Pioglitazone C: Other antidiabetic drugs |
1.53/1.64 years | Composite of all-cause death, nonfatal cerebral infarction, and nonfatal myocardial infarction |
| DEVOTE494 | NCT01959529 | 2013–2014 | 3 | 7637 |
I: Insulin Degludec C: Insulin Glargine |
1.99 years | Death from cardiovascular causes, nonfatal myocardial infarction, nonfatal stroke |
| ORIGINALE495,496 | NCT00069784 | 2012–2014 | 3 | 4718 |
C: Glargine I: Standard care |
2.7 years | Death from cardiovascular causes or myocardial infarction or stroke and any of these three outcomes or hospitalization for heart failure or carotid, coronary, or peripheral revascularization |
| ACCORD497 | NCT00000620 | 2001–2005 | 3 | 10251 |
I: intensive glycemic control C: standard glycemic control |
3.7 years | Composite cardiovascular outcome, cardiovascular and total mortality, nonfatal myocardial infarction |
| Steno-2498,499 | NCT00320008 | 1993–2006 | 3 | 160 |
I: intensive glycemic control C: standard glycemic control |
13.3 years |
Death from any cause Stroke |
| VADT500 | NCT00032487 | 2000–2008 | 3 | 1791 |
I: intensive glycemic control C: standard glycemic control |
5.6 years | Composite of major cardiovascular events |
| Look AHEAD77 | NCT00017953 | 2001–2012 | 3 | 5145 |
I: intensive lifestyle intervention C: receive diabetes support and education |
9.6 years | Composite cardiovascular outcome |
| AleCardio501 | NCT01042769 | 2010–2012 | 3 | 7226 |
I: Aleglitazar C: placebo |
2.5 years | Composite of cardiovascular mortality, nonfatal myocardial infarction, nonfatal stroke |
MACE major adverse cardiovascular events, NA no official trial name
Diabetic encephalopathy (DE)
DM is significantly associated with an increased risk of several intracranial diseases, including cerebral macro- and microangiopathy.46–48 Broadly speaking, the intracranial complication of DM includes stroke, which can also manifest as depression, mild cognitive impairment (MCI), and dementia.46,49–51 Such vascular lesions can involve large carotid and vertebral arteries, small intracerebral perforating arteries, micro-arteries, capillaries, micro-venules, and small veins. They are also involved in disrupting the integrity of the blood-brain barrier (BBB) and neurodegeneration.52,53 The cerebral microvasculature facilitates intracranial nutrient delivery and waste removal, supports neuronal activity, maintains the interstitial environment, and reduces and stabilizes blood flow.54 Diabetic macroangiopathy and microangiopathy mutually promote each other, and the occlusion of macrovessels can cause chronic perfusion insufficiency in the brain and microvascular disorders.55 Microvascular function can affect collateral circulation in macrovessels56,57 and increase the risk of stroke58 as well as a poor prognosis.59–61 Neurons injury,62 Alzheimer’s like pathologies,63 and abnormal activity of neurotransmitter receptors64 are also closely associated with cerebrovascular lesions, and jointly cause brain function impairment in patients.
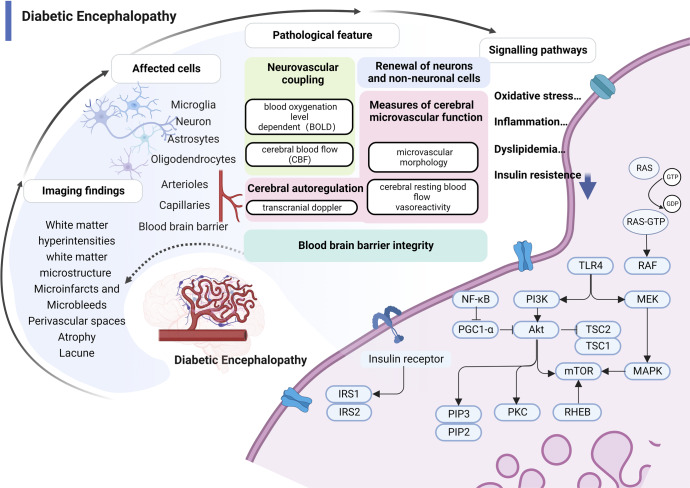
Elevated blood glucose is a risk factor for pathological changes in the brain and brain function impairment.65,66 Not only DM but also pre-DM can promote the development of dementia.67 However, the imaging changes do not correspond to the degree of cognitive impairment, and its mechanism deserves further exploration.68 Diabetic cerebral microangiopathy has multiple complex changes in images (cerebral atrophy, subcortical microinfarcts, cerebral white matter hyperintensity, lacunar infarction, perivascular space, and cerebral microhemorrhage),69 with diffuse adverse effects.70,71 Meanwhile, advances are being made in the measurement of cerebral microangiopathy, along with more precise MRI interpretations and artificial intelligence that have revealed more DM-induced cerebrovascular pathologies.72
Intracranial pathological changes of microangiopathy include endothelial dysfunction, platelet aggregation impairment, and increased inflammation.73–75 The expression of vascular endothelial growth factor (VEGF) and endothelin nitric oxide synthase (eNOS) are decreased in DE, which impairs cerebral artery endothelial function and results in decreased vascular autoregulatory response.76 Disorders of platelet aggregation and inflammation reduce cerebral blood flow and increase the risk of DE.77 Central nervous system is highly dependent on glucose for energy supply.78–80 Disturbance of carbohydrate metabolism can cause an intracranial energy metabolism imbalance and promote lesion development.81,82 Aldose reductase activity is significantly and systemically increased with hyperactivation of the sorbitol pathway in diabetes.83 This leads to insulin resistance, resulting in widespread oxidative stress and increased inflammatory cytokines. The insulin receptor signaling system plays an important role in maintaining normal brain and cognitive functions84 by regulating GLP-1 receptors,84,85 insulin receptor substrate (IRS) receptors.86,87 In other tissues, insulin activates the glucose transporter (GLUT) family of glucose transport proteins, but in the skull GLUT is directly regulated by glucose or cAMP. Activation of IRS or GLUT promotes glucose utilization in the Phosphatidylinositide 3-kinases (PI3K), protein kinase B (Akt), and β-arrestin/ extracellular regulated protein kinases (ERK) pathways. Insulin resistance causes chronic inflammation and increased oxidative stress, imbalanced energy metabolism.88–92 This leads to neuroinflammation and the apoptosis of pericytes and microglial cells,88,89,92–96 thus disrupting vascular endothelial tight junctions and causing damage to the BBB. The accumulation of AGEs also affects various cellular constituents of the BBB, resulting in increased BBB permeability and cognitive impairment.97–99 Vascular endothelial dysfunction further promotes the production of inflammatory mediators that disrupt the BBB,59,63,64,100 which exposes the brain parenchyma to potentially neurotoxic proteins.101 Classical inflammatory mediators such as IL-1β, IL-6, IL-10, TNF-α, vascular cell adhesion protein-1 (VCAM-1), and matrix metalloproteinases (MMP)-2 and -9 suggest vascular neuroinflammation. Intracranial-specific inflammatory signals, estrogen receptors promote increased expression of membrane Rα and ERβ in the hippocampus and promote hippocampal apoptosis;102 P38 activates mitogen-activated protein kinase (MAPK) pathway to promote neuronal cell death, and microglia activation.103,104 Hyperglycemia stimulates inflammatory signals and adaptive signals, accelerating ER stress and mitochondrial dysfunction.105 In addition, glutamate is a key excitatory neurotransmitter in the central nervous system. Glutamate receptors, including N-methyl-d-aspartic acid receptors (NMDA), may regulate neurogenesis and synaptic plasticity.106,107 Upregulation of NMDA receptors had beneficial effects on learning and memory in diabetic rats108 (Fig. 3).
Fig. 3.
Pathology and molecular mechanisms of DE. (1) Structural brain changes from MRI studies in diabetes are the primary diagnostic basis of DE, including microinfarcts and microbleeds, perivascular spaces, white matter hyperintensities, white matter microstructure, lacunes, and atrophy; (2) Pathologies related to imaging findings include blood-brain barrier permeability, perfusion defects, hypoxia, and increased angiogenesis that can involve brain microvessels, multiple nerve cells, and the blood-brain barrier; (3) Microvascular dysfunction manifested by impaired neurovascular coupling and impaired neuronal function. Neurovascular coupling links transient local neural activity to subsequently increased blood flow; (4) The molecular pathways of hyperglycemic damage to brain microvasculature are closely related to oxidative stress, inflammation, abnormal lipid metabolism, and insulin resistance. RAS rat sarcoma protein, GTP guanosine triphosphate, GDP guanosine diphosphate, TLR4 Toll-like receptor 4, MEK mitogen-activated protein kinase, PI3K phosphoinositide 3-kinase, Akt protein kinase B, TSC tuberous sclerosis complex, MAPK mitogen-activated protein kinases, mTOR mammalian target of rapamycin, RHEB Ras homolog protein enriched in brain, PKC protein kinase C, PGC1-α PPAR-gamma co-activator-1 alpha, PIP2,3 phosphatidylinositol bisphosphate2, 3, IRS-1,2 insulin receptor substrate1, 2, NFκB nuclear factor kappa-B
The main therapeutic approaches target brain microvascular endothelium and the BBB, microvascular function, neuroinflammation, and antiplatelet agents.109,110 Maintaining normal blood glucose levels is essential, but no evidence supports intensive glucose-lowering regimens for patients with diabetes and cognitive impairment or dementia.111–113 Moreover, the risk of hypoglycemic events is associated with cognitive decline and increased risk of dementia.81,82,114 Although lowering glucose is generally ineffective for preventing stroke and cognitive impairment,113 some drugs do provide these benefits in addition to controlling glucose levels. Metformin, pioglitazone, and GLP-1 agonizts that can cross the BBB are of interest in the treatment of DE.115 Metformin might improve cognitive impairment associated with stroke or Alzheimer’s disease,116,117 and prevent dementia in persons aged <75 years more effectively than sulfonylurea hypoglycemic agents.111 Pioglitazone might reduce the risk of dementia by 47% in populations with diabetes.48 Pioglitazone and glitazones can activate peroxisome proliferator-activated receptor-γ (PPAR-γ),118 which can improve cell adhesion factors and inflammatory factors in brain cells. This receptor can also act on other tissues and regulate glucose metabolism and overall energy homeostasis.119 Abundant GLP-1α is expressed in the brain, and GLP-1 receptor agonizts have a good safety profile, neuroprotective effects, and can improve cognitive impairment. The new glucose-lowering agent SGLT-2i has cardio-renal protective effects, but it might increase the risk of stroke, which makes its use controversial.120–122 Furthermore, the mechanism of SGLT-2i actions is unknown. Meanwhile, new benefits have been confirmed for some traditional hypoglycemic agents, such as glibenclamide, which can reduce hemispheric edema after stroke when intravenously injected.123,124 Among non-hypoglycemic drugs, phosphodiesterase type III inhibitor Cilostazole125 improves oxidative stress and regulates cerebrovascular damage. NMDA receptor agonizts, neurotrophic factors, and mitochondrial function modifiers are also under development.126
Imaging modalities such as MRI are significantly more important than serum markers for identifying encephalopathy compared with other diabetic vascular lesions. However, current imaging protocols for diagnosing DE are not sufficiently specific. The correlation between imaging and corresponding molecular mechanisms is not yet clear. To overcome this limitation, large clinical trials targeted other vascular lesions and glycaemic control need to be conducted, to identify the population with diabetes that is at high risk of developing cerebrovascular lesions.82
Diabetic kidney disease (DKD)
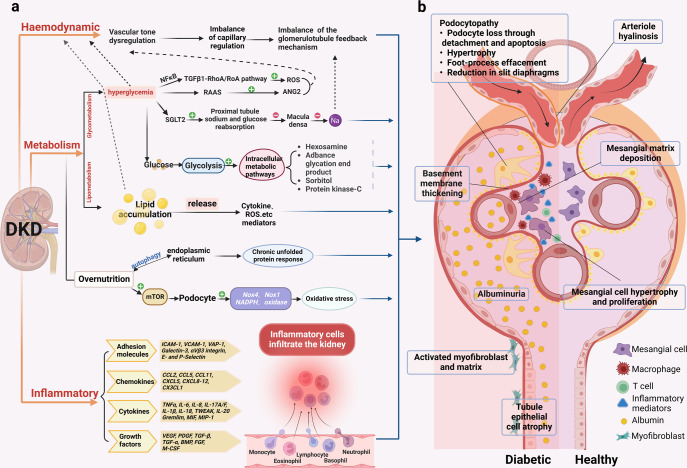
Between 5 and 40% of patients with diabetes eventually develop diabetic kidney disease (DKD),127 and the number of those with chronic DKD is increasing by 2.62 million annually, with chronic DKD being the leading cause of end-stage renal disease.128 DKD is a microvascular complication characterized by glomerular hypertrophy, basement membrane thickening, and damage.129 The complex renal vascular system, including the renal arteries and their branches, as well as glomerular and peritubular capillary networks, form the basis for maintaining normal renal function. The mechanism through which DM causes kidney damage is complex and might involve hemodynamic, metabolic, and inflammatory pathways and targets130,131 (Fig. 4). A hyperglycemic environment induces hypertension, which increases the disturbed renal perfusion pressure and indirectly causes microvascular damage in renal arteries, glomerular and tubulointerstitial capillaries.132–134 AS causes thickening of renal artery walls and lumen narrowing.135,136 Upregulated SGLT expression promotes glucose uptake, which affects the tubular-glomerular feedback mechanism, leading to glomerular hypertension.137,138 Plasma levels of kallikrein, thrombin, and coagulation factors are elevated in hyperglycemic states. A chronically activated coagulation system is closely associated with a vascular injury in patients with DKD.139
Fig. 4.
Pathology of the glomerulus and tubules in DKD. a The classical pathological mechanisms of DKD. It mainly includes hemodynamic, metabolic disturbances, and inflammation, which often interact with each other. (1). Hemodynamic disturbances lead to dysregulation of tubulobulbar feedback balance. (2). Metabolic disorders are crucial to the pathogenesis of DKD. Hyperglycemia affects pathways such as TGFβ1-RhoA/Roa pathway, RAAS, proximal tubular sodium and glucose reabsorption, and intracellular metabolism; abnormal lipid metabolism can affect the release of mediators such as cytokines and ROS; in the presence of nutrient overload in the organism, endoplasmic reticulum autophagy leads to a chronic unfolded protein response, and mTOR also disturbs the podocytes leading to oxidative stress. (3). Inflammation promotes the release of inflammatory mediators such as adhesion molecules, chemokines, cytokines, and growth factors, causing renal infiltration of inflammatory cells. b Schematic representation of the pathological damage of DKD. Differences in structural changes of glomeruli and tubules in the diabetic setting and in the healthy state. Diabetic glomerulopathy is characterized by arterial hyalinization, thylakoid stromal deposition, basement membrane thickening, glomerular thylakoid cell hypertrophy and proliferation, podocytosis, proteinuria, tubular epithelial atrophy, activated myofibroblasts, and stromal accumulation. NFκB nuclear factor kappa-B, TGFβ transforming growth factor-β, ROS reactive oxygen species, RAAS renin-angiotensin-aldosterone system, ANG2 angiotensin II, SGLT2 sodium-dependent glucose transporters 2, mTOR mammalian target of rapamycin, NADPH nicotinamide adenine dinucleotide phosphate, NOX NADPH oxidase, ICAM-1 intercellular cell adhesion molecule-1, VCAM-1 vascular cell adhesion molecule-1, VAP-1 vascular adhesion protein-1, CCL CC chemokine ligand, CXCL C-X-C motif chemokine ligand, TNF tumor necrosis factor, IL interleukin, TWEAK tumor necrosis factor-like weak inducer of apoptosis, MIF macrophage migration inhibitory factor, MIP-1 macrophage inflammatory protein-1, VEGF vascular endothelial growth factor, PDGF platelet-derived growth factor, BMP bone morphogenetic protein, FGF fibroblast growth factor, M-CSF macrophage colony-stimulating factor
The most prevalent diagnostic markers for DKD are the estimated glomerular filtration rate (eGFR) and proteinuria calculated based on serum creatinine or cystatin C.140 However, tissue damage is often irreversible by the time a diagnosis is confirmed, and new biomarkers are needed to diagnose DKD earlier and to stratify risk factors. Current new biomarkers mainly target diagnoses of inflammation, endothelial damage, fibrosis, endothelial dysfunction, and kidney damage,141 including TNF-α receptor,142 intercellular adhesion molecule-1,143 endostatin,144 copeptin,145 kidney injury molecule-1,146,147 monocyte chemoattractant protein-1,148 and neutrophil gelatinase-associated lipocalins.149 Extracellular vesicles have recently attracted interest,150 because they are essentially exosomes and particles151 that transport miRNA,152 mRNA,153,154 and proteins,155 and might serve as early biomarkers of DKD. In addition to individual biomarkers, other approaches such as proteomics,156 genomics,157 and metabolomics158 all play roles in screening, especially in studies of chronic kidney disease (CKD)159 which can predict the development of proteinuria.160 Despite many studies of novel biomarkers, only eGFR and proteinuria are routinely applied to clinically diagnose DKD, and the specificity and accuracy of these awaits clarification in future controlled clinical trials with large samples.
In addition, imbalanced pro- and anti-angiogenic factors can disrupt the vascular network in DKD. Hyperglycemia increases glomerular capillary pressure through a RAAS-mediated increase in angiotensin II.161,162 Hyperglycemia-mediated changes in glomerular capillary autoregulation can cause endothelial dysfunction and inflammation by increasing transforming growth factorβ-1 (TGFβ-1) mediated ROS that dilates glomerular afferent and efferent arterioles.163 Lipid metabolism is often abnormal in patients with diabetes, and this can also contribute to glomerular and tubulointerstitial vascular injury through mediators such as cytokines, ROS, and hemodynamic changes.164,165 Hypoxia in renal tissues due to reduced density of tubulointerstitial capillaries is also a major cause of renal disease progression.166,167 Although scant vascular endothelial growth factor A (VEGFA) is expressed in renal tubular capillaries, the absence of specific VEGFA expression leads to a significant decrease in peritubular capillary density in mice.168 However, overexpression causes dilation of peritubular capillaries.169
Inflammatory mediators (chemokines, cytokines, and adhesion molecules)170,171 are often released due to hyperglycemia and hemodynamic abnormalities, which lead to nephron damage through ultrafiltration, mechanical stress, oxidative stress, glycocalyx dysfunction, and endothelial activation.172,173 These mediators cause renal microvascular dilation or altered permeability through thylakoid proliferation, podocyte or tubular injury,174 and inflammatory cell infiltration.175 In addition, oxidative stress is closely associated with inflammation and causes endothelial dysfunction by activating NF-κB176,177 and adapter protein complex-1178,179 to induce pro-inflammatory factors and, in turn, mediate the inflammatory response, thus inducing renal fibrosis.180 Therefore, the main causes of vasculopathy in DKD are inflammation, hemodynamic, and metabolic disorders. Lesions are mostly concentrated on the glomerular and tubulointerstitial microvasculature and also in other vessels such as renal arteries, and glomerular afferent and efferent arterioles. The pathogenesis and therapeutic strategies need further exploration.
The current strategies for treating DKD mainly comprise controlling blood sugar and blood pressure and blocking the RAAS140 (Table 2). After controlling various risk factors such as hyperglycemia, hyperlipidemia, hypertension, and uric acid,181,182 the risk of DKD is reduced, but the vascular disease continues to progress. Blood pressure control and fenofibrate can increase the risk of renal adverse events such as decreased eGFR, suggesting a need to explore more effective treatment modalities.183,184 Novel hypoglycemic agents might protect the kidney through combined actions against hyperglycemia, hypertension, lipotoxicity,185 abnormal tubuloglomerular feedback,186 hypoxia,187 endothelial dysfunction, and renal fibrosis.188 The SGLT-2i dapagliflozin exerts renoprotective effects, possibly by affecting the hemodynamics of patients mainly through post-glomerular vasodilation to normalize the eGFR.189 In addition to stimulating insulin secretion from pancreatic β-cells to control blood glucose, incretin might also bind to GLP-1 receptors to inhibit endothelial damage and thus exert positive effects.190 The large FIDELIO-DKD clinical trial found that finerenone, a novel nonsteroidal mineralocorticoid receptor antagonist combined with a RAAS inhibitor, reduced the risk of cardiac and renal outcomes while reducing the incidence of hyperkalemia.191 MiRNAs play an important role in maintaining optimal vascular homeostasis and regulating microvasculature disorders.192 The miR-132 inhibitor CDR132L improves HF and affects cardiac fibrosis biomarkers193 and might also have a therapeutic effect on renal fibrosis. Anticoagulants might hamper the progression of DKD,194 but the clinical application requires further validation and tests, as some anticoagulants such as vorapaxar might increase bleeding risk (Table 2).195
Table 2.
Renal outcomes trials in diabetes mellitus
| Clinical trials | Clinical trials’ number | Year | Phase | Paticipants (n) | Intervention | Follow-up | Main outcome |
|---|---|---|---|---|---|---|---|
| Dipeptidyl peptidase-4 (DPP-4) inhibitors | |||||||
| CARMELINA502 | NCT01897532 | 2013–2018 | 4 | 6991 |
I: Linagliptin C: Placebo |
2.2 years | Reduced proteinuria, control blood glucose. |
| Glucagon-like peptide-1 receptor (GLP-1) agonizts | |||||||
| REWIND503 | NCT01394952 | 2011–2018 | 3 | 9901 |
I: Dulaglutide C: placebo |
5.4 years | Reduced compound renal outcome. |
| PIONEER 5504 | NCT02827708 | 2016–2018 | 3 | 424 |
I: Semaglutide C: Placebo |
0.54 years | Effective in patients with type 2 diabetes and moderate renal impairment, but with higher adverse events. |
| BETENT-4505 | NCT03730662 | 2018–2021 | 3 | 2002 |
I: Tirzepatide C: Insulin Glargine |
2 years | Slowed down eGFR decline rate, reduced UACR (urinary albumin creatinine ratio). |
| Sodium-glucose cotransporter-2 (SGLT2) inhibitors | |||||||
| DAPA-CKD506 | NCT03036150 | 2017–2020 | 3 | 4304 |
I: Dapagliflozin C: Placebo |
3.2 years | Reduced the risk of GFR and major renal and cardiovascular adverse events in diabetic and non-diabetic patients with chronic kidney disease. |
| CREDENCE487 | NCT02065791 | 2014–2018 | 3 | 4401 |
I: Canagliflozin C: Placebo |
2.62 years | Reduced the risk of kidney failure and cardiovascular events. |
| SCORED507 | NCT03315143 | 2017–2020 | 3 | 10584 |
I: Sotagliflozin C: Placebo |
1.3 years | Reduced risk of cardiovascular-related hospitalization and death from diabetes and CKD, but associated with adverse events. |
| VERTIS CV508 | NCT01986881 | 2013–2019 | 3 | 8223 |
I: Ertugliflozin C: Placebo |
3.5 years | Ertugliflozin reduced the risk of composite renal end points and was associated with reduced eGFR and UACR. |
| Mineralcorticoid receptor antagonists | |||||||
| FIDELIO-DKD191 | NCT02540993 | 2015–2021 | 3 | 5734 |
I: Finerenone C: Placebo |
2.6 years | Reduced the risk of the cardio-renal outcome. |
| PRIORITY509 | NCT02040441 | 2014–2018 | 2/3 | 209 |
I: Spironolactone C: Placebo Standard care |
2.5 years | Can’t prevent disease progression of high-risk patients with DKD. |
| SONAR510 | NCT01858532 | 2013–2018 | 3 | 2648 |
I: Atrasentan C: Placebo Standard care |
4.4 years | Reduced the risk of renal events in patients with diabetes and CKD. |
| Others | |||||||
| CKD-FIX181 | ACTRN12611000791932 | 2014–2016 | 3 | 369 |
I: Allopurinol C: Placebo Standard care |
2.17 years | Decreased serum urate but did not affect the renal outcome and did not alleviate the decline in eGFR. |
| ALBUM197 | NCT02358096 | 2015–2017 | 2 | 125 |
I:ASP8232 C: Placebo |
2 years | Reduced albuminuria in DKD patients, safe and well tolerated. |
| NA196 | NCT01683409 | 2012–2017 | 2 | 130 |
I: Baricitinib C: Placebo |
0.46 years | Reduced albuminuria. |
| NA | NCT03804879 | 2018–2021 | 2 | 83 |
I: Nidufexor C: Placebo |
0.54 years | UACR and 24-hour urinary albumin were decreased in DKD patients. |
| NA201 | NCT03016832 | 2017–2021 | 1 | 413 |
I: HuangKui capsule C: Irbesartan tablets |
0.46 years | The combination of Huangkui capsule and irbesartan had the best effect on reducing ACR in DKD patients. |
NA no official trial name
Some pathways associated with kidney injury have emerged as novel targets for treating DKD. For example, baricitinib is an oral small-molecule inhibitor that selectively inhibits the Janus kinase (JAK) protein tyrosine kinase family members JAK1 and JAK2,196 which inhibits the JAK-mediated inflammatory pathways and reduces proteinuria. Small-molecule inhibitors associated with kidney damage, such as the vascular adhesion protein-1 inhibitor ASP8232, reduce proteinuria through local effects on glomeruli and podocytes, offering potential multi-target interventions.197 Another promising therapeutic target is gut flora, as the renal disease causes the dysbiosis of various gut microbes. Inhibiting phenyl sulfate (a metabolite derived from gut microbiota) reduces proteinuria in mice with DKD.198 Supplementing patients with diabetes who are on hemodialysis with probiotics improves glucose and lipid metabolism, as well as biomarkers of inflammation and oxidative stress.199 Orally administered Faecalibacterium exerts both anti-inflammatory and renoprotective effects on patients with CKD through butyrate-mediated G protein-coupled receptor-43 signaling.200 Moreover, traditional Chinese medicine has also achieved considerable progress in DKD treatment, as a combination of Huangkui capsules and irbesartan was found to be more effective than either of the medications alone in reducing albumin-to-creatinine ratio in patients with DKD,201 suggesting the potential role of Chinese medicine in future DKD therapy.
Renal microvasculature is an important target of DPD. Intensive management of DM, including controlling blood sugar and blood pressure and blocking the RAAS, will reduce the incidence of CKD and delay its progression. Current and future health resource requirements for DKD treatment are difficult to estimate. Thus innovative therapeutic strategies are needed to prevent, block, treat, and reverse DKD.
Diabetic retinopathy (DR)
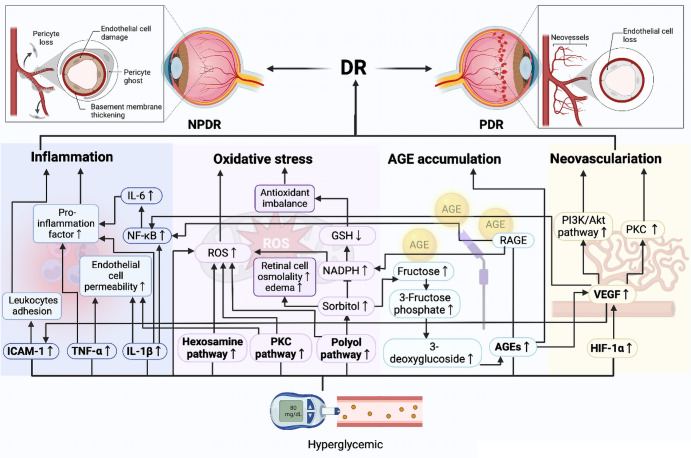
DR is one of the most common microvascular complications of DM.202,203 Pathological changes of DR start with the loss of retinal neuronal. The series of events include early loss of neurovascular coupling, retinal neurodegeneration, and subsequent gliosis, finally leading to retinal vasculopathy. Microvasculopathy in the DR retina are manifested as loss of retinal capillary epithelial cells, decreased capillary elasticity, and increased vascular permeability, exudation, local inflammation, and growth factors promoting neovascularization.204,205 DR is clinically classified as non-proliferative (NPDR) or proliferative (PDR) in the absence or presence of retinal neovascularization, respectively. The NPDR type progresses to PDR and eventually develops into macular edema, with the latter being an important cause of vision loss or blindness in patients with diabetes.206,207 Such patients must undergo regular fundus examinations to detect vision-threatening stages of DR, such as PDR and diabetic macular edema, as early as possible to treat them before vision loss becomes irreversible.
Delayed diagnosis and treatment are the most common causes of visual impairment in patients with diabetes. Therefore, the early detection and prevention of lesions in DR is the key to stop DR progression. Biomarker-related biomic and artificial intelligence (AI) investigations will play increasingly important roles in the risk assessment, early diagnosis, and treatment of the disease. Homocysteine levels are significantly high in the serum of patients with diabetes and should become a screening and diagnostic indicator of DR, as its prevention and treatment can be targeted by increasing homocysteine clearance.208 The main factor controlling neovascularization is VEGF, levels of which increase in vitreous and tear fluids from patients with DR and correlate positively with DR severity. Retinol-binding protein 3 (RBP3) is a retinol transporter protein secreted by photoreceptors, and high levels of RBP3 in the vitreous body of patients with diabetes slow DR progression.209 Elevated RBP3 expression can alleviate hyperglycemia-induced DR by inhibiting glucose uptake by glucose transporter protein-1 and reducing the expression of inflammatory cytokines and VEGF.210 Therefore, RBP3 could serve as a biomarker and therapeutic strategy in preventing the progression of DR. MiRNAs are involved in retinal neovascularization and the inflammatory response in DR. The relative expression of serum miRNAs was measured in 80 patients with T2DM comprising an NPDR group with normal, mild, moderate, or severe symptoms and a PDR group. The results showed that the relative expression of serum miR-146a and miR-21 increased, whereas that of miR-34a decreased with worsening DR severity.211 miR-125a-5p significantly attenuated vascular leakage in DR.212 These findings suggested that miR-146a, miR-21, miR-34a, and miR-125a-5p could serve as promising biomarkers for DR.211 AI has recently become a research hotspot in auxiliary medical diagnosis. Ophthalmic AI integrates imaging databases with deep learning (DL) technology to automatically measure and analyze the characteristic biological structures of the eyes of patients with DR to assist with diagnosis. We found different features of Hematoxylin-eosin-stained retinal sections from diabetic mouse models based on changes in nerve fiber layers and ganglion cells during the early stage of the disease. We then identified these features using image recognition and DL, and consequently identified changes in ganglion cells and the nerve fiber layer that could be applied to the early quantitative diagnosis of DR.213 Another AI-based study by our team quantified the pathological changes of retinal neurons and synapses in mice with diabetes induced by monosodium glutamate (MSG). We found that MSG-induced DR was closely associated with neurotransmitter abnormalities and had important features of retinal neurodegeneration, providing an effective animal model and technique for quantifying retinal neuron pathology.214
A thorough knowledge of the mechanism of DR is essential for its prevention and treatment. Hyperglycemia can lead to inflammation, oxidative stress, and increased glycosylation product and VEGF contents during the late stages. These can increase retinal permeability and alter retinal hemodynamics, leading to retinal leakage and the development of DR. Serine racemase (SR) promotes the formation of d-serine, which activates NMDA receptors and has multiple effects on neuron.215 Overexpression of SR in diabetic retinopathy leads to retinal neurodegeneration. Hyperglycemia subsequently leads to vascular EC damage, and in turn, leukocyte aggregation/adhesion to vessel walls.216 Leukocyte adhesion and aggregation activate a massive amount of neutrophils that adhere to EC and form a reticular network and aggravate tissue hypoxia, causing vascular remodeling and neovascularization.217 The main protein that mediates intercellular adhesion is intercellular cell adhesion molecule-1 (ICAM-1).218 Under inflammation, ICAM-1 is abundantly expressed in retinal EC, where it binds to receptors. This induces leukocytes to penetrate the endothelium and become adherent, indicating that ICAM-1 is an important mediator of the inflammatory response in DR.219,220 The inflammatory response of the retina in DR involves the production and release of various inflammatory factors.221 In particular, the release of massive amounts of IL-1β can lead to the apoptosis of retinal pigment epithelial cells, which damages the integrity of photoreceptors. IL-1β activates NF-κB and oxidative stress, leading to the apoptosis of capillary EC and increasing EC permeability.222 IL-1β also promotes IL-6 secretion and induces capillary angiogenesis by activating the NF-κB pathway and p38/MAPK.223 The pro-inflammatory factor TNF-α can cause EC damage and increase EC permeability, resulting in vascular leakage.224 Inflammatory factors induce each other via cascade amplification, mediating the inflammatory response and exacerbating DR. Oxidative stress leads to nerve, vascular, and retinal tissue damage and, in turn, the development of DR.225,226 Chronic hyperglycemia causes oxidative stress mainly through PKC, polyol, hexosamine, and AGEs formation pathways.225 Hyperglycemia can regulate vascular cell permeability, the extracellular matrix, cell growth, neovascularization, cytokine response, and leukocyte adhesion through the diacylglycerol-PKC pathway, leading to structural and functional changes of the retinal vasculature.227,228 Activation of the polyol pathway produces oxidative stress, which increases the consumption of reduced coenzyme II (NADPH) oxidase through the production of sorbitol by aldose reductase (AR), thus affecting the production of the antioxidant reduced glutathione and causing an oxidative-antioxidative imbalance.229–231 The production and accumulation of sorbitol under hyperglycemic conditions increases retinal osmotic pressure, cell edema, metabolic disorders, and microvascular damage,232,233 consequently aggravating DR. Advanced glycation end-products promote NF-kB activation by interacting with the cellular RAGEs on the cell surface.234 This leads to retinal pericyte apoptosis and elevated expression of VEGF, inflammatory cytokines, and adhesion molecules.235 Inhibition of ACEs can improve hyperglycemia-induced blood-retinal barrier leakage and reduce retinal EC proliferation, migration, and neovascularization,236 thus alleviating DR. The main hallmark of PDR is neovascularization. The most important inflammatory factor that stimulates neovascularization and causes vascular leakage is VEGF.209 Hypoxia-inducible factor-1α is activated under hyperglycemic and hypoxic conditions, which leads to increased secretion of VEGF, and overexpressed VEGF, in turn promotes neovascularization through activation of the PI3K/Akt,237–239 PKC,240,241 and NF-κB242,243 signal pathways. The expression of ICAM and nitric oxide synthase induced by VEGF promotes leukocyte adhesion and causes changes in vascular permeability and pathological neovascularization.244–246 The pathogenesis of DR is complex, with numerous factors that synergistically interact with each other during the development and progression of DR (Fig. 5).
Fig. 5.
Pathology and molecular mechanisms of DR. Multiple mechanisms are involved in the pathogenesis of DR. Hyperglycemia can promote oxidative stress through the polyol pathway, accumulation of advanced glycosylation end-products (AGEs), the protein kinase C (PKC) pathway, and the hexosamine pathway, and exacerbate inflammation and abnormal angiogenesis by stimulating the secretion of inflammatory factors and vascular endothelial growth factor, inducing retinal dysfunction until vision loss
DR can be treated mainly by laser photocoagulation and vitreous injections of antibodies.247 Retinal laser photocoagulation can prevent further vision deterioration, but cannot restore damaged vision. Intravitreally injected ranibizumab and bevacizumab reduce the recurrence of active neovascularization and risk of retinal detachment. However, frequent intraocular administrations due to a short half-life increase the risk of retinitis, retinal obstruction, and patient pain and might facilitate the development of drug resistance.248,249 Intraocular glucocorticoid injection is often used to treat persistent and refractory diabetic macular edema, which improves vision but increases the incidence of cataracts and glaucoma.250 Drugs such as ranibizumab, aflibercept, and fenofibrate have been included in clinical trials to evaluate their effectiveness and safety (Table 3), thus providing new ideas for treating DR.
Table 3.
Interventional trials in diabetic retinopathy
| Clinical trials | Clinical trials’ number | Year | Phase | Participants (n) | Intervention | Follow-up | Main outcome | Adverse events |
|---|---|---|---|---|---|---|---|---|
| Sodium-glucose cotransporter-2 (SGLT2) inhibitors | ||||||||
| Dapagliflozin511 | NCT02919345 (Completed) | 2017 | / | 97 |
I: Dapagliflozin C: glibenclamide |
12 weeks | Central retinal thickness | Not reported |
| Dapagliflozin plus another oral hypoglycemic agent | NCT05310916 (Ongoing) | 2022 | 3 | 60 |
I:Dapagliflozin 10 mg Tab plus another oral hypoglycemic agent C:Two oral hypoglycemic agents other than dapagliflozin |
12 weeks | Severity of retinopathy | / |
| Others | ||||||||
| Faricimab512 | NCT03622580 (Completed) | 2018 | 3 | 1891 |
I:faricimab C:faricimab per personalized treatment interval or aflibercept |
1 year | Mean change in best-corrected visual acuity | Intraocular inflammation |
| Brolucizumab513 | NCT03321513 (Completed) | 2017 | 3a | 270 (312 eyes) |
I:Brolucizumab C: aflibercept |
2 years | Mean best-corrected visual acuity; Retinal central subfield thickness and visual acuity | Retinal vasculitis and retinal vascular occlusion |
| Abicipar514 |
NCT02462486 (Completed) |
2015 | 3 | 1888 |
I: Abicipar C: ranibizumab |
52 weeks | Stable vision, best-corrected visual acuity, central retinal thickness | Intraocular inflammation |
| Fenofibrate515 | NCT01927315 (Completed) | 2013 | 4 | 41 |
I: Fenofibrate C: placebo |
12 weeks | Change in the levels of circulating hematopoietic stem/progenitor cells | Ischaemic stroke related to pre-existing conditions |
| PDS implant pre-filled with ranibizumab | NCT04503551 (Ongoing) | 2020 | 3 | 174 |
I: PDS implant pre-filled with ranibizumab C: Intravitreal ranibizumab |
52 weeks | Early treatment diabetic retinopathy study, diabetic retinopathy severity scale | / |
| OPL-0401 | NCT05393284 (Ongoing) | 2022 | 2 | 120 |
I: OPL-0401 C: Placebo |
24 weeks | Improvement in diabetic retinopathy severity scale | / |
| Calcium Dobesilate | NCT04283162 (Ongoing) | 2020 | 4 | 1200 |
I:Calcium dobesilate + conventional treatment C:conventional treatment |
12 months | The rate of the progression of diabetic retinopathy | / |
| Sinemet CR | NCT05132660 (Ongoing) | 2022 | 1 | 244 |
I:Sinemet CR C:placebo |
24 months | Electroretinogram | / |
| Aflibercept | NCT04708145 (Ongoing) | 2021 | 4 | 150 | Eyes without panretinal photocoagulation (PRP) and eyes with PRP, Drug: Aflibercept Injection | 112 weeks | Improvement in diabetic retinopathy severity scale | / |
Diabetic peripheral vasculopathy (DPVD)
DPVD is often overlooked, yet it is one of the most important and common vascular complications in patients with T2DM.251 In such patients, DPVD increases the risk of not only coronary atherosclerotic events,252 but also major adverse limb events such as amputation.253 DPVD can manifest as diabetic foot syndrome and peripheral arterial disease (PAD), which seriously affect the quality of life of patients with diabetes. Peripheral arterial disease is traditionally considered to be dominated by large artery AS.254 In fact, PAD is often accompanied by local and systemic microangiopathy.255,256 Multivessel endothelial dysfunction can manifest as microangiopathy (either exclusively or with other diseases), such as capillary basement membrane thickening, endothelial hyperplasia, oxygen tension reduction, and hypoxia, affecting peripheral nerve function.257 Pre-DM can affect blood vessels and accompanying nerves.258,259 The chronic course of DM might have further adverse effects under poor glycemic control.260,261 An increase in postprandial glucose plays an important role in the development of peripheral vascular disease in DM.262
The pathogenesis of DPVD overlaps with that of other AS and microvascular endothelial injuries. IL-6, high-sensitivity C-reactive protein, lipoprotein-associated phospholipase A2, and high-molecular-weight lipocalin biomarkers serve as indicators of the risk of cardiovascular disease and peripheral vascular disease.263–267 Specific markers for DPVD are unknown, but many biomarkers of vascular injury are available.268,269 For example, circulating levels of ICAM and sE-selectin indicate EC activation and vascular inflammation, and thus have potential as diagnostic markers of DPVD.270,271
The molecular mechanisms of AS in DPVD can be found in the section on coronary AS. Microvasculopathy in DPVD is highly concomitant with neuropathy, as microvessels form a neurovascular network with accompanying nerves.272 Neurons and Schwann cells are highly susceptible to hyperglycemia.273,274 Energy and inflammation, oxidative stress,275 insulin resistance,276 AGEs,277 nerve growth factors,278 activation of the polyol pathway,279 and activation of the hexosamine and PKC pathways280 are core pathological factors and processes similar to those of other DM vascular complications.281,282 Glucose and fatty acid metabolism,283 neural metabolism,284 and exosome regulation have recently attracted attention.285,286 Much more is known about peripheral neuropathy than about DPVD. Glucose overload and high fatty acid metabolism lead to decreased ATP production, excessive ROS formation, and impaired mitochondrial function, which further increases oxidative stress, leading to the formation of AGEs from the glycosylation of various proteins. The vicious cycle of these events further promotes ROS formation and ER stress, resulting in DNA damage and apoptosis of various cells. Abnormal neurometabolism in DM manifests as changes in sphingolipid metabolism, wherein sphingolipids are biologically active and important structural components of plasma cell membranes and are important signaling molecules. Abnormal sphingolipid metabolism causes neurotoxicity in patients with hyperglycemia.287 All of these pathways eventually manifest as increased pro-inflammatory factors that further induce AGEs production, leading to oxidative stress and endothelial dysfunction.288 These interactive processes simultaneously place EC and neurons in a state of oxidative stress and inflammation.
The endpoint of DPVD is amputation, the occurrence of which is closely associated with infection and trauma. Therefore, care and lifestyle changes play an important role in its treatment, which greatly differs from the preventive measures for other vascular pathologies. Normal or near-normal glycemic control is a primary therapeutic goal. Intensified hypoglycemic therapy reduces the incidence of peripheral neuropathy in patients with T1DM but has a little additional benefit for those with T2DM.289,290 Systemic antioxidant and anti-inflammatory therapy might also provide some benefits.291 New glucose-lowering drugs can reduce blood glucose without increasing the risk of amputation. In a subgroup of patients with T2DM combined with PAD, empagliflozin reduced rates of mortality, hospitalization for HF, and the progression of kidney disease.252 Patients treated with the new glucose-lowering drugs SGLT-2i, GLP-1RA, and DPP-4i have low risks of amputation with a good safety profile.292 Some glucose-lowering agents have conferred advantages for patients with DPVD and other multivessel diseases. The results of the LEADER trial suggested that liraglutide could be used in diabetic multivessel diseases.293 These treatment options could improve the overall quality of life of patients.
Temporal progression of DPDs
The time of onset of panvascular complications in patients with diabetes is closely associated with patient age,294 race,295 genetic background,296 DM staging,297 treatment regimen,298,299 and level of glycemic control.300,301 The natural course of DPDs is not completely clear, but diabetic microangiopathy generally precedes diabetic macroangiopathy.302 Clinical trials of patients with insulin-dependent DM have found that DR develops within the first 2 years of DM in conventionally treated patients, and that by the fifth year, 25–40% of these patients develop retinal, renal, and peripheral microangiopathy.303 A Chinese cohort study has shown that >20% of patients develop moderate retinopathy and >40% develop mild proteinuria within 7 years after the onset of DM.304 According to the ADVANCED study, the incidence of macroangiopathy at the fifth year of DM does not exceed 10%.305 Complications with panvascular disease appear earlier and more frequently in patients with T2DM, than in those with T1DM.306,307 After adjusting for age and the duration of DM, the risk of peripheral neuropathy is found to be significantly higher in patients with T2DM than in those with T1DM.306 The median elapsed time to the onset of microproteinuria is also significantly shorter in adolescents with T2DM than in those with T1DM (1.3 vs. 6.8 years).307
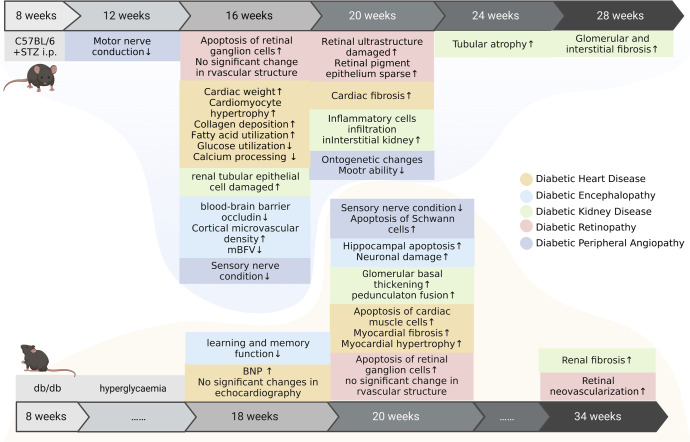
The natural course of DPDs in rodents from the same genetic background would provide insights into the pathological changes in various tissues at the same time. Streptozotocin induced in C57BL/6 mice develop microangiopathy in various organs at 4‒20 (mostly 8‒10) weeks after the onset of DM;308–310 db/db mice simulating type 2 diabetes develop microangiopathy in various organs at 16‒40 (mostly 20) weeks of age.311,312 The temporal progression of diabetic microvascular complications in mice are Concurrent regardless of the modeling modality (Fig. 6). DKD is the earliest to appear, with peripheral motor nerve conduction velocity decelerating at 4 weeks of DM (C57BL/6 mice).313 Microvascular function in diabetic mice partially changes around 8–10 weeks after DM onset (C57BL/6 mice)310,314–318 or 20 weeks of age (db/db mice),268,311,319,320 manifested as pathological staining of various organs changes. After 8 weeks of hyperglycemic state, further pathological changes of diabetic C57B/6 mice in microvascular structure can occur, including expansion of the mesangial matrix of renal microvasculature and rupture of the renal tubular epithelium321 The retinal ultrastructure changes, infoldings in retinal pigment epithelium layers are decreased, and balance or proprioception are impaired due to neurovascular disease.322 At 12‒20 weeks of DM, late panvascular disease can occur, with advanced vasculopathy of the heart, retina, or brain.323–325 occurring slightly before that of the kidneys.326 Diabetic macroangiopathy does not develop spontaneously in rats after DM modeling; rather, it is generally induced through specific diet control or vascular occlusion of the DM model rats.327 Thus, after simply constructing animal models of diabetes, studies on microangiopathy and macroangiopathy in the same models are scant. Rodents have different lipoprotein metabolism from humans and are highly resistant to AS. C57BL/6 mice are relatively too resistant to intraperitoneal injections of STZ to construct a model of DKD, and various diabetic complications require different animals for optimal modeling. Therefore, the optimal animal model for DPD studies awaits further investigation and clinical evidence.
Fig. 6.
The natural course of diabetic vasculopathy in mice. Streptozotocin induced in C57BL/6 mice simulate type 1 diabetes in human; db/db mice simulate type 2 diabetes in human. Vasculopathy in different organs appears over a period of time, In C57BL/6 mice Streptozotocin induced in C57BL/6 mice develop typical microangiopathy mostly at 8‒10 weeks after the onset of DM and develop advanced microangiopathy at 12–16 weeks. db/db mice develop microangiopathy at about 20 weeks of age and develop advanced microangiopathy at 34 weeks
Relationships among DPDs (Fig. 7) outcomes in vital organs (heart, kidney, and brain) are mutually predictive. DR is associated with coronary atherosclerotic heart disease, macrovascular events (including stroke), and all-cause mortality.328 Screening retinal microvessels have a potential role in the risk identification of cerebrovascular and neurodegenerative diseases.329 In T2DM, retinal parameters and a genome-wide polygenic risk score for coronary heart disease have independent and incremental prognostic value compared with conventional cardiovascular risk assessment.330 Risk of macrovascular complications in patients with diabetes and retinal artery occlusion for at least 5 years after an obstruction event is increased compared with those who do not have such occlusion. Therefore, retinopathy can predict cardiovascular risk in patients with T2DM.331 DR is closely associated with stroke332–336 and cerebral microangiopathy.337 During embryonic development, the diencephalon is homologous to the retina and optic nerve, especially the capillary-linked microglia and neuronal synapses, which are abundant in the retina and brain and sensitive to blood glucose.338 The microvasculature of the retina and the axonal function of retinal ganglion cells can be detected using fundus photography and optical coherence tomography to assist with the diagnosis of cerebrovascular neuropathy.339 Retinal microvascular imaging findings closely correlate with cerebral infarction and white matter lesions, parapapillary retinal nerve fiber layer thickness, macular thickness, and volume being indicators of stroke risk.340 Changes in the retinal vasculature can predict various stroke subtypes, suggesting that retinal vascular changes reflect specific cerebral microangiopathy and might even distinguish stroke from other causes of focal neurological deficits.341 In contrast, qualitative retinal vascular signs and quantitative retinal vascular measurements of narrowing small retinal arteries and widening small retinal veins, might indicate a cognitive decline.342 Compared to that with stroke, the association of DR with dementia and cognitive decline is more limited, suggesting a need for further prospective studies. In addition, because risk factors for stroke differ between patients with and without diabetes, stroke risk prediction models should include data on DR and DKD,335 which is a topic for future studies. DKD increases the risk of stroke, cerebral infarction, and cerebral hemorrhage.334 However, some subclinical cardiovascular complications of DM are not associated with stroke.343 Retrospective studies have shown that DR is associated with the development of DKD and the decline of renal function.344–346 Subsequent retrospective studies have found a positive association between DR and the risk of DKD progression.347,348 Retrospective findings of narrowing small retinal arteries and widening small veins both suggested the development of DKD,349 and this was later confirmed by a cross-sectional study350 and several prospective studies.351–353 Diabetic macroangiopathy and neuropathy are also important risk factors for DKD. Prospective studies have shown that carotid plaques and aortic stiffness are associated with DKD progression.354,355 A retrospective cohort study found that coronary artery calcification played a similar predictive role.356 Two retrospective cohort studies found that peripheral neuropathy and cardiac autonomic neuropathy are strong predictors of DKD.357,358 As discussed, vasculopathy due to various diseases, especially DR, is generally predictive of DKD progression, providing evidence supporting the panvascular nature of DM.
Fig. 7.
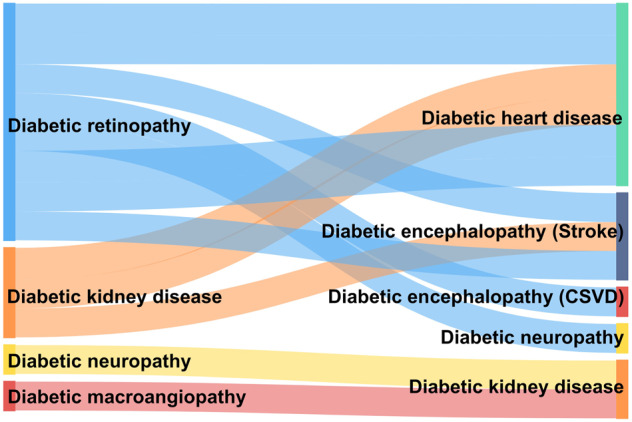
Predictive relationships among DPDs. CSVD cerebral small vessel diseases
Molecular mechanism and signaling pathway of DPDs
Cellular energy metabolism
Cellular energy metabolism requires energy supply from glucose, fatty acids, amino acids, etc.359,360 In the diabetic state, abnormalities in cellular energy metabolism affect macrovascular and microvascular lesions,361–363 including abnormalities in substrate delivery to vascular EC or target organ cells (e.g., cardiac myocytes, thylakoid cells in glomeruli, and neurons and Schwann cells in peripheral nerves), conversion of the ratio of cell-specific fuel sources between glucose intermediates, fatty acids, and amino acids, changes in respiratory chain protein function, and uncoupling of respiratory chains.364–366 The hyperosmolar state and abnormal energy metabolism of diabetes increase the PKC pathway, endothelial xanthine oxidase, and the eNOS uncoupling pathway, promoting increased ROS levels.367,368 The mechanisms of energy metabolism imbalance vary slightly between target organs with different mitochondrial content and different major energy supply substances.369
Glucose metabolism
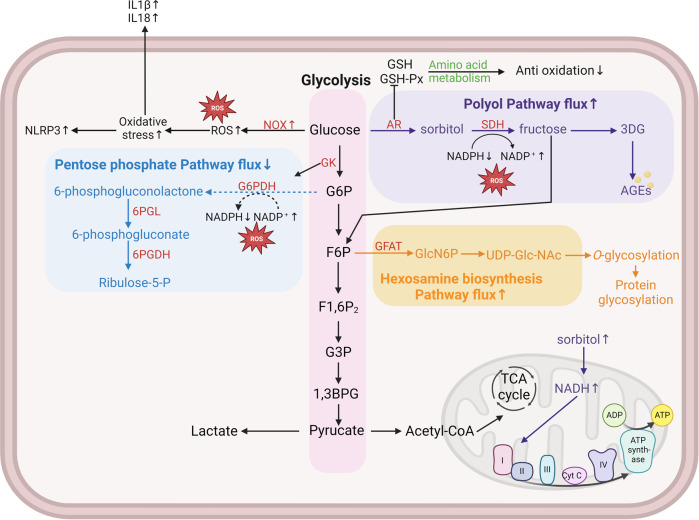
Glucose metabolism is the main factor affecting cellular energy metabolism. The “unification hypothesis”226 suggests that several seemingly independent biochemical pathways that are overactivated in diabetes are actually caused by excessive intracellular glucose flux (Fig. 8). EC are extensively damaged in DPDs, and EC produce ATP mainly by glycolysis.370 In hyperglycemic states, changes in the metabolic pathways of sugar (increased flux of the hexosamine pathway, increased flux of the polyol pathway, decreased flux of pentose phosphate and glycolytic pathways) lead to decreased production of NADPH and increased ROS, exacerbating oxidative stress.371 In the pentose phosphate pathway, glucokinase/hexokinase is involved, and this enzyme also regulates glucose transport into cells, as well as glycogen metabolism and gluconeogenesis. Activation of glucokinases (Dorzagliatin, PB-201, AZD-1656, etc.) has a regulatory effect on glucose homeostasis,372–377 but no additional vascular protective value has been reported yet.378,379
Fig. 8.
Schematic diagram of cellar sugar metabolic pathways. Cells obtain energy through multiple gluconeogenic pathways. These include glycolysis, polyol, hexosamine, and pentose phosphate pathways. In the diabetic environment, excessive intracellular glucose causes abnormal activation of the polyol and hexosamine pathways, and inhibition of the major glycolytic and pentose phosphate pathways, resulting in continued accumulation of reactive oxygen species, which ultimately leads to increased oxidative stress loss in cells and induces the development of DPDs. Different metabolic pathways are distinguished by different colors, with pink representing the glycolytic pathway, purple representing the polyol pathway, blue representing the pentose phosphate pathway, and yellow representing the hexosamine pathway. All enzymes are indicated in red. Solid arrows indicate that this process is promoted and dashed arrows indicate that this process is inhibited. IL interleukin, GSH glutathione, GSH-px glutathione peroxidase, AR aldose reductase, SDH sorbitol dehydrogenase, 3DG 3-deoxyglucosone, AGE advanced glycosylation end, NOX reduced nicotinamide adenine dinucleotide phosphate oxidase, ROS reactive oxygen species, NLRP3 NOD-like receptor thermal protein domain associated protein 3, GK glucokinase, G6P glucose-6-phosphate, F6P d-fructose-6-phosphate disodium salt hydrate, F1,6P2 fructose 1,6-bisphosphate, G3P glyceraldehyde 3-phosphate, 1,3BPG 1,3-bisphosphoglycerate, G6PDH glucose-6-phosphate dehydrogenase, 6PGL 6-phosphogluconolactonase, 6PGDH 6-phosphogluconate dehydrogenase, GFAT glutamine-fructose-6-phosphate aminotransferase, TCA tricarboxylic acid cycle
Hyperglycemia reduces glucose-6-phosphate dehydrogenase (G6PDH)-mediated entry of glucose into the pentose phosphate pathway, but rather into the polyol pathway through conversion to sorbitol by the rate-limiting enzyme AR, and these processes are accompanied by a decrease in the rate of NADPH (an important intracellular reducing agent) production.380 Meanwhile, high glucose induces activation of NADPH oxidase (NOX) to produce ROS, increases oxidative stress levels, and promotes NLRP3/IL-1β and IL-18 to increase inflammation levels.381 Diabetes promotes the accumulation of fructose-6-phosphate (F6P), which can lead to an increase in the hexosamine (HBP) pathway and overproduction of UDPn-acetylglucosamine.382 UDPn-acetylglucosamine is involved in intracellular protein regulation, especially for post-translational modifications of proteins such as O-GlcNAcylation.383 The HBP pathway accounts for a small percentage of glucose metabolism and does not affect tissue energy supply, but regulates glucose transporter protein and insulin signal transduction, regulates glycogen synthesis and elevates cellular O-glycosylation levels, stimulates cytokines, etc.
The sorbitol/polyol pathway is similarly increased in the high glucose state, where sorbitol is, in turn, converted to fructose by sorbitol dehydrogenase (SDH), resulting in the production of 3-deoxyglucosone (3DG), a highly reactive α-oxo-aldehyde, and promoting the production of AGEs.384 AR is also a key enzyme in this pathway. AR promotes the conversion of glucose to sorbitol and further to fructose with the participation of NADPH (produced by the pentose phosphate pathway). In the hyperglycemic state, AR depletes NADPH while increasing the accumulation of F6P, so there is a close influence between several glucose metabolic pathways. Not only that, AR decreases glutathione levels and glutathione peroxidase activity, and reduces cellular antioxidant capacity through amino acid metabolism. Elevated intracellular sorbitol also provides excess nicotinamide adenine dinucleotide (NADH) to the mitochondrial electron transport chain, which is a substrate for complex I (substrate for complex I) in mitochondria and closely affects mitochondrial function and cellular energy metabolism.385 Overexpression of AR in mice increases susceptibility to diabetes-induced AS and ischemia/reperfusion injury.386,387 In contrast, aldose reductase inhibitors (ARIs) and AR gene-deficient animals have vascular protective effects in DPDs.83,388 Some natural compounds and plant extracts have shown the inhibitory effect of aldose reductase 2 (ALR2), which can improve inflammation and protect the vascular endothelium, but in recent years ARIs have not been successfully available for widespread clinical use.389
Amino acid metabolism
Amino acid metabolism plays an important role in diabetes and complications, and branched-chain amino acids may serve as new biomarkers as well as signaling pathways suggesting the risk of DPDs.390,391 Glutamine is a branched-chain amino acid that is synthesized by glutamine synthetase (GS) and hydrolyzed by glutaminase (GLS).392,393 Glutamine levels were negatively associated with BMI and insulin resistance index (HOMA-IR) in men with type 2 diabetes.394 Glutamine in plasma binds the bridging protein GRB-10394 and improves the insulin resistance of cells. It also promotes the secretion of GLP-1 and GLP-2 secretion from intestinal cells.395,396 Vascular EC expresses glutaminase (GLS) and break down glutamine, and glutamine deficiency or inhibition of endothelial GLS1 can cause impaired EC proliferation and reduced vascular neogenesis.397 Increased glutamine synthetase (GS) transcription or increased glutamine levels promote macrophage polarization and atherosclerotic plaque formation.398 Chromosome 1q25 single nucleotide polymorphism (SNP) variants cause reduced GS expression in EC. In these populations, patients with diabetes have an increased risk of CAD but not patients with diabetes, and the causal mechanism remains to be determined.399
Glutamine metabolism in cells holds two branches: glutamine catabolism and asparagine synthetase/gamma-aminobutyric acid (ASNS-GABA) shunting. These two pathways independently regulate the AMP-activated protein kinase/mechanistic target of the rapamycin kinase complex (AMPK/mTORC) pathway, mediating cellular autophagy.400,401 Also, glutamine provides anaplerotic substrates for the TCA cycle.397,402 In EC, 30% of the tricarboxylic acid carbon comes from glutamine, comparable to that produced by the glycolytic pathway, and the novel hypoglycemic agent SGLT-2i can regulate mitochondrial oxidative phosphorylation and improve cellular energy metabolism through the above pathway.403 Glutamine metabolites are involved in intracellular oxygen reduction regulation. EC produces glutathione via glutamine, which regulates redox homeostasis, and its depletion makes EC vulnerable to ROS-induced damage. Glutamine catabolism produces glutamate that is converted to ornithine404 and aspartate397 and these generated amino acids are also involved in the proliferation of EC.405 EC proliferation is reduced after silencing of asparagine synthetase (ASNS), and supplementation with asparagine and α-ketoglutarate reverses the EC damage caused by glutamine deficiency.397,401 Thus, asparagine is also an important part of amino acid metabolism in DPDs.406
Amino acid metabolism also affects oxidative stress in EC via the arginine metabolic pathway.407 Vascular protective NO is produced via endothelial-type nitric oxide synthase (eNOS).408,409 In vitro experiments, arginine deficiency leads to EC eNOS dysfunction.410 Elevated arginase levels lead to L-arginine depletion, decreased output of NO, increased ROS, and impaired endothelium-dependent vasodilation.411 On the other hand, EC can absorb serine directly or produce serine in the reaction intermediate 3-phosphoglycerate of the glycolytic pathway412 for nucleotide biosynthesis and redox homeostasis.413 The serine pathway is activated in the high glucose state and it synergizes with the pentose phosphate pathway with single carbon metabolism to alter chromatin status and promote inflammation.414
Fatty acid metabolism
Diabetic patients often have abnormal lipid metabolism, and hyperlipidemia can lead to increased cellular uptake of fatty acids through passive diffusion and protein-mediated pathways. A cluster of differentiation 36 (CD36) and the fatty acid binding protein (FABP) family mediate fatty acid uptake into tissues,415 and soluble CD36 expression is increased in diabetic patients.416 Fatty acid uptake and transport by EC is extremely important for many cellular processes, including membrane synthesis, intracellular signaling, ATP production, and post-translational modification of proteins.417 Imbalance of fatty acid metabolism in EC does not lead to significant abnormalities in energy supply or disturbance of redox homeostasis, but can impair de novo nucleotide synthesis for DNA replication.418 For example, DNA repair factors such as Polyadp-ribose polymerase (PARP) are involved in fatty acid metabolism.419 In a high glucose state, vascular damage can be aggravated by PARP1.420
Fatty acid metabolism plays an important role in high-energy-demand cells and is closely associated with diabetic heart disease in DPDs.421,422 Overexpression of GLUT-1 in the myocardium increased glucose levels in cardiomyocytes and revealed that FAO in the heart was inhibited and that a high fatty acid diet failed to upregulate FAO in these hearts, while glucose supply was significantly increased, further leading to activation of p38 mitogen-activated protein kinase and increased oxidative stress in the heart.423
Mitochondrial dysfunction
An imbalance in energy metabolic pathways causes impaired mitochondrial function, most often manifested as increased mitochondrial autophagy and ROS production.424 Mitochondrial function plays an important role in cellular energy homeostasis. Alterations in glycolytic pathways, fatty acid oxidation, and some amino acid metabolism in the high glucose state can affect mitochondrial oxidative phosphorylation processes. AGEs production in the hyperglycemic state and AGE-RAGE-induced increase in cytoplasmic ROS promote the production of mitochondrial superoxide and the development of diabetic microangiopathy in the condition of hyperglycemia.425,426 Most ROS are derived from complexes I and III in mitochondria.427 In addition to complexes I and III, the NOX family also promotes the mitochondrial production of ROS.428,429 NOX4 is the highest expressed member of the NOX family and is upregulated by a variety of agonizts and cellular stress.429,430 Administration of novel mitochondria-targeted drugs helps to improve the mitochondrial ROS/NLRP3 axis and attenuate mesangial tubular injury in DKD;431 similar manifestations exist in the myocardium, attenuating diabetic myocardial ischemia-reperfusion injury68. The intramitochondrial protein p66Shc can promote increased reactive oxygen species in mitochondria by interfering with Ras activation or binding to cytochrome C and other.432
Mitochondria can store calcium ions and act in concert with the endoplasmic reticulum and extracellular matrix to control the dynamic balance of calcium ion concentration in cells and regulate the cell cycle and apoptosis.433 High glucose can affect myocardial contractile function by upregulating sarcolipin (SLN) promotes calcium sparks.434 Therapeutically, metformin inhibits mitochondrial respiratory chain complex-1 and regulates cellular energy metabolism.435 In addition, metformin and GLP-1 agonizts regulate the glucagon-lowering hormone glucagon-like peptide-1 and the bile acid pathway and alter the composition of the gut microbiota, which may also indirectly affect mitochondrial function.362,436
Mitochondrial energy metabolism differs among target organs, and myocardial mitochondrial content is abundant. Diabetic mice have altered mitochondrial function in the myocardium earlier than in the kidney, brain, and liver.437 Further in-depth mechanistic exploration is worthwhile in tissues with high mitochondrial content.
Insulin resistance
Insulin resistance is the most common and widespread molecular mechanism of diabetic complications, not only in the high glucose state with extensive activation of insulin receptor signaling pathways, such as the regulation of glucose uptake by GLUT.438 Even in the presence of normal blood glucose, insulin resistance is still harmful, and the lack of insulin receptor signaling pathways in renal pedal cells induces a disease state similar to diabetic nephropathy.439
The most common downstream mechanisms of insulin resistance include inhibitory phosphorylation of IRS, growth factor receptor binding protein-2 (GRB-2), GRB-10, SHC transforming protein (SHC), and SH2B adapter protein-2 (SH2B-2) through induced insulin receptor.439,440 Selective glucose transporters exist in different target organs, such as the renal SGLT2 receptor;441 the distribution of GLUT receptors in different target organs and the pathways also differ.78,442 This paper summarizes the new advances in insulin signaling receptor pathways and their roles from 2018 to date (Table 4).
Table 4.
List of novel targets with emerging implications related to insulin receptor signaling
| Insulin receptor signaling | Target tissue | Effect/ potential role | Reference |
|---|---|---|---|
| HCF-1 | Hepatocyte (human, mice) | HCF-1-dependent pathway regulating Glucose and lipid metabolism | 516 |
| IRS/PTP1B | Cerebral microvascular endothelial cells (mice) | Hyperinsulinemia affects insulin receptor signaling and internalization of endothelial cells | 517 |
| SMPDL3b/C1P/Cav-1 | kidney cortexes (rats), podocytes (human) | Inducing glucose and lipid metabolism, protein synthesis | 518 |
| IRS/IGF1/SRF | Myocardium (mice) | Affecting autophagy and apoptosis of cardiomyocytes | 519 |
| SDF-1/CXCR4 | Primary endothelial cells (human), CD31 + cells (mice) | Affecting immune cell recruitment to the vascular wall or tissue parenchyma | 520 |
| miR-15b, miR-16, miR-30b/IRS2 | Endothelial cells (human) | IRS2 and eNOS in ECs are ceRNAs and related to the Akt signal pathway | 521 |
| IRS | Renal hemodynamics (mice) | stimulation of renal functions and renal hemodynamics | 522 |
| IRS/p53/KLF4 | Vascular smooth muscle cells (mice) | IRS-1/p53 affects the progression of atherosclerotic lesions in hyperglycemia | 523 |
| QKI-7 | Endothelial cells (human) | Promotes mRNA degradation of essential genes for EC function | 524 |
| EPDR1 | Endothelial cells (mice) | Mediate pathological angiogenesis during hyperinsulinemia and insulin resistance | 525 |
| IRS-1 rs956115 genotypes | (Human) | IRS-1 CG/GG genotype are at higher risk of major adverse cardiovascular events | 526 |
| Therapeutic effect | |||
| Amlexanox inhibition of TBK1/IKKe | Serum (human) | Lowering HBA1c, fructosamine, ameliorates metabolic dysfunctions | 527 |
| Loganin inhibition of JNK-IRS-1/Akt/GSK3β | Peripheral nerve (rats), SH-SY5Y cell (human) | Inhibiting oxidative stress-provoked inflammation, improved Nerve pain behaviors | 528 |
| SGLT-2i inhibition of JunD/PPAR-γ, IRS-1, IRS2 | Endocardiomyocytes (human) | Inhibiting JunD/PPAR-γ overexpression and lipid accumulation, ameliorate diabetic cardiomyopathy | 529 |
C1P ceramide-1-phosphate, IRS insulin receptor substrate, PTP1B protein tyrosine phosphatase, non-receptor type 1, SMPDL3b sphingomyelin phosphodiesterase acid-like 3b, Cav-1 caveolin-1, IGF1 insulin-like growth factor 1, SRF serum response factor, SDF-1 stromal cell-derived factor-1, CXCR4 CXC receptor 4, CD31 endothelial cell adhesion molecule-1, eNOS endothelial nitric oxide synthase, ECs endothelial cells, ceRNAs competing endogenous RNAs, Akt protein kinase B, KLF4 Krüppel-like factor 4, QKI-7 quaking-7, EPDR1 ependymin-related protein-1, TBK1 TANK binding kinase 1, IKKe IkappaB kinase, HBA1c glycated hemoglobin, JNK c-Jun N-terminal kinase, GSK3β glycogen synthase kinase-3β, JunD jund proto-oncogene subunit
Glycosylation end-products
As the end-products of dysfunctional EC metabolism, AGEs have profound effects on the immediate extracellular environment of EC as well as on other cell types. AGEs can bind key proteins (such as laminin, elastin, and collagen) in the extracellular matrix (ECM) basement membrane, leading to increased vascular stiffness promoting DPDs.443–445 AGEs may also affect coagulation and hemodynamics, cause increased vascular permeability and induce tissue factor expression.446,447 The extensive intracellular action of circulating AGEs is mediated through the attachment of receptors for RAGE, which is expressed in monocytes, smooth muscle cells (SMC), and EC.448,449
RAGE induces an inflammatory cascade response by activating the transcription factor NF-κB, which promotes the expression of growth factors and adhesion molecules. On the other hand, RAGE activates NADPH oxidase to increase oxidative stress; RAGE also binds to tissue-specific proteins to promote local vascular injury,450,451 such as binding AGEs or S100/calmodulin or β-amyloid peptide to exacerbate cerebral hemorrhage;452 RAGE binds to EC to promote increased NADPH expression leading to inflammatory responses and a prothrombotic state by activating diaphanous-related 1 (DIAPH 1), ERK1, ERK2, and PKC.451,453 AGE/RAGE on mononuclear macrophages increases CD36 expression, promotes OX-LDL uptake while decreasing high-density lipoprotein (HDL) efflux, and promotes foam cell formation; AGE/RAGE action on vascular smooth muscle cells (VSMC) induces autophagy through the ERK/Akt pathway454 and increases ROS and NOS levels through activation of NOX and NF-κB,455,456 increases oxidative stress, and accelerates atherosclerotic plaque progression.447 Excessive AGE formation and overactivation of the hexosamine pathway induce angiopoietin-2 transcription by inhibiting transcriptional co-repressor complex binding and silencing the angiopoietin-2 promoter.457 The formation of AGEs may partially explain the “hyperglycemic memory” of tissue damage, i.e., the vascular damage that occurs during hyperglycemia can continue into the normoglycemic cycle.458 Hyperglycemia may alter cellular epigenetics, such as DNA and protein modifications, DNA methylation, non-coding RNA, or histone modifications by receptor-mediated mechanisms to alter cellular function.459 Thus, epigenetics, as well as AGEs, are important targets for intervention outside of glycemic control.
Prospects
DPD has become a major public health problem that requires urgent attention, as 11% of patients with T2DM complicated with AS have a multivessel disease.460 Patients with T2DM complicated with the multivessel disease have a significantly higher risk of ischemic events and overall mortality than those with T2DM with single-vessel disease. The significant cardio-renal benefits of metformin and SGLT-2i beyond glycemic control have led to the emergence of an integrated treatment model that can be applied to manage DPD.461 The pathogenesis of DPD involves insulin resistance, inflammation, oxidative stress, and AGEs, with a wide prevalence of hyperglycemic “metabolic memory” and some epigenetic alterations in various vascular pathologies.462,463 The latest drugs in development are ARIs, tyrosine phosphatase 1B inhibitors, PPAR-γ agonizts, regulators of the glucagon system, and mitochondrial energy modulators. Antisense oligonucleotides and monoclonal antibodies are widely developed.464,465 New biomarkers and drug intervention targets to improve the prognosis of DPD hold enormous potential for clinical applications.
Diabetic microangiopathy and macroangiopathy significantly differ, and simultaneously developing them in animal models is challenging. However, clinical practice has often found coexisting interactive macroangiopathy and microangiopathy.303 The predictive relationship among DPDs also suggests that diabetic microangiopathy is not the only indicator of the risk for macroangiopathy, and that the various types of diabetic microangiopathy are also closely interrelated.460 In recent years, several molecular pathways with simultaneously large/microvascular protective effects have been identified (PPAR-γ, CXCR4, etc.), but most of them are still in animal experiments or phase I clinical stage.
DPD research also faces several challenges. Concepts should be integrated across disciplines that focus on specific aspects of DM and angiopathy management in different organs. Research results of new technology might be redundant. For example, miRNA-based studies of diagnosis or regulation have found that miR-21 plays an important role in diabetic vascular complications;211,273,466–470 however, the role of miR-21 in organs and at various points during disease progression remains unknown because concepts across disciplines have not been fully integrated. New technologies such as induced pluripotent stem cells should be developed to enrich experimental findings of DPD and construct model human blood vessel organoids.129 Multi-omic studies471,472 should explore the pathogenesis of DPD. Furthermore, new cross-disciplinary, integrated prevention and treatment models should also be developed. Cross-collaborations among medical and research institutions and academic organizations are needed to narrow gaps among research results, new technologies, and clinical applications. Cross-collaborations among medical institutions at all levels are needed to fully integrate the resources of primary- and higher-level care. Cross-collaborations among doctors, nursing staff, and patients are needed to ensure personalized treatment for patients. Cross-collaborations among clinical disciplines, including endocrinology, cardiovasology, geriatrics, neurology, nephrology, vascular surgery, and nutrition are also needed to integrate comprehensive evidence. Traditional Chinese medicine also has considerable potential for the prevention and treatment of DPD.473,474 A comprehensive DPD prevention and treatment system should be established with Chinese characteristics, promoted cooperation, and accelerated clinical translation to improve the overall prevention and control of chronic diseases such as diabetes.
Acknowledgements
This work was supported by the National Outstanding Youth Natural Science Foundation of China (82022076) and the Young Qihuang Scholar of the “Tens of millions” talent project of China. All figures were created with Biorender.com.
Author contributions
Y.L., L.H., and K.C. designed the manuscript. Y.W.L., Y.F.L., and S.L. did the literature search, wrote the manuscript, and drafted figures. M.G., W.W., and Y.W.L. revised the manuscript. All authors listed have made a substantial contribution to the work. All authors have read and approved the article.
Competing interests
The authors report no commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
These authors contributed equally: Yiwen Li, Yanfei Liu, Shiwei Liu
Contributor Information
Keji Chen, Email: kjchenvip@163.com.
Luqi Huang, Email: Huangluqi01@126.com.
Yue Liu, Email: liuyueheart@hotmail.com.
References
- 1.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pr. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 2.Harding JL, Pavkov ME, Magliano DJ, Shaw JE, Gregg EW. Global trends in diabetes complications: a review of current evidence. Diabetologia. 2019;62:3–16. doi: 10.1007/s00125-018-4711-2. [DOI] [PubMed] [Google Scholar]
- 3.Jia W, et al. Standards of medical care for type 2 diabetes in China 2019. Diabetes Metab. Res. Rev. 2019;35:e3158. doi: 10.1002/dmrr.3158. [DOI] [PubMed] [Google Scholar]
- 4.Bruno G, et al. Incidence of type 1 and type 2 diabetes in adults aged 30-49 years: the population-based registry in the province of Turin, Italy. Diabetes Care. 2005;28:2613–2619. doi: 10.2337/diacare.28.11.2613. [DOI] [PubMed] [Google Scholar]
- 5.Avogaro A, Fadini GP. Microvascular complications in diabetes: a growing concern for cardiologists. Int J. Cardiol. 2019;291:29–35. doi: 10.1016/j.ijcard.2019.02.030. [DOI] [PubMed] [Google Scholar]
- 6.Catrina SB, Zheng X. Hypoxia and hypoxia-inducible factors in diabetes and its complications. Diabetologia. 2021;64:709–716. doi: 10.1007/s00125-021-05380-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Demir, S., Nawroth, P. P. & Herzig, S. & Ekim Üstünel, B. Emerging targets in type 2 diabetes and diabetic complications. Adv. Sci. 8, e2100275 (2021). [DOI] [PMC free article] [PubMed]
- 8.Kaplovitch E, et al. Rivaroxaban and aspirin in patients with symptomatic lower extremity peripheral artery disease: a subanalysis of the COMPASS randomized clinical trial. JAMA Cardiol. 2021;6:21–29. doi: 10.1001/jamacardio.2020.4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou, X., Yu, L., Zhao, Y. & Ge, J. Panvascular medicine: an emerging discipline focusing on atherosclerotic diseases. Eur. Heart. J. 43, 4528–4531 (2022). [DOI] [PubMed]
- 10.Dal Canto E, et al. Diabetes as a cardiovascular risk factor: an overview of global trends of macro and micro vascular complications. Eur. J. Prev. Cardiol. 2019;26:25–32. doi: 10.1177/2047487319878371. [DOI] [PubMed] [Google Scholar]
- 11.Sandoval-Garcia E, et al. Retinal arteriolar tortuosity and fractal dimension are associated with long-term cardiovascular outcomes in people with type 2 diabetes. Diabetologia. 2021;64:2215–2227. doi: 10.1007/s00125-021-05499-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Braunwald E. Diabetes, heart failure, and renal dysfunction: the vicious circles. Prog. Cardiovasc. Dis. 2019;62:298–302. doi: 10.1016/j.pcad.2019.07.003. [DOI] [PubMed] [Google Scholar]
- 13.Kotte AN, van Leeuwen GM, Lagendijk JJ. Modelling the thermal impact of a discrete vessel tree. Phys. Med. Biol. 1999;44:57–74. doi: 10.1088/0031-9155/44/1/006. [DOI] [PubMed] [Google Scholar]
- 14.van Kuijk JP, et al. Long-term prognosis of patients with peripheral arterial disease with or without polyvascular atherosclerotic disease. Eur. Heart J. 2010;31:992–999. doi: 10.1093/eurheartj/ehp553. [DOI] [PubMed] [Google Scholar]
- 15.Jacob M, Chappell D, Becker BF. Regulation of blood flow and volume exchange across the microcirculation. Crit. Care. 2016;20:319. doi: 10.1186/s13054-016-1485-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freeman K, Tao W, Sun H, Soonpaa MH, Rubart M. In situ three-dimensional reconstruction of mouse heart sympathetic innervation by two-photon excitation fluorescence imaging. J. Neurosci. Methods. 2014;221:48–61. doi: 10.1016/j.jneumeth.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Polovina M, et al. Type 2 diabetes increases the long-term risk of heart failure and mortality in patients with atrial fibrillation. Eur. J. Heart Fail. 2020;22:113–125. doi: 10.1002/ejhf.1666. [DOI] [PubMed] [Google Scholar]
- 18.Kozakova M, Morizzo C, Fraser AG, Palombo C. Impact of glycemic control on aortic stiffness, left ventricular mass and diastolic longitudinal function in type 2 diabetes mellitus. Cardiovasc. Diabetol. 2017;16:78. doi: 10.1186/s12933-017-0557-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grubić Rotkvić P, et al. The mystery of diabetic cardiomyopathy: from early concepts and underlying mechanisms to novel therapeutic possibilities. Int. J. Mol. Sci. 2021;22:5973. doi: 10.3390/ijms22115973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jia G, Whaley-Connell A, Sowers JR. Diabetic cardiomyopathy: a hyperglycaemia- and insulin-resistance-induced heart disease. Diabetologia. 2018;61:21–28. doi: 10.1007/s00125-017-4390-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu L, Yan X, Tang Z, Feng B. Association between circulating oxidized OxLDL/LDL-C ratio and the severity of coronary atherosclerosis, along with other emerging biomarkers of cardiovascular disease in patients with type 2 diabetes. Diabetes Res. Clin. Pract. 2022;191:110040. doi: 10.1016/j.diabres.2022.110040. [DOI] [PubMed] [Google Scholar]
- 22.Gordin D, et al. Osteopontin is a strong predictor of incipient diabetic nephropathy, cardiovascular disease, and all-cause mortality in patients with type 1 diabetes. Diabetes Care. 2014;37:2593–2600. doi: 10.2337/dc14-0065. [DOI] [PubMed] [Google Scholar]
- 23.Platt DE, et al. Type II diabetes mellitus and hyperhomocysteinemia: a complex interaction. Diabetol. Metab. Syndr. 2017;9:19. doi: 10.1186/s13098-017-0218-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu T, et al. Plasma fingerprint of free fatty acids and their correlations with the traditional cardiac biomarkers in patients with type 2 diabetes complicated by coronary heart disease. Front. Cardiovasc. Med. 2022;9:903412. doi: 10.3389/fcvm.2022.903412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kobiyama K, Ley K. Atherosclerosis. Circ. Res. 2018;123:1118–1120. doi: 10.1161/CIRCRESAHA.118.313816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kimura T, et al. Regulatory CD4(+) T cells recognize major histocompatibility complex class II molecule-restricted peptide epitopes of apolipoprotein B. Circulation. 2018;138:1130–1143. doi: 10.1161/CIRCULATIONAHA.117.031420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sherer Y, et al. Early atherosclerosis and autoantibodies to heat-shock proteins and oxidized LDL in systemic sclerosis. Ann. N. Y Acad. Sci. 2007;1108:259–267. doi: 10.1196/annals.1422.028. [DOI] [PubMed] [Google Scholar]
- 28.Govea-Alonso DO, Beltrán-López J, Salazar-González JA, Vargas-Morales J, Rosales-Mendoza S. Progress and future opportunities in the development of vaccines against atherosclerosis. Expert Rev. Vaccines. 2017;16:337–350. doi: 10.1080/14760584.2017.1258309. [DOI] [PubMed] [Google Scholar]
- 29.Kenny HC, Abel ED. Heart failure in type 2 diabetes mellitus. Circ. Res. 2019;124:121–141. doi: 10.1161/CIRCRESAHA.118.311371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jia G, DeMarco VG, Sowers JR. Insulin resistance and hyperinsulinaemia in diabetic cardiomyopathy. Nat. Rev. Endocrinol. 2016;12:144–153. doi: 10.1038/nrendo.2015.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin K-H, et al. Carboxyl terminus of HSP70-interacting protein attenuates advanced glycation end products-induced cardiac injuries by promoting NFκB proteasomal degradation. J. Cell. Physiol. 2022;237:1888–1901. doi: 10.1002/jcp.30660. [DOI] [PubMed] [Google Scholar]
- 32.Su S-C, et al. Cilostazol inhibits hyperglucose-induced vascular smooth muscle cell dysfunction by modulating the RAGE/ERK/NF-κB signaling pathways. J. Biomed. Sci. 2019;26:68. doi: 10.1186/s12929-019-0550-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y, et al. MD2 activation by direct AGE interaction drives inflammatory diabetic cardiomyopathy. Nat. Commun. 2020;11:2148. doi: 10.1038/s41467-020-15978-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang Y-C, et al. Pkcδ activation is involved in ROS-mediated mitochondrial dysfunction and apoptosis in cardiomyocytes exposed to advanced glycation end products (Ages) Aging Dis. 2018;9:647–663. doi: 10.14336/AD.2017.0924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hussain S, et al. Hyperglycemia induces myocardial dysfunction via epigenetic regulation of JunD. Circ. Res. 2020;127:1261–1273. doi: 10.1161/CIRCRESAHA.120.317132. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Q, et al. LncDACH1 promotes mitochondrial oxidative stress of cardiomyocytes by interacting with sirtuin3 and aggravates diabetic cardiomyopathy. Sci. China Life Sci. 2022;65:1198–1212. doi: 10.1007/s11427-021-1982-8. [DOI] [PubMed] [Google Scholar]
- 37.Kizub IV, Pavlova OO, Johnson CD, Soloviev AI, Zholos AV. Rho kinase and protein kinase C involvement in vascular smooth muscle myofilament calcium sensitization in arteries from diabetic rats. Br. J. Pharmacol. 2010;159:1724–1731. doi: 10.1111/j.1476-5381.2010.00666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kizub IV, Klymenko KI, Soloviev AI. Protein kinase C in enhanced vascular tone in diabetes mellitus. Int. J. Cardiol. 2014;174:230–242. doi: 10.1016/j.ijcard.2014.04.117. [DOI] [PubMed] [Google Scholar]
- 39.Li G, et al. Syringaresinol protects against type 1 diabetic cardiomyopathy by alleviating inflammation responses, cardiac fibrosis, and oxidative stress. Mol. Nutr. Food Res. 2020;64:e2000231. doi: 10.1002/mnfr.202000231. [DOI] [PubMed] [Google Scholar]
- 40.García-Díez E, et al. Supplementation with a cocoa-carob blend, alone or in combination with metformin, attenuates diabetic cardiomyopathy, cardiac oxidative stress and inflammation in Zucker diabetic rats. Antioxidants. 2022;11:432. doi: 10.3390/antiox11020432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Song S, et al. Sirtuin 3 deficiency exacerbates diabetic cardiomyopathy via necroptosis enhancement and NLRP3 activation. Acta Pharmacol. Sin. 2021;42:230–241. doi: 10.1038/s41401-020-0490-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rajagopalan S, Brook R. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N. Engl. J. Med. 2017;377:2098–2099. doi: 10.1056/NEJMc1712572. [DOI] [PubMed] [Google Scholar]
- 43.Jorsal A, et al. Effect of liraglutide, a glucagon-like peptide-1 analogue, on left ventricular function in stable chronic heart failure patients with and without diabetes (LIVE)-a multicentre, double-blind, randomised, placebo-controlled trial. Eur. J. Heart Fail. 2017;19:69–77. doi: 10.1002/ejhf.657. [DOI] [PubMed] [Google Scholar]
- 44.Marso SP, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 2016;375:1834–1844. doi: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 45.Scirica BM, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N. Engl. J. Med. 2013;369:1317–1326. doi: 10.1056/NEJMoa1307684. [DOI] [PubMed] [Google Scholar]
- 46.Biessels GJ, Staekenborg S, Brunner E, Brayne C, Scheltens P. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol. 2006;5:64–74. doi: 10.1016/S1474-4422(05)70284-2. [DOI] [PubMed] [Google Scholar]
- 47.Xu WL, von Strauss E, Qiu CX, Winblad B, Fratiglioni L. Uncontrolled diabetes increases the risk of Alzheimer’s disease: a population-based cohort study. Diabetologia. 2009;52:1031–1039. doi: 10.1007/s00125-009-1323-x. [DOI] [PubMed] [Google Scholar]
- 48.Xue M, et al. Diabetes mellitus and risks of cognitive impairment and dementia: a systematic review and meta-analysis of 144 prospective studies. Ageing Res. Rev. 2019;55:100944. doi: 10.1016/j.arr.2019.100944. [DOI] [PubMed] [Google Scholar]
- 49.van Sloten TT, Sedaghat S, Carnethon MR, Launer LJ, Stehouwer CDA. Cerebral microvascular complications of type 2 diabetes: stroke, cognitive dysfunction, and depression. Lancet Diabetes Endocrinol. 2020;8:325–336. doi: 10.1016/S2213-8587(19)30405-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Crane PK, et al. Glucose levels and risk of dementia. N. Engl. J. Med. 2013;369:540–548. doi: 10.1056/NEJMoa1215740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kloppenborg RP, van den Berg E, Kappelle LJ, Biessels GJ. Diabetes and other vascular risk factors for dementia: which factor matters most? A systematic review. Eur. J. Pharm. 2008;585:97–108. doi: 10.1016/j.ejphar.2008.02.049. [DOI] [PubMed] [Google Scholar]
- 52.Jellinger KA. Pathomechanisms of vascular depression in older adults. Int. J. Mol. Sci. 2021;23:308. doi: 10.3390/ijms23010308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cannistraro RJ, et al. CNS small vessel disease: a clinical review. Neurology. 2019;92:1146–1156. doi: 10.1212/WNL.0000000000007654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Masi S, et al. Assessment and pathophysiology of microvascular disease: recent progress and clinical implications. Eur. Heart J. 2021;42:2590–2604. doi: 10.1093/eurheartj/ehaa857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Washida K, Hattori Y, Ihara M. Animal models of chronic cerebral hypoperfusion: from mouse to primate. Int. J. Mol. Sci. 2019;20:6176. doi: 10.3390/ijms20246176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lin MP, et al. Collateral recruitment is impaired by cerebral small vessel disease. Stroke. 2020;51:1404–1410. doi: 10.1161/STROKEAHA.119.027661. [DOI] [PubMed] [Google Scholar]
- 57.Shi L, et al. Mapping the contribution and strategic distribution patterns of neuroimaging features of small vessel disease in poststroke cognitive impairment. J. Neurol. Neurosurg. Psychiatry. 2018;89:918–926. doi: 10.1136/jnnp-2017-317817. [DOI] [PubMed] [Google Scholar]
- 58.Kim GM, et al. Extensive leukoaraiosis is associated with high early risk of recurrence after ischemic stroke. Stroke. 2014;45:479–485. doi: 10.1161/STROKEAHA.113.003004. [DOI] [PubMed] [Google Scholar]
- 59.Wei C, et al. Cerebral small vessel disease combined with cerebral collaterals to predict the prognosis of patients with acute large artery atherosclerotic stroke. Front. Neurol. 2022;13:969637. doi: 10.3389/fneur.2022.969637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou JY, et al. Beyond collaterals: brain frailty additionally improves prediction of clinical outcome in acute ischemic stroke. Eur. Radio. 2022;32:6943–6952. doi: 10.1007/s00330-022-08792-6. [DOI] [PubMed] [Google Scholar]
- 61.Ryu WS, et al. Stroke outcomes are worse with larger leukoaraiosis volumes. Brain. 2017;140:158–170. doi: 10.1093/brain/aww259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tsukuda K, et al. Diabetes-associated cognitive impairment is improved by a calcium channel blocker, nifedipine. Hypertension. 2008;51:528–533. doi: 10.1161/HYPERTENSIONAHA.107.101634. [DOI] [PubMed] [Google Scholar]
- 63.Khandelwal M, Manglani K, Upadhyay P, Azad M, Gupta S. AdipoRon induces AMPK activation and ameliorates Alzheimer’s like pathologies and associated cognitive impairment in APP/PS1 mice. Neurobiol. Dis. 2022;174:105876. doi: 10.1016/j.nbd.2022.105876. [DOI] [PubMed] [Google Scholar]
- 64.Prabhakar NR, Peng YJ, Nanduri J. Hypoxia-inducible factors and obstructive sleep apnea. J. Clin. Invest. 2020;130:5042–5051. doi: 10.1172/JCI137560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Reijmer YD, Leemans A, Brundel M, Kappelle LJ, Biessels GJ. Disruption of the cerebral white matter network is related to slowing of information processing speed in patients with type 2 diabetes. Diabetes. 2013;62:2112–2115. doi: 10.2337/db12-1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang J, et al. Disrupted white matter network and cognitive decline in type 2 diabetes patients. J. Alzheimers Dis. 2016;53:185–195. doi: 10.3233/JAD-160111. [DOI] [PubMed] [Google Scholar]
- 67.Chau ACM, et al. Impaired cerebral blood flow in type 2 diabetes mellitus - A comparative study with subjective cognitive decline, vascular dementia and Alzheimer’s disease subjects. Neuroimage Clin. 2020;27:102302. doi: 10.1016/j.nicl.2020.102302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Iadecola C. The neurovascular unit coming of age: a journey through neurovascular coupling in health and disease. Neuron. 2017;96:17–42. doi: 10.1016/j.neuron.2017.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Blevins BL, et al. Brain arteriolosclerosis. Acta Neuropathol. 2021;141:1–24. doi: 10.1007/s00401-020-02235-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang J, et al. White matter integrity disruptions associated with cognitive impairments in type 2 diabetic patients. Diabetes. 2014;63:3596–3605. doi: 10.2337/db14-0342. [DOI] [PubMed] [Google Scholar]
- 71.Zhou X, et al. Aggravated cognitive and brain functional impairment in mild cognitive impairment patients with type 2 diabetes: a resting-state functional MRI study. J. Alzheimers Dis. 2014;41:925–935. doi: 10.3233/JAD-132354. [DOI] [PubMed] [Google Scholar]
- 72.Gurol ME. Atrial fibrillation and FLAIR/T2 white matter hyperintensities on MRI. J. Neurol. Neurosurg. Psychiatry. 2018;89:1–2. doi: 10.1136/jnnp-2017-316290. [DOI] [PubMed] [Google Scholar]
- 73.Villano A, et al. Endothelial dysfunction and cardiovascular outcome in asymptomatic patients with type 2 diabetes: a pilot study. Diabetes Metab. Res. Rev. 2020;36:e3215. doi: 10.1002/dmrr.3215. [DOI] [PubMed] [Google Scholar]
- 74.Rom S, et al. Hyperglycemia-driven neuroinflammation compromises BBB leading to memory loss in both diabetes mellitus (DM) type 1 and type 2 mouse models. Mol. Neurobiol. 2019;56:1883–1896. doi: 10.1007/s12035-018-1195-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yu H, et al. Platelet biomarkers identifying mild cognitive impairment in type 2 diabetes patients. Aging Cell. 2021;20:e13469. doi: 10.1111/acel.13469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bogush M, Heldt NA, Persidsky Y. Blood brain barrier injury in diabetes: unrecognized effects on brain and cognition. J. Neuroimmune Pharm. 2017;12:593–601. doi: 10.1007/s11481-017-9752-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wing RR, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N. Engl. J. Med. 2013;369:145–154. doi: 10.1056/NEJMoa1212914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Banks WA, Owen JB, Erickson MA. Insulin in the brain: there and back again. Pharm. Ther. 2012;136:82–93. doi: 10.1016/j.pharmthera.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fernandez AM, et al. Insulin regulates neurovascular coupling through astrocytes. Proc. Natl Acad. Sci. USA. 2022;119:e2204527119. doi: 10.1073/pnas.2204527119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pan W, Kastin AJ. Interactions of IGF-1 with the blood-brain barrier in vivo and in situ. Neuroendocrinology. 2000;72:171–178. doi: 10.1159/000054584. [DOI] [PubMed] [Google Scholar]
- 81.Geijselaers SLC, Sep SJS, Stehouwer CDA, Biessels GJ. Glucose regulation, cognition, and brain MRI in type 2 diabetes: a systematic review. Lancet Diabetes Endocrinol. 2015;3:75–89. doi: 10.1016/S2213-8587(14)70148-2. [DOI] [PubMed] [Google Scholar]
- 82.Exalto LG, et al. Risk score for prediction of 10 year dementia risk in individuals with type 2 diabetes: a cohort study. Lancet Diabetes Endocrinol. 2013;1:183–190. doi: 10.1016/S2213-8587(13)70048-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Huang YK, et al. The role of aldose reductase in beta-amyloid-induced microglia activation. Int. J. Mol. Sci. 2022;23:15088. doi: 10.3390/ijms232315088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Baum P, Toyka KV, Blüher M, Kosacka J, Nowicki M. Inflammatory mechanisms in the pathophysiology of diabetic peripheral neuropathy (DN)-new aspects. Int. J. Mol. Sci. 2021;22:10835. doi: 10.3390/ijms221910835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cantini G, Mannucci E, Luconi M. Perspectives in GLP-1 research: new targets, new receptors. Trends Endocrinol. Metab. 2016;27:427–438. doi: 10.1016/j.tem.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 86.Talbot K, et al. Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J. Clin. Invest. 2012;122:1316–1338. doi: 10.1172/JCI59903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Haeusler RA, McGraw TE, Accili D. Biochemical and cellular properties of insulin receptor signalling. Nat. Rev. Mol. Cell Biol. 2018;19:31–44. doi: 10.1038/nrm.2017.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cui Y, et al. Melatonin prevents diabetes-associated cognitive dysfunction from microglia-mediated neuroinflammation by activating autophagy via TLR4/Akt/mTOR pathway. FASEB J. 2021;35:e21485. doi: 10.1096/fj.202002247RR. [DOI] [PubMed] [Google Scholar]
- 89.Gu HF, et al. Nicotinate-curcumin ameliorates cognitive impairment in diabetic rats by rescuing autophagic flux in CA1 hippocampus. CNS Neurosci. Ther. 2019;25:430–441. doi: 10.1111/cns.13059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mohamed MAE, Abdel-Rahman RF, Mahmoud SS, Khattab MM, Safar MM. Metformin and trimetazidine ameliorate diabetes-induced cognitive impediment in status epileptic rats. Epilepsy Behav. 2020;104:106893. doi: 10.1016/j.yebeh.2019.106893. [DOI] [PubMed] [Google Scholar]
- 91.Rizzo MR, et al. Cognitive impairment and type 2 diabetes mellitus: focus of SGLT2 inhibitors treatment. Pharm. Res. 2022;176:106062. doi: 10.1016/j.phrs.2022.106062. [DOI] [PubMed] [Google Scholar]
- 92.Tian J, et al. Ginkgo biloba leaf extract attenuates atherosclerosis in streptozotocin-induced diabetic ApoE-/- mice by inhibiting endoplasmic reticulum stress via restoration of autophagy through the mTOR signaling pathway. Oxid. Med. Cell Longev. 2019;2019:8134678. doi: 10.1155/2019/8134678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kong FJ, et al. Endoplasmic reticulum stress/autophagy pathway is involved in diabetes-induced neuronal apoptosis and cognitive decline in mice. Clin. Sci. 2018;132:111–125. doi: 10.1042/CS20171432. [DOI] [PubMed] [Google Scholar]
- 94.Liu Y, Weng W, Gao R, Liu Y. New insights for cellular and molecular mechanisms of aging and aging-related diseases: herbal medicine as potential therapeutic approach. Oxid. Med. Cell Longev. 2019;2019:4598167. doi: 10.1155/2019/4598167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Natunen T, et al. Diabetic phenotype in mouse and humans reduces the number of microglia around β-amyloid plaques. Mol. Neurodegener. 2020;15:66. doi: 10.1186/s13024-020-00415-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tian J, et al. Interplay between exosomes and autophagy in cardiovascular diseases: novel promising target for diagnostic and therapeutic application. Aging Dis. 2019;10:1302–1310. doi: 10.14336/AD.2018.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dybjer E, et al. Incretin hormones, insulin, glucagon and advanced glycation end products in relation to cognitive function in older people with and without diabetes, a population-based study. Diabet. Med. 2020;37:1157–1166. doi: 10.1111/dme.14267. [DOI] [PubMed] [Google Scholar]
- 98.Lotan R, et al. Design and feasibility of a randomized controlled pilot trial to reduce exposure and cognitive risk associated with advanced glycation end products in older adults with type 2 diabetes. Front. Nutr. 2021;8:614149. doi: 10.3389/fnut.2021.614149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rom S, et al. Hyperglycemia and advanced glycation end products disrupt BBB and promote occludin and claudin-5 protein secretion on extracellular microvesicles. Sci. Rep. 2020;10:7274. doi: 10.1038/s41598-020-64349-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lee MMY, et al. Effect of empagliflozin on left ventricular volumes in patients with type 2 diabetes, or prediabetes, and heart failure with reduced ejection fraction (SUGAR-DM-HF) Circulation. 2021;143:516–525. doi: 10.1161/CIRCULATIONAHA.120.052186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Toyama K, et al. Apoptosis signal-regulating kinase 1 is a novel target molecule for cognitive impairment induced by chronic cerebral hypoperfusion. Arterioscler. Thromb. Vasc. Biol. 2014;34:616–625. doi: 10.1161/ATVBAHA.113.302440. [DOI] [PubMed] [Google Scholar]
- 102.Tang SS, et al. ERα and/or ERβ activation ameliorates cognitive impairment, neurogenesis and apoptosis in type 2 diabetes mellitus mice. Exp. Neurol. 2019;311:33–43. doi: 10.1016/j.expneurol.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 103.Wei TH, Hsieh CL. Effect of acupuncture on the p38 signaling pathway in several nervous system diseases: a systematic review. Int. J. Mol. Sci. 2020;21:4693. doi: 10.3390/ijms21134693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang J, et al. TREM-2-p38 MAPK signaling regulates neuroinflammation during chronic cerebral hypoperfusion combined with diabetes mellitus. J. Neuroinflammation. 2020;17:2. doi: 10.1186/s12974-019-1688-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zeeshan HM, Lee GH, Kim HR, Chae HJ. Endoplasmic reticulum stress and associated ROS. Int. J. Mol. Sci. 2016;17:327. doi: 10.3390/ijms17030327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Otter S, Lammert E. Exciting times for pancreatic islets: glutamate signaling in endocrinecells. Trends Endocrinol. Metab. 2016;27:177–188. doi: 10.1016/j.tem.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 107.Li Y, et al. Treatment of cerebral ischemia through NMDA receptors: metabotropic signaling and future directions. Front. Pharm. 2022;13:831181. doi: 10.3389/fphar.2022.831181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Grzeda E, Wiśniewska RJ, Wiśniewski K. Effect of an NMDA receptor agonist on T-maze and passive avoidance test in 12-week streptozotocin-induced diabetic rats. Pharm. Rep. 2007;59:656–663. [PubMed] [Google Scholar]
- 109.Bath PM, Wardlaw JM. Pharmacological treatment and prevention of cerebral small vessel disease: a review of potential interventions. Int. J. Stroke. 2015;10:469–478. doi: 10.1111/ijs.12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Patrone C, Eriksson O, Lindholm D. Diabetes drugs and neurological disorders: new views and therapeutic possibilities. Lancet Diabetes Endocrinol. 2014;2:256–262. doi: 10.1016/S2213-8587(13)70125-6. [DOI] [PubMed] [Google Scholar]
- 111.Orkaby AR, Cho K, Cormack J, Gagnon DR, Driver JA. Metformin vs sulfonylurea use and risk of dementia in US veterans aged ≥65 years with diabetes. Neurology. 2017;89:1877–1885. doi: 10.1212/WNL.0000000000004586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Areosa Sastre A, Vernooij RW, González-Colaço Harmand M, Martínez G. Effect of the treatment of type 2 diabetes mellitus on the development of cognitive impairment and dementia. Cochrane Database Syst. Rev. 2017;6:Cd003804. doi: 10.1002/14651858.CD003804.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.de Galan BE, et al. Cognitive function and risks of cardiovascular disease and hypoglycaemia in patients with type 2 diabetes: the action in diabetes and vascular disease: preterax and diamicron modified release controlled evaluation (ADVANCE) trial. Diabetologia. 2009;52:2328–2336. doi: 10.1007/s00125-009-1484-7. [DOI] [PubMed] [Google Scholar]
- 114.Haroon NN, et al. Risk of dementia in seniors with newly diagnosed diabetes: a population-based study. Diabetes Care. 2015;38:1868–1875. doi: 10.2337/dc15-0491. [DOI] [PubMed] [Google Scholar]
- 115.Shan Y, et al. The glucagon-like peptide-1 receptor agonist reduces inflammation and blood-brain barrier breakdown in an astrocyte-dependent manner in experimental stroke. J. Neuroinflammation. 2019;16:242. doi: 10.1186/s12974-019-1638-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Luchsinger JA, et al. Metformin in amnestic mild cognitive impairment: results of a pilot randomized placebo controlled clinical trial. J. Alzheimers Dis. 2016;51:501–514. doi: 10.3233/JAD-150493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Samaras K, et al. Metformin use is associated with slowed cognitive decline and reduced incident dementia in older adults with type 2 diabetes: the Sydney Memory and Ageing Study. Diabetes Care. 2020;43:2691–2701. doi: 10.2337/dc20-0892. [DOI] [PubMed] [Google Scholar]
- 118.Kumar AP, et al. Glitazones, PPAR-γ and neuroprotection. Mini Rev. Med. Chem. 2021;21:1457–1464. doi: 10.2174/1389557521666210304112403. [DOI] [PubMed] [Google Scholar]
- 119.Geldhof V, et al. Single cell atlas identifies lipid-processing and immunomodulatory endothelial cells in healthy and malignant breast. Nat. Commun. 2022;13:5511. doi: 10.1038/s41467-022-33052-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Palmer SC, et al. Sodium-glucose cotransporter protein-2 (SGLT-2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists for type 2 diabetes: systematic review and network meta-analysis of randomised controlled trials. BMJ. 2021;372:m4573. doi: 10.1136/bmj.m4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zannad F, et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-reduced and DAPA-HF trials. Lancet. 2020;396:819–829. doi: 10.1016/S0140-6736(20)31824-9. [DOI] [PubMed] [Google Scholar]
- 122.Zhou Z, et al. Effect of SGLT2 inhibitors on stroke and atrial fibrillation in diabetic kidney disease: results from the CREDENCE trial and meta-analysis. Stroke. 2021;52:1545–1556. doi: 10.1161/STROKEAHA.120.031623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Khanna A, Walcott BP, Kahle KT, Simard JM. Effect of glibenclamide on the prevention of secondary brain injury following ischemic stroke in humans. Neurosurg. Focus. 2014;36:E11. doi: 10.3171/2013.10.FOCUS13404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Vorasayan P, et al. Intravenous glibenclamide reduces lesional water uptake in large hemispheric infarction. Stroke. 2019;50:3021–3027. doi: 10.1161/STROKEAHA.119.026036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wada T, et al. Cilostazol ameliorates systemic insulin resistance in diabetic db/db mice by suppressing chronic inflammation in adipose tissue via modulation of both adipocyte and macrophage functions. Eur. J. Pharm. 2013;707:120–129. doi: 10.1016/j.ejphar.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 126.Hamed SA. Brain injury with diabetes mellitus: evidence, mechanisms and treatment implications. Expert Rev. Clin. Pharm. 2017;10:409–428. doi: 10.1080/17512433.2017.1293521. [DOI] [PubMed] [Google Scholar]
- 127.Papadopoulou-Marketou N, Kanaka-Gantenbein C, Marketos N, Chrousos GP, Papassotiriou I. Biomarkers of diabetic nephropathy: a 2017 update. Crit. Rev. Clin. Lab Sci. 2017;54:326–342. doi: 10.1080/10408363.2017.1377682. [DOI] [PubMed] [Google Scholar]
- 128.Deng Y, et al. Global, regional, and national burden of diabetes-related chronic kidney disease from 1990 to 2019. Front. Endocrinol. 2021;12:672350. doi: 10.3389/fendo.2021.672350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wimmer RA, et al. Human blood vessel organoids as a model of diabetic vasculopathy. Nature. 2019;565:505–510. doi: 10.1038/s41586-018-0858-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pérez-Morales RE, et al. Inflammation in diabetic kidney disease. Nephron. 2019;143:12–16. doi: 10.1159/000493278. [DOI] [PubMed] [Google Scholar]
- 131.Mora-Fernández, C. et al. Diabet. kidney disease: from physiology to therapeutics. J. Physiol. 592, 3997–4012 (2014). [DOI] [PMC free article] [PubMed]
- 132.Carlstrom M, Wilcox CS, Arendshorst WJ. Renal autoregulation in health and disease. Physiol. Rev. 2015;95:405–511. doi: 10.1152/physrev.00042.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bidani AK, Polichnowski AJ, Loutzenhiser R, Griffin KA. Renal microvascular dysfunction, hypertension and CKD progression. Curr. Opin. Nephrol. Hypertens. 2013;22:1–9. doi: 10.1097/MNH.0b013e32835b36c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Roman RJ, Fan F. Genetic susceptibility to hypertension-induced renal injury. Hypertension. 2018;71:559–560. doi: 10.1161/HYPERTENSIONAHA.118.10773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Balafa O, Kalaitzidis R, Siamopoulos KC. Optimal medical management in patients with renovascular hypertension. Am. J. Cardiovasc. Drugs. 2013;13:71–78. doi: 10.1007/s40256-013-0011-x. [DOI] [PubMed] [Google Scholar]
- 136.Paneni F, Beckman JA, Creager MA, Cosentino F. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part I. Eur. Heart J. 2013;34:2436–2443. doi: 10.1093/eurheartj/eht149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Neuen BL, et al. SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2019;7:845–854. doi: 10.1016/S2213-8587(19)30256-6. [DOI] [PubMed] [Google Scholar]
- 138.Neal B, Perkovic V, Matthews DR. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N. Engl. J. Med. 2017;377:2099. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 139.Pan L, et al. Clinical significance of hemostatic parameters in the prediction for type 2 diabetes mellitus and diabetic nephropathy. Dis. Markers. 2018;2018:5214376. doi: 10.1155/2018/5214376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Thomas MC, et al. Diabetic kidney disease. Nat. Rev. Dis. Prim. 2015;1:15018. doi: 10.1038/nrdp.2015.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Colhoun HM, Marcovecchio ML. Biomarkers of diabetic kidney disease. Diabetologia. 2018;61:996–1011. doi: 10.1007/s00125-018-4567-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gohda T, et al. Clinical predictive biomarkers for normoalbuminuric diabetic kidney disease. Diabetes Res. Clin. Pr. 2018;141:62–68. doi: 10.1016/j.diabres.2018.04.026. [DOI] [PubMed] [Google Scholar]
- 143.Liu JJ, et al. Vascular cell adhesion molecule-1, but not intercellular adhesion molecule-1, is associated with diabetic kidney disease in Asians with type 2 diabetes. J. Diabetes Complications. 2015;29:707–712. doi: 10.1016/j.jdiacomp.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 144.Chauhan K, et al. Plasma endostatin predicts kidney outcomes in patients with type 2 diabetes. Kidney Int. 2019;95:439–446. doi: 10.1016/j.kint.2018.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Noor T, et al. Relation of copeptin with diabetic and renal function markers among patients with diabetes mellitus progressing towards diabetic nephropathy. Arch. Med. Res. 2020;51:548–555. doi: 10.1016/j.arcmed.2020.05.018. [DOI] [PubMed] [Google Scholar]
- 146.Coca SG, et al. Plasma biomarkers and kidney function decline in early and established diabetic kidney disease. J. Am. Soc. Nephrol. 2017;28:2786–2793. doi: 10.1681/ASN.2016101101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Moresco RN, et al. Urinary kidney injury molecule-1 in renal disease. Clin. Chim. Acta. 2018;487:15–21. doi: 10.1016/j.cca.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 148.Wu L, et al. Associations of urinary epidermal growth factor and monocyte chemotactic protein-1 with kidney involvement in patients with diabetic kidney disease. Nephrol. Dial. Transpl. 2020;35:291–297. doi: 10.1093/ndt/gfy314. [DOI] [PubMed] [Google Scholar]
- 149.Phanish MK, et al. Evaluation of urinary biomarkers of proximal tubular injury, inflammation, and fibrosis in patients with albuminuric and nonalbuminuric diabetic kidney disease. Kidney Int. Rep. 2021;6:1355–1367. doi: 10.1016/j.ekir.2021.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lu Y, Liu D, Feng Q, Liu Z. Diabetic nephropathy: perspective on extracellular vesicles. Front. Immunol. 2020;11:943. doi: 10.3389/fimmu.2020.00943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kanakalakshmi ST, et al. Microparticles in diabetic kidney disease. Clin. Chim. Acta. 2022;531:418–425. doi: 10.1016/j.cca.2022.05.001. [DOI] [PubMed] [Google Scholar]
- 152.Xu YX, et al. Exosomal ncRNAs: Novel therapeutic target and biomarker for diabetic complications. Pharm. Res. 2022;178:106135. doi: 10.1016/j.phrs.2022.106135. [DOI] [PubMed] [Google Scholar]
- 153.Feng Y, et al. Urinary small extracellular vesicles derived CCL21 mRNA as biomarker linked with pathogenesis for diabetic nephropathy. J. Transl. Med. 2021;19:355. doi: 10.1186/s12967-021-03030-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hashemi E, et al. WT1 and ACE mRNAs of blood extracellular vesicle as biomarkers of diabetic nephropathy. J. Transl. Med. 2021;19:299. doi: 10.1186/s12967-021-02964-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ding X, et al. A systematic review and Meta-analysis of urinary extracellular vesicles proteome in diabetic nephropathy. Front. Endocrinol. 2022;13:866252. doi: 10.3389/fendo.2022.866252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Dubin RF, Rhee EP. Proteomics and metabolomics in kidney disease, including insights into etiology, treatment, and prevention. Clin. J. Am. Soc. Nephrol. 2020;15:404–411. doi: 10.2215/CJN.07420619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kato M, Natarajan R. Epigenetics and epigenomics in diabetic kidney disease and metabolic memory. Nat. Rev. Nephrol. 2019;15:327–345. doi: 10.1038/s41581-019-0135-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhang Y, Zhang S, Wang G. Metabolomic biomarkers in diabetic kidney diseases-A systematic review. J. Diabetes Complications. 2015;29:1345–1351. doi: 10.1016/j.jdiacomp.2015.06.016. [DOI] [PubMed] [Google Scholar]
- 159.Good DM, et al. Naturally occurring human urinary peptides for use in diagnosis of chronic kidney disease. Mol. Cell Proteom. 2010;9:2424–2437. doi: 10.1074/mcp.M110.001917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Argiles A, et al. CKD273, a new proteomics classifier assessing CKD and its prognosis. PLoS ONE. 2013;8:e62837. doi: 10.1371/journal.pone.0062837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Raij L. The pathophysiologic basis for blocking the renin-angiotensin system in hypertensive patients with renal disease. Am. J. Hypertens. 2005;18:95S–99S. doi: 10.1016/j.amjhyper.2004.11.040. [DOI] [PubMed] [Google Scholar]
- 162.Jeansson M, et al. Angiopoietin-1 is essential in mouse vasculature during development and in response to injury. J. Clin. Invest. 2011;121:2278–2289. doi: 10.1172/JCI46322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sharma K, Cook A, Smith M, Valancius C, Inscho EW. TGF-beta impairs renal autoregulation via generation of ROS. Am. J. Physiol. Ren. Physiol. 2005;288:F1069–F1077. doi: 10.1152/ajprenal.00345.2004. [DOI] [PubMed] [Google Scholar]
- 164.Yang W, et al. Ectopic lipid accumulation: potential role in tubular injury and inflammation in diabetic kidney disease. Clin. Sci. 2018;132:2407–2422. doi: 10.1042/CS20180702. [DOI] [PubMed] [Google Scholar]
- 165.Fu Y, et al. Elevation of JAML promotes diabetic kidney disease by modulating podocyte lipid metabolism. Cell Metab. 2020;32:1052–1062 e1058. doi: 10.1016/j.cmet.2020.10.019. [DOI] [PubMed] [Google Scholar]
- 166.Zafrani L, Ince C. Microcirculation in acute and chronic kidney diseases. Am. J. Kidney Dis. 2015;66:1083–1094. doi: 10.1053/j.ajkd.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 167.Kramann R, Wongboonsin J, Chang-Panesso M, Machado FG, Humphreys BD. Gli1(+) pericyte loss induces capillary rarefaction and proximal tubular injury. J. Am. Soc. Nephrol. 2017;28:776–784. doi: 10.1681/ASN.2016030297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Dimke H, et al. Tubulovascular cross-talk by vascular endothelial growth factor a maintains peritubular microvasculature in kidney. J. Am. Soc. Nephrol. 2015;26:1027–1038. doi: 10.1681/ASN.2014010060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hakroush S, et al. Effects of increased renal tubular vascular endothelial growth factor (VEGF) on fibrosis, cyst formation, and glomerular disease. Am. J. Pathol. 2009;175:1883–1895. doi: 10.2353/ajpath.2009.080792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Navarro-Gonzalez JF, Mora-Fernandez C. The role of inflammatory cytokines in diabetic nephropathy. J. Am. Soc. Nephrol. 2008;19:433–442. doi: 10.1681/ASN.2007091048. [DOI] [PubMed] [Google Scholar]
- 171.Pichler R, Afkarian M, Dieter BP, Tuttle KR. Immunity and inflammation in diabetic kidney disease: translating mechanisms to biomarkers and treatment targets. Am. J. Physiol. Ren. Physiol. 2017;312:F716–F731. doi: 10.1152/ajprenal.00314.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Anders HJ, Huber TB, Isermann B, Schiffer M. CKD in diabetes: diabetic kidney disease versus nondiabetic kidney disease. Nat. Rev. Nephrol. 2018;14:361–377. doi: 10.1038/s41581-018-0001-y. [DOI] [PubMed] [Google Scholar]
- 173.Boels MG, et al. Atrasentan reduces albuminuria by restoring the glomerular endothelial glycocalyx barrier in diabetic nephropathy. Diabetes. 2016;65:2429–2439. doi: 10.2337/db15-1413. [DOI] [PubMed] [Google Scholar]
- 174.Jung CY, Yoo TH. Pathophysiologic mechanisms and potential biomarkers in diabetic kidney disease. Diabetes Metab. J. 2022;46:181–197. doi: 10.4093/dmj.2021.0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Agere SA, Kim EY, Akhtar N, Ahmed S. Syndecans in chronic inflammatory and autoimmune diseases: pathological insights and therapeutic opportunities. J. Cell Physiol. 2018;233:6346–6358. doi: 10.1002/jcp.26388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ke G, et al. Receptor activator of NF-kappaB mediates podocyte injury in diabetic nephropathy. Kidney Int. 2021;100:377–390. doi: 10.1016/j.kint.2021.04.036. [DOI] [PubMed] [Google Scholar]
- 177.Wang H, et al. Apolipoprotein C3 aggravates diabetic nephropathy in type 1 diabetes by activating the renal TLR2/NF-kappaB pathway. Metabolism. 2021;119:154740. doi: 10.1016/j.metabol.2021.154740. [DOI] [PubMed] [Google Scholar]
- 178.Nam JS, et al. The activation of NF-kappaB and AP-1 in peripheral blood mononuclear cells isolated from patients with diabetic nephropathy. Diabetes Res. Clin. Pr. 2008;81:25–32. doi: 10.1016/j.diabres.2008.01.032. [DOI] [PubMed] [Google Scholar]
- 179.Zhang C, et al. Positive and negative regulatory effects of transcription factor activator protein 1 (AP1) on the expression of antimicrobial peptides in Macrobrachium nipponense. Fish. Shellfish Immunol. 2020;98:130–137. doi: 10.1016/j.fsi.2020.01.002. [DOI] [PubMed] [Google Scholar]
- 180.Garcia-Garcia PM, Getino-Melian MA, Dominguez-Pimentel V, Navarro-Gonzalez JF. Inflammation in diabetic kidney disease. World J. Diabetes. 2014;5:431–443. doi: 10.4239/wjd.v5.i4.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Badve SV, et al. Effects of allopurinol on the progression of chronic kidney disease. N. Engl. J. Med. 2020;382:2504–2513. doi: 10.1056/NEJMoa1915833. [DOI] [PubMed] [Google Scholar]
- 182.Doria A, et al. Serum urate lowering with allopurinol and kidney function in type 1 diabetes. N. Engl. J. Med. 2020;382:2493–2503. doi: 10.1056/NEJMoa1916624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Prattichizzo F, de Candia P, Ceriello A. Diabetes and kidney disease: emphasis on treatment with SGLT-2 inhibitors and GLP-1 receptor agonists. Metabolism. 2021;120:154799. doi: 10.1016/j.metabol.2021.154799. [DOI] [PubMed] [Google Scholar]
- 184.Mottl AK, et al. Long-term effects of intensive glycemic and blood pressure control and fenofibrate use on kidney outcomes. Clin. J. Am. Soc. Nephrol. 2018;13:1693–1702. doi: 10.2215/CJN.06200518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Heerspink HJ, Perkins BA, Fitchett DH, Husain M, Cherney DZ. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation. 2016;134:752–772. doi: 10.1161/CIRCULATIONAHA.116.021887. [DOI] [PubMed] [Google Scholar]
- 186.Kidokoro K, et al. Evaluation of glomerular hemodynamic function by empagliflozin in diabetic mice using in vivo imaging. Circulation. 2019;140:303–315. doi: 10.1161/CIRCULATIONAHA.118.037418. [DOI] [PubMed] [Google Scholar]
- 187.Hesp AC, et al. The role of renal hypoxia in the pathogenesis of diabetic kidney disease: a promising target for newer renoprotective agents including SGLT2 inhibitors? Kidney Int. 2020;98:579–589. doi: 10.1016/j.kint.2020.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zhang Y, et al. A sodium-glucose cotransporter 2 inhibitor attenuates renal capillary injury and fibrosis by a vascular endothelial growth factor-dependent pathway after renal injury in mice. Kidney Int. 2018;94:524–535. doi: 10.1016/j.kint.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 189.van Bommel EJM, et al. The renal hemodynamic effects of the SGLT2 inhibitor dapagliflozin are caused by post-glomerular vasodilatation rather than pre-glomerular vasoconstriction in metformin-treated patients with type 2 diabetes in the randomized, double-blind RED trial. Kidney Int. 2020;97:202–212. doi: 10.1016/j.kint.2019.09.013. [DOI] [PubMed] [Google Scholar]
- 190.Tanaka T, Higashijima Y, Wada T, Nangaku M. The potential for renoprotection with incretin-based drugs. Kidney Int. 2014;86:701–711. doi: 10.1038/ki.2014.236. [DOI] [PubMed] [Google Scholar]
- 191.Bakris GL, et al. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N. Engl. J. Med. 2020;383:2219–2229. doi: 10.1056/NEJMoa2025845. [DOI] [PubMed] [Google Scholar]
- 192.Zhang Y, Sun X, Icli B, Feinberg MW. Emerging roles for microRNAs in diabetic microvascular disease: novel targets for therapy. Endocr. Rev. 2017;38:145–168. doi: 10.1210/er.2016-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Täubel J, et al. Novel antisense therapy targeting microRNA-132 in patients with heart failure: results of a first-in-human Phase 1b randomized, double-blind, placebo-controlled study. Eur. Heart J. 2021;42:178–188. doi: 10.1093/eurheartj/ehaa898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Oe Y, et al. Coagulation factor Xa and protease-activated receptor 2 as novel therapeutic targets for diabetic nephropathy. Arterioscler Thromb. Vasc. Biol. 2016;36:1525–1533. doi: 10.1161/ATVBAHA.116.307883. [DOI] [PubMed] [Google Scholar]
- 195.Ungar L, et al. Stroke outcomes with vorapaxar versus placebo in patients with acute coronary syndromes: insights from the TRACER trial. J. Am. Heart Assoc. 2018;7:e009609. doi: 10.1161/JAHA.118.009609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Tuttle KR, et al. JAK1/JAK2 inhibition by baricitinib in diabetic kidney disease: results from a Phase 2 randomized controlled clinical trial. Nephrol. Dial. Transpl. 2018;33:1950–1959. doi: 10.1093/ndt/gfx377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.de Zeeuw D, et al. Efficacy of a novel inhibitor of vascular adhesion protein-1 in reducing albuminuria in patients with diabetic kidney disease (ALBUM): a randomised, placebo-controlled, phase 2 trial. Lancet Diabetes Endocrinol. 2018;6:925–933. doi: 10.1016/S2213-8587(18)30289-4. [DOI] [PubMed] [Google Scholar]
- 198.Kikuchi K, et al. Gut microbiome-derived phenyl sulfate contributes to albuminuria in diabetic kidney disease. Nat. Commun. 2019;10:1835. doi: 10.1038/s41467-019-09735-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Soleimani A, et al. Probiotic supplementation in diabetic hemodialysis patients has beneficial metabolic effects. Kidney Int. 2017;91:435–442. doi: 10.1016/j.kint.2016.09.040. [DOI] [PubMed] [Google Scholar]
- 200.Li HB, et al. Faecalibacterium prausnitzii attenuates CKD via butyrate-renal GPR43 axis. Circ. Res. 2022;131:e120–e134. doi: 10.1161/CIRCRESAHA.122.320184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Zhao J, et al. Efficacy of combined abelmoschus manihot and irbesartan for reduction of albuminuria in patients with type 2 diabetes and diabetic kidney disease: a multicenter randomized double-blind parallel controlled clinical trial. Diabetes Care. 2022;45:e113–e115. doi: 10.2337/dc22-0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Das T, et al. Recently updated global diabetic retinopathy screening guidelines: commonalities, differences, and future possibilities. Eye. 2021;35:2685–2698. doi: 10.1038/s41433-021-01572-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376:124–136. doi: 10.1016/S0140-6736(09)62124-3. [DOI] [PubMed] [Google Scholar]
- 204.Wang W, Lo ACY. Diabetic retinopathy: pathophysiology and treatments. Int. J. Mol. Sci. 2018;19:1816. doi: 10.3390/ijms19061816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Khansari MM, et al. Automated deformation-based analysis of 3D optical coherence tomography in diabetic retinopathy. IEEE Trans. Med. Imaging. 2020;39:236–245. doi: 10.1109/TMI.2019.2924452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Musch DC, Chew EY. Evidence for step therapy in diabetic macular edema. N. Engl. J. Med. 2022;387:751–752. doi: 10.1056/NEJMe2208454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Lechner J, O’Leary OE, Stitt AW. The pathology associated with diabetic retinopathy. Vis. Res. 2017;139:7–14. doi: 10.1016/j.visres.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 208.Elsherbiny NM, et al. Homocysteine induces inflammation in retina and brain. Biomolecules. 2020;10:393. doi: 10.3390/biom10030393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Fickweiler W, et al. Elevated retinol binding protein 3 concentrations are associated with decreased vitreous inflammatory cytokines, VEGF, and progression of diabetic retinopathy. Diabetes Care. 2022;45:2159–2162. doi: 10.2337/dc22-0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Yokomizo H, et al. Retinol binding protein 3 is increased in the retina of patients with diabetes resistant to diabetic retinopathy. Sci. Transl. Med. 2019;11:eaau6627. doi: 10.1126/scitranslmed.aau6627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Helal HG, Rashed MH, Abdullah OA, Salem TI, Daifalla A. MicroRNAs (-146a, -21 and -34a) are diagnostic and prognostic biomarkers for diabetic retinopathy. Biomed. J. 2021;44:S242–S251. doi: 10.1016/j.bj.2020.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Hwang SJ, et al. miR-125a-5p attenuates macrophage-mediated vascular dysfunction by targeting Ninjurin1. Cell Death Differ. 2022;29:1199–1210. doi: 10.1038/s41418-021-00911-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Xie Q, et al. An innovative method for screening and evaluating the degree of diabetic retinopathy and drug treatment based on artificial intelligence algorithms. Pharmacol. Res. 2020;159:104986. doi: 10.1016/j.phrs.2020.104986. [DOI] [PubMed] [Google Scholar]
- 214.Liu Y, et al. Monosodium glutamate-induced mouse model with unique diabetic retinal neuropathy features and artificial intelligence techniques for quantitative evaluation. Front. Immunol. 2022;13:862702. doi: 10.3389/fimmu.2022.862702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Jiang H, Zhang H, Jiang X, Wu S. Overexpression of D-amino acid oxidase prevents retinal neurovascular pathologies in diabetic rats. Diabetologia. 2021;64:693–706. doi: 10.1007/s00125-020-05333-y. [DOI] [PubMed] [Google Scholar]
- 216.Sergeys J, et al. Longitudinal in vivo characterization of the streptozotocin-induced diabetic mouse model: focus on early inner retinal responses. Invest. Ophthalmol. Vis. Sci. 2019;60:807–822. doi: 10.1167/iovs.18-25372. [DOI] [PubMed] [Google Scholar]
- 217.Binet F, et al. Neutrophil extracellular traps target senescent vasculature for tissue remodeling in retinopathy. Science. 2020;369:eaay5356. doi: 10.1126/science.aay5356. [DOI] [PubMed] [Google Scholar]
- 218.Sans M, et al. VCAM-1 and ICAM-1 mediate leukocyte-endothelial cell adhesion in rat experimental colitis. Gastroenterology. 1999;116:874–883. doi: 10.1016/S0016-5085(99)70070-3. [DOI] [PubMed] [Google Scholar]
- 219.Lessieur EM, et al. ICAM-1 on the luminal surface of endothelial cells is induced to a greater extent in mouse retina than in other tissues in diabetes. Diabetologia. 2022;65:1734–1744. doi: 10.1007/s00125-022-05719-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Portillo J-AC, et al. Disruption of retinal inflammation and the development of diabetic retinopathy in mice by a CD40-derived peptide or mutation of CD40 in Müller cells. Diabetologia. 2022;65:2157–2171. doi: 10.1007/s00125-022-05775-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Giblin MJ, et al. Nuclear factor of activated T-cells (NFAT) regulation of IL-1β-induced retinal vascular inflammation. Biochim. Biophys. Acta Mol. Basis Dis. 2021;1867:166238. doi: 10.1016/j.bbadis.2021.166238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Giblin MJ, Ontko CD, Penn JS. Effect of cytokine-induced alterations in extracellular matrix composition on diabetic retinopathy-relevant endothelial cell behaviors. Sci. Rep. 2022;12:12955. doi: 10.1038/s41598-022-12683-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Liu X, et al. IL-1β induces IL-6 production in retinal Müller cells predominantly through the activation of p38 MAPK/NF-κB signaling pathway. Exp. Cell Res. 2015;331:223–231. doi: 10.1016/j.yexcr.2014.08.040. [DOI] [PubMed] [Google Scholar]
- 224.Feng S, et al. Levels of inflammatory cytokines IL-1, IL-6, IL-8, IL-17A, and TNF- in aqueous humour of patients with diabetic retinopathy. J. Diabetes Res. 2018;2018:8546423. doi: 10.1155/2018/8546423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Kang Q, Yang C. Oxidative stress and diabetic retinopathy: Molecular mechanisms, pathogenetic role and therapeutic implications. Redox Biol. 2020;37:101799. doi: 10.1016/j.redox.2020.101799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ. Res. 2010;107:1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Geraldes P, et al. Activation of PKC-delta and SHP-1 by hyperglycemia causes vascular cell apoptosis and diabetic retinopathy. Nat. Med. 2009;15:1298–1306. doi: 10.1038/nm.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Suo L, et al. METTL3-mediated -methyladenosine modification governs pericyte dysfunction during diabetes-induced retinal vascular complication. Theranostics. 2022;12:277–289. doi: 10.7150/thno.63441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Chang K-C, Snow A, LaBarbera DV, Petrash JM. Aldose reductase inhibition alleviates hyperglycemic effects on human retinal pigment epithelial cells. Chem. Biol. Interact. 2015;234:254–260. doi: 10.1016/j.cbi.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Chen H, et al. MD2 blockade prevents modified LDL-induced retinal injury in diabetes by suppressing NADPH oxidase-4 interaction with Toll-like receptor-4. Exp. Mol. Med. 2021;53:681–694. doi: 10.1038/s12276-021-00607-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Liu XY, et al. Shabyar ameliorates high glucose induced retinal pigment epithelium injury through suppressing aldose reductase and AMPK/mTOR/ULK1 autophagy pathway. Front. Pharmacol. 2022;13:852945. doi: 10.3389/fphar.2022.852945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Mahmoud AM, Abd El-Twab SM, Abdel-Reheim ES. Consumption of polyphenol-rich Morus alba leaves extract attenuates early diabetic retinopathy: the underlying mechanism. Eur. J. Nutr. 2017;56:1671–1684. doi: 10.1007/s00394-016-1214-0. [DOI] [PubMed] [Google Scholar]
- 233.Winges A, et al. Osmotic expression of aldose reductase in retinal pigment epithelial cells: involvement of NFAT5. Graefe’s Arch. Clin. Exp. Ophthalmol. 2016;254:2387–2400. doi: 10.1007/s00417-016-3492-x. [DOI] [PubMed] [Google Scholar]
- 234.Xue J, et al. The receptor for advanced glycation end products (RAGE) specifically recognizes methylglyoxal-derived AGEs. Biochemistry. 2014;53:3327–3335. doi: 10.1021/bi500046t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Sun L, et al. Advanced glycation end products promote VEGF expression and thus choroidal neovascularization via Cyr61-PI3K/AKT signaling pathway. Sci. Rep. 2017;7:14925. doi: 10.1038/s41598-017-14015-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Kang M-K, et al. Chrysin ameliorates malfunction of retinoid visual cycle through blocking activation of AGE-RAGE-ER stress in glucose-stimulated retinal pigment epithelial cells and diabetic eyes. Nutrients. 2018;10:1046. doi: 10.3390/nu10081046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Cui J, Gong R, Hu S, Cai L, Chen L. Gambogic acid ameliorates diabetes-induced proliferative retinopathy through inhibition of the HIF-1α/VEGF expression via targeting PI3K/AKT pathway. Life Sci. 2018;192:293–303. doi: 10.1016/j.lfs.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 238.Xie W, et al. Corrigendum: ginsenoside Re attenuates high glucose-induced RF/6A injury regulating PI3K/AKT inhibited HIF-1a/VEGF signaling pathway. Front. Pharmacol. 2020;11:1312. doi: 10.3389/fphar.2020.01312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Wang Y, et al. Calcium dobesilate restores autophagy by inhibiting the VEGF/PI3K/AKT/mTOR signaling pathway. Front. Pharmacol. 2019;10:886. doi: 10.3389/fphar.2019.00886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Fahmideh F, et al. Effect of troxerutin in counteracting hyperglycemia-induced VEGF upregulation in endothelial cells: a new option to target early stages of diabetic retinopathy? Front. Pharmacol. 2022;13:951833. doi: 10.3389/fphar.2022.951833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Zhang H, et al. The effect of total lignans from Fructus Arctii on streptozotocin-induced diabetic retinopathy in Wistar rats. J. Ethnopharmacol. 2020;255:112773. doi: 10.1016/j.jep.2020.112773. [DOI] [PubMed] [Google Scholar]
- 242.Abu El-Asrar AM, et al. Evaluation of proteoforms of the transmembrane chemokines CXCL16 and CX3CL1, their receptors, and their processing metalloproteinases ADAM10 and ADAM17 in proliferative diabetic retinopathy. Front. Immunol. 2020;11:601639. doi: 10.3389/fimmu.2020.601639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Abu El-Asrar AM, et al. The proinflammatory and proangiogenic macrophage migration inhibitory factor is a potential regulator in proliferative diabetic retinopathy. Front. Immunol. 2019;10:2752. doi: 10.3389/fimmu.2019.02752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Li Y, et al. Thermostable small-molecule inhibitor of angiogenesis and vascular permeability that suppresses a pERK-FosB/ΔFosB-VCAM-1 axis. Sci. Adv. 2020;6:eaaz7815. doi: 10.1126/sciadv.aaz7815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Thounaojam MC, et al. Protective effects of agonists of growth hormone-releasing hormone (GHRH) in early experimental diabetic retinopathy. Proc. Natl Acad. Sci. USA. 2017;114:13248–13253. doi: 10.1073/pnas.1718592114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Jung S-H, Kim YS, Lee Y-R, Kim JS. High glucose-induced changes in hyaloid-retinal vessels during early ocular development of zebrafish: a short-term animal model of diabetic retinopathy. Br. J. Pharmacol. 2016;173:15–26. doi: 10.1111/bph.13279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Liu K, et al. Intravitreal conbercept for diabetic macular oedema: 2-year results from a randomised controlled trial and open-label extension study. Br. J. Ophthalmol. 2022;106:1436–1443. doi: 10.1136/bjophthalmol-2020-318690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Uludag G, et al. Efficacy and safety of intravitreal anti-VEGF therapy in diabetic retinopathy: what we have learned and what should we learn further? Expert Opin. Biol. Ther. 2022;22:1275–1291. doi: 10.1080/14712598.2022.2100694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Jhaveri CD, et al. Aflibercept monotherapy or bevacizumab first for diabetic macular edema. N. Engl. J. Med. 2022;387:692–703. doi: 10.1056/NEJMoa2204225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Gu X, et al. Glucocorticoids promote extracellular matrix component remodeling by activating YAP in human retinal capillary endothelial cells. Front. Cell Dev. Biol. 2021;9:738341. doi: 10.3389/fcell.2021.738341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Shah AD, et al. Type 2 diabetes and incidence of cardiovascular diseases: a cohort study in 1·9 million people. Lancet Diabetes Endocrinol. 2015;3:105–113. doi: 10.1016/S2213-8587(14)70219-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Verma S, et al. Cardiovascular outcomes and safety of empagliflozin in patients with type 2 diabetes mellitus and peripheral artery disease: a subanalysis of EMPA-REG OUTCOME. Circulation. 2018;137:405–407. doi: 10.1161/CIRCULATIONAHA.117.032031. [DOI] [PubMed] [Google Scholar]
- 253.Anand SS, et al. Major adverse limb events and mortality in patients with peripheral artery disease: the COMPASS trial. J. Am. Coll. Cardiol. 2018;71:2306–2315. doi: 10.1016/j.jacc.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 254.Aday AW, Matsushita K. Epidemiology of peripheral artery disease and polyvascular disease. Circ. Res. 2021;128:1818–1832. doi: 10.1161/CIRCRESAHA.121.318535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Lim JZ, Ng NS, Thomas C. Prevention and treatment of diabetic foot ulcers. J. R. Soc. Med. 2017;110:104–109. doi: 10.1177/0141076816688346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Mohammedi K, et al. Presentations of major peripheral arterial disease and risk of major outcomes in patients with type 2 diabetes: results from the ADVANCE-ON study. Cardiovasc Diabetol. 2016;15:129. doi: 10.1186/s12933-016-0446-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Ergul A. Endothelin-1 and diabetic complications: focus on the vasculature. Pharm. Res. 2011;63:477–482. doi: 10.1016/j.phrs.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Kirthi V, et al. Prevalence of peripheral neuropathy in pre-diabetes: a systematic review. BMJ Open Diabetes Res. Care. 2021;9:e002040. doi: 10.1136/bmjdrc-2020-002040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Vas PRJ, Alberti KG, Edmonds ME. Prediabetes: moving away from a glucocentric definition. Lancet Diabetes Endocrinol. 2017;5:848–849. doi: 10.1016/S2213-8587(17)30234-6. [DOI] [PubMed] [Google Scholar]
- 260.Nanayakkara N, et al. Impact of age at type 2 diabetes mellitus diagnosis on mortality and vascular complications: systematic review and meta-analyses. Diabetologia. 2021;64:275–287. doi: 10.1007/s00125-020-05319-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Hicks CW, Selvin E. Epidemiology of peripheral neuropathy and lower extremity disease in diabetes. Curr. Diab Rep. 2019;19:86. doi: 10.1007/s11892-019-1212-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Bongaerts BW, et al. Postchallenge hyperglycemia is positively associated with diabetic polyneuropathy: the KORA F4 study. Diabetes Care. 2012;35:1891–1893. doi: 10.2337/dc11-2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Held C, et al. Inflammatory biomarkers interleukin-6 and C-reactive protein and outcomes in stable coronary heart disease: experiences from the STABILITY (stabilization of atherosclerotic plaque by initiation of darapladib therapy) trial. J. Am. Heart Assoc. 2017;6:e005077. doi: 10.1161/JAHA.116.005077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Wallentin L, et al. Lipoprotein-associated phospholipase A2 activity is a marker of risk but not a useful target for treatment in patients with stable coronary heart disease. J. Am. Heart Assoc. 2016;5:e003407. doi: 10.1161/JAHA.116.003407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Garg PK, et al. Lipoprotein-associated phospholipase A2 and incident peripheral arterial disease in older adults: the cardiovascular health study. Arterioscler. Thromb. Vasc. Biol. 2016;36:750–756. doi: 10.1161/ATVBAHA.115.306647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Cheng Z, Zhang C, Mi Y. IL-6 gene rs1800795 polymorphism and diabetes mellitus: a comprehensive analysis involving 42,150 participants from a meta-analysis. Diabetol. Metab. Syndr. 2022;14:95. doi: 10.1186/s13098-022-00851-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Danielsson P, Truedsson L, Eriksson KF, Norgren L. Inflammatory markers and IL-6 polymorphism in peripheral arterial disease with and without diabetes mellitus. Vasc. Med. 2005;10:191–198. doi: 10.1191/1358863x05vm617oa. [DOI] [PubMed] [Google Scholar]
- 268.Sharma K, McCue P, Dunn SR. Diabetic kidney disease in the db/db mouse. Am. J. Physiol. Ren. Physiol. 2003;284:F1138–F1144. doi: 10.1152/ajprenal.00315.2002. [DOI] [PubMed] [Google Scholar]
- 269.Bönhof GJ, et al. Emerging biomarkers, tools, and treatments for diabetic polyneuropathy. Endocr. Rev. 2019;40:153–192. doi: 10.1210/er.2018-00107. [DOI] [PubMed] [Google Scholar]
- 270.Roustit M, Loader J, Deusenbery C, Baltzis D, Veves A. Endothelial dysfunction as a link between cardiovascular risk factors and peripheral neuropathy in diabetes. J. Clin. Endocrinol. Metab. 2016;101:3401–3408. doi: 10.1210/jc.2016-2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Schamarek I, et al. Adiponectin, markers of subclinical inflammation and nerve conduction in individuals with recently diagnosed type 1 and type 2 diabetes. Eur. J. Endocrinol. 2016;174:433–443. doi: 10.1530/EJE-15-1010. [DOI] [PubMed] [Google Scholar]
- 272.Chapouly C, et al. Impaired Hedgehog signalling-induced endothelial dysfunction is sufficient to induce neuropathy: implication in diabetes. Cardiovasc. Res. 2016;109:217–227. doi: 10.1093/cvr/cvv263. [DOI] [PubMed] [Google Scholar]
- 273.Wang L, et al. Exosomes derived from Schwann cells ameliorate peripheral neuropathy in type 2 diabetic mice. Diabetes. 2020;69:749–759. doi: 10.2337/db19-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Feldman EL, Nave KA, Jensen TS, Bennett DLH. New horizons in diabetic neuropathy: mechanisms, bioenergetics, and pain. Neuron. 2017;93:1296–1313. doi: 10.1016/j.neuron.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Yu JW, et al. Metformin improves the angiogenic functions of endothelial progenitor cells via activating AMPK/eNOS pathway in diabetic mice. Cardiovasc. Diabetol. 2016;15:88. doi: 10.1186/s12933-016-0408-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Akintoye OO, et al. Diabetic neuropathy is associated with increased pain perception, low serum beta-endorphin and increase insulin resistance among Nigerian cohorts in Ekiti State. Heliyon. 2020;6:e04377. doi: 10.1016/j.heliyon.2020.e04377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Singh VP, Bali A, Singh N, Jaggi AS. Advanced glycation end products and diabetic complications. Korean J. Physiol. Pharm. 2014;18:1–14. doi: 10.4196/kjpp.2014.18.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Kessler JA, et al. Gene therapy for diabetic peripheral neuropathy: a randomized, placebo-controlled phase III study of VM202, a plasmid DNA encoding human hepatocyte growth factor. Clin. Transl. Sci. 2021;14:1176–1184. doi: 10.1111/cts.12977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Cortese A, et al. Biallelic mutations in SORD cause a common and potentially treatable hereditary neuropathy with implications for diabetes. Nat. Genet. 2020;52:473–481. doi: 10.1038/s41588-020-0615-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Das SK, Yuan YF, Li MQ. Specific PKC βII inhibitor: one stone two birds in the treatment of diabetic foot ulcers. Biosci. Rep. 2018;38:BSR20171459. doi: 10.1042/BSR20171459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Elafros MA, et al. Towards prevention of diabetic peripheral neuropathy: clinical presentation, pathogenesis, and new treatments. Lancet Neurol. 2022;21:922–936. doi: 10.1016/S1474-4422(22)00188-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Singh R, Kishore L, Kaur N. Diabetic peripheral neuropathy: current perspective and future directions. Pharm. Res. 2014;80:21–35. doi: 10.1016/j.phrs.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 283.Rumora AE, et al. The divergent roles of dietary saturated and monounsaturated fatty acids on nerve function in murine models of obesity. J. Neurosci. 2019;39:3770–3781. doi: 10.1523/JNEUROSCI.3173-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Zuellig RA, et al. Deoxysphingolipids, novel biomarkers for type 2 diabetes, are cytotoxic for insulin-producing cells. Diabetes. 2014;63:1326–1339. doi: 10.2337/db13-1042. [DOI] [PubMed] [Google Scholar]
- 285.Guo SC, et al. Exosomes derived from platelet-rich plasma promote the re-epithelization of chronic cutaneous wounds via activation of YAP in a diabetic rat model. Theranostics. 2017;7:81–96. doi: 10.7150/thno.16803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Akbar N, et al. Rapid neutrophil mobilisation by VCAM-1+ endothelial extracellular vesicles. Cardiovasc. Res. 2022;4:cvac012. doi: 10.1093/cvr/cvac012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Penno A, et al. Hereditary sensory neuropathy type 1 is caused by the accumulation of two neurotoxic sphingolipids. J. Biol. Chem. 2010;285:11178–11187. doi: 10.1074/jbc.M109.092973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Wassmann S, et al. Interleukin-6 induces oxidative stress and endothelial dysfunction by overexpression of the angiotensin II type 1 receptor. Circ. Res. 2004;94:534–541. doi: 10.1161/01.RES.0000115557.25127.8D. [DOI] [PubMed] [Google Scholar]
- 289.Lachin JM, et al. Association of glycemic variability in type 1 diabetes with progression of microvascular outcomes in the diabetes control and complications trial. Diabetes Care. 2017;40:777–783. doi: 10.2337/dc16-2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Qaseem A, et al. Hemoglobin A1c targets for glycemic control with pharmacologic therapy for nonpregnant adults with type 2 diabetes mellitus: a guidance statement update from the American College of Physicians. Ann. Intern. Med. 2018;168:569–576. doi: 10.7326/M17-0939. [DOI] [PubMed] [Google Scholar]
- 291.Li H, Horke S, Förstermann U. Oxidative stress in vascular disease and its pharmacological prevention. Trends Pharm. Sci. 2013;34:313–319. doi: 10.1016/j.tips.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 292.Paul SK, Bhatt DL, Montvida O. The association of amputations and peripheral artery disease in patients with type 2 diabetes mellitus receiving sodium-glucose cotransporter type-2 inhibitors: real-world study. Eur. Heart J. 2021;42:1728–1738. doi: 10.1093/eurheartj/ehaa956. [DOI] [PubMed] [Google Scholar]
- 293.Verma S, et al. Effect of liraglutide on cardiovascular events in patients with type 2 diabetes mellitus and polyvascular disease: results of the LEADER trial. Circulation. 2018;137:2179–2183. doi: 10.1161/CIRCULATIONAHA.118.033898. [DOI] [PubMed] [Google Scholar]
- 294.Sarwar N, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215–2222. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Mohan V, Deepa R, Rani SS, Premalatha G. Prevalence of coronary artery disease and its relationship to lipids in a selected population in South India: the Chennai Urban Population Study (CUPS No. 5) J. Am. Coll. Cardiol. 2001;38:682–687. doi: 10.1016/S0735-1097(01)01415-2. [DOI] [PubMed] [Google Scholar]
- 296.Cole JB, Florez JC. Genetics of diabetes mellitus and diabetes complications. Nat. Rev. Nephrol. 2020;16:377–390. doi: 10.1038/s41581-020-0278-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Dabelea D, et al. Association of type 1 diabetes vs type 2 diabetes diagnosed during childhood and adolescence with complications during teenage years and young adulthood. JAMA. 2017;317:825–835. doi: 10.1001/jama.2017.0686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Nassif ME, et al. The SGLT2 inhibitor dapagliflozin in heart failure with preserved ejection fraction: a multicenter randomized trial. Nat. Med. 2021;27:1954–1960. doi: 10.1038/s41591-021-01536-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Oyama K, et al. Obesity and effects of dapagliflozin on cardiovascular and renal outcomes in patients with type 2 diabetes mellitus in the DECLARE-TIMI 58 trial. Eur. Heart J. 2022;43:2958–2967. doi: 10.1093/eurheartj/ehab530. [DOI] [PubMed] [Google Scholar]
- 300.Duckworth W, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N. Engl. J. Med. 2009;360:129–139. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 301.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N. Engl. J. Med. 2008;359:1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 302.Holman N, Young B, Gadsby R. Current prevalence of type 1 and type 2 diabetes in adults and children in the UK. Diabet. Med. 2015;32:1119–1120. doi: 10.1111/dme.12791. [DOI] [PubMed] [Google Scholar]
- 303.Nathan DM, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 304.Chi ZS, Lee ET, Lu M, Keen H, Bennett PH. Vascular disease prevalence in diabetic patients in China: standardised comparison with the 14 centres in the WHO multinational study of vascular disease in diabetes. Diabetologia. 2001;44:S82–S86. doi: 10.1007/PL00002944. [DOI] [PubMed] [Google Scholar]
- 305.Clarke PM, et al. Event rates, hospital utilization, and costs associated with major complications of diabetes: a multicountry comparative analysis. PLoS Med. 2010;7:e1000236. doi: 10.1371/journal.pmed.1000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Abbott CA, Malik RA, van Ross ER, Kulkarni J, Boulton AJ. Prevalence and characteristics of painful diabetic neuropathy in a large community-based diabetic population in the U.K. Diabetes Care. 2011;34:2220–2224. doi: 10.2337/dc11-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Eppens MC, et al. Prevalence of diabetes complications in adolescents with type 2 compared with type 1 diabetes. Diabetes Care. 2006;29:1300–1306. doi: 10.2337/dc05-2470. [DOI] [PubMed] [Google Scholar]
- 308.Riehle C, Bauersachs J. Of mice and men: models and mechanisms of diabetic cardiomyopathy. Basic Res Cardiol. 2018;114:2. doi: 10.1007/s00395-018-0711-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Al-Awar A, et al. Experimental diabetes mellitus in different animal models. J. Diabetes Res. 2016;2016:9051426. doi: 10.1155/2016/9051426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Poittevin M, et al. Diabetic microangiopathy: impact of impaired cerebral vasoreactivity and delayed angiogenesis after permanent middle cerebral artery occlusion on stroke damage and cerebral repair in mice. Diabetes. 2015;64:999–1010. doi: 10.2337/db14-0759. [DOI] [PubMed] [Google Scholar]
- 311.Fan B, et al. Mesenchymal stromal cell-derived exosomes ameliorate peripheral neuropathy in a mouse model of diabetes. Diabetologia. 2020;63:431–443. doi: 10.1007/s00125-019-05043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Daniels A, et al. Impaired cardiac functional reserve in type 2 diabetic db/db mice is associated with metabolic, but not structural, remodelling. Acta Physiol. 2010;200:11–22. doi: 10.1111/j.1748-1716.2010.02102.x. [DOI] [PubMed] [Google Scholar]
- 313.Yorek MS, et al. Effect of glycemic control on corneal nerves and peripheral neuropathy in streptozotocin-induced diabetic C57Bl/6J mice. J. Peripher. Nerv. Syst. 2014;19:205–217. doi: 10.1111/jns.12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Elmadbouh I, Singla DK. BMP-7 attenuates inflammation-induced pyroptosis and improves cardiac repair in diabetic cardiomyopathy. Cells. 2021;10:2640. doi: 10.3390/cells10102640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.An D, Rodrigues B. Role of changes in cardiac metabolism in development of diabetic cardiomyopathy. Am. J. Physiol. Heart Circ. Physiol. 2006;291:H1489–H1506. doi: 10.1152/ajpheart.00278.2006. [DOI] [PubMed] [Google Scholar]
- 316.Boudina S, et al. Contribution of impaired myocardial insulin signaling to mitochondrial dysfunction and oxidative stress in the heart. Circulation. 2009;119:1272–1283. doi: 10.1161/CIRCULATIONAHA.108.792101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Kim SM, et al. Targeting T helper 17 by mycophenolate mofetil attenuates diabetic nephropathy progression. Transl. Res. 2015;166:375–383. doi: 10.1016/j.trsl.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 318.Patschan D, et al. eEOC-mediated modulation of endothelial autophagy, senescence, and EnMT in murine diabetic nephropathy. Am. J. Physiol. Ren. Physiol. 2014;307:F686–F694. doi: 10.1152/ajprenal.00650.2013. [DOI] [PubMed] [Google Scholar]
- 319.Komeno M, et al. Cardio- and reno-protective effects of dipeptidyl peptidase III in diabetic mice. J. Biol. Chem. 2021;296:100761. doi: 10.1016/j.jbc.2021.100761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Sharma G, Ashhar MU, Aeri V, Katare DP. Development and characterization of late-stage diabetes mellitus and -associated vascular complications. Life Sci. 2019;216:295–304. doi: 10.1016/j.lfs.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 321.Jeong HY, et al. Chloroquine and amodiaquine enhance AMPK phosphorylation and improve mitochondrial fragmentation in diabetic tubulopathy. Sci. Rep. 2018;8:8774. doi: 10.1038/s41598-018-26858-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Platania CBM, et al. Retinal and circulating miRNA expression patterns in diabetic retinopathy: an in silico and in vivo approach. Br. J. Pharm. 2019;176:2179–2194. doi: 10.1111/bph.14665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Bucciarelli LG, et al. RAGE and modulation of ischemic injury in the diabetic myocardium. Diabetes. 2008;57:1941–1951. doi: 10.2337/db07-0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Zhao F, Gao X, Ge X, Cui J, Liu X. Cyanidin-3-o-glucoside (C3G) inhibits vascular leakage regulated by microglial activation in early diabetic retinopathy and neovascularization in advanced diabetic retinopathy. Bioengineered. 2021;12:9266–9278. doi: 10.1080/21655979.2021.1996512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Rossi S, et al. Activation of melanocortin receptors MC 1 and MC 5 attenuates retinal damage in experimental diabetic retinopathy. Mediators Inflamm. 2016;2016:7368389. doi: 10.1155/2016/7368389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Azushima K, Gurley SB, Coffman TM. Modelling diabetic nephropathy in mice. Nat. Rev. Nephrol. 2018;14:48–56. doi: 10.1038/nrneph.2017.142. [DOI] [PubMed] [Google Scholar]
- 327.Moriya J, et al. Platelet-derived growth factor C promotes revascularization in ischemic limbs of diabetic mice. J. Vasc. Surg. 2014;59:1402–1409.e1401-1404. doi: 10.1016/j.jvs.2013.04.053. [DOI] [PubMed] [Google Scholar]
- 328.Barrot J, et al. Diabetic retinopathy as a predictor of cardiovascular morbidity and mortality in subjects with type 2 diabetes. Front. Med. 2022;9:945245. doi: 10.3389/fmed.2022.945245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Cabrera DeBuc D, Somfai GM, Koller A. Retinal microvascular network alterations: potential biomarkers of cerebrovascular and neural diseases. Am. J. Physiol. Heart Circ. Physiol. 2017;312:H201–h212. doi: 10.1152/ajpheart.00201.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Mordi IR, et al. Prediction of major adverse cardiovascular events from retinal, clinical, and genomic data in individuals with type 2 diabetes: a population cohort study. Diabetes Care. 2022;45:710–716. doi: 10.2337/dc21-1124. [DOI] [PubMed] [Google Scholar]
- 331.Ørskov M, Vorum H, Larsen TB, Larsen M, Skjøth F. Retinal artery occlusion as an early indicator of macrovascular complications in diabetes. Am. J. Med. 2022;136:179–185. doi: 10.1016/j.amjmed.2022.09.012. [DOI] [PubMed] [Google Scholar]
- 332.Petitti DB, Bhatt H. Retinopathy as a risk factor for nonembolic stroke in diabetic subjects. Stroke. 1995;26:593–596. doi: 10.1161/01.STR.26.4.593. [DOI] [PubMed] [Google Scholar]
- 333.Cheung N, et al. Is diabetic retinopathy an independent risk factor for ischemic stroke? Stroke. 2007;38:398–401. doi: 10.1161/01.STR.0000254547.91276.50. [DOI] [PubMed] [Google Scholar]
- 334.Hägg S, et al. Incidence of stroke according to presence of diabetic nephropathy and severe diabetic retinopathy in patients with type 1 diabetes. Diabetes Care. 2013;36:4140–4146. doi: 10.2337/dc13-0669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Hägg S, et al. Different risk factor profiles for ischemic and hemorrhagic stroke in type 1 diabetes mellitus. Stroke. 2014;45:2558–2562. doi: 10.1161/STROKEAHA.114.005724. [DOI] [PubMed] [Google Scholar]
- 336.Lip GYH, Clementy N, Pierre B, Boyer M, Fauchier L. The impact of associated diabetic retinopathy on stroke and severe bleeding risk in diabetic patients with atrial fibrillation: the loire valley atrial fibrillation project. Chest. 2015;147:1103–1110. doi: 10.1378/chest.14-2096. [DOI] [PubMed] [Google Scholar]
- 337.Ong YT, et al. Microvascular structure and network in the retina of patients with ischemic stroke. Stroke. 2013;44:2121–2127. doi: 10.1161/STROKEAHA.113.001741. [DOI] [PubMed] [Google Scholar]
- 338.Mills SA, et al. Fractalkine-induced microglial vasoregulation occurs within the retina and is altered early in diabetic retinopathy. Proc. Natl Acad. Sci. USA. 2021;118:e2112561118. doi: 10.1073/pnas.2112561118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Cheung CY, Ikram MK, Chen C, Wong TY. Imaging retina to study dementia and stroke. Prog. Retin Eye Res. 2017;57:89–107. doi: 10.1016/j.preteyeres.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 340.Hughes AD, et al. Association of retinopathy and retinal microvascular abnormalities with stroke and cerebrovascular disease. Stroke. 2016;47:2862–2864. doi: 10.1161/STROKEAHA.116.014998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Vuong LN, et al. Ocular fundus photography of patients with focal neurologic deficits in an emergency department. Neurology. 2015;85:256–262. doi: 10.1212/WNL.0000000000001759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Little K, et al. Common pathways in dementia and diabetic retinopathy: understanding the mechanisms of diabetes-related cognitive decline. Trends Endocrinol. Metab. 2022;33:50–71. doi: 10.1016/j.tem.2021.10.008. [DOI] [PubMed] [Google Scholar]
- 343.Bertoni AG, et al. Diabetic cardiomyopathy and subclinical cardiovascular disease: the Multi-Ethnic Study of Atherosclerosis (MESA) Diabetes Care. 2006;29:588–594. doi: 10.2337/diacare.29.03.06.dc05-1501. [DOI] [PubMed] [Google Scholar]
- 344.Lee WJ, et al. Ischemic diabetic retinopathy as a possible prognostic factor for chronic kidney disease progression. Eye. 2014;28:1119–1125. doi: 10.1038/eye.2014.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Kramer CK, Retnakaran R. Concordance of retinopathy and nephropathy over time in Type 1 diabetes: an analysis of data from the Diabetes Control and Complications Trial. Diabet. Med. 2013;30:1333–1341. doi: 10.1111/dme.12296. [DOI] [PubMed] [Google Scholar]
- 346.Moriya T, et al. Diabetic retinopathy and microalbuminuria can predict macroalbuminuria and renal function decline in Japanese type 2 diabetic patients: Japan Diabetes Complications Study. Diabetes Care. 2013;36:2803–2809. doi: 10.2337/dc12-2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Yamanouchi M, et al. Retinopathy progression and the risk of end-stage kidney disease: results from a longitudinal Japanese cohort of 232 patients with type 2 diabetes and biopsy-proven diabetic kidney disease. BMJ Open Diabetes Res. Care. 2019;7:e000726. doi: 10.1136/bmjdrc-2019-000726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Park HC, et al. Diabetic retinopathy is a prognostic factor for progression of chronic kidney disease in the patients with type 2 diabetes mellitus. PLoS ONE. 2019;14:e0220506. doi: 10.1371/journal.pone.0220506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Broe R, et al. Retinal vessel calibers predict long-term microvascular complications in type 1 diabetes: the Danish Cohort of Pediatric Diabetes 1987 (DCPD1987) Diabetes. 2014;63:3906–3914. doi: 10.2337/db14-0227. [DOI] [PubMed] [Google Scholar]
- 350.Lee WJ, et al. The relationship between diabetic retinopathy and diabetic nephropathy in a population-based study in Korea (KNHANES V-2, 3) Invest. Ophthalmol. Vis. Sci. 2014;55:6547–6553. doi: 10.1167/iovs.14-15001. [DOI] [PubMed] [Google Scholar]
- 351.Bjerg L, et al. Development of microvascular complications and effect of concurrent risk factors in type 1 diabetes: a multistate model from an observational clinical cohort study. Diabetes Care. 2018;41:2297–2305. doi: 10.2337/dc18-0679. [DOI] [PubMed] [Google Scholar]
- 352.Lin HT, et al. Diabetic retinopathy as a risk factor for chronic kidney disease progression: a multicenter case(-)control study in Taiwan. Nutrients. 2019;11:509. doi: 10.3390/nu11030509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Nusinovici S, et al. Retinal microvascular signs and risk of diabetic kidney disease in asian and white populations. Sci. Rep. 2021;11:4898. doi: 10.1038/s41598-021-84464-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Seo DH, et al. Presence of carotid plaque is associated with rapid renal function decline in patients with type 2 diabetes mellitus and normal renal function. Diabetes Metab. J. 2019;43:840–853. doi: 10.4093/dmj.2018.0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Cardoso CRL, Leite NC, Salles GC, Ferreira MT, Salles GF. Aortic stiffness and ambulatory blood pressure as predictors of diabetic kidney disease: a competing risks analysis from the Rio de Janeiro type 2 diabetes cohort study. Diabetologia. 2018;61:455–465. doi: 10.1007/s00125-017-4484-z. [DOI] [PubMed] [Google Scholar]
- 356.Bjornstad P, et al. Predictors of early renal function decline in adults with type 1 diabetes: the coronary artery calcification in type 1 diabetes and the Pittsburgh epidemiology of diabetes complications studies. Diabet. Med. 2017;34:1532–1540. doi: 10.1111/dme.13430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Teliti M, et al. Risk factors for the development of micro-vascular complications of type 2 diabetes in a single-centre cohort of patients. Diab. Vasc. Dis. Res. 2018;15:424–432. doi: 10.1177/1479164118780808. [DOI] [PubMed] [Google Scholar]
- 358.Orlov S, et al. Cardiac autonomic neuropathy and early progressive renal decline in patients with nonmacroalbuminuric type 1 diabetes. Clin. J. Am. Soc. Nephrol. 2015;10:1136–1144. doi: 10.2215/CJN.11441114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Groh L, Keating ST, Joosten LAB, Netea MG, Riksen NP. Monocyte and macrophage immunometabolism in atherosclerosis. Semin. Immunopathol. 2018;40:203–214. doi: 10.1007/s00281-017-0656-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Chen MY, et al. Profile of crosstalk between glucose and lipid metabolic disturbance and diabetic cardiomyopathy: Inflammation and oxidative stress. Front. Endocrinol. 2022;13:983713. doi: 10.3389/fendo.2022.983713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Abe Y, et al. Bioenergetic characterization of mouse podocytes. Am. J. Physiol. Cell Physiol. 2010;299:C464–C476. doi: 10.1152/ajpcell.00563.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Akude E, et al. Diminished superoxide generation is associated with respiratory chain dysfunction and changes in the mitochondrial proteome of sensory neurons from diabetic rats. Diabetes. 2011;60:288–297. doi: 10.2337/db10-0818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Nakayama Y, Mukai N, Kreitzer G, Patwari P, Yoshioka J. Interaction of ARRDC4 With GLUT1 mediates metabolic stress in the ischemic heart. Circ. Res. 2022;131:510–527. doi: 10.1161/CIRCRESAHA.122.321351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.McCrimmon A, et al. Redox phospholipidomics analysis reveals specific oxidized phospholipids and regions in the diabetic mouse kidney. Redox Biol. 2022;58:102520. doi: 10.1016/j.redox.2022.102520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Jha AK, et al. Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immunity. 2015;42:419–430. doi: 10.1016/j.immuni.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 366.Shirai T, et al. The glycolytic enzyme PKM2 bridges metabolic and inflammatory dysfunction in coronary artery disease. J. Exp. Med. 2016;213:337–354. doi: 10.1084/jem.20150900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Rohlenova K, Veys K, Miranda-Santos I, De Bock K, Carmeliet P. Endothelial cell metabolism in health and disease. Trends Cell Biol. 2018;28:224–236. doi: 10.1016/j.tcb.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 368.Pingle SC, et al. Osmotic diuretics induce adenosine A1 receptor expression and protect renal proximal tubular epithelial cells against cisplatin-mediated apoptosis. J. Biol. Chem. 2004;279:43157–43167. doi: 10.1074/jbc.M405666200. [DOI] [PubMed] [Google Scholar]
- 369.Groschner LN, Waldeck-Weiermair M, Malli R, Graier WF. Endothelial mitochondria-less respiration, more integration. Pflug. Arch. 2012;464:63–76. doi: 10.1007/s00424-012-1085-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.De Bock K, et al. Role of PFKFB3-driven glycolysis in vessel sprouting. Cell. 2013;154:651–663. doi: 10.1016/j.cell.2013.06.037. [DOI] [PubMed] [Google Scholar]
- 371.Iacobini C, Vitale M, Pesce C, Pugliese G, Menini S. Diabetic complications and oxidative stress: a 20-year voyage back in time and back to the future. Antioxidants. 2021;10:727. doi: 10.3390/antiox10050727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Klein KR, et al. The SimpliciT1 Study: a randomized, double-blind, placebo-controlled phase 1b/2 adaptive study of TTP399, a hepatoselective glucokinase activator, for adjunctive treatment of type 1 diabetes. Diabetes Care. 2021;44:960–968. doi: 10.2337/dc20-2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Vella A, et al. Targeting hepatic glucokinase to treat diabetes with TTP399, a hepatoselective glucokinase activator. Sci. Transl. Med. 2019;11:eaau3441. doi: 10.1126/scitranslmed.aau3441. [DOI] [PubMed] [Google Scholar]
- 374.Yang W, et al. Dorzagliatin add-on therapy to metformin in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled phase 3 trial. Nat. Med. 2022;28:974–981. doi: 10.1038/s41591-022-01803-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Zhu D, et al. Dorzagliatin monotherapy in Chinese patients with type 2 diabetes: a dose-ranging, randomised, double-blind, placebo-controlled, phase 2 study. Lancet Diabetes Endocrinol. 2018;6:627–636. doi: 10.1016/S2213-8587(18)30105-0. [DOI] [PubMed] [Google Scholar]
- 376.Zhu D, et al. Dorzagliatin in drug-naïve patients with type 2 diabetes: a randomized, double-blind, placebo-controlled phase 3 trial. Nat. Med. 2022;28:965–973. doi: 10.1038/s41591-022-01802-6. [DOI] [PubMed] [Google Scholar]
- 377.Zhu XX, et al. Dorzagliatin (HMS5552), a novel dual-acting glucokinase activator, improves glycaemic control and pancreatic β-cell function in patients with type 2 diabetes: A 28-day treatment study using biomarker-guided patient selection. Diabetes Obes. Metab. 2018;20:2113–2120. doi: 10.1111/dom.13338. [DOI] [PubMed] [Google Scholar]
- 378.Lei L, et al. Antidiabetic potential of a novel dual-target activator of glucokinase and peroxisome proliferator activated receptor-γ. Metabolism. 2015;64:1250–1261. doi: 10.1016/j.metabol.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 379.Xu X, et al. Glucokinase in stellate ganglia cooperates with P2X3 receptor to develop cardiac sympathetic neuropathy in type 2 diabetes rats. Brain Res. Bull. 2020;165:290–297. doi: 10.1016/j.brainresbull.2020.10.004. [DOI] [PubMed] [Google Scholar]
- 380.Du X, et al. Inhibition of GAPDH activity by poly(ADP-ribose) polymerase activates three major pathways of hyperglycemic damage in endothelial cells. J. Clin. Invest. 2003;112:1049–1057. doi: 10.1172/JCI18127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Mittal M, Siddiqui MR, Tran K, Reddy SP, Malik AB. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014;20:1126–1167. doi: 10.1089/ars.2012.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Chatham JC, Young ME, Zhang J. Role of O-linked N-acetylglucosamine (O-GlcNAc) modification of proteins in diabetic cardiovascular complications. Curr. Opin. Pharm. 2021;57:1–12. doi: 10.1016/j.coph.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Caon I, et al. Cell energy metabolism and hyaluronan synthesis. J. Histochem. Cytochem. 2021;69:35–47. doi: 10.1369/0022155420929772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Eelen G, de Zeeuw P, Simons M, Carmeliet P. Endothelial cell metabolism in normal and diseased vasculature. Circ. Res. 2015;116:1231–1244. doi: 10.1161/CIRCRESAHA.116.302855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Zhao RZ, Jiang S, Zhang L, Yu ZB. Mitochondrial electron transport chain, ROS generation and uncoupling (Review) Int J. Mol. Med. 2019;44:3–15. doi: 10.3892/ijmm.2019.4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Hwang YC, et al. Central role for aldose reductase pathway in myocardial ischemic injury. FASEB J. 2004;18:1192–1199. doi: 10.1096/fj.03-1400com. [DOI] [PubMed] [Google Scholar]
- 387.Vikramadithyan RK, et al. Human aldose reductase expression accelerates diabetic atherosclerosis in transgenic mice. J. Clin. Invest. 2005;115:2434–2443. doi: 10.1172/JCI24819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Mauer SM, Steffes MW, Azar S, Brown DM. Effects of sorbinil on glomerular structure and function in long-term-diabetic rats. Diabetes. 1989;38:839–846. doi: 10.2337/diab.38.7.839. [DOI] [PubMed] [Google Scholar]
- 389.Grewal AS, Bhardwaj S, Pandita D, Lather V, Sekhon BS. Updates on aldose reductase inhibitors for management of diabetic complications and non-diabetic diseases. Mini Rev. Med. Chem. 2016;16:120–162. doi: 10.2174/1389557515666150909143737. [DOI] [PubMed] [Google Scholar]
- 390.White PJ, et al. The BCKDH kinase and phosphatase integrate BCAA and lipid metabolism via regulation of ATP-citrate lyase. Cell Metab. 2018;27:1281–1293.e1287. doi: 10.1016/j.cmet.2018.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Yoneshiro T, et al. BCAA catabolism in brown fat controls energy homeostasis through SLC25A44. Nature. 2019;572:614–619. doi: 10.1038/s41586-019-1503-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Coqueiro AY, Rogero MM, Tirapegui J. Glutamine as an anti-fatigue amino acid in sports nutrition. Nutrients. 2019;11:863. doi: 10.3390/nu11040863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Cruzat V, Macedo Rogero M, Noel Keane K, Curi R, Newsholme P. Glutamine: metabolism and immune function, supplementation and clinical translation. Nutrients. 2018;10:1564. doi: 10.3390/nu10111564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Dollet L, et al. Glutamine regulates skeletal muscle immunometabolism in type 2 diabetes. Diabetes. 2022;71:624–636. doi: 10.2337/db20-0814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Samocha-Bonet D, Chisholm DJ, Holst JJ, Greenfield JR. L-glutamine and whole protein restore first-phase insulin response and increase glucagon-like peptide-1 in type 2 diabetes patients. Nutrients. 2015;7:2101–2108. doi: 10.3390/nu7042101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Wang Y, et al. Berberine slows the progression of prediabetes to diabetes in Zucker diabetic fatty rats by enhancing intestinal secretion of glucagon-like peptide-2 and improving the gut microbiota. Front. Endocrinol. 2021;12:609134. doi: 10.3389/fendo.2021.609134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Huang H, et al. Role of glutamine and interlinked asparagine metabolism in vessel formation. EMBO J. 2017;36:2334–2352. doi: 10.15252/embj.201695518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Tavakoli S, et al. Characterization of macrophage polarization states using combined measurement of 2-deoxyglucose and glutamine accumulation: implications for imaging of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2017;37:1840–1848. doi: 10.1161/ATVBAHA.117.308848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Qi L, et al. Association between a genetic variant related to glutamic acid metabolism and coronary heart disease in individuals with type 2 diabetes. JAMA. 2013;310:821–828. doi: 10.1001/jama.2013.276305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Napolitano G, et al. A substrate-specific mTORC1 pathway underlies Birt-Hogg-Dubé syndrome. Nature. 2020;585:597–602. doi: 10.1038/s41586-020-2444-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Bodineau C, Tomé M, Murdoch PDS, Durán RV. Glutamine, MTOR and autophagy: a multiconnection relationship. Autophagy. 2022;18:2749–2750. doi: 10.1080/15548627.2022.2062875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Mathew AV, et al. Impaired amino acid and TCA metabolism and cardiovascular autonomic neuropathy progression in type 1 diabetes. Diabetes. 2019;68:2035–2044. doi: 10.2337/db19-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Zügner E, et al. Differential in vitro effects of SGLT2 inhibitors on mitochondrial oxidative phosphorylation, glucose uptake and cell metabolism. Int. J. Mol. Sci. 2022;23:7966. doi: 10.3390/ijms23147966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Kucharzewska P, Welch JE, Svensson KJ, Belting M. Ornithine decarboxylase and extracellular polyamines regulate microvascular sprouting and actin cytoskeleton dynamics in endothelial cells. Exp. Cell Res. 2010;316:2683–2691. doi: 10.1016/j.yexcr.2010.05.033. [DOI] [PubMed] [Google Scholar]
- 405.Kövamees O, et al. Arginase inhibition improves microvascular endothelial function in patients with type 2 diabetes mellitus. J. Clin. Endocrinol. Metab. 2016;101:3952–3958. doi: 10.1210/jc.2016-2007. [DOI] [PubMed] [Google Scholar]
- 406.Park SY, et al. Dietary glutamic acid and aspartic acid as biomarkers for predicting diabetic retinopathy. Sci. Rep. 2021;11:7244. doi: 10.1038/s41598-021-83165-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Viribay A, Burgos J, Fernández-Landa J, Seco-Calvo J, Mielgo-Ayuso J. Effects of arginine supplementation on athletic performance based on energy metabolism: a systematic review and meta-analysis. Nutrients. 2020;12:1300. doi: 10.3390/nu12051300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Leo F, et al. Red blood cell and endothelial eNOS independently regulate circulating nitric oxide metabolites and blood pressure. Circulation. 2021;144:870–889. doi: 10.1161/CIRCULATIONAHA.120.049606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Zhang G, et al. A ternary synergistic eNOS gene delivery system based on calcium ion and L-arginine for accelerating angiogenesis by maximizing NO production. Int. J. Nanomed. 2022;17:1987–2000. doi: 10.2147/IJN.S363168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Palmer RM, Ashton DS, Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988;333:664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- 411.Kovamees O, Shemyakin A, Eriksson M, Angelin B, Pernow J. Arginase inhibition improves endothelial function in patients with familial hypercholesterolaemia irrespective of their cholesterol levels. J. Intern Med. 2016;279:477–484. doi: 10.1111/joim.12461. [DOI] [PubMed] [Google Scholar]
- 412.Amelio I, Cutruzzolá F, Antonov A, Agostini M, Melino G. Serine and glycine metabolism in cancer. Trends Biochem. Sci. 2014;39:191–198. doi: 10.1016/j.tibs.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Tibbetts AS, Appling DR. Compartmentalization of mammalian folate-mediated one-carbon metabolism. Annu. Rev. Nutr. 2010;30:57–81. doi: 10.1146/annurev.nutr.012809.104810. [DOI] [PubMed] [Google Scholar]
- 414.Yu W, et al. One-carbon metabolism supports S-adenosylmethionine and histone methylation to drive inflammatory macrophages. Mol. Cell. 2019;75:1147–1160.e1145. doi: 10.1016/j.molcel.2019.06.039. [DOI] [PubMed] [Google Scholar]
- 415.Liao B, et al. Adipocyte fatty acid-binding protein exacerbates cerebral ischaemia injury by disrupting the blood-brain barrier. Eur. Heart J. 2020;41:3169–3180. doi: 10.1093/eurheartj/ehaa207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Bernal-Lopez RM, et al. Modulation of human monocyte CD36 by type 2 diabetes mellitus and other atherosclerotic risk factors. Eur. J. Clin. Invest. 2011;41:854–862. doi: 10.1111/j.1365-2362.2011.02475.x. [DOI] [PubMed] [Google Scholar]
- 417.Li X, Kumar A, Carmeliet P. Metabolic pathways fueling the endothelial cell drive. Annu. Rev. Physiol. 2019;81:483–503. doi: 10.1146/annurev-physiol-020518-114731. [DOI] [PubMed] [Google Scholar]
- 418.Schoors S, et al. Fatty acid carbon is essential for dNTP synthesis in endothelial cells. Nature. 2015;520:192–197. doi: 10.1038/nature14362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Genois MM, et al. CARM1 regulates replication fork speed and stress response by stimulating PARP1. Mol. Cell. 2021;81:784–800.e788. doi: 10.1016/j.molcel.2020.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Sun J, et al. PARP1 is upregulated by hyperglycemia via N6-methyladenosine modification and promotes diabetic retinopathy. Disco. Med. 2022;34:115–129. [PubMed] [Google Scholar]
- 421.Soppert J, Lehrke M, Marx N, Jankowski J, Noels H. Lipoproteins and lipids in cardiovascular disease: from mechanistic insights to therapeutic targeting. Adv. Drug Deliv. Rev. 2020;159:4–33. doi: 10.1016/j.addr.2020.07.019. [DOI] [PubMed] [Google Scholar]
- 422.Zhao M, et al. Gut microbiota production of trimethyl-5-aminovaleric acid reduces fatty acid oxidation and accelerates cardiac hypertrophy. Nat. Commun. 2022;13:1757. doi: 10.1038/s41467-022-29060-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Yan J, et al. Increased glucose uptake and oxidation in mouse hearts prevent high fatty acid oxidation but cause cardiac dysfunction in diet-induced obesity. Circulation. 2009;119:2818–2828. doi: 10.1161/CIRCULATIONAHA.108.832915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Tang X, Luo YX, Chen HZ, Liu DP. Mitochondria, endothelial cell function, and vascular diseases. Front. Physiol. 2014;5:175. doi: 10.3389/fphys.2014.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Coughlan MT, et al. RAGE-induced cytosolic ROS promote mitochondrial superoxide generation in diabetes. J. Am. Soc. Nephrol. 2009;20:742–752. doi: 10.1681/ASN.2008050514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Gu MJ, et al. Glycolaldehyde, an advanced glycation end products precursor, induces apoptosis via ROS-mediated mitochondrial dysfunction in renal mesangial cells. Antioxidants. 2022;11:934. doi: 10.3390/antiox11050934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Dromparis P, Michelakis ED. Mitochondria in vascular health and disease. Annu. Rev. Physiol. 2013;75:95–126. doi: 10.1146/annurev-physiol-030212-183804. [DOI] [PubMed] [Google Scholar]
- 428.Kim YM, et al. ROS-induced ROS release orchestrated by Nox4, Nox2, and mitochondria in VEGF signaling and angiogenesis. Am. J. Physiol. Cell Physiol. 2017;312:C749–c764. doi: 10.1152/ajpcell.00346.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Xirouchaki CE, et al. Skeletal muscle NOX4 is required for adaptive responses that prevent insulin resistance. Sci. Adv. 2021;7:eabl4988. doi: 10.1126/sciadv.abl4988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Chen C, Li L, Zhou HJ, Min W. The role of NOX4 and TRX2 in angiogenesis and their potential cross-talk. Antioxidants. 2017;6:42. doi: 10.3390/antiox6020042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Han Y, et al. Reactive oxygen species promote tubular injury in diabetic nephropathy: the role of the mitochondrial ros-txnip-nlrp3 biological axis. Redox Biol. 2018;16:32–46. doi: 10.1016/j.redox.2018.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Ciciliot S, Fadini GP. Modulation of obesity and insulin resistance by the redox enzyme and adaptor protein p66(Shc) Int. J. Mol. Sci. 2019;20:985. doi: 10.3390/ijms20040985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Bravo-Sagua R, et al. Calcium transport and signaling in mitochondria. Compr. Physiol. 2017;7:623–634. doi: 10.1002/cphy.c160013. [DOI] [PubMed] [Google Scholar]
- 434.Liu Z, et al. Diabetes mellitus exacerbates post-myocardial infarction heart failure by reducing sarcolipin promoter methylation. ESC Heart Fail. 2020;7:1935–1948. doi: 10.1002/ehf2.12789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Wheaton WW, et al. Metformin inhibits mitochondrial complex I of cancer cells to reduce tumorigenesis. Elife. 2014;3:e02242. doi: 10.7554/eLife.02242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Foretz M, Guigas B, Viollet B. Understanding the glucoregulatory mechanisms of metformin in type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2019;15:569–589. doi: 10.1038/s41574-019-0242-2. [DOI] [PubMed] [Google Scholar]
- 437.Bugger H, et al. Tissue-specific remodeling of the mitochondrial proteome in type 1 diabetic akita mice. Diabetes. 2009;58:1986–1997. doi: 10.2337/db09-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Yaribeygi H, Farrokhi FR, Butler AE, Sahebkar A. Insulin resistance: review of the underlying molecular mechanisms. J. Cell Physiol. 2019;234:8152–8161. doi: 10.1002/jcp.27603. [DOI] [PubMed] [Google Scholar]
- 439.Welsh GI, et al. Insulin signaling to the glomerular podocyte is critical for normal kidney function. Cell Metab. 2010;12:329–340. doi: 10.1016/j.cmet.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Song W, et al. SH2B regulation of growth, metabolism, and longevity in both insects and mammals. Cell Metab. 2010;11:427–437. doi: 10.1016/j.cmet.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Osorio H, et al. Effect of phlorizin on SGLT2 expression in the kidney of diabetic rats. J. Nephrol. 2010;23:541–546. [PubMed] [Google Scholar]
- 442.Zhang H, et al. Podocyte-specific overexpression of GLUT1 surprisingly reduces mesangial matrix expansion in diabetic nephropathy in mice. Am. J. Physiol. Ren. Physiol. 2010;299:F91–F98. doi: 10.1152/ajprenal.00021.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Chang KC, Liang JT, Tsai PS, Wu MS, Hsu KL. Prevention of arterial stiffening by pyridoxamine in diabetes is associated with inhibition of the pathogenic glycation on aortic collagen. Br. J. Pharm. 2009;157:1419–1426. doi: 10.1111/j.1476-5381.2009.00309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Kass DA, et al. Improved arterial compliance by a novel advanced glycation end-product crosslink breaker. Circulation. 2001;104:1464–1470. doi: 10.1161/hc3801.097806. [DOI] [PubMed] [Google Scholar]
- 445.Shapiro BP, et al. Advanced glycation end products accumulate in vascular smooth muscle and modify vascular but not ventricular properties in elderly hypertensive canines. Circulation. 2008;118:1002–1010. doi: 10.1161/CIRCULATIONAHA.108.777326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Thijssen DH, Carter SE, Green DJ. Arterial structure and function in vascular ageing: are you as old as your arteries? J. Physiol. 2016;594:2275–2284. doi: 10.1113/JP270597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Goldin A, Beckman JA, Schmidt AM, Creager MA. Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation. 2006;114:597–605. doi: 10.1161/CIRCULATIONAHA.106.621854. [DOI] [PubMed] [Google Scholar]
- 448.Zhang L, et al. Knockout RAGE alleviates cardiac fibrosis through repressing endothelial-to-mesenchymal transition (EndMT) mediated by autophagy. Cell Death Dis. 2021;12:470. doi: 10.1038/s41419-021-03750-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Ott C, et al. Role of advanced glycation end products in cellular signaling. Redox Biol. 2014;2:411–429. doi: 10.1016/j.redox.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Wautier JL, Wautier MP. Cellular and molecular aspects of blood cell-endothelium interactions in vascular disorders. Int. J. Mol. Sci. 2020;21:5315. doi: 10.3390/ijms21155315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Yan SF, Ramasamy R, Schmidt AM. The receptor for advanced glycation endproducts (RAGE) and cardiovascular disease. Expert Rev. Mol. Med. 2009;11:e9. doi: 10.1017/S146239940900101X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Li H, et al. Expression and cell distribution of receptor for advanced glycation end-products in the rat cortex following experimental subarachnoid hemorrhage. Brain Res. 2014;1543:315–323. doi: 10.1016/j.brainres.2013.11.023. [DOI] [PubMed] [Google Scholar]
- 453.Wautier JL, Schmidt AM. Protein glycation: a firm link to endothelial cell dysfunction. Circ. Res. 2004;95:233–238. doi: 10.1161/01.RES.0000137876.28454.64. [DOI] [PubMed] [Google Scholar]
- 454.Hu P, Lai D, Lu P, Gao J, He H. ERK and Akt signaling pathways are involved in advanced glycation end product-induced autophagy in rat vascular smooth muscle cells. Int. J. Mol. Med. 2012;29:613–618. doi: 10.3892/ijmm.2012.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Qin J, et al. AKF-PD alleviates diabetic nephropathy via blocking the RAGE/AGEs/NOX and PKC/NOX Pathways. Sci. Rep. 2019;9:4407. doi: 10.1038/s41598-018-36344-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Hu R, et al. Salidroside ameliorates endothelial inflammation and oxidative stress by regulating the AMPK/NF-κB/NLRP3 signaling pathway in AGEs-induced HUVECs. Eur. J. Pharm. 2020;867:172797. doi: 10.1016/j.ejphar.2019.172797. [DOI] [PubMed] [Google Scholar]
- 457.Hammes HP. Diabetic retinopathy: hyperglycaemia, oxidative stress and beyond. Diabetologia. 2018;61:29–38. doi: 10.1007/s00125-017-4435-8. [DOI] [PubMed] [Google Scholar]
- 458.Zhan J, Chen C, Wang DW, Li H. Hyperglycemic memory in diabetic cardiomyopathy. Front. Med. 2022;16:25–38. doi: 10.1007/s11684-021-0881-2. [DOI] [PubMed] [Google Scholar]
- 459.Kowluru RA, Mohammad G. Epigenetic modifications in diabetes. Metabolism. 2022;126:154920. doi: 10.1016/j.metabol.2021.154920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Gutierrez JA, et al. Prevalence and outcomes of polyvascular (coronary, peripheral, or cerebrovascular) disease in patients with diabetes mellitus (from the SAVOR-TIMI 53 Trial) Am. J. Cardiol. 2019;123:145–152. doi: 10.1016/j.amjcard.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 461.Chinese College of Cardiovascular Physicians, t.P.o.C.E.C.o.t.R.A.a.M.o.P.D.i.P.w.T.D.M.e. Chinese expert consensus on the risk assessment and management of panvascular disease in patients with type 2 diabetes mellitus (2022 edition). 14, 1017-1034 (2022).
- 462.Natarajan R. Epigenetic mechanisms in diabetic vascular complications and metabolic memory: the 2020 Edwin Bierman Award Lecture. Diabetes. 2021;70:328–337. doi: 10.2337/dbi20-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Prattichizzo F, de Candia P, De Nigris V, Nicolucci A, Ceriello A. Legacy effect of intensive glucose control on major adverse cardiovascular outcome: systematic review and meta-analyses of trials according to different scenarios. Metabolism. 2020;110:154308. doi: 10.1016/j.metabol.2020.154308. [DOI] [PubMed] [Google Scholar]
- 464.Artasensi A, Pedretti A, Vistoli G, Fumagalli L. Type 2 diabetes mellitus: a review of multi-target drugs. Molecules. 2020;25:1987. doi: 10.3390/molecules25081987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Kerru N, Singh-Pillay A, Awolade P, Singh P. Current anti-diabetic agents and their molecular targets: a review. Eur. J. Med. Chem. 2018;152:436–488. doi: 10.1016/j.ejmech.2018.04.061. [DOI] [PubMed] [Google Scholar]
- 466.Goswami SK, Ranjan P, Dutta RK, Verma SK. Management of inflammation in cardiovascular diseases. Pharm. Res. 2021;173:105912. doi: 10.1016/j.phrs.2021.105912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Jin ZQ. MicroRNA targets and biomarker validation for diabetes-associated cardiac fibrosis. Pharm. Res. 2021;174:105941. doi: 10.1016/j.phrs.2021.105941. [DOI] [PubMed] [Google Scholar]
- 468.Lhamyani S, et al. miR-21 mimic blocks obesity in mice: a novel therapeutic option. Mol. Ther. Nucleic Acids. 2021;26:401–416. doi: 10.1016/j.omtn.2021.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Liu L, et al. BMP-7 inhibits renal fibrosis in diabetic nephropathy via miR-21 downregulation. Life Sci. 2019;238:116957. doi: 10.1016/j.lfs.2019.116957. [DOI] [PubMed] [Google Scholar]
- 470.Lu JM, Zhang ZZ, Ma X, Fang SF, Qin XH. Repression of microRNA-21 inhibits retinal vascular endothelial cell growth and angiogenesis via PTEN dependent-PI3K/Akt/VEGF signaling pathway in diabetic retinopathy. Exp. Eye Res. 2020;190:107886. doi: 10.1016/j.exer.2019.107886. [DOI] [PubMed] [Google Scholar]
- 471.Faulkner A, et al. Multi-omics analysis of diabetic heart disease in the db/db model reveals potential targets for treatment by a longevity-associated gene. Cells. 2020;9:1283. doi: 10.3390/cells9051283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Lotta LA, et al. A cross-platform approach identifies genetic regulators of human metabolism and health. Nat. Genet. 2021;53:54–64. doi: 10.1038/s41588-020-00751-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Fu Q, et al. Traditional Chinese medicine foot bath combined with acupoint massage for the treatment of diabetic peripheral neuropathy: a systematic review and meta-analysis of 31 RCTs. Diabetes Metab. Res. Rev. 2020;36:e3218. doi: 10.1002/dmrr.3218. [DOI] [PubMed] [Google Scholar]
- 474.Wang J, et al. Research progress on traditional Chinese medicine syndromes of diabetes mellitus. Biomed. Pharmacother. 2020;121:109565. doi: 10.1016/j.biopha.2019.109565. [DOI] [PubMed] [Google Scholar]
- 475.Rosenstock J, et al. Effect of linagliptin vs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk: the CARMELINA randomized clinical trial. JAMA. 2019;321:69–79. doi: 10.1001/jama.2018.18269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.Gantz I, et al. A randomized, placebo-controlled study of the cardiovascular safety of the once-weekly DPP-4 inhibitor omarigliptin in patients with type 2 diabetes mellitus. Cardiovasc. Diabetol. 2017;16:112. doi: 10.1186/s12933-017-0593-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Green JB, et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 2015;373:232–242. doi: 10.1056/NEJMoa1501352. [DOI] [PubMed] [Google Scholar]
- 478.Ferrannini G, et al. Similar cardiovascular outcomes in patients with diabetes and established or high risk for coronary vascular disease treated with dulaglutide with and without baseline metformin. Eur. Heart J. 2021;42:2565–2573. doi: 10.1093/eurheartj/ehaa777. [DOI] [PubMed] [Google Scholar]
- 479.Husain M, et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 2019;381:841–851. doi: 10.1056/NEJMoa1901118. [DOI] [PubMed] [Google Scholar]
- 480.Holman RR, et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 2017;377:1228–1239. doi: 10.1056/NEJMoa1612917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Marso SP, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 2016;375:311–322. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Pfeffer MA, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N. Engl. J. Med. 2015;373:2247–2257. doi: 10.1056/NEJMoa1509225. [DOI] [PubMed] [Google Scholar]
- 483.Fitchett D, et al. Heart failure outcomes with empagliflozin in patients with type 2 diabetes at high cardiovascular risk: results of the EMPA-REG OUTCOME® trial. Eur. Heart J. 2016;37:1526–1534. doi: 10.1093/eurheartj/ehv728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484.Rådholm K, et al. Canagliflozin and heart failure in type 2 diabetes mellitus: results from the CANVAS program. Circulation. 2018;138:458–468. doi: 10.1161/CIRCULATIONAHA.118.034222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485.Neal B, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N. Engl. J. Med. 2017;377:644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 486.Solomon SD, et al. Baseline characteristics of patients with HF with mildly reduced and preserved ejection fraction: DELIVER trial. JACC Heart Fail. 2022;10:184–197. doi: 10.1016/j.jchf.2021.11.006. [DOI] [PubMed] [Google Scholar]
- 487.Perkovic V, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N. Engl. J. Med. 2019;380:2295–2306. doi: 10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
- 488.Wiviott SD, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 2019;380:347–357. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 489.Zinman B, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N. Engl. J. Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 490.Bergmark BA, et al. Metformin use and clinical outcomes among patients with diabetes mellitus with or without heart failure or kidney dysfunction: observations from the SAVOR-TIMI 53 trial. Circulation. 2019;140:1004–1014. doi: 10.1161/CIRCULATIONAHA.119.040144. [DOI] [PubMed] [Google Scholar]
- 491.Vaccaro O, et al. Effects on the incidence of cardiovascular events of the addition of pioglitazone versus sulfonylureas in patients with type 2 diabetes inadequately controlled with metformin (TOSCA.IT): a randomised, multicentre trial. Lancet Diabetes Endocrinol. 2017;5:887–897. doi: 10.1016/S2213-8587(17)30317-0. [DOI] [PubMed] [Google Scholar]
- 492.Erdmann E, Harding S, Lam H, Perez A. Ten-year observational follow-up of PROactive: a randomized cardiovascular outcomes trial evaluating pioglitazone in type 2 diabetes. Diabetes Obes. Metab. 2016;18:266–273. doi: 10.1111/dom.12608. [DOI] [PubMed] [Google Scholar]
- 493.Yoshii H, et al. Effects of pioglitazone on macrovascular events in patients with type 2 diabetes mellitus at high risk of stroke: the PROFIT-J study. J. Atheroscler. Thromb. 2014;21:563–573. [PubMed] [Google Scholar]
- 494.Marso SP, et al. Efficacy and safety of degludec versus glargine in type 2 diabetes. N. Engl. J. Med. 2017;377:723–732. doi: 10.1056/NEJMoa1615692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 495.Cukierman-Yaffe T, et al. Effects of basal insulin glargine and omega-3 fatty acid on cognitive decline and probable cognitive impairment in people with dysglycaemia: a substudy of the ORIGIN trial. Lancet Diabetes Endocrinol. 2014;2:562–572. doi: 10.1016/S2213-8587(14)70062-2. [DOI] [PubMed] [Google Scholar]
- 496.Gerstein HC, et al. Basal insulin and cardiovascular and other outcomes in dysglycemia. N. Engl. J. Med. 2012;367:319–328. doi: 10.1056/NEJMoa1203858. [DOI] [PubMed] [Google Scholar]
- 497.The ACCORD Study Group. Nine-year effects of 3.7 years of intensive glycemic control on cardiovascular outcomes. Diabetes Care39, 701–708 (2016). [DOI] [PMC free article] [PubMed]
- 498.Gæde P, et al. Beneficial impact of intensified multifactorial intervention on risk of stroke: outcome of 21 years of follow-up in the randomised Steno-2 Study. Diabetologia. 2019;62:1575–1580. doi: 10.1007/s00125-019-4920-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499.Oellgaard J, et al. Reduced risk of heart failure with intensified multifactorial intervention in individuals with type 2 diabetes and microalbuminuria: 21 years of follow-up in the randomised Steno-2 study. Diabetologia. 2018;61:1724–1733. doi: 10.1007/s00125-018-4642-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 500.Reaven PD, et al. Intensive glucose control in patients with type 2 diabetes - 15-year follow-up. N. Engl. J. Med. 2019;380:2215–2224. doi: 10.1056/NEJMoa1806802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501.Lincoff AM, et al. Effect of aleglitazar on cardiovascular outcomes after acute coronary syndrome in patients with type 2 diabetes mellitus: the AleCardio randomized clinical trial. JAMA. 2014;311:1515–1525. doi: 10.1001/jama.2014.3321. [DOI] [PubMed] [Google Scholar]
- 502.Perkovic V, et al. Effects of linagliptin on cardiovascular and kidney outcomes in people with normal and reduced kidney function: secondary analysis of the CARMELINA randomized trial. Diabetes Care. 2020;43:1803–1812. doi: 10.2337/dc20-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503.Gerstein HC, et al. Dulaglutide and renal outcomes in type 2 diabetes: an exploratory analysis of the REWIND randomised, placebo-controlled trial. Lancet. 2019;394:131–138. doi: 10.1016/S0140-6736(19)31150-X. [DOI] [PubMed] [Google Scholar]
- 504.Mosenzon O, et al. Efficacy and safety of oral semaglutide in patients with type 2 diabetes and moderate renal impairment (PIONEER 5): a placebo-controlled, randomised, phase 3a trial. Lancet Diabetes Endocrinol. 2019;7:515–527. doi: 10.1016/S2213-8587(19)30192-5. [DOI] [PubMed] [Google Scholar]
- 505.Ludvik B, et al. Once-weekly tirzepatide versus once-daily insulin degludec as add-on to metformin with or without SGLT2 inhibitors in patients with type 2 diabetes (SURPASS-3): a randomised, open-label, parallel-group, phase 3 trial. Lancet. 2021;398:583–598. doi: 10.1016/S0140-6736(21)01443-4. [DOI] [PubMed] [Google Scholar]
- 506.Heerspink HJL, et al. Dapagliflozin in patients with chronic kidney disease. N. Engl. J. Med. 2020;383:1436–1446. doi: 10.1056/NEJMoa2024816. [DOI] [PubMed] [Google Scholar]
- 507.Bhatt DL, et al. Sotagliflozin in patients with diabetes and chronic kidney disease. N. Engl. J. Med. 2021;384:129–139. doi: 10.1056/NEJMoa2030186. [DOI] [PubMed] [Google Scholar]
- 508.Cherney DZI, et al. Effects of ertugliflozin on kidney composite outcomes, renal function and albuminuria in patients with type 2 diabetes mellitus: an analysis from the randomised VERTIS CV trial. Diabetologia. 2021;64:1256–1267. doi: 10.1007/s00125-021-05407-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 509.Tofte, N. et al. Early detection of diabetic kidney disease by urinary proteomics and subsequent intervention with spironolactone to delay progression (PRIORITY): a prospective observational study and embedded randomised placebo-controlled trial. Lancet Diab Endocrinol.8, 301–312 (2020). [DOI] [PubMed]
- 510.Heerspink, H. J. L. et al. Atrasentan and renal events in patients with type 2 diabetes and chronic kidney disease (SONAR): a double-blind, randomised, placebo-controlled trial. Lancet393, 1937–1947 (2019). [DOI] [PubMed]
- 511.Fernandes VHR, et al. Dapagliflozin increases retinal thickness in type 2 diabetic patients as compared with glibenclamide: a randomized controlled trial. Diabetes Metab. 2021;47:101280. doi: 10.1016/j.diabet.2021.101280. [DOI] [PubMed] [Google Scholar]
- 512.Wykoff CC, et al. Efficacy, durability, and safety of intravitreal faricimab with extended dosing up to every 16 weeks in patients with diabetic macular oedema (YOSEMITE and RHINE): two randomised, double-masked, phase 3 trials. Lancet. 2022;399:741–755. doi: 10.1016/S0140-6736(22)00018-6. [DOI] [PubMed] [Google Scholar]
- 513.Khanani AM, et al. MERLIN: phase 3a, multicenter, randomized, double-masked trial of brolucizumab in participants with neovascular age-related macular degeneration and persistent retinal fluid. Ophthalmology. 2022;129:974–985. doi: 10.1016/j.ophtha.2022.04.028. [DOI] [PubMed] [Google Scholar]
- 514.Kunimoto D, et al. Efficacy and safety of abicipar in neovascular age-related macular degeneration: 52-week results of phase 3 randomized controlled study. Ophthalmology. 2020;127:1331–1344. doi: 10.1016/j.ophtha.2020.03.035. [DOI] [PubMed] [Google Scholar]
- 515.Bonora BM, et al. Fenofibrate increases circulating haematopoietic stem cells in people with diabetic retinopathy: a randomised, placebo-controlled trial. Diabetologia. 2021;64:2334–2344. doi: 10.1007/s00125-021-05532-1. [DOI] [PubMed] [Google Scholar]
- 516.Hancock ML, et al. Insulin receptor associates with promoters genome-wide and regulates gene expression. Cell. 2019;177:722–736.e722. doi: 10.1016/j.cell.2019.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 517.Watson LS, et al. Hyperinsulinemia alters insulin receptor presentation and internalization in brain microvascular endothelial cells. Diab. Vasc. Dis. Res. 2022;19:14791641221118626. doi: 10.1177/14791641221118626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 518.Mitrofanova A, et al. SMPDL3b modulates insulin receptor signaling in diabetic kidney disease. Nat. Commun. 2019;10:2692. doi: 10.1038/s41467-019-10584-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519.Riehle C, et al. Insulin receptor substrates differentially exacerbate insulin-mediated left ventricular remodeling. JCI Insight. 2020;5:e134920. doi: 10.1172/jci.insight.134920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 520.Rathjen T, et al. Endothelial cell insulin signaling regulates CXCR4 (C-X-C motif chemokine receptor 4) and limits leukocyte adhesion to endothelium. Arterioscler. Thromb. Vasc. Biol. 2022;42:e217–e227. doi: 10.1161/ATVBAHA.122.317476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 521.Sun X, et al. Commutative regulation between endothelial NO synthase and insulin receptor substrate 2 by microRNAs. J. Mol. Cell Biol. 2019;11:509–520. doi: 10.1093/jmcb/mjy055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 522.Hashimoto S, et al. Roles of insulin receptor substrates (IRS) in renal function and renal hemodynamics. PLoS ONE. 2020;15:e0242332. doi: 10.1371/journal.pone.0242332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 523.Xi G, Shen X, Wai C, White MF, Clemmons DR. Hyperglycemia induces vascular smooth muscle cell dedifferentiation by suppressing insulin receptor substrate-1-mediated p53/KLF4 complex stabilization. J. Biol. Chem. 2019;294:2407–2421. doi: 10.1074/jbc.RA118.005398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524.Yang C, et al. Targeting QKI-7 in vivo restores endothelial cell function in diabetes. Nat. Commun. 2020;11:3812. doi: 10.1038/s41467-020-17468-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 525.Ahmed, T. et al. EPDR1 is a noncanonical effector of insulin-mediated angiogenesis regulated by an endothelial-specific TGF-β receptor complex. J. Biol. Chem.298, 102297 (2022). [DOI] [PMC free article] [PubMed]
- 526.Zong, J. et al. Impact of Insulin Receptor Substrate-1 rs956115 and CYP2C19 rs4244285 Genotypes on Clinical Outcome of Patients Undergoing Percutaneous Coronary Intervention. J. Am. Heart Assoc.11, e025058 (2022). [DOI] [PMC free article] [PubMed]
- 527.Oral EA, et al. Inhibition of IKKɛ and TBK1 Improves Glucose Control in a Subset of Patients with Type 2 Diabetes. Cell Metab. 2017;26:157–170.e157. doi: 10.1016/j.cmet.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 528.Cheng, Y. C., Chiu, Y. M., Dai, Z. K. & Wu, B. N. Loganin Ameliorates Painful Diabetic Neuropathy by Modulating Oxidative Stress, Inflammation and Insulin Sensitivity in Streptozotocin-Nicotinamide-Induced Diabetic Rats. Cells10, (2021). [DOI] [PMC free article] [PubMed]
- 529.Marfella, R. et al. Sodium/glucose cotransporter 2 (SGLT2) inhibitors improve cardiac function by reducing JunD expression in human diabetic hearts. Metabolism127, 154936 (2022). [DOI] [PubMed]