Abstract
Background
There is limited knowledge about T cell responses in patients with multiple sclerosis (MS) after 3 doses of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) mRNA vaccine.
Objectives
Assess the SARS-CoV-2 spike antibody and T cell responses in MS patients and healthy controls (HCs) after 2 doses (2-vax) and 3 doses (3-vax) of SARS-CoV-2 mRNA vaccination.
Methods
We studied seroconversion rates and T cell responses by flow cytometry in HC and MS patients on fingolimod or ocrelizumab.
Results
After 2-vax, 8/33 (24.2%) patients in ocrelizumab group, 5/7 (71.4%) in fingolimod group, and 29/29 (100%) in HC group (P = 5.7 × 10−11) seroconverted. After 3-vax, 9/22 (40.9%) patients in ocrelizumab group, 19/21 (90.5%) in fingolimod group, and 7/7 (100%) in HC group seroconverted (P = 0.0003). The percentage of SARS-CoV-2 peptide reactive total CD4+ T cells increased in HC and ocrelizumab group but not in fingolimod group after 2-vax and 3-vax (P < 0.0001). The percentage of IFNγ and TNFα producing total CD4+ and CD8+ T cells increased in fingolimod group as compared to HC and ocrelizumab group after 2-vax and 3-vax (P < 0.0001).
Conclusions
MS patients on ocrelizumab and fingolimod had attenuated humoral responses, but preserved cytokine producing T cell responses compared to HCs after SARS-CoV-2 mRNA vaccination.
Clinical Trials Registration
Keywords: Multiple sclerosis, COVID-19, mRNA vaccine, T cell response, disease modifying therapies
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the disease caused by it, coronavirus disease 2019 (COVID-19), has posed many challenges for patients with multiple sclerosis (MS) especially those on high efficacy immunotherapies. Vaccination against COVID-19 is safe in patients with MS,1,2 but patients on certain disease modifying therapies (DMTs) may have decreased humoral and cell-mediated responses to vaccines. Prior studies have shown that humoral responses to vaccination are decreased in patients on anti-CD20 therapy with preserved cell-mediated responses, and that patients on sphingosine 1-phosphate (S1P) receptor-targeting therapies have decreased humoral and cell-mediated responses after 2 doses of vaccine.3–7
Third doses of vaccine in MS patients on a variety of DMTs were safe without increasing the risk of relapse activity. 8 However, the impact of a 3rd dose of vaccine on humoral and cell-mediated responses in patients on high efficacy therapies is still under investigation. Studies to date have shown that in patients on anti-CD20 therapy and fingolimod a 3rd dose may modestly increase anti-SARS-CoV-2 spike antibody levels. 9 A recent study that examined the capacity of T cells from ocrelizumab treated patients to respond to Delta and Omicron spike protein variants showed that the T cell response was increased after the 3rd dose. 10
The goal of our study is to characterize humoral and cell-mediated responses after 2 doses (2-vax) and 3 doses (3-vax) of mRNA vaccination in MS patients on high efficacy immunotherapy and in healthy controls (HC). We also specifically evaluated memory T cell subsets and cytokine producing CD4 and CD8 T cells, which provides more detailed analyses than previously reported.
Materials and methods
Participants and blood samples
Subjects were selected from a research study at the Brigham's Multiple Sclerosis Center approved by the Massachusetts General Brigham Human Research Committee (IRB # 2021P001156). The inclusion criteria for the study were: MS patients meeting 2017 McDonald Criteria, 11 aged 18–65 on fingolimod or ocrelizumab for at least 3 months prior to their 1st mRNA vaccine (either BNT162b2, Pfizer-BioNTech, or mRNA-1273, Moderna) and HCs who also received 2 or 3 doses of mRNA vaccines. After informed consent, blood samples were collected 2–3 months after the 2nd mRNA vaccine dose (2-vax) and were assessed for SARS-CoV-2 spike antibody, nucleocapsid and immunoglobulin levels (Supplementary Material, Appendix A). Some MS patients and HC received a 3rd mRNA vaccine (3-vax) and had additional blood samples collected 1–2 months later. Patients who developed COVID-19 infection or had a positive nucleocapsid antibody (<1.00 COI) were excluded. Demographic information for each subject, DMT and MS history was extracted from the Harvard Multiple Sclerosis Patient Database (2002P001045).
Cell stimulation assay and FACS analysis
Peripheral blood mononuclear cells (PBMCs) from HC-2-vax (n = 8), fingolimod-2-vax (n = 6), ocrelizumab-2-vax (n = 10), HC-3-vax (n = 5) fingolimod-3-vax (n = 10) and ocrelizumab-3-vax (n = 9) were isolated by density gradient centrifugation and stimulated with 4 μg/ml of PepTivator® SARS-CoV-2 Prot_S. Cell stimulation assay and flow cytometric analysis is described in Supplementary Materials, Appendix B. Graphs were made using GraphPad Prism version 8.4.2 (464).
Statistical analysis
The difference in spike antibody levels between groups was assessed with the Kruskal–Wallis test for three groups comparisons. The difference in seroconversion rates between groups was assessed with the Fisher's exact test. Paired samples after 2-vax and 3-vax were compared using a Wilcoxon signed rank test and McNemar's test. Association between spike antibody levels and immunoglobulins was examined using Spearman's correlation coefficient. Differences in T cell responses between groups was determined with ordinary one-way ANOVA and Sidak's multiple comparisons test. We used P < 0.0001, P < 0.0003, P < 0.005, and P < 0.05 to indicate statistical significance. Statistical analyses were performed in the statistical package R (http://www.r-project.org/) for antibody responses and GraphPad Prism version 8.4.2 (464) for T cell responses.
Results
Baseline demographics of enrolled participants
Second dose vaccine group
The 2-vax cohort consisted of 33 ocrelizumab patients, 7 fingolimod patients and 29 HC. Blood samples were drawn at a median [IQR] of 83 [24] days after 2nd dose of mRNA vaccine for the ocrelizumab group, 95 [19] days for the fingolimod group, and 76 [24] days for the HC group. Additional participant demographic data and information on the subset of participants that were examined for T cell responsivity are shown in Table 1.
Table 1.
Demographics of participants.
| Ocrelizumab patients after 2nd vaccine | Ocrelizumab patients after 3rd vaccination | Fingolimod patients after 2nd vaccine | Fingolimod patients after 3rd vaccine | Healthy controls after 2nd vaccine | Healthy controls after 3rd vaccine | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All (n = 33) | T-cell (n = 10) | All (n = 22) | T-cell (n = 9) | All (n = 7) | T-cell (n = 6) | All (n = 21) | T-cell (n = 10) | All (n = 29) | T-cell (n = 8) | All (n = 7) | T-cell (n = 5) | |
| Age, median [IQR] years | 54.9 [12.3] | 47.2 [12.7] | 54.5 [13.0] | 53.9 [9] | 51.1 [4.6] | 49.9 [4.2] | 51.8 [9.9] | 50.5 [11.25] | 36.6 [25.4] | 52.0 [19.5] | 27.8 [15.4] | 27.8 [5.4] |
| Female, n (%) | 24 (72.7%) | 7 (70%) | 16 (72.7%) | 6 (66.7%) | 5 (71.4%) | 4 (66.7%) | 16 (76.2%) | 8 (80%) | 13 (44.8%) | 5 (62.5%) | 6 (85.7%) | 4 (80%) |
| EDSS, median [IQR] | 1.5 [4.5] | 2.5 [3.9] | 2.5 [1.9] | 3 [2] | 1.5 [1] | 1.25 [0.5] | 1.5 [1] | 1.75 [0.5] | NA | NA | NA | NA |
| Time on DMT prior to first vaccine, median [IQR] years | 2.07 [1.8] | 2.54 [1.8] | 2.47 [1.1] | 3.01 [0.7] | 8.24 [1.6] | 7.79 [1.4] | 8.0 [3.8] | 5.24 [4.2] | NA | NA | NA | NA |
| Type of MS | ||||||||||||
| RRMS | 22 | 6 | 17 | 6 | 6 | 6 | 21 | 10 | NA | NA | NA | NA |
| SPMS | 8 | 3 | 4 | 2 | 1 | 0 | 0 | 0 | NA | NA | NA | NA |
| PPMS | 3 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | NA | NA | NA | NA |
| Time sample after 2nd dose vaccine, median [IQR] days | 83 [24] | 89.5 [19] | NA | NA | 95 [19] | 91.5 [25] | NA | NA | 76 [24] | 78.5 [17.5] | NA | NA |
| Time sample after 3rd dose vaccine, median [IQR] days | NA | NA | 62 [39.8] | 83 [17] | NA | NA | 62 [4] | 63 [5.8] | NA | NA | 70 [7] | 64 [6] |
| Time between 2nd and 3rd dose vaccine, median [IQR] days | NA | NA | 115.5 [32.5] | 104 [48] | NA | NA | 132 [39] | 127 [34.25] | NA | NA | 275 [7.5] | 276 [2] |
| Time between ocrelizumab infusion and 1st vaccine, median [IQR] days | 90 [42] | 92 [17] | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Time between ocrelizumab infusion and 3rd vaccine, median [IQR] days | NA | NA | 88 [43] | 84 [88] | NA | NA | NA | NA | NA | NA | NA | NA |
| Vaccine type, primary | ||||||||||||
| mRNA-1273 | 9 | 3 | 7 | 5 | 1 | 1 | 8 | 4 | 10 | 2 | 5 | 3 |
| BNT162b2 | 24 | 7 | 15 | 4 | 6 | 5 | 13 | 6 | 19 | 6 | 2 | 2 |
| Vaccine type, 3rd dose | ||||||||||||
| mRNA-1273 a | NA | NA | 6 | 3 | NA | NA | 8 | 4 | NA | NA | 5 | 3 |
| BNT162b2 | NA | NA | 16 | 6 | NA | NA | 13 | 6 | NA | NA | 2 | 2 |
Of the 14 mRNA-1273 3rd doses, 9 patients received 0.5 mL (100 mcg) the other 5 patients had unknown quantities.
NA: not applicable; RRMS: relapsing remitting MS; SPMS: secondary progressive MS; PPMS: primary progressive MS.
Third dose vaccine group
The 3-vax cohort consisted of 22 ocrelizumab patients, 21 fingolimod patients, and 7 HC. Blood samples were drawn a median [IQR] of 62 [39.8] days after the 3rd dose of mRNA vaccine for the ocrelizumab group, 62 [4] days for the fingolimod group, and 70 [7] days for the HC group. Additional participant demographic data and information on the subset of participants that were examined for T cell responsivity are shown in Table 1.
Anti-SARS-CoV-2 humoral response is reduced in MS patients compared to HCs
Within the 2-vax group, 13/40 (32.5%) of MS patients seroconverted compared to 29/29 (100%) in the HC group (Fisher's exact test, P = 1.2 × 10−9). When stratified by DMT, the proportion who seroconverted in the ocrelizumab group was 8/33 (24.2%), compared to 5/7 (71.4%) in the fingolimod group, and 29/29 (100%) in the HC group (Fisher's exact test, P = 5.7 × 10−11) (Supplementary Table 1).
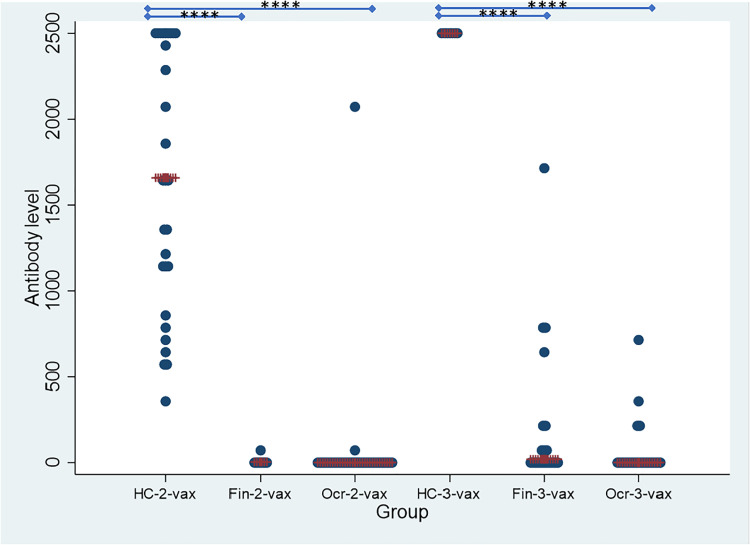
The median [IQR] spike antibody level after 2-vax was <0.4 [0] U/mL in the ocrelizumab group, 3.82 [4.18] U/mL in the fingolimod group, which was significantly different from the HC group which had a median [IQR] spike antibody level of 1659 [1374] U/mL (Kruskal–Wallis test, P = 8.1 × 10−12) (Figure 1 and Supplementary Table 1).
Figure 1.
Spike antibody levels (U/mL) in HC, fingolimod and ocrelizumab groups after 2nd and 3rd dose of vaccine shown on the y-axis. Patients are stratified by groups on the X axis. HC-2 vax (n = 29) indicates the HC group after 2 doses of vaccine; HC-3 vax (n = 7) indicates the HC group after 3 doses of vaccine. Fin-2 vax (n = 7) indicates the fingolimod group after 2 doses of vaccine; Fin-3 vax (n = 21) indicates the fingolimod group after 3 doses of vaccine; Ocr-2 vax (n = 33) indicates the ocrelizumab group after 2 doses of vaccine; Ocr-3 vax (n = 22) indicates the ocrelizumab group after 3 doses of vaccine. Statistical significance between groups is shown with **** symbolizing p < 0.0001.
After the 3rd vaccination, 28/43 (65.1%) of MS patients seroconverted as compared to 7/7 (100%) of HC (Fisher's exact test, P = 0.08). The proportion who seroconverted in the ocrelizumab group was 9/22 (40.9%), compared to 19/21 (90.5%) in the fingolimod group, and 7/7 (100%) in the HC group (Fisher's exact test for difference, P = 0.0003) (Supplementary Table 1).
The median [IQR] spike antibody level after 3-vax was <0.4 [20.1] U/mL in the ocrelizumab group, 19.3 [215.4] U/mL in the fingolimod group, which was significantly different when compared to the HC group who had a median [IQR] spike antibody level of >2500 [0] U/mL (Kruskal–Wallis test, P = 8.6 × 10−6) (Figure 1 and Supplementary Table 1).
In the ocrelizumab patients, there was no correlation between spike antibody level and serum IgG (rs = 0.194; P = 0.386), IgM (rs = 0.262; P = 0.238), or IgA (rs = 0.210; P = 0.347).
The potential for the 3rd vaccination to augment seroconversion rates was measured in a set of 13 ocrelizumab treated patients and 3 fingolimod patients. Five out of 13 (38.5%) ocrelizumab patients seroconverted after 2-vax, compared to 6/13 (46.1%) after 3-vax (McNemar's test, P =1, Wilcoxon signed rank test for change in level P = 0.035). The median [IQR] change in the ocrelizumab group was 0 [21.9]. 1/3 (33.3%) fingolimod patients seroconverted after 2-vax, compared to 2/3 (66.7%) after 3-vax (McNemar's test, P = 1, Wilcoxon signed rank test for change in level P = 0.37). The median [IQR] change in the fingolimod group was 4.18 [16.24].
Total T cells and SARS-Cov-2 Prot_S peptide reactive activation of T cells compared to the unstimulated condition
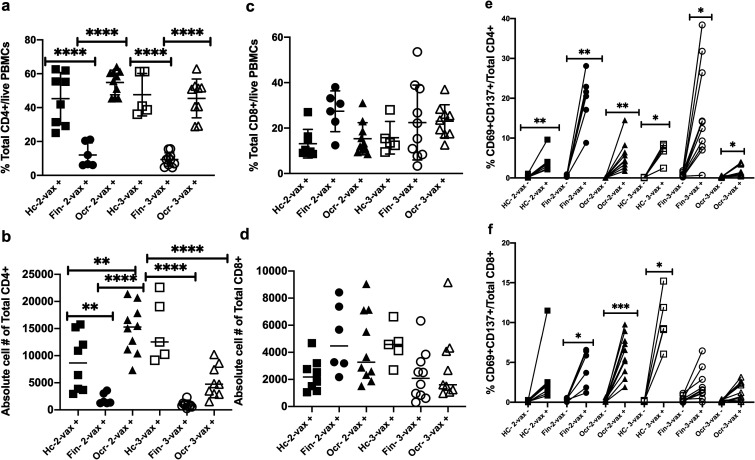
There was a significant increase in the percentage of total SARS-CoV-2 Prot_S reactive CD4+ T cells in the HC and ocrelizumab group compared to the fingolimod group after 2-vax and 3-vax (P < 0.0001, Sidak's multiple comparisons test) (Figure 2(a)). There was a significant increase in the absolute number of CD4+ T cells in the HC group as compared to the fingolimod group after 2-vax (P = 0.0091) and after 3-vax (P < 0.0001). There was also a significant difference in the absolute number of CD4+ T cells in the HC group as compared to the ocrelizumab group after 2-vax (P = 0.0094) and after 3-vax (P = 0.0003). There was a significant increase in the absolute number of CD4+ T cells in the ocrelizumab group as compared to the fingolimod group after 2-vax but did not reach significance after 3-vax (P < 0.0001) Sidak's multiple comparisons test) (Figure 2(b)). There was no significant difference in the percentage and absolute number of total CD8+ T cells for the three groups after 2-vax and 3-vax (Figure 2(c) and (d)).
Figure 2.
Peptivator® SARS-CoV-2 Prot_S peptide reactive percentage, absolute cell number and activated total CD4+ and CD8+ T cell from PBMCs of the HC, fingolimod and ocrelizumab groups after 2nd and 3rd dose of vaccine shown on the y-axis. Patients are stratified by groups on the X axis. HC-2 vax (n = 8) indicates the HC group after 2 doses of vaccine, Fin-2 vax (n = 6) indicates the fingolimod group after 2 doses of vaccine, Ocr-2 vax (n = 10) indicates the ocrelizumab group after 2 doses of vaccine, HC-3 vax (n = 5) indicates the HC group after 3 doses of vaccine, Fin-3 vax (n = 10) indicates the fingolimod group after 3 doses of vaccine, Ocr-3 vax (n = 9) indicates the ocrelizumab group after 3 doses of vaccine. Plus (+) indicates presence of PepTivator® SARS-CoV-2 Prot_S peptide and minus (−) indicates absence of PepTivator® SARS-CoV-2 Prot_S peptide (negative control). PBMCs were stimulated with SARS-CoV-2 peptide: PepTivator® SARS-CoV-2 Prot_S at a concentration of 4 μg/ml and BrefeldinA at a concentration of 10 μg/ml for 18 h and unstimulated cells containing only media were used as negative control, followed by staining with antibodies and flow cytometry. (A) Percentage of total CD4+ T cells (B) Absolute number of total CD4+ T cells (C) Percentage of total CD8+ T cell (D) Absolute number of total CD8+ T cells © Percentage of PepTivator® SARS-CoV-2 Prot_S peptide reactive activated total CD4+ T cells compared to negative control (F) Percentage of PepTivator® SARS-CoV-2 Prot_S peptide reactive activated total CD8+ T cells compared to negative control. Sidak's multiple comparisons test, ****P < 0.0001, ***P < 0.0003, **P < 0.005, *P < 0.05.
SARS-CoV-2 Prot_S reactive CD4+ and CD8+ T cell activation (CD69/CD137++) was evaluated by comparing with the unstimulated condition. There was a significant increase in the percentage of SARS-CoV-2 Prot_S reactive activation of CD4+ T cells as compared to the unstimulated condition among all three groups after 2-vax and 3-vax (CD4-HC 2-vax− vs HC 2-vax+: P = 0.008, CD4-Fin 2-vax− vs Fin 2-vax+: P = 0.0043, CD4-Ocr 2-vax− vs Ocr 2-vax+: P = 0.0064, CD4-HC 3-vax− vs HC 3-vax+: P = 0.023, CD4-Fin 3-vax− vs Fin 3-vax+: P = 0.014, CD4-Ocr 3-vax− vs Ocr 3-vax+: P = 0.029, Sidak's multiple comparisons test). There was significant increase in the percentage of SARS-CoV-2 Prot_S reactive activation of CD8+ T cells as compared to the unstimulated condition in the fingolimod and ocrelizumab groups after 2-vax and HC after 3-vax (CD8-Fin 2-vax− vs Fin 2-vax+: P = 0.0043, CD8-Ocr 2-vax− vs Ocr 2-vax+: P = 0.0003, CD8-HC 3-vax− vs HC 3-vax+: P = 0.01, Sidak's multiple comparisons test) (Figure 2(e) and (f)).
IFNγ and TNFα producing total T cells
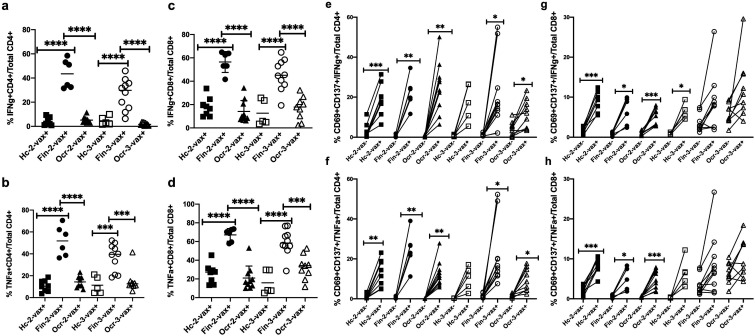
The percentage of SARS-CoV-2 Prot_S reactive IFNγ and TNFα producing CD4+ and CD8+ T cells in the fingolimod group was increased compared to HC and the ocrelizumab group after 2-vax and 3-vax (P < 0.0001, Sidak's multiple comparisons test) (Figure 3(a)–(d)).
Figure 3.
Peptivator® SARS-CoV-2 Prot_S peptide reactive percentage and activated IFNγ and TNFα producing total CD4+ and CD8+ T cells from PBMCs of HC, fingolimod and ocrelizumab groups after 2nd and 3rd dose of vaccine shown on the y-axis. Patients are stratified by groups on the X axis. HC-2 vax (n = 8) indicates HC group after 2 doses of vaccine, Fin-2 vax (n = 6) indicates fingolimod group after 2 doses of vaccine, Ocr-2 vax (n = 10) indicates ocrelizumab group after 2 doses of vaccine, HC-3 vax (n = 5) indicates HC group after 3 doses of vaccine, Fin-3 vax (n = 10) indicates fingolimod group after 3 doses of vaccine, Ocr-3 vax (n = 9) indicates ocrelizumab group after 3 doses of vaccine. Plus (+) indicates presence of PepTivator® SARS-CoV-2 Prot_S peptide and minus (−) indicates absence of PepTivator® SARS-CoV-2 Prot_S peptide (negative control). PBMCs were stimulated with SARS-CoV-2 peptide: PepTivator® SARS-CoV-2 Prot_S at a concentration of 4 μg/ml and BrefeldinA at a concentration of 10 μg/ml for 18 h and unstimulated cells containing only media were used as negative control, followed by staining with antibodies and flow cytometry as described in materials and methods. Data was analyzed using FlowJo software version 10.7.1 and the graphs were made using GraphPad Prism version 8.4.2 (464). (A) Percentage of IFNγ producing CD4+ T cells (B) Percentage of TNFα producing CD4+ T cells (C) Percentage of IFNγ producing CD8+ T cells (D) Percentage of TNFα producing CD8+ T cell©(E) Percentage of PepTivator® SARS-CoV-2 Prot_S peptide reactive activated IFNγ producing CD4+ T cells compared to negative control (F) Percentage of PepTivator® SARS-CoV-2 Prot_S peptide reactive activated TNFα producing CD4+ T cells compared to negative control (G) Percentage of PepTivator® SARS-CoV-2 Prot_S peptide reactive activated IFNγ producing CD8+ T cells compared to negative control (H) Percentage of PepTivator® SARS-CoV-2 Prot_S peptide reactive activated TNFα producing CD8+ T cells compared to negative control. Sidak's multiple comparisons test, ****P < 0.0001, ***P < 0.0003, **P < 0.005, *P < 0.05.
SARS-CoV-2 Prot_S reactive activation (CD69/CD137++) of IFNγ and TNFα producing CD4+ and CD8+ T cells was evaluated by comparing with the unstimulated condition. There was a significant increase in the percentage of SARS-CoV-2 Prot_S reactive activation of IFNγ and TNFα producing CD4+ T cells after 2-vax among all three groups and after 3-vax in fingolimod and ocrelizumab groups as compared to the unstimulated condition (IFNγ HC 2-vax− vs HC 2-vax+: P = 0.0009, IFNγ Fin 2-vax− vs Fin 2-vax+: P = 0.0043, IFNγ Ocr 2-vax− vs Ocr 2-vax+: P = 0.0010, IFNγ Fin 3-vax− vs Fin 3-vax+: P = 0.023, IFNγ Ocr 3-vax− vs Ocr 3-vax+: P = 0.040, TNFα HC 2-vax− vs HC 2-vax+: P = 0.005, TNFα Fin 2-vax− vs Fin 2-vax+: P = 0.0062, TNFα Ocr 2-vax− vs Ocr 2-vax+: P = 0.0014, TNFα Fin 3-vax− vs Fin 3-vax+: P = 0.014, TNFα Ocr 3-vax− vs Ocr 3-vax+: P = 0.019, Sidak's multiple comparisons test) (Figure 3(e) and (f)). There was a significant increase in the percentage of SARS-CoV-2 Prot_S reactive activation of IFNγ and TNFα producing CD8+ T cells after 2-vax among all three groups but did not reach significance after 3-vax as compared to the unstimulated condition (IFNγ HC 2-vax− vs HC 2-vax+: P = 0.0002, IFNγ Fin 2-vax− vs Fin 2-vax+: P = 0.034, IFNγ Ocr 2-vax− vs Ocr 2-vax+: P = 0.0007, IFNγ HC 3-vax− vs HC 3-vax+: P = 0.01, TNFα HC 2-vax− vs HC 2-vax+: P = 0.0004, TNFα Fin 2-vax− vs Fin 2-vax+: P = 0.034, TNFα Ocr 2-vax− vs Ocr 2-vax+: P = 0.0010, Sidak's multiple comparisons test) (Figure 3(g) and (h)).
The absolute number of IFNγ producing CD4+ T cells was higher in fingolimod and ocrelizumab groups as compared to HC after 2-vax (IFNγ Fin 2-vax vs HC 2-vax: P = 0.012, IFNγ Ocr 2-vax vs HC 2-vax: P = 0.03, Sidak's multiple comparisons test) (Supplementary Figure 1(a)). The absolute number of TNFα producing CD4+ T cells was higher in the ocrelizumab as compared to fingolimod group and HC after 2-vax (TNFα Ocr 2-vax vs Fin 2-vax: P = 0.02, TNFα Ocr 2-vax vs HC 2-vax: P = 0.005, Sidak's multiple comparisons test) (Supplementary Figure 1(b)). The absolute number of IFNγ and TNFα producing CD8+ T cells was higher in fingolimod as compared to ocrelizumab group and HC after 2-vax (P < 0.0001, Sidak's multiple comparisons test) (Supplementary Figure 1(c) and (d)). There was no statistical difference in the absolute numbers of IFNγ and TNFα producing CD4+ and CD8+ T cells across all three groups after 3-vax.
IFNγ and TNFα producing memory T cells
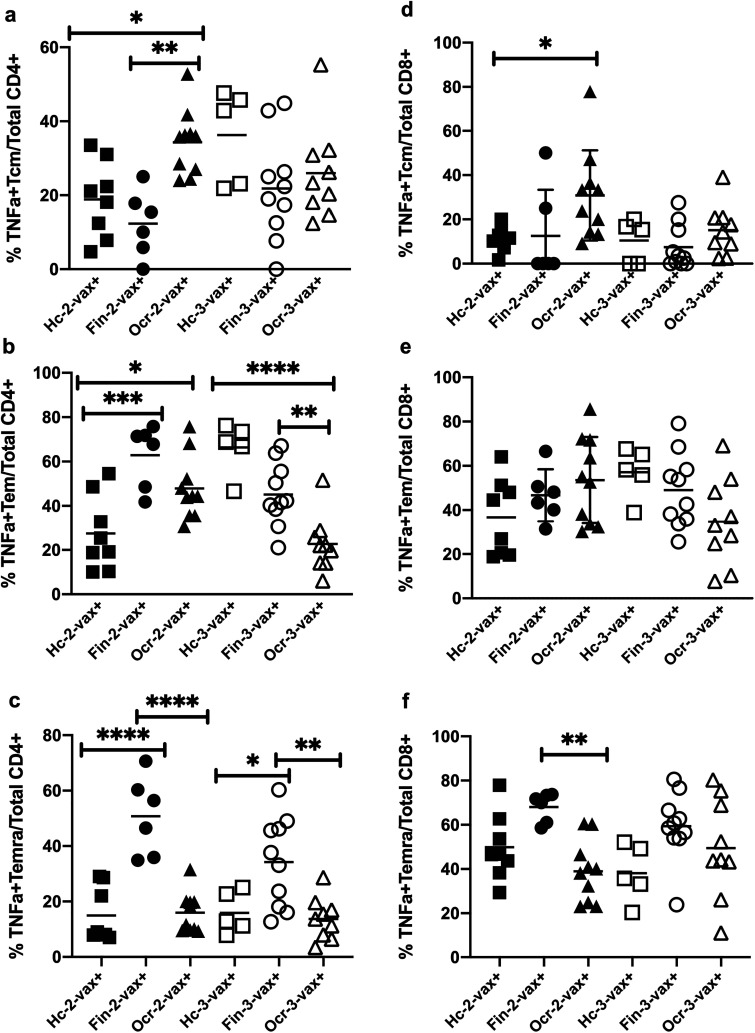
We found a significant increase in the percentage of SARS-CoV-2 Prot_S reactive TNFα producing central memory (Tcm) CD4+ cells in ocrelizumab group as compared to fingolimod group (P = 0.004) and HC (P = 0.04) by Sidak's multiple comparisons test after 2-vax but not after 3-vax (Figure 4(a)). There was no significant difference in the percentage of IFNγ producing Tcm CD4+ cells across all three groups after 2-vax and 3-vax (Supplementary Figure 2(a))
Figure 4.
Peptivator® SARS-CoV-2 Prot_S peptide reactive percentage of TNFα producing memory CD4+ and CD8+ T cells from PBMCs of HC, fingolimod and ocrelizumab groups after 2nd and 3rd dose of vaccine shown on the y-axis. Patients are stratified by groups on the X axis. HC-2 vax (n = 8) indicates HC group after 2 doses of vaccine, Fin-2 vax (n = 6) indicates fingolimod group after 2 doses of vaccine, Ocr-2 vax (n = 10) indicates ocrelizumab group after 2 doses of vaccine, HC-3 vax (n = 5) indicates HC group after 3 doses of vaccine, Fin-3 vax (n = 10) indicates fingolimod group after 3 doses of vaccine, Ocr-3 vax (n = 9) indicates ocrelizumab group after 3 doses of vaccine. Plus (+) indicates presence of PepTivator® SARS-CoV-2 Prot_S peptide. PBMCs were stimulated with SARS-CoV-2 peptide: PepTivator® SARS-CoV-2 Prot_S at a concentration of 4 μg/ml and BrefeldinA at a concentration of 10 μg/ml for 18 h followed by staining with antibodies and flow cytometry as described in materials and methods. Data was analyzed using FlowJo software version 10.7.1 and the graphs were made using GraphPad Prism version 8.4.2 (464). (A) Percentage of TNFα producing central memory CD4+ T cells (Tcm) (B) Percentage of TNFα producing effector memory CD4+ T cells (Tem) (C) Percentage of TNFα producing terminally differentiated effector memory CD4+ T cells (Temra) (D) Percentage of TNFα producing central memory CD8+ T cells (Tcm) (E) Percentage of TNFα producing effector memory CD8+ T cells (Tem) (F) Percentage of TNFα producing terminally differentiated effector memory CD8+ T cells (Temra). Sidak's multiple comparisons test, ****P < 0.0001, ***P < 0.0003, **P < 0.005, *P < 0.05.
The percentage of TNFα producing effector memory (Tem) CD4+ cells was significantly higher in ocrelizumab (P = 0.0252) and fingolimod groups (P = 0.0002) as compared to HC after 2-vax. After 3-vax, HC (P < 0.0001) and fingolimod groups (P = 0.008), both showed an increased percentage of TNFα producing Tem CD4+ cells as compared to ocrelizumab group, Sidak's multiple comparisons test (Figure 4(b)). There was a significant increase in the percentage of IFNγ producing Tem CD4+ cells in fingolimod as compared to ocrelizumab group and HC after 2-vax. (P < 0.0001). After 3-vax, HC (P = 0.003) and fingolimod groups (P < 0.0001), both showed an increase in the percentage of IFNγ producing Tem CD4+ cells as compared to ocrelizumab group, Sidak's multiple comparisons test (Supplementary Figure 2(b)).
The percentage of TNFα and IFNγ producing terminally differentiated effector memory (Temra) CD4+ cells was significantly higher in the fingolimod group as compared to ocrelizumab group and HC after 2-vax (P < 0.0001) as well as 3-vax, (TNFα Fin 3-vax vs Ocr 3-vax: P = 0.0014, TNFα Fin 3-vax vs HC 3-vax: P = 0.02, IFNγ Fin 3-vax vs Ocr 3-vax: P = 0.0006, IFNγ Fin 3-vax vs HC 3-vax: P = 0.0021, Sidak's multiple comparisons test) (Figure 4(c) and Supplementary Figure 2(c)).
The percentage of TNFα producing Tcm CD8+ cells was significantly higher in ocrelizumab group as compared to HC (P = 0.03, Sidak's multiple comparisons test) after 2-vax but not after 3-vax (Figure 4(d)). There was no significant difference in the percentage IFNγ producing Tcm CD8+ cells across all three groups after 2-vax and 3-vax (Supplementary Figure 2(d)). There was no significant difference in the percentage of TNFα and IFNγ producing Tem CD8+ cells across all three groups after 2-vax and 3-vax (Figure 4(e) and Supplementary Figure 2(e)). However, there was a significant increase in the percentage of TNFα and IFNγ producing Temra CD8+ cells in fingolimod group as compared to ocrelizumab group (TNFα P = 0.0055, IFNγ P = 0.0048, Sidak's multiple comparisons test) after 2-vax but not after 3-vax (Figure 4(f) and Supplementary Figure 2(f)).
Discussion
In this study, we found that MS patients on ocrelizumab and fingolimod had lower seroconversion rates and median spike antibody levels after 2-vax and 3-vax doses of mRNA vaccines when compared to a cohort of HC and that 3rd dose of mRNA vaccine may increase seroconversion rates in patients on ocrelizumab and fingolimod. We also found relatively preserved T cell responses. The ocrelizumab group and HC had higher percentage of CD4+ T cells specific to SARS-CoV-2 Prot_S peptide as compared to fingolimod patients after 2-vax and 3-vax. There was also increased CD4+ T cell activation indicated by CD69/CD137++ cells in response to SARS-CoV-2 Prot_S peptide in all three groups after 2-vax and 3-vax as compared to the unstimulated condition. We found a higher percentage of IFNγ and TNFα producing CD4+ and CD8+ T cells in the fingolimod group as compared to ocrelizumab group and HC after 2-vax and 3-vax. Importantly, these subsets of cytokine producing CD4+ and CD8+ T cells have been associated with effective COVID vaccine responses,12–14 and our results suggest that effector T cell responses are preserved with both therapies, and higher in fingolimod-treated patients.
In line with the above observations, we found that Tem responses including IFNγ producing Tem CD4+ cells were higher in fingolimod group as compared to ocrelizumab group and HC, and the percentage of TNFα producing Tem CD4+ cells were increased in ocrelizumab and fingolimod patients as compared to HC after 2-vax. After 3-vax, fingolimod group as well as HC showed an increase in the IFNγ and TNFα producing Tem CD4+ cells but ocrelizumab group did not.
In contrast to the increased Tem response seen with fingolimod, the Tcm response was decreased. The preserved Tem response is in line with the medication's mechanism of action of trapping naïve and Tcm cells in secondary lymphoid organs without affecting Tem cells.15–17 Prior studies have shown that Tem CD4+ cells were the dominant activated T cell phenotype upon viral exposure, and that these responses are durable over time.18,19
Our results add to prior work which has demonstrated that a 3rd dose of vaccine may modestly increase spike antibody response in patients on anti-CD20 and S1P receptor-targeting therapies. 9 Prior studies have shown that ocrelizumab treated patients have preserved SARS-CoV-2 specific T cell responses after a series of 2 mRNA vaccines20–22 and that 3rd dose of vaccine in ocrelizumab treated patients may increase cellular immune response.10,23 However, emerging data has shown that use of B-cell depleting medications is a risk factor for developing a breakthrough infection, which argues that T cell responses alone may not be sufficient in this population to provide immunity. 24
Our results differ from prior studies on fingolimod-treated patients which have shown impaired cellular and humoral immune responses to vaccination.7,25–27 This may be because we examined different subsets of T cells including total CD4+ and CD8+ T cells, IFNγ and TNFα producing CD4+ and CD8+ T cells as well as memory T cells. Preserved T cell responses are of increasing clinical relevance especially considering new variants which may escape antibody neutralization. Multiple studies have found that despite Omicron's numerous mutations and reduced susceptibility to neutralizing antibodies, T cell responses were overall preserved.28,29
Our study is limited by a relatively small sample size, specifically with regards to the group that had longitudinal data to follow comparing 2-vax vs 3-vax. We acknowledge that biospecimens were collected at 2–3 months after vaccination for 2-vax group and 1–2 months for 3-vax group, which could lead to some differences in the counts observed. Though our study analyzed seroconversion rates and spike-antibody levels, we did not specifically assess safety data for 3-vax or analyze subsequent COVID-19 infection. We also acknowledge that our MS group of patients was older than the control group, which is a limitation because response to vaccination declines with age. 30 We also did not evaluate the correlation between T cell and humoral responses or relationship with CD19 count.
In conclusion, our data shows that 3rd dose of mRNA COVID-19 vaccines may increase seroconversion rates and spike antibody levels in MS patients treated with anti-CD20 and S1P receptor-targeting therapies and that T cell responses are overall preserved in this patient population.
Supplemental Material
Supplemental material, sj-docx-1-mso-10.1177_20552173231165196 for Preserved T cell but attenuated antibody response in MS patients on fingolimod and ocrelizumab following 2nd and 3rd SARS-CoV-2 mRNA vaccine by Sarah Conway, Shrishti Saxena, Clare Baecher-Allan, Rajesh Krishnan, Maria Houtchens, Bonnie Glanz, Taylor J Saraceno, Mariann Polgar-Turcsanyi, Gauruv Bose, Rohit Bakshi, Shamik Bhattacharyya, Kristin Galetta, Tamara Kaplan, Christopher Severson, Tarun Singhal, Lynn Stazzone, Jonathan Zurawski, Anu Paul, Howard L Weiner, Brian C Healy and Tanuja Chitnis in Multiple Sclerosis Journal – Experimental, Translational and Clinical
Supplemental material, sj-docx-2-mso-10.1177_20552173231165196 for Preserved T cell but attenuated antibody response in MS patients on fingolimod and ocrelizumab following 2nd and 3rd SARS-CoV-2 mRNA vaccine by Sarah Conway, Shrishti Saxena, Clare Baecher-Allan, Rajesh Krishnan, Maria Houtchens, Bonnie Glanz, Taylor J Saraceno, Mariann Polgar-Turcsanyi, Gauruv Bose, Rohit Bakshi, Shamik Bhattacharyya, Kristin Galetta, Tamara Kaplan, Christopher Severson, Tarun Singhal, Lynn Stazzone, Jonathan Zurawski, Anu Paul, Howard L Weiner, Brian C Healy and Tanuja Chitnis in Multiple Sclerosis Journal – Experimental, Translational and Clinical
Footnotes
Author contributions: Significant contribution to conception and design of the study (SC, SS, TC), acquisition and analysis of data (SC, SS, RK, BH, TC), participation in drafting a significant portion of the manuscript or figures (SC, SS, CBA, TC), critical review of the manuscript (SC, SS, CBA, RK, MH, BG, TJS, MPT, GB, RB, SB, KG, TK, CS, TS, LS, JZ, AP, HLW, BH, TC). All authors approved the final version of the manuscript.
Author disclosures: S.C. has received advisory board fees from Bristol-Myers Squibb.; S.S. reports no disclosures; C.B.A. reports funding from the National MS Society and Department of Defense; R.K. reports no disclosures; M.H. has served as a consultant for Biogen, Roche-Genentech, Novartis, Genzyme, she has received research support from Biogen, Roche-Genentech, Novartis and Genzyme; B.G. has received research support from Verily Life Sciences and Merck Serono; T.J.S. reports no disclosures; M.P.T. reports no disclosures; G.B. has received an MS Post-Doctoral Fellowship award from the Multiple Sclerosis Society of Canada; R.B. has received consulting fees from Bristol-Myers Squibb and EMD Serono and research support from Bristol-Myers Squibb, EMD Serono, and Novartis; S.B. has received research support from NIH and Alexion Pharmaceuticals; consulting from Alexion Pharmaceuticals and Teladoc Health, publishing honorarium from UpToDate; K.G. has received consulting compensation from Glaxo Smith Kline that is not relevant to this project; T.K. has received consulting and advisory board fees from Biogen, Genzyme-Sanofi, Roche-Genentech, Novartis, and Bristol-Myers Squibb; C.S. has consulted for Biogen, Novartis, Roche-Genentech, and Genzyme, and has received grant support from the NMSS; T.S. has received consulting compensation from Novartis Pharmaceuticals and research support from Novartis Pharmaceuticals and Genzyme-Sanofi; L.S. reports no disclosures; J.Z. has received research support from Novartis Pharmaceuticals and the Race to Erase MS Foundation; A.P. reports no disclosures; H.L.W. has received personal fees for advisory board activities from Guthy Jackson Charitable Foundation, Teva Pharmaceutical Industries Ltd, Biogen Idec, Novartis, Sanofi-aventis, Tilos Therapeutics, Tiziana Life Sciences, CBridge Capital, IM Therapeutics, Magnolia Therapeutics, Genentech, Genzyme, vTv Therapeutics, MedDay Pharmaceuticals, Weston Foundation, and I-MAB Biopharma, consulting fees from Genentech, Inc, IM Therapeutics, I-MAB Biopharma, MedDay Pharmaceuticals, Tiziana Life Sciences, and vTv Therapeutics, and holds stocks with vTv Therapeutics, as a member of the Board of Directors; B.H. has received research support from Analysis Group, Celgene (Bristol-Myers Squibb), Verily Life Sciences, Merck-Serono, Novartis and Genzyme; T.C. has received compensation for consulting from Banner Life Sciences, Biogen, Bristol-Myers Squibb, Novartis Pharmaceuticals, Roche Genentech, and Sanofi Genzyme. She has received research support from the National Institutes of Health, National MS Society, US Department of Defense, Sumaira Foundation, Brainstorm Cell Therapeutics, Bristol-Myers Squibb, EMD Serono, I-Mab Biopharma, Mallinckrodt ARD, Novartis Pharmaceuticals, Octave Bioscience, Roche Genentech, Sanofi Genzyme, and Tiziana Life Sciences.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Novartis Pharmaceuticals Corporation (COMB157GUS19T).
ORCID iDs: Sarah Conway https://orcid.org/0000-0002-2873-7368
Maria Houtchens https://orcid.org/0000-0001-6077-0654
Bonnie Glanz https://orcid.org/0000-0002-4344-3456
Taylor J Saraceno https://orcid.org/0000-0002-2605-8505
Rohit Bakshi https://orcid.org/0000-0001-8601-5534
Jonathan Zurawski https://orcid.org/0000-0002-2896-8369
Tanuja Chitnis https://orcid.org/0000-0002-9897-4422
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Sarah Conway, Department of Neurology, Brigham Multiple Sclerosis Center, Brigham and Women's Hospital, Boston, MA, USA; Harvard Medical School, Boston, MA, USA.
Shrishti Saxena, Department of Neurology, Brigham Multiple Sclerosis Center, Brigham and Women's Hospital, Boston, MA, USA.
Clare Baecher-Allan, Department of Neurology, Brigham Multiple Sclerosis Center, Brigham and Women's Hospital, Boston, MA, USA; Harvard Medical School, Boston, MA, USA.
Rajesh Krishnan, Department of Neurology, Brigham Multiple Sclerosis Center, Brigham and Women's Hospital, Boston, MA, USA.
Maria Houtchens, Department of Neurology, Brigham Multiple Sclerosis Center, Brigham and Women's Hospital, Boston, MA, USA; Harvard Medical School, Boston, MA, USA.
Bonnie Glanz, Department of Neurology, Brigham Multiple Sclerosis Center, Brigham and Women's Hospital, Boston, MA, USA; Harvard Medical School, Boston, MA, USA.
Gauruv Bose, Department of Neurology, Brigham Multiple Sclerosis Center, Brigham and Women's Hospital, Boston, MA, USA; Harvard Medical School, Boston, MA, USA.
Rohit Bakshi, Department of Neurology, Brigham Multiple Sclerosis Center, Brigham and Women's Hospital, Boston, MA, USA; Harvard Medical School, Boston, MA, USA.
Shamik Bhattacharyya, Department of Neurology, Brigham Multiple Sclerosis Center, Brigham and Women's Hospital, Boston, MA, USA; Harvard Medical School, Boston, MA, USA.
Kristin Galetta, Department of Neurology, Brigham Multiple Sclerosis Center, Brigham and Women's Hospital, Boston, MA, USA; Harvard Medical School, Boston, MA, USA.
Tamara Kaplan, Department of Neurology, Brigham Multiple Sclerosis Center, Brigham and Women's Hospital, Boston, MA, USA; Harvard Medical School, Boston, MA, USA.
Christopher Severson, Department of Neurology, Brigham Multiple Sclerosis Center, Brigham and Women's Hospital, Boston, MA, USA; Harvard Medical School, Boston, MA, USA.
Tarun Singhal, Department of Neurology, Brigham Multiple Sclerosis Center, Brigham and Women's Hospital, Boston, MA, USA; Harvard Medical School, Boston, MA, USA.
Lynn Stazzone, Department of Neurology, Brigham Multiple Sclerosis Center, Brigham and Women's Hospital, Boston, MA, USA.
Jonathan Zurawski, Department of Neurology, Brigham Multiple Sclerosis Center, Brigham and Women's Hospital, Boston, MA, USA; Harvard Medical School, Boston, MA, USA.
Anu Paul, Department of Neurology, Brigham Multiple Sclerosis Center, Brigham and Women's Hospital, Boston, MA, USA.
Howard L Weiner, Department of Neurology, Brigham Multiple Sclerosis Center, Brigham and Women's Hospital, Boston, MA, USA; Harvard Medical School, Boston, MA, USA.
Brian C Healy, Department of Neurology, Brigham Multiple Sclerosis Center, Brigham and Women's Hospital, Boston, MA, USA; Harvard Medical School, Boston, MA, USA.
Tanuja Chitnis, Department of Neurology, Brigham Multiple Sclerosis Center, Brigham and Women's Hospital, Boston, MA, USA; Harvard Medical School, Boston, MA, USA.
References
- 1.Lotan I, Wilf-Yarkoni A, Friedman Yet al. et al. Safety of the BNT162b2 COVID-19 vaccine in multiple sclerosis (MS): early experience from a tertiary MS center in Israel. Eur J Neurol 2021; 28: 3742–3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ciampi E, Uribe-San-Martin R, Soler B, et al. Safety and humoral response rate of inactivated and mRNA vaccines against SARS-CoV-2 in patients with multiple sclerosis. Mult Scler Relat Disord 2022; 59: 103690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Achiron A, Mandel M, Dreyer-Alster S, et al. Humoral immune response to COVID-19 mRNA vaccine in patients with multiple sclerosis treated with high-efficacy disease-modifying therapies. Ther Adv Neurol Disord 2021; 14: 17562864211012835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iannetta M, Landi D, Cola G, et al. B- and T-cell responses after SARS-CoV-2 vaccination in patients with multiple sclerosis receiving disease modifying therapies: immunological patterns and clinical implications. Front Immunol 2021; 12: 796482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Katz JD, Bouley AJ, Jungquist RMet al. et al. Humoral and T-cell responses to SARS-CoV-2 vaccination in multiple sclerosis patients treated with ocrelizumab. Mult Scler Relat Disord 2022; 57: 103382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Novak F, Nilsson AC, Nielsen C, et al. Humoral immune response following SARS-CoV-2 mRNA vaccination concomitant to anti-CD20 therapy in multiple sclerosis. Mult Scler Relat Disord 2021; 56: 103251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tallantyre EC, Vickaryous N, Anderson V, et al. COVID-19 vaccine response in people with multiple sclerosis. Ann Neurol 2022; 91: 89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dreyer-Alster S, Menascu S, Mandel M, et al. COVID-19 vaccination in patients with multiple sclerosis: safety and humoral efficacy of the third booster dose. J Neurol Sci 2022; 434: 120155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.König M, Torgauten HM, Tran TT, et al. Immunogenicity and safety of a third SARS-CoV-2 vaccine dose in patients with multiple sclerosis and weak immune response after COVID-19 vaccination. JAMA Neurol 2022; 79: 307–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Madelon N, Heikkilä N, Sabater Royo I, et al. Omicron-specific cytotoxic T-cell responses after a third dose of mRNA COVID-19 vaccine among patients with multiple sclerosis treated with ocrelizumab. JAMA Neurol 2022; 79: 399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 2018; 17: 162–173. [DOI] [PubMed] [Google Scholar]
- 12.Sahin U, Muik A, Derhovanessian E, et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature 2020; 586: 594–599. [DOI] [PubMed] [Google Scholar]
- 13.Widge AT, Rouphael NG, Jackson LA, et al. Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. N Engl J Med 2021; 384: 80–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mateus J, Dan JM, Zhang Z, et al. Low-dose mRNA-1273 COVID-19 vaccine generates durable memory enhanced by cross-reactive T cells. Science 2021; 374: eabj9853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hawke S, Zinger A, Juillard PGet al. et al. Selective modulation of trans-endothelial migration of lymphocyte subsets in multiple sclerosis patients under fingolimod treatment. J Neuroimmunol 2020; 349: 577392. [DOI] [PubMed] [Google Scholar]
- 16.Sica F, Centonze D, Buttari F. Fingolimod immune effects beyond its sequestration ability. Neurol Ther 2019; 8: 231–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hjorth M, Dandu N, Mellergård J. Treatment effects of fingolimod in multiple sclerosis: selective changes in peripheral blood lymphocyte subsets. PLoS One 2020; 15: e0228380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaaijk P, Pimentel VO, Emmelot ME, et al. Children and adults with mild COVID-19: dynamics of the memory T cell response up to 10 months. Front Immunol 2022; 13: 817876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vo HTM, Maestri A, Auerswald H, et al. Robust and functional immune memory up to 9 months after SARS-CoV-2 infection: a Southeast Asian longitudinal cohort. Front Immunol 2022; 13: 817905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brill L, Rechtman A, Zveik O, et al. Humoral and T-cell response to SARS-CoV-2 vaccination in patients with multiple sclerosis treated with ocrelizumab. JAMA Neurol 2021; 78: 1510–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iannetta M, Landi D, Cola G, et al. T-cell responses to SARS-CoV-2 in multiple sclerosis patients treated with ocrelizumab healed from COVID-19 with absent or low anti-spike antibody titers. Mult Scler Relat Disord 2021; 55: 103157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Apostolidis SA, Kakara M, Painter MM, et al. Cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy. Nat Med 2021; 27: 1990–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brill L, Raposo C, Rechtman A, et al. SARS-CoV-2 third vaccine immune response in MS patients treated with ocrelizumab. Ann Neurol 2022; 91: 796–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schiavetti I, Cordioli C, Stromillo ML, et al. Breakthrough SARS-CoV-2 infections in MS patients on disease-modifying therapies. Mult Scler 2022; 28: 2106–2111. [DOI] [PubMed] [Google Scholar]
- 25.Bock H, Juretzek T, Handreka R, et al. Humoral and cellular immune responses to SARS CoV-2 vaccination in people with multiple sclerosis and NMOSD patients receiving immunomodulatory treatments. Mult Scler Relat Disord 2022; 59: 103554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tortorella C, Aiello A, Gasperini C, et al. Humoral- and T-cell-specific immune responses to SARS-CoV-2 mRNA vaccination in patients with MS using different disease-modifying therapies. Neurology 2022; 98: e541–e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Achiron A, Mandel M, Gurevich M, et al. Immune response to the third COVID-19 vaccine dose is related to lymphocyte count in multiple sclerosis patients treated with fingolimod. J Neurol 2022: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keeton R, Tincho MB, Ngomti A, et al. T cell responses to SARS-CoV-2 spike cross-recognize Omicron. Nature 2022; 603: 488–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen H, Rotem S, Elia U, et al. T cell response following anti-COVID-19 BNT162b2 vaccination is maintained against the SARS-CoV-2 omicron B.1.1.529 variant of concern. Viruses 2022; 14: 347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Allen JC, Toapanta FR, Chen Wet al. et al. Understanding immunosenescence and its impact on vaccination of older adults. Vaccine 2020; 38: 8264–8272. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-mso-10.1177_20552173231165196 for Preserved T cell but attenuated antibody response in MS patients on fingolimod and ocrelizumab following 2nd and 3rd SARS-CoV-2 mRNA vaccine by Sarah Conway, Shrishti Saxena, Clare Baecher-Allan, Rajesh Krishnan, Maria Houtchens, Bonnie Glanz, Taylor J Saraceno, Mariann Polgar-Turcsanyi, Gauruv Bose, Rohit Bakshi, Shamik Bhattacharyya, Kristin Galetta, Tamara Kaplan, Christopher Severson, Tarun Singhal, Lynn Stazzone, Jonathan Zurawski, Anu Paul, Howard L Weiner, Brian C Healy and Tanuja Chitnis in Multiple Sclerosis Journal – Experimental, Translational and Clinical
Supplemental material, sj-docx-2-mso-10.1177_20552173231165196 for Preserved T cell but attenuated antibody response in MS patients on fingolimod and ocrelizumab following 2nd and 3rd SARS-CoV-2 mRNA vaccine by Sarah Conway, Shrishti Saxena, Clare Baecher-Allan, Rajesh Krishnan, Maria Houtchens, Bonnie Glanz, Taylor J Saraceno, Mariann Polgar-Turcsanyi, Gauruv Bose, Rohit Bakshi, Shamik Bhattacharyya, Kristin Galetta, Tamara Kaplan, Christopher Severson, Tarun Singhal, Lynn Stazzone, Jonathan Zurawski, Anu Paul, Howard L Weiner, Brian C Healy and Tanuja Chitnis in Multiple Sclerosis Journal – Experimental, Translational and Clinical