Abstract
In the study, Wheat germ, Hops and Grape seed extracts were made into a mixture (BX). The BX was supplemented in AA + broilers diets to investigate the effects of BX on broiler growth performance, blood indicators, microbiota, and noxious gas emissions in faeces. Four hundred and eighty 1-day-old AA + male broilers with an average initial body weight (44.82 ± 0.26) were randomly divided into four dietary treatments of six replicates each, with 20 birds per replicate. The experimental groups consisted of a group fed a basal diet and groups fed basal diet supplemented with 0.05%, 0.1%, and 0.2% BX. The trail was 42 days. The results showed that supplementing the dietary with graded levels of BX linearly increased ADG and ADFI from days 22–42 and 1–42. When dietarys supplemented with 0.2% BX significantly increased ADG and ADFI on days 22–42 and 1–42 (p < 0.05). The addition of BX reduced H2S and NH3 emissions in the faeces; the levels of E. coli and Salmonella in the faeces were significantly reduced and the levels of Lactobacillus were increased (p < 0.05). In this trial, when the diet was supplemented with 0.2% BX, faecal levels of E. coli and Salmonella were consistently at their lowest levels and Lactobacillus were at their highest. At the same time, NH3 and H2S emissions from broiler faecal also had been at their lowest levels. Conclusion: Dietary supplementation with a 0.2% BX could improve the growth performance of broilers and also reduced faecal H2S and NH3 emissions, as well as faecal levels of E. coli and Salmonella, and increased levels of Lactobacillus. Thus, BX made by Wheat germ, Hops and Grape seed extract is expected to be an alternative to antibiotics. And based on the results of this trial, the recommended dose for use in on-farm production was 0.2%.
Keywords: wheat germ, hops extract, grape seed extract, broilers, growth performance, blood indicators, faecal microbiota, noxious gas
Introduction
A complete grain of wheat consists of bran, endosperm and germ, with the germ making up about 3% of the grain weight (Fardet, 2010). Wheat germ (WG) was the life source of wheat and was a major by-product of the wheat flour industry. WG contains approximately 10%–15% fat, 26%–35% protein, 17% sugar, 1.5%–4.5% fibre, and 4% minerals (Brandolini and Hidalgo, 2012). Matsui et al. (2000) obtained an angiotensin-converting enzyme inhibitory peptide with the sequence Ile-Val-Tyr by isolating a peptide produced by the enzymatic digestion of wheat germ protein. Meanwhile, many researchers had explored the hypolipidemic (Liaqat et al., 2021), anti-aging (Zhao et al., 2021), anti-inflammatory (Sui et al., 2020), and antioxidant effects (Wang et al., 2020) of WG. Studies had shown that broilers fed wheat germ oil achieve higher body weights (Arshad et al., 2013a). When combined with a-lipoic acid, wheat germ oil could help improve lipid distribution in broilers (Arshad et al., 2013b). To date, no papers have been published on the effects of crushing wheat germ and feeding it directly to broilers.
Hops (Humulus lupulus) is a perennial plant of the Cannabaceae family, which has only two genera. The genus Humulus and the genus Cannabis. The hops plant plays an important role in human nutrition and culture, as its female inflorescences are used to produce beer. Extracts of the hops plant had an antibacterial effect on Gram-positive bacteria (Teuber and Schmalreck, 1973; Srinivasan et al., 2004). The addition of beta-acids from hops to broiler diets had been reported to improve the overall redox stability and nutritional properties of broilers (Zawadzki et al., 2018). It had been shown that extracts of hops (xanthohumol) could upregulate the expression of phase II enzymes while increasing the protein, activity and glutathione levels of these enzymes (Yao et al., 2015). And, the anti-inflammatory effect of xanthohumol might be related to its modulation of Nrf2-ARE signalling (Lee et al., 2011).
Grape by-products, mainly consisting of skins, seeds and stems, are rich in polyphenols. Grape Seed Extract (GSE) contains mainly proanthocyanidins (PA), catechins, quercetin and tannins. Of these, PA is the most abundant and purest, and is the main substance in the GSE that possesses a biological function. Studies had shown that GSE had antibacterial (Ahn et al., 2004), anti-inflammatory (Carini et al., 2001), antioxidant (Luther et al., 2007), and anticancer (Cheah et al., 2014) activities, with protective effects on host skin (Yamakoshi et al., 2003), cardiovascular (Nunes et al., 2016), liver (Hassan and Al-Rawi, 2013), and nervous system (Mahmoud, 2013). Farahat et al. (2017) reported that GSE increased Newcastle disease virus antibody potency in broiler serum and effectively reduced malondialdehyde levels in meat tissue. In addition, the negative effects of aflatoxin B1 could be effectively reduced by adding a certain amount of GSE to broiler diets (Ali Rajput et al., 2017).
Many studies had reported that the use of botanical preparations in animal feed as dietary supplements could effectively regulate animal metabolism and influence animal welfare and meat quality (Patra and Saxena, 2011; Zawadzki et al., 2017). Many researchers had reported on the physiological properties and beneficial effects of wheat germ, hops and grape seed extracts. However, the effect of adding a mixture of wheat germ, hops and grape seed extract to broiler dietary as a feed additive on broiler production has not been reported. Therefore, in this trial, wheat germ, hops and grape seed extract mixture (BX) was supplemented in AA + broiler diets to study its effects on broiler growth performance, blood indicators, faecal microbiota and noxious gas emissions. It is hoped that this will provide a theoretical basis for the future use of plant additives in poultry production.
Materials and methods
Ethics statement
The Animal Conservation and Utilisation Committee of the JZMU approved the animal use agreement.
Prebiotic sources
BX was supplied by Liaoning Kaiwei Biotechnology Co., Ltd. and consisted mainly of 20% wheat germ, 25% hops extract, 30% grape seed extract and 25% inert carrier (silica). Wheat germ was supplied in powder form. Hops extract contained ≥70% xanthohumol. Grape seed extract contained ≥90% proanthocyanidins.
Animals and experimental design
The trial was a completely randomised group design. Four hundred and eighty 1-day-old AA + male broilers of similar weight (44.82 ± 0.26) and health were selected and randomly divided into four groups of six replicates each, with 20 broilers in each replicate. The experimental group consisted of a base diet group fed a basic basal diet and a base diet group fed 0.05%, 0.1% and 0.2% of BX.
Animals feeding management
All birds were housed in the experimental cages (1.25 × 0.80 × 0.50 m/cage) in a test broilers room. The test broilers house was a fully enclosed house with an automatic environmental control system to ensure optimum temperature and humidity (Temperature was started at 33°C and reduced by 3°C every week up to 22°C, and 65% relative humidity). Birds were free to feed and drink. The lighting programme on days 1–7 and 36–42 was 24 h per day throughout the trial period. The lighting programme on days 8–30 provided 20 h per day and 4 h of darkness. After day 31, the darkness hours were gradually reduced. Diets were formulated to meet the nutrient requirements recommend by the National Research Council, (1994) and provided in mash form (Table 1).
TABLE 1.
Composition and nutrient levels of the basal diet.
| Items | Contents | |
|---|---|---|
| Days 1–21 | Days 22–42 | |
| Ingredients (%) a | ||
| Corn | 60.4 | 64.05 |
| Soybean meal | 34.4 | 30 |
| CaHPO4 | 1.40 | 1.30 |
| CaCO3 | 1.21 | 1.12 |
| NaCl | 0.25 | 0.25 |
| Soybean oil | 1.00 | 2.00 |
| Choline chloride | 0.05 | 0.05 |
| Lysine | 0.08 | 0.10 |
| DL-Met | 0.21 | 0.13 |
| Premix | 1 | 1 |
| Total | 100.00 | 100.00 |
| Nutrient levels (%) b | ||
| ME (MJ/Kg) | 12.14 | 12.51 |
| CP | 21.17 | 19.24 |
| Available phosphorus | 0.38 | 0.36 |
| Lys | 1.29 | 1.15 |
| Met | 0.67 | 0.48 |
| Met + Cys | 1.00 | 0.72 |
| Ca | 0.92 | 0.87 |
Each kg of premix provides: VA, 5000 IU; VD, 10,000 IU; VE, 75.0 IU; VK3, 18.8 mg; VB1, 9.8 mg; VB2, 28.8 mg; VB6, 19.6 mg; VB12, 0.1 mg; Biotin, 2.5 mg; Folic Acid, 4.9 mg; d-Pantothenic acid, 58.8 mg; Nicotinic acid, 196.0 mg; Zn, 37.6 mg; Fe, 40.0 mg; Cu, 4.0 mg; Mn, 50.0 mg; I, 0.2 mg; Se, 0.2 mg.
The nutrient levels were calculated values.
Test indicator determination
Growth performance
Birds were weighed at 1, 21, and 42 days. Feed intake was recorded in replicates throughout the trial and average daily feed intake (ADG), average daily weight gain (ADFI) and meat to feed ratio (F/G) were calculated.
Blood indicators
On days 21 and 42 of the trial, 4 ml of blood was collected from the broiler’s lower wing vein, left to stand for 30 min and centrifuged at 1,200 r/min for 15 min to extract the supernatant. The potency of serum antibodies to Newcastle disease and avian influenza H9 is determined by a haemagglutination inhibition test. Serum levels of albumin (ALB), total protein (TP), globulin (GLOB), alanine transaminase (ALT), alkaline phosphatase (ALP), and glucose (GLU) were measured using a fully automated biochemical analyser.
Faecal microbiota
On day 21 and 42 of the trial, a 1 g sample of broiler manure from each replicate was collected and transported on ice to the laboratory following the method of Dang et al. (2020). Each replicate of 1 g faecal sample was diluted and mixed with 9 ml of 1% peptone broth. The viable counts of E. coli, Lactobacillus and Salmonella in faecal samples were determined on McConkey agar plates, MRS agar plates and BS agar plates (in a 10 g/L peptide solution) in a biosafety cabinet. The microbial count is ultimately expressed as log10 colony forming units per Gram of faeces.
Noxious gas emissions
On day 21 and 42 of the trial, fresh broiler manure was collected from each replicate and ammonia and hydrogen sulphide emissions from the manure were determined using the method of Dang et al. (2020). The manure was placed in a 2 L plastic box with small holes attached to the side and fermented at room temperature (25°C) for 12, 24, and 48 h. The air sample is then collected with a gas collection pump from above the small holes on either side. NH3 and H2S concentrations are measured in the range of 0.00–100.00 mg/m3.
Data analysis
The data was designed using a completely randomised grouping design. Replicate cage serves as the experimental unit. Multiple comparisons of significant differences in means were performed using the one-way ANOVA LSD method in SPSS 25.0 and visualisation was completed using Graphpad Prism 8. Orthogonal contrasts were used to examine the linear and quadratic effects in response to increasing dietary BX levels. Results are expressed as mean and standard deviation, with p < 0.05 indicating a significant difference.
Results
Growth performance
As shown in Table 2, Dietary supplementation with graded levels of BX linearly increased ADG and ADFI in broilers during days 22–42 days and 1–42 (p < 0.05), and there was a quadratic effect on ADG in broilers during days 22–42 and 1–42 (p < 0.05). Dietarys supplemented with 0.2% BX significantly increased ADG and ADFI at 22–42 and 1–42 days compared to controls (p < 0.05). There was no significant effect of dietary supplementation with BX on F/G in all periods (p > 0.05).
TABLE 2.
Effects of dietary supplementation of Wheat germ, Hops and Grape seed extract mixture (BX) on growth performance in broilers.
| Items | Dietary BX levels, % | p-Value | ||||
|---|---|---|---|---|---|---|
| 0 | 0.05 | 0.1 | 0.2 | Linear | Quadratic | |
| ADG, g/d | ||||||
| Days 1–21 | 41.96 ± 0.50 | 41.93 ± 0.46 | 42.04 ± 0.46 | 42.04 ± 0.46 | 0.670 | 0.913 |
| Days 22–42 | 79.23 ± 2.89b | 80.29 ± 2.50ab | 82.30 ± 3.05ab | 83.16 ± 2.51a | 0.009 | 0.035 |
| Days 1–42 | 60.60 ± 1.60b | 61.11 ± 1.42ab | 62.17 ± 1.70ab | 62.60 ± 1.45a | 0.016 | 0.058 |
| ADFI, g/d | ||||||
| Days 1–21 | 52.71 ± 0.55 | 52.57 ± 0.75 | 52.73 ± 0.43 | 52.72 ± 0.66 | 0.874 | 0.951 |
| Days 22–42 | 134.48 ± 3.78b | 136.10 ± 3.81ab | 138.93 ± 3.70ab | 139.81 ± 3.84a | 0.010 | 0.036 |
| Days 1–42 | 93.60 ± 2.10b | 94.34 ± 2.08ab | 95.83 ± 1.98ab | 96.27 ± 2.14a | 0.016 | 0.058 |
| F/G | ||||||
| Days 1–21 | 1.25 ± 0.01 | 1.26 ± 0.01 | 1.25 ± 0.01 | 1.25 ± 0.01 | 0.748 | 0.924 |
| Days 22–42 | 1.70 ± 0.02 | 1.70 ± 0.02 | 1.69 ± 0.02 | 1.68 ± 0.01 | 0.076 | 0.214 |
| Days 1–42 | 1.55 ± 0.01 | 1.54 ± 0.01 | 1.54 ± 0.02 | 1.54 ± 0.01 | 0.156 | 0.374 |
ADG, average daily gain; ADFI, average daily feed intake; F/G feed-to-weight ratio.
a,bMeans in the same row with different superscripts are significantly different (p < 0.05).
Blood indicators
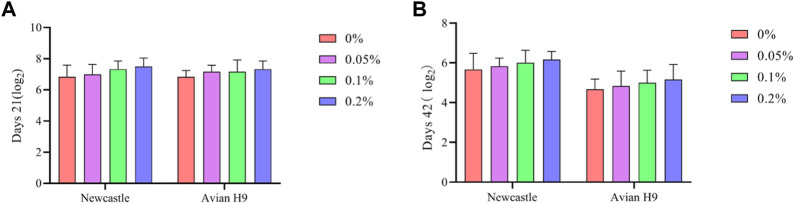
As shown in Figure 1, Dietary supplementation with graded levels of BX increased the potency of Newcastle disease and avian influenza H9 antibodies in broiler serum at days 21 and 42, but the statistical results did not reach significant levels (p > 0.05).
FIGURE 1.
Effects of dietary supplementation of Wheat germ, Hops and Grape seed extract mixture (BX) on serum antibody potency in broilers. (A) Newcastle disease and Avian influenza H9 antibody potency in 21 days broiler sera; (B) Newcastle disease and Avian influenza H9 antibody potency in 42 days broiler sera.
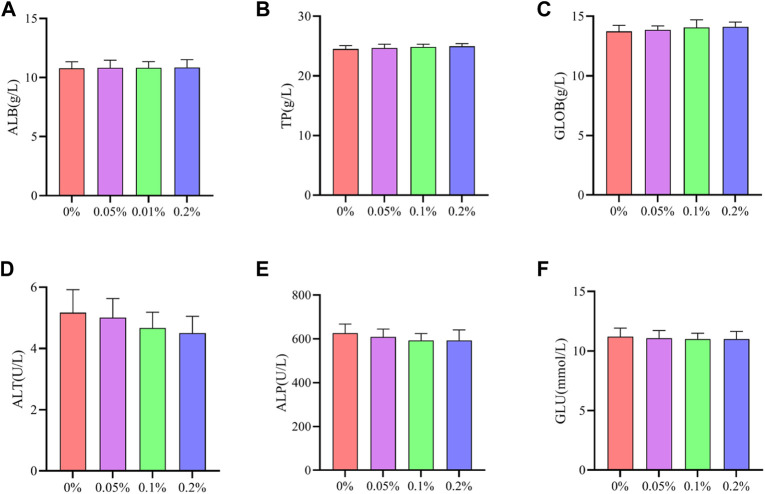
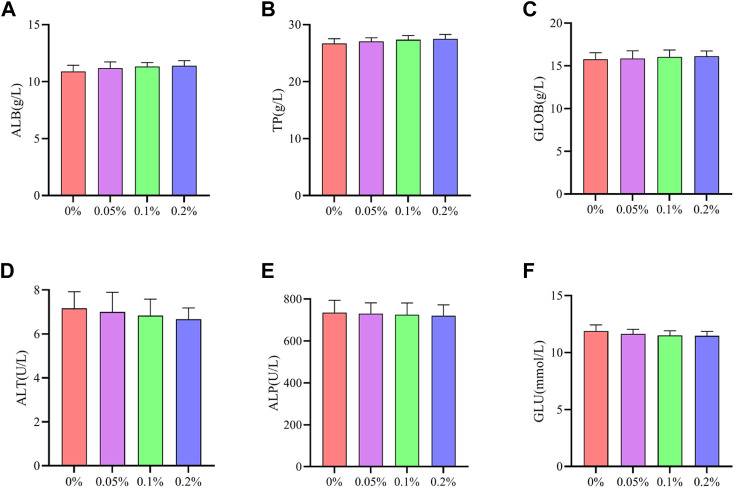
As shown in Figures 2, 3, Dietary supplementation with graded levels of BX increased broiler serum levels of 21-day GLOB and 42-day ALB, and decreased serum levels of ALT at days 21 and 42, but the statistical results did not reach significant levels (p > 0.05).
FIGURE 2.
Effects of dietary supplementation of Wheat germ, Hops and Grape seed extract mixture (BX) on serum biochemical parameters in 21 days broilers. (A) Albumin; (B) Total protein; (C) Globulin; (D) Alanine transaminase; (E) Alkaline phosphatase; (F) Glucose.
FIGURE 3.
Effects of dietary supplementation of Wheat germ, Hops and Grape seed extract mixture (BX) on serum biochemical parameters in 42 days broilers. (A) Albumin; (B) Total protein; (C) Globulin; (D) Alanine transaminase; (E) Alkaline phosphatase; (F) Glucose.
Faecal microbiota
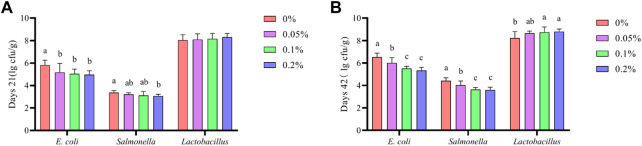
As shown in Figure 4, Dietary supplementation with graded levels of BX significantly reduced E. coli levels in broiler faeces at 21 days (p < 0.05). Salmonella levels in 21-day broiler faeces was significantly reduced when supplemented with 0.2% BX compared to the control group (p < 0.05). Dietary supplementation with graded levels of BX significantly reduced E. coli and Salmonella levels in broiler faeces at 42 days (p < 0.05). Lactobacillus levels in 42-day broiler faeces was significantly increased relative to the control when supplemented at 0.1% and 0.2% (p < 0.05).
FIGURE 4.
Effects of dietary supplementation of Wheat germ, Hops and Grape seed extract mixture (BX) on faecal microbiota in broilers. (A) Days 21; (B) Days 42. a,b,c,dMeans in the different groups with different superscripts are significantly different (p < 0.05). The same as below.
Noxious gas emissions
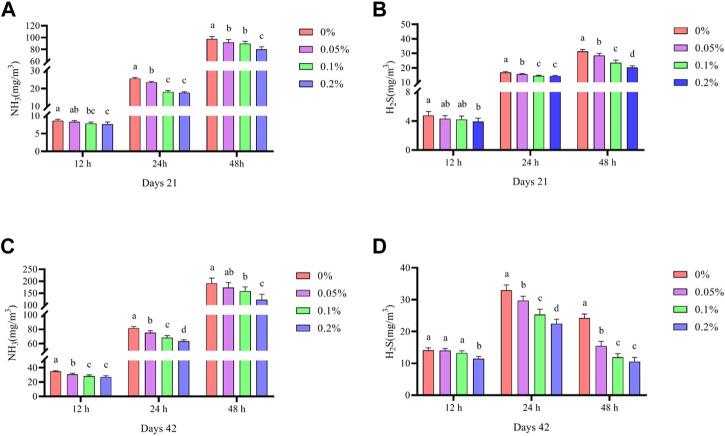
As shown in Figure 5, Dietary supplementation with graded levels of BX significantly reduced NH3 and H2S emissions in faeces at days 21 and 42 (p < 0.05). In contrast, at 0.2% BX supplementation, NH3 and H2S emissions were at their lowest values at each fermentation time.
FIGURE 5.
Effects of dietary supplementation of Wheat germ, Hops and Grape seed extract mixture (BX) on noxious gas emissions in broilers. (A) Days 21 NH3 emissions; (B) Days 21 H2S emissions; (C) Days 42 NH3 emissions; (D) Days 21 H2S emissions.
Discussion
The results of this trial showed that supplementing the broiler diet with wheat germ, hops and grape seed extract mixture (BX) mainly improved the growth performance of AA + broilers in the later stages of production (22–42 days). And as the amount added increases, so does the ADG and ADFI. Although there were few reports on whether wheat germ, hops and grape seed extracts promote broiler growth, some reports suggested positive effects (Abu and Ibrahim, 2018; Goluch et al., 2023). Possible reasons for this were that natural antioxidants protect the intestinal mucosa from oxidative damage and pathogens, while limiting peristaltic activity in digestive disorders, and that some reduction in intestinal motility might lead to better nutrient absorption (Kermauner and Laurenčič, 2008). To date, there had been much evidence that wheat germ, hops and grape seed extracts had antioxidant, antibacterial and anti-inflammatory effects. However, further research is needed to determine whether they promote growth, or whether they do so in other ways.
This pilot study found that although BX increased Newcastle disease antibody potency, ALB and GLOB levels and reduced ALT levels in broiler serum, none of these reached statistically significant levels. The increased potency of Newcastle disease antibodies in serum might be due to the immunostimulatory function of grape seed extract (GSE), mainly due to its antioxidant and free radical scavenging properties, which increased the integrity and added value of B lymphocytes that differentiate into antibody-producing plasma cells (Hamilos et al., 1989). Previous studies had shown that the addition of GSE to the diet could activate the Nrf2 signalling pathway and improved resistance to oxidative stress in broilers (Long et al., 2016; Rajput et al., 2019). Ali Rajput et al. (2017) found that the addition of GSE (250 and 500 mg/kg) to the diet significantly increased serum TP, ALB, and GLOB levels. This is similar to the results of this test.
In this study, the faecal microbiota of broilers were counted at 21 and 42 days and harmful gas emissions were measured. The results showed that dietary supplementation with BX significantly reduced E. coli and Salmonella counts in broiler faeces. At the same time, the number of Lactobacillus increased, suggesting that the addition of BX to the diet could selectively inhibit the growth of pathogens. There was also a significant reduction in NH3 and H2S emissions from faeces. The antimicrobial activity of BX was attributed to lectins, tannins, flavonoids, ß-acids, and xanthohumol. These bioactive components exert their antimicrobial action through different mechanisms that had been reported by most researchers. Studies had confirmed that the flavonoids in GSE could promote the growth of beneficial intestinal bacteria and inhibit certain pathogenic bacteria such as E. coli, Candida albicans, and S. aureus (Silván et al., 2013; Brenes et al., 2016). Cao et al. (2020) reported that the polyphenols in GSE could show positive prebiotic effects by promoting the growth of Lactobacillus and Bifidobacterium to maintain intestinal health. In addition, lectins from wheat germ had been shown to be viable options for various biomedical and therapeutic applications (Ryva et al., 2019). The ß-acid of hops was dipentylated at the C-6 site and also has antibacterial activity (Forino et al., 2016). The results of several studies had shown that faecal odour and ammonia emissions are associated with nutrient utilisation and the gut microbial ecosystem (Misiukiewicz et al., 2021). Along with Pseudomonas, Citrobacter, Aeromonas and Salmonella, E. coli was identified as the most promising H2S producing bacteria (Maker and Washington, 1974). Fecal NH3 and H2S emissions were also reduced due to the addition of BX to the dietary reducing the number of E. coli and Salmonella in broiler faeces. Based on the results of this trial, we hypothesize that one of the reasons for the increased growth performance of broilers at days 22–42 due to BX supplementation in the diet is the antioxidant properties of BX. Is this also due to the antibacterial properties of BX? The reduction in harmful bacteria and the increase in beneficial bacteria maintains a healthy gut and further promotes digestion and absorption of the diet in broilers, thus improving growth performance. This conjecture has yet to be tested through subsequent studies.
Conclusion
Dietary supplementation with Wheat germ, Hops and Grape seeds mixture (BX) improved the growth performance of AA + broilers in the later stages of growth (22–42 days). Dietary supplementation with BX reduced the levels of E. coli and Salmonella in broiler faeces, as well as the emission of NH3 and H2S. and lead to increased levels of Lactobacillus.
Acknowledgments
We thank the College of Animal Husbandry and Veterinary Medicine of Jinzhou Medical University for its support of the experiment. We thank Liaoning Kaiwei Biological Co., Ltd. for providing the breeding base for this trial. We thank Jinzhou Zhongke Genetic Testing Service Co., Ltd. for providing the antigens for antibody detection, and for assisting us with microbiological testing.
Funding Statement
This work was financed by the grants from the central government-guided local science and technology development fund projects (Grant No. 2021001).
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was reviewed and approved by Animal Ethics Committee of Jinzhou Medical University. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Abu S. H., Ibrahim S. A. (2018). Effect of dietary polyphenol-rich grape seed on growth performance, antioxidant capacity and ileal microflora in broiler chicks. J. animal physiology animal Nutr. 102 (1), 268–275. 10.1111/jpn.12688 [DOI] [PubMed] [Google Scholar]
- Ahn J., Grün I. U., Mustapha A. (2004). Antimicrobial and antioxidant activities of natural extracts in vitro and in ground beef. J. food Prot. 67 (1), 148–155. 10.4315/0362-028x-67.1.148 [DOI] [PubMed] [Google Scholar]
- Ali Rajput S., Sun L., Zhang N., Mohamed Khalil M., Gao X., Ling Z., et al. (2017). Ameliorative effects of grape seed proanthocyanidin extract on growth performance, immune function, antioxidant capacity, biochemical constituents, liver histopathology and aflatoxin residues in broilers exposed to aflatoxin B₁. Toxins 9 (11), 371. 10.3390/toxins9110371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arshad M. S., Anjum F. M., Khan M. I., Shahid M., Akhtar S., Sohaib M. (2013a). Wheat germ oil enrichment in broiler feed with α-lipoic acid to enhance the antioxidant potential and lipid stability of meat. Lipids health Dis. 12, 164. 10.1186/1476-511X-12-164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arshad M. S., Anjum F. M., Khan M. I., Shahid M. (2013b). Wheat germ oil and α-lipoic acid predominantly improve the lipid profile of broiler meat. J. Agric. food Chem. 61 (46), 11158–11165. 10.1021/jf4041029 [DOI] [PubMed] [Google Scholar]
- Brandolini A., Hidalgo A. (2012). Wheat germ: Not only a by-product. Int. J. food Sci. Nutr. 63 (1), 71–74. 10.3109/09637486.2011.633898 [DOI] [PubMed] [Google Scholar]
- Brenes A., Viveros A., Chamorro S., Arija I. (2016). Use of polyphenol-rich grape by-products in monogastric nutrition. a review. Animal Feed Sci. Technol. 211, 1–17. 10.1016/j.anifeedsci.2015.09.016 [DOI] [Google Scholar]
- Cao G., Zeng X., Liu J., Yan F., Xiang Z., Wang Y., et al. (2020). Change of serum metabolome and cecal microflora in broiler chickens supplemented with grape seed extracts. Front. Immunol. 11, 610934. 10.3389/fimmu.2020.610934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carini M., Stefani R., Aldini G., Ozioli M., Facino R. M. (2001). Procyanidins from Vitis vinifera seeds inhibit the respiratory burst of activated human neutrophils and lysosomal enzyme release. Planta medica 67 (8), 714–717. 10.1055/s-2001-18353 [DOI] [PubMed] [Google Scholar]
- Cheah K. Y., Howarth G. S., Bastian S. E. (2014). Grape seed extract dose-responsively decreases disease severity in a rat model of mucositis; concomitantly enhancing chemotherapeutic effectiveness in colon cancer cells. PloS one 9 (1), e85184. 10.1371/journal.pone.0085184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang D. X., Yong M. K., Kim I. H. (2020). Effects of a root extract from Achyranthes Japonica Nakai on the growth performance, blood profile, fecal microbial community, fecal gas emission, and meat quality of finishing pigs. Livest. Sci. 239, 104160. 10.1016/j.livsci.2020.104160 [DOI] [Google Scholar]
- Farahat M. H., Abdallah F. M., Ali H. A., Hernandez-Santana A. (2017). Effect of dietary supplementation of grape seed extract on the growth performance, lipid profile, antioxidant status and immune response of broiler chickens. Animal Int. J. animal Biosci. 11 (5), 771–777. 10.1017/S1751731116002251 [DOI] [PubMed] [Google Scholar]
- Fardet A. (2010). New hypotheses for the health-protective mechanisms of whole-grain cereals: What is beyond fibre? Nutr. Rev. 23 (1), 65–134. 10.1017/S0954422410000041 [DOI] [PubMed] [Google Scholar]
- Forino M., Pace S., Chianese G., Santagostini L., Werner M., Weinigel C., et al. (2016). Humudifucol and bioactive prenylated polyphenols from hops (Humulus lupulus cv. "cascade"). J. Nat. Prod. 79 (3), 590–597. 10.1021/acs.jnatprod.5b01052 [DOI] [PubMed] [Google Scholar]
- Goluch Z., Słupczyńska M., Okruszek A., Haraf G., Wereńska M., Wołoszyn J. (2023). The energy and nutritional value of meat of broiler chickens fed with various addition of wheat germ expeller. Animals open access J. MDPI 13 (3), 499. 10.3390/ani13030499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilos D. L., Zelarney P., Mascali J. J. (1989). Lymphocyte proliferation in glutathione-depleted lymphocytes: Direct relationship between glutathione availability and the proliferative response. Immunopharmacology 18 (3), 223–235. 10.1016/0162-3109(89)90020-9 [DOI] [PubMed] [Google Scholar]
- Hassan H. A., Al-Rawi M. M. (2013). Grape seeds proanthocyanidin extract as a hepatic-reno-protective agent against gibberellic acid induced oxidative stress and cellular alterations. Cytotechnology 65 (4), 567–576. 10.1007/s10616-012-9506-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kermauner A., Laurenčič A. (2008). “Supplementation of rabbit diet with chestnut wood extract: Effect on in vitro gas production from two sources of protein,” in Proceedings of the 9thWorld Rabbit Congress, Verona, Italy, 10 - 13 Jun, 2008, 689–693. [Google Scholar]
- Lee I. S., Lim J., Gal J., Kang J. C., Kim H. J., Kang B. Y., et al. (2011). Anti-inflammatory activity of xanthohumol involves heme oxygenase-1 induction via NRF2-ARE signaling in microglial BV2 cells. Neurochem. Int. 58 (2), 153–160. 10.1016/j.neuint.2010.11.008 [DOI] [PubMed] [Google Scholar]
- Liaqat H., Kim K. J., Park S. Y., Jung S. K., Park S. H., Lim S., et al. (2021). Antioxidant effect of wheat germ extracts and their antilipidemic effect in palmitic acid-induced steatosis in HepG2 and 3T3-L1 cells. Foods (Basel, Switz. 10 (5), 1061. 10.3390/foods10051061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long M., Zhang Y., Li P., Yang S. H., Zhang W. K., Han J. X., et al. (2016). Intervention of grape seed proanthocyanidin extract on the subchronic immune injury in mice induced by aflatoxin B1. Int. J. Mol. Sci. 17 (4), 516. 10.3390/ijms17040516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luther M., Parry J., Moore J., Meng J, H., Zhang Y, F., Cheng Z, h., et al. (2007). Inhibitory effect of chardonnay and black raspberry seed extracts on lipid oxidation in fish oil and their radical scavenging and antimicrobial properties. Food Chem. 104, 1065–1073. 10.1016/j.foodchem.2007.01.034 [DOI] [Google Scholar]
- Mahmoud Y. I. (2013). Grape seed extract neutralizes the effects of Cerastes cerastes cerastes post-synaptic neurotoxin in mouse diaphragm. Micron 44, 298–302. Oxford, England : 1993. 10.1016/j.micron.2012.07.007 [DOI] [PubMed] [Google Scholar]
- Maker M. D., Washington J. A., 2nd (1974). Hydrogen sulfide-producing variants of Escherichia coli . Appl. Microbiol. 28 (2), 303–305. 10.1128/am.28.2.303-305.1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui T., Li C. H., Tanaka T., Maki T., Osajima Y., Matsumoto K. (2000). Depressor effect of wheat germ hydrolysate and its novel angiotensin I-converting enzyme inhibitory peptide, Ile-Val-Tyr, and the metabolism in rat and human plasma. Biol. Pharm. Bull. 23 (4), 427–431. 10.1248/bpb.23.427 [DOI] [PubMed] [Google Scholar]
- Misiukiewicz A., Gao M., Filipiak W., Cieslak A., Patra A. K., Szumacher-Strabel M. (2021). Review: Methanogens and methane production in the digestive systems of nonruminant farm animals. Animal Int. J. animal Biosci. 15 (1), 100060. 10.1016/j.animal.2020.100060 [DOI] [PubMed] [Google Scholar]
- National Research Council (1994). Nutrient requirements of poultry ninth revised. Edition. Washington, D.C: National Academies Press, 145. [Google Scholar]
- Nunes M, A., Pimentel F., Costa A, S, G., Alves R, C., Oliveira M, B, P, P., Beatriz P, P. (2016). Cardioprotective properties of grape seed proanthocyanidins: An update. Trends Food Sci. Technol. 57, 31–39. 10.1016/j.tifs.2016.08.017 [DOI] [Google Scholar]
- Patra A. K., Saxena J. (2011). Exploitation of dietary tannins to improve rumen metabolism and ruminant nutrition. J. Sci. food Agric. 91 (1), 24–37. 10.1002/jsfa.4152 [DOI] [PubMed] [Google Scholar]
- Rajput S. A., Sun L., Zhang N. Y., Khalil M. M., Ling Z., Chong L., et al. (2019). Grape seed proanthocyanidin extract alleviates AflatoxinB₁-induced immunotoxicity and oxidative stress via modulation of NF-κB and Nrf2 signaling pathways in broilers. Toxins 11 (1), 23. 10.3390/toxins11010023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryva B., Zhang K., Asthana A., Wong D., Vicioso Y., Parameswaran R. (2019). Wheat germ agglutinin as a potential therapeutic agent for leukemia. Front. Oncol. 9, 100. 10.3389/fonc.2019.00100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silván J, M., Mingo E., Hidalgo M., De Pascual-Teresa S., Carrascosa A. V., Martinez-Rodriguez A. J. (2013). Antibacterial activity of a grape seed extract and its fractions against campylobacter spp. Food control 29 (1), 25–31. 10.1016/j.foodcont.2012.05.063 [DOI] [Google Scholar]
- Srinivasan V., Goldberg D., Hass G. J. (2004). Contributions to the antimicrobial spectrum of hop constituents. Econ. Bot. 58, S230–S238. 10.1663/0013-0001(2004)58[s230:cttaso]2.0.co;2 [DOI] [Google Scholar]
- Sui H., Wang F., Weng Z., Song H., Fang Y., Tang X., et al. (2020). A wheat germ-derived peptide YDWPGGRN facilitates skin wound-healing processes. Biochem. biophysical Res. Commun. 524 (4), 943–950. 10.1016/j.bbrc.2020.01.162 [DOI] [PubMed] [Google Scholar]
- Teuber M., Schmalreck A. F. (1973). Membrane leakage in Bacillus subtilis 168 induced by the hop constituents lupulone, humulone, isohumulone and humulinic acid. Arch. fur Mikrobiol. 94 (2), 159–171. 10.1007/BF00416690 [DOI] [PubMed] [Google Scholar]
- Wang F., Weng Z., Lyu Y., Bao Y., Liu J., Zhang Y., et al. (2020). Wheat germ-derived peptide ADWGGPLPH abolishes high glucose-induced oxidative stress via modulation of the PKCζ/AMPK/NOX4 pathway. Food & Funct. 11 (8), 6843–6854. 10.1039/d0fo01229g [DOI] [PubMed] [Google Scholar]
- Yamakoshi J., Otsuka F., Sano A., Tokutake S., Saito M., Kikuchi M., et al. (2003). Lightening effect on ultraviolet-induced pigmentation of Guinea pig skin by oral administration of a proanthocyanidin-rich extract from grape seeds. Pigment Cell Res. 16 (6), 629–638. 10.1046/j.1600-0749.2003.00093.x [DOI] [PubMed] [Google Scholar]
- Yao J., Zhang B., Ge C., Peng S., Fang J. (2015). Xanthohumol, a polyphenol chalcone present in hops, activating Nrf2 enzymes to confer protection against oxidative damage in PC12 cells. J. Agric. food Chem. 63 (5), 1521–1531. 10.1021/jf505075n [DOI] [PubMed] [Google Scholar]
- Zawadzki A., Alloo C., Grossi A. B., do Nascimento E. S. P., Almeida L. C., Bogusz Junior S., et al. (2018). Effect of hop β-acids as dietary supplement for broiler chickens on meat composition and redox stability. Food Res. Int. Ott. Ont.) 105, 210–220. 10.1016/j.foodres.2017.10.072 [DOI] [PubMed] [Google Scholar]
- Zawadzki A., Arrivetti L. O. R., Vidal M. P., Catai J. R., Nassu R. T., Tullio R. R., et al. (2017). Mate extract as feed additive for improvement of beef quality. Food Res. Int. Ott. Ont.) 99 (1), 336–347. 10.1016/j.foodres.2017.05.033 [DOI] [PubMed] [Google Scholar]
- Zhao Y., Liao A.-M., Liu N., Huang J.-H., Lv X., Yang C.-R., et al. (2021). Potential anti-aging effects of fermented wheat germ in aging mice. Food Biosci. 42, 101182. 10.1016/j.fbio.2021.101182 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.