Abstract
Objective
To examine the characteristics of those who fulfil the recent National Institute of Neurological Disease and Stroke (NINDS) Consensus Diagnostic Criteria for Traumatic Encephalopathy Syndrome (TES) and test whether they show differences in MRI-based regional brain volumes, cognitive domains, and certain plasma biomarkers.
Methods
Professional fighters 35 years of age or older and/or retired were included. Participants were categorised as either having TES (TES+) or not (non-TES). TES+ participants were further subtyped by their cognitive profile. Multiple linear regression models were used to compare MRI-based regional brain volumes, cognitive performance, plasma tau and neurofilament light levels between TES– and TES+ groups.
Results
176 participants (110 boxers and 66 MMA) were included in the analysis. 72 (41%)/176 were categorised as having TES, the likelihood of TES increasing with age. TES+ participants tended to be boxers, started fighting at a younger age, had more professional fights and knocked out more frequently. The TES+ group had lower regional brain volumes including both grey and white matter structures. TES+ also had lower scores on simple and choice reaction time, psychomotor speed and Trails A.
Conclusion
The new TES criteria does distinguish a group of fighters with differences in regional brain volumes and reduced cognitive function. Our findings support the use of the NINDS criteria for TES in further research of the long-term effects of repetitive head impacts.
Keywords: boxing, magnetic resonance imaging, observational study, brain concussion
What is already known on this topic?
The first consensus criteria for the diagnosis of traumatic encephalopathy syndrome (TES) was recently developed but has yet to be applied to groups exposed to repetitive head impacts.
What this study adds?
This study demonstrates, in a cohort of professional fighters, that the National Institute of Neurological Disease and Stroke (NINDS) Consensus Diagnostic Criteria for TES does differentiate a group who differ in MRI regional volumes and cognitive measures.
How this study might affect research, practice or policy?
This study suggests that the NINDS TES criteria can be useful in research related to the long-term effects of repetitive head impacts.
Chronic traumatic encephalopathy (CTE) is a neurodegenerative disorder described in athletes with previous exposure to repetitive head impacts (RHIs).1 Currently, the diagnosis can only be made by pathological examination.2 Despite the media attention it has received, much is still unknown about the disease including its incidence/prevalence, natural history and risk factors.3
One of the difficulties in studying CTE had been the lack of an agreed on clinical diagnostic criteria. However, guided by accumulating clinical and pathological data, the first National Institute of Neurological Disease and Stroke (NINDS) Consensus Diagnostic Criteria for Traumatic Encephalopathy Syndrome (TES) was recently published.4 The TES criteria was designed to represent the clinical syndrome of CTE and consists of core features including substantial exposure to RHI, impairment in cognitive function and/or neurobehavioural dysregulation, a progressive course, and the absence of any other process that could account for the symptoms.
While intended to be used for research purposes, the TES consensus criteria has not yet been validated. The data that guided the TES consensus criteria came mainly from clinical and pathological findings of former professional American football players.5 Thus, it is not yet known how the criteria would perform in various populations exposed to RHI and if those who are categorised as TES have distinctive features on neuroimaging or cognitive measures, or ultimately the neuropathological changes described in CTE.
The Professional Fighters Brain Health study is a longitudinal observational study of professional boxers and mixed martial artists.6 Utilising this well characterised cohort, we examined the TES criteria in a cohort of fighters and test whether TES+ individuals show differences in MRI-based regional brain volumes, cognitive domains and plasma biomarkers.
Methods
The study cohort was drawn from the Professional Athletes Brain Health Study (PABHS), a convenience sample of active and retired professional fighters (boxers and mixed martial arts (MMAs) fighters), along with age-matched and education-matched controls. Active fighters were required to have at least one professional fight within 2 years of enrolment and be training with the intent to compete; information about the study was disseminated by the Nevada Athletic Commission, fight promoters and local training facilities. Retired fighters were included if they had been boxers or MMA fighters, had a minimum of 10 professional fights, had no sanctioned fights for at least 2 years and did not intend to return to competition. Both active and retired fighters could not have any history of other neurological or psychiatric disorders. Enrolment in the PFBHS began in 2011 and has been continuous since then. Each participant is seen on an annual basis, and for active fighters, not sooner than 45 days from a sanctioned fight. If a participant missed a visit, the subsequent study visit was conducted as soon as the participant was available. The PFBHS was approved by the Cleveland Clinic Institutional Review Board and written informed consent was obtained from all participants. More detailed methods of recruitment and study procedures have been described previously.6
At baseline and each annual visit, a battery of tests and information are acquired including MRI brain, computerised cognitive testing and exposure history. Participants answer questionnaires with the assistance of the study coordinator who collect information on demographics; educational attainment; medical history including concurrent illnesses and prescribed medications; previous head trauma, both related and unrelated to athletic activities; and prior involvement in other contact sports. Number of professional fights was ascertained by review of commonly recognised databases (boxrec.com for boxers, sherdog.com for MMA fighters).
Patient and public involvement
Patients or the public were not involved in the design, conduct, reporting or dissemination plans of our research.
Participant selection
All retired fighters and active fighters 35 years of age or older were included in the study. This age cut-off for active fighters was chosen to allow older active fighters who have had a significant duration of exposure to RHI.7 While the extent of exposure to RHI may be somewhat different between boxers and MMA fighters, there is overlap in the type of exposure that both experience; including both disciplines of fighting allowed a comparison regarding prevalence of TES.
TES diagnosis
Utilising the NINDS Consensus Clinical Criteria for TES, a panel of four clinicians adjudicated a diagnosis of (TES+) or no TES (non-TES) for each participant included in the study. The core features for TES diagnosis include: (1) substantial exposure to RHIs, (2) cognitive impairment (involving either memory or executive function) and/or behavioural dysregulation, (3) progressive course of symptoms and (4) no other condition that could otherwise account for the symptoms.4 The panel reviewed data from the first visit that the individual met inclusion criteria for this study. Based on previous work, a cut-off of 10 or greater professional fights was used as a threshold for substantial exposure to RHI.8 The core clinical feature of cognitive dysfunction was determined by evaluating memory and executive performance on the PABHS cognitive test battery. The PABHS test battery takes approximately 1 hour to complete and is comprised of largely computer-based tests from CNS Vital Signs and C3 Logix.9 10 Memory was assessed by the CNS Vital Signs verbal recognition memory test. Executive functioning was evaluated using data from a computerised version of Trail Making Test B (C3), Stroop (CNS Vitals), Digit Symbol Substitution task (CNS Vital Signs) and Controlled Oral Word Association Test. The determination of cognitive impairment was based on either the participant or clinician noting the presence of a cognitive complaint and performance below 1.5 SD for age-matched norms on either the memory test or at least one of the executive tests. If only one test score was below the cut-off, it was ascertained that the score was not an outlier (possibly indicating poor understanding of the test rules or poor effort). Behavioural dysregulation was evaluated using participant self-reports of individual items on the Barrett Impulsivity Scale II (questions #2, 6, 12, 15, 21) input from the clinical interview, and/or from additional source of information (collateral input from families or medical records).11 Progression of symptoms was based on performance on prior cognitive tests (if available), participant’s subjective experience and study-clinician input. Finally, the panel considered self-reported medical history, study-clinician impressions (from interview and examination), MRI findings and scores on Patient Health Questionnaire 9 (PHQ-9) to rule out other contributing disorders. For this analysis, we required evidence of cognitive impairment for a TES+ diagnosis. Because not all of the supportive features for TES are evaluated in PABHS, the consensus panel was not able to assign provisional levels of CTE pathology that are outlined in the TES criteria.
A high-resolution T1-weighted anatomical MRI was obtained on all fighters at each annual visit. A 3T MRI scanner (Siemens, Munich, Germany, Verio from April 2011 through October 2015 and Siemens Skyra from December 2016 to the present) with a 32-channel head coil was used to acquire structural 3D T1-weighted magnetisation-prepared rapid acquisition gradient echo images (repetition time ms/echo time ms, 2300/2.98; resolution, 1×1 × 1.2 mm3). Only data with high-quality cortical reconstruction from FreeSurfer were utilised. This was ensured through a quality control procedure outlined by FreeSurfer’s quality analysis tools (surfer.nmr.mgh.harvard.edu/fswiki/QATools), and only data with signal-to-noise ratio of at least 16 were included in this study (surfer.nmr.mgh.harvard.edu/pub/dist/freesurfer/tutorial_packages/OSX/freesurfer/bi n/wm-anat-snr). Volumes of the hippocampus and amygdala and subcortical grey matter including thalamus, caudate and putamen, along with corpus callosum, lateral ventricle, total grey and white matter volumes were calculated using the automated full brain segmentation process in Freesurfer V.6 software. These regions have been shown in pathologic series and our prior work to be affected in those with extensive RHI.8 12 13 The volumes of each structure were measured in both hemispheres separately and adjusted for total intracranial volume.
Several other cognitive measures were obtained as part of the PABHS protocol that were not considered in determining TES status. The CNS Vital Signs includes a finger tapping test and in combination with the other tests determines scores in categories termed psychomotor speed and reaction time. The C3 Logix battery also contains a simple and choice reaction time test.
Blood samples were collected annually in EDTA tubes and centrifuged at 3200 rpm for 10 min to separate plasma from blood cells. The supernatant was aliquoted in 2 mL portions that were immediately frozen and stored at −80° pending analysis. Plasma tau and neurofilament light chain (NF-L) concentrations were measured using ultrasensitive Single molecule array (Simoa) assays as previously described in detail.14 15 The lower limits of quantification for tau and NF-L were 1.22 pg/mL and 6.9 pg/mL, respectively, and intra-assay coefficients of variation were just above or below 10%. All analyses were performed by board-certified laboratory technicians who were blinded to clinical data.
Apolipoprotein E (APOE) genotyping was based on one single-nucleotide polymorphism (SNP) for allelic discrimination of APOE ε4 allele carriers from non-carriers. Allelic discrimination was performed using the 7500 Real Time PCR System and TaqMan SNP Genotyping Assays (Thermo Fisher). The allelic discrimination analyses utilised the APOE SNP rs429358 (Thermo Fisher). Each predesigned TaqMan assay includes two allele-specific TaqMan MGB probes that contain distinct fluorescent dyes and a PCR primer pair to detect the SNP target. The genotypes were determined (based on sample clustering) using the auto-caller function of the genotyping application within the browser-based Thermo Fisher Cloud software.
Statistical analysis
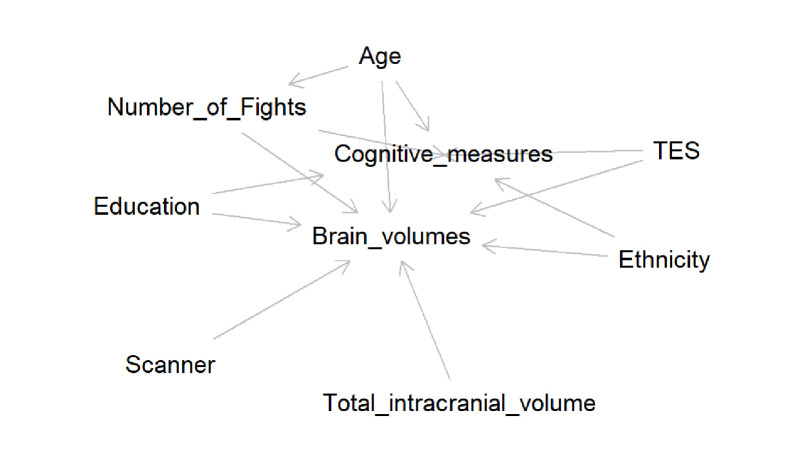
For participants’ characteristics, we calculated mean and SD for continuous variables (eg, age, number of fights) and computed the p value for comparing the non-TES group and the TES+ group using the independent two-sample t-test. Shapiro-Wilk’s test was used to test the normality assumption and Levene’s test was used for testing the homogeneity of variance assumption. For continuous outcome, we also checked the normality assumption from the Q–Q plots. When these assumptions are not satisfied, the non-parametric Wilcoxon rank-sum test was used for comparing two independent groups.16 For categorical variables, the number of events and the associated proportion were computed with the p value from the Fisher’s exact test. The Fisher’s exact test was chosen to avoid the assumptions from asymptotic tests.17 All these tests were 2-sided at α level of 0.05. All statistical analyses were performed using SAS statistical software (V.9.4; SAS Institute, Cary, North Carolina, USA). Multiple linear regression models were used for comparing the brain volumes and cognitive measures between the non-TES group and the TES+ group. The main effect of these models is the TES status, and the covariates include age, education years, ethnicity and number of fights; these covariates were chosen due to their known influence on brain volume and cognitive performance. For brain volumes, we added scanner type and total intracranial volume in the covariates as the scanner for the study was upgraded a few years ago. Figure 1 displays a directed acyclic graph to show the set of confounders used as covariates in the statistical model.18 We checked the following model assumptions: linearity, homoscedasticity and normality in the linear regression model. These assumptions were checked by inspection of residuals versus predicted value plots and Q–Q plots. None of the assumptions were violated. Similar statistical models were used when comparing the non-TES group and the two TES+ subgroups.
Figure 1.
Directed acyclic graph shows a minimally sufficient set of confounders as covariates in the statistical models.
A logistic regression model was used to investigate the performance of using number of fights to classify TES status. The Youden index that maximise the sum of sensitivity and specificity was used to identify the optimal number of fights as the threshold value, where Youden index=sensitivity + specificity − 1.19
Results
A total of 176 participants in the PABHS met criteria for inclusion in our analysis and had sufficient data to be included in the cohort. One hundred and ten of those adjudicated were boxers and 66 were MMA. Most fighters had retired at the time of their clinical visit (109/176). Among the 176 participants undergoing the adjudication process, 72/176=41% (95% CI: 34% to 48%) were categorised as having TES. Among the boxers, 55% met criteria for TES whereas only 18% of the MMA fighters did.
Demographics and exposure to RHI
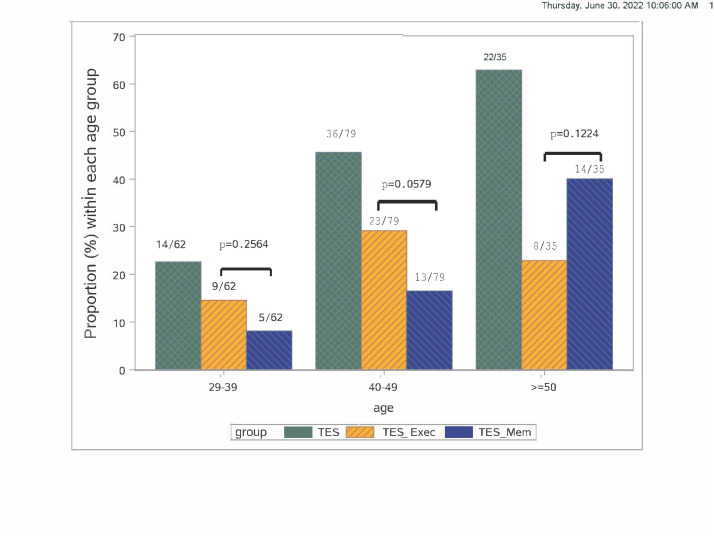
Compared with the non-TES group, the percentage of fighters who met criteria for TES+ increased with age (figure 2). In regard to exposure to RHI, those TES+ started fighting at a younger age, had more professional fights and had been knocked out more frequently. Boxers made up a higher percentage of those categorised as TES (boxers, 83% vs MMA, 17%; table 1).
Figure 2.
Age distribution of participants adjudicated as having TES. Bars represent the percentage of fighters within each age group who were adjudicated as having TES (green) and subtypes: memory (blue) and executive only (orange). TES, traumatic encephalopathy syndrome.
Table 1.
Characteristics of fighters who were adjudicated as either having TES or not. mean (SD) or median (IQR) for continuous variables and number (%) for categorical variables
| Non-TES | TES+ | P value | |
| N | 104 | 72 | |
| Age in years | 41.25 (8.21) | 47.31 (9.02) | <0.0001 |
| Years of education | 13.48 (3.10) | 12.83 (2.57) | 0.0302 |
| Number of fights, median | 16 (5, 35) | 33.50 (21.50, 48.50) | <0.0001 |
| Professional years | 8.96 (5.67) | 12.99 (5.41) | <0.0001 |
| Boxer (%) | 50 (48%) | 60 (83%) | <0.0001 |
| MMA (%) | 54 (52%) | 12 (17%) | <0.0001 |
| Active (%) | 59 (57%) | 8 (11%) | <0.0001 |
| Race | 0.0081 | ||
| Black | 19 (18%) | 27 (38%) | |
| White | 64 (62%) | 29 (40%) | |
| Other | 21 (20%) | 16 (22%) | |
| Age start competing in years | 19.46 (8.18) | 14.19 (6.30) | <0.0001 |
| Knock outs, median | 1.5 (0, 3.5) | 3.0 (2.0, 5.0) | 0.0011 |
| APOE ε4+ (%) | 25/82 (30%) | 12/52 (23%) | 0.4292 |
MMA, mixed martial art; TES, traumatic encephalopathy syndrome.
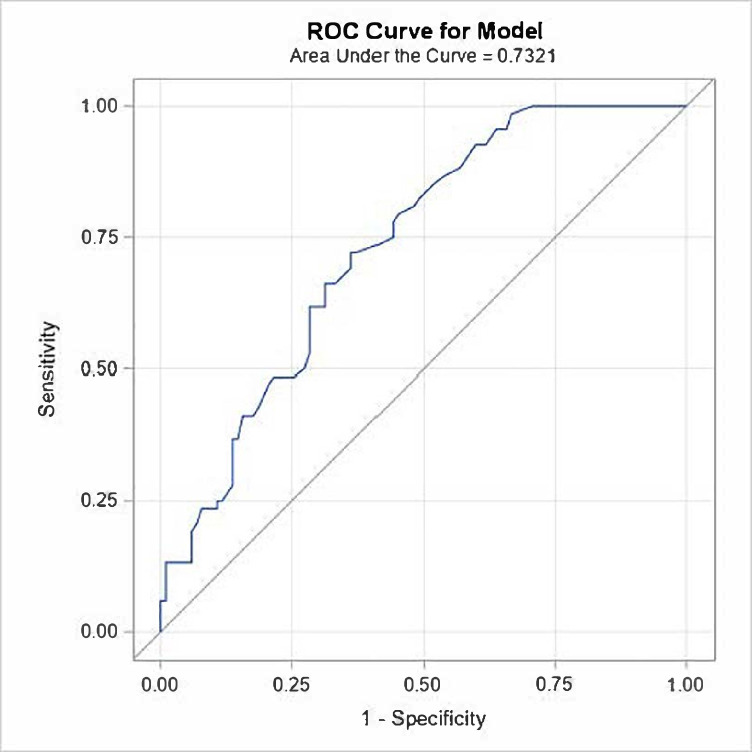
In order to determine the number of professional fights that may increase the likelihood of developing TES, we built a logistic regression model. Because only a few MMA fighters were diagnosed with TES+we restricted our analysis to boxers. From the fitted logistic regression model, a receiver operating characteristic (ROC) curve was plotted from the statistical software to obtain the area under the curve (AUC) which was estimated as 0.73 (95% CI: 0.66 to 0.81) (figure 3). The optimal threshold value for number of fights from the Youden index was 25 fights. A fighter with 25 or more fights is more likely to be TES+. With that threshold, the estimated sensitivity was 72% (95% CI: 60% to 81%) and the specificity was 64% (95% CI: 54% to 72%).
Figure 3.
ROC curve for assessment of the number of professional fights that is associated with TES+ status. Area under the curve estimated as 0.73 (95% CI: 0.66 to 0.81). TES, traumatic encephalopathy syndrome; ROC, receiver operating characteristic.
Brain volumes, cognitive performance and biomarkers
In most of the brain volumes investigated, the TES+ group had lower regional brain volumes compared with the non-TES group, including the thalamus, hippocampus, posterior corpus callosum, white matter volume, total and subcortical grey matter volume with increased lateral ventricle volume (table 2). With sample sizes of 104 in the non-TES group and 72 in the TES+ group, the 95% CI for the difference in volume of thalamus is from −613 to −120 cubic mm with the non-TES group as the reference group (table 2).
Table 2.
MRI regional volumes by TES status
| TES vs non-TES | TES+E vs non-TES | TES+ME vs non-TES | ||||
| Estimate (95% CI) | P value | Estimate (95% CI) | P value | Estimate (95% CI) | P value | |
| Thalamus | 366 (−613 to −120) | 0.004 | −252 (−527 to 22) | 0.072 | −553 (−874 to −233) | 0.0008 |
| Putamen | −120 (−317 to 77) | 0.230 | 57 (−156 to 269) | 0.598 | −410 (−658 to −163) | 0.0013 |
| Hippocampus | −235 (−388 to −82) | 0.003 | −97 (−262 to 68) | 0.245 | −460 (−652 to −268) | <0.0001 |
| Lateral ventricle | 2706 (311 to 5102) | 0.027 | 230 (−2313 to 2772) | 0.859 | 6778 (3817 to 9740) | <0.0001 |
| Inferior lateral ventricle | 161 (−16 to 339) | 0.075 | −56 (−240 to 127) | 0.547 | 519 (305 to 733) | <0.0001 |
| CC_posterior | −89 (−161 to −16) | 0.017 | −50 (−131 to 31) | 0.225 | −152 (−246 to −58) | 0.0017 |
| CC_mid posterior | −54 (−104 to −4) | 0.035 | −56 (−113 to 0) | 0.051 | −50 (−116 to 16) | 0.1355 |
| Left cerebral white matter | −8176 (−14 775 to −1576) | 0.016 | −4170 (−11 469 to 3129) | 0.261 | −14 763 (−23 264 to −6263) | 0.0008 |
| Right cerebral white matter | −8700 (−15 054 to −2346) | 0.008 | −5002 (−12 041 to 2037) | 0.162 | −14 782 (−22 980 to −6583) | 0.0005 |
| Cerebral white matter | −16 876 (−29 750 to −4003) | 0.011 | −9172 (−23 418 to 5074) | 0.205 | −29 545 (−46 137 to −12 953) | 0.0006 |
| Subcortical grey | 2390 (−3916 to −865) | 0.002 | −1020 (−2665 to 625) | 0.222 | −4644 (−6559 to −2728) | <0.0001 |
| Total grey | −15 531 (−27 808 to −3254) | 0.014 | −7881 (−21 444 to 5682) | 0.253 | −28 110 (7987) | 0.0006 |
Volumes of the thalamus, putamen and hippocampus represent average of both hemispheres (in cubic mm). TES+E = TES with executive impairment, TES+ME = TES with both memory and executive impairment.
CC, corpus callosum; TES, traumatic encephalopathy syndrome.
Restricting the analysis to only the retired fighters (eliminating the active fighters 35 years of age or older) yielded similar results.
In addition, those with TES+ had lower scores on a variety of cognitive measures not used in defining the groups including simple and choice reaction time, psychomotor speed and Trails A.
Levels of NF-L or total plasma tau were similar between the groups.
Memory impairment versus executive impairment in participants with TES
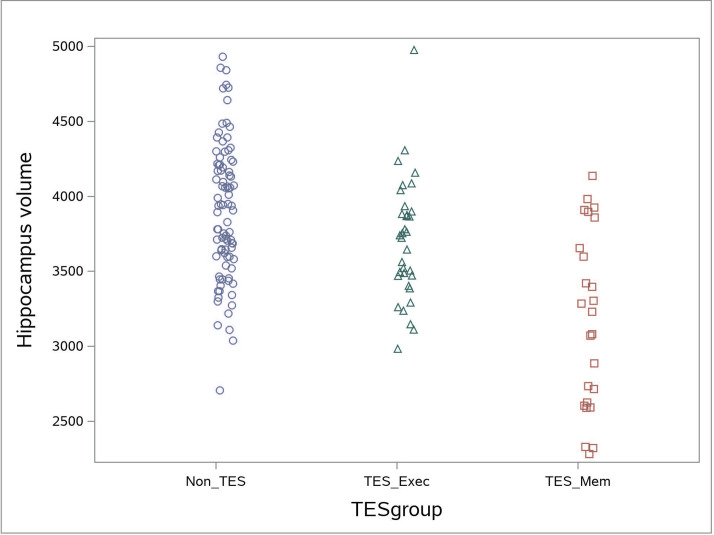
In regard to cognitive test performance, we found that we could group patients into three groups: (1) those without cognitive impairment (non-TES), (2) those with executive impairment (TES+E) and (3) those with both memory and executive impairment (TES+ME). Only a small percentage of individuals had isolated memory impairments (without executive impairment). TES+ME were older, had more fights and had been knocked out more frequently. TES+ME had more atrophy in most brain volumes compared with TES+E (table 2). Differences in regional brain volumes between non-TES and TES+ seemed to be driven almost entirely by the TES+ME subgroup, this is especially notable for the hippocampus (figure 4). Both the TES+E and TES+ME had worse test performance on reaction time test (simple and choice) compared with the non-TES group.
Figure 4.
Distribution of hippocampal volumes (averaging right and left sides) of each fighter separated by group: non-TES, TES memory and TES executive only. TES, traumatic encephalopathy syndrome.
Discussion
The recently published NINDS consensus clinical criteria for TES is intended to define the clinical syndrome associated with CTE and aid in clinical research by providing a common diagnostic criterion. Applying these criteria to a well characterised cohort of professional fighters, our study found that those who were categorised as TES had both greater exposure to RHI (earlier age of fighting, more professional fights, more times being knocked out) and lower regional brain volumes and cognitive function. Furthermore, those differences were primarily driven by a smaller subset of individuals within TES group who had both recognition memory and executive impairments.
Prevalence of TES
One of the outstanding questions regarding CTE is the incidence and prevalence in various groups exposed to RHI. The prevalence of TES in our entire cohort of retired professional fighters and those over the age of 35 was 41% (95% CI: 34% to 48%) and increases with age. More than 60% of retired fighters above the age of 50 met criteria for TES. It is important to emphasise that it is currently unknown what percentage of individuals who fulfil the criteria for TES actually harbour CTE pathology. Though the TES criteria provide for the determination of levels of certainty for CTE, we were not able to apply this to our cohort because this study does not collect data on functional status. Furthermore, while the TES criteria does allow for individuals to be classified as having TES if they have behavioural dysregulation and no cognitive impairment, we required the presence of cognitive impairment for several reasons: (1) cognitive features are among the most common clinical symptoms identified in those with autopsy verified CTE,4 (2) the term neurobehavioural dysregulation is a relatively new construct and there were not sufficient standardised measures to adequately assess for this included in the PABHS when it began enrolment in 2011 and (3) the PABHS did not formally collect collateral information from study partners whose input may be more informative in defining this syndrome
The heterogeneity of previous cohorts studied makes it difficult to compare our findings to prevalence rates reported in the literature. In the initial description of CTE, Martland claimed that 50% of boxers had symptoms of what was termed ‘punch drunk’.20 One of the most extensive epidemiological study of boxers performed over 50 years ago on 250 randomly selected retired fighters in Great Britain found that 17% had what would be considered CTE and 40% with some neurological findings or alcoholism.21 Using the NINDS Consensus pathological criteria, postmortem studies have indicated rates of CTE ranging from 32% of individuals who had a history of playing contact sports in the Mayo Clinic Brain Bank, to 99% of former National Football League players in the Boston University Brain Bank.5 22 In assessing these varying rates, factors such as age of the cohort, the criteria utilised for classification, whether clinical or pathological features were used, amount of exposure to RHI and selection bias need to be considered.
Risk factors
Aside from age, we found the major factor associated with TES+ in our group was amount of exposure to RHI. The TES criteria require substantial exposure to RHI and designates only for former football players a specific minimum amount (5 years of play). Looking at boxers in our cohort, we found that a threshold of 25 or more fights predicted whether a patient was categorised as TES+, though the sensitivity and specificity of this was relatively low (72 and 64, respectively). This finding may help guide required exposure levels for combat sports in future iterations of the TES criteria, but further data modelling may be improved by incorporating other factors that can be easily assessed such as frequency of competitions, age of starting fighting or number of knock outs.
While it may be that anyone given enough exposure to RHI will develop TES, other factors have been reported to interact with exposure to determine an individual’s long-term neurological outcome from RHI.23 Of note, and similar to previous findings in our cohort and others, age at which one starts exposure to RHI may be a risk factor for worse performance and TES.24 On the other hand, though some reports in the literature suggest APOE 4 to be a risk factor for poorer outcome in those exposed to RHI, there was no difference in rates of APOE 4 carriers between the TES+ and non-TES group.25 This finding is consistent with other studies that have also not clearly shown that APOE 4 confers any increased risk of neurological impairment in those exposed to RHI.26 27
Clinical and biomarkers
While TES currently is defined by clinical features, it does seem to separate a group that show volumetric differences on MRI imaging. A variety of cortical and subcortical structures had significantly lower volumes in the TES+ group including thalamus, hippocampus, corpus callosum and both total white matter and grey matter. This is similar to prior findings from our cohort that indicate a relationship between exposure and lower volumes in these regions; if this is found to be a consistent result in other cohorts of individuals with TES, MRI volumetric patterns may play a role as a biomarker to complement the clinical criteria.8 13
A core feature of TES is cognitive impairment in either memory or executive function. As we were adjudicating cases, we saw a distinct pattern emerge in which participants we classified as having TES+ could be further grouped into one of two groups: those with executive impairments only (TES+E) and those with both executive and memory impairments (TES+ME). In general, most (but not all) participants we subcategorised into the TES+ME group were older, more likely to be high level boxers (championship level), and had significantly more RHI (based on number of professional fights as a surrogate for exposure to RHI). Interestingly, when we analysed this group separately, we found that the subgroup of TES+ME was largely driving the group differences between TES+ and non-TES. In fact, the TES+E group actually looked more similar to the non-TES group than they did the TES+ME group. Several explanations for these findings could be considered. It is possible that TES+E is simply an early stage in the TES spectrum. In this model, exposure to RHI may initially preferentially affect frontal and subcortical structures, perhaps due to response to axonal injury, which is the start of a pathology that will progress by spread of tau over time to include temporal lobe and greater cortical involvement. In applying this model to our cohort, we would expect to see all participants with TES+E eventually develop TES+ME. Our previous study of longitudinal MRI changes in combat fighters would support this hypothesis.13 Alternatively, those with primarily dysexecutive function may represent a group with lower levels of pathology that are less likely to significantly worsen over time. Some authors have questioned whether CTE is necessarily a progressive disease for most individuals exposed to RHI.28 Whether TES+ fighters in our cohort have progressive decline is unknown. Clearly, longitudinal studies are needed to understand the prognostic value of these separate clinical presentations, but our data could indicate that it may be important to focus on studying younger individuals with memory impairment as they could be at higher risk for significant decline.
We did not find any relationship between TES status and plasma levels of neurofilament light or total tau. Neurofilament light is highly expressed in large-calibre myelinated axons of white matter and has been shown to increase in response to acute traumatic injury.28 29 Tau is a CNS-enriched protein with greater expression in unmyelinated cortical axons.30 We had previously found that NF-L levels did not differ between retired fighters and controls nor were related to cognitive impairment.31 Similarly, despite the fact that tau deposits are required for the pathological diagnosis of CTE, a previous study indicated that plasma levels of total tau was not able to distinguish retired fighters from controls.32 Further research is needed to see if other more specific tau species are related to TES diagnosis.33–35
Limitations
Within the new TES criteria, if TES is present, then there is an algorithm to determine the likelihood of CTE pathology based on additional factors such as level of functioning and presence or absence of delay in onset of symptoms, motoric or psychiatric symptoms. Because we did not have complete data in all those fields, we were not able to make this further assessment. Other limitations of this study include the absence of thorough measures of memory function which could lead to an underestimate of participants who did have memory impairment, use of population-based norms to determine cognitive impairment that may not apply to this cohort and lead to misclassification, and lack of a complete set of standardised questionnaires to identify behavioural dysregulation. The PABHS cohort is a convenience sample which could bias the generalisability of prevalence of TES in a fighter’s cohort. However, earlier in the study we did compare those who entered the study as active fighters with a random sample of individuals who were licensed professional combatants in Nevada over the same time and there were no significant differences in age, number of fights or win/loss record.6 Furthermore, previous work by our group did not show any difference in the prevalence of depression in our cohort compared with the general population. Because the sample size was limited to those already enrolled in the PABHS, it is possible that analysis of a larger sample might alter the results. All the data we collect ares from the participant and self-reported; we did not collect collateral information from an informant for verification. This study was also conducted entirely in professional athletes engaged in combat sports, and thus generalisability to athletes from other sports is not possible. Finally, there may have been other unmeasured confounding factors that differentiate the TES+ and TES− groups and contribute to our findings.
Conclusion
We report the first application of the new NINDS TES criteria to a cohort of professional fighters. Our results suggest that the criterion does distinguish a group with clear differences in regional brain volumes. While ultimately clinical and pathological studies are needed to validate the accuracy of the criteria to identify those with CTE, these findings support its proposed use in further research of the long-term effects of RHI.
Footnotes
Contributors: AR contributed to the design, data collection, analysis and writing of the manuscript. CB contributed to the design, data collection, analysis, writing of the manuscript and acting as guarantor. AM and RR contributed to the data collection and review of the manuscript. GS contributed to the design, analysis and writing of the manuscript.
Funding: Funding for this project came from the UFC, Top Rank Promotions, Haymon Boxing, Bellator Promotions.
Competing interests: Dr Bernick has received research funding for the Professional Athletes Brain Health Study from UFC, Top Rank promotions, Belator promotions and Haymon Boxing. The other authors do not have any competing interests.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Limited deidentified data are available upon reasonable request from bernick@uw.edu
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The study was approved by the Cleveland Clinic Institutional Review Board, #10-944.
References
- 1. Alosco ML, Stern RA. The long-term consequences of repetitive head impacts: chronic traumatic encephalopathy. Handb Clin Neurol 2019;167:337–55. 10.1016/B978-0-12-804766-8.00018-2 [DOI] [PubMed] [Google Scholar]
- 2. McKee AC, Cairns NJ, Dickson DW, et al. The first NINDS/NIBIB consensus meeting to define neuropathological criteria for the diagnosis of chronic traumatic encephalopathy. Acta Neuropathol 2016;131:75–86. 10.1007/s00401-015-1515-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Asken BM, Sullan MJ, DeKosky ST, et al. Research gaps and controversies in chronic traumatic encephalopathy: a review. JAMA Neurol 2017;74:1255–62. 10.1001/jamaneurol.2017.2396 [DOI] [PubMed] [Google Scholar]
- 4. Katz DI, Bernick C, Dodick DW, et al. National Institute of neurological disorders and stroke consensus diagnostic criteria for traumatic encephalopathy syndrome. Neurology 2021;96:848–63. 10.1212/WNL.0000000000011850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mez J, Daneshvar DH, Kiernan PT, et al. Clinicopathological evaluation of chronic traumatic encephalopathy in players of American football. JAMA 2017;318:360–70. 10.1001/jama.2017.8334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bernick C, Banks S, Phillips M, et al. Professional fighters brain health study: rationale and methods. Am J Epidemiol 2013;178:280–6. 10.1093/aje/kws456 [DOI] [PubMed] [Google Scholar]
- 7. Jordan BD: neurologic aspects of boxing. Arch Neurol 1987;44:453–9. [DOI] [PubMed] [Google Scholar]
- 8. Bernick C, Banks SJ, Shin W, et al. Repeated head trauma is associated with smaller thalamic volumes and slower processing speed: the professional fighters' brain health study. Br J Sports Med 2015;49:1007–11. 10.1136/bjsports-2014-093877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gualtieri CT, Johnson LG. Reliability and validity of a computerized neurocognitive test battery, CNS vital signs. Arch Clin Neuropsychol 2006;21:623–43. 10.1016/j.acn.2006.05.007 [DOI] [PubMed] [Google Scholar]
- 10. Simon M, Maerlender A, Metzger K, et al. Reliability and concurrent validity of select C3 Logix test components. Dev Neuropsychol 2017;42:446–59. 10.1080/87565641.2017.1383994 [DOI] [PubMed] [Google Scholar]
- 11. Potts GF, George MRM, Martin LE, et al. Reduced punishment sensitivity in neural systems of behavior monitoring in impulsive individuals. Neurosci Lett 2006;397:130–4. 10.1016/j.neulet.2005.12.003 [DOI] [PubMed] [Google Scholar]
- 12. McKee AC, Stein TD, Kiernan PT, et al. The neuropathology of chronic traumatic encephalopathy. Brain Pathol 2015;25:350–64. 10.1111/bpa.12248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bernick C, Shan G, Zetterberg H, et al. Longitudinal change in regional brain volumes with exposure to repetitive head impacts. Neurology 2020;94:e232–40. 10.1212/WNL.0000000000008817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rohrer JD, Woollacott IOC, Dick KM, et al. Serum neurofilament light chain protein is a measure of disease intensity in frontotemporal dementia. Neurology 2016;87:1329–36. 10.1212/WNL.0000000000003154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mattsson N, Zetterberg H, Janelidze S, et al. Plasma tau in Alzheimer disease. Neurology 2016;87:1827–35. 10.1212/WNL.0000000000003246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.pp.Wilding GE, Shan G, Hutson AD. Exact two-stage designs for phase II activity trials with rank-based endpoints. Contemp Clin Trials 2012;33:332–41. 10.1016/j.cct.2011.10.008 [DOI] [PubMed] [Google Scholar]
- 17. Shan G. Exact statistical inference for categorical data. London, UK: Academic Press, 2016. [Google Scholar]
- 18. Textor J, van der Zander B, Gilthorpe MS, et al. Robust causal inference using directed acyclic graphs: the R package 'dagitty'. Int J Epidemiol 2016;45:1887–94. 10.1093/ije/dyw341 [DOI] [PubMed] [Google Scholar]
- 19. Shan G. Improved confidence intervals for the Youden index. PLoS One 2015;10:e0127272. 10.1371/journal.pone.0127272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Martland H. Punch drunk. JAMA 1928;91:1103–7. [Google Scholar]
- 21. Roberts A. Brain damage in boxers: a study of prevalence of traumatic encephalopathy among ex-professional boxers. London: Pitman Medical Scientific Publishing Co, 1969. [Google Scholar]
- 22. Bieniek KF, Ross OA, Cormier KA, et al. Chronic traumatic encephalopathy pathology in a neurodegenerative disorders brain bank. Acta Neuropathol 2015;130:877–89. 10.1007/s00401-015-1502-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stephen SJ, Shan G, Banks SJ, et al. The relationship between fighting style, cognition, and regional brain volume in professional Combatants: a preliminary examination using brief neurocognitive measures. J Head Trauma Rehabil 2020;35:E280–7. 10.1097/HTR.0000000000000540 [DOI] [PubMed] [Google Scholar]
- 24. Turk KW, Budson AE. Chronic traumatic encephalopathy. Continuum 2019;25:187–207. 10.1212/CON.0000000000000686 [DOI] [PubMed] [Google Scholar]
- 25. Bryant BR, Narapareddy BR, Bray MJC, et al. The effect of age of first exposure to competitive fighting on cognitive and other neuropsychiatric symptoms and brain volume. Int Rev Psychiatry 2020;32:89–95. 10.1080/09540261.2019.1665501 [DOI] [PubMed] [Google Scholar]
- 26. Abdolmohammadi B, Dupre A, Evers L, et al. Genetics of chronic traumatic encephalopathy. Semin Neurol 2020;40:420–9. 10.1055/s-0040-1713631 [DOI] [PubMed] [Google Scholar]
- 27. McKee AC, Stern RA, Nowinski CJ, et al. The spectrum of disease in chronic traumatic encephalopathy. Brain 2013;136:43–64. 10.1093/brain/aws307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Iverson GL, Gardner AJ, Shultz SR, et al. Chronic traumatic encephalopathy neuropathology might not be inexorably progressive or unique to repetitive neurotrauma. Brain 2019;142:3672–93. 10.1093/brain/awz286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Banks SJ, Miller JB, Rissman RA, et al. Lack of influence of apolipoprotein E status on cognition or brain structure in professional fighters. J Neurotrauma 2017;34:380–4. 10.1089/neu.2016.4453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zetterberg H, Blennow K. Fluid biomarkers formild traumatic brain injury and relate disorders. Nat Rev 2016;12:563–74. [DOI] [PubMed] [Google Scholar]
- 31. Shahim P, Zetterberg H, Tegner Y, et al. Serum neurofilament light as a biomarker for mild traumatic brain injury in contact sports. Neurology 2017;88:1788–94. 29.. 10.1212/WNL.0000000000003912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bernick C, Zetterberg H, Shan G, et al. Longitudinal performance of plasma neurofilament light and tau in professional fighters: the professional fighters brain health study. J Neurotrauma 2018;35:2351–6. 10.1089/neu.2017.5553 [DOI] [PubMed] [Google Scholar]
- 33. Rubenstein R, Chang B, Yue JK, et al. Comparing plasma phospho tau, total tau, and phospho Tau-Total tau ratio as acute and chronic traumatic brain injury biomarkers. JAMA Neurol 2017;74:1063–72. 10.1001/jamaneurol.2017.0655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Trojanowski JQ, Schuck T, Schmidt ML, et al. Distribution of tau proteins in the normal human central and peripheral nervous system. J Histochem Cytochem 1989;37:209–15. 10.1177/37.2.2492045 [DOI] [PubMed] [Google Scholar]
- 35. Lee B, Bennett LL, Bernick C, et al. The relations among depression, cognition, and brain volume in professional boxers: a preliminary examination using brief clinical measures. J Head Trauma Rehabil 2019;34:E29–39. 10.1097/HTR.0000000000000495 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Limited deidentified data are available upon reasonable request from bernick@uw.edu