Abstract
Background
Timing of disease-modifying therapy affects clinical disability in multiple sclerosis, but it is not known whether patient reported outcomes are also affected. This study investigates the relationship between treatment timing and patient-reported symptoms and health-related quality of life.
Methods
This was a nationwide observational cohort study of adults with relapsing multiple sclerosis, with disease onset between 2001 and 2016, and commenced on disease-modifying treatment within 4 years from disease onset. Patients commencing treatment within 0–2 years were compared with patients commencing treatment at 2–4 years. Indication bias was mitigated by propensity matching. Outcomes were patient-reported symptoms and health-related quality of life as measured by the Multiple Sclerosis Impact Scale (MSIS-29) and EuroQol-5 Dimensions-3 Level (EQ-5D). The follow-up period was 4–10 years from disease onset.
Results
There were 2648 patients (69% female, median age 32.8) eligible for matching. Mean follow-up time was 3.7 years. Based on 780 matched patients, each year of treatment delay was associated with a worse MSIS physical score by 2.75 points (95% CI 1.29 to 4.20), and worse MSIS psychological score by 2.02 points (95% CI 0.03 to 3.78), in the adjusted models.
Among 690 matched patients, earlier treatment start was not associated with EQ-5D score during the follow-up.
Conclusions
Earlier commencement of disease-modifying treatment was associated with better patient-reported physical symptoms when measured using a disease-specific metric; however, general quality of life was not affected. This indicates that other factors may inform patients’ overall quality of life.
Keywords: multiple sclerosis, neuroepidemiology, quality of life
WHAT IS ALREADY KNOWN ON THIS TOPIC
Earlier treatment start was associated with more favourable long-term clinical outcomes, as measured by the expanded disability severity scale score, in relapsing multiple sclerosis.
WHAT THIS STUDY ADDS
Earlier treatment is also associated with more favourable patient reported symptoms of multiple sclerosis but not overall quality of life.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
This study supports the importance of early treatment start in patients with relapsing multiple sclerosis with regard to improving physical symptom burden, but their quality of life may be determined by other factors.
Introduction
Advances in multiple sclerosis (MS) treatment over the last 20 years have been measured by improvements in clinical metrics of disease, including relapse rates, disability scores and radiographic disease activity. Indeed, the contemporary goal of treatment, ‘no evidence of disease activity’, is defined by optimisation of these clinical parameters. There is, however, growing awareness of the need to include the patient experience as an independent endpoint in research and clinical practice, alongside these traditional metrics.1–3
An established principle in MS management is the effect of early start of disease-modifying therapy (DMT) in reducing long-term disability accumulation4–11 and cost of illness,12 but it is not known whether this also has a beneficial effect from the patients’ perspective.
The Swedish MS register is a national database that has collected longitudinal patient-reported outcome measures (PROMs) since 2007.13 Two distinct PROMs are routinely collected: the EuroQol-5 Dimensions-3 Level (EQ-5D-3L), a generic, disease-invariant PROM that assesses function across five domains as well as general health-related quality of life,14 and the MSIS-29 (Multiple Sclerosis Impact Scale), a disease-specific PROM, which measures the impact of physical and psychological symptoms of MS.15
This study aimed to investigate whether commencing DMT early (at 0–2 years from disease onset) affected patient-reported outcomes, as measured by EQ-5D and MSIS-29, compared with commencing later (2–4 years after disease onset).
Methods
Study design and participants
This was an observational cohort study using data from the Swedish MS registry, which contains prospectively recorded individual patient information from neurology clinics across Sweden (2001 to present). The registry captures approximately 80% of all prevalent cases of MS in the population.13 Participation is voluntary and all patients provide consent for their data to be used for clinical and research purposes. Date of extract for this study was 29 June 2020.
Inclusion criteria were: adult-onset (≥18 years old) relapsing MS; disease onset between 1 January 2001 and 30 June 2016; and DMT initiation within 4 years of disease onset. Patients were required to have a complete minimum dataset, which included birthdate, sex, date of symptom onset and at least one pretreatment Expanded Disability Status Scale (EDSS) score recorded during the first 2 years of disease. Further, for each outcome, participants were required to have at least one measure (EQ-5D, or MSIS-29) recorded during the follow-up period.
Exposure
The exposure of interest was time to commencement of DMT, defined as the duration between MS onset and start date of initial DMT. Persons were categorised into early-treated (DMT initiation within 2 years of onset) and late-treated (DMT initiation 2–4 years after onset) groups. This binarisation of patients into early start vs late start was based in recent international guidelines that advocate rapid referral, diagnosis and DMT initiation within less than 12 months,16 17 while at the same time capturing an adequately large range of DMT start times that represent real world start times. In the Swedish cohort of patients with relapsing MS with onset 2001–2016, 86.3% of treated patients commenced DMT within the study window of 0–4 years. DMTs included injectable therapies (interferon beta, glatiramer acetate), oral preparations (fingolimod, dimethyl fumarate, teriflunomide, cladribine) and monoclonal therapies (rituximab, ocrelizumab, natalizumab, alemtuzumab). Treatment with haematopoietic stem cell transplantation was also considered a form of DMT.
Outcomes
Outcomes were measured in the 4–10 year period from disease onset, and included the (1) MSIS-29 and (2) EQ-5D-3L.
The MSIS is a disease-specific 29-item questionnaire used to report physical (20 items) and psychological (9 items) symptoms of MS that the patient has experienced in the last 2 weeks. The physical and psychological scores are divided by the maximum denominator to obtain a percentage score out of 100, with higher values indicating more severe disability. The EQ-5D is a generic, disease-invariant measure of health-related quality of life, consisting of self-assessed function in five domains (mobility, pain, self-care, ability to complete usual activities and mood) on a three-level ordinal scale (level 1: no problems, level 2: some problems, level 3: severe problems) as well as a Visual Analogue Scale (VAS) for self-assessment of general health (0–100, higher values indicating better health).
As a positive control, clinical disability as measured by the EDSS was also included as a secondary outcome. This is an ordinal scale from 0 (no disability) to 10 (dead), with the smallest increment being 0.5. Score is determined by clinical examination by a neurologist or trained practitioner.
Each of these outcomes were repeatedly measured at regular intervals. EDSS was measured and reported by clinicians at the time of clinical visit. Patients in the registry could complete the two PROMS at any time through an online patient portal and were invited to do so preceding their clinical visit.
Statistical analysis
To account for potential indication bias, patients in the early-treated group were propensity-score matched to the late DMT group based on the following variables: age at disease onset, sex, baseline EDSS (within the first 2 years of disease and either prior to or less than 6 months from initiation of DMT) and relapse rate at baseline (first 2 years following disease onset).
Matching was performed separately for each outcome analysis, using only those patients for whom the respective outcome was recorded. The size of the early-treated cohort was more than 10-fold that of the late-treated cohort for each analysis; thus, each person in the late-treated cohort was matched with up to 5 persons in the early-treated cohort using nearest neighbour matching without replacement and a calliper of 0.1. The variable matching ratio was accounted for in outcome analyses through weighting, such that the total weight of all early-treated matches for each late-treated person was 1. Adequacy of matching was assessed by comparing baseline characteristics between matched early and late-treated groups using standardised mean differences (see table 1 for baseline characteristics of cohorts used for outcomes analyses and online supplemental material S1 for comparison of matched and unmatched cohorts).
Table 1.
Baseline characteristics of matched patient cohorts
| Variable | Early | Late | SMD |
| A: Patients with at least one MSIS-29 measurement during follow-up | |||
| n | 650 | 130 | |
| Male sex, n (%) | 177 (27.2) | 34 (26.2) | 0.024 |
| Age at onset (mean (SD)) | 34.36 (9.52) | 34.49 (8.85) | 0.014 |
| Calendar year (median (IQR)) | 2011 (2009–2013) | 2009 (2007–2011) | 0.551 |
| Baseline EDSS (median (IQR)) | 1.50 (0.81–2.00) | 1.50 (0.56–2.00) | 0.003 |
| Baseline number of relapses (mean (SD)) | 0.72 (0.94) | 0.75 (0.94) | 0.036 |
| Delay to first DMT, years (mean (SD)) | 0.69 (0.53) | 2.63 (0.57) | 3.531 |
| B: Patients with at least one EQ-5D measurement during follow-up | |||
| n | 575 | 115 | |
| Male sex, n (%) | 172 (29.9) | 32 (27.8) | 0.046 |
| Age at onset (mean (SD)) | 33.72 (8.54) | 34.32 (8.75) | 0.068 |
| Calendar year at onset (median (IQR)) | 2012 (2009–2013) | 2009 (2007–2012) | 0.552 |
| Baseline EDSS (median (IQR)) | 1.50 (0.62–2.00) | 1.50 (1.00–2.00) | 0.025 |
| Baseline number of relapses (mean (SD)) | 0.68 (0.86) | 0.72 (0.82) | 0.045 |
| Delay to first DMT, years (mean (SD)) | 0.64 (0.51) | 2.62 (0.56) | 3.658 |
| C: Patients with at least one EDSS measurement during followup | |||
| n | 886 | 179 | |
| Male sex, n (%) | 256 (28.9) | 50 (27.9) | 0.021 |
| Age at onset (mean (SD)) | 35.32 (9.99) | 35.23 (9.37) | 0.009 |
| Calendar year (median (IQR)) | 2010 (2007–2013) | 2008 (2005–2011) | 0.476 |
| Baseline EDSS (median (IQR)) | 1.50 (0.50–2.00) | 1.00 (0.00–2.00) | 0.010 |
| Baseline number of relapses (mean (SD)) | 0.84 (1.10) | 0.79 (0.95) | 0.041 |
| Delay to first DMT, years (mean (SD)) | 0.70 (0.52) | 2.65 (0.55) | 3.612 |
Baseline EDSS and number of relapses measured 0-2 years following disease onset.
DMT, disease-modifying therapy; EDSS, Expanded Disability Status Scale; EQ-5D, EuroQol-5 Dimensions; SMD, standardised mean difference.
jnnp-2022-330169supp001.pdf (289.5KB, pdf)
The primary analysis assessed for differences in PROMs between the early-treated versus late-treated groups. The continuous response variables included the EQ-5D VAS, and the MSIS physical and psychological scores. To account for the right-skewed distribution of these response variables and the dependence of repeated measures, these were modelled using generalised linear mixed models with a gamma link function. The estimated effect size of the model predictors, with regard to gamma-distributed response variables, were reported as multiples of the reference mean value, with 95% CIs. The individual EQ-5D domains are ordinal but were modelled using logistic mixed models by binarising outcomes in each domain as presence (levels 2 and 3) or absence (level 1) of any problems. Estimates of the model predictors were reported as ORs (with 95% CIs) of having problems in each domain, compared with the reference predictor value (absence of any problems).
As a positive control, EDSS was compared between groups using multilevel mixed models. EDSS was modelled as a continuous numeric outcome. All scores were included regardless of relapse status.
All analyses modelled patient and clinic ID as random effects and were adjusted for calendar year and disease duration at the time of each outcome measurement.
Secondary analyses using the same matched cohort repeated the primary analysis but modelled time to first DMT as a continuous rather than categorical variable. A further sensitivity analysis used the full unmatched cohort for each of the three outcome analyses, including the match variables as model covariates (see online supplemental material S2 for model specification of sensitivity analyses and outcomes).
Results
Of 7849 adult patients with relapsing MS and disease onset between 2001 and 2016, 2648 met inclusion criteria and were eligible for matching (see figure 1 for flow chart of the patient selection process). Baseline characteristics of all eligible patients are provided in online supplemental material S1, while baseline characteristics of matched cohorts are provided in table 1.
Figure 1.
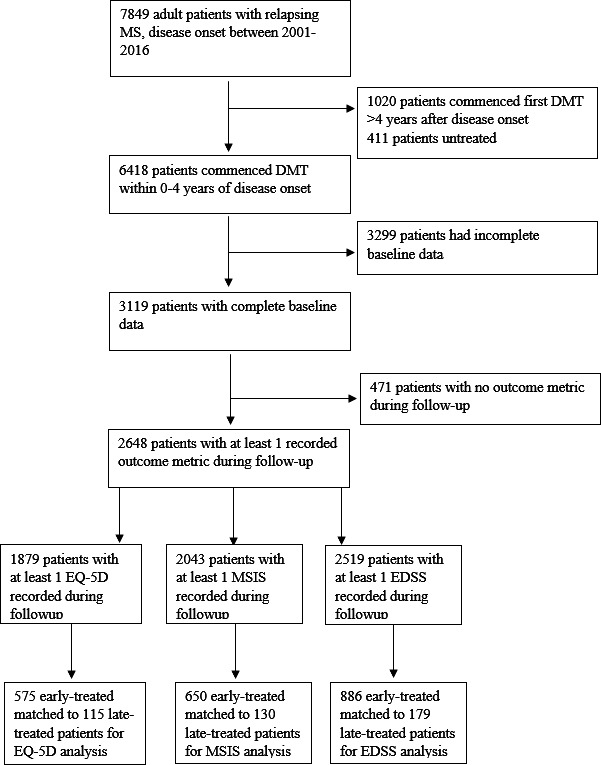
CONSORT chart of patient selection. CONSORT, Consolidated Standards of Reporting Trials; DMT, disease-modifying therapy; EDSS, Expanded Disability Status Scale; EQ-5D, EuroQol-5 Dimensions; MSIS, Multiple Sclerosis Impact Scale.
There were no statistically significant differences in DMT category (used between the early-treated and late-treated groups for any of the analyses (see online supplemental material S3).
For the analysis of MSIS physical and psychological scores, 650 patients in the early-treated group were matched to 130 patients in the late-treated group (see table 1A). The median (IQR) number of MSIS records per patient during follow-up was 3 (2, 5). The mean (SD) follow-up was 3.4 (1.9) years from start of follow-up (4 years postonset), equating to 7.4 years from disease onset. Patients in the early-treated group had a mean MSIS physical score of 17.78 (95% CI 13.39 to 23.60). Late-treated patients had a mean score of 23.29 (95% CI 19.38, 28.09), which was a 1.31-fold increase compared with the early group (95% CI 1.09 to 1.58), indicating greater problems with physical symptoms.
Patients in the early-treated group had a mean MSIS psychological score of 26.41 (95% CI 20.71 to 33.68). Late-treated patients had a mean score of 30.11 (95% CI 25.62 to 35.39), which was a 1.14-fold increase compared with early treatment, indicating greater problems with psychological symptoms; however, the estimates were imprecise (95% CI 0.97 to 1.34). Disease duration showed no significant association with either physical or psychological scores during follow-up.
For the analysis of EQ-5D, 575 patients in the early-treated group were matched to 115 in the late-treated group (see table 1B). The median (IQR) number of EQ-5Ds completed was 3 (1, 4). Mean (SD) follow-up was 3.4 years from the start of the follow-up period, or 7.4 years from disease onset. Patients in the early-treated group had a mean score of 71.63 (95% CI 67.02 to 76.56). Patients in the late-treated group had mean EQ-5D VAS score of 66.62 (95% CI 60.89 to 72.35), or 0.93 (95% CI 0.85 to 1.01) times that of the early-treated group, indicating poorer subjective general health status, but estimates were imprecise (see table 2A). There was no difference between groups on any of the five individual health domains (see table 3).
Table 2.
Effect of treatment delay on PROMs during the 4–10 years follow-up period: results of the multivariate mixed models regression
| A: Binarised exposure (late (2–4 years) vs early (0–2 years)) DMT initiation | ||||
| Outcome | Early mean score (95% CI) | Late mean score (95% CI) | Coefficient (late vs early) (95% CI) | P value |
| EQ-5D VAS | 71.63 (67.02 to 76.56) | 66.62 (60.89 to 72.35) | 0.93 (0.85 to 1.01) | 0.089 |
| MSIS physical | 17.78 (13.39 to 23.60) | 23.29 (19.38 to 28.09) | 1.31 (1.09 to 1.58) | 0.004 |
| MSIS psychological | 26.41 (20.71 to 33.68) | 30.11 (25.62 to 35.39) | 1.14 (0.97 to 1.34) | 0.102 |
| EDSS | 1.27 (1.12 to 1.42) | 1.62 (1.37 to 1.86) | 0.35 (0.10 to 0.59) | 0.005 |
| B: Continuous exposure (years from MS onset to first DMT) | ||||
| Outcome | Reference score (95% CI) | Change in score per year of DMT delay (95% CI) | Coefficient per year of DMT delay (95% CI) | P value |
| EQ-5D VAS | 72.55 (67.58 to 77.90) | −2.18 (−4.35 to 0.07) | 0.97 (0.94 to 1.01) | 0.146 |
| MSIS physical | 16.15 (12.11 to 21.55) | 2.75 (1.29 to 4.20) | 1.17 (1.08 to 1.26) | <0.001 |
| MSIS psychological | 25.19 (19.67 to 32.27) | 2.02 (0.03 to 3.78) | 1.08 (1.01 to 1.15) | 0.024 |
| EDSS | 1.17 (0.99 to 1.35) | 0.15 (0.05 to 0.25) | 0.15 (0.05 to 0.25) | 0.004 |
Each outcome was adjusted for disease duration; coefficients not shown.
Higher values indicate more severe disease burden in EDSS and MSIS; higher values indicate better quality of life in EQ5D-VAS2.
Coefficients are additive for EDSS (values >0 indicate higher scores).
DMT, disease-modifying therapy; EDSS, Expanded Disability Status Scale; EQ-5D, EuroQol-5 Dimensions; MS, multiple sclerosis; MSIS, Multiple Sclerosis Impact Scale; PROMs, patient-reported outcome measures; VAS, Visual Analogue Scale.
Table 3.
Effect of treatment delay on EQ-5D functional domains during 4–10 years follow-up period: results of the logistic mixed models regression
| Binarised time to DMT intiation (late (2–4 years) vs early (0–2 years)) | |||
| Functional domain | OR for having difficulties (late vs early) | 95% CI (lower limit) | 95% CI (upper limit) |
| Mobility | 1.59 | 0.38 | 6.66 |
| Self-care | 1.77 | 0.22 | 14.44 |
| Usual activities | 1.57 | 0.43 | 5.77 |
| Mood | 1.40 | 0.77 | 2.53 |
| Pain | 1.72 | 0.65 | 4.57 |
| Continuous time to DMT initiation (years from MS onset to first DMT) | |||
| Functional domain | OR for having difficulties (per year of DMT delay) | 95% CI (lower limit) | 95% CI (upper limit) |
| Mobility | 1.13 | 0.62 | 2.08 |
| Self-care | 1.28 | 0.52 | 3.17 |
| Usual activities | 1.19 | 0.68 | 2.06 |
| Mood | 1.10 | 0.86 | 1.41 |
| Pain | 1.45 | 0.96 | 2.20 |
DMT, disease-modifying therapy; EQ-5D, EuroQol-5 Dimensions; MS, multiple sclerosis.
For the analysis of EDSS, 886 patients in the early-treated group were matched to 179 in the late-treated group (see table 1C). Later start was associated with a higher EDSS by 0.23 points (95% CI 0.01 to 0.47) during follow-up (mean (SD) 3.7 (1.9) years from start of follow-up). Disease duration was significantly associated with EDSS worsening during follow-up, estimated at 0.07 points per year (95% CI 0.06 to 0.08).
Secondary analyses modelling time to DMT as a continuous variable (delay to first DMT, in years) confirmed the results of the primary analyses (see table 2B). Of note, time to DMT had a statistically significant effect on psychological symptoms as measured by the MSIS-psychological subscale (2.02 points per year of delay, 95% CI 0.03 to 3.78). Effect sizes were lower, as this model considered differences in outcome with every additional year of delay, compared with a delay of approximately 2 years between early and late groups in the primary analysis.
A sensitivity analysis included all eligible patients without propensity matching, with the same model specifications but included matching variables as covariates. The results of the main analyses were replicated in full (see online supplemental material S2 for results).
Full model specifications and estimates for the main analysis can be found in online supplemental material S4.
Discussion
Our study aimed to assess the impact of delay to DMT on patient-reported outcomes. An earlier start of DMTs had a favourable effect on MS physical symptoms, and to a lesser extent on psychological symptoms. However, there was no effect of early initiation of DMT on the general health-related quality of life. This suggests that patients’ experience of physical MS symptoms may be congruent to their clinician-determined disease severity, and benefit from early initiation of treatment.
Two patient-reported outcomes were used in this study. The MSIS is a disease-specific questionnaire that was developed in consultation with patients and advocacy groups, with a view to optimise validity, reliability and responsiveness.15 18–20 The psychometric properties of the MSIS have been upheld in numerous validation studies, with the minimum clinically important change in the physical subscale reported to be 7.5–8.19 20 In our study, the effect size of treatment delay on MSIS physical subscale was 3.7 per year, suggesting that, on a group level, a delayed start time of >3 years would produce a clinically important change on the physical scale.
This study found a small effect of treatment delay on the psychological subscale of MSIS, which was not statistically significant in the main analysis. Previous studies have demonstrated this subscale to be less responsive compared with other PROMs for psychological symptoms, and confirm that there is limited correlation to clinical disability.18 21 Nevertheless, they suggest that despite both the objective and subjectively reported improvements in physical health associated with early treatment, patients do not experience correlating improvements to their psychological health.
The EQ-5D is a disease-invariant measure of health-related quality of life,14 developed initially within the field of health economics. The five domains of health are thus preselected and not necessarily indicative of a holistic picture of a person’s health. Nevertheless, we found that in all five domains, the ORs for having ‘any problems’ (vs ‘no problems’) were increased, but none met statistical significance. It is possible that we were underpowered to detect a difference, or that the effect of delayed treatment initiation is not consequential when it comes to quality of life. The lack of treatment effect seen in individual domains may be due to lack of response granularity.
The VAS of general health has greater response granularity (with scores between 0 and 100), but likewise showed minimal change with earlier treatment despite detectable change on the MSIS. This may suggest that overall health-related quality of life may not be influenced by treatment timing, but may instead be determined by other factors such as comorbidity, socioeconomic status and individual disposition.22–25 There is also a trade-off between generalisability of generic PROMS and sensitivity of disease-specific PROMs,2 26–28 such that meaningful changes in symptoms and health utilities may not be adequately captured using the EQ-5D alone.
The study cohort represents contemporary practice in Sweden, which has a universal healthcare system offering equitable access to quality healthcare. The vast majority of patients received treatment in the ‘early’ time period. Within this context, treatment delay demonstrated changes in some but not all outcomes studied.
As the study used observational data, indication bias is likely to result in systematic differences between patients who commenced treatment early and late. Propensity score matching aimed to mitigate this bias by matching on variables observed during the baseline period. During this period, all patients included in the study had an opportunity to start DMT. Despite this, there may remain residual bias in unobserved variables such as MRI findings or fluid biomarkers. The direction of this bias is such that earlier treatment is clinically indicated in more severe disease and would likely dilute our findings. The true effect of earlier treatment may therefore be underestimated by our study.
While EQ-5D and MSIS are used in a limited number of MS studies, they do not enjoy the same widespread use and routine measurement as the EDSS. They are nonetheless among the most widely used PROMs among a multitude of others.29 A standard instrument for measuring PROs in MS has not been agreed on. Additional PROMs are currently still under development, with a view to better reflecting patients' priorities and better psychometric properties within the target population of people with MS.1 30 The development, acceptance and routine longitudinal collection of such a PROM in future studies will help to build on the results presented here.
Conclusion
In relapsing MS, delay to DMT start has a detrimental effect on long-term patient-reported physical and psychological symptoms, as measured by a disease specific PROM. Generic health-related quality of life does not appear to be sensitive to timing of DMT.
Footnotes
Contributors: All authors contributed to the conception, design and interpretation of data, gave critical revisions for intellectual content and final approval of the version to be published, and are accountable for all aspects of the work. Additionally, AH, TS, AM and KM contributed to data analysis; AH and KM drafted the manuscript. AH is responsible for overall content as guarantor, and accepts full responsibility for the finished work and the conduct of the study, had access to the data, and controlled the decision to publish.
Funding: JH is funded by the Swedish Research Council and the Swedish Brain foundation and receivesJH is funded by the Swedish Research Council and the Swedish Brain foundation and receivesJH is funded by the Swedish Research Council and the Swedish Brain foundation and receives
Competing interests: TS received compensation for serving on scientific advisory boards, honoraria for consultancy and funding for travel from Biogen. JH has received honoraria for serving on advisory boards for Biogen, Celgene, Sanofi-Genzyme, Merck KGaA, Novartis and Sandoz and speaker’s fees from Biogen, Novartis, Merck KGaA, Teva and Sanofi-Genzyme; he has served as P.I. for projects, or received unrestricted research support from, Biogen, Celgene, Merck KGaA, Novartis, Roche and Sanofi-Genzyme.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. Data are available to parties with ethical permission for access to the Swedish multiple sclerosis registry, for the specified purposes of that permission.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by Stockholm County Ethical Review Board, Reference numbers: Dnr 2019-02819Dnr 2017/1378-31. Participants gave informed consent to participate in the study before taking part.
References
- 1. Brichetto G, Zaratin P. Measuring outcomes that matter most to people with multiple sclerosis: the role of patient-reported outcomes. Curr Opin Neurol 2020;33:295–9. 10.1097/WCO.0000000000000821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. D'Amico E, Haase R, Ziemssen T. Review: patient-reported outcomes in multiple sclerosis care. Mult Scler Relat Disord 2019;33:61–6. 10.1016/j.msard.2019.05.019 [DOI] [PubMed] [Google Scholar]
- 3. Berrigan LI, Fisk JD, Patten SB, et al. Health-related quality of life in multiple sclerosis: direct and indirect effects of comorbidity. Neurology 2016;86:1417–24. 10.1212/WNL.0000000000002564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. He A, Merkel B, Brown JWL, et al. Timing of high-efficacy therapy for multiple sclerosis: a retrospective observational cohort study. Lancet Neurol 2020;19:307–16. 10.1016/S1474-4422(20)30067-3 [DOI] [PubMed] [Google Scholar]
- 5. Brown JWL, Coles A, Horakova D, et al. Association of initial disease-modifying therapy with later conversion to secondary progressive multiple sclerosis. JAMA 2019;321:175–87. 10.1001/jama.2018.20588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Johnson KP, Ford CC, Lisak RP, et al. Neurologic consequence of delaying glatiramer acetate therapy for multiple sclerosis: 8-year data. Acta Neurol Scand 2005;111:42–7. 10.1111/j.1600-0404.2004.00351.x [DOI] [PubMed] [Google Scholar]
- 7. Trojano M, Pellegrini F, Paolicelli D, et al. Real-Life impact of early interferon beta therapy in relapsing multiple sclerosis. Ann Neurol 2009;66:513–20. 10.1002/ana.21757 [DOI] [PubMed] [Google Scholar]
- 8. Kappos L, O'Connor P, Radue E-W, et al. Long-Term effects of fingolimod in multiple sclerosis: the randomized freedoms extension trial. Neurology 2015;84:1582–91. 10.1212/WNL.0000000000001462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. PRISMS Study Group and the University of British Columbia MS/MRI Analysis Group. . PRISMS-4: long-term efficacy of interferon-beta-1a in relapsing MS. Neurology 2001;56:1628–36. 10.1212/WNL.56.12.1628 [DOI] [PubMed] [Google Scholar]
- 10. Wandall-Holm MF, Buron MD, Kopp TI, et al. Time to first treatment and risk of disability pension in relapsing-remitting multiple sclerosis. J Neurol Neurosurg Psychiatry 2022;93:858–64. 10.1136/jnnp-2022-329058 [DOI] [PubMed] [Google Scholar]
- 11. Iaffaldano P, Lucisano G, Butzkueven H, et al. Early treatment delays long-term disability accrual in RRMS: results from the BMSD network. Mult Scler 2021;27:1543–55. 10.1177/13524585211010128 [DOI] [PubMed] [Google Scholar]
- 12. Karampampa K, Gyllensten H, Murley C, et al. Early vs. late treatment initiation in multiple sclerosis and its impact on cost of illness: a register-based prospective cohort study in Sweden. Mult Scler J Exp Transl Clin 2022;8:205521732210924. 10.1177/20552173221092411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hillert J, Stawiarz L. The Swedish MS registry – clinical support tool and scientific resource. Acta Neurol Scand 2015;132:11–19. 10.1111/ane.12425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol group. Ann Med 2001;33:337–43. 10.3109/07853890109002087 [DOI] [PubMed] [Google Scholar]
- 15. Hobart J, Lamping D, Fitzpatrick R, et al. The multiple sclerosis impact scale (MSIS-29): a new patient-based outcome measure. Brain 2001;124:962–73. 10.1093/brain/124.5.962 [DOI] [PubMed] [Google Scholar]
- 16. Hobart J, Bowen A, Pepper G, et al. International consensus on quality standards for brain health-focused care in multiple sclerosis. Mult Scler 2019;25:1809–18. 10.1177/1352458518809326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Giovannoni G, Butzkueven H, Dhib-Jalbut S, et al. Brain health: time matters in multiple sclerosis. Mult Scler Relat Disord 2016;9 Suppl 1:S5–48. 10.1016/j.msard.2016.07.003 [DOI] [PubMed] [Google Scholar]
- 18. Hobart JC, Riazi A, Lamping DL, et al. How responsive is the multiple sclerosis impact scale (MSIS-29)? A comparison with some other self report scales. J Neurol Neurosurg Psychiatry 2005;76:1539–43. 10.1136/jnnp.2005.064584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Phillips GA, Wyrwich KW, Guo S, et al. Responder definition of the multiple sclerosis impact scale physical impact subscale for patients with physical worsening. Mult Scler 2014;20:1753–60. 10.1177/1352458514530489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Costelloe L, O'Rourke K, Kearney H, et al. The patient knows best: significant change in the physical component of the multiple sclerosis impact scale (MSIS-29 physical). J Neurol Neurosurg Psychiatry 2007;78:841–4. 10.1136/jnnp.2006.105759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hoogervorst ELJ, Zwemmer JNP, Jelles B, et al. Multiple sclerosis impact scale (MSIS-29): relation to established measures of impairment and disability. Mult Scler 2004;10:569–74. 10.1191/1352458504ms1078oa [DOI] [PubMed] [Google Scholar]
- 22. Steptoe A, Deaton A, Stone AA. Subjective wellbeing, health, and ageing. Lancet 2015;385:640–8. 10.1016/S0140-6736(13)61489-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Teni FS, Gerdtham U-G, Leidl R, et al. Inequality and heterogeneity in health-related quality of life: findings based on a large sample of cross-sectional EQ-5D-5L data from the Swedish general population. Qual Life Res 2022;31:697-712. 10.1007/s11136-021-02982-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mackenbach JP, Stirbu I, Roskam A-JR, et al. Socioeconomic inequalities in health in 22 European countries. N Engl J Med 2008;358:2468–81. 10.1056/NEJMsa0707519 [DOI] [PubMed] [Google Scholar]
- 25. Spronk I, Haagsma JA, Lubetkin EI, et al. Health inequality analysis in Europe: exploring the potential of the EQ-5D as outcome. Front Public Health 2021;9:744405. 10.3389/fpubh.2021.744405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rowen D, Brazier J, Ara R, et al. The role of Condition-Specific Preference-Based measures in health technology assessment. Pharmacoeconomics 2017;35:33–41. 10.1007/s40273-017-0546-9 [DOI] [PubMed] [Google Scholar]
- 27. Longworth L, Yang Y, Young T, et al. Use of generic and condition-specific measures of health-related quality of life in NICE decision-making: a systematic review, statistical modelling and survey. Health Technol Assess 2014;18:1–224. 10.3310/hta18090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Goodwin E, Green C. A quality-adjusted Life-Year measure for multiple sclerosis: developing a patient-reported health state classification system for a multiple sclerosis-specific Preference-Based measure. Value Health 2015;18:1016–24. 10.1016/j.jval.2015.07.002 [DOI] [PubMed] [Google Scholar]
- 29. Khurana V, Sharma H, Afroz N, et al. Patient-Reported outcomes in multiple sclerosis: a systematic comparison of available measures. Eur J Neurol 2017;24:1099–107. 10.1111/ene.13339 [DOI] [PubMed] [Google Scholar]
- 30. The Lancet Neurology . Patient-Reported outcomes in the spotlight. Lancet Neurol 2019;18:981. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jnnp-2022-330169supp001.pdf (289.5KB, pdf)
Data Availability Statement
Data are available on reasonable request. Data are available to parties with ethical permission for access to the Swedish multiple sclerosis registry, for the specified purposes of that permission.


