Abstract
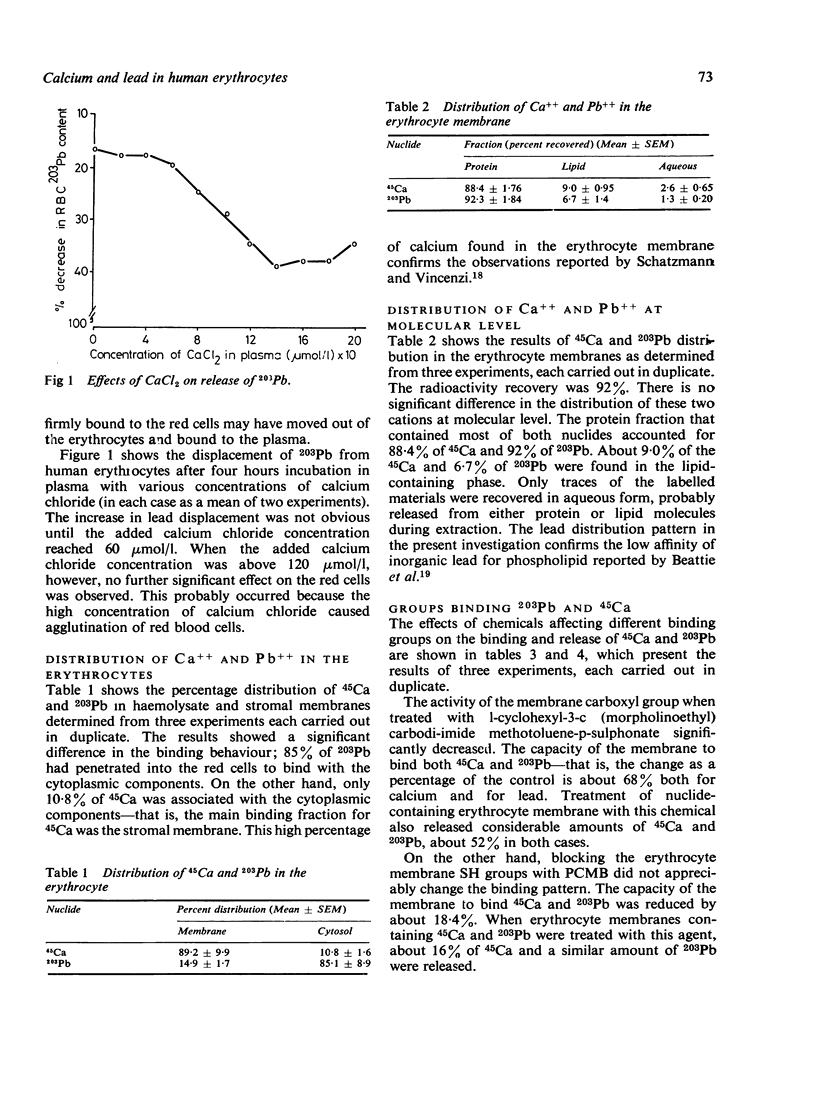
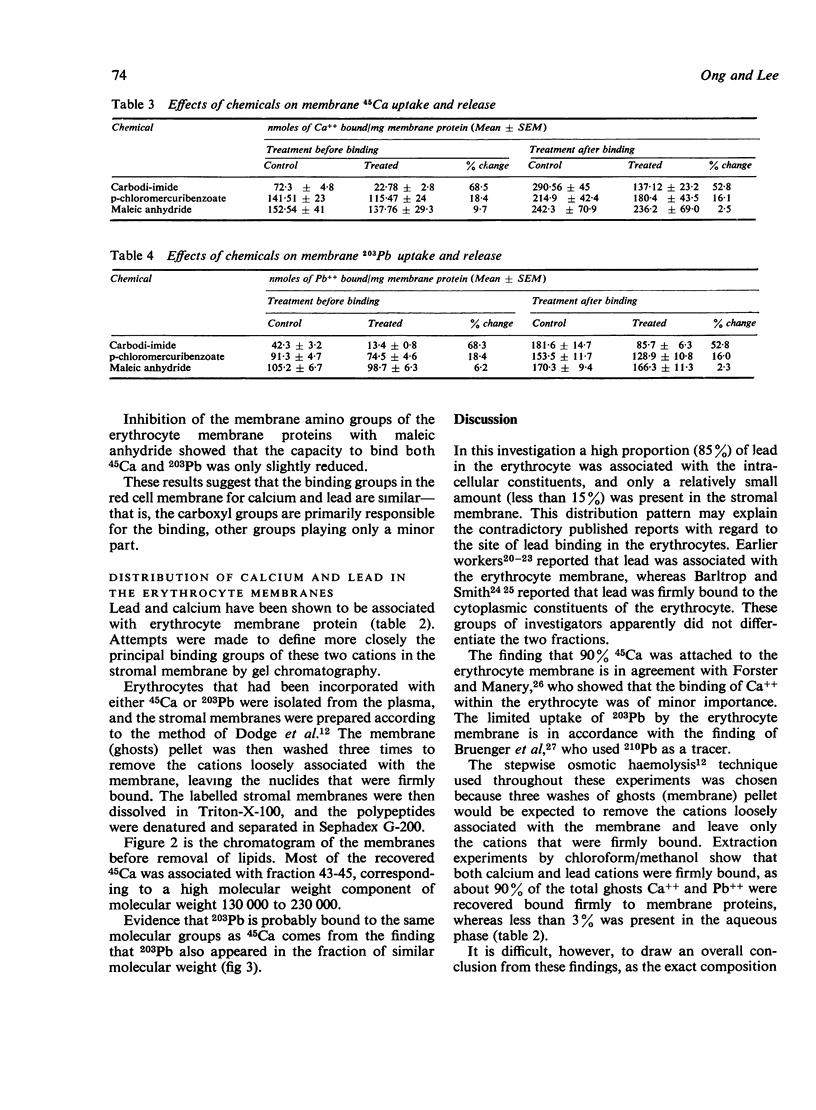
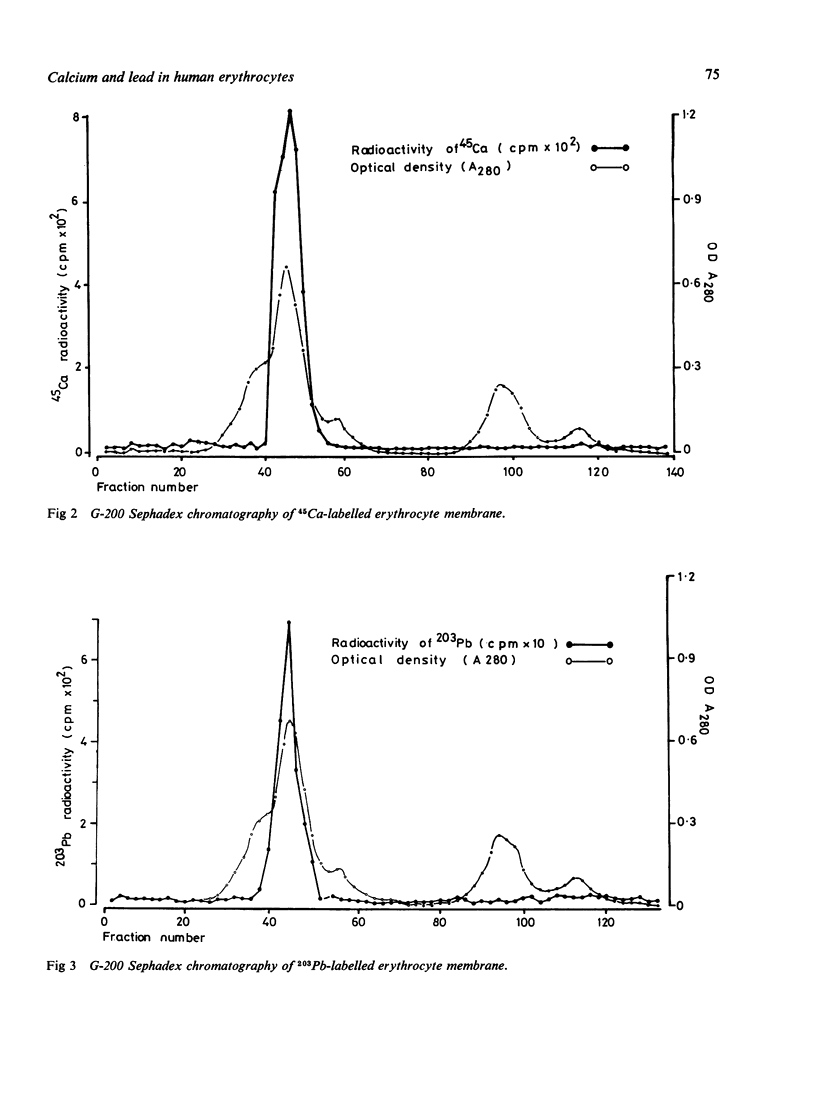
The interactions of calcium and lead on the human erythrocytes have been studied in vitro using 45Ca and 203Pb as tracers. The chemical groups binding calcium and lead on the erythrocytes were also investigated. Calcium ions in the plasma were shown to be capable of replacing the 203Pb on the red cells. More than 85% of the 203Pb in the erythrocyte was associated with the cytoplasmic components, and the rest was bound to the stromal membrane. About 90% of 45Ca was attached to erythrocyte membrane. Extraction of 45Ca and 203Pb-labelled erythrocyte membranes with chloroform/methanol mixture showed that the distribution patterns of these two nuclides are similar, with over 88% protein bound, less than 10% lipid bound, and traces in the aqueous phase. Chemical modification of erythrocyte membrane proteins with carbodi-imide, p-chloromercuribenzoate (PCMB), and maleic anhydride suggested that the carboxyl groups are responsible for binding lead and calcium to the red cell membrane. The SH groups may have a minor role in the binding for both cations. Amino groups did not appear to affect the binding of these cations. Gel chromatography of 45Ca-labelled erythrocyte membrane indicated that Ca++ bound to the same fraction of membrane proteins as 203Pb, corresponding to a molecular weight of about 130 000 to 230 000. A possible implication of these findings is that lead and calcium may compete for the same binding site(s) on the erythrocyte.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. J., Rack P. H. Changes in obsessive/compulsive patients as measured by the Leyton Inventory before and after treatment with clomipramine. Scott Med J. 1975;20(1 Suppl):41–44. doi: 10.1177/00369330750200S109. [DOI] [PubMed] [Google Scholar]
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barltrop D., Smith A. Interaction of lead with erythrocytes. Experientia. 1971 Jan 15;27(1):92–93. doi: 10.1007/BF02137760. [DOI] [PubMed] [Google Scholar]
- Barltrop D., Smith A. Lead binding to human haemoglobin. Experientia. 1972 Jan 15;28(1):76–77. doi: 10.1007/BF01928273. [DOI] [PubMed] [Google Scholar]
- Beattie A. D., Moore M. R., Goldberg A. Tetraethyl-lead poisoning. Lancet. 1972 Jul 1;2(7766):12–15. doi: 10.1016/s0140-6736(72)91276-7. [DOI] [PubMed] [Google Scholar]
- Bruenger F. W., Stevens W., Stover B. J. The association of 210Pb with constituents of erythrocytes. Health Phys. 1973 Jul;25(1):37–42. doi: 10.1097/00004032-197307000-00004. [DOI] [PubMed] [Google Scholar]
- CLARKSON T. W., KENCH J. E. Uptake of lead by human erythrocytes in vitro. Biochem J. 1958 Jul;69(3):432–439. doi: 10.1042/bj0690432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstner J., Manery J. F. Calcium binding by human erythrocyte membranes. Biochem J. 1971 Sep;124(3):563–571. doi: 10.1042/bj1240563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyer R. A., Rhyne B. C. Pathological effects of lead. Int Rev Exp Pathol. 1973;12:1–77. [PubMed] [Google Scholar]
- Hanahan D. J., Ekholm J. E., Luthra M. G. Is lipid lost during preparation of erythrocyte membranes? Biochim Biophys Acta. 1974 Sep 6;363(2):283–286. doi: 10.1016/0005-2736(74)90068-6. [DOI] [PubMed] [Google Scholar]
- KOSTIAL K., VOUK V. B. Lead ions and synaptic transmission in the superior cervical ganglion of the cat. Br J Pharmacol Chemother. 1957 Jun;12(2):219–222. doi: 10.1111/j.1476-5381.1957.tb00123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober T. E., Cooper G. P. Lead competitively inhibits calcium-dependent synaptic transmission in the bullfrog sympathetic ganglion. Nature. 1976 Aug 19;262(5570):704–705. doi: 10.1038/262704a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Manalis R. S., Cooper G. P. Letter: Presynaptic and postsynaptic effects of lead at the frog neuromuscular junction. Nature. 1973 Jun 8;243(5406):354–356. doi: 10.1038/243354a0. [DOI] [PubMed] [Google Scholar]
- Mitchell C. D., Hanahan D. J. Solubilization of certain proteins from the human erythrocyte stroma. Biochemistry. 1966 Jan;5(1):51–57. doi: 10.1021/bi00865a008. [DOI] [PubMed] [Google Scholar]
- REDDI K. K. Isolation of thorium B (lead)-binding substance from the erythrocytes of rabbit blood. Nature. 1953 Aug 1;172(4370):202–202. doi: 10.1038/172202a0. [DOI] [PubMed] [Google Scholar]
- Rosen J. F., Trinidad E. E. Significance of plasma lead levels in normal and lead-intoxicated children. Environ Health Perspect. 1974 May;7:139–144. doi: 10.1289/ehp.747139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatzmann H. J., Vincenzi F. F. Calcium movements across the membrane of human red cells. J Physiol. 1969 Apr;201(2):369–395. doi: 10.1113/jphysiol.1969.sp008761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secchi G. C., Alessio L., Cambiaghi G. Na plus-K plus-ATPase activity of erythrocyte membranes: in urban populations not occupationally exposed to lead. Arch Environ Health. 1973 Dec;27(6):399–400. doi: 10.1080/00039896.1973.10666412. [DOI] [PubMed] [Google Scholar]
- Whyman A. E. Measurement of tritium in neat plasma and urine with a toluene/triton X-100/hyamine 10-X scintillant. Int J Appl Radiat Isot. 1970 Feb;21(2):81–86. doi: 10.1016/0020-708x(70)90015-3. [DOI] [PubMed] [Google Scholar]


