Abstract
Background
The decline of humoral response to COVID-19 vaccine led to authorise a booster dose. Here, we characterised the kinetics of B-cell and T-cell immune responses in patients with multiple sclerosis (PwMS) after the booster dose.
Methods
We enrolled 22 PwMS and 40 healthcare workers (HCWs) after 4–6 weeks from the booster dose (T3). Thirty HCWs and 19 PwMS were also recruited 6 months (T2) after the first dose. Antibody response was measured by anti-receptor-binding domain (RBD)-IgG detection, cell-mediated response by an interferon (IFN)-γ release assay (IGRA), Th1 cytokines and T-cell memory profile by flow cytometry.
Results
Booster dose increased anti-RBD-IgG titers in fingolimod-treated, cladribine-treated and IFN-β-treated patients, but not in ocrelizumab-treated patients, although antibody titres were lower than HCWs. A higher number of fingolimod-treated patients seroconverted at T3. Differently, T-cell response evaluated by IGRA remained stable in PwMS independently of therapy. Spike-specific Th1-cytokine response was mainly CD4+ T-cell-mediated, and in PwMS was significantly reduced (p<0.0001) with impaired IL-2 production compared with HCWs at T3. In PwMS, total Th1 and IFN-γ CD4+ T-cell responders to spike protein were increased from T2 to T3.
Compared with HCWs, PwMS presented a higher frequency of CD4+ and CD8+ terminally differentiated effector memory cells and of CD4+ effector memory (TEM) cells, independently of the stimulus suggesting the association of this phenotype with MS status. CD4+ and CD8+ TEM cell frequency was further increased at T3 compared with T2.
Conclusions
COVID-19 vaccine booster strengthens humoral and Th1-cell responses and increases TEM cells in PwMS.
Keywords: COVID-19, multiple sclerosis, immunology, interferon, infectious diseases
WHAT IS ALREADY KNOWN ON THIS TOPIC
Most of the studies have investigated the serological and/or cell-mediated response to SARS-CoV-2 vaccination focusing only on anti-CD20-treated or fingolimod-treated patients. Other studies investigating a wide range of disease-modifying therapies (DMTs) have evaluated only one of the adaptive responses and/or involved subjects before the booster dose. There is a lack of studies focusing on a longitudinal characterisation of both serological and T-cell response in patients with multiple sclerosis before and after the COVID-19 booster dose. The few studies available did not perform any in-depth analysis of the cytokine response or the memory profile of T cells in patients treated with an array of different immunotherapies, which are important to understand the vaccine-induced immunity in the MS population.
WHAT THIS STUDY ADDS
To the best of our knowledge, this is the first longitudinal prospective study characterising the humoral response and deeply investigating the T-cell response, in terms of cytokine and memory profile, before and after the COVID-19 vaccine booster dose in patients with multiple sclerosis (PwMS) using different DMTs.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Our results highlight the beneficial effects of the booster dose as it strengthens the humoral and the T-cell response, in terms of Th1 cytokine production, in most of the PwMS.
PwMS showed a significant increase of effector memory (TEM) and terminally differentiated effector memory (TEMRA) cells after the booster dose. The demonstration of these kinetic changes is important to understand the memory response induced by COVID-19 vaccination in this vulnerable population.
Introduction
The COVID-19 pandemic represents a serious concern for human global health, particularly for patients with multiple sclerosis (PwMS). MS is a chronic inflammatory autoimmune disease causing neuroinflammation and myelin neurodegeneration.1 Most PwMS are treated with immunomodulatory or immunosuppressive disease-modifying therapies (DMTs), including interferon (IFN)-β, fingolimod, ocrelizumab and cladribine. Considered their mechanism of action, DMTs might be to some extent associated with an increased risk of infection or COVID-19 severity and mortality.2–4
To date, large-scale vaccination represents the most powerful tool to control COVID-19 pandemic and to prevent a severe outcome. Several studies have demonstrated the immunogenicity of mRNA vaccines after the first vaccination cycle in healthy individuals.5 6
In PwMS, mRNA vaccines induce both humoral and T-cell specific immune responses with a lower magnitude than healthy subjects according to DMTs.7 8 Particularly, T-cell and antibody responses are both reduced in fingolimod-treated patients whereas ocrelizumab reduces the antibody response; differently, they are mostly preserved in those under cladribine or IFN-β.7–11
The waning of the humoral response led public health services to authorise the administration of a booster dose to restore the protection against COVID-19.12–14
In healthy subjects, the efficacy of the booster dose has been largely demonstrated.15 16 In PwMS, the SARS-CoV-2 vaccine booster dose increases antibody titers according to therapy.17 18 Indeed, evidence show that the booster does not improve the already low serological response in those under fingolimod or ocrelizumab treatments, and neither the impaired cellular responses of patients under fingolimod therapy.13 19 20 Similarly, in ocrelizumab-treated patients SARS-CoV-2 booster dose has no additive effect on the maximal T-cell response observed after the first vaccination cycle.21 To date, in PwMS there is a lack of longitudinal characterisation of both serological and T-cell response before and after the COVID-19 vaccine booster dose. The few studies available did not perform any in-depth analysis of the cytokine or memory profile of T cells,19 20 which is important to understand the vaccine-induced immunity in PwMS. Therefore, we aimed to assess the adaptive immune response after the booster dose in PwMS treated with different DMTs, deeply investigating the cytokine and memory profiles within the T-cell compartment.
Materials and methods
The extended version of materials and methods is included as online supplemental information.
jnnp-2022-330175supp001.pdf (310.2KB, pdf)
Study design and participants
This longitudinal prospective study involved the enrolment of PwMS and healthcare workers (HCWs) at the MS Centre of the Department of Neurosciences of San Camillo Forlanini Hospital (Rome, Italy) and at the National Institute for Infectious Diseases (INMI)-Lazzaro Spallanzani-IRCCS (Rome, Italy). For PwMS, the inclusion criteria were: (1) diagnosis of relapsing-remitting MS based on McDonald 2017 criteria22; (2) ongoing DMTs with ocrelizumab, fingolimod, cladribine or IFN-β for at least 6 months prior to the study entry; (3) completion of the first vaccination cycle of mRNA vaccines (BNT162b2 or mRNA-1273), and booster dose performance within the previous 4–6 weeks and (4) absence of relapses and/or steroids treatment during the last 3 months before study entry.
For PwMS undergoing ocrelizumab and cladribine therapy, the vaccination timing after the last DMT administration was established according to the European Academy of Neurology for COVID-19 vaccination guidelines. In detail, ocrelizumab was provided after 3 months, while cladribine with at least 4 weeks of delay. IFN-β and fingolimod therapies were not interrupted at the time of vaccination.
HCWs were used as healthy control group (some were included in our previous study).23 Inclusion criteria for their enrolment were: no immunosuppression condition and having received the completed SARS-CoV-2 vaccination cycle and the booster dose as reported above for PwMS.
Exclusion criteria for both cohorts were: previous SARS-CoV-2 infection, HIV infection, age <18 years.
The follow-up study was performed on 30 HCWs and 19 PwMS providing blood samples after both 6 months from the first vaccine dose (T2) and 4–6 weeks from the booster dose (T3) (figure 1A).
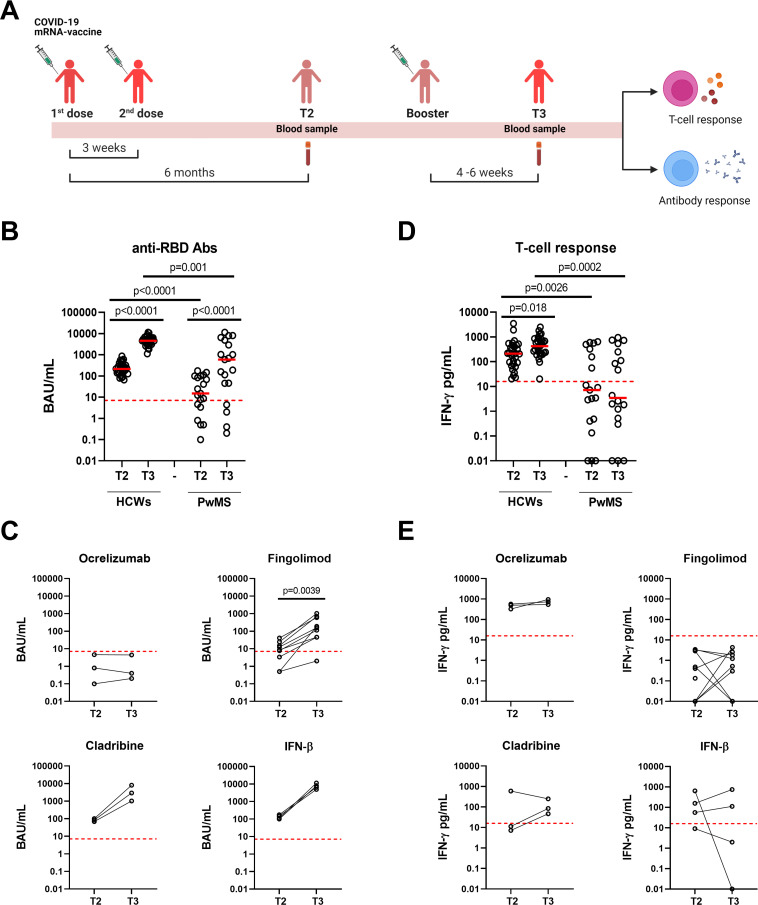
Figure 1.
Kinetic of the antibody and T-cell responses to COVID-19 vaccine in HCWs and PwMS. (A) Timeline of COVID-19 vaccination and study enrolment. For the analyses, blood samples of both HCWs and PwMS were collected after 6 months from the first vaccine dose (T2) and after 4–6 weeks from the booster dose (T3). Anti-RBD antibodies (B, C) and T-cell response (D, E) were evaluated in HCWs (n=30) and PwMS followed over time (n=19). (C, E) Antibody and T-cell responses were stratified according to DMTs: ocrelizumab (n=3), fingolimod (n=9), cladribine (n=3) and IFN-β (n=4). (B, C) Anti-RBD-IgG were measured in sera samples and expressed as binding antibody units (BAU)/mL. The cut-off was set at 7.1 BAU/mL (red dashed line). (D, E) For T-cell response, IFN-γ levels were quantified using an automatic ELISA and reported after subtracting the unstimulated control value. The cut-off was set at 16 pg/mL (red dashed lines). Data were analysed using Mann-Whitney and Wilcoxon matched pairs signed rank test and p values <0.05 were considered statistically significant. Each dot represents a different individual. Abs, antibodies; BAU, binding antibody units; DMTs, disease modifying therapies; HCWs, healthcare workers; IFN-γ, interferon gamma; IFN-β, interferon beta; PwMS, patients with multiple sclerosis; RBD, receptor-binding domain.
Experimental design
Antibody response was evaluated by measuring antinucleoprotein-immunoglobulin G (Anti-N-IgG) and anti-receptor-binding domain (RBD)-IgG. Anti-RBD-IgG were indicated as positive when ≥7.1 BAU/mL. For the T-cell response evaluated by IFN-γ release assay (IGRA), whole blood was overnight stimulated with a peptide mix (0.1 µg/mL of each peptide pool) covering the SARS-CoV-2 spike protein (Miltenyi Biotec, Germany) or with the staphylococcal enterotoxin B (SEB) at 200 ng/mL, as positive control.24 25 Plasma IFN-γ levels were measured using an automatic ELISA (ELLA, protein simple, R&D Systems, Minnesota, USA). IFN-γ levels ≥16 pg/mL were considered positive.
For flow cytometry, fresh whole blood (600 µL) was overnight stimulated with spike protein or SEB together with a-CD28 and a-CD49d (1 µg/mL each). Cells were then stained for intracellular cytokines and T-cell phenotype as previously described12 23 (online supplemental table 1 and figure 1).
Statistical analysis
Data were analysed using GraphPad software (GraphPad Prism V.9.3.1). Continuous and categorical variables were reported, respectively, as median and IQR and count and proportion. The following non-parametric statistical inference tests were performed: Friedman test for comparisons among groups followed by Dunn’s multiple comparisons test, Mann-Whitney and Wilcoxon signed-rank tests for pairwise comparisons, χ2 and McNemar tests for proportions. Two-tailed p values <0.05 were considered significant.
Results
Characteristics of the enrolled subjects
We prospectively enrolled 62 vaccinated subjects: 22 PwMS and 40 HCWs. Demographical and clinical characteristics are summarised in table 1.
Table 1.
Demographic and clinical characteristics of the enrolled participants
| Characteristics | HCWs | PwMS | P value |
| Total, N (%) | 40 (100) | 22 (100) | |
| Age, years (IQR) | 43 (29–51) | 51 (47–58) | 0.0013* |
| Male, N (%) | 11 (27.5) | 5 (22.72) | 0.681† |
| Origin | |||
| Western Europe | 39 | 22 | 0.445† |
| Asia | 1 | 0 | |
| BMI kg/m2 | 23.90 | ||
| Treatment duration, years (IQR) | 5.26 (2.1–9.2) | ||
| Time to last relapse, y (IQR) | 6.5 (2.4–10.3) | ||
| CD4:CD8 T cell ratio‡ | 2.1 (0.8–3.4) | 1.9 (1.1–2.6) | 0.842* |
| MS treatment | |||
| Ocrelizumab, N (%) | 5 (22.72) | ||
| Fingolimod, N (%) | 9 (40.9) | ||
| Cladribine, N (%) | 3 (13.63) | ||
| IFN-β, N (%) | 5 (22.72) |
*Mann-Whitney U statistic test.
†χ2 test
‡CD4:CD8 T cell ratio is a marker of immune-senescence.26
BMI, body mass index; HCWs, healthcare workers; IFN-β, interferon beta; N, Number; PwMS, patients with multiple sclerosis.
Age significantly differed between the two cohorts (p=0.0013). Nevertheless, CD4:CD8 T-cell ratio, which is inverted in people older than 60 years,26 was comparable between HCWs and PwMS (p=0.842). Among PwMS, five were treated with ocrelizumab, nine with fingolimod, three with cladribine and five with IFN-β. Fourteen PwMS have been previously treated with other DMTs (nine underwent IFN-β, three glatiramer acetate, one with dymethil fumarate and one with azathioprine). All enrolled subjects were naïve for SARS-CoV-2 infection as confirmed by undetectable anti-N antibodies (data not shown). For the kinetic study, a proportion of PwMS (n=19) and HCWs (n=30) was longitudinally sampled at T2 and T3 (figure 1A).
Kinetic of humoral and T-cell-specific responses in PwMS
Humoral and IFN-γ-spike-specific T-cell responses were monitored in HCWs (n=30) and PwMS (n=19) sampled at T2 and T3. We found that the booster dose significantly increased anti-RBD-IgG titers in both HCWs and PwMS (p<0.0001 for both), and in PwMS induced a higher seroconversion rate compared with T2 (T3: 15/19, 78.9% vs T2: 13/19, 68.4%), although not significant (figure 1B). However, compared with HCWs, PwMS showed significant lower anti-RBD titers at both T2 (p<0.0001) and T3 (p=0.001). Stratifying patients according to DMTs, we observed that antibody titers significantly differed from T2 to T3 in fingolimod-treated patients (T2: 8.60 BAU/mL, IQR: 1.95–19.85 and T3: 157.1 BAU/mL, IQR: 45.45–640.1, p=0.0039). Conversely, no significant differences were observed for cladribine- or IFN-β-treated patients, likely due to the small sample size; however, an increasing trend of anti-RBD-IgG titers was found. To note, patients under ocrelizumab did not present an antibody response (figure 1C).
In contrast, in PwMS the T-cell response, evaluated by IGRA, remained stable over time without significant differences between T2 and T3, although persisted significantly lower than in HCWs (T2: p=0.0026 and T3: p=0.0002) (figure 1D). This result was consistent across all treatments (figure 1E). Differently, in HCWs a significant increase was observed from T2 to T3 (p=0.018) (figure 1D). Sex and age did not show any effect on antibody or T-cell response in either of our cohorts (online supplemental table 2).
CD4+ and CD8+ T-cell responses after COVID-19 booster
To characterise the T-cell response to SARS-CoV-2 vaccine at T3, we analysed the frequency of CD4+ and CD8+ T cells producing IFN-γ, IL-2, or TNF-α in the 22 PwMS and a portion of HCWs (n=15).
First, we assessed the cytokine response to spike. In the CD4+ T-cell compartment of both cohorts, we found a higher response rate for either total or single Th1 cytokines compared with CD8+ T cells (table 2).
Table 2.
Number of CD4+ and CD8+ T-cell responders among HCWs and PwMS at T3
| Stimulation and type of T-cell response evaluation | Cytokine produced | T3 responders over total N (%) | ||
| HCWs 15 (100) |
PwMS 22 (100) |
P value* | ||
| Spike-specific CD4+ T cells | Any Th1 | 13 (86.6) | 5 (22.7) | 0.0001 |
| IFN-γ | 7 (46.6) | 5 (22.7) | 0.1267 | |
| TNF-α | 9 (60) | 0 (0) | <0.0001 | |
| IL-2 | 9 (60) | 0 (0) | <0.0001 | |
| Spike-specific CD8+ T cells | Any Th1 | 4 (26.6) | 2 (9.1) | 0.1544 |
| IFN-γ | 4 (26.6) | 2 (9.1) | 0.1544 | |
| TNF-α | 1 (6.6) | 0 (0) | 0.2195 | |
| IL-2 | 1 (6.6) | 0 (0) | 0.2195 | |
| CD4+ T cells response to SEB | Any Th1 | 15 (100) | 22 (100) | n/a |
| IFN-γ | 15 (100) | 22 (100) | n/a | |
| TNF-α | 15 (100) | 21 (91.3) | 0.4025 | |
| IL-2 | 12 (80) | 12 (54.5) | <0.0001 | |
| CD8+ T cells response to SEB | Any Th1 | 15 (100) | 22 (100) | n/a |
| IFN-γ | 15 (100) | 22 (100) | n/a | |
| TNF-α | 13 (86.7) | 21 (95.5) | 0.3363 | |
| IL-2 | 14 (93.3) | 3 (13.6) | <0.0001 | |
In bold are indicated the significant values.
*χ2 test.
HCWs, healthcare care workers; N, Number; n/a, not available; PwMS, patients with multiple sclerosis; SEB, staphylococcal enterotoxin B.
PwMS showed the highest number of CD4+ T-cell responders for IFN-γ compared with TNF-α or IL-2. Compared with HCWs, the proportions of TNF-α-, IL-2- and total Th1-specific-CD4+ T-cell responders were significantly reduced in PwMS (p<0.0001, p<0.0001 and p=0.0001, respectively) (table 2). Particularly, CD4+ T cells from PwMS failed to produce IL-2. Analysing the quantitative response, significant lower frequencies of IFN-γ, TNF-α-, IL-2 and Th1-specific CD4+ T cells were observed in PwMS (p=0.0129, p<0.0001, p=0.0002 and p<0.0001, respectively) (figure 2A).
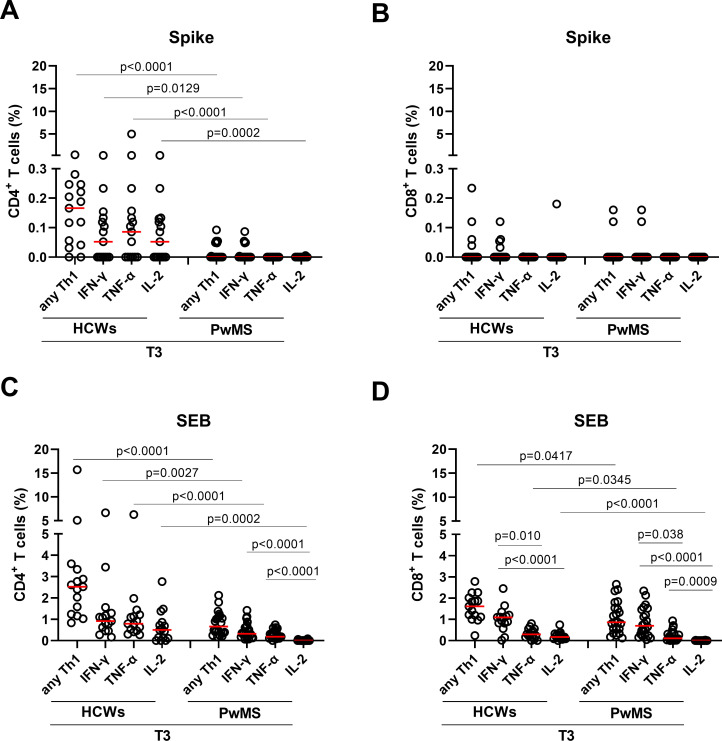
Figure 2.
CD4+ and CD8+ T cell cytokine profiles in response to spike and SEB after the booster dose (T3). (A, B) CD4+ and CD8+ T-cell cytokine responses to spike at T3. Graphs show cytokine production, in terms of total Th1 and single cytokines (IFN-γ, TNF-α and IL-2) in HCWs (n=15) and PwMS (n=22) within the CD4+ (A) and CD8+ (B) T-cell compartments. (C, D) CD4+ and CD8+ T-cell responses to the non-specific stimulus of SEB. Graphs show total Th1 and single cytokine (IFN-γ, TNF-α and IL-2) producing cells in HCWs (n=15) and PwMS (n=22) within the CD4+ (C) and CD8+ (D) T-cell compartments. Frequencies were reported after subtracting the cytokine production of the unstimulated condition. Statistical analysis was performed using Mann-Whitney U test to compare the frequency of the T cells expressing IFN-γ, IL-2 or TNF-α between HCWs and PwMS and Friedman test followed by Dunn’s multiple comparison test for intragroup analyses. P values <0.05 were considered significant. Red lines indicate medians and each dot represents a different HCW or PwMS. HCWs, healthcare workers; IFN-γ, interferon gamma; IFN-β, interferon beta; PwMS, patients with multiple sclerosis; SEB, staphylococcal enterotoxin B.
Regarding the cytokine response within the CD8+ T-cell compartment, the highest number of responders was observed for IFN-γ. However, no significant differences were observed in terms of magnitude or response rate, neither for total nor for single Th1 cytokine response between the two cohorts, likely due to the low number of responders (figure 2B and table 2).
Then, we investigated the ability of T cells to produce cytokines in response to SEB, a non-specific stimulus. Compared with spike, both cohorts showed a higher number of responders in terms of total and single Th1 cytokine response in both CD4+ and CD8+ T-cell subset (table 2).
Within the SEB response, all subjects showed a CD4 and CD8 Th1 cytokine response (table 2); however, PwMS presented a frequency of both CD4+ and CD8+ Th1 response significantly lower than HCWs (p<0.0001 and p=0.0417, respectively) (figure 2C, D). Evaluating the single cytokine response, we confirmed with SEB that the highest response rate in both cohorts and T-cell compartments was observed for IFN-γ. No significant difference was found in the number of TNF-α-specific CD4+ or CD8+ T-cell responders between HCWs and PwMS. Differently, the proportion of IL-2-specific CD4+ and CD8+ T-cell responders was significantly reduced in PwMS compared with HCWs (p<0.0001 for both) (table 2). Regarding the magnitude of the response, CD4+ T cells of PwMS showed lower frequencies of IFN-γ (p=0.0027), TNF-α (p<0.0001) and IL-2 (p=0.0002) producing cells than controls (figure 2C). Similar results were found within the CD8+ T-cell compartment for TNF-α (p=0.034) and IL-2 (p<0.0001), whereas the IFN-γ-specific CD8+ T cells showed a trend of reduction, although not significant (figure 2D). Within the PwMS cohort, the IFN-γ T-cell frequency was significantly higher than that of IL-2 and TNF-α in both CD4+ and CD8+ T-cell compartments (figure 2C, D). Similar results were found in the CD8+ T-cell compartment of HCWs.
Cd4+ and CD8+ T-cell memory phenotype after booster
We characterised the CD4+ and CD8+ memory T cells at T3 according to the expression of CD45RA and CCR7.
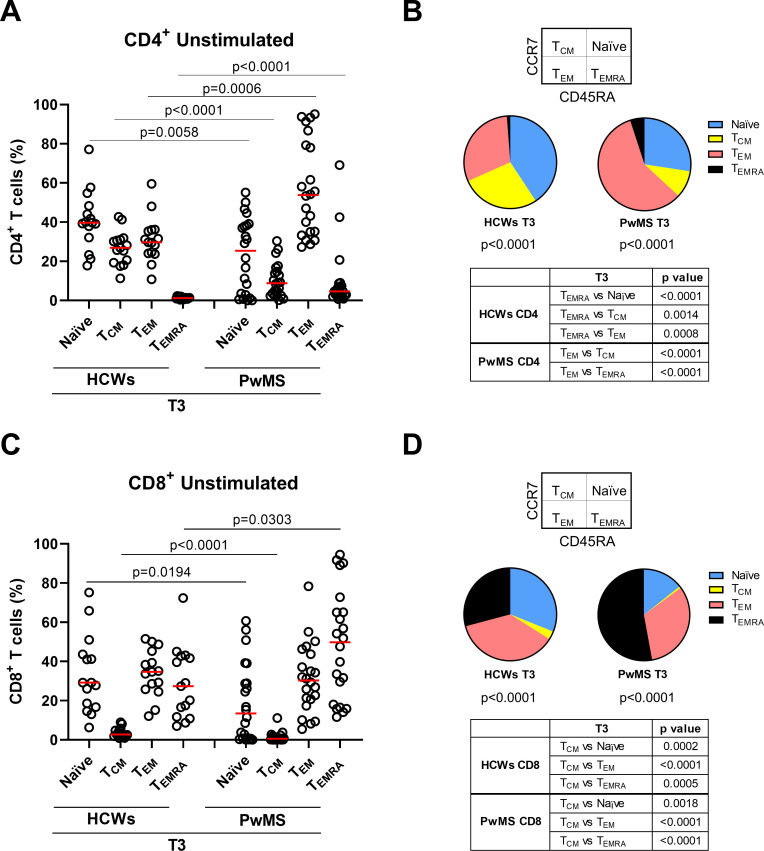
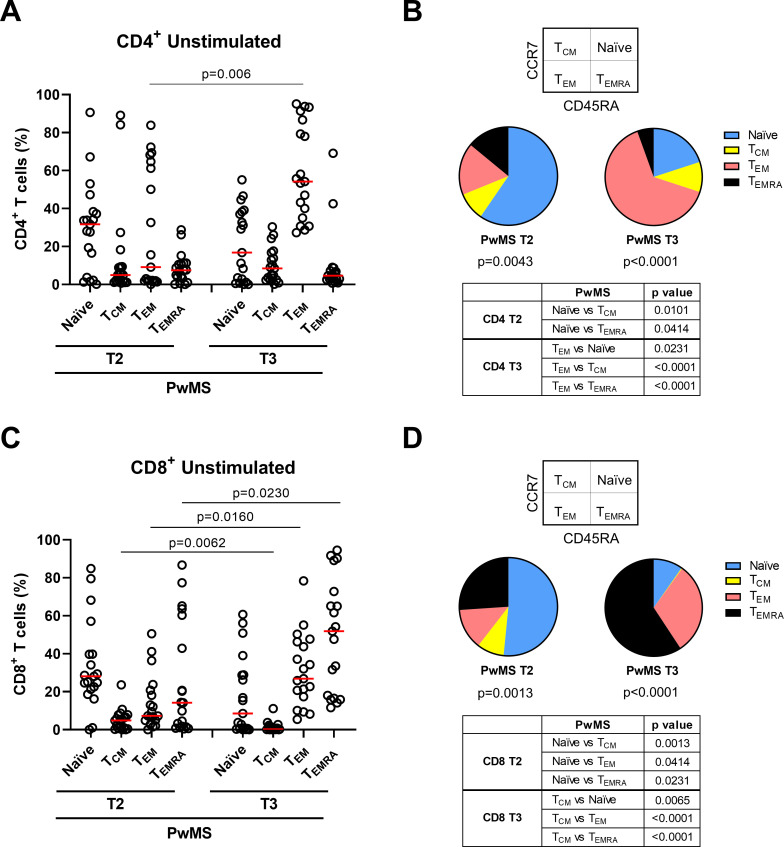
In the CD4+ T-cell subset, naïve (CD45RA+CCR7+) and central memory (TCM, CD45RA-CCR7+) cells were significantly reduced in PwMS compared with HCWs (p=0.0058 and p<0.0001, respectively). Differently, effector memory (TEM, CD45RA-CCR7-) and terminally differentiated effector memory T cells (TEMRA, CD45RA+CCR7-) increased in PwMS (p=0.0006 and p<0.0001, respectively) (figure 3A, B). Within the CD4+ T cells of PwMS, TEM cells were the most represented; differently, the naïve population was more represented in HCWs. In both cohorts, the lowest proportion was represented by the TEMRA subpopulation (figure 3B).
Figure 3.
Memory T-cell phenotype in CD4+ and CD8+ T cells after the booster dose (T3). (A–D) T-cell phenotype in HCWs (n=15) and PwMS (n=22) in unstimulated condition. Frequency of CD4+ (A) and CD8+ T cells (C) gated according to CD45RA and CCR7 expression. Pie charts show the proportion of CD4+ (B) and CD8+ (D) T-cell subpopulations in both cohorts. Statistical analyses were performed using Mann-Whitney U test and p values <0.05 were considered significant. Friedman test followed by Dunn’s multiple comparison test was performed for pie charts analyses and pie graphs were generated using the median frequency. Red lines indicate medians. Each dot represents a different HCW or PwMS. HCWs, healthcare workers; PwMS, patients with multiple sclerosis; TCM, central memory; TEM, effector memory; TEMRA, terminally differentiated effector memory.
Similarly, within the CD8+ T-cell compartment, significant reduction of both naïve and TCM subset (p=0.0194 and p<0.0001) and significant increase of TEMRA cells (p=0.0303) were observed in PwMS compared with HCWs (figure 3C, D). The frequency of TEM cells was comparable between the two cohorts. Within each cohort, the proportion among memory T-cell subsets significantly differed (p<0.0001). In PwMS, TEMRA and TEM populations were the most represented within the CD8+ T cells, whereas in HCWs TEM and naïve populations showed the highest frequency (figure 3D). In both cohorts, the lowest proportion was represented by the TCM population.
Temporal evolution of the T-cell response in PwMS
To monitor the over time evolution of the Th1 cytokine response to SARS-CoV-2 vaccination in PwMS, 19 subjects were analysed at T2 and T3.
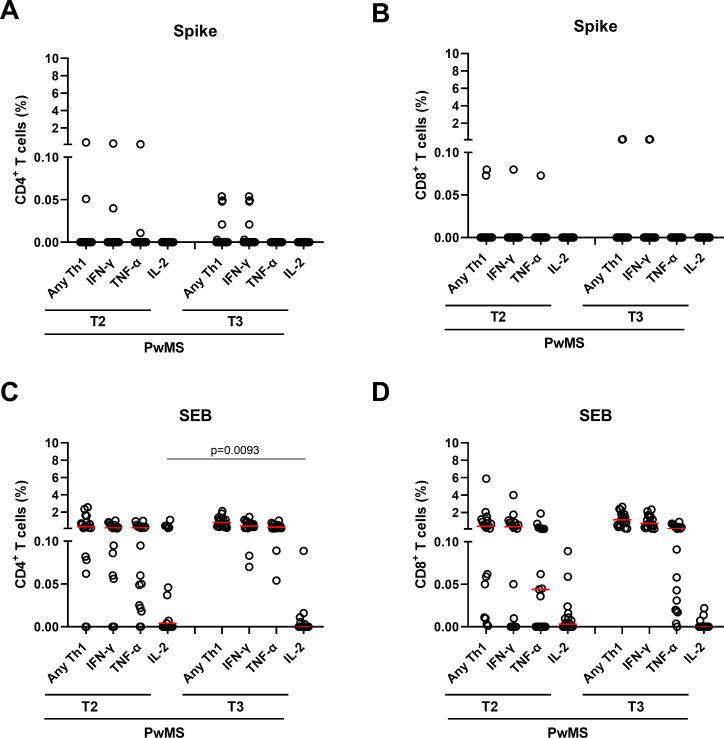
In response to spike, comparing T2 and T3 no significant differences were observed in terms of magnitude or response rate neither for total nor for single Th1 cytokine responses in both T-cell subsets (figure 4A, B). However, higher proportions of Th1 and IFN-γ-specific CD4+ T-cell responders were observed from T2 to T3 (table 3). Differently, no IL-2-specific CD4+ or CD8+ T- cell responders were observed in either T2 or T3. The few TNF-α-specific responders at T2 were lost at T3.
Figure 4.
Temporal evolution of CD4+ and CD8+ T cell cytokine profiles in response to spike and SEB stimuli. (A, B) CD4+ and CD8+ T-cell cytokine responses to spike in the PwMS (n=19) longitudinally sampled after 6 months from the first vaccine dose (T2) and after 4–6 weeks from the booster dose (T3). Graphs show cytokine production, in terms of total Th1 and single cytokines (IFN-γ, TNF-α and IL-2) within the CD4+ (A) and CD8+ (B) T-cell compartments. (C, D) CD4+ and CD8+ T-cell responses to SEB in the PwMS followed over time. Graphs show the total Th1 and single cytokine (IFN-γ, TNF-α and IL-2) producing cells within the CD4+ (C) and CD8+ (D) T-cell compartments. Frequencies were reported after subtracting the cytokine production of the unstimulated condition. Statistical analysis was performed using Wilcoxon matched pairs signed rank test to compare the frequency of the T cells expressing IFN-γ, IL-2 or TNF-α between T2 and T3. P values <0.05 were considered significant. Red lines indicate the medians and each dot represents a different PwMS. IFN-γ, interferon gamma; IFN-β, interferon beta; PwMS, patients with multiple sclerosis; SEB, staphylococcal enterotoxin B.
Table 3.
Number of CD4+ and CD8+ T-cell responders among PwMS at T2 and T3
| Stimulation and type of T-cell response evaluation | Cytokine produced | Responders over total N (%) | ||
| T2 19 (100) | T3 19 (100) | P value* | ||
| Spike-specific CD4+ T cells | Any Th1 | 2 (10.5) | 5 (26.31) | 0.248 |
| IFN-γ | 2 (10.5) | 5 (26.31) | 0.248 | |
| TNF-α | 2 (10.5) | 0 (0) | 0.479 | |
| IL-2 | 0 (0) | 0 (0) | n/a | |
| Spike-specific CD8+ T cells | Any Th1 | 2 (10.5) | 2 (10.5) | n/a |
| IFN-γ | 1 (5.2) | 2 (10.5) | >0.9999 | |
| TNF-α | 1 (5.2) | 0 (0) | >0.9999 | |
| IL-2 | 0 (0) | 0 (0) | n/a | |
| CD4+ T cells response to SEB | Any Th1 | 17 (89.5) | 19 (100) | 0.479 |
| IFN-γ | 16 (84.2) | 19 (100) | 0.248 | |
| TNF-α | 17 (89.5) | 19 (100) | 0.479 | |
| IL-2 | 10 (60.1) | 5 (26.3) | 0.182 | |
| CD8+ T cells response to SEB | Any Th1 | 19 (100) | 19 (100) | n/a |
| IFN-γ | 16 (84.2) | 19 (100) | 0.248 | |
| TNF-α | 11 (57.9) | 18 (94.7) | 0.045 | |
| IL-2 | 11 (57.9) | 3 (15.8) | 0.026 | |
In bold are indicated the significant values.
*McNemar’s test.
N, Number; n/a, not available; PwMS, Patients with Multiple Sclerosis; SEB, staphylococcal enterotoxin B.
In response to SEB, we also found that Th1, IFN-γ and TNF-α CD4+ T-cell responses were stable over time without significant differences in terms of magnitude or number of responders (figure 4C). Differently, from T2 to T3 we observed a significant reduction of the frequency of CD4+ T cells producing IL-2 (p=0.0093) (figure 4C). Moreover, also the number of IL-2-specific CD4+ T-cell responders decreased, although the difference was not significant (table 3).
Similarly, CD8+ T cells in response to SEB showed a significant reduction of the proportion of IL-2 responders (p=0.026) from T2 to T3 (table 3). No significant differences were found in terms of magnitude or response rate for total Th1 and IFN-γ cytokine responses, whereas the proportion of TNF-α responders significantly increased (p=0.045).
Kinetic of CD4+ and CD8+ T-cell memory phenotype within PwMS
To evaluate whether the COVID-19 vaccine booster could influence the memory T-cell compartment in PwMS, we compared the T-cell phenotype of the patients longitudinally sampled at T2 and T3.
CD4+ memory T-cell subpopulations significantly differed in frequency at both time points (T2: p=0.0043 and T3: p<0.0001) (figure 5A, B). Particularly, at T3 TEM population frequency was significantly increased compared with T2 (p=0.006), thus making TEM cells the most represented among all CD4+ memory T cells. By contrast, no differences were found for naïve, TCM or TEMRA cells between T2 and T3 (figure 5A).
Figure 5.
Over time evolution of memory T-cell phenotypes in CD4+ and CD8+ T cells. (A–D) T-cell phenotype in PwMS (n=19) longitudinally sampled after 6 months from the first vaccine dose (T2) and after 4–6 weeks from the booster dose (T3) in unstimulated condition. Frequency of CD4+ (A) and CD8+ T cells (C) gated according to CD45RA and CCR7 expression. Pie charts show the proportion of CD4+ (B) and CD8+ (D) T-cell subpopulations at both time points. Statistical analyses were performed using Wilcoxon matched pairs signed rank test and p values <0.05 were considered significant. Friedman test followed by Dunn’s multiple comparison test was used for pie charts analyses and pie graphs were generated using the median frequency. Red lines indicate the medians. Each dot represents a different PwMS. PwMS, patients with multiple sclerosis; TCM, central memory; TEM, effector memory; TEMRA, terminally differentiated effector memory.
Within the CD8+ T-cell compartment, memory T-cell subpopulations significantly differed from each other in terms of frequency at both time points (T2: p=0.0013 and T3: p<0.0001) and all of them significantly varied over time. Indeed, the frequency of TEM and TEMRA subsets increased (p=0.016 and p=0.023), whereas TCM cells decreased (p=0.0062) from T2 to T3 (figure 5C, D). Also, naïve cells showed a reduction trend, although not significant. Consequently, while at T2 the naïve population presented the highest frequency among all CD8+ memory cells, after the booster dose TEM and TEMRA populations were predominant.
Considering only the CD4+ Th1-specific responders (n=5), we observed a similar increasing trend for TEM and TEMRA populations at T3, although not significant (online supplemental figure 2). These results confirmed what was observed in the whole population independently of the positive response.
A similar memory T-cell phenotype was observed also after spike or SEB stimulation (data not shown), thus demonstrating that the memory profile was independent of the stimulus used.
Discussion
To the best of our knowledge, this is the first longitudinal prospective study characterising the humoral response and deeply investigating the T-cell response and memory profile, before and after the COVID-19 vaccine booster dose in PwMS treated with different DMTs. Our results highlight the beneficial effects of the booster dose showing increased anti-RBD-IgG titers in fingolimod, cladribine and IFN-β-treated patients, and an increased seroconversion rate for fingolimod-treated patients, although the magnitude of the response is lower than in healthy controls. As expected, no seroconversion was observed for ocrelizumab-treated patients due to CD20+ B cells depletion.27 Differently, the T-cell response evaluated by IGRA remained mostly stable over time in PwMS, whereas an increasing trend was observed in HCWs. Spike-specific T-cell response analysis by flow cytometry confirmed that the response is mainly CD4+ T cell-mediated.7 Moreover, after the booster dose, we showed that the magnitude of the specific CD4+ T-cell response was significantly reduced in PwMS compared with controls and characterised by an impaired IL-2 production. Regarding the T-cell memory phenotype, within the CD4+ T-cell compartment PwMS showed a significant reduction of naïve and TCM cells compared with HCWs, while TEM and TEMRA cells increased. A similar memory profile was observed within the CD8+ T-cell compartment, except for TEM cells whose frequency was comparable between the two cohorts. Overall, the booster dose increases the humoral response and the T-cell response in terms of Th1 cytokine production. These effects can be particularly relevant to prevent COVID-19 in the MS population.
Evaluation of both humoral and cell-mediated response is pivotal to accurately estimate the immune response to SARS-CoV-2 vaccines. Several studies demonstrated that antibody titers rapidly decrease over time.12 14 Differently, CD4+ T cells may contribute to SARS-CoV-2 long-term protection being recalled after antigen re-exposure even after a long period from the booster dose.28 Moreover, T-cell response cross-recognises also SARS-CoV-2 variants.29 30 Due to the immune response waning over 6 months following the second vaccine dose,10 13 the booster dose administration was authorised. Several studies have investigated the adaptive immune responses to SARS-CoV-2 vaccination focusing only on anti-CD20-, fingolimod-treated patients9 11 13 or on a wide range of DMTs but before the booster dose.17 31 32 This study confirms that fingolimod and anti-CD20-therapies elicit reduced cellular and humoral responses also after SARS-CoV-2 booster dose. Similarly, in ocrelizumab-treated patients no additive effect was observed on the maximal T-cell response, despite the presence of a boost response.21
IL-2 production was impaired in PwMS independently of the booster dose, whereas HCWs showed an IL-2 specific T-cell response. IL-2 is involved in the cell activation associated with a worsening of the MS disease status33; therefore, the IL-2 production impairment may indirectly explain the lack of MS worsening symptoms after SARS-CoV-2 vaccination. Interestingly, an impaired IL-2 production was also found in COVID-19 vaccinated patients with other autoimmune diseases, including rheumatoid arthritis.34
After the booster, an increased number of spike-specific Th1 T-cell responders, particularly IFN-γ responders, was observed, as previously shown.35 Despite the small number of spike responders, the greatest response to the non-specific stimulus of SEB supports the specificity and value of our data. The low number of responders could be partially explained by the highest number of PwMS under fingolimod, which reduces T lymphocytes bioavailability by retaining T cells in lymph nodes, thus affecting the T-cell response.36
In PwMS, the CD4+ T-cell memory phenotype presented a significant reduction of naïve and TCM cells, while TEM and TEMRA cells increased compared with HCWs in unstimulated conditions or after spike/SEB stimulation, suggesting an association with MS condition independently of COVID-19 vaccination, as reported.37 The chronic autoantigen exposure in MS favours the development of TEM rather than TCM cells.38 Moreover, fingolimod acts by reversibly retaining CD4+ naïve and TCM cells in lymph nodes.39 These might explain the differences observed. Interestingly, the booster dose in PwMS further increases both CD4+ and CD8+ TEM cells, making them particularly represented within memory T cells. We also confirmed that the booster dose increases CD8+ TEMRA cells, as reported.16 Memory T cells expand if re-challenged and contribute to prompt immune responses limiting initial viral replication, including that of the current variants,40 41 and dissemination in the host, thereby preventing severe disease. Indeed, reduced rates of hospitalisation and complications following extensive vaccination were reported in immunocompetent and immunocompromised individuals.42
Some limitations are acknowledged. Although PwMS were well characterised both immunologically and clinically, the robustness of the data might be limited by the small sample size that did not allow to study in detail the impact of the different DMTs. The lower number of responders observed in the flow cytometry analysis compared with other studies43 can be ascribed to the different experimental setting, including SARS-CoV-2 peptide composition/concentrations and the use of whole blood samples instead of peripheral blood mononuclear cells.44 Furthermore, the limited number of HCWs analysed at T2 by flow cytometry did not allow us to perform comparison analysis with PwMS. Finally, the lacking of untreated PwMS did not allow us to verify whether the memory phenotype might be affected by DMTs. The significantly different age between HCWs and PwMS did not represent a bias for the study, as we already demonstrated that the immune impairment is associated with ongoing DMTs and/or the MS disease status more than with age.7 Furthermore, other studies reported an age impact on the immune response in MS patients older than 60 years,45 46 whereas our cohort is younger than 60 years.
The main strength of this study is the longitudinal observation of both T-cell and humoral response as well as the extensive characterisation of the T-cell memory phenotype before and after COVID-19 mRNA-vaccine booster dose in PwMS and HCWs.
In conclusion, this study demonstrates the benefits of the booster dose in most PwMS as it strengthens the humoral response and the T-cell response in terms of Th1 cytokine production. The increase of effector memory T cells after the booster dose highlights the value of implementing COVID-19 vaccine booster strategies in the MS population. Accordingly, COVID-19 vaccination should be strongly recommended for PwMS, and clinicians should evaluate the appropriate timing for vaccine administration and additional protective measures, particularly in anti-CD20 and fingolimod-treated patients, who may have reduced immune responses, potentially limiting vaccine efficacy.
Acknowledgments
The authors gratefully acknowledge the Nurses of MS Centre of the San Camillo Forlanini Hospital and all patients who helped to conduct this study.
Footnotes
AA and AC contributed equally.
Contributors: DG and CG conceived and designed the study. AA, AC and DG analysed, interpreted data and wrote the manuscript. AC, CF, AMGA, AS, VV and LP processed samples for IGRA test and/or flow cytometry analysis. SM, DL and AB performed serologic tests. CT, GC, SH, LP, SG, MEQ, SR, CG, CA and VP enrolled the subjects and collected clinical data. All the authors critically revised the article and approved the final version of the manuscript. AA and AC equally contributed to this work and shared the first authorship with SR. DG acts as the guarantor.
Funding: This work was supported by INMI 'Lazzaro Spallanzani' Ricerca Corrente on emerging infections funded by the Italian Ministry of Health and by generous liberal donation/funding for COVID-19 research from Camera di Commercio, Industria e Artigianato di Roma (resolution number 395 on 25 May 2021).
Disclaimer: The funders were not involved in the study design, collection, analysis, and interpretation of data, the writing of this article, or the decision to submit it for publication.
Competing interests: CT and CG received honoraria for speaking, manuscript writing or educational events from Merck, Biogen, Roche, Novartis Sanofi, Celgene, and Almirall. LP (Luca Prosperini) received consulting fees and/or speaker honoraria from Biogen, Celgene, Genzyme, Merck-Serono, Novartis and Teva, travel grants from Biogen, Genzyme, Novartis and Teva, research grants from the Italian MS Society (Associazione Italiana Sclerosi Multipla) and Genzyme. SH received travel funding and/or speaker honoraria from Biogen, Roche, Genzyme, Novartis and CSL Behring. SG received honoraria for speaking and travel grants from Biogen, Sanofi-Aventis, Merck Serono, Bayer-Schering, Teva, Genzyme, Almirall and Novartis. SR has received honoraria from Biogen, Merck Serono, Novartis and Teva for consulting services, speaking and/or travel support. EN participates on a data safety monitoring board or advisory board and receives fees for educational training from Gilead, Eli Lilly, GS, SOBI and Roche. EN has a patent pending for raloxifene use in COVID-19 with Dompè Pharmaceutical. DG is a member of the advisory board of Biomerieux and Eli Lilly and received fees for educational training or consultancy from Almirall, Biogen, Celgene, Diasorin, Janssen, Qiagen and Quidel. All the other authors declare that the research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as online supplementary information.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The study was approved by the Ethical Committee of INMI 'L. Spallanzani'-IRCCS (approval numbers 247/2021, 297/2021 and 319/2021). Study protocols followed the ethics principles for human experimentation in agreement with the Declaration of Helsinki. A written informed consent was signed by all participants before the study procedures. Participants gave informed consent to participate in the study before taking part.
References
- 1. Hauser SL, Cree BAC. Treatment of multiple sclerosis: a review. Am J Med 2020;133:1380–90. 10.1016/j.amjmed.2020.05.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Iyer RB, S R, M JN, et al. COVID-19 outcomes in persons with multiple sclerosis treated with rituximab. Mult Scler Relat Disord 2022;57:103371. 10.1016/j.msard.2021.103371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Foerch C, Friedauer L, Bauer B, et al. Severe COVID-19 infection in a patient with multiple sclerosis treated with fingolimod. Mult Scler Relat Disord 2020;42:102180. 10.1016/j.msard.2020.102180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Simpson-Yap S, De Brouwer E, Kalincik T, et al. Associations of disease-modifying therapies with COVID-19 severity in multiple sclerosis. Neurology 2021;97:e1870–85. 10.1212/WNL.0000000000012753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Angyal A, Longet S, Moore SC, et al. T-Cell and antibody responses to first BNT162b2 vaccine dose in previously infected and SARS-CoV-2-naive UK health-care workers: a multicentre prospective cohort study. Lancet Microbe 2022;3:e21–31. 10.1016/S2666-5247(21)00275-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Agrati C, Castilletti C, Goletti D, et al. Coordinate induction of humoral and spike specific T-cell response in a cohort of Italian health care workers receiving BNT162b2 mRNA vaccine. Microorganisms 2021;9:1315. 10.3390/microorganisms9061315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tortorella C, Aiello A, Gasperini C, et al. Humoral- and T-cell-specific immune responses to SARS-CoV-2 mRNA vaccination in patients with MS using different disease-modifying therapies. Neurology 2022;98:e541–54. 10.1212/WNL.0000000000013108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sabatino JJ, Mittl K, Rowles WM, et al. Multiple sclerosis therapies differentially affect SARS-CoV-2 vaccine-induced antibody and T cell immunity and function. JCI Insight 2022;7. 10.1172/jci.insight.156978. [Epub ahead of print: 22 Feb 2022]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Apostolidis SA, Kakara M, Painter MM, et al. Cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy. Nat Med 2021;27:1990–2001. 10.1038/s41591-021-01507-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Achiron A, Mandel M, Dreyer-Alster S, et al. Humoral immune response to COVID-19 mRNA vaccine in patients with multiple sclerosis treated with high-efficacy disease-modifying therapies. Ther Adv Neurol Disord 2021;14:17562864211012836. 10.1177/17562864211012835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Habek M, Željko C, Savić Mlakar A, et al. Humoral and cellular immunity in convalescent and vaccinated COVID-19 people with multiple sclerosis: effects of disease modifying therapies. Mult Scler Relat Disord 2022;59:103682. 10.1016/j.msard.2022.103682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Farroni C, Picchianti-Diamanti A, Aiello A, et al. Kinetics of the B- and T-cell immune responses after 6 months from SARS-CoV-2 mRNA vaccination in patients with rheumatoid arthritis. Front Immunol 2022;13:846753. 10.3389/fimmu.2022.846753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bajwa HM, Novak F, Nilsson AC, et al. Persistently reduced humoral and sustained cellular immune response from first to third SARS-CoV-2 mRNA vaccination in anti-CD20-treated multiple sclerosis patients. Mult Scler Relat Disord 2022;60:103729. 10.1016/j.msard.2022.103729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Agrati C, Castilletti C, Goletti D, et al. Persistent Spike-specific T cell immunity despite antibody reduction after 3 months from SARS-CoV-2 BNT162b2-mRNA vaccine. Sci Rep 2022;12:6687. 10.1038/s41598-022-07741-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Herzberg J, Fischer B, Becher H, et al. Cellular and Humoral Immune Response to a Third Dose of BNT162b2 COVID-19 Vaccine - A Prospective Observational Study. Front Immunol 2022;13:896151. 10.3389/fimmu.2022.896151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu Y, Zeng Q, Deng C, et al. Robust induction of B cell and T cell responses by a third dose of inactivated SARS-CoV-2 vaccine. Cell Discov 2022;8:10. 10.1038/s41421-022-00373-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dreyer-Alster S, Menascu S, Mandel M, et al. COVID-19 vaccination in patients with multiple sclerosis: safety and humoral efficacy of the third booster dose. J Neurol Sci 2022;434:120155. 10.1016/j.jns.2022.120155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brill L, Rechtman A, Shifrin A, et al. Longitudinal humoral response in MS patients treated with cladribine tablets after receiving the second and third doses of SARS-CoV-2 mRNA vaccine. Mult Scler Relat Disord 2022;63:103863. 10.1016/j.msard.2022.103863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Milo R, Staun-Ram E, Karussis D, et al. Humoral and cellular immune responses to SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis: an Israeli multi-center experience following 3 vaccine doses. Front Immunol 2022;13:868915. 10.3389/fimmu.2022.868915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Meyer-Arndt L, Braun J, Fauchere F, et al. SARS-cov-2 mrna vaccinations fail to elicit humoral and cellular immune responses in patients with multiple sclerosis receiving fingolimod. J Neurol Neurosurg Psychiatry 2022;93:960–71. 10.1136/jnnp-2022-329395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Palomares Cabeza V, Kummer LYL, Wieske L, et al. Longitudinal T-cell responses after a third SARS-CoV-2 vaccination in patients with multiple sclerosis on Ocrelizumab or fingolimod. Neurol Neuroimmunol Neuroinflamm 2022;9:e1178. 10.1212/NXI.0000000000001178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 2018;17:162–73. 10.1016/S1474-4422(17)30470-2 [DOI] [PubMed] [Google Scholar]
- 23. Farroni C, Aiello A, Picchianti-Diamanti A. Booster dose of SARS-CoV-2 mRNA vaccines strengthens the specific immune response of patients with rheumatoid arthritis: a prospective multicenter longitudinal study. International Journal of Infectious Diseases Published Online First: 1 November 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Aiello A, Najafi Fard S, Petruccioli E, et al. Spike is the most recognized antigen in the whole-blood platform in both acute and convalescent COVID-19 patients. Int J Infect Dis 2021;106:338–47. 10.1016/j.ijid.2021.04.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Aiello A, Coppola A, Vanini V. Accuracy of QuantiFERON SARS-CoV-2 RUO assay and characterization of the CD4+ and CD8+ T-cell-SARS-CoV-2 response: comparison with a homemade IFN-γ release assay. Int J Infect Dis 2022;S1201-9712:00444–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Muller GC, Gottlieb MGV, Luz Correa B, et al. The inverted CD4:CD8 ratio is associated with gender-related changes in oxidative stress during aging. Cell Immunol 2015;296:149–54. 10.1016/j.cellimm.2015.05.006 [DOI] [PubMed] [Google Scholar]
- 27. Hauser SL, Bar-Or A, Comi G, et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med 2017;376:221–34. 10.1056/NEJMoa1601277 [DOI] [PubMed] [Google Scholar]
- 28. Wragg KM, Lee WS, Koutsakos M, et al. Establishment and recall of SARS-CoV-2 spike epitope-specific CD4+ T cell memory. Nat Immunol 2022;23:768–80. 10.1038/s41590-022-01175-5 [DOI] [PubMed] [Google Scholar]
- 29. Petrone L, Picchianti-Diamanti A, Sebastiani GD, et al. Humoral and cellular responses to spike of δ SARS-CoV-2 variant in vaccinated patients with immune-mediated inflammatory diseases. Int J Infect Dis 2022;121:24–30. 10.1016/j.ijid.2022.04.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Petrone L, Tortorella C, Aiello A, et al. Humoral and cellular response to spike of delta SARS-CoV-2 variant in vaccinated patients with multiple sclerosis. Front Neurol 2022;13:881988. 10.3389/fneur.2022.881988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Maglione A, Morra M, Meroni R, et al. Humoral response after the booster dose of anti-SARS-CoV-2 vaccine in multiple sclerosis patients treated with high-efficacy therapies. Mult Scler Relat Disord 2022;61:103776. 10.1016/j.msard.2022.103776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Iannetta M, Landi D, Cola G, et al. B- and T-cell responses after SARS-CoV-2 vaccination in patients with multiple sclerosis receiving disease modifying therapies: immunological patterns and clinical implications. Front Immunol 2021;12:796482. 10.3389/fimmu.2021.796482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sadeghi Hassanabadi N, Broux B, Marinović S, et al. Innate Lymphoid Cells - Neglected Players in Multiple Sclerosis. Front Immunol 2022;13:909275. 10.3389/fimmu.2022.909275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dayam RM, Law JC, Goetgebuer RL, et al. Accelerated waning of immunity to SARS-CoV-2 mRNA vaccines in patients with immune-mediated inflammatory diseases. JCI Insight 2022;7:e159721. 10.1172/jci.insight.159721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Madelon N, Heikkilä N, Sabater Royo I, Royo S, et al. Omicron-Specific cytotoxic T-cell responses after a third dose of mRNA COVID-19 vaccine among patients with multiple sclerosis treated with Ocrelizumab. JAMA Neurol 2022;79:399–404. 10.1001/jamaneurol.2022.0245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Achiron A, Mandel M, Gurevich M, et al. Immune response to the third COVID-19 vaccine dose is related to lymphocyte count in multiple sclerosis patients treated with fingolimod. J Neurol 2022;269:2286–92. 10.1007/s00415-022-11030-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nielsen BR, Ratzer R, Börnsen L, et al. Characterization of naïve, memory and effector T cells in progressive multiple sclerosis. J Neuroimmunol 2017;310:17–25. 10.1016/j.jneuroim.2017.06.001 [DOI] [PubMed] [Google Scholar]
- 38. Raphael I, Joern RR, Forsthuber TG. Memory CD4+ T Cells in Immunity and Autoimmune Diseases. Cells 2020;9:531. 10.3390/cells9030531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hunter SF, Bowen JD, Reder AT. The direct effects of fingolimod in the central nervous system: implications for relapsing multiple sclerosis. CNS Drugs 2016;30:135–47. 10.1007/s40263-015-0297-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol 2002;2:251–62. 10.1038/nri778 [DOI] [PubMed] [Google Scholar]
- 41. Goel RR, Painter MM, Apostolidis SA, et al. mRNA vaccines induce durable immune memory to SARS-CoV-2 and variants of concern. Science 2021;374:abm0829. 10.1126/science.abm0829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tenforde MW, Self WH, Gaglani M, et al. Effectiveness of mRNA Vaccination in Preventing COVID-19-Associated Invasive Mechanical Ventilation and Death - United States, March 2021-January 2022. MMWR Morb Mortal Wkly Rep 2022;71:459–65. 10.15585/mmwr.mm7112e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gao Y, Cai C, Grifoni A, et al. Ancestral SARS-CoV-2-specific T cells cross-recognize the omicron variant. Nat Med 2022;28:472–6. 10.1038/s41591-022-01700-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hoffmeister B, Bunde T, Rudawsky IM, et al. Detection of antigen-specific T cells by cytokine flow cytometry: the use of whole blood may underestimate frequencies. Eur J Immunol 2003;33:3484–92. 10.1002/eji.200324223 [DOI] [PubMed] [Google Scholar]
- 45. Zuroff L, Rezk A, Shinoda K, et al. Immune aging in multiple sclerosis is characterized by abnormal CD4 T cell activation and increased frequencies of cytotoxic CD4 T cells with advancing age. EBioMedicine 2022;82:104179. 10.1016/j.ebiom.2022.104179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Keegan AP, Joshi U, Abdullah L, et al. Characterization of immune profile in an aging multiple sclerosis clinic population. Mult Scler Relat Disord 2022;63:103818. 10.1016/j.msard.2022.103818 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jnnp-2022-330175supp001.pdf (310.2KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as online supplementary information.