Abstract
Objective
Most earlier studies on occupational risk of COVID-19 covering the entire workforce are based on relatively rare outcomes such as hospital admission and mortality. This study examines the incidence of SARS-CoV-2 infection by occupational group based on real-time PCR (RT-PCR) tests.
Methods
The cohort includes 2.4 million Danish employees, 20–69 years of age. All data were retrieved from public registries. The incidence rate ratios (IRRs) of first-occurring positive RT-PCR test from week 8 of 2020 to week 50 of 2021 were computed by Poisson regression for each four-digit Danish Version of the International Standard Classification of Occupations job code with more than 100 male and 100 female employees (n=205). Occupational groups with low risk of workplace infection according to a job exposure matrix constituted the reference group. Risk estimates were adjusted by demographic, social and health characteristics including household size, completed COVID-19 vaccination, pandemic wave and occupation-specific frequency of testing.
Results
IRRs of SARS-CoV-2 infection were elevated in seven healthcare occupations and 42 occupations in other sectors, mainly social work activities, residential care, education, defence and security, accommodation and transportation. No IRRs exceeded 2.0. The relative risk in healthcare, residential care and defence/security declined across pandemic waves. Decreased IRRs were observed in 12 occupations.
Discussion
We observed a modestly increased risk of SARS-CoV-2 infection among employees in numerous occupations, indicating a large potential for preventive actions. Cautious interpretation of observed risk in specific occupations is needed because of methodological issues inherent in analyses of RT-PCR test results and because of multiple statistical tests.
Keywords: epidemiology, occupational health, viruses
WHAT IS ALREADY KNOWN ON THIS TOPIC
Epidemiological studies suggest that the workplace contributes to the COVID-19 pandemic.
Results are mostly based on studies of less frequent outcomes such as COVID-19 morbidity or mortality, which limit inference about risk in specific occupations.
WHAT THIS STUDY ADDS
The risk of COVID-19 infection was increased in 49 out of 205 specific occupations.
The majority of employees at risk were working within health care and residential care, social work, education, defence and security, accommodation, transportation and various service activities.
The relative risk in healthcare, residential care and defence/security declined across pandemic waves, while the relative risk in education and social work increased during the pandemic.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Preventive actions targeting the workplace may contribute substantially to alleviate disease occurrence in the ongoing COVID-19 and similar future pandemics.
Introduction
Less than 3 years have elapsed since the still ongoing COVID-19 pandemic started. During this short time span, numerous epidemiological studies have consistently demonstrated that a range of occupations are associated with an increased risk of being infected with SARS-CoV-2.1–5 The reasons may include higher frequencies of close social contacts at the workplace, caring for infected patients and less possibility to maintain sufficient distancing and efficiently use personal protective equipment.6 7 Other risk factors such as mode of transportation when commuting to work might also be of importance. Previously identified at-risk occupations include healthcare workers, teachers, bus and taxi drivers, meat packers, retail sale workers, bartenders and police officers.1–4 8 The majority of studies have investigated risk across occupations using relatively rare outcomes such as COVID-19-related admission to hospital9–11 or mortality.4 9 10 12–16 However, these approaches fail to address less severe non-hospital demanding cases and may thus underestimate risk of infection. Considering reports on delayed return to work17 and of post-COVID-19 conditions with persistent ill health following cases without acute critical disease,18 we find that this is of importance, not the least in occupations with many young and healthy employees who are less likely to be hospitalised but at equal or higher risk of infection.
We have recently reported increased occupational risk of severe COVID-19 in terms of COVID-19-related hospital admission among workers in the healthcare, social care and transportation sectors.8 Based on the same cohort and time period, in this paper, we unravel the risk of asymptomatic and less severe COVID-19 using real-time PCR (RT-PCR) test as the outcome. Hereby, a strong gain is achieved in statistical power, which enables detection of potentially increased risk in occupations with too few (or too young and healthy) employees to allow analyses of hospital admissions. The capacity for free and on-demand COVID-19 RT-PCR testing covering all residents in Denmark was established during autumn 2020. By late spring 2021, the number of tests peaked at 0.8 million/week among 2.4 million employees (own data). While the cumulative incidence for COVID-19-related hospital admission in the workforce during the first almost 2 years of the pandemic in Denmark was 0.17%,8 the corresponding rate of at least one positive PCR test was 10.7% (own data).
The objective of this paper was to estimate the relative risk of SARS-CoV-2 infection conferred by specific occupations independently of COVID-19 vaccination and PCR test frequency. Infection was defined by a positive PCR test and the reference group by an independent expert-rated job exposure matrix (JEM).
Methods
Population and data
The study population was a nationwide register-based cohort of all Danish residents 20–69 years of age, who by 31 December 2019 were gainfully employed (n=2 451 542). It was extricated as a subset of the Danish Occupational Cohort with exposure data,19, which includes a range of demographic, social, occupational and health characteristics. Records were merged by the 10-digit unique personal identification number assigned by the Danish authorities. Additional information on vital data, SARS-CoV-2 tests and COVID-19 vaccinations was provided by Statistics Denmark and the National Board of Health Data from baseline week 8, 2020, to the end of follow-up week 50, 2021. Details on the cohort and its key variables have been published in a previous paper.8
Occupational data
Jobs are classified by Statistics Denmark according to the Danish Version of the International Standard Classification of Occupations (DISCO)-08,20 which is close to identical with the international classification ISCO-08 (423 four-digit codes, coverage 86% of all gainfully employed) and the Danish version of the Statistical Classification of Economic Activities in the European Communities (DB07)20 (22 one-digit sections and 88 two-digit divisions, coverage >99%).
Population characteristics
Population characteristics retrieved from Statistics Denmark by 31 December 2019 included sex, age, duration of education in years, country of birth, hospital admission for 1 or more of 11 chronic diseases during 2010–2019, geographical residential area and the number of household members sharing the same residence including children. Information on health behaviours is not available in public registries with national coverage. We assigned sex-specific and age-specific probability of current smoking and estimates of body mass index (kg/m2) using data from large Danish survey samples executed in 2010 and 2013.21 These JEM-derived variables predict mortality and ischaemic heart disease independently of other risk factors in the Danish population of employees.22
SARS-CoV-2 test results
SARS-CoV-2 infection was identified by RT-PCR tests performed at Statens Serum Institut in Copenhagen or at an accredited hospital laboratory.23 The specificity of the RT-PCR test is extremely high (99.9%),24 while the sensitivity in the general Danish population is unknown but believed to be above 80%.24 The sensitivity mainly depends on stage of infection and sampling procedure. In the early phase of the pandemic, RT-PCR tests were carried out for diagnostic purposes and for tracing of close contacts, but from autumn 2020, the test capacity was expanded and tests free of charge were offered to the entire population regardless of symptoms and contacts.24 Some 29.1 million tests were conducted in the study population until 14 December 2021, of which 1% were positive (own data).
Antigen tests (rapid home tests)
Antigen tests (rapid home tests) have in particular been recommended in periods and geographical regions with a high load of viral transmission, and in age and occupational groups considered at high risk. The antigen test targets viral proteins. It has been suggested by a Danish authority (Statens Serum Institut) that both sensitivity and specificity are inferior compared with the RT-PCR test.25 Therefore, it is recommended that a positive antigen test is confirmed by a RT-PCR test. While antigen tests done as part of the nationwide and publicly funded test set-up are included in the national database of test results, results of privately bought and used antigen tests were not. In this population, only 72% of positive antigen test results were confirmed when a PCR test was performed within 2 days. In order to give priority to specificity over sensitivity, results of antigen tests were not included in the outcome measure.
Reference group
The reference group was defined by an independent expert-rated COVID-19 JEM.6 It includes eight workplace characteristics, each rated on a scale from 0 (low SARS-CoV-2 exposure) to 3 (high exposure). The initial protocol for this study used a sum score across all eight dimensions of 0 to define the reference group. However, following external reviews and the publication of a JEM-validation study,26 only the four dimensions with the strongest association with the independent measure of infection defined the reference group (sum score 0 of the four dimensions that directly address risk of virus transmission at the workplace: number and nature of social contacts at the workplace, contamination of workspaces and work outside or home).
Statistical analysis
We used Poisson regression to examine the incidence rate ratios (IRRs) of first-occurring positive SARS-CoV-2 RT-PCR test across all waves from week 8 of 2020 to week 50 of 2021 for each of the 205 non-reference four-digit DISCO-08 job codes with more than 100 male and 100 female employees. Employees with missing DISCO-08 codes and small occupations were kept as separate categories. The time unit was 1 week, and weeks after the first positive RT-PCR test, death, emigration or retirement were censored. The applied Poisson regression can be seen as a special case of Cox regression to model time to event data by including risk time as an offset in the model. Hereby, it is assumed that the baseline hazard is constant within each wave of the COVID-19 pandemic in Denmark. Since we do not have repeated observations—each individual contributes only once to the outcome—there is no need for robust estimation techniques.
Analyses were adjusted by a priori selected characteristics regardless of association with exposures27: age, duration of education, country of birth, geographical area, chronic disease, size of the household, body mass index, smoking and pandemic wave defined as calendar periods delimited by weeks with the lowest number of positive RT-PCR tests between pandemic peaks (for covariate categories, see table 1). In order to estimate the direct effect of occupation independent of COVID-19 vaccination (two doses with at least 21 days in between) and average occupational test frequency, we also adjusted for these variables. An outline of assumed causal pathways is provided in online supplemental figure 1.
Table 1.
Prevalence of at least one positive SARS-CoV-2 RT-PCR test by characteristics of Danish male and female employees during the COVID-19 pandemic from week 8 of 2020 to week 52 of 2021
| Characteristic | Men | Women | ||
| Total (N) | Ever positive RT-PCR test (%) | Total (N) | Ever positive RT-PCR test (%) | |
| Sex, % ever testing RT-PCR positive | 1 274 370 | 10.3 | 1 178 172 | 10.9 |
| Age (years) | ||||
| 20–<30 | 260 918 | 13.6 | 236 569 | 14.1 |
| 30–<40 | 275 776 | 11.2 | 249 010 | 12.3 |
| 40–<50 | 298 547 | 10.6 | 284 747 | 11.5 |
| 50–<60 | 297 951 | 8.5 | 287 463 | 8.5 |
| 60+ | 141 178 | 6.5 | 120 383 | 6.5 |
| Geographical region | ||||
| Capital | 410 947 | 15.2 | 401 203 | 15.7 |
| Zealand | 176 827 | 10.2 | 162 902 | 11.1 |
| South | 238 305 | 7.1 | 212 381 | 7.3 |
| Central | 297 423 | 7.9 | 267 526 | 8.1 |
| North | 150 868 | 7.7 | 133 160 | 7.9 |
| Duration of education (years) | ||||
| ≤10 | 295 236 | 11.0 | 394 354 | 11.2 |
| >10–13 | 569 720 | 9.3 | 444 206 | 9.7 |
| >13–16 | 280 763 | 12.5 | 277 423 | 12.5 |
| >16 | 97 509 | 9.5 | 43 613 | 11.5 |
| Missing | 31 142 | 9.8 | 17 576 | 10.9 |
| Country of birth | ||||
| Denmark | 1 095 062 | 9.5 | 1 027 040 | 10.0 |
| Other Western countries | 39 782 | 9.6 | 33 592 | 10.4 |
| Eastern Europe | 57 468 | 13.9 | 45 736 | 15.4 |
| Low-income countries | 81 899 | 20.7 | 70 718 | 21.6 |
| Missing | 159 | 27.0 | 86 | 25.6 |
| Number of hospital admissions, 2010–2020 4 | ||||
| 0 | 1 107 771 | 10.6 | 950 570 | 11.1 |
| 1 | 126 235 | 9.4 | 188 757 | 10.5 |
| ≥2 | 30 364 | 8.6 | 37 845 | 9.9 |
| Probability of tobacco smoking (JEM assigned) | ||||
| <10% | 26 352 | 10.7 | 72 229 | 9.9 |
| 10–<20% | 475 984 | 10.1 | 618 042 | 10.6 |
| 20%+ | 556 205 | 10.5 | 351 505 | 11.8 |
| Missing | 215 929 | 10.9 | 135 396 | 10.9 |
| Body mass index (kg/m2) (JEM assigned) | ||||
| <25 | 217 499 | 13.4 | 531 232 | 12.0 |
| ≥25 | 840 942 | 9.4 | 510 544 | 9.8 |
| Missing | 215 929 | 10.7 | 135 396 | 10.9 |
| Number of household members | ||||
| 1 | 244 767 | 8.7 | 175 841 | 9.5 |
| 2 | 409 460 | 9.2 | 419 905 | 9.3 |
| 3 | 233 389 | 10.7 | 230 766 | 11.1 |
| 4+ | 386 754 | 12.5 | 351 160 | 13.4 |
| Average number of RT-PCR tests in the employee’s occupation (four-digit DISCO-08 code) during follow-up, quintiles | ||||
| 0–9 | 327 945 | 8.8 | 55 889 | 8.2 |
| >9–10 | 339 452 | 10.8 | 265 947 | 11.0 |
| >10–12 | 269 442 | 10.3 | 191 274 | 10.1 |
| >12–14 | 228 990 | 11.1 | 287 274 | 10.6 |
| >14–28 | 108 541 | 12.9 | 376 788 | 11.9 |
| Second COVID-19 vaccination obtained | ||||
| 1 January 2021–30 June 2021 | 373 343 | 8.2 | 436 546 | 8.7 |
| 1 July 2021–14 December 2021 | 752 894 | 9.9 | 631 132 | 10.4 |
| Less than two vaccinations by 14 December 2021 | 148 133 | 18.7 | 109 494 | 22.8 |
DISCO, Danish Version of the International Standard Classification of Occupations; JEM, job exposure matrix; RT-PCR, real-time PCR.
oemed-2022-108713supp001.pdf (9.4MB, pdf)
Risk estimates were first adjusted for age and sex, then by the full list of the forementioned covariates except occupational testing frequency. In a final step, risk estimates were also adjusted for the average RT-PCR test frequency during the follow-up period for each four-digit DISCO-08 occupational code.
In supplementary analyses, we explored the risk of SARS-CoV-2 infection in each pandemic wave using the same statistical model as outlined previously except that vaccination, which in Denmark was initiated at the end of December 2020, was not included in analyses of the first two waves. Analyses were restricted to occupations with overall elevated risk grouped into main economic sectors (n=49).
Considering the many comparisons and risk of type II error, we applied a significance level of 0.01 rather than the conventional 0.05. All analyses were carried out in SAS V.9.4 by access to a secured platform at Statistics Denmark.
Results
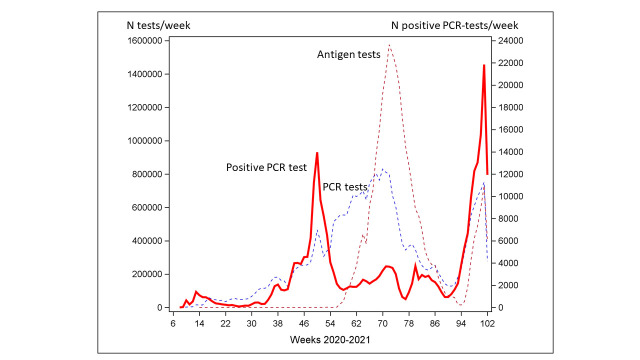
The study population included 2 451 542 employees who were traced for SARS-CoV-2 test results through 220 633 049 person-weeks of follow-up. The RT-PCR testing frequency and the proportion of employees testing positive at least once varied strongly during the study period (figure 1), as did the average testing frequency across occupational groups (data not shown). The average number of RT-PCR tests per employee was 11.9 (median 8.0, range 0–223). In the entire study population, 10.7% tested positive at least once during the follow-up period (table 1). Among the 261 203 employees with a positive RT-PCR test, only 1917 (0.01%) had a second positive test later than 90 days (to exclude the possibility of ongoing first infection) after the first positive test (71% were tested on average 9.5 months later).
Figure 1.
Weekly number of SARS-CoV-2 RT-PCR and antigen tests (left axis, dashed lines) and number of positive RT-PCR tests (right axis, solid line) among employees (n=2 451 542) in Denmark from week 8 of 2020 to week 50 of 2021. RT-PCR, real-time PCR.
The reference population comprised 461 762 employees working in 77 occupations with a balanced sex distribution (49.4% men). Various types of office clerks and administrative jobs comprised the majority of reference occupations (online supplemental table 1). With few exceptions, the occupation-specific testing frequency was lower in men than in women (the average number of tests per employee across all reference occupations during follow-up was 13.3 in women and 10.5 in men).
While the infection rate was within the same range among men and women, it was strongly related to several covariates in both sexes—lower in the high age range and in employees with chronic disease, higher in the capital region, among foreign-born employees from non-Western countries and among employees with high probability of low body mass index. Moreover, the infection rate increased with the number of household members and with the average number of RT-PCR tests performed in the occupational group of the employee (table 1).
Among the 205 non-reference four-digit DISCO-08 occupational codes with more than 100 male and 100 female employees (n=1 569 737 employees with 141 235 382 weeks of follow-up), the fully adjusted risk was increased in 63 occupations without adjustment for the average occupation-specific RT-PCR test frequency and in 49 with adjustment for test frequency. An overview of the latter is provided in figure 2 with occupations grouped according to descending size within two-digit economic sector codes (DB07). Details with respect to number of male and female employees, occupational test frequency and risk estimates before and after adjustment for covariates and test frequency are listed in online supplemental table 2, which also includes occupations with elevated fully adjusted risk before but not after adjustment for occupational test frequency.
Figure 2.
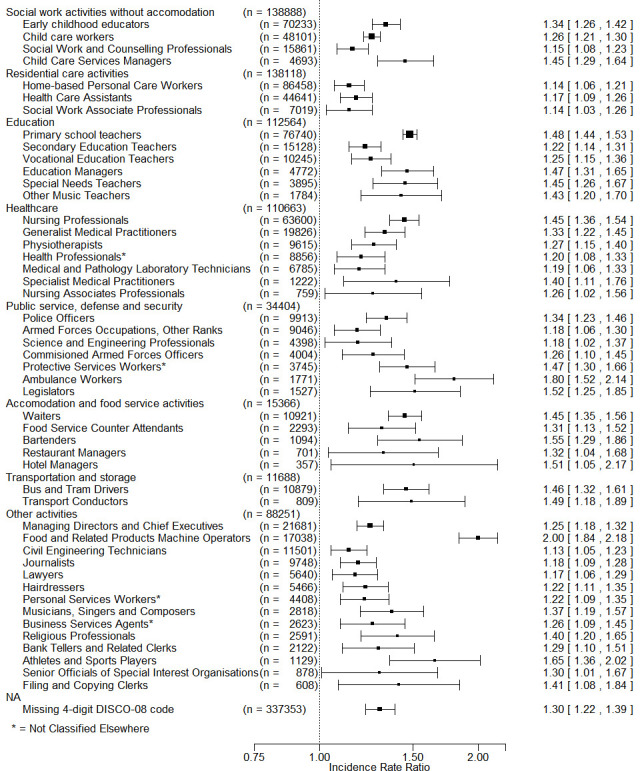
Adjusted* incidence rate ratios above 1.0 and p value of <0.01 for first positive SARS-CoV-2 real-time PCR test during the pandemic in 2020–2021 by four-digit DISCO-08 codes. †Ordered by main economic sector (two-digit DB07) and descending number of employees in occupational groups within economic sectors. *Adjusted for sex, age, education, hospital admissions for chronic disease, country of birth, geographical region, number of household members, tobacco smoking, body mass index, COVID-19 vaccination, pandemic period and occupational test frequency. †The reference is low-level exposed employees according to a COVID-19 job exposure matrix. DISCO, Danish Version of the International Standard Classification of Occupations.
Most employees at risk were working in social activities (eg, early childhood educators equivalent to child pedagogues), residential care (eg, home-based personal care workers), education (eg, primary school teachers), healthcare (eg, nursing professionals) and defence and security (eg, police officers), but several occupations within other sectors were also associated with increased risk of SARS-CoV-2 infection. These included—listed in order of descending adjusted risk estimate—food machine operators, athletes and sports players, bartenders, transport conductors, bus and tram drivers, religious professionals and hairdressers, among several others (figure 1 and online supplemental table 2). The magnitude of adjusted increased IRRs ranged from 1.13 to 2.00. According to these fully adjusted risk estimates, 32.1% of the male workforce and 49.0% of the female workforce were at increased risk of SARS-CoV-2 infection, given the selected reference group and the chosen statistical model.
Some occupations were associated with decreased risk of SARS-CoV-2 infection—38 before adjustment for test frequency, 12 after adjustment. Most of these employees were manual workers within construction (with the DISCO-08 code label civil engineering labourers), electricians and heavy truck and lorry drivers (online supplemental table 2). Most occupations with decreased risk that vanished after adjustment for test frequency were within the construction and manufacturing sectors (online supplemental table 3).
Adjustment of the sex-adjusted and age-adjusted risk estimates by all other covariates resulted in a slight average increase of the IRR by 0.019 but with large variation (SD 0.138). Further adjustment of with the occupational PCR test frequency reduced the risk estimate by an average 0.032 (SD 0.120).
Risk across pandemic waves is displayed in figure 3 (overview) and online supplemental table 4 (details) for occupations with elevated fully adjusted risk grouped by main economic sector. The relative risk in healthcare, residential care and defence/security declined across waves and approached or reached the background level in the latest wave, while the relative risk in education and social work increased during the pandemic.
Figure 3.
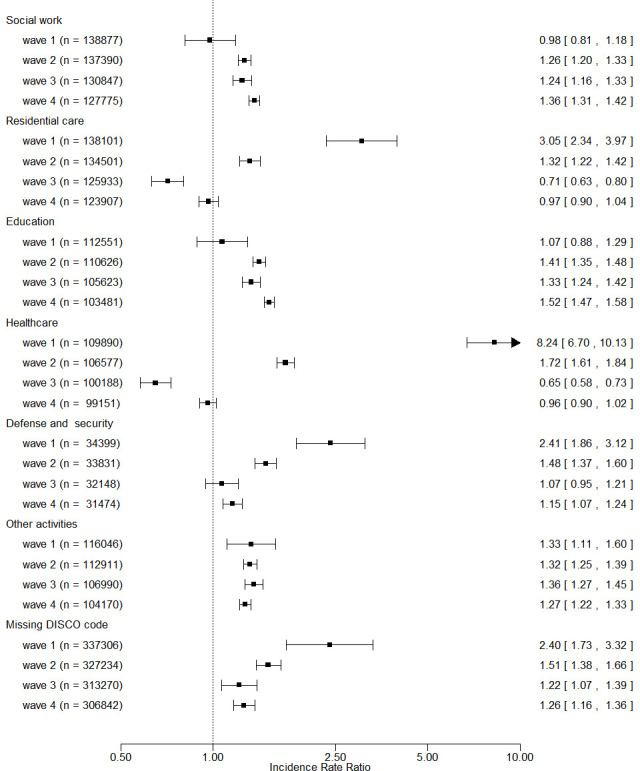
Adjusted incidence rate ratio (with 99% CI) of SARS-CoV-2 infection across pandemic waves for at-risk1 occupations grouped by economic sector. 1At-risk occupations include 49 occupations with elevated, fully adjusted risk estimate (including adjustment for occupational PCR test frequency). The reference group is low-level exposed employees according a COVID-19 job exposure matrix. DISCO, Danish Version of the International Standard Classification of Occupations.
Discussion
In this study of the entire Danish work force, the IRR of SARS-CoV-2 infection during 2020–2021 was elevated in 49 occupational groups comprising 40.3% of the workforce. All elevated adjusted IRR estimates were up to but not exceeding doubling of the relative risk. Decreased risk was observed in 12 occupations. Increased risk was mainly apparent in child and social care, health, education and services entailing many and/or close contacts, but occupations with increased risk were encountered in most of the major economic sectors. Findings are not directly comparable to results in other studies that typically use broader grouping of employees—for instance, into essential and all other workers lumped together—but obvious discrepancies are not apparent.
Strengths of the study are primarily a large cohort with national coverage through the first almost 2 years of the pandemic and, with few exceptions, complete data from independent data sources. In the following, the focus is on various limitations.
Adjustment for testing frequency across occupations did not take indications for testing into account. Higher testing frequency will identify more employees with infection and should be adjusted for if a high testing frequency is caused by request to be tested in some occupations more than in others. For instance, a negative test may have been requested repeatedly by health authorities or employers at some workplaces more than others. On the contrary, if a high testing frequency is reflecting a high rate of SARS-CoV-2 infection in a particular occupation, adjustment will erroneously attenuate risk estimates. In our study, adjustment for test frequency attenuated otherwise fully adjusted risk estimates to insignificant levels in 14 out of 63 occupations. Some of these, therefore, may be subject to overadjustment and may be truly associated with increased risk of infection. On the other hand, some of the 49 occupations that survived adjustment for occupational test frequency may falsely be associated with increased risk because of residual confounding.
For the same reasons, estimates for some occupational groups may be biased towards decreased risk of infection. While it is conceivable that working may in some instances protect the employee from background infection if work is performed with limited contact to other people as, for instance, in lorry drivers (online supplemental table 2), residual confounding relating to testing behaviour may be part of the explanation. Therefore, the apparently low infection rates in some occupations in construction and manufacturing may be caused by higher testing frequencies in asymptomatic individuals in the reference group. This would fit with the observation that adjustment for testing strongly attenuated risk estimates to non-significant levels for many occupations in the construction sector (online supplemental table 3). It is also possible, however, that the risk is in fact reduced in some of these occupations because construction workers often are working outdoors28 and may for that reason be less exposed. The JEM-based reference group does not include outdoor workers (online supplemental table 1).
Restricting analyses to persons who ever performed a test during specified periods (labelled test-negative design29 30) is not a promising option in this dataset with tremendous variation of test frequencies (figure 1). Conditioning on testing can result in collider bias and will inevitably produce spurious associations,31 which cannot be resolved unless all employees are tested repeatedly and systematically or tested completely at random.32
In spite of unresolved methodological limitations related to analyses of non-random SARS-CoV-2 test results, findings are in fair agreement with risk of COVID-19-related hospital admission estimated from analyses of the same cohort.8 Among the 16 occupations with more than 2000 employees who were found to be at risk of COVID-19-related hospital admission, 12 were also found to be at risk according to RT-PCR test results or, expressed by number of employees, 95.8% working in occupations at increased risk of hospital admission were also at increased risk of testing positive (384 648/401 651). On the other hand, 37 occupations were at increased risk according to RT-PCR testing but not according to hospital admission. This is most likely explained by the much stronger statistical power of RT-PCR test analyses compared with analysis of the relatively rare hospital admissions. These occupations include several specific jobs within healthcare, prison and security guards, police officers, waiters and bartenders, luggage porters, musicians and many others. It should be acknowledged, however, that some of these associations may be random findings due to multiple testing and uncontrolled bias because of wide differences in reasons for testing and in testing frequency.
At the bottom line, unresolved limitations inherently related to analysis of testing results call for cautious interpretation of risk for specific occupations which must be construed in the light of findings in earlier studies applying other methodological approaches2 3 10 33–36 and independent assessment of workplace risk factors, for instance, using job exposure matrices.6 37
It is well established that men are more likely to develop serious COVID-19 than women,8 38 but there are no indications that men more easily become infected with SARS-CoV-2. That substantially more women than men seem to be at risk from workplace exposure simply reflects that many at-risk occupations are dominated by women. However, the extent of occupational exposure may differ between men and women within the same occupational group. Even at the four-digit level, the DISCO-08 codes may include several different specific occupations. There are 423 four-digit codes but about 2200 specific occupational titles entailing different work tasks and potentially different risk of exposure. The sex distribution across these occupational titles within DISCO-08 groups may not be balanced.
The relative risk in healthcare workers declined substantially across the pandemic, a development also observed in other studies.5 37 This may be explained by increased access to and use of adequate personal protection gear and early vaccination. The same applies to residential care activity. That the relative risk in education and social work (including childcare) seemed to increase after the first wave may be related to easing of the initial strict lockdown. It should be kept in mind that the background level of infections in the society is adjusted for by computation of the wave-specific relative risk. Therefore, a lower relative risk at later waves in periods with a higher prevalence of infection in the background population may be associated with a higher absolute risk of infection.
Despite methodological limitations, the study corroborates most findings of occupationally increased risk of COVID-19-related hospital admissions and adds a number of occupational groups to the list of potential at-risk jobs—at least partly due to higher statistical power allowing estimation of risk in more rare occupations and among younger and more healthy employees.
Conclusion
The study corroborates some earlier findings on increased occupational risk of COVID-19 and indicates a modestly increased risk of SARS-CoV-2 infection among employees in several occupations that have not been identified in earlier studies using more rare outcomes. Methodological limitations call for cautious interpretation of risk of specific occupations which must be assessed in the light of findings in earlier studies. Nevertheless, the study indicates that risk of SARS-CoV-2 infection is far from limited to healthcare, social care and other essential occupations and that preventive action is warranted for a sizeable proportion of in particular the female workforce during possible forthcoming pandemics.
Acknowledgments
Læge Sofus Carl Emil Friis og Hustru Olga Doris Friis' Legat and Interreg Øresund-Kattegat-Skagerrak (ÄrendeID: NYPS 20303383) are thanked for generous grants that proved crucial for undertaking this project.
Footnotes
Contributors: JPEB acted as the guanrantor of the study. KKUP, SST and JPEB drafted research protocols and obtained grants. JPEB and EMF obtained permissions. JPEB performed data analyses and drafted the manuscript. EMF was the statistical adviser. All authors contributed to development of the study design and reviewed the manuscript.
Funding: This study was funded by Læge Sofus Carl Emil Friis og Hustru Olga Doris Friis' Legat; Interreg Øresund-Kattegat-Skagerrak (Ärende ID: NYPS 20303383).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Author note: Permissions to retrieve and analyse pseudonymised data through Statistics Denmark were obtained from the Knowledge Centre on Data Protection Compliance under the records of processing regarding health science research projects within the Capitol Region of Denmark (P-2020-897), Statistics Denmark (P-708121) and the National Board of Health Data (FSEID-00005368).
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data may be obtained from a third party and are not publicly available. The pseudonymised database used for the presented analyses is hosted by Statistics Denmark and is not publicly available. Permission to access the database can be granted by researchers at a research institution authorised by Statistics Denmark. On request, the corresponding author can assist interested researchers to gain access.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1. Murti M, Achonu C, Smith BT, et al. COVID-19 workplace outbreaks by industry sector and their associated household transmission, ontario, canada, january to june, 2020. J Occup Environ Med 2021;63:574–80. 10.1097/JOM.0000000000002201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. van der Plaat DA, Madan I, Coggon D, et al. Risks of COVID-19 by occupation in NHS workers in england. Occup Environ Med 2022;79:176–83. 10.1136/oemed-2021-107628 [DOI] [PubMed] [Google Scholar]
- 3. Magnusson K, Nygård K, Methi F, et al. Occupational risk of COVID-19 in the first versus second epidemic wave in norway, 2020. Euro Surveill 2021;26:2001875. 10.2807/1560-7917.ES.2021.26.40.2001875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nafilyan V, Pawelek P, Ayoubkhani D, et al. Occupation and COVID-19 mortality in england: a national linked data study of 14.3 million adults. Occup Environ Med 2022;79:433–41. 10.1136/oemed-2021-107818 [DOI] [PubMed] [Google Scholar]
- 5. Rhodes S, Wilkinson J, Pearce N, et al. Occupational differences in SARS-cov-2 infection: analysis of the UK ONS COVID-19 infection survey. J Epidemiol Community Health 2022;76:841–6. 10.1136/jech-2022-219101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Oude Hengel KM, Burdorf A, Pronk A, et al. Exposure to a SARS-cov-2 infection at work: development of an international job exposure matrix (COVID-19-JEM). Scand J Work Environ Health 2022;48:61–70. 10.5271/sjweh.3998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fadel M, Gilbert F, Legeay C, et al. Association between COVID-19 infection and work exposure assessed by the mat-O-COVID job exposure matrix in the CONSTANCES cohort. Occup Environ Med 2022;79:782–9. 10.1136/oemed-2022-108436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bonde JPE, Sell L, Flachs EM, et al. Occupational risk of COVID-19 related hospital admission in denmark 2020-2021: a follow-up study. Scand J Work Environ Health 2023;49:84–94. 10.5271/sjweh.4063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mutambudzi M, Niedwiedz C, Macdonald EB, et al. Occupation and risk of severe COVID-19: prospective cohort study of 120 075 UK biobank participants. Occup Environ Med 2020;78:307–14. 10.1136/oemed-2020-106731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chea N, Brown CJ, Eure T, et al. Risk factors for SARS-cov-2 infection among US healthcare personnel, may-december 2020. Emerg Infect Dis 2022;28:95–103. 10.3201/eid2801.211803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alderling M, Albin M, Ahlbom A, et al. Risk att sjukhusvårdas för covid-19 i olika yrken. In: Rapport. Stockholm: Centrum for arbets- och miljømedicin, 2021: 02. [Google Scholar]
- 12. Chen Y-H, Glymour M, Riley A, et al. Excess mortality associated with the COVID-19 pandemic among Californians 18-65 years of age, by occupational sector and occupation: March through November 2020. PLoS One 2021;16:e0252454. 10.1371/journal.pone.0252454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Windsor-Shellard B, Rabiya N. Coronavirus (COVID-19) related deaths by occupation, england and wales: deaths registered up to and including 20 April 2020. Statistical bulletin. London: Office for national statistics, 2020. [Google Scholar]
- 14. Hawkins D, Davis L, Kriebel D. COVID-19 deaths by occupation, massachusetts, march 1-july 31, 2020. Am J Ind Med 2021;64:238–44. 10.1002/ajim.23227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rimmer A. Covid-19: two thirds of healthcare workers who have died were from ethnic minorities. BMJ 2020;369:m1621. 10.1136/bmj.m1621 [DOI] [PubMed] [Google Scholar]
- 16. Billingsley S, Brandén M, Aradhya S, et al. COVID-19 mortality across occupations and secondary risks for elderly individuals in the household: a population register-based study. Scand J Work Environ Health 2022;48:52–60. 10.5271/sjweh.3992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gualano MR, Rossi MF, Borrelli I, et al. Returning to work and the impact of post COVID-19 condition: a systematic review. Work 2022;73:405–13. 10.3233/WOR-220103 [DOI] [PubMed] [Google Scholar]
- 18. Franco JVA, Garegnani LI, Oltra GV, et al. Long-term health symptoms and sequelae following SARS-cov-2 infection: an evidence MAP. Int J Environ Res Public Health 2022;19:9915. 10.3390/ijerph19169915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Flachs EM, Petersen SEB, Kolstad HA, et al. Cohort profile: DOC*X: a nationwide Danish occupational cohort with exposure data-an open research resource. Int J Epidemiol 2019;48:1413–1413k. 10.1093/ije/dyz110 [DOI] [PubMed] [Google Scholar]
- 20. Statistics Denmark . Dansk branchekode DB07, v3: 2014-. Copenhagen: Statistics Denmark, 2014. Available: https://www.dst.dk/da/Statistik/dokumentation/nomenklaturer/db07 [Google Scholar]
- 21. Bondo Petersen S, Flachs EM, Prescott EIB, et al. Job-exposure matrices addressing lifestyle to be applied in register-based occupational health studies. Occup Environ Med 2018;75:890–7. 10.1136/oemed-2018-104991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bonde JPE, Flachs EM, Madsen IE, et al. Acute myocardial infarction in relation to physical activities at work: a nationwide follow-up study based on job-exposure matrices. Scand J Work Environ Health 2020;46:268–77. 10.5271/sjweh.3863 [DOI] [PubMed] [Google Scholar]
- 23. Corman VM, Landt O, Kaiser M, et al. Detection of 2019 novel coronavirus (2019-ncov) by real-time RT-PCR. Euro Surveill 2020;25:2000045. 10.2807/1560-7917.ES.2020.25.3.2000045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vogels CBF, Brito AF, Wyllie AL, et al. Analytical sensitivity and efficiency comparisons of SARS-cov-2 RT-qpcr primer-probe sets. Nat Microbiol 2020;5:1299–305. 10.1038/s41564-020-0761-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Serum Instituttet (In Danish) . Falske antigen test copenhagen. 2021. Available: https://www.ssi.dk/aktuelt/nyheder/2021/antigentest-gav-47-falsk-negative-svar [Google Scholar]
- 26. Rhodes S, Beale S, Wilkinson J, et al. Exploring the relationship between job characteristics and infection: application of a COVID-19 job exposure matrix to SARS-cov-2 infection data in the United Kingdom. Scand J Work Environ Health 2022:4076. 10.5271/sjweh.4076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. VanderWeele TJ. Principles of confounder selection. Eur J Epidemiol 2019;34:211–9. 10.1007/s10654-019-00494-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vested A, Schlünssen V, Burdorf A, et al. A quantitative general population job exposure matrix for occupational daytime light exposure. Ann Work Expo Health 2019;63:666–78. 10.1093/annweh/wxz031 [DOI] [PubMed] [Google Scholar]
- 29. Vandenbroucke JP, Brickley EB, Pearce N, et al. The evolving usefulness of the test-negative design in studying risk factors for COVID-19. Epidemiology 2022;33:e7–8. 10.1097/EDE.0000000000001438 [DOI] [PubMed] [Google Scholar]
- 30. Vandenbroucke JP, Brickley EB, Vandenbroucke-Grauls CMJE, et al. A test-negative design with additional population controls can be used to rapidly study causes of the SARS-cov-2 epidemic. Epidemiology 2020;31:836–43. 10.1097/EDE.0000000000001251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Griffith GJ, Morris TT, Tudball MJ, et al. Collider bias undermines our understanding of covid-19 disease risk and severity. Nat Commun 2020;11:5749. 10.1038/s41467-020-19478-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Burstyn I, Goldstein ND, Gustafson P. It can be dangerous to take epidemic curves of COVID-19 at face value. Can J Public Health 2020;111:397–400. 10.17269/s41997-020-00367-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Eyre DW, Lumley SF, O’Donnell D, et al. Differential occupational risks to healthcare workers from SARS-cov-2 observed during a prospective observational study. Elife 2020;9:e60675. 10.7554/eLife.60675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shah VP, Breeher LE, Alleckson JM, et al. Occupational exposure to severe acute respiratory coronavirus virus 2 (SARS-cov-2) and risk of infection among healthcare personnel. Infect Control Hosp Epidemiol 2022;43:1785–9. 10.1017/ice.2021.533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dusefante A, Negro C, D’Agaro P, et al. Occupational risk factors for SARS-cov-2 infection in hospital health care workers: a prospective nested case-control study. Life (Basel) 2022;12:263. 10.3390/life12020263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Baker MG, Peckham TK, Seixas NS. Estimating the burden of United States workers exposed to infection or disease: a key factor in containing risk of COVID-19 infection. PLoS One 2020;15:e0232452. 10.1371/journal.pone.0232452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bonde JPE, Sell L, Johan Høy JH, et al. Occupational risk of COVID-19 across pandemic waves: a two-year national follow-up study of hospital admissions. Scand J Work Environ Health 2022;48:672–7. 10.5271/sjweh.4056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yoshida Y, Chu S, Fox S, et al. Sex differences in determinants of COVID-19 severe outcomes-findings from the National COVID cohort collaborative (N3C). BMC Infect Dis 2022;22:784. 10.1186/s12879-022-07776-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
oemed-2022-108713supp001.pdf (9.4MB, pdf)
Data Availability Statement
Data may be obtained from a third party and are not publicly available. The pseudonymised database used for the presented analyses is hosted by Statistics Denmark and is not publicly available. Permission to access the database can be granted by researchers at a research institution authorised by Statistics Denmark. On request, the corresponding author can assist interested researchers to gain access.