Abstract
Background
Accumulating evidence indicates that some non-absorbed food additives, including emulsifiers carboxymethylcellulose (CMC) and polysorbate 80 (P80), can negatively impact intestinal microbiota, leading to microbiota encroachment, chronic low-grade intestinal inflammation and, subsequently, promotion of metabolic dysregulations. Detrimental impacts of emulsifier consumption on gut microbiota include depletion of the health-associated mucus-fortifying bacteria, Akkermansia muciniphila.
Objective
Investigate, in mice, the potential of administration of exogenous A. muciniphila as a means to protect against detrimental impacts of emulsifiers.
Results
Daily oral administration of A. muciniphila prevented phenotypic consequences of consumption of both CMC and P80, including hyperphagia, weight gain and dysglycaemia. A. muciniphila administration also counteracted the low-grade intestinal inflammation-induced CMC and P80. Furthermore, A. muciniphila supplementation prevented the proximal impacts of CMC and P80 on gut microbiota that are thought to drive low-grade chronic inflammation and metabolic dysregulations. Specifically, A. muciniphila prevented alterations in species composition and encroachment of gut microbiota that were otherwise induced by CMC and P80. Remarkably, we finally report that CMC and P80 altered the colonic transcriptome, while A. muciniphila largely protected against these alterations.
Conclusion
Daily administration of A. muciniphila protects against the detrimental impact of emulsifiers on both the microbiota and host. These results support the notion that use of A. muciniphila as a probiotic can help maintain intestinal and metabolic health amidst the broad array of modern stresses that can promote chronic inflammatory diseases.
Keywords: MUCUS, INFLAMMATION, INTESTINAL MICROBIOLOGY, PROBIOTICS
WHAT IS ALREADY KNOWN ON THIS TOPIC.
Previous findings reported that commonly used dietary emulsifiers alter the intestinal microbiota and promote chronic intestinal inflammation and metabolic dysregulations.
Microbiota encroachment is a central step in emulsifier-induced detrimental consequences.
Akkermansia muciniphila is a next-generation beneficial probiotic able to reinforce the intestinal barrier and prevent metabolic dysregulations.
WHAT THIS STUDY ADDS
A. muciniphila supplementation prevents metabolic dysregulations that are otherwise induced by emulsifier consumption.
A. muciniphila prevent alterations in species composition and encroachment of gut microbiota that are otherwise induced by carboxymethylcellulose and polysorbate 80.
Dietary emulsifiers alter the colonic transcriptome, while A. muciniphila largely protects against these alterations.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Our findings underlie A. muciniphila as a therapeutic approach to protect against the detrimental impact of emulsifiers on both the microbiota and host.
A. muciniphila might help to maintain intestinal and metabolic health in the context of modern stresses that normally promote chronic inflammatory diseases.
Introduction
Humanity is faced with a stark increase in the constellation of metabolic disorders referred to as metabolic syndrome, the cardinal features of which include obesity and insulin resistance. Metabolic syndrome is associated with alterations in gut microbiota composition.1 Faecal microbiota transplant studies in mice and humans argue that such alterations do not merely mark, but rather promote, dysregulated metabolism.2 While mechanisms by which altered microbiota promote metabolic dysregulation are not entirely clear, the inverse correlation of microbiota-epithelial distance with extent of dysglycaemia3 suggests an important role for microbiota that encroach into the normally near-sterile inner mucus layer, perhaps reflecting that such encroaching bacteria promote low-grade inflammation, which can subsequently dysregulate metabolism.
A variety of factors can induce microbiota dysbiosis and encroachment, including consumption of a high-fat low-fibre ‘western-style’ diet4 and the class of food additives known as emulsifiers.5 Emulsifiers are incorporated into many processed foods to extend shelf life and improve organoleptic properties6 7 and they are suspected to be a significant driver of the association of consumption of ultraprocessed foods consumption with development of chronic inflammatory diseases.8 9 Some emulsifiers, for example, lecithin, are natural dietary components, while others, including carboxymethylcellulose (CMC) and polysorbate 80 (P80) are human synthetic creations. Consumption of CMC and P80 alter gut microbiota composition and induce microbiota encroachment in mice, and a recent report suggests CMC acts in a similar manner in humans.10 Such microbiota dysbiosis and encroachment associate with chronic low-grade intestinal inflammation that manifest as metabolic dysregulations in wild-type mice and potentiation of colitis in mice genetically prone to this disorder.5 Studies using in vitro microbiota models suggest that CMC and P80 may perturb host–microbiota homeostasis as a result of their direct action on microbiota.11 12
The ubiquity of emulsifiers, and other additives, in processed foods, which provide a significant portion of human food consumption, makes avoiding these additives challenging. Hence, as a possible countermeasure to emulsifiers, we turned to the bacterium Akkermansia muciniphila, which exhibits reduced abundance in metabolic syndrome and, moreover, can protect against this state when exogenously administered.13 14 A. muciniphila fortifies the mucosal barrier by stimulating mucus production, leading to a thicker mucus layer under a mucus-disruptive high-fat diet15 16 and, furthermore, by inducing production of antimicrobial peptides such as Reg3γ.17 Beneficial effects of A. muciniphila can be observed when using the intact bacteria, its pasteurised (but not autoclaved) form, or its outer membrane and secreted proteins,15 18–20 suggesting that its beneficial properties are linked to surface/secreted molecules, rather than its metabolic activity, including mucus digestion. A pilot clinical study of A. muciniphila suggested benefits in humans, including a trend towards lowered fat-mass gain and decreased hip circumference, enhanced insulin sensitivity and reductions in endotoxaemia and inflammation in overweight subjects.21 Thus, the goal of this study was to investigate the potential of A. muciniphila to prevent emulsifier disturbance of host-microbiota homeostasis as well as its impact on low-grade inflammation and metabolism. We found that daily administration of A. muciniphila protects mice from emulsifier-induced metabolic dysregulations and the low-grade intestinal inflammation thought to drive this state. Furthermore, A. muciniphila prevents emulsifier-induced shifts in microbiota composition and localisation, as well as protects against colonic transcriptome alterations. Such ability of A. muciniphila supports its use as a countermeasure to combat modern stressors that perturb host–microbiota interactions to promote metabolic syndrome and other chronic inflammatory diseases.
Results
Impact of dietary emulsifier consumption on the faecal abundance of A. muciniphila
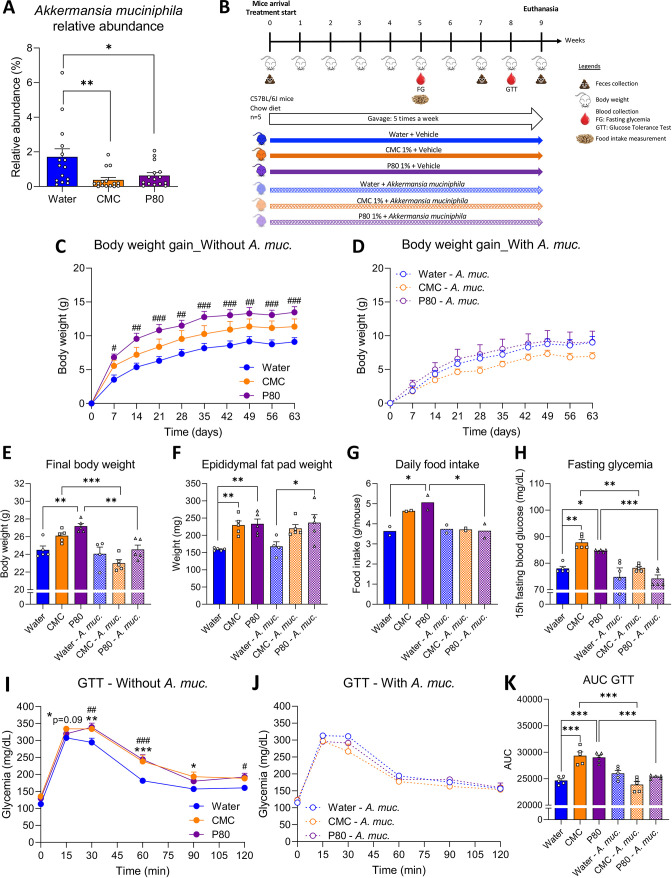
Some dietary emulsifiers, including the synthetic compounds CMC and P80, have the potential to disrupt host–microbiota interactions resulting in low-grade intestinal inflammation and dysregulated metabolism.5 11 15 Impacts of these emulsifiers on microbiota include numerous alterations in relative species abundance, as well as depletion of beneficial bacteria.5 10 In accord with this notion, our analysis here of microbiota composition of emulsifier-treated mice revealed that chronic consumption of either CMC or P80 significantly decreased relative faecal abundance of A. muciniphila (figure 1A). Considering the well-established probiotic health potential of this bacterium,16–20 including its ability to fortify mucus, we hypothesised that supplementing microbiota via exogenous administration of A. muciniphila might counteract deleterious impacts of emulsifier consumption.
Figure 1.
Akkermansia muciniphila administration prevented emulsifier-induced metabolic deregulations. (A) Relative abundance of faecal A. muciniphila in mice chronically exposed to drinking water, CMC 1.0% or P80 1.0%. (B) Schematic representation of the experimental design. Mice were exposed to drinking water (blue) containing 1.0% of CMC (orange) or p80 (purple) for 9 weeks, and gavaged 5 days per week with either vehicle (sterile PBS, solid lines and bars) or A. muciniphila (A. muc., hatched lines and bars). Body weight gain over time of mice orally receiving (C) vehicle or (D) A. muciniphila. (E) Final body weight, (F) epididymal fat pad weight, (G) daily food intake measurement and (H) 15 hours fasting blood glucose level. (I–K) At week 8, mice were 5 hours fasted, challenged with an intraperitoneal bolus of glucose (2 g/kg of body weight). Glycaemic response was measured after 15, 30, 60, 90, 120 min in mice receiving (I) vehicle or (J) A. muciniphila. (K) Areas under the curves obtained from the glucose tolerance test. Data are represented as means±SEM. n=4–5. For bar graphs, statistical analyses were performed using a one-way ANOVA followed by a Bonferroni post hoc test and significant differences were recorded as follows: *p<0.05, **p<0.01, ***p<0.001. For line charts, a two-way ANOVA or a mixed model (if missing values) was performed, followed by a Bonferroni post hoc test, and significant differences were recorded as follows: CMC vs water, *p<0.05, **p<0.01, ***p<0.001; P80 vs water, #p<0.05, ##p<0.01, ###p<0.001. Exact p values for trends (0.05≤p<0.10) are recorded on graphs for additional indication. ANOVA, analysis of variance; CMC, carboxymethylcellulose; P80, polysorbate 80.
Akkermansia muciniphila administration prevents emulsifier-induced metabolic dysregulations
C57/Bl6 mice were exposed to water, CMC (1%) or P80 (1%) for 9 weeks, while concomitantly treated with either phosphate buffered saline (PBS) - vehicle or A. muciniphila by oral gavage 5 days per week (figure 1B), using the #BAA-835 (ATCC) A. muciniphila strain, isolated by Derrien et al.13 Culture purity was confirmed, after bacteria growth, washing and aliquoting (cf. Method section for details), via 16S rRNA gene amplification and sequencing (online supplemental figure S1A). We subsequently used these verified A. muciniphila aliquots to treat mice daily with 2.5×108 CFU, an approach that mildly, but nonetheless significantly, increased A. muciniphila faecal relative abundance, as assessed by qPCR approach (online supplemental figure S1B).
gutjnl-2021-326835supp004.pdf (10.3MB, pdf)
As previously reported, P80 induced a greater body weight gain in mice compared with the control group, with a similar trend observed for CMC-treated mice (figure 1C). This body weight gain was abrogated in mice receiving A. muciniphila, with all A. muciniphila-treated group presenting similar final body weights as non-emulsifier-treated control mice (figure 1D, E). In contrast, A. muciniphila did not alter body weight in water-treated animals (figure 1D, E, p=0.80), consistent with the possibility that A. muciniphila was ameliorating a dysbiotic state rather than directly impacting host metabolism. Impact of emulsifiers on body weight were generally associated with impact on fat pad weight, as presented figure 1F. Furthermore, A. muciniphila administration completely abrogated CMC and P80’s induction of overeating and hyperglycaemia (figure 1G, H). To better evaluate the impact of A. muciniphila administration on glucose homoeostasis, an intraperitoneal glucose tolerance test (GTT) was performed after 8 weeks of emulsifier exposure. As presented in figure 1I, J both CMC- and P80-treated mice exhibited significant alteration in their glucose excursion curve (figure 1I), while no differences were observed in mice receiving A. muciniphila (figure 1J), with both CMC-treated and P80-treated groups aligning with the water-only treated group. Measure of areas under the curves further supported that emulsifier-induced glucose intolerance was fully prevented with daily administration of A. muciniphila (figure 1K). Altogether, these data demonstrate that A. muciniphila administration was sufficient to largely prevent emulsifier promotion of metabolic dysregulations.
A. muciniphila administration prevents emulsifier-induced low-grade intestinal inflammation
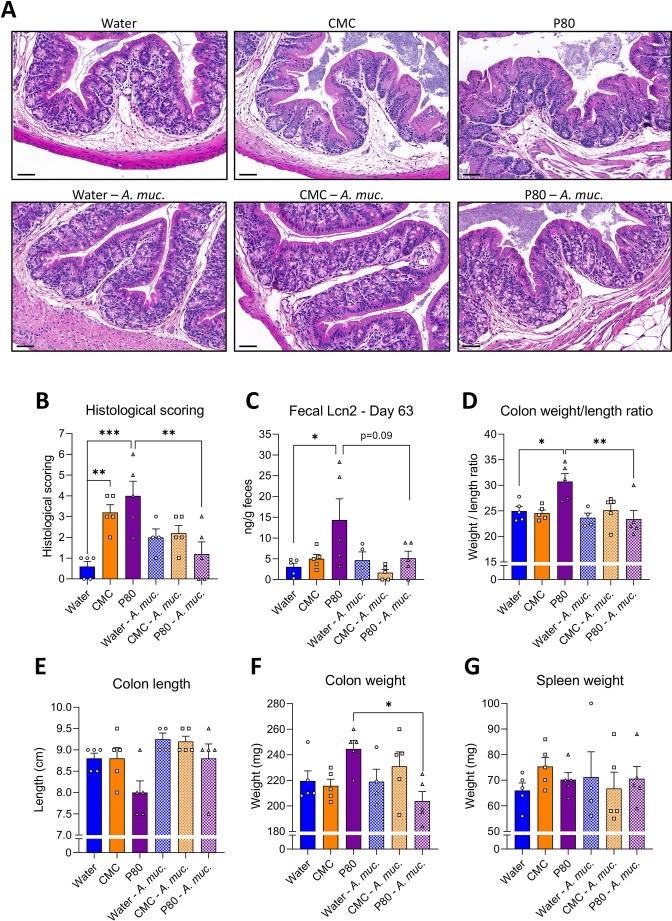
The negative impacts of emulsifiers on metabolism are thought to be driven by low-grade intestinal inflammation, which can be assayed by histopathological analysis, measure of inflammatory markers such as lipocalin-2 (Lcn2) and may manifest in gross morphological changes in colon and/or spleen. Accordingly, consumption of both CMC and P80 resulted in subtle but nonetheless histopathologically evident colon inflammation, particularly increased numbers of inflammatory cells infiltrating the mucosa and the submucosa (figure 2A, B, online supplemental table S1). Other indices of inflammation were more variable in that P80 also induced elevations in faecal Lcn2 and colon weight/length ratio, while CMC induced mild splenomegaly (figure 2C–G). A. muciniphila by itself did not significantly impact these parameters in water-treated mice. However, induction of low-grade inflammation by both CMC and P80 was abrogated by administration of A. muciniphila, suggesting this bacterium may broadly prevent negative impacts of emulsifiers.
Figure 2.
Akkermansia muciniphila administration prevents emulsifier-induced low-grade intestinal inflammation. Mice were exposed to drinking water (blue) containing 1.0% of CMC (orange) or P80 (purple) for 9 weeks, and gavaged 5 days per week with either vehicle (sterile PBS, solid bars) or A. muciniphila (A. muc., hatched bars). (A) Representative images of (B) the histopathological score of H&E-stained colonic sections; scale bar, 100 µm. (C) Faecal lipocalin-2 (Lcn2) level at day 63, (D) weight/length ratio, (E) colon length, (F) colon weight, and (G) spleen weight. Data are represented as means±SEM. n=4–5. Statistical analyses were performed using a one-way ANOVA followed by a Bonferroni post hoc test and significant differences were recorded as follows: *p<0.05, **p<0.01, ***p<0.001. Exact p values for trends (0.05≤p< 0.10) are recorded on graphs for additional indication. ANOVA, analysis of variance; CMC, carboxymethylcellulose; P80, polysorbate 80.
gutjnl-2021-326835supp001.xlsx (13.4KB, xlsx)
A. muciniphila administration prevents emulsifier-induced alterations in microbiota composition
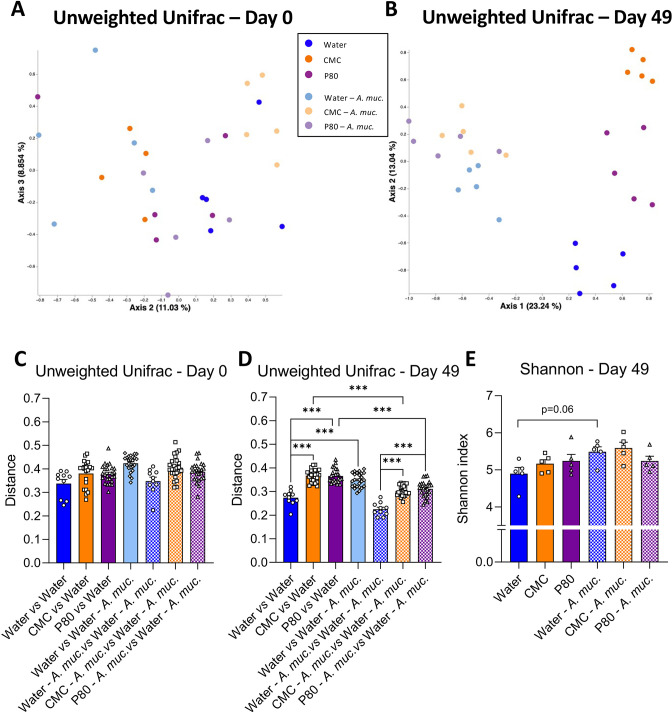
The impacts of emulsifiers on intestinal microbiota play a central role in promoting intestinal inflammation and its downstream consequences.5 11 Hence, we next examined the extent to which A. muciniphila might ameliorate emulsifier-induced changes in gut microbiota composition. Use of 16S sequencing followed by principal coordinate analysis (PCoA) of the unweighted Unifrac distances revealed that mice used here had homogeneous baseline microbiotas prior to the start of treatment (day 0, figure 3A). In contrast, such analysis showed that 7 weeks exposure to CMC or P80 resulted in clear treatment-based microbiota clustering (day 49, figure 3A, B) indicating that both CMC and P80 markedly shifted microbiota composition. This clear alteration in microbiota composition was confirmed by quantification of the unweighted Unifrac distance, exhibiting highly significant impact of CMC and P80 consumption on microbiota composition compared with water-treated mice (figure 3C, D). A. muciniphila administration, by itself, also clearly impacted microbiota composition with a clear distinct clustering (figure 3B) and a significant increase in unweighted Unifrac distance separating mice from these two groups (figure 3D), while no effect was observed on the microbiota alpha diversity (figure 3E). However, consumption of CMC and P80 amidst A. muciniphila administration had only slight impacts on microbiota composition. Specifically, all A. muciniphila-treated groups were observed to tightly cluster together, irrespective of emulsifier treatment while measure of unweighted Unifrac distance showed slight shifts that were much less than that induced by CMC and P80 in the absence of A. muciniphila, thus demonstrating that A. muciniphila almost fully prevented microbiota disturbances that were otherwise induced by consuming these emulsifiers. Finally, to ensure A. muciniphila-based clustering was not solely due to its DNA being excreted in faeces, unweighted Unifrac was computed after removing all Qiime2-generated amplicon sequence variants (ASVs) related to the Verrucomicrobia phylum (online supplemental figure S2). This approach had no impact on the above-described clustering, indicating that daily A. muciniphila had impacted the intestinal microbiota composition independently of its own phylum.
Figure 3.
Akkermansia muciniphila administration dampens emulsifier-induced alterations in microbiota composition. Mice were exposed to drinking water (blue) containing 1.0% of CMC (orange) or P80 (purple) for 9 weeks, and gavaged 5 days per week with either sterile vehicle (sterile PBS, solid bars) or A. muciniphila (A. muc., hatched bars). Bacterial DNA was extracted from faeces collected at days 0 and 49 and subjected to 16S rRNA gene sequencing. (A, B) Principal coordinates analysis (PcoA) of the unweighted Unifrac matrix of microbiota assessed by 16S rRNA gene sequencing at days (A) 0 and (B) 49. Each dot represents an individual animal and is colour coded (blue, water; orange, CMC; purple, P80, light blue, water—A. muciniphila; light orange, CMC—A. muciniphila; light purple, P80—A. muciniphila). (C, D) Unweighted Unifrac distance separating mice from different groups at (C) day 0 and (D) day 49. (E) Shannon alpha-diversity index at day 49. Data are represented as means±SEM. n=10–25 for the Unweighted Unifrac metric, and n=4–5 for the Shannon index. Statistical analyses were performed using a one-way ANOVA followed by a Bonferroni post hoc test and significant differences were recorded as follows: *p<0.05, **p<0.01, ***p<0.001. ANOVA, analysis of variance; CMC, carboxymethylcellulose; P80, polysorbate 80.
We next performed MaAsLin2 (Microbiome Multivariable Associations with Linear Models) analysis to identify features which are the more significantly impacted by emulsifier consumption (online supplemental figure S3), by comparing water-treated animals to CMC-treated or P80-treated animals.21 Among those, 2 belonged to the Allobaculum genus (online supplemental figure S3A, B), 2 to the Clostridiaceae family (online supplemental figure S3C, D), 10 to the S24-7 family (online supplemental figure S3E–N), 2 to the Rikenellaceae family (online supplemental figure S3O–P) and the rest to the Turicibacter, Prevotella, Odoribacter genera and Ruminococcaceae family (online supplemental figure S3Q–T). Interestingly, A. muciniphila administration prevented emulsifier consumption-induced alteration of most of these taxa, while only few differences were not restored to baseline (water-treatment) levels in the context of daily A. muciniphila. For example, various members of belonging to S24-7 family were significantly increased by CMC and P80 consumption. In contrast, these OTUs were not altered by CMC or P80 amidst daily administration of A. muciniphila (online supplemental figure S3E–I). Moreover, we observed disappearance of a Prevotella-related feature in emulsifier-treated mice, while A. muciniphila administration fully prevented such depletion (online supplemental figure S3R). Hence, these data demonstrate that A. muciniphila is having a marked impact on intestinal microbiota composition that makes microbiota refractory to emulsifier-induced alterations.
A. muciniphila administration prevents emulsifier-induced intestinal abnormalities and microbiota encroachment
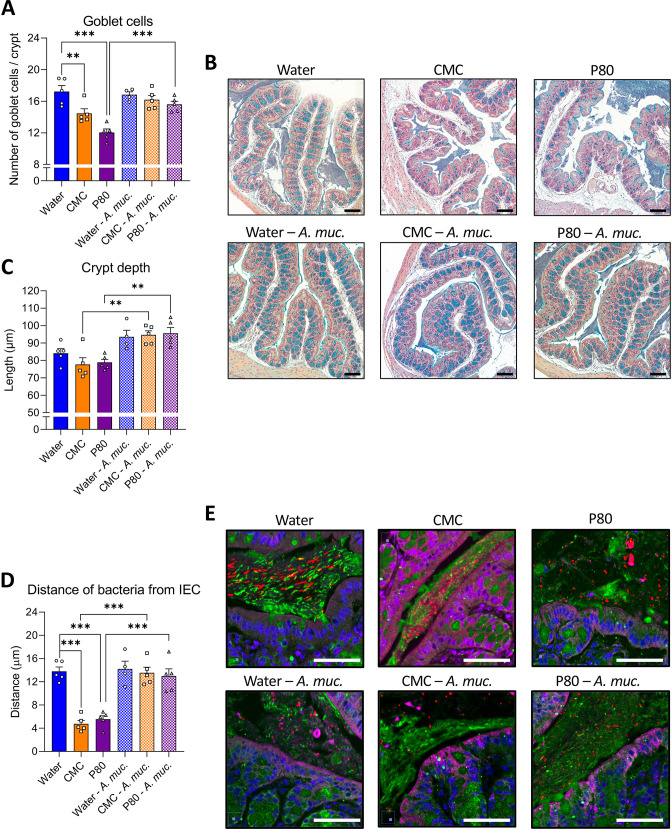
Some changes in microbiota composition, including those induced by CMC and P80, can impact levels of proinflammatory agonists such as flagellin and LPS.5 Thus, we next measured functional levels of these agonists in faeces via use of TLR5 and TLR4 reporter cells. While a trend of emulsifiers resulting in elevated flagellin (FliC) and LPS was observed, it did not reach statistical significance (online supplemental figure S4A, B). Guided by previous studies,22–24 we sought colonic morphological alterations in animals consuming CMC and P80, and observed a decreased number of goblets cells per crypt (figure 4A, B). In contrast, animals receiving daily A. muciniphila administration were fully protected against emulsifier’s impact on goblet cells. Moreover, while emulsifier consumption alone was not sufficient to impact colonic crypt anatomy, A. muciniphila-treated mice exhibited increased crypt depth (figure 4A–C), as previously described.22–24 Another consequence of emulsifier consumption is to induce microbiota to penetrate the mucus later manifesting as a decrease in the epithelium/microbiota distance.5 11 25 Such microbiota encroachment is hypothesised to play a central role in emulsifier-induced chronic low-grade intestinal inflammation and metabolic dysregulations through the activation of various innate and adaptive immune signalling. Hence, we next examined microbiota encroachment by measuring the distance separating microbiota members from the surface of the epithelium using confocal imaging of Carnoy-fixed colon specimen. This approach recapitulated reports that both CMC or P80 consumption induce microbiota encroachment, with the average bacteria/epithelium being reduced from 13.80 µm in water-treated mice to 4.75 µm in CMC-treated mice (p<0.001) and 5.55 µm in P80-treated mice (<0.001) (figure 4D, E). Such microbiota encroachment was not associated with any impact on circulating immunoreactivity towards FliC and LPS (online supplemental figure S4C, D). A. muciniphila administration by itself did not alter bacterial-epithelial distance but, remarkably, A. muciniphila administration fully prevented emulsifier-induced microbiota encroachment, with distances of 14.21 µm, 13.56 µm and 12.99 µm being observed in water-treated, CMC-treated and P80-treated groups, respectively (figure 4D, E). Thus, A. muciniphila prevents emulsifier-induced microbiota encroachment, which is a cardinal feature of gut inflammation.
Figure 4.
Akkermansia muciniphila administration prevents emulsifier-induced intestinal abnormalities and microbiota encroachment. Mice were exposed to drinking water (blue) containing 1.0% of CMC (orange) or P80 (purple) for 9 weeks, and gavaged 5 days per week with either vehicle (sterile PBS, solid bars) or A. muciniphila (A. muc., hatched bars). Colon was subjected to immunostaining paired with fluorescent in situ hybridisation (FISH) followed by confocal microscopy analysis of microbiota localisation. (A, B) Colonic sections were stained with Alcian blue, and 17–23 crypts (3–5 per colonic sections) were randomly selected per animal to determine goblet cell number per crypt (A) as well as crypt depth (B). (C) Representative pictures obtained from 5 biological replicates, Alcian blue staining. Scale bar, 100 µm. (D) Distances of closest bacteria to intestinal epithelial cells (IEC) per condition over five high-powered fields per mouse. (E) Representative pictures obtained from 5 biological replicates, microbiota and mucus staining. n=4–5. MUC2, green; actin, purple; bacteria, red; and DNA, blue. Scale bar, 50 µm. Statistical analyses were performed using a one-way ANOVA followed by a Bonferroni post-hoc test and significant differences were recorded as follows: **p<0.01, ***p<0.001. ANOVA, analysis of variance; CMC, carboxymethylcellulose; P80, polysorbate 80.
A. muciniphila administration prevented emulsifier-induced alteration of the colonic transcriptome
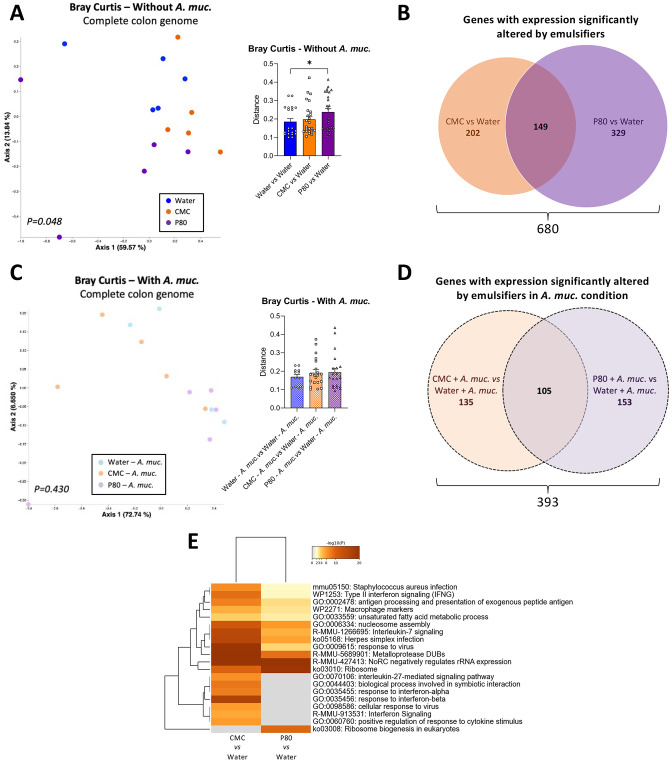
We next examined the extent to which A. muciniphila amelioration of emulsifier-induced changes in microbiota composition would impact on intestinal gene expression. We performed untargeted RNA-seq analysis to identify the impact of emulsifier consumption on colonic gene expression, as well as the potential modulatory role played by A. muciniphila supplementation. As revealed by PCoA of the Bray Curtis distance using the entire RNA-seq dataset, we observed that both CMC and P80 consumption significantly impacted the colonic transcriptome (figure 5A, Permanova p=0.048), with CMC and P80 significantly altering expression of 351 and 478 genes, respectively (figure 5B, C, Cuffdiff cut-off q-value<0.05). We also observed an effect of A. muciniphila administration on colonic gene expression of water-only treated mice, with 296 significantly altered genes (online supplemental figure S5A), concurring with previous observations.26 27 Moreover, A. muciniphila supplementation drastically reduced, but not completely abrogate, emulsifier-induced transcriptome alteration (figure 5D and online supplemental figure S5) (Permanova p=0.430) (figure 5E). Deeper analysis of differentially expressed genes (DEGs) revealed that CMC and P80 induced shared and compound-specific alterations, with 202 genes impacted by CMC, 329 genes impacted by P80, and 149 genes impacted by both emulsifiers (figure 5B, online supplemental figure 5B, C, online supplemental tables S 2 and S 3). Interestingly, based on the number of variables studied and the percentage of impacted genes, only 8 common genes, instead of 149, should have been observed if CMC and P80 were impacting colon gene expression through independent mechanism, supporting that these two compounds drive common alterations—likely related to mucosal inflammation. This was further supported by PCoA of the Bray Curtis distances focusing on these genes, which displayed strong differential clustering between water-treated and emulsifier-treated groups along PC1, and to a less extent between CMC and P80 groups along PC2 (online supplemental figure S5F, Permanova p=0.006). Moreover, A. muciniphila supplementation fully prevented this clustering, as presented online supplemental figure S5G (Permanova p=0.150), indicating that A. muciniphila administration was able to counteract both CMC-induced and P80-induced transcriptomic alterations.
Figure 5.
Akkermansia muciniphila administration prevented emulsifier-induced alteration of the colonic transcriptome. Mice were exposed to drinking water containing 1.0% of CMC or P80 for 9 weeks, and gavaged 5 days per week with either sterile PBS or A. muciniphila (A. muc.). Colon RNA was extracted and subjected to NextSeq sequencing. (A) Principal coordinates analysis (PCoA) of the Bray-Curtis distance matrix of the colonic transcriptome (all genes included) with dot coloured by treatment (water=blue; CMC=orange; P80=purple). Bray-Curtis distance separating samples from various group is also presented. (B) Venn diagram presenting the number of genes with significantly altered expression induced by CMC (orange) and/or P80 (purple). (C) PCoA of the Bray-Curtis distance matrix of the colonic transcriptome (all genes included) with dot coloured by treatment (water—A. muciniphila=light blue; CMC—A. muciniphila=light orange; P80—A. muciniphila=light purple). Bray-Curtis distance separating samples from various group is also presented. (D) Venn diagram presenting the number of genes with significantly altered expression induced by CMC (orange) and/or P80 (purple) in A. muciniphila-treated groups. (E) Heatmaps representing altered pathways/functions for CMC versus water and P80 versus water comparisons. PERMANOVA p values are indicated in the bottom of each PCoA. CMC, carboxymethylcellulose; P80, polysorbate 80.
gutjnl-2021-326835supp002.xlsx (8.6MB, xlsx)
gutjnl-2021-326835supp003.xlsx (204.1KB, xlsx)
At the functional level, the DEGs altered in response to emulsifiers comprise an array of functions including inflammatory (macrophage markers, antigen processing and presentation, interleukin-7 and interleukin-27 signalling pathways, regulation of response to cytokine stimulus) and metabolic (unsaturated fatty acid metabolic process, regulation of lipid metabolic process secretion) processes (figure 5C and online supplemental figure S6). That daily A. muciniphila administration restored basal levels of expression for these genes and processes, as presented online supplemental figure S6, S7, further supported the notion that this bacterium promotes a healthy mucosal environment in contexts that normally associate with chronic intestinal inflammation and metabolic dysregulation.
Discussion
Microbiota dysbiosis is thought to play a central role in driving intestinal inflammation and, consequently, numerous chronic inflammatory diseases.28 29 Features of microbiota dysbiosis include alterations in species composition with depletion of beneficial bacteria as well as microbiota encroachment, which is defined by increased bacterial penetrance into the normally near-sterile inner mucus layer.3 5 Such encroaching microbiota are thought to play an outsize role in driving gut inflammation.3 5 While there are likely a broad array of underlying causes for microbiota dysbiosis and encroachment, the increased incidence of chronic inflammatory diseases supports a major role for environmental (ie, non-genetic) determinants.30 For example, we and others have shown that consumption of dietary emulsifiers can induce altered microbiota composition and encroachment that most commonly results in low-grade inflammation and metabolic syndrome.5 12 15 31 32 Here, we examined a possible means of preventing such emulsifier-induced phenotypes, namely via direct administration of the mucus-fortifying bacteria A. muciniphila, which is depleted in metabolic syndrome and other chronic inflammatory diseases.33–36 We observed that endogenous A. muciniphila was depleted by emulsifiers, while administration of exogenous A. muciniphila fully prevented emulsifier impacts on microbiota and host. Specifically, the stark impacts of both CMC and P80 on microbiota composition, microbiota localisation, colon gene expression, inflammatory indices and metabolism were all absent in A. muciniphila-treated mice. Thus, A. muciniphila administration may be one means to avoid detrimental consequences of emulsifier consumption.
First isolated in 2004 by Derrien et al,13 this bacterium, present in mice and humans, has subsequently gained attention with the observation of its ability to prevent metabolic dysregulations in both preclinical and clinical studies.16–18 20 Mechanisms by which A. muciniphila benefits these disorders have not been entirely elucidated yet, but are thought to involve the ability of this mucus-loving bacterium to stimulate mucus production, potentially through its ability to digest it, but also most likely, by upregulating host defenses and mucus secretion via surface and/or secreted molecules, thus speeding mucus turnover, and potentially fortifying it.17 37 The mechanism by which A. muciniphila protects the intestine from emulsifiers remains to be defined but might involve membrane-associated Amuc_100,17 38 secreted P9,19 membrane-associated phospholipid diacyl phosphatidylethanolamine,39 and/or the ADP-heptose-like molecule, recently identified as being released by A. muciniphila with the ability to modulate the NF-kB signalling pathway.40 Moreover, accumulating evidence suggest that A. muciniphila interaction with the host involves TLR2-signalling pathways17 39 as well as modulation of IL10 and IL22 cytokines.41 42 Thus, future studies to identify the mechanism at play during protection against emulsifier-induced metabolic deregulations are warranted.43
The current study, together with previous reports that emulsifier can directly impact in vitro human microbiota,11 12 led us to hypothesise that A. muciniphila might prevent emulsifier-induced microbiota encroachment and its impacts on inflammation and metabolism without a direct impact on microbiota composition. However, A. muciniphila also surprisingly appears to prevent emulsifier-induced changes of microbiota composition. Hence, a possible explanation for our results is that the primary mechanism of action for A. muciniphila is indeed through the fortification of the mucus barrier, as suggested by its ability to reverse emulsifier-induced depletion in colonic goblet cells, and that, in vivo, altered microbiota composition is a consequence of encroachment-induced inflammation rather than the reflection of a direct emulsifier-microbiota interaction, which had been suggested by our in vitro studies. However, arguing against this possibility, we did not observe alteration in mucus gene expression in response to A. muciniphila, by itself or in presence of emulsifiers. Therefore, we propose an alternate and/or additional potential mechanism of A. muciniphila action. Specifically, we postulate that A. muciniphila might act directly on microbiota, shifting its composition to one that is resistant to emulsifier’s perturbation. Indeed, our data accords with this suggested mechanism, but further studies are needed to understand how A. muciniphila can possibly protect microbiota against emulsifiers. We envision use of ex vivo colonic explants to study the dynamic of mucus secretion and function,44 which, together with, longitudinal investigation of microbiota composition evolution during A. muciniphila supplementation, will elucidate impact of this probiotic on the mucus–microbiota relationship. Furthermore, it remains important to investigate the specificity of A. muciniphila-mediated protection by analysing the impacts of other commensal bacteria. This includes other microbiota members that are detrimentally impacted by emulsifier exposure, such as Bifidobacterium, Prevotella and Faecalibacterium,5 10 as well as bacterium with the ability to modulate mucus layer homoeostasis, such as Bacteroides thetaiotaomicron.45 Collectively, we anticipate these studies will yield mechanistic understanding of how A. muciniphila protects against dietary emulsifier consumption.
While this study primarily focused on A. muciniphila, in the course of studying its action, we also performed the first non-targeted study of emulsifier-induced microbiota encroachment impacts on colon transcriptome via RNA-seq analysis. This approach revealed profound host response induced by both emulsifiers, and while 22% of the deregulated genes were common between CMC-treated and P80-treated mice, 78% were specific to only one compound, supporting our previous observations that these two emulsifiers act through both common and specific mechanisms on the host–microbiota interface. Further in-dept characterisation of emulsifier-induced intestinal inflammation await investigation. For example, use of flow cytometry for immune cell phenotyping and/or single cell RNA-sequencing approaches to investigate the impact of emulsifier consumption on the transcriptome at the cell level should lead to a better understanding of the host response to emulsifier consumption, as well as the impact of A. muciniphila administration in this context.
While emulsifier-induced metabolic dysregulation serves as a tractable model to potential means of remediating dysbiosis, the protection afforded by A. muciniphila in this model may prove broadly applicable to other triggers of inflammation. Indeed, while CMC and P80 promote metabolic dysregulations in WT mice, they increase incidence and severity of colitis and cancer in mice genetically predisposed to these disorders.5 15 25 Regardless of what mechanism is ultimately ascribed to A. muciniphila’s protection against emulsifier-induced metabolic dysregulations, we predict it would likely extend to these other disorders. Moreover, we report here that similar protection was conferred by A. muciniphila on CMC or P80 exposure, both of which are non-metabolisable46 47 and act on the intestinal microbiota via different mechanisms,11 suggesting that the protection observed is not compound-specific. It nonetheless remains necessary to investigate the ability of A. muciniphila supplementation to protect against other additives, such as carrageenan emulsifier found to have a stark detrimental impact on microbiota composition and proinflammatory potential.12 Similarly, we would anticipate that either a more stable microbiota and/or a more rapidly renewing mucus layer might offer protection against a variety of modern stressors that might otherwise induce microbiota dysbiosis and, consequently, inflammation. This view accords with findings by Cani's laboratory and collaborators, founding that live or pasteurised A. muciniphila ameliorated metabolic parameters not only in mice but also in a small proof-of concept clinical trial in which the underlying metabolic syndrome of the study subjects can be presumed to have resulted from a variety of multifactorial underlying causes.16–18 20 That said, given the increasing recognised associations of consumption of ultraprocessed foods, which often contain multiple emulsifiers, we posit that consumers of such foods would be particularly likely to benefit from A. muciniphila supplementation. While designing a practical strategy to deliver such protection will require better understanding of underlying mechanism, it may ultimately prove to be a means of mitigating some of the negative aspects of these foods.
Material and methods
Materials
Sodium CMC (average MW ~250 000) and P80 were purchased from Sigma (Sigma, St. Louis, MO). A. muciniphila previously isolated by Derrien et al 13 was purchased from ATCC (Reference #BAA-835). Following ATCC recommendation, A. muciniphila was grown in Brain Heart Infusion broth for 72 hours at 37°C under strict anaerobic conditions. Bacteria were then pelleted by centrifugation 15 min at 4500 g, washed twice in sterile PBS, and aliquoted at 6.32×108 bacteria per mL (determined by serial dilution and platting on Brain Heart Infusion agar plates) before storage at −80°C. The purity of the obtained aliquot was determined by bacterial DNA extraction and 16S rRNA gene sequencing, revealing the absence of environmental contamination in the A. muciniphila suspension (online supplemental figure S1A).
Mice
The 5–6 week-old wild-type C57BL/6 male mice were purchased from The Jackson Laboratory (Reference # 000664). Mice were randomly grouped housed (n=5 per cage) at Georgia State University under institutionally approved protocol (Institutional Animal Care and Use Committee No A18006) and kept on LabDiet rodent chow diet #5001 ad libitum and reverse-osmosis treated Atlanta city water ad libitum. Mice were exposed to water (control group, N=10), CMC (N=10) or P80 (N=10) diluted in the drinking water (1.0% w/v and v/v, respectively) for 9 weeks, with solutions changed every week. For each group, half of the mice (n=5) were treated 5 days per week with 400 µL of sterile PBS (vehicle) and half of the mice (N=5) were treated 5 days per week with 400 µL of PBS containing 2.528×108 colony-forming units of A. muciniphila. Body weights were measured every week. Food intake was measured twice during the same week by placing groups of mice in a clean cage with a known amount of food, for 24 hours, at which point the remaining food was weighted. Food consumption was expressed as gram per mouse per 24 hours. Fresh faeces were collected at days 0, 49 and 63 for downstream analysis. After 9 weeks of treatment (day 63), mice were euthanised, and one side of the epididymal fat pad weight, spleen weight, colon weight and colon length were measured. Tissues were collected for downstream analysis, as detailed below. The detailed experimental design is represented in figure 1B.
Fasting blood glucose measurement
After 5 weeks of treatment, mice were placed in a clean cage and fasted for 15 hours. Blood glucose concentration was then determined using a Nova Max Plus Glucose Metre and expressed in mg/dL.
Oral GTT
After 8 weeks of treatment, mice were 15 hours fasted and underwent a GTT. A bolus of glucose (2 g/kg of body weight) was intraperitoneally administered to the animals and glycaemia was recorded before the glucose challenge and after 15, 30, 60, 90, 120 min using a Nova Max plus Glucose metre.
Quantification of faecal lipocalin-2 (Lcn-2) by ELISA
For quantification of faecal Lcn-2 by ELISA, frozen faecal samples were reconstituted in PBS containing 0.1% Tween 20 to a final concentration of 100 mg/mL and vortexed for 20 min to get a homogeneous faecal suspension.48 These samples were then centrifuged for 10 min at 14 000 g and 4°C. Clear supernatants were collected and stored at −20°C until analysis. Lcn-2 levels were estimated in the supernatants using Duoset murine Lcn-2 ELISA kit (R&D Systems, Minneapolis, Minnesota, USA) using the colorimetric peroxidase substrate tetramethylbenzidine, and optical density was read at 450 nm (Versamax microplate reader).
Microbiota analysis by 16S rRNA gene sequencing using illumina technology
Microbiota analyses were performed before (day 0) and after (day 49) dietary emulsifier exposure. A. muciniphila relative abundance presented figure 1A were measured in a previous protocol, following 16 weeks of dietary emulsifier exposure. 16S rRNA gene amplification and sequencing were done using the Illumina MiSeq technology following the protocol of Earth Microbiome Project with their modifications to the MOBIO PowerSoil DNA Isolation Kit procedure for extracting DNA (https://press.igsb.anl.gov/earthmicrobiome).49 50 Bulk DNA was extracted from frozen faeces using a PowerSoil-htp kit from MoBio Laboratories (Carlsbad, California, USA) with mechanical disruption (bead-beating). The 16S rRNA genes, region V4, were PCR amplified from each sample using a composite forward primer and a reverse primer containing a unique 12-base barcode, designed using the Golay error-correcting scheme, which was used to tag PCR products from respective samples50). We used the forward primer 515F 5’- AATGATACGGCGACCACCGAGATCTACACGCTXXXXXXXXXXXXTATGGTAATTGT GTGYCAGCMGCCGCGGTAA-3’: the italicised sequence is the 5’ Illumina adaptor, the 12 X sequence is the golay barcode, the bold sequence is the primer pad, the italicised and bold sequence is the primer linker, and the underlined sequence is the conserved bacterial primer 515F. The reverse primer 806R used was 5’-CAAGCAGAAGACGGCATACGAGATAGTCAGCCAGCC GGACTACNVGGGTWTCTAAT-3’: the italicised sequence is the 3’ reverse complement sequence of Illumina adaptor, the bold sequence is the primer pad, the italicised and bold sequence is the primer linker and the underlined sequence is the conserved bacterial primer 806R. PCR reactions consisted of Hot Master PCR mix (Quantabio, Beverly, Massachusetts, USA), 0.2 mM of each primer, 10–100 ng template and reaction conditions were 3 min at 95°C, followed by 30 cycles of 45 s at 95°C, 60 s at 50°C and 90 s at 72°C on a Biorad thermocycler. PCRs products were purified with Ampure magnetic purification beads (Agencourt, Brea, California, USA), and visualised by gel electrophoresis. Products were then quantified (BIOTEK Fluorescence Spectrophotometer) using Quant-iT PicoGreen dsDNA assay. A master DNA pool was generated from the purified products in equimolar ratios. The pooled products were quantified using Quant-iT PicoGreen dsDNA assay and then sequenced using an Illumina MiSeq sequencer (paired-end reads, 2×250 bp) at Cornell University, Ithaca.
16S rRNA gene sequence analysis
16S rRNA sequences were analysed using QIIME2—version 2019.51 Sequences were demultiplexed and quality filtered using the Dada2 method52 with QIIME2 default parameters in order to detect and correct Illumina amplicon sequence data, and a table of Qiime 2 artefact was generated. A tree was next generated, using the align-to-tree- mafft-fasttree command, for phylogenetic diversity analyses, and alpha and beta diversity analyses were computed using the core-metrics-phylogenetic command. PCoA plots were used to assess the variation between the experimental group (beta diversity). For taxonomy analysis, features were assigned to operational taxonomic units (OTUs) with a 99% threshold of pairwise identity to the Greengenes reference database 13_8.53 Unprocessed sequencing data are deposited in the Genome Sequence Archive (GSA) in BIG Data Centre, Beijing Institute of Genomics, Chinese Academy of Sciences, under accession number XXXXX, publicly accessible at http://bigd.big.ac.cn/gsa.
Quantitative PCR analysis
Bacterial DNA was extracted from serially diluted A. muciniphila stock using the QIAamp Fast DNA Stool Mini kit, following manufacturer instruction (Qiagen). Quantitative PCR was subsequently performed on a LigthCycler 480 instrument (Roche Molecular Systems) on DNA from serially diluted A. muciniphila stock, as well as on DNA extracted from faecal samples collected at day 28. The QuantiFast SYBR Green PCR kit (Qiagen) was used with the following A. muciniphila-specific primers: forward A. muciniphila, CAGCACGTGAAGGTGGGGAC, reverse A. muciniphila, CCTTGCGGTTGGCTTCAGAT, as previously reported.16 Results are expressed in A. muciniphila /mg faeces based on a standard curve obtained from the serially diluted A. muciniphila stock.
Staining of colonic tissue and histopathologic analysis
Mouse proximal colons were placed in methanol-Carnoy’s fixative solution (60% methanol, 30% chloroform, 10% glacial acetic acid) for a minimum of 3 hours at room temperature and stored at 4°C. Tissues were then washed in methanol 2×30 min, absolute ethanol 2×15 min, ethanol/xylene (1:1) 15 min and xylene 2×15 min, followed by embedding in Paraffin with a vertical orientation. Tissues were then sectioned at 4 µm thickness. For histological score, slides were stained with H&E using standard protocols. Images were acquired using a Lamina Slide Scanner (Perkin Elmer) at the Hist’IM platform (INSERM U1016, Paris, France). Histological scoring (ranging from 0 to 36) was blindly determined on each colon as previously described.48 54 Briefly, each colon was assigned four scores based on the degree of epithelial damage and inflammatory infiltrate in the mucosa, submucosa and muscularis/serosa.54 Each of the four scores was multiplied by a coefficient 1 if the change was focal, 2 if it was patchy and 3 if it was diffuse48 and the 4 individual scores per colon were added.
Colonic sections (4 µm) were also stained with Alcian Blue, preferentially staining mucopolysaccharides, and 17–23 crypts (3–5 per colonic sections) were randomly selected per animal to determine goblet cell number per crypt as well as crypt depth.
Statistical analysis
Data are expressed as means±SEM and statistical analyses were performed using GraphPad Prism software (V.8). Significance was determined using a one-way analysis of variance (ANOVA), followed by a Bonferroni post hoc test for bar graphs and noted as follows: *p<0.05, **p<0.01, ***p<0.001. For data collected at different timepoints in line chart form, a two-way repeated measures ANOVA or a mixed-effects model (if missing values) with a Bonferroni post hoc test was performed and significance was noted as follows: CMC vs water, *p<0.05, **p<0.01, ***p<0.001; P80 vs water, #p<0.05, ##p<0.01, ###p<0.001. Results were considered significant at p<0.05. For statistical analysis of microbiota data, the 20 most significantly differentially abundant features were identified using Microbiome Multivariable Associations with Linear Models (MaAsLin 2).21 Threshold for Volcano plots was set at q<0.05.
Please see online supplemental material for Supplementary Methods.
gutjnl-2021-326835supp005.pdf (165.5KB, pdf)
Acknowledgments
The authors thank the Hist’IM and the Genom’IC platforms (INSERM U1016, Paris, France) for their help. We thank Dr. Emilie Viennois and Dr. Omry Koren for critical review of the manuscript and/or helpful discussions.
Twitter: @NoemieDaniel2, @BenoitChassaing
Contributors: ND, ATG and BC contributed to the conception and design of the study. ND and BC performed and analysed the experiments. ND and BC performed the statistical analysis. All authors contributed to the article and approved the submitted version. BC is the guarantor of this study.
Funding: This work was supported by a Starting Grant from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (grant agreement No. ERC-2018-StG- 804135), a Chaire d’Excellence from IdEx Université de Paris - ANR-18-IDEX-0001, an Innovator Award from the Kenneth Rainin Foundation, an award from the Fondation de l'avenir (AP-RM-21-032), ANR grants EMULBIONT (ANR-21-CE15-0042-01) and DREAM (ANR-20-PAMR-0002) and the national program 'Microbiote' from INSERM. No funders had any role in the design of the study and data collection, analysis and interpretation, nor in manuscript writing.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY. Published by BMJ.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
The key words are the following: Microbiota, Mucus, Emulsifier, Metabolism, Inflammation, Probiotic, Akkermansia muciniphila, Intestinal transcriptome
Data availability statement
Data are available upon reasonable request. Unprocessed sequencing data are deposited in the European Nucleotide Archive under accession number PRJEB57855.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1. Koh A, Bäckhed F. From association to causality: the role of the gut microbiota and its functional products on host metabolism. Mol Cell 2020;78:584–96. 10.1016/j.molcel.2020.03.005 [DOI] [PubMed] [Google Scholar]
- 2. Turnbaugh PJ, Ley RE, Mahowald MA, et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006;444:1027–31. 10.1038/nature05414 [DOI] [PubMed] [Google Scholar]
- 3. Chassaing B, Raja SM, Lewis JD, et al. Colonic microbiota Encroachment correlates with dysglycemia in humans. Cell Mol Gastroenterol Hepatol 2017;4:205–21. 10.1016/j.jcmgh.2017.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zou J, Chassaing B, Singh V, et al. Fiber-mediated Nourishment of gut microbiota protects against diet-induced obesity by restoring IL-22-mediated colonic health. Cell Host Microbe 2018;23:41–53. 10.1016/j.chom.2017.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chassaing B, Koren O, Goodrich JK, et al. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature 2015;519:92–6. 10.1038/nature14232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Halmos EP, Mack A, Gibson PR. Review article: emulsifiers in the food supply and implications for gastrointestinal disease. Aliment Pharmacol Ther 2019;49:41–50. 10.1111/apt.15045 [DOI] [PubMed] [Google Scholar]
- 7. Chazelas E, Deschasaux M, Srour B, et al. Food additives: distribution and co-occurrence in 126,000 food products of the French market. Sci Rep 2020;10:3980. 10.1038/s41598-020-60948-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Laster J, Bonnes SL, Rocha J. Increased use of emulsifiers in processed foods and the links to obesity. Curr Gastroenterol Rep 2019;21:61. 10.1007/s11894-019-0723-4 [DOI] [PubMed] [Google Scholar]
- 9. Narula N, Wong ECL, Dehghan M, et al. Association of ultra-processed food intake with risk of inflammatory bowel disease: prospective cohort study. BMJ 2021;374:n1554. 10.1136/bmj.n1554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chassaing B, Compher C, Bonhomme B, et al. Randomized controlled-feeding study of dietary emulsifier carboxymethylcellulose reveals detrimental impacts on the gut microbiota and metabolome. Gastroenterology 2022;162:03728–8. 10.1053/j.gastro.2021.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chassaing B, Van de Wiele T, De Bodt J, et al. Dietary emulsifiers directly alter human microbiota composition and gene expression ex vivo potentiating intestinal inflammation. Gut 2017;66:1414–27. 10.1136/gutjnl-2016-313099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Naimi S, Viennois E, Gewirtz AT, et al. Direct impact of commonly used dietary emulsifiers on human gut microbiota. Microbiome 2021;9:66. 10.1186/s40168-020-00996-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Derrien M, Vaughan EE, Plugge CM, et al. Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. Int J Syst Evol Microbiol 2004;54:1469–76. 10.1099/ijs.0.02873-0 [DOI] [PubMed] [Google Scholar]
- 14. Ottman N, Davids M, Suarez-Diez M, et al. Genome-scale model and omics analysis of metabolic capacities of akkermansia muciniphila reveal a preferential mucin-degrading lifestyle. Appl Environ Microbiol 2017;83:e01014–7. 10.1128/AEM.01014-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jin G, Tang Q, Ma J, et al. Maternal emulsifier p80 intake induces gut dysbiosis in offspring and increases their susceptibility to colitis in adulthood. mSystems 2021;6:e01337–20. 10.1128/mSystems.01337-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Everard A, Belzer C, Geurts L, et al. Cross-talk between akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A 2013;110:9066–71. 10.1073/pnas.1219451110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Plovier H, Everard A, Druart C, et al. A purified membrane protein from akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat Med 2017;23:107–13. 10.1038/nm.4236 [DOI] [PubMed] [Google Scholar]
- 18. Depommier C, Van Hul M, Everard A, et al. Pasteurized akkermansia muciniphila increases whole-body energy expenditure and fecal energy excretion in diet-induced obese mice. Gut Microbes 2020;11:1231–45. 10.1080/19490976.2020.1737307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yoon HS, Cho CH, Yun MS, et al. Akkermansia muciniphila secretes a glucagon-like peptide-1-inducing protein that improves glucose homeostasis and ameliorates metabolic disease in mice. Nat Microbiol 2021;6:563–73. 10.1038/s41564-021-00880-5 [DOI] [PubMed] [Google Scholar]
- 20. Depommier C, Everard A, Druart C, et al. Supplementation with akkermansia muciniphila in overweight and obese human volunteers: a proof-of-concept exploratory study. Nat Med 2019;25:1096–103. 10.1038/s41591-019-0495-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mallick H, Rahnavard A, McIver LJ, et al. Multivariable association discovery in population-scale meta-omics studies. PLoS Comput Biol 2021;17:e1009442. 10.1371/journal.pcbi.1009442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ashrafian F, Shahriary A, Behrouzi A, et al. Akkermansia muciniphila-derived extracellular vesicles as a mucosal delivery vector for amelioration of obesity in mice. Front Microbiol 2019;10:2155. 10.3389/fmicb.2019.02155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ashrafian F, Keshavarz Azizi Raftar S, Lari A, et al. Extracellular vesicles and pasteurized cells derived from Akkermansia muciniphila protect against high-fat induced obesity in mice. Microb Cell Fact 2021;20:219. 10.1186/s12934-021-01709-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Choi Y, Bose S, Seo J, et al. Effects of live and pasteurized forms of akkermansia from the human gut on obesity and metabolic dysregulation. Microorganisms 2021;9:9. 10.3390/microorganisms9102039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Viennois E, Bretin A, Dubé PE, et al. Dietary emulsifiers directly impact adherent-invasive E. coli gene expression to drive chronic intestinal inflammation. Cell Rep 2020;33:108229. 10.1016/j.celrep.2020.108229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. van der Lugt B, van Beek AA, Aalvink S, et al. Akkermansia muciniphila ameliorates the age-related decline in colonic mucus thickness and attenuates immune activation in accelerated aging Ercc1−/Δ7 mice. Immun Ageing 2019;16:6. 10.1186/s12979-019-0145-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kostopoulos I, Aalvink S, Kovatcheva-Datchary P, et al. A Continuous battle for host-derived glycans between a mucus specialist and a glycan generalist in vitro and in vivo. Front Microbiol 2021;12:632454. 10.3389/fmicb.2021.632454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Caruso R, Lo BC, Núñez G. Host-microbiota interactions in inflammatory bowel disease. Nat Rev Immunol 2020;20:411–26. 10.1038/s41577-019-0268-7 [DOI] [PubMed] [Google Scholar]
- 29. Viennois E, Gewirtz AT, Chassaing B. Chronic inflammatory diseases: are we ready for microbiota-based dietary intervention? Cell Mol Gastroenterol Hepatol 2019;8:61–71. 10.1016/j.jcmgh.2019.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rothschild D, Weissbrod O, Barkan E, et al. Environment dominates over host genetics in shaping human gut microbiota. Nature 2018;555:210–5. 10.1038/nature25973 [DOI] [PubMed] [Google Scholar]
- 31. Viennois E, Merlin D, Gewirtz AT, et al. Dietary emulsifier-induced low-grade inflammation promotes colon carcinogenesis. Cancer Res 2017;77:27–40. 10.1158/0008-5472.CAN-16-1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Miclotte L, De Paepe K, Rymenans L, et al. Dietary emulsifiers alter composition and activity of the human gut microbiota in vitro, irrespective of chemical or natural emulsifier origin. Front Microbiol 2020;11:577474. 10.3389/fmicb.2020.577474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dao MC, Everard A, Aron-Wisnewsky J, et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut 2016;65:426–36. 10.1136/gutjnl-2014-308778 [DOI] [PubMed] [Google Scholar]
- 34. Dao MC, Belda E, Prifti E, et al. Akkermansia muciniphila abundance is lower in severe obesity, but its increased level after bariatric surgery is not associated with metabolic health improvement. Am J Physiol Endocrinol Metab 2019;317:E446–59. 10.1152/ajpendo.00140.2019 [DOI] [PubMed] [Google Scholar]
- 35. Zhang J, Ni Y, Qian L, et al. Decreased abundance of akkermansia muciniphila leads to the impairment of insulin secretion and glucose homeostasis in lean type 2 diabetes. Adv Sci 2021;8:2100536. 10.1002/advs.202100536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang T, Ji X, Lu G, et al. The potential of akkermansia muciniphila in inflammatory bowel disease. Appl Microbiol Biotechnol 2021;105:5785–94. 10.1007/s00253-021-11453-1 [DOI] [PubMed] [Google Scholar]
- 37. Derrien M, Belzer C, de Vos WM. Akkermansia muciniphila and its role in regulating host functions. Microb Pathog 2017;106:171–81. 10.1016/j.micpath.2016.02.005 [DOI] [PubMed] [Google Scholar]
- 38. Wang L, Tang L, Feng Y, et al. A purified membrane protein from akkermansia muciniphila or the pasteurised bacterium blunts colitis associated tumourigenesis by modulation of CD8+ T cells in mice. Gut 2020;69:1988–97. 10.1136/gutjnl-2019-320105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bae M, Cassilly CD, Liu X, et al. Akkermansia muciniphila phospholipid induces homeostatic immune responses. Nature 2022;608:168–73. 10.1038/s41586-022-04985-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Martin-Gallausiaux C, Garcia-Weber D, Lashermes A, et al. Akkermansia muciniphila upregulates genes involved in maintaining the intestinal barrier function via ADP-heptose-dependent activation of the ALPK1/TIFA pathway. Gut Microbes 2022;14:2110639. 10.1080/19490976.2022.2110639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gu Z, Pei W, Shen Y, et al. Akkermansia muciniphila and its outer protein amuc_1100 regulates tryptophan metabolism in colitis. Food Funct 2021;12:10184–95. 10.1039/D1FO02172A [DOI] [PubMed] [Google Scholar]
- 42. Bachmann R, Van Hul M, Baldin P, et al. Akkermansia muciniphila reduces peritonitis and Improves intestinal tissue wound healing after a colonic transmural defect by a myD88-dependent mechanism. Cells 2022;11:2666. 10.3390/cells11172666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cani PD, Depommier C, Derrien M, et al. Akkermansia muciniphila: paradigm for next-generation beneficial microorganisms. Nat Rev Gastroenterol Hepatol 2022;19:625–37. 10.1038/s41575-022-00631-9 [DOI] [PubMed] [Google Scholar]
- 44. Gustafsson JK, Ermund A, Johansson MEV, et al. An ex vivo method for studying mucus formation, properties, and thickness in human colonic biopsies and mouse small and large intestinal explants. Am J Physiol Gastrointest Liver Physiol 2012;302:G430–8. 10.1152/ajpgi.00405.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Luis AS, Jin C, Pereira GV, et al. A single sulfatase is required to access colonic mucin by a gut bacterium. Nature 2021;598:332–7. 10.1038/s41586-021-03967-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP), Bampidis V, Azimonti G, et al. Safety and efficacy of sodium carboxymethyl cellulose for all animal species. Efsa J 2020;18:e06211. 10.2903/j.efsa.2020.6211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Levy G, Perälä A. Effect of polysorbate 80 and oleic acid on drug absorption from the rat intestine. J Pharm Sci 1970;59:874–5. 10.1002/jps.2600590640 [DOI] [PubMed] [Google Scholar]
- 48. Chassaing B, Srinivasan G, Delgado MA, et al. Fecal lipocalin 2, a sensitive and broadly dynamic non-invasive biomarker for intestinal inflammation. PLoS One 2012;7:e44328. 10.1371/journal.pone.0044328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gilbert JA, Meyer F, Jansson J, et al. The earth microbiome project: meeting report of the "1 EMP meeting on sample selection and acquisition" at argonne national laboratory october 6 2010. Stand Genomic Sci 2010;3:249–53. 10.4056/aigs.1443528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Caporaso JG, Lauber CL, Walters WA, et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. Isme J 2012;6:1621–4. 10.1038/ismej.2012.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bolyen E, Rideout JR, Dillon MR, et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol 2019;37:852–7. 10.1038/s41587-019-0209-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Callahan BJ, McMurdie PJ, Rosen MJ, et al. DADA2: high-resolution sample inference from illumina amplicon data. Nat Methods 2016;13:581–3. 10.1038/nmeth.3869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. McDonald D, Price MN, Goodrich J, et al. An improved greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. Isme J 2012;6:610–8. 10.1038/ismej.2011.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Katakura K, Lee J, Rachmilewitz D, et al. Toll-like receptor 9-induced type I IFN protects mice from experimental colitis. J Clin Invest 2005;115:695–702. 10.1172/JCI22996 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
gutjnl-2021-326835supp004.pdf (10.3MB, pdf)
gutjnl-2021-326835supp001.xlsx (13.4KB, xlsx)
gutjnl-2021-326835supp002.xlsx (8.6MB, xlsx)
gutjnl-2021-326835supp003.xlsx (204.1KB, xlsx)
gutjnl-2021-326835supp005.pdf (165.5KB, pdf)
Data Availability Statement
Data are available upon reasonable request. Unprocessed sequencing data are deposited in the European Nucleotide Archive under accession number PRJEB57855.