Abstract
Objectives
Clinical studies revealed that early-life adverse events contribute to the development of IBS in adulthood. The aim of our study was to investigate the relationship between prenatal stress (PS), gut microbiota and visceral hypersensitivity with a focus on bacterial lipopeptides containing γ-aminobutyric acid (GABA).
Design
We developed a model of PS in mice and evaluated, in adult offspring, visceral hypersensitivity to colorectal distension (CRD), colon inflammation, barrier function and gut microbiota taxonomy. We quantified the production of lipopeptides containing GABA by mass spectrometry in a specific strain of bacteria decreased in PS, in PS mouse colons, and in faeces of patients with IBS and healthy volunteers (HVs). Finally, we assessed their effect on PS-induced visceral hypersensitivity.
Results
Prenatally stressed mice of both sexes presented visceral hypersensitivity, no overt colon inflammation or barrier dysfunction but a gut microbiota dysbiosis. The dysbiosis was distinguished by a decreased abundance of Ligilactobacillus murinus, in both sexes, inversely correlated with visceral hypersensitivity to CRD in mice. An isolate from this bacterial species produced several lipopeptides containing GABA including C14AsnGABA. Interestingly, intracolonic treatment with C14AsnGABA decreased the visceral sensitivity of PS mice to CRD. The concentration of C16LeuGABA, a lipopeptide which inhibited sensory neurons activation, was decreased in faeces of patients with IBS compared with HVs.
Conclusion
PS impacts the gut microbiota composition and metabolic function in adulthood. The reduced capacity of the gut microbiota to produce GABA lipopeptides could be one of the mechanisms linking PS and visceral hypersensitivity in adulthood.
Keywords: LIPIDS, IRRITABLE BOWEL SYNDROME, ENTERIC BACTERIAL MICROFLORA, LACTIC ACID BACTERIA, VISCERAL HYPERSENSITIVITY
WHAT IS ALREADY KNOWN ON THIS TOPIC.
Intestinal microbiota dysbiosis is associated with IBS symptoms.
Perinatal stress is a major risk for the development of IBS in adulthood.
A bacterial lipopeptide, C12AsnGABA, possesses analgesic properties against capsaicin-induced visceral hypersensitivity in mice.
WHAT THIS STUDY ADDS
In mice, prenatal stress at the end of the gestation induced IBS-like symptoms in adulthood.
Abundance of Ligilactobacillus murinus is inversely correlated to visceral sensitivity to colorectal distension.
L. murinus produce several lipopeptides containing GABA, whose concentration is decreased in prenatally stressed mice.
GABA-lipopeptide concentration is decreased in faeces of patients with IBS compared with healthy volunteers.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Treatment of patients with IBS with lipopeptides containing GABA could be used to decrease visceral pain.
Quantification of lipopeptides containing GABA could be used to develop personal therapy.
Lipopeptide-producing bacteria cultured with GABA could represent a novel therapeutic approach in IBS.
Introduction
IBS affects ~11% of the world population and is one of the most common causes of gastroenterology consultation.1 This functional intestinal disorder is characterised by repeated periods of abdominal pain and transit changes (constipation, diarrhoea or an alternate of both). Risk factors for IBS encompass infection, female sex and stress. Indeed, stressful events deeply impact body functions and have been linked to increased IBS symptom severity.2–4 Recent literature has shown that early-life adverse events have consequences on the onset of chronic non-communicable diseases in adulthood such as IBS.5–7 In 2012, Bradford et al showed that patients with IBS reported more early-life stressors than healthy subjects, linking early-life adverse events and IBS onset in adulthood.8 Because events occurring before birth can hardly be included in questionnaires, the effects of in utero events on intestinal dysfunction and IBS symptoms remain unexplored.
In murine models, stress in pregnancy results in gut microbiota dysbiosis in mother and offspring.9 In patients with IBS, dysbiosis has been reported, but there is a discrepancy in the bacterial composition between studies. For instance, in IBS-D, Tap and colleagues quantified an increase in Bacteroides,10 while Su and collaborators described a decrease in the same IBS subtype.11 In IBS, a lot of studies investigated the taxonomic composition of the microbiota, but few studies have investigated active genes, proteins or metabolites.12 However, to better understand the role played by microbiota in host function, studying the molecules produced by the microbiota rather than composition may be more relevant. Indeed, although the taxonomic composition of the human microbiota varies tremendously across individuals, its functional capacity is highly conserved.13 In a previous study, we highlighted that a probiotic, Escherichia coli Nissle 1917, produces a lipopeptide, the C12-asparagine-γ-aminobutyric acid (C12AsnGABA) that decreases both neuronal activation induced by pro-nociceptive molecules in vitro and capsaicin-induced hypersensitivity in vivo.14 We then determined lipoamino acid (LpAA) and GABA–lipopeptide structures by high-resolution mass spectrometry and developed a quantitative method of newly identified LpAA and lipopeptide–GABA by liquid chromatography coupled to mass spectrometry (LC-MS/MS) in different strains of bacteria.15 16
We hypothesised that visceral hypersensitivity in adulthood may originate from functional intestinal microbiota dysbiosis induced by stress in pregnancy. Based on our previous studies demonstrating the ability of lipids to regulate sensory neuron activation,14 17 18 we assumed that bacteria-derived GABA–lipopeptides may be the link between functional dysbiosis and IBS symptoms. We show that prenatal stress (PS) induces in adult mouse offspring microbiota dysbiosis characterised by a decrease in GABA-containing lipopeptides and visceral hypersensitivity. This decrease is also observed in faeces of patients with IBS.
Methods
Animal experiments
Six to ten-week-old C57BL/6 J mice (Janvier, Saint Quentin Fallavier, France) were used. Mice were raised in sanitary conditions without pathogens, with free access to water and food, and submitted to alternating cycles of 12 hours of light and darkness. After mating (three males and two females per cage), C57BL/6J dams, two mice per cage, were randomly assigned to receive stress from day 13 to day 18 of gestation. The pregnant mice assigned to the stress group experienced bright light (100 W) coupled to restraint in a drilled falcon tube (50 mL; Fischer Scientific, Illkirch, France) for 30 min, 3 times a day, with at least 3 hours between each stress session. Stress efficacy was assessed by controlling faecal output (>4 faecal pellets during the first stress session for the first 2 days). The pregnant mice assigned to the control group were not manipulated. On the last day, gestating mice were put one per cage for natural delivery. The pups were weighed every 3 days to monitor their growth. On postnatal days 21–28, the pups were weaned from their mothers. The offspring between 8 weeks and 11 weeks of age, both male and female, were assessed for visceral sensitivity to CRD, paracellular permeability, colon inflammation, plasma corticosterone concentration, colonic concentration of GABA-containing lipopeptides, and taxonomic, predicted functional analysis and biogeography of the gut microbiota (online supplemental methods). In a second set of experiments, visceral sensitivity to CRD was assessed in PS mice before and 30 min after intracolonic administration of C14AsnGABA (10 µM).
gutjnl-2022-328084supp001.pdf (3.7MB, pdf)
Patients
Patients with IBS and diarrhoea (IBS-D) were recruited according to Rome IV criteria.19 Clinical examination and standard biological tests were normal. Total colonoscopy and additional tests when necessary had excluded organic disease. Demographic data were prospectively recorded (table 1). IBS symptom severity was assessed by IBS Severity Scoring System (IBS-SSS).20 This score allows classification of patients with mild (75–174), moderate (175–299) or severe symptoms (>300) (table 1). Healthy volunteers (HVs) were recruited by public advertisement using the gastrointestinal symptom rating scale (GSRS) questionnaire according to European consensus (European cost project).21 Patients were included at the gastroenterology department of the tertiary care centre (Rouen University Hospital, France) between March 2017 and June 2019 and at the outpatient clinic of the University Hospitals Leuven in Belgium between January 2019 and October 2021.
Table 1.
Demographic and clinical characteristics of the human cohort
| HV | IBS | |
| N | 18 | 43 |
| Female, n (%) | 15 (75) | 32 (78) |
| Age, mean±SD | 31.9±12.1 | 29.6±8.6 |
| Mild IBS, n (%) 75<IBS-SSS<149 | 7 (16) | |
| IBS–moderate, n (%) 150<IBS-SSS<299 | 18 (42) | |
| IBS–severe, n (%) IBS-SSS>300 | 18 (42) |
HV, healthy volunteer; IBS-SSS, IBS Severity Scoring System.
Results
PS induces visceral hypersensitivity in the adult offspring
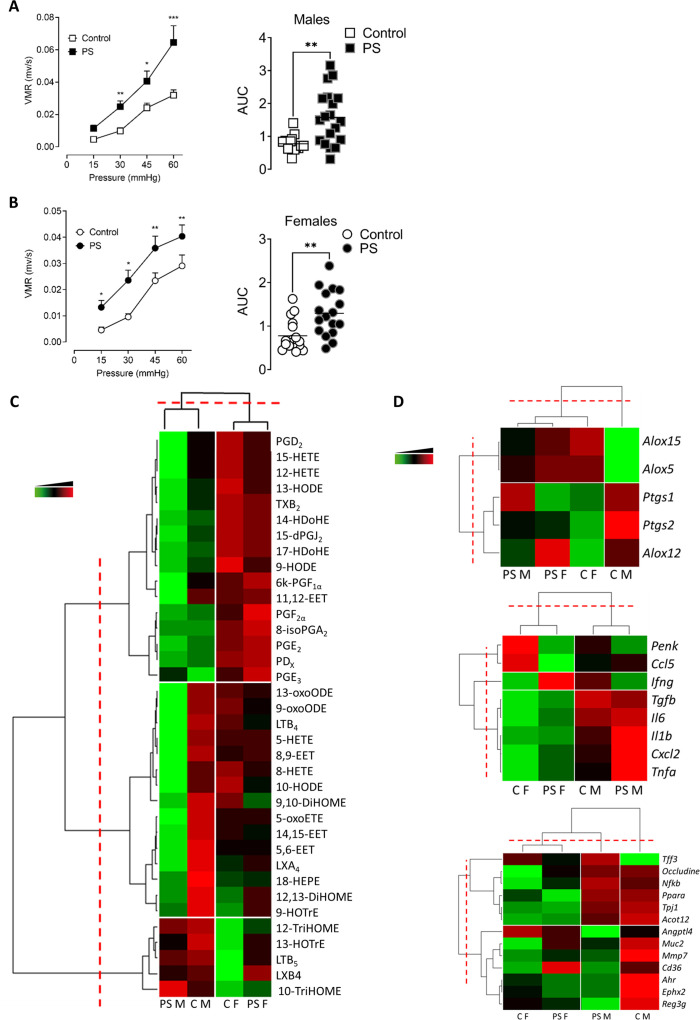
We measured the impact of PS on visceral sensitivity in adulthood by measuring visceromotor responses (VMRs) to colorectal distension (CRD). In PS male and female offspring, the VMR and the AUC of the VMR was significantly increased compared with control mice (figure 1A, B). No significant differences in visceral paracellular permeability (online supplemental figure S1A), colon thickness (online supplemental figure S1B) and inflammatory scores at the macroscopic and microscopic level (online supplemental figure S1B) were observed in offspring from stressed dams as compared with controls. PS altered neither basal plasma corticosterone nor the expression of corticosterone receptors in the colon of the adult offspring (online supplemental figure S1C).
Figure 1.
PS induces visceral hypersensitivity in the adult offspring. VMR to colorectal distensions in control (white) or PS (black) mice in response to increasing pressures of distension (15, 30, 45 and 60 mm Hg) was measured in both male (A) and female (B) offspring. Data are expressed as mean±SEM (n=14–19 mice/group, three independent experiments). Statistical analysis was performed using two-way analysis of variance and subsequent Sidak multiple comparison test. *P<0.05, **P<0.01, ***P<0.001 significantly different from the control group. The results are also expressed as AUC presented as scatter dot plot with the mean. Statistical analysis was performed using a Mann-Whitney test. **P<0.01, significantly different from the control group. (C) Heat map of PUFA metabolites quantified by liquid chromatography coupled to mass spectrometry. Data are shown in a matrix format: each row represents a single PUFA metabolite, and each column represents a group of mice: C M=control males, PS M=PS males, C F=control females, PS F=PS females. each colour patch represents the normalised quantity of PUFA metabolites (row) in a group of mice (column), with a continuum of quantity from bright green (lowest) to bright red (highest). The pattern and length of the branches in the dendrogram on the left, reflect the relatedness of the PUFA metabolites, on top the relatedness of the mouse groups. The dashed red line is the dendrogram distance used to cluster PUFA metabolites and mouse groups. (n=15 mice/group, 2 independent experiments). (D) Heatmap of mRNA expression of genes coding for enzymes implicated in PUFA metabolism (top panel), colonic immune response (middle panel) and gut homeostasis (bottom panel). Data are shown in a matrix format: each row represents a single PUFA metabolite, and each column represents a group of mice: CM, CF, PSM and PSF. Each colour patch represents the normalised gene expression (row) in a group of mice (column), with a continuum of quantity from bright green (lowest) to bright red (highest). The pattern and length of the branches in the dendrogram on the left reflect the relatedness of the gene expression on top the relatedness of the mouse groups. The dashed red line is the dendrogram distance used to cluster genes and mouse groups (n=15 mice/group, three independent experiments). AUC, area under the curve; CF, control female; CM, control male; PS, prenatal stress; PSF, prenatal stress male female; PSM, prenatal stress male; PUFA, polyunsaturated fatty acid; VMR, visceromotor response.
At the molecular level, polyunsaturated fatty acid (PUFA) metabolites were quantified in the mouse colons using LC-MS/MS. Mice hierarchical clustering showed that the mice were not separated by their stress status but by their sex (figure 1C), demonstrating that PS had little or no impact on colonic PUFA metabolism. PUFA metabolites hierarchical clustering revealed three different clusters (figure 1C). The first one regrouped products derived from the metabolism of PUFAs by cyclooxygenases (COXs) (PGE2, TXB2, 6k-PGF1∝, PGF2∝, PGD2, 8-isoPGA2, 15-dPGJ2 and PGE3), by lipoxygenases (LOXs) (15-HETE, 12-HETE, 9-HODE, 13-HODE, PDx and 14-HDoHE) and by cytochromes epoxygenases (11,12-EET). The means of these metabolites’ concentrations were higher in females than in males, but no statistical differences were observed between PS and control mice, except for the 11,12-EET, which was significantly decreased in PS male versus control (online supplemental table S1). The second cluster was composed of LOX metabolites (13-oxoODE, 9-oxoODE, 9-HOTrE, LTB4, LXA4, 5-HETE, 5-oxoETE, 8-HETE and 18-HEPA), CYP metabolites (8,9-EET, 9,10-DiHOME, 14,15-EET, 5,6-EET, 12,13-DiHOME) and by the 10-HODE. In this cluster, the concentration of 5-oxoETE, 5-HETE, 8-HETE, LXA4, 10-HODE, 8,9-EET and 14–15-EET was significantly decreased in PS male offspring compared with control mice (online supplemental table S1). Other PUFA metabolites from the LOX pathway (10-TriHOME, 12-TriHOME, LTB5, LXB4 and 13-HOTrE) were portrayed in the last cluster, and their mean concentrations were increased in males compared with females, but no statistical differences were observed (online supplemental table S1). In addition, we quantified mRNA expression of genes coding for the main enzymes implicated in PUFA metabolism. Heatmap of the expression of PUFA metabolising enzymes showed a hierarchical separation of the control males from the three other experimental groups. 5-LOX and 15-LOX expression clustered apart form 12-LOX, COX-1 and COX-2 mRNA expression. Only Alox5, which codes for the 5-LOX, was increased in PS males compared with control (online supplemental table S2). PS increases neither proinflammatory lipid mediators nor the enzymes implicated in their synthesis, with the exception of the 5-LOX, in adult male mice.
Then, we assessed the mRNA expression of genes implicated in colonic immune response and homeostasis (figure 1D). Again, hierarchical clustering showed that mice were not separated by their stress status but by their sex and that genes were clustered in three groups Penk and Ccl5 (cluster 1), Ifng (cluster 2), and Tgfb, Il6, Il1b, Cxcl2 and Tnfa (cluster 3). No statistical differences were observed between PS and control adult male and female mice. In agreement, the percentages of CD8+ and CD4+ T cells, regardless of their conventional or memory phenotype, were not significantly different between control and PS mice (online supplemental figure S1D). The expression of genes significant for the epithelium homeostasis grouped the female mice together, unlike males that were separated by the hierarchical clustering, depending on their stress status. Control male mice were represented by an increase in the means of the expression of Ocln, Nfkb, Ppara, Tjp1, Acot12, Angptl4, Muc2, Mmp7, Cd36, Ahr, Ephx2 and Reg3g, whereas PS males were represented by an increase of Tff3, Ocln, Nfkb, Ppara, Tjp1 and Acot12 (figure 1D). In female mice, the means of expression of these genes were lower than in male mice. No statistical difference between control and PS mice was observed (online supplemental table S2).
In conclusion, PS induced visceral hypersensitivity in the absence of any sign of colonic inflammation in adulthood.
PS induces a gut microbiota dysbiosis and alters gut microbiota spatial organisation in adulthood
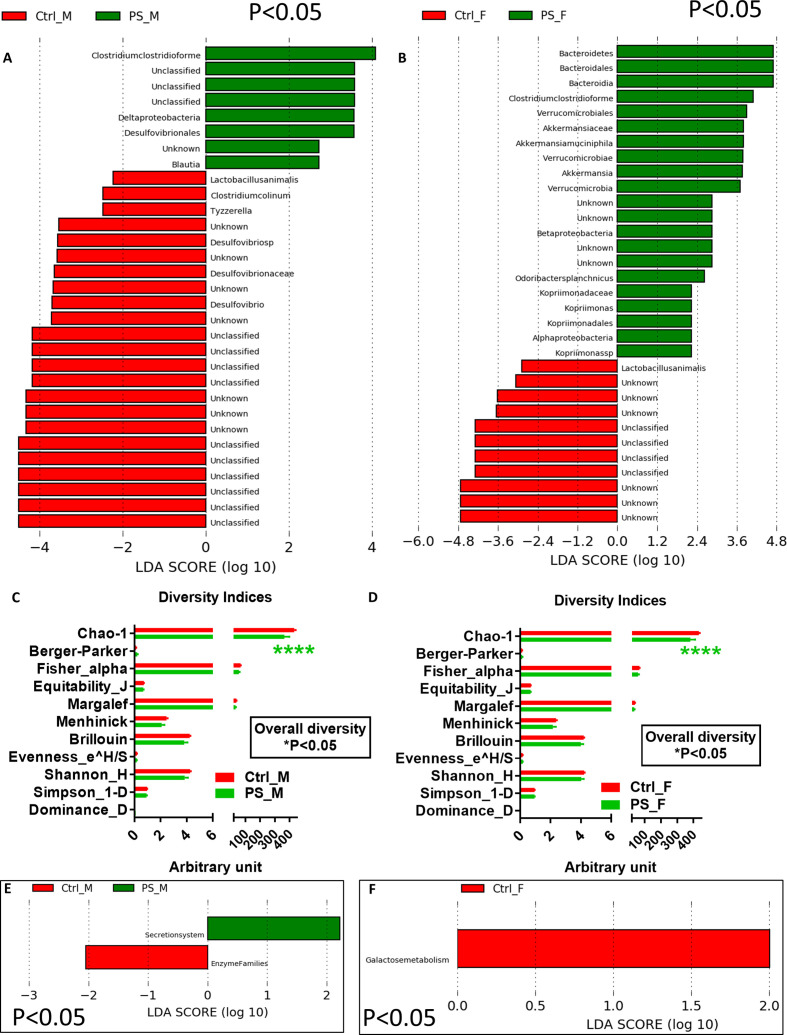
The 16S RNA analyses of the faeces revealed a shift in the gut microbiota composition in PS mice that differed in male and female offspring. In males, PS mice showed a higher relative abundance of Clostridium clostridioforme, Deltaproteobacteria, Desulfovibrionales and Blautia, while the relative abundance of Desulfovibrio and Desulfovibrionaceae as well as Tyzzerella and C. colinum was higher in control mice (figure 2A). In females, the relative abundances of taxa from Bacteroidetes and Verrucomicrobia phyla were higher than in control mice (figure 2B). In both PS male and female offspring, the relative abundance of C. clostridioforme was higher, whereas the relative abundance of Lactobacillus animalis was lower (figure 2A, B). These taxonomical differences were in accordance with a significant different and lower overall diversity, mostly based on Chao-1 index, related to rare species, for both PS male and female mice (figure 2C, D). We also performed a phylogenetic investigation of communities by reconstruction of unobserved states (PICRUSt)-based predictive analysis of gut microbial functions. In males, PS mice showed a higher function related to secretion system, whereas control mice displayed higher undefined enzyme families (figure 2E). In females, the predictive gut microbiome did not vary compared with control mice, which showed a higher galactose metabolism (figure 2F).
Figure 2.
PS induces a gut microbiota dysbiosis both in male and female mice. LDA score in male (A) and female (B) PS mice versus control mice; diversity indices in male (C) and female (D) PS mice versus control mice; PICRUSt-based predicted gut microbial functions in male (E) and female (F) PS mice versus control mice (n=15 mice/group). **** P<0.0001 significantly different from the control group. Ctrl, control; LDA, linear discriminant analysis; PS, prenatal stress; PSF, prenatal stress male female; PSM, prenatal stress male.
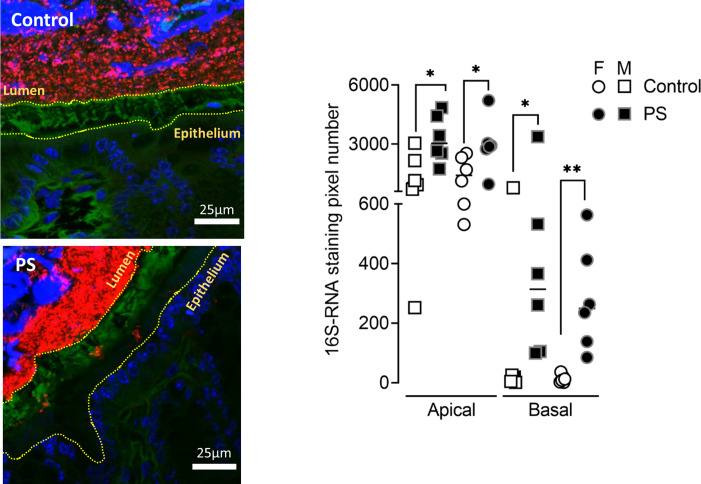
Beyond taxonomic and functional alteration of the gut microbiota, PS induced a spatial organisational change of the colonic microbiota. In control mice of either sex, bacteria were separated from the epithelial cells by a dense sterile mucus layer (figure 3). In contrast, in PS mice, some bacteria from the gut lumen invaded this layer (figure 3). By quantifying the 16S RNA-labelled pixels inside the mucus layer, we showed that in PS mice, more bacteria crossed the border with the lumen and moved close to the epithelium (figure 3).
Figure 3.
PS alters gut microbiota spatial organisation in adulthood. Bacteria were labelled with the universal probe Eub338 (red); wheat germ agglutinin-Fluorescein-5-isothiocyanate (FITC) was used to stain the polysaccharide-rich mucus layer (green); and the epithelial cell nucleus was stained with 4',6-diamidino-2-phénylindole (DAPI; blue). Bacteria penetration into the mucus was measured in both M (square) and F (circle) control (white symbols) and PS (black symbols) mice by image processing on Fiji by quantifying the number of 16S RNA-labelled pixel between the edge of the lumen and the middle of the mucus (apical) and between the middle of the mucus and the edge of the epithelium (basal). The results are expressed as scatter dot plot with the mean. Statistical analysis was performed using Mann-Whitney test. * P<0.05, **P<0.01, significantly different from the control group. (n=12 mice/group; four images/mice, two independent experiments). F, female; M, male; PS, prenatal stress.
Overall, these data show that PS induces gut microbiota dysbiosis and biogeographical changes in adulthood.
Abundance of L. animalis is inversely correlated to visceral hypersensitivity
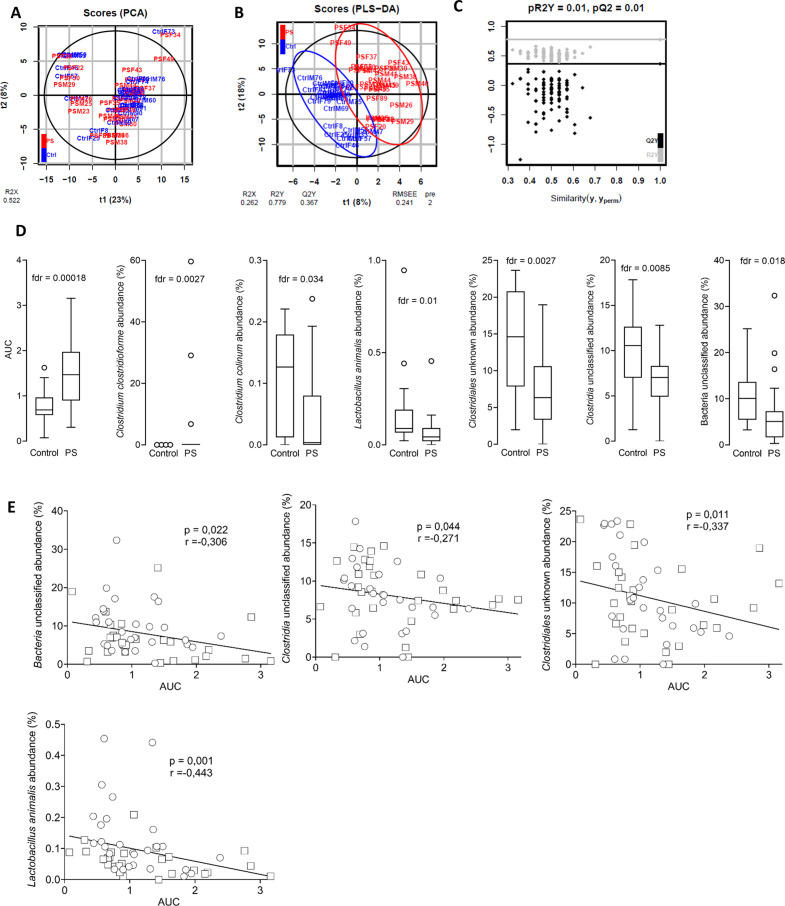
To extract the important differences between prenatally stressed and control mice, we first ran a principal component analysis (PCA) model followed by a partial least-squares discriminant analysis (PLS-DA) model. In both models, the mice were mainly located in within the 95% CIs (black ellipse) (figure 4A, B). The plotted PCA scores showed the aggregation and dispersion of the mice, and no outliers were identified (figure 4). The PLS-DA score plot was fitted on unit variance-scaled data and displays the classification effect. It showed a clear discrimination between the control and PS groups (figure 4B), and the underlying model was validated by a robust (p<0.05) permutation test (figure 4C). Seven variables had a variable importance in projection value of ≥1 and a p value of ≤0.05, showing their importance for group separation in the PLS-DA model. Besides visceral sensitivity (AUC), six bacterial species were identified as important: Clostridia unclassified, L. animalis, C. clostridioforme, C. colinum, Clostridiales unknown and Bacteria unclassified (figure 4D). However, when similar analysis were done on each sex separately, visceral sensitivity was a common variable for separating Ctrl and PS mice, while significant bacteria abundances were different in male and female (online supplemental figure S2). We performed Spearman correlations between these variables to consolidate the link between the identified bacterial species and visceral hypersensitivity. Clostridia unclassified, L. animalis, Clostridiales unknown and Bacteria unclassified were inversely correlated to visceral sensitivity (figure 4E). The highest correlation coefficient was reached with L. animalis (figure 4E).
Figure 4.
The abundance of Lactobacillus animalis is inversely correlated to visceral hypersensitivity in adulthood. Multivariate analysis of the complete cohort: (A) two-dimensional PCA (R2=52%) score plot of data generated from 56 samples (control, n=28, blue; PS, n=28, red). each dot corresponds to an individual. (B) two-dimensional PLS-DA (R2X=26.2%, R2Y=77.9%, Q2=0.367) score plot of data generated from 53 samples (control, n=26, blue; PS, n=27, red). Each dot corresponds to an individual. The black ellipse corresponds to a 95% CI based on the Hotelling’s T2. RMSEE, root mean square error (C) Permutation test result for PLS-DA model validation. (D) Box plots of discriminant (variable importance in projection >1) and significant (false discovery rate (FDR)-corrected p value of Wilcoxon test <0.05) variables. (E) Spearman correlations were used to analyse the correlation between the faecal microbiota abundances and visceral motor response to colorectal distension expressed in AUC, in male (square) and female (circle) offspring. P and R values are indicated on each graph. AUC, area under the curve; PS, prenatal stress.
In conclusion, visceral hypersensitivity and microbiota composition were the main drivers of the separation between control and PS mice and visceral hypersensitivity strongly correlated to the decreased abundance of L. animalis.
Ligilactobacillus murinus concentration is decreased in adult PS faeces
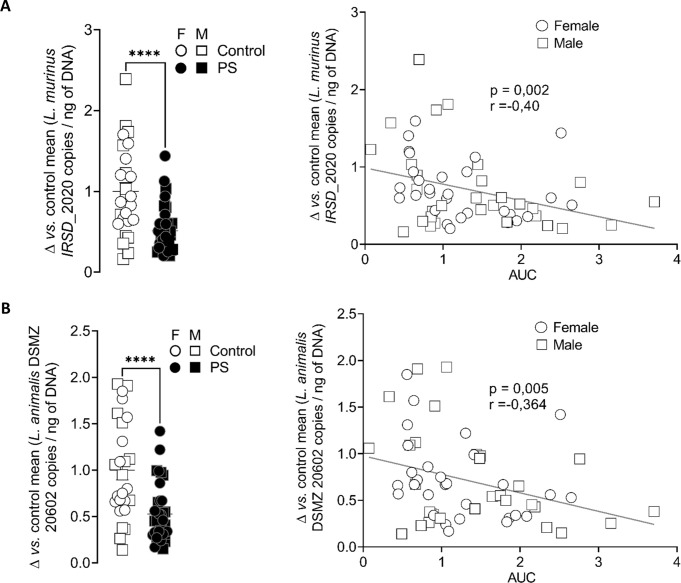
In the mucus of control but not PS mice, we identified by matrix assisted laser desorption ionisation-time of flight (MALDI-TOF) the presence of L. murinus, L. reuteri and L. johnsonii/gasseri. In 2020, the Lactobacillus genera regrouped 261 species very diverse from each other on the phenotypical, ecological and genotypical levels. Zheng et al re-evaluated the taxonomy of Lactobacillaceae based on whole-genome sequencing and reclassified them into 25 genera.22 On this basis, Lactobacillus reuteri is now classified as Limosilactobacillus reuteri and L. murinus as L. murinus. The latter is closely related to another species, L. animalis and differentiating them from one another remains an issue using the current 16S RNA sequencing pipelines.23 The same observation is done for probe designing as it is difficult to find species-specific 16S RNA locations. Therefore, it is now recommended to identify them as a single species named L. murinus/animalis. As L. murinus/animalis was the only identified bacterial species to be correlated with visceral sensitivity, we focused our attention on the role of this bacterium in intestinal homeostasis. Whole-genome sequencing of L. murinus, identified in the mice and named strain IRSD_2020, allowed us to determine that it possessed few sequences different from the reference strain DSMZ-20602 (online supplemental figure S3). We then quantified L. murinus IRSD_2020 from mice faecal samples by Taqman real-time PCR. L. murinus IRSD_2020 quantity was lower in the faeces of PS mice in adulthood and was inversely correlated to the AUC of the visceral sensitivity (online supplemental figure 5A and table S4). The same results were obtained with primers and probe designed for the L. animalis strain DSMZ-20602 (figure 5B).
Figure 5.
Ligilactobacillus murinus IRSD_2020 concentration is decreased in PS mouse faeces. The levels of the L. murinus strain IRSD_2020 (A) and of the reference L. murinus/animalis strain DSMZ 20602 (B) were quantified by TaqMan real-time PCR in the faeces of control mice (white) or PS mice (black), in the M (square) and F(circle) offspring. Data are expressed as scatter dot plot with the mean. Statistical analysis was performed using Mann-Whitney test. ****P<0.0001, significantly different from the control group (n=27–36 mice/group, three independent experiments). Spearman correlations were used to analyse the correlation between the faecal bacteria quantity and visceral motor response to colorectal distension expressed in AUC, in M (square) and F (circle) offspring. P and R values are indicated on each graph. AUC, area under the curve; F, female; M, male; PS, prenatal stress
L. murinus produces analgesic lipopeptides
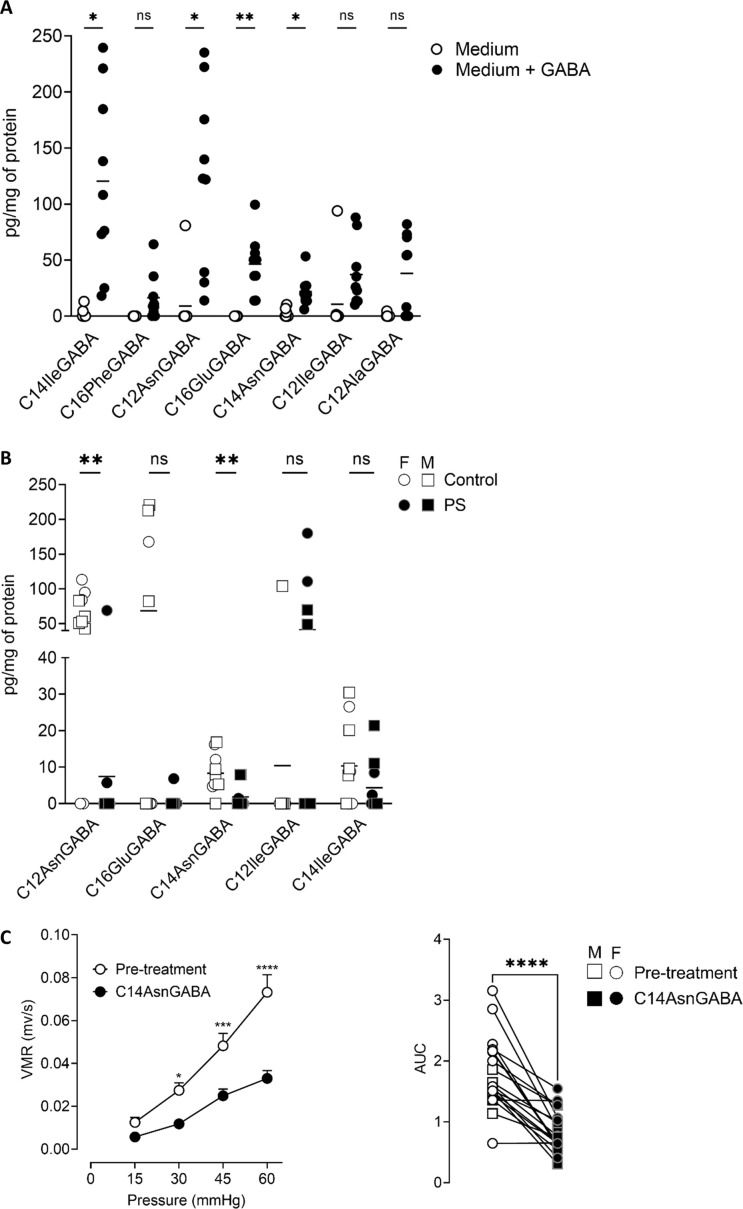
As the abundance of L. murinus IRSD_2020 was inversely correlated to the visceral sensitivity, we hypothesised that these bacteria produced a metabolite decreasing visceral sensitivity. Based on our previous study where we described the production of an analgesic GABA-containing lipopeptide by E. coli strain14 14 and on our studies identifying several lipoamines in bacteria,15 16 we developed a method to quantify the concentration of lipopeptides containing GABA: C12AlaGABA, C12AsnGABA, C14AsnGABA, C14:1AlaGABA, C12ValGABA, C12LeuGABA, C14GABA, C12IleGABA, C14IleGABA, C16LeuGABA, C16PheGABA and C16GluGABA (online supplemental methods). In L. murinus IRSD_2020, as no clear production of lipopeptides-GABA was observed (online supplemental figure 6A and table S5) and as these bacteria did not express the enzymes implicated in GABA synthesis, we cultured L. murinus IRSD_2020 in the presence of GABA. Under this condition, L. murinus IRSD_2020 produced several lipopeptides containing GABA: C16PheGABA, C12AsnGABA, C16GluGABA, C14AsnGABA, C12IleGABA, C14IleGABA and C12AlaGABA (online supplemental figure 6A and table S5). C14:1AlaGABA, C12ValGABA, C12LeuGABA, C14GABA and C16LeuGABA were not quantifiable in L. murinus IRSD_2020. The concentrations of C12AsnGABA, C16GluGABA, C14AsnGABA and C14IleGABA were significantly increased in bacteria cultured with 4 mg/mL of GABA (figure 6A). In mouse colons, we quantified C12AsnGABA, C16GluGABA, C14AsnGABA, C12IleGABA and C14IleGABA (online supplemental figure 6B and table S6). C16PheGABA, C12AlaGABA, C14:1AlaGABA, C12ValGABA, C12LeuGABA, C14GABA and C16LeuGABA were not quantifiable in mouse colons. In the colon of PS mice, the concentrations of C12AsnGABA and C14AsnGABA were significantly decreased compared with control (online supplemental figure 6B and table S5). Finally, in the last set of experiments, CRD experiments were performed before and 30 min after intracolonic administration of 10 µM, as already described for the C12AsnGABA,14 of C14AsnGABA in male and female adult PS mice. Treatment by the lipopeptide decreased the visceral hypersensitivity induced by PS (online supplemental figure 6C and table S5). We identified that L. murinus IRSD_2020 produced lipopeptides containing GABA in the presence of GABA. The treatment of sensitive mouse by C14AsnGABA, whose concentration was decreased in their colon, restored a normal visceral sensitivity.
Figure 6.
Ligilactobacillus murinus IRSD_2020 produces an analgesic lipopeptide. (A) Concentration of lipopeptides quantified by LC-MS/MS in the bacterial pellets of L. murinus IRSD_2020 cultivated without (white circle) or with (4 mg/mL) (black circle) GABA. Data are expressed as scatter dot plot with the mean (n=9). Statistical analysis was performed using Mann-Whitney test. *P<0.05, **P<0.01, significantly different from the corresponding L. murinus IRSD_2020 without GABA. (B) Concentration of lipopeptides quantified by LC-MS/MS in the colon of M (square) and F (circle) control (white) and PS mice (black). Data are expressed as scatter dot plot with the mean (n=9–10). Statistical analysis was performed using Mann-Whitney test. **P<0.01, significantly different from the corresponding control group. (C) VMR to colorectal distensions in response to increasing pressures of distension (15, 30, 45 and 60 mm Hg) was measured in both M and FM PS offspring. Measurements were done before (white) and after intracolonic administrations of C14AsnGABA (black). Data are expressed as mean±SEM (n=19 mice/group, two independent experiments). Statistical analysis was performed using two-way analysis of variance and subsequent Sidak multiple comparison test. *P<0.05, ***P<0.001, ****P<0.0001 significantly different from the pretreatment group. The results are also expressed as area under the curve (AUC) presented as scatter dot plot with the mean for M (square) and F (circle) mice. statistical analysis was perform using a Wilcoxon test. ****p<0.0001, significantly different from the pretreatment group. F, female; GABA, LC-MS/MS, iquid chromatography–tandem mass spectrometry; M, male; ns, not significant; PS, prenatal stress; VMR, visceromotor response.
Concentration of neuronal activation inhibitory lipopeptides is decreased in faeces of patients with IBS
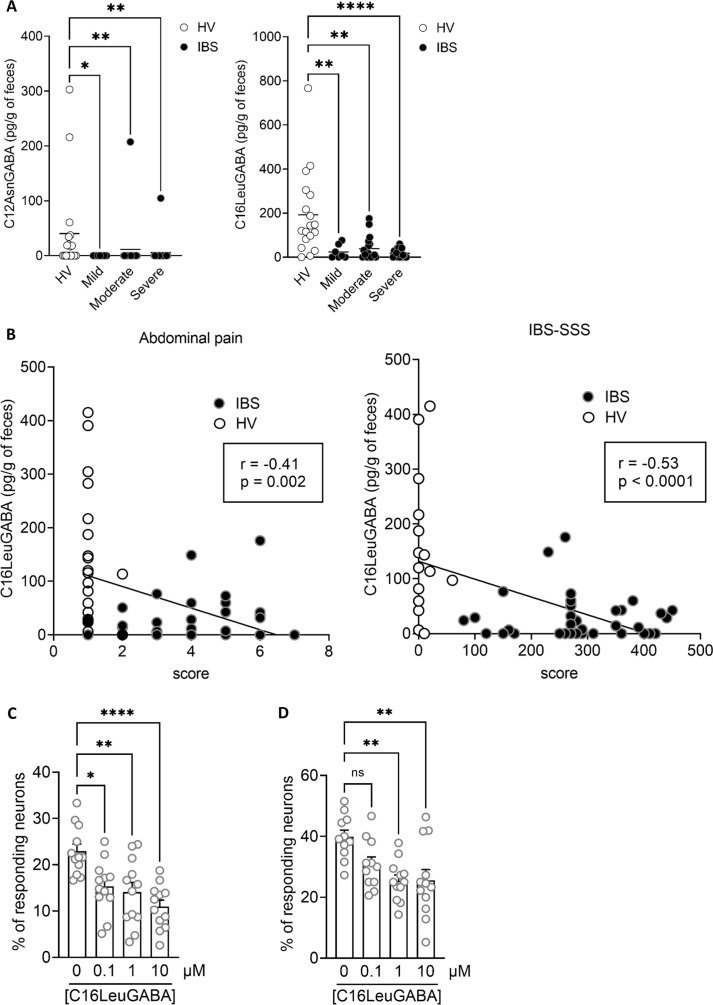
GABA–lipopeptides were quantified in faeces from patients with IBS and HVs using LC-MS/MS. In contrast to L. murinus supplemented with GABA and mouse colon, only the C12AsnGABA and the C16LeuGABA were quantifiable in human faeces. C12AsnGABA was quantifiable in 8 out of 18 HVs and was significantly decreased in patients with IBS (figure 7A). C16LeuGABA was quantifiable in 17 out of 18 HVs and was significantly decreased in patients with IBS (figure 7A). The concentration of C16LeuGABA was significantly inversely correlated to the IBS-SSS and to the abdominal pain score (figure 7B), and did not correlate with SF-36 (online supplemental filgure S8). As C16LeuGABA has never been quantified before, its role on sensory nerves activation is unknown. We next determined whether C16LeuGABA decreases neuronal activation, as previously described for C12AsnGABA.14 Primary cultures of mouse dorsal root ganglion neurons, activated either by an agonist of the receptor calcium channel TRPV1 (capsaicin) or by a mix of agonists (histamine, serotonin and bradykinin) for G protein-coupled receptors (GPCRs), were treated with C16LeuGABA. Neuron exposure to either capsaicin (500 nM) or the GPCR agonists mix (histamine, bradykinin and serotonin, 10 µM each) induced an increase in calcium flux as shown by the higher % of responding neurons (figure 7C, D). The calcium flux increase induced by both nociceptive stimuli was prevented by C16LeuGABA pretreatment in a dose-dependent manner (figure 7).
Figure 7.
Lipopeptide–GABA concentrations are decreased in the faeces of patients with IBS. (A) 16LeuGABA (left panel) and C12AsnGABA (right panel) concentrations quantified by liquid chromatography–tandem mass spectrometry; in the faeces of HVs (n=18, white) and patients with IBS (black) with an IBS-SSS corresponding to mild (n=7), moderate (n=18) or severe (n=18) IBS. Data are expressed as scatter dot plots with the mean. Statistical analysis was performed using Kruskal-Wallis analysis of variance and subsequent Dunn multiple comparison test. **P<0.01, **P<0.001 significantly different from HVs. (B) Spearman correlations were used to analyse the correlation between the concentration of CL16LeuGABA and abdominal pain score (left panel) or IBS-SSS (right panel) in HVs (white) and patients with IBS (black). P and R values are indicated on each graph. (C) Percentage of responding neurons pretreated with increasing amounts of C16LeuGABA or vehicle (HBSS/MeOH 0.06%, 0 µM) and treated with capsaicin (500 nM) or (D) a mix of GPCR agonists (histamine, serotonin and bradykinin, 10 µM each). Data are represented as mean±SEM; n=4 independent experiments of two to three wells per condition and 20–50 neurons per well. Statistical analysis was performed using Kruskal–Wallis analysis of variance and subsequent Dunn post hoc test. *P<0.05, **P<0.01, ****P<0.0001 significantly different from capsaicin or GPCR mix. GPCR, G protein-coupled receptor; IBS-SSS, IBS Severity Scoring System; ns, not significant; HV, healthy volunteer.
Discussion
The onset of IBS in adulthood is associated with a higher number of early-life adverse events.8 However, due to the lack of data on prenatal period in humans, it is difficult to establish the causal link between stressful events during pregnancy and intestinal homeostasis disruption in adulthood. Here, in a mouse model of PS, we show that the male and female offspring of stressed mothers present the main characteristics of IBS in adulthood: visceral hypersensitivity, no overt colonic inflammation and a gut microbiota dysbiosis. Our results confirmed previous observations made in a rat model of PS where an increase in visceral sensitivity and no change of paracellular permeability have been described.24 25 In contrast to stress performed in adulthood,26 we did not observe an increase in plasma corticosterone concentration in PS mice as previously observed in PS rodents.24 25 27 28 So, corticosterone is probably not implicated in the increase in visceral sensitivity observed in this model at adulthood. Nevertheless, we cannot exclude a long-lasting impact of corticosterone during foetal development and infancy. The gut microbiota of PS mice was, however, significantly altered in both male and female offspring, characterised by an increased abundance of C. clostridioforme and a decreased abundance of L. murinus/animalis. C. clostridioforme, reclassified as Enterocloster clostridioformis in 2019,29 is hardly dissociable from two other bacteria: C. bolteae and C. hathewayi, all found in human faeces.30 These three species are therefore usually considered as the C. clostridioforme group. A higher abundance of this group in the faeces has been linked to the onset of autism spectrum disorders (ASDs) in children.31 32 In rodents, PS has also been associated with an increase of ASD-like behaviours in adulthood,33–35 but, in the absence of microbiota analysis, a direct link between PS, C. clostridioforme and ASD is still unknown. More studies are needed to decipher the implication of C. clostridioforme in IBS, but its correlation with behavioural disorders is indicative of a role in psychological comorbidities such as anxiety and depression.
The decreased abundance of the Lactobacillus genera seems to be a universal response to chronic stress as this observation has been made in various chronic stress models, applied either in adulthood or in early life, and in different species.24 27 36 37 In humans, studies looking at the correlation between stress in pregnancy and the baby’s gut microbiota have also highlighted that infants of stressed mothers present a decreased abundance of Lactobacillus in their faeces.38 However, how chronic stress impacts the Lactobacillus population and its duration remain to be determined. Given that we previously showed analgesic properties of C12AsnGABA, a lipopeptide containing GABA in a model of visceral pain,14 the inverse correlation between L. murinus/animalis abundance and visceral sensitivity led us to hypothesise that this bacterium was implicated in the maintenance of normosensitivity by producing analgesic molecules. As expected, L. murinus IRSD_2020, in the presence of GABA, produced GABA-containing lipopeptides, such as C16PheGABA, C12AsnGABA, C16GluGABA, C14AsnGABA, C12IleGABA, C14IleGABA and C12AlaGABA, highlighting the redundancy to produce lipopeptides linked to GABA.
GABA production is widely distributed in all three life kingdoms: animals, plants and bacteria. In bacteria, the most studied producers belong to the lactic acid-producing bacteria, a group that includes Lactobacilli.39 40 GABA is synthetisd by a pyridocal-5′-phosphate-dependent enzyme glutamate decarboxylase (EC 4.1.1.15) by irreversible α-decarboxylation of L-glutamate and consumption of one cytoplasmic proton.41 These enzymes are encoded by gadB and gadC genes.42 L. murinus/animalis does not possess the enzymes required to produce GABA. Nevertheless, when cultivated in a GABA-enriched medium, this species forms lipopeptides linked to GABA. In vivo GABA could be provided to L. murinus/animalis by two other hardly differentiable Lactobacillus species that we isolated from the mucus of control but not from PS mice, L. reuteri and the L. johnsonii/gasseri cluster. In the literature, these two latter species tend to form dual-species biofilm in the gut and are often clustering with L. murinus/animalis.43 L. reuteri possesses the GABA production machinery,43 and the close interactions between these species in the gut could be one of the mechanisms through which L. murinus/animalis acquires the GABA to produce lipopeptides containing GABA. Interestingly, we quantified a decrease of C16LeuGABA concentration in the faeces of patients with IBS compared with HVs. C16LeuGABA was not quantifiable in L. murinus/animalis or in mouse colon, meaning that in human, this lipopeptide could be produced by other species of lactobacillus or even other species of bacteria. The diversity in lipopeptide composition could be dependent on the culture conditions or on the diet as the leucine is an essential fatty acid, which cannot be produced by the organism. In addition, bacterial environment could also have an impact on lipopeptide synthesis as, for the example, the size of fatty acids in bacteria is dependent on culture conditions and temperature.44 45 A prospective clinical study unifying microbiota analyses and lipopeptides quantification is needed to identify bacteria implicated in the production of C16LeuGABA in the human gut microbiota. As this GABA–lipopeptide decreased capsaicin-induced and GPCR agonist-induced neuronal activation, the contribution of GABA–lipopeptides to visceral pain could be relevant in patients. Quantification of GABA–lipopeptides in patients would allow identification of patients which could be treated directly by the lipopeptide or by a probiotic supplemented with GABA.
GABA analogues such as gabapentine or pregabalin decrease abdominal pain in patients with IBS, but their serious side effects (hepatotoxicity and neurotoxicity) prevent their chronic use.46–48 The analgesic properties of GABA lipopeptides, as demonstrated here for C14AsnGABA and C16LeuGABA, show that they could represent a novel therapeutic track to explore in patients with IBS. Our results show that the production of lipopeptides–GABA by commensal bacteria could be one of the mechanisms of communication between the host and its gut microbiota implicated in the upkeep of intestinal homeostasis.
Acknowledgments
We gratefully acknowledge the animal care facility, Genetoul, anexplo, US006/INSERM, Toulouse, MetaToul (Toulouse metabolomics and fluxomics facilities, www.metatoul.fr) which is part of the French National Infrastructure for Metabolomics and Fluxomics MetaboHUB and the platform Aninfimip, an EquipEx (‘Equipement d’Excellence’) supported by the French government through the Investments for the Future programme. We acknowledge the technical assistance provided by the personnel of flow cytometry Infinity core facility and the cellular imaging facility of INSERM 1291 connected to the ‘Toulouse Réseau Imagerie’ network.
Footnotes
Contributors: CP, PLF and NC designed the research studies; conducted the experiments; and acquired, analysed the data and wrote the manuscript. SM, GP and MSdesigned the research studies, conducted the experiments, and acquired and analysed the data. AH, BA RM and FA conducted the experiments and acquired and analysed the data. GL, J-PM, PF, SL and AD acquired and analysed the data. MT-F analysed the data. JMG, AG and TD designed and synthesised the lipopeptides. GG, JB-M, AS, EO PB and GD designed the research studies and wrote the manuscript. HH and LD provided patient samples and clinical scores. CM and GB provided patient samples and clinical scores and wrote the manuscript. PLF and NC are responsible for the overall content as guarantor.
Funding: Funding from the Agence Nationale de la Recherche (ANR): ANR-18-CE14-0039 for N.C, ANR-20-CE14-0011 for NC & JBM, ANR-17-EURE-022 for EURIDOL Graduate School of Pain, ANR-11-INBS-0010 for Metabohub, ANR-11-EQPX-0003 for the platform Aninfimip. Funding from the Fédération pour la Recherche sur le Cerveau (FRC) FRC20200411001 for AS and NC. AS, PP and GG are supported by Centre National de la Recherche Scientifique. PP and GG received support from CNRS and University of Strasbourg. GG, CP and SM received a scholarship from Ministère de l’Enseignement Supérieur, de la Recherche et de l’Innovation.
Competing interests: PP is a senior fellow of the Institut Universitaire de France. CM has been awarded the UEG Research Award 2020 for her stay at The University of Gothenburg and by the FARE Fellowship of the French Gastroenterology Society in 2015.
Patient and public involvement: Patients and/or the public were not involved in the design, conduct, reporting or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants. For French patients, the use of informatics data was declared to the CNIL (number 817.917) and the biological collection was declared to the French Ministry (numbers DC 2016-2637 and AC 2019-3840). Collection of samples from healthy volunteers and patients with IBS in Leuven was approved by the medical ethics committee of the University Hospitals Leuven (B) (S51573). The research was performed according to the Declaration of Helsinki. All patients gave their written informed consent. For animal experiments, all procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals of the European Council and were approved by the Animal Care and Ethics Committee of US006/CREFE (CEEA-122; application number APAFIS #16385CE2018080222083660V3).
References
- 1. Enck P, Aziz Q, Barbara G, et al. Irritable bowel syndrome. Nat Rev Dis Primers 2016;2:16014. 10.1038/nrdp.2016.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Surdea-Blaga T, Băban A, Dumitrascu DL. Psychosocial determinants of irritable bowel syndrome. World J Gastroenterol 2012;18:616. 10.3748/wjg.v18.i7.616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Blanchard EB, Lackner JM, Jaccard J, et al. The role of stress in symptom exacerbation among IBS patients. J Psychosom Res 2008;64:119–28. 10.1016/j.jpsychores.2007.10.010 [DOI] [PubMed] [Google Scholar]
- 4. Chang L. The role of stress on physiologic responses and clinical symptoms in irritable bowel syndrome. Gastroenterology 2011;140:761–5. 10.1053/j.gastro.2011.01.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Caparros-Gonzalez RA, Torre-Luque Adela, Romero-Gonzalez B, et al. Stress during pregnancy and the development of diseases in the offspring: a Systematic-Review and meta-analysis. Midwifery 2021;97:102939. 10.1016/j.midw.2021.102939 [DOI] [PubMed] [Google Scholar]
- 6. Olén O, Stephansson O, Backman A-S, et al. Pre- and perinatal stress and irritable bowel syndrome in young adults - A nationwide register-based cohort study. Neurogastroenterol Motil 2018;30:e13436. 10.1111/nmo.13436 [DOI] [PubMed] [Google Scholar]
- 7. Low EXS, Mandhari MNKA, Herndon CC, et al. Parental, perinatal, and childhood risk factors for development of irritable bowel syndrome: a systematic review. J Neurogastroenterol Motil 2020;26:437–46. 10.5056/jnm20109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bradford K, Shih W, Videlock EJ, et al. Association between early adverse life events and irritable bowel syndrome. Clin Gastroenterol Hepatol 2012;10:385–90. 10.1016/j.cgh.2011.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jašarević E, Howard CD, Morrison K, et al. The maternal vaginal microbiome partially mediates the effects of prenatal stress on offspring gut and hypothalamus. Nat Neurosci 2018;21:1061–71. 10.1038/s41593-018-0182-5 [DOI] [PubMed] [Google Scholar]
- 10. Tap J, Derrien M, Törnblom H, et al. Identification of an intestinal microbiota signature associated with severity of irritable bowel syndrome. Gastroenterology 2017;152:111–23. 10.1053/j.gastro.2016.09.049 [DOI] [PubMed] [Google Scholar]
- 11. Su T, Liu R, Lee A, et al. Altered Intestinal Microbiota with Increased Abundance of Prevotella Is Associated with High Risk of Diarrhea-Predominant Irritable Bowel Syndrome. Gastroenterol Res Pract 2018;2018:1–9. 10.1155/2018/6961783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lepage P, Leclerc MC, Joossens M, et al. A metagenomic insight into our gut's microbiome. Gut 2013;62:146–58. 10.1136/gutjnl-2011-301805 [DOI] [PubMed] [Google Scholar]
- 13. HuttenhowerC, GeversD, KnightR, et al. Structure, function and diversity of the healthy human microbiome. Nature 2012;486:207–14. 10.1038/nature11234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pérez-Berezo T, Pujo J, Martin P, et al. Identification of an analgesic lipopeptide produced by the probiotic Escherichia coli strain Nissle 1917. Nat Commun 2017;8:1314. 10.1038/s41467-017-01403-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hueber A, Gimbert Y, Langevin G, et al. Identification of bacterial lipo-amino acids: origin of regenerated fatty acid carboxylate from dissociation of lipo-glutamate anion. Amino Acids 2022;54:241–50. 10.1007/s00726-021-03109-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hueber A, Petitfils C, Le Faouder P, et al. Discovery and quantification of lipoamino acids in bacteria. Anal Chim Acta 2022;1193:339316. 10.1016/j.aca.2021.339316 [DOI] [PubMed] [Google Scholar]
- 17. Bautzova T, Hockley JRF, Perez-Berezo T, et al. 5-oxoETE triggers nociception in constipation-predominant irritable bowel syndrome through Mas-related G protein-coupled receptor D. Sci Signal 2018;11:eaal2171. 10.1126/scisignal.aal2171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cenac N, Bautzova T, Le Faouder P, et al. Quantification and potential functions of endogenous agonists of transient receptor potential channels in patients with irritable bowel syndrome. Gastroenterology 2015;149:433–44. 10.1053/j.gastro.2015.04.011 [DOI] [PubMed] [Google Scholar]
- 19. Lacy BE, Mearin F, Chang L, et al. Bowel disorders. Gastroenterology 2016;150:1393–407. 10.1053/j.gastro.2016.02.031 [DOI] [PubMed] [Google Scholar]
- 20. Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther 1997;11:395–402. 10.1046/j.1365-2036.1997.142318000.x [DOI] [PubMed] [Google Scholar]
- 21. Boeckxstaens GE, Drug V, Dumitrascu D, et al. Phenotyping of subjects for large scale studies on patients with IBS. Neurogastroenterol Motil 2016;28:1134–47. 10.1111/nmo.12886 [DOI] [PubMed] [Google Scholar]
- 22. Zheng J, Wittouck S, Salvetti E, et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int J Syst Evol Microbiol 2020;70:2782–858. 10.1099/ijsem.0.004107 [DOI] [PubMed] [Google Scholar]
- 23. Park SH, Itoh K. Species-specific oligonucleotide probes for the detection and identification of Lactobacillus isolated from mouse faeces. J Appl Microbiol 2005;99:51–7. 10.1111/j.1365-2672.2005.02584.x [DOI] [PubMed] [Google Scholar]
- 24. Golubeva AV, Crampton S, Desbonnet L, et al. Prenatal stress-induced alterations in major physiological systems correlate with gut microbiota composition in adulthood. Psychoneuroendocrinology 2015;60:58–74. 10.1016/j.psyneuen.2015.06.002 [DOI] [PubMed] [Google Scholar]
- 25. Wang H-J, Xu X, Xie R-H, et al. Prenatal maternal stress induces visceral hypersensitivity of adult rat offspring through activation of cystathionine- β -synthase signaling in primary sensory neurons. Mol Pain 2018;14:174480691877740. 10.1177/1744806918777406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bernatova I, Puzserova A, Balis P, et al. Chronic stress produces persistent increases in plasma corticosterone, reductions in brain and cardiac nitric oxide production, and delayed alterations in endothelial function in young prehypertensive rats. Front Physiol 2018;9:1179. 10.3389/fphys.2018.01179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jašarević E, Howard CD, Misic AM, et al. Stress during pregnancy alters temporal and spatial dynamics of the maternal and offspring microbiome in a sex-specific manner. Sci Rep 2017;7:44182. 10.1038/srep44182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. van Bodegom M, Homberg JR, Henckens MJAG. Modulation of the hypothalamic-pituitary-adrenal axis by early life stress exposure. Front Cell Neurosci 2017;11:87. 10.3389/fncel.2017.00087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Haas KN, Blanchard JL. Reclassification of the Clostridium clostridioforme and Clostridium sphenoides clades as Enterocloster gen. nov. and Lacrimispora gen. nov., including reclassification of 15 taxa. Int J Syst Evol Microbiol 2020;70:23–34. 10.1099/ijsem.0.003698 [DOI] [PubMed] [Google Scholar]
- 30. Finegold SM, Song Y, Liu C, et al. Clostridium clostridioforme: a mixture of three clinically important species. Eur J Clin Microbiol Infect Dis 2005;24:319–24. 10.1007/s10096-005-1334-6 [DOI] [PubMed] [Google Scholar]
- 31. Finegold SM, Molitoris D, Song Y, et al. Gastrointestinal microflora studies in late-onset autism. Clin Infect Dis 2002;35:S6–16. 10.1086/341914 [DOI] [PubMed] [Google Scholar]
- 32. Song Y, Liu C, Finegold SM. Real-Time PCR quantitation of clostridia in feces of autistic children. Appl Environ Microbiol 2004;70:6459–65. 10.1128/AEM.70.11.6459-6465.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Weinstock M. Prenatal stressors in rodents: effects on behavior. Neurobiol Stress 2017;6:3–13. 10.1016/j.ynstr.2016.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kinney DK, Munir KM, Crowley DJ, et al. Prenatal stress and risk for autism. Neurosci Biobehav Rev 2008;32:1519–32. 10.1016/j.neubiorev.2008.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Weinstock M. The long-term behavioural consequences of prenatal stress. Neurosci Biobehav Rev 2008;32:1073–86. 10.1016/j.neubiorev.2008.03.002 [DOI] [PubMed] [Google Scholar]
- 36. Hantsoo L, Zemel BS. Stress gets into the belly: early life stress and the gut microbiome. Behav Brain Res 2021;414:113474. 10.1016/j.bbr.2021.113474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mackos AR, Varaljay VA, Maltz R, et al. Role of the intestinal microbiota in host responses to stressor exposure. Int Rev Neurobiol 2016;131:1–19. 10.1016/bs.irn.2016.08.002 [DOI] [PubMed] [Google Scholar]
- 38. Zijlmans MAC, Korpela K, Riksen-Walraven JM, et al. Maternal prenatal stress is associated with the infant intestinal microbiota. Psychoneuroendocrinology 2015;53:233–45. 10.1016/j.psyneuen.2015.01.006 [DOI] [PubMed] [Google Scholar]
- 39. Dhakal R, Bajpai VK, Baek K-H. Production of gaba (γ - Aminobutyric acid) by microorganisms: a review. Braz J Microbiol 2012;43:1230–41. 10.1590/S1517-83822012000400001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Seok J-H, Park K-B, Kim Y-H. Production and characterization of kimchi with enhanced levels of γ-aminobutyric acid;7. [Google Scholar]
- 41. Ueno H. Enzymatic and structural aspects on glutamate decarboxylase. J Mol Catal B Enzym 2000;10:67–79. 10.1016/S1381-1177(00)00114-4 [DOI] [Google Scholar]
- 42. Ma D, Lu P, Yan C, et al. Structure and mechanism of a glutamate-GABA antiporter. Nature 2012;483:632–6. 10.1038/nature10917 [DOI] [PubMed] [Google Scholar]
- 43. Lin XB, Wang T, Stothard P, et al. The evolution of ecological facilitation within mixed-species biofilms in the mouse gastrointestinal tract. Isme J 2018;12:2770–84. 10.1038/s41396-018-0211-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Núñez-Cardona MT. Influence of culture conditions on the fatty acid composition of green and purple photosynthetic sulphure bacteria. IntechOpen 2014. 10.5772/57389 [DOI] [Google Scholar]
- 45. Suutari M, Laakso S. Microbial fatty acids and thermal adaptation. Crit Rev Microbiol 1994;20:285–328. 10.3109/10408419409113560 [DOI] [PubMed] [Google Scholar]
- 46. Cui Y, Miao K, Niyaphorn S, et al. Production of gamma-aminobutyric acid from lactic acid bacteria: a systematic review. Int J Mol Sci 2020;21:995. 10.3390/ijms21030995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhang M-M, Liu S-B, Chen T, et al. Effects of NB001 and gabapentin on irritable bowel syndrome-induced behavioral anxiety and spontaneous pain. Mol Brain 2014;7:47. 10.1186/1756-6606-7-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Saito YA, Almazar AE, Tilkes KE, et al. Randomised clinical trial: pregabalin vs placebo for irritable bowel syndrome. Aliment Pharmacol Ther 2019;49:389–97. 10.1111/apt.15077 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
gutjnl-2022-328084supp001.pdf (3.7MB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.