Abstract
Objective
Digital healthcare systems could provide insights into the global prevalence of heart failure (HF). We designed the CardioRenal and Metabolic disease (CaReMe) HF study to estimate the prevalence, key clinical adverse outcomes and costs of HF across 11 countries.
Methods
Individual level data from a contemporary cohort of 6 29 624 patients with diagnosed HF was obtained from digital healthcare systems in participating countries using a prespecified, common study plan, and summarised using a random effects meta-analysis. A broad definition of HF (any registered HF diagnosis) and a strict definition (history of hospitalisation for HF) were used. Event rates were reported per 100 patient years. Cumulative hospital care costs per patient were calculated for a period of up to 5 years.
Results
The prevalence of HF was 2.01% (95% CI 1.65 to 2.36) and 1.05% (0.85 to 1.25) according to the broad and strict definitions, respectively. In patients with HF (broad definition), mean age was 75.2 years (95% CI 74.0 to 76.4), 48.8% (40.9–56.8%) had ischaemic heart disease and 34.5% (29.4–39.6%) had diabetes. In 51 442 patients with a recorded ejection fraction (EF), 39.1% (30.3–47.8%) had a reduced, 18.8% (13.5–24.0%) had a mildly reduced and 42.1% (31.5–52.8%) had a preserved left ventricular EF. In 1 69 518 patients with recorded estimated glomerular filtration rate, 49% had chronic kidney disease (CKD) stages III–V. Event rates were highest for cardiorenal disease (HF or CKD) and all cause mortality (19.3 (95% CI 11.3 to 27.1) and 13.1 (11.1 to 15.1), respectively), and lower for myocardial infarction, stroke and peripheral artery disease. Hospital care costs were highest for cardiorenal diseases.
Conclusions
We estimate that 1–2% of the contemporary adult population has HF. These individuals are at significant risk of adverse outcomes and associated costs, predominantly driven by hospitalisations for HF or CKD. There is considerable public health potential in understanding the contemporary burden of HF and the importance of optimising its management.
Keywords: Heart Failure, Epidemiology
What is already known on this topic
Few studies have assessed the burden of heart failure (HF) using both healthcare data from electronic healthcare records and national registries, and of those that have, highly selected patient populations that might not be representative of today’s problem have been described.
What this study adds
This study shows that the contemporary prevalence of heart failure is 2% when a broad definition of HF was used and 1% when a strict definition was applied, similar across several countries.
The most frequent comorbidities were ischaemic heart disease and chronic kidney disease (CKD) stages III– V. Patients with HF have high risks of cardiorenal complications (HF or CKD) and all cause mortality.
Furthermore, hospital care costs were highest for cardiorenal diseases, higher than those stemming from atherosclerotic cardiovascular diseases.
How this study might affect research, practice or policy
The cardiorenal burden, risks and costs in HF patients highlights an urgent need for improved risk management and an area that policy makers need to prioritise when planning healthcare for patients with HF.
Introduction
Heart failure affects up to 64 million people worldwide and its incidence is expected to rise with ageing populations and improved diagnostic methods.1 Heart failure already places an enormous economic burden on healthcare systems, with Europe and the US each allocating 1–2% of their annual healthcare budgets towards it.2
Heart failure management is changing rapidly following pivotal clinical trials,3–8 which are shaping treatment guidelines.9–11 Consequently, the population with heart failure is also evolving quickly. Multinational studies of the characteristics and outcomes in persons with heart failure are scarce, often describing highly selected patient groups and likely unrepresentative of today’s patient.12–14 Hence there is a need for a comprehensive understanding of the contemporary patient with heart failure. The CardioRenal and Metabolic disease (CaReMe) Heart Failure study collected detailed contemporaneous data from healthcare systems in 11 nations to determine the prevalence of heart failure and to detail patient characteristics, risks and costs associated with heart failure across the participating countries.
Materials and methods
Study setting and data sources
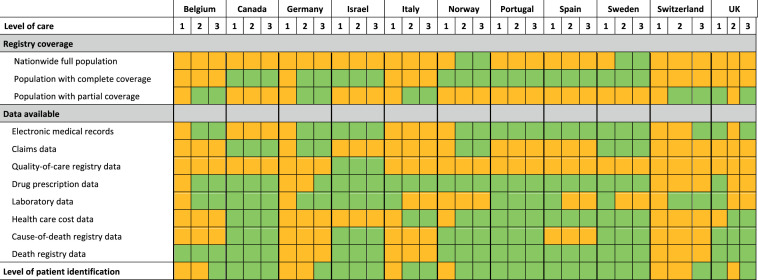
The multinational, observational CaReMe study used data from healthcare registries, including patient records from routine clinical practice across Belgium, Canada, Germany, Israel, Italy, Norway, Portugal, Spain, Sweden, Switzerland, and the UK (figure 1).14 A description of the data sources is provided in the online supplemental material (3–6) online supplemental material (pages 3–6). A heat map describing the coverage of the registries, data availability and healthcare level at which heart failure was identified is illustrated in figure 2. Permissions were obtained from ethics authorities before the start of the study in each participating country that required it. Approval numbers are available in the online supplemental materials (3-6).
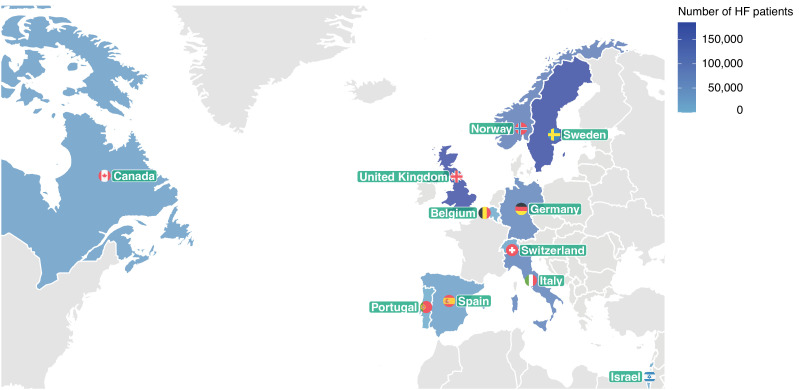
Figure 1.
Number of included patients with heart failure (HF) in each of the 11 participating countries.
Figure 2.
Description of data sources used across the participating countries. Data extractions are from the following levels of healthcare: (1) primary healthcare, (2) secondary healthcare (specialist or outpatient hospital care) and (3) tertiary healthcare (inhospital care). Green colour, Data available and utilized; Orange colour, Data not available.
heartjnl-2022-321702supp001.pdf (257KB, pdf)
Study population
To define the patient population, diagnoses of heart failure were searched for in all data available prior to the index date (online supplemental table S1). Prevalence was determined using a broad and a strict definition of heart failure. The broad definition included patients with a diagnosis of heart failure in a primary care or hospital setting.15 The strict definition was restricted to patients with history of a hospital admission where heart failure was the main diagnosis, reflecting the prevalence of validated heart failure diagnoses.15
Index years and follow-up time
Three cohorts were formed in each country to describe: cohort 1 (cross sectional), the most contemporary patient characteristics; cohort 2 (longitudinal risks), 1 year event rates; and cohort 3 (longitudinal costs), hospital healthcare costs over a period of up to 5 years. All patients were indexed on 1 January in the year that their country of residence entered the study (online supplemental table S2). The index year varied between nations to ensure that the most recent data available in each participating country were used, and thus that the most contemporary patient populations were formed. For cohorts 2 and 3, indexing was adjusted to allow sufficient follow-up.
Baseline characteristics
In cohort 1, comorbidities and laboratory variables were searched for in all available data prior to the index, except for cancer, where diagnoses were identified in the 5 year period prior to the index. Medication use (renin–angiotensin–aldosterone system inhibitors, beta blockers, mineralocorticoid receptor antagonists, angiotensin receptor–neprilysin inhibitors and sodium–glucose cotransporter 2 (SGLT-2) inhibitors) indicated by a filled drug prescription was searched for in the year prior to the index.
Outcomes
Clinical outcomes
In cohort 2, 1 year hospital event rates per 100 patient years from index year were calculated for hospitalisations with a main diagnosis of heart failure, chronic kidney disease (including diagnoses of chronic, acute, unspecified, diabetic, hypertensive, glomerular, tubulo-intestinal or dialysis), myocardial infarction, stroke, peripheral artery disease and all cause death (online supplemental table S3).
Hospital healthcare costs
In cohort 3, the cumulative costs were calculated for each patient for a period of up to 5 years, including costs for all first and repeated hospitalisations. Costs were extracted from registered diagnose related groups that were weighted and calculated within each country (eg, the actual reimbursement claims to the local payer).
Statistical analysis
Analyses were performed separately in each country according to a prespecified common statistical analysis plan. Baseline characteristics were described using mean and SD for numerical variables, and frequencies and percentages for categorical variables. Random effect estimates were used when pooling data, assuming some heterogeneity between countries. The pooled estimates from the random effects models are presented with 95% CIs. Tau was used to describe this heterogeneity, which corresponds to the estimated SD in the underlying distribution of true results across participating countries. All analyses were conducted using R statistical software (R V.3.5.0). The meta-analyses of means and proportions were performed using metamean and metaprop functions, respectively, in the meta package, and tau was estimated using a restricted maximum-likelihood estimator.
Event rates
Event rates were calculated as events per 100 patient years based on time to first event, and patients were censored at death or 1 year after the index. Patients without an event were censored at the end of follow-up or when leaving the database. All analyses of the cumulative incidence are descriptive and formal comparisons between countries were not performed.
Hospital healthcare costs
Costs were summarised annually within each patient as the total cost per year per diagnosis, and then summarised further within country as the mean cost per patient per year. Costs were censored from death onwards, whereas patients leaving the database were not included in the denominator from the year after leaving the database. Results are presented separately for each country and there was no standardisation or formal comparisons between countries. All diagnoses were analysed independently from other diagnoses and hospitalisations, given that more than one of the targeted diagnoses contributes costs to each of the included diagnoses. Therefore, one cannot add the hospital healthcare costs of two diagnoses to form a combined cost.
Patient and public involvement
Patients and the public were not involved in the design, conduct, reporting or dissemination plans of this study.
Results
Prevalence of heart failure
In a background population of >32 million adults, the pooled prevalence of heart failure was 2.01% (95% CI 1.65 to 2.36) and 1.05% (95% CI 0.85 to 1.25) according to the broad and strict heart failure definitions, respectively (table 1). The highest prevalence (broad definition) was in Portugal (2.9%) and the lowest in the UK (1.4%). In countries with nationwide coverage (Norway and Sweden), the prevalence of heart failure (broad definition) was 1.8% and 2.2%, respectively.
Table 1.
Prevalence of heart failure in 32 million patients across multiple countries in Asia, Europe and North America, 2018–20
| Canada | Israel | Italy | Norway* | Portugal | Spain | Sweden* | UK | Total | Pooled prevalence (95% CI) |
Tau | |
| Prevalence of heart failure | |||||||||||
| Broad definition (%) | 2.26 | n/a | 1.54 | 1.84 | 2.86 | 1.88 | 2.22 | 1.44 | 1.77 | 2.01 (1.65 to 2.36) | 0.48 |
| Strict definition (%) | 1.06 | 0.60 | 0.82 | 1.13 | 1.43 | n/a | 1.27 | 1.05 | 1.07 | 1.05 (0.85 to 1.25) | 0.27 |
| No of patients with heart failure | |||||||||||
| Strict definition (n) | 11 243 | 9759 | 35 660 | 46 840 | 1840 | n/a | 103 182 | 74 055 | 282 579 | ||
| Broad definition (n) | 23 953 | n/a | 67 369 | 76 561 | 3681 | 21 851 | 180 727 | 165 244 | 539 386 | ||
| Background population >18 years (n) | 1 060 153 | 1 622 570 | 4 363 833 | 4 153 579 | 128 605 | 1 189 003 | 8 147 081 | 11 496 448 | 32 161 272 |
Broad definition of heart failure=numbers of patients with a registered heart failure diagnosis in any available healthcare records. Strict definition of heart failure=only patients hospitalised with heart failure as the main diagnosis.
*Countries with nationwide coverage of patients with heart failure and background populations. Background populations were estimated based on the coverage of the healthcare registries for countries in which this information was available. Random effect estimates were used to calculate pooled values and tau describes the estimated SD of the underlying data across countries.
n/a, not available.
Baseline characteristics
A total of 6 29 440 patients with prevalent heart failure (broad definition) were identified between 2018 and 2020 (mean age 75.2 years (95% CI 74.0 to 76.4); 44.8% (95% CI 41.1 to 48.6) women; 48.8% (95% CI 40.9 to 56.8) had ischaemic heart disease; 44.1% (95% CI 39.1 to 49.0) had atrial fibrillation; and 34.5% (95% CI 29.4 to 39.6) had diabetes) (table 2). Most patients (74%) had a New York Heart Association (NYHA) class II or class III functional classification, whereas NYHA class I (13%) and class IV (13%) were less frequent. Regarding disease modifying medical treatment, 65.8% (95% CI 60.3 to 671.3) of patients were being treated with renin–angiotensin–aldosterone system inhibitors, 69.3% (95% CI 62.5 to 76.1) with beta blockers and 30.2% (95% CI 16.8 to 43.6) with mineralocorticoid receptor antagonists. Of the novel heart failure medications, 3.8% (95% CI 1.9 to 5.7) of patients were treated with angiotensin receptor–neprilysin inhibitors and 2.9% (95% CI 1.6 to 4.2) with SGLT-2 inhibitors. Device treatment was registered in 8.2% (95% CI 4.3–12.1) of patients.
Table 2.
Baseline characteristics of 629 440 contemporary patients with heart failure across 11 countries between 2018 and 2020
| Belgium | Canada | Germany*† | Israel | Italy | Norway | Portugal | Spain | Sweden | Switzerland* | UK | Pooled baseline (95% CI) |
Tau | |
| No of patients | 2379 | 23 953 | 63 712 | 9759 | 67 369 | 76 561 | 3681 | 21 851 | 180 727 | 14 204 | 165 244 | n/a | n/a |
| Index year | 2018 | 2019 | 2019 | 2020 | 2018 | 2020 | 2019 | 2019 | 2019 | 2019 | 2020 | ||
| Age (years) (mean (SD)) | 72 (17) | 75 (14) | 75 (12) | 74 (13) | 78 (12) | 74 (13) | 78 (12) | 78 (11) | 75 (13) | 74 (13) | 74 (13) | 75.2 (74.0 to 76.4) | 2.00 |
| Women (n (%)) | 932 (39) | 11 993 (50) | 27 892 (44) | 3681 (38) | 33 987 (50) | 30 746 (40) | 2171 (59) | 10 261 (47) | 77 791 (43) | 5612 (40) | 71 862 (43) | 44.8 (41.1 to 48.6) | 6.29 |
| NYHA functional classification (n (%)) | |||||||||||||
| I | 101 (9) | n/a | 2810 (5) | n/a | n/a | n/a | n/a | 2781 (13) | n/a | 436 (8) | 8768 (32) | 13.4 (3.8 to 23.0) | 10.92 |
| II | 472 (43) | n/a | 15 427 (27) | n/a | n/a | n/a | n/a | 9716 (45) | n/a | 1532 (27) | 12 668 (47) | 37.7 (29.0 to 46.4) | 9.94 |
| III | 419 (38) | n/a | 25 441 (45) | n/a | n/a | n/a | n/a | 8172 (38) | n/a | 2299 (40) | 5427 (20) | 36.2 (27.8 to 44.5) | 9.48 |
| IV | 105 (10) | n/a | 13 398 (23) | n/a | n/a | n/a | n/a | 821 (4) | n/a | 1446 (25) | 358 (1) | 12.7 (3.0 to 22.4) | 11.10 |
| Ischaemic heart disease (n (%)) | 1424 (60) | 13 850 (58) | 33 711 (53) | 5812 (60) | 19 720 (29) | 41 933 (55) | 1546 (42) | 4769 (22) | 87 152 (48) | n/a | 70 379 (43) | 48.8 (40.9 to 56.8) | 12.16 |
| Myocardial infarction (n (%)) | 883 (37) | 7042 (29) | 13 041 (20) | 3132 (32) | 7665 (11) | 23 160 (30) | 673 (18) | 3130 (14) | 62 768 (35) | n/a | 45 022 (27) | 25.5 (20.0 to 31.0) | 8.84 |
| Unstable angina (n (%)) | 9 (0) | 6126 (26) | 3399 (5) | 299 (3) | 2624 (4) | n/a | 437 (12) | 1034 (5) | 20 255 (11) | n/a | 5118 (3) | 7.7 (2.7 to 12.7) | 7.69 |
| Angina pectoris (n (%)) | 764 (32) | 13 282 (55) | 2978 (5) | 256 (3) | 19 080 (28) | 37 117 (48) | 1296 (35) | 1735 (8) | 63 302 (35) | n/a | 30 119 (18) | 26.8 (15.6 to 38.1) | 18.15 |
| Stroke (n (%)) | 428 (18) | 4133 (17) | 5112 (8) | 1147 (12) | 7297 (11) | 2298 (3) | 466 (13) | 2401 (11) | 28 415 (16) | n/a | 29 805 (18) | 12.6 (9.6 to 15.6) | 4.82 |
| Atrial fibrillation/flutter (n (%)) | 1258 (53) | 11 886 (50) | 29 675 (47) | 4144 (42) | 20 655 (31) | 39 544 (52) | 1482 (40) | 7246 (33) | 95 330 (53) | n/a | 67 552 (41) | 44.1 (39.1 to 49.0) | 7.97 |
| Peripheral artery disease (n (%)) | 236 (10) | 2729 (11) | 6547 (10) | 332 (3) | 5115 (8) | 7881 (10) | 216 (6) | 1050 (5) | 14 010 (8) | n/a | 11 985 (7) | 7.8 (6.2 to 9.5) | 2.62 |
| Diabetes (n (%)) | 865 (36) | 10 549 (44) | 21 564 (34) | 4868 (50) | 25 103 (37) | 16 039 (21) | 1540 (42) | 7371 (34) | 45 134 (25) | 3922 (28) | 48 533 (29) | 34.5 (29.4 to 39.6) | 8.61 |
| CKD diagnosis (n (%)) | 1515 (64) | 9766 (41) | 34 784 (55) | 6146 (63) | 11 082 (16) | 21 398 (28) | 898 (24) | 6143 (28) | 32 669 (18) | 6389 (45) | 60 331 (37) | 38.0 (28.0 to 48.0) | 16.91 |
| Cancer (n (%)) | 439 (18) | 3271 (14) | 1798 (3) | 2437 (25) | 7665 (11) | 19 637 (26) | 956 (26) | 2417 (11) | 53 011 (29) | n/a | 20 830 (13) | 17.6 (12.2 to 22.9) | 8.61 |
| Disease modifying HF drug treatment (n (%)) | 2379 (100) | 18 547 (77) | 5529 (95) | 9039 (93) | 56 895 (84) | 65 470 (86) | 3020 (82) | 19 407 (89) | 163 686 (91) | n/a | 150 758 (91) | 88.7 (84.7 to 92.8) | 6.56 |
| RAAS inhibitor (n (%)) | 1445 (61) | 13 827 (58) | 5007 (86) | 6697 (69) | 43 575 (65) | 50 879 (66) | 1837 (50) | 14 446 (66) | 132 989 (74) | 8409 (59) | 117 198 (71) | 65.8 (60.3 to 71.3) | 9.36 |
| ACE inhibitor (n (%)) | 1368 (58) | 9174 (38) | 2578 (44) | 3488 (36) | 23 926 (36) | 30 913 (40) | 1380 (37) | 6840 (31) | 74 681 (41) | 5746 (40) | 81 713 (49) | 41.0 (36.8 to 45.3) | 7.19 |
| ARB (n (%)) | 77 (3) | 4653 (19) | 1938 (33) | 3738 (38) | 23 033 (34) | 21 653 (28) | 487 (13) | 7606 (35) | 62 741 (35) | 3363 (24) | 40 177 (24) | 26.1 (19.8 to 32.5) | 10.77 |
| Beta blocker (n (%)) | 1914 (80) | 13 541 (57) | 4944 (85) | 7940 (81) | 36 842 (55) | 56 186 (73) | 1939 (53) | 15 160 (69) | 142 418 (79) | 8823 (62) | 113 060 (68) | 69.3 (62.5 to 76.1) | 11.46 |
| MRA (n (%)) | 2077 (87) | 2942 (12) | 1878 (32) | 3389 (35) | 15 474 (23) | 12 828 (17) | 452 (12) | 6816 (31) | 50 363 (28) | n/a | 40 299 (24) | 30.2 (16.8 to 43.6) | 21.59 |
| Sacubitril–valsartan (n (%)) | 138 (6) | 185 (1) | 595 (10) | n/a | 593 (1) | 2678 (3) | 41 (1) | 1632 (7) | 3887 (2) | 509 (4) | 5003 (3) | 3.8 (1.9 to 5.7) | 3.08 |
| SGLT-2i (n (%)) | 39 (2) | 568 (2) | 268 (5) | 840 (9) | 499 (1) | 2472 (3) | 73 (2) | 797 (4) | 3677 (2) | 164 (1) | 3086 (2) | 2.9 (1.6 to 4.2) | 2.18 |
| Other HF treatments (n (%)) | |||||||||||||
| Loop diuretics | 1360 (57) | 12 548 (52) | 4077 (70) | 5948 (61) | 37 532 (56) | 34 688 (45) | 1964 (53) | 15 680 (72) | 95 881 (53) | n/a | 85 769 (52) | 57.1 (52.0 to 62.3) | 8.24 |
| Digoxin | 591 (25) | 2095 (9) | 8 (0) | 556 (6) | 6851 (10) | 5221 (7) | n/a | 1676 (8) | 19 338 (11) | n/a | 19 842 (12) | 9.6 (5.3 to 13.9) | 6.61 |
| Device therapy* | n/a | 1145 (5) | 1021 (2) | 1683 (17) | 460 (1) | 7429 (10) | n/a | 1430 (7) | 28 702 (16) | 768 (5) | 20 036 (12) | 8.2 (4.3 to 12.1) | 5.92 |
| Nitrates (n (%)) | 77 (3) | 2999 (13) | 107 (2) | 975 (10) | 5805 (9) | 10 177 (13) | 443 (12) | 2411 (11) | 37 700 (21) | n/a | 32 047 (19) | 11.3 (7.5 to 15.0) | 6.02 |
| Warfarin (n (%)) | 648 (27) | 3331 (14) | 1108 (19) | 834 (9) | 11 107 (16) | 9786 (13) | 227 (6) | 4096 (19) | 39 739 (22) | n/a | 26 196 (16) | 16.0 (12.2 to 19.9) | 6.15 |
| Receptor P2Y12 antagonists (n (%)) | 627 (26) | 2782 (12) | 1563 (27) | 2092 (21) | 7636 (11) | 7722 (10) | 274 (7) | 2137 (10) | 15 040 (8) | n/a | 22 768 (14) | 14.7 (10.1 to 19.2) | 7.32 |
Random effect estimates were used to calculate pooled values and tau describes the estimated SD of underlying data across countries.
*Patients identified following a first hospitalisation for heart failure in a specified time period in Germany and Switzerland due to data availability, during 2019 and 2015–2019, respectively
†Laboratory and drug treatment data from one hospital, Leipzig Heart Centre, Leipzig, Germany.
ACE, angiotensin converting enzyme; ARB, angiotensin receptor blockers; CKD, chronic kidney disease; HF, heart failure; MRA, mineralocorticoid receptor antagonist; n/a, not available; NYHA, New York Heart Association; RAAS, renin–angiotensin–aldosterone system inhibitor; SGLT-2i, sodium–glucose cotransporter 2 inhibitors.
Baseline left ventricular ejection fraction and estimated glomerular filtration rate
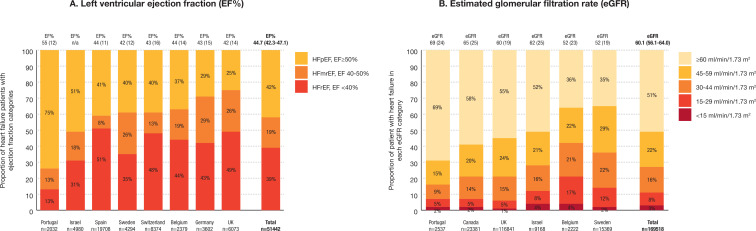
Measured left ventricular ejection fraction and estimated glomerular filtration rate (eGFR) were reported in 51 442 and 1 69 518 patients, respectively, representing 20% and 62% of patients with available electronic health records (online supplemental table S4). Left ventricular ejection fraction was reduced in 39.1% (95% CI 30.3 to 47.8), mildly reduced in 18.8% (95% CI 13.5 to 24.0) and preserved in 42.1% (95% CI 31.5 to 52.8) of those patients (figure 3A and online supplemental table S5). Of the 1 69 518 patients with a measured eGFR value, 49% had chronic kidney disease, stages III–V (eGFR of <60 mL/min/1.73 m2; figure 3B and online supplemental table S5).
Figure 3.
Baseline measurements of left ventricular ejection fraction (n=51 422) and estimated glomerular filtration rate (eGFR, n=1 69 518) across participating countries from data sources including these variables. (A) The proportion of 51 442 patients with heart failure and reduced (HFrEF), mildly reduced (HFmrEF) and preserved (HFpEF) left ventricular ejection fraction. Mean (SD) ejection fraction (EF%) is shown for each country on top of each bar. (B) The 1 69 518 patients with heart failure and a recorded eGFR value. Mean (SD) eGFR is shown for each country on top of each bar. Chronic kidney disease defined as eGFR <60 mL/min/1.73 m2.
Event rates and hospital healthcare costs
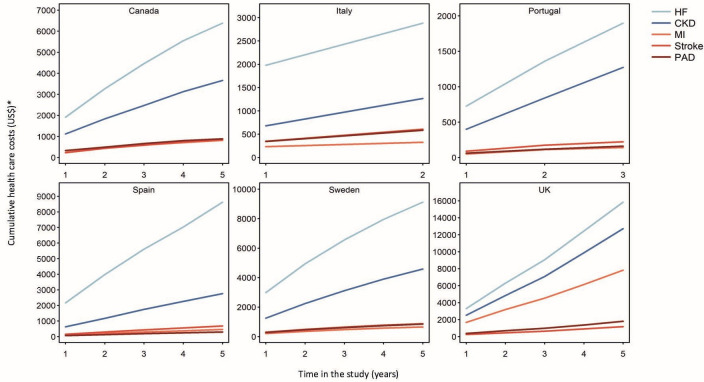
Patterns of events per 100 patient years in persons with prevalent heart failure were similar across countries, and highest for cardiorenal disease (19.3 events (95% CI 11.3 to 27.2)) and all cause mortality (13.10 events (95% CI 11.1 to 15.1)) (table 3). When the components of cardiorenal disease were assessed separately, event rates for heart failure and chronic kidney disease were 15 and 6 events per 100 patients years, respectively. Events per 100 patient years for myocardial infarction (2.7 events (95% CI 1.3 to 3.9)), stroke (1.8 events (95% CI 1.2 to 2.5)) and peripheral artery disease (1.4 events (95% CI 0.8 to 2.0)) were lower, with similar incidence patterns between countries. During the first year, 13.1% died. Hospital healthcare costs were available from six countries covering 462 825 (74%) patients in the population. Baseline and cumulative costs were highest for heart failure, followed by chronic kidney disease. In comparison, costs for atherosclerotic cardiovascular diseases were lower (figure 4 and online supplemental table S6).
Table 3.
One year event rates per 100 patient years in a contemporary multinational population with prevalent heart failure
| Belgium | Canada | Germany* | Israel | Italy | Norway | Portugal | Spain | Sweden | UK | Pooled event rates (95% CI) |
Tau | |
| Cardiorenal disease | n/a | 4735 (21.9) | 10 974 (18.6) | 1243 (13.3) | 14 017 (23.5) | 7848 (11.8) | 128 (13.3) | 8846 (48.8) | 14 106 (8.8) | 8750 (13.1) | 19.3 (11.3 to 27.2) | 12.09 |
| Heart failure | 256 (19.7) | 2918 (13.5) | 9722 (16.6) | 770 (8.2) | 9987 (16.1) | 6343 (9.5) | 107 (11.1) | 6512 (37.2) | 12 271 (7.6) | 6869 (10.2) | 15.0 (9.5 to 20.4) | 8.75 |
| Chronic kidney disease | 251 (19.1) | 1817 (8.4) | 1280 (2.2) | 482 (5.2) | 1531 (2.3) | 1996 (2.9) | 38 (4.0) | 2334 (11.5) | 2425 (1.5) | 2644 (3.8) | 6.0 (2.7 to 9.4) | 5.40 |
| Myocardial infarction | 113 (8.1) | 517 (2.4) | 652 (1.1) | 67 (0.7) | 1401 (2.1) | 1541 (2.3) | 21 (2.2) | 1060 (5.0) | 2289 (1.4) | 1320 (1.9) | 2.7 (1.4 to 3.9) | 2.06 |
| Stroke | 45 (3.1) | 375 (1.7) | 579 (1.0) | 56 (0.6) | 1699 (2.6) | 282 (0.4) | 18 (1.9) | 765 (3.6) | 2784 (1.7) | 1368 (2.0) | 1.8 (1.2 to 2.4) | 1.02 |
| Peripheral artery disease | 51 (3.5) | 284 (1.3) | 1619 (2.8) | 65 (0.7) | 846 (1.3) | 933 (1.4) | 1 (0.1) | 445 (2.1) | 1331 (0.8) | 451 (0.6) | 1.4 (0.8 to 2.0) | 0.98 |
| All cause death | 172 (10.7) | 2649 (12.1) | n/a | 1115 (11.9) | n/a | 7920 (11.6) | 114 (11.9) | 2677 (13.1) | 21 966 (13.2) | 13 869 (19.9) | 13.1 (11.1 to 15.1) | 2.89 |
Values are number of events (event rate per 100 patient years).
Random effect estimates were used to calculate pooled values, and tau describes the estimated SD of the underlying data across countries. High heart failure event rates in Spain is partly explained by physicians being prone to admit a patient earlier instead of ambulatory outpatient clinic follow-up.
*Patients identified following a first hospitalisation for heart failure in a specified time period in Germany and Switzerland due to data availability, during 2019 and 2015–2019, respectively.
†Countries with inhospital mortality death only.
n/a, not available.
Figure 4.
Cumulative hospital healthcare costs per patient in 3 62 825 patients with heart failure (HF) from six countries. Hospital healthcare cost data were available from Canada, Italy, Portugal, Spain, Sweden and the UK. Costs are in US$ per patient at index and cumulatively over a period of up to 5 years (from 2014 in Sweden, the UK and Canada; from 2015 in Spain; from 2017 in Portugal; and from 2018 in Italy). The x axis is the number of years (year 0 to 1 almost not illustrated). *For the purpose of currency conversion to US Dollars, US$1=0.77 Canadian Dollars, 1.13 Euros and 8.56 Swedish Krona. Fixed currency rates were used and variations over time were not accounted for. CKD, chronic kidney disease; MI, myocardial infarction; PAD, peripheral artery disease.
Discussion
From a contemporary routine clinical practice setting that included a background population of approximately 32 million people, this study characterised more than 600 000 patients with heart failure using digital healthcare registries in 11 countries, and estimated the total cost of heart failure in healthcare systems across Europe, Israel and North America. The prevalence of heart failure varied between 1% and 2%, dependent on whether a strict or broad definition of heart failure was applied. Those with heart failure had numerous comorbidities, with ischaemic heart disease and chronic kidney disease stages III–V being higher than previously reported. Despite large heterogeneity in phenotypes of heart failure between countries, mainly explained by variations in the data sources, similar event rates and cost patterns from heart failure were observed. Modern treatment with angiotensin receptor–neprilysin inhibitors, SGLT-2 inhibitors and devices was generally still low. Most healthcare costs were attributable to cardiorenal events, higher than those stemming from atherosclerotic cardiovascular diseases, illustrating high rates of repeated heart failure events and mortality following heart failure. Patients with heart failure were also at high risk of death (13% died after 1 year).
Prevalence of heart failure
The prevalence of heart failure (1–2%) is consistent with several European focused cohort studies conducted over the past two decades.16 However, as recently highlighted, heart failure often goes undiagnosed, and thus its prevalence could be as high as 4%.16 By applying a broader definition of heart failure, it can be expected that not only a higher prevalence would be estimated than that using the strict definition, but also increased discrepancy between countries. The recent European Heart Failure Atlas Survey also found variations in prevalence between countries (1.2–3.9%),16 potentially due to varying reporting practices and diagnostic tools, variation in the population’s average age and, perhaps more importantly, differences in the clusters of risk factors.
A population burdened by comorbidities
The average age (75 years) of the patients in this study was higher than that of the populations included in several randomised clinical trials and cohort studies focused on heart failure.4–8 Although the burden of comorbidities differed between countries, this study demonstrated that overall, around 50% of patients had ischaemic heart disease, one third had diabetes and about 50% had eGFR verified stage III–V chronic kidney disease (eGFR <60 mL/min/1.73 m2), of which most (78%) were stage IIIa or stage IIIb. This indicates that contemporary patients with heart failure in clinical practice are generally older and burdened with more comorbidities than previously reported in single country studies (routine healthcare settings) that are now ageing.11 13 17 This might partly be explained by a general trend of increasing survival, highlighting the importance of access to contemporary data to better understand the current population with heart failure.
Cardiorenal syndrome (heart failure and chronic kidney disease) has been associated with a substantially higher mortality risk than atherosclerotic cardiovascular diseases.18 19 This study reports a high prevalence of cardiorenal syndrome. The highest hospitalisation rates after the first year were related to cardiorenal causes, further emphasising the deleterious interaction between heart failure and chronic kidney disease, and highlighting the importance of detecting chronic kidney disease in patients with heart failure.19
Heart failure phenotypes
The overall distribution of heart failure with reduced (39%), mildly reduced (19%) and preserved (42%) left ventricular ejection fraction (HFrEF, HFmrEF and HFpEF, respectively) in routine clinical practice differs from other studies with highly selected populations in terms of HFrEF (56–60%) and HFpEF (16–23%),20 21 but is consistent with reports of increasing proportions of HFpEF in ageing populations.1 16 For instance, HFrEF is often reported to be more common in populations with acute heart failure.22 However, HFpEF or HFmrEF were most common (61%) phenotypes in the present study where data were collected in a routine clinical setting (at any healthcare level, both primary and hospital care, and not following an acute hospitalisation for heart failure). Proportions varied between countries, with higher incidences of HFpEF in countries with older populations, variations that might also be explained by how patients were referred or diagnosed (eg, availability of cardiologist examinations, accuracy of echocardiography measurements etc).
Risks
Event rates for heart failure and mortality were higher in this study compared with those reported by recent clinical trials in heart failure with reduced and preserved heart failure.4–8 This might be explained by a population identified in clinical practice, which was older in age, versus those formed in randomised clinical trials, indirectly highlighting the need for clinical trials in an older, more representative, patient population.4–8
Hospital healthcare costs in a population with heart failure
The cumulative costs analyses account for repeated events, rather than the time to first event. This provided the capacity to demonstrate that, over a 5 year period, hospital healthcare costs in patients with heart failure were mainly driven by cardiorenal events, and to a lesser extent by atherosclerotic cardiovascular disease events, further highlighting the need for improved cardiorenal prevention and management.
Observational data collected from contemporary, real world, routine, clinical practice settings at all healthcare levels are of increasing importance given that heart failure management is rapidly changing due to paradigm shifting trials3–8 and updated guidelines.9–11 Hence real time understanding of the characteristics of patients with heart failure, as well as its burden and treatment, in routine real world clinical practice is warranted to understand unmet clinical needs and the current implementation of new guidelines.23 24 For instance, it displays a truer comorbidity pattern of patients in need of intensified prevention, and thus informs how healthcare resources could be optimised. Further, it illustrates more realistic patterns and event rates resulting from heart failure than does the clinical trial setting, including more per protocol follow-up or disease specific registries where patients are often selected based on hospitalisation for heart failure. Moreover, data from the present study have been collected by all types of healthcare professionals interacting with patients with heart failure, and not only in a cardiology setting. Indeed, event rates in the present study were also higher than those in the most recent HFrEF trials, as discussed above. Finally, for researchers planning and interpreting clinical trial findings, the understanding of differences in characteristics and event rates across countries might be important to acknowledge if unexpected heterogeneity is seen in relation to treatment effects.25
This study used digital healthcare data to characterise over 600 000 patients with heart failure who were in routine clinical care. The recorded diagnoses for heart failure and chronic kidney disease used in that protocol have been validated previously, demonstrating high sensitivity and specificity (online supplemental material (3–6)).
Despite the strengths of this study, the findings should be interpreted with caution. The generalisability of our results to populations with very different circumstances in terms of race, resources or care is unknown. The prevalence of heart failure was not obtained in three of the 11 participating countries since estimation of the background population was missing. However, the robustness of the findings were supported by their consistency across heterogenous data sources (figure 2), representative population data (all countries) and different ethnicities (American, Asian and European; figure 1). Undetected and unreported heart failure in patients was not possible to assess in this study and might therefore underestimate the true prevalence. This study only assessed outcomes requiring hospital care, which might have also underestimated event rates with less severe conditions (eg, those managed in primary care). Some variables were not available in the registries (eg, ejection fraction (available in 20% of the population), eGFR (available in 62%), hypertension history, diabetes duration, body mass index, smoking, alcohol consumption, diet, physical activity, stress, socioeconomic and environmental factors), limiting the descriptive capacity of this study. Further, data sources were limited to high income countries.
Although hospital healthcare costs were obtained in six out of the 11 participating countries, the available data covers 74% of the total population with heart failure, providing an indication of what healthcare costs could amount to across all countries in the analysis. It was assumed that the national healthcare and reimbursement structure specifics would affect different diseases similarly, and that within country ranking of costs for different diseases would therefore be possible. Renal replacement therapy costs were handled differently in different countries and this is likely to affect some within country rankings; notably, rankings were nonetheless quite similar between countries. However, ultimately, total healthcare costs are likely to be underestimated in this study as most costs are attributed to hospital care and do not account for non-hospital related costs (eg, primary care, drugs, indirect disease burden (eg, sick leave), etc).
Conclusion
In this contemporary population from a routine clinical practice setting, the prevalence of heart failure was 1–2% in Europe, Canada and Israel. Of these, more than half (>60%) had mildly reduced or preserved heart failure and almost half showed signs of kidney failure. These individuals are at significant risk of adverse outcomes and associated costs, predominantly driven by hospitalisations for heart failure or chronic kidney disease. With rapidly improving treatments for heart failure, there is considerable public health potential in understanding the contemporary burden of heart failure and the importance of optimising its management.
Acknowledgments
This study is the result of the contributions from many collaborating investigators, statisticians and project managers from all participating countries, for which we are deeply grateful. We thank the scientific and statistical support from the the CaReMe Heart Failure Investigator group, Imke Masuy, LynxCare Clinical Research, Leuven; Monika Beles, Cardiovascular Center, Onze-Lieve-Vrouw Ziekenhuis Aalst, Aalst; Sebastian König PhD, Heart Center Leipzig at University of Leipzig and Leipzig Heart Institute, Leipzig; Vincent Pellissier PhD and Anne Nitsche, Leipzig Heart Institute, Leipzig; Cheli Melzer Cohen MD PhD, Maccabi Institute for Research and Innovation, Maccabi Healthcare Services, Tel Aviv; Letizia Dondi MSc, Fondazione ReS Ricerca e Salute, Casalecchio di Reno, Bologna; KI Birkeland MD PhD, Oslo University Hospital and University of Oslo, Oslo; Cristina Gavina MD PhD, Department of Cardiology, Hospital Pedro Hispano USLM; Roberto Alcazar MD, University Hospital Infanta Leonor, Madrid; Antonio Hormigo MD, Primary Care Center Puerta Blanca, Malaga; Nicolás Manito MD, University Hospital Bellvitge, Hospitalet de Llobregat, Barcelona; Jan W Eriksson MD PhD, Department of Medical Sciences, Clinical Diabetes and Metabolism, Uppsala University, Uppsala; Thomas Cars PhD, SENCE Research AB, Uppsala; Valentina Gonzalez-Jaramillo MD MSc and Professor Taulant Muka MD PhD, Institute of Social and Preventive Medicine (ISPM), University of Bern, Bern; Ruiqi Zhang PhD and Jil Billy Mamza PhD, Medical and Scientific Affairs, BioPharmaceuticals Medical, AstraZeneca, Cambridge. The study would not have been possible without the valuable management and support from Isabelle Fovel, Eef Vandendriessche, Zarha Vermeulen PhD and Marieke De Boeck PhD, AstraZeneca, Brussels; Navid Shobeiri PhD and Sheena Kayaniyil PhD, AstraZeneca, Ontario; Antje Arnold and Marija Halbach, AstraZeneca, Hamburg; Maya Greenbloom, AstraZeneca, Tel Aviv; Marco Gnesi PhD, Francesca Pluchinotta MD and Lavinia Narici, AstraZeneca, Milan; Mário Almeida, Hugo Martinho and Filipa Bernardo, AstraZeneca, Lisbon; Carlos Escobar Cervantes MD, University Hospital La Paz, Madrid; Beatriz Palacios PhD and Luis Varela MD, AstraZeneca, Madrid; Peter Langer, AstraZeneca, Bern. Special thanks to Susanna Jerström and Helena Goike PhD, AstraZeneca Nordic, Södertälje, for international coordination and publication support. The authors thank Jordan Loader PhD of Sence, Uppsala, Sweden, for providing medical writing support/editorial support, which was funded by AstraZeneca, Stockholm, Sweden, in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3). All authors are guarantors of the manuscript. Data from the Norwegian Patient Register, Norwegian Cause of Death Registry, and Norwegian Prescription Database have been used in this publication. The interpretation and reporting of these data are the sole responsibility of the authors, and no endorsement by the Norwegian patient register is intended nor should be inferred.
Footnotes
Twitter: @amibanerjee1
Contributors: All authors participated in the research design. MT performed the data management and statistical analyses for all countries after discussion with all authors. Statistical analyses were separately performed in Belgium, Canada, Germany, Israel, Italy, Norway, Portugal, Spain, Sweden and the UK. All authors participated in data interpretation and in writing the manuscript. AN, ABo and JB drafted the first manuscript with further adjustments from all authors. All authors took final responsibility in the decision to submit for publication. JB is the guarantor of this work and, as such, had full access to all the data in the study takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding: This work was sponsored by AstraZeneca. AstraZeneca, JB, supported the design, interpretation of results, writing of the manuscript and publication of this study together with the investigators. Study management and data extraction was coordinated by AstraZeneca in all countries.
Map disclaimer: The inclusion of any map (including the depiction of any boundaries therein), or of any geographic or locational reference, does not imply the expression of any opinion whatsoever on the part of BMJ concerning the legal status of any country, territory, jurisdiction or area or of its authorities. Any such expression remains solely that of the relevant source and is not endorsed by BMJ. Maps are provided without any warranty of any kind, either express or implied.
Competing interests: AN has received honoraria from MSD, AstraZeneca, Eli Lilly, Boehringer Ingelheim and Novo Nordisk. JB holds a full time position at AstraZeneca as an epidemiologist. NT reports grants and personal fees from AstraZeneca, grants and personal fees from Janssen, grants and personal fees from BI-Lilly, grants and personal fees from Otsuka, grants, personal fees and other from Tricida, personal fees and other from Pulsedata, personal fees and other from Mesentech, personal fees and other from Renibus, and other from ClinPredict, outside the submitted work; NT has a patent for a microfluidic device for point of care detection of urine albumin pending. AK has received research grants and speaking honoraria from Astrazeneca, Novonordisk and Boehringer Ingelheim. APM reports receiving fees for serving on study committees from AstraZeneca, Novartis, Bayer and Fresenius, outside the present work. TT-G declares speaker and consulting fees from AstraZeneca, BIAL, Daiichi-Sankyo, MSD, Medinfar and Novartis; TT-G holds shares in MTG. MB has received honoraria from Astra Zeneca, Janssen, Lilly, Boehringer Ingelheim, Sanofi, Amgen and Novo Nordisk. MT holds a full time position by an independent statistical consultant company, Statisticon AB, Uppsala, Sweden, of which AstraZeneca Nordic is a client. JS reports stock ownership in companies providing services to Itrim, Amgen, Janssen, Novo Nordisk, Eli Lilly, Boehringer, Bayer, Pfizer, Takeda and AstraZeneca, outside the submitted work. AB is supported by research funding from NIHR, British Medical Association, AstraZeneca and UK Research and Innovation. ABo is part of the BigData@Heart Consortium, funded by the Innovative Medicines Initiative-2 Joint Undertaking under grant agreement No 116074; this joint undertaking receives support from the European Union’s Horizon 2020 research and innovation programme and EFPIA. KAS has received speaking honoraria from Astrazeneca, Novonordisk, Sanofi and Boehringer Ingelheim.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
No data are available. The data sources used in the present are all underlying local, ethical and privacy restrictions for data transfer abroad or into public domain, limiting data availability on request. Therefore, the data that support the findings of this study are not available on request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by countries where applicable. Canada: the study was approved by the University of Manitoba Health Research Ethics Board (ethics file number HS223414 (H2019:454)). Norway: the study was approved by the Regional Ethics Committee, Helse Sør-Øst (reference Nos 2015/1337/REK sør-øst A and 11744) and was authorised by the Norwegian Data Inspectorate (Datatilsynet). Portugal: the study was approved by the ethics committee and the Data Protection Officer of USLM-EPE. Spain: the study was approved by the Investigation Ethics Committee of Consorci Sanitari from Terrassa. Sweden: the study was approved by the Stockholm Regional Ethics Committee (reference Nos 2020-05714 and 2013/2206-31); the study was also approved by the Ethical Review Authority (reference No 2020-03850). Switzerland: the study was approved for quality assurance by the ethics committee of the Canton Bern study (KEK-Nr. Req-2020-00980). UK: the overall study protocol was approved by the Independent Scientific Advisory Committee (ISAC) of CPRD; protocol reference No 19_264AR3; this was a secondary data study and data were fully anonymised and dissociated from patients.
References
- 1. Savarese G, Lund LH, Department of Cardiology, Karolinska University Hospital, Stockholm, Sweden . Global public health burden of heart failure. Card Fail Rev 2017;03:7–11. 10.15420/cfr.2016:25:2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stewart S, Jenkins A, Buchan S, et al. The current cost of heart failure to the National Health Service in the UK. Eur J Heart Fail 2002;4:361–71. 10.1016/S1388-9842(01)00198-2 [DOI] [PubMed] [Google Scholar]
- 3. Solomon SD, McMurray JJV, Claggett B, et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med 2022;387:1089–98. 10.1056/NEJMoa2206286 [DOI] [PubMed] [Google Scholar]
- 4. McMurray JJV, Packer M, Desai AS, et al. Angiotensin–neprilysin inhibition versus enalapril in heart failure. N Engl J Med Overseas Ed 2014;371:993–1004. 10.1056/NEJMoa1409077 [DOI] [PubMed] [Google Scholar]
- 5. McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019;381:1995–2008. 10.1056/NEJMoa1911303 [DOI] [PubMed] [Google Scholar]
- 6. Packer M, Anker SD, Butler J, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 2020;383:1413–24. 10.1056/NEJMoa2022190 [DOI] [PubMed] [Google Scholar]
- 7. Anker SD, Butler J, Filippatos G, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med 2021;385:1451–61. 10.1056/NEJMoa2107038 [DOI] [PubMed] [Google Scholar]
- 8. Heidenreich PA, Bozkurt B, Aguilar D. AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022;2022:e895–1032. 10.1161/CIR.0000000000001063 [DOI] [PubMed] [Google Scholar]
- 9. McDonagh TA, Metra M, Adamo M. Esc guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;2021:3599–726. 10.1093/eurheartj/ehab368 [DOI] [PubMed] [Google Scholar]
- 10. McDonald M, Virani S, Chan M, et al. CCS/CHFS heart failure guidelines update: defining a new pharmacologic standard of care for heart failure with reduced ejection fraction. Can J Cardiol 2021;37:531–46. 10.1016/j.cjca.2021.01.017 [DOI] [PubMed] [Google Scholar]
- 11. Conrad N, Judge A, Tran J, et al. Temporal trends and patterns in heart failure incidence: a population-based study of 4 million individuals. Lancet 2018;391:572–80. 10.1016/S0140-6736(17)32520-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Seferović PM, Vardas P, Jankowska EA, et al. The Heart Failure Association Atlas: heart failure epidemiology and management statistics 2019. Eur J Heart Fail 2021;23:906–14. 10.1002/ejhf.2143 [DOI] [PubMed] [Google Scholar]
- 13. Gerber Y, Weston SA, Redfield MM, et al. A contemporary appraisal of the heart failure epidemic in Olmsted County, Minnesota, 2000 to 2010. JAMA Intern Med 2015;175:996–1004. 10.1001/jamainternmed.2015.0924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sundström J, Bodegard J, Bollmann A, et al. Prevalence, outcomes, and cost of chronic kidney disease in a contemporary population of 2·4 million patients from 11 countries: the CaReMe CKD study. Lancet Reg Health Eur 2022;20:100438. 10.1016/j.lanepe.2022.100438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ingelsson E, Arnlöv J, Sundström J, et al. The validity of a diagnosis of heart failure in a hospital discharge register. Eur J Heart Fail 2005;7:787–91. 10.1016/j.ejheart.2004.12.007 [DOI] [PubMed] [Google Scholar]
- 16. Rosano GMC, Seferovic P, Savarese G, et al. Impact analysis of heart failure across European countries: an ESC‐HFA position paper. ESC Heart Fail;26. 10.1002/ehf2.14076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lindmark K, Boman K, Olofsson M, et al. Epidemiology of heart failure and trends in diagnostic work-up: a retrospective, population-based cohort study in Sweden. Clin Epidemiol 2019;11:231–44. 10.2147/CLEP.S170873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Birkeland KI, Bodegard J, Eriksson JW, et al. Heart failure and chronic kidney disease manifestation and mortality risk associations in type 2 diabetes: a large multinational cohort study. Diabetes Obes Metab 2020;22:1607–18. 10.1111/dom.14074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rangaswami J, Bhalla V, Blair JEA, et al. Cardiorenal syndrome: classification, pathophysiology, diagnosis, and treatment strategies: a scientific statement from the American Heart Association. Circulation 2019;139:e840–78. 10.1161/CIR.0000000000000664 [DOI] [PubMed] [Google Scholar]
- 20. Chioncel O, Lainscak M, Seferovic PM, et al. Epidemiology and one-year outcomes in patients with chronic heart failure and preserved, mid-range and reduced ejection fraction: an analysis of the ESC heart failure long-term registry. Eur J Heart Fail 2017;19:1574–85. 10.1002/ejhf.813 [DOI] [PubMed] [Google Scholar]
- 21. Savarese G, Settergren C, Schrage B, et al. Comorbidities and cause-specific outcomes in heart failure across the ejection fraction spectrum: a blueprint for clinical trial design. Int J Cardiol 2020;313:76–82. 10.1016/j.ijcard.2020.04.068 [DOI] [PubMed] [Google Scholar]
- 22. Chioncel O, Mebazaa A, Harjola V-P, et al. Clinical phenotypes and outcome of patients hospitalized for acute heart failure: the ESC heart failure long-term registry. Eur J Heart Fail 2017;19:1242–54. 10.1002/ejhf.890 [DOI] [PubMed] [Google Scholar]
- 23. Savarese G, Bodegard J, Norhammar A, et al. Heart failure drug titration, discontinuation, mortality and heart failure hospitalization risk: a multinational observational study (US, UK and Sweden). Eur J Heart Fail 2021;23:1499–511. 10.1002/ejhf.2271 [DOI] [PubMed] [Google Scholar]
- 24. Savarese G, Kishi T, Vardeny O, et al. Heart failure drug Treatment-Inertia, titration, and discontinuation: a multinational observational study (evolution HF). JACC Heart Fail 2022. 10.1016/j.jchf.2022.08.009 [DOI] [PubMed] [Google Scholar]
- 25. Lam CSP, Ferreira JP, Pfarr E, et al. Regional and ethnic influences on the response to empagliflozin in patients with heart failure and a reduced ejection fraction: the EMPEROR-Reduced trial. Eur Heart J 2021;42:4442–51. 10.1093/eurheartj/ehab360 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
heartjnl-2022-321702supp001.pdf (257KB, pdf)
Data Availability Statement
No data are available. The data sources used in the present are all underlying local, ethical and privacy restrictions for data transfer abroad or into public domain, limiting data availability on request. Therefore, the data that support the findings of this study are not available on request.