Abstract
Background
A thrombus in the M1 segment of the middle cerebral artery (MCA) can occlude this main stem only or extend into the M1-M2 bifurcation. The occlusion pattern may affect endovascular treatment (EVT) success, as a bifurcated thrombus may be more prone to fragmentation during retrieval.
Objective
To investigate whether bifurcated thrombus patterns are associated with EVT procedural and clinical outcomes.
Methods
Occlusion patterns of MCA thrombi on CT angiography from MR CLEAN Registry patients were classified into three groups: main stem occlusion, bifurcation occlusion extending into one M2 branch, and bifurcation occlusion extending into both M2 branches. Procedural parameters, procedural outcomes (reperfusion grade and embolization to new territory), and clinical outcomes (24-48 hour National Institutes of Health Stroke Scale [NIHSSFU] score, change in NIHSS scores between 24 and 48 hours and baseline [NIHSS], and 90-day modified Rankin Scale [mRS] scores) were compared between occlusion patterns.
Results
We identified 1023 patients with an MCA occlusion of whom 370 (36%) had a main stem occlusion, 151 (15%) a single branch, and 502 (49%) a double branch bifurcation occlusion. There were no statistically significant differences in retrieval method, procedure time, number of retrieval attempts, reperfusion grade, and embolization to new territory between occlusion patterns. Patients with main stem occlusions had lower NIHSSFU scores than patients with single (7 vs 11, p=0.01) or double branch occlusions (7 vs 9, p=0.04). However, there were no statistically significant differences in NIHSS or in 90-day mRS scores.
Conclusions
In our population, EVT procedural and long-term clinical outcomes were similar for MCA bifurcation occlusions and MCA main stem occlusions.
Keywords: Stroke, Thrombectomy, Stent, CT Angiography
Introduction
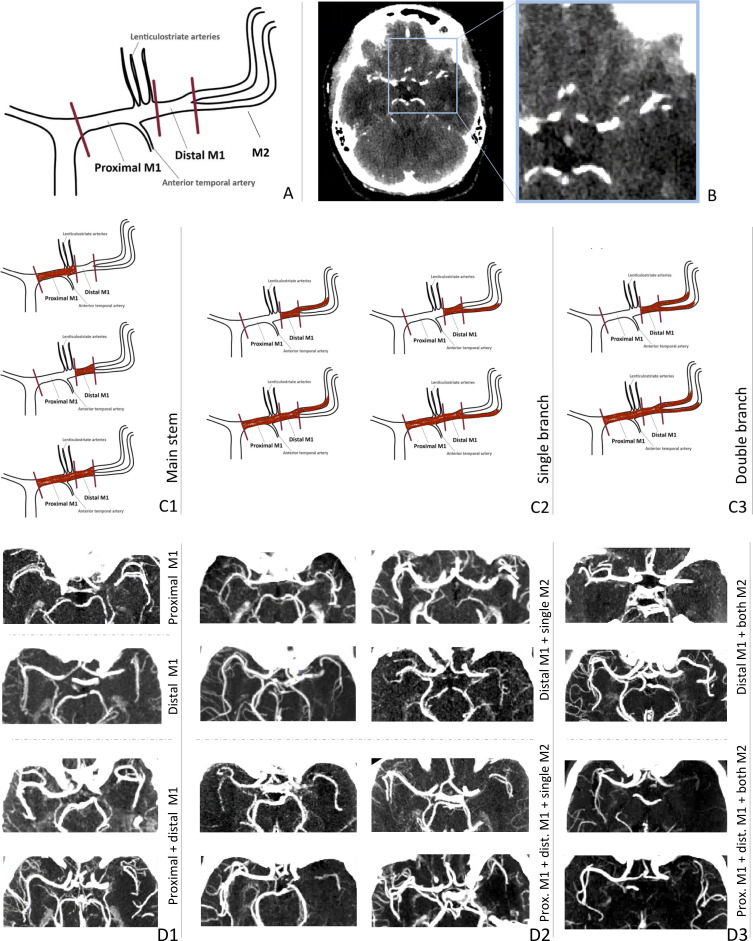
Middle cerebral artery (MCA) occlusions are the most common large vessel occlusions in patients with acute ischemic stroke (AIS).1 The configuration of the MCA is highly variable across patients.2 Most often, the M1 main stem bifurcates into two M2 segments: the frontoparietal M2 and the temporal M2 (figure 1A).
Figure 1.
(A) Middle cerebral artery (MCA) configuration: proximal M1, distal M1 and the M2 bifurcation: frontoparietal M2 and temporal M2. (B) Axial view of a CT angiography (CTA) scan showing a proximal M1 occlusion, identifiable by a sudden stop of contrast filling. (C) Classification of MCA occlusion patterns: C1, main stem; C2, single branch; and C3, double branch occlusions. (D) Examples of thin-slab maximum intensity projections of CTA scans showing each occlusion pattern. Some scans are mirrored so all occlusions can be found on the right. (D1) Main stem occlusions where proximal M1, distal M1, or both proximal and distal M1 are occluded, from top to bottom. (D2) Single branch occlusion where the proximal and/or distal M1 and only one M2 branch (temporal or frontoparietal) are occluded. (D3) Double branch occlusion where the proximal and/or distal M1 and both M2 branches are occluded.
A thrombus in the MCA can occlude the main stem only or extend into one or two of the M2 branches. This difference in occlusion pattern may affect endovascular treatment (EVT) success. For example, when the thrombus extends into both M2 branches, one stent retriever (or aspiration device) cannot capture both branches simultaneously, as it can only be extended into one branch. During EVT, the interventionalist positions the treatment device in one branch, expecting that the thrombus segment occluding the other branch will come along. However, these bifurcated thrombi can be refractory to retrieval, requiring multiple retrieval attempts aimed at alternating branches.3–5 Increasing the number of retrieval attempts may reduce the chance of good outcome by increasing the procedure time, the risk of thrombus fragmentation and of other procedural complications.6
The use of two stent retrievers simultaneously has been reported for difficult cases where the bifurcated thrombus cannot be removed by a single stent retriever.3–5 7–9 However, this novel technique has been reported only in individual case studies, which makes it questionable whether it is a safe technique to use.
In this study, we aim to investigate whether MCA bifurcation occlusions are different from MCA main stem occlusions for EVT procedural and clinical outcomes. Such information may support the development of (and need for) novel EVT techniques.
Methods
Patient selection
Patients included in this study were recruited from the MR CLEAN Registry, a multicenter prospective observational registry of all patients undergoing EVT for AIS in the Netherlands.10 This registry was approved by the central medical ethics committee of the Erasmus Medical Center Rotterdam, which served as the review board of all participating centers and granted permission to carry out the study as a registry (MEC-2014-235). All patients or legal representatives were provided with oral and written information on the registry and had the opportunity to withdraw consent to use their data.
We included patients with AIS aged 18 years or older, with an MCA occlusion, who underwent EVT with a stent retriever (with or without aspiration) or aspiration device between March 2014 and November 2017. Patients without contraindications received 0.9 mg/kg of intravenous alteplase prior to EVT. The EVT approach and choice of material was left to the individual interventionalist. Source data for this study are not available owing to privacy regulations, but analysis methods, codes, and results are available from the corresponding author on reasonable request.
Data collection
All patients underwent a standard stroke imaging protocol at baseline, consisting of baseline non-contrast computed tomography (NCCT) and CT angiography (CTA). The MR CLEAN Registry imaging core laboratory, which has been involved in all major clinical studies of the MR CLEAN Registry, assessed occlusion location, clot burden score (CBS), Alberta Stroke Program Early CT Score (ASPECTS), and collateral score. Core laboratory members were blinded to all clinical information except symptom side.10
Occlusion pattern classification
MCA segments were defined as follows: proximal M1, distal M1, M2-frontoparietal, and M2-temporal (figure 1A). M3 and more distal MCA segments were not considered in this study. The MR CLEAN Registry central imaging core laboratory assessed which MCA segments were occluded based on the contrast-filling defects found on baseline CTA (figure 1B). The central imaging core laboratory in charge of the CTA modality consisted of 31 interventional neuroradiologists with at least 5 years of experience assessing CTA scans in daily clinical practice. Each observer assessed a subset of the total number of these CTA scans. For the scoring, three views (axial, sagittal, and coronal) were used, aided with a maximum intensity projection of the CTA scan, if available. To ensure scoring-homogeneity, the observers were provided with training and guidelines of relevant definitions of each occlusion segment, including the scheme shown in figure 1A. The scoring of bifurcating M2 branches was performed based on the perfusion territory (frontoparietal vs temporal). This criterion was also applied for special cases like trifurcations or early bifurcations. If the scoring was not clear, the scoring was reviewed by a senior radiologist. Based on the scored occluded segment(s), we distinguished between patients with:
An MCA main stem occlusion, where the proximal M1, distal M1, or both segments are occluded, but no M2 branches are occluded.
A bifurcated thrombus with occlusion of the M1 segment and only one M2 branch (temporal or frontoparietal), i.e., single branch occlusion.
A bifurcated thrombus with occlusion of the M1 segment and both M2 branches (temporal and frontoparietal), i.e., double branch occlusion.
A complete overview of these patterns is displayed in figure 1C, D.
EVT outcomes
For each occlusion pattern group, we compared the retrieval method (stent retriever vs aspiration), duration of EVT procedure, number of retrieval attempts, reperfusion grade, and presence of an embolus in a new (previously unaffected) vascular territory (ENT). Both reperfusion grade and ENT were assessed by the MR CLEAN Registry imaging core laboratory. Reperfusion grade after EVT was scored on digital subtraction angiography (DSA) according to the expanded Thrombolysis in Cerebral Infarction (eTICI) scale, ranging from 0 (no reperfusion) to 3 (complete reperfusion) and including a 2c score (99% reperfusion).10 If only one DSA view was available a maximum of 2a was scored. The presence of ENT was identified on the last DSA run and defined as a remaining occlusion not matching the primary target occlusion on the first DSA run.
Functional outcome
Stroke severity at 24 to 48 hours' follow-up was assessed using the National Institutes of Health Stroke Scale (NIHSSFU).11 Change in NIHSS score between hospital presentation and follow-up was calculated according to: NIHSS=NIHSSFU - NIHSSBL. A negative NIHSS value implies clinical improvement and a positive value means clinical deterioration. Patients’ functional outcome was assessed with the modified Rankin scale (mRS) at 90 days through telephone or in-person interviews by trained nurses.12 Presence of symptomatic intracranial hemorrhage (sICH) on follow-up NCCT was assessed by the MR CLEAN Registry core laboratory using the Heidelberg bleeding criteria for hemorrhage classification.13
Statistical analyses
In this exploratory study, we compared baseline clinical characteristics, EVT outcomes, and patient functional outcomes for the three occlusion patterns. Numerical data were reported as medians with IQR, and categorical data as numbers and percentages. The Kruskal-Wallis test was used to compare numerical data and the test (or Fisher exact test) for categorical data. Statistical significance was set at p<0.05. If a variable was statistically significantly different between the three groups, a post hoc analysis was performed to assess the pairwise differences: Mann-Whitney U test for numerical data, and pairwise test (with Bonferroni corrections) for categorical data. To further evaluate the effect of observed baseline differences, we performed a subgroup analysis to investigate if similar trends in outcome variables are observed after stratification based on age. We further performed a subgroup analysis where we stratified the population based on the used first-line EVT approach (stent retriever (with or without aspiration) or aspiration alone). In addition, for each occlusion pattern, we assessed the impact of proximal M1 occlusions. All analyses were performed with IBM SPSS statistics package software (version 26.0).
Results
Our study population consisted of 1023 patients. The patient inclusion flow chart can be found in the online supplemental figure S1. Of all included patients, 370/1023 (36%) patients had an MCA main stem occlusion, 151/1023 (15%) had a single branch occlusion, and 502/1023 (49%) had a double branch occlusion (online supplemental table S1).
neurintsurg-2021-018560supp001.pdf (196.9KB, pdf)
Baseline characteristics
Baseline characteristics of the three subgroups can be found in table 1. Patients with main stem occlusions were slightly younger than patients with single branch (70 years vs 74 years, p=0.03) and double branch occlusions (70 years vs 73 years, p=0.03). Patients with main stem occlusions had a higher collateral score than patients with single branch and double branch occlusions (p<0.01). Main stem occlusions resulted in higher CBS than single branch (8 vs 7, p<0.01) and double branch occlusions (8 vs 6, p<0.01).
Table 1.
Baseline characteristics of the three occlusion pattern groups
| Baseline characteristics | Main stem (n=370) | Single branch (n=151) | Double branch (n=502) | P value |
| Age – median (IQR) | 70 (59–79) | 74 (62–82) | 73 (63–80) | 0.03 |
| Sex, female – no./total (%) | 174/370 (47) | 73/151 (48) | 255/502 (51) | 0.54 |
| Medical history – no./total (%) | ||||
| Previous stroke | 60/368 (16) | 27/149 (18) | 93/500 (19) | 0.67 |
| Diabetes mellitus | 63/369 (17) | 18/150 (12) | 87/500 (17) | 0.27 |
| Hypertension | 185/364 (51) | 83/147 (56) | 274/495 (55) | 0.33 |
| Atrial fibrillation | 87/367 (24) | 37/147(25) | 132/499 (26) | 0.66 |
| Pre-stroke mRS score – no./total (%) | 0.43 | |||
| 0 | 243/363 (67) | 91/147 (62) | 327/491 (67) | |
| 1 | 57/363 (16) | 25/147 (17) | 63/491 (13) | |
| 2 | 26/363 (7) | 8/147 (5) | 35/491 (7) | |
| ≥3 | 37/363 (10) | 23/147 (16) | 66/491 (13) | |
| Systolic blood pressure† (mm Hg) – median (IQR) | 150 (130–166) | 149 (130–165) | 146 (130–162) | 0.77 |
| Diastolic blood pressure‡ (mm Hg) – median (IQR) | 80 (71–91) | 80 (70–90) | 80 (70–90) | 0.44 |
| NIHSSBL§ – median (IQR) | 15 (11–18) | 16 (12–20) | 15 (11–19) | 0.11 |
| ASPECTSBL¶ – median (IQR) | 9 (8–10) | 9 (8–10) | 9 (8–10) | 0.99 |
| CS – no./total (%) | <0.01 | |||
| 0 | 8/358 (2) | 0/150 (0) | 28/492 (6) | |
| 1 | 75/358 (21) | 56/150 (37) | 179/492 (36) | |
| 2 | 143/358 (40) | 80/150 (53) | 209/492 (42) | |
| 3 | 132/358 (37) | 14/150 (10) | 76/492 (15) | |
| CBS | 8 (6–8) | 7 (5–7) | 6 (4–6) | <0.01 |
| Treatment and workflow | ||||
| IVT – no./total (%) | 277/370 (75) | 112/151 (74) | 365/502 (73) | 0.66 |
| Transferred patients – no./total (%) | 191/370 (52) | 89/151 (59) | 281/502 (56) | 0.24 |
| Stroke onset* to first hospital presentation** (min) – median (IQR) | 55 (38–104) | 57 (40–105) | 57 (40–101) | 0.72 |
| Hospital presentation to IVT†† (min) – median (IQR) | 25 (19–32) | 23 (17–33) | 24 (18–33) | 0.41 |
| Stroke onset* to groin puncture (min) – median (IQR) | 193 (150–255) | 209 (160–270) | 192 (147–255) | 0.12 |
*Time of stroke onset was defined as the time of witnessed symptom onset or, if unknown, as the time the patient was last seen well.
†Missing value: 26.
‡Missing value: 31.
§Missing value: 13.
¶Missing value: 1.
**Missing value: 185.
††Missing value: 429
ASPECTS, Alberta Stroke Programme Early CT Score; BL, baseline; CBS, clot burden score; CS, collateral score; CT, computed tomography; IQR, interquartile range; IVT, intravenous treatment with alteplase; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale.
EVT outcomes
Stent retrievers were used as a first-line device in 72% of patients with main stem occlusions, in 75% of patients with single branch, and in 73% of patients with double branch occlusions (p=0.77). There were no statistically significant differences in duration of EVT procedure, number of retrieval attempts, reperfusion grade, and presence of ENT between the three groups (table 2).
Table 2.
EVT outcomes: first-line device used, duration of EVT procedure, number of retrieval attempts, eTICI scores, and ENT
| EVT outcomes | Main stem, n=370 | Single branch, n=151 | Double branch, n=502 | P value |
| First-line device | 0.77 | |||
| Stent retriever | 268/370 (72) | 114/151 (75) | 368/502 (73) | |
| Aspiration | 102/370 (28) | 37/151 (25) | 134/502 (27) | |
| Procedural time* (min) – median (IQR) | 55 (36–80) | 60 (37–89) | 56 (38–83) | 0.62 |
| Retrieval attempts – no./total (%) | 0.38 | |||
| 1 | 146/343 (42) | 67/144 (47) | 194/476 (41) | |
| 2 | 68/343 (20) | 28/144 (19) | 127/476 (27) | |
| 3 | 61/343 (18) | 24/144 (17) | 72/476 (15) | |
| 4 | 33/343 (10) | 10/144 (7) | 34/476 (7) | |
| ≥5 | 35/343 (10) | 15/144 (10) | 49/476 (10) | |
| eTICI scores – no./total (%) | 0.55 | |||
| 0 | 27/362 (7) | 14/148 (9) | 35/494 (7) | |
| 1 | 14/362 (4) | 7/148 (5) | 8/494 (2) | |
| 2a | 72/362 (20) | 30/148 (20) | 105/494 (21) | |
| 2b | 73/362 (20) | 31/148 (21) | 107/494 (22) | |
| 2c | 43/362 (12) | 14/148 (9) | 67/494 (14) | |
| 3 | 133/362 (37) | 52/148 (35) | 172/494 (35) | |
| ENT – no./total (%) | 11/336 (3) | 6/138 (4) | 22/465 (5) | 0.59 |
*Missing values: 71.
ENT, embolization to new (previously unaffected) vascular territory; eTICI, expanded Thrombolysis in Cerebral Infarction; EVT, endovascular treatment; IQR, interquartile range.
Functional outcome
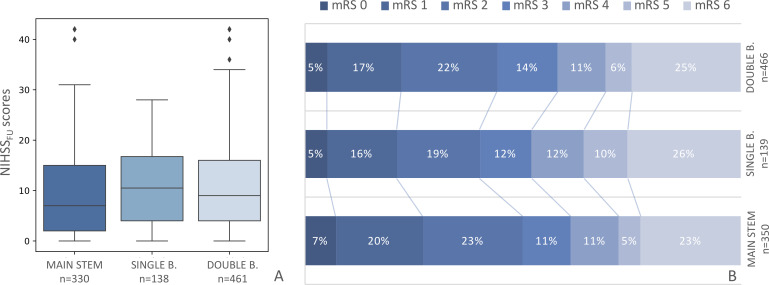
Patients with a main stem occlusion had lower NIHSSFU scores than patients with single branch (7 vs 11, p=0.01) and double branch occlusions (7 vs 9, p=0.04) (figure 2A). However, there were no statistically significant differences in the NIHSS scores for the three occlusion patterns (online supplemental table S2). Patients with main stem thrombi had slightly lower mRS scores at 90 days than patients with bifurcated thrombi (figure 2B), although these differences were not statistically significant (p=0.58). There were no significant differences in the occurrence of sICH (online supplemental file 2).
Figure 2.
2(A) Neurological deficit assessed by the National Institutes of Health Stroke Scale (NIHSS) scores at 24–48 hours (NIHSSFU) for patients with main stem, single branch, and double branch middle cerebral artery (MCA) occlusions. Patients with main stem occlusions have lower NIHSSFU scores than patients with single branch (p=0.01) and double branch (p=0.04) occlusions. (B) Functional outcome assessed by the modified Rankin scale (mRS) at 90 days for patients with a main stem, single branch, and double branch MCA occlusions. Between-group differences are not significant (p=0.58).
neurintsurg-2021-018560supp002.pdf (2MB, pdf)
The subgroup analysis, stratified by the median age of the study population, showed similar trends in older patients (age >72 years): main stem occlusion had lower NIHSSFU scores than patients with single branch occlusions (8 vs 13, p=0.01), but were not significantly different from double branch occlusions (8 vs 11, p=0.07) after adjusting with Bonferroni corrections. For younger patients (age <72 years): the three groups showed a median NIHSSFU score of 7 (p=0.46) (online supplemental table S3).
Subgroup analysis: first-line EVT approach
Stent retriever
The results of the stent-retriever subgroup analysis can be found in online supplemental table S4. We found no differences in EVT outcomes. Patients with main stem occlusions had lower NIHSSFU scores than patients with single branch occlusions (7 vs 10, p=0.03), but were not significantly different from double branch occlusions (7 vs 9, p=0.07) after Bonferroni corrections. There were no differences in NIHSS or in mRS scores. We found differences in the occurrence of sICH between the three groups; however these differences did not remain when performing a post hoc analysis with Bonferroni corrections.
Aspiration
We found no differences in EVT or functional outcomes for the three occlusion patterns for patients treated with aspiration. Results of the aspiration subgroup analysis can be found in online supplemental table S5.
Impact of proximal M1 occlusions
We found similar EVT procedural and functional outcomes in main stem and single branch patients with and without an occluded proximal M1 segment. The results can be found in the online supplemental tables S6 and S7.
We found significant differences in the distribution of eTICI scores in patients with a double branch occlusion: double branch patients with a proximal M1 occlusion had less often 2a scores than patients without a proximal M1 occlusion (12% vs 25%, p=0.04 after Bonferroni corrections). Double branch patients with a proximal M1 occlusion also had higher median NIHSSFU scores than patients without a proximal M1 occlusion (11 vs 8, p<0.01). However, we found similar NIHSS and 90-day mRS scores in both groups. The results can be found in the online supplemental table S8.
Discussion
In this study, we found no differences in EVT procedural outcomes based on MCA occlusion pattern groups, when comparing main stem, single branch, and double branch occlusions. We found that patients with main stem occlusions had better NIHSS scores at 24-48 hours than patients with bifurcated thrombi; however, there were no differences in NIHSS scores or in long-term functional outcomes as measured with the 90-day mRS.
MCA main stem and bifurcation occlusions have previously been compared in 62 patients, where lower rates of successful recanalization were found in patients with bifurcated thrombi than in main stem thrombi, but no differences in long-term functional outcomes.14 Studies comparing M1 and M2 occlusions have reported similar results for recanalization and complication rates.15 16 Proximal and distal M1 occlusions have also been associated with similar post-EVT clinical outcomes.17 Other studies showed that shorter distance from the internal cerebral artery terminus to an MCA occlusion is related to higher rates of successful recanalization after EVT.18
Successful recanalization of an MCA artery depends on multiple factors besides the occlusion pattern/location - to enumerate a few: MCA tortuosity has been associated with reduced stent-retriever thrombectomy success.19 Smaller thrombi have been related to fewer EVT attempts, higher rates of successful recanalization, and better functional outcomes after EVT.20 21 Red blood cell-rich thrombi may be more prone to fragmentation during retrieval than fibrin-rich thrombi.22 The occlusion dynamics (ie, the way the thrombus occludes the vessel) might affect thrombus removal: emboli can be bent and folded while traveling along the vascular system before they obstruct a vessel.23 24 Ultimately, all these thrombus characteristics result in a wide spectrum of biomechanical responses, which may affect thrombus-device interaction, and therefore thrombus retrieval.25–27
We found higher NIHSSFU scores in patients with MCA main stem thrombi than in those with bifurcated thrombi, while the 90-day mRS scores remained similar. NIHSS scores at 24-48 hours may better reflect consequences of the procedure than mRS scores at 3 months. The mRS score lacks specificity compared with the NIHSS score, since it accounts for all patient disabilities (even if they are not related to the AIS) and is a more coarse scale, including 7 rather than 42 levels.28 The longer the time between treatment and outcome assessment, the more probable that the outcome score will be affected by other factors, such as patient comorbidities or adverse effects at a later stage.
On the other hand, given that the procedural parameters were similar between the three groups, the observed difference in NIHSSFU scores might be due to other between-group differences, e.g., age, and not because of the occlusion patterns. The stratification analysis showed that when comparing patients with similar ages, the distribution of NIHSSFU scores remain different only for older patients.
The occlusion pattern can affect the length and complexity of procedures (which are associated with functional outcome), and it can also directly affect the functional outcome of the patient, regardless of the treatment. Main stem occlusions are less extensive (higher CBS) than bifurcation occlusions. A higher CBS has previously been related to better patient functional outcome.29 However, we found no such differences in functional outcome among the occlusion pattern groups.
In patients with double branch occlusions, we found that patients without an occluded proximal M1 segment had more eTICI 2a scores than patients with an occluded proximal M1, while the other eTICI categories remained similar. An eTICI score of 2a implies partial reperfusion (≤50%) of the target vascular territory. A bifurcated thrombus also occluding the proximal M1 segment could potentially better integrate with the stent since it has a larger stent-thrombus contact area, and therefore has a lower chance of fracture during retrieval than a shorter thrombus occluding only the distal M1 segment and the bifurcation. On the other hand, this finding could also be due to other factors, e.g., an uneven distribution of the single DSA views over the patients might also have caused this difference in eTICI 2a scores. Nevertheless, the ∆NIHSS and 90-day mRS scores remained similar for both groups.
The use of dual stents has been reported in the literature as a solution for refractory thrombi located in bifurcations.3–5 This approach was not used in our patient cohort. In our patient cohort, bifurcation occlusions are not different from main stem occlusions for EVT outcomes, and therefore our results do not a priori encourage the use of a double stents for bifurcated thrombi. However, the thrombus imaging and histological characteristics of these refractory thrombi should be further studied and considered when developing novel EVT techniques.
Limitations
Occlusion patterns were scored on single-phase CTA by human eye. As such, some inaccuracy and interobserver variability might have been introduced. Poor distal contrast filling might have caused misclassification. Patients with a double branch occlusion might be misclassified if there is a main stem occlusion in combination with poor collateral distal filling. This might be reflected in the EVT outcomes: patients with double branch occlusions had similar outcomes to patients with main stem occlusions, and had better outcomes than patients with single branch occlusions. Assessment on multiphase CTA could improve the classification of the occlusion pattern. Combined CTA- and NCCT-based thrombus segmentations, although time consuming, could more accurately assess the occlusion pattern and additionally provide information on the clot burden. However, the occlusion segment classification presented in this study was performed as done in current clinical practice: based only on CTA scans.
Interobserver variability was not assessed in this study. However, previous studies have shown that, among experienced observers (>5 years), interobserver agreement was substantial for the assessment of CTA scans.30
The occlusion patterns illustrated in this study are an oversimplification of all the complex patterns that can be found.
Conclusion
In our population, EVT procedural and clinical outcomes are similar for patients with MCA main stem and patients with MCA bifurcation occlusions. More research should be conducted to justify the use of a more aggressive EVT approach for bifurcation thrombi.
Footnotes
Collaborators: MR CLEAN Registry investigators: Executive committee: Diederik WJ Dippel (Department of Neurology, Erasmus MC University Medical Center); Aad van der Lugt (Radiology, Erasmus MC University Medical Center); Charles BLM Majoie (Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam); Yvo BWEM Roos (Neurology, Amsterdam UMC, University of Amsterdam, Amsterdam); Robert J van Oostenbrugge (Department of Neurology, Maastricht University Medical Center and Cardiovascular Research Institute Maastricht (CARIM)); Wim H van Zwam (Radiology, Maastricht University Medical Center and Cardiovascular Research Institute Maastricht (CARIM)); Jelis Boiten (Neurology, Haaglanden MC, the Hague); Jan Albert Vos (Radiology, Sint Antonius Hospital, Nieuwegein). Study coordinators: Ivo GH Jansen (Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam); Maxim JHL Mulder(Department of Neurology, Erasmus MC University Medical Center; Radiology, Erasmus MC University Medical Center); Robert- Jan B Goldhoorn (Department of Neurology, Maastricht University Medical Center and Cardiovascular Research Institute Maastricht (CARIM); Radiology, Maastricht University Medical Center and Cardiovascular Research Institute Maastricht (CARIM)); Kars CJ Compagne (Radiology, Erasmus MC University Medical Center); Manon Kappelhof (Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam); Josje Brouwer (Neurology, Amsterdam UMC, University of Amsterdam, Amsterdam); Sanne J den Hartog (Department of Neurology, Radiology, Public Health, Erasmus MC University Medical Center); Wouter H Hinsenveld (Department of Neurology, Maastricht University Medical Center and Cardiovascular Research Institute Maastricht (CARIM); Radiology, Maastricht University Medical Center and Cardiovascular Research Institute Maastricht (CARIM)),. Local principal investigators: Diederik WJ Dippel (Department of Neurology, Erasmus MC University Medical Center); Bob Roozenbeek (Department of Neurology, Erasmus MC University Medical Center); Aad van der Lugt (Radiology, Erasmus MC University Medical Center); Adriaan CGM van Es (Radiology, Erasmus MC University Medical Center); Charles BLM Majoie (Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam); Yvo BWEM Roos (Neurology, Amsterdam UMC, University of Amsterdam, Amsterdam); Bart J Emmer (Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam); Jonathan M Coutinho (Neurology, Amsterdam UMC, University of Amsterdam, Amsterdam); Wouter J Schonewille (Department of Neurology, Sint Antonius Hospital, Nieuwegein); Jan Albert Vos (Radiology, Sint Antonius Hospital, Nieuwegein); Marieke JH Wermer; Marianne AA van Walderveen (Department of Neurology, Radiology, Leiden University Medical Center); Julie Staals (Department of Neurology, Maastricht University Medical Center and Cardiovascular Research Institute Maastricht (CARIM)); Robert J van Oostenbrugge (Department of Neurology, Maastricht University Medical Center and Cardiovascular Research Institute Maastricht (CARIM)); Wim H van Zwam (Radiology, Maastricht University Medical Center and Cardiovascular Research Institute Maastricht (CARIM)); Jeannette Hofmeijer; Jasper M Martens (Department of Neurology, Radiology, Rijnstate Hospital, Arnhem); Geert J Lycklama à Nijeholt (Department of Radiology, Haaglanden MC, the Hague); Jelis Boiten (Neurology, Haaglanden MC, the Hague); Sebastiaan F de Bruijn (Department of Neurology, HAGA Hospital, the Hague); Lukas C van Dijk (Radiology, HAGA Hospital, the Hague); H Bart van der Worp (Department of Neurology, University Medical Center Utrecht); Rob H Lo (Radiology, University Medical Center Utrecht); Ewoud J van Dijk (Department of Neurology, Radboud University Medical Center, Nijmegen); Hieronymus D Boogaarts (Neurosurgery, Radboud University Medical Center, Nijmegen); J de Vries (Department of Neurology, Isala Klinieken, Zwolle); Paul LM de Kort (Department of Neurology, Elisabeth-TweeSteden ziekenhuis, Tilburg); Julia van Tuijl (Department of Neurology, Elisabeth-TweeSteden ziekenhuis, Tilburg); Jo P Peluso (Radiology, Elisabeth-TweeSteden ziekenhuis, Tilburg); Puck Fransen (Department of Neurology, Isala Klinieken, Zwolle); Jan SP van den Berg (Department of Neurology, Isala Klinieken, Zwolle); Boudewijn AAM van Hasselt (Radiology, Isala Klinieken, Zwolle); Leo AM Aerden (Department of Neurology, Reinier de Graaf Gasthuis, Delft); René J Dallinga (Radiology, Reinier de Graaf Gasthuis, Delft); Maarten Uyttenboogaart (Department of Neurology, University Medical Center Groningen); Omid Eschgi (Radiology, University Medical Center Groningen); Reinoud PH Bokkers (Radiology, University Medical Center Groningen); Tobien HCML Schreuder (Department of Neurology, Atrium Medical Center, Heerlen); Roel JJ Heijboer (Radiology, Atrium Medical Center, Heerlen); Koos Keizer (Department of Neurology, Catharina Hospital, Eindhoven); Lonneke SF Yo (Radiology, Catharina Hospital, Eindhoven); Heleen M den Hertog (Department of Neurology, Isala Klinieken, Zwolle); Tomas Bulut (Radiology, Medisch Spectrum Twente, Enschede); Paul JAM Brouwers (Department of Neurology, Medisch Spectrum Twente, Enschede). Imaging assessment committee: Charles BLM Majoie (Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam) (chair); Wim H van Zwam (Radiology, Maastricht University Medical Center and Cardiovascular Research Institute Maastricht (CARIM)); Aad van der Lugt (Radiology, Erasmus MC University Medical Center); Geert J Lycklama à Nijeholt (Department of Radiology, Haaglanden MC, the Hague); Marianne AA van Walderveen (Radiology, Leiden University Medical Center); Marieke ES Sprengers (Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam); Sjoerd FM Jenniskens (Radiology, Radboud University Medical Center, Nijmegen); René van den Berg (Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam); Albert J Yoo (Department of Radiology, Texas Stroke Institute, Texas, United States of America); Ludo FM Beenen (Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam); Alida A Postma (Radiology, Maastricht University Medical Center and Cardiovascular Research Institute Maastricht (CARIM)); Stefan D Roosendaal (Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam); Bas FW van der Kallen (Department of Radiology, Haaglanden MC, the Hague); Ido R van den Wijngaard (Department of Radiology, Haaglanden MC, the Hague); Adriaan CGM van Es (Radiology, Erasmus MC University Medical Center); Bart J Emmer (Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam); Jasper M. Martens (Radiology, Rijnstate Hospital, Arnhem); Lonneke SF Yo (Radiology, Catharina Hospital, Eindhoven); Jan Albert Vos (Radiology, Sint Antonius Hospital, Nieuwegein); Joost Bot (Department of Radiology, Amsterdam UMC, Vrije Universiteit van Amsterdam, Amsterdam); Pieter-Jan van Doormaal (Radiology, Erasmus MC University Medical Center); Anton Meijer (Radiology, Radboud University Medical Center, Nijmegen); Elyas Ghariq (Department of Radiology, Haaglanden MC, the Hague); Reinoud PH Bokkers (Radiology, University Medical Center Groningen); Marc P van Proosdij37; G Menno Krietemeijer (Radiology, Catharina Hospital, Eindhoven); Jo P Peluso (Radiology, Elisabeth-TweeSteden ziekenhuis, Tilburg); Hieronymus D Boogaarts (Neurosurgery, Radboud University Medical Center, Nijmegen); Rob Lo (Radiology, University Medical Center Utrecht); Wouter Dinkelaar (Radiology, Erasmus MC University Medical Center); Auke PA Appelman (Radiology, University Medical Center Groningen); Bas Hammer (Radiology, HAGA Hospital, the Hague); Sjoert Pegge (Radiology, Radboud University Medical Center, Nijmegen); Anouk van der Hoorn (Radiology, University Medical Center Groningen); Saman Vinke (Neurosurgery, Radboud University Medical Center, Nijmegen). Writing committee: Diederik WJ Dippel (Department of Neurology, Erasmus MC University Medical Center) (chair); Aad van der Lugt (Radiology, Erasmus MC University Medical Center); Charles BLM Majoie (Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam); Yvo BWEM Roos (Neurology, Amsterdam UMC, University of Amsterdam, Amsterdam); Robert J van Oostenbrugge (Department of Neurology, Maastricht University Medical Center and Cardiovascular Research Institute Maastricht (CARIM)); Wim H van Zwam (Radiology, Maastricht University Medical Center and Cardiovascular Research Institute Maastricht (CARIM)); Geert J Lycklama à Nijeholt (Department of Radiology, Haaglanden MC, the Hague); Jelis Boiten (Neurology, Haaglanden MC, the Hague); Jan Albert Vos (Radiology, Sint Antonius Hospital, Nieuwegein); Wouter J Schonewille (Department of Neurology, Sint Antonius Hospital, Nieuwegein); Jeannette Hofmeijer (Department of Neurology, Rijnstate Hospital, Arnhem); Jasper M Martens (Radiology, Rijnstate Hospital, Arnhem); H Bart van der Worp (Department of Neurology, University Medical Center Utrecht); Rob H Lo (Radiology, University Medical Center Utrecht). Adverse event committee: Robert J van Oostenbrugge (Department of Neurology, Maastricht University Medical Center and Cardiovascular Research Institute Maastricht (CARIM)) (chair); Jeannette Hofmeijer (Department of Neurology, Rijnstate Hospital, Arnhem); H Zwenneke Flach (Radiology, Isala Klinieken, Zwolle) Trial methodologist: Hester F Lingsma (Public Health, Erasmus MC University Medical Center) Research nurses / local trial coordinators: Naziha el Ghannouti (Department of Neurology, Erasmus MC University Medical Center); Martin Sterrenberg (Department of Neurology, Erasmus MC University Medical Center); Wilma Pellikaan (Department of Neurology, Sint Antonius Hospital, Nieuwegein); Rita Sprengers (Neurology, Amsterdam UMC, University of Amsterdam, Amsterdam); Marjan Elfrink (Department of Neurology, Rijnstate Hospital, Arnhem); Michelle Simons (Department of Neurology, Rijnstate Hospital, Arnhem); Marjolein Vossers (Radiology, Rijnstate Hospital, Arnhem); Joke de Meris (Neurology, Haaglanden MC, the Hague); Tamara Vermeulen (Neurology, Haaglanden MC, the Hague); Annet Geerlings (Department of Neurology, Radboud University Medical Center, Nijmegen); Gina van Vemde (Department of Neurology, Isala Klinieken, Zwolle); Tiny Simons (Department of Neurology, Atrium Medical Center, Heerlen); Gert Messchendorp (Department of Neurology, University Medical Center Groningen); Nynke Nicolaij (Department of Neurology, University Medical Center Groningen); Hester Bongenaar (Department of Neurology, Catharina Hospital, Eindhoven); Karin Bodde (Department of Neurology, Reinier de Graaf Gasthuis, Delft); Sandra Kleijn (Department of Neurology, Medisch Spectrum Twente, Enschede); Jasmijn Lodico (Department of Neurology, Medisch Spectrum Twente, Enschede); Hanneke Droste (Department of Neurology, Medisch Spectrum Twente, Enschede); Maureen Wollaert (Department of Neurology, Maastricht University Medical Center and Cardiovascular Research Institute Maastricht (CARIM)); Sabrina Verheesen (Department of Neurology, Maastricht University Medical Center and Cardiovascular Research Institute Maastricht (CARIM)); D Jeurrissen (Department of Neurology, Maastricht University Medical Center and Cardiovascular Research Institute Maastricht (CARIM)); Erna Bos (Department of Neurology, Leiden University Medical Center); Yvonne Drabbe (Department of Neurology, HAGA Hospital, the Hague); Michelle Sandiman (Department of Neurology, HAGA Hospital, the Hague); Nicoline Aaldering (Department of Neurology, Rijnstate Hospital, Arnhem); Berber Zweedijk (Department of Neurology, University Medical Center Utrecht); Jocova Vervoort (Department of Neurology, Elisabeth-TweeSteden ziekenhuis, Tilburg); Eva Ponjee (Department of Neurology, Isala Klinieken, Zwolle); Sharon Romviel (Department of Neurology, Radboud University Medical Center, Nijmegen); Karin Kanselaar (Department of Neurology, Radboud University Medical Center, Nijmegen); Denn Barning (Radiology, Leiden University Medical Center). PhD / Medical students: Esmee Venema (Public Health, Erasmus MC University Medical Center); Vicky Chalos (Department of Neurology, Erasmus MC University Medical Center; Public Health, Erasmus MC University Medical Center),; Ralph R Geuskens (Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam); Tim van Straaten (Department of Neurology, Radboud University Medical Center, Nijmegen); Saliha Ergezen (Department of Neurology, Erasmus MC University Medical Center); Roger RM Harmsma (Department of Neurology, Erasmus MC University Medical Center); Daan Muijres (Department of Neurology, Erasmus MC University Medical Center); Anouk de Jong (Department of Neurology, Erasmus MC University Medical Center); Olvert A Berkhemer (Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam; (Department of Neurology, Erasmus MC University Medical Center); Radiology, Maastricht University Medical Center and Cardiovascular Research Institute Maastricht (CARIM)); Anna MM Boers (Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam; Biomedical Engineering & Physics, Amsterdam UMC, University of Amsterdam, Amsterdam); J Huguet (Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam); PFC Groot (Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam); Marieke A Mens (Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam); Katinka R van Kranendonk (Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam); Kilian M Treurniet (Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam); Manon L Tolhuisen (Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam; Biomedical Engineering & Physics, Amsterdam UMC, University of Amsterdam, Amsterdam); Heitor Alves (Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam); Annick J Weterings (Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam), Eleonora LF Kirkels (Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam), Eva JHF Voogd (Department of Neurology, Rijnstate Hospital, Arnhem); Lieve M Schupp (Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam); Sabine L Collette (Department of Neurology, University Medical Center Groningen; Radiology, University Medical Center Groningen); Adrien ED Groot (Neurology, Amsterdam UMC, University of Amsterdam, Amsterdam); Natalie E LeCouffe (Neurology, Amsterdam UMC, University of Amsterdam, Amsterdam); Praneeta R Konduri (Biomedical Engineering & Physics, Amsterdam UMC, University of Amsterdam, Amsterdam); Haryadi Prasetya (Biomedical Engineering & Physics, Amsterdam UMC, University of Amsterdam, Amsterdam); Nerea Arrarte-Terreros (Biomedical Engineering & Physics, Amsterdam UMC, University of Amsterdam, Amsterdam); Lucas A Ramos (Biomedical Engineering & Physics, Amsterdam UMC, University of Amsterdam, Amsterdam).
Contributors: NAT developed the study, performed the data analysis, wrote the manuscript, and is the guarantor of the study. AAEB, HvV, and PRK contributed to the study design. IGHJ, MK, MLT, and NB helped with data acquisition and interpretation. DWJD, AvdL, WHvZ, RJvO, BvdW, BJE, FJAM, and YBWEMR contributed to the clinical assessment of the data. EvB, HAM, and CBLMM contributed to the research development and project supervision. All authors contributed to the final version of the manuscript.
Funding: This project has received funding from the European Union’s Horizon 2020 research and innovation program under grant agreement No 777 072 (INSIST project), and the AMC medical Research BV, Amsterdam UMC, location AMC, under project No 21 937. The MR CLEAN Registry was partly funded by TWIN Foundation, Erasmus MC University Medical Center, Maastricht University Medical Center, and Amsterdam UMC.
Competing interests: This project has received funding from the European Union’s Horizon 2020 research and innovation program under grant agreement No 777072 (INSIST project), and the AMC medical Research BV, Amsterdam UMC, location AMC, under project No 21937. The MR CLEAN registry is partially funded by unrestricted grants from the Applied Scientific Institute for Neuromodulation (Toegepast Wetenschappelijk Instituut voor Neuromodulatie), Erasmus Medical Center, Amsterdam University Medical Center and Maastricht University Medical Center. HAM reports co-founder and shareholder of Nicolab, a company that focuses on the use of artificial intelligence for medical image analysis. CBLMM reports grants from European Commission during the conduct of the study; grants from CVON/Dutch Heart Foundation, TWIN Foundation, and Stryker, outside the submitted work; and shareholder of Nicolab. IGHJ reports shareholder of Nicolab. DWJD and AvdL report unrestricted grants from Stryker, Penumbra, Medtronic, Cerenovus, Thrombolytic Science, LLC, Dutch Heart Foundation, Brain Foundation Netherlands, The Netherlands Organization for Health Research and Development, Health Holland Top Sector Life Sciences and Health, and Thrombolytic Science, LLC for research, paid to institution. BvdW received funds from Bayer and LivaNova for consultancy, paid to his institution, and grants from the Dutch Heart Foundation, the Horizon 2020 programme, and Stryker, paid to his institution, all outside the work submitted. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Contributor Information
MR CLEAN Registry investigators:
Diederik WJ Dippel, Aad van der Lugt, Charles BLM Majoie, Yvo BWEM Roos, Robert J van Oostenbrugge, Wim H van Zwam, Jelis Boiten, Jan Albert Vos, Ivo GH Jansen, Maxim JHL Mulder, Robert Jan Goldhoorn, Kars CJ Compagne, Manon Kappelhof, Josje Brouwer, Sanne J den Hartog, Wouter H Hinsenveld, Bob Roozenbeek, Adriaan CGM van Es, Bart J Emmer, Jonathan M Coutinho, Wouter J Schonewille, Marieke JH Wermer, Marianne AA van Walderveen, Julie Staals, Jeannette Hofmeijer, Jasper M Martens, Geert Lycklama Nijeholt, Sebastiaan F de Bruijn, Lukas C van Dijk, H Bart van der Worp, Rob H Lo, Ewoud J van Dijk, Hieronymus D Boogaarts, J de Vries, Paul LM de Kort, Julia van Tuijl, Jo P Peluso, Puck Fransen, Jan SP van den Berg, Boudewijn AAM van Hasselt, Leo AM Aerden, René J Dallinga, Maarten Uyttenboogaart, Omid Eschgi, Reinoud PH Bokkers, Tobien HCML Schreuder, Roel JJ Heijboer, Koos Keizer, Lonneke SF Yo, Heleen M den Hertog, Tomas Bulut, Paul JAM Brouwers, Marieke ES Sprengers, Sjoerd FM Jenniskens, René van den Berg, Albert J Yoo, Ludo FM Beenen, Alida A Postma, Stefan D Roosendaal, Bas FW van der Kallen, Ido R van den Wijngaard, Joost Bot, Pieter-Jan van Doormaal, Anton Meijer, Elyas Ghariq, Marc P van Proosdij, G Menno Krietemeijer, Wouter Dinkelaar, Auke PA Appelman, Bas Hammer, Sjoert Pegge, Anouk van der Hoorn, Saman Vinke, H Zwenneke Flach, Hester F Lingsma, Naziha el Ghannouti, Martin Sterrenberg, Wilma Pellikaan, Rita Sprengers, Marjan Elfrink, Michelle Simons, Marjolein Vossers, Joke de Meris, Tamara Vermeulen, Annet Geerlings, Gina van Vemde, Tiny Simons, Gert Messchendorp, Nynke Nicolaij, Hester Bongenaar, Karin Bodde, Sandra Kleijn, Jasmijn Lodico, Hanneke Droste, Maureen Wollaert, Sabrina Verheesen, D Jeurrissen, Erna Bos, Yvonne Drabbe, Michelle Sandiman, Nicoline Aaldering, Berber Zweedijk, Jocova Vervoort, Eva Ponjee, Sharon Romviel, Karin Kanselaar, Denn Barning, Esmee Venema, Vicky Chalos, Ralph R Geuskens, Tim van Straaten, Saliha Ergezen, Roger RM Harmsma, Daan Muijres, Anouk de Jong, Olvert A Berkhemer, Anna MM Boers, J Huguet, PFC Groot, Marieke A Mens, Katinka R van Kranendonk, Kilian M Treurniet, Manon L Tolhuisen, Heitor Alves, Annick J Weterings, Eleonora LF Kirkels, Eva JHF Voogd, Lieve M Schupp, Sabine L Collette, Adrien ED Groot, Natalie E LeCouffe, Praneeta R Konduri, Haryadi Prasetya, Nerea Arrarte-Terreros, and Lucas A Ramos
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information. Source data for this study are not available due to privacy regulations, but analysis methods, codes, and results are available from the corresponding author upon reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
These patients are part of the MR CLEAN Registry, a multicenter prospective observational registry of all patients undergoing EVT for AIS in the Netherlands. This registry was approved by the central medical ethics committee of the Erasmus Medical Center Rotterdam, which served as the review board of all participating centers (MEC-2014–235). The requirement for written informed consent was waived, but all patients or legal representatives were provided with oral and written information on the registry, and had the opportunity to withdraw consent to use their data via an opt-out form, conforming to the European Union General Data Protection Regulation.
References
- 1. Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 2016;387:1723–31. 10.1016/S0140-6736(16)00163-X [DOI] [PubMed] [Google Scholar]
- 2. Shapiro M, Raz E, Nossek E, et al. Neuroanatomy of the middle cerebral artery: implications for thrombectomy. J Neurointerv Surg 2020;12:768–73. 10.1136/neurintsurg-2019-015782 [DOI] [PubMed] [Google Scholar]
- 3. Moreu M, Pérez-García C, Gómez-Escalonilla C, et al. Dual save technique for mechanical thrombectomy rescue on MCA bifurcation clots. J Neurointerv Surg 2020;12:1034. 10.1136/neurintsurg-2020-016061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Asadi H, Brennan P, Martin A, et al. Double stent-retriever technique in endovascular treatment of middle cerebral artery saddle embolus. J Stroke Cerebrovasc Dis 2016;25:e9–11. 10.1016/j.jstrokecerebrovasdis.2015.10.005 [DOI] [PubMed] [Google Scholar]
- 5. Jiang C, Li Y, Hao F, et al. Y-configuration double-stent-retriever thrombectomy for refractory thrombus in middle cerebral artery bifurcation: a case report. Medicine 2021;100:e24993. 10.1097/MD.0000000000024993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Loh Y, Jahan R, McArthur DL, et al. Recanalization rates decrease with increasing thrombectomy attempts. AJNR Am J Neuroradiol 2010;31:935–9. 10.3174/ajnr.A1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Imahori T, Miura S, Sugihara M, et al. Double stent retriever (SR) technique: a novel mechanical thrombectomy technique to facilitate the device-clot interaction for refractory acute cerebral large vessel occlusions. World Neurosurg 2020;141:175–83. 10.1016/j.wneu.2020.05.268 [DOI] [PubMed] [Google Scholar]
- 8. Klisch J, Sychra V, Strasilla C, et al. Double Solitaire mechanical thrombectomy in acute stroke: effective rescue strategy for refractory artery occlusions? AJNR Am J Neuroradiol 2015;36:552–6. 10.3174/ajnr.A4133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Patro SN, Iancu D. Dual-stent retrieval for mechanical thrombectomy of refractory clot in acute stroke as a rescue technique. CMAJ 2017;189:E634–7. 10.1503/cmaj.160472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jansen IGH, Mulder MJHL, Goldhoorn R-JB, et al. Endovascular treatment for acute ischaemic stroke in routine clinical practice: prospective, observational cohort study (Mr CLEAN Registry). BMJ 2018;360:k949. 10.1136/bmj.k949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kwah LK, Diong J. National Institutes of Health Stroke Scale (NIHSS). J Physiother 2014;60:61. 10.1016/j.jphys.2013.12.012 [DOI] [PubMed] [Google Scholar]
- 12. Broderick JP, Adeoye O, Elm J. Evolution of the modified Rankin Scale and its use in future stroke trials. Stroke 2017;48:2007–12. 10.1161/STROKEAHA.117.017866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. von Kummer R, Broderick JP, Campbell BCV, et al. The Heidelberg bleeding classification: classification of bleeding events after ischemic stroke and reperfusion therapy. Stroke 2015;46:2981–6. 10.1161/STROKEAHA.115.010049 [DOI] [PubMed] [Google Scholar]
- 14. Guenego A, Fahed R, Sussman ES, et al. Impact of clot shape on successful M1 endovascular reperfusion. Front Neurol 2021;12:1–8. 10.3389/fneur.2021.642877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Compagne KCJ, van der Sluijs PM, van den Wijngaard IR, et al. Endovascular treatment: the role of dominant caliber M2 segment occlusion in ischemic stroke. Stroke 2019;50:419–27. 10.1161/STROKEAHA.118.023117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Saber H, Narayanan S, Palla M, et al. Mechanical thrombectomy for acute ischemic stroke with occlusion of the M2 segment of the middle cerebral artery: a meta-analysis. J Neurointerv Surg 2018;10:620–4. 10.1136/neurintsurg-2017-013515 [DOI] [PubMed] [Google Scholar]
- 17. Kim SH, Kim SW, Lee H, et al. Differences between proximal and distal M1 occlusions after mechanical thrombectomy. J Clin Neurosci 2021;87:1–7. 10.1016/j.jocn.2021.01.053 [DOI] [PubMed] [Google Scholar]
- 18. Pavabvash S, Taleb S, Majidi S, et al. Correlation of acute M1 middle cerebral artery thrombus location with endovascular treatment success and clinical outcome. J Vasc Interv Neurol 2017;9:17–22. [PMC free article] [PubMed] [Google Scholar]
- 19. Schwaiger BJ, Gersing AS, Zimmer C, et al. The curved MCA: influence of vessel anatomy on recanalization results of mechanical thrombectomy after acute ischemic stroke. AJNR Am J Neuroradiol 2015;36:971–6. 10.3174/ajnr.A4222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Baek J-H, Yoo J, Song D, et al. Predictive value of thrombus volume for recanalization in stent retriever thrombectomy. Sci Rep 2017;7:1–8. 10.1038/s41598-017-16274-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Soize S, Barbe C, Kadziolka K, et al. Predictive factors of outcome and hemorrhage after acute ischemic stroke treated by mechanical thrombectomy with a stent-retriever. Neuroradiology 2013;55:977–87. 10.1007/s00234-013-1191-4 [DOI] [PubMed] [Google Scholar]
- 22. Fereidoonnezhad B, Dwivedi A, Johnson S. Blood clot fracture properties are dependent on red blood cell and fibrin content. Acta Biomater 2020;127:213–28. [DOI] [PubMed] [Google Scholar]
- 23. Cerenovus . Neuravi Thromboembolic Initiative - Occlusion dynamics [Internet], 2021. Available: https://www.jnjmedicaldevices.com/en-EMEA/neuravi-thromboembolic-initiative
- 24. McCarthy R, Mirza M, Johnson S, et al. Aspects of ischemic stroke biomechanics derived using ex-vivo and in-vitro methods relating to mechanical thrombectomy. J Biomech 2022;131:110900. 10.1016/j.jbiomech.2021.110900 [DOI] [PubMed] [Google Scholar]
- 25. Johnson S, Chueh J, Gounis MJ, et al. Mechanical behavior of in vitro blood clots and the implications for acute ischemic stroke treatment. J Neurointerv Surg 2020;12:853–7. 10.1136/neurintsurg-2019-015489 [DOI] [PubMed] [Google Scholar]
- 26. Johnson S, Duffy S, Gunning G, et al. Review of mechanical testing and modelling of thrombus material for vascular implant and device design. Ann Biomed Eng 2017;45:2494–508. 10.1007/s10439-017-1906-5 [DOI] [PubMed] [Google Scholar]
- 27. Chueh JY, Wakhloo AK, Hendricks GH, et al. Mechanical characterization of thromboemboli in acute ischemic stroke and laboratory embolus analogs. AJNR Am J Neuroradiol 2011;32:1237–44. 10.3174/ajnr.A2485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chalos V, van der Ende NAM, Lingsma HF, et al. National Institutes of Health Stroke Scale: an alternative primary outcome measure for trials of acute treatment for ischemic stroke. Stroke 2020;51:282–90. 10.1161/STROKEAHA.119.026791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Treurniet KM, Yoo AJ, Berkhemer OA, et al. Clot burden score on baseline computerized tomographic angiography and intra-arterial treatment effect in acute ischemic stroke. Stroke 2016;47:2972–8. 10.1161/STROKEAHA.116.014565 [DOI] [PubMed] [Google Scholar]
- 30. Mair G, von Kummer R, Adami A, et al. Observer reliability of CT angiography in the assessment of acute ischaemic stroke: data from the Third International Stroke Trial. Neuroradiology 2015;57:1–9. 10.1007/s00234-014-1441-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
neurintsurg-2021-018560supp001.pdf (196.9KB, pdf)
neurintsurg-2021-018560supp002.pdf (2MB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information. Source data for this study are not available due to privacy regulations, but analysis methods, codes, and results are available from the corresponding author upon reasonable request.