Abstract
The aim of the present study was to investigate the role of estrogen receptor (ER)α and ERβ, and galectin-3 (GAL-3) in migration and invasion of androgen-independent DU-145 prostate cancer cells, and to examine the regulation of the expression of GAL-3 by the activation of these receptors. Wound healing and cell invasion assays were performed using the control (basal level of cellular function) and treated DU-145 cells. At 24 h of treatment, 17β-estradiol (E2), the ERα-selective agonist, 4,4′,4”-(4-propyl-(1H)-pyrazole-1,3,5-triyl)trisphenol (PPT), or the ERβ-selective agonist, 2,3-bis(4-hydroxyphenyl)-propionitrile (diarylprepionitrile; DPN), increased the migration and invasion of the DU-145 cells. Pre-treatment with the ERα- and ERβ-selective antagonists blocked these effects, indicating that ERα and ERβ are upstream receptors regulating these processes. Western blot analysis and immunofluorescence staining for the detection of the GAL-3 were performed using the control and treated DU-145 cells. Treatment of the DU-145 cells with E2, PPT or DPN for 24 h increased the expression of the GAL-3 compared to the control. Furthermore, a specific inhibitor of GAL-3 (VA03) inhibited the migration and invasion of DU-145 cells, indicating the involvement of the complex ERα/GAL-3 and ERβ/GAL-3 in the regulation of these processes. On the whole, the present study demonstrates that the activation of both ERs increases the expression and signaling of GAL-3, and promotes the migration and invasion of DU-145 cells. The findings of the present study provide novel insight into the signatures and molecular mechanisms of ERα and ERβ in DU-145 cells.
Keywords: estrogen receptor, galectin-3, migration and invasion, prostate cancer cells
Introduction
Radical prostatectomy or radiotherapy with or without androgen deprivation therapy are the current treatments for localized prostate cancer (1). However, disease recurrence and the emergence of castration-resistant prostate cancer (CRPC) are frequent occurrences following therapy, and treatment options for these remain insufficient; in addition, the survival rate for patients with advanced disease remains low (2), highlighting the importance of basic research required for this disease.
The overexpression of galectins (GALs), carbohydrate binding proteins, with affinity for N-acetylglucosamine, their roles in cancer progression (3) and their potential as therapeutic targets have been demonstrated in various tumor types over the past 10 years (4). The role of GAL in prostate cancer has previously been described (5,6). These previous studies have mainly focused on GAL-1 and GAL-3, although the importance of GAL-4, GAL-7, GAL-8 and GAL-9 has also been highlighted in this disease (7). The expression of GAL-3 at the mRNA and protein level in the tumor decreases during prostate cancer progression (8–17). However, cytoplasmic overexpression in tumors cells has also been shown to be positively associated with disease progression (11), suggesting the dual role of GAL-3 in prostate cancer cells, depending on its subcellular localization (18).
In vitro studies have revealed that GAL-3 inhibits the apoptosis of prostate cancer cells (19–21), and induces T-cell apoptosis (22) and tumor cell adhesion to endothelial cells (23,24). It has also been demonstrated that in androgen-independent (PC-3 cells) and androgen-dependent prostate cancer cells (LNCaP) transfected with GAL-3 (LNCaP-GAL-3 cells), GAL-3 induces proliferation, migration and invasion (13,25). These findings were corroborated in vivo using tumor xenograft mouse models, in which GAL-3 inhibition with pharmacological or RNA interference (RNAi) strategies impaired tumor growth (13,25) angiogenesis (22) and metastasis (21,22,26).
LNCaP cells do not express GAL-3, whereas the androgen-independent prostate cancer cells, DU-145 and PC-3, highly express GAL-3 (21,25). As previously demonstrated, the overexpression of GAL-3 in LNCaP cells (21,25) or the knockdown of GAL-3 in PC-3 cells does not alter the expression level of the androgen receptor (AR) (21); similarly, it has been demonstrated that the overexpression of AR in PC-3 cells has no regulatory effect on the expression of GAL-3 (21).
The molecular regulatory mechanisms responsible for the expression of GAL-3 in tumor cells are not yet clear (27,28). The expression of GAL-3, at both the transcriptional and translational level, can be regulated by various stimuli (27,28). It has been suggested that promoter methylation is not the only factor regulating the expression of GAL-3 (29). The expression of GAL-3 is increased by transcription factors, such as the RUNX protein family, homeodomain-interacting protein kinase 2, cAMP-response element-binding protein, the NF-κB transcription factor, hypoxia-inducible factor-1α, and inflammatory cytokines and the Ras/MAPK pathway (27,28). Several of these transcription factors and signaling pathways are activated by estrogen receptors (ERs) or interact with ERs (30,31). The regulatory effects of ERs on the expression of GAL-3 remain to be explored in prostate cancer cells.
The authors have previously demonstrated the presence of the ERs, ERα (ESR1) and ERβ (ESR2), in the androgen-independent PC-3 and DU-145 prostate cancer cells, and these receptors are mostly located outside the cell nucleus (32,33). The activation of ERα and ERβ can activate rapid cell signaling pathways in these cells, including an increase in the phosphorylation of ERK1/2 in PC-3 and DU-145 cells (32,33), and SRC and AKT in PC-3 cells (34,35). It is noteworthy that the expression of ERα (unpublished data, Fig. S1) and ERβ (33) is higher in DU-145 than in PNT1A and PC-3 cells, so the present study focused on DU-145 cells.
The present study aimed to examine the roles of ERα, ERβ and GAL-3 in the migration and invasion of DU-145 cells, and to determine the regulatory effects of the activation of these receptors on the expression of the GAL-3.
Materials and methods
Cells and cell culture
The human post-pubertal prostate epithelial cell line, PNT1A, was obtained from Public Health England Culture Collections (lot 11B010; cat. no. 95012614). The DU-145 (derived from brain metastasis) and PC-3 (derived from bone metastasis) cell lines were obtained from ATCC (DU-145 cells, lot7000 9869, cat. no. HTB-81; and PC-3 cells, lot BCRJ:0269, cat. no. CRL-1435; deposited at the Rio de Janeiro Cell Bank). Mycoplasma testing was carried out for all cell lines used. The PNT1A, DU-145 and PC-3 cells were cultured as previously described (32–36). The culture medium was replaced by serum free medium for 24 h before the assays. All experimental procedures (cell culture, western blot analysis, immunofluorescence, wound healing, cell invasion and cell viability analyses, and statistical analysis) were described, submitted, analyzed and approved by the Research Ethics Committee at the Paulista School of Medicine (EPM), Federal University of São Paulo (UNIFESP; no. 3527220917).
Western blot analysis for the detection of GAL-3 and ERα (ESR1)
The PNT1A and PC-3 (used in some experiments) and the DU-145 cells were incubated in the absence (control, untreated cells) or presence of 17β-estradiol (E2, 10 nM; MilliporeSigma), the ERα-selective agonist, 4,4′,4”-(4-propyl-(1H)-pyrazole-1,3,5-triyl)trisphenol (PPT; 10 nM, MilliporeSigma) or the ERβ-selective agonist, 2,3-bis(4-hydroxyphenyl)-propionitrile (diarylprepionitrile; DPN; 10 nM, MilliporeSigma) for 30 min, 1, 2 and 4 h at 37°C. At these concentrations, the agonists are highly selective, as previously reported (32,37,38).
Total cell lysates (20 or 50 µg of protein/lane), SDS/PAGE and western blot analysis were performed as previously described (33,39). The protein concentration was determined using the Bio-Rad protein assay, using bovine serum albumin as standard (Bio Rad Laboratories, Inc.). Briefly, rabbit polyclonal antibody raised against a peptide mapping at the carboxyterminal of ERα of mouse origin, similar to human ERα [MC-20, sc-542, Santa Cruz Biotechnology, Inc.; diluted at 1:200 in Tris-buffered saline containing 0.2% Tween-20 (TBS-T) (Sigma Chemical Co.) and 10% non-fat dry milk (Nestle), pH 7.6, overnight at 4°C] and anti-GAL-3 [hybridoma M3/38.1.2.8 HL.2, TIB-166™, ATCC; donated by Professor Roger Chammas, Center for Translational Research in Oncology, Instituto do Cancer do Estado de São Paulo, São Paulo, SP, Brazil; diluted at 1:100 in phosphate-buffered saline containing 0.1% Tween-20 (PBS-T) (Sigma Chemical Co.) and 5% non-fat dry milk (Nestle), pH 7.2, for 1 h at room temperature], were used. Proteins were visualized by enhanced chemiluminescence reagent (ECL, GE Healthcare), after incubation for 1 h at room temperature, with the appropriate HRP-conjugated secondary antibody (GE Healthcare) diluted in TBS-T at 1:3,000 or in PBS-T 1:3,500. The band intensities of ERα, GAL-3, β-tubulin and GAPDH from individual experiments were quantified using the densitometric analysis of linear-range autoradiograms, using an Epson Expression 1680 scanner (Epson America, Inc.) and the quick Scan 2000 WIN software (Helena Laboratories Co.). β-tubulin or GAPDH were used as protein loading controls. The results were normalized to the respective expression of β-tubulin or GAPDH, expressed in relation to the control (C=1) or in arbitrary unit and plotted (mean ± SEM) from three to six independent experiments. The blots are representative of three to six independent experiments.
Immunofluorescence staining for the detection of the GAL-3
The DU-145 cells were incubated in the absence (control) or presence of E2, 10 nM); PPT (10 nM) or DPN (10 nM) for 2, 4 and 24 h at 37°C. The cells were also untreated or pre-treated with the ERα-selective antagonist, 1,3-bis(4-hydrox- yphenyl)-4-methyl-5-[4-(2-piperidinylethoxy)phenol]-1H-pyrazole dihydrochloride (MPP; 10 nM, MilliporeSigma) and the ERβ-selective antagonist, 4-[2-phenyl-5,7-bis(trifluoromethyl)pyrazolo[1,5-a]pyrimidin-3-yl]phenol (PHTPP; 10 nM, Tocris Bioscience) for 30 min at 37°C. Incubation was continued in the absence or presence of E2 (10 nM), PPT (10 nM) or DPN (10 nM) for 2 and 4 h at 37°C, as previously described (33). Subsequently, the DU-145 cells were washed with ice-cold PBS, fixed in 2% formalin (formaldehyde EM grade, Electron Microscopy Sciences) for 20 min at room temperature, and washed with PBS containing 0.1 M glycine (Sigma Chemical Co). The immunofluorescence assays were performed as previously described (32,33). Briefly, rat monoclonal antibody raised against GAL-3, at 1:50 dilution, in PBS containing 0.01% saponin (Sigma Chemical Co.) and 1% BSA (Sigma Chemical Co.), for 1 h at room temperature. The cells were also incubated with Alexa Fluor 594-labeled secondary antibody (anti-rat; 1:300; Molecular Probes®, Invitrogen; Thermo Fisher Scientific, Inc.). Nuclear staining was performed with DAPI (4′,6-diamidino-2-phenylindole, Sigma Chemical Co.). Negative controls were performed in the absence of primary antibodies. The immunostaining of GAL-3 was visualized under a confocal microscope Leica Microsystems TCSSP8 (Leica Microsystems GmbH). Images of five random microscope fields containing ~20 cells were captured, in duplicate, in each assay (three independent experiments) and analyzed using LAS-X software version: 3.7.0.20979 (Leica Microsystems CMS GmbH). Images are representative of two to four independent experiments performed in duplicate. The fluorescence intensity of whole cell was obtained and analyzed using ImageJ software 1.53t (National Institutes of Health) from the control and treated cells and expressed in arbitrary units.
Wound healing assay
The DU-145 cells in culture medium without serum containing a blocking DNA replication mitomycin C (10 µg/l; MilliporeSigma) to avoid cell proliferation, were wounded using 200 µl sterile pipette tips as previously described (40,41). The DU-145 cells were incubated in the absence (control, basal level of cellular function) or presence of E2 (10 nM), PPT (10 nM) DPN (10 nM) for 24 h at 37°C. The cells were also untreated or pre-treated with MPP (10 nM), PHTTP (10 nM), simultaneously with MPP (10 nM) and PHTPP (10 nM), or with the inhibitor of GAL-3, 1,2,3-triazole-galactosyl arylsulfadimethoxine [VA03; donated by Professor Vanessa Leiria Campo Barão de Mauá University Center (CBM), Ribeirão Preto, SP, Brazil. 200 µM] (42) for 30 min at 37°C. Incubation was continued in the absence or presence of E2 (10 nM), PPT (10 nM) or DPN (10 nM) for 24 h at 37°C. Wound healing analysis was performed as previously described (40,41). For measuring the closure of the wound in the control and treated cells, images of the same area of the wound were obtained at 0 and 24 h. Images were captured using an inverted optical microscope Axio Observer Z1 (Zeiss Nikon Eclipse, Zeiss GmbH) and ZEN 3.3 blue edition software (eiss Nikon Eclipse, Zeiss GmbH). The areas that were occupied by migrating cells after 24 h of incubation (control and treated cells) were calculated by subtracting the background levels at 0 h. The experiments were quantified using ImageJ software 1.53t (National Institutes of Health). The results were expressed in relation to the control (C=100%) and plotted (mean ± SEM) from three to five independent experiments, in duplicate. Images are representative of three to five independent experiments performed in duplicate.
Cell invasion assay
The DU-145 cells in culture medium without serum were seeded in Thincert® chambers (Greiner Bio-One) with polyethylene terephthalate membranes (8 µm pore size) pre-coated with 50 µl of phenol red-free Matrigel (1:10, Corning, Inc.). These chambers were placed in 24-well plates containing culture medium with 10% of fetal bovine serum in the lower chamber. The DU-145 cells in the upper chamber were incubated in the absence (control) or presence of E2 (10 nM), PPT (10 nM) or DPN (10 nM) for 24 h at 37°C. The cells were also untreated or pre-treated with MPP (10 nM), PHTPP (10 nM), simultaneously with MPP (10 nM) and PHTPP (10 nM), or VA03 (200 µM) for 30 min at 37°C (42). Incubation was continued in the absence or presence of E2 (10 nM), PPT (10 nM) or DPN (10 nM), for 24 h at 37°C. Cell invasion analysis was performed as previously described (35,41). Briefly, the membranes were washed thoroughly with 10 mM PBS (Sigma Chemical Co.), fixed in 4% paraformaldehyde (Electron Microscopy Science) for 30 min, and stained with 0.2% crystal violet (Merck KGaA) for 10 min (35,41). Non-invading cells from the membrane upper surface were removed using a sterile cotton swab. The membranes containing the invaded cells (under the surface of membrane), were photographed. Images of three random microscope fields, in duplicate, were captured using an inverted optical microscope (Nikon Eclipse, Nikon Corporation). The areas of invaded cells were analyzed using Micrometrics SE Premium 4 software (Nikon Eclipse, Nikon Corporation). The experiments were quantified using ImageJ software1.53t (National Institutes of Health). The results were expressed in relation to the control (C=100%) and plotted (mean ± SEM) from three to six independent experiments, in duplicate. Images are representative of three to six independent experiments performed in duplicate.
MTT cell viability assay
The DU-145 cells were incubated in the absence (control) or presence of VA03 (20 and 200 µM) for 24 h at 37°C. Cell viability assay was performed using MTT assay (Thermo Fisher Scientific Inc.) as previously described (44). The cells were washed with ice-cold PBS, replaced with 100 µl of fresh culture medium containing MTT (2.4 mM), and incubated for 2 h at 37°C. The medium was removed, and the formazan product was dissolved in DMSO (100 µl to each well) at room temperature for 10 min with intermittent shaking. Each sample was mixed again and the absorbance at 595 nm was read using the ELx800 absorbance microplate reader (Biotek ELX800, BioTek Instruments, Inc.). Each assay was repeated at least three times in triplicate. The negative control was supplemented with 100 µl DMSO (Sigma Chemical Co.; without cells). Each sample from the control and treated cells was subtracted from the negative control, and the results were plotted as the mean ± SEM.
Statistical analysis
Data are expressed as the mean ± SEM. Statistical analysis was performed using one-way ANOVA followed by the Newman-Keuls test or Tukey's post hoc test for multiple comparisons. P-values <0.05 were considered to indicate statistically significant differences.
Results
The activation of ERα and ERβ promotes the migration and invasion of DU-145 cells
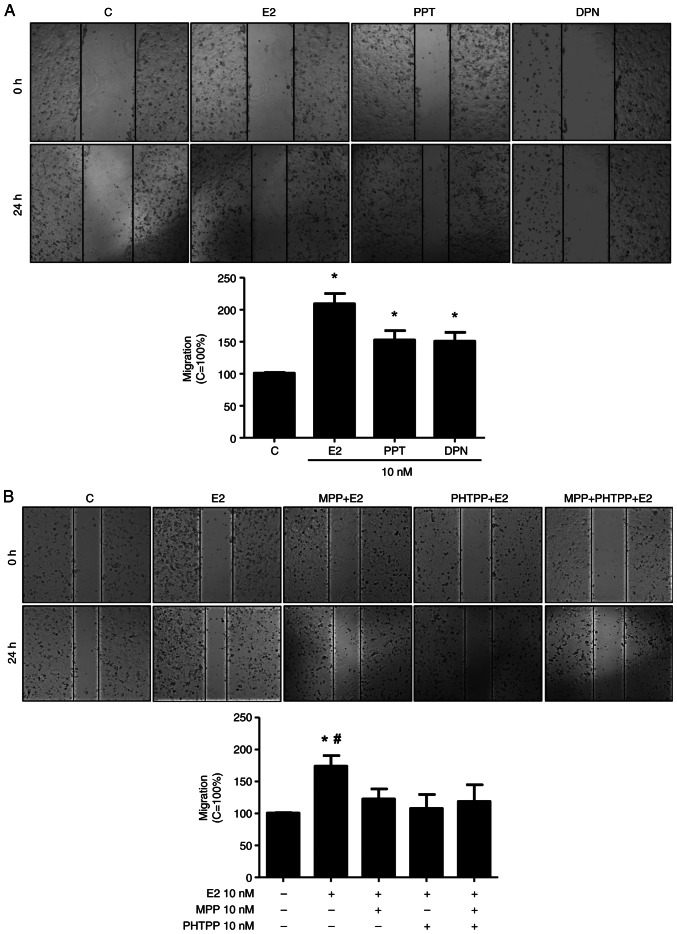
The present study analyzed various cellular characteristics of tumor development in vitro, using DU-145 cells. At 24 h of treatment E2 (10 nM), the ERα-selective agonist, PPT (10 nM), or the ERβ-selective agonist, DPN (10 nM), increased the migration of the DU-145 cells (2.0-, 1.5- and 1.5-fold, respectively) compared to the control (Fig. 1A), suggesting the involvement of the ERs, ERα and ERβ, in this process. Of note, the increase in DU-145 cell migration induced by E2 (10 nM) at 24 h was blocked by the ERα-selective antagonist (MPP, 10 nM), the ERβ-selective antagonist (PHTPP, 10 nM) or simultaneous pre-treatment with both MPP (10 nM) and PHTPP (10 nM) (Fig. 1B). Pre-treatment with MPP, PHTPP or both MPP and PHTPP, in the absence of the E2, yielded results similar to those of the control (data not shown).
Figure 1.
Effects of E2, the ERα-selective agonist, PPT, and the ERβ-selective agonist, DPN, on the migration of the DU-145 cells. (A) Cells, in the same culture plate in different wells, were wounded and then incubated in the absence (C, control) or presence of E2 (10 nM), ERα-selective agonist PPT (10 nM) or ERβ-selective agonist DPN (B) for 24 h at 37°C. (B) Cells were also untreated or pre-treated with the ERα-selective antagonist MPP (10 nM), ERβ-selective antagonist PHTPP (10 nM) or with both antagonists, MPP (10 nM) and PHTPP (10 nM) for 30 min. Incubation was continued in the presence of E2 (10 nM) for 24 h at 37°C. Wound healing assay was performed as described in the Materials and methods. The results are expressed in relation to the control (C=100%) and plotted (mean ± SEM) from four to five independent experiments, in duplicate (bar graphs). Images (×100 magnification) are representative of four to five independent experiments performed in duplicate. *P<0.05, significantly different from the control; #P<0.05, significantly different from the MPP + E2, PHTPP + E2, or MPP + PHTPP + E2 groups (determined using ANOVA and Tukey's post hoc test). E2, 17β-estradiol; ER, estrogen receptor; PPT, 4,4′,4”-(4-propyl- (1H)-pyrazole-1,3,5-triyl)trisphenol; DPN, 2,3-bis(4-hydroxyphenyl)-propionitrile; MPP, 1,3-bis(4-hydroxyphenyl)-4-methyl-5-[4-(2-piperidinylethoxy)phenol]-1H-pyrazole dihydrochloride; PHTPP, 4-[2-phenyl-5,7-bis(trifluoromethyl)pyrazolo[1,5-a]pyrimidin-3-yl]phenol.
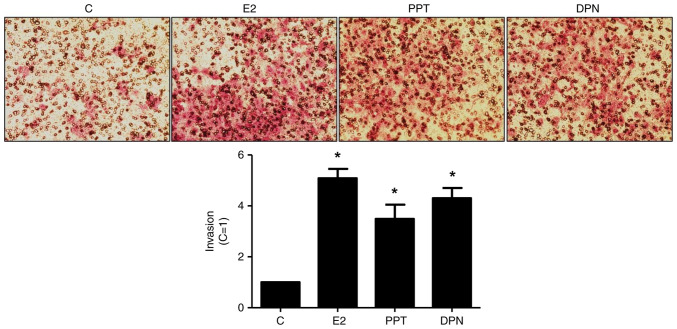
Treatment with E2 (10 nM), PPT (10 nM) or DPN (10 nM) for 24 h led to an enhancement of the invasion of the DU-145 cells (5-, 3,5- and 4-fold, respectively) (Fig. 2). The increase in DU-145 cell invasion induced by E2 was blocked by simultaneous pre-treatment with both MPP and PHTPP (Fig. S2A), suggesting that ERα and ERβ may play a role in the DU-145 cells invasion. To confirm the involvement of these receptors, the DU-145 cells were also untreated or pre-treated with MPP (10 nM) or PHTPP (10 nM) and the incubation was continued in the absence or presence of PPT (10 nM) or DPN (10 nM). The increase in DU-145 cell invasion induced by PPT or DPN was blocked, respectively, by MPP or PHTPP (Fig. S2B and C), confirming that ERα and ERβ are upstream receptors regulating this process. Pre-treatment with MPP, PHTPP or simultaneous pre-treatment with both antagonists, in the absence of E2, PPT or DPN, yielded results similar to those of the control (Fig. 1).
Figure 2.
Effects of E2, the ERα-selective agonist, PPT, and the ERβ-selective agonist, DPN, on the invasion of the DU-145 cells. Cells in culture medium without serum were seeded in ThincertR chambers with polyethylene terephthalate membranes pre-coated with phenol red-free Matrigel. These chambers were placed in 24-well plates containing culture medium with 10% FBS in the lower chamber. Cells in upper chambers of the same culture plate were incubated in the absence (C, control) and the presence of E2 (10 nM), ERα-selective agonist PPT (10 nM) or ERβ-selective agonist DPN (10 nM) for 24 h at 37°C. Cell invasion assay was performed as described in the Materials and methods. The results are expressed in relation to control (C=1) and plotted (mean ± SEM) from five to six independent experiments, in duplicate (bar graphs). Images (×200 magnification) are representative of five to six independent experiments performed in duplicate. *P<0.05, significantly different from the control (determined using ANOVA and Tukey's post hoc test). E2, 17β-estradiol; ER, estrogen receptor; PPT, 4,4′,4”-(4-propyl-(1H)-pyrazole-1,3,5-triyl)trisphenol; DPN, 2,3-bis(4-hydroxyphenyl)-propionitrile.
The activation of ERα and ERβ for 24 h increases the expression of GAL-3 in DU-145 cells
GAL-3 was detected as a single protein band of 31 kDa in the total cell extracts of the PNT1A, PC-3 and DU-145 cells (Fig. 3). The expression of GAL-3 was higher in the DU-145 and PNT1A cells than in the PC-3 cells (Fig. 3), suggesting that the AR is not involved in the regulation of GAL-3. No difference was observed in the expression of the GAPDH among the three cells, used as protein loading control (Fig. 3). Thus, the androgen-independent prostate cancer cells, DU-145, were used in the analyses of the regulatory effects of the activation of the ERs on the expression of GAL-3.
Figure 3.
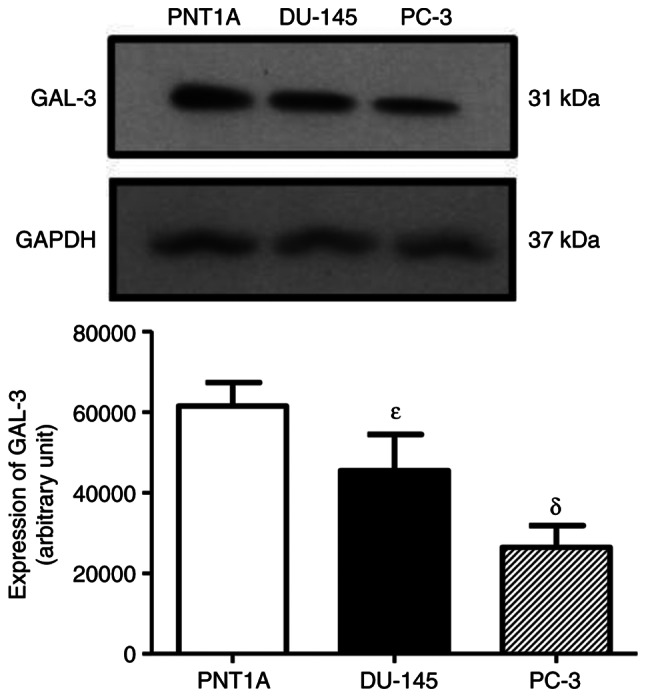
Expression of GAL-3 in PNT1A, DU-145 and PC-3 cells. Western blot analysis was performed for the detection of GAL-3 in PNT1A, DU-145 and PC-3 cells, using 50 µg of protein/lane and antibody specific for GAL-3 (top row) or antibody that recognizes GAPDH (bottom row). The protein sizes of GAL-3 and GAPDH are shown on the right. The data shown are representative of six independent experiments. Results of the densitometric analysis of the western blots were normalized to the respective expression of GAPDH, expressed in arbitrary units and plotted (mean ± SEM) from four independent experiments (bar graph). δP>0.05, significantly different from PNT1A cells; εP>0.05, significantly different from PC-3 cells (determined using ANOVA and the Newman-Keuls test, n=6). GAL-3, galectin-3.
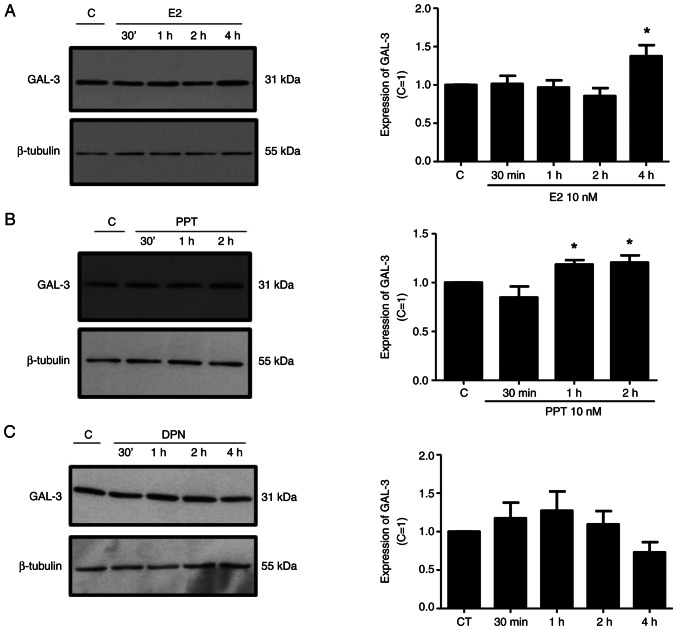
Treatment of the DU-145 cells with E2 (10 nM) for 4 h or with PPT (10 nM) for 1 and 2 h increased the expression of GAL-3 compared to the control DU-145 cells (untreated cells) (Fig. 4A and B). On the other hand, treatment of the DU-145 cells with DPN (10 nM) for 30 min, 1, 2 and 4 h did not have any marked effects on the expression of GAL-3 compared to the control (Fig. 4C). No difference was observed in the expression of β-tubulin under any of these conditions, used as the protein loading control (Fig. 4).
Figure 4.
Effects of E2, the ERα-selective agonist, PPT, and the ERβ-selective agonist, DPN, on the expression of GAL-3 in DU-145 cells. Cells were incubated in the absence (control, C) or presence of (A) E2 (10 nM), (B) ER α-selective agonist PPT (10 nM) or (C) ERβ-selective agonist DPN (10 nM) for different periods of time at 37°C. Western blot analysis for the detection of the GAL-3 in PNT1A, DU-145 and PC-3 cells was performed as described in the Materials and methods, using 20 µg of protein/lane and antibody specific for GAL-3 (top row) or antibody that recognizes β-tubulin (bottom row). The protein sizes of GAL-3 and β-tubulin proteins are shown at the right. The data shown are representative of three to five independent experiments. Results of the densitometric analysis of the western blots were normalized to the respective expression of β-tubulin, expressed in relation to the control (C=1) and plotted (mean ± SEM) from three to five independent experiments (bar graphs). *P<0.05, significantly different from the control (determined using ANOVA and Tukey's post hoc test). E2, 17β-estradiol; ER, estrogen receptor; PPT, 4,4′,4”-(4-propyl-(1H)-pyrazole-1,3,5-triyl)trisphenol; DPN, 2,3-bis(4-hydroxyphenyl)-propionitrile.
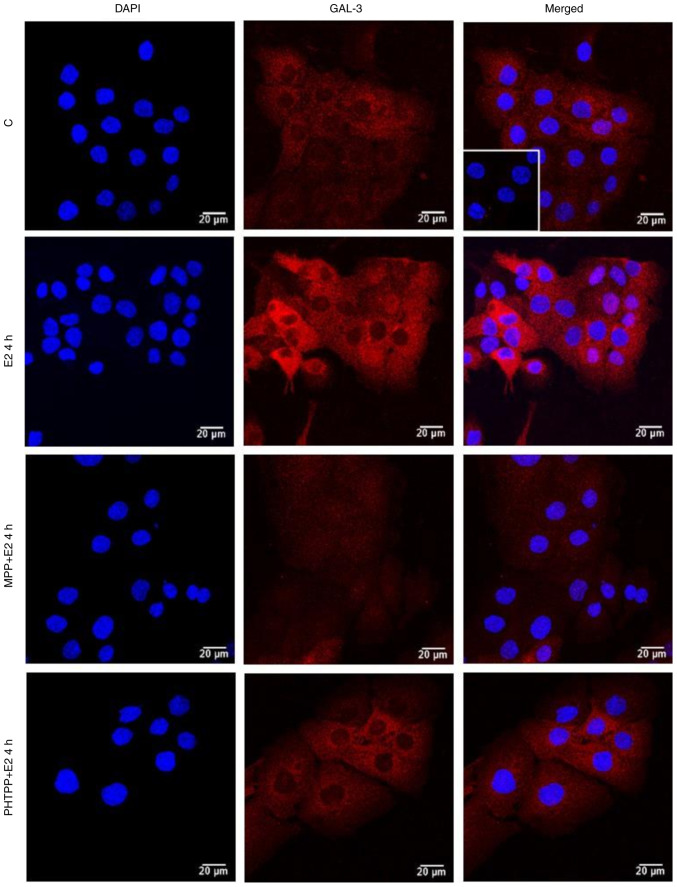
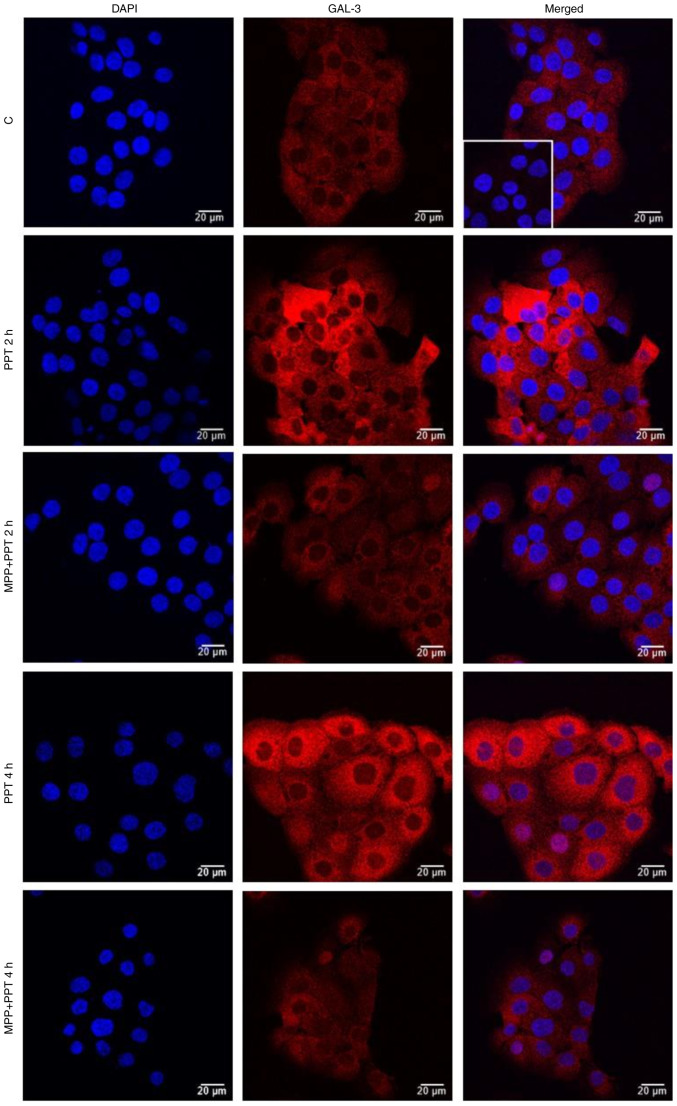
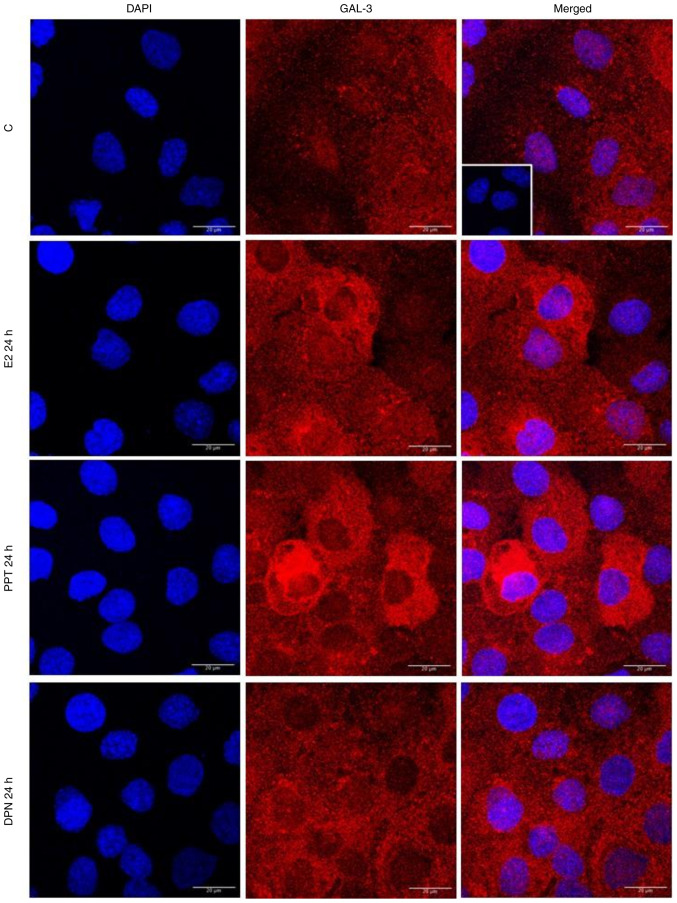
The localization and expression of GAL-3 were determined using immunofluorescence assays. In the control DU-145 cells (Fig. 5, Fig. 6, Fig. 7 and S3), the immunostaining of GAL-3 was predominantly found in the cytoplasm, although immunostaining in some nuclei was also observed. Treatment of these cells with E2 (10 nM) for 4 h (Figs. 5 and S4) or PPT (10 nM) for 2 and 4 h (Figs. 6 and S4) increased the expression of GAL-3 in the cytoplasm and nuclei. On the other hand, treatment of the DU-145 cells with DPN (10 nM) for 2 and 4 h did not have any marked effects on the expression of GAL-3 compared to the control (Fig. S3).
Figure 5.
Effects of treatment with E2 for 4 h on the expression and localization of the GAL-3 in DU-145 cells. Cells were incubated in the absence (control, C) or presence of E2 (10 nM) for 4 h at 37°C. Cells were also untreated or pre-treated with the ERα-selective antagonist, MPP, (10 nM) or the ERβ-selective antagonist, PHTPP (10 nM), for 30 min. Incubation was continued in the absence or presence of E2 (10 nM) for 4 h at 37°C. Immunostaining for GAL-3 (red) was detected as described in the Materials and methods. Nuclei were stained with DAPI (blue). Negative control was performed in the absence of primary antibody (insert). Scale bars, 20 µm. Images are representative of two independent experiments. E2, 17β-estradiol; GAL-3, galectin-3; ER, estrogen receptor; MPP, 1,3-bis(4-hydroxyphenyl)-4-methyl-5-[4-(2-piperidinylethoxy)phenol]-1H-pyrazole dihydrochloride; PHTPP, 4-[2-phenyl-5,7-bis(trifluoromethyl)pyrazolo[1,5-a]pyrimidin-3-yl]phenol.
Figure 6.
Effects of the ERα-selective agonist, PPT, for 2 and 4 h on the expression and localization of GAL-3 in DU-145 cells. Cells were incubated in the absence (control, C) or presence of ERα-selective agonist PPT (10 nM) for 2 h and 4 h at 37°C. Cells were also untreated or pre-treated with ERα-selective antagonist MPP (10 nM) for 30 min. Incubation was continued in the absence or presence of PPT (10 nM) for 4 h at 37°C. Immunostaining for GAL-3 (red) was detected as described in the Materials and methods. Nuclei were stained with DAPI (blue). Negative control was performed in the absence of primary antibody (insert). Scale bars, 20 µm. Images are representative of four independent experiments. ER, estrogen receptor; PPT, 4,4′,4”-(4-propyl-(1H)-pyrazole- 1,3,5-triyl)trisphenol; DPN, 2,3-bis(4-hydroxyphenyl)-propionitrile; GAL-3, galectin-3; MPP, 1,3-bis(4-hydroxyphenyl)-4-methyl-5-[4-(2-piperidinylethoxy)phenol]-1H-pyrazole dihydrochloride.
Figure 7.
Effects of treatment with E2, the ERα-selective agonist, PPT, or the ERβ-selective agonist, DPN, for 24 h on the expression and localization of the GAL-3 in DU-145 cells. Cells were incubated in the absence (control, C) or presence of E2 (10 nM), ERα-selective agonist PPT (10 nM) or ERβ-selective agonist DPN (10 nM) for 24 h at 37°C. Immunostaining for GAL-3 (red) was detected as described in the Materials and methods. Nuclei were stained with DAPI (blue). Negative control was performed in the absence of primary antibody (insert). Scale bars, 20 µm. Images are representative of two independent experiments. E2, 17β-estradiol; ER, estrogen receptor; GAL-3, galectin-3; PPT, 4,4′,4”-(4-propyl-(1H)-pyrazole-1,3,5-triyl)trisphenol; DPN, 2,3-bis(4-hydroxyphenyl)-propionitrile.
The effects of treatment of the DU-145 cells with E2 (10 nM) for 2 h were blocked by pre-treatment with MPP (10 nM) and partially blocked by PHTPP (10 nM) (Figs. 5 and S4). The expression of GAL-3 induced by 2 or 4 h of treatment with PPT (10 nM) was blocked by pre-treatment with MPP (10 nM) (Figs. 6 and S4). Treatment with MPP or PHTPP alone did not have any marked effects on the expression of the GAL-3, and the effects were similar to those of the control (data not shown).
It is important to mention that at 24 h of treatment with E2, PPT or DPN, the expression of GAL-3 increased compared to the control (Fig. 7). The analysis of these findings using ImageJ software revealed that treatment with E2, PPT and DPN for 24 h increased the fluorescence intensity of GAL-3 by 25, 39 and 28%, respectively in the whole DU-145 cells compared to the control (Fig. S4). No immunostaining was observed in the negative control, performed in the absence of primary antibodies for GAL-3 (Fig. 5, Fig. 6, Fig. 7 and S2, inserts).
GAL-3 is involved in the migration and invasion of DU-145 cells
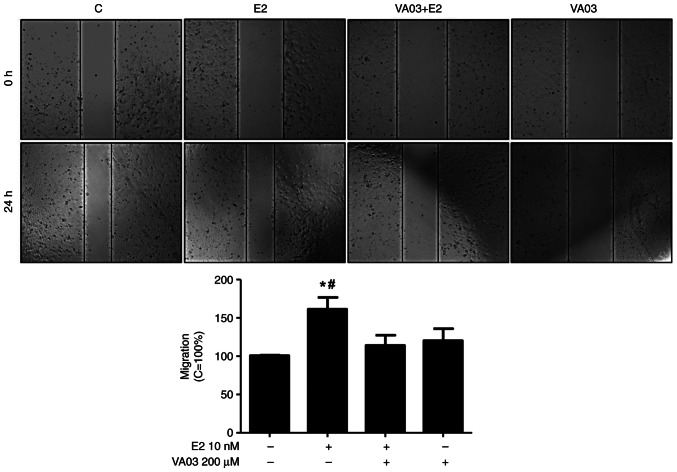
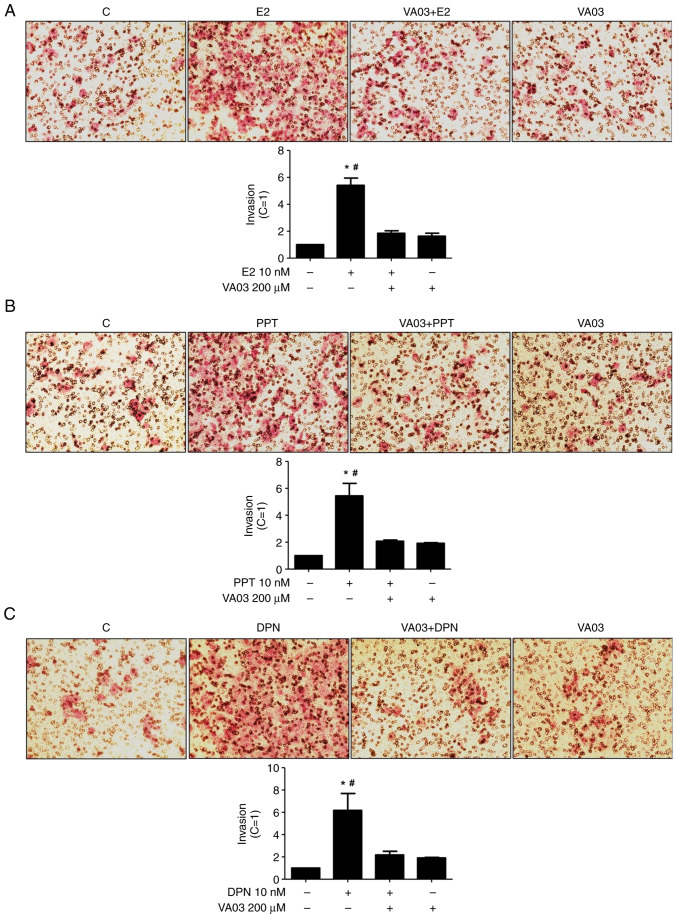
To explore the involvement of GAL-3 in the migration and invasion of DU-145 cells, VA03 (a specific inhibitor of GAL-3) was used at 200 µM (Figs. 8 and 9). Pre-treatment with VA03 inhibited the migration of the DU-145 cells induced by E2 (10 nM) (Fig. 8), PPT (10 nM) or DPN (10 nM) (data not shown). Pre-treatment with VA03 inhibited the invasion of the DU-145 cells induced by E2 (10 nM), PPT (10 nM) or DPN (10 nM) (Fig. 9), indicating the involvement of the complex ERα/GAL-3 and ERβ/GAL-3 in the regulation of the migration and invasion of DU-145 cells. Treatment with VA03 alone did not have any marked effects on the migration or invasion of DU-145 cells (Figs. 8 and 9). In addition, treatment with VA03 (20 and 200 µM) for 24 h did not have any notable effects on the number and viability of the DU-145 cells compared to the control cells (Fig. S5).
Figure 8.
Effects of the specific inhibitor of GAL-3 (VA03) on the migration of DU-145 cells induced by E2. Cells, in the same culture plate in different wells, were wounded and then incubated in the absence (C, control) or presence of E2 (10 nM) for 24 h at 37°C. Cells were also untreated or pretreated with a specific inhibitor of GAL-3 (VA03, 200 µM) for 30 min. Incubation was continued in the absence or presence of E2 for 24 h at 37°C. Wound healing assay was performed as described in the Materials and methods. Results are expressed in relation to control (C=100%) and plotted (mean ± SEM) from three independent experiments, in duplicate (bar graph). Images (×100 magnification) are representative of four different experiments. *P<0.05, significantly different from the control; #P<0.05, significantly different from the VA03 + E2 group (determined using ANOVA and Tukey's post hoc test). E2, 17β-estradiol; GAL-3, galectin-3.
Figure 9.
Effects of a specific inhibitor of GAL-3 (VA03) on the invasion of DU-145 cells induced by E2, PPT and DPN. Cells in culture medium without serum were seeded in ThincertR chambers with polyethylene terephthalate membranes pre-coated with phenol red-free Matrigel. These chambers were placed in 24-well plates containing culture medium with 10% FBS in the lower chamber. Cells in the upper chambers of the same culture plate were incubated in the absence (C, control) or presence of (A) E2 (10 nM), (B) the ERα-selective agonist, PPT (10 nM), or (C) the ERβ-selective agonist, DPN (10 nM), for 24 h at 37°C. Cells were also untreated or pre-treated with a specific inhibitor of GAL-3 (VA03, 200 µM) for 30 min. Incubation was continued in the absence or presence of (A) E2, (B) PPT or (C) DPN for 24 h at 37°C. Cell invasion analysis was performed as described in the Materials and methods. Results are expressed in relation to the control (C=1) and plotted (mean ± SEM) from three to four independent experiments, in duplicate (bar graphs). Images (×200 magnification) are representative of three to four different experiments. *P<0.05, significantly different from the control; #P<0.05, significantly different from the VA03 + E2; VA03 + PPT, VA03 + DPN and VA03 groups (determined using ANOVA and Tukey's post hoc test). GAL-3, galectin-3; E2, 17β-estradiol; ER, estrogen receptor; PPT, 4,4′,4”-(4-propyl-(1H)-pyrazole-1,3,5-triyl)trisphenol; DPN, 2,3-bis(4-hydroxyphenyl)-propionitrile.
Discussion
The expression of the ERs, ERα and ERβ, changes in the different stages of prostate cancer and conflicting findings on the expression, regulation and roles of these receptors in prostate cancer development have been found (45–48). It is recognized that there is wide variability in the sensitivity and specificity of ERβ antibodies, which may contribute to the uncertainties surrounding its molecular action and tissue expression. Nelson et al (49) published a study advising about which antibodies are acceptable against ERβ. Using the antibody previously reported (49), the authors previously demonstrated the presence of ERβ in PNT1A, PC-3 and DU-145 cells (33). The expression of ERα also was shown in these cells (32,33, Fig. S1). Taken together, these results confirm that the expression of ERα and ERβ is higher in the DU-145 cells than in the PNT1A and PC-3 cells, suggesting that distinct androgen-independent mechanisms are involved in the regulation of these receptors. These mechanisms remain to be explored.
The activation of ERα and ERβ promoted an increase in the migration and invasion of the DU-145 cells. In the PC-3 cells, the activation of ERβ and ERα increased the invasion and anchorage-independent growth of these cells (40,50). The activation of ERβ by DPN has also been shown to promote the survival and migration of the CPEC cell line (cells expressing prostate-specific antigens), established from patients with prostate cancer (51). Furthermore, the expression of the ERβ5 (ERβ splice variant) in PC-3 cells increased the cell migration, and the expression of ERβ2 and ERβ5 increased the invasion, but did not affect the proliferation of the cells (52). It is important to emphasize that ERβ splice variants do not bind ligands (53), although dimers may be observed with ERβ (ERβ1). ERα/β heterodimers formation was observed in DU-145 cells (33). Taken together, these results support an oncogenic role for ERα and ERβ in DU-145 cells.
In the present study, the activation of ERα by PPT, but not ERβ by DPN, for 2 h increased the expression of the GAL-3 in DU-145 cells. However, at 24 h of treatment, DPN also increased the expression of GAL-3 compared to the control (basal level of cellular function). Taken together, these results indicate that ERα and ERβ are involved in the regulation of the expression of the GAL-3. The promoter region of the human LGALS3 gene contains several regulatory elements: Five putative Sp1 binding sites (GC boxes), five cAMP-dependent response element motifs, four AP-1- and one AP-4-like sites, two NF-κB-like sites, one sis-inducible element and a consensus basic helix-loop-helix core sequence (27,54). Several of these transcription factors interact with ERs (30) and induce the genomic signaling. Furthermore, ERs also activate two major pathways regulating cell proliferation and survival, SRC/MAPK and PI3K/AKT pathways (rapid or non-genomic signaling) (31). Indeed, in the DU-145 cells, the activation of ERα and ERβ can activate rapid cell signaling pathways in these cells, including an increase in the phosphorylation of ERK1/2 (33). Thus, the transcriptional regulation (genomic activity) combined with direct activation of signaling cascades (non-genomic activity) by ERs may be involved in the expression of the GAL-3 in DU-145 cells. These mechanisms remain to be explored in DU-145 and other prostate cancer cells.
It is important to mention that E2 and progesterone have also been shown to induce the upregulation of GAL-3 expression in RL95-2 epithelial cells from the human endometrium, which in turn decreases the apoptotic rate of these cells (55). Furthermore, in a preliminary study, ERα and GAL-3 were shown as markers of aggressiveness and prognosis in prolactinoma (56).
In the present study, in the DU-145 cells (control), the immunostaining of GAL-3 was predominantly found in the cytoplasm, although immunostaining in some nuclei was also observed. Treatment of these cells for 24 h with E2, PPT or DPN increased the expression of the GAL-3 in the cytoplasm and nuclei. Several cytosolic molecules were identified, as GAL-3 ligands and these proteins are involved in the regulation of cell proliferation, differentiation, survival and death (27). In addition, GAL-3 interacts with nuclear factors to regulate the expression of multiple genes related to tumor plasticity (57). For example, GAL-3 interacts with the factor activator protein 1 (AP1); this GAL-3-AP1 complex binds to the matrix metalloproteinase-1 promoter and mediates its transcription, which facilitates the migration and invasion of melanoma cells (58).
Herein, to explore the involvement of GAL-3 in migration and invasion of the DU-145 cells induced by activation of ERs, VA03 (a specific inhibitor of GAL-3) was used. Pre-treatment with VA03 inhibited the migration and invasion of the DU-145 cells induced by the activation of ERs, indicating that the complex ERα/GAL-3 and ERβ/GAL-3 plays a role in these processes.
The regulation of the expression of GAL-3 by ERs in other prostate cancer cell lines and in different stages of the prostate cancer remains to be explored. It is important to mention that in PC-3 cells, the activation of ERs induces an increase of the active non-phosphorylated β-catenin, and these proteins are involved in the proliferation, migration, invasion and colony formation of these cells (34,40). It has been shown that β-catenin can co-localize with GAL-3 in other cancer cells (59). Taken together, these results indicate that the process is more complex that should be addressed in near future using different prostate cancer cell lines and prostate cancer tissues.
GAL-3 inhibitors have shown promising results in preclinical studies (22,60). Notably, TFD100, a GAL3-binding glycopeptide, has been shown to block GAL3-induced T-cell apoptosis, and to impair angiogenesis and metastasis in xenograft models (22). In addition, G3-C12-modified copolymers (targeting GAL-3) have been shown to improve the antitumor activity of 5-fluorouracil in prostate cancer xenograft mouse models (60), and modified citrus pectin, a natural dietary fiber soluble polysaccharide, that plays a role as an antagonist of extracellular GAL-3 (61), sensitizes prostate cancer cells to radiotherapy, and reduces their migratory and invasive capabilities (62).
Overall, although GAL-3 levels in the tumor are decreased during prostate cancer progression, the cytoplasmic overexpression of this protein has been reported during progression, and in vitro data and preclinical xenograft models have shown that strategies targeting GAL-3 may be effective in impairing prostate cancer progression in vivo. However, several important challenges need to be addressed before anti-GAL-based strategies can be translated to clinical settings.
In conclusion, the activation of both ERs increases the expression and signaling of the GAL-3, and induces the migration and invasion of DU-145 cells. The findings of the present study provide novel insight into the signatures and molecular mechanisms of ERα and ERβ in DU-145 cells.
Supplementary Material
Acknowledgements
The authors would like to thank Dr Elizabeth Kanashiro and Dr Caroline Zito Romera [Paulista School of Medicine (EPM), Federal University of São Paulo (UNIFESP)] for providing technical assistance. The Leica Microsystems TCSSP8 confocal microscope is a facility from the Instituto Nacional de Farmacologia e Biologia Molecular (INFAR), EPM-UNIFESP, and it was supported by Financiadora de Estudos e Projetos (FINEP) and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP). The research fellowship of CSP and doctoral fellowship of CM were supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). The doctoral fellowship of DSS was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
Funding Statement
The present study was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, grant no. 2020/01285-2).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
DSS conceived and designed the study, collected the data, performed the analysis and wrote the manuscript. CM and RPC contributed to data analysis. CMV conceived and designed the study, contributed data or analysis tools and performed the analyses. VLC performed the synthesis of the galectin-3 inhibitor. CSP conceived and designed the study, and performed the revision of the manuscript. DSS and CSP confirm the authenticity of all the raw data. All authors have read and approved the submitted version of the manuscript.
Ethics approval and consent to participate
All experimental procedures were approved by the Research Ethical Committee at EPM-UNIFESP (#3527220917).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Sathianathen NJ, Konety BR, Crook J, Saad F, Lawrentschuk N. Landmarks in prostate cancer. Nat Rev Urol. 2018;15:627–642. doi: 10.1038/s41585-018-0060-7. [DOI] [PubMed] [Google Scholar]
- 2.Baciarello G, Gizzi M, Fizazi K. Advancing therapies in metastatic castration-resistant prostate cancer. Expert Opin Pharmacother. 2018;19:1797–1804. doi: 10.1080/14656566.2018.1527312. [DOI] [PubMed] [Google Scholar]
- 3.Liu FT, Rabinovich GA. Galectins as modulators of tumour progression. Nat Rev Cancer. 2005;5:29–41. doi: 10.1038/nrc1527. [DOI] [PubMed] [Google Scholar]
- 4.Thijssen VL, Heusschen R, Caers J, Griffioen AW. Galectin expression in cancer diagnosis and prognosis: A systematic review. Biochim Biophys Acta. 2015;1855:235–247. doi: 10.1016/j.bbcan.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Laderach DJ, Gentilini LD, Giribaldi L, Delgado VC, Nugnes L, Croci DO, Al Nakouzi N, Sacca P, Casas G, Mazza O, et al. A unique galectin signature in human prostate cancer progression suggests galectin-1 as a key target for treatment of advanced disease. Cancer Res. 2013;73:86–96. doi: 10.1158/0008-5472.CAN-12-1260. [DOI] [PubMed] [Google Scholar]
- 6.Compagno D, Gentilini LD, Jaworski FM, Pérez IG, Contrufo G, Laderach DJ. Glycans and galectins in prostate cancer biology, angiogenesis, and metastasis. Glycobiology. 2014;24:899–906. doi: 10.1093/glycob/cwu055. [DOI] [PubMed] [Google Scholar]
- 7.Martínez-Bosch N, Rodriguez-Vida A, Juanpere N, Lloreta J, Rovira A, Albanell J, Bellmunt J, Navarro P. Galectins in prostate and bladder cancer: Tumorigenic roles and clinical opportunities. Nat Rev Urol. 2019;16:433–445. doi: 10.1038/s41585-019-0183-5. [DOI] [PubMed] [Google Scholar]
- 8.Ahmed H, Cappello F, Rodolico V, Vasta GR. Evidence of heavy methylation in the galectin 3 promoter in early stages of prostate adenocarcinoma: Development and validation of a methylated marker for early diagnosis of prostate cancer. Transl Oncol. 2009;2:146–156. doi: 10.1593/tlo.09118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ellerhorst J, Troncoso P, Xu XC, Lee J, Lotan R. Galectin-1 and galectin-3 expression in human prostate tissue and prostate cancer. Urol Res. 1999;27:362–367. doi: 10.1007/s002400050164. [DOI] [PubMed] [Google Scholar]
- 10.Pacis RA, Pilat MJ, Pienta KJ, Wojno K, Raz A, Hogan V, Cooper CR. Decreased galectin-3 expression in prostate cancer. Prostate. 2000;44:118–123. doi: 10.1002/1097-0045(20000701)44:2<118::AID-PROS4>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 11.van den Brûle FA, Waltregny D, Liu FT, Castronovo V. Alteration of the cytoplasmic/nuclear expression pattern of galectin-3 correlates with prostate carcinoma progression. Int J Cancer. 2000;89:361–367. doi: 10.1002/1097-0215(20000720)89:4<361::AID-IJC8>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 12.Merseburger AS, Kramer MW, Hennenlotter J, Simon P, Knapp J, Hartmann JT, Stenzl A, Serth J, Kuczyk MA. Involvement of decreased Galectin-3 expression in the pathogenesis and progression of prostate cancer. Prostate. 2008;68:72–77. doi: 10.1002/pros.20688. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Nangia-Makker P, Tait L, Balan V, Hogan V, Pienta KJ, Raz A. Regulation of prostate cancer progression by galectin-3. Am J Pathol. 2009;174:1515–1523. doi: 10.2353/ajpath.2009.080816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Melo-Júnior MR, Araújo-Filho JL, Lins CA, de Pontes-Filho NT, de Carvalho LB., Jr Immobilization of anti-galectin-3 onto polysiloxane-polyvinyl alcohol disks for tumor prostatic diseases diagnosis. Appl Biochem Biotechnol. 2010;160:2198–2207. doi: 10.1007/s12010-009-8753-2. [DOI] [PubMed] [Google Scholar]
- 15.Knapp JS, Lokeshwar SD, Vogel U, Hennenlotter J, Schwentner C, Kramer MW, Stenzl A, Merseburger AS. Galectin-3 expression in prostate cancer and benign prostate tissues: Correlation with biochemical recurrence. World J Urol. 2013;31:351–358. doi: 10.1007/s00345-012-0925-y. [DOI] [PubMed] [Google Scholar]
- 16.Geisler C, Gaisa NT, Pfister D, Fuessel S, Kristiansen G, Braunschweig T, Gostek S, Beine B, Diehl HC, Jackson AM, et al. Identification and validation of potential new biomarkers for prostate cancer diagnosis and prognosis using 2D-DIGE and MS. Biomed Res Int. 2015;2015:454256. doi: 10.1155/2015/454256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahmed H, Banerjee PP, Vasta GR. Differential expression of galectins in normal, benign, and malignant prostate epithelial cells: Silencing of galectin-3 expression in prostate cancer by its promoter methylation. Biochem Biophys Res Commun. 2007;358:241–246. doi: 10.1016/j.bbrc.2007.04.114. [DOI] [PubMed] [Google Scholar]
- 18.Califice S, Castronovo V, Bracke M, van den Brûle F. Dual activities of galectin-3 in human prostate cancer: Tumor suppression of nuclear galectin-3 vs tumor promotion of cytoplasmic galectin-3. Oncogene. 2004;23:7527–7536. doi: 10.1038/sj.onc.1207997. [DOI] [PubMed] [Google Scholar]
- 19.Fukumori T, Oka N, Takenaka Y, Nangia-Makker P, Elsamman E, Kasai T, Shono M, Kanayama HO, Ellerhorst J, Lotan R, Raz A. Galectin-3 regulates mitochondrial stability and antiapoptotic function in response to anticancer drug in prostate cancer. Cancer Res. 2006;66:3114–3119. doi: 10.1158/0008-5472.CAN-05-3750. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, Nangia-Makker P, Balan V, Hogan V, Raz A. Calpain activation through galectin-3 inhibition sensitizes prostate cancer cells to cisplatin treatment. Cell Death Dis. 2010;1:e101. doi: 10.1038/cddis.2010.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y, Balan V, Gao X, Reddy PG, Kho D, Tait L, Raz A. The significance of galectin-3 as a new basal cell marker in prostate cancer. Cell Death Dis. 2013;4:e753. doi: 10.1038/cddis.2013.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guha P, Kaptan E, Bandyopadhyaya G, Kaczanowska S, Davila E, Thompson K, Martin SS, Kalvakolanu DV, Vasta GR, Ahmed H. Cod glycopeptide with picomolar affinity to galectin-3 suppresses T-cell apoptosis and prostate cancer metastasis. Proc Natl Acad Sci USA. 2013;110:5052–5057. doi: 10.1073/pnas.1202653110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glinsky VV, Glinsky GV, Rittenhouse-Olson K, Huflejt ME, Glinskii OV, Deutscher SL, Quinn TP. The role of Thomsen-Friedenreich antigen in adhesion of human breast and prostate cancer cells to the endothelium. Cancer Res. 2001;61:4851–4857. [PubMed] [Google Scholar]
- 24.Glinsky VV, Glinsky GV, Glinskii OV, Huxley VH, Turk JR, Mossine VV, Deutscher SL, Pienta KJ, Quinn TP. Intravascular metastatic cancer cell homotypic aggregation at the sites of primary attachment to the endothelium. Cancer Res. 2003;63:3805–3811. [PubMed] [Google Scholar]
- 25.Dondoo TO, Fukumori T, Daizumoto K, Fukawa T, Kohzuki M, Kowada M, Kusuhara Y, Mori H, Nakatsuji H, Takahashi M, Kanayama HO. Galectin-3 is implicated in tumor progression and resistance to anti-androgen drug through regulation of androgen receptor signaling in prostate cancer. Anticancer Res. 2017;37:125–134. doi: 10.21873/anticanres.11297. [DOI] [PubMed] [Google Scholar]
- 26.Farhad M, Rolig AS, Redmond WL. The role of Galectin-3 in modulating tumor growth and immunosuppression within the tumor microenvironment. Oncoimmunology. 2018;7:e1434467. doi: 10.1080/2162402X.2018.1434467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dumic J, Dabelic S, Flögel M. Galectin-3: An open-ended story. Biochim Biophys Acta. 2006;1760:616–635. doi: 10.1016/j.bbagen.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 28.Wang L, Guo XL. Molecular regulation of galectin-3 expression and therapeutic implication in cancer progression. Biomed Pharmacother. 2016;78:165–171. doi: 10.1016/j.biopha.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 29.Ruebel KH, Jin L, Qian X, Scheithauer BW, Kovacs K, Nakamura N, Zhang H, Raz A, Lloyd RV. Effects of DNA methylation on galectin-3 expression in pituitary tumors. Cancer Res. 2005;65:1136–1140. doi: 10.1158/0008-5472.CAN-04-3578. [DOI] [PubMed] [Google Scholar]
- 30.Heldring N, Pike A, Andersson S, Matthews J, Cheng G, Hartman J, Tujague M, Ström A, Treuter E, Warner M, Gustafsson JA. Estrogen receptors: How do they signal and what are their targets. Physiol Rev. 2007;87:905–931. doi: 10.1152/physrev.00026.2006. [DOI] [PubMed] [Google Scholar]
- 31.Thiebaut C, Vlaeminck-Guillem V, Trédan O, Poulard C, Le Romancer M. Non genomic signaling of steroid receptors in cancer. Mol Cell Endocrinol. 2021;538:111453. doi: 10.1016/j.mce.2021.111453. [DOI] [PubMed] [Google Scholar]
- 32.Pisolato R, Lombardi AP, Vicente CM, Lucas TF, Lazari MF, Porto CS. Expression and regulation of the estrogen receptors in PC-3 human prostate cancer cells. Steroids. 2016;107:74–86. doi: 10.1016/j.steroids.2015.12.021. [DOI] [PubMed] [Google Scholar]
- 33.Souza DS, Lombardi A, Vicente CM, Lucas T, Erustes AG, Pereira G, Porto CS. Estrogen receptors localization and signaling pathways in DU-145 human prostate cancer cells. Mol Cell Endocrinol. 2019;483:11–23. doi: 10.1016/j.mce.2018.12.015. [DOI] [PubMed] [Google Scholar]
- 34.Lombardi AP, Pisolato R, Vicente CM, Lazari MF, Lucas TF, Porto CS. Estrogen receptor beta (ERβ) mediates expression of β-catenin and proliferation in prostate cancer cell line PC-3. Mol Cell Endocrinol. 2016;430:12–24. doi: 10.1016/j.mce.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 35.Lombardi A, Cavalheiro RP, Porto CS, Vicente CM. Estrogen receptor signaling pathways involved in invasion and colony formation of androgen-independent prostate cancer cells PC-3. Int J Mol Sci. 2021;22:1153. doi: 10.3390/ijms22031153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Silva RS, Lombardi A, Souza DS, Vicente CM, Porto CS. Activation of estrogen receptor beta (ERβ) regulates the expression of N-cadherin, E-cadherin and β-catenin in androgen-independent prostate cancer cells. Int J Biochem Cell Biol. 2018;96:40–50. doi: 10.1016/j.biocel.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 37.Stauffer SR, Coletta CJ, Tedesco R, Nishiguchi G, Carlson K, Sun J, Katzenellenbogen BS, Katzenellenbogen JA. Pyrazole ligands: Structure-affinity/activity relationships and estrogen receptor-alpha-selective agonists. J Med Chem. 2000;43:4934–4947. doi: 10.1021/jm000170m. [DOI] [PubMed] [Google Scholar]
- 38.Meyers MJ, Sun J, CarlsonK E, Marriner GA, Katzenellenbogen BS, Katzenellenbogen JA. Estrogen receptor-beta potency-selective ligands: Structure-activity relationship studies of diarylpropionitriles and their acetylene and polar analogues. J Med Chem. 2001;44:4230–4251. doi: 10.1021/jm010254a. [DOI] [PubMed] [Google Scholar]
- 39.Bustos SO, da Silva Pereira GJ, de Freitas Saito R, Gil CD, Zanatta DB, Smaili SS, Chammas R. Galectin-3 sensitized melanoma cell lines to vemurafenib (PLX4032) induced cell death through prevention of autophagy. Oncotarget. 2018;9:14567–14579. doi: 10.18632/oncotarget.24516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lombardi A, Vicente CM, Porto CS. Estrogen receptors promote migration, invasion and colony formation of the androgen-independent prostate cancer cells PC-3 through β-catenin pathway. Front Endocrinol. 2020;11:184. doi: 10.3389/fendo.2020.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vicente CM, Lima MA, Yates EA, Nader HB, Toma L. Enhanced tumorigenic potential of colorectal cancer cells by extracellular sulfatases. Mol Cancer Res. 2015;13:510–523. doi: 10.1158/1541-7786.MCR-14-0372. [DOI] [PubMed] [Google Scholar]
- 42.Marchiori MF, Riul TB, Oliveira Bortot L, Andrade P, Junqueira GG, Foca G, Doti N, Ruvo M, Dias-Baruffi M, Carvalho I, Campo VL. Binding of triazole-linked galactosyl arylsulfonamides to galectin-3 affects Trypanosoma cruzi cell invasion. Bioorg Med Chem. 2017;25:6049–6059. doi: 10.1016/j.bmc.2017.09.042. [DOI] [PubMed] [Google Scholar]
- 43.Wang Y, Jing Y, Ding L, Zhang X, Song Y, Chen S, Zhao X, Huang X, Pu Y, Wang Z, et al. Epiregulin reprograms cancer-associated fibroblasts and facilitates oral squamous cell carcinoma invasion via JAK2-STAT3 pathway. J Exp Clin Cancer Res. 2019;38:274. doi: 10.1186/s13046-019-1277-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Macheroni C, Gameiro Lucas TF, Souza DS, Vicente CM, Pereira GJDS, Junior IDSV, Juliano MA, Porto CS. Activation of estrogen receptor ESR1 and ESR2 induces proliferation of the human testicular embryonal carcinoma NT2/D1 cells. Mol Cell Endocrinol. 2022;554:111708. doi: 10.1016/j.mce.2022.111708. [DOI] [PubMed] [Google Scholar]
- 45.Nelson AW, Tilley WD, Neal DE, Carroll JS. Estrogen receptor beta in prostate cancer: Friend or foe? Endocr Relat Cancer. 2014;21:T219–T234. doi: 10.1530/ERC-13-0508. [DOI] [PubMed] [Google Scholar]
- 46.Lau KM, To KF. Importance of estrogenic signaling and its mediated receptors in prostate cancer. Int J Mol Sci. 2016;17:1434. doi: 10.3390/ijms17091434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kowalska K, Piastowska-Ciesielska AW. Oestrogens and oestrogen receptors in prostate cancer. Springer Plus. 2016;5:522. doi: 10.1186/s40064-016-2185-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Warner M, Fan X, Strom A, Wu W, Gustafsson JÅ. 25 years of ERβ: A personal journey. J Mol Endocrinol. 2021;68:R1–R9. doi: 10.1530/JME-21-0121. [DOI] [PubMed] [Google Scholar]
- 49.Nelson AW, Groen AJ, Miller JL, Warren AY, Holmes KA, Tarulli GA, Tilley WD, Katzenellenbogen BS, Hawse JR, Gnanapragasam VJ, Carroll JS. Comprehensive assessment of estrogen receptor beta antibodies in cancer cell line models and tissue reveals critical limitations in reagent specificity. Mol Cell Endocrinol. 2017;440:138–150. doi: 10.1016/j.mce.2016.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Semenas J, Wang T, Sajid Syed Khaja A, Firoj Mahmud A, Simoulis A, Grundström T, Fällman M, Persson JL. Targeted inhibition of ERα signaling and PIP5K1α/Akt pathways in castration-resistant prostate cancer. Mol Oncol. 2021;15:968–986. doi: 10.1002/1878-0261.12873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rossi V, Di Zazzo E, Galasso G, De Rosa C, Abbondanza C, Sinisi AA, Altucci L, Migliaccio A, Castoria G. Estrogens modulate somatostatin receptors expression and synergize with the somatostatin analog pasireotide in prostate cells. Front Pharmacol. 2019;10:28. doi: 10.3389/fphar.2019.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leung YK, Lam HM, Wu S, Song D, Levin L, Cheng L, Wu CL, Ho SM. Estrogen receptor beta2 and beta5 are associated with poor prognosis in prostate cancer and promote cancer cell migration and invasion. Endocr Relat Cancer. 2010;17:675–689. doi: 10.1677/ERC-09-0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gustafsson JA, Strom A, Warner M. Update on ERbeta. J Steroid Biochem Mol Biol. 2019;191:105312. doi: 10.1016/j.jsbmb.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 54.Kadrofske MM, Openo KP, Wang JL. The human LGALS3 (galectin 3) gene: Determination of the gene structure and functional characterization of the promoter. Arch Biochem Biophys. 1998;349:7–20. doi: 10.1006/abbi.1997.0447. [DOI] [PubMed] [Google Scholar]
- 55.Yang H, Lei C, Cheng C, Feng Y, Zhang W, Petracco RG, Sak S. The Antiapoptotic effect of Galectin-3 in human endometrial cells under the regulation of estrogen and progesterone. Biol Reprod. 2012;87:39. doi: 10.1095/biolreprod.112.099234. [DOI] [PubMed] [Google Scholar]
- 56.Bima C, Chiloiro S, Giampietro A, Gessi M, Mattogno PP, Lauretti L, Anile C, Rindi G, Pontecorvi A, De Marinis L, Bianchi A. Galectin-3 and estrogen receptor alpha as prognostic markers in prolactinoma: Preliminary results from a pilot study. Front Endocrinol (Lausanne) 2021;12:684055. doi: 10.3389/fendo.2021.684055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mourad-Zeidan AA, Melnikova VO, Wang H, Raz A, Bar-Eli M. Expression profiling of Galectin-3-depleted melanoma cells reveals its major role in melanoma cell plasticity and vasculogenic mimicry. Am J Pathol. 2008;173:1839–1852. doi: 10.2353/ajpath.2008.080380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang YG, Kim SJ, Baek JH, Lee HW, Jeong SY, Chun KH. Galectin-3 increases the motility of mouse melanoma cells by regulating matrix metalloproteinase-1 expression. Exp Mol Med. 2012;44:387–393. doi: 10.3858/emm.2012.44.6.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shimura T, Takenaka Y, Tsutsumi S, Hogan V, Kikuchi A, Raz A. Galectin-3, a novel binding partner of beta-catenin. Cancer Res. 2004;64:6363–6367. doi: 10.1158/0008-5472.CAN-04-1816. [DOI] [PubMed] [Google Scholar]
- 60.Yang Y, Zhou Z, He S, Fan T, Jin Y, Zhu X, Chen C, Zhang ZR, Huang Y. Treatment of prostate carcinoma with (galectin-3)-targeted HPMA copolymer-(G3-C12)-5-Fluorouracil conjugates. Biomaterials. 2012;33:2260–2271. doi: 10.1016/j.biomaterials.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 61.Glinsky VV, Raz A. Modified citrus pectin anti-metastatic properties: One bullet, multiple targets. Carbohydr Res. 2009;344:1788–1791. doi: 10.1016/j.carres.2008.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Conti S, Vexler A, Hagoel L, Kalich-Philosoph L, Corn BW, Honig N, Shtraus N, Meir Y, Ron I, Eliaz I, Lev-Ari S. Modified citrus pectin as a potential sensitizer for radiotherapy in prostate cancer. Integr Cancer Ther. 2018;17:1225–1234. doi: 10.1177/1534735418790382. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.