Abstract
Prostate cancer is one of the most heritable cancers. Hundreds of germline polymorphisms have been linked to prostate cancer diagnosis and prognosis. Polygenic risk scores can predict genetic risk of a prostate cancer diagnosis. Although these scores inform the probability of developing a tumor, it remains unknown how germline risk influences the tumor molecular evolution. We cultivated a cohort of 1250 localized European-descent patients with germline and somatic DNA profiling. Men of European descent with higher genetic risk were diagnosed earlier and had less genomic instability and fewer driver genes mutated. Higher genetic risk was associated with better outcome. These data imply a polygenic “two-hit” model where germline risk reduces the number of somatic alterations required for tumorigenesis. These findings support further clinical studies of polygenic risk scores as inexpensive and minimally invasive adjuncts to standard risk stratification. Further studies are required to interrogate generalizability to more ancestrally and clinically diverse populations.
Prostate cancer is the second-most common malignancy in men and one of the most heritable (1). An estimated 57% of the variability in prostate cancer diagnosis is explained by genetic factors (1). Elevated risk of prostate cancer diagnosis can be attributed to rare germline variants in DNA damage repair genes (2) or transcription factors (3) as well as aggregated weak effects from common variants (4,5). Polygenic risk scores (PRS) leverage common variants and predict risk of diagnosis (4,5).
There is mounting evidence that germline variation influences the somatic evolution of prostate cancer. Localized tumors in BRCA2 carriers molecularly resemble metastatic castrate-resistant disease (6). Specific germline single nucleotide polymorphisms are associated with PTEN deletion (7) and point mutations in SPOP (8), and the prostate cancer epigenome is influenced by the germline genome (9,10). It remains unknown how inherited genetic risk influences somatic evolution of a tumor.
We assembled a cohort of 427 localized prostate cancer patients with whole-genome sequencing (11-14). All patients had localized disease at diagnosis, were genetically confirmed to be of European descent, and were treated by image-guided radiotherapy or surgery. All samples were treatment naïve and macro-dissected by a genitourinary pathologist to obtain 70% tumor cellularity (Supplementary Table 1, available online). For replication we compiled a 552-patient cohort of treatment-naïve tumors arising in men of European descent from The Cancer Genome Atlas (15) supplemented by 140 primary prostate cancers (Supplementary Table 2, available online).
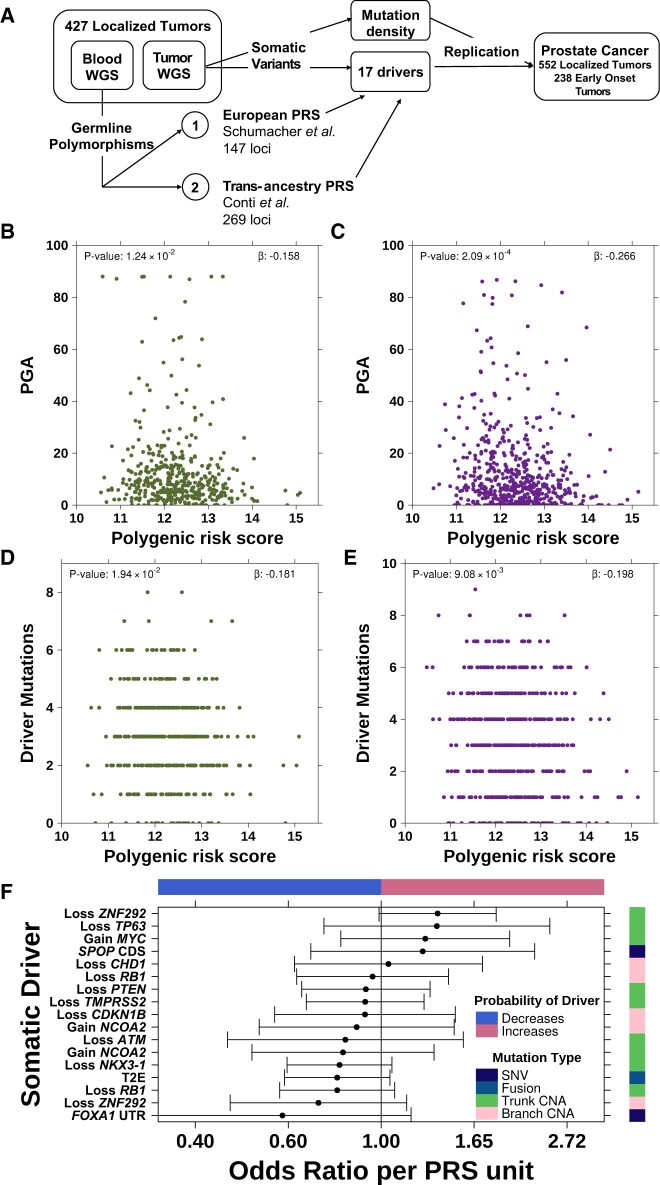
To identify if inherited genetic risk is associated with somatic evolution, we considered a 147-variant prostate cancer PRS (4) (Figure 1, A). This PRS was inversely associated with genomic instability, that is, proportion of the genome with a copy number aberration (PGA). This relationship held in both discovery and replication cohorts (βdiscovery = .16, Pdiscovery = .01; βreplication = −.27, Preplication < .001; Figure 1, B and C) and was not driven by extremely unstable tumors (PGA > 80%; Supplementary Figure 1, A, available online). The PRS–PGA association was stronger for subclonal (β= −.15, P = .005; Supplementary Figure 1, B, available online) than clonal copy number aberrations (CNAs) (β = .003; P = .95; Supplementary Figure 1, C, available online) and was independent of tumor ploidy (Supplementary Figure 1, D, available online) and subclone number (Supplementary Figure 1, E, available online) (16). This relationship replicated for a 269-variant transancestry prostate cancer PRS (βdiscovery = −.011; Pdiscovery = .02; βreplication = .019; Preplication = .002; Figure 1, A;Supplementary Figure 1, F and G, available online) (5). The negative association between PGA and genetic risk was significantly stronger than a null distribution created by randomly permuting 10 000 PRS (P = .005; Supplementary Figure 1, H, available online). The mutation density of single nucleotide variants (SNVs), Indels, and genomic rearrangements was not associated with PRS (17) (Supplementary Figure 1, I-K, available online).
Figure 1.
Genetic risk inversely associated with somatic mutation burden. A) Schematic of polygenic risk score (PRS) associations with the somatic mutational landscape of prostate cancer. The PRS was negatively associated with percent genome altered in both the discovery cohort (B) and the replication cohort (C). Green dots indicate discovery cohort and purple dots indicate replication cohort. The PRS was also negatively associated with the number of driver mutations in both the discovery (D) and replication (E) cohorts. F) PRS is not significantly associated with any individual somatic driver (false discovery rate [FDR] > 0.05). Forest plot shows odds ratio along with 95% confidence interval (x-axis) of PRS associated with each somatic driver (y-axis). Covariate on right indicates the mutation type of each somatic driver while the covariate along the top indicates the direction of effect (ie, whether high inherited risk prevents or promotes the acquisition of each driver mutation). WGS = whole genome sequencing; PGA = percent genome copy number altered; SNV = single nucleotide variant; CNA = copy number aberration.
Tumors arising in individuals with higher PRS harbored fewer driver mutations (βdiscovery = −.18, Pdiscovery = .02; βreplication = −.20, Preplication = .009; Figure 1, D and E). Driver mutations were defined as significantly recurrently mutated genes observed in more than 5% of the discovery cohort (n = 17; Supplementary Table 3, available online; see Methods). A similar trend was observed with a transancestry PRS (βdiscovery = −.009, Pdiscovery = .10; βreplication = −.008, Preplication = .16; Supplementary Figure 1, L and M, available online) (5), and the effect was greater for subclonal vs clonal drivers (βsubclonal = −.019 vs βclonal = −.017). No individual driver was associated with PRS (false discovery rate [FDR] > 0.1; Figure 1, F;Supplementary Table 4, available online). Thus, elevated genetic risk was associated with less genomic instability and fewer driver mutations at diagnosis.
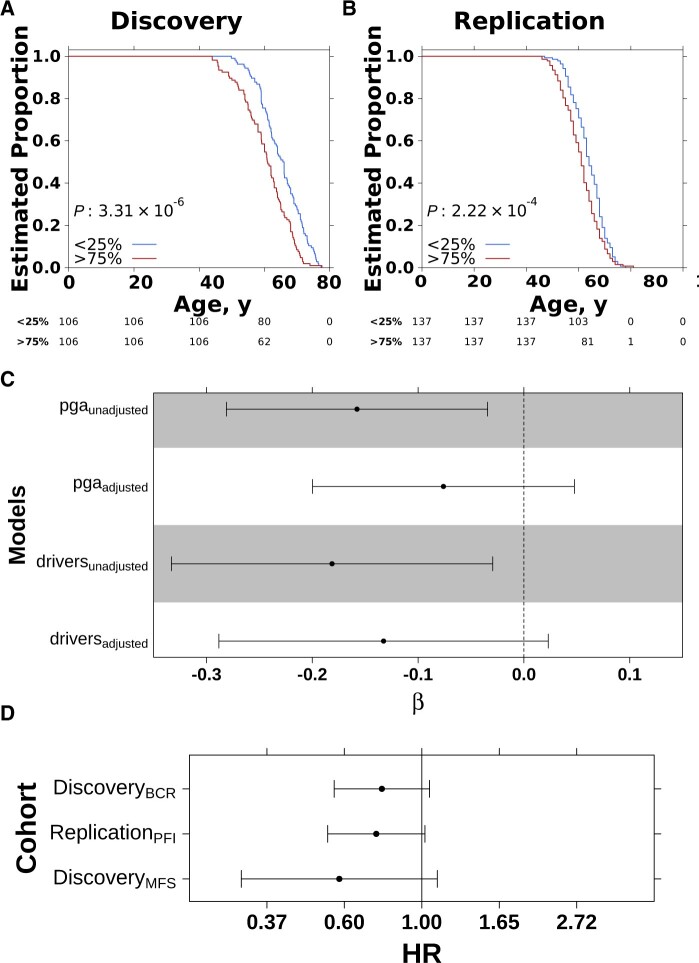
The association between PRS and somatic mutation burden may be mediated by age. Patients with higher genetic risk were diagnosed at a younger age in both the discovery and replication cohort (median difference: discovery = 4.9 years; replication = 3.0 years; Figure 2, A and B). PRS–PGA and PRS–driver associations were no longer statistically significant after adjusting for age, although with clear trends (βPGA = −.08, PPGA = .23; βdrivers = −.13, Pdrivers = .09; Figure 2, C). These data suggest younger age at diagnosis partially mediates the negative association between inherited risk and genomic instability.
Figure 2.
Genetic risk inversely associated with age at diagnosis. Kaplan-Meier curves of time to diagnosis (age) stratified by high (>75%) vs low (<25%) polygenic risk score (PRS) in discovery (A) vs replication (B). C) Coefficients from linear model quantifying association between PRS and proportion of the genome with a copy number aberration (PGA) (Box Cox-transformed) or number of driver mutations with or without adjustment of age of diagnosis. Error bars show 95% confidence interval and background shading reflects P value less than .05. D) PRS is negatively associated with relapse after primary treatment in both the discovery and replication cohort. Scatterplot shows the hazard ratio and 95% confidence intervals from a CoxPH model correcting for the first 5 genetic principal components and age. Biochemical recurrence (BCR), progression-free survival (PFI), and metastasis-free survival (MFS) were used as endpoints. HR = hazard ratio.
Early-onset prostate tumors (EOPC; diagnosis <55 years) provide a natural experiment to explore the interaction of inherited genetic risk, age of diagnosis, and genomic instability. PRS was negatively associated with PGA in 238 additional EOPC tumors (18) (βEOPC = −.35, PEOPC = .04; Supplementary Figure 1, N, available online), and was fully mediated by age (P = .36).
Finally, given the strong link between genomic instability and disease aggression, we evaluated if inherited risk was linked to relapse (19). Previous studies have shown prostate cancer PRS is not associated with tumor grade at diagnosis (20) or likelihood of metastasis or death (21), but to our knowledge there have been no studies linking PRS with relapse after primary treatment. Although we did not observe associations between PRS and T category or pretreatment PSA (Supplementary Figures 1, O-R, available online), we observed a weak negative association between PRS and International Society of Urological Pathology (ISUP) grade group in the discovery cohort (ρ = −0.06, P = .25), which replicated in the replication cohort (ρ = −0.16, P < .001) (Supplementary Figure 1, S and T, available online). Compared with the discovery cohort, which was enriched for intermediate-risk prostate cancer by design, the replication cohort had a more even distribution of risk categories and was better powered for these analyses. PRS was negatively associated with relapse in both the discovery and replication cohorts, controlling for age and primary treatment (hazard ratio [HR]discoveryBCR = 0.77, 95% confidence interval [CI] = 0.57 to 1.05, PdiscoveryBCR = .10; HRreplicationPFI = 0.75, 95% CI = 0.54 to 1.02, PreplicationPFI = .07) (Figure 2, D). PRS showed a trend toward negative association with metastasis-free survival in the discovery cohort (HRdiscoveryMFS = 0.58, 95% CI = 0.31 to 1.10, PdiscoveryMFS = .10) (Figure 2, D). These data suggest germline risk influences the number of somatic alterations required for tumorigenesis, leading to lower age at diagnosis and favorable prognosis (22).
Knudson (23) discovered that germline loss-of-function variants in RB1 required one somatic alteration to trigger retinoblastoma compared with sporadic cases, which required 2 somatic loss-of-function alterations. Hereditary retinoblastoma cases were diagnosed at a younger age than sporadic cases. Our data are analogous to a polygenic version of Knudson’s “two-hit” hypothesis. Though not focused on one gene, germline variants that increase prostate cancer risk require fewer somatic alterations to trigger tumorigenesis and lead to a younger age at diagnosis. The association is stronger for subclonal vs clonal PGA, suggesting risk variants may exert selection pressures facilitating clonal sweeps. Given deletions are preferentially clonal and gains are preferentially subclonal (16), it is also possible PRS may associate differently with varying types of DNA damage. These findings suggest European men with high inherited risk may have more favorable outcomes given their more stable somatic genomes. Presently, these findings are limited to individuals of European descent. Well-documented ancestry-related variability in clinical and molecular presentation (24) motivates further interrogation of the utility of prostate cancer PRS in prognosis in larger and more diverse populations.
Supplementary Material
Contributor Information
Kathleen E Houlahan, Department of Human Genetics, University of California, Los Angeles, CA, USA; Jonsson Comprehensive Cancer Center, University of California, Los Angeles, CA, USA; Department of Medical Biophysics, University of Toronto, Toronto, Canada; Institute for Precision Health, University of California, Los Angeles, CA, USA; Ontario Institute for Cancer Research, Toronto, Canada; Vector Institute, Toronto, Canada.
Julie Livingstone, Department of Human Genetics, University of California, Los Angeles, CA, USA; Jonsson Comprehensive Cancer Center, University of California, Los Angeles, CA, USA; Institute for Precision Health, University of California, Los Angeles, CA, USA; Department of Urology, University of California, Los Angeles, CA, USA.
Natalie S Fox, Department of Human Genetics, University of California, Los Angeles, CA, USA; Jonsson Comprehensive Cancer Center, University of California, Los Angeles, CA, USA; Department of Medical Biophysics, University of Toronto, Toronto, Canada; Institute for Precision Health, University of California, Los Angeles, CA, USA; Ontario Institute for Cancer Research, Toronto, Canada.
Natalie Kurganovs, Australian Prostate Cancer Research Centre Epworth, Richmond, VIC, Australia; Department of Surgery, The University of Melbourne, Parkville, VIC, Australia; Princess Margaret Cancer Centre, University Health Network, Toronto, Canada.
Helen Zhu, Department of Medical Biophysics, University of Toronto, Toronto, Canada; Vector Institute, Toronto, Canada; Princess Margaret Cancer Centre, University Health Network, Toronto, Canada.
Jocelyn Sietsma Penington, Bioinformatics Division, Walter and Eliza Hall Institute, Parkville, VIC, Australia.
Chol-Hee Jung, Melbourne Bioinformatics, The University of Melbourne, Parkville, VIC, Australia.
Takafumi N Yamaguchi, Department of Human Genetics, University of California, Los Angeles, CA, USA; Jonsson Comprehensive Cancer Center, University of California, Los Angeles, CA, USA; Institute for Precision Health, University of California, Los Angeles, CA, USA; Department of Urology, University of California, Los Angeles, CA, USA.
Lawrence E Heisler, Ontario Institute for Cancer Research, Toronto, Canada.
Richard Jovelin, Ontario Institute for Cancer Research, Toronto, Canada.
Anthony J Costello, Division of Urology, Royal Melbourne Hospital, Parkville, VIC, Australia.
Bernard J Pope, Department of Surgery, The University of Melbourne, Parkville, VIC, Australia; Melbourne Bioinformatics, The University of Melbourne, Parkville, VIC, Australia; Department of Clinical Pathology, The University of Melbourne, Parkville, VIC, Australia; Department of Medicine, Central Clinical School, Faculty of Medicine Nursing and Health Sciences, Monash University, Melbourne, VIC, Australia.
Amar U Kishan, Jonsson Comprehensive Cancer Center, University of California, Los Angeles, CA, USA; Department of Radiation Oncology, University of California, Los Angeles, CA, USA.
Niall M Corcoran, Australian Prostate Cancer Research Centre Epworth, Richmond, VIC, Australia; Department of Surgery, The University of Melbourne, Parkville, VIC, Australia; Division of Urology, Royal Melbourne Hospital, Parkville, VIC, Australia; Department of Urology, Peninsula Health, Frankston, VIC, Australia; The Victorian Comprehensive Cancer Centre, Parkville, VIC, Australia.
Robert G Bristow, Department of Medical Biophysics, University of Toronto, Toronto, Canada; Princess Margaret Cancer Centre, University Health Network, Toronto, Canada; Manchester Cancer Research Centre, Manchester, UK.
Sebastian M Waszak, Centre for Molecular Medicine Norway (NCMM), Nordic EMBL Partnership, University of Oslo, and Oslo University Hospital, Oslo, Norway; Department of Pediatric Research, Division of Paediatric and Adolescent Medicine, Rikshospitalet, Oslo University Hospital, Oslo, Norway; Department of Neurology, University of California, San Francisco, San Francisco, CA, USA.
Joachim Weischenfeldt, Biotech Research and Innovation Centre (BRIC), University of Copenhagen, Copenhagen, Denmark; Finsen Laboratory, Rigshospitalet, Copenhagen, Denmark; Department of Urology, Charité-Universitätsmedizin Berlin, Berlin, Germany.
Housheng H He, Department of Medical Biophysics, University of Toronto, Toronto, Canada; Princess Margaret Cancer Centre, University Health Network, Toronto, Canada.
Rayjean J Hung, Prosserman Centre for Population Health Research, Lunenfeld-Tanenbaum Research Institute, Sinai Health System, Toronto, Canada; Epidemiology Division, Dalla Lana School of Public Health, University of Toronto, Toronto, Canada.
Christopher M Hovens, Australian Prostate Cancer Research Centre Epworth, Richmond, VIC, Australia; Department of Surgery, The University of Melbourne, Parkville, VIC, Australia.
Paul C Boutros, Department of Human Genetics, University of California, Los Angeles, CA, USA; Jonsson Comprehensive Cancer Center, University of California, Los Angeles, CA, USA; Department of Medical Biophysics, University of Toronto, Toronto, Canada; Institute for Precision Health, University of California, Los Angeles, CA, USA; Ontario Institute for Cancer Research, Toronto, Canada; Vector Institute, Toronto, Canada; Department of Urology, University of California, Los Angeles, CA, USA; Department of Pharmacology and Toxicology, University of Toronto, Toronto, Canada.
Funding
This work was supported by Prostate Cancer Canada and is proudly funded by the Movember Foundation (grant #RS2014-01 to P.C.B.). P.C.B. was supported by a Terry Fox Research Institute New Investigator Award and a Canadian Institutes of Health Research (CIHR) New Investigator Award. This project was supported by Genome Canada through a Large-Scale Applied Project contract to P.C.B., R. Morin and S. P. Shah. K.E.H was supported by a CIHR Vanier Fellowship. This work was supported by the National Institutes of Health/National Cancer Institute (NIH/NCI) under award number P30CA016042, by an operating grant from the National Cancer Institute Early Detection Research Network (U01CA2141941) and by support from the Informatics Technology for Cancer Research (ITCR) (U24CA248265). H.H.H. holds Joey and Toby Tanenbaum Brazilian Ball Chair in Prostate Cancer. This work is supported by a Terry Fox New Frontiers Program Project Grant (1090 P3 to H.H.H.). This work was supported by a Prostate Cancer Foundation Special Challenge Award to PCB (Award ID #: 20CHAS01) made possible by the generosity of Mr Larry Ruvo. B.J.P. was supported by a Victorian Health and Medical Research Fellowship. S.M.W. was supported by the Research Council of Norway (187615), the South-Eastern Norway Regional Health Authority, and the University of Oslo.
Notes
Role of the funder: The funders had no role in the study design; data collection, analysis, or interpretation of the data; decision to publish, or preparation of the manuscript.
Disclosures: A.U.K. has received personal fees from Varian Medical Systems, Inc, ViewRay, Inc, Janssen, Inc, and Intelligent Automation, Inc. P.C.B. sits on the Scientific Advisory Boards of Sage Bionetworks, BioSymetrics Inc and Intersect Diagnostics Inc. All other authors declare they have no conflicts of interest. At the time of publication, N.S.F was an employee of Hoffman-La Roche Limited (Roche Canada). All contributions by N.S.F were completed prior to this employment. P.C.B., a JNCI Associate Editor and co-author of this brief communication was not involved in the editorial review or decision to accept it for publication.
Author contributions: Kathleen E. Houlahan (Conceptualization, Formal analysis, Writing – original draft, Writing – review & editing); Julie Livingstone, (Formal analysis, Writing – review & editing); Natalie S. Fox (Formal analysis, Writing – review & editing); Natalie Kurganovs (Data curation, Writing – review & editing); Helen Zhu (Formal analysis, Writing – review & editing); Jocelyn Sietsma Penington, (Formal analysis, Writing – review & editing); Chol-Hee Jung (Formal analysis, Writing – review & editing); Takafumi N. Yamaguchi (Formal analysis, Writing – review & editing); Lawrence E. Heisler (Formal analysis, Writing – review & editing); Richard Jovelin (Formal analysis, Writing – review & editing); Anthony J. Costello (Data curation, Writing – review & editing); Bernard J. Pope (Supervision, Writing – review & editing); Amar U. Kishan (Supervision, Writing – review & editing); Niall M. Corcoran (Supervision, Writing – review & editing); Robert G. Bristow (Supervision, Writing – review & editing); Sebastian M. Waszak (Formal analysis, Supervision, Writing – review & editing); Joachim Weischenfeldt (Formal analysis, Supervision, Writing – review & editing); Housheng H. He (Supervision, Writing – review & editing); Rayjean J. Hung (Supervision, Writing – review & editing); Christopher M. Hovens (Supervision, Writing – review & editing); Paul C. Boutros (Conceptualization, Supervision, Writing – review & editing).
Acknowledgements: The authors thank all members of the Boutros lab for helpful suggestions and support. The results described here are based in part upon data generated by the The Cancer Genome Atlas (TCGA) Research Network: http://cancergenome.nih.gov/.
Data availability
Raw sequencing data are available in the European Genome-phenome Archive under accession EGAS00001000900 (https://www.ebi.ac.uk/ega/studies/EGAS00001000900). Processed variant calls are available through the International Cancer Genome Consortium (ICGC) Data Portal under the project PRAD-CA (https://dcc.icgc.org/projects/PRAD-CA). TCGA whole genome and whole exome sequencing (WGS/WES) data are available at Genomic Data Commons Data Portal (https://gdc-portal.nci.nih.gov/projects/TCGA-PRAD).
References
- 1. Mucci LA, Hjelmborg JB, Harris JR, et al. ; Nordic Twin Study of Cancer (NorTwinCan) Collaboration. Familial risk and heritability of cancer among twins in Nordic countries. JAMA. 2016;315(1):68-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pritchard CC, Mateo J, Walsh MF, et al. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N Engl J Med. 2016;375(5):443-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ewing CM, Ray AM, Lange EM, et al. Germline mutations in HOXB13 and prostate-cancer risk. N Engl J Med. 2012;366(2):141-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schumacher FR, Al Olama AA, Berndt SI; Genetic Associations and Mechanisms in Oncology (GAME-ON)/Elucidating Loci Involved in Prostate Cancer Susceptibility (ELLIPSE) Consortium. Association analyses of more than 140,000 men identify 63 new prostate cancer susceptibility loci. Nat Genet. 2018;50(7):928-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Conti DV, Darst BF, Moss LC, et al. Trans-ancestry genome-wide association meta-analysis of prostate cancer identifies new susceptibility loci and informs genetic risk prediction. Nat Genet. 2021;53(1):65-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Taylor RA, Fraser M, Livingstone J, et al. Germline BRCA2 mutations drive prostate cancers with distinct evolutionary trajectories. Nat Commun. 2017;8:13671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Briollais L, Ozcelik H, Xu J, et al. Germline mutations in the Kallikrein 6 region and predisposition for aggressive prostate cancer. J Natl Cancer Inst. 2017;109:djw258. doi: 10.1093/jnci/djw258. [DOI] [PubMed] [Google Scholar]
- 8. Romanel A, Garritano S, Stringa B, et al. Inherited determinants of early recurrent somatic mutations in prostate cancer. Nat Commun. 2017;8(1):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Heyn H, Sayols S, Moutinho C, et al. Linkage of DNA methylation quantitative trait loci to human cancer risk. Cell Rep. 2014;7(2):331-338. [DOI] [PubMed] [Google Scholar]
- 10. Houlahan KE, Shiah Y-J, Gusev A, et al. Genome-wide germline correlates of the epigenetic landscape of prostate cancer. Nat Med. 2019;25(10):1615-1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Baca SC, Prandi D, Lawrence MS, et al. Punctuated evolution of prostate cancer genomes. Cell. 2013;153(3):666-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Berger MF, Lawrence MS, Demichelis F, et al. The genomic complexity of primary human prostate cancer. Nature. 2011;470(7333):214-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wedge DC, Gundem G, Mitchell T, et al. ; TCGA Consortium. Sequencing of prostate cancers identifies new cancer genes, routes of progression and drug targets. Nat Genet. 2018;50(5):682-692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Weischenfeldt J, Simon R, Feuerbach L, et al. Integrative genomic analyses reveal an androgen-driven somatic alteration landscape in early-onset prostate cancer. Cancer Cell. 2013;23(2):159-170. [DOI] [PubMed] [Google Scholar]
- 15. The Cancer Genome Atlas Research Network. The molecular taxonomy of primary prostate cancer. Cell. 2015;163:1011-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Espiritu SMG, Liu LY, Rubanova Y, et al. The evolutionary landscape of localized prostate cancers drives clinical aggression. Cell. 2018;173(4):1003-1013.e15. [DOI] [PubMed] [Google Scholar]
- 17. Ciriello G, Miller ML, Aksoy BA, et al. Emerging landscape of oncogenic signatures across human cancers. Nat Genet. 2013;45(10):1127-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gerhauser C, Favero F, Risch T, et al. Molecular evolution of early-onset prostate cancer identifies molecular risk markers and clinical trajectories. Cancer Cell. 2018;34(6):996-1011.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lalonde E, Ishkanian AS, Sykes J, et al. Tumour genomic and microenvironmental heterogeneity for integrated prediction of 5-year biochemical recurrence of prostate cancer: a retrospective cohort study. Lancet Oncol. 2014;15(13):1521-1532. [DOI] [PubMed] [Google Scholar]
- 20. Bakshi A, Riaz M, Orchard SG, et al. A polygenic risk score predicts incident prostate cancer risk in older men but does not select for clinically significant disease. Cancers. 2021;13:5815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Klein RJ, Vertosick E, Sjoberg D, et al. Prostate cancer polygenic risk score and prediction of lethal prostate cancer. NPJ Precis Onc. 2022;6:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Qing T, Mohsen H, Marczyk M, et al. Germline variant burden in cancer genes correlates with age at diagnosis and somatic mutation burden. Nat Commun. 2020;11(1):2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Knudson AG. Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci USA. 1971;68(4):820-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yuan J, Hu Z, Mahal BA, et al. Integrated analysis of genetic ancestry and genomic alterations across cancers. Cancer Cell. 2018;34(4):549-560.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw sequencing data are available in the European Genome-phenome Archive under accession EGAS00001000900 (https://www.ebi.ac.uk/ega/studies/EGAS00001000900). Processed variant calls are available through the International Cancer Genome Consortium (ICGC) Data Portal under the project PRAD-CA (https://dcc.icgc.org/projects/PRAD-CA). TCGA whole genome and whole exome sequencing (WGS/WES) data are available at Genomic Data Commons Data Portal (https://gdc-portal.nci.nih.gov/projects/TCGA-PRAD).