Abstract
Background
Cancer rates in rural areas across the United States have different patterns than in urban areas. This study examines associations between rurality and incidence for the top 5 cancers in California and evaluates whether these associations vary jointly by sex, race, and ethnicity.
Methods
We used 2015-2019 California Cancer Registry data to compare incidence rate ratios (IRRs) and trends for breast, prostate, lung, colorectal, and skin (melanoma) cancers. We leveraged census tract aggregation zones and 7 levels of percentage rural population (0%, >0% to <10%, 10% to <20%, 20% to <30%, 30% to <40%, 40% to <50%, and 50+%).
Results
Zones with higher proportions of rural population were significantly associated with lower incidence of female breast cancer and prostate cancer, though the trends were not statistically significant overall. Zones with higher proportions of rural population were significantly associated with higher incidence of lung cancer and melanoma. There were no statistically significant trends for colorectal cancer overall. Comparing areas with 50% and over rural population with areas with 0% rural population, the IRR for lung cancer in Hispanic females was higher (IRR = 1.43, 95% confidence interval [CI] = 1.17 to 1.74) than in Hispanic males (IRR = 0.90, 95% CI = 0.72 to 1.11). Also, in areas with 50% or more rural population, the IRR for melanoma was higher in Hispanic females (IRR = 1.75, 95% CI = 1.23 to 2.45) than non-Hispanic White females (IRR = 0.87, 95% CI = 0.80 to 0.95).
Conclusions
Our findings show that rurality is associated with cancer incidence and underscore the importance of jointly examining rural disparities with sex, race, and ethnicity by cancer site.
Cancer rates in rural areas across the United States have different patterns than in urban areas. Rural areas often report lower incidence rates for breast and prostate cancers and higher rates for lung and colorectal cancers (1-4). Rural disparities in cancer incidence have been attributed to a complex combination of risk factors, including access to health care, screening rates, smoking, obesity, poverty, and neighborhood disinvestment (1,5). However, rural subpopulations may experience risks differently, resulting in variable cancer incidence. These differences confound efforts to interpret findings and generalize about rural cancer disparities (2,6).
Rural cancer research to date has most often defined “rural” in broad terms, using classification schemes based on Rural-Urban Continuum Codes (RUCC) or Rural-Urban Commuting Area (RUCA) codes, both developed by the US Department of Agriculture Economic Research Service (3,4,7-9). In the RUCC scheme, metropolitan counties are coded according to population size, and non-metropolitan counties are coded by population size and adjacency to a metro area. A 9-point coding system comprises the RUCC scheme, with counties often categorized as “urban” (codes 1-3) or “rural” (codes 4-9). The RUCA scheme uses a 10-point system in which census tracts are coded according to population density, urbanization, and daily commuting patterns and then categorized in various ways, such as urban, large rural town, or small rural town (10). Although both the RUCC and RUCA schemes are practical for many research purposes, population size in low-density counties may be too small for the granular statistical analyses necessary to understand cancer disparities within rural populations. Also, collapsing codes into rural vs urban dichotomies masks the considerable variation across levels of rurality.
Recent cancer control studies have begun to examine rurality more as a continuum rather than as a rural–urban dichotomy. Some researchers have used RUCC or RUCA codes to create 4 categories rather than 2 (1,6). Others have explored the use of all 9 RUCC categories, although the use of nominal categories as an ordinal continuum presents limitations in statistical analyses (10,11). Researchers have also explored using an index of rurality with a continuum ranging from 0 to 10 (10). The US Census provides data on proportion of rural residents in a census tract. The US Census defines urban areas as densely developed residential, commercial, and other nonresidential areas; rural areas are defined as all regions not included within an urban area (12,13). However, no consensus has yet emerged about the most feasible and effective way to operationalize a continuum of rurality. In addition, almost no research has examined incidence rates by race and ethnicity and sex across a continuum of rurality (14).
We used a novel approach based on census tract aggregation zones, developed through a collaboration with the National Cancer Institute and Westat (15). Census tract aggregation zones were created by combining adjacent census tracts according to demographic and socioeconomic factors (15). Census tract aggregation zones may comprise multiple counties in sparsely populated areas or subcounty units in densely populated areas. This approach allows for reporting results at the subcounty level in more densely populated areas and reduces suppression of results in less densely populated areas. These zones are primarily structured around population and neighborhood attributes rather than relying on geopolitical boundaries, thereby creating geographies that are more homogeneous and potentially more meaningful for assessing cancer risk. Moreover, zones can be characterized based on US Census Bureau data for census tracts that comprise them, including providing a more nuanced measure of rurality.
California has 578 census tract aggregation zones with population sizes ranging from 51 229 to 98 764 and number of census tracts from 5 to 25 (15). We used these zones and California Cancer Registry (CCR) data to examine associations between a 7-level ordinal measure of rurality and incidence rates for the top 5 cancers by sex, race, and ethnicity. California’s large, diverse population and varied geography make it possible to investigate nuanced patterns of cancer incidence across rural areas and identify disparities.
Methods
Data source
We used CCR data to estimate 5-year incidence rates (2015-2019) for female breast cancer (International Classification of Diseases [ICD]-O-3 = C50.0-C50.9), colorectal cancer (ICD-O-3 = C18.0, C18.2-C18.9, C19.9, C20.9), lung cancer (ICD-O-3 = C34.1-C34.9), melanoma (ICD-O-3 = C449), and male prostate cancers (ICD-O-3 = C61.9) (16). Analysis was limited to cases reported as male or female sex. This study received University of California San Francisco institutional review board approval as a part of the protocol for the Greater Bay Area Cancer Registry.
Study variables
Rurality was defined as the proportion of residents in rural areas within zones and for the analysis was categorized into 7 levels: 0% (not rural), >0% to <10%, 10% to <20%, 20% to <30%, 30% to <40%, 40% to <50%, and 50+%. We used publicly available census tract–level data on rurality from the US Census, which is defined based on percent of residents who reside in blocks that are designated as rural (12,13). Zone-level rurality was calculated using population-weighted census tract–level data on rurality from Census 2010. Seven levels of rurality were chosen to maximize the granularity of the rural measure while still retaining enough power (ie, cases) to calculate incidence rates.
Zones were generated using a software zone design program called AZTool to create geographically compact areas similar in terms of minority population, poverty, and urban or rural status with a minimum population of 50 000 (17). Counties with larger populations were divided into multiple zones; counties with smaller populations were combined to form zones (18). Cancer incidence rates for the most common invasive cancer sites in California can be viewed by zone at https://www.californiahealthmaps.org/ (15).
Race and ethnicity were defined as non-Hispanic American Indian/Alaska Native, Hispanic, non-Hispanic Asian American/Pacific Islander, non-Hispanic Black, non-Hispanic White, and Other/Unknown. The Hispanic group included people of all races. Other/Unknown includes individuals with other or unknown race and ethnicity. The source of the race and ethnicity data in cancer registry records is taken from patient medical records (which may be self-reported by the patient or noted by the provider or other staff). CCR additionally applies the North American Association of Central Cancer Registries’ identification algorithms for Hispanic and non-Hispanic Asian American/Pacific Islander population groups based on ethnicity, ancestry, birthplace, and/or surnames to improve quality of this data (19,20). Sex was defined as male and female and extracted from patient medical records.
Statistical analysis
We computed age-adjusted incidence rates (per 100 000 population) for each of the 7 levels of rurality and incidence rate ratios (IRRs) with reference to 0% (not rural). The 95% confidence intervals (CIs) for age-adjusted incidence rates and IRRs were calculated using the Tiwari et al. (21) modification. Annual population counts for incidence calculations were estimated using linear interpolation and extrapolation of 2017 census tract population estimates produced by Information Management Services with support from the National Cancer Institute (22). We conducted tests for the linear trend of incidence rates across rurality levels by using weighted linear regression, with the inverse of the incidence rate variance as the weight. The analyses were stratified by sex, race and ethnicity, and cancer site combined groups. Data for non-Hispanic American Indian/Alaska Native cases were too sparse to report rates. As population estimates were not available for “Other/Unknown” race and ethnicity, rates were not calculated for this group.
We performed all analyses using SAS software, version 9.4 (SAS institute Inc, Cary, NC, USA). All statistical tests were 2-sided, with P < .05 indicating statistical significance.
Results
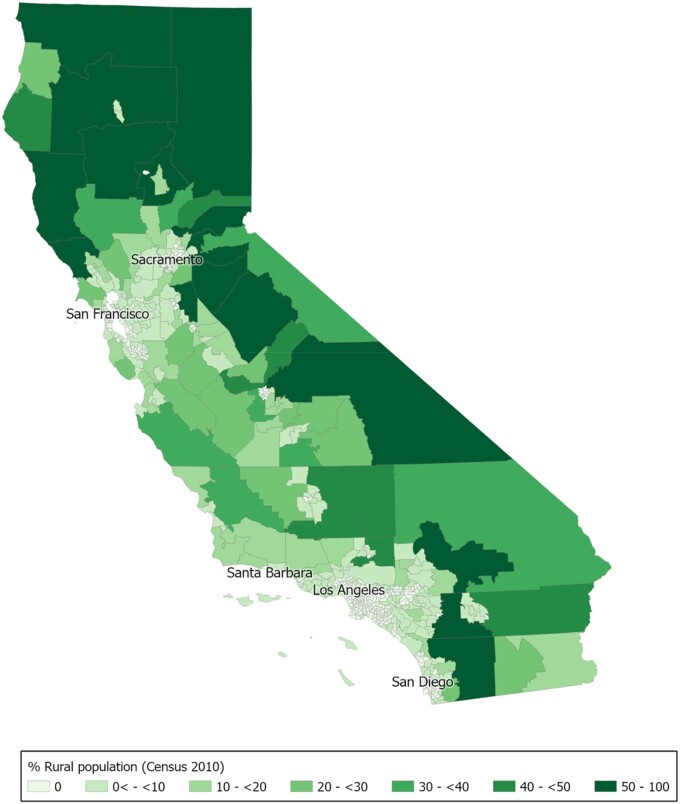
In California, rural areas are located in the north, Central Coast, Central Valley, and southeastern desert regions, and urban areas are centered around Sacramento, San Francisco, Los Angeles, and San Diego (Figure 1). A total of 450 194 cancer patients diagnosed in California from 2015 to 2019 were included in this analysis. Median age at diagnosis was 67 years (interquartile range = 58-75 years) (Tables 1 and 2).
Figure 1.
Map of census tract aggregation zones in California by percentage rural population. Rurality was defined as the proportion of residents in rural areas within zones and for the analysis was categorized into 7 levels: 0% (not rural), >0% to <10%, 10% to <20%, 20% to <30%, 30% to <40%, 40% to <50%, and 50+% (12). We used census tract–level data on rurality, which the US Census defines based on percent of residents who reside in blocks that are designated as rural. Zone-level rurality was calculated using population-weighted census tract–level data on rurality from Census 2010.
Table 1.
Characteristics of female breast, prostate, and lung cancer cases in California (2015-2019)a
| Characteristic | Female breast | Male prostate | Male lung | Female lung |
|---|---|---|---|---|
| No. (%) | No. (%) | No. (%) | No. (%) | |
| All | 139 119 (100.0) | 101 161 (100.0) | 42 518 (100.0) | 42 780 (100.0) |
| Year of diagnosis | ||||
| 2015 | 27 141 (19.5) | 18 509 (18.3) | 8711 (20.5) | 8607 (20.1) |
| 2016 | 26 911 (19.3) | 18 995 (18.8) | 8608 (20.2) | 8541 (20.0) |
| 2017 | 27 850 (20.0) | 21 047 (20.8) | 8454 (19.9) | 8712 (20.4) |
| 2018 | 28 250 (20.3) | 20 773 (20.5) | 8329 (19.6) | 8403 (19.6) |
| 2019 | 28 967 (20.8) | 21 837 (21.6) | 8416 (19.8) | 8517 (19.9) |
| Age at diagnosis, y | ||||
| 0-29 | 811 (0.6) | 7 (0.0) | 82 (0.2) | 85 (0.2) |
| 30-39 | 6036 (4.3) | 31 (0.0) | 196 (0.5) | 236 (0.6) |
| 40-49 | 19 961 (14.3) | 1487 (1.5) | 798 (1.9) | 952 (2.2) |
| 50-59 | 31 061 (22.3) | 17 043 (16.8) | 4445 (10.5) | 4613 (10.8) |
| 60-69 | 38 486 (27.7) | 42 752 (42.3) | 12 164 (28.6) | 11 248 (26.3) |
| 70-79 | 27 861 (20.0) | 29 097 (28.8) | 14 786 (34.8) | 14 632 (34.2) |
| 80+ | 14 903 (10.7) | 10 744 (10.6) | 10 047 (23.6) | 11 014 (25.7) |
| Race/ethnicity | ||||
| American Indian/Alaska Native | 831 (0.6) | 465 (0.5) | 280 (0.7) | 310 (0.7) |
| Hispanic | 29 488 (21.2) | 18 766 (18.6) | 5558 (13.1) | 5346 (12.5) |
| Non-Hispanic AAPI | 20 765 (14.9) | 8366 (8.3) | 6433 (15.1) | 5536 (12.9) |
| Non-Hispanic Black | 8549 (6.1) | 9079 (9.0) | 3207 (7.5) | 3121 (7.3) |
| Non-Hispanic White | 77 529 (55.7) | 57 830 (57.2) | 26 777 (63.0) | 28 243 (66.0) |
| Other/unknown | 1957 (1.4) | 6655 (6.6) | 263 (0.6) | 224 (0.5) |
| SEER summary stage | ||||
| Localized | 89 288 (64.2) | 38 633 (38.2) | 9027 (21.2) | 11 504 (26.9) |
| Regional | 37 801 (27.2) | 8725 (8.6) | 8274 (19.5) | 8095 (18.9) |
| Distant | 7689 (5.5) | 5342 (5.3) | 21 666 (51.0) | 19 998 (46.7) |
| Unknown | 4341 (3.1) | 48 461 (47.9) | 3551 (8.4) | 3183 (7.4) |
| Rural | ||||
| 0% | 76 783 (55.2) | 52 270 (51.7) | 22 865 (53.8) | 22 527 (52.7) |
| 0% to <10% | 39 785 (28.6) | 29 965 (29.6) | 11 403 (26.8) | 12 151 (28.4) |
| 10% to <20% | 10 386 (7.5) | 8122 (8.0) | 3278 (7.7) | 3315 (7.7) |
| 20% to <30% | 3560 (2.6) | 2861 (2.8) | 1242 (2.9) | 1097 (2.6) |
| 30% to <40% | 1945 (1.4) | 1803 (1.8) | 886 (2.1) | 854 (2.0) |
| 40% to <50% | 1902 (1.4) | 1773 (1.8) | 705 (1.7) | 738 (1.7) |
| 50%+ | 4758 (3.4) | 4367 (4.3) | 2139 (5.0) | 2098 (4.9) |
AAPI = Asian American/Pacific Islander; SEER = Surveillance, Epidemiology, and End Results.
Table 2.
Characteristics of CRC and melanoma cases in California (2015-2019)a
| Characteristic | Male CRC | Female CRC | Male melanoma | Female melanoma |
|---|---|---|---|---|
| No. (%) | No. (%) | No. (%) | No. (%) | |
| All | 39 211 (100.0) | 35 329 (100.0) | 30 144 (100.0) | 19 932 (100.0) |
| Year of diagnosis | ||||
| 2015 | 7831 (20.0) | 7039 (19.9) | 5846 (19.4) | 3893 (19.5) |
| 2016 | 7674 (19.6) | 7086 (20.1) | 5939 (19.7) | 3807 (19.1) |
| 2017 | 7743 (19.7) | 7064 (20.0) | 6044 (20.1) | 3921 (19.7) |
| 2018 | 7942 (20.3) | 7040 (19.9) | 6085 (20.2) | 4074 (20.4) |
| 2019 | 8021 (20.5) | 7100 (20.1) | 6230 (20.7) | 4237 (21.3) |
| Age at diagnosis, y | ||||
| 0-29 | 426 (1.1) | 497 (1.4) | 387 (1.3) | 658 (3.3) |
| 30-39 | 1167 (3.0) | 1115 (3.2) | 992 (3.3) | 1577 (7.9) |
| 40-49 | 3424 (8.7) | 2956 (8.4) | 1842 (6.1) | 2181 (10.9) |
| 50-59 | 8784 (22.4) | 6641 (18.8) | 4856 (16.1) | 3879 (19.5) |
| 60-69 | 10 600 (27.0) | 8055 (22.8) | 8155 (27.1) | 4963 (24.9) |
| 70-79 | 8278 (21.1) | 7565 (21.4) | 8036 (26.7) | 3724 (18.7) |
| 80+ | 6532 (16.7) | 8500 (24.1) | 5876 (19.5) | 2950 (14.8) |
| Race/ethnicity | ||||
| American Indian/Alaska Native | 272 (0.7) | 262 (0.7) | 87 (0.3) | 68 (0.3) |
| Hispanic | 9536 (24.3) | 8248 (23.3) | 1155 (3.8) | 1619 (8.1) |
| Non-Hispanic AAPI | 5638 (14.4) | 5113 (14.5) | 182 (0.6) | 229 (1.1) |
| Non-Hispanic Black | 2479 (6.3) | 2404 (6.8) | 66 (0.2) | 55 (0.3) |
| Non-Hispanic White | 20 663 (52.7) | 18 739 (53.0) | 26 084 (86.5) | 15 875 (79.6) |
| Other/unknown | 623 (1.6) | 563 (1.6) | 2570 (8.5) | 2086 (10.5) |
| SEER summary stage | ||||
| Localized | 13 857 (35.3) | 12 690 (35.9) | 21 788 (72.3) | 14 943 (75.0) |
| Regional | 13 887 (35.4) | 12 489 (35.4) | 2679 (8.9) | 1437 (7.2) |
| Distant | 8464 (21.6) | 7280 (20.6) | 1471 (4.9) | 649 (3.3) |
| Unknown | 3003 (7.7) | 2870 (8.1) | 4206 (14.0) | 2903 (14.6) |
| Rural | ||||
| 0% | 21 842 (55.7) | 19 787 (56.0) | 13 844 (45.9) | 9255 (46.4) |
| 0% to <10% | 10 465 (26.7) | 9687 (27.4) | 9926 (32.9) | 6444 (32.3) |
| 10% to <20% | 2956 (7.5) | 2524 (7.1) | 2775 (9.2) | 1876 (9.4) |
| 20% to <30% | 1146 (2.9) | 915 (2.6) | 918 (3.0) | 622 (3.1) |
| 30% to <40% | 677 (1.7) | 534 (1.5) | 518 (1.7) | 350 (1.8) |
| 40% to <50% | 578 (1.5) | 488 (1.4) | 564 (1.9) | 377 (1.9) |
| 50%+ | 1547 (3.9) | 1394 (3.9%) | 1599 (5.3%) | 1008 (5.1%) |
AAPI = Asian American/Pacific Islander; CRC = colorectal cancer; SEER = Surveillance, Epidemiology, and End Results.
The study sample included 0.6% non-Hispanic American Indian/Alaska Native cases (n = 2575), 17.7% Hispanic cases (n = 79 716), 11.6% non-Hispanic Asian American/Pacific Islander cases (n = 52 262), 6.4% non-Hispanic Black cases (n = 28 960), 60.4% non-Hispanic White cases (n = 271 740), and 3.9% cases with other or unknown race or ethnicity (n = 14 941). Most cancer cases (53.1%) included in this analysis were located in nonrural zones (0% rural population) (Tables 1 and 2).
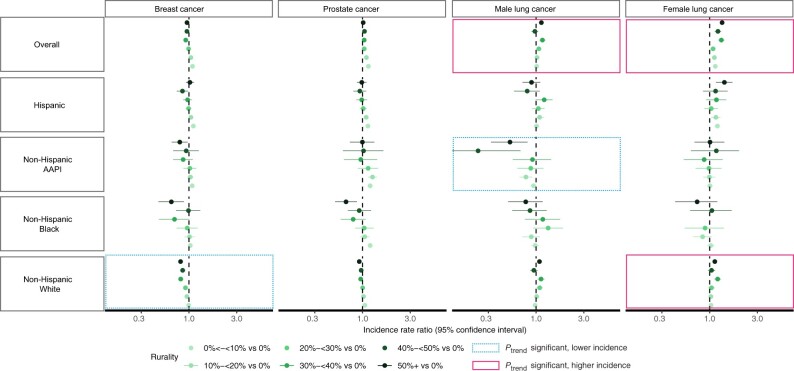
Overall, areas with a greater proportion of rural residents had lower rates of female breast cancer, though this trend was only statistically significant for non-Hispanic White females. Non-Hispanic Black females living in areas with 50% or more rural population had statistically significantly lower incidence of breast cancer compared with those in nonrural areas (Figures 2 and 3; Supplementary Tables 1-5, available online).
Figure 2.
Age-adjusted incidence rate ratios for breast, prostate, and lung cancer by sex, race, and ethnicity in California (2015-2019). The figure is plotted on log scale; however, axis labels for incidence rate ratios are not on log scale for interpretability. Some confidence intervals extend beyond the plot limit. Rurality was defined as the proportion of residents in rural areas within zones and for the analysis was categorized into 7 levels: 0% (not rural), >0% to <10%, 10% to <20%, 20% to <30%, 30% to <40%, 40% to <50%, and 50+% (12). We used census tract–level data on rurality, which the US Census defines based on percent of residents who reside in blocks that are designated as rural. Zone-level rurality was calculated using population-weighted census tract–level data on rurality from Census 2010. AAPI = Asian American/Pacific Islander.
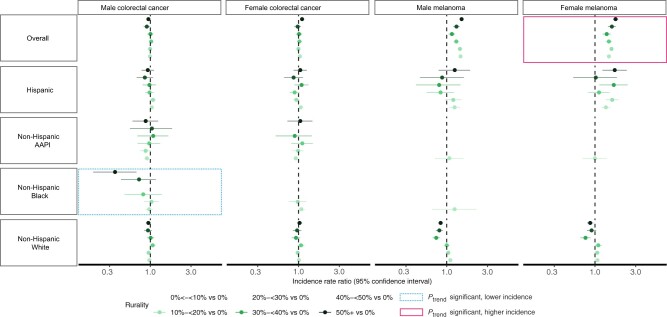
Figure 3.
Age-adjusted incidence rate ratios for colorectal cancer and melanoma by sex, race, and ethnicity in California (2015-2019). The figure is plotted on log scale; however, axis labels for incidence rate ratios are not on log scale for interpretability. Some confidence intervals extend beyond the plot limit. Rurality was defined as the proportion of residents in rural areas within zones and for the analysis was categorized into 7 levels: 0% (not rural), >0% to <10%, 10% to <20%, 20% to <30%, 30% to <40%, 40% to <50%, and 50+% (12). We used census tract–level data on rurality, which the US Census defines based on percent of residents who reside in blocks that are designated as rural. Zone-level rurality was calculated using population-weighted census tract–level data on rurality from Census 2010. AAPI = Asian American/Pacific Islander.
Areas with a greater proportion of rural residents had lower prostate cancer rates, though this trend was not statistically significant for any groups. However, non-Hispanic Black males living in areas with 50% or more rural population had statistically significantly lower incidence of prostate cancer compared with those living in nonrural areas (Figures 2 and 3; Supplementary Tables 1-5, available online).
Increasing rurality was associated with a statistically significant trend in higher lung cancer incidence overall for both sexes, with variations across racial and ethnic groups. In areas with 50% or more rural population, the IRR for lung cancer in Hispanic females was higher (IRR = 1.43, 95% CI = 1.17 to 1.74) than rates in Hispanic males (IRR = 0.90, 95% CI = 0.72 to 1.11). Increasing rurality was associated with a trend of lower incidence of lung cancer in non-Hispanic Asian American/Pacific Islander males and higher incidence for non-Hispanic White females (Figures 2 and 3; Supplementary Tables 1-5, available online).
There was no association between rurality and colorectal cancer overall. However, increasing rurality was associated with a trend in lower incidence of colorectal cancer for non-Hispanic Black males, and living in areas with 50% or more rural population had particularly low incidence of colorectal cancer (Figures 2 and 3; Supplementary Tables 1-5, available online).
A rural disadvantage for melanoma incidence was found among females and males overall, and there was a statistically significant trend in the association between increasing rurality and higher incidence for melanoma in all females. In areas with 50% or more rural population, the IRR for melanoma was higher in Hispanic females (IRR = 1.75, 95% CI = 1.23 to 2.45) compared with non-Hispanic White females (IRR = 0.87, 95% CI = 0.80 to 0.95) (Figures 2 and 3; Supplementary Tables 1-5, available online).
Discussion
Overall, we found evidence of a rural disadvantage for lung cancer and melanoma incidence. We found more complex patterns in melanoma, breast, lung, and colorectal cancer incidence when race, ethnicity, and sex were considered.
Within rural regions, racial and ethnic minoritized populations often have higher cancer incidence compared with non-Hispanic White populations (14,23). In particular, non-Hispanic Black and non-Hispanic American Indian/Alaska Native populations have notable urban and rural disparities in cancer incidence in the United States (14,23). This disparity is likely due to a combination of factors that rural and minoritized racial and ethnic populations tend to experience, such as poverty and limited access to health care (1,5). Across the United States, of the counties in persistent poverty (sustained level of poverty over 4 decades), 83% were rural (24). Furthermore, the impact of historical and current structural racism on minoritized racial and ethnic populations can also lead to differential access to housing, health care, and socioeconomic opportunities and, ultimately, poorer health outcomes (25-27).
Regional differences are important to consider when evaluating urban and rural disparities. Zahnd et al. (5) analyzed data from the North American Association of Central Cancer Registries public use data set (which represents 93% of the US population and includes data from Center for Disease Control’s National Program of Cancer Registries, CCR’s Provincial and Territorial Registries, and the National Cancer Institute’s Surveillance, Epidemiology, and End Results Registries) and found that incidence of tobacco-associated, human papillomavirus-associated, and lung and bronchus cancers was highest in rural areas, except for the Midwest, where there were no urban and rural differences. Henley et al. (1) looked at cancer incidence data from the National Program of Cancer Registries and the Surveillance, Epidemiology, and End Results Program and found that colorectal cancer incidence rates were higher in rural areas, except in the western United States, where there was no difference. However, Hispanic residents in the rural western United States had a higher rate of colorectal cancer than Hispanic residents in urban areas (1). In California, patterns in cancer incidence rates are different than in other parts of the United States. Cancer incidence rates in California are among the lowest in the nation, in large part due to low smoking rates (6,7). Data on urban and rural cancer disparities in California are relatively sparse; however, Hofer et al. (28) examined rural–urban variations in California cancer cases diagnosed 2006-2015 and found, as we did, incidence of lung cancer and melanoma were significantly higher among residents of rural areas compared with residents of urban areas. In addition, rural males had higher incidence of urinary bladder cancer and rural females had higher incidence of kidney cancer.
Our findings corroborate other breast and prostate cancer incidence studies, which consistently report lower incidence rates in rural areas compared with urban areas (1,5,28-30). This likely reflects the challenges that rural areas face in availability, accessibility, and affordability of health care leading to lower rates for commonly screened cancers (14). Additionally, areas with more urban populations might have higher incidence rates due to overdiagnosis from screening, given higher access to health care in urban areas (1,4,29,31,32). Using national data, Zahnd et al. (4) found that breast and prostate cancer incidence rates were lower in rural settings among non-Hispanic White, non-Hispanic Black, and Hispanic populations. Hofer et al. (28) similarly found lower 10-year incidence of breast and prostate cancers in rural populations across racial and ethnic groups in California.
For lung cancer, a consistent rural disadvantage has been found in numerous studies across the United States, which may be attributed to higher smoking rates in rural populations (1,5,7,11,29,30,33-35). PLACES data show an increasing trend in smoking for more rural areas in California, but these data are not available by race and ethnicity (15,36). We found similar results in the overall population and in non-Hispanic White populations for lung cancer incidence. However, for non-Hispanic Asian American/Pacific Islander males in California, increasing rurality was associated with lower incidence of lung cancer.
Across the United States, a consistent rural disadvantage in colorectal cancer incidence rates has been reported (30). This may be related to higher smoking rates in rural populations or decreased access or acceptance of colorectal cancer screening modalities relative to breast and prostate cancer (1,5,23,29,34,37,38). Rural regions also have higher rates of obesity (39-44), which is associated with increased colorectal cancer incidence (45-47). However, studies have found no urban or rural difference for colorectal cancer incidence in the western United States (1,5). We also found that there is no rural disadvantage for colorectal cancer incidence in California for racial and ethnic groups in our study. This may reflect improved colorectal cancer screening rates or different patterns in obesity in California (39,48-50). Colorectal cancer screening rates in California are among the highest in the nation, and screening rates have improved in recent years (39,51-55). However, it should also be noted that colorectal cancer rates were lower among non-Hispanic Black men in rural areas compared with those in urban areas. This may be driven by differences in lower screening rates due to reduced health-care access or possibly by lifestyle habits such as physical activity or diet (1,4,28).
Two studies on urban and rural differences for melanoma show an urban disadvantage in melanoma incidence rates overall (1,4). Our analysis shows a rural disadvantage for melanoma incidence, especially for Hispanic females living in areas with 50% or more rural population. However, these results are based on relatively small numbers of Hispanic females with melanoma (n = 41) and should be interpreted with caution.
The higher incidence of lung cancer and melanoma in Hispanic females but not males in areas with 50% or more rural population may reflect differences in occupation and access to care. Although Hofer et al. (28) also reported higher lung cancer rates and melanoma in rural Hispanic populations in California, the data were not analyzed by sex. In contrast, Zahnd et al. (5) found that both Hispanic males and females in rural counties had higher rates of lung cancer and melanoma nationwide as well as in the western United States. However, because this study used a dichotomous measure for rurality, gradient in incidence rates across levels of rurality may have been masked (5).
By examining multiple levels of rurality, we were able to identify lower rates for breast cancer in non-Hispanic Black females and lower rates of prostate and colorectal cancer in non-Hispanic Black males living in areas with 50% or more rural population. Additionally, by looking at effects by sex, race, and ethnicity, we were able to identify a higher incidence of lung cancer and melanoma in Hispanic females living in areas with 50% or more rural population, which may have been masked by looking at Hispanic males and females combined.
Limitations of our study include focusing on only the most common cancer sites and relatively low numbers of cases for some groups. Assessing rates of other cancers such as cervical cancer could further lend insight into how screening affects cancer incidence in rural California. However, lower cases for rarer cancers would lead to suppression of data because California Department of Public Health guidelines restrict reporting of cancer incidence rates if based on less than 15 cancer cases and/or a population of less than 10 000 to ensure confidentiality and stable statistical rates. Although we could not present results for granular age categories due to suppression, we conducted a sensitivity analysis on our age-adjusted results by comparing results for those younger than 65 years with those 65 years and older and found generally similar patterns. However, decreasing trends for non-Hispanic Black females with breast cancer, increasing trends in non-Hispanic White females with lung cancer, and increasing trends for Hispanic females with melanoma were limited to those younger than 65 years old (data not shown). Another limitation is that we did not look at regional differences likely masking some of the heterogeneity across these communities, which ranges from predominantly non-Hispanic White mountain communities to predominantly Hispanic agricultural regions.
Our study uncovered previously masked patterns, raising new questions about factors contributing to rural advantage or disadvantage. Although some differences are likely due to variation in health behaviors and access to health care, there is still much to be learned about pathways through which rurality differently influences cancer incidence by site, sex, and racial and ethnic group as well how these pathways manifest in other parts of the country. A more granular understanding of rurality and cancer incidence is needed to effectively direct cancer control efforts to rural areas and subpopulations where they are most needed.
Supplementary Material
Contributor Information
Debora L Oh, Department of Epidemiology and Biostatistics, University of California, San Francisco, USA; Greater Bay Area Cancer Registry, University of California, San Francisco, USA.
Karen Schumacher, Department of Physiological Nursing, University of California, San Francisco, CA, USA; Helen Diller Family Comprehensive Cancer Center, University of California, San Francisco, USA.
Juan Yang, Department of Epidemiology and Biostatistics, University of California, San Francisco, USA; Greater Bay Area Cancer Registry, University of California, San Francisco, USA.
Katarina Wang, Department of Epidemiology and Biostatistics, University of California, San Francisco, USA.
Katherine Lin, Department of Epidemiology and Biostatistics, University of California, San Francisco, USA; Greater Bay Area Cancer Registry, University of California, San Francisco, USA.
Scarlett Lin Gomez, Department of Epidemiology and Biostatistics, University of California, San Francisco, USA; Greater Bay Area Cancer Registry, University of California, San Francisco, USA; Helen Diller Family Comprehensive Cancer Center, University of California, San Francisco, USA.
Salma Shariff-Marco, Department of Epidemiology and Biostatistics, University of California, San Francisco, USA; Greater Bay Area Cancer Registry, University of California, San Francisco, USA; Helen Diller Family Comprehensive Cancer Center, University of California, San Francisco, USA.
Funding
This work is supported by the California Department of Public Health, National Cancer Institute’s Surveillance, Epidemiology and End Results Program, and Centers for Disease Control and Prevention’s (CDC) National Program of Cancer Registries. The collection of cancer incidence data used in this study was supported by the California Department of Public Health pursuant to California Health and Safety Code Section 103885; Centers for Disease Control and Prevention’s (CDC) National Program of Cancer Registries, under cooperative agreement 5NU58DP006344; the National Cancer Institute’s Surveillance, Epidemiology and End Results Program under contract HHSN261201800032I awarded to the University of California, San Francisco, contract HHSN261201800015I awarded to the University of Southern California, and contract HHSN261201800009I awarded to the Public Health Institute, Cancer Registry of Greater California. The ideas and opinions expressed herein are those of the author(s) and do not necessarily reflect the opinions of the State of California, Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors.
Notes
Role of the funder: The California Department of Public Health, National Cancer Institute’s Surveillance, Epidemiology and End Results Program, and Centers for Disease Control and Prevention’s (CDC) National Program of Cancer Registries supported the collection of data and staff effort for analysis, interpretation of the data, and writing of the manuscript.
Disclosures: DLO, JY, KL, SLG, and SSM have received funding from the National Cancer Institute for support of the Greater Bay Area Cancer Registry. KS and KW have no conflicts of interest to disclose. SLG, who is a JNCI Associate Editor and coauthor on this article, was not involved in the editorial review or decision to publish the manuscript.
Author contributions: DLO (Project administration, Visualization, Writing — original draft, Writing — review & editing); KS (Conceptualization, Methodology, Writing — original draft, Writing — review & editing); JY (Conceptualization, Data curation, Formal analysis, Methodology, Visualization, Writing — review & editing); KW (Writing — original draft, writing — review & editing); KL (Visualization, Writing — review & editing); SLG (Conceptualization, Methodology, Supervision, Writing — review & editing); SSM (Conceptualization, Methodology, Supervision, Writing — review & editing).
Acknowledgements: We acknowledge Greater Bay Area Cancer Registry (University of California, San Francisco), Los Angeles Cancer Surveillance Program (University of Southern California), National Cancer Institute, Westat, Inc for their role in the development of the California census tract aggregation zones.
Prior presentations: 6/16—Salma Shariff-Marco, Juan Yang, Debora Oh, Katherine Lin, Katarina Wang, Scarlett Lin Gomez, and Karen Schumacher—Cancer incidence and stage of diagnosis by rurality in California (North American Association of Central Cancer Registries Summer Forum).
Data availability
California Cancer Registry data is available by request at: ccrcal.org.
References
- 1. Henley SJ, Anderson RN, Thomas CC, Massetti GM, Peaker B, Richardson LC.. Invasive cancer incidence, 2004-2013, and deaths, 2006-2015, in nonmetropolitan and metropolitan counties - United States. MMWR Surveill Summ. 2017;66(14):1-13. doi: 10.15585/mmwr.ss6614a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Blake KD, Moss JL, Gaysynsky A, Srinivasan S, Croyle RT.. Making the case for investment in rural cancer control: an analysis of rural cancer incidence, mortality, and funding trends. Cancer Epidemiol Biomarkers Prev. 2017;26(7):992-997. doi: 10.1158/1055-9965.EPI-17-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yao N, Alcalá HE, Anderson R, Balkrishnan R.. Cancer disparities in rural Appalachia: incidence, early detection, and survivorship. J Rural Health. 2017;33(4):375-381. doi: 10.1111/jrh.12213. [DOI] [PubMed] [Google Scholar]
- 4. Zahnd WE, James AS, Jenkins WD, et al. Rural-urban differences in cancer incidence and trends in the United States. Cancer Epidemiol Biomarkers Prev. 2018;27(11):1265-1274. doi: 10.1158/1055-9965.EPI-17-0430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zahnd WE, Fogleman AJ, Jenkins WD.. Rural-urban disparities in stage of diagnosis among cancers with preventive opportunities. Am J Prev Med. 2018;54(5):688-698. doi: 10.1016/j.amepre.2018.01.021. [DOI] [PubMed] [Google Scholar]
- 6. McLafferty S, Wang F.. Rural reversal? Rural-urban disparities in late-stage cancer risk in Illinois. Cancer. 2009;115(12):2755-2764. doi: 10.1002/cncr.24306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. O'Neil ME, Henley SJ, Rohan EA, Ellington TD, Gallaway MS.. Lung cancer incidence in nonmetropolitan and metropolitan counties - United States, 2007-2016. MMWR Morb Mortal Wkly Rep. 2019;68(44):993-998. doi: 10.15585/mmwr.mm6844a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. United States Department of Agriculture Economic Research Service. Rural-urban continuum codes. https://www.ers.usda.gov/data-products/rural-urban-continuum-codes.aspx. Accessed March 22, 2022.
- 9. United States Department of Agriculture Economic Research Service. Rural-urban commuting area codes. https://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes/. Accessed March 22, 2022.
- 10. Yaghjyan L, Cogle C, Deng G, et al. Continuous rural-urban coding for cancer disparity studies: is it appropriate for statistical analysis? Int J Environ Res Public Health. 2019;16(6):1076. doi: 10.3390/ijerph16061076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Atkins GT, Kim T, Munson J.. Residence in rural areas of the United States and lung cancer mortality. Disease incidence, treatment disparities, and stage-specific survival. Ann Am Thorac Soc. 2017;14(3):403-411. doi: 10.1513/AnnalsATS.201606-469OC. [DOI] [PubMed] [Google Scholar]
- 12. United States Census Bureau. 2010 Census urban and rural classification and urban area criteria: P2. Urban and rural. https://www2.census.gov/programs-surveys/decennial/2010/technical-documentation/complete-tech-docs/summary-file/sf1.pdf. Accessed July 19, 2023.
- 13. United States Census Bureau. 2010 Census urban and rural classification and urban area criteria: rural definition. https://www.census.gov/programs-surveys/geography/guidance/geo-areas/urban-rural/2010-urban-rural.html. Accessed October 11, 2022.
- 14. Zahnd WE, Murphy C, Knoll M, et al. The intersection of rural residence and minority race/ethnicity in cancer disparities in the United States. Int J Environ Res Public Health. 2021;18(4):1384. doi: 10.3390/ijerph18041384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Greater Bay Area Cancer Registry. California health maps. https://www.californiahealthmaps.org/. Accessed January 18, 2022.
- 16. Brant M, Hansen D. California cancer reporting system standards, Volume I: abstracting and coding procedures. California Cancer Registry. https://www.ccrcal.org/wpfd_file/california-cancer-reporting-system-standards-volume-i-abstracting-and-coding-procedures-updated-12-3-19-new/. Accessed September 7, 2022.
- 17. Martin D, Cockings S, Harfoot A. AZTool. http://aztool.geodata.soton.ac.uk/. Accessed December 9, 2021.
- 18. Tatalovich Z, Stinchcomb DG, Ng D, et al. Developing geographic areas for cancer reporting using automated zone design. Am J Epidemiol. 2022;191(12):2109-2119. doi: 10.1093/aje/kwac155. [DOI] [PubMed] [Google Scholar]
- 19. Howe H. NAACCR guideline for enhancing Hispanic-Latino identification: revised NAACCR Hispanic/Latino identification algorithm [NHIA v2.2.1]. https://www.naaccr.org/wp-content/uploads/2016/11/NHIA-v2.2.1.pdf. Published 2011. Accessed March 15, 2022.
- 20. Boscoe F. NAACCR Asian/Pacific Islander identification algorithm [NAPIIA v1.2.1]: enhancing the specificity of identification. https://www.naaccr.org/wp-content/uploads/2016/11/NAPIIA_v1_2_1_08122011.pdf. Published 2011. Accessed March 15, 2022.
- 21. Tiwari RC, Clegg LX, Zou Z.. Efficient interval estimation for age-adjusted cancer rates. Stat Methods Med Res. 2006;15(6):547-569. doi: 10.1177/0962280206070621. [DOI] [PubMed] [Google Scholar]
- 22. Surveillance, Epidemiology, and End Results (SEER) Program. SEERStat Database: Populations - total U.S. (2006-2019), census tract estimates by race/origin controlling to vintage 2019 <2010 Tract geographies>, National Cancer Institute, DCCPS, Surveillance Research Program, released October 2021. Source: Woods and Poole Economics, Inc. Washington, DC copyright, 2021. https://www.seer.cancer.gov. Accessed January 18, 2022.
- 23. Zahnd WE, Gomez SL, Steck SE, et al. Rural-urban and racial/ethnic trends and disparities in early-onset and average-onset colorectal cancer. Cancer. 2021;127(2):239-248. doi: 10.1002/cncr.33256. [DOI] [PubMed] [Google Scholar]
- 24. Moss JL, Pinto CN, Srinivasan S, Cronin KA, Croyle RT.. Persistent poverty and cancer mortality rates: an analysis of county-level poverty designations. Cancer Epidemiol Biomarkers Prev. 2020;29(10):1949-1954. doi: 10.1158/1055-9965.EPI-20-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bailey ZD, Feldman JM, Bassett MT.. How structural racism works - racist policies as a root cause of U.S. racial health inequities. N Engl J Med. 2021;384(8):768-773. doi: 10.1056/NEJMms2025396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hardeman RR, Murphy KA, Karbeah J, Kozhimannil KB.. Naming institutionalized racism in the public health literature: a systematic literature review. Public Health Rep. 2018;133(3):240-249. doi: 10.1177/0033354918760574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lynch EE, Malcoe LH, Laurent SE, Richardson J, Mitchell BC, Meier HCS.. The legacy of structural racism: associations between historic redlining, current mortgage lending, and health. SSM Popul Health. 2021;14:100793. doi: 10.1016/j.ssmph.2021.100793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hofer B, Parikh-Patel A, Maguire F, Kizer K, Morris C, Movsisyan A.. Rural-Urban Variations in Cancer Incidence, Detection, and Survival in California. UC Davis Institute for Population Health Management. https://health.ucdavis.edu/cancer/pdfs/RuralUrbanVariationsInCancerIncidenceDetectionSurvivalInCA.pdf. Accessed December 6, 2021. [Google Scholar]
- 29. Batai K, Gachupin FC, Estrada AL, Garcia DO, Gomez J, Kittles RA.. Patterns of cancer related health disparities in Arizona. Cancer Health Disparities. 2019;3:1-20. [PMC free article] [PubMed] [Google Scholar]
- 30. Bhatia S, Landier W, Paskett ED, et al. Rural-urban disparities in cancer outcomes: opportunities for future research. J Natl Cancer Inst. 2022;114(7):940-952. doi: 10.1093/jnci/djac030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chandak A, Nayar P, Lin G.. Rural-urban disparities in access to breast cancer screening: a spatial clustering analysis. J Rural Health. 2019;35(2):229-235. doi: 10.1111/jrh.12308. [DOI] [PubMed] [Google Scholar]
- 32. Hashibe M, Kirchhoff AC, Kepka D, et al. Disparities in cancer survival and incidence by metropolitan versus rural residence in Utah. Cancer Med. 2018;7(4):1490-1497. doi: 10.1002/cam4.1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zahnd WE, Mueller GS, Fogleman AJ, Jenkins WD.. Intrastate variations in rural cancer risk and incidence: an Illinois case study. J Public Health Manag Pract. 2016;22(5):472-478. doi: 10.1097/PHH.0000000000000310. [DOI] [PubMed] [Google Scholar]
- 34. Gallaway MS, Henley SJ, Steele CB, et al. Surveillance for cancers associated with tobacco use - United States, 2010-2014. MMWR Surveill Summ. 2018;67(12):1-42. doi: 10.15585/mmwr.ss6712a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liu L, Edland S, Myers MG, Hofstetter CR, Al-Delaimy WK.. Smoking prevalence in urban and rural populations: findings from California between 2001 and 2012. Am J Drug Alcohol Abuse. 2016;42(2):152-161. doi: 10.3109/00952990.2015.1125494. [DOI] [PubMed] [Google Scholar]
- 36. Centers for Disease Control and Prevention. PLACES: local data for better health. https://www.cdc.gov/places/index.html. Published April 4, 2022. Accessed May 5, 2022.
- 37. Liang PS, Mayer JD, Wakefield J, Ko CW.. Temporal trends in geographic and sociodemographic disparities in colorectal cancer among Medicare patients, 1973-2010. J Rural Health. 2017;33(4):361-370. doi: 10.1111/jrh.12209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Adams SA, Zahnd WE, Ranganathan R, et al. Rural and racial disparities in colorectal cancer incidence and mortality in South Carolina, 1996–2016. J Rural Health. 2022;38(1):34-39. doi: 10.1111/jrh.12580. [DOI] [PubMed] [Google Scholar]
- 39. Gong S, Wang K, Li Y, Alamian A.. Geographic differences in obesity prevalence and its risk factors among Asian Americans: findings from the 2013-2014 California Health Interview Survey. Sci Rep. 2018;8(1):12510. doi: 10.1038/s41598-018-29906-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jackson JE, Doescher MP, Jerant AF, Hart LG.. A national study of obesity prevalence and trends by type of rural county. J Rural Health. 2005;21(2):140-148. doi: 10.1111/j.1748-0361.2005.tb00074.x. [DOI] [PubMed] [Google Scholar]
- 41. Befort CA, Nazir N, Perri MG.. Prevalence of obesity among adults from rural and urban areas of the United States: findings from NHANES (2005-2008). J Rural Health. 2012;28(4):392-397. doi: 10.1111/j.1748-0361.2012.00411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Patterson PD, Moore CG, Probst JC, Shinogle JA.. Obesity and physical inactivity in rural America. J Rural Health. 2004;20(2):151-159. doi: 10.1111/j.1748-0361.2004.tb00022.x [DOI] [PubMed] [Google Scholar]
- 43. Wang Y, Beydoun MA.. The obesity epidemic in the United States--gender, age, socioeconomic, racial/ethnic, and geographic characteristics: a systematic review and meta-regression analysis. Epidemiol Rev. 2007;29:6-28. doi: 10.1093/epirev/mxm007. [DOI] [PubMed] [Google Scholar]
- 44. Hill JL, You W, Zoellner JM.. Disparities in obesity among rural and urban residents in a health disparate region. BMC Public Health. 2014;14:1051. doi: 10.1186/1471-2458-14-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schumacher AJ, Chen Q, Attaluri V, McLemore EC, Chao CR.. Metabolic risk factors associated with early-onset colorectal adenocarcinoma: a case-control study at Kaiser Permanente Southern California. Cancer Epidemiol Biomarkers Prev. 2021;30(10):1792-1798. doi: 10.1158/1055-9965.EPI-20-1127. [DOI] [PubMed] [Google Scholar]
- 46. Juo YY, Gibbons MAM, Dutson E, et al. Obesity is associated with early onset of gastrointestinal cancers in California. J Obes. 2018;2018:7014073. doi: 10.1155/2018/7014073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Meyerhardt JA, Kroenke CH, Prado CM, et al. Association of weight change after colorectal cancer diagnosis and outcomes in the Kaiser Permanente Northern California population. Cancer Epidemiol Biomarkers Prev. 2017;26(1):30-37. doi: 10.1158/1055-9965.EPI-16-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schwarte L, Samuels SE, Capitman J, Ruwe M, Boyle M, Flores G.. The Central California Regional Obesity Prevention Program: changing nutrition and physical activity environments in California’s heartland. Am J Public Health. 2010;100(11):2124-2128. doi: 10.2105/AJPH.2010.203588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lopez-Zetina J, Lee H, Friis R.. The link between obesity and the built environment. Evidence from an ecological analysis of obesity and vehicle miles of travel in California. Health Place. 2006;12(4):656-664. doi: 10.1016/j.healthplace.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 50. Matsuzaki M, Sánchez BN, Acosta ME, Sanchez-Vaznaugh EV.. Competitive food and beverage policies and obesity among middle school students: variability by urbanicity in California. Child Obes. 2022;18(1):41-49. doi: 10.1089/chi.2021.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Joseph DA, King JB, Dowling NF, Thomas CC, Richardson LC.. Vital signs: colorectal cancer screening test use - United States, 2018. MMWR Morb Mortal Wkly Rep. 2020;69(10):253-259. doi: 10.15585/mmwr.mm6910a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rico J, Miguelino-Keasling V, Darsie B, Davis S, Kwong S, Snipes K. Colorectal cancer in California, 1988–2012. https://www.ccrcal.org/retrieve-data/data-for-the-public/cancer-statistics-and-reports/. Published Online 2016. Accessed December 6, 2021.
- 53. American Cancer Society. Colorectal cancer facts and figures 2020-2022. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/colorectal-cancer-facts-and-figures/colorectal-cancer-facts-and-figures-2020-2022.pdf. Published 2020. Accessed December 6, 2021.
- 54. Centers for Disease Control and Prevention. Colorectal Cancer Screening Test Use. https://www.cdc.gov/cancer/ncccp/screening-test-use/index.htm. Published August 31, 2021. Accessed December 6, 2021.
- 55. Wang KS, Liu X, Ategbole M, et al. Generalized linear mixed model analysis of urban-rural differences in social and behavioral factors for colorectal cancer screening. Asian Pac J Cancer Prev. 2017;18(9):2581-2589. doi: 10.22034/APJCP.2017.18.9.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
California Cancer Registry data is available by request at: ccrcal.org.