Abstract
Introduction
Based on expert opinion, the length of antibiotic treatment for pleural infection in adults is typically recommended to be a minimum of 4 weeks. This clinical trial aimed to assess whether shorter antibiotic courses lead to more treatment failures than standard longer courses.
Methods
In an open-label randomised controlled trial, adult patients with pleural infection who were medically treated and stabilised within 14 days of admission were randomised to either a short antibiotic course (total course 14–21 days) or a long antibiotic course (total course 28–42 days). Patients were excluded if their baseline RAPID score was >4 (high-risk category). The primary outcome was the incidence of treatment failure by 6 weeks post-admission. Secondary outcomes were total length of antibiotic treatment, proportion of patients who resumed normal activity levels within 6 weeks post-admission, time from discharge to resuming normal activity levels and incidence of antibiotic-related adverse reactions.
Results
Between September 2020 and October 2021, 50 patients (mean±sd age 46±13.7 years; 35 (70%) males) were recruited to the trial and randomly assigned to the short course group (n=25) or the long course group (n=25), with outcome data available for 24 patients in each study group. Treatment failure occurred in four (16.7%) patients in the short course group and three (12.5%) patients in the long course group. In the intention-to-treat analysis the OR for treatment failure in the long course group was 0.714 (95% CI 0.142–3.600; p=0.683). The median (interquartile range) duration of antibiotic treatment in the short course group was 20.5 (18–22.5) days compared with 34.5 (32–38) days in the long course group (p<0.001). There were no statistically significant differences in the other outcomes.
Conclusions
In medically treated adult patients with pleural infection a long course of antimicrobial therapy did not lead to fewer treatment failures compared with a shorter course. These findings need to be confirmed in a larger multicentre trial.
Short abstract
In medically treated adult patients with pleural infection, a long course of antimicrobial therapy does not lead to fewer treatment failures compared with a shorter course https://bit.ly/3JJHvxn
Introduction
Infection of the pleural space is a serious condition associated with substantial morbidity and mortality [1]. The core management involves drainage of the infected fluid and antimicrobial therapy [2]. Based on expert opinion, a minimum of 4 weeks of antibiotics is used to treat pleural infection, although frequently this is extended to up to 6 weeks in routine clinical practice [1, 3–5].
A key principle of antimicrobial stewardship is administering the minimum effective duration of an antibiotic course that aims to reduce the risk of antimicrobial resistance and drug toxicity, both associated with unnecessarily long courses [6, 7]. In lower respiratory tract infections, randomised controlled trial studies have shown that short courses of antibiotics lead to good clinical outcomes [8, 9]. However, in pleural infection there is a paucity of good quality evidence to inform on the optimal duration of antibiotic therapy in adults. A retrospective study of 91 patients with pleural infection reported that antibiotics were prescribed for an average of 4 weeks but were extended to longer durations in some patients [5]. The authors of that study concluded that 3 weeks of antibiotics was sufficient to treat empyema. The only clinical trial studying the optimum length of antibiotic therapy in pleural infection found that short and long antibiotic regimens had similar risks of treatment failure [10]. However, due to the exclusion criteria for participation in that trial (which led to the exclusion of two-thirds of screened patients) the results are not applicable to a substantial proportion of adult patients with pleural infection.
The RAPID score (comprising renal function, age, purulence of pleural fluid, infection source and dietary factors) has been validated as a robust tool to predict 3-month mortality in adult patients with pleural infection [11]. The score is stratified into three tiers, with a predicted 3-month mortality of 3%, 9% and 30% in the low-, intermediate- and high-risk categories, respectively [12]. The clinical utility of this score is yet to be determined. However, a robust tool for predicting outcomes is appealing to help identify patients who may be safely treated with shorter antibiotic courses.
This pilot study aimed to investigate whether prescribing shorter courses of antibiotics (2–3 weeks) versus standard longer courses (4–6 weeks) would lead to a difference in rates of treatment failure (defined as the requirement for further management) in adult patients with pleural infection at low-to-intermediate risk of mortality (RAPID score 0–4) who can be safely discharged home within 14 days of hospitalisation.
Methods
Study design
The Short versus Long antibiotic course for pleural Infection Management (SLIM) trial was a pilot open-label pragmatic randomised controlled clinical trial that recruited adult participants admitted with pleural infection to a university hospital (Alexandria University, Alexandria, Egypt). The protocol of the study was approved by the Alexandria University Faculty of Medicine Ethics Committee (0304785) and was registered at ClinicalTrials.gov with identifier number NCT04615286. A copy of the protocol is included in the supplementary material.
Consecutive patients admitted with pleural infection were invited to take part in an observational study (Pleural Infection Cohort Study (PICS)) and consenting to the SLIM trial occurred at the same time (using the “trials within cohorts” study design [13]). All patients provided written informed consent for the observational study and the trial, but were only assigned a trial number at the point of discharge (see the Randomisation section).
Participants
The trial included adult participants (≥18 years old) admitted to hospital for treatment of non-tuberculous pleural infection (both parapneumonic and primary pleural infections included). Pleural infection was defined by the presence of at least one of the following: presence of pus in the pleural space, positive pleural fluid Gram stain or culture, or pleural fluid pH <7.2 or pleural fluid glucose <40 mg·dL−1 in the setting of acute respiratory infection. Included participants had to have a low-to-intermediate RAPID score (0–4) at admission and to be fit for discharge within 14 days of admission. Participants were excluded if they needed referral for surgery during admission, if their admission was due to a recurrent ipsilateral pleural infection within the last 3 months, or the infected pleural collection was not amenable to drainage at time of diagnosis or a large residual collection persisted despite drainage and therefore a prolonged antibiotic course was deemed necessary by the treating clinician.
Randomisation
Eligible participants were recruited to the trial immediately before discharge and randomised in a 1:1 ratio to either the short course group or the long course group. Randomisation via permuted blocks with variable sizes was used and was unstratified. The sequence was generated using the online platform Sealed Envelope (www.sealedenvelop.com). At the point of randomisation, a member of the study team logged into the website to obtain the treatment allocation after confirmation of eligibility criteria. The allocation was unblinded to participants and team members performing study assessments.
Procedures
The study intervention was the modification of the length of the antibiotic course at the point of discharge from hospital. All other aspects of management were carried out according to standards of care.
Baseline data including patient demographics, admission blood and pleural fluid results, thoracic ultrasound (TUS) and computed tomography (CT) findings, and treatments received were recorded on a case report form (supplementary material). The initial choice of the antibiotic regimen was based on international guidelines for treating pleural infection [4, 14, 15], local patterns of microbiology and known participants’ drug allergies. The regimen was subsequently modified according to results of microbiological studies where necessary.
At discharge, repeat blood tests and TUS were performed. In the intervention arm (short course antibiotic group), participants were discharged with a minimum of 7 days oral antibiotics and a maximum period of 21 days (total number of antibiotic days 14–21 days, including the intravenous course). In the control arm (long course antibiotic group), participants were prescribed an oral antibiotic course for a minimum of 14 days and up to a maximum period of 42 days (total number of antibiotic days 28–42 days).
Follow-up occurred at two visits: 2 weeks post-discharge and 6 weeks post-admission. While these were encouraged to be face to face, for participants unable to attend both appointments, the first or the second follow-up was via telephone. At each visit participants underwent a clinical review for symptom recurrence, treatment adherence was confirmed and adverse antibiotic reactions were recorded. In addition, during each physical visit, TUS with/without chest radiography was performed, and blood samples were sent for white cell count and C-reactive protein (CRP). The size of residual collection on TUS was quantified using the maximum effusion depth (in cm) and the height in rib spaces.
Outcomes
The primary outcome was the incidence of treatment failure defined by the requirement for further management (additional antibiotics and/or drainage procedure and/or surgical intervention) within 6 weeks post-index admission. Treatment failure was determined by 1) clinical deterioration (i.e. worsening or recurrence of symptoms) plus either 2) biochemical parameters (worsening of white cell count (by 2000 mm−3) or CRP (by >20%) from discharge values) or 3) radiological parameters (chest radiography and/or TUS evidence of increasing or new pleural collection).
Secondary outcomes included the total length of antibiotic treatment, proportion of participants who returned to normal activity levels within 6 weeks post-admission, time from discharge to resuming normal activity levels and incidence of antibiotic-related adverse reactions in the study groups. The following outcomes were explored post hoc: number of participants with residual pleural collection on thoracic imaging at 6 weeks post-admission and number of participants with persistent respiratory symptoms 6 weeks post-admission.
Statistics
This was a pilot study and therefore no formal power calculation was made. A sample size of 50 participants (25 per group) was chosen by the study team to simulate sizes chosen by trials examining outcomes in pleural infection in adult and paediatric populations [3, 16].
Continuous variables were summarised using mean and standard deviation or median and interquartile range (IQR) according to whether data were normally distributed. Categorical variables were summarised using frequencies and percentages.
The primary outcome was analysed both in the intention-to-treat population (including all participants with known outcome) and the per-protocol population using the Chi-squared test and Fisher's exact test. The per-protocol population excluded participants who received antibiotic durations that were discordant with the prespecified durations for their allocated group. An analysis of the primary outcome adjusted for the RAPID score (low versus intermediate score), infection source (community- versus hospital-acquired), presence of sonographic septations and whether the split pleura sign was seen on CT scan was carried out using a binary logistic regression model. The adjusted odds ratio (aOR) with 95% confidence interval for treatment failure in the long course group was calculated from the regression model.
For secondary outcomes, continuous variables were compared between the study groups using the Mann–Whitney U-test. Categorical variables were compared using the Chi-squared test or Fisher's exact test as appropriate. A significance level of p<0.05 was used throughout the analyses.
Results
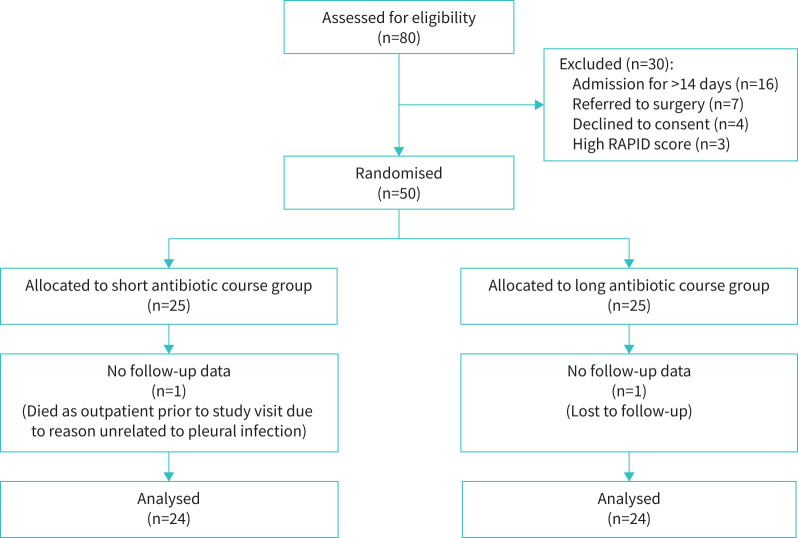
Between 28 September 2020 and 30 October 2021, 80 participants were recruited to the observational study. Of these, 50 participants were recruited to the SLIM trial: 25 participants randomised to the short antibiotic course group and 25 participants randomised to the long antibiotic course group. Exclusions are summarised in figure 1. The final SLIM study visit was conducted on 9 December 2021, after which the dataset was locked.
FIGURE 1.
Flow of participants through the trial.
Baseline and discharge data are summarised in table 1. The median (IQR) age of participants in the short course group was 43 (34–49) years and in the long course group was 52 (39–59) years (p=0.153). 72% of the short course group participants and 68% of the long course group participants were males. More participants in the short course group (68%) were in the low RAPID score category than in the long course group (40%). In addition, sonographic evidence of septations was more common in the long course group (77.7%) than in the short course group (28.6%). Participants in the long course group received more days of intravenous antibiotics (mean difference 2 (95% CI 0.3–3.8) days).
TABLE 1.
Admission and in-hospital data of study participants
| Short antibiotic course (n=25) | Long antibiotic course (n=25) | |||
| Participants with available data | Result | Participants with available data | Result | |
| Age, years | 25 | 43 (34–49) | 25 | 52 (39–59) |
| Male | 25 | 18 (72) | 25 | 17 (68) |
| Symptom duration before admission, days | 24 | 17 (9.25–39) | 24 | 16.5 (10–27.5) |
| Any comorbidity or risk factor# | 25 | 23 | 25 | 24 |
| Poor dentition | 13 | 11 | ||
| Intravenous drug user | 6 | 5 | ||
| Cardiovascular disease | 4 | 4 | ||
| Diabetes | 5 | 7 | ||
| Respiratory disease | 4 | 3 | ||
| Septations on ultrasound | 14 | 4 (28.6) | 18 | 14 (77.7) |
| CT signs | ||||
| Split pleura sign | 22 | 18 (81.1) | 24 | 17 (70.8) |
| Volume loss | 22 | 3 (13.6) | 24 | 2 (8.3) |
| Multiloculated | 25 | 10 (40) | 24 | 10 (41.7) |
| Infection source | 25 | 25 | ||
| Community-acquired | 20 (80) | 22 (88) | ||
| Hospital-acquired | 5 (20) | 3 (12) | ||
| Infection type | 23 | 25 | ||
| Primary pleural | 19 (82.6) | 18 (72) | ||
| Parapneumonic | 4 (17.4) | 7 (28) | ||
| Purulent pleural fluid | 25 | 20 (80) | 25 | 16 (64) |
| RAPID category | 25 | 25 | ||
| Low | 17 (68) | 10 (40) | ||
| Intermediate | 8 (32) | 15 (60) | ||
| Admission blood tests | ||||
| Albumin, mg·dL−1 | 25 | 3.01±0.58 | 25 | 2.71±0.61 |
| WCC, ×109 L−1 | 24 | 12.3 (8.77–17.23) | 25 | 12.8 (10.2–18.85) |
| Haemoglobin, g·dL−1 | 24 | 11.19±1.99 | 25 | 10.66±2.15 |
| Platelets, mm−3 | 24 | 328.5 (213.25–444) | 25 | 376 (312–484) |
| CRP, mg·L−1 | 20 | 158.68±94.95 | 15 | 163.60±92.64 |
| Drain size | 20 | 21 | ||
| <18 Fr | 9 (45) | 12 (57) | ||
| ≥18 Fr | 11 (55) | 9 (43) | ||
| Duration of drainage, days | 18 | 9 (6–12) | 18 | 7 (4–11) |
| Discharge blood tests | ||||
| WCC, ×109 L−1 | 24 | 9.09±3.44 | 25 | 9.68±3.93 |
| Platelets, mm−3 | 24 | 362 (276–486.25) | 25 | 508 (287–596) |
| CRP, mg·L−1 | 23 | 32 (15.9–44.7) | 24 | 21.9 (16.5–32.7) |
| Length of intravenous antibiotic course, days | 25 | 9.12±3.56 | 25 | 11.20±2.51 |
| Length of stay, days | 25 | 10.52±3.28 | 25 | 11.63±2.58 |
| Status at discharge | 25 | 25 | ||
| No residual collection | 11 (44) | 11 (44) | ||
| Residual collection | 14 (56) | 13 (52) | ||
| Outpatient drainage | 0 (0) | 1 (4) | ||
| Inpatient antibiotics | 25 | 25 | ||
| Ampicillin-sulbactam/metronidazole | 15 (60) | 14 (56) | ||
| Cephalosporin/metronidazole | 3 (12) | 3 (12) | ||
| Other | 7 (28) | 8 (32) | ||
| Outpatient antibiotics | 25 | 25 | ||
| Co-amoxiclav/metronidazole | 6 (24) | 10 (40) | ||
| Moxifloxacin | 6 (24) | 6 (24) | ||
| Moxifloxacin/metronidazole | 7 (28) | 0 (0) | ||
| Co-amoxiclav | 0 (0) | 4 (24) | ||
Data are presented as n, median (interquartile range), n (%) or mean±sd, unless otherwise stated. CT: computed tomography; WCC: white cell count; CRP: C-reactive protein. #: some participants had more than one comorbidity or risk factor.
One participant in the long course group was lost to follow-up and one participant from the short course group died from a malignancy-related pulmonary embolism before attending the first follow-up visit. Therefore, outcome data were available for 24 participants for each study group. There were four instances of protocol deviation, all in the short course group, where participants received antibiotic courses ranging between 24 and 30 days.
Outcomes
All participants attended physically for the first follow-up visit. The second follow-up visit was attended physically by 93% of the participants and the remaining undertook the visit via telephone. The primary outcome, i.e. treatment failure, occurred in four (16.7%) participants in the short course group and three (12.5%) participants in the long course group (table 2). In the intention-to-treat population, the OR for treatment failure in the long course group was 0.714 (95% CI 0.142–3.600; p=0.683). After adjusting for other covariates, the aOR was 0.542 (95% CI 0.070–4.227; p=0.559). The difference in treatment failure was not statistically significant in the analysis of the per-protocol population (table 2).
TABLE 2.
Primary and secondary outcomes for the study groups
| Short antibiotic course (n=24) | Long antibiotic course (n=24) | p-value | |||
| Participants with available data | Result | Participant with available data | Result | ||
| Treatment failure | |||||
| ITT population | 24 | 4 (16.7) | 24 | 3 (12.5) | χ2=0.167 (p=0.683), Fisher's exact p=1 |
| PP population | 20 | 4 (20) | 24 | 3 (12.5) | χ2=0.459 (p=0.498), Fisher's exact p=0.684 |
| Total antibiotic duration, days | 24 | 20.5 (18–22.5) | 24 | 34.5 (32–38) | <0.001 |
| Returned to ADL | 20 | 11 (55) | 21 | 9 (42.9) | 0.436 |
| Time to return to ADL from admission, days | 11 | 10 (4.5–18) | 9 | 13 (6–18) | 0.970 |
| Antibiotic-related adverse events | 21 | 0 (0) | 24 | 2 (8.3) | Fisher's exact p=0.488 |
| Residual pleural collection | 20 | 4 (20) | 21 | 2 (9.5) | Fisher exact p=0.409 |
| Persistent symptoms at 6 weeks post-admission | 20 | 9 (45) | 21 | 11 (52.4) | χ2=0.382 (p=0.537) |
Data are presented as n, n (%) or median (interquartile range), unless otherwise stated. ADL: activities of daily living; ITT: intention-to-treat; PP: per-protocol.
Treatment failure was diagnosed at the first follow-up visit in all four participants in the short course group. In the long course group, treatment failure was diagnosed at the first follow-up visit in one participant, while the two other treatment failures were diagnosed at the second follow-up visit. Of those with treatment failure, two participants from the short course group required another chest tube insertion, while two participants of the short course group and all those in long course group with treatment failure were referred to surgery.
Table 2 summarises the results of the secondary outcomes. The median (IQR) duration of antibiotic treatment in the short course group was 20.5 (18–22.5) days compared with 34.5 (32–38) days in the long course group (p<0.001) (figure 2). There were no statistically significant differences in the other outcomes. Notably, in the 41 participants with treatment success at 6 weeks post-admission, 20 (48.8%) participants had at least one persistent respiratory symptom: nine (45%) participants from the short course group and 11 (52.4%) participants from the long course group.
FIGURE 2.
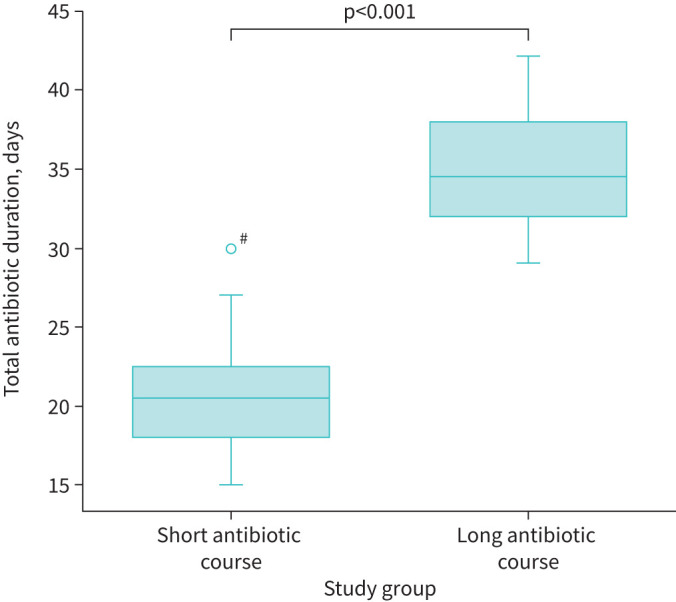
Difference of antibiotic duration between the study groups. #: the data point above the left box plot refers to trial participant number 10 who was randomised to the short course group but received 30 days of antibiotic therapy (a trial deviation).
Discussion
The results of this pilot randomised trial confirm the feasibility of randomising adult patients with low-to-intermediate risk pleural infection to shorter courses of antibiotic. There was no statistically significant difference in short-term outcomes between short and long courses of antibiotics, but a larger study power is needed to definitively rule out the superiority of longer antibiotic courses. Despite an antibiotic course that was on average reduced by 14 days, there were no significant differences in time taken by patients to return to their normal levels of activity or the rate of persistent respiratory symptoms by 6 weeks from diagnosis.
There has been a paucity of evidence for the optimum length for antimicrobial therapy for adult pleural infection to inform guidelines, which is highlighted in the recent European Respiratory Society/European Society of Thoracic Surgeons statement on pleural infection [17]. This study is an attempt to tackle this gap in evidence and the results support the feasibility of prescribing a 3-week treatment course. It is noteworthy that the study excluded patients who required hospital admission for a duration of >14 days (20% of screened patients). Therefore, the results of the study are not applicable to those with longer periods of hospitalisation.
Shorter durations of antimicrobials are associated with lower future risk of infection by antibiotic-resistant organisms, a problem that was responsible for 1.27 million deaths globally in 2019 [18]. Avoiding longer durations of antimicrobial therapy has been shown to improve mortality in severe community-acquired pneumonia [19]. Another implication of a shorter antibiotic course in treating pleural infection is to prevent the delay in declaring failure of medical treatment and referral for definitive management. In two of the three participants who failed medical therapy in the long course group in this study, the decision to refer to surgery was delayed until they completed >4 weeks of antibiotics.
This study utilised the RAPID score to stratify participants according to the severity of infection, to exclude participants at high risk of mortality, who may be more vulnerable to complications with a shorter antimicrobial treatment course. Unlike predictive scores in community-acquired pneumonia, such as CURB-65 which is used to inform the site of care [20] and the choice of empirical antibiotic therapy [21], the RAPID score has not been studied as a tool to guide management. To the best of our knowledge, this is the first study to suggest a clinically meaningful use for the RAPID score, where patients with low-to-intermediate scores can be given shorter outpatient antimicrobial courses. Future studies looking into other clinical/biochemical factors to guide the intensity and length of antimicrobial therapy in pleural infection would be useful. Indeed, in other respiratory infections, guidance by sputum bacterial load [22] or serum biomarkers [23] has been successfully used to guide antimicrobial therapy with improved outcomes.
The results of this trial are in agreement with the only published randomised trial studying antibiotic duration in adult pleural infection. The trial included 55 participants with community-acquired parapneumonic pleural infection and no excess treatment failure with a 3- versus 2-week antibiotic course was observed [10]. However, in that trial all patients were treated with co-amoxiclav and therefore patients who were penicillin-allergic were excluded. In our trial, the choice of any suitable antibiotic regimen was modifiable according to microbiological results and informed by patients’ drug intolerances, to make the study more pragmatic and the results more generalisable. Additionally, and unlike the aforementioned trial, our study included patients with both community- and hospital-acquired pleural infections, and both parapneumonic and primary pleural infections. Our results also agree with a retrospective study of predominantly surgically treated patients [5], where it was found that 3 weeks of antibiotics was sufficient to treat empyema.
Despite randomisation in this trial, between study groups there were baseline differences in the number of participants in each RAPID score category and in the proportion of participants with sonographic septations. However, these imbalances were subsequently adjusted for in the analysis of the primary outcome. Furthermore, it remains unclear whether higher RAPID scores or the presence of septations on ultrasound are predictors of treatment failure [11, 24]. The median age of the subjects included was relatively younger than commonly reported in pleural infection. However, this partially reflects different patient demographics where the trial recruited and inclusion of a lower risk tier of the RAPID score spectrum. It is worth noting that imaging findings suggestive of evolving fibrosis (split pleura sign, multiloculation and volume loss) were not different between study groups.
Important limitations of this study are that the study was conducted in a single centre and the sample size was relatively small. This small size may have not provided enough study power to detect significant change in the primary outcome between the study groups. The findings will need to be replicated in a larger multicentre study. Another limitation of the study was the open-label design; however, ensuring generalisability with a pragmatic flexible choice of antibiotic regimens did not make blinding possible. The short course group had a number of protocol deviations in terms of antibiotic course length. Finally, the study did not collect long-term data, such as 1-year mortality, which is another limitation of the study.
Conclusions
This single-centre pilot randomised controlled trial showed that there was no statistically significant difference between long and short courses of antimicrobial therapy in terms of incidence of treatment failure in medically treated adult patients with pleural infection who are stabilised within 14 days of admission and who have a low-to-intermediate risk of mortality per the RAPID score. Due to the pilot nature of this trial, a larger multicentre study is needed to confirm these findings. Further studies are needed to identify clinical and/or biochemical factors to help triage suitable patients to ambulatory or less invasive management with shorter antimicrobial courses.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00635-2022.SUPPLEMENT (425.3KB, pdf)
Acknowledgements
We would like to thank the patients and their families for their involvement in the trial. We would also like to thank the resident doctors at Alexandria University Hospitals (Alexandria, Egypt) who helped with screening and other trial activities.
Provenance: Submitted article, peer reviewed.
This study is registered at ClinicalTrials.gov with identifier number NCT04615286. Request to access study data by other teams will be expected via e-mail to the corresponding author and access will be granted if the request is deemed reasonable.
Author contributions: M. Hassan conceived the study idea. M. Hassan and A.S. Sadaka wrote the protocol. All authors contributed to data acquisition and interpretation. M. Hassan wrote the first draft of the manuscript, with critical revision from C. Daneshvar and A.S. Sadaka. All authors reviewed and approved the final manuscript.
Conflict of interest: The authors have nothing to disclose.
Support statement: The trial was sponsored by Alexandria University. No funding was received for the conduct of the trial.
References
- 1.Davies HE, Davies RJO, Davies CWH. Management of pleural infection in adults: British Thoracic Society pleural disease guideline 2010. Thorax 2010; 65: Suppl. 2, ii41–ii53. doi: 10.1136/thx.2010.137000 [DOI] [PubMed] [Google Scholar]
- 2.Hassan M, Patel S, Sadaka AS, et al. . Recent insights into the management of pleural infection. Int J Gen Med 2021; 14: 3415–3429. doi: 10.2147/IJGM.S292705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hooper CE, Edey AJ, Wallis A, et al. . Pleural irrigation trial (PIT): a randomised controlled trial of pleural irrigation with normal saline versus standard care in patients with pleural infection. Eur Respir J 2015; 46: 456–463. doi: 10.1183/09031936.00147214 [DOI] [PubMed] [Google Scholar]
- 4.Bhatnagar R, Skouras VS, Rahman NM, et al. . Antibiotics for pleural infections. In: Aliberti S, Chalmers JD, Pletz MW, eds. Anti-infectives and the Lung (ERS Monograph). Sheffield, European Respiratory Society, 2017; pp. 253–263. [Google Scholar]
- 5.Birkenkamp K, O'Horo JC, Kashyap R, et al. . Empyema management: a cohort study evaluating antimicrobial therapy. J Infect 2016; 72: 537–543. doi: 10.1016/j.jinf.2016.02.009 [DOI] [PubMed] [Google Scholar]
- 6.Li Q, Liu B, Liu L. Successfully controlling the incidence of multidrug-resistant Pseudomonas aeruginosa through antibiotic stewardship and infection control programmes at a Chinese university hospital. J Clin Pharm Ther 2021; 46: 1357–1366. doi: 10.1111/jcpt.13446 [DOI] [PubMed] [Google Scholar]
- 7.Doron S, Davidson LE. Antimicrobial stewardship. Mayo Clin Proc 2011; 86: 1113–1123. doi: 10.4065/mcp.2011.0358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goss CH, Heltshe SL, West NE, et al. . A randomized clinical trial of antimicrobial duration for cystic fibrosis pulmonary exacerbation treatment. Am J Respir Crit Care Med 2021; 204: 1295–1305. doi: 10.1164/rccm.202102-0461OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruffles TJC, Goyal V, Marchant JM, et al. . Duration of amoxicillin–clavulanate for protracted bacterial bronchitis in children (DACS): a multi-centre, double blind, randomised controlled trial. Lancet Respir Med 2021; 9: 1121–1129. doi: 10.1016/S2213-2600(21)00104-1 [DOI] [PubMed] [Google Scholar]
- 10.Porcel JM, Ferreiro L, Rumi L, et al. . Two vs. three weeks of treatment with amoxicillin–clavulanate for stabilized community-acquired complicated parapneumonic effusions. A preliminary non-inferiority, double-blind, randomized, controlled trial. Pleura Peritoneum 2020; 5: 20190027. doi: 10.1515/pp-2019-0027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corcoran JP, Psallidas I, Gerry S, et al. . Prospective validation of the RAPID clinical risk prediction score in adult patients with pleural infection: the PILOT study. Eur Respir J 2020; 56: 2000130. doi: 10.1183/13993003.00130-2020 [DOI] [PubMed] [Google Scholar]
- 12.Rahman NM, Kahan BC, Miller RF, et al. . A clinical score (RAPID) to identify those at risk for poor outcome at presentation in patients with pleural infection. Chest 2014; 145: 848–855. doi: 10.1378/chest.13-1558 [DOI] [PubMed] [Google Scholar]
- 13.Kim SY, Flory J, Relton C. Ethics and practice of trials within cohorts: an emerging pragmatic trial design. Clin Trials 2018; 15: 9–16. doi: 10.1177/1740774517746620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hassan M, Cargill T, Harriss E, et al. . The microbiology of pleural infection in adults: a systematic review. Eur Respir J 2019; 54: 1900542. doi: 10.1183/13993003.00542-2019 [DOI] [PubMed] [Google Scholar]
- 15.Shen KR, Bribriesco A, Crabtree T, et al. . The American Association for Thoracic Surgery consensus guidelines for the management of empyema. J Thorac Cardiovasc Surg 2017; 153: e129–e146. doi: 10.1016/j.jtcvs.2017.01.030 [DOI] [PubMed] [Google Scholar]
- 16.Tagarro A, Otheo E, Baquero-Artigao F, et al. . Dexamethasone for parapneumonic pleural effusion: a randomized, double-blind, clinical trial. J Pediatr 2017; 185: 117–123. doi: 10.1016/j.jpeds.2017.02.043 [DOI] [PubMed] [Google Scholar]
- 17.Bedawi EO, Ricciardi S, Hassan M, et al. . ERS/ESTS statement on the management of pleural infection in adults. Eur Respir J 2022; 61: 2201062. doi: 10.1183/13993003.01062-2022 [DOI] [PubMed] [Google Scholar]
- 18.Murray CJ, Ikuta KS, Sharara F, et al. . Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet 2022; 399: 629–655. doi: 10.1016/S0140-6736(21)02724-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niederman MS. Antibiotic use in sepsis: how and why less can really mean more (survival). Am J Respir Crit Care Med 2021; 203: 157–158. doi: 10.1164/rccm.202008-3294ED [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lim WS, Baudouin SV, George RC, et al. . BTS guidelines for the management of community acquired pneumonia in adults: update 2009. Thorax 2009; 64: Suppl. 3, iii1–iii55. doi: 10.1136/thx.2009.121434 [DOI] [PubMed] [Google Scholar]
- 21.Chalmers JD, Singanayagam A, Akram AR, et al. . Safety and efficacy of CURB65-guided antibiotic therapy in community-acquired pneumonia. J Antimicrob Chemother 2011; 66: 416–423. doi: 10.1093/jac/dkq426 [DOI] [PubMed] [Google Scholar]
- 22.Kyriazopoulou E, Liaskou-Antoniou L, Adamis G, et al. . Procalcitonin to reduce long-term infection-associated adverse events in sepsis. A randomized trial. Am J Respir Crit Care Med 2021; 203: 202–210. doi: 10.1164/rccm.202004-1201OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bedi P, Cartlidge MK, Zhang Y, et al. . Feasibility of shortening intravenous antibiotic therapy for bronchiectasis based on bacterial load: a proof-of-concept randomised controlled trial. Eur Respir J 2021; 58: 2004388. doi: 10.1183/13993003.04388-2020 [DOI] [PubMed] [Google Scholar]
- 24.Bedawi E, Hassan M, Harriss E, et al. . S57 Sonographic septations in pleural infection – what do they actually mean? Thorax 2009; 2018; 73: A35.. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00635-2022.SUPPLEMENT (425.3KB, pdf)