Abstract
The prevalence of the gastro-oesophageal reflux disease (GORD) in the western world is increasing. Uncontrolled GORD can lead to harmful long-term sequela such as oesophagitis, stricture formation, Barrett’s oesophagus and oesophageal adenocarcinoma. Moreover, GORD has been shown to negatively impact quality of life. The current treatment paradigm for GORD consists of lifestyle modification, pharmacological control of gastric acid secretion or antireflux surgery. In recent years, several minimally invasive antireflux endoscopic therapies (ARET) have been developed which may play a role in bridging the unmet therapeutic gap between the medical and surgical treatment options. To ensure optimal patient outcomes following ARET, considered patient selection is crucial, which requires a mechanistic understanding of individual ARET options. Here, we will discuss the differences between ARETs along with an overview of the current evidence base. We also outline future research priorities that will help refine the future role of ARET.
Keywords: anti-reflux therapy, gastroesophageal reflux disease, oesophageal reflux, endoscopy, diagnostic and therapeutic endoscopy
Introduction
The prevalence of gastro-oesophageal reflux disease (GORD) in the western world is gradually increasing. This trend is reflective of the growing obesity epidemic compounded by an overall ageing population.1 Current data suggest a global prevalence for GORD that equates to roughly 14%.1 2
Long-term GORD can lead to oesophagitis, oesophageal strictures and ulceration.3 Furthermore 10%–15% of patients with chronic GORD develop Barrett’s oesophagus (BO).4 BO is considered a premalignant condition with non-dysplastic BO having a 0.2%–0.5% risk of developing oesophageal adenocarcinoma per year.5
In addition to clinical consequences GORD can negatively affect health-related quality of life (HRQL) and this has been demonstrated through several descriptive studies.6 A number of instruments have been developed and validated to enable assessment of quality of life metrics.6
The current mainstay of treatment is spearheaded by lifestyle modification, followed by pharmacological treatment in the form of antacids, proton pump inhibitors and H2-Receptor blockers. Although most consider short term proton pump inhibitor (PPI) use to be safe, several studies have reported risks associated with long-term use. These include chronic kidney disease, acute interstitial nephritis, clostridium difficile infection, fracture risk, dementia and cardiovascular disease. Although some risks are contested, it is important to consider an individualised risk benefit analysis for each patient especially in a younger cohort where prolonged treatment is required.7
Unfortunately, a significant proportion of patients have symptoms that remain refractory to medical therapy with a further cohort affected by side effects and pill burden. Here antireflux surgery (ARS) can be considered. ARS focuses on restoring the integrity of the lower oesophageal sphincter while undertaking concomitant hiatal hernia repair. The gold standard is laparoscopic fundoplication with patient satisfaction rates up to 95%.8
ARS in well-selected patients with typical GORD-related symptoms is a very effective treatment but can be associated with complications in some patients. Early complications are usually very few and tend to be related to technical failure of the procedure. These include acute-onset dysphagia, usually from over tightening of the crural repair, slipped wrap following profound retching and vomiting.9 These are indications for reoperation. Late complications include dysphagia, which usually improves over ensuing months, breakdown of the fundoplication leading to recurrent symptoms.10 Given some limitations of fundoplication, some patients may seek non-operative, less invasive approaches to reflux treatment.
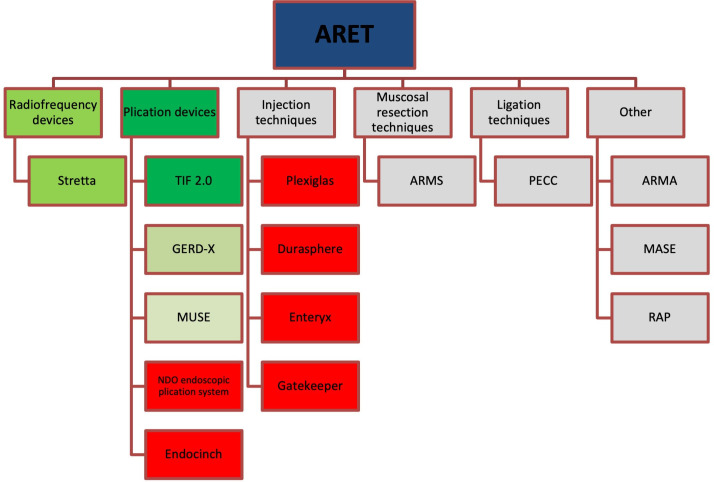
In recent years, several minimally invasive antireflux endoscopic therapies (ARET) have been developed which may play a role in bridging the unmet therapeutic gap between the medical and surgical treatment. A number of these endoscopic options are no longer available either due to issues surrounding long-term efficacy or safety concerns, but lessons learnt from early devices undoubtedly influenced the development of current promising options that provide hope to fulfilling this unmet need (figure 1).
Figure 1.
Overview of historical, current and emerging therapy for GORD. Red=Historical therapies that are no longer available. Green=current therapies with evidence base (darker green=Stronger evidence base), grey=emerging/experimental therapies with limited evidence base or safety profile data. ARET, antireflux endoscopic therapies; ARMA, antireflux mucosal ablation; ARMS, antireflux mucosectomy; MASE, mucosal ablation and suturing at the oesophagogastric; MUSE, medigus ultrasonic surgical endostapler; PECC, peroral endoscopic cardial constriction; RAP, resection and plication; TIF, transoral incisionless fundoplication.
The endoscopic options available can broadly be split into radiofrequency (RF) devices, plications devices, injection techniques, mucosal resection techniques, ligation techniques and other. These will be discussed below.
Patient selection for ARET
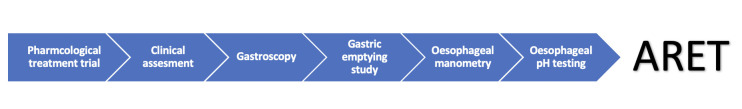
To ensure optimal outcomes following ARET careful patient selection is crucial. We suggest a standardised approach (figure 2) akin to that seen for patients undergoing ARS.
Figure 2.
Patient selection process for ARET. ARET, antireflux endoscopic therapies.
Pharmacological treatment trial
Prior to considering ARET patients should be treated with maximal medical therapy for at least a period of 6 months. This allows for an adequate treatment trial without subjecting patients to the associated long-term side effects of pharmacological antireflux therapy. Following adequate treatment trial ARET can be considered for those patients who have refractory symptoms.
Clinical assessment and patient discussion
As part of the workup for ARET, a clinical assessment should be undertaken taking into consideration patient fitness. It should be noted that some of the currently available devices are of large calibre and require pre procedure balloon dilatation of the cricopharynx to allow for easy intubation.
Patients with atypical symptoms as a primary presentation such as cough without concomitant typical symptoms such as heartburn or regurgitation should not be selected for ARET.
Patients with high body mass index (BMI) are not recommended to undergo ARET and may be best served by undergoing bariatric procedures such as Roux-en-Y gastric bypass. Many current devices are not recommended for patients with a BMI >35 kg/m2.
Finally, a through patient discussion is recommended explaining the merits and drawbacks of these novel treatment options and highlighting that surgical fundoplication is a time-tested effective treatment option for a large cohort of patients and currently remains the gold standard.
Gastroscopy
All patients should undergo a high-quality diagnostic gastroscopy. This allows for the detection of pathology that contravenes endoscopic intervention, such as large hiatal hernias, severe erosive oesophagitis (grade C/D), BO or stricturing disease.
We recommend thorough assessment of hiatal hernias with documentation of longitudinal and axial length and associated Hill grade. Patients with large hiatal hernias are not amenable to ARET and should be considered for ARS instead.
Gastric emptying studies
A multifactorial relationship exists between gastroparesis and GORD. Studies have demonstrated that the rate of proximal stomach emptying can impact postprandial oesophageal acid exposure and the number of reflux episodes per hour.11 The rate of gastric emptying can be evaluated through gastric emptying studies and should be undertaken as part of the ARET workup.
Manometric testing
Oesophageal manometry allows for the detection of motility disorders which can be misdiagnosed as GORD and subsequently lead to poor symptom response post ARET.12
Physiology studies
The advent of gastrointestinal (GI) physiology has allowed us to further characterise and appreciate the phenotypic spectrum of GORD. Fifty per cent of patients with reflux symptoms have no evidence of oesophagitis at endoscopy. Those with pathological oesophageal acid exposure on ambulatory pH testing without oesophagitis are considered to have non-erosive reflux disease (NERD). If there is positive reflux symptom association without pathological oesophageal acid exposure on physiological testing this is considered oesophageal hypersensitivity. Finally, if both acid exposure and symptom association are negative this is considered functional heartburn.13 14
Prior to performing ARET objective physiology testing is warranted to confirm the presence and severity of GORD. This can be conducted via 24-hour catheter-based studies or via wireless capsule pH studies up to 96 hours. Those with functional heartburn and hypersensitive oesophagus should not be selected for ARET.
RF techniques
Stretta RF ablation (Restech, Houston, Texas, USA)
The Stretta system is an endoscopic-assisted procedure that deploys RF thermal energy at several locations above, at and below the gastrooesophageal junction (GOJ). This is achieved through four radially placed needles at the distal end of the catheter (figure 3). Although the exact mechanism is unclear the delivery of thermal energy is postulated to cause tissue hypertrophy and remodelling, which results in improved barrier function and subsequent reduction in GORD symptoms 15–17
Figure 3.

The Stretta device.
Evidence base for Stretta
A 2014 systematic review and meta-analysis assessing evidence from controlled trials comparing Stretta therapy to sham or PPI found stretta to not be superior for improving physiological parameters, stopping PPI therapy or HRQL scores. Data were collated and reviewed from four trials which included a total of 165 patients. Three trials compared stretta to sham with one comparing stretta to PPI therapy.18 The society of American GI and endoscopic surgeons disagreed with the methodological approach and conclusions reached in the systematic review.19
A further systematic review and meta-analysis conducted by a separate group in 2017 involving 2468 patients across 28 studies (4 randomised controlled trials, 23 cohort studies and 1 registry) in contrast found Stretta to significantly improve HRQL scores, PPI dependence at follow-up, as well as the incidence of erosive oesophagitis with reduced oesophageal acid exposure (p<0.001 across all comparisons).20
Most of the evidence for Stretta is confined to trials undertaken over a 12-month period with limited high-quality long-term data. However, two single arm trials have assessed the long-term efficacy of Stretta. Dughera et al evaluated outcomes post Stretta at 4 and 8 years in 26 patients finding 76.9% remaining off PPI, with a significant decrease in gastroesophageal reflux diesease-health related quality of life (GERD-HRQL) scores (p=0.003) at the 8-year point.21 Another prospective study evaluated outcomes on 99 patients at 10 years and found 72% had improvement in GERD-HRQL scores with 41% remaining off PPI.22
Due to heterogeneous outcome data, the current 2021 American College of Gastroenterology (ACG) guidelines for the diagnosis and management of GORD do not recommend Stretta as an antireflux procedure.23
Plication devices
Esophyx
Transoral incisionless fundoplication (TIF) was first introduced in 2006 as a non-surgical minimally invasive endoscopic option for treating reflux. TIF involves restoring a flap valve at the GOJ by pulling up the fundus and fastening it to oesophagus. The technique is most akin to the surgical gold standard of Nissen fundoplication in terms of anatomical alteration.24
The procedure is undertaken using the EsophyX device, which has undergone three revisions over time. In addition to device changes, the technique has also evolved over time.24 The current iteration (TIF 2.0) involves reconstructing the gastro-oesophageal valve by creating a 2–3 cm 270° fundoplication, with plication performed above the Z line (figure 4). The plication is achieved through the deployment of roughly 20 polypropylene non absorbable H shaped fasteners that have the equivalent strength of 3–0 sutures.25
Figure 4.
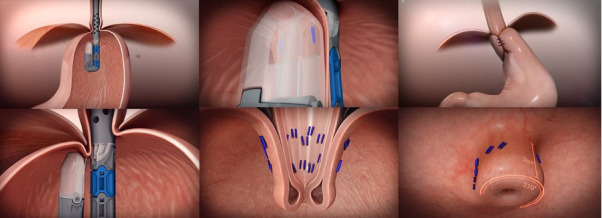
This figure demonstrates the steps for performing a 270°, 3 cm fundoplication with the EsophyX Z+device.
TIF 2.0 is suitable for patients with Hiatal hernia’s equal or less than 2 cm in size with a Hill grade equal or less than 2. TIF is being evaluated as an option for patients with larger hiatal hernias as part of a concomitant TIF/laparoscopic hiatal hernia repair.26
Evidence base for TIF
Several randomised controlled trials have evaluated the role of TIF 2.0 in the treatment of GORD. The RESPECT study was a randomised placebo-controlled trial comparing TIF to sham. Patients were randomised to either TIF plus 6 months of placebo treatment (n=87) or Sham plus 6 months of omeprazole treatment (n=42). Thirty-six per cent of patients in the sham group had suffered early failure at 3 months in comparison to 11% who had undergone TIF (p=0.004). (Early failure was defined as moderate to severe regurgitation 12 weeks after intervention despite doubling of PPI treatment or placebo). Both arms of the trial showed improvement in GORD reported outcomes with TIF eliminating troublesome regurgitation in a greater proportion of patients (67% vs 45%, p=0.023).
Oesophageal pH improved following TIF. The mean per cent total time pH <4 went from 9.3% to 6.4% post TIF (p<0.001). The mean DeMeester score fell from 33.6 to 23.9 post-TIF (p<0.001). No improvement was observed following sham surgery.
Significant adverse events were reported in three patients who underwent TIF (abdominal pain, chest pain and musculoskeletal pain) and one who underwent the sham procedure (Nausea).27
The TEMPO trial was a randomised trial comparing TIF 2.0 using the EsophyX device, to PPI therapy. Sixty-three patients were randomised to either undergo TIF (n=40) or medical therapy with PPI (n=23). At 6 months follow-up, 97% of patients in the TIF group (off PPI) were no longer experiencing troublesome regurgitation as assessed by the reflux disease questionnaire vs 50% in the PPI group (RR 1.9, 95% CI 1.2 to 3.1, p=0.006). At 6 months, 90% of patients (35/39, 95% CI 0.76 to 0.97) in the TIF group had stopped taking PPI with 3% consuming PPI on demand (1/39, 95% CI <0.0001 to 0.14) and 8% taking daily PPI (3/39, 95% CI 0.02 to 0.21). 54% (21/39) of patients in the TIF group had normalised oesophageal acid exposure in comparison to 52% (11/21) in the placebo arm (p=0.914). However, significant reductions were noted in the TIF group for number of reflux episodes, number of reflux episodes >5 min, %pH time less than 4 and DeMeester scores on 48 hour wireless Bravo testing (p<0.001).28 Long-term follow-up of the TEMPO trial has shown GERD-HRQL scores improving from 22.2 to 6.8 at 5 years (p<0.001).29
Håkansson et al conducted a double-blind sham-controlled trial with patients randomised to either TIF 2.0 (n=22) or sham (n=22). At 6-month follow-up, patients who had undergone TIF 2.0 had improved GORD symptom scores (p=0.005), whereas no significant change was noted in the sham arm. Ambulatory 24 hours pH monitoring revealed a significant reduction in total acid exposure time in the TIF 2.0 arm (7.8% pre-TIF vs 3.6% 6 months post-TIF, (p=0.003)), with no effect observed in the sham group (presham 13.1% vs 9.8% 6 months postsham, (p=0.147)).30
A 2018 systematic review and meta-analysis of 32 studies incorporating 1475 patients who underwent TIF revealed significant improvements in GERD-HRQL scores, gastro-oesophageal reflux symptom scale and reflux symptoms index post-TIF. (p<0.001 for all). There was also a reduction in hiatal hernia size, in addition to discontinuation of PPI therapy post procedure. (p<0.001). Significant reduction in DeMeester scores was also observed (mean difference 10.22; p<0.001).31
Long-term outcomes of TIF 2.0 have been reported and show durable improvements in quality of life metrics, symptom response as well as reduction/interruption in PPI dosage up to 10 years.32–35
GERD-x
GERD-x (G-SURG, Germany) is a endoscopic device introduced in 2014 that allows for full thickness plication.36 Following intubation with the GERD-X device and an ultrathin gastroscope, the GERD-X device is retroflexed, and the arms of the device are opened under direct endoscopic visualisation. A screw allows for tissue capture, and the arms of the device are closed enabling suture application via a stapler (figure 5).37 38
Figure 5.

Overview of the GERD-X device.
A recent single-centre, randomised, sham-controlled trial evaluated GERD-X in 70 PPI dependant patients who were randomised to either sham procedure or GERD-X in a 1:1 ratio. The primary endpoint was ≥50% improvement in GERD-HRQL score at 3 months. This was met by 65.7% of patients in the GERD-x group (2.9% in the sham group) (p<0.001). 62.8% of patients were PPI free at 12 months (11.4% in the sham group) (p<0.001). Furthermore, no serious adverse events (SAE)s were recorded in relation to the procedure.36
Medigus ultrasonic surgical endostapler
The Medigus ultrasonic surgical endostapler (MUSE) device contains a miniature camera, stapling anvil and ultrasound at the endoscopic tip. The device is retroflexed and retracted in the stomach with tissue clamped between the distal scope tip and body of the MUSE device. Ultrasound is used to assess tissue thickness prior to firing staples during plication.
A 2015 multicentre prospective study evaluated outcomes and safety data for MUSE in 66 patients. Six months postprocedure HRQL scores improved by >50% in 73% of patients with 64.6% no longer taking daily PPI therapy. Significant improvements in oesophageal acid exposure times were also noted (p<0.001). Eight AEs were reported in the initial 24 patients which included pneumothorax, oesophageal leak and GI haemorrhage. A protocol review led to future procedures requiring additional staples to reduce tension on individual staple sites.39 A 2019 prospective observational trial of MUSE found symptom improvement at 1-year postprocedure and 90% of patients stopping or halving PPI consumption.40
Endocinch
Endocinch (Bard) was a suction-based plication device that applied sutures to the gastric mucosa. Long-term results failed to prove durability, presumably due to suture loss from lack of full thickness plication.41–43
NDO endoscopic plication system
The NDO plication system allowed for full thickness plication of the gastric cardia to the oesophagus via a transmural suture. A 2006 randomised sham-controlled trial found significant improvements in QOL metrics, PPI dependence and oesophageal acid exposure with long-term durability.44 45 Due to the manufacturer financial difficulties this device is no longer available.46
Injection techniques
It has been postulated that submucosal injection of inert substances into the GOJ causes tissue remodelling at the lower oesophagus resulting in favourable outcomes for GORD by creating an enhanced physiological barrier to gastric acid. A number of injectable agents have been evaluated however many are no longer available due to poor long-term efficacy and/or safety concerns.47–52
Mucosal resection
Antireflux mucosectomy
Antireflux mucosectomy (ARMS) was born from a case report of a patient who underwent endoscopic resection for high-grade dysplastic BO. The resection margin extended to include a portion of the gastric cardia. The resultant scarring led to satisfactory reflux control up to 10 years postprocedure.53
Inoue et al 54 reported on findings from a case series on 10 GORD patients who underwent ARMS, finding improvements in DeMeester scores, acid exposure as well as Hill grade (3.2–1.2, p=0.0152). A recent prospective feasibility study has further explored ARMS as a treatment option.55 The group used a cap assisted EMR based technique for mucosectomy (ARMS-C) on 33 patients. A 6-month follow-up revealed 63% of patients no longer required antireflux medication with 30% reducing PPI dosage. GORD symptom scores had also significantly decreased. Improvements were also noted in pH studies, valve grade scores and GOJ distensibility as assessed by the endoFLIP device. Two patients required balloon dilatation for strictures and no SAEs were noted.
Ligation techniques
Peroral endoscopic cardial constriction
Peroral endoscopic cardial constriction (PECC) is a ligation based endoscopic technique. Although variations of the procedure have been studied the general premise involves ligating various locations across the GOJ and/or cardia with a combination of bands and/or clips. The resultant narrowing creates an enhanced physiological barrier for refluxate.56
Hu et al evaluated PECC through a preliminary feasibility study involving 13 patients. Significant improvements were noted in GERD-HRQL and DeMeester scores (p<0.01), with no SAE.56 PECC-b is an adaptation of early PECC that uses solely bands for ligation deployed via a multiring ligator. A preliminary feasibility study involving PECC-b on 68 patients, found reduced symptom scores as assessed by the reflux diagnostic questionnaire (p<0.05) at 12 months with 77.9% of patients completely discontinuing PPI therapy, furthermore no SAE were reported.57
Antireflux band mucosectomy
Band ligation of the cardia continues to be evaluated and a recent case series on four patients has found that band ligation can lead to significant resolution of symptoms with all patients discontinuing PPI 4 weeks postprocedure.58
Other techniques
Mucosal ablation and suturing at the oesophagogastric junction
The Apollo Overstitch endoscopic suturing system allows endoscopists to place full thickness sutures through the endoscope. This device has been used for several applications including defect closure, stent fixation and gastric bypass revisions.
Mucosal ablation and suturing at the oesophagogastric junction is a technique first reported by Han et al. Initial treatment with sutures suffered from a lack of durability due to suture loss.59 60 Modification of the technique by ablating the mucosa with APC prior to suture placement reduced suture loss. Out of 27 patients, 59% were able to discontinue PPI, with 14% reducing their daily dosage.60
Resection and plication anti-reflux method
Benias et al evaluated the resection and plication (RAP) method in a pilot study of 10 patients. RAP involves creating a limited mucosectomy at the LOS, followed by plication with full thickness sutures at the mucosectomy site. All patients demonstrated significant improvement in GERD-HRQL scores (p<0.0001) with eight patients discontinuing PPI use. One patient developed dysphagia related to stricture formation that required dilatation.61
Antireflux mucosal ablation
Antireflux mucosal ablation (ARMA) was developed by Inoue et al after trialling the technique on a patient with refractory GORD following the ARMS procedure. The technique involves ablating the gastric side of the cardia. Ablation is conducted up to the submucosa, with indigo carmine injection enabling appreciation of depth. A 2020 single arm pilot study found significant improvement in DeMeester scores and GERD-HRQL scores among 12 patients.62 A 2021 meta-analysis of non-randomised studies revealed similar levels of efficacy between ARMS and ARMA with acceptable safety profile.63
Future research priorities
The current body of evidence for ARET is associated with several limitations which has hampered widespread adoption into current treatment paradigms for GORD. Current ACG guidance only recommends TIF as a treatment option for patients with milder forms of GORD (<LA grade C oesophagitis) who do not wish to undergo ARS. Due to it having a heterogeneous evidence base Stretta is not recommended. Other ARET are also not recommended due to a limited evidence base.
We propose several research priorities that will help refine the future role of ARET.
-
High-quality randomised controlled trials
The placebo response for GORD can be as high as 50%.64 65 Sham-controlled trials can help address, but within this space such trials are limited in number with low participant numbers impacting generalisability of results.
-
Trials comparing endoscopic therapy to the surgical gold standard
High-quality studies comparing endotherapy directly against Nissen fundoplication will help define the position of ARET within the current treatment paradigm.
-
Head-to-head comparative studies
Head-to-head comparative trials are lacking but can help assess individual merits of ARETs and empower decision makers as well as inform future guidelines.
-
Long-term data
GORD is chronic ailment therefore long-term data is vital for assessing the durability of ARETs. A significant proportion of trials in this field involve follow-up data to 12 months with limited high quality randomised data evaluating patient response at 5 years or above.
-
Mechanistic data
Subjective improvement in reflux symptoms post endotherapy does not necessarily correlate with objective markers such as oesophageal acid exposure time or LOS pressure. It has previously been suggested that subjective improvement could be related to a disruption of sensory fibres, which results in decreased oesophageal sensitivity to gastric acid.66
Conclusions
The endoluminal treatment of GORD is an area of growing interest. Over the last two decades a number of devices and techniques have been developed and evaluated with many being withdrawn or not reaching mainstream popularity. The gastro-oesophageal junction is a complex zone that is still not anatomically or physiologically understood in its entirety.67
Currently, ARETs cannot be recommended over first-line ARS for many patients but do represent an alternative option for very high-risk surgical candidates with smaller hiatus hernias and milder forms of GORD. To ensure successful outcomes patient selection is key and a considered patient selection process is warranted. TIF 2.0 and Stretta have the largest body of evidence of all the ARETs, however, several novel therapies remain in the pipeline and require ongoing review (table 1). Future research needs to incorporate randomised controlled trials evaluating long-term outcomes, which will ultimately define future treatment algorithms for GORD.
Table 1.
Overview of current endoscopic therapies
| Device/technique | Advantage | Disadvantage | Reported adverse events |
| Stretta Radiofrequency |
Single operator Does not preclude future antireflux surgery Good safety profile |
Limited mechanistic data Heterogeneous evidence base with variable response rates Limited improvement in physiological parameters Requires proprietary equipment |
Chest pain Erosive oesophagitis Gastroparesis |
| EsophyX Z+ Plication |
Does not preclude future anti-reflux surgery Can be revised if required Being evaluated as an option for concomitant laparoscopic HH repair (C-TIF) for larger HH |
Two operator technique Requires proprietary equipment Large calibre device can make oesophageal intubation difficult |
Pneumothorax Perforation Bleeding |
| GERD-X Plication |
Relatively short operating time36
Faster learning curve36 |
Two operator technique Requires proprietary equipment Limited long-term data |
Chest pain |
| ARMS Mucosal resection |
Does not require proprietary equipment Can be conducted without General anaesthetic (GA) |
Steep learning curve High risk of perforation and bleeding Non standardised technique |
Dysphagia Bleeding Perforation |
| ARMA Ablation |
Does not require expensive proprietary equipment Less technical than ARMS Can be performed without GA |
Relatively new technique with limited evidence base Non standardised technique |
Dysphagia |
| PECC/ARBM Ligation |
Uses a technique and equipment that most gastroenterologists are well versed with Shorter procedure time Less technically challenging |
Limited data Non standardised technique |
Retrosternal discomfort Dysphagia |
| MASE/RAP Suturing |
Trialled in patients with altered anatomy post-surgery | Limited evidence base Non-standardised technique Steep learning curve Requires proprietary equipment |
Dysphagia |
ARBM, antireflux band mucosectomy; ARMA, antireflux mucosal ablation; ARMS, antireflux mucosectomy; c-TIF, concomitant transoral incisionless fundoplication; MASE, mucosal ablation and suturing at the esophagogastric; PECC, peroral endoscopic cardial constriction; RAP, resection and plication.
Footnotes
Twitter: @dr_nas_aslam, @barrettsonline
Contributors: NA conceptualised and drafted the initial manuscript. VS, RS, LBL, AT and RH revised critically for intellectual content. NA, VS, RS, LBL, AT and RH were involved in the review of the final manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: RH receives educational grants to support research infrastructure from Medtronic. Cook endoscopy (fellowship support), Pentax Europe, C2 therapeutics, Beamline diagnostic and Fractyl. VS receives honorarium for professional services from Pentax Europe, Medtronic, Astra Zeneca and Pharmacosmos. RS receives honoraria for running course and speaking at symposia for Medtronic, Johnson & Johnson, Falk Pharma, Medispar. HE is also on the advisory board for Johnson and Johnson and Falk Pharma.
Provenance and peer review: Not commissioned; externally peer reviewed.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1. Sweis R, Fox M. The global burden of gastro-oesophageal reflux disease: more than just heartburn and regurgitation. Lancet Gastroenterol Hepatol 2020;5:519–21. 10.1016/S2468-1253(20)30002-9 [DOI] [PubMed] [Google Scholar]
- 2. Nirwan JS, Hasan SS, Babar ZUD, et al. Global prevalence and risk factors of gastro-oesophageal reflux disease (GORD): systematic review with meta-analysis. Sci Rep 2020;10:5814. 10.1038/s41598-020-62795-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Scott M, Gelhot AR. Gastroesophageal reflux disease: diagnosis and management. Am Fam Physician 1999;59:1161–9. [PubMed] [Google Scholar]
- 4. Patel A, Gyawali CP. Screening for Barrett’s esophagus: balancing clinical value and cost-effectiveness. J Neurogastroenterol Motil 2019;25:181–8. 10.5056/jnm18156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. A narrative review of barrett’s esophagus in 2020, molecular and clinical update - dam - annals of translational medicine. Available: https://atm.amegroups.com/article/view/51164/html [Accessed 13 Jul 2021]. [DOI] [PMC free article] [PubMed]
- 6. Irvine EJ. Quality of life assessment in gastro-oesophageal reflux disease. Gut 2004;53 Suppl 4:iv35–9. 10.1136/gut.2003.034314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yibirin M, De Oliveira D, Valera R, et al. Adverse effects associated with proton pump inhibitor use. Cureus 2021;13:e12759. 10.7759/cureus.12759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Moore M, Afaneh C, Benhuri D, et al. Gastroesophageal reflux disease: a review of surgical decision making. World J Gastrointest Surg 2016;8:77–83. 10.4240/wjgs.v8.i1.77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yadlapati R, Hungness ES, Pandolfino JE. Complications of antireflux surgery. Am J Gastroenterol 2018;113:1137–47. 10.1038/s41395-018-0115-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Richter JE. Gastroesophageal reflux disease treatment: side effects and complications of fundoplication. Clin Gastroenterol Hepatol 2013;11:465–71. 10.1016/j.cgh.2012.12.006 [DOI] [PubMed] [Google Scholar]
- 11. Stacher G, Lenglinger J, Bergmann H, et al. Gastric emptying: a contributory factor in gastro-oesophageal reflux activity? Gut 2000;47:661–6. 10.1136/gut.47.5.661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jobe BA, Richter JE, Hoppo T, et al. Preoperative diagnostic workup before antireflux surgery: an evidence and experience-based consensus of the esophageal diagnostic advisory panel. J Am Coll Surg 2013;217:586–97. 10.1016/j.jamcollsurg.2013.05.023 [DOI] [PubMed] [Google Scholar]
- 13. Galmiche JP, Clouse RE, Bálint A, et al. Functional esophageal disorders. Gastroenterology 2006;130:1459–65. 10.1053/j.gastro.2005.08.060 [DOI] [PubMed] [Google Scholar]
- 14. Hershcovici T, Fass R. Nonerosive reflux disease (NERD)-an update. J Neurogastroenterol Motil 2010;16:8–21. 10.5056/jnm.2010.16.1.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. NICE . The technology | stretta system for gastro-oesophageal reflux disease | advice. Available: https://www.nice.org.uk/advice/mib74/chapter/The-technology [Accessed 16 Aug 2021].
- 16. Triadafilopoulos G. Stretta: a valuable endoscopic treatment modality for gastroesophageal reflux disease. World J Gastroenterol 2014;20:7730–8. 10.3748/wjg.v20.i24.7730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sowa P, Samarasena JB. Nonablative radiofrequency treatment for gastroesophageal reflux disease (STRETTA). Gastrointest Endosc Clin N Am 2020;30:253–65. 10.1016/j.giec.2019.12.006 [DOI] [PubMed] [Google Scholar]
- 18. Lipka S, Kumar A, Richter JE. No evidence for efficacy of radiofrequency ablation for treatment of gastroesophageal reflux disease: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2015;13:1058–67. 10.1016/j.cgh.2014.10.013 [DOI] [PubMed] [Google Scholar]
- 19. Richardson WS, Stefanidis D, Fanelli RD. Society of american gastrointestinal and endoscopic surgeons response to “no evidence for efficacy of radiofrequency ablation for treatment of gastroesophageal reflux disease: a systematic review and meta-analysis.” Clin Gastroenterol Hepatol 2015;13:1700–1. 10.1016/j.cgh.2015.02.007 [DOI] [PubMed] [Google Scholar]
- 20. Fass R, Cahn F, Scotti DJ, et al. Systematic review and meta-analysis of controlled and prospective cohort efficacy studies of endoscopic radiofrequency for treatment of gastroesophageal reflux disease. Surg Endosc 2017;31:4865–82. 10.1007/s00464-017-5431-2 [DOI] [PubMed] [Google Scholar]
- 21. Dughera L, Rotondano G, De Cento M, et al. Durability of stretta radiofrequency treatment for GERD: results of an 8-year follow-up. Gastroenterol Res Pract 2014;2014:531907. 10.1155/2014/531907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Noar M, Squires P, Noar E, et al. Long-Term maintenance effect of radiofrequency energy delivery for refractory GERD: a decade later. Surg Endosc 2014;28:2323–33. 10.1007/s00464-014-3461-6 [DOI] [PubMed] [Google Scholar]
- 23. Katz PO, Dunbar KB, Schnoll-Sussman FH, et al. Acg clinical guideline for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol 2022;117:27–56. 10.14309/ajg.0000000000001538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ihde GM. The evolution of TIF: transoral incisionless fundoplication. Therap Adv Gastroenterol 2020;13:1756284820924206. 10.1177/1756284820924206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. EndoGastric Solut . EsophyX device for transoral reconstructive surgery. Available: https://www.endogastricsolutions.com/technology/esophyx-device [Accessed 1 Aug 2021].
- 26. Gisi C, Wang K, Khan F, et al. Efficacy and patient satisfaction of single-session transoral incisionless fundoplication and laparoscopic hernia repair. Surg Endosc 2021;35:921–7. 10.1007/s00464-020-07796-x [DOI] [PubMed] [Google Scholar]
- 27. Hunter JG, Kahrilas PJ, Bell RCW, et al. Efficacy of transoral fundoplication vs omeprazole for treatment of regurgitation in a randomized controlled trial. Gastroenterology 2015;148:324–33. 10.1053/j.gastro.2014.10.009 [DOI] [PubMed] [Google Scholar]
- 28. Trad KS, Barnes WE, Simoni G, et al. Transoral incisionless fundoplication effective in eliminating GERD symptoms in partial responders to proton pump inhibitor therapy at 6 months: the tempo randomized clinical trial. Surg Innov 2015;22:26–40. 10.1177/1553350614526788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Trad KS, Barnes WE, Prevou ER, et al. The tempo trial at 5 years: transoral fundoplication (TIF 2.0) is safe, durable, and cost-effective. Surg Innov 2018;25:149–57. 10.1177/1553350618755214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Håkansson B, Montgomery M, Cadiere GB, et al. Randomised clinical trial: transoral incisionless fundoplication vs. sham intervention to control chronic GERD. Aliment Pharmacol Ther 2015;42:1261–70. 10.1111/apt.13427 [DOI] [PubMed] [Google Scholar]
- 31. McCarty TR, Itidiare M, Njei B, et al. Efficacy of transoral incisionless fundoplication for refractory gastroesophageal reflux disease: a systematic review and meta-analysis. Endoscopy 2018;50:708–25. 10.1055/a-0576-6589 [DOI] [PubMed] [Google Scholar]
- 32. Bell RCW, Freeman K, Heidrick R, et al. Transoral incisionless fundoplication demonstrates durability at up to 9 years. Therap Adv Gastroenterol 2021;14:17562848211004827. 10.1177/17562848211004827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Testoni S, Hassan C, Mazzoleni G, et al. Long-Term outcomes of transoral incisionless fundoplication for gastro-esophageal reflux disease: systematic-review and meta-analysis. Endosc Int Open 2021;9:E239–46. 10.1055/a-1322-2209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Testoni PA, Testoni S, Distefano G, et al. Transoral incisionless fundoplication with EsophyX for gastroesophageal reflux disease: clinical efficacy is maintained up to 10 years. Endosc Int Open 2019;7:E647–54. 10.1055/a-0820-2297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chimukangara M, Jalilvand AD, Melvin WS, et al. Long-Term reported outcomes of transoral incisionless fundoplication: an 8-year cohort study. Surg Endosc 2019;33:1304–9. 10.1007/s00464-018-6403-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kalapala R, Karyampudi A, Nabi Z, et al. Endoscopic full-thickness plication for the treatment of PPi-dependent GERD: results from a randomised, sham controlled trial. Gut 2022;71:686–94. 10.1136/gutjnl-2020-321811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. G-SURG. Available: http://www.g-surg.com [Accessed 22 Jul 2021].
- 38. Iannetti A. Updates on management of GERD disease. J Gastrointest Dig Syst 2017;07:04. 10.4172/2161-069X.1000523 [DOI] [Google Scholar]
- 39. Zacherl J, Roy-Shapira A, Bonavina L, et al. Endoscopic anterior fundoplication with the Medigus Ultrasonic Surgical Endostapler (MUSETM) for gastroesophageal reflux disease: 6-month results from a multi-center prospective trial. Surg Endosc 2015;29:220–9. 10.1007/s00464-014-3731-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Testoni PA, Testoni S, Mazzoleni G, et al. Transoral incisionless fundoplication with an ultrasonic surgical endostapler for the treatment of gastroesophageal reflux disease: 12-month outcomes. Endoscopy 2020;52:469–73. 10.1055/a-1124-3187 [DOI] [PubMed] [Google Scholar]
- 41. Schiefke I, Zabel-Langhennig A, Neumann S, et al. Long term failure of endoscopic gastroplication (endocinch). Gut 2005;54:752–8. 10.1136/gut.2004.058354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Montgomery M, Håkanson B, Ljungqvist O, et al. Twelve months’ follow-up after treatment with the endocinch endoscopic technique for gastro-oesophageal reflux disease: a randomized, placebo-controlled study. Scand J Gastroenterol 2006;41:1382–9. 10.1080/00365520600735738 [DOI] [PubMed] [Google Scholar]
- 43. Parker M, Smith CD. Comparing the effectiveness of endoscopic full-thickness plication and endoscopic radiofrequency treatments for patients with GERD. Expert Rev Gastroenterol Hepatol 2010;4:387–90. 10.1586/egh.10.46 [DOI] [PubMed] [Google Scholar]
- 44. Rothstein R, Filipi C, Caca K, et al. Endoscopic full-thickness plication for the treatment of gastroesophageal reflux disease: a randomized, sham-controlled trial. Gastroenterology 2006;131:704–12. 10.1053/j.gastro.2006.07.004 [DOI] [PubMed] [Google Scholar]
- 45. Pleskow D, Rothstein R, Kozarek R, et al. Endoscopic full-thickness plication for the treatment of GERD: five-year long-term multicenter results. Surg Endosc 2008;22:326–32. 10.1007/s00464-007-9667-0 [DOI] [PubMed] [Google Scholar]
- 46. Yew KC, Chuah SK. Antireflux endoluminal therapies: past and present. Gastroenterol Res Pract 2013;2013:481417. 10.1155/2013/481417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Feretis C, Benakis P, Dimopoulos C, et al. Endoscopic implantation of plexiglas (PMMA) microspheres for the treatment of GERD. Gastrointest Endosc 2001;53:423–6. 10.1067/mge.2001.113912 [DOI] [PubMed] [Google Scholar]
- 48. Kushner BS, Awad MM, Mikami DJ, et al. Endoscopic treatments for GERD. Ann N Y Acad Sci 2020;1482:121–9. 10.1111/nyas.14511 [DOI] [PubMed] [Google Scholar]
- 49. Chen D, Barber C, McLoughlin P, et al. Systematic review of endoscopic treatments for gastro-oesophageal reflux disease. Br J Surg 2009;96:128–36. 10.1002/bjs.6440 [DOI] [PubMed] [Google Scholar]
- 50. Fockens P, Cohen L, Edmundowicz SA, et al. Prospective randomized controlled trial of an injectable esophageal prosthesis versus a sham procedure for endoscopic treatment of gastroesophageal reflux disease. Surg Endosc 2010;24:1387–97. 10.1007/s00464-009-0784-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dumitru V, Hoara P, Dumitru D, et al. Invasive treatment options for gastro-esophageal reflux disease. J Med Life 2020;13:442–8. 10.25122/jml-2020-0160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lemperle G, Lemperle SM. Gastroesophageal reflux disease (GERD): an overview of current minimal-invasive treatment potentials. AJBSR 2019;2:253–64. 10.34297/AJBSR.2019.02.000619 Available: https://biomedgrid.com/volume2-issue6.php [DOI] [Google Scholar]
- 53. Satodate H, Inoue H, Yoshida T, et al. Circumferential EMR of carcinoma arising in Barrett’s esophagus: case report. Gastrointest Endosc 2003;58:288–92. 10.1067/mge.2003.361 [DOI] [PubMed] [Google Scholar]
- 54. Inoue H, Ito H, Ikeda H, et al. Anti-reflux mucosectomy for gastroesophageal reflux disease in the absence of hiatus hernia: a pilot study. Ann Gastroenterol 2014;27:346–51. [PMC free article] [PubMed] [Google Scholar]
- 55. Yoo IK, Ko WJ, Kim HS, et al. Anti-Reflux mucosectomy using a cap-assisted endoscopic mucosal resection method for refractory gastroesophageal disease: a prospective feasibility study. Surg Endosc 2020;34:1124–31. 10.1007/s00464-019-06859-y [DOI] [PubMed] [Google Scholar]
- 56. Hu H-Q, Li H-K, Xiong Y, et al. Peroral endoscopic cardial constriction in gastroesophageal reflux disease. Medicine (Baltimore) 2018;97:e0169. 10.1097/MD.0000000000010169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Li Z-T, Ji F, Han X-W, et al. Endoscopic cardial constriction with band ligation in the treatment of refractory gastroesophageal reflux disease: a preliminary feasibility study. Surg Endosc 2021;35:4035–41. 10.1007/s00464-021-08397-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Deshmukh A, Parsa N, Elmeligui A, et al. Antireflux band mucosectomy: a novel minimally invasive approach for the treatment of refractory gastroesophageal reflux disease. VideoGIE 2022;7:340–3. 10.1016/j.vgie.2022.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Han J, Chin M, Fortinsky KJ, et al. Endoscopic augmentation of gastroesophageal junction using a full-thickness endoscopic suturing device. Endosc Int Open 2018;6:E1120–5. 10.1055/a-0603-3693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chang KJ. Endoscopic foregut surgery and interventions: the future is now. The state-of-the-art and my personal journey. World J Gastroenterol 2019;25:1–41. 10.3748/wjg.v25.i1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Benias PC, D’Souza L, Lan G, et al. Initial experience with a novel resection and plication (RAP) method for acid reflux: a pilot study. Endosc Int Open 2018;6:E443–9. 10.1055/s-0044-101453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Inoue H, Tanabe M, de Santiago ER, et al. Anti-Reflux mucosal ablation (armA) as a new treatment for gastroesophageal reflux refractory to proton pump inhibitors: a pilot study. Endosc Int Open 2020;8:E133–8. 10.1055/a-1031-9436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Rodríguez de Santiago E, Sanchez-Vegazo CT, Peñas B, et al. Antireflux mucosectomy (arms) and antireflux mucosal ablation (armA) for gastroesophageal reflux disease: a systematic review and meta-analysis. Endosc Int Open 2021;9:E1740–51. 10.1055/a-1552-3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Cremonini F, Ziogas DC, Chang HY, et al. Meta-Analysis: the effects of placebo treatment on gastro-oesophageal reflux disease. Aliment Pharmacol Ther 2010;32:29–42. 10.1111/j.1365-2036.2010.04315.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hogan WJ. Clinical trials evaluating endoscopic GERD treatments is it time for a moratorium on the clinical use of these procedures? Am J Gastroenterology 2006;101:437–9. 10.1111/j.1572-0241.2006.00523.x [DOI] [PubMed] [Google Scholar]
- 66. Wenzel G, Kuhlbusch R, Heise J, et al. Relief of reflux symptoms after endoscopic gastroplication may be associated with reduced esophageal acid sensitivity: a pilot study. Endoscopy 2005;37:236–9. 10.1055/s-2005-860992 [DOI] [PubMed] [Google Scholar]
- 67. Sugano K, Spechler SJ, El-Omar EM, et al. Kyoto international consensus report on anatomy, pathophysiology and clinical significance of the gastro-oesophageal junction. Gut 2022;71:1488–514. 10.1136/gutjnl-2022-327281 [DOI] [PMC free article] [PubMed] [Google Scholar]