Abstract
Background
Obstructive sleep apnoea (OSA) is associated with increased risk of type 2 diabetes. However, results from large population-based prospective cohort studies are rare. The main aim of the present study was to investigate the relative risk of 8-year incident type 2 diabetes in relation to OSA severity in a prospective cohort study of middle-aged and older adults.
Methods
A total of 2918 participants (mean age 59 years) of the Korean Genome and Epidemiology Study (KoGES), who underwent home-based overnight polysomnography at baseline examination between 2011 and 2014, were followed up 4-yearly between 2015–2018 and 2019–2021. A total of 1697 participants were present in both follow-ups. After excluding participants who had diabetes at baseline (n=481), a total of 1216 participants were eligible for the analyses.
Results
OSA at baseline was categorised by apnoea–hypopnoea index levels as non-OSA (0–4.9 events·h−1), mild OSA (5.0–14.9 events·h−1) and moderate–severe OSA (≥15.0 events·h−1). Incident type 2 diabetes was identified at each follow-up. Compared with non-OSA, participants with moderate–severe OSA had 1.5 times higher risk of developing type 2 diabetes at the end of the 8-year follow-up after adjusting for potential covariates (relative risk 1.50, 95% CI 1.02–2.21). In the same analytical models for 4-year relative risk of incident type 2 diabetes, none of the OSA groups were at significantly higher risk compared with the non-OSA group.
Conclusion
Moderate–severe OSA, a modifiable risk factor, poses a higher risk of incident type 2 diabetes compared with non-OSA over an 8-year period in general middle-aged and older adults.
Short abstract
In this large population-based study, moderate–severe obstructive sleep apnoea was an independent risk factor for incident type 2 diabetes in the middle-aged and older, which may be a potential target for intervention in prevention strategies for diabetes https://bit.ly/3x55zmD
Introduction
Type 2 diabetes continues to increase in prevalence and incidence, and is a leading cause of morbidity and mortality [1]. The International Diabetes Federation has projected that 629 million people will be diagnosed with type 2 diabetes globally by 2045, compared with 425 million in 2017 [2]. The dramatic increase in newly diagnosed cases of type 2 diabetes and its associated morbidity and mortality urgently demand identifying potentially modifiable risk factors for primary prevention of diabetes in the general population. Obstructive sleep apnoea (OSA), the recurrent episodic disruption of normal breathing during sleep, is highly prevalent in the general adult population, ranging from 6% to 17% (as high as 49% in advanced age groups) [3]. OSA is a disorder that has also been found to be associated with type 2 diabetes. The mechanisms by which OSA may lead to diabetes can be explained by disruptions of the sleep pattern, intermittent hypoxia and oxidative stress, as well as obesity and cardiovascular comorbidities [4].
A growing number of studies have shown that OSA is associated with insulin resistance and glucose intolerance independent of other known risk factors [5–7]. Also, there are data suggesting a higher prevalence of diabetes in subjects with OSA independent of age, gender and obesity [8]. However, few prospective studies have investigated OSA as an independent risk factor for the future development of diabetes in the general population. A causal relationship of OSA and type 2 diabetes incidence has been reported in a clinic population from a historical cohort study [9], although prospective population-based study results remained mixed [8, 10, 11]. The Australian population-based cohort study reported that moderate–severe sleep apnoea was a significant risk factor for incident diabetes, although the confidence intervals were so wide that studies with greater power were suggested to verify the relationship [10]. However, from self-reported diabetes data, severe OSA was found to be associated with a greater risk of incident diabetes, independent of adiposity, in a community-based sample of participants during a median follow-up of 13 years [11]. The Wisconsin Sleep Cohort measured sleep apnoea by in-laboratory polysomnography (PSG) and found a non-significant association between moderate–severe sleep apnoea and incident physician-diagnosed diabetes after 4 years (OR 1.62, 95% CI 0.67–3.65) [8].
In this 8-year prospective follow-up study, we investigated the cumulative incidence and relative risk of type 2 diabetes by OSA severity in Korean general middle-aged and older adults. Based on cross-sectional data, we have previously reported that habitual snoring may affect glucose and insulin metabolism, independent of diabetes and hypertension, in non-obese Korean middle-aged men [12].
Methods
Study design and participants
The study participants were from the Korean Genome and Epidemiology Study (KoGES) [13]. The KoGES-Ansan Aging Study is a subcohort of KoGES, which is an ongoing prospective investigation (figure 1) that was designed to undertake overnight in-home PSG. Details of the KoGES-Ansan Aging Study and sampling method have been provided in previous reports [14, 15]. Briefly, at the baseline examination between 2011 and 2014, a total of 2918 participants (mean age 59 years) completed home unattended PSG and were followed up 4-yearly with a scheduled site visit for similar interviews, comprehensive health examination and collection procedures for bio-specimens. Two follow-ups were performed during 2015–2018 and 2019–2021 (figure 1). Written informed consent was obtained from all participants and the study protocol was approved by the institutional ethics committee of Korea University Ansan Hospital (Ansan, Republic of Korea).
FIGURE 1.
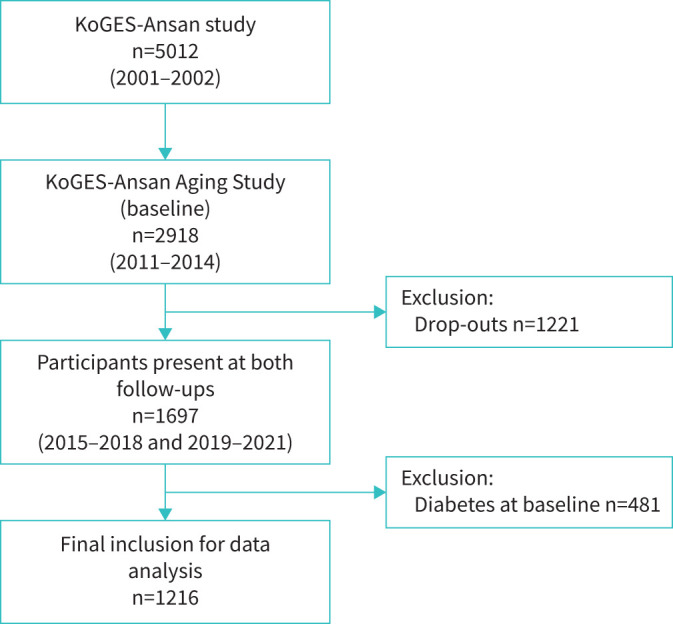
Flowchart of participants in the KoGES-Ansan study, 2001–2021. KoGES: Korean Genome and Epidemiology Study.
Polysomnography
Overnight PSG was performed with the portable device (Embletta X-100; Embla Systems, Broomfield, CO, USA) at home. A trained technologist connected the device to the patient at bedtime and data were retrieved in the morning after the unattended overnight recording [16]. All PSG results were manually scored using standard criteria [17]. Further details of PSG are provided in the supplementary material.
Definition of incident type 2 diabetes
All study participants underwent a fasting and 2-h 75-g oral glucose tolerance test (OGTT) at each follow-up visit [18]. Incident type 2 diabetes was defined as a fasting glucose concentration ≥126 mg·dL−1 or a post 2-h glucose after the 75-g OGTT ≥200 mg·dL−1 based on World Health Organization criteria [19, 20]. Regardless of glucose values, participants who reported currently being under antidiabetic medications were considered to have type 2 diabetes.
Other variables
Demographic data, alcohol consumption, physical activity and medical conditions were obtained via questionnaire. Physical activity was assessed using a scale consisting of five categories for activity intensity (sedentary, very light, light, moderate and vigorous) as measured by hours spent in a typical day per intensity level. The total metabolic equivalent (MET·week−1) score was calculated by multiplying the hours spent at a particular activity intensity by the MET value [21, 22]. Alcohol consumption status was determined by asking the participants whether they had ever consumed alcoholic beverages in their lifetime, whether there was a time in their life when they regularly consumed alcohol and whether they drank in the past 30 days. Total alcohol consumption (g·day−1) was then calculated based on detailed information of pattern, volume, frequency and type of beverages [22]. Self-reported smoking status (never-smoker, ex-smoker or current smoker) was collected and pack-years of smoking was calculated. Blood pressure, height, body weight, and neck and waist circumference of participants were measured using standard methods. Mean arterial pressure was calculated by: (systolic blood pressure/3)+(diastolic blood pressure×2/3). Body mass index (BMI) was calculated as weight divided by height squared (kg·m−2). We calculated percent change of BMI and waist circumference from baseline over time (4- and 8-year follow-up) by ratio of the follow-up measurement divided by the baseline measurement (e.g. (BMIfollow-up/BMIbaseline)×100). All blood samples were obtained in the morning after a 12-h overnight fast and were immediately stored at −80°C for subsequent assays. Plasma concentrations of glucose, total cholesterol, triglycerides and high-density lipoprotein (HDL) cholesterol were measured enzymatically using a 747 Chemistry Analyser (Hitachi, Tokyo, Japan). Low-density lipoprotein (LDL) cholesterol levels were estimated using the Friedewald formula [23]. Body fat percentage was measured by an InBody 720 body composition analyser (Biospace, Seoul, Republic of Korea) that uses the principle of bioelectrical impedance [24]. Body composition was measured at a fasting state and after voiding in the morning. Excessive daytime sleepiness (EDS) was measured by the Korean version of the Epworth Sleepiness Scale (KESS) questionnaire that is widely used to measure the general level of daytime sleepiness [25]. We defined EDS when the KESS score was ≥11 [26].
Statistical analysis
All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA). Demographic, lifestyle, clinical and sleep characteristics of the study participants were expressed as mean with standard deviation or number and percentage as per the OSA categories defined by apnoea–hypopnoea index (AHI) levels as non-OSA (AHI 0–4.9 events·h−1), mild OSA (AHI 5.0–14.9 events·h−1) and moderate–severe OSA (AHI ≥15.0 events·h−1). For continuous variables, one-way ANOVA was used; for categorical variables, the Chi-squared test was used. We used relative risk estimation by “Poisson regression with robust error variance” [27] to estimate relative risk and 95% confidence interval of incident type 2 diabetes in OSA groups, holding non-OSA as reference category. The robust error variance was estimated by using the repeated statement and the subject identifier in the PROC GENMOD procedure. To account for within-subject correlation for subjects, an unstructured correlation matrix was used. We examined both univariate and multivariable models. In multivariable models, relative risks were examined with adjustment for age, sex, occupation and income first. We found that waist circumference had a higher correlation with fasting blood glucose levels compared with the other body habitus measures, which is also consistent with a previous report [28]. When including both BMI and waist circumference in models, BMI was no longer significant; therefore, we chose to use waist circumference as our body habitus measure to be adjusted in the further models. However, we conducted sensitivity analyses with BMI included in the models. Finally, other potentially confounding variables measured at baseline (physical activity, alcohol consumption, smoking, mean arterial pressure, total cholesterol, history of cardiovascular disease, total sleep time and napping) were adjusted to determine if those significantly changed the OSA–diabetes relationship. Two-tailed p-values <0.05 were considered to indicate statistical significance.
Results
In total, 1697 participants were present in both follow-ups. After excluding participants who had diabetes at baseline (n=481), a total of 1216 participants (age range 49–79 years) were analysed (figure 1). The baseline characteristics of the study participants in the three OSA groups are shown in table 1. Mean±sd age was 56.6±4.9, 58.6±5.9 and 59.5±6.4 years in the non-OSA, mild OSA and moderate–severe OSA group, respectively. The proportion of women was 60.7%, 48.9% and 25.9% in the non-OSA, mild OSA and moderate–severe OSA group, respectively. Body habitus measures (BMI and waist circumference) were highest among the moderate–severe OSA group, and were significantly different among the groups (table 1). Although regular exercise proportions did not differ significantly, median physical activity (MET·week−1) was lowest in the moderate–severe OSA group. Current alcohol drinkers were proportionately higher in the moderate–severe OSA group; however, current alcohol consumption amount was higher in the mild OSA group. Total cholesterol and LDL cholesterol were not significantly different among the OSA groups; however, HDL cholesterol was lower in the moderate–severe OSA group. Mean arterial pressure and history of cardiovascular disease were higher among the moderate–severe OSA group.
TABLE 1.
Baseline general characteristics of the study participants (n=1216) by obstructive sleep apnoea (OSA) severity
| Total (n=1216) | OSA category# | p-value ¶ | |||
| Non-OSA (n=717) | Mild OSA (n=356) | Moderate–severe OSA (n=143) | |||
| Age, years | 57.5±5.5 | 56.6±4.9 | 58.6±5.9 | 59.5±6.4 | <0.001 |
| Women | 646 (53.1) | 435 (60.7) | 174 (48.9) | 37 (25.9) | <0.001 |
| Occupation | 0.042 | ||||
| White collar | 352 (29.0) | 199 (27.8) | 98 (27.5) | 55 (38.5) | |
| Blue collar | 390 (32.0) | 230 (32.1) | 118 (33.2) | 42 (29.4) | |
| Housekeeper | 474 (39.0) | 288 (40.2) | 140 (39.3) | 46 (32.2) | |
| Low income+ | 84 (6.9) | 46 (6.5) | 27 (7.6) | 11 (7.7) | 0.859 |
| BMI, kg·m−2 | 24.5±2.9 | 23.8±2.7 | 25.1±2.7 | 26.2±3.1 | <0.001 |
| Obesity (BMI categories) | <0.001 | ||||
| Normal weight (BMI <25 kg·m−2) | 711 (58.5) | 488 (68.0) | 177 (49.7) | 46 (32.2) | |
| Overweight (BMI 25– <30 kg·m−2) | 470 (38.7) | 223 (31.1) | 164 (46.1) | 83 (58.0) | |
| Obese (BMI ≥30 kg·m−2) | 35 (2.9) | 6 (0.8) | 15 (4.2) | 14 (9.8) | |
| Neck circumference, cm | 34.8±3.7 | 34.0±3.3 | 35.4±4.2 | 37.1±3.2 | <0.001 |
| Waist circumference, cm | 81.3±8.4 | 79.1±8.0 | 83.3±7.8 | 87.3±8.1 | <0.001 |
| Body fat, % | 27.7±7.0 | 27.5±7.0 | 28.1±7.2 | 27.7±6.5 | 0.416 |
| Alcohol consumption status | 0.001 | ||||
| Never-drinker | 586 (48.2) | 369 (51.5) | 170 (47.8) | 47 (32.9) | |
| Ex-drinker | 59 (4.9) | 28 (3.9) | 18 (5.1) | 13 (9.1) | |
| Current drinker | 571 (47.0) | 320 (44.6) | 168 (47.2) | 83 (58.0) | |
| Current alcohol consumption§, g·week−1 | 52.7 (16.1–165.3) | 44.2 (15.2–145.2) | 81.0 (20.3–249.9) | 52.7 (13.7–162.0) | 0.007 |
| Smoking status | <0.001 | ||||
| Never-smoker | 780 (64.1) | 489 (68.2) | 222 (62.4) | 69 (48.3) | |
| Ex-smoker | 304 (25.0) | 156 (21.7) | 94 (26.4) | 54 (37.8) | |
| Current smoker | 132 (10.9) | 72 (10.0) | 40 (11.2) | 20 (14.0) | |
| Current smoking, pack-years | 27.6±19.1 | 27.0±21.3 | 26.9±14.8 | 30.8±18.9 | 0.717 |
| Regular exercise | 411 (33.8) | 231 (32.2) | 136 (38.2) | 44 (30.8) | 0.541 |
| Physical activity§, MET·week−1 | 540 (0–1260) | 540 (0–1260) | 630 (0–1485) | 360 (0–1080) | 0.024 |
| Fasting blood glucose, mg·dL−1 | 90.0±7.5 | 89.4±7.7 | 90.2±7.0 | 92.2±7.8 | <0.001 |
| Post 2-h OGTT glucose, mg·dL−1 | 127.8±29.6 | 124.9±29.2 | 128.4±29.4 | 140.2±29.0 | <0.001 |
| Glycated haemoglobin A1c, % | 5.5±0.3 | 5.5±0.3 | 5.5±0.3 | 5.6±0.3 | <0.001 |
| Fasting blood insulin, IU·L−1 | 8.1±3.4 | 7.7±3.0 | 8.4±3.6 | 9.6±4.4 | <0.001 |
| Post 2-h OGTT insulin, IU·L−1 | 44.3±37.0 | 41.3±34.6 | 45.8±38.9 | 54.7±41.7 | <0.001 |
| HOMA-IR | 1.8±0.9 | 1.7±0.8 | 1.9±0.9 | 2.2±1.1 | <0.001 |
| Systolic blood pressure, mmHg | 113.7±13.8 | 112.2±13.9 | 114.7±13.7 | 118.3±12.7 | <0.001 |
| Diastolic blood pressure, mmHg | 75.1±9.5 | 74.2±9.5 | 75.6±9.6 | 78.4±8.9 | <0.001 |
| Mean arterial pressure, mmHg | 87.9±10.4 | 86.8±10.4 | 88.6±10.4 | 91.7±9.4 | <0.001 |
| History of CVD | 75 (6.2) | 34 (4.7) | 25 (7.0) | 16 (11.2) | 0.003 |
| Total cholesterol, mg·dL−1 | 202.1±35.5 | 204.1±35.6 | 199.2±32.9 | 199.8±40.9 | 0.074 |
| HDL cholesterol, mg·dL−1 | 49.7±12.7 | 51.0±12.8 | 48.9±12.4 | 44.9±11.6 | <0.001 |
| LDL cholesterol, mg·dL−1 | 126.6±31.9 | 128.3±32.6 | 123.6±29.1 | 125.7±34.3 | 0.076 |
| Sleep and PSG variables | |||||
| EDS | 58 (4.78) | 30 (4.2) | 19 (5.3) | 9 (6.3) | 0.217 |
| Napping | 457 (37.6) | 270 (37.7) | 132 (37.1) | 55 (38.5) | 0.951 |
| Self-reported sleep time, h | 6.07±1.2 | 6.1±1.2 | 6.0±1.2 | 6.1±1.1 | 0.809 |
| Mean oxygen saturation, % | 95.78±1.3 | 96.2±1.1 | 95.4±1.2 | 94.6±1.2 | <0.001 |
| Lowest oxygen saturation, % | 87.53±6.2 | 90.0±5.4 | 85.3±4.7 | 80.8±6.4 | <0.001 |
| ODI, events·h−1 | 6.04±7.9 | 1.7±1.3 | 7.7±2.9 | 23.2±10.0 | <0.001 |
Data are presented as mean±sd or median (interquartile range) for continuous variables and n (%) for categorical variables. BMI: body mass index; MET: metabolic equivalent; OGTT: oral glucose tolerance test; HOMA-IR: homeostasis model assessment of insulin resistance; CVD: cardiovascular disease; HDL: high-density lipoprotein; LDL: low-density lipoprotein; PSG: polysomnography; EDS: excessive daytime sleepiness; ODI: oxygen desaturation index. #: categories are defined by apnoea–hypopnoea index 0–4.9, 5.0–14.9 and ≥15.0 events·h−1 as non-OSA, mild OSA and moderate–severe OSA, respectively; ¶: p-values are based on one-way ANOVA for continuous variables and the Chi-squared test for categorical variables; +: average monthly wage below KRW 1 million (USD 770), which approximately corresponds to the government-set minimum wage for a family of three; §: statistical significance was estimated after logarithmic transformation.
Sleep characteristics of the study participants by OSA severity are presented in the table 1. EDS was 4.2%, 5.3% and 6.3% in the non-OSA, mild OSA and moderate–severe OSA group, respectively, but did not differ significantly among the OSA groups. Self-reported sleep time also was not significantly different among the OSA groups. Mean oxygen saturation was 96.2%, 95.4% and 94.6% in the non-OSA, mild OSA and moderate–severe OSA group, respectively, and was significantly different among the OSA groups (p<0.001).
The 4- and 8-year cumulative incidence rates of type 2 diabetes were 7.9% and 14%, respectively (table 2). Compared with the non-OSA group, the 4-year relative risk of type 2 diabetes in both the mild OSA and moderate–severe OSA groups was not significantly higher in the unadjusted model and in models after adjusting for potential covariates (table 3). The 8-year relative risk of type 2 diabetes in the mild OSA compared with the non-OSA group was also not significantly higher (table 4). However, the moderate–severe OSA compared with the non-OSA group had increased risk of developing type 2 diabetes at the end of the 8-year follow-up in the unadjusted model (relative risk 1.89, 95% CI 1.31–2.72). The risk persisted similar in the model adjusted for age, sex, occupation and income (relative risk 1.99, 95% CI 1.37–2.91). The relative risk was attenuated but remained significant after further adjustment for waist circumference and percent change of waist circumference (relative risk 1.53, 95% CI 1.04–2.24) and for other covariates (relative risk 1.50, 95% CI 1.02–2.21) (table 4). In sensitivity analyses including BMI and percent change of BMI overtime (for both 4 and 8 years) in the models, results did not change essentially (supplementary tables S3 and S4). Although the lower 95% CI limit of 8-year relative risk was barely >1 (relative risk 1.47, 95% CI 1.003–2.17; p=0.048), the risk remained positive (supplementary table S4). Full regression results including coefficient estimates for all other covariates are shown in supplementary tables S3 and S4.
TABLE 2.
Cumulative incidence rate of type 2 diabetes among the study participants (n=1216) by obstructive sleep apnoea (OSA) categories#
| Participants, n | Cumulative incidence rate, n (%) | ||
| 4-year | 8-year | ||
| Non-OSA | 717 | 43 (6.0) | 85 (11.9) |
| Mild OSA | 356 | 33 (9.3) | 53 (14.9) |
| Moderate–severe OSA | 143 | 20 (14.0) | 32 (22.4) |
| All | 1216 | 96 (7.9) | 170 (14.0) |
#: categories are defined by apnoea–hypopnoea index 0–4.9, 5.0–14.9 and ≥15.0 events·h−1 as non-OSA, mild OSA and moderate–severe OSA, respectively.
TABLE 3.
Four-year relative risk of type 2 diabetes of the study participants (n=1216) by obstructive sleep apnoea (OSA) categories#
| Participants, n | Relative risk (95% CI) ¶ | ||||
| Unadjusted model | Model 1 | Model 2 | Model 3 | ||
| Non-OSA | 717 | 1.0 | 1.0 | 1.0 | 1.0 |
| Mild OSA | 356 | 1.25 (0.91–1.72) | 1.47 (0.96–2.27) | 1.24 (0.80–1.90) | 1.27 (0.82–1.97) |
| Moderate–severe OSA | 143 | 1.89 (1.31–2.72) | 2.13 (1.27–3.57) | 1.56 (0.93–2.60) | 1.58 (0.95–2.63) |
Model 1: adjusted for age, sex, occupation and income; Model 2: adjusted for Model 1 plus waist circumference (WC) and WC change (=percent change of WC from baseline to 4-year follow-up); Model 3: adjusted for Model 2 plus exercise (metabolic equivalent·week−1), drinking alcohol (g·week−1), smoking (pack-years), mean arterial pressure, total cholesterol, cardiovascular disease history, total sleep time (self-reported) and napping. #: categories are defined by apnoea–hypopnoea index 0–4.9, 5.0–14.9 and ≥15.0 events·h−1 as non-OSA, mild OSA and moderate–severe OSA, respectively; ¶: relative risk estimated by Poisson regression with robust error variance.
TABLE 4.
Eight-year relative risk of type 2 diabetes of the study participants (n=1216) by obstructive sleep apnoea (OSA) categories#
| Participants, n | Relative risk (95% CI) ¶ | ||||
| Unadjusted model | Model 1 | Model 2 | Model 3 | ||
| Non-OSA | 717 | 1.0 | 1.0 | 1.0 | 1.0 |
| Mild OSA | 356 | 1.25 (0.91–1.72) | 1.28 (0.93–1.76) | 1.10 (0.80–1.51) | 1.12 (0.82–1.54) |
| Moderate–severe OSA | 143 | 1.89 (1.31–2.72) | 1.99 (1.37–2.91) | 1.53 (1.04–2.24) | 1.50 (1.02–2.21) |
Model 1: adjusted for age, sex, occupation and income; Model 2: adjusted for Model 1 plus waist circumference (WC) and WC change (=percent change of WC from baseline to 8-year follow-up); Model 3: adjusted for Model 2 plus exercise (metabolic equivalent·week−1), drinking alcohol (g·week−1), smoking (pack-years), mean arterial pressure, total cholesterol, cardiovascular disease history, total sleep time (self-reported) and napping. #: categories are defined by apnoea–hypopnoea index 0–4.9, 5.0–14.9 and ≥15.0 events·h−1 as non-OSA, mild OSA and moderate–severe OSA, respectively; ¶: relative risk estimated by Poisson regression with robust error variance.
Discussion
In this KoGES-Ansan Aging Study cohort, moderate–severe OSA was an independent risk factor for 8-year incident type 2 diabetes in general middle-aged and older adults. A short-term (4-year) follow-up did not yield significant association, indicating the effect of moderate–severe OSA on increased risk of incident diabetes takes time. To the best of our knowledge, this is one of the few studies to report a significant independent risk between moderate–severe OSA and incident diabetes (ascertained clinically) from a large population-based cohort study over a long-term follow-up period. These data are also the first from an Asian population-based cohort that is ethnically distinct from the Caucasian populations from where the data currently exist.
Our findings are consistent with previous longitudinal studies [10, 11]. However, our study was strengthened in several different ways over the previous studies. In our study, incident diabetes was ascertained clinically with standard laboratory methods that may have prevented us from any misclassification due to potential measurement errors when using self-reported data [11]. Compared with the Australian population-based cohort study that also reported moderate–severe OSA as a significant risk factor for incident diabetes, our study was performed in a substantially larger population [10]. In contrast to the longitudinal analysis of the Wisconsin Sleep Cohort, our study yielded a significant association between sleep apnoea and diabetes after adjustment for age, sex and waist circumference [8]. The higher average age and the relatively larger sample size of our study population may have given more power to detect a significant relationship.
There are several potential pathophysiological mechanisms for developing type 2 diabetes due to OSA. Numerous studies reveal a connection between OSA and abnormal glucose metabolism, insulin resistance, onset and progression of type 2 diabetes. Additionally, Bulcun et al. [29] observed that progression from snoring and mild OSA to severe OSA led to an increased frequency of abnormal glucose metabolism. Our previous study suggested that habitual snorers tend to be more glucose intolerant and insulin resistant compared with non-habitual snorers [12]. One of the proposed molecular mechanisms is based on the oxygen-sensitive α-subunit of hypoxia-inducible factor (HIF)-1, a key regulator of oxygen metabolism. HIF-1α is involved in the regulation of metabolic processes and mediates the development of insulin resistance and diabetes mellitus [30]. A previous study found patients with OSA (resulting in a chronic decrease in oxygen saturation of haemoglobin) present with increased HIF-1α serum protein concentration compared with healthy controls [30].
In recent years, clinical trials on whether treatment of OSA with continuous positive airway pressure (CPAP) improves insulin resistance and glycaemic control in non-diabetic and diabetic patients with OSA have yielded favourable results. In patients with pre-diabetes, 8-h nightly CPAP treatment for 2 weeks improved glucose metabolism compared with placebo [31], but this level of treatment adherence is rarely found in clinical practice. A longer CPAP treatment (6 months) resulted in improved glycaemic control and insulin resistance in type 2 diabetes patients compared with a control group [32]. However, negative studies have also been reported. In a substudy of 888 participants in the Sleep Apnea cardioVascular End-points (SAVE) trial in which patients with OSA and stable cardiovascular disease were randomised to receive CPAP plus usual care or usual care alone [33], in those with pre-existing diabetes (n=274), there was no significant difference between the CPAP and usual care groups in serum glucose, glycated haemoglobin A1c or antidiabetic medications during a median follow-up of 4.3 years. In another randomised, double-blind crossover study (based on sleep clinical referrals), 50 subjects with moderate–severe sleep apnoea (AHI >15 events·h−1) and impaired glucose tolerance were randomised to 8 weeks of CPAP or sham CPAP, followed by the alternate therapy after a 1-month washout [34]. The primary outcome was normalisation of the mean 2-h OGTT and the study did not show that impaired glucose tolerance normalises after CPAP in subjects with moderate sleep apnoea.
We did not find the risk of incident type 2 diabetes increasing with increasing severity of OSA. One previous study reported a graded inverse relationship between OSA severity and glucose control in patients with type 2 diabetes [35]. Compared with patients without OSA, the adjusted mean glycated haemoglobin A1c was increased in patients with mild, moderate and severe OSA gradually. Intuitively, a similar relationship might be expected with OSA and incidence of diabetes. However, our study, as well as previous studies, has not found a significant risk of incident diabetes in the mild OSA compared with the non-OSA group. Since diabetes is a multifactorial disease, it is possible that only moderate–severe OSA contributes to the additional risk and not a mild level of OSA. Another possibility could be that the latent period for the development of type 2 diabetes in the mild OSA group could extend beyond the duration of our study period [8]. Additionally, those with prevalent type 2 diabetes at baseline were excluded in the current study (figure 1), who also had significantly higher prevalent OSA (both mild and moderate–severe OSA compared with the included participants; supplementary table S2), leaving participants who were possibly more “resistant” against diabetes development. Although mild OSA was not found to be a significant risk for incident diabetes, the point estimates were higher in all three models (tables 3 and 4), which is also consistent with findings from previous studies. Thus, we cannot rule out the potential effect of a mild level of OSA on diabetes incidence. Future cohort studies with greater power need to investigate this observation since mild OSA is more common than moderate–severe OSA and thus might be more important from a public health perspective [36].
Our study has several limitations. First, due to the observational nature of the study, participants with moderate–severe OSA were not offered any treatment or intervention, although we informed them fully about their PSG results. Consequently, the present study did not consider any effects that might have occurred due to any treatment sought by the participants during the follow-up period. However, we assume that the number of participants seeking treatment for OSA would be very low given that <3% of the study participants had an AHI ≥30 events·h−1 (data not shown) and were mostly asymptomatic (table 1). On the other hand, our data might serve as important “real-world” data on the natural course of OSA showing what happens if patients are left untreated or not intervened. Second, we cannot rule out selection bias in the study due to the large number of drop-outs and the exclusion of participants who had diabetes at baseline (figure 1). Additionally, some of the general characteristics were significantly different between the cohort and non-participants, as evident from supplementary table S1. However, this is one of the biggest population-based cohorts that we have followed up for a relatively long duration. One aspect relevant to this point, however, is that our study did not consider the exact interdependence between the development of OSA and the time (both prior to and after the index date of inclusion) lived with the diagnosis of OSA. Third, incident diabetes cases were considered to be type 2 diabetes, therefore a possible misclassification could not be completely ruled out due to late-onset type 1 diabetes. However, we assumed that the number would be low especially because the incidence of adult-onset type 1 diabetes has been found to be lowest predominantly in East-Asian countries [37]. Fourth, we did not have information about visceral obesity, which is an important risk factor for the development of diabetes. Although we adjusted for waist circumference, which is a better indicator than BMI for visceral obesity, we cannot completely rule out some residual confounding. However, we performed a sensitivity analysis using body fat percentage in the model and the results were essentially the same (data not shown). Our results, however, may not hold in populations with greater overt obesity and high BMI. Racial differences in genetic and epigenetic mechanisms may also limit generalisation to other populations. Finally, our results may need to be interpreted with caution because of lacking data for some disease conditions (e.g. non-alcoholic fatty liver disease, gestational diabetes mellitus, polycystic ovary syndrome, mental health conditions, etc.) and medication usage (e.g. lipid-lowering agents such as nicotinic acid, epinephrine, glucocorticoids, thiazide diuretics, antipsychotics, etc.) that are potential risk factors for type 2 diabetes or could alter the glycaemic index.
Conclusions
In summary, our results provide evidence that moderate–severe OSA is an independent risk factor for incident type 2 diabetes in the middle-aged and older general population over a long-term period. While future studies are warranted to investigate the effects of treatment of moderate–severe OSA on prevention of incident type 2 diabetes, it seems prudent that long-term monitoring and timely intervention or treatment of OSA could be useful in diabetes prevention strategies.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary methods 00401-2022.SUPPLEMENT (102.2KB, pdf)
Supplementary tables 00401-2022.SUPPLEMENT2 (173.3KB, pdf)
Footnotes
Provenance: Submitted article, peer reviewed.
Author contributions: C. Shin had full access to all the data in the study, and takes responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: A.T. Siddiquee, S. Kim and C. Shin. Acquisition, analysis or interpretation of data: all authors. Drafting of the manuscript: A.T. Siddiquee, S. Kim, R.J. Thomas and C. Shin. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: A.T. Siddiquee. Administrative, technical or material support: R.J. Thomas, M-H. Lee and S.K. Lee. Supervision: C. Shin.
Conflict of interest: R.J. Thomas reports personal consulting fees from GLG Councils, Guidepoint Global and Jazz Pharmaceuticals, outside the submitted work. In addition, R.J. Thomas has a patent (ECG-spectrogram) with royalties paid by MyCardio, LLC, a patent (PAPGAM) issued and a patent (Auto CPAP) licensed to DeVilbiss-Drive. No other disclosures were reported.
Support statement: This research is supported by grants from the Korea Centers for Disease Control and Prevention (2011-E71004-00, 2012-E71005-00, 2013-E71005-00, 2014-E71003-00, 2015-P71001-00, 2016-E71003-00, 2017-E71001-00, 2018-E7101-00, 2019-E7104-00, 2019-E7104-01 and 2021-E0602-00). The funding sources had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review or approval of the manuscript; and decision to submit the manuscript for publication. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Khan MAB, Hashim MJ, King JK, et al. . Epidemiology of type 2 diabetes – global burden of disease and forecasted trends. J Epidemiol Glob Health 2020; 10: 107–111. doi: 10.2991/jegh.k.191028.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saeedi P, Petersohn I, Salpea P, et al. . Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract 2019; 157: 107843. doi: 10.1016/j.diabres.2019.107843 [DOI] [PubMed] [Google Scholar]
- 3.Heinzer R, Vat S, Marques-Vidal P, et al. . Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med 2015; 3: 310–318. doi: 10.1016/S2213-2600(15)00043-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reutrakul S, Mokhlesi B. Obstructive sleep apnea and diabetes: a state of the art review. Chest 2017; 152: 1070–1086. doi: 10.1016/j.chest.2017.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Punjabi NM, Shahar E, Redline S, et al. . Sleep-disordered breathing, glucose intolerance, and insulin resistance: the Sleep Heart Health Study. Am J Epidemiol 2004; 160: 521–530. doi: 10.1093/aje/kwh261 [DOI] [PubMed] [Google Scholar]
- 6.Resnick HE, Redline S, Shahar E, et al. . Diabetes and sleep disturbances: findings from the Sleep Heart Health Study. Diabetes Care 2003; 26: 702–709. doi: 10.2337/diacare.26.3.702 [DOI] [PubMed] [Google Scholar]
- 7.Tasali E, Mokhlesi B, Van Cauter E. Obstructive sleep apnea and type 2 diabetes: interacting epidemics. Chest 2008; 133: 496–506. doi: 10.1378/chest.07-0828 [DOI] [PubMed] [Google Scholar]
- 8.Reichmuth KJ, Austin D, Skatrud JB, et al. . Association of sleep apnea and type II diabetes: a population-based study. Am J Respir Crit Care Med 2005; 172: 1590–1595. doi: 10.1164/rccm.200504-637OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kendzerska T, Gershon AS, Hawker G, et al. . Obstructive sleep apnea and incident diabetes. A historical cohort study. Am J Respir Crit Care Med 2014; 190: 218–225. doi: 10.1164/rccm.201312-2209OC [DOI] [PubMed] [Google Scholar]
- 10.Marshall NS, Wong KK, Phillips CL, et al. . Is sleep apnea an independent risk factor for prevalent and incident diabetes in the Busselton Health Study? J Clin Sleep Med 2009; 5: 15–20. doi: 10.5664/jcsm.27387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagayoshi M, Punjabi NM, Selvin E, et al. . Obstructive sleep apnea and incident type 2 diabetes. Sleep Med 2016; 25: 156–161. doi: 10.1016/j.sleep.2016.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shin C, Kim J, Kim J, et al. . Association of habitual snoring with glucose and insulin metabolism in nonobese Korean adult men. Am J Respir Crit Care Med 2005; 171: 287–291. doi: 10.1164/rccm.200407-906OC [DOI] [PubMed] [Google Scholar]
- 13.Kim Y, Han B-G, KoGES Group . Cohort profile: the Korean Genome and Epidemiology Study (KoGES) consortium. Int J Epidemiol 2017: 46: e29. doi: 10.1093/ije/dyx316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shin C, Kim REY, Thomas RJ, et al. . Neck circumference and cerebral gray matter volume. Alzheimer Dis Assoc Disord 2020; 34: 306–312. doi: 10.1097/WAD.0000000000000386 [DOI] [PubMed] [Google Scholar]
- 15.Siddiquee AT, Kim S, Abbott RD, et al. . Implications of age and sex in relation to obstructive sleep apnea severity spectrum: Korean Genome and Epidemiology-Ansan Aging Study. Ann Am Thorac Soc 2022; 19: 1069–1072. doi: 10.1513/AnnalsATS.202112-1339RL [DOI] [PubMed] [Google Scholar]
- 16.Kim S, Lee KY, Kim NH, et al. . Relationship of obstructive sleep apnoea severity and subclinical systemic atherosclerosis. Eur Respir J 2020; 55: 1900959. doi: 10.1183/13993003.00959-2019 [DOI] [PubMed] [Google Scholar]
- 17.Berry RB, Budhiraja R, Gottlieb DJ, et al. . Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med 2012; 8: 597–619. doi: 10.5664/jcsm.2172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seo JA, Lee DY, Yu JH, et al. . Habitual late sleep initiation is associated with increased incidence of type 2 diabetes mellitus in Korean adults: the Korean Genome and Epidemiology Study. Sleep 2019; 42: zsz090. doi: 10.1093/sleep/zsz090 [DOI] [PubMed] [Google Scholar]
- 19.American Diabetes Association . Diagnosis and classification of diabetes mellitus. Diabetes Care 2011; 34: Suppl. 1, S62–S69. doi: 10.2337/dc11-S062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 1998; 15: 539–553. doi: [DOI] [PubMed] [Google Scholar]
- 21.Ainsworth BE, Haskell WL, Whitt MC, et al. . Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc 2000; 32: Suppl. 9, S498–S504. doi: 10.1097/00005768-200009001-00009 [DOI] [PubMed] [Google Scholar]
- 22.Baik I, Shin C. Prospective study of alcohol consumption and metabolic syndrome. Am J Clin Nutr 2008; 87: 1455–1463. doi: 10.1093/ajcn/87.5.1455 [DOI] [PubMed] [Google Scholar]
- 23.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972; 18: 499–502. doi: 10.1093/clinchem/18.6.499 [DOI] [PubMed] [Google Scholar]
- 24.Kyle UG, Bosaeus I, De Lorenzo AD, et al. . Bioelectrical impedance analysis – part II: utilization in clinical practice. Clin Nutr 2004; 23: 1430–1453. doi: 10.1016/j.clnu.2004.09.012 [DOI] [PubMed] [Google Scholar]
- 25.Cho YW, Lee JH, Son HK, et al. . The reliability and validity of the Korean version of the Epworth sleepiness scale. Sleep Breath 2011; 15: 377–384. doi: 10.1007/s11325-010-0343-6 [DOI] [PubMed] [Google Scholar]
- 26.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 1991; 14: 540–545. doi: 10.1093/sleep/14.6.540 [DOI] [PubMed] [Google Scholar]
- 27.Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004; 159: 702–706. doi: 10.1093/aje/kwh090 [DOI] [PubMed] [Google Scholar]
- 28.Wang Y, Rimm EB, Stampfer MJ, et al. . Comparison of abdominal adiposity and overall obesity in predicting risk of type 2 diabetes among men. Am J Clin Nutr 2005; 81: 555–563. doi: 10.1093/ajcn/81.3.555 [DOI] [PubMed] [Google Scholar]
- 29.Bulcun E, Ekici M, Ekici A. Disorders of glucose metabolism and insulin resistance in patients with obstructive sleep apnoea syndrome. Int J Clin Pract 2012; 66: 91–97. doi: 10.1111/j.1742-1241.2011.02795.x [DOI] [PubMed] [Google Scholar]
- 30.Gabryelska A, Szmyd B, Szemraj J, et al. . Patients with obstructive sleep apnea present with chronic upregulation of serum HIF-1α protein. J Clin Sleep Med 2020; 16: 1761–1768. doi: 10.5664/jcsm.8682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pamidi S, Wroblewski K, Stepien M, et al. . Eight hours of nightly continuous positive airway pressure treatment of obstructive sleep apnea improves glucose metabolism in patients with prediabetes. A randomized controlled trial. Am J Respir Crit Care Med 2015; 192: 96–105. doi: 10.1164/rccm.201408-1564OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martínez-Cerón E, Barquiel B, Bezos AM, et al. . Effect of continuous positive airway pressure on glycemic control in patients with obstructive sleep apnea and type 2 diabetes. A randomized clinical trial. Am J Respir Crit Care Med 2016; 194: 476–485. doi: 10.1164/rccm.201510-1942OC [DOI] [PubMed] [Google Scholar]
- 33.Loffler KA, Heeley E, Freed R, et al. . Continuous positive airway pressure treatment, glycemia, and diabetes risk in obstructive sleep apnea and comorbid cardiovascular disease. Diabetes Care 2020; 43: 1859–1867. doi: 10.2337/dc19-2006 [DOI] [PubMed] [Google Scholar]
- 34.Weinstock TG, Wang X, Rueschman M, et al. . A controlled trial of CPAP therapy on metabolic control in individuals with impaired glucose tolerance and sleep apnea. Sleep 2012; 35: 617–625. doi: 10.5665/sleep.1816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aronsohn RS, Whitmore H, Van Cauter E, et al. . Impact of untreated obstructive sleep apnea on glucose control in type 2 diabetes. Am J Respir Crit Care Med 2010; 181: 507–513. doi: 10.1164/rccm.200909-1423OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med 2002; 165: 1217–1239. doi: 10.1164/rccm.2109080 [DOI] [PubMed] [Google Scholar]
- 37.Harding JL, Wander PL, Zhang X, et al. . The incidence of adult-onset type 1 diabetes: a systematic review from 32 countries and regions. Diabetes Care 2022; 45: 994–1006. doi: 10.2337/dc21-1752 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary methods 00401-2022.SUPPLEMENT (102.2KB, pdf)
Supplementary tables 00401-2022.SUPPLEMENT2 (173.3KB, pdf)


