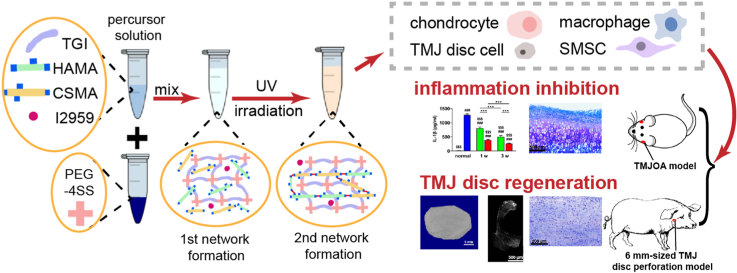
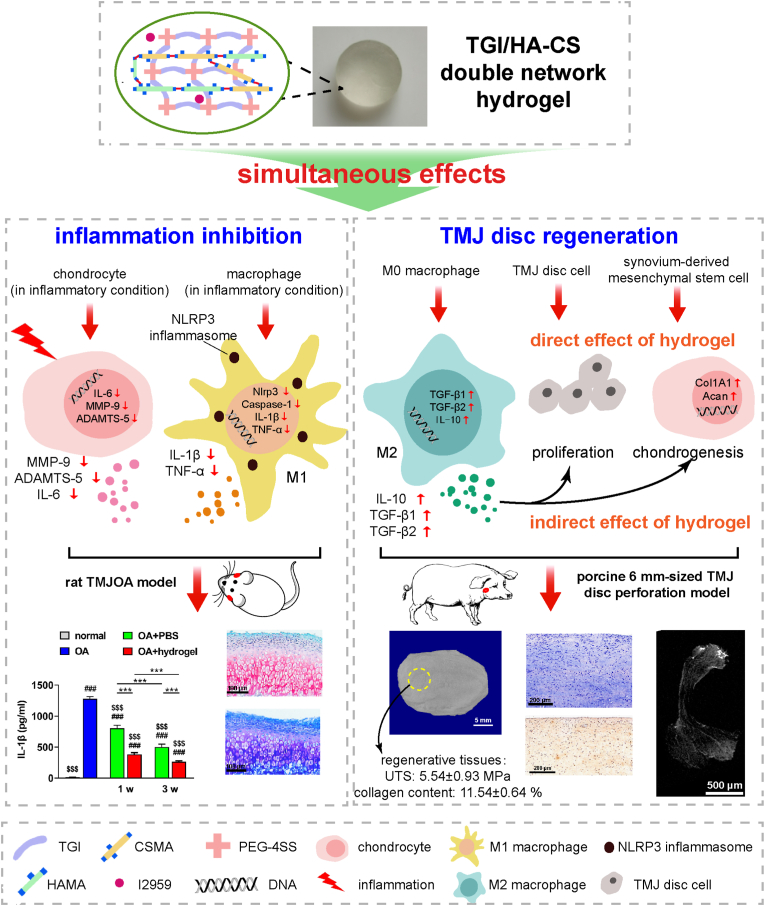
Abstract
Due to the lack of an ideal material for TMJ (temporomandibular joint) disc perforation and local inflammation interfering with tissue regeneration, a functional TGI/HA-CS (tilapia type I gelatin/hyaluronic acid-chondroitin sulfate) double network hydrogel was constructed in this paper. It was not only multiply bionic in its composition, structure and mechanical strength, but also endowed with the ability to immunomodulate microenvironment and simultaneously induce in situ repair of defected TMJ discs. On the one hand, it inhibited inflammatory effects of inflammasome in macrophages, reduced the extracellular matrix (ECM)-degrading enzymes secreted by chondrocytes, reversed the local inflammatory state, promoted the proliferation of TMJ disc cells and induced fibrochondrogenic differentiation of synovium-derived mesenchymal stem cells (SMSCs). On the other hand, it gave an impetus to repairing a relatively-large (6 mm-sized) defect in mini pigs’ TMJ discs in a rapid and high-quality manner, which suggested a promising clinical application.
Keywords: Bioinspired double network hydrogel, TMJ disc Perforation, Inflammasome, Immunomodulation, Induced regeneration
Graphical abstract
Highlights
-
•
The bionic and functional TGI/HA-CS double network hydrogel had advantages in physical, chemical and mechanical properties.
-
•
It inhibited inflammatory effects of inflammasome and reduced secreted ECM-degrading enzymes, thus reversing the local inflammatory state.
-
•
It promoted proliferation of TMJ disc cells and induced fibrochondrogenic differentiation of SMSCs via direct and indirect ways.
1. Introduction
Temporomandibular joint disc perforation, known as one of the severe complications of temporomandibular joint disorder (TMD), is often accompanied with inflammation in the joint cavity [[1], [2], [3], [4]]. At present, there is no biomaterial for defects larger than 1/3 of the whole disc size, which are clinically treated by autologous transplantation, but with undesirable prognosis such as dislocation of implanted tissues and infected donor site [5]. Although a study released in 2019 reported a scaffold-free implant obtained from in-vitro cultured porcine chondrocytes was able to repair a 3 mm-sized (which was relatively small) TMJ disc fenestration and the tensile fracture strength of neo-tissues reached 1.1 Mpa at 8 w postoperatively [2]. It was clear that regenerative tissue had insufficient mechanical strength and limited repairing area. Nor had it taken into consideration the influence on regeneration exerted by the local inflammatory microenvironment. Therefore, based on characteristics of clinical treatment for TMJ disc perforation, it is immensely necessary to develop a bioinspired and high-strengthed biomaterial that can actively reverse local inflammatory microenvironment as well as simultaneously induce TMJ disc in situ regeneration via its immunomodulatory effects. The material is not only critical to solve the bottleneck problem in repairing TMJ disc perforation, but also of vital clinical significance and practical value.
From the perspective of bio-mimetic design, physiologically the TMJ disc is formed by highly porous, hydrogel-like fibrocartilage composed chiefly of water (about 70%), type I collagen (about 25%), hyaluronic acid and chondroitin sulfate (approximately 1.5% in total) [6,7]. However, hydrogels composed mainly of collagen and hyaluronic acid, are usually not so strong, with a compressive strength of no more than 4 MPa [8,9], which is far from what TMJ disc needs to bear during mouth opening (7 MPa [10]). Consequently, when constructing a bionic TMJ disc repairing material, besides constituent factors, priorities should be given to the discrepancy between mechanical strength of hydrogels and clinical requirements. Since J. P. Gong pioneered the synthetic polymeric double network hydrogel composed of poly(acrylamide) and poly(2-acrylamido-2-methylpropanesulfonic acid) in 2003, mechanical strength was improved remarkably due to the introduction of the second network [11]. Double network hydrogels composed of natural polymers such as modified gutta and gelatin have been reported with compressive fracture strength up to 6.9 MPa [12], indicating that even natural polymers with relatively low strength when made into hydrogel will have a large increase in mechanical properties after proper modification and formation of a double network structure. It inspiringly provides theoretical basis for the design and construction of our conception of a bio-imitated, bio-originated and high-strengthed material for treating perforated TMJ discs.
The persistent inflammation in the joint cavity during TMJ disc perforation significantly affects regeneration of local tissues. That is because enzymes and pro-inflammatory cytokines in cartilage or joint fluid not only further accelerate ECM-degradation, but also induce assembly of inflammasome in macrophages, both of which continuously aggravate the microenvironment. In recent years, some oral drugs have been developed to inhibit the formation of inflammasome [13,14], but the local efficacy is extremely limited. Accordingly, if the biomaterials for TMJ disc perforation can simultaneously exert dual functions of improving or reversing the inflammatory microenvironment as well as actively inducing in situ tissue regeneration, it will be a strategy for treating disc perforation. Previous studies of our team found that hydrolyzed tilapia type I collagen inhibited TNF-α secreted by macrophages in an inflammatory state [15]. In vivo studies by Hu et al. confirmed oligopeptides from tilapia type I collagen were able to reduce oxidative stress, thus were endowed with antioxidative, anti-inflammatory and anti-apoptotic abilities [16]. These studies suggested components of tilapia type I collagen not only had anti-inflammatory effects, but also could reduce side effects associated with drugs.
In summary, based on the conception of bio-mimicry, this research attempted to construct a bionic double network hydrogel composed mainly of tilapia type I gelatin, hyaluronic acid and chondroitin sulfate. Operational window time and enhancement of mechanical strength were attained through double curing (chemical-light curing). Furthermore, PCR, ELISA, WB, immunofluorescence and some other methods were employed to reveal the pleiotropic effects of the hydrogel in attenuating pro-inflammatory actions of inflammasome, down-regulating the release of ECM-degrading enzymes as well as direct and indirect induction in disc regeneration. Finally, in vitro results about anti-inflammation and pro-reparation of the hydrogel were verified on a rat temporomandibular osteoarthritis (TMJOA) model and a porcine TMJ disc perforation model, respectively.
2. Materials and methods
2.1. Preparation of HAMA and CSMA
Methacrylic anhydride-modified hyaluronic acid (HAMA) and methacrylic anhydride-modified chondroitin sulfate (CSMA) were prepared in reference to our previous work with minor modifications [17]. Briefly, 1% hyaluronic acid (90–100 kDa, Shanghai Yuanye Bio-Technology Co., Ltd., China) or chondroitin sulfate (50–60 kDa, Sigma, USA) solution was prepared. 5 M NAOH and methacrylic anhydride (Sigma, USA) were then added. The mixture was stirred at 0–4 °C for one day, centrifuged to remove solid impurities, dialyzed (MWCO = 5 kDa) for 2 days and obtained after lyophilization for 2 days. Formula for the synthesis of HAMA and CSMA were described in supporting information Fig. S1 and the substitute rate of MA-was measured with 1H-NMR (Bruker, USA).
2.2. Preparation of TGI/HA-CS double network hydrogel
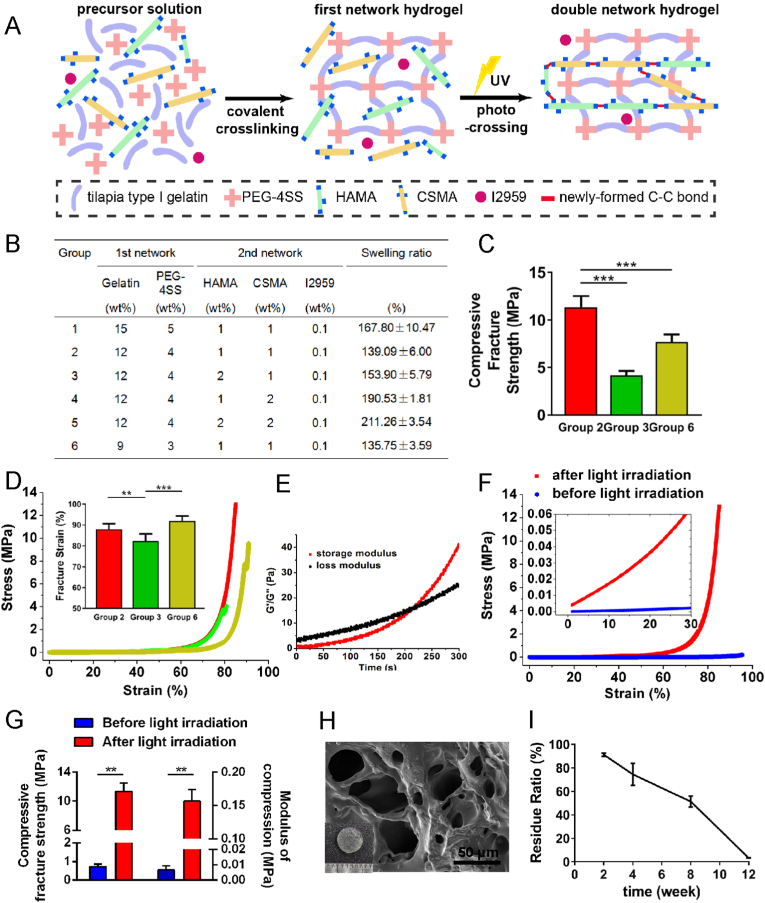
HAMA, CSMA and tilapia type I gelatin (a kind gift from Shanghai Fisheries Research Institute) was dissolved in deionized water. Irgacure 2959 (2-hydroxy-1-[4-(2-hydroxyethoxy) phenyl]-2-methyl-1-propanone, I2959, Sigma, USA) was also added as photoinitiator. pH of this precursor solution was adjusted to around 7.4. PEG-4SS (4arm-polyethylene glycol-succinimidyl succinate, MW = 10 kDa, XIAMEN SINOPEG BIOTECH CO., LTD., China) was also dissolved. After a mixture of precursor solution and PEG-4SS solution, the first network of TGI/HA-CS double network hydrogel was constructed through amide bond condensation between gelatin and PEG-4SS. The second network of the hydrogel was attained by exposing it to 365 nm-ultraviolet (UV, 20 mW/cm2) light for 3 min, photoinitiating crosslinking between HAMA and CSMA (reaction mechanism was shown in Fig. 1A and chemical formula were in supporting information Fig. S2).
Fig. 1.
Gelling mechanisms and characteristics of TGI/HA-CS double network hydrogel.
(A) Gelling mechanisms, (B) swelling ratio, (C) compressive fracture strength, (D) compressive stress-strain curves (the inserted picture represented fracture strain) of different groups. (E) Time-dependent rheological behaviour, (F) compressive stress-strain curves, (G) compressive fracture strength and modulus before or after irradiation, (H) SEM and (I) residue ratio of Group 2. **p < 0.01, ***p < 0.001, bar = 50 μm.
2.3. Characterization of hydrogel
2.3.1. Swelling and degradation in vitro
Swelling ratios and degradation of fabricated hydrogels were assessed in a gravimetric method. TGI/HA-CS double network hydrogel samples (in cylindrical shapes, d = 10 mm, h = 5 mm) were prepared, weighed (M0) and then immersed in PBS (4 mL, pH 7.4) at 37 °C. At predetermined time points (24 h to reach swelling equilibrium for evaluating swelling ratios while 2, 4, 8, 12 w for residue ratios), samples were carefully blotted with filter paper to remove buffer on the surface and weighed again (M1). The swelling and residue ratios were calculated as follows (n = 5):
| Swelling ratio (%)=(M1-M0)/M0 × 100% |
| Residue ratio (%) = (M1)/M0 × 100% |
The concentration of CS and free amino acids in the extraction 1 d and 2 w after immersion were measuerd with HPLC-DAD (high performance liquid chromatography-diode array detector, Agilent, USA) and Amino Acid Analyser (HITACHI, Japan).
2.3.2. Mechanical properties of TGI/HA-CS double network hydrogel
Mechanical properties of TGI/HA-CS DN hydrogel were gauged with a universal material testing machine (HY-1080, Shangai Hengyi testing instruments Co., Ltd., China). Briefly, hydrogel samples, in cylindrical shapes (d = 10 mm, h = 5 mm), were compressed at a speed of 1 mm/min. The compressive fracture strength was recorded and modulus referred to the average slope of the linear area (elastic range, ε = 5–20%) in stress-strain curves. Each test has 5 parallel samples, and data were shown as mean ± standard deviation (SD). Cyclic compressive loading-unloading experiments for five cycles with a maximum strain of 60% were also conducted at 1 mm/min.
2.3.3. Rheometry
The gelling process of our hydrogel was monitored on a modular conpact rheometer (MCR302, Anton Paar, Austria). The diameter of parallel plates was 25 mm and the gap between was set at 1 mm. Hydrogels for rheological experiments were quickly injected onto the bottom plate. Time-sweep tests were conducted at 1 Hz frequency and 5% strain until gelation process was completed. The gelation point was defined as the time point when storage modulus (G′) surpassed loss modulus (G″). Strain-sweep tests were performed after gelatin, at 1 Hz and from 0.1% to 100% strain.
2.3.4. SEM and contact angle
Hydrogel samples were lyophilized, sputter-coated with gold prior to SEM observation (Mira3, Tescan, Czech Republic). In addition, contact angle was measured with SL250 (Kino, USA).
2.4. In vitro studies
2.4.1. Cell culture and treatment
Macrophages were generated from 6-week-old male C57BL/6j mice. Bone marrow (BM) was flushed and since then, red blood cells were lysed. Whole BM cells were cultured on plates in DMEM complete medium containing 10% fetal bovine serum (Capricorn Scientific GmbH, Germany), penicillin and streptomycin (Hyclone, USA) at 37 °C with 5% CO2. 40 ng/mL of murine M-CSF (Peprotech, USA) were supplemented. Nonadherent cells were removed on the third day of culture, followed by aspirating of the medium every two days. On day 8, resting macrophages (M0) were harvested by trypsin digestion. For inflammasome activation, M0 were cultured on 24-well plates, treated with 500 ng/ml LPS (Solarbio, China) for 4 h and 20 μM Nigericin (MCE, China) for 30 min. Then cells were planted on the surface of different volume of hydrogels which had been previously coated on the plates (cells planted without hydrogels were employed as positive controls, while cells not stimulated with LPS and Nigericin as negative controls). 24 h later, macrophages in various groups were harvested and supernatant was collected for cytokine analysis. Additionally, in order to prove the anti-inflammation of CS, macrophage, equally stimulated with LPS and Nigericin, were cultured with 200 μg/mL CS for 24 h.
For coculturing experiments, macrophages were planted on plates with or without hydrogels for 24 h, which were deemed as hydrogel + macrophage group or macrophage group (cells on blank plates were referred to as the negative control, while only hydrogel in the equal amount of medium as hydrogel group). Supernatant was also collected and centrifuged for 5 min to obtain conditioned mediums (CMs).
Chondrocytes were separated from condyle cartilages using type I collagenase and then cultured in 1640 with 10% FBS, penicillin and streptomycin. Passages 3–5 were used for subsequent experiments. 10 ng/mL IL-1β (Peprotech, USA) was employed to create inflammatory environment. Then these chondrocytes were planted on hydrogels (hydrogel group) or blank plates (positive group). Additionally, the negative group referred to cells that had not been cultured with IL-1β.
TMJ disc cells were obtained from TMJ discs in a digestion method as well and cultured in the same medium as chondrocytes.
Synovium-derived mesenchymal stem cells (SMSCs) were harvested from synovium of mouse TMJ and cultured in DMEM with 10% FBS, penicillin and streptomycin. After characterization, the stem cells were diluted (1:3) when reached 90% confluence.
2.4.2. Cell flow cytometry and immunofluorescence assay
Macrophages were washed with cold PBS and then stained with PE-labeled anti-CD86 (BD, USA) or AF488-labeled anti-CD206 (eBionscience, USA) in the dark at 4 °C (CD206 needed a kit (BD, USA) for prefixation and permeabilization first), then analyzed with a flow cytometer (FACSCelesta, BD, USA).
For immunofluorescence assays, cells were fixed, permeabilized and blocked at the very beginning. Inflammasome activated macrophages were then incubated with FITC-labeled ASC antibody (Novus, USA) overnight. Chondrcytes were incubated with specific primary antibodies against MMP-9 (abcam, UK) or ADAMTS-5 (Novus, USA) and Cy3-conjugated secondary antibodies (Beyotime, China). DAPI (Beyotime, China) was employed to stain nucleus. Fluorophore-conjugated antibody (eBionscience, USA) was also used to detect expression of CD206 in macrophages. Images was taken with Multi-Mode Cell Imaging Reader (Cytation 3, BioTek Instruments, USA).
2.4.3. Quantitative real-time PCR (qRT-PCR) analysis
Extraction and reverse-transcription of mRNA were achieved with RNeasy Mini kit (Qiagen, USA) and PrimeScript RT reagent Kit (Takara, Japan) following protocols provided by manufacturers. qRT-PCR was conducted with the help of SYBR Premix EX Taq (TaKaRa, Japan). The expression of detected genes was calculated in the 2−ΔΔCT method and normalized against housekeeping gene. Specific primer sequences of the genes were listed in supporting information Table S1.
2.4.4. Western blot
Cellular proteins were extracted with RIPA (Epizyme, China) supplemented with protease inhibitor cocktail (Abmole, China). Resolved by electrophoresis, proteins were then transferred and blocked. Membranes were then incubated with primary antibodies against nlrp3, pro-caspase-1 and β-actin (1:1000, Abcam, UK) at 4 °C overnight. Next, secondary antibody (1:5000, CST, USA) was incubated for 1 h at room temperature. Finally, detected with electrochemiluminescence (ECL) reagents (Millipore, USA), the target protein bands were photographed with FlourChem E (Proteinsimple, USA).
2.4.5. Enzyme-linked immunosorbent assay (ELISA)
ELISA was employed to detect cytokines in the cell-free supernatant or tissue homogenate, including: ① proinflammatory factors such as IL-1β, TNF-ɑ (MultiSciences, China), and IL-6 (RD, USA); ② pro-regenerative ones such as IL-10 (RD, USA), TGF-β1 and TGF-β2 (MultiSciences, China); ③ enzymes related to degradation of extracellular matrix (ECM) such as MMP-9 (RD, USA) and ADAMTS-5 (Abbexa, USA).
2.4.6. Alcian blue staining
Murine SMSCs, having been cultured in conditioned mediums for 1 or 2 w, were fixed with paraformaldehyde and then stained with Alcian blue (Solarbio, China) in order to observe glycosaminoglycan (GAG).
2.5. In vivo studies
2.5.1. Animals care
6-8 w female Sprague Dawley rat and male mini pigs (skeletally mature, 18–22 kg) were purchased from SLACCAS Laboratory Animal Co, Ltd. (Shanghai, China). Animal procedures had all received approval from the Independent Ethics Committee of Shanghai Ninth Peopleʹs Hospital, Shanghai Jiao Tong University School of Medicine (SH9H-2020-A297-1). Researches were conducted under the instructions of Division of Laboratory Animal Medicine guidelines.
2.5.2. Animal experiments
For rats, TMJOA was induced by monosodium iodoacetate (MIA, Sigma, USA) injection, the most commonly-used chemical compound to induce OA-like lesions in joints. In this model, 50 μL PBS containing 0.5 mg MIA was injected into the upper compartment of both temporomandibular joints. 2 weeks after OA induction (when we defined as day 0), rats were given weekly intra-articular injections of 50 μL hydrogel (in the left side, as the hydrogel-treated group, with no UV-irradiation due to the narrow space and blocking of soft tissues) or PBS (in the right side, as the untreated group) for 1 or 3 weeks (n = 5 for each time point). Five intact animals served as the normal group.
For mini pigs, perforated TMJ discs were achieved by making 6 mm-sized fenestration in the lateral part of the disc and on both sides of TMJ (n = 3). Hydrogels were injected into the left side (hydrogel-treated group) while the right side remained untreated (untreated group). The synovium, masseter and skins were sutured layer by layer. One pig, with only surgically perforation-making (recorded as model group, with neither regeneration nor inflammation), was sacrificed immediately after surgery in order to evaluate the original size of perforation in MRI and CT analyses. At 2 and 8 weeks post-treatment, animals were euthanized with bilateral TMJs removed surgically. Normal tissue from the lateral part of discs (the same position as the surgical modeling) from another intact pig was also harvested for histopathological and immunohistochemical analyses (normal group).
2.5.3. Preparation of homogenates
Soft tissues from rats in different groups, including the joint capsule, synovial membrane, retrodiscal tissue, TMJ disc, and maybe a small part of pterygoid muscle, were stored at −80 °C or excised and weighted on ice. Homogenates were prepared in PBS added with protease inhibitor cocktail with a homogenizer. After being centrifuged at 15000 rpm at 0 °C, supernatants were assayed for IL-1β and TNF-ɑ (Multi Sciences, China). Total protein content from various groups were quantified with the BCA protein assay (Thermo, USA) so that factor concentrations could be normalized to total protein.
2.5.4. Biochemical and biomechanical testing
Surgically removed TMJ from pigs were injected with 200 μL saline into the lower compartment. After mild shaking, liquid was extract out for hydroxyproline analysis (Solarbio, China).
In order to evaluate the quality of regeneration at 8 w, dumbbell-shaped samples (which were parallel to anteroposterior axis of the disc and with the newly-formed tissues approximately in the middle) were trimmed from TMJ discs. Normal controls were obtained from the symmetrical site of the perforation, in the medial part of the disc. The ultimate tensile strength (UTS) was tested with the universal material testing machine.
Repair tissues for collagen content measurement was first weighed, cut into small pieces, lyophilized for 48 h and then digested in acetic acid and papain (MCE, China) at 4 °C. Collagen content was determined with the Sircol collagen assay (S1000, Biocolor, UK).
2.5.5. Computed tomography (CT) and magnetic resonance imaging (MRI) evaluation
At pre-determined time intervals, dissected TMJ discs from mini pigs were fixed and scanned by a high resolution CT (Skyscan 1076, Bruker, USA) or MRI (BioSpec70/20 USR, Bruker, USA). CT data were analyzed with CT analyser, DataViewer, and CTvox.
2.5.6. Histopathological and immunohistochemical analyses
For histopathological and immunohistochemical observations, samples were decalcified, embedded, sectioned and stained with hematoxylin and eosin (H&E) for general morphology, as well as Toluidine and Safranin O-Fast Green (Solarbio, China) for ECM (glycosaminoglycan and collagen, respectively). Immunohistochemistry was performed to detect ADAMTS-5 (NOVUS, USA). CD163 (NOVUS, USA) was also analyzed as a biomarker for M2 in TMJ discs of mini pigs.
2.6. Statistical analysis
Statistics in this study were analyzed with SPSS 20.0 software (Chicago, USA) and results were expressed in the form of mean ± standard deviation (SD). One-way analysis of variance (ANOVA) was employed to identify significant differences between various groups. Statistical significance was set as p < 0.05.
3. Results and discussion
Local inflammation interfering with tissue regeneration during TMJ disc perforation is an urgent clinical problem. In this paper, we developed a new multifunctional hydrogel and found it not only inhibited inflammasome-ralated genes and proteins in macrophages, but also reduced ECM-degrading enzymes secreted by chondrocytes that could cause cartilage loss. In addition, direct and indirect (through inducing polarization of macrophages from M0 to M2) promotion of TMJ disc cells' proliferation and SMSCs’ chondrogenic differentiation were also observed. Promisingly, the pleiotropic immunomodulatory properties of the hydrogel were demonstrated in animal models. This study proposed a bionic, high-strengthed, multifunctional, light-curing, double-network and tilapia gelatin-based hydrogel with immunomodulatory functions and in situ pro-regeneration effects on TMJ discs. It is of great importance to clinical treatment for TMJ disc perforation.
3.1. Bio-mimetic component design and characteristics of TGI/HA-CS double network hydrogel
3.1.1. Bio-mimetic component design of TGI/HA-CS double network hydrogel
Bearing the conception of bio-mimicry in mind, hydrogels, with type I collagen as the main component and a small amount of HA and CS, were first considered to simulate the composition and structure of TMJ discs. To circumvent zoonoses, religious conflicts, and potential risks of immunogenicity that might be brought about by collagens from terrestrial mammals (e.g., pigs, cattle, etc.) [18], we chose tilapia type I collagens with superiorly-low immunogenicity [19]. And taking the more cross-linking sites and fewer structural restrictions into consideration, tilapia type I gelatin, which was consistent with tilapia type I collagen in composition, was selected as raw materials for our hydrogel. To solve the problem of insufficient mechanical strength, we designed a double network hydrogel that could cure at room temperature (see Fig. 1A and supporting information Fig. S2 for gelling mechanisms). TGI and PEG-4SS first underwent amide condensation reaction to form the first network, which required neither specific temperature or catalyst, nor initiator. Subsequently, HAMA (77.57% substitute rate, supporting information Fig. S3A) and CSMA(49.36% substitute rate, supporting information Fig. S3B) were crosslinked for the second network under the influence of a safe, non-toxic and biocompatible initiator (I2959) and the clinically-common 365 nm UV light. In 2021 Ren et al. proposed a double network hydrogel with maleamide-modified HA and methacrylic anhydride-modified gelatin as its main compositions [20]. However, most of the reaction had to be kept in a constant temperature of 37 °C (apparently its clinical suitability was not so good) and employed dithiothreitol (DTT), which had potential damage to DNA backbone [21], as its catalyst. Therefore, compared with Ren's work, the hydrogel proposed in this study not only had a composition mimicking the TMJ disc itself, but also had the advantage of biosafety, simplicity, and clinically suitability.
3.1.2. Swelling characteristics of TGI/HA-CS double network hydrogel
One of the major characteristics of hydrogels is water absorption and swelling to a larger volume. The hydrogel for repairing TMJ disc perforation is anticipated to keep its physical shape and volume relatively stable in limited space of the joint cavity, so as to avoid adverse effects on tissue repair exerted by dislocation or rupture. As a result, we first focused on the proportion of hydrogel components in order to evaluate and select the one with suitable swelling property for clinical applications. Data in Fig. 1B showed Group 1, 4 and 5 had higher swelling rates than the other groups, which might be attributed to higher mass percentage of polymers (especially HAMA and CSMA). It is ubiquitously known that water absorption of hydrogels is positively correlated to mass fraction of polymers (e.g., Group 1). Additionally, Gao et al. suggested that HA with excellent water absorption can greatly increase the swelling rate of hydrogels [22](e.g., Group 5 is higher than Group 4). Besides, the higher CS content also led to an increase in the number of ionized groups (carboxylates and amides) and repulsive forces between them, which resulted in an increase in swelling rate [23] (e.g., Group 4 and Group 5, both had relatively higher swelling ratio). In summary, as suggested by the swelling test results mentioned above, volumetric stability of hydrogels in Group 2, 3 and 6 are preferable. Thus they could be considered for subsequent mechanical properties tests.
3.1.3. Mechanical properties of TGI/HA-CS double network hydrogel
Since TMJ discs play a role as “cushions” between the temporal glenoid fossa and mandibular condyle to absorb force, reduce impact and protect the skull and brain, requirements for compressive capacity are put forward for disc repair materials. Li et al. concluded through finite element analysis that the TMJ disc needs to endure up to 7 MPa during physical mouth-opening [10]. Therefore, we were pleased to see among the three groups of hydrogels, Group 2 exhibited the compressive fracture strength of 11.34 ± 1.18 MPa, which was significantly higher than Groups 3 and 6 (Fig. 1C–D). It was probably because the total mass fraction of both TGI and PEG-4SS in Group 6 was less than that of Group 2. And higher HAMA to lower compressive fracture strength. Although the rigid second network could enhance strength of the material, it also increased brittleness, giving rise to an earlier fracture point (and maybe lower compression fracture strength). This phenomenon was consistent with that one reported by Hyeongho Shin in 2012 [12]. The composition ratio of Group 2 in this study (1% for both HAMA and CSMA) could achieve a balance of robustness and brittleness, hence showing best mechanical properties. In the cyclic compressive loading-unloading tests (supporting information Fig. S4), it can be clearly seen that from 1st cycle to 5th cycle, the stress-strain curve almost overlapped with each other (except for a slight stress decrease). It showed TGI/HA-CS double network hydrogel not only had a stable network structure, but also recovered its original shape when the pressure disappeared, showing excellent fatigue resistance.
3.1.4. Application performance of TGI/HA-CS double network hydrogel
Based on above-mentioned findings, we selected Group 2 for TMJ disc repair. By adjusting pH of the hydrogel precursor solution, approximately 3–5 min time window was obtained (Fig. 1E), during which the first network formed and surgeons could shape it to fill it tightly into irregular and scattered TMJ disc perforations. A strain-sweep showed both storage modulus (G′) and loss modulus (G″) remained almost unchanged as strain increased from 0.1% to 100% (supporting information Fig. S5), indicating TGI/HA-CS double network hydrogel could assume large deformation after the first network formed and was a desirably elastic material. Then, after the hydrogel was filled, the carbon-carbon double bond addition between HAMA and CSMA was triggered by UV light and I2959 to form the second network. By analyzing mechanical properties of TGI/HA-CS double network hydrogel before and after UV irradiation, it was found compressive modulus increased 22 times and compressive fracture strength increased 15 times after exposure (Fig. 1F–G). The good compressive properties were achieved mainly thanks to the two interpenetrating networks in the hydrogel. The rigid network of HAMA and CSMA protected the flexible network formed by TGI and PEG-4SS, allowing stress to be dispersed uniformly and effectively and thus preventing the expansion of cracks inside, indicating the double network structure was essential for enhancing mechanical properties of hydrogels [24,25]. In addition, from Fig. 1G we know that the compression modulus of TGI/HA-CS double network hydrogel was 0.16 ± 0.02 MPa. Though it was lower than 1.79 ± 0.34 MPa of autologous tissue [26], it was superior to the hydrogels formed by natural polymers such as gelatin, HA and CS reported in recent years, the elastic modulus of which was basically in the range of several to tens of kPa [27,28].
3.1.5. Surface morphology, hydrophilicity and degradation of TGI/HA-CS double network hydrogel
Next, in order to characterize the properties related to cell growth and tissue regeneration, we investigated the microstructure, contact angle, and degradation behavior of TGI/HA-CS double network hydrogel. The result of scanning electron microscopy (SEM) was exhibited in Fig. 1H. The high water content and three-dimensional scaffold was conducive to cell crawling, nutrient transport, and metabolic waste discharge. Contact angle of 38.03 ± 3.61° (supporting information Fig. S6) suggested good hydrophilicity and helped with cell adhesion. Moreover, degradation tests revealed (Fig. 1I) residue mass of the hydrogel at 4 w, 8 w and 12 w was 74.52%, 51.27% and 3.26%, respectively. It might be attributed to the fact that compared with small molecule cross-linkers, PEG-4SS, as a polymer cross-linker with multiple gelling sites and relatively high molecular weight, brought an evident increase in the length and molecular weight of cross-linked chains [29], thereby slowing down degradation and making it match the rate of disc regeneration. As a result, maintenance of hydrogel's scaffold structure for longer time provided necessary physical support for cell crawling and growing. These findings demonstrated the biomolecule-based double network hydrogel developed in this paper had obvious advantages in swelling, mechanical properties, microstructure, hydrophilicity, degradation, and clinical suitability.
3.2. Inhibitory effect of TGI/HA-CS double network hydrogel on local inflammation in the joint cavity
TMJ disc perforation is usually accompanied by self-amplified aseptic inflammation in the joint cavity, at which time chondrocytes are stimulated and pro-inflammatory cytokines are secreted. Therefore, inflammasome forms in macrophages, then inflammatory cytokines such as IL-1β are released in large quantities, further inducing chondrocytes to produce matrix metalloproteinases (MMP), a disintegrin and metalloprotease with thrombospond (ADAMTS) and IL-6 [30,31]. Accordingly, in order to successfully repair TMJ discs, it is of necessity to consider alleviating or controlling the local inflammatory state at the same time. Previous studies of our group found that hydrolysis of tilapia type I collagen could release glycine, reduce intracellular calcium ion levels in macrophages under inflammatory conditions and acted as an anti-inflammatory agent [15]. Additionally, in vivo studies by Hu et al. confirmed that oligopeptides from tilapia type I collagen was anti-inflammatory and anti-apoptotic by reducing oxidative stress levels [16]. Nevertheless, whether the TGI-based hydrogel constructed in this paper could also reverse local inflammatory microenvironment still needed to be fully validated, especially in aspects of its immunomodulation to inflammasome in macrophages, as well as its reversal of ECM loss caused by chondrocytes.
3.2.1. Inhibitory effect of TGI/HA-CS double network hydrogel on inflammasome's pro-inflammatory function and its immunomodulation to local microenvironment
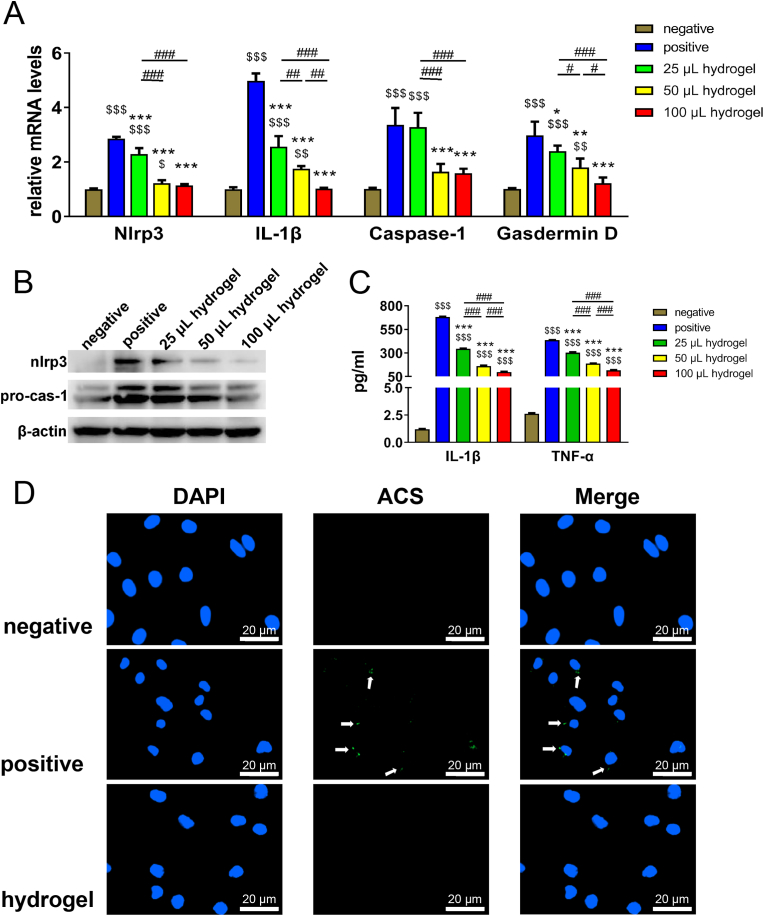
It is well known to all that resting macrophages (M0), when stimulated by inflammation, first assemble inflammasome intracellularly, resulting in polarization towards the pro-inflammatory type (M1). NLRP3 (NLR family, pyrin domain containing 3) complex is the most representative inflammasome. Moreover, it has been documented that when osteoarthritis emerges, M0 shows high expression of NLRP3 complexes, eventually leading to pyroptosis and triggering an intense local inflammatory response [32]. Therefore, if some biomaterial can simultaneously inhibit the pro-inflammatory capacity of NLRP3 complexes when repairing tissue defects, it will not only limit deterioration and spread of existing inflammation from the very beginning, but also reverse the local inflammatory microenvironment, thus showing great importance to tissue regeneration. However, unfortunately, there are few studies in this area. In view of this point, our research firstly investigated the inhibitory effect of TGI/HA-CS double network hydrogel on NLRP3 complexes. Surprisingly, we found it significantly down-regulated the expression and secretion levels of inflammasome-related genes (NLRP3, IL-1β, caspase-1, Gasdermin D), proteins (NLRP3, pro-caspase-1) and cytokines (IL-1β, TNF-ɑ) (Fig. 2A–C). Moveover, the degree of reduction was positively correlated with the hydrogel volume. Results of immunofluorescence also supported this conclusion (Fig. 2D). To probe possible causes of these effects, we further investigated the release properties. Data suggested the hydrogel extract contains a variety of amino acids (such as glycine) and CS after immersing for 1 day (supporting information Table S2). According to our previous studies and Hartog et al., hydrolyzed collagen could inhibit immune responses by activating glycine receptors for inflammation-controlling purposes [15,33]. Additionally, CS also has anti-inflammatory functions (supporting information Fig. S7 and reference [34]). Therefore, it could be hypothesized the anti-inflammatory ability of TGI/HA-CS double network hydrogel was related to glycine and CS in its composition. Results of this study showed TGI/HA-CS double network hydrogel inhibited NLRP3 inflammasome assembly and reduced caspase-1 levels, hence cutting down IL-1β secretion. It exerted a unique immunomodulational effect on the local microenvironment so as to lighten the inflammatory state of the joint cavity.
Fig. 2.
Inhibitory effects of TGI/HA-CS double network hydrogel on inflammasome.
(A) Expression of inflammasome-related genes and (B) proteins in macrophages cocultured with different volume of hydrogels. (C) Concentration of pro-inflammatory cytokines (IL-1β and TNF-α) released by macrophages. (D) Immunofluorescence of ACS (one of the main parts of NLRP3 inflammasome, and was pointed out with white arrows, bar = 20 μm). $p < 0.05, $$p < 0.01, $$$p < 0.001 compared with the negative group. *p < 0.05, **p < 0.01, ***p < 0.001 compared with the positive group. #p < 0.05, ##p < 0.01, ###p < 0.001.
3.2.2. Modulational function of TGI/HA-CS double network hydrogel on ECM degradation caused by chondrocytes
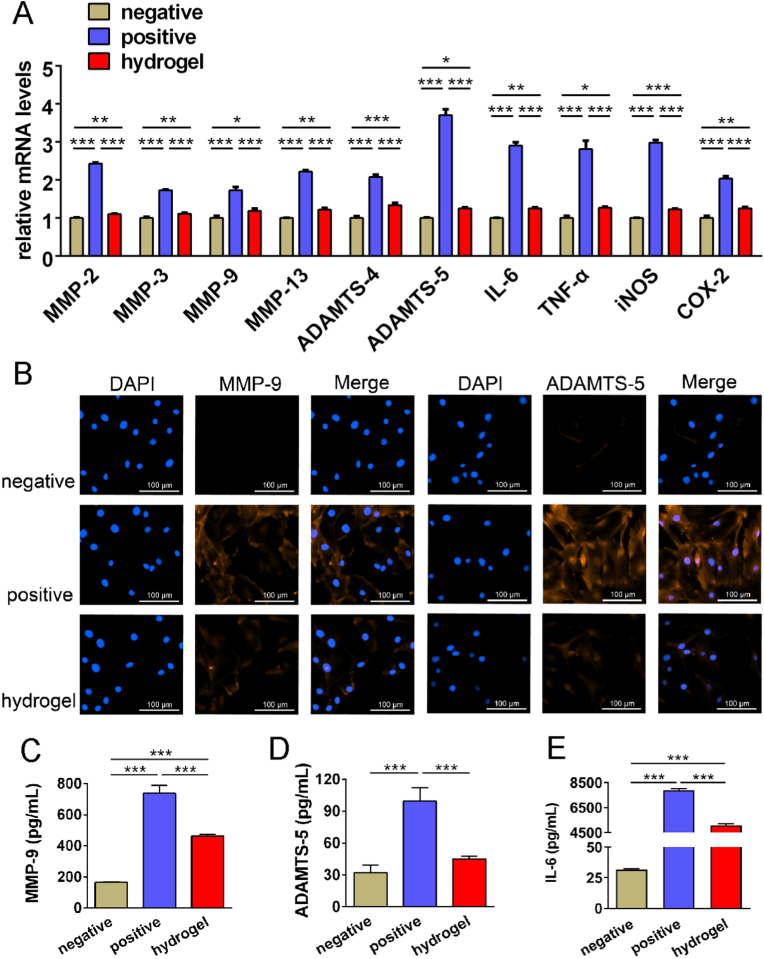
As mentioned above, the large amount of MMP and ADAMTS released by chondrocytes during disc perforation leads to ECM degradation and cartilage damage. Among them, MMP-9 degrades not only collagen, the main component of the ECM, but also proteoglycan. So MMP-9 is an important biomarker in the pathological process of OA [35]. Furthermore, ADAMTS-5 is a major proteoglycan-degrading enzyme in humans [36]. They both play a pivotal role in the development of cartilage inflammation. It was found in our research that TGI/HA-CS double network hydrogel significantly inhibited the expression of several genes in MMP and ADAMTS families in chondrocytes (Fig. 3A), with MMP-9 and ADAMTS-5 in particular (Fig. 3B–D). Furthermore, TGI/HA-CS double network hydrogel also down-regulated the expression of inflammatory factors (IL-6 and TNF-ɑ) and inflammation-related enzymes (iNOS and COX-2) at the same time (Fig. 3A and E). All these were vital for reversing the local inflammatory state and attenuating M1-polarization of macrophages. In 2021 Huang Y et al. reported a scaffold that could resist inflammation by down-regulating gene expression of IL-6 and TNF-ɑ [37]. And once iNOS and COX-2 were declined, the pathological development of OA would be suppressed [38]. As a result, it could be concluded that TGI/HA-CS double network hydrogel inhibited both inflammatory factors (IL-1β and TNF-ɑ) and imflammation-concerned enzymes (iNOS and COX-2) released by macrophages and chondrocytes, reduced the secretion of cartilage ECM-degrading enzymes (MMP-9 and ADAMTS-5) as well, ultimately preventing the loss of ECM and providing a favorable immune microenvironment for in situ tissue regeneration.
Fig. 3.
Inhibitory effects of TGI/HA-CS double network hydrogel on ECM degradation.
(A) Gene expression of ECM-degrading enzymes in MMP or ADAMTS family, inflammatory factors (IL-1β and TNF-ɑ) and inflammation-related enzymes (iNOS and COX-2) in chondrocytes under inflammatory conditions. (B) Immunofluorescence of MMP-9 and ADAMTS-5, bar = 100 μm. Quntification of (C) MMP-9, (D) ADAMTS-5 and (E) IL-6. *p < 0.05, **p < 0.01, ***p < 0.001.
3.2.3. Inhibitory effects of TGI/HA-CS double network hydrogel on rat TMJOA models
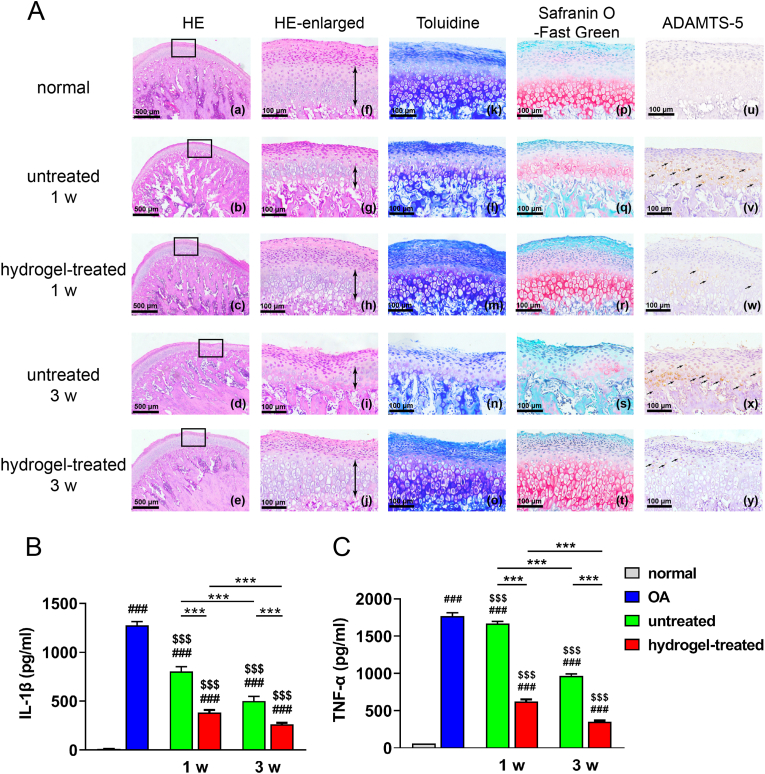
To fully validate the results obtained from in vitro studies, we erected a rat TMJOA model and found at 1 w and 3 w after injection that the TGI/HA-CS double network hydrogel significantly promoted repair of inflammation-induced ECM loss over time, as evidenced by increased continuity of condyle surface (Fig. 4A (a-e), which represented the whole defects), increased condylar cartilage thickness (especially thickness of mature layer + hypertrophic layer, marked by the double-headed arrow in Fig. 4A (f-j)), collagen and proteoglycan levels (Fig. 4A (k-o) and (p-t) respectively), and a gradual normalization in the number of ADAMTS-5+ chondrocytes (Fig. 4A (u-y)). In contrast, the untreated group exhibited exacerbating inflammation, with massive ECM-degradation and dense array of ADAMTS-5+ chondrocytes at 3 w, indicating more severe damage to condylar cartilage. Our results were consistent with the ones reported by Shipin Zhang et al., who used macrophage exosomes to treat TMJOA in 2019 [39]. After quantifying pro-inflammatory factors (IL-1β and TNF-ɑ) in local tissues of the joint (Fig. 4B–C), it was discovered the concentration of hydrogel-treated group was significantly lower at both two time points, fully validating the effect of TGI/HA-CS double network hydrogel in suppressing local inflammation.
Fig. 4.
Inhibitory effects of TGI/HA-CS double network hydrogel on rats.
(A) HE ((a-e) represented the whole defects while (f-j) represented the local enlarged ones and the thickness of mature layer + hypertrophic layer was marked by the double-headed arrow), (k–o) Toluidine, (p–t) Safranin O-Fast Green and (u–y) immunohistochemical staining of ADAMTS-5 (bar = 500 or 100 μm). Quntification of (B) IL-1β and (C) TNF-α in joint tissues. ###p < 0.001 compared with the negative group. $$$p < 0.001 compared with the positive group. ***p < 0.001.
3.3. Effects of TGI/HA-CS double network hydrogel on regeneration of perforated TMJ disc
The series of in vivo and ex vivo findings demonstrated immunomodulatory effects of TGI/HA-CS double network hydrogel on local inflammatory environment, which contributed a lot to creating favorable microenvironment for in situ regeneration. Next, we focused on the topic of TMJ disc perforation repairing. On the one hand, immunomodulatory effects of hydrogel on macrophages' M2-polarization was investigated. On the other hand, we revealed the promotion effect of TGI/HA-CS double network hydrogel on chondrocytes' proliferation and SMSCs’ differentiation through direct and indirect pathways. Finally, the in vivo regenerative effect was verified on a 6 mm-sized porcine TMJ disc perforation model.
3.3.1. Induction of TGI/HA-CS double network hydrogel on M2-polarization
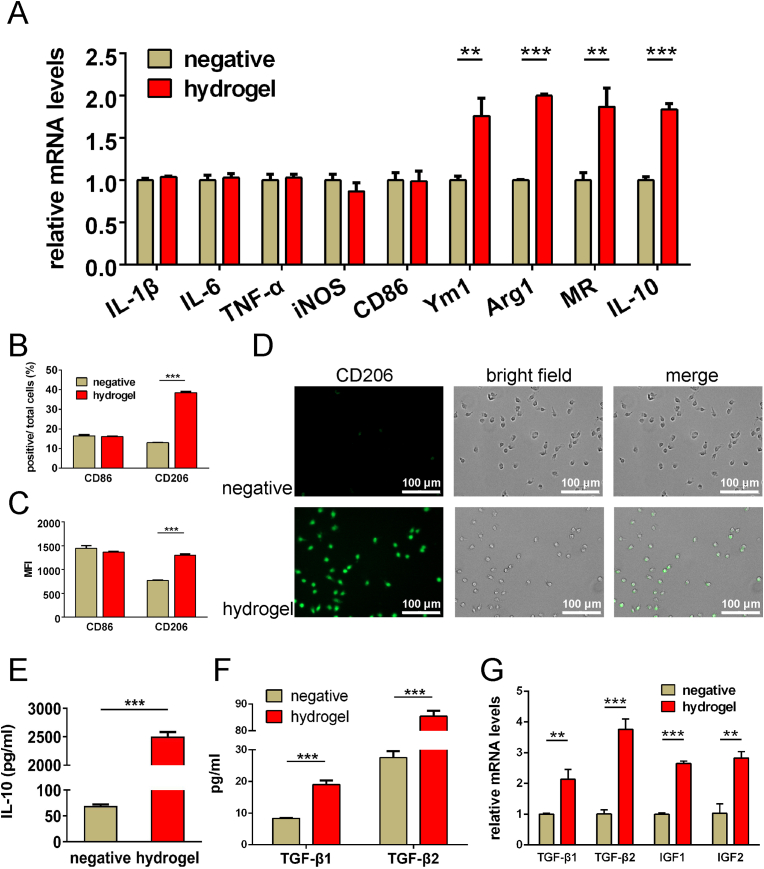
Generally speaking, when stimulated by materials, M0 macrophages in local tissues show M1-(pro-inflammatory) or M2-polarization (anti-inflammation). M2-polarization has been agreed to have an important role in promoting regeneration [[40], [41], [42]]. Previous studies of our team successively reported that glycine, histidine, proline and hydroxyproline from hydrolyzed or degraded components of type II squid collagen and gelatin, had the ability to induce M2-polarization in macrophages [17]. Therefore, did the tilapia type I gelatin present in TGI/HA-CS double network hydrogels have the same function? This aroused our great interest. According to the composition analysis of TGI/HA-CS double network hydrogel extraction for 1 day, free amino acids such as glycine and hydroxyproline were present in the liquid (supporting information Table S2). Further studies showed the hydrogel could up-regulate the expression of characteristic genes of M2 macrophages (Ym1, Arg1, MR, IL-10), proteins (CD206) and cytokines (IL-10, TGF-β1, TGF-β2, IGF1 and IGF2, Fig. 5A–G), thus fully demonstrating the TGI/HA-CS double network hydrogel could directly induce M2-polarization, and this immunomodulatory effect on macrophages was likely related to the free amino acids released during degradation. Moreover, the results also suggested the hydrogel had no obvious stimulating effect on the genes (IL-1β, IL-6, TNF-ɑ, iNOS, CD86) and surface proteins (CD86) related to M1-polarization, which once again confirmed the TGI/HA-CS double network hydrogel only promoted M0 macrophages to the tissue regeneration-friendly (M2) type, without inducing the pro-inflammatory (M1) type. It would create a favorable environment for disc perforation repairing.
Fig. 5.
Induction of TGI/HA-CS double network hydrogel on M2-polarization of macrophages.
(A) Expression of M1-related (IL-1β, IL-6, TNF-ɑ, iNOS, CD86) and M2-related (Ym1, Arg1, MR, IL-10) genes. (B) Percent of CD86-and CD206-positive cells and (C) mean fluorescence intensity (MFI) of these two markers. (D) Immunofluorescence of CD206 (Bar = 100 μm). Quntification of (E) IL-10, (F) TGF-β1 and TGF-β2. (G) Gene expression of cytokines that induce fibrochondrogenic differentiation (TGF-β1, TGF-β2, IGF1, IGF2). **p < 0.01, ***p < 0.001.
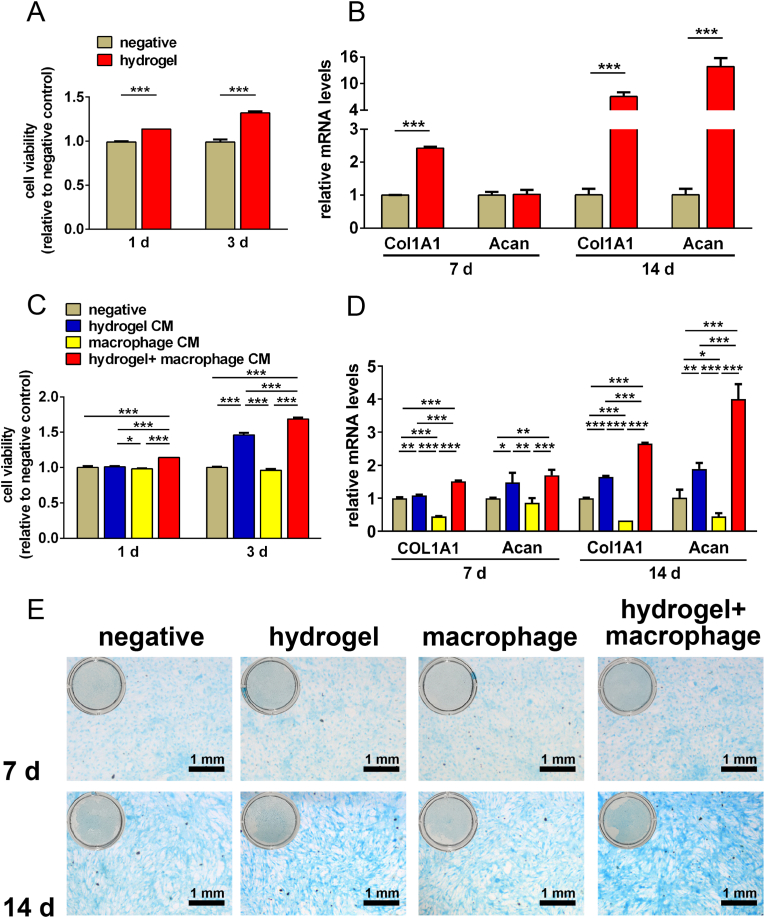
3.3.2. Direct and indirect effects of TGI/HA-CS double network hydrogels on TMJ disc cells and SMSCs
Regeneration of TMJ disc perforations is dependent on both TMJ disc cells and SMSCs. To verify the reparation-inducing ability of hydrogel, we first investigated direct influence of the hydrogel on these two types of cells. As it was shown in Fig. 6A and B that the hydrogel could promote proliferation of TMJ disc cells. And gene expression of both type I collagen (Col1A1) and aggrecan (acan, a protein expressed at the late stage of cartilage differentiation) were significantly increased in SMSCs at 14 days, indicating TGI/HA-CS double network hydrogel had the ability to induce stem cells to differentiate towards fibrochondrocytes. It has been found that type I collagen induces chondrogenic differentiation of bone marrow mesenchymal stem cells from equine [43] and CS up-regulates acan gene expression levels [44]. Since TGI/HA-CS double network hydrogel produced large amounts of glycine and CS during degradation (supporting information Table S2), it could be hypothesized that the fibrochondrogenic differentiation of SMSCs was directly related to its constituents.
Fig. 6.
Effects of TGI/HA-CS double network hydrogels on TMJ disc cells and SMSCs.
The hydrogel directly (A) promoted proliferation of TMJ disc cells and (B) induced fibrochondrogenic differentiation of SMSCs. TGI/HA-CS double network hydrogel (C) fastened disc cells growth and (D) induced chondrogenic differentiation of SMSCs through indirect ways. (E) Alcian staining of SMSCs cultured with different supernatant (bar = 1 mm). **p < 0.01, ***p < 0.001.
As it was shown in Fig. 5 that TGI/HA-CS double network hydrogel could promote M2-polarization of macrophages, thus secreting a series of cytokines related to tissue regeneration, such as TGF-β1. So would these factors exert positive biological effects on TMJ disc cells and SMSCs? To answer this question, we collected conditioned mediums (CMs) from the macrophages cocultured with hydrogel and then cultured TMJ disc cells or SMSCs with these CMs (we deemed it as the indirect contact between hydrogel and disc cells or SMSCs). We found that cytokines in the CMs secreted by M2-macrophages not only promoted faster proliferation of disc cells, but also caused SMSCs to differentiate into fibrochondrocytes (Fig. 6C–E). It has been widely reported that TGF-β1, TGF-β2, IGF1 and IGF2 are influential in inducing chondrogenic differentiation, promoting cartilage ECM formation and reducing its degradation [[45], [46], [47], [48]]. In addition, TGF-β has been shown to have a positive effect on the proliferation of a variety of cells [[49], [50], [51]].
The series of findings mentioned above suggested TGI/HA-CS double network hydrogel could promote the proliferation of TMJ disc cells and fibrochondrogenic differentiation of SMSCs via direct and indirect ways, aiming at in situ regeneration of defected TMJ discs.
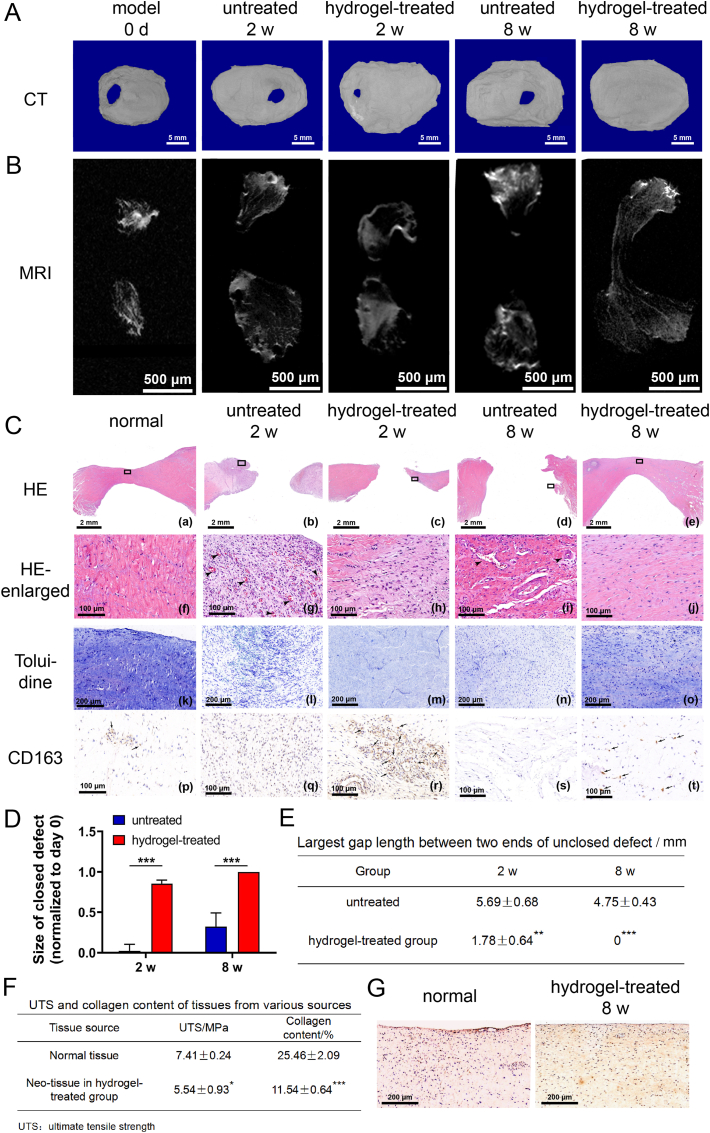
3.3.3. Effect of TGI/HA-CS double network hydrogel on regeneration of 6 mm-sized perforation in porcine TMJ discs
The most promising aspect of this study was TGI/HA-CS double network hydrogel's function in promoting regeneration on a mini-pig TMJ disc perforation model. Although in vitro studies had adequately demonstrated the influence of hydrogels in immunomodulating the local inflammatory microenvironment, promoting proliferation as well as inducing differentiation, the clinical therapeutic effect was the most convincing evidence. Through Micro-CT, MRI and histopathological-staining results, we were surprised to see the healing of porcine TMJ disc perforation was obviously better at different time points in the hydrogel-treated group, with complete closure of defective areas at 8 w (Fig. 7A–B, C (a-e) and 7D-E). Further histopathological observations supported this result, as shown in Fig. 7C. At 2 w, local infiltrated inflammatory cells in the hydrogel-treated group were obviously reduced, with new-born collagens densely arranged and regularly oriented, and a certain amount of CS deposited in the new tissues. There was also an increase in the number of M2 macrophages observed through immunohistochemical staining. At 8 w, there was no local inflammation present and orientation of the new-born collagen bundles was consistent with normal ones. CS deposition in the neo-tissue was more obvious than 2 w, and a small amount of M2 was still visible locally, indicating that the repair was still continuing at this time. In the untreated group, a great quantity of inflammatory cells infiltrated and capillaries appeared at 2 w. The level of ECM deposition was low, with almost no M2 macrophages. At 8 w, the defective area was unhealed with a small number of inflammatory cells still seen locally. In addition, collagens in the new-born tissues were loosely arranged and disorganized, with blood vessels, extremely low level of ECM and a large number of fissures.
Fig. 7.
Effect of TGI/HA-CS double network hydrogel on regeneration of perforated discs.
(A) CT, (B) MRI and (C) HE ((a-e) represented the whole tissue with or without defects, while (f-j) represented the local enlarged ones), (k–o) Toluidine as well as (p–t) immunohistochemical staining of CD163 (bar = 5 mm, 500 μm, 2 mm, 100 μm or 200 μm, respectively). Quntification of (D) healed size in Fig. 7A and largest unclosed gap between two ends in Fig. 7B. (F) Ultimate tensile strength and collagen content of normal tissues and new-born ones at 8 w. (G) Immunohistochemical staining of type I collagen in normal and neo-tissues (bar = 200 μm). *p < 0.05, **p < 0.01, ***p < 0.001.
In addition, though no hydrogel block was seen, flushing fluid of the joint cavity was relatively viscous at 8 w. To further analyze whether the hydrogel continued to function at this time, we tested hydroxyproline level of the flushing fluid of bilateral joint cavity. Results showed the hydroxyproline concentration in the hydrogel-treated group was significantly higher than the untreated group (189.85 ± 3.07 μg/ml vs 8.43 ± 0.82 μg/ml, p < 0.001). Since hydroxyproline was one of the important amino acids that constituted the collagen skeleton structure [52], its higher local content indicated although the hydrogel underwent degradation, its active ingredients continued to positively regulate tissue regeneration, which explained its excellent effect in promoting disc repair.
To further assess the quality of new-born tissues, we trimmeded TMJ disc samples at 8 w for mechanical testing and compositional analysis. Although the tensile fracture strength and percentage of collagen of new-born tissues in hydrogel-treated group were less than normal ones (see Fig. 7F and G), they were better than those reported by Natalia Vapniarsky et al. [2]. Li et al. reported the maximum tensile stress tolerated by human TMJ discs during mouth opening was 4 MPa [10], while the neonatal disc tissue reached 5.54 ± 0.93 MPa, indicating the tissue was basically able to withstand it. To conclude, results of the studies on a 6 mm-sized porcine TMJ disc perforation model fully demonstrated TGI/HA-CS double network hydrogel could significantly and rapidly promote functional regeneration of TMJ disc, both from the aspect of macroscopic morphological recovery and the aspect of microscopic structure (collagen and CS content, as well as collagen arrangement).
3.4. Mechanisms for regeneration of TMJ disc perforation by TGI/HA-CS double network hydrogel
Based on our findings, the mechanisms for disc regeneration could be included as follows (Fig. 8). Firstly, the material could regulate local immune microenvironment. On the one hand, it reduced the release of IL-1β and TNF-α by lowering the level of Nlrp3 and caspase-1. On the other hand, it down-regulated MMP-9, ADAMTS-5 and IL-6 secreted by chondrocytes, which is conducive to reversal of local inflammatory state and provision of a good microenvironment for in situ tissue regeneration. Secondly, the TGI/HA-CS double network hydrogel urged the proliferation of TMJ disc cells and enhanced expression of Col I and Acan in SMSCs through both direct and indirect pathways (inducing macrophages to exhibit a pro-repair M2 phenotype and secrete cytokines such as IL-10, TGF-β1 and TGF-β2), which facilitated tissue regeneration. Finally, two parts of animal experiments revealed the hydrogel not only effectively decreased concentrations of inflammatory factors (IL-1β and TNF-α), cut down the loss of ECM and reversed the pathological progression of TMJOA, but also rapidly promoted regeneration and healing of defective areas in a mini pig 6 mm-sized TMJ disc perforation model. The macroscopic (shape), microscopic (tissue structure) and functional (strength and collagen content) bionic repair confirmed positive effects of the hydrogel. Taken together, these mechanisms explained why TGI/HA-CS double network hydrogel could achieve in situ self-regeneration while reversing immune microenvironment.
Fig. 8.
Schematic illustration of TGI/HA-CS double network hydrogels' effect on inflammation inhibition as well as in situ TMJ disc regeneration via pleiotropic immunomodulation.
4. Conclusion
To address the two pressing clinical problems of lacking an ideal material for TMJ disc perforation and local inflammation interfering with tissue regeneration, a bionic TGI/HA-CS double network hydrogel with natural polymers as its main components was constructed. It not only had an ideal compressive strength (11 MPa, higher than the stress on TMJ disc during mouth opening) and clinical adaptability (could be shaped intraoperatively, with 3–5 min for operating), but also, surprisingly, had the ability to evidently reverse local inflammatory microenvironment and simultaneously promote rapid regeneration of TMJ disc. A series of in vivo and ex vivo studies demonstrated the TGI/HA-CS double network hydrogel could, on the one hand, inhibit pro-inflammatory actions of inflammasome, down-regulate secretion of inflammatory factors and reduce destructive influences of ECM-degradating enzymes, thus controlling the inflammatory state in the joint cavity. On the other hand, the hydrogel could promote cell proliferation and fibrochondrogenic differentiation through direct and indirect ways. Particularly, taking account of tensile strength, collagen content and histological characteristics of new-born tissues, the hydrogel accomplished a rapid and satisfactory in situ regeneration with high-quality on a 6 mm-sized mini pig TMJ disc perforation model. Findings of this study provided a new treatment for large perforation in TMJ disc with broad clinical application.
CRediT authorship contribution statement
Xiao Xu: Investigation, Data curation, Writing – original draft. Baiyan Sui: Methodology, Formal analysis, Software. Xin Liu: Methodology, Project administration, Supervision. Jiao Sun: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Authors thank Lan He and Xiuyu Guo (Shanghai Fisheries Research Institute) for their kind help and practical suggestions. This work was sponsored by grants from the National Nature Science Foundation of China (82072070), the National Key Research and Development Program of China (2018YFC1105202), and Science and Technology Commission of Shanghai Municipality (21DZ2291700, 18DZ2290300).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bioactmat.2022.07.006.
Contributor Information
Xin Liu, Email: liuxin0556@163.com.
Jiao Sun, Email: jiaosun59@shsmu.edu.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Donahue R.P., Hu J.C., Athanasiou K.A. Remaining hurdles for tissue-engineering the temporomandibular joint disc. Trends Mol. Med. 2019;25(3):241–256. doi: 10.1016/j.molmed.2018.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Natalia V., Huwe L.W., Boaz A., Houghton M.K., Wong M.E., Wilson J.W., Hatcher D.C., Hu J.C., Athanasiou K.A. Tissue engineering toward temporomandibular joint disc regeneration. Transl. Med. 2018;10(446) doi: 10.1126/scitranslmed.aaq1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murphy M.K., MacBarb R.F., Wong M.E., Athanasiou K.A. Temporomandibular disorders: a review of etiology, clinical management, and tissue engineering strategies. Int. J. Oral Maxillofac. Implants. 2013;28(6):e393–e414. doi: 10.11607/jomi.te20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luo P., Peng S., Yan Y., Ji P., Xu J. IL-37 inhibits M1-like macrophage activation to ameliorate temporomandibular joint inflammation through the NLRP3 pathway. Rheumatology. 2020;59(10):3070–3080. doi: 10.1093/rheumatology/keaa192. [DOI] [PubMed] [Google Scholar]
- 5.Tuncel U., Kostakoglu N., Turan A., Markoç F., Gokçe E., Erkorkmaz U. The use of temporalis muscle graft, fresh and cryopreserved amniotic membrane in preventing temporomandibular joint ankylosis after discectomy in rabbits. J. Cranio-Maxillo-Facial Surg. 2014;42(8):1868–1876. doi: 10.1016/j.jcms.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 6.Gutman S., Kim D., Tarafder S., Velez S., Jeong J., Lee C.H. Regionally variant collagen alignment correlates with viscoelastic properties of the disc of the human temporomandibular joint. Arch. Oral Biol. 2018;86:1–6. doi: 10.1016/j.archoralbio.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eder J., Szomolanyi P., Schmid-Schwap M., Bristela M., Skolka A., Pittschieler E., Piehslinger E., Trattnig S. Early diagnosis of degenerative changes in the articular/fibrocartilaginous disc of the temporomandibular joint in patients with temporomandibular disorders using delayed gadolinium-enhanced MRI at 3 Tesla – preliminary results. Magn. Reson. Imag. 2020;67:24–27. doi: 10.1016/j.mri.2019.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Bao Z., Gao M., Fan X., Cui Y., Yang J., Peng X., Xian M., Sun Y., Nian R. Development and characterization of a photo-cross-linked functionalized type-I collagen (Oreochromis niloticus) and polyethylene glycol diacrylate hydrogel. Int. J. Biol. Macromol. 2020;155:163–173. doi: 10.1016/j.ijbiomac.2020.03.210. [DOI] [PubMed] [Google Scholar]
- 9.Ren P., Zhang H., Dai Z., Ren F., Wu Y., Hou R., Zhu Y., Fu J. Stiff micelle-crosslinked hyaluronate hydrogels with low swelling for potential cartilage repair. J. Mater. Chem. B. 2019;7(36):5490–5501. doi: 10.1039/c9tb01155b. [DOI] [PubMed] [Google Scholar]
- 10.Li Q., Ren S., Ge C., Sun H., Lu H., Duan Y., Rong Q. Effect of jaw opening on the stress pattern in a normal human articular disc: finite element analysis based on MRI images. Head Face Med. 2014;10:24. doi: 10.1186/1746-160X-10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gong J.P., Katsuyama Y., Kurokawa T., Osada Y. Double-network hydrogels with extremely high mechanical strength. Adv. Mater. 2003;15(14):1155–1158. [Google Scholar]
- 12.Shin H., Olsen B.D., Khademhosseini A. The mechanical properties and cytotoxicity of cell-laden double-network hydrogels based on photocrosslinkable gelatin and gellan gum biomacromolecules. Biomaterials. 2012;33(11):3143–3152. doi: 10.1016/j.biomaterials.2011.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo Z., Zeng X., Zheng Y. Zafirlukast ameliorates Docetaxel-induced activation of NOD-like receptor protein 3 (NLRP3) inflammasome, mediated by Sirtuin1 (SIRT1) in hepatocytes. Bioengineered. 2021;12(2):11030–11040. doi: 10.1080/21655979.2021.2005895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ambati M., Apicella I., Wang S.-B., Narendran S., Leung H., Pereira F., Nagasaka Y., Huang P., Varshney A., Baker K.L., Marion K.M., Shadmehr M., Stains C.I., Werner B.C., Sadda S.R., Taylor E.W., Sutton S.S., Magagnoli J., Gelfand B.D. Identification of fluoxetine as a direct NLRP3 inhibitor to treat atrophic macular degeneration. Proc. Natl. Acad. Sci. U.S.A. 2021;118(41) doi: 10.1073/pnas.2102975118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu C., Liu X., Xue Y., Ding T., Sun J. Hydrolyzed tilapia fish collagen modulates the biological behavior of macrophages under inflammatory conditions. RSC Adv. 2015;5(39):30727–30736. [Google Scholar]
- 16.Hu J., Liu R., Yu X., Li Z., Liu X., Hao Y., Zhu N., Kang J., Li Y. Protective effects of small-molecule oligopeptides isolated from Tilapia fish scale on ethanol-induced gastroduodenal injury in rats. Nutrients. 2021;13(6):2078. doi: 10.3390/nu13062078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dai M., Sui B., Hua Y., Zhang Y., Bao B., Lin Q., Liu X., Zhu L., Sun J. A well defect-suitable and high-strength biomimetic squid type II gelatin hydrogel promoted in situ costal cartilage regeneration via dynamic immunomodulation and direct induction manners. Biomaterials. 2020;240 doi: 10.1016/j.biomaterials.2020.119841. [DOI] [PubMed] [Google Scholar]
- 18.Zhou T., Wang N., Xue Y., Ding T., Liu X., Mo X., Sun J. Electrospun tilapia collagen nanofibers accelerating wound healing via inducing keratinocytes proliferation and differentiation. Colloids Surf. B Biointerfaces. 2016;143:415–422. doi: 10.1016/j.colsurfb.2016.03.052. [DOI] [PubMed] [Google Scholar]
- 19.Xu X., Sui B., Liu X., Sun J. Superior low-immunogenicity of tilapia type I collagen based on unique secondary structure with single calcium binding motif over terrestrial mammals by inhibiting activation of DC intracellular Ca2+-mediated STIM1-Orai1/NF-кB pathway. Mater. Sci. Eng. C. 2021;131 doi: 10.1016/j.msec.2021.112503. [DOI] [PubMed] [Google Scholar]
- 20.Ren Y., Zhang Y., Zhang H., Wang Y., Liu L., Zhang Q. A gelatin-hyaluronic acid double cross-linked hydrogel for regulating the growth and dual dimensional cartilage differentiation of bone marrow mesenchymal stem cells. J. Biomed. Nanotechnol. 2021;17(6):1044–1057. doi: 10.1166/jbn.2021.3088. [DOI] [PubMed] [Google Scholar]
- 21.Fjelstrup S., Andersen M.B., Thomsen J., Wang J., Stougaard M., Pedersen F.S., Ho Y.-P., Hede M.S., Knudsen B.R. The effects of dithiothreitol on DNA. Sensors. 2017;17(6):1201. doi: 10.3390/s17061201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao Y., Liu Q., Kong W., Wang J., He L., Guo L., Lin H., Fan H., Fan Y., Zhang X. Activated hyaluronic acid/collagen composite hydrogel with tunable physical properties and improved biological properties. Int. J. Biol. Macromol. 2020;164:2186–2196. doi: 10.1016/j.ijbiomac.2020.07.319. [DOI] [PubMed] [Google Scholar]
- 23.Khalid I., Ahmad M., Usman Minhas M., Barkat K. Synthesis and evaluation of chondroitin sulfate based hydrogels of loxoprofen with adjustable properties as controlled release carriers. Carbohydr. Polym. 2018;181:1169–1179. doi: 10.1016/j.carbpol.2017.10.092. [DOI] [PubMed] [Google Scholar]
- 24.Dhand A.P., Galarraga J.H., Burdick J.A. Enhancing biopolymer hydrogel functionality through interpenetrating networks. Trends Biotechnol. 2021;39(5):519–538. doi: 10.1016/j.tibtech.2020.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khalesi H., Lu W., Nishinari K., Fang Y. New insights into food hydrogels with reinforced mechanical properties: a review on innovative strategies. Adv. Colloid Interface Sci. 2020;285 doi: 10.1016/j.cis.2020.102278. [DOI] [PubMed] [Google Scholar]
- 26.Detamore M.S., Athanasiou K.A. Motivation, characterization, and strategy for tissue engineering the temporomandibular joint disc. Tissue Eng. 2003;9(6):1065–1087. doi: 10.1089/10763270360727991. [DOI] [PubMed] [Google Scholar]
- 27.Ye J., Yang G., Zhang J., Xiao Z., He L., Zhang H., Liu Q. Preparation and characterization of gelatin-polysaccharide composite hydrogels for tissue engineering. PeerJ. 2021;9 doi: 10.7717/peerj.11022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shariatzadeh F.J., Solouk A., Khoulenjani S.B., Bonakdar S., Mirzadeh H. Injectable and reversible preformed cryogels based on chemically crosslinked gelatin methacrylate (GelMA) and physically crosslinked hyaluronic acid (HA) for soft tissue engineering. Colloids Surf. B Biointerfaces. 2021;203 doi: 10.1016/j.colsurfb.2021.111725. [DOI] [PubMed] [Google Scholar]
- 29.Lu J., Chen Y., Ding M., Fan X., Hu J., Chen Y., Li J., Li Z., Liu W. A 4arm-PEG macromolecule crosslinked chitosan hydrogels as antibacterial wound dressing. Carbohydr. Polym. 2022;277 doi: 10.1016/j.carbpol.2021.118871. [DOI] [PubMed] [Google Scholar]
- 30.Malemud C.J. Inhibition of MMPs and ADAM/ADAMTS. Biochem. Pharmacol. 2019;165:33–40. doi: 10.1016/j.bcp.2019.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Millerand M., Berenbaum F., Jacques C. Danger signals and inflammaging in osteoarthritis. Clin. Exp. Rheumatol. 2019;37(5):48–56. Suppl 120. [PubMed] [Google Scholar]
- 32.An S., Hu H., Li Y., Hu Y. Pyroptosis plays a role in osteoarthritis. Aging Disease. 2020;11(5):1146. doi: 10.14336/AD.2019.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hartog A., Cozijnsen M., de Vrij G., Garssen J. Collagen hydrolysate inhibits zymosan-induced inflammation. Exp. Biol. Med. (Maywood, NJ, U. S.) 2013;238(7):798–802. doi: 10.1177/1535370213480740. [DOI] [PubMed] [Google Scholar]
- 34.Zheng H.X., Chen D.J., Zu Y.X., Wang E.Z., Qi S.S. Chondroitin sulfate prevents STZ induced diabetic osteoporosis through decreasing blood glucose, AntiOxidative stress, anti-inflammation and OPG/RANKL expression regulation. Int. J. Mol. Sci. 2020;21(15):5303. doi: 10.3390/ijms21155303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Slovacek H., Khanna R., Poredos P., Poredos P., Jezovnik M., Hoppensteadt D., Fareed J., Hopkinson W. Interrelationship of MMP-9, proteoglycan-4, and inflammation in osteoarthritis patients undergoing total hip arthroplasty. Clinical Appl. Thrombosis/Hemostasis. 2021;27 doi: 10.1177/1076029621995569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Santamaria S. ADAMTS-5: a difficult teenager turning 20. Int. J. Exp. Pathol. 2020;101(1–2):4–20. doi: 10.1111/iep.12344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang Y., Zhang L., Song R., Mao X., Tang S. A carrageenan/agarose composite sponge and its immunomodulatory activities toward RAW264.7. J. Biomed. Mater. Res., Part A. 2021;109(6):829–839. doi: 10.1002/jbm.a.37070. [DOI] [PubMed] [Google Scholar]
- 38.Huang C.Y., Hung L.F., Liang C.C.T., Ho L.J. COX-2 and iNOS are critical in advanced glycation end product-activated chondrocytes in vitro. Eur. J. Clin. Invest. 2009;39(5):417–428. doi: 10.1111/j.1365-2362.2009.02106.x. [DOI] [PubMed] [Google Scholar]
- 39.Zhang S., Teo K.Y.W., Chuah S.J., Lai R.C., Lim S.K., Toh W.S. MSC exosomes alleviate temporomandibular joint osteoarthritis by attenuating inflammation and restoring matrix homeostasis. Biomaterials. 2019;200:35–47. doi: 10.1016/j.biomaterials.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 40.Qiu P., Li M., Chen K., Fang B., Chen P., Tang Z., Lin X., Fan S. Periosteal matrix-derived hydrogel promotes bone repair through an early immune regulation coupled with enhanced angio- and osteogenesis - ScienceDirect. Biomaterials. 2020;227 doi: 10.1016/j.biomaterials.2019.119552. [DOI] [PubMed] [Google Scholar]
- 41.Qiu P., Li M., Chen K., Fang B., Chen P., Tang Z., Lin X., Fan S. Periosteal matrix-derived hydrogel promotes bone repair through an early immune regulation coupled with enhanced angio- and osteogenesis. Biomaterials. 2020;227 doi: 10.1016/j.biomaterials.2019.119552. [DOI] [PubMed] [Google Scholar]
- 42.Zhu Y., Liang H., Liu X., Wu J., Yang C., Wong T.M., Kwan K.Y.H., Cheung K.M.C., Wu S., Yeung K.W.K. Regulation of macrophage polarization through surface topography design to facilitate implant-to-bone osteointegration. Sci. Adv. 2021;7(14) doi: 10.1126/sciadv.abf6654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kremer A., Ribitsch I., Reboredo J., Dürr J., Egerbacher M., Jenner F., Walles H. Three-dimensional coculture of meniscal cells and mesenchymal stem cells in collagen type I hydrogel on a small intestinal matrix-A pilot study toward equine meniscus tissue engineering. Tissue Eng. 2017;23(9–10):390–402. doi: 10.1089/ten.TEA.2016.0317. [DOI] [PubMed] [Google Scholar]
- 44.Corradetti B., Taraballi F., Minardi S., Van Eps J., Cabrera F., Francis L.W., Gazze S.A., Ferrari M., Weiner B.K., Tasciotti E. Chondroitin sulfate immobilized on a biomimetic scaffold modulates inflammation while driving chondrogenesis. Stem Cells Translat. Med. 2016;5(5):670–682. doi: 10.5966/sctm.2015-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Almarza A.J., Athanasiou K.A. Evaluation of three growth factors in combinations of two for temporomandibular joint disc tissue engineering. Arch. Oral Biol. 2006;51(3):215–221. doi: 10.1016/j.archoralbio.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 46.Wen C., Xu L., Xu X., Wang D., Liang Y., Duan L. Insulin-like growth factor-1 in articular cartilage repair for osteoarthritis treatment. Arthritis Res. Ther. 2021;23(1):277. doi: 10.1186/s13075-021-02662-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Uchimura T., Hollander J.M., Nakamura D.S., Liu Z., Rosen C.J., Georgakoudi I., Zeng L. An essential role for IGF2 in cartilage development and glucose metabolism during postnatal long bone growth. Development. 2017;144(19):3533–3546. doi: 10.1242/dev.155598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tekari A., Luginbuehl R., Hofstetter W., Egli R.J. Transforming growth factor beta signaling is essential for the autonomous formation of cartilage-like tissue by expanded chondrocytes. PLoS One. 2015;10(3) doi: 10.1371/journal.pone.0120857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shu J., Hu L., Wu Y., Chen L., Huang K., Wang Z., Liang M. Daidzein suppresses TGF-β1-induced cardiac fibroblast activation via the TGF-β1/SMAD2/3 signaling pathway. Eur. J. Pharmacol. 2022;919 doi: 10.1016/j.ejphar.2022.174805. [DOI] [PubMed] [Google Scholar]
- 50.Fan J., Gong Y., Ren L., Varshney R.R., Cai D., Wang D.-A. In vitro engineered cartilage using synovium-derived mesenchymal stem cells with injectable gellan hydrogels. Acta Biomater. 2010;6(3):1178–1185. doi: 10.1016/j.actbio.2009.08.042. [DOI] [PubMed] [Google Scholar]
- 51.Ding Q., Wei Q., Sheng G., Wang S., Jing S., Ma T., Zhang R., Wang T., Li W., Tang X., Wu H., Liu C. The preventive effect of decorin on epidural fibrosis and epidural adhesions after laminectomy. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.774316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.An B., Lin Y.-S., Brodsky B. Collagen interactions: drug design and delivery. Adv. Drug Deliv. Rev. 2016;97:69–84. doi: 10.1016/j.addr.2015.11.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.