Abstract
Background
To evaluate the long‐term efficacy and safety of continued repeated low‐level red‐light (RLRL) therapy on myopia control over 2 years, and the potential rebound effect after treatment cessation.
Methods
The Chinese myopic children who originally completed the one‐year randomised controlled trial were enrolled. Children continued RLRL‐therapy were defined as RLRL‐RLRL group, while those who stopped and switched to single‐vision spectacle (SVS) in the second year were RLRL‐SVS group. Likewise, those who continued to merely wear SVS or received additional RLRL‐therapy were SVS‐SVS and SVS‐RLRL groups, respectively. RLRL‐therapy was provided by an at‐home desktop light device emitting red‐light of 650 nm and was administered for 3 min at a time, twice a day and 5 days per week. Changes in axial length (AL) and cycloplegic spherical equivalence refraction (SER) were measured.
Results
Among the 199 children who were eligible, 138 (69.3%) children attended the examination and 114 (57.3%) were analysed (SVS‐SVS: n = 41; SVS‐RLRL: n = 10; RLRL‐SVS: n = 52; RLRL‐RLRL: n = 11). The baseline characteristics were balanced among four groups. In the second year, the mean changes in AL were 0.28 ± 0.14 mm, 0.05 ± 0.24 mm, 0.42 ± 0.20 mm and 0.12 ± 0.16 mm in SVS‐SVS, SVS‐RLRL, RLRL‐SVS and RLRL‐RLRL group, respectively (p < 0.001). The respective mean SER changes were −0.54 ± 0.39D, −0.09 ± 0.55D, −0.91 ± 0.48D, and −0.20 ± 0.56D (p < 0.001). Over the 2‐year period, axial elongation and SER progression were smallest in RLRL‐RLRL group (AL: 0.16 ± 0.37 mm; SER: −0.31 ± 0.79D), followed by SVS‐RLRL (AL: 0.44 ± 0.37 mm; SER: −0.96 ± 0.70D), RLRL‐SVS (AL: 0.50 ± 0.28 mm; SER: −1.07 ± 0.69D) and SVS‐SVS group (AL: 0.64 ± 0.29 mm; SER: −1.24 ± 0.63D). No self‐reported adverse events, functional or structural damages were noted.
Conclusions
Continued RLRL therapy sustained promising efficacy and safety in slowing myopia progression over 2 years. A modest rebound effect was noted after treatment cessation.
Keywords: axial length, clinical trials, myopia control, repeated low‐level red‐light therapy, spherical equivalent refraction
1. INTRODUCTION
Myopia continues to be a worldwide public concern with increasing prevalence over the past decades, especially in East Asia. 1 It is estimated that 54% and 10% of the world's population will have myopia and high myopia by 2050, respectively. 1 The increasing prevalence of myopia, together with the irreversible vision impairment and costs due to myopia‐related complications pose a tremendous burden to both individuals and society. 2 Various interventions have been proposed for myopia control, 3 however, the feasibility of the current strategies remains to be limited by the adverse effects and inconvenience. 4 , 5
Repeated low‐level red‐light (RLRL) therapy has emerged as a novel myopia control treatment modality recently. A 12‐month multicenter randomised controlled trial (RCT) conducted by our research team was thought to be the first to systematically evaluate the treatment of myopic children using RLRL therapy. 6 The trial demonstrated that RLRL therapy was effective on myopia control, reducing axial elongation and spherical equivalence refraction (SER) progression by 69.4% and 76.6% compared with single vision spectacle (SVS) over a 12‐month period, respectively. The promising efficacy of RLRL therapy has been further confirmed in other studies. 7 , 8 In addition, satisfactory user acceptability and no documented functional and structural damages were observed.
Myopia generally progresses throughout childhood and hence a study duration of 1 year is not sufficient to widely adopt the RLRL therapy as a treatment strategy for myopia control. The sustainability of treatment efficacy, rebound phenomenon upon cessation of treatment, and potential risks and adverse effects in myopic children with longer‐term RLRL therapy, remain to be fully elucidated.
Thus, the aims of this post‐trial follow‐up study were to invite the participants to come back for a 24‐month visit and to investigate the long‐term efficacy and safety of continued RLRL therapy as well as the potential rebound effect following RLRL treatment cessation.
2. METHODS
2.1. Study design
The study design of the 12‐month RCT has been described in detail previously. 6 In brief, children aged 8–13 years with cycloplegic SER between −1.00 and −5.00 diopters (D), astigmatism ≤2.5D, anisometropia ≤1.50D, and best‐corrected visual acuity (BCVA) ≥ Snellen20/20 in either eye were enrolled at five study sites from four tertiary hospitals in China between July and August 2019. In the first year, a total of 264 children were randomly allocated to the intervention group (n = 119) receiving RLRL therapy in addition to SVS or the control group (n = 145) receiving SVS alone, with follow‐ups at 1, 3, 6 and 12 months. This trial was completed in September 2020.
After the 12‐month follow‐up visit when the RCT was completed, a real‐world study (RWS) was initiated to observe the long‐term efficacy and safety of the treatment. The study participants, in either intervention or control arm of the RCT, were invited to voluntarily participate this RWS study.
Three out of five study sites (Ouzhuang, Zhongshan Ophthalmic Center; Zhujiang New Town, Zhongshan Ophthalmic Center; The Second People's Hospital of Foshan) participated in the post‐trial follow‐up study. All participants (N = 199) originally enrolled from these sites were eligible and invited. A total of 138 (69.3%) participants returned to the 24‐month follow‐up visit (Figure 1). All children wore SVS throughout the study period for optical correction and updated their spectacles if needed. Children who received RLRL therapy along with SVS in the first year and continued the RLRL therapy were defined as the RLRL‐RLRL group, while those who stopped RLRL therapy and only wore SVS in the second year were the RLRL‐SVS group. Children who wore SVS for 2 years were defined as the SVS‐SVS group, whereas children received additional RLRL therapy in the second year were the SVS‐RLRL group. The original study was a 12‐month RCT and therefore when the 12‐month visit was completed, the participants were required to pay for the device. The 24‐month follow‐up was performed between July and September 2021. All examinations were performed using the same protocol and equipments as the first phase of the study.
FIGURE 1.
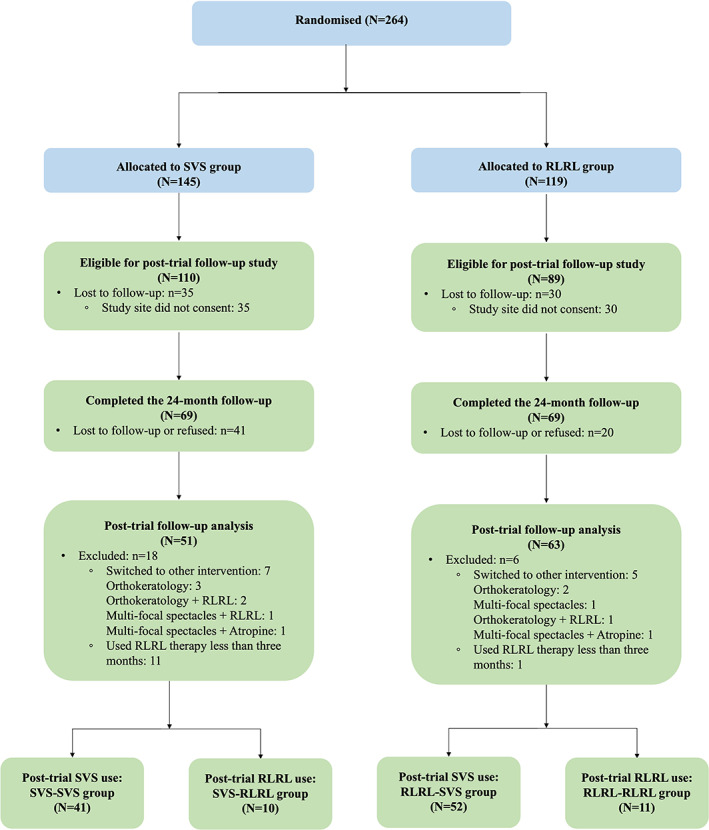
Study design and flow diagram of the post‐trial follow‐up study. The first year was the in‐trial period, after which time the randomization was stopped and all eligible participants were invited to voluntarily participate a real‐world study to use the RLRL therapy in the second year. Blue boxes indicate the in‐trial period. Green boxes indicate the post‐trial period. RLRL, repeated low‐level red‐light; SVS, single vision spectacle.
The original RCT and the post‐trial follow‐up study were registered with ClinicalTrials.gov (NCT04073238) and were approved by the Ethics Committee of Zhongshan Ophthalmic Centre (2019KYPJ093). The RWS was approved by the Ethics Committee of Zhongshan Ophthalmic Centre (2020KYPJ107). Verbal and written informed consents were obtained from participating children and their parents/guardians, respectively. All procedures adhered to Good Clinical Practice guidelines, the tenets of the Declaration of Helsinki, and all applicable regulations.
2.2. RLRL therapy
RLRL therapy was provided with the desktop light therapy device (Eyerising, Suzhou Xuanjia Optoelectronics Technology, Jiangsu, China). Details of the device have been published previously. 6 Briefly, the desktop light therapy device has been available in the Chinese market and widely used for the treatment of amblyopia in children during the past decade. It emits low‐level red‐light with a wavelength of 650 ± 10 nm. An independent lab verified that the light power entering a 4‐mm pupil is classified as Class I, which is considered safe for direct ocular exposure. 9 The RLRL therapy was conducted at home under the supervision of parents/guardians. The treatment was scheduled for 3 min per session, 2 treatment sessions per day, with a minimum interval of 4 h between sessions and 5 days per week. The device was connected to the internet with an automated diary function to record treatment sessions and to monitor patient compliance. Reminder messages were sent to guardians/parents and trial managers if the recorded treatment sessions were less than 8 per week.
2.3. Ophthalmic examinations
The ophthalmic examinations conducted in the 24‐month follow‐up visit were the same as for the previous RCT. 6 Uncorrected visual acuity (UCVA) and BCVA were assessed at 4 m by optometrists using the Early Treatment Diabetic Retinopathy Study visual acuity chart (Precision Vision, Villa Park, Illinois, USA). Ocular biometric parameters, including axial length (AL), corneal curvature (CC), and anterior chamber depth (ACD), were measured before cycloplegia using IOLMaster (Carl Zeiss 500, Meditec, Oberkochen, Germany). Five measures were performed and averaged until the desired precision (i.e., ≤0.05 mm for AL) was achieved. Otherwise, measurements with signal‐to‐noise ratios of less than 10 were deleted and the examiner repeated the measurement.
Cycloplegia was performed using 1 drop of 0.5% Alcaine (Alcon, Puurs, Belgium) and 1 drop of 1% cyclopentolate (Alcon, Puurs, Belgium) to both eyes. Two more drops of cyclopentolate were administered at 5 and 20 min. If the pupillary light reflex was absent and the pupil size was 6 mm or greater after an additional 15 min, full cycloplegia was achieved. Cycloplegic refraction data were obtained using an autorefractor (KR‐8800, Topcon, Tokyo, Japan) with an average of three measurements within a minimum deviation of 0.25D for spherical and cylinder power and 5° for axis. SER was defined as the spherical power plus half of the cylinder power.
Radial scans of 12.0 mm (resolution: 1024 × 12) centered at the fovea were conducted after cycloplegia and under standardised mesopic light conditions by a swept‐source optical coherence tomography (SS‐OCT, DRI‐OCT Triton, Topcon, Tokyo, Japan). The quality of the scans was indicated by an automated display mode. Choroidal thickness (ChT), the distance between outer choroid‐sclera margin and retinal pigment epithelium‐Bruch's complex, was obtained automatically with the assistance of the built‐in software.
Data from the IOLMaster and autorefractor were automatically extracted into an electronic data capture system (EDC, Solomon, Vision Tech Medical Technology, Guangzhou, China). Other data were initially recorded in the printed case report forms and manually entered into the EDC system on the same day of examination. Data completeness and integrity were checked by trial managers every week.
2.4. Adverse events
A questionnaire on potential adverse events, including but not limited to sudden vision loss of more than two lines, scotoma, dazzling, short‐term glare, flash blindness, and afterimages, was collected from children or their parents/guardians. Two ophthalmologists independently screened the available OCT data to detect any possible structural damages (XRL and ZZT). These were recorded in the case report forms and further entered into the EDC system.
2.5. Outcomes
Outcomes included changes of AL, SER, UCVA, ChT and other ocular parameters during the second year, cumulative changes of these parameters over the course of 2 years, and the potential rebound effect. Adverse events were assessed based on the adverse events questionnaire, visual function indicated by BCVA, and structural changes observed in OCT.
2.6. Statistical analyses
Data from right eyes were used for analyses if right eyes met the enrolment criteria, otherwise, left eyes data were used (n = 2). Missing data were not imputed. Participants who were switched to or combining with myopia treatment other than RLRL and SVS were not included in the analysis.
Axial elongation and SER progression were calculated as the difference between the baseline and the designated follow‐up visit. To ensure the accuracy, only SER data with full cycloplegia were included in the analysis. Treatment efficacy was calculated by dividing the difference between the SVS‐SVS and the other three groups, with the corresponding value in the SVS‐SVS group. BCVA was categorised into two groups: Snellen ≥ 20/20 and Snellen = 20/25 (no participants presented with BCVA < 20/25). All adverse events were recorded in detail.
Data were reported as means with standard deviations (SDs) and medians with interquartile ranges (IQRs) for continuous data, and numbers with percentages for categorical data. Characteristics of participants were compared using unpaired t‐tests, one‐way ANOVA, Mann–Whitney U tests, and Kruskal–Wallis H tests for continuous data and the chi‐square tests for categorical data, as appropriate. One‐way ANOVA was performed to detect the difference among four groups, in terms of changes in AL, SER, and other parameters. Paired t‐tests were conducted to test the difference between the first‐ and the second‐year changes of outcomes in each group. Bonferroni method was used to correct for multiple comparisons. A two‐sided p value less than 0.05 was considered statistically significant. All statistical analyses were conducted using Stata version 15.1 (StataCorp LP, College Station, TX, USA) and R version 4.0.4 (R Foundation for Statistical Computing, www.R-project.org).
3. RESULTS
A total of 138 participants (69.3%) returned to the 24‐month follow‐up visit. Among 138 participants, 12 switched to myopia control treatment other than RLRL and SVS (orthokeratology: n = 5; multi‐focal spectacles: n = 1; combination therapy: n = 6), and 12 used RLRL therapy less than 3 months, both groups were excluded, leaving 114 (57.3% of the eligible) participants in the final analysis (SVS‐SVS: n = 41; SVS‐RLRL: n = 10; RLRL‐SVS: n = 52; RLRL‐RLRL: n = 11; Figure 1). Baseline characteristics of participants included and excluded in the present analysis are shown in Table S1. There were no significant differences in baseline characteristics between those included and excluded in the analysis.
3.1. Characteristics of participants
Table 1 presents the baseline characteristics of participants stratified by the four groups. Participants in the RLRL‐RLRL group were less myopic at the baseline compared to those in the other three groups (RLRL‐RLRL: −1.77 ± 0.57D; SVS‐SVS: −2.76 ± 1.15D; SVS‐RLRL: −2.57 ± 1.11D; RLRL‐SVS: −2.50 ± 0.83D; p = 0.042). The distributions of baseline age, gender, UCVA and AL were well balanced. The first‐year rate of axial elongation and SER progression was slowest for participants in the RLRL‐RLRL group (AL: 0.04 ± 0.25 mm, SER: −0.11 ± 0.58D), followed by those in the RLRL‐SVS (AL: 0.08 ± 0.20 mm, SER: −0.19 ± 0.50D), SVS‐SVS (AL: 0.38 ± 0.19 mm, SER: −0.71 ± 0.42D) and SVS‐RLRL groups (AL: 0.39 ± 0.20 mm, p < 0.001; SER: −0.97 ± 0.42D, p < 0.001), but the difference between the RLRL‐RLRL and RLRL‐SVS groups, and the difference between the SVS‐SVS and SVS‐RLRL groups, were not statistically significant. Participants in the RLRL‐RLRL and RLRL‐SVS groups had similar treatment compliance as in the first year (p = 0.259).
TABLE 1.
Baseline characteristics for the participants stratified by the four groups
| Characteristics | SVS‐SVS | SVS‐RLRL | RLRL‐SVS | RLRL‐RLRL | p Value a |
|---|---|---|---|---|---|
| Number | 41 | 10 | 52 | 11 | |
| Age, years | |||||
| Mean | 10.79 (1.55) | 9.99 (1.53) | 10.46 (1.33) | 11.18 (1.67) | 0.202 |
| Median | 11.16 (9.39, 11.89) | 10.00 (8.41, 11.33) | 10.39 (9.37, 11.46) | 10.76 (10.35, 12.97) | 0.253 |
| Gender | |||||
| Female | 26 (63.41) | 5 (50.00) | 26 (50.00) | 4 (36.36) | 0.358 |
| Male | 15 (36.59) | 5 (50.00) | 26 (50.00) | 7 (63.64) | |
| UCVA, logMAR | |||||
| Mean | 0.29 (0.20) | 0.23 (0.11) | 0.25 (0.14) | 0.25 (0.11) | 0.541 |
| Median | 0.20 (0.20, 0.40) | 0.20 (0.16, 0.25) | 0.20 (0.16, 0.32) | 0.25 (0.12, 0.32) | 0.850 |
| AL, mm | |||||
| Mean | 24.58 (0.94) | 24.75 (0.71) | 24.51 (0.58) | 24.89 (0.94) | 0.450 |
| Median | 24.61 (23.87, 25.16) | 24.99 (24.20, 25.07) | 24.57 (24.05, 24.85) | 24.48 (24.19, 25.58) | 0.499 |
| SER, D | |||||
| Mean | ‐2.76 (1.15) | ‐2.57 (1.11) | ‐2.50 (0.83) | −1.77 (0.57) | 0.042 |
| Median | −2.75 (−3.62, −1.62) | −2.62 (−3.00, −2.25) | −2.38 (−3.25, −1.88) | −1.81 (−2.12, −1.12) | 0.052 |
| 1st year change in AL, mm | |||||
| Mean | 0.38 (0.19) | 0.39 (0.20) | 0.08 (0.20) | 0.04 (0.25) | <0.001 |
| Median | 0.34 (0.24, 0.50) | 0.39 (0.27, 0.53) | 0.08 (−0.05, 0.21) | −0.01 (−0.13, 0.25) | <0.001 |
| 1st year change in SER, D | |||||
| Mean | −0.71 (0.42) | −0.97 (0.42) | −0.19 (0.50) | −0.11 (0.58) | <0.001 |
| Median | −0.62 (−0.88, −0.50) | −0.94 (−1.25, −0.69) | −0.13 (−0.50, 0.12) | −0.19 (−0.50, 0.38) | <0.001 |
| 1st year compliance, % | |||||
| Mean | ‐ | ‐ | 76.28 (26.52) | 85.78 (16.38) | 0.259 |
| Median | ‐ | ‐ | 80.80 (69.88, 92.23) | 86.51 (71.98, 95.53) | 0.365 |
Note: Data are mean (SD), number (%), or median (IQR). Bold values denote statistical significance at the p < 0.05 level.
Abbreviations: RLRL, repeated low‐level red‐light; SVS, single vision spectacle; AL, axial length; SER, spherical equivalent refraction; UCVA, uncorrected visual acuity; D, diopter.
p Values were comparisons among participants in the four groups based on one‐way ANOVA or Kruskal–Wallis H tests for continuous variables, and chi‐square tests for categorical variables.
3.2. Changes in AL and SER in the second year
As shown in Table 2, the mean changes in AL during the second year were 0.28 ± 0.14 mm, 0.05 ± 0.24 mm, 0.42 ± 0.20 mm and 0.12 ± 0.16 mm in the SVS‐SVS, SVS‐RLRL, RLRL‐SVS and RLRL‐RLRL groups, respectively (p < 0.001). The respective mean SER changes were − 0.54 ± 0.39D, −0.09 ± 0.55D, −0.91 ± 0.48D, and − 0.20 ± 0.56D (p < 0.001). Compared with participants in the SVS‐SVS group, axial elongation was significantly reduced for those in the SVS‐RLRL group (p = 0.005). The slowed progression of SER in the SVS‐RLRL group was also noted when compared with that in the SVS‐SVS group, but the significant difference disappeared after Bonferroni correction (P raw = 0.021, p = 0.123). Similar axial elongation and SER progression in the second year were observed between the SVS‐RLRL and RLRL‐RLRL group (p > 0.05). After the cessation of RLRL treatment in the second year, significantly faster axial elongation and SER progression in the RLRL‐SVS group were found compared to those in the SVS‐SVS (p = 0.002 and 0.003, respectively), SVS‐RLRL (p < 0.001 and <0.001, respectively), and RLRL‐RLRL (p < 0.001 and <0.001, respectively) groups.
TABLE 2.
Changes in ocular parameters between the first year and the second year
| (1) SVS‐SVS | (2) SVS‐RLRL | (3) RLRL‐SVS | (4) RLRL‐RLRL | p Value a (overall, 1 vs. 2, 1 vs. 3, 1 vs. 4, 2 vs. 3, 2 vs. 4, 3 vs. 4) | |
|---|---|---|---|---|---|
| AL, mm | |||||
| Baseline to 24 months | 0.64 (0.29) | 0.44 (0.37) | 0.50 (0.28) | 0.16 (0.37) | <0.001, 0.366, 0.156, <0.001, 1.000, 0.206, 0.005 |
| Baseline to 12 months | 0.38 (0.19) | 0.39 (0.20) | 0.08 (0.20) | 0.04 (0.25) | <0.001, 1.000, <0.001, <0.001, <0.001, 0.001, 1.000 |
| 12–24 months | 0.28 (0.14) | 0.05 (0.24) | 0.42 (0.20) | 0.12 (0.16) | <0.001, 0.005, 0.002, 0.083, <0.001, 1.000, <0.001 |
| p Value b | <0.001 | 0.002 | <0.001 | 0.223 | |
| SER, D | |||||
| Baseline to 24 months | −1.24 (0.63) | −0.96 (0.70) | −1.07 (0.69) | −0.31 (0.79) | 0.003, 1.000, 1.000, 0.001, 1.000, 0.325, 0.010 |
| Baseline to 12 months | −0.71 (0.42) | −0.97 (0.42) | −0.19 (0.50) | −0.11 (0.58) | <0.001, 0.931, <0.001, 0.004, <0.001, 0.001, 1.000 |
| 12–24 months | −0.54 (0.39) | −0.09 (0.55) | −0.91 (0.48) | −0.20 (0.56) | <0.001, 0.123, 0.003, 0.258, <0.001, 1.000, <0.001 |
| p Value b | 0.074 | 0.014 | <0.001 | 0.746 | |
| UCVA, logMAR | |||||
| Baseline to 24 months | −0.10 (0.17) | −0.05 (0.11) | −0.06 (0.13) | −0.03 (0.11) | 0.363, 1.000, 1.000, 0.928, 1.000, 1.000, 1.000 |
| Baseline to 12 months | −0.07 (0.16) | −0.02 (0.18) | 0.01 (0.12) | 0.02 (0.09) | 0.033, 1.000, 0.035, 0.288, 1.000, 1.000, 1.000, |
| 12–24 months | −0.03 (0.08) | −0.03 (0.10) | −0.07 (0.10) | −0.04 (0.06) | 0.183, 1.000, 0.209, 1.000, 1.000, 1.000, 1.000 |
| p Value b | 0.122 | 0.859 | 0.004 | 0.160 | |
| ChT, μm | |||||
| Baseline to 24 months | −16.09 (19.37) | −2.93 (25.64) | −8.66 (24.68) | 21.49 (36.21) | 0.004, 1.000, 1.000, 0.002, 1.000, 0.292, 0.012 |
| Baseline to 12 months | −8.28 (9.72) | −15.57 (12.79) | 5.47 (22.89) | 20.86 (27.69) | <0.001, 1.000, 0.204, 0.005, 0.102, 0.004, 0.261 |
| 12–24 months | −7.46 (14.78) | 8.25 (25.01) | −18.23 (23.14) | 12.34 (18.78) | 0.001, 0.798, 0.711, 0.233, 0.041, 1.000, 0.003 |
| p Value b | 0.587 | 0.109 | 0.002 | 0.789 | |
| ACD, mm | |||||
| Baseline to 24 months | 0.02 (0.07) | 0.06 (0.05) | 0.02 (0.08) | 0.04 (0.08) | 0.331, 0.547, 1.000, 1.000, 0.549, 1.000, 1.000 |
| Baseline to 12 months | 0.00 (0.06) | 0.07 (0.09) | 0.01 (0.07) | 0.04 (0.07) | 0.027, 0.032, 1.000, 0.961, 0.055, 1.000, 1.000 |
| 12–24 months | 0.02 (0.09) | −0.01 (0.07) | 0.01 (0.07) | 0.00 (0.06) | 0.781, 1.000, 1.000, 1.000, 1.000, 1.000, 1.000 |
| p Value b | 0.448 | 0.184 | 0.816 | 0.250 | |
| CC, D | |||||
| Baseline to 24 months | −0.03 (0.24) | −0.14 (0.12) | 0.01 (0.21) | −0.05 (0.12) | 0.252, 0.891, 1.000, 1.000, 0.311, 1.000, 1.000 |
| Baseline to 12 months | 0.02 (0.26) | −0.02 (0.14) | 0.03 (0.23) | −0.01 (0.11) | 0.911, 1.000, 1.000, 1.000, 1.000, 1.000, 1.000 |
| 12–24 months | −0.05 (0.15) | −0.12 (0.14) | −0.02 (0.16) | −0.04 (0.10) | 0.308, 1.000, 1.000, 1.000, 0.385, 1.000, 1.000 |
| p Value b | 0.231 | 0.276 | 0.304 | 0.623 | |
Note: Bold values denote statistical significance at the p < 0.05 level. Abbreviations: RLRL, repeated low‐level red‐light; SVSL, single vision spectacle; AL, axial length; SER, spherical equivalent refraction; D, diopter; UCVA, uncorrected visual acuity; ACD, anterior chamber depth; CC, corneal curvature; ChT, choroidal thickness.
p Values were the comparisons among the four groups based on one‐way ANOVA and Bonferroni correction was applied for the pairwise comparisons.
p Values were the comparisons between values of baseline to 12 months and 12–24 months based on paired t‐tests.
3.3. Comparison between the second and the first year
Axial elongation and SER progression were reduced in the SVS‐SVS group between the second and the first year (axial elongation: p < 0.001, SER progression: p = 0.074, Table 3). Similar myopia progression was observed in the RLRL‐RLRL group over 2 years (axial elongation: p = 0.223, SER progression: p = 0.746, Table 3). Axial elongation and SER progression were significantly decreased for those who wore SVS in the first year and switched to RLRL therapy in the second year (axial elongation: p = 0.002, SER progression: p = 0.014). On the contrary, significantly increased rates of progression in AL and SER were found after the cessation of RLRL therapy in the second year (all ps <0.001).
TABLE 3.
Changes of best corrected visual acuity from baseline to 24 months
| Time (months) | N (%) | |||
|---|---|---|---|---|
| SVS‐SVS | SVS‐RLRL | RLRL‐SVS | RLRL‐RLRL | |
| BCVA ≥ 20/20 | ||||
| 0 | 41 (100.00) | 10 (100.00) | 52 (100.00) | 11 (100.00) |
| 12 | 38 (95.00) | 9 (90.00) | 50 (96.15) | 11 (100.00) |
| 24 | 39 (95.12) | 10 (100.00) | 51 (98.08) | 11 (100.00) |
| BCVA = 20/25 | ||||
| 0 | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| 12 | 2 (5.00) | 1 (10.00) | 2 (3.85) | 0 (0.00) |
| 24 | 2 (4.88) | 0 (0.00) | 1 (1.92) | 0 (0.00) |
Abbreviations: RLRL, repeated low‐level red‐light; SVS, single vision spectacle; BCVA, best‐corrected visual acuity.
3.4. Changes in AL and SER over 2 years
The cumulative mean changes in AL and SER over 2 years are shown in Table 2, Figure 2, and Table S2. The overall axial elongation was the smallest in the RLRL‐RLRL group (0.16 ± 0.37 mm), followed by SVS‐RLRL (0.44 ± 0.37 mm), RLRL‐SVS (0.50 ± 0.28 mm) and SVS‐SVS groups (0.64 ± 0.29 mm; p < 0.001). Relative to the SVS‐SVS group, the efficacy of myopia control regarding axial elongation were 31.3%, 21.9% and 75.0% in the SVS‐RLRL, RLRL‐SVS and RLRL‐RLRL groups.
FIGURE 2.
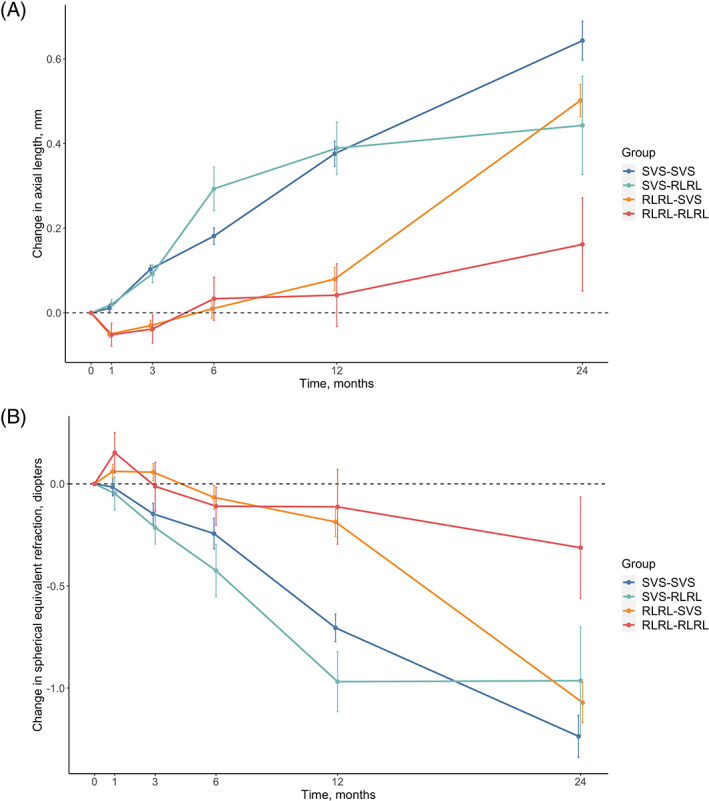
Mean changes in axial length and cycloplegic spherical equivalent refractions from baseline to 24 months. (A) For axial elongation; (B) for myopia progression. RLRL, repeated low‐level red‐light; SVS, single vision spectacle
The cumulative mean changes in SER were −1.24 ± 0.63D, −0.96 ± 0.70D, −1.07 ± 0.69D and − 0.31 ± 0.79D in the SVS‐SVS, SVS‐RLRL, RLRL‐SVS, and RLRL‐RLRL groups, respectively (p = 0.003). Similarly, the RLRL‐RLRL group achieved the best efficacy in controlling SER progression (75.0%) when compared with the SVS‐SVS group, followed by the SVS‐RLRL (22.6%) and RLRL‐SVS (13.7%) groups.
3.5. Changes in other ocular parameters
The changes in other ocular parameters are summarised in Table 2. Changes in UCVA, ACD and CC were similar across the four groups not only during the second year but also during the 2 years. During the second year, there were improvements in ChT in the SVS‐RLRL (8.25 ± 25.01 μm) and RLRL‐RLRL (12.34 ± 18.78 μm) groups, while the RLRL‐SVS (−18.23 ± 23.14 μm) and SVS‐SVS (−7.46 ± 14.78 μm) groups had reductions in ChT. Comparing the second year with the first year, ChT changes in the RLRL‐RLRL (12.34 ± 18.78 μm vs. 20.86 ± 27.69 μm, p = 0.789) and SVS‐SVS (−7.46 ± 14.78 μm vs. −8.28 ± 9.72 μm, p = 0.587) groups were comparable. However, in the RLRL‐SVS group, ChT was significantly decreased in the second year compared with that in the first year (−18.23 ± 23.14 μm vs. 5.47 ± 22.89 μm, p = 0.002). ChT was increased in the second year in the SVS‐RLRL group compared with that in the first year (8.25 ± 25.01 μm vs. −15.57 ± 12.79 μm), but the difference did not reach the statistical significance (p = 0.109). Over the 2 years, ChT increased on average by 21.49 ± 36.21 μm in the RLRL‐RLRL group, while the thinning in ChT was noted in the other three groups.
3.6. Adverse events
In the 24‐month follow‐up, none of the participants reported having side effects or a severe adverse event. All participants in the RLRL‐RLRL and SVS‐RLRL groups achieved a BCVA of at least 20/20 (Table 3). One participant in the RLRL‐SVS group and two in the SVS‐SVS group had the BCVA of 20/25. No structural damage on the photosensory layer was found for participants with available OCT images (n = 78).
4. DISCUSSION
Over 2 years, continued RLRL therapy (i.e., RLRL‐RLRL group) achieved the best control on axial elongation and SER progression (75.0% and 75.0% efficacy, respectively), followed by the SVS‐RLRL (31.3% and 22.6%, respectively) and RLRL‐SVS (21.9% and 13.7%, respectively) groups, compared with the SVS‐SVS group. Our study also showed that a modest rebound effect was noted after cessation of RLRL therapy for 1 year. In addition, continued RLRL therapy over the 2 years was well tolerated and no documented functional and structural damages were observed.
4.1. Continuing RLRL therapy in the second year
Compared with the participants who discontinued RLRL therapy in the second year, participants who continued RLRL therapy showed the best efficacy in slowing axial elongation and SER progression. In the present study, the baseline mean age of the children was 10.46 and 11.18 years for the RLRL‐SVS and RLRL‐RLRL groups, respectively. Myopia progression continues and may not be stabilised until the age of 16 years for Asian children. 10 Therefore, the continuation of RLRL therapy may confer further benefits for myopia control.
4.2. Reduction in myopia progression after switching to RLRL therapy
Participants in the SVS‐RLRL group were found to have a reduction in axial elongation and SER progression after starting the RLRL therapy during the second year. The second‐year myopia progression in the SVS‐RLRL group (AL: 0.05 mm, SER: −0.09D) was similar to the first‐year progression in the RLRL‐RLRL (AL: 0.04 mm, SER: −0.11D) and the RLRL‐SVS groups (AL: 0.08 mm, SER: −0.19D). Relative to the SVS‐SVS group, the treatment efficacies over 2 years were 31.3% and 22.6% in the SVS‐RLRL group, in terms of axial elongation and SER progression, respectively. These findings suggested that a comparable myopia control effect was achieved even though the RLRL therapy was started 1 year later.
4.3. Efficacy in the first versus second year
The RLRL treatment efficacy in axial elongation during the first and the second year was 89.5% (RLRL‐RLRL: 0.04 mm, SVS‐SVS: 0.38 mm) and 57.1% (RLRL‐RLRL: 0.12 mm, SVS‐SVS: 0.28 mm), respectively. Similarly, the efficacy in slowing the SER progression reduced from 84.5% (RLRL‐RLRL: −0.11D, SVS‐SVS: −0.71D) to 63.0% (RLRL‐RLRL:‐0.20D, SVS‐SVS:‐0.54D). The loss of the treatment efficacy over time was in line with the pattern observed in a recent pooled analysis of different myopia interventions, where absolute efficacy in slowing axial elongation was decreased after year one. 11 Specifically, the first‐year reduction in axial elongation was 0.34 and 0.19 mm and then was reduced to 0.06 and 0.07 mm in the second year for atropine 1% 12 and orthokeratology, 13 respectively. The natural deceleration of AL growth with age might partly explain the loss of absolute treatment efficacy, as evidenced by the significant reduction in axial elongation in the second year in the SVS‐SVS group compared with that in the first year (0.38 vs. 0.28 mm, p < 0.001). However, the relative efficacy of RLRL therapy also decreased by 22%–32% in the second year, suggesting other as yet unknown mechanisms might also contribute to the diminishing efficacy.
4.4. Two‐year efficacy in comparison with other interventions
The 2‐year changes in AL and SER in the RLRL‐RLRL group were 0.16 ± 0.37 mm and −0.31 ± 0.79D, respectively. The cumulative, absolute reduction in axial elongation and SER progression was 0.48 mm and 0.93D compared with the SVS‐SVS group, representing the treatment efficacy of 75.0% and 75.0%, respectively. Compared with other myopia control interventions, RLRL seems to have better efficacy in slowing myopia progression. Atropine 0.05% produced a 64.5% reduction in myopia progression over 2 years. 14 The cumulative, absolute reduction in axial elongation with a range of 0.27–0.32 mm of orthokeratology has been reported, representing treatment efficacy ranging from 43% to 63%. 15 , 16 The efficacy of Defocus Incorporated Multiple Segments spectacles in slowing axial elongation and SER progression was 62%(0.34 mm) and 52%(0.44D), respectively. 17 MiSight lens reduced the axial elongation and SER progression by 38.4% and 37.8%, respectively. 18 In general, soft multifocal contact lenses have shown to be less efficacious in slowing myopia progression with a relative efficacy of 25%–60%. 18 , 19 , 20 , 21 Direct comparison of the current study with other studies should be done with caution due to differences in the study design, characteristics of the study sample, and selection of the control group. Further, the possibility of overestimating the efficacy of RLRL therapy cannot be ruled out, as the non‐randomization in the second year resulted in those who had better efficacy in myopia control and/or better compliance to the treatment in the first year being more likely to continue the RLRL therapy. This was supported by the evidence that the axial elongation and SER progression during the first year in the RLRL‐RLRL group (0.04 ± 0.25 mm and −0.11 ± 0.58D, respectively) were less than that in the RLRL group (0.12 ± 0.23 mm and −0.18 ± 0.54D, respectively) from the original RCT study, and that the high level of compliance in the RLRL‐RLRL group during the second year (99.25%) was noted.
4.5. Rebound effect
The observed second‐year axial elongation was 0.42 mm in the RLRL‐SVS group when the RLRL treatment was stopped. Similarly, the SER progression in this group was −0.91D. This rate of myopia progression was greater than the observed second year progression (AL: 0.28 mm; SER: −0.54D) but similar to the first year progression (AL: 0.38 mm, SER: −0.71D) in the SVS‐SVS group, which suggested a modest rebound effect. A rebound phenomenon has been observed in other myopia interventions. 11 , 22 , 23 , 24 , 25 After the discontinuation of atropine 1% for 1 year, the mean elongation in AL was 0.31 mm. 12 , 24 Acceleration of axial elongation (0.15 mm) was also demonstrated after cessation of orthokeratology for 7 months. 23 The greater magnitude of rebound effect noted for the RLRL therapy might be explained by its greater efficacy, supported by the findings from the Atropine for the Treatment of Myopia and the Low‐concentration atropine for myopia progression study. 12 , 14 , 22 , 24 , 25 Alternatively, the faster inherent physiological progression in the present study might also contribute to the greater rebound effect.
4.6. Safety
In this 2‐year study, the RLRL therapy was well‐tolerated. Children who initiated the RLRL therapy in the second year or those who continued the RLRL therapy did not report any side effects or severe adverse events. In addition, no functional vision loss indicated by BCVA or structural damage seen on OCT was documented. Altogether, our findings suggested that the RLRL therapy was well tolerated. However, since there are no longer‐term studies on the effect of the RLRL therapy on retinal structure and functions, continued vigilance is necessary. Nevertheless, the device used for the RLRL therapy was originally used for amblyopia treatment and has been widely used in the past decade in China, ano long‐term adverse effects associated with its use have been reported.
4.7. Clinical perspective
An ultimate goal of myopia control is to slow axial elongation and SER progression when myopia is highly progressive, thus leading to a reduction in the incidence of high myopia and its related blinding complications. Our results firstly indicated that RLRL therapy for periods up to 2 years slowed the myopia progression by 75% and was a clinically viable treatment of myopia with a sustained effect on myopia progression. The cumulative treatment efficacy indicated by comparing the 2‐year progression among the RLRL‐RLRL and SVS‐SVS groups appears to be maintained over time. In addition, the good user acceptability and safety for the period of 2 years, together with the fact that the device has been widely used in the past decade indicates that the use of the RLRL therapy for up to 2 years remains safe. Secondly, the rebound effect observed in children stopping the RLRL therapy for 1 year indicated that abrupt cessation of RLRL therapy is not appropriate, although the rebound effect did not completely obliterate the earlier benefits from the RLRL therapy (RLRL‐SVS: 0.50 ± 0.28 mm; SVS‐SVS: 0.64 ± 0.29 mm). A gradual reduction in the treatment session is likely beneficial for minimising the rebound effect, as has been suggested for atropine. 22 , 26
4.8. Strengths and limitations
To the best of our knowledge, this was the first study to investigate the long‐term efficacy and safety of RLRL therapy over 2 years for myopia control. This was also the first to date to evaluate the rebound effect of RLRL therapy following treatment cessation. A strength of the present study was the presence of a control group on the natural myopia progression over 2 years. Several limitations should also be noted. Firstly, the current study is a post‐trial follow‐up research, and the participants were not randomly assigned to continuation or cessation of the treatment. This could introduce selection bias, if, for example, those who experienced better treatment efficacy were more likely to voluntarily stay in the treatment arm. However, the baseline characteristics across the four groups were well balanced, except for less myopic SER in the RLRL‐RLRL group, which might bias the results. Secondly, the number of participants on RLRL therapy, either in the RLRL‐RLRL or SVS‐RLRL group, were significantly smaller. This problem might compromise the power to detect the differences among groups and identification of the risk factors. Large‐scale and well‐designed studies are needed to corroborate our findings in the future. Finally, the current study was limited to Chinese children, therefore the generalizability of our findings to other ethnicities remained to be explored.
In conclusion, it was highly suggestive that RLRL therapy had sustained efficacy in controlling myopia progression and was well tolerated in Chinese children aged 8–13 years over 2 years. A modest rebound effect was found after the cessation of RLRL therapy. Further evidence is required to elucidate the mechanisms of the RLRL therapy, to explore the efficacy and safety of the RLRL therapy beyond 2 years, to identify the determinants of the rebound effect, and to investigate the optimal dosimetric parameters for the RLRL therapy with a good balance between efficacy and rebound effect.
CONFLICT OF INTEREST
MH and ZZT are listed as inventors on the patents and patent applications related to the study (CN201910490186.6). MH is director and shareholder in Eyerising Ltd and Eyerising International Pty Ltd. No other potential conflicts of interest relevant to this article were reported.
Supporting information
Table S1: Comparison between the baseline characteristics for the participants included and excluded in the analysis.
Table S2: Mean changes in the axial length and cycloplegic spherical equivalent refraction from baseline to 24 months at each timepoint.
ACKNOWLEDGEMENTS
We are indebted to the participants who were involved in this trial, without whom the trial would not have been possible. We also thank members of the Data Safety Monitoring Committee, who provided valuable input throughout the study: Ian G. Morgan, Leon B. Ellwein, Robert Chang and Lei Zhang. Open access publishing facilitated by The University of Melbourne, as part of the Wiley ‐ The University of Melbourne agreement via the Council of Australian University Librarians.
Xiong R, Zhu Z, Jiang Y, et al. Sustained and rebound effect of repeated low‐level red‐light therapy on myopia control: A 2‐year post‐trial follow‐up study. Clin Experiment Ophthalmol. 2022;50(9):1013‐1024. doi: 10.1111/ceo.14149
Ruilin Xiong, Zhuoting Zhu, Yu Jiang and Xiangbin Kong contributed equally to this work.
Funding information Fundamental Research Funds of the State Key Laboratory of Ophthalmology, Grant/Award Number: 303060202400362; National Natural Science Foundation of China, Grant/Award Numbers: 81271037, 81420108008
REFERENCES
- 1. Holden BA, Fricke TR, Wilson DA, et al. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. 2016;123:1036‐1042. [DOI] [PubMed] [Google Scholar]
- 2. Sankaridurg P, Tahhan N, Kandel H, et al. IMI impact of myopia. Invest Ophthalmol Vis Sci. 2021;62:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huang J, Wen D, Wang Q, et al. Efficacy comparison of 16 interventions for myopia control in children: a network meta‐analysis. Ophthalmology. 2016;123:697‐708. [DOI] [PubMed] [Google Scholar]
- 4. Gong Q, Janowski M, Luo M, et al. Efficacy and adverse effects of atropine in childhood myopia: a meta‐analysis. JAMA Ophthalmol. 2017;135:624‐630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bullimore MA, Johnson LA. Overnight orthokeratology. Cont Lens Anterior Eye. 2020;43:322‐332. [DOI] [PubMed] [Google Scholar]
- 6. Jiang Y, Zhu Z, Tan X, et al. Effect of repeated low‐level red‐light therapy for myopia control in children: a multicenter randomized controlled trial. Ophthalmology. 2022;129:509‐519. [DOI] [PubMed] [Google Scholar]
- 7. Xiong F, Mao T, Liao H, et al. Orthokeratology and low‐intensity laser therapy for slowing the progression of myopia in children. Biomed Res Int. 2021;2021:8915867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhou L, Xing C, Qiang W, Hua C, Tong L. Low‐intensity, long‐wavelength red light slows the progression of myopia in children: an eastern China‐based cohort. Ophthalmic Physiol Opt. 2022;42:335‐344. [DOI] [PubMed] [Google Scholar]
- 9. International Electrotechnical Commission %J 3IEC G. IEC 60825–1: 2014 Safety of laser products‐Part 1: Equipment classification and requirements; 2014.
- 10. Group C . Myopia stabilization and associated factors among participants in the correction of myopia evaluation trial (COMET). Invest Ophthalmol Vis Sci. 2013;54:7871‐7884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brennan NA, Toubouti YM, Cheng X, Bullimore MA. Efficacy in myopia control. Prog Retin Eye Res. 2021;83:100923. [DOI] [PubMed] [Google Scholar]
- 12. Chua WH, Balakrishnan V, Chan YH, et al. Atropine for the treatment of childhood myopia. Ophthalmology. 2006;113:2285‐2291. [DOI] [PubMed] [Google Scholar]
- 13. Hiraoka T, Kakita T, Okamoto F, Takahashi H, Oshika T. Long‐term effect of overnight orthokeratology on axial length elongation in childhood myopia: a 5‐year follow‐up study. Invest Ophthalmol Vis Sci. 2012;53:3913‐3919. [DOI] [PubMed] [Google Scholar]
- 14. Yam JC, Li FF, Zhang X, et al. Two‐year clinical trial of the low‐concentration atropine for myopia progression (LAMP) study: phase 2 report. Ophthalmology. 2020;127:910‐919. [DOI] [PubMed] [Google Scholar]
- 15. Charm J, Cho P. High myopia‐partial reduction ortho‐k: a 2‐year randomized study. Optom Vis Sci. 2013;90:530‐539. [DOI] [PubMed] [Google Scholar]
- 16. Cho P, Cheung SW. Retardation of myopia in orthokeratology (ROMIO) study: a 2‐year randomized clinical trial. Invest Ophthalmol Vis Sci. 2012;53:7077‐7085. [DOI] [PubMed] [Google Scholar]
- 17. Lam CSY, Tang WC, Tse DY, et al. Defocus incorporated multiple segments (DIMS) spectacle lenses slow myopia progression: a 2‐year randomised clinical trial. Br J Ophthalmol. 2020;104:363‐368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ruiz‐Pomeda A, Perez‐Sanchez B, Valls I, Prieto‐Garrido FL, Gutierrez‐Ortega R, Villa‐Collar C. MiSight assessment study Spain (MASS). A 2‐year randomized clinical trial. Graefes Arch Clin Exp Ophthalmol. 2018;256:1011‐1021. [DOI] [PubMed] [Google Scholar]
- 19. Lam CS, Tang WC, Tse DY, Tang YY, To CH. Defocus incorporated soft contact (DISC) lens slows myopia progression in Hong Kong Chinese schoolchildren: a 2‐year randomised clinical trial. Br J Ophthalmol. 2014;98:40‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chamberlain P, Peixoto‐de‐Matos SC, Logan NS, Ngo C, Jones D, Young G. A 3‐year randomized clinical trial of MiSight lenses for myopia control. Optom Vis Sci. 2019;96:556‐567. [DOI] [PubMed] [Google Scholar]
- 21. Walline JJ, Walker MK, Mutti DO, et al. Effect of high add power, medium add power, or single‐vision contact lenses on myopia progression in children: the BLINK randomized clinical trial. JAMA. 2020;324:571‐580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yam JC, Zhang XJ, Zhang Y, et al. Three‐year clinical trial of low‐concentration atropine for myopia progression (LAMP) study: continued versus washout. Phase 3 report. Ophthalmology. 2022;129(3):308–21. [DOI] [PubMed] [Google Scholar]
- 23. Cho P, Cheung SW. Discontinuation of orthokeratology on eyeball elongation (DOEE). Cont Lens Anterior Eye. 2017;40:82‐87. [DOI] [PubMed] [Google Scholar]
- 24. Tong L, Huang XL, Koh AL, Zhang X, Tan DT, Chua WH. Atropine for the treatment of childhood myopia: effect on myopia progression after cessation of atropine. Ophthalmology. 2009;116:572‐579. [DOI] [PubMed] [Google Scholar]
- 25. Chia A, Chua WH, Wen L, Fong A, Goon YY, Tan D. Atropine for the treatment of childhood myopia: changes after stopping atropine 0.01%, 0.1% and 0.5%. Am J Ophthalmol. 2014;157(451–7):e1. [DOI] [PubMed] [Google Scholar]
- 26. Chia A, Lu QS, Tan D. Five‐year clinical trial on atropine for the treatment of myopia 2: myopia control with atropine 0.01% Eyedrops. Ophthalmology. 2016;123:391‐399. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Comparison between the baseline characteristics for the participants included and excluded in the analysis.
Table S2: Mean changes in the axial length and cycloplegic spherical equivalent refraction from baseline to 24 months at each timepoint.


