Abstract
Objective: Repeated pregnancy loss has been shown to be related to decidual immune imbalance. Metformin has been found to promote a shift in the Th17/Treg balance towards immune tolerance. Our research aims to evaluate and obtain further information on the role and potential mechanism of metformin on Th17/Treg balance in the early pregnancy decidua. Methods: Decidual immune cells from normal pregnancy women were treated with metformin, pro-inflammatory cytokines or metformin + cytokines respectively. The mRNA expression levels of STAT3, STAT5, RORC and Foxp3 were detected by qRT-PCR. The proportions of Th17 and Treg cells, the stability of Treg cells, and the STATs phosphorylation levels of T cells were evaluated by flow cytometry. The cytokine concentrations in the culture medium were detected by ELISA. Results: After treated with metformin, indicators related to immune tolerance, including the mRNA expression and phosphorylation levels of STAT5, mRNA expression level of Foxp3, the proportion of Treg cell, and the IL-10 concentration increased significantly. Indicators related to immune rejection including the mRNA expression level of STAT3, the proportion of Th17 cell, and the IL-17A concentration showed a significant decrease. In inflammatory conditions, the proportion of Th-like Treg cells increased. Metformin promoted CD25 expression to maintain Treg cell stability. Conclusion: Metformin has beneficial effects on immunological tolerance at the maternal-foetal interface in early pregnancy. The underlying mechanism may be that metformin restores the Th17/Treg balance by changing the expression of STATs, which is conducive to establishing maternal-foetal immune tolerance.
Keywords: Metformin, regulatory T cells, T helper 17 cells, repeated pregnancy loss, decidual immune cells, STATs
Introduction
Metformin is a kind of biguanide antidiabetic drug and one of the most generally used drugs for the treatment of type 2 diabetes and insulin resistance-related diseases. Over the last few years, with the deepening of research, the indications of metformin have been expanded to weight loss, gestational diabetes-related infertility, polycystic ovary syndrome (PCOS), etc. It was first discovered in 2001 that metformin could improve fertility outcomes in PCOS patients who were suffering insulin resistance [1]. Metformin treatment improves fertility through multiple mechanisms, including restoring the menstrual cycle, reducing the incidence of caesarean section and limiting preterm birth [2]. Furthermore, it has been reported that metformin improves metabolism, endocrine function and angiogenesis to prevent early pregnancy loss [3].
The definition of recurrent pregnancy loss (RPL) is the failure of two or more clinically recognized pregnancies before 20-24 weeks of gestation, and the prevalence is approximately 2-5% in women trying to conceive [4-6]. The aetiology of RPL is multifactorial, including antiphospholipid syndrome, anatomical uterine structural anomalies, endocrine disorders, inherited thrombophilia, poor endometrial receptivity and parental chromosomal anomalies [7,8]. However, in 40-50% of the cases, the cause cannot be determined, and patients are diagnosed with idiopathic or unexplained RPL [4]. Immune disorders appear to be the risk factors in numerous cases of RPL and are considered to play a vital part in the aetiology [9,10].
It has been reported that CD4+ T cells differentiate into Th17 cells under the action of IL-6 and transforming growth factor-β1 (TGF-β1), and differentiate into Treg cells under the stimulation of TGF-β1 alone [11]. Another study showed that IL-23 was involved in the functional maintenance of human decidual immune cells (DICs) and the promotion of their differentiation into Th17 cells [12]. The works of our group [13] and other scholars [14] have used this multi-cytokine activation as a reasonable model to simulate the pathological changes of RPL DICs and to analyse the relationship between these changes and maternal-foetal immune rejection. To study the underlying mechanism, we stimulated DICs with IL-23, IL-6 and TGF-β1 to mimic the immune-inflammatory environment in the uterine cavity of patients with RPL and then studied how metformin affects the Th17/Treg balance in early pregnancy.
Metformin has been found to enhance the Treg cell bias in the Treg/Th17 balance in leukaemia, fibrosarcoma, melanoma [15], non-small cell lung carcinoma, intestinal carcinoma [16], and Behcet’s disease [17]. Since these immune-related diseases, including RPL, share a common pathogenesis of Treg/Th17 imbalance, we hypothesized that metformin may have a potential therapeutic value in pregnancy disorders [11,18]. To test this hypothesis, we used human DICs to examine how metformin impacts the balance between Treg cells and Th17 cells in first-trimester pregnancy and to explore potential molecular mechanisms.
Materials and methods
Human decidual tissue collection
The research protocol was approved by the Ethics Committee of Guangxi Medical University First Affiliated Hospital, and written informed consent was obtained from all participants. Decidual samples were collected from 12 healthy women with a normal pregnancy (mean age 26.47±3.68, mean gestational age 50.8±3.38 days) who sought an artificial abortion due to unintended pregnancy at the Department of Obstetrics and Gynecology, Guangxi Medical University First Affiliated Hospital between November 2017 and October 2018. The following eligibility criteria were used: women who received an artificial abortion due to unintended pregnancy; women with age of 25-30 years; women with regular menstruation before pregnancy; women with confirmed intrauterine pregnancy and a gestational age between 42 and 63 days; women with no symptoms and signs of threatened abortion, such as abdominal pain or vaginal bleeding; women with observed foetal heartbeat by transvaginal ultrasound. The exclusion criteria included: women with a history of menstrual disorder; women with harmful substances during pregnancy; women with contact to radioactive substances; women with spontaneous abortion, hereditary disease, chronic systemic disease, immunological disease, abdominal pain, vaginal bleeding, chills and fever; women with multiple pregnancy; women with abnormal examination, reproductive system anatomy or couple ABO blood group incompatibility; women whose partner had semen abnormalities. Before performing the induced abortion by vacuum aspiration, no patients had been treated with misoprostol or mifepristone.
After the artificial abortion procedures, decidua from aborted tissue was collected and rinsed twice with sterile phosphate buffered saline. The decidua was stored in prechilled RPMI 1640 medium (Invitrogen, USA) containing antibiotics (100 IU/mL penicillin and 100 mg/mL streptomycin) before DICs were isolated. The villous tissue and necrotic tissue were removed carefully.
Isolation and treatment of human first-trimester DICs
DIC separation was performed as described previously [19]. In short, DICs were aggregated with a density ranging from 1.056 to 1.077 g/ml after enzymatic digestion and discontinuous gradient centrifugation. Considering the phenotypic changes in different subjects, there was no mixing of DICs from different patients.
After coating with 10 μg/ml anti-CD3 antibody (eBioscience, USA) at 4°C overnight, 2.0 × 106/well DICs were seeded on 6-well plates. Then, recombinant human IL-2 (rhIL-2, 50 ng/ml; PeproTech, USA) and anti-CD28 antibodies (1 mg/ml; eBioscience, USA) were added to the medium to stimulate DIC activation. To study the effect of metformin on DICs, the wells were divided into 4 groups and cultured for 72 hours. The metformin (MET) group received 1 μM metformin. The pro-inflammatory cytokine (CTK) group received 10 ng/ml IL-23, 5 ng/ml TGF-β1 and 20 ng/ml IL-6. The metformin + cytokines (MET+CTK) group was treated with the pro-inflammatory cytokines mentioned previously for 24 h and then with 1 μM metformin for the next 48 h. The control group did not receive any special treatments. All the experiments were performed in triplicate.
Real-time quantitative polymerase chain reaction (qPCR)
After treatment for 72 h, 2 × 106 DICs were harvested. By using TRIzol reagent (Invitrogen), total cell RNA was extracted. Following the manufacturer’s detailed instructions, complementary DNA (cDNA) was generated using 5 × All-In-One RT MasterMix (ABM, Canada). Real-time qPCR was performed on an Agilent Strata Gene Mx3005 (Stratagene, USA) by using commercial primers specific for human Foxp3 (primer ID HQP055129), human RORC (primer ID HQP016379), human STAT3 (primer ID HQP017767), human STAT5 (primer ID HQP017774) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH; primer ID HQP006940) (GeneCopoeia Inc., USA). The expression levels were standardized to the GAPDH level. The specificity of all the primers was verified before marketing by the manufacturer. The samples were subjected to 40 amplification cycles using the EvaGreen 5 × qPCR MasterMix (ABM, Canada) with a reaction volume of 20 μl. The reaction conditions were set to 95°C for 10 minutes, and then 40 cycles at 95°C for 15 s and 60°C for 60 s. Fluorescence data were collected at 72-95°C to reduce the number of non-specific signals, and amplification of specific transcripts was identified by evaluating the melting curve at the end of each PCR. Data from each sample were obtained in triplicate. The relative quantity of target mRNA was calculated from the ΔCt values of the evaluated target and endogenous reference gene GAPDH by using the 2-ΔΔCt cycle threshold method.
Flow cytometry
All the reagents and antibodies used for flow cytometry were manufactured by BD Biosciences unless otherwise stated.
For Th17 cell analysis, cells were stimulated with phorbol 12-myristate 13-acetate (25 ng/ml), ionomycin (1 μg/ml) and GolgiStop (2 μl) for 5 h before staining. Then, the DICs were stained with a PerCP/Cy 5.5-conjugated anti-CD4 antibody and a phycoerythrin (PE)-conjugated anti-human IL-17 antibody according to the manual instructions and procedures.
For Treg cell analysis, the cells were stained with a PerCP-Cy5.5-conjugated anti-human CD4 antibody, a PE-conjugated anti-human CD25 antibody and a monoclonal antibody Alexa Fluor 647-conjugated anti-Foxp3.
For flow cytometry analysis of phosphorylated STAT3 and STAT5, cells were fixed, permeabilized, washed, and then incubated with a PerCP-Cy5.5-conjugated anti-human CD4 antibody and PE-conjugated anti-human STAT3 (pY705) or AlexaFluor®488-conjugated anti-STAT5 (pY694) antibody.
All stained cells were detected using CellQuest software and analysed using FlowJo v10 software (TreeStar, USA).
Enzyme-linked immunosorbent assay (ELISA)
All the culture medium was collected and centrifuged for 5 min at 450 g after 72 hours of cultivation. According to the manufacturer’s instructions, the collected supernatant was used to measure the levels of IL-10 and IL-17A by ELISA (RayBiotech, USA). Samples were tested in triplicate. The ELISA kit sensitivities were as follows: > 1 pg/ml (IL-10) and > 80 pg/ml (IL-17A).
Statistical analysis
Statistical analysis was performed with SPSS 22.0 (IBM SPSS Inc., USA). Before analysis, the Levene’s test was used to evaluate the homogeneity of variance in the data. Results of IL-17A and IL-10 expression were presented as the median, interquartile range and mean in nonparametric tests. Parametric test results, which include the percentages of Th17, Treg, pSTAT3, and pSTAT5 cells and the mRNA expression of STAT3, STAT5, Foxp3, and RORC, were shown as mean ± SD. In parametric tests, comparisons among multiple groups were conducted by one-way ANOVA. For nonparametric analyses, rank-sum test was used to compare the expression of IL-17A or IL-10, and the Kruskal-Wallis method was used to compare between groups. P < 0.05 was considered as statistically significant.
Results
Metformin promoted the differentiation of DICs into Treg cells and inhibited the differentiation of DICs into Th17 cells
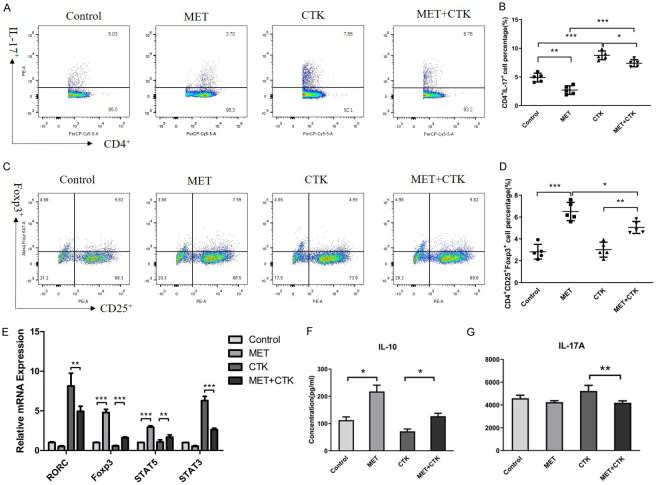
The percentages of Treg and Th17 cells in DICs treated with metformin and cytokines were compared. The cell percentage of Th17/CD4+ in the CTK group was significantly higher than that in the control group (P < 0.001; Figure 1A, 1B). This suggests that pro-inflammatory cytokines can aggrandize the population of Th17 cells, which is consistent with the immune changes in the decidua of patients with RPL, indicating that the treatment can effectively simulate these pathological conditions. The percentage of Th17 cells in the MET group and the MET+CTK group was significantly lower than that in the control group and the CTK group, respectively (Figure 1A). This implies that metformin can significantly reduce the percentage of Th17 cells in both normal conditions and the inflammatory environment. In addition, the percentage of Th17 cells in the MET+CTK group was remarkably higher than that in the MET group.
Figure 1.
Effect of metformin on the Th17/Treg cell of decidual immune cells in early pregnancy. Effects of metformin and pro-inflammatory cytokines on the ratio of Treg and Th17 cells in DICs. (A) Representative flow cytometric analysis of Th17 cells in each group. (B) In the normal state and inflammatory environment, metformin reduced the proportion of the Th17 cells. (C) Representative flow cytometric analysis of Treg cells in each group. CD4+CD25+Foxp3+ T cells were gated by the CD4+ population. (D) The proportion of Treg cells increased in both the MET and MET+CTK groups compared with that in the control and CTK groups, respectively. Relative mRNA levels of Foxp3, STAT5, STAT3 and RORC. (E) Effect of metformin on the expression of STAT3, STAT5, RORC, Foxp3 mRNA. Expression levels were normalized to the GAPDH. The results presented are from three independent experiments. (F, G) The concentrations of IL-17A and IL-10 in the culture medium of each group. Comparisons in (A-E) groups were conducted by one-way ANOVA. Comparisons in (F and G) groups were conducted by rank-sum test and the Kruskal-Wallis test. *P < 0.05; **P < 0.01; ***P < 0.001.
The percentage of Treg/CD4+ in the MET group was remarkably higher than that in the control group (P < 0.001; Figure 1C, 1D). This indicates that the proportion of Treg cells can be significantly augmented by metformin. Furthermore, the percentage of Treg/CD4+ in the MET+CTK group was remarkably higher than that in the CTK group (P < 0.01; Figure 1C, 1D). This indicates that metformin can restrain the inhibitory effects of pro-inflammation cytokines on DIC differentiation into Treg cells.
The ratios of Treg/Th17 were statistically different between the control and MET (P < 0.001), CTK and MET+CTK (P < 0.01), and MET and MET+CTK (P < 0.01) groups.
Metformin restrained the mRNA expression of STAT3 and RORC in an inflammatory environment and facilitated the mRNA expression of STAT5 and Foxp3
Differentiation of DICs into Th17 cells is controlled by a “master-regulator” transcription factor, ROR γt (related orphan receptor γt) encoded by the RORC gene in human [20]. Thus, the relative expressions of RORC mRNA in the control, MET, CTK and MET+CTK groups were detected using RT-PCR to evaluate whether metformin influences the RORC mRNA expression (Figure 1E). There was no significant difference in RORC mRNA expression between the MET group and the control group (P = 0.498). The expression level of RORC mRNA in the CTK group was significantly higher than that in the MET+CTK group (P < 0.001).
The transcription factor Foxp3 is the most specific functional and phenotypic marker of Treg cells. Therefore, the Foxp3 mRNA relative expression levels in the control, MET, CTK, and MET+CTK groups were detected using RT-PCR (Figure 1E). The expression of Foxp3 mRNA in the MET group was approximately 4.8 times higher than that in the control group (P < 0.001). The mRNA expression of Foxp3 in the MET+CTK group was 2.8-fold higher than that in the CTK group (P < 0.001).
STAT5 activates the transcription of Foxp3 expression in Treg cells. STAT3 plays a vital role in the differentiation of DICs into Th17 cells, modulating T cell proliferation and survival. Therefore, the relative expression levels of STAT5 and STAT3 mRNA in the four groups were measured to assess whether metformin affects STAT5 and STAT3 transcription (Figure 1E). The STAT5 mRNA expression level in the MET group was elevated and remarkably higher than that in the control group (P < 0.001). The expression level of STAT5 mRNA in the MET+CTK group was also remarkably higher than that in the CTK group (P < 0.01). There was no remarkable difference in STAT3 mRNA expression between the MET group and the control group (P = 0.072). The expression level of STAT3 mRNA in the MET+CTK group was remarkably lower than that in the CTK group (P < 0.001).
Metformin increased the secretion of IL-10 and decreased the secretion of IL-17A in an inflammatory environment
Decidual Treg cells protect the foetus against the maternal immune system. The expression of multiple immunomodulatory cytokines, such as IL-10 and TGF-β1, is one of the characteristics of this tolerogenic microenvironment [21]. Therefore, the IL-10 concentrations in culture supernatant were measured to analyse the function of Treg cells (Table 1 and Figure 1F). We did not detect the level of TGF-β1 because this cytokine was added to the medium as a T cell-stimulating factor. Parallel changes were found between the concentrations of IL-10 and the size of the Treg cell population. The results turned out that the IL-10 concentration in the MET group and the MET+CTK group was remarkably higher than that in the control group and the CTK group, respectively (P < 0.05).
Table 1.
Concentrations of IL-10 and IL-17A in decidual immune cells culture supernatant (pg/ml)
| Group | IL-10 | IL-17A | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Median | IQR | Mean | Median | IQR | Mean | |
| Control | 114.6443 | 16.33 | 112.5657 | 4573.2649 | 367.32 | 4590.4581 |
| MET | 208.1495 | 42.54 | 217.5990† | 4248.9481 | 189.58 | 4247.2637 |
| CTK | 71.4350 | 12.01 | 71.3075 | 5250.5461 | 887.43 | 5229.0450 |
| MET+CTK | 126.1301 | 21.84 | 127.0752‡ | 4224.1384 | 241.26 | 4195.3262§ |
IQR = interquartile range; MET = metformin; CTK = cytokines.
Compared with the control group;
P < 0.05.
compared with the CTK group;
P < 0.05.
compared with the CTK group;
P < 0.01.
IL-17A is an important cytokine produced by Th17 cells and associated with specific inflammation and placental development [22]. Therefore, the concentrations of IL-17A in cell culture supernatant were detected to analyse the function of Th17 cells (Table 1 and Figure 1G). There was no remarkable difference in the IL-17A concentration between the control group and the MET group (P = 0.435), while the concentration of IL-17A in the MET+CTK group was significantly lower than that in the CTK group (P < 0.01).
Metformin inhibited the phosphorylation of STAT3 and promoted the phosphorylation of STAT5 in DICs
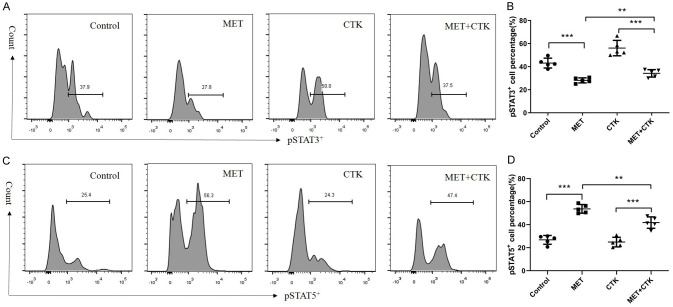
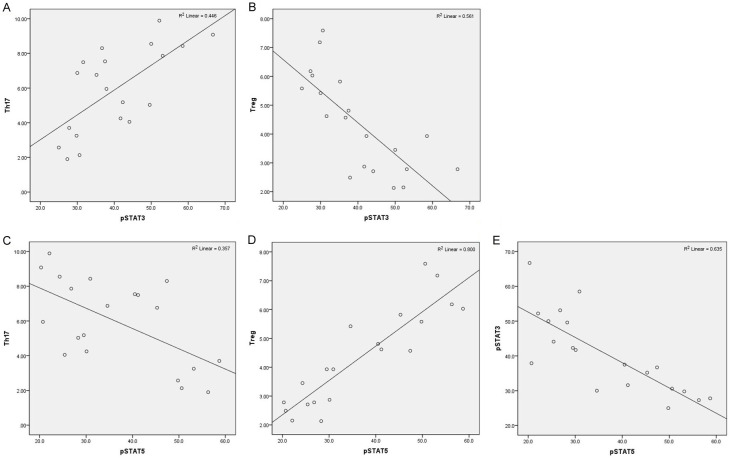
Our previous study found that the phosphorylation of STAT3 in DICs at the maternal-foetal interface affected the transformation of naïve T cells into Treg cells [12]. Therefore, we tested the phosphorylation level of STAT3 in DICs before and after metformin treatment to explore the potential mechanism by which metformin affects the Th17/Treg cell balance (Figure 2A, 2B). We found that metformin inhibited STAT3 phosphorylation in both normal conditions and inflammatory conditions (P < 0.001). The numbers of Th17 cells and pSTAT3+ cells were mutually related (P < 0.01, R = 0.668, Figure 3A). There was a notable negative correlation between Treg cells and pSTAT3+ cells (P < 0.001, R = -0.749, Figure 3B).
Figure 2.
Effect of metformin on the phosphorylation levels of STATs in decidual immune cells collected during early pregnancy. A. Representative flow cytometry analysis of pSTAT3+ CD4+ T cells in each group. B. Metformin reduced the ratio of pSTAT3+/CD4+ T cells in normal and inflammatory environments. C. Representative flow cytometry analysis of pSTAT5+/CD4+ T cells in each group. D. Metformin increased the ratio of pSTAT5+/CD4+ T cells in both normal and inflammatory environments. Comparisons among multiple groups were conducted by one-way ANOVA. *P < 0.05; **P < 0.01; ***P < 0.001.
Figure 3.
Correlations of various cells in the whole T cell population analysed by Pearson correlation analysis. A. Correlation between the number of Th17 cells and pSTAT3+ cells. B. Correlation between the number of Treg cells and pSTAT3+ cells. C. Correlation between the number of Th17 cells and pSTAT5+ cells. D. Correlation between the number of Treg cells and pSTAT5+ cells. E. Correlation between the number of pSTAT3 and pSTAT5 cells. Pearson correlation coefficient was used in this section to show statistical dependence or correlation between two variables.
STAT5 is the key transcriptional regulator of Treg cells. In addition, the relative activities of STAT3 and STAT5 modulate Th17 cell expansion [23]. Therefore, we detected the proportion of CD4+ pSTAT5+ cells in the four groups (Figure 2B). There were notable distinctions between the control and MET groups (P < 0.001) and the CTK and MET+CTK groups (P < 0.001). The MET+CTK group had a lower proportion of CD4+ pSTAT5+ cells than the MET group (P < 0.01). Th17 cells were significantly negatively correlated with pSTAT5+ cells (P = 0.005, R = -0.597, Figure 3C). There was a significant positive relationship between Treg cells and pSTAT5+ cells (P < 0.0001, R = 0.895, Figure 3D). Furthermore, the correlation between pSTAT3 and pSTAT5 (P < 0.0001, R = -0.797, Figure 3E) was clearly negative.
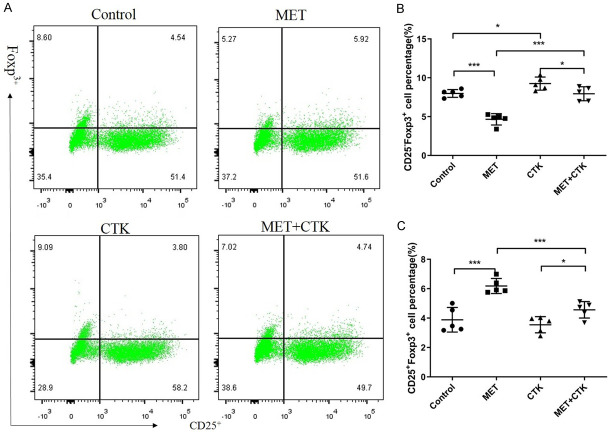
Metformin preserved the stability of Treg cells, reduced the ratio of CD25-Foxp3+ Treg cells and increased the proportion of CD25+Foxp3+ Treg cells
In an inflammatory environment, Treg cells can alter their immunophenotype to secrete pro-inflammatory cytokines to participate in the inflammatory response, which plays a role in immune rejection. This change is called Treg plasticity [24]. This kind of plastic Treg cells express the CD4+CD25lo/-Foxp3+ phenotype and are called Th-like Treg cells. We gated on CD4 positivity and detected the influences of metformin and pro-inflammatory cytokines on the stability of Treg cells (Figure 4). When DICs were stimulated with pro-inflammatory cytokines, the ratio of Th-like Treg cells was significantly increased (P < 0.05). After DICs were treated with metformin under either control conditions or inflammatory conditions, we found that Treg cells upregulated CD25 expression to expand the population of stable Treg cells and reduce the population of Th-like Treg cells (P < 0.01).
Figure 4.
Effect of metformin on the stability of Treg cells. A. Representative flow cytometry analysis of Th-like (CD25-Foxp3+) Treg cells and conventional (CD25+Foxp3+) Treg cells in each group. T cells were gated within the CD4+ population. B. The influence of metformin treatment on the number of Th-like Treg cells. C. The influence of metformin treatment on the number of conventional Treg cells. Comparisons among multiple groups were conducted by one-way ANOVA. *P < 0.05; ***P < 0.001.
Discussion
Metformin reduces the incidence of spontaneous abortion in early pregnancy and improves clinical pregnancy outcomes [2,3]. Some studies have demonstrated that metformin has an immunomodulatory effect, but it is still unclear how this insulin sensitizer acts on the maternal-foetal interface [25]. Previous studies have focused on metformin for treating infertility women with PCOS, obesity or type 2 diabetes and shown that metformin significantly increases the rate of pregnancy and lowers the rate of abortion [26]. Many studies have reported that the Th17 cell proportions in peripheral blood and decidua of RPL patients are increased and that the Treg cell proportion are decreased [11,27,28]. Therefore, it is possible to improve pregnancy outcomes by restoring the homeostasis between Th17 and Treg cells. This research studied the effects of metformin on human decidual lymphocytes in early pregnancy and provided evidence that metformin increased Treg cell numbers, reduced Th17 cell numbers, regulated inflammatory cytokines levels, maintained Treg cell stability, and thereby restored the immune balance of the maternal-foetal interface. These findings will help the development of therapeutic strategies for RPL.
Treg cells, which express the specific markers CD4, CD25 and Foxp3, are involved in the maintenance of immune equilibrium and self-tolerance by limiting abnormal or excessive inflammation. Foxp3 is a marker of differentiation and function of Treg cells. The mechanism that conventional Treg cells play an inhibitory effect includes not only cell-cell contact-dependent and contact-independent effects but also the secretion of anti-inflammatory cytokines, including IL-10, TGF-β1 and IL-35 [29]. However, Treg cells display plasticity, secrete pro-inflammatory cytokines and play a pro-inflammatory role in an inflammatory environment [24]. The developmental and functional plasticity of Treg cells is influenced by inflammatory signals in the surrounding environment. A typical kind of plastic Treg cells, which has the phenotype of CD4+CD25-Foxp3+, stably expresses Foxp3, loses CD25 expression and produces pro-inflammatory cytokines simultaneously [30,31]. In several autoimmune diseases, these Th-like Treg cells function as pathological effector T cells, leading to tissue damage and inflammatory activity [30,32,33]. In another study, we found an elevated proportion of Th17-like Treg cells in the decidua of unexplained RPL patients and a reduction in the proportion of conventional Treg cells (data not published). A recent study found that after metformin treatment, Th-like Treg cell levels were decreased, and conventional Treg cell levels were increased under both normal conditions and inflammatory conditions. These changes are conducive to embryo implantation, maternal-foetal immune tolerance and pregnancy maintenance.
Th17 cells are main effector T cells that secrete pro-inflammatory cytokines. These cells receive multiple stimulatory signals to induce inflammation and play a vital role in anti-infection immunity by inducing neutrophil infiltration and stimulating the secretion of other pro-inflammatory cytokines and chemokines [11]. Th17 cells are directly involved in pathological immune response, including transplant rejection, allergy, pregnancy disorders and autoimmunity [18,34-36]. It has been reported that women with unexplained recurrent spontaneous miscarriage have higher expression of IL-17A than women with normal pregnancies [37,38]. Our results showed that metformin reduced STAT3 and RORC mRNA expression during inflammation. In addition, in an uninflamed state, although the STAT3 mRNA expression did not change, the population of Th17 cells was significantly reduced. This suggests that metformin can not only regulate STAT3 and RORC mRNA expression during inflammation to inhibit the differentiation of the Th17 cells but also regulate the differentiation of DICs by other mechanisms (such as phosphorylation of STATs) in non-inflammatory situations.
STATs are versatile cytoplasmic signalling mediators and nuclear transcription factors. After cytokines or growth factors combine with transmembrane receptors upstream of STATs, these signalling mediators are activated to directly convert extracellular signals into transcriptional reactions in the nucleus. STAT protein biosynthetic autoregulating loops have important influences on many cell functions, with both antagonistic and synergistic relationships being observed [39]. The combination of a single cytokine-receptor pair can produce completely different or even opposite biological effects, depending on the activity of downstream STAT proteins. This activity often depends on the environment around the cells. For example, STAT3 and STAT5 attach to multiple common sites throughout the Il17 genetic locus. IL-2 induces STAT5 to attach to these sites and reduces STAT3 attachment, which leads to the downregulation of related active epigenetic markers [23]. In addition, STAT proteins can also be post-translationally modified through various mechanisms, such as phosphorylation, acetylation, methylation, SUMOylation and ubiquitination, although not all STAT proteins have all modifications. It has been confirmed that the increased levels of pro-inflammatory factors, such as IL-6, IL-1β, IL-17 and TNF-α, can promote the STAT3 phosphorylation [12,40]. An important requirement for inhibiting the inflammatory response is suppressing the amount of pro-inflammatory cytokines and pSTAT3. In our study, the anti-inflammatory activity of metformin inhibited the phosphorylation of STAT3, which was combined with an augment in the phosphorylated STAT5 level. As a result of these changes, the Treg cell proportion and IL-10 production were increased, while the secretion of IL-17A and the proportion of Th17 cells were decreased.
However, we only studied Treg/Th17 cells in DICs. The composition of DICs is complex, including NK cells, macrophages, B cells, Th1 cells, Th2 cells and other immune cells. It is necessary to further study the effects of metformin on other cell types. In addition, the mechanism by which metformin affects the stability of Treg cells is still expected to be further studied in molecular biology.
Conclusion
In summary, metformin has an anti-inflammatory activity in DICs. Our study gives clues to the immunoregulatory mechanisms of metformin and provides new evidence for the application of metformin in the reproductive field.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant number 82160295), the Natural Science Foundation of Guangxi (grant number 2021GXNSFAA196018, 2020GXNSFBA238007 and 2018JJB140171), Development and Application of Appropriate Medical and Health Technologies in Guangxi (grant number S2018111), Self-funded Project of Guangxi Health Commission (grant number Z20190512), the Research Capacity Improvement Project of College Teacher in Guangxi (grant number 2020KY03026), the Key Research and Development Plan of Science and Technology Project in Qingxiu District in Nanning City (grant number 2020054), and Medical Excellence Award Funded by the Creative Research Development Grant from the First Affiliated Hospital of Guangxi Medical University (2020, 2021).
All subjects provided written informed consent. All authors and patients agreed to publish.
Disclosure of conflict of interest
None.
References
- 1.Glueck CJ, Phillips H, Cameron D, Sieve-Smith L, Wang P. Continuing metformin throughout pregnancy in women with polycystic ovary syndrome appears to safely reduce first-trimester spontaneous abortion: a pilot study. Fertil Steril. 2001;75:46–52. doi: 10.1016/s0015-0282(00)01666-6. [DOI] [PubMed] [Google Scholar]
- 2.Faure M, Bertoldo MJ, Khoueiry R, Bongrani A, Brion F, Giulivi C, Dupont J, Froment P. Metformin in reproductive biology. Front Endocrinol (Lausanne) 2018;9:675. doi: 10.3389/fendo.2018.00675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Biate MA. Effect of metformin on early pregnancy loss in women with polycystic ovary syndrome. Taiwan J Obstet Gynecol. 2015;54:266–269. doi: 10.1016/j.tjog.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 4.ESHRE Guideline Group on RPL, Bender Atik R, Christiansen OB, Elson J, Kolte AM, Lewis S, Middeldorp S, Nelen W, Peramo B, Quenby S, Vermeulen N, Goddijn M. ESHRE guideline: recurrent pregnancy loss. Hum Reprod Open. 2018;2018:hoy004. doi: 10.1093/hropen/hoy004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaiser J, Branch DW. Recurrent pregnancy loss: generally accepted causes and their management. Clin Obstet Gynecol. 2016;59:464–473. doi: 10.1097/GRF.0000000000000214. [DOI] [PubMed] [Google Scholar]
- 6.Simon A, Laufer N. Repeated implantation failure: clinical approach. Fertil Steril. 2012;97:1039–1043. doi: 10.1016/j.fertnstert.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 7.Zhang T, Huang C, Du Y, Lian R, Mo M, Zeng Y, Mor G. Successful treatment with intrauterine delivery of dexamethasone for repeated implantation failure. Am J Reprod Immunol. 2017;78 doi: 10.1111/aji.12766. [DOI] [PubMed] [Google Scholar]
- 8.Diejomaoh MF, Bello Z, Al Jassar W, Jirous J, Karunakaran K, Mohammed AT. Consecutive successful pregnancies subsequent to intravenous immunoglobulin therapy in a patient with recurrent spontaneous miscarriage. Int Med Case Rep J. 2015;8:337–344. doi: 10.2147/IMCRJ.S93159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christiansen OB. Immunological causes of ovarian infertility and repeated implantation failure-two aspects of the same problem? Hum Reprod. 1997;12:638–639. doi: 10.1093/oxfordjournals.humrep.a019577. [DOI] [PubMed] [Google Scholar]
- 10.Diejomaoh MF. Recurrent spontaneous miscarriage is still a challenging diagnostic and therapeutic quagmire. Med Princ Pract. 2015;24(Suppl 1):38–55. doi: 10.1159/000365973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saifi B, Rezaee SA, Tajik N, Ahmadpour ME, Ashrafi M, Vakili R, Soleimani Asl S, Aflatoonian R, Mehdizadeh M. Th17 cells and related cytokines in unexplained recurrent spontaneous miscarriage at the implantation window. Reprod Biomed Online. 2014;29:481–489. doi: 10.1016/j.rbmo.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 12.Liu B, Wu H, Huang Q, Li M, Fu X. Phosphorylated STAT3 inhibited the proliferation and suppression of decidual Treg cells in unexplained recurrent spontaneous abortion. Int Immunopharmacol. 2020;82:106337. doi: 10.1016/j.intimp.2020.106337. [DOI] [PubMed] [Google Scholar]
- 13.Cai JY, Li MJ. Interleukin 23 regulates the functions of human decidual immune cells during early pregnancy. Biochem Biophys Res Commun. 2016;469:340–344. doi: 10.1016/j.bbrc.2015.11.118. [DOI] [PubMed] [Google Scholar]
- 14.Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, Levy DE, Leonard WJ, Littman DR. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 15.Kunisada Y, Eikawa S, Tomonobu N, Domae S, Uehara T, Hori S, Furusawa Y, Hase K, Sasaki A, Udono H. Attenuation of CD4(+)CD25(+) regulatory T cells in the tumor microenvironment by metformin, a type 2 diabetes drug. EBioMedicine. 2017;25:154–164. doi: 10.1016/j.ebiom.2017.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eikawa S, Nishida M, Mizukami S, Yamazaki C, Nakayama E, Udono H. Immune-mediated antitumor effect by type 2 diabetes drug, metformin. Proc Natl Acad Sci U S A. 2015;112:1809–1814. doi: 10.1073/pnas.1417636112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Y, Luo D, Lin CH, Shen Y, Cai JF, Guan JL. Efficacy and safety of metformin for Behcet’s disease and its effect on Treg/Th17 balance: a single-blinded, before-after study. Nan Fang Yi Ke Da Xue Xue Bao. 2019;39:127–133. doi: 10.12122/j.issn.1673-4254.2019.02.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang WJ, Hao CF, Qu QL, Wang X, Qiu LH, Lin QD. The deregulation of regulatory T cells on interleukin-17-producing T helper cells in patients with unexplained early recurrent miscarriage. Hum Reprod. 2010;25:2591–2596. doi: 10.1093/humrep/deq198. [DOI] [PubMed] [Google Scholar]
- 19.Fu XQ, Cai JY, Huang QY, Li DJ, Li N, Li MJ. Prednisone may induce immunologic tolerance by activating the functions of decidual immune cells in early pregnancy. Oncotarget. 2017;8:102191–102198. doi: 10.18632/oncotarget.22188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kimura A, Kishimoto T. Th17 cells in inflammation. Int Immunopharmacol. 2011;11:319–322. doi: 10.1016/j.intimp.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 21.Guerin LR, Prins JR, Robertson SA. Regulatory T-cells and immune tolerance in pregnancy: a new target for infertility treatment? Hum Reprod Update. 2009;15:517–535. doi: 10.1093/humupd/dmp004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu HX, Jin LP, Xu B, Liang SS, Li DJ. Decidual stromal cells recruit Th17 cells into decidua to promote proliferation and invasion of human trophoblast cells by secreting IL-17. Cell Mol Immunol. 2014;11:253–262. doi: 10.1038/cmi.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang XP, Ghoreschi K, Steward-Tharp SM, Rodriguez-Canales J, Zhu J, Grainger JR, Hirahara K, Sun HW, Wei L, Vahedi G, Kanno Y, O’Shea JJ, Laurence A. Opposing regulation of the locus encoding IL-17 through direct, reciprocal actions of STAT3 and STAT5. Nat Immunol. 2011;12:247–254. doi: 10.1038/ni.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sakaguchi S. Immunology: conditional stability of T cells. Nature. 2010;468:41–42. doi: 10.1038/468041a. [DOI] [PubMed] [Google Scholar]
- 25.Putilin DA, Kamyshnyi AM. Distribution of FoxP3+regulatory T-cells in rat’s pancreatic lymph nodes under streptozotocin-induced diabetes and metformin administration. Pathology. 2015;1:39–43. [Google Scholar]
- 26.Hyer S, Balani J, Shehata H. Metformin in pregnancy: mechanisms and clinical applications. Int J Mol Sci. 2018;19:1954. doi: 10.3390/ijms19071954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu YS, Wu L, Tong XH, Wu LM, He GP, Zhou GX, Luo LH, Luan HB. Study on the relationship between Th17 cells and unexplained recurrent spontaneous abortion. Am J Reprod Immunol. 2011;65:503–511. doi: 10.1111/j.1600-0897.2010.00921.x. [DOI] [PubMed] [Google Scholar]
- 28.Zenclussen AC, Hammerling GJ. Cellular regulation of the uterine microenvironment that enables embryo implantation. Front Immunol. 2015;6:321. doi: 10.3389/fimmu.2015.00321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao H, Liao X, Kang Y. Tregs: where we are and what comes next? Front Immunol. 2017;8:1578. doi: 10.3389/fimmu.2017.01578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Komatsu N, Okamoto K, Sawa S, Nakashima T, Oh-Hora M, Kodama T, Tanaka S, Bluestone JA, Takayanagi H. Pathogenic conversion of Foxp3+ T cells into TH17 cells in autoimmune arthritis. Nat Med. 2014;20:62–68. doi: 10.1038/nm.3432. [DOI] [PubMed] [Google Scholar]
- 31.Miyao T, Floess S, Setoguchi R, Luche H, Fehling HJ, Waldmann H, Huehn J, Hori S. Plasticity of Foxp3(+) T cells reflects promiscuous Foxp3 expression in conventional T cells but not reprogramming of regulatory T cells. Immunity. 2012;36:262–275. doi: 10.1016/j.immuni.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 32.Joller N, Kuchroo VK. Good guys gone bad: exTreg cells promote autoimmune arthritis. Nat Med. 2014;20:15–17. doi: 10.1038/nm.3439. [DOI] [PubMed] [Google Scholar]
- 33.Chen HH, Handel N, Ngeow J, Muller J, Huhn M, Yang HT, Heindl M, Berbers RM, Hegazy AN, Kionke J, Yehia L, Sack U, Blaser F, Rensing-Ehl A, Reifenberger J, Keith J, Travis S, Merkenschlager A, Kiess W, Wittekind C, Walker L, Ehl S, Aretz S, Dustin ML, Eng C, Powrie F, Uhlig HH. Immune dysregulation in patients with PTEN hamartoma tumor syndrome: analysis of FOXP3 regulatory T cells. J Allergy Clin Immunol. 2017;139:607–620. e615. doi: 10.1016/j.jaci.2016.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shi YH, Shi GC, Wan HY, Jiang LH, Ai XY, Zhu HX, Tang W, Ma JY, Jin XY, Zhang BY. Coexistence of Th1/Th2 and Th17/Treg imbalances in patients with allergic asthma. Chin Med J (Engl) 2011;124:1951–1956. [PubMed] [Google Scholar]
- 35.Lee SK, Kim JY, Lee M, Gilman-Sachs A, Kwak-Kim J. Th17 and regulatory T cells in women with recurrent pregnancy loss. Am J Reprod Immunol. 2012;67:311–318. doi: 10.1111/j.1600-0897.2012.01116.x. [DOI] [PubMed] [Google Scholar]
- 36.Wang D, Huang S, Yuan X, Liang J, Xu R, Yao G, Feng X, Sun L. The regulation of the Treg/Th17 balance by mesenchymal stem cells in human systemic lupus erythematosus. Cell Mol Immunol. 2017;14:423–431. doi: 10.1038/cmi.2015.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee SK, Kim JY, Hur SE, Kim CJ, Na BJ, Lee M, Gilman-Sachs A, Kwak-Kim J. An imbalance in interleukin-17-producing T and Foxp3(+) regulatory T cells in women with idiopathic recurrent pregnancy loss. Hum Reprod. 2011;26:2964–2971. doi: 10.1093/humrep/der301. [DOI] [PubMed] [Google Scholar]
- 38.Wang WJ, Hao CF, Yi L, Yin GJ, Bao SH, Qiu LH, Lin QD. Increased prevalence of T helper 17 (Th17) cells in peripheral blood and decidua in unexplained recurrent spontaneous abortion patients. J Reprod Immunol. 2010;84:164–170. doi: 10.1016/j.jri.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 39.Afzali B, John S. Encyclopedia of inflammatory diseases. 2014. Janus kinases (JAKs)/STAT pathway; pp. 1–13. [Google Scholar]
- 40.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]