Abstract
Macrophages are the core of the pathophysiology of rheumatoid arthritis (RA). They participate in specific and non-specific immunological responses, have phagocytosis, chemotaxis and immune regulatory functions, and are involved in the onset and progression of RA. In recent years, research on the pathophysiology of RA has focused on the polarization and functions of classically activated M1 and selectively activated M2 macrophage subtypes. M1 macrophages release different proinflammatory cytokines, thus driving the chronic proinflammatory, tissue destruction and pain response in RA. M2 macrophages play an anti-inflammatory role. Because of the important role of monocyte-macrophage in RA, drug research targeting monocyte-macrophage can bring us more hope for treatment of RA. This study reviewed the characteristics, plasticity, molecular activation mechanism and relationship of RA with mononuclear macrophages, as well as the transformative potential of macrophages in developing new therapeutic drugs for clinical practice.
Keywords: Mononuclear macrophages, rheumatoid arthritis, characteristics, polarization, targeted therapy, clinical application
Introduction
Rheumatoid arthritis (RA) is a chronic systemic inflammatory autoimmune disease with an unknown etiology. The global overall incidence rate is as high as 1%, with a large affected population [1]. Synovitis is the main pathological feature of the disease. The normal synovium is relatively cell-free, with scattered macrophages and sparse blood vessels in the interstitial tissue. Synovial tissue is proliferative and hypertrophic in RA, with many infiltrating inflammatory cells. The aggregated immune cells directly participate in the development of synovitis by promoting the proliferation of synovial fibroblasts and activating osteoclasts [2,3].
According to the classic theoretical model, CD4+ T cells have a wide range of pathological activities, leading the overall disease process of RA, and have been extensively investigated. The innate immune system represented by mononuclear macrophages has been neglected for a long time. In addition to phagocytosing and killing pathogenic microorganisms, mononuclear macrophages also produce several proinflammatory chemokines and cytokines that contribute to the pathogenesis of RA [4]. Macrophage phenotype and function are heterogeneous. Under the influence of various factors, macrophages exhibit distinct phenotypes and activities, namely M1 and M2 macrophages. This is known as macrophage polarization [5,6]. During RA progression, many factors disrupt the dynamic equilibrium of M1/M2 macrophages, causing an imbalance in their number and proportion, resulting in a constant rise of M1-type proinflammatory macrophages and an intensified inflammatory response [6,7]. Therefore, effective intervention to change M1 to M2 macrophages will restore M1/M2 balance and promote tissue repair and the regression of inflammation. Therefore, this study elaborates on the mononuclear macrophages’ characteristics, polarization features, molecular mechanism of activation and relationship with RA, as well as the direction of RA drug development.
Characteristics of mononuclear macrophages
The mononuclear macrophage system has been discovered for over 100 years. It has recently been found that the mononuclear macrophage system plays a crucial role in the onset and progression of several diseases, as well as in homeostasis, immunological surveillance and anti-infection. In terms of developmental origin, the mononuclear macrophage system members of adult individuals not only originate from bone marrow hematopoiesis but also from embryonic primitive hematopoiesis. In terms of function, the mononuclear macrophage system shows a wide range of heterogeneity. It has been found in recent years that this system has an important immune regulation function.
Characteristics of monocytes
Monocytes, the largest cells in the human body, account for 3%-8% of peripheral white blood cells. In a stable state, monocytes are non-proliferating cells that circulate in the peripheral blood, spleen and bone marrow [8,9]. During infection, monocytes infiltrate tissues and lymph nodes in large numbers. Monocytes have two main differentiation fates during their migration to tissues: macrophages and dendritic cells (DCs). The inflammatory microenvironment and pattern recognition receptors may influence tissue migration and differentiation into inflammatory DCs or macrophages [8].
CD14 is a lipopolysaccharide (LPS) pattern recognition receptor that recognizes and binds LPS or the LPS-binding protein complex and mediates innate immune response [10]. CD16 can bind the Fc segment of IgG to regulate antibody-dependent cell-mediated cytotoxicity [11]. To some extent, the difference in CD14 and CD16 expression in monocyte subsets may reflect the above different effects. According to the distribution status of LPS helper receptor CD14 and scavenger receptor CD16, monocytes can be divided into three subtypes: CD14++CD16+ classical monocytes (CMs), CD14-CD16++ non-classical monocytes (NCMs) and CD14+CD16+ intermediate monocytes (IMs). Many studies have shown that changes in monocyte subsets are important for disease diagnosis and treatment (Table 1).
Table 1.
Classification and characteristics of human monocytes
| Subset | CMs | IMs | NCMs |
|---|---|---|---|
| Markers | CD14++CD16- | CD14+CD16+ | CD14-CD16++ |
| Chemokine receptors | CCR2hi | CCR2hi | CCR2hi |
| CX3CR1lo | CX3CR1hi | CX3CR1lo | |
| HLA-DR + | HLA-DR +++ | HLA-DR ++ | |
| Function | Phagocytosis | Phagocytosis and inflammatory | Patrol |
Notes: Classical monocytes (CMs); Intermediate monocytes (IMs); Non-classical monocytes (NCMs); Chemokine (C-C motor) receptor 2 (CCR2); Chemokine (C-X3-C motor) receptor 1 (CX3CR1).
CMs account for 90% of peripheral blood monocytes in healthy people and can be quickly recruited to inflammatory sites to play a role in phagocytosis, but they lack typical inflammatory characteristics [12]. The adhesion molecule CD62 ligand (CD62L) can combine with endothelial cell peripheral node, mediating monocyte migration [13]. CD62L is almost exclusively expressed in CMs cells in the corresponding stable state. At the same time, the high expression of chemokine (C-X-C motor) receptor 1 (CXCR1), CXCR2 and chemokine (C-C motor) receptor 2 (CCR2) is also conducive to their migration [14,15]. CMs also highly express phagocytic-function-related proteins CD93, CD64, CD11b and CD36. Functional experiments have also shown that its phagocytosis is stronger than IMs and NCMs [14]. This also demonstrates that CMs are important cell scavengers and contribute to the first-line innate immune defense. CMs cells can secrete an excess of granulocyte colony-stimulating factor, chemokine (C-C motif) ligand 2 (CCL2), interleukin 6 (IL-6) and IL-10 [14].
IMs are transitional monocytes with both phagocytic and inflammatory functions. They express CCR2 and chemokine (C-X3-C motor) receptor 1 (CX3CR1). They are capable of rapidly recruiting to inflammatory sites and participating in the inflammatory response. They also express HLA-DR, CD163, CCR5 and other antigens [16]. CX3CR1 and CCR2 are related to monocytes’ recruitment, activation, tissue infiltration and target organ damage [13]. Overexpression of antigen processing and presentation-related molecules HLA-DR, HLA-DO, CD74, and costimulatory molecules CD40 and CD54 in IMs has the strongest ability to stimulate CD4+ T cell proliferation in vitro. Additionally, MHC class I molecules are highly expressed by both CD14+CD16++ monocytes and IMs cells, which may contribute to the activation of CD8+ T cells [17].
NCMs are usually called “patrol monocytes”, which can show antigen presentation and “inflammation” characteristics after activation. Its low expression of CCR2 and high expression of CX3CR1 may be related to its “patrol” function. NCMs were transferred into mice by adoptive transfer and crawled along the blood vessel wall of the mice, showing the “patrol” function [18]. In vitro experiments also found that NCMs could crawl long distances through the front-extended lamellar pseudopods [19].
Characteristics of macrophages
Macrophages have strong heterogeneity and plasticity in function. Macrophages are classified as M1 or M2 based on their cytokine secretion, surface molecule expression, arginine metabolism pathway and other characteristics [6,20,21]. The macrophages activated by Th1 cytokines, including Interferon-γ (IFN-γ), are called classically activated macrophages (M1 macrophages). Th2 cytokines (IL-4 and IL-13) activated macrophages, are known as alternatively activated macrophages, or M2 macrophages [22].
M1 macrophages are primarily activated by granulocyte-macrophage colony-stimulating factors (GM-CSF), LPS and other factors. They are capable of secreting several proinflammatory cytokines, such as tumor necrosis factor-α (TNF-α), IL-6, IL-1β, IL-23 and others. They express CD80, CD86, HLA-DR and other molecules, enhancing antigen presentation ability, promoting T cell activation, Th1 immune response and inflammation, accelerating extracellular matrix degradation and apoptosis [21]. Macrophage colony-stimulating factor (M-CSF), IL-4 and other stimuli are principally responsible for the selective activation of M2 macrophages. They suppress T cell proliferation and activation, regulate Th2 type immune responses, and play an anti-inflammatory role by highly expressing anti-inflammatory cytokines IL-10, transforming growth factor-β (TGF-β), arginase-1 (ARG1), CD206, CD163 and others [21]. In addition, according to different activating molecules, M2 can be divided into four subtypes: M2a, M2b, M2c and M2d: (1) IL-4 and/or IL-13 induce M2a macrophages, which can promote Th2 immune response and participate in tissue repair [23]; (2) M2b macrophages are induced by Toll-like receptors (TLRs) agonists, IL-1R ligands or immune complexes and release high quantities of inflammatory cytokines (TNF-α, IL-6 and others) [23]; (3) M2c macrophages are triggered by IL-10, glucocorticoids and others, and highly express TGF-β and IL-10, inhibit immune and inflammatory responses, and participate in cell debris clearance and immune regulation [23]; (4) M2d macrophages are co induced by TLRs and adenosine A2a receptor agonist to inhibit the production of proinflammatory cytokines, induce anti-inflammatory cytokines (IL-10 and IL-12) and vascular endothelial growth factor secretion, participate in tumor angiogenesis, and promote the development and metastasis of tumors [23,24] (Table 2).
Table 2.
Classification and characteristics of human macrophages
| Subset | M1 type | M2 type |
|---|---|---|
| Activated by | LPS, IFN-γ, and GM-CSF | Immune complexes, IL-4, IL-13, c-Myc, and IRF-4 |
| Markers | CD80, CD86, MHC II, CD64, CD40, CD11c, TLR2, and TLR4 | CD206, CD163, CD209, CD301, CD36, CD200R, CD32, D16 and CD23 |
| Cytokine production | TNF, IL-1β, and IL-6 (proinflammatory) | IL-10, IL-1RII, and IL-1RA (anti-inflammatory) |
| Chemokine production | CXCL-1, CXCL-10, and CCL-5 | CCL-17, CCL-18, CCL-22, and CCL-24 |
| Function | Inflammatory, bactericidal, pathogen clearing, and anti-tumor effects | Anti-inflammation, immune regulation, allergy, tissue repair, and tumor promotion |
Notes: Lipopolysaccharide (LPS); Interferon-γ (IFN-γ); Granulocyte-macrophage colony-stimulating factors (GM-CSF); Interleukin 4 (IL-4); V-Myc Avian Myelocytomatosis Viral Oncogene Homolog (c-Myc); Interferon regulatory factor 4 (IRF4); MHC class II molecules (MHC II); Toll-like receptor 2 (TLR2); Tumor necrosis factor (TNF); Chemokine (C-X-C motif) ligand 1 (CXCL-1); Chemokine (C-C motif) ligand 5 (CCL-5).
Macrophage polarization regulation
Different signaling pathways control macrophage polarization, including Janus kinase (JAK)-signal transducers and activators of transcription (STAT), phosphoinositide 3-kinases (PI3K)/AKT, TLR4/nuclear factor kappa-B (NF-κB), Notch and TGF-β (Figure 1). M1 macrophages are primarily associated with AKT2, Reconciliation Signal Binding Protein-Jkappa (RBP-J), STAT1, p38 and NF-κB, whereas M2 macrophages are primarily associated with AKT1, STAT6 and SMAD2/3/4. In 2014, Wang et al. [25] showed that lncRNAs (such as lncRNA E330013P06) could also regulate the polarization state of macrophages. Despite some understanding of macrophage polarization and the roles that different macrophage types play, the precise mechanism of macrophage polarization and mutual transformation remains unknown.
Figure 1.
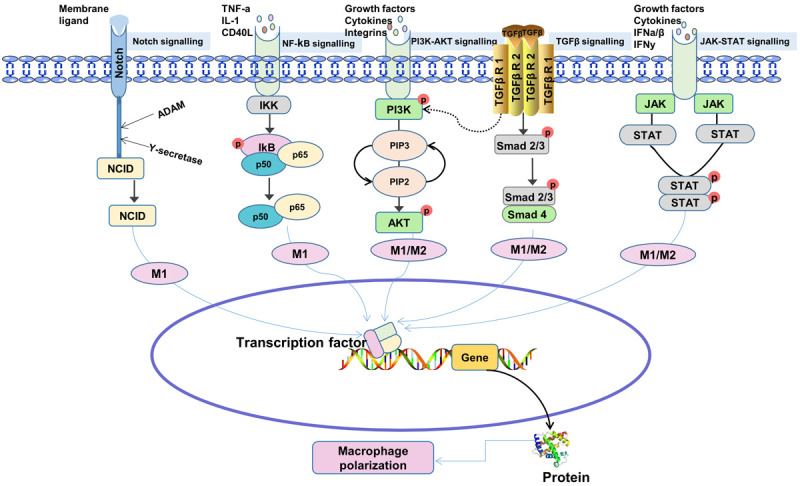
Signaling pathways involved in macrophage regulation. The ligand combines with Notch receptor to form a complex to activate A disintegrin and metalloprotease (ADAM) and γ-secretase, resulting in Notch intercellular domain (NICD) entering the nucleus to regulate transcription of downstream target genes. Nuclear factor kappa-B (NF-κB) signal path is composed of NF-κB, NF-κB inhibitor (IκB) and IκB kinase (IKK) [41]. When the ligand binds to the relevant receptor, the receptor configuration changes and activates IKK, which leads to phosphorylation of IκB. Phosphorylated IκB is recognized and degraded by the 26S proteasome after ubiquitination by ubiquitin ligase. NF-κB is then released from the cytoplasmic NF-κB/IκB complex and activated. The upstream ligand activates phosphoinositide 3-kinases (PI3K), which catalyzes the phosphorylation of PI(4)P and PI(4,5)P2 at site 3 to PI(3,4)P2 and PI(3,4,5)P3, respectively, which then activates the protein kinase AKT. Transforming growth factor-β (TGF-β) binds to its receptor, downstream SMAD2/3 protein is phosphorylated, and p-SMAD2/3 recruits Smad4 to form complexes and translocate to the nucleus, co-regulating transcription of downstream target genes. Cytokine interactions phosphorylate Janus kinase (JAK) and further promote signal transducers and activators of transcription (STAT) dimerization, which then enters the nucleus to regulate transcription of downstream target genes.
M1 polarization signaling pathway
NF-κB is a key transcription regulator of TLR4-induced M1 macrophage polarization [26,27]. STAT1 is an important transcription factor of IFN-γ induced M1 macrophage polarization. IFN-γ ligands bind to their receptors to induce JAK1/2-mediated tyrosine phosphorylation and subsequent STAT1 dimerization. STAT1, as a homodimer, binds to a cis-element named IFN-γ activation site in the M1 polarization-related gene promoter and eventually promotes M1 macrophage polarization [28]. The Notch signaling pathway is also involved in macrophage M1 polarization. According to studies, the ligand Delta-like Ligand-4 (Dll4) combines with the Notch1 receptor to form a complex to activate the downstream A disintegrin and metalloprotease (ADAM) and γ-secretase, leading to Notch intercellular domain (NICD) entering the nucleus and interacting with the transcription factor RBP-J. Thus, M1 polarization is promoted [29]. It has also been found that the Notch signaling pathway of M1 macrophages from mouse bone marrow is activated. Notch inhibitors can reduce the number of M1 macrophages induced by TNF-α and increase the number of M2 macrophages, thereby improving joint damage in mice [30]. In addition to activating the classic D drosophila mothers against decapentaplegic (SMAD) dependent signaling transduction pathway, the TGF-β receptor can also activate SMAD-independent signal transduction, such as mitogen-activated protein kinase and PI3K pathways. The TGF-β-SMAD independent pathway can activate proinflammatory proteins and transcription factors, such as c-Jun N-terminal kinase (JNK), p38 and NF-κB. Thus, macrophages are “reprogrammed” to the M1 type [31]. PI3K produces phosphatidylinositol 3,4,5-triphosphate, which activates the protein kinase AKT. AKT is consisted of three subtypes: AKT1, AKT2 and AKT3 [32]. AKT2 promotes M1 macrophage polarization while inhibiting M2 macrophage polarization [33].
M2 polarization signaling pathway
IL-4 and IL-13 can activate STAT6 and promote polarization of M2 macrophages, whereas STAT6 acetylation inhibits this polarization [34]. The SMAD-dependent pathway activated by TGF-β promotes the expression of M2 polarization-related genes and reprograms macrophages into M2 type [35]. Macrophages lacking AKT1 expression have high levels of inducible nitric oxide synthase (iNOS), IL-6 and TNF-α. This shows that AKT1 promotes M2 macrophage polarization while inhibiting M1 macrophage polarization [33]. In addition, studies have shown that the activation of sirtuin 1 (SIRT1) promotes the high expression of M2-related genes such as IL-10 and can also down-regulate the expression of M1-related genes such as CCL2, iNOS, and the activity of NF-κB, suggesting that SIRT1 promotes the polarization of macrophages to the M2 type and plays an anti-inflammatory role [36].
The role of mononuclear macrophages in RA pathogenesis
Compared to that in healthy people, the proportion of CMs in peripheral blood in RA patients decreased, while the proportion of CD16+ monocyte subsets increased (including NCMs and IMs), and the proportion of such cells was positively correlated with disease activity [37,38]. Under pathological conditions, CD16+ monocytes have a unique receptor expression profile, which can promote the development of RA. STAT1 pathway is down-regulated, and RA-derived monocytes secrete a large amount of TNF-α [39]. This cytokine has strong proinflammatory immune activity, and over synthesis is the main feature of the disorder of the cytokine network in RA patients, which is directly related to the progress of the disease. Further research clarified the pathological function of mononuclear macrophages from synovial tissues (Figure 2). In RA patients, the turnover rate of monocytes increases, and the blood circulation time decreases, showing a significant tendency toward joint aggregation [40]. After migration into the RA synovium, monocytes can polarize into M1 macrophages to play an inflammatory role and can also polarize into M2 macrophages, which may be related to the regression of inflammation. Compared to that of healthy people, the ratio of M1/M2 in the synovial tissue of RA patients increases [41]. M1 macrophages express MHC-II, TLR and Fc gamma receptors. They can promote T cell activation through antigen presentation and activate B cells to produce antibodies. On the one hand, M1 macrophages can promote CD4+ T cell activation [42]. The overflow of inflammatory factors caused by T cell activation directly feeds back to monocytes and promotes the generation of CD16 and IgG immune complexes, promoting their differentiation into osteoclasts [43]. On the other hand, macrophages provide an environment for the long-term survival of B cells and promote their production of anti-citrullinated protein antibodies (ACPAs), diagnostic markers of RA [44]. In ACPA-positive patients, mononuclear macrophages have enhanced their proinflammatory capacity and can secrete more inflammatory factors, while the aggravated immune disorder promotes the differentiation and activation of osteoclasts [45]. This feedback mechanism shows that M1 macrophages are an important driver of joint immune homeostasis destruction and bone damage in RA. M1 macrophages secrete cytokines, including IL-1 and TNF-α, recruit inflammatory cells, stimulate fibroblast-like synoviocytes (FLS), induce osteoclast activation, and promote inflammatory response and bone destruction [27]. Synovial M1 macrophages can also secrete chemokine (C-X-C motif) ligand 4 (CXCL4), CXCL7, recruit monocytes and neutrophils, secrete several matrix metalloproteinases (MMPs), and promote synovitis and articular cartilage tissue destruction [46].
Figure 2.
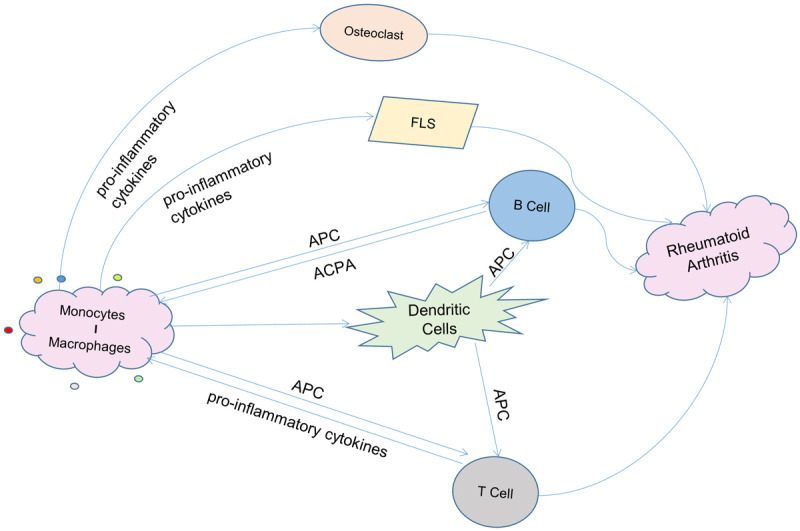
Mononuclear macrophages’ role in the pathogenesis of rheumatoid arthritis. Firstly, mononuclear macrophages can act as antigen presenting cells (APC) to promote the activation of T cells and B cells. B cells can produce autoantibodies such as anti-citrullinated protein antibodies (ACPA). The immune complex formed by the combination of autoantibodies produced by B cells and antigens or the pro-inflammatory factors produced by T cells can feedback on mononuclear macrophages, further amplifying the pro-inflammatory function of mononuclear macrophages. In addition, mononuclear macrophages also secrete inflammatory cytokines to recruit inflammatory cells, stimulate fibroblast-like synoviocytes (FLS) and induce osteoclast activation, promoting inflammatory response and bone destruction. The interaction of mononuclear macrophages, other immune cells and immune factors ultimately leads to the progression of rheumatoid arthritis.
M2 macrophages play a protective role in RA. In the advanced stages of inflammation, macrophages undergo apoptosis or transform into an anti-inflammatory phenotype, namely the M2 phenotype, which secretes several anti-inflammatory factors, inhibits the inflammatory reaction, promotes tissue repair, remodeling and angiogenesis, and maintains internal balance to counteract the potential tissue damage from the inflammatory macrophage reaction [47]. However, macrophage polarization is dynamically reversible, which means that when the microenvironment changes, M1 macrophages can turn to M2 macrophages [48]. Some studies have found that in the presence of a large number of TLR2 ligands, the anti-inflammatory activity of M2 macrophages decreases, but there is no significant change in cell surface markers, implying that the classic M1/M2 model cannot fully represent the function of macrophages under inflammatory conditions [49,50].
The relationship between macrophages and targeted treatment of RA
Antigen-activated T cells produce pro-inflammatory cytokines, which stimulate macrophages, monocytes and synovial fibroblasts to produce additional pro-inflammatory cytokines, IL-1, IL-6 and TNF-α. This will lead to the recruitment of more immune cells (such as B cells and NK cells), thus secreting immunoglobulins, such as rheumatoid factor and IFN-γ. These pro-inflammatory cytokines can make an overall contribution to joint inflammation [27]. Identifying the molecular mechanism of the pathological changes of mononuclear macrophages that dominate the progress of RA is conducive to developing a new scheme for the precise treatment of RA [51].
Currently, many RA drugs in clinical use have inhibitory effects on macrophages, which can effectively improve the disease condition. However, there are still refractory RA patients with poor efficacy and a variety of adverse reactions after the drug treatments. Therefore, developing new therapeutic targets is particularly important. Given the mononuclear macrophages’ role in RA pathogenesis, research on mononuclear macrophage-related targets can bring more hope. In view of the relationship between mononuclear macrophages and RA, we focused on biotherapeutic drugs that target cytokines, cytokine receptors and signal pathways that regulate macrophage polarization (Table 3).
Table 3.
Approved and under-development drugs for the treatment of mononuclear macrophages in RA
| Target | Name | Clinical stage |
|---|---|---|
| Cytokines | Sinoacutine | Approval |
| MTX | Approval | |
| TNF-α | Infliximab | Approval |
| Etanercept | Approval | |
| Adalimumab | Approval | |
| Cetrolizumab-pegol | Approval | |
| Golimumab | Approval | |
| IL-6 | Sarilumab | Approval |
| Tocilizumab | ||
| IL-1 | Anakinra | Approval |
| IL-1 | Canakinumab | Approval |
| GM-CSF | Mavrilimumab | Phase III |
| JAK | Tofacitinib | Approval |
| Baricitinib | Approval | |
| Upadacitinib | Phase III | |
| Filogtinib | Phase III | |
| NF-κB | Iguramod | Approval |
| Denosumab | Approval | |
| PI3K | PBT-6 | -- |
| ZSTK474 | ||
| GS9901 |
Notes: Methotrexate (MTX); Tumor necrosis factor-α (TNF-α); Interleukin 6 (IL-6); Granulocyte-macrophage colony-stimulating factors (GM-CSF); Janus kinase (JAK); Nuclear factor kappa-B (NF-κB); Phosphoinositide 3-kinases (PI3K).
Biotherapeutics targeting cytokines and their receptors
Proinflammatory cytokines are the core driving factors for the onset and progression of RA. Many key cytokines, such as TNF-α, IL-1 and IL-6, are primarily secreted by inflammatory mononuclear macrophages, whereas other cytokines can interfere with the polarization and function of mononuclear macrophages, affecting their immune function. Sinomenine has been shown to reduce the peripheral IM distribution ratio in RA patients and inhibit the production of inflammatory cytokines, including GM-CSF, TNF-α, CXCL1, M-CSF, IL-1β and others, thereby alleviating local inflammation and tissue destruction dominated by mononuclear macrophages [52]. Methotrexate treatment can effectively regulate cytokine production and membrane receptor expression in RA patients’ monocytes [53].
In this context, it is not surprising that many classic RA drugs intervene mononuclear macrophage secretion. TNF-α is the first cytokine targeted in RA treatment. Successfully blocking TNF-α in RA patients confirmed its core role as an inflammatory mediator. TNF-α is the target of nearly 20% of biological agents targeting cytokines in the treatment of RA. Five anti-TNF-α drugs have been approved for marketing, including infliximab, etanercept, adalimumab, certolizumab-pegol and golimumab, which regulate macrophage polarization toward M2 type and inhibit the inflammatory response of M1 macrophages [54].
Selective blocking of other monocyte-related cytokines shows similar therapeutic potential. IL-6 is a critical cytokine in RA pathogenesis and functions in synovial tissues similarly to TNF-α. IL-6 participates in various inflammatory reactions by activating immune cells, endothelial cells and osteoclasts and generating acute phase proteins, including C-reactive protein [55]. Biological agents targeting IL-6 cytokines have been approved for marketing. Tocilizumab is a recombinant humanized anti-IL-6R monoclonal antibody that can prevent IL-6 from binding to soluble IL-6 receptors and membrane-bound IL-6 receptors, thereby alleviating IL-6-mediated inflammatory responses [56]. Sarilumab is a fully human monoclonal antibody drug that targets IL-6 receptors and blocks IL-6-mediated signal transduction [57]. The primary role of IL-1 in RA is destructing the bone and cartilage. IL-1 participates in the generation of osteoclasts and MMPs, which leads to cartilage degradation by stimulating the human nuclear factor KB receptor activator ligand. In patients with RA, high levels of IL-1 (IL-1α and IL-1β) were detected in synovial tissue and tissue fluid [58]. Anakinra is a typical drug that targets the IL-1 pathway and reduces the activity of IL-1α and IL-1β by binding to IL-1 receptors [59]. Targeting IL-1β with canakinumab can also inhibit the signal transduction of IL-1 [60]. GM-CSF has been implicated in the regulation of M1 macrophage polarization [61]. GM-CSF is an immune regulatory cytokine and a new therapeutic target for RA. Mavrilimumab is a monoclonal antibody that can be administered intravenously to block GM-CSF receptors. It has been shown to significantly reduce the RA activity and is currently in the clinical development stage [62].
Biotherapeutics targeting signaling pathways
The JAK-STAT pathway is an intracellular signaling transduction pathway stimulated by cytokines, through which many RA proinflammatory cytokines, such as IL-6, conduct intracellular signaling transduction and play their roles. The JAK-STAT pathway also includes the cytokine synthesis process and is identified as one of the key signaling transduction pathways involved in RA inflammatory lesions [63]. Tofacitinib and baricitinib are two new synthetic oral small-molecule drugs for treating RA. They block the intracellular signal of cytokines and inhibit the transmission of abnormal immune signals by inhibiting the JAK-STAT pathway [64,65]. Other JAK inhibitors in clinical development include filgotinib and upadacitinib, which are highly selective JAK1 inhibitors [66].
NF-κB expression is significantly increased in the synovial tissue of RA patients. Highly activated NF-κB can increase the production of proinflammatory cytokines, including IL-1β, TNF-α and IL-6, and accelerate the progression of RA. Through positive feedback regulation, the upregulation of proinflammatory cytokines can activate NF-κB, forming a vicious circle that accelerates the progression of RA [67,68]. Iguratimod is a new class of disease-modifying anti-rheumatic drugs (DMARDs) that inhibit NF-κB activation and is exclusively approved in China and Japan for treating RA [69]. Iguratimod was recommended as the guiding drug for RA patients by the Asia Pacific League of Associations for Rheumatology [70]. Denosumab was able to decrease NF-κB receptor activity and partially restore bone degradation in RA patients, according to the findings. Denosumab can be administered in combination with DMARDs for RA patients with progressive bone degradation [71].
PI3K/AKT inhibits FLS autophagy, promotes synovial cell proliferation cells, and aggravates RA by acting on the mammalian target of rapamycin (mTOR) [72]. In addition to being engaged in synovial inflammation and FLS cell aberrant proliferation, the PI3K/AKT signaling pathway also affects osteoclast differentiation and production, which eventually results in joint deformity and exacerbates the progression of RA. PI3K is assumed to be a viable therapeutic target for RA because it participates in the inflammatory process. PI3KC2γ expression in synovial tissue fluid, tissue and peripheral blood mononuclear cells of RA patients is significantly higher than that in healthy people, and PBT-6 inhibits PI3KC2γ expression in synovial fibroblasts and macrophages [73]. In vivo and in vitro studies have shown that ZSTK474 can inhibit PI3K activity, thereby inhibiting synovitis and bone destruction in RA patients and that this inhibition is far stronger than that of the commonly used PI3K inhibitor LY294002 [74]. GS9901 is a highly selective oral PI3Kδ inhibitor for RA treatment [75].
Discussion
According to the distribution of CD14 and CD16, monocytes can be divided into three subclasses: CMs, IMs and NCMs. Monocytes can be activated into macrophages by cytokines. Macrophages activated by Th1 cytokines are called M1 macrophages, and those activated by Th2 cytokines are called M2 macrophages [22]. During the development of RA disease, many factors can break the dynamic balance of M1/M2 macrophages, causing an imbalance of quantity and proportion, leading to the continuous increase of M1 pro-inflammatory macrophages, thus exacerbating the inflammatory reaction [6].
Peripheral monocytes, when stimulated, can move into RA synovium, polarize to the M1 type, and play an inflammatory function. M2 polarization of peripheral monocytes may be associated with the reversal of inflammation [7]. Mononuclear macrophages play a critical role in RA pathogenesis, with M1-type proinflammatory macrophages predominating [5]. Macrophages release proinflammatory cytokines through antigen presentation, activate FLS, recruit inflammatory cells, secrete MMPs, and transform into osteoclasts to destruct cartilage and bone. Although conventional RA drugs can alleviate symptoms, they cannot stop the disease progression and have obvious toxic and adverse effects. Recent biotherapies have a well-defined mechanism of action and target, as well as a considerable short-term clinical benefit. Identifying the molecular mechanism of the pathological changes of mononuclear macrophages that dominate the progress of RA is conducive to developing a new scheme for precise treatment of RA [51,76]. In recent years, the role of biological agents in RA patients has become increasingly prominent, but with the occurrence of adverse reactions cannot be ignored. Infection, as one of the adverse reactions [76], may be related to the immune disorder and the immune dysfunction [77], and is one of the common causes of death [78]. We should pay more attention to RA secondary infection and provide early diagnosis and treatment to improve patients’ prognoses and alleviate their pain. Therefore, it is meaningful to continue to explore the research related to RA and infection.
To sum up, the pathophysiological process of RA is very complex, and mononuclear macrophages play a crucial role in the pathogenesis of RA. An in-depth study of macrophages can help further clarify the pathogenesis of RA and explore new therapeutic targets.
Acknowledgements
This work was supported by Henan Science and Technology Research Project (No. LHGJ20200686), Henan Science and Technology Innovation Project (No. XTCX2021-12), Science Research of Traditional Chinese Medicine of Henan Province (No. 2021JDZX2090) and General program of Henan Natural Science Foundation (No. 212300410393).
Disclosure of conflict of interest
None.
References
- 1.Rheumatoid arthritis. Nat Rev Dis Primers. 2018;4:18002. doi: 10.1038/nrdp.2018.2. [DOI] [PubMed] [Google Scholar]
- 2.Alivernini S, Peluso G, Fedele AL, Tolusso B, Gremese E, Ferraccioli G. Tapering and discontinuation of TNF-alpha blockers without disease relapse using ultrasonography as a tool to identify patients with rheumatoid arthritis in clinical and histological remission. Arthritis Res Ther. 2016;18:39. doi: 10.1186/s13075-016-0927-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pitzalis C, Kelly S, Humby F. New learnings on the pathophysiology of RA from synovial biopsies. Curr Opin Rheumatol. 2013;25:334–44. doi: 10.1097/BOR.0b013e32835fd8eb. [DOI] [PubMed] [Google Scholar]
- 4.Rana AK, Li Y, Dang Q, Yang F. Monocytes in rheumatoid arthritis: circulating precursors of macrophages and osteoclasts and, their heterogeneity and plasticity role in RA pathogenesis. Int Immunopharmacol. 2018;65:348–359. doi: 10.1016/j.intimp.2018.10.016. [DOI] [PubMed] [Google Scholar]
- 5.Tardito S, Martinelli G, Soldano S, Paolino S, Pacini G, Patane M, Alessandri E, Smith V, Cutolo M. Macrophage M1/M2 polarization and rheumatoid arthritis: a systematic review. Autoimmun Rev. 2019;18:102397. doi: 10.1016/j.autrev.2019.102397. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y, Han CC, Cui D, Li Y, Ma Y, Wei W. Is macrophage polarization important in rheumatoid arthritis? Int Immunopharmacol. 2017;50:345–352. doi: 10.1016/j.intimp.2017.07.019. [DOI] [PubMed] [Google Scholar]
- 7.Cutolo M, Campitiello R, Gotelli E, Soldano S. The role of M1/M2 macrophage polarization in rheumatoid arthritis synovitis. Front Immunol. 2022;13:867260. doi: 10.3389/fimmu.2022.867260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Auffray C, Sieweke MH, Geissmann F. Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu Rev Immunol. 2009;27:669–92. doi: 10.1146/annurev.immunol.021908.132557. [DOI] [PubMed] [Google Scholar]
- 9.Sanchez-Martin L, Estecha A, Samaniego R, Sanchez-Ramon S, Vega MA, Sanchez-Mateos P. The chemokine CXCL12 regulates monocyte-macrophage differentiation and RUNX3 expression. Blood. 2011;117:88–97. doi: 10.1182/blood-2009-12-258186. [DOI] [PubMed] [Google Scholar]
- 10.Alves PT, Fujimura PT, Morais LD, Goulart LR. Revisiting the CD14: epitope mapping by phage display. Immunobiology. 2014;219:822–9. doi: 10.1016/j.imbio.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Ben MS, Aloulou M, Benhamou M, Monteiro RC. Role of FcgammaRIIIA (CD16) in IVIg-mediated anti-inflammatory function. J Clin Immunol. 2014;34(Suppl 1):S46–50. doi: 10.1007/s10875-014-0031-6. [DOI] [PubMed] [Google Scholar]
- 12.Mukherjee R, Kanti BP, Kumar TP, Tripathy R, Kumar DB, Ravindran B. Non-classical monocytes display inflammatory features: validation in sepsis and systemic lupus erythematous. Sci Rep. 2015;5:13886. doi: 10.1038/srep13886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol. 2011;11:762–74. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong KL, Tai JJ, Wong WC, Han H, Sem X, Yeap WH, Kourilsky P, Wong SC. Gene expression profiling reveals the defining features of the classical, intermediate, and nonclassical human monocyte subsets. Blood. 2011;118:e16–31. doi: 10.1182/blood-2010-12-326355. [DOI] [PubMed] [Google Scholar]
- 15.Jung H, Mithal DS, Park JE, Miller RJ. Localized CCR2 activation in the bone marrow niche mobilizes monocytes by desensitizing CXCR4. PLoS One. 2015;10:e0128387. doi: 10.1371/journal.pone.0128387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu B, Dhanda A, Hirani S, Williams EL, Sen HN, Martinez EF, Ling D, Thompson I, Casady M, Li Z, Si H, Tucker W, Wei L, Jawad S, Sura A, Dailey J, Hannes S, Chen P, Chien JL, Gordon S, Lee RW, Nussenblatt RB. CD14++CD16+ monocytes are enriched by glucocorticoid treatment and are functionally attenuated in driving effector T cell responses. J Immunol. 2015;194:5150–60. doi: 10.4049/jimmunol.1402409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zawada AM, Rogacev KS, Rotter B, Winter P, Marell RR, Fliser D, Heine GH. SuperSAGE evidence for CD14++CD16+ monocytes as a third monocyte subset. Blood. 2011;118:e50–61. doi: 10.1182/blood-2011-01-326827. [DOI] [PubMed] [Google Scholar]
- 18.Cros J, Cagnard N, Woollard K, Patey N, Zhang SY, Senechal B, Puel A, Biswas SK, Moshous D, Picard C, Jais JP, D’Cruz D, Casanova JL, Trouillet C, Geissmann F. Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity. 2010;33:375–86. doi: 10.1016/j.immuni.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collison JL, Carlin LM, Eichmann M, Geissmann F, Peakman M. Heterogeneity in the locomotory behavior of human monocyte subsets over human vascular endothelium in vitro. J Immunol. 2015;195:1162–70. doi: 10.4049/jimmunol.1401806. [DOI] [PubMed] [Google Scholar]
- 20.Zhang YH, He M, Wang Y, Liao AH. Modulators of the balance between M1 and M2 macrophages during pregnancy. Front Immunol. 2017;8:120. doi: 10.3389/fimmu.2017.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shapouri-Moghaddam A, Mohammadian S, Vazini H, Taghadosi M, Esmaeili SA, Mardani F, Seifi B, Mohammadi A, Afshari JT, Sahebkar A. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. 2018;233:6425–6440. doi: 10.1002/jcp.26429. [DOI] [PubMed] [Google Scholar]
- 22.Vogel DY, Glim JE, Stavenuiter AW, Breur M, Heijnen P, Amor S, Dijkstra CD, Beelen RH. Human macrophage polarization in vitro: maturation and activation methods compared. Immunobiology. 2014;219:695–703. doi: 10.1016/j.imbio.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 23.Chistiakov DA, Bobryshev YV, Nikiforov NG, Elizova NV, Sobenin IA, Orekhov AN. Macrophage phenotypic plasticity in atherosclerosis: the associated features and the peculiarities of the expression of inflammatory genes. Int J Cardiol. 2015;184:436–445. doi: 10.1016/j.ijcard.2015.03.055. [DOI] [PubMed] [Google Scholar]
- 24.Ferrante CJ, Pinhal-Enfield G, Elson G, Cronstein BN, Hasko G, Outram S, Leibovich SJ. The adenosine-dependent angiogenic switch of macrophages to an M2-like phenotype is independent of interleukin-4 receptor alpha (IL-4Ralpha) signaling. Inflammation. 2013;36:921–31. doi: 10.1007/s10753-013-9621-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang N, Liang H, Zen K. Molecular mechanisms that influence the macrophage m1-m2 polarization balance. Front Immunol. 2014;5:614. doi: 10.3389/fimmu.2014.00614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li H, Jiang T, Li MQ, Zheng XL, Zhao GJ. Transcriptional regulation of macrophages polarization by MicroRNAs. Front Immunol. 2018;9:1175. doi: 10.3389/fimmu.2018.01175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Siouti E, Andreakos E. The many facets of macrophages in rheumatoid arthritis. Biochem Pharmacol. 2019;165:152–169. doi: 10.1016/j.bcp.2019.03.029. [DOI] [PubMed] [Google Scholar]
- 28.Ivashkiv LB. IFNgamma: signalling, epigenetics and roles in immunity, metabolism, disease and cancer immunotherapy. Nat Rev Immunol. 2018;18:545–558. doi: 10.1038/s41577-018-0029-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu H, Zhu J, Smith S, Foldi J, Zhao B, Chung AY, Outtz H, Kitajewski J, Shi C, Weber S, Saftig P, Li Y, Ozato K, Blobel CP, Ivashkiv LB, Hu X. Notch-RBP-J signaling regulates the transcription factor IRF8 to promote inflammatory macrophage polarization. Nat Immunol. 2012;13:642–50. doi: 10.1038/ni.2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun W, Zhang H, Wang H, Chiu YG, Wang M, Ritchlin CT, Kiernan A, Boyce BF, Xing L. Targeting notch-activated M1 macrophages attenuates joint tissue damage in a mouse model of inflammatory arthritis. J Bone Miner Res. 2017;32:1469–1480. doi: 10.1002/jbmr.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hata A, Chen YG. TGF-beta signaling from receptors to smads. Cold Spring Harb Perspect Biol. 2016;8:a022061. doi: 10.1101/cshperspect.a022061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fruman DA, Chiu H, Hopkins BD, Bagrodia S, Cantley LC, Abraham RT. The PI3K pathway in human disease. Cell. 2017;170:605–635. doi: 10.1016/j.cell.2017.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vergadi E, Ieronymaki E, Lyroni K, Vaporidi K, Tsatsanis C. Akt signaling pathway in macrophage activation and M1/M2 polarization. J Immunol. 2017;198:1006–1014. doi: 10.4049/jimmunol.1601515. [DOI] [PubMed] [Google Scholar]
- 34.Yu T, Gan S, Zhu Q, Dai D, Li N, Wang H, Chen X, Hou D, Wang Y, Pan Q, Xu J, Zhang X, Liu J, Pei S, Peng C, Wu P, Romano S, Mao C, Huang M, Zhu X, Shen K, Qin J, Xiao Y. Modulation of M2 macrophage polarization by the crosstalk between Stat6 and Trim24. Nat Commun. 2019;10:4353. doi: 10.1038/s41467-019-12384-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malyshev I, Malyshev Y. Current concept and update of the macrophage plasticity concept: intracellular mechanisms of reprogramming and M3 macrophage “switch” phenotype. Biomed Res Int. 2015;2015:341308. doi: 10.1155/2015/341308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park SY, Lee SW, Lee SY, Hong KW, Bae SS, Kim K, Kim CD. SIRT1/adenosine monophosphate-activated protein kinase alpha signaling enhances macrophage polarization to an anti-inflammatory phenotype in rheumatoid arthritis. Front Immunol. 2017;8:1135. doi: 10.3389/fimmu.2017.01135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rossol M, Kraus S, Pierer M, Baerwald C, Wagner U. The CD14(bright) CD16+ monocyte subset is expanded in rheumatoid arthritis and promotes expansion of the Th17 cell population. Arthritis Rheum. 2012;64:671–7. doi: 10.1002/art.33418. [DOI] [PubMed] [Google Scholar]
- 38.Tsukamoto M, Seta N, Yoshimoto K, Suzuki K, Yamaoka K, Takeuchi T. CD14(bright)CD16+ intermediate monocytes are induced by interleukin-10 and positively correlate with disease activity in rheumatoid arthritis. Arthritis Res Ther. 2017;19:28. doi: 10.1186/s13075-016-1216-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smiljanovic B, Grun JR, Biesen R, Schulte-Wrede U, Baumgrass R, Stuhlmuller B, Maslinski W, Hiepe F, Burmester GR, Radbruch A, Haupl T, Grutzkau A. The multifaceted balance of TNF-alpha and type I/II interferon responses in SLE and RA: how monocytes manage the impact of cytokines. J Mol Med (Berl) 2012;90:1295–309. doi: 10.1007/s00109-012-0907-y. [DOI] [PubMed] [Google Scholar]
- 40.Smiljanovic B, Radzikowska A, Kuca-Warnawin E, Kurowska W, Grun JR, Stuhlmuller B, Bonin M, Schulte-Wrede U, Sorensen T, Kyogoku C, Bruns A, Hermann S, Ohrndorf S, Aupperle K, Backhaus M, Burmester GR, Radbruch A, Grutzkau A, Maslinski W, Haupl T. Monocyte alterations in rheumatoid arthritis are dominated by preterm release from bone marrow and prominent triggering in the joint. Ann Rheum Dis. 2018;77:300–308. doi: 10.1136/annrheumdis-2017-211649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu W, Li X, Fang S, Zhang X, Wang Y, Zhang T, Li Z, Xu Y, Qu S, Liu C, Gao F, Pan H, Wang G, Li H, Sun B. Anti-citrullinated protein antibodies induce macrophage subset disequilibrium in RA patients. Inflammation. 2015;38:2067–75. doi: 10.1007/s10753-015-0188-z. [DOI] [PubMed] [Google Scholar]
- 42.Tu J, Huang W, Zhang W, Mei J, Zhu C. A tale of two immune cells in rheumatoid arthritis: the crosstalk between macrophages and T cells in the synovium. Front Immunol. 2021;12:655477. doi: 10.3389/fimmu.2021.655477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prajzlerova K, Krystufkova O, Komarc M, Mann H, Hulejova H, Petrovska N, Gregova M, Hanova P, Pavelka K, Vencovsky J, Senolt L, Filkova M. The dysregulation of monocyte subpopulations in individuals at risk of developing rheumatoid arthritis. Rheumatology (Oxford) 2021;60:1823–1831. doi: 10.1093/rheumatology/keaa518. [DOI] [PubMed] [Google Scholar]
- 44.Kerkman PF, Kempers AC, van der Voort EI, van Oosterhout M, Huizinga TW, Toes RE, Scherer HU. Synovial fluid mononuclear cells provide an environment for long-term survival of antibody-secreting cells and promote the spontaneous production of anti-citrullinated protein antibodies. Ann Rheum Dis. 2016;75:2201–2207. doi: 10.1136/annrheumdis-2015-208554. [DOI] [PubMed] [Google Scholar]
- 45.Fukui S, Iwamoto N, Takatani A, Igawa T, Shimizu T, Umeda M, Nishino A, Horai Y, Hirai Y, Koga T, Kawashiri SY, Tamai M, Ichinose K, Nakamura H, Origuchi T, Masuyama R, Kosai K, Yanagihara K, Kawakami A. M1 and M2 monocytes in rheumatoid arthritis: a contribution of imbalance of M1/M2 monocytes to osteoclastogenesis. Front Immunol. 2017;8:1958. doi: 10.3389/fimmu.2017.01958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yeo L, Adlard N, Biehl M, Juarez M, Smallie T, Snow M, Buckley CD, Raza K, Filer A, Scheel-Toellner D. Expression of chemokines CXCL4 and CXCL7 by synovial macrophages defines an early stage of rheumatoid arthritis. Ann Rheum Dis. 2016;75:763–71. doi: 10.1136/annrheumdis-2014-206921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen H, Shi H, Liu Y, Ren X, He S, Chang X, Yin Y. Activation of corticotropin-releasing factor receptor 1 aggravates dextran sodium sulphate-induced colitis in mice by promoting M1 macrophage polarization. Mol Med Rep. 2018;17:234–242. doi: 10.3892/mmr.2017.7909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu W, Zhao X, Daha MR, van Kooten C. Reversible differentiation of pro- and anti-inflammatory macrophages. Mol Immunol. 2013;53:179–86. doi: 10.1016/j.molimm.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 49.Abdel RA, Ashmalla GA, Gaballa G, Nada N. Pilot study of ultrasound parotid imaging reporting and data system (PIRADS): inter-observer agreement. Eur J Radiol. 2015;84:2533–8. doi: 10.1016/j.ejrad.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 50.Vogelpoel LT, Hansen IS, Rispens T, Muller FJ, van Capel TM, Turina MC, Vos JB, Baeten DL, Kapsenberg ML, de Jong EC, den Dunnen J. Fc gamma receptor-TLR cross-talk elicits pro-inflammatory cytokine production by human M2 macrophages. Nat Commun. 2014;5:5444. doi: 10.1038/ncomms6444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Romano C, Esposito S, Ferrara R, Cuomo G. Tailoring biologic therapy for real-world rheumatoid arthritis patients. Expert Opin Biol Ther. 2021;21:661–674. doi: 10.1080/14712598.2021.1847268. [DOI] [PubMed] [Google Scholar]
- 52.Liu W, Zhang Y, Zhu W, Ma C, Ruan J, Long H, Wang Y. Sinomenine inhibits the progression of rheumatoid arthritis by regulating the secretion of inflammatory cytokines and monocyte/macrophage subsets. Front Immunol. 2018;9:2228. doi: 10.3389/fimmu.2018.02228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chara L, Sanchez-Atrio A, Perez A, Cuende E, Albarran F, Turrion A, Chevarria J, Del BA, Sanchez MA, Monserrat J, Prieto A, de la Hera A, Sanz I, Diaz D, Alvarez-Mon M. The number of circulating monocytes as biomarkers of the clinical response to methotrexate in untreated patients with rheumatoid arthritis. J Transl Med. 2015;13:2. doi: 10.1186/s12967-014-0375-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dutton AE, McElnea EM, Rubinstein TJ, Curragh DS, McKelvie PA, Selva D, Rose GE, McNab AA. Lacrimal gland orbital lobe cysts associated with lymphoid hyperplasia or mucosa-associated lymphoid tissue lymphoma in patients with chronic autoimmune disease. Ophthalmic Plast Reconstr Surg. 2019;35:e59–e62. doi: 10.1097/IOP.0000000000001344. [DOI] [PubMed] [Google Scholar]
- 55.Narazaki M, Tanaka T, Kishimoto T. The role and therapeutic targeting of IL-6 in rheumatoid arthritis. Expert Rev Clin Immunol. 2017;13:535–551. doi: 10.1080/1744666X.2017.1295850. [DOI] [PubMed] [Google Scholar]
- 56.Shetty A, Hanson R, Korsten P, Shawagfeh M, Arami S, Volkov S, Vila O, Swedler W, Shunaigat AN, Smadi S, Sawaqed R, Perkins D, Shahrara S, Sweiss NJ. Tocilizumab in the treatment of rheumatoid arthritis and beyond. Drug Des Devel Ther. 2014;8:349–64. doi: 10.2147/DDDT.S41437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Crotti C, Biggioggero M, Becciolini A, Favalli EG. Sarilumab: patient-reported outcomes in rheumatoid arthritis. Patient Relat Outcome Meas. 2018;9:275–284. doi: 10.2147/PROM.S147286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Striz I. IL-1 family cytokines in chronic inflammatory disorders. Vnitr Lek. 2019;65:81–85. [PubMed] [Google Scholar]
- 59.Mertens M, Singh JA. Anakinra for rheumatoid arthritis. Cochrane Database Syst Rev. 2009:CD005121. doi: 10.1002/14651858.CD005121.pub3. [DOI] [PubMed] [Google Scholar]
- 60.Cota-Arce JM, Cota J, De Leon-Nava MA, Hernandez-Caceres A, Moncayo-Salazar LI, Valle-Alvarado F, Cordero-Moreno VL, Bonfil-Solis KL, Bichara-Figueroa JE, Hernandez-Hernandez J, Villela L. Efficacy and safety of canakinumab in the treatment of adult-onset Still’s disease: a systematic review. Semin Arthritis Rheum. 2021;51:1282–1290. doi: 10.1016/j.semarthrit.2021.08.007. [DOI] [PubMed] [Google Scholar]
- 61.Hamilton JA. GM-CSF in inflammation. J Exp Med. 2020;217:e20190945. doi: 10.1084/jem.20190945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Crotti C, Biggioggero M, Becciolini A, Agape E, Favalli EG. Mavrilimumab: a unique insight and update on the current status in the treatment of rheumatoid arthritis. Expert Opin Investig Drugs. 2019;28:573–581. doi: 10.1080/13543784.2019.1631795. [DOI] [PubMed] [Google Scholar]
- 63.Simon LS, Taylor PC, Choy EH, Sebba A, Quebe A, Knopp KL, Porreca F. The Jak/STAT pathway: a focus on pain in rheumatoid arthritis. Semin Arthritis Rheum. 2021;51:278–284. doi: 10.1016/j.semarthrit.2020.10.008. [DOI] [PubMed] [Google Scholar]
- 64.Ikari Y, Isozaki T, Tsubokura Y, Kasama T. Peficitinib inhibits the chemotactic activity of monocytes via proinflammatory cytokine production in rheumatoid arthritis fibroblast-like synoviocytes. Cells. 2019;8:561. doi: 10.3390/cells8060561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kubo S, Nakayamada S, Sakata K, Kitanaga Y, Ma X, Lee S, Ishii A, Yamagata K, Nakano K, Tanaka Y. Janus kinase inhibitor baricitinib modulates human innate and adaptive immune system. Front Immunol. 2018;9:1510. doi: 10.3389/fimmu.2018.01510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Semerano L, Decker P, Clavel G, Boissier MC. Developments with investigational Janus kinase inhibitors for rheumatoid arthritis. Expert Opin Investig Drugs. 2016;25:1355–1359. doi: 10.1080/13543784.2016.1249565. [DOI] [PubMed] [Google Scholar]
- 67.Maracle CX, Kucharzewska P, Helder B, van der Horst C, Correa DSP, Noort AR, van Zoest K, Griffioen AW, Olsson H, Tas SW. Targeting non-canonical nuclear factor-kappaB signalling attenuates neovascularization in a novel 3D model of rheumatoid arthritis synovial angiogenesis. Rheumatology (Oxford) 2017;56:294–302. doi: 10.1093/rheumatology/kew393. [DOI] [PubMed] [Google Scholar]
- 68.Noort AR, Tak PP, Tas SW. Non-canonical NF-kappaB signaling in rheumatoid arthritis: Dr Jekyll and Mr Hyde? Arthritis Res Ther. 2015;17:15. doi: 10.1186/s13075-015-0527-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xie S, Li S, Tian J, Li F. Iguratimod as a new drug for rheumatoid arthritis: current landscape. Front Pharmacol. 2020;11:73. doi: 10.3389/fphar.2020.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jiang H, Gao H, Wang Q, Wang M, Wu B. Molecular mechanisms and clinical application of Iguratimod: a review. Biomed Pharmacother. 2020;122:109704. doi: 10.1016/j.biopha.2019.109704. [DOI] [PubMed] [Google Scholar]
- 71.Yue J, Griffith JF, Xiao F, Shi L, Wang D, Shen J, Wong P, Li EK, Li M, Li TK, Zhu TY, Hung VW, Qin L, Tam LS. Repair of bone erosion in rheumatoid arthritis by denosumab: a high-resolution peripheral quantitative computed tomography study. Arthritis Care Res (Hoboken) 2017;69:1156–1163. doi: 10.1002/acr.23133. [DOI] [PubMed] [Google Scholar]
- 72.Qu Y, Wu J, Deng JX, Zhang YP, Liang WY, Jiang ZL, Yu QH, Li J. MicroRNA-126 affects rheumatoid arthritis synovial fibroblast proliferation and apoptosis by targeting PIK3R2 and regulating PI3K-AKT signal pathway. Oncotarget. 2016;7:74217–74226. doi: 10.18632/oncotarget.12487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim J, Jung KH, Yoo J, Park JH, Yan HH, Fang Z, Lim JH, Kwon SR, Kim MK, Park HJ, Hong SS. PBT-6, a novel PI3KC2gamma inhibitor in rheumatoid arthritis. Biomol Ther (Seoul) 2020;28:172–183. doi: 10.4062/biomolther.2019.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Toyama S, Tamura N, Haruta K, Karakida T, Mori S, Watanabe T, Yamori T, Takasaki Y. Inhibitory effects of ZSTK474, a novel phosphoinositide 3-kinase inhibitor, on osteoclasts and collagen-induced arthritis in mice. Arthritis Res Ther. 2010;12:R92. doi: 10.1186/ar3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Patel L, Chandrasekhar J, Evarts J, Forseth K, Haran AC, Ip C, Kashishian A, Kim M, Koditek D, Koppenol S, Lad L, Lepist EI, McGrath ME, Perreault S, Puri KD, Villasenor AG, Somoza JR, Steiner BH, Therrien J, Treiberg J, Phillips G. Discovery of orally efficacious phosphoinositide 3-kinase delta inhibitors with improved metabolic stability. J Med Chem. 2016;59:9228–9242. doi: 10.1021/acs.jmedchem.6b01169. [DOI] [PubMed] [Google Scholar]
- 76.Canete JD, Pablos JL. Biologic therapy in rheumatoid arthritis. Curr Top Med Chem. 2013;13:752–9. doi: 10.2174/15680266113139990093. [DOI] [PubMed] [Google Scholar]
- 77.Listing J, Gerhold K, Zink A. The risk of infections associated with rheumatoid arthritis, with its comorbidity and treatment. Rheumatology (Oxford) 2013;52:53–61. doi: 10.1093/rheumatology/kes305. [DOI] [PubMed] [Google Scholar]
- 78.Matsui T, Ohsumi K, Ozawa N, Shimada K, Sumitomo S, Shimane K, Kawakami M, Nakayama H, Sugii S, Ozawa Y, Tohma S. CD64 on neutrophils is a sensitive and specific marker for detection of infection in patients with rheumatoid arthritis. J Rheumatol. 2006;33:2416–24. [PubMed] [Google Scholar]


