Abstract
Objective: Endometrial cancer is one of the most common tumors of the female reproductive system, and the existing treatment options for advanced and metastatic endometrial cancer have certain limitations. The antitumor activity of luteolin has been gradually discovered. The purpose of this study was to predict the potential of luteolin in the treatment of endometrial cancer and to provide reference for future clinical drug use. Methods: The target gene database of luteolin and differential gene dataset of uterine corpus endometrial carcinoma (UCEC) have been constructed to obtain the differential genes (DR-DEGs) for luteolin and UCEC. The Gene Set Enrichment Analysis (GSEA), Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis are performed at the same time. Genes associated with prognosis in DR-DEGs were screened and validated using univariate and multivariate COX risk regression analysis so as to construct a prognostic model. Genes are divided into high-risk and low-risk groups according to risk scores for survival analysis and the predictive effect of the model is evaluated. The role of immune function in UCEC is investigated by immune infiltration and immune checkpoint analysis Finally, Transwell experiment was conducted to investigate the effect of luteolin on the migration ability of endometrial cancer cells, and the expression changes of MMP1, IL-17 and VEGF were detected by q-PCR. Results: Through the GO, KEGG and GSEA enrichment analysis, we have found a significant enrichment in “IL 17 signaling (IL-17) pathway”, “oxidative stress response” and “HOMOLOGOUS_RECOMBINATION”. Through multivariate COX risk regression analysis, four genes associated with the prognosis are harvested, including “PRSS1, MMP1, ERBB2 and NUF2” which belong to high-risk genes. Kaplan-Meier analysis shows that the survival rate in the high risk group is lower than that in the low risk group, and the receiver operating characteristic (ROC) curve reveals that the predictive effect of the model is good and stable (area under 1-year curve (AUC) 0.569, two-year AUC 0.628 and three-year AUC 0.653). Immune infiltration and immune checkpoint analysis suggest that “CD40”, “T cells regulatory (Tregs)”, “dendritic cells resting” and “dendritic cells activated” are correlated with survival and prognosis in UCEC patients. In in vitro experiments, we found that the migration ability of endometrial cancer cells was significantly reduced after luteolin treatment, and the expressions of MMP1, IL-17 and VEGF were all decreased. Conclusion: Through bioinformatic analysis, we found that luteolin could slow down the progression of UCEC by inhibiting the production of inflammatory mediators such as IL-17 and oxidative stress, and constructed genetic prognostic models associated with them: PRSS1, MMP1, ERBB2 and NUF2, respectively. In addition, we found that luteolin has an inhibitory effect on the migration of endometrial cancer cells and can reduce the expressions of MMP1, IL-17 and VEGF, thus easing the progression of endometrial cancer.
Keywords: Luteolin, UCEC, bioinformatics analysis, experimental verification, treatment
Introduction
Uterine corpus endometrial carcinoma (UCEC) mostly occurs in menopausal women with an average age of about 60 years. It is one of the most common tumors of the female reproductive system [1]. According to the estimation of the American Cancer Society, there will be about 65,950 new UCEC patients in the United States in 2022, and nearly 130,000 women will die from the disease in 2022 [2]. More than 600,000 UCEC patients still exist in the United States until today [2]. In recent years, the morbidity and mortality of UCEC are increasing [3,4]. It not only seriously affects their quality of life and their life and health, but also increases the medical burden of the society. At present, the treatment for UCEC patients is mainly surgical. The main methods include complete hysterectomy, bilateral salpingo-oophorectomy, paraaortic and pelvic lymphadenectomy, etc. It can also be treated through non-surgical therapy such as radiotherapy, chemotherapy and other adjuvant therapies [5,6]. However, the adjuvant treatment for UCEC is still complex and controversial. There is relatively little room for the treatment options of advanced and metastatic cancers [7]. Considering that the morbidity and mortality of UCEC are increasing in recent years and the treatment protocols have certain limitations and controversies for the advanced and metastatic UCEC, it is, therefore, very important to explore new treatment directions and establish new prognostic models to accurately and conveniently predict the survival time of UCEC patients.
In China, traditional Chinese medicine (TCM) has been widely accepted as an alternative cancer therapy. Moreover, it has been proved that cancer patients may widely benefit from the TCM treatment [8,9]. Compared with traditional anti-cancer drugs with obvious adverse effects, many natural small molecules in traditional Chinese medicine have attracted the attention of researchers in the anti-cancer drug R&D due to their potential tumor selectivity and low cytotoxicity [10,11]. As a small molecule compound of flavonoids of natural traditional Chinese medicine, luteolin is widely found in a variety of plants such as carrots, onion and broccoli [12], with strong antioxidant and anti-inflammatory activities [13]. Many studies have found that the anti-inflammatory effect of luteolin is related to the anti-tumor effect to some extent [14,15]. It has a certain inhibitory effect on breast cancer, colon cancer, liver cancer and other carcinoma cell lines [16-18]. Recent studies have also discovered that flavonoids such as luteolin may play a role in prevention of cancer by regulating the microbiota and immune function in the digestive system [19]. However, there is a lack of further studies on luteolin in the treatment of UCEC. In this context, we have explored the potential mechanism of luteolin in the UCEC treatment and tested it experimentally, and established a new prognostic model of drug-related differentially expressed genes for UCEC.
Methods
Construction of target gene dataset for luteolin
First, we searched drug target proteins of small molecule luteolin through the Traditional Chinese Medicine Systems Pharmacology (TCMSP) database. Gene transformation and standardization of drug target proteins were performed using the uniport [https://www.uniprot.org/] database, and the relevant target gene dataset of luteolin was constructed.
Construction of differential gene dataset for UCEC
The raw gene sequencing (RNA seq) dataset and clinical data of uterine corpus endometrial cancer (UCEC) were downloaded from the Cancer Genome Atlas (TCGA) website (https://portal.gdc.cancer.gov). A total of 23 normal samples and 552 tumor samples were obtained. The data were extracted and standardized by using R software (R 4.1.2). In order to identify and screen differentially expressed genes (DEGs), we screened them via “limma” R package, with parameters set as P<0.05 and false discovery rate (FDR)<0.05.
Construction and analysis of drug-related DGEs (DR-DEGs)
The intersection of luteolin target genes and UCEC-DEGs was performed through the Venny2.1.0 website (https://bioinfogp.cnb.csic.es/tools/venny/). A total of 22 DR-DGEs were harvested. A protein interaction network of 22 DR-DEGs was constructed using the String (https://string-db.org/) database. It was further visualized by using Cytoscape (version3.9.0) software according to the degree of interaction of each protein node. Gene Ontology (GO) enrichment analyses were performed for DR-DEGs via the “clusterProfiler” R package, including biological processes, cellular components and molecular functions. Moreover, Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses were carried out for DR-DEGs using the same tool.
Construction and validation of prognostic models of DR-DEGs
For further exploration of the prognostic value of DR-DEGs associated with UCEC, we combined the expression levels of DR-DEGs with clinical data using perl language (perl 5.30.1) and excluded the samples with incomplete clinical data and zero or negative survival time. Univariate COX proportional hazards regression analysis was conducted for 22 DR-DEGs in relation to survival status using the coxph function (P<0.05) of the “survival” R package in order to identify genes that were significantly correlated with the prognosis of UCEC patients. In order to avoid overfitting, the prognosis-related DR-DEGs were brought into the least absolute shrinkage and selection operator (LASSO) -penalized Cox regression using the “glmnet” R package. A multivariate COX proportional hazards regression analysis was continuously used to finally acquire four DR-DEGs associated with prognosis and build a prognostic model. Risk score for each patient: risk score = ∑Xλ*coefλ. The risk score formula is based on the relative expression level of each gene and the coefficient of each gene from multivariate COX proportional risk regression analysis, where Xλ is the standardized relative expression level of each drug differential gene and coefλ is the coefficient. The patients were divided into high-risk and low-risk groups according to the median risk score of the tumor group. With respect to the determination of whether the high-risk and low-risk patients were well divided into two groups as well as the investigation of the distribution of the low-risk group, the Principal Component Analysis (PCA) was performed using “Rtsne” R package. The t-SNE test and grouping visualization were also carried out. In order to determine the prognostic role of risk scores in predicting clinical UCEC patients, a survival analysis for life cycle was performed between the high-risk and low-risk groups and the results were shown by Kaplan-Meier curves. Meanwhile, a time-dependent receiver operating characteristic (ROC) curve analysis was conducted using the “survivalROC” R package to test the specificity and sensitivity of the prognostic model for survival.
Immune correlation analysis and tumor microenvironment analysis
The infiltration scores of 22 immune cells in tumor patients were calculated separately by CIBERSORT algorithm. The expression of immune checkpoints related genes in the high and low UCEC risk groups was calculated using the “limma”, ”reshape2” package of R software and then visualized. The total scores of stromal cells and immune cells in the high-risk and low-risk groups in the tumor microenvironment were calculated using the “estimate” package of R software. The correlation of stromal cell and immune cell scores and risk scores was investigated respectively.
Gene set enrichment analysis (GSEA)
Relevant pathways and biological processes were introduced to detect the high-risk populations. Expressed gene sets of low-risk or high-risk populations and these collected marker gene sets from the Kyoto Encyclopedia of Genes and Genomes (KEGG) database V7.5.1 were analyzed using GSEA (4.2.1) software. FDR<0.05 was considered as statistical significance.
Transwell invasion experiment
500 μl of DEME complete medium was added to the Transwell chambers under the plate without matrigel. The Transwell chambers were placed therein. The culture plate was then placed in a cell incubator for activation for 15 minutes. Cells were diluted to 1×106/ml with serum-free DMEM medium containing 0 and 10 µM of luteolin, adding 100 µl in each of the different chambers. The cells and medium inside of the Transwell upper chambers were completely and gently wiped off with a cotton ball after 24 hours, and then fixed in pre-cooled paraformaldehyde for 30 minutes. The Transwell chambers were inverted on the tabletop. A drop of crystal violet solution was dropped in the chambers and stained for 20 minutes. After 20 minutes, the crystal violet solution was washed out with PBS. The inverted Transwell chambers were dried on the tabletop and then observed under an inverted microscope. The number of cells traversed was observed under a 10-fold microscope in each group for statistical analysis.
The q-PCR experiment
These cells were inoculated in the six-well plate to 80% of confluence. The original medium was replaced with complete DMEM containing 0, 5, 10 and 15 µM of luteolin for intervention. According to instructions of the RNA extraction kit of FeJet Biotech, RNA was then extracted from a six-well plate in the blank group (cultured in the complete DMEM for 24 h) and the drug group (a complete DMEM containing 5, 10 and 15 µM luteolin) after 24 hours. The reverse transcription kit (China, Genecopoeia) was used for cDNA synthesis under 25°C, 5 min; 42°C, 15 min; 85°C, 5 min; and 4°C, holding setup program. After acquiring cDNA, the primer sequences are shown in Table 1. The PCR reaction system is as follows: the Premix Ex Taq (loading dye Mix) 10 μl, 0.5 μl each in the upstream and downstream primers, cDNA 1 μL and ddH2O supplemented to the total system 20 μl. The reaction conditions: 1. Pre-denaturation under 95°C for 30 s; 2. denaturation under 95°C for 30 s; 3. annealing under 60°C for 30 s; 4. extension under 65°C for 1 min. The reaction was ended after 35 cycles under 65°C with final extension of 5 minutes for observation. The glyceraldehyde-3-phosphate dehydrogenase gene was used as an endogenous control. Fold change was calculated as 2-ΔΔCT.
Table 1.
The primer sequences for real-time PCR
| Gene | Forward | Reverse |
|---|---|---|
| MMP1 | AAAGGG AATAAGTACTGGGC | CAGTGTTTTCCTCAGAAAGAG |
| GAPDH | GCACCGTCAAGGCTGAGAAC | TGGTGAAGACGCCAGTGGA |
| IL-17 | ACCAATCCCAAAAGGTCCTC | GGGGACAGAGTTCATGTGGT |
| VEGF | ATGAACTTTCTGCTGTCTTGGGT | TGGCCTTGGTGAGGTTTGATCC |
Results
Results of DR-DEGs related to luteolin and UCEC
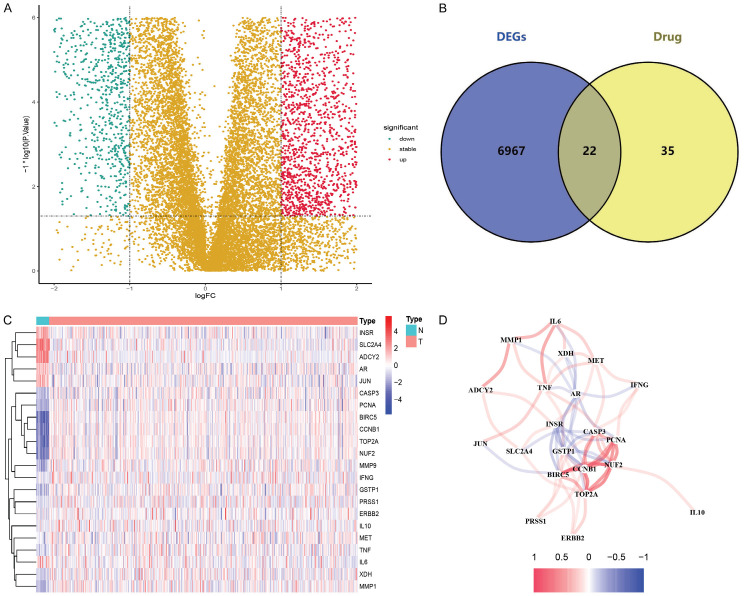
The analysis flow chart is shown in Figure 1. The TCMSP database was used to search for target proteins of small molecules of luteolin, which were transformed and standardized through the uniport database, thereby yielding a total of 57 drug target genes. Data obtained from the TCGA web site were screened by “limma” package, showing 6,989 genes that were differentially expressed in 23 normal and 552 tumor groups (Figure 2A). The intersection of DEGs and drug target genes was acquired using the website Venny2.1.0 and a total of 22 DR-DEGs were yielded (Figure 2B). The expression levels of these genes were visualized in the normal and tumor groups by heatmap (Figure 2C), including DR-DEGs: INSR, SLC2A4, ADCY2, AR and JUN which were highly expressed in the normal group, and 17 remaining DR-DEGs: CASP3, PCNA, BIRC5, CCNB1, TOP2A, NUF2, MMP9, IFNG, GSTP1, PRSS1, ERBB2, IL10, MET, TNF, IL6, XDH and MMP1 which were highly expressed in the tumor group. An expression relationship map of genes was also further plotted (Figure 2D).
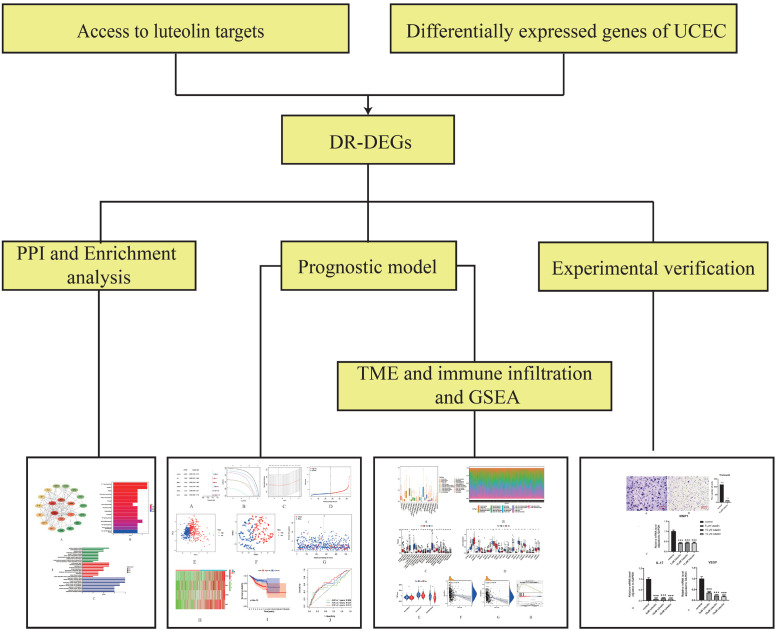
Figure 1.
Flow chart. The workflow of data analysis in this study.
Figure 2.
Drug-related differential genes of endometrial cancer and interactions. A. Volcano plot of 6989 differential-drug genes in endometrial cancer; B. Venny plot of 22 drug-related differential genes (DR-DEGs); C. Heatmap of 22 DR-DEGs (N: normal group, T: tumor group; red represents high gene expression, blue represents low gene expression, and the depth of color represents the level of expression); D. The relationship between 22 DR-DEGs (red line is positive correlation, blue line is negative correlation, and the depth of color represents the strength of the correlation).
Results of protein interaction network and functional enrichment analysis
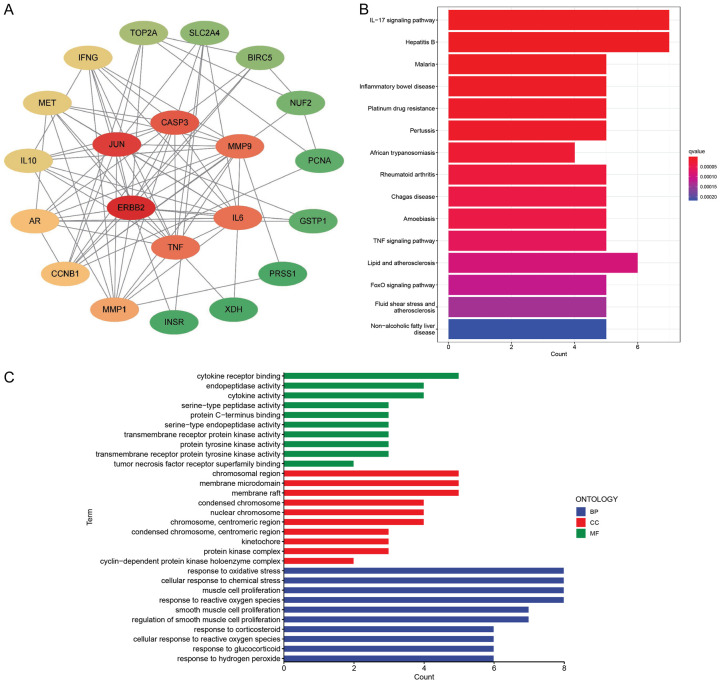
In order to explore the relationships among target proteins of 22 DR-DEGs, a gene target protein interaction network diagram was established using the string database. After deleting a target protein with no relationship to other nodes, the interaction network data were imported into Cytoscape software (version3.9.0) for further analysis. Finally, 21 target protein interaction network diagrams of DR-DEGs were obtained (Figure 3A). Of which, the genes “ERBB2, JUN, CASP3, MMP9, IL6 and TNF” were at the center of the interaction network, indicating that they were more connected with other nodes in the network and can be the core genes of the network. In order to explore the potential mechanism of 22 DR-DEGs and UCEC, the GO and KEGG enrichment analysis was performed. The GO enrichment analysis showed that it was highly enriched in the “response to reactive oxygen species, muscle cell proliferation, cellular response to chemical stress and response to oxidative stress” (Figure 3C). KEGG enrichment analysis revealed that it was highly enriched in the “IL-17 signaling pathway and Hepatitis B” (Figure 3B).
Figure 3.
Protein-protein interaction (PPI) network and Gene Ontology (GO), Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis. A. Protein-protein interaction networks (red means more interaction with other proteins, green means less interaction with other proteins); B. KEGG enrichment of 22 DRG-DEGs analysis chart; C. GO enrichment analysis diagram of 22 DRG-DEGs.
Results of construction and validation of prognostic models
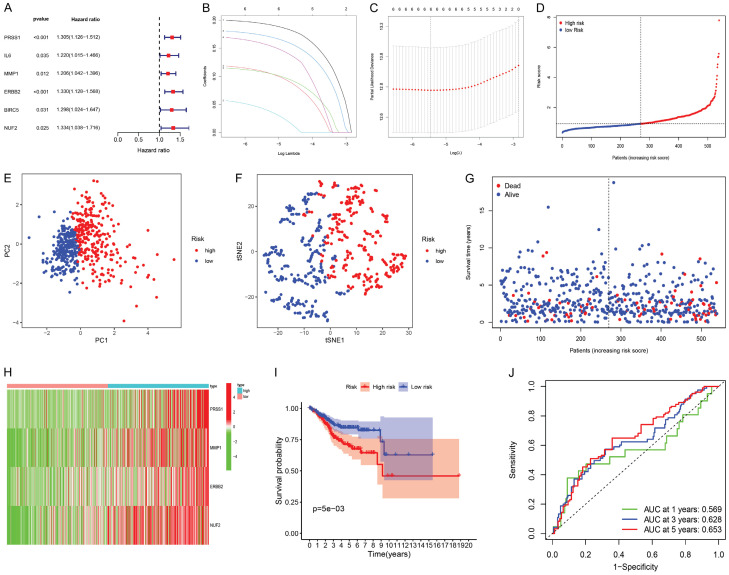
In order to obtain the UCEC prognostic model associated with DR-DEGs, the univariate COX proportional hazards regression analysis was performed for 22 DR-DEGs expression and their clinical data, yielding a total of 6 DR-DEGs related to UCEC prognosis, as well as drawing the forest plots (Figure 4A). The hazard ratio was greater than 1.2, indicating that these genes were all high-risk genes. Six prognosis-related DR-DEGs were validated by LASSO-penalized Cox regression and the results revealed that these genes showed a stable and good performance (Figure 4B, 4C). After that, the multivariate COX proportional hazards regression analysis was carried out for these six DR-DEGs, finally obtaining four DR-DEGs associated with the prognosis, namely PRSS1, MMP1, ERBB2 and NUF2. Risk scores were calculated for each patient based on the relative expression levels and risk coefficient of four prognosis-related DR-DEGs in order to predict the patient’s prognosis. The final risk score for each patient is PRSS1’s expression value * 0.204891906336637 + MMP1’s expression value * 0.151587628101162 + ERBB2’s expression value * 0.183491564882044 + NUF2’s expression value * 0.22194538428941. They were then divided into high-risk and low-risk groups according to the median value (Figure 4D). PCA analysis and t-SNE test were also performed (Figure 4E, 4F). The test results suggest that the high-risk and low-risk patients were well divided into two groups with significantly different directions. The relationship of the survival status and time in UCEC patients was evaluated between the high-risk and low-risk groups (Figure 4G). We found that the deaths in the high-risk group were more centralized than these in the lower-risk group as risk scores increased. Heat maps were drawn to show the gene expression profile of UCEC patients in the high-risk and low-risk groups (Figure 4H). We discovered that the four prognosis-related DR-DEGs were highly expressed in the tumor group. They were high-risk genes and consistent with the results of previous forest plots. According to the Kaplan-Meier curve (Figure 4I), the survival rate of the high-risk group was lower than that of the low-risk group at different times. P=0.005 and the results were statistically significant. Finally, the predictive effect of the prognostic model was evaluated by the time-dependent ROC curve (Figure 4J). The results indicated that an one-year area under curve (AUC) was 0.569, a two-year AUC was 0.628 and a three-year AUC was 0.653, indicating that the predictive effect of the prognostic model was good and stable.
Figure 4.
Construction and validation of a genetic prognostic model. A. Forest plot of 6 DR-DEGs associated with endometrial cancer survival was constructed by univariate regression analysis; B. Least absolute shrinkage and selection operator (LASSO) of 6 genes analysis coefficient spectrum distribution; C. Cross-validation of optimal parameter selection in LASSO regression. D. Distribution map of patients in high and low risk groups based on risk score; E. Principal Component Analysis (PCA) plot based on risk score; F. t-distributed Stochastic Neighbor Embedding (t-SNE) map based on risk score; G. Survival status map of patients in high and low risk groups (left side of the dotted line, low risk group; right side of the dotted line, high risk group); H. Heatmap of DR-DEGs associated with prognostic features of endometrial cancer; I. Kaplan-Meier analysis curve of prognosis related to high and low risk groups; J. Time-dependent receiver operating characteristic (ROC) curve, the area under the curve (AUC) at 1 year, 3 years, and 5 years is 0.569, 0.628, and 0.653.
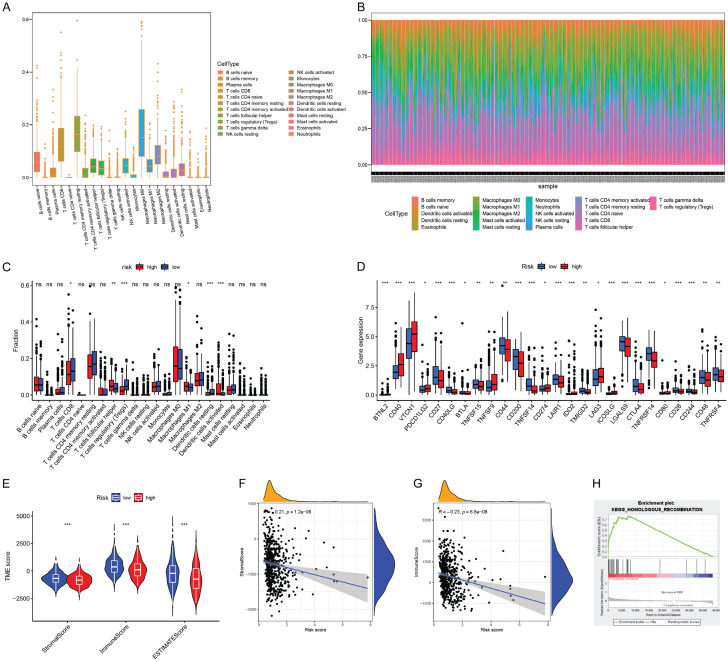
Results of immunology and tumor microenvironment analysis
The CIBERSORT algorithm was used to obtain the infiltration scores of 22 immune cells in each tumor patient sample. Figure 5A showed the distribution of each individual immune cell in the total number of UCEC patients. Figure 5B showed the levels of individual immune cells in each UCEC tumor patient. Figure 5C showed infiltration scores in the high and low risk groups, of which the T cells regulatory (Tregs) and dendritic cells activated infiltration scores were significantly different. Compared with the immune cell infiltration scores, most of the immune checkpoint gene expression levels were significantly different (Figure 5D), such as “BTNL2, CD40, VTCN1, CD27 and CD40LG”, etc. At the same time, the scores of stromal cells and immune cells and the total scores were obtained in the tumor microenvironment in the high-risk and low-risk groups using the “estimate” package of R software (Figure 5E). The results showed that the differences in the scores of stromal cells, immune cells and the total scores were significant between the high-risk and low-risk groups. Moreover, the scores were higher in the low-risk group. At the same time, the relationships between the scores of stromal cells, immune cells and the risk scores were explored (Figure 5F, 5G). We found that both were negatively correlated with risk scores.
Figure 5.
tumor microenvironment (TME) and immune infiltration and Gene Set Enrichment Analysis (GSEA). A. The distribution of each individual immune cell in the total number of UCEC patients; B. The levels of individual immune cells in each UCEC tumor patient; C. Infiltration scores in the high and low risk groups; D. The immune checkpoint gene expression level in the high and low risk groups. E. The tumor microenvironment of the high and low risk groups; F, G. Correlation between immune cell scores and risk scores; H. The “homologous recombination” pathway is more active in the high-risk group.
Results of GSEA pathway for screening and analysis
Through enrichment analysis of gene sets in the population of the high-risk and low-risk groups, 123 pathways were obtained in the high-risk group and 55 pathways in the low-risk group. We found that “homologous recombination” was correlated with UCEC and scores more concentrated in the high-risk group after screening (Figure 5H).
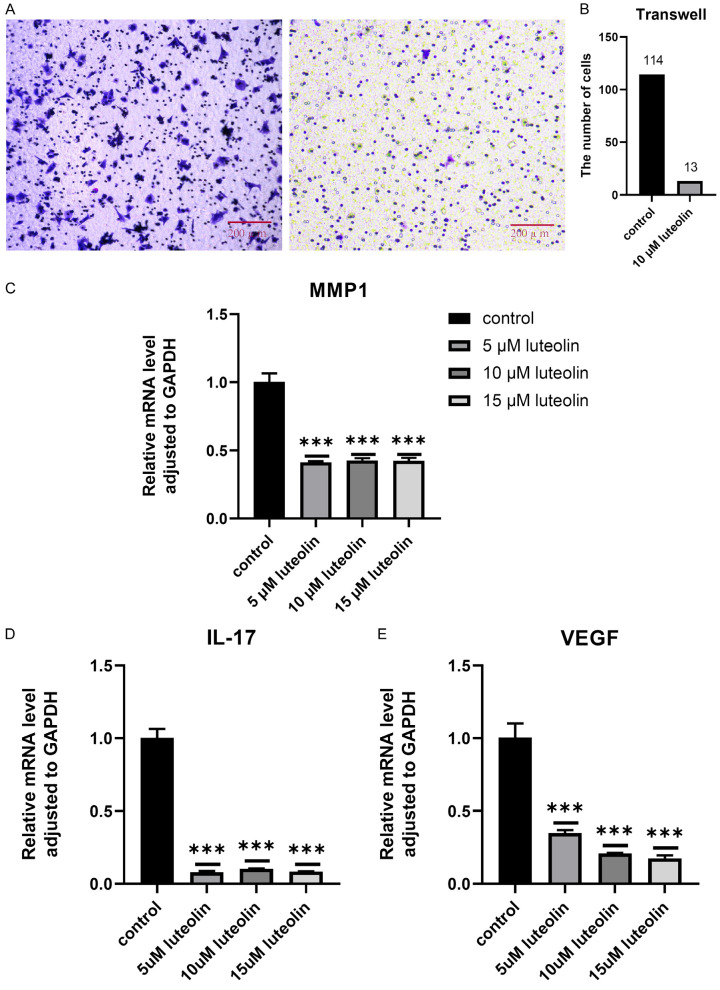
Impacts of luteolin on the motility of PC-3 cells tested by transwell
After different concentrations of luteolin were applied to AN3-CA cells for 24 hours, the results showed that the number of perforated cells in AN3-CA cells at luteolin concentrations of 0 and 10 μM was 114,13 (Figure 6A, 6B).
Figure 6.
In vitro experiments. A, B. Impacts of luteolin on the motility of PC-3 cells tested by Transwell; C-E. q-PCR.
q-PCR results
Compared with the blank DMEM group, the expression levels of MMP1 and IL-17 were significantly decreased in all experimental groups treated with luteolin, but there seemed to be no significant difference between 5-15 μM. In addition, we also examined the effect of luteolin on VEGF expression in endometrial cancer cells, and the results showed that VEGF expression level was significantly reduced after luteolin treatment. With the increase of drug concentration, the expression levels of VEGF tended to decrease. P<0.05 is considered that the difference is significant (Figure 6C-E).
Discussion
As one of the most common gynecological cancers in developed countries, the incidence of UCEC has been on the rise in recent years [20]. The situation in developing countries remains critical. Their ratio of mortality to morbidity is much higher than that of developed countries [21]. For early premenopausal UCEC patients, it is more important to preserve fertility through non-surgical and other adjuvant treatments [24]. Therefore, it is necessary to explore the direction of the new UCEC treatment and to construct a new prognostic model. Previous studies have demonstrated the therapeutic mechanism of capsaicin and puerarin on endometrial cancer, indicating that small drug molecules have potential research value for UCEC [22,23]. As a small molecule TCM drug luteolin may play an anti-tumor role by inhibiting the metastasis and generation of tumor cells, etc. [25]. However, its efficacy on UCEC has not been deeply studied. In this study, we have investigated the possibility and potential mechanism of luteolin in the treatment of UCEC through bioinformatics, and established a new drug-related prognostic model via experimental validation.
We performed GO and KEGG enrichment analysis of DR-DEGs by R software. The results suggest that it is relatively significant in the “oxidative stress response” in the GO pathway. Oxidative stress is a reaction of the imbalance between the reactive oxygen species and antioxidants in tissues, which increases reactive oxygen species and produces damage [26]. Many studies have found that the reactive oxygen species may induce the production of tumor cells through the form of signaling molecules [27] or result in DNA damages and abnormal repair mechanism so as to increase the probability of tumor formation [28,29]. The KEGG enrichment analysis reveals a significant enrichment effect in the “IL-17 signaling pathway”. Interleukin 17 (IL-17) is a pro-inflammatory cytokine that plays an important role in combating pathogens, autoimmune diseases and inflammatory responses [38,39]. Recent studies have proved that it may promote tumor occurrence and growth by maintaining chronic inflammatory microenvironment and promoting angiogenesis [40-42]. In certain circumstances, we have also observed that it is closely related to gynecological cancer, such as causing the growth of cervical cancer and ovarian cancer cells [43-45]. By studying IL-17 knockout mice, Wang et al. found that IL-17 disruption reduced tumorigenesis [46]. Whereas luteolin can significantly inhibit various inflammatory mediators, including “IL-17, IL-1 and Il-6, etc.” and regulate inflammation-related signaling pathways [47,48]. Therefore, we speculate that luteolin may inhibit the related pathways by suppressing the production of reactive oxygen species and inflammatory mediators, thereby altering the environment for UCEC cell production and growth and inhibiting tumor growth.
Serine protease 1 (PRSS1) is located on chromosome 7 in the human body and can be digestion-promoting by encoding human cationic trypsinogen 1 [49-51]. In recent years, some studies reveal that its expression increases in various cancer tissues such as pancreatic cancer, cervical cancer and gastric cancer, etc. [50,52,53]. Ye et al. have found that silencing PRSS1 expression activates the EPK signaling pathway, thereby inhibiting the growth and proliferation of cancer cells [51]. Thus, in addition to its presence as a prognostic gene for PRSS1, we believe that it has the potential to serve as a therapeutic target for UCEC cells. The matrix metalloproteinases (MMPs) are a family of proteases closely linked to tumorigenesis, in which MMP1 is one of the zinc-dependent protein endonuclease family members. It may be associated with tumor metastasis and invasion [54]. It is found that MMP1 expression is increased in various tumor tissues, such as gastric cancer, liver cancer and breast cancer, etc. [55-57]. Other studies have shown that it is also correlated with the tumor proliferation process to some extent, which may be related to the activation of the PI3K/AKT pathway [58]. Recently, it is found that MMP1 expression is increased in the UCEC tissues, and the inhibition of MMP1 by lapatinib through EGFR and HER2 and its downstream ERK and AKT signaling pathways may have potential research value for the treatment of UCEC [59]. Human epidermal growth factor receptor 2 gene (ERBB2), also known as HER2 (Erb-B2 Receptor Tyrosine Kinase 2), is a gene that may cause the occurrence and development of a variety of cancer cells, such as lung cancer and breast cancer, etc. It can also be a therapeutic target and biomarker [60-63]. At present, ERBB2 has been used in a large number of ERBB2-positive gastric cancer and breast cancer patients. It has greatly improved the survival rate [64,65]. ERBB2 has also been studied in the targeted treatment of UCEC. For example, Amanda has found that the targeted treatment of uterine serous carcinoma, one of the ERBB2-positive subtypes of UCEC, may improve the progression-free survival of patients [66]. Therefore, we speculate that it also has a better potential to predict survival and therapeutic applications in UCEC. Ndc80 kinetochore complex component (NUF2), a protein complex component associated with the centromere [67], is connected with spindle binding and chromosome segregation. Therefore, regulating cell proliferation and apoptosis is closely related to the cell life cycle [68]. Many studies have found that NUF2 is highly expressed in the cells of various cancer lines. It exists as a therapeutic target and prognostic marker [69-71]. HU et al. have found that high expression of NUF2 is associated with poor prognosis of pancreatic cancer patients [68]. Shiraishi et al. have also found that the survival in patients with high NUF2 expression is significantly shortened [72]. Therefore, we believe that, in addition to being a prognostic gene, RSS1, MMP1, and ERBB2 have the potential to be called therapeutic targets, while the therapeutic potential of NUF2 remains to be further explored.
Immune checkpoints are receptor-ligand pairs that play a role in immunity. They can be classified into stimulatory immune checkpoint molecules and inhibitory immune checkpoint molecules [30]. Among them, enhancing stimulatory immune checkpoints and blocking inhibitory immune checkpoints have been widely used in the immune response to tumors [31,32]. CD40 belongs to the tumor necrosis factor receptor family, and CD40 ligand and its CD40L receptor are stimulatory checkpoints, which may promote the immune process of cellular and humoral immune responses and play a certain anti-tumor role [33]. It is shown that CD40/CD40L interaction may induce malignant B cells expressing the death receptors CD95, DR5 and TNF-R1 [34,35], thereby making the cells more prone to apoptosis in the inflammatory microenvironment [34,35]. It has been reported that CD40/CD40L may promote the apoptosis of malignant tumor cells, such as breast cancer and kidney cancer [36,37]. Although there are few studies on its relationship with UCEC, we speculate that it can also participate in the process of apoptosis. However, some studies also suggest that the role of CD40 in UCEC seems to be bidirectional. In addition to inhibiting EC cells, it also promotes EC cell invasion and involves in the expression of some genes for tumor promotion and metastasis, such as IL-6, LIF and TNF- [73]. Interestingly, our prognostic model also shows that the score of more active CD40 is higher in the high-risk group, and correlated with its outcome. Therefore, we believe that it remains to be further investigated whether it has a positive value for patients with UCEC although CD40 can be used as a direction for most tumor treatment.
Through the GSEA data analysis, we have found that the “HOMOLOGOUS_RECOMBINATION” pathway is significantly expressed in the high-risk group. “Homologous recombination” may accurately repair the damage caused by double-strand breaks of DNA in eukaryotic cells in the S and G2 phases in the presence of sister chromosomes [74]. False DNA repair may lead to chromosomal displacement and gene mutations, and accelerate the occurrence of the tumor [75,76]. It is found that the expression of homologous recombination is up-regulated in various tissues of cancer cell lines compared with surrounding normal tissues [77-79]. In terms of daughter UCEC treatment, Hu et al. have found that the inhibition of the homologous recombination pathway by diosmetin may suppress the proliferation of UCEC cells and improve the sensitivity to radiotherapy [80], thus assisting the treatment of UCEC. We believe that the expression of HOMOLOGOUS_RECOMBINATION is significant in the high risk group, which may be associated with the poor prognosis of patients. Its pathway expression inhibition may have positive significance for the treatment and prognosis of patients.
As a small molecule with anti-inflammatory and anti-antitumor effects, luteolin’s therapeutic potential for UCEC has not been deeply studied. In this study, we have investigated its potential targets and mechanism of action on UCEC by obtaining DE-DEGs of luteolin and UCEC and using enriched function and immunity, acquired the genes related to the prognosis and established a prognostic model in order to explore the new direction of UCEC treatment and prognosis. In vitro experiments, we found that the migration ability of endometrial cancer cells was significantly reduced after luteolin treatment, and the expressions of MMP1, IL-17 and VEGF were all decreased. However, due to the lack of clinical studies of luteolin on UCEC, whether it has a definite therapeutic effect on UCEC has not been determined. In addition, the efficacy of the pathway mechanism of luteolin targets on UCEC cells requires further experiments to confirm on the basis of the literature support.
Some studies have shown that IL-17 signal pathway can promote tumor progression, increase the expression of VEGF and promote tumor angiogenesis [81]. We observed that after the use of luteolin, the expression of IL-17 in endometrial cancer cells was inhibited, and the expression of MMP1 and VEGF was decreased. This may indicate that luteolin can inhibit the invasion of endometrial carcinoma by inhibiting IL-17 signal pathway and reducing the expression of MMP1, and reduce angiogenesis by reducing VEGF, thus improving the prognosis of patients with endometrial carcinoma.
Limitations and prospective
There are some limitations in this study. In vivo experiments of luteolin in animals and humans have not been performed. We plan to perform additional experiments and explore this in depth in subsequent studies.
Conclusion
In summary, our study provides a new direction for the treatment of UCEC, new genetic markers for the prognosis of UCEC and a basis for the R&D of luteolin as a drug in the treatment of UCEC.
Acknowledgements
The APC was funded by Junde Zhao.
Disclosure of conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.Paleari L, Pesce S, Rutigliani M, Greppi M, Obino V, Gorlero F, Vellone VG, Marcenaro E. New insights into endometrial cancer. Cancers (Basel) 2021;13:1496. doi: 10.3390/cancers13071496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Cancer Society. Cancer Statistics for USA. 2021. Available online: https://www.cancer.org/cancer/endometrial-cancer/about/key-statistics.html.
- 3.Onstad MA, Schmandt RE, Lu KH. Addressing the role of obesity in endometrial cancer risk, prevention, and treatment. J. Clin. Oncol. 2016;34:4225–4230. doi: 10.1200/JCO.2016.69.4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McAlpine JN, Temkin SM, Mackay HJ. Endometrial cancer: not your grandmother’s cancer. Cancer. 2016;122:2787–2798. doi: 10.1002/cncr.30094. [DOI] [PubMed] [Google Scholar]
- 5.Braun MM, Overbeek-Wager EA, Grumbo RJ. Diagnosis and management of endometrial cancer. Am Fam Physician. 2016;93:468–474. [PubMed] [Google Scholar]
- 6.van den Heerik ASVM, Horeweg N, de Boer SM, Bosse T, Creutzberg CL. Adjuvant therapy for endometrial cancer in the era of molecular classification: radiotherapy, chemoradiation and novel targets for therapy. Int J Gynecol Cancer. 2021;31:594–604. doi: 10.1136/ijgc-2020-001822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brooks RA, Fleming GF, Lastra RR, Lee NK, Moroney JW, Son CH, Tatebe K, Veneris JL. Current recommendations and recent progress in endometrial cancer. CA Cancer J Clin. 2019;69:258–279. doi: 10.3322/caac.21561. [DOI] [PubMed] [Google Scholar]
- 8.Cui Y, Shu XO, Gao Y, Wen W, Ruan ZX, Jin F, Zheng W. Use of complementary and alternative medicine by Chinese women with breast cancer. Breast Cancer Res Treat. 2004;85:263–270. doi: 10.1023/B:BREA.0000025422.26148.8d. [DOI] [PubMed] [Google Scholar]
- 9.Chen Z, Gu K, Zheng Y, Zheng W, Lu W, Shu XO. The use of complementary and alternative medicine among Chinese women with breast cancer. J Altern Complement Med. 2008;14:1049–1055. doi: 10.1089/acm.2008.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang X, Chen LX, Ouyang L, Cheng Y, Liu B. Plant natural compounds: targeting pathways of autophagy as anti-cancer therapeutic agents. Cell Prolif. 2012;45:466–476. doi: 10.1111/j.1365-2184.2012.00833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eritja N, Yeramian A, Chen BJ, Llobet-Navas D, Ortega E, Colas E, Abal M, Dolcet X, Reventos J, Matias-Guiu X. Endometrial carcinoma: specific targeted pathways. Adv Exp Med Biol. 2017;943:149–207. doi: 10.1007/978-3-319-43139-0_6. [DOI] [PubMed] [Google Scholar]
- 12.Lim SH, Jung SK, Byun S, Lee EJ, Hwang JA, Seo SG, Kim YA, Yu JG, Lee KW, Lee HJ. Luteolin suppresses UVB-induced photoageing by targeting JNK1 and p90 RSK2. J Cell Mol Med. 2013;17:672–680. doi: 10.1111/jcmm.12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boeing T, de Souza P, Speca S, Somensi LB, Mariano LNB, Cury BJ, Ferreira Dos Anjos M, Quintão NLM, Dubuqoy L, Desreumax P, da Silva LM, de Andrade SF. Luteolin prevents irinotecan-induced intestinal mucositis in mice through antioxidant and anti-inflammatory properties. Br J Pharmacol. 2020;177:2393–2408. doi: 10.1111/bph.14987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harborne JB, Williams CA. Advances in flavonoid research since 1992. Phytochemistry. 2000;55:481–504. doi: 10.1016/s0031-9422(00)00235-1. [DOI] [PubMed] [Google Scholar]
- 15.Birt DF, Hendrich S, Wang W. Dietary agents in cancer prevention: flavonoids and isoflavonoids. Pharmacol Ther. 2001;90:157–177. doi: 10.1016/s0163-7258(01)00137-1. [DOI] [PubMed] [Google Scholar]
- 16.Wu HT, Lin J, Liu YE, Chen HF, Hsu KW, Lin SH, Peng KY, Lin KJ, Hsieh CC, Chen DR. Luteolin suppresses androgen receptor-positive triple-negative breast cancer cell proliferation and metastasis by epigenetic regulation of MMP9 expression via the AKT/mTOR signaling pathway. Phytomedicine. 2021;81:153437. doi: 10.1016/j.phymed.2020.153437. [DOI] [PubMed] [Google Scholar]
- 17.Monti E, Marras E, Prini P, Gariboldi MB. Luteolin impairs hypoxia adaptation and progression in human breast and colon cancer cells. Eur J Pharmacol. 2020;881:173210. doi: 10.1016/j.ejphar.2020.173210. [DOI] [PubMed] [Google Scholar]
- 18.Lee Y, Kwon YH. Regulation of apoptosis and autophagy by luteolin in human hepatocellular cancer Hep3B cells. Biochem Biophys Res Commun. 2019;517:617–622. doi: 10.1016/j.bbrc.2019.07.073. [DOI] [PubMed] [Google Scholar]
- 19.Cassidy A, Minihane AM. The role of metabolism (and the microbiome) in defining the clinical efficacy of dietary flavonoids. Am J Clin Nutr. 2017;105:10–22. doi: 10.3945/ajcn.116.136051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 21.Gu B, Shang X, Yan M, Li X, Wang W, Wang Q, Zhang C. Variations in incidence and mortality rates of endometrial cancer at the global, regional, and national levels, 1990-2019. Gynecol Oncol. 2021;161:573–580. doi: 10.1016/j.ygyno.2021.01.036. [DOI] [PubMed] [Google Scholar]
- 22.Lin Z, Sui X, Jiao W, Chen C, Zhang X, Zhao J. Mechanism investigation and experiment validation of capsaicin on uterine corpus endometrial carcinoma. Front Pharmacol. 2022;13:953874. doi: 10.3389/fphar.2022.953874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin Z, Sui X, Jiao W, Wang Y, Zhao J. Exploring the mechanism and experimental verification of puerarin in the treatment of endometrial carcinoma based on network pharmacology and bioinformatics analysis. BMC Complement Med Ther. 2022;22:150. doi: 10.1186/s12906-022-03623-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Westin SN, Fellman B, Sun CC, Broaddus RR, Woodall ML, Pal N, Urbauer DL, Ramondetta LM, Schmeler KM, Soliman PT, Fleming ND, Burzawa JK, Nick AM, Milbourne AM, Yuan Y, Lu KH, Bodurka DC, Coleman RL, Yates MS. Prospective phase II trial of levonorgestrel intrauterine device: nonsurgical approach for complex atypical hyperplasia and early-stage endometrial cancer. Am J Obstet Gynecol. 2021;224:191.e1–191.e15. doi: 10.1016/j.ajog.2020.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin Y, Shi R, Wang X, Shen HM. Luteolin, a flavonoid with potential for cancer prevention and therapy. Curr Cancer Drug Targets. 2008;8:634–646. doi: 10.2174/156800908786241050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sies H. Oxidative stress: oxidants and antioxidants. Exp Physiol. 1997;82:291–295. doi: 10.1113/expphysiol.1997.sp004024. [DOI] [PubMed] [Google Scholar]
- 27.Ranjan P, Anathy V, Burch PM, Weirather K, Lambeth JD, Heintz NH. Redox-dependent expression of cyclin D1 and cell proliferation by Nox1 in mouse lung epithelial cells. Antioxid Redox Signal. 2006;8:1447–1459. doi: 10.1089/ars.2006.8.1447. [DOI] [PubMed] [Google Scholar]
- 28.Martindale JL, Holbrook NJ. Cellular response to oxidative stress: signaling for suicide and survival. J Cell Physiol. 2002;192:1–15. doi: 10.1002/jcp.10119. [DOI] [PubMed] [Google Scholar]
- 29.Matsui A, Ikeda T, Enomoto K, Hosoda K, Nakashima H, Omae K, Watanabe M, Hibi T, Kitajima M. Increased formation of oxidative DNA damage, 8-hydroxy-2’-deoxyguanosine, in human breast cancer tissue and its relationship to GSTP1 and COMT genotypes. Cancer Lett. 2000;151:87–95. doi: 10.1016/s0304-3835(99)00424-3. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y, Zheng J. Functions of immune checkpoint molecules beyond immune evasion. Adv Exp Med Biol. 2020;1248:201–226. doi: 10.1007/978-981-15-3266-5_9. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Q, Vignali DA. Co-stimulatory and co-inhibitory pathways in autoimmunity. Immunity. 2016;44:1034–1051. doi: 10.1016/j.immuni.2016.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He X, Xu C. Immune checkpoint signaling and cancer immunotherapy. Cell Res. 2020;30:660–669. doi: 10.1038/s41422-020-0343-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang T, Cheng X, Truong B, Sun L, Yang X, Wang H. Molecular basis and therapeutic implications of CD40/CD40L immune checkpoint. Pharmacol Ther. 2021;219:107709. doi: 10.1016/j.pharmthera.2020.107709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dicker F, Kater AP, Prada CE, Fukuda T, Castro JE, Sun G, Wang JY, Kipps TJ. CD154 induces p73 to overcome the resistance to apoptosis of chronic lymphocytic leukemia cells lacking functional p53. Blood. 2006;108:3450–3457. doi: 10.1182/blood-2006-04-017749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dicker F, Kater AP, Fukuda T, Kipps TJ. Fas-ligand (CD178) and TRAIL synergistically induce apoptosis of CD40-activated chronic lymphocytic leukemia B cells. Blood. 2005;105:3193–3198. doi: 10.1182/blood-2003-10-3684. [DOI] [PubMed] [Google Scholar]
- 36.Gomes EM, Rodrigues MS, Phadke AP, Butcher LD, Starling C, Chen S, Chang D, Hernandez-Alcoceba R, Newman JT, Stone MJ, Tong AW. Antitumor activity of an oncolytic adenoviral-CD40 ligand (CD154) transgene construct in human breast cancer cells. Clin Cancer Res. 2009;15:1317–1325. doi: 10.1158/1078-0432.CCR-08-1360. [DOI] [PubMed] [Google Scholar]
- 37.Ibraheem K, Yhmed AMA, Qayyum T, Bryan NP, Georgopoulos NT. CD40 induces renal cell carcinoma-specific differential regulation of TRAF proteins, ASK1 activation and JNK/p38-mediated, ROS-dependent mitochondrial apoptosis. Cell Death Discov. 2019;5:148. doi: 10.1038/s41420-019-0229-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lowes MA, Kikuchi T, Fuentes-Duculan J, Cardinale I, Zaba LC, Haider AS, Bowman EP, Krueger JG. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J Invest Dermatol. 2008;128:1207–1211. doi: 10.1038/sj.jid.5701213. [DOI] [PubMed] [Google Scholar]
- 39.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alshaker HA, Matalka KZ. IFN-γ, IL-17 and TGF-β involvement in shaping the tumor microenvironment: the significance of modulating such cytokines in treating malignant solid tumors. Cancer Cell Int. 2011;11:33. doi: 10.1186/1475-2867-11-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Numasaki M, Fukushi J, Ono M, Narula SK, Zavodny PJ, Kudo T, Robbins PD, Tahara H, Lotze MT. Interleukin-17 promotes angiogenesis and tumor growth. Blood. 2003;101:2620–2627. doi: 10.1182/blood-2002-05-1461. [DOI] [PubMed] [Google Scholar]
- 42.Pongcharoen S, Niumsup P, Sanguansermsri D, Supalap K, Butkhamchot P. The effect of interleukin-17 on the proliferation and invasion of JEG-3 human choriocarcinoma cells. Am J Reprod Immunol. 2006;55:291–300. doi: 10.1111/j.1600-0897.2006.00366.x. [DOI] [PubMed] [Google Scholar]
- 43.Kryczek I, Banerjee M, Cheng P, Vatan L, Szeliga W, Wei S, Huang E, Finlayson E, Simeone D, Welling TH, Chang A, Coukos G, Liu R, Zou W. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood. 2009;114:1141–1149. doi: 10.1182/blood-2009-03-208249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tartour E, Fossiez F, Joyeux I, Galinha A, Gey A, Claret E, Sastre-Garau X, Couturier J, Mosseri V, Vives V, Banchereau J, Fridman WH, Wijdenes J, Lebecque S, Sautès-Fridman C. Interleukin 17, a T-cell-derived cytokine, promotes tumorigenicity of human cervical tumors in nude mice. Cancer Res. 1999;59:3698–3704. [PubMed] [Google Scholar]
- 45.Lai T, Wang K, Hou Q, Zhang J, Yuan J, Yuan L, You Z, Xi M. Interleukin 17 induces up-regulation of chemokine and cytokine expression via activation of the nuclear factor κB and extracellular signal-regulated kinase 1/2 pathways in gynecologic cancer cell lines. Int J Gynecol Cancer. 2011;21:1533–1539. doi: 10.1097/IGC.0b013e31822d2abd. [DOI] [PubMed] [Google Scholar]
- 46.Wang L, Yi T, Kortylewski M, Pardoll DM, Zeng D, Yu H. IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. J Exp Med. 2009;206:1457–1464. doi: 10.1084/jem.20090207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gendrisch F, Esser PR, Schempp CM, Wölfle U. Luteolin as a modulator of skin aging and inflammation. Biofactors. 2021;47:170–180. doi: 10.1002/biof.1699. [DOI] [PubMed] [Google Scholar]
- 48.Shi F, Zhou D, Ji Z, Xu Z, Yang H. Anti-arthritic activity of luteolin in Freund’s complete adjuvant-induced arthritis in rats by suppressing P2X4 pathway. Chem Biol Interact. 2015;226:82–87. doi: 10.1016/j.cbi.2014.10.031. [DOI] [PubMed] [Google Scholar]
- 49.Sahin-Tóth M. Human mesotrypsin defies natural trypsin inhibitors: from passive resistance to active destruction. Protein Pept Lett. 2005;12:457–464. doi: 10.2174/0929866054395356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang F, Hu YL, Feng Y, Guo YB, Liu YF, Mao QS, Xue WJ. High-level expression of PRSS3 correlates with metastasis and poor prognosis in patients with gastric cancer. J Surg Oncol. 2019;119:1108–1121. doi: 10.1002/jso.25448. [DOI] [PubMed] [Google Scholar]
- 51.Ye D, Li Y, Zhang H, Zhou Z, Tang Y, Wu P, Zhao Q, Zhang Z. Silencing PRSS1 suppresses the growth and proliferation of gastric carcinoma cells via the ERK pathway. Int J Biol Sci. 2021;17:957–971. doi: 10.7150/ijbs.52591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Song JY, Bae HS, Koo do H, Lee JK, Jung HH, Lee KW, Lee NW. Candidates for tumor markers of cervical cancer discovered by proteomic analysis. J Korean Med Sci. 2012;27:1479–1485. doi: 10.3346/jkms.2012.27.12.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zeng K, Liu QC, Lin JH, Lin XH, Zhuang ZH, Gao F, Ou QS. Novel mutations of PRSS1 gene in patients with pancreatic cancer among Han population. Chin Med J (Engl) 2011;124:2065–2067. [PubMed] [Google Scholar]
- 54.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bao W, Fu HJ, Jia LT, Zhang Y, Li W, Jin BQ, Yao LB, Chen SY, Yang AG. HER2-mediated upregulation of MMP-1 is involved in gastric cancer cell invasion. Arch Biochem Biophys. 2010;499:49–55. doi: 10.1016/j.abb.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 56.Armstrong DA, Phelps LN, Vincenti MP. CCAAT enhancer binding protein-beta regulates matrix metalloproteinase-1 expression in interleukin-1beta-stimulated A549 lung carcinoma cells. Mol Cancer Res. 2009;7:1517–1524. doi: 10.1158/1541-7786.MCR-09-0082. [DOI] [PubMed] [Google Scholar]
- 57.Park YH, Jung HH, Ahn JS, Im YH. Ets-1 upregulates HER2-induced MMP-1 expression in breast cancer cells. Biochem Biophys Res Commun. 2008;377:389–394. doi: 10.1016/j.bbrc.2008.09.135. [DOI] [PubMed] [Google Scholar]
- 58.Liu M, Hu Y, Zhang MF, Luo KJ, Xie XY, Wen J, Fu JH, Yang H. MMP1 promotes tumor growth and metastasis in esophageal squamous cell carcinoma. Cancer Lett. 2016;377:97–104. doi: 10.1016/j.canlet.2016.04.034. [DOI] [PubMed] [Google Scholar]
- 59.Lin CY, Chao A, Wang TH, Hsueh S, Lee YS, Wu TI, Chao AS, Huang HJ, Chou HH, Chang TC, Lai CH. A dual tyrosine kinase inhibitor lapatinib suppresses overexpression of matrix metallopeptidase 1 (MMP1) in endometrial cancer. J Mol Med (Berl) 2014;92:969–981. doi: 10.1007/s00109-014-1163-0. [DOI] [PubMed] [Google Scholar]
- 60.Llobet D, Pallares J, Yeramian A, Santacana M, Eritja N, Velasco A, Dolcet X, Matias-Guiu X. Molecular pathology of endometrial carcinoma: practical aspects from the diagnostic and therapeutic viewpoints. J Clin Pathol. 2009;62:777–785. doi: 10.1136/jcp.2008.056101. [DOI] [PubMed] [Google Scholar]
- 61.Kris MG, Johnson BE, Berry LD, Kwiatkowski DJ, Iafrate AJ, Wistuba II, Varella-Garcia M, Franklin WA, Aronson SL, Su PF, Shyr Y, Camidge DR, Sequist LV, Glisson BS, Khuri FR, Garon EB, Pao W, Rudin C, Schiller J, Haura EB, Socinski M, Shirai K, Chen H, Giaccone G, Ladanyi M, Kugler K, Minna JD, Bunn PA. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA. 2014;311:1998–2006. doi: 10.1001/jama.2014.3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Arcila ME, Chaft JE, Nafa K, Roy-Chowdhuri S, Lau C, Zaidinski M, Paik PK, Zakowski MF, Kris MG, Ladanyi M. Prevalence, clinicopathologic associations, and molecular spectrum of ERBB2 (HER2) tyrosine kinase mutations in lung adenocarcinomas. Clin Cancer Res. 2012;18:4910–4918. doi: 10.1158/1078-0432.CCR-12-0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ferreira D, Soares M, Correia J, Adega F, Ferreira F, Chaves R. Assessment of ERBB2 and TOP2α gene status and expression profile in feline mammary tumors: findings and guidelines. Aging (Albany NY) 2019;11:4688–4705. doi: 10.18632/aging.102079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, Pegram M, Oh DY, Diéras V, Guardino E, Fang L, Lu MW, Olsen S, Blackwell K EMILIA Study Group. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367:1783–1791. doi: 10.1056/NEJMoa1209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, Lehle M, Rüschoff J, Kang YK ToGA Trial Investigators. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 66.Fader AN, Roque DM, Siegel E, Buza N, Hui P, Abdelghany O, Chambers SK, Secord AA, Havrilesky L, O’Malley DM, Backes F, Nevadunsky N, Edraki B, Pikaart D, Lowery W, ElSahwi KS, Celano P, Bellone S, Azodi M, Litkouhi B, Ratner E, Silasi DA, Schwartz PE, Santin AD. Randomized phase II trial of carboplatin-paclitaxel versus carboplatin-paclitaxel-trastuzumab in uterine serous carcinomas that overexpress human epidermal growth factor receptor 2/neu. J. Clin. Oncol. 2018;36:2044–2051. doi: 10.1200/JCO.2017.76.5966. [DOI] [PubMed] [Google Scholar]
- 67.Nabetani A, Koujin T, Tsutsumi C, Haraguchi T, Hiraoka Y. A conserved protein, Nuf2, is implicated in connecting the centromere to the spindle during chromosome segregation: a link between the kinetochore function and the spindle checkpoint. Chromosoma. 2001;110:322–334. doi: 10.1007/s004120100153. [DOI] [PubMed] [Google Scholar]
- 68.Hu P, Chen X, Sun J, Bie P, Zhang LD. siRNA-mediated knockdown against NUF2 suppresses pancreatic cancer proliferation in vitro and in vivo. Biosci Rep. 2015;35:e00170. doi: 10.1042/BSR20140124. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 69.Xu W, Wang Y, Wang Y, Lv S, Xu X, Dong X. Screening of differentially expressed genes and identification of NUF2 as a prognostic marker in breast cancer. Int J Mol Med. 2019;44:390–404. doi: 10.3892/ijmm.2019.4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fu HL, Shao L. Silencing of NUF2 inhibits proliferation of human osteosarcoma Saos-2 cells. Eur Rev Med Pharmacol Sci. 2016;20:1071–1079. [PubMed] [Google Scholar]
- 71.Xie X, Jiang S, Li X. Nuf2 Is a prognostic-related biomarker and correlated with immune infiltrates in hepatocellular carcinoma. Front Oncol. 2021;11:621373. doi: 10.3389/fonc.2021.621373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shiraishi T, Terada N, Zeng Y, Suyama T, Luo J, Trock B, Kulkarni P, Getzenberg RH. Cancer/testis antigens as potential predictors of biochemical recurrence of prostate cancer following radical prostatectomy. J Transl Med. 2011;9:153. doi: 10.1186/1479-5876-9-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dumas G, Dufresne M, Asselin É, Girouard J, Carrier C, Reyes-Moreno C. CD40 pathway activation reveals dual function for macrophages in human endometrial cancer cell survival and invasion. Cancer Immunol Immunother. 2013;62:273–283. doi: 10.1007/s00262-012-1333-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mao Z, Bozzella M, Seluanov A, Gorbunova V. DNA repair by nonhomologous end joining and homologous recombination during cell cycle in human cells. Cell Cycle. 2008;7:2902–2906. doi: 10.4161/cc.7.18.6679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chapman JR, Taylor MR, Boulton SJ. Playing the end game: DNA double-strand break repair pathway choice. Mol Cell. 2012;47:497–510. doi: 10.1016/j.molcel.2012.07.029. [DOI] [PubMed] [Google Scholar]
- 76.Scully R, Panday A, Elango R, Willis NA. DNA double-strand break repair-pathway choice in somatic mammalian cells. Nat Rev Mol Cell Biol. 2019;20:698–714. doi: 10.1038/s41580-019-0152-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mao Z, Jiang Y, Liu X, Seluanov A, Gorbunova V. DNA repair by homologous recombination, but not by nonhomologous end joining, is elevated in breast cancer cells. Neoplasia. 2009;11:683–691. doi: 10.1593/neo.09312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Maacke H, Jost K, Opitz S, Miska S, Yuan Y, Hasselbach L, Lüttges J, Kalthoff H, Stürzbecher HW. DNA repair and recombination factor Rad51 is over-expressed in human pancreatic adenocarcinoma. Oncogene. 2000;19:2791–2795. doi: 10.1038/sj.onc.1203578. [DOI] [PubMed] [Google Scholar]
- 79.Raderschall E, Stout K, Freier S, Suckow V, Schweiger S, Haaf T. Elevated levels of Rad51 recombination protein in tumor cells. Cancer Res. 2002;62:219–225. [PubMed] [Google Scholar]
- 80.Hu Z, Cai B, Wang M, Wen X, Geng A, Hu X, Xue R, Mao Z, Jiang Y, Wan X. Diosmetin enhances the sensitivity of radiotherapy by suppressing homologous recombination in endometrial cancer. Cell Cycle. 2020;19:3115–3126. doi: 10.1080/15384101.2020.1831257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li X, Bechara R, Zhao J, McGeachy MJ, Gaffen SL. IL-17 receptor-based signaling and implications for disease. Nat Immunol. 2019;20:1594–1602. doi: 10.1038/s41590-019-0514-y. [DOI] [PMC free article] [PubMed] [Google Scholar]