Abstract
In patients with interstitial lung disease (ILD) complicating classical or amyopathic idiopathic inflammatory myopathy (IIM), lung transplantation outcomes might be affected by the disease and treatments. Here, our objective was to assess survival and prognostic factors in lung transplant recipients with IIM‐ILD. We retrospectively reviewed data for 64 patients who underwent lung transplantation between 2009 and 2021 at 19 European centers. Patient survival was the primary outcome. At transplantation, the median age was 53 [46–59] years, 35 (55%) patients were male, 31 (48%) had classical IIM, 25 (39%) had rapidly progressive ILD, and 21 (33%) were in a high‐priority transplant allocation program. Survival rates after 1, 3, and 5 years were 78%, 73%, and 70%, respectively. During follow‐up (median, 33 [7–63] months), 23% of patients developed chronic lung allograft dysfunction. Compared to amyopathic IIM, classical IIM was characterized by longer disease duration, higher‐intensity immunosuppression before transplantation, and significantly worse posttransplantation survival. Five (8%) patients had a clinical IIM relapse, with mild manifestations. No patient experienced ILD recurrence in the allograft. Posttransplantation survival in IIM‐ILD was similar to that in international all‐cause‐transplantation registries. The main factor associated with worse survival was a history of muscle involvement (classical IIM). In lung transplant recipients with idiopathic inflammatory myopathy, survival was similar to that in all‐cause transplantation and was worse in patients with muscle involvement compared to those with the amyopathic disease.
Keywords: connective tissue disease, idiopathic inflammatory myopathy, interstitial lung disease, lung transplantation
Short abstract
A retrospective multicenter study shows that patient survival after lung transplantation for myositis‐related interstitial lung disease is comparable to that reported in international registries for all causes combined.
Abbreviations
- 95%CI
95% confidence interval
- a‐IIM
amyopathic idiopathic inflammatory myopathy
- c‐IIM
classical idiopathic inflammatory myopathy
- CT
computed tomography
- HR
hazard ratio
- IIM
idiopathic inflammatory myopathy
- ILD
interstitial lung disease
- IPF
idiopathic pulmonary fibrosis
- LTx
lung transplantation
- PFT
pulmonary function tests
- RP‐ILD
rapidly progressive interstitial lung disease
1. INTRODUCTION
The idiopathic inflammatory myopathies (IIMs), first defined by Bohan and Peter 1 , 2 in 1975, constitute a heterogeneous group of connective tissue diseases characterized chiefly by varying frequencies and degrees of acquired skeletal‐muscle inflammation. In adults, they include polymyositis, dermatomyositis (DM), inclusion‐body myositis, and necrotizing auto‐immune myositis. 3 It was recognized recently that DM may occur without muscle involvement and that amyopathic DM is part of the IIM spectrum. 4
Interstitial lung disease (ILD) is the most common non‐muscular manifestation of IIM. 5 Importantly, IIM‐associated ILD (IIM‐ILD) is associated with impaired quality of life and higher mortality, 6 , 7 even when the muscle and/or skin disease respond well to immunosuppressive therapy. Although the first symptoms of ILD usually occur after the diagnosis of IIM, they are inaugural in a substantial minority of patients, in whom appropriate immunosuppressive therapy may thus be delayed and outcomes poorer. 8 In addition, rapidly progressive ILD (RP‐ILD) is a specific manifestation of IIM in which life‐threatening hypoxemic respiratory failure develops over a few weeks or months. 9
Lung transplantation (LTx) may be an option when lung function declines either progressively or acutely despite optimal immunosuppressive therapy. However, LTx is rarely performed in patients with IIM‐ILD. According to the International Society for Heart and Lung Transplantation (ISHLT) registry, patients with ILD related to connective tissue diseases accounted for only 564/63530 (0.9%) of all LTx procedures done between 1995 and 2018. 10 A major concern is that the complex and often severe extrapulmonary manifestations of IIM may decrease survival after LTx. More specifically, myositis may involve the diaphragm and impair swallowing, which may increase postoperative morbidity. Moreover, previous immunosuppressant exposure combined with an often poor nutritional status may negatively impact LTx outcomes. Many centers are thus reluctant to offer LTx to patients with IIM‐ILD. Nonetheless, several case reports 11 , 12 and a retrospective observational cohort study 13 suggest that selected patients with IIM‐ILD may experience acceptable short‐ and long‐term outcomes after LTx and that this option may be under‐used. However, large gaps exist in our understanding of the evaluation and selection of patients with IIM‐ILD for LTx.
The objectives of this study were to assess patient selection for LTx to treat IIM‐ILD, evaluate survival, and identify prognostic factors. To this end, we conducted a retrospective observational study of 64 patients who underwent LTx at 19 European transplant centers.
2. METHODS
This retrospective study complied with the Declaration of Helsinki and was approved by the Paris‐Saint Joseph Hospital institutional review board (Groupe Éthique en Recherche Médicale, # IRB548). In addition, approval was obtained from the local ethics committee. All data were anonymized and compiled in compliance with the requirements of the data protection authority of each participating country.
2.1. Study design and population
We identified 68 transplant centers in 23 European countries via the International Society for Heart and Lung Transplantation (ISHLT) registry and by personal knowledge. Among these centers, 23 responded and 19 reported patients who met the study inclusion criteria, namely, the presence of diagnostic criteria for IIM 14 , 15 and IIM‐ILD, 16 , 17 including positive myositis‐specific antibody titers at some point of the disease; cadaveric LTx between 2009 and 2021; available thoracic computed tomography (CT) data.
2.2. Collection of baseline data
We used standardized forms to record the following information: demographic data; medical history; physical findings; World Health Organization functional class; routine blood‐test results; 6‐min walking distance 18 ; most recent pulmonary function test (PFT) results before LTx including forced vital capacity, forced expiratory volume in 1 s, 19 and diffusing capacity for carbon monoxide, measured according to established protocols and reported as percent of predicted values. We also collected IIM characteristics, distinguishing between patients with (classical IIM, c‐IIM) vs. without (amyopathic IIM, a‐IIM) muscle involvement at any time between the diagnosis of IIM and LTx.
RP‐ILD was defined as rapidly progressive dyspnea and hypoxemia with worsening radiologic interstitial lung changes within 3 months after the onset of respiratory symptoms. 20
IIM‐related pulmonary hypertension was sought during right heart catheterization and defined as mean pulmonary artery pressure > 20 mm Hg, pulmonary capillary wedge pressure ≤ 15 mm Hg, and pulmonary vascular resistance >3 WU, in the absence of other known causes of PH. 21 Severe PH was defined as mean pulmonary artery pressure ≥ 35 mm Hg or as mean pulmonary artery pressure ≥ 25 mm Hg with a cardiac index ≤2.0 L/min/m2. 22
ILD was diagnosed by retrospective review of the last thoracic CT performed before LTx, at each participating center. Published criteria for ILD were used. 23
2.3. Patient management
Details on patient management are available in the online data supplement.
2.4. Outcome measure
Patient survival was the outcome measure. Information on vital status was collected by each center and was available for all patients. Survival analyses were performed in the overall population and the sub‐groups with c‐IIM vs. a‐IIM.
2.5. Statistical analysis
Baseline demographics were described as median [1st–3rd quartiles] for continuous variables and as percentages for categorical variables. Baseline features were compared among groups using ANOVA, Fisher's exact test, or Kruskal‐Wallis ANOVA depending on distribution and sample size. Survival was evaluated using the Kaplan–Meier method. No patient underwent re‐transplantation. Cox proportional hazards models were built for univariate analyses of patient survival after verifying that the proportionality assumption was met. The number of deaths was too small for multivariate analysis to look for independent predictors of patient survival. Since the numbers of patients with heart and diaphragm involvement, subcutaneous calcinosis, or a history of cancer or pneumomediastinum were very limited, these variables were dropped from the univariate analyses. For the univariate analyses of continuous variables, estimations of the risk over time were described by computing the hazard ratios (HRs) with their 95% confidence intervals (95%CIs). Two‐tailed p‐values smaller than .05 were considered significant. No patient was lost to follow‐up. All statistical analyses and graphs were performed using R v3.6.0 with the “ggplot2,” “survival,” “cmprsk,” and “kmi” packages.
3. RESULTS
3.1. Patients
Between September 2009 and September 2021, 70 patients with IIM‐ILD underwent LTx at the 19 participating centers. We excluded the six patients with no thoracic CT (n = 4) or PFT (n = 2) data (Figure 1). The remaining 64 patients were included in the study. Among them, eight underwent single‐LTx and 56 double‐LTx (Table 1).
FIGURE 1.
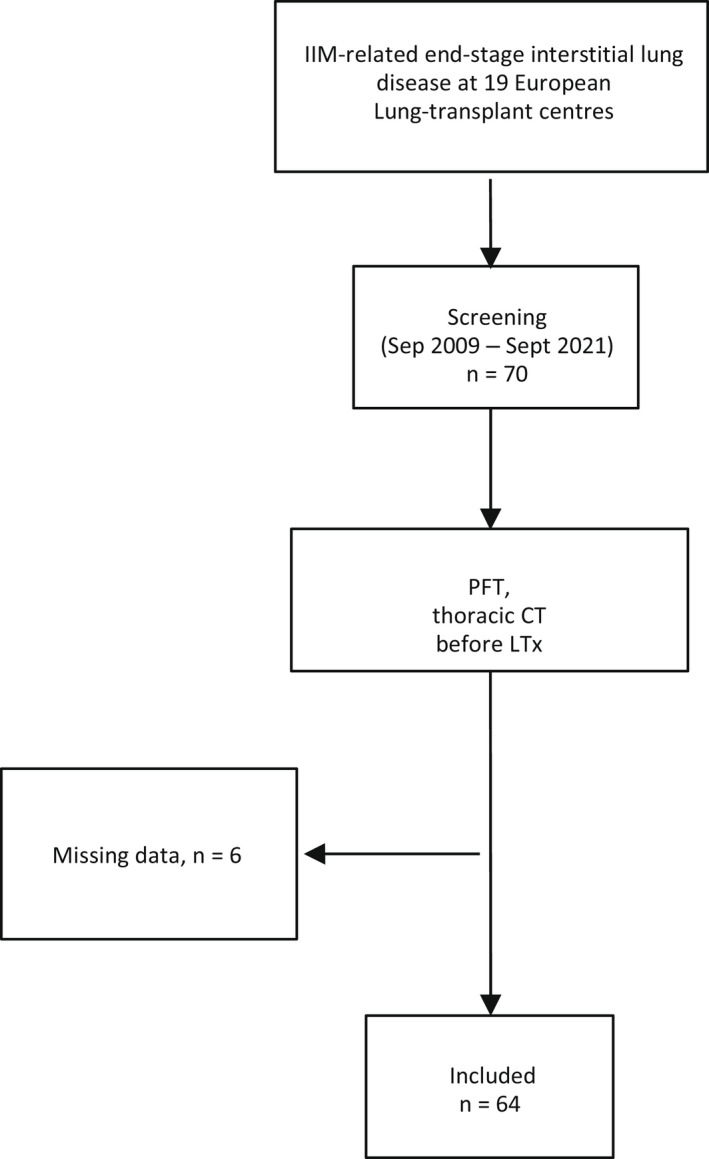
Patient flowchart. PFT, pulmonary function tests; CT, computed tomography.
TABLE 1.
Main features of the 64 study patients, including 31 with classical idiopathic inflammatory myositis (c‐IIM) and 33 with amyopathic idiopathic inflammatory myositis (a‐IIM)
| Overall n = 64 | c‐IIM n = 31 | a‐IIM n = 33 | p‐value | |
|---|---|---|---|---|
| Male, n (%) | 35 (55) | 14 (45) | 21 (64) | .22 |
| Recipient age (years), med [IQR] | 53 [46–59] | 55 [46–59] | 52 [46–56] | .71 |
| Body mass index, kg/m2 | 26 [23–29] | 25 [24–28] | 27 [23–29] | .29 |
| Caucasian, n (%) | 49 (77) | 22 (71) | 27 (82) | .10 |
| Smoking history (pack‐years), med [IQR], n = 44 | 12 [0–20] | 15 [10–20] | 6 [0–21] | .22 |
| CMV: donor positive / recipient negative, n (%), n = 56 | 4 (7%) | 0 | 4 (13) | .11 |
| Blood group, A/O/B/AB, %, n = 55 | 40/34/17/9 | 38/46/12/4 | 38/34/17/11 | .92 |
| Lung transplantation procedure: double/single, n (%) | 56 (87)/8 (13) | 27 (87)/4 (13) | 29 (87)/4 (13) | .99 |
| High‐priority transplant allocation program, n (%) | 21 (33) | 10 (32) | 11 (33) | .99 |
| Pulmonary hypertension, n (%), n = 39 | 29 (74) | 17 (89) | 12 (60) | .07 |
| Severe pulmonary hypertension, n (%), n = 39 | 13 (33) | 7 (37) | 6 (30) | .91 |
| Preoperative extracorporeal membrane oxygenation, n (%) | 13 (21) | 4 (13) | 9 (27) | .21 |
| Intraoperative extracorporeal membrane oxygenation, n (%) | 32 (50) | 12 (39) | 20 (61) | .13 |
| Postoperative extracorporeal membrane oxygenation, n (%) | 25 (39) | 12 (39) | 13 (39) | .99 |
| Induction, n (%) | 28 (44) | 14 (45) | 14 (42) | .99 |
| Dialysis during intensive care unit stay, n (%) | 12 (19) | 7 (23) | 5 (15) | .53 |
| Primary graft dysfunction Grade 3 at 72 h, n (%) | 17 (29) | 10 (36) | 7 (23) | .46 |
| Ventilation time during intensive care unit stay (days), med [IQR] | 6 [1–22] | 4 [1–16] | 11 [3–22] | .13 |
| Hemothorax, n (%), n = 63 | 14 (22) | 7 (23) | 7 (22) | .99 |
| Pneumonia requiring hospitalization during the first posttransplantation year, n (%), n = 50 | 25 (50) | 12 (46) | 13 (54) | .78 |
| Acute cellular rejection ≥grade 2 during the first posttransplantation year, n (%), n = 53 | 5 (9) | 2 (7) | 3 (12) | .63 |
| Preformed donor‐specific antibody, n (%) | 12 (19) | 7 (23) | 5 (16) | .75 |
| Antibody‐mediated rejection during the first posttransplantation year, n (%), n = 59 | 6 (10) | 1 (3) | 5 (17) | .11 |
| Chronic lung allograft dysfunction at last follow‐up, n (%), n = 57 | 13 (23) | 8 (29) | 5 (17) | .52 |
| Bronchiolitis obliterans syndrome | 10 (77) | 6 (76) | 4 (80) | |
| Restrictive allograft syndrome | 2 (15) | 1 (12) | 0 | |
| Mixed | 1 (8) | 1 (12) | 1 (20) | |
| Cancer after transplantation, n (%) | 3 (5) | 3 (10) | 0 | .11 |
| Deaths, n (%) | 19 (30) | 16 (52) | 3 (9) | <.01 |
| In‐hospital mortality, n (%) | 11 (17) | 8 (26) | 3 (9) | .10 |
| Causes of death, n (%) | .49 | |||
| Septic shock | 5 (26) | 3 (19) | 2 (67) | |
| Primary graft dysfunction | 4 (21) | 4 (25) | 0 | |
| Pneumonia | 4 (21) | 4 (25) | 0 | |
| Other | 6 (32) | 5 (31) | 1 (33) |
Abbreviations: a‐IIM, amyopathic idiopathic inflammatory myositis; c‐IIM, classical idiopathic inflammatory myositis; CMV, cytomegalovirus.
Numbers of inclusions by center were as follows: Suresnes (France), n = 10; Le Plessis‐Robinson (France), n = 7; Hanover (Germany), n = 7; Santander (Spain), n = 6; Barcelona (Spain), n = 6; Leuven (Belgium), n = 4; Paris‐Bichat (France), n = 4; Gothenburg (Sweden), n = 3; Pavia (Italy), n = 2; Bologna (Italy), n = 2; Vienna (Austria), n = 2; Bordeaux (France), n = 2; Milano (Italy), n = 2; Brussels (Belgium), n = 2; Copenhagen (Denmark), n = 1; Lausanne (Switzerland), n = 1; Paris‐Georges Pompidou (France), n = 1; Marseille (France), n = 1; and Lyon (France), n = 1.
Trends toward a larger proportion of patients with pulmonary hypertension were found in the group with c‐IIM compared to the group with a‐IIM (Table 1).
3.2. Features of idiopathic inflammatory myositis (IIM) at listing
Of the 64 patients, 33 (52%) had a‐IIM, with a significantly shorter disease duration at listing compared to the c‐IIM group (Table 2). Similar proportions of patients in the a‐IIM and c‐IIM sub‐groups had positive antisynthetase antibody titers, whereas the proportion of patients with positive anti‐MDA5 titers was significantly higher in the a‐IIM sub‐group (Table 2 ). Compared to the a‐IIM group, impaired swallowing was more common in the c‐IIM group, in which the number of immunosuppressive lines was higher. In the c‐IIM group, we found a trend toward a higher proportion of patients being diagnosed with cancer after transplantation.
TABLE 2.
Main idiopathic inflammatory myositis features at listing for lung transplantation
| Overall n = 64 | c‐IIM n = 31 | a‐IIM n = 33 | p‐value | |
|---|---|---|---|---|
| IIM duration at transplantation (months), med [IQR] | 52 [18–140] | 105 [49–168] | 29 [9–66] | <.01 |
| Rapidly progressive interstitial lung disease, n (%) | 25 (39) | 11 (35) | 14 (42) | .75 |
| History of pneumomediastinum, n (%) | 2 (3) | 1 (3) | 1 (3) | .99 |
| Antibody positivity, n (%) | ||||
| MDA5 | 13 (20) | 2 (6) | 11 (33) | .01 |
| Jo1 | 31 (48) | 18 (56) | 13 (39) | .21 |
| PL12 | 10 (16) | 6 (19) | 4 (12) | .50 |
| PL7 | 4 (6) | 2 (6) | 2 (6) | .99 |
| Other | 6 (9) | 3 (13) | 3 (10) | .99 |
| Positive muscle biopsy, n (%), n = 30 | 15 (50) | 15 (88) | 0 | <.01 |
| Muscle involvement, n (%) | <.01 | |||
| Weakness of lower and/or upper extremities | 28 (44) | 28 (91) | 0 | |
| Weakness of lower and/or upper extremities and heart involvement | 2 (4) | 2 (6) | 0 | |
| Weakness of lower and/or upper extremities and diaphragm involvement | 1 (2) | 1 (3) | 0 | |
| Skin involvement, n (%) | 23 (36) | 13 (42) | 10 (30) | .48 |
| Raynaud's phenomenon, n (%) | 21 (33) | 10 (32) | 11 (33) | .99 |
| Esophageal dysmotility, n (%) | 4 (6) | 3 (10) | 1 (3) | .35 |
| Gastroesophageal reflux disease, n (%) | 21 (33) | 10 (32) | 11 (33) | .99 |
| Swallowing impairment, n (%) | 7 (11) | 6 (19) | 1 (3) | .05 |
| Immunosuppressive lines, med [IQR], n = 53 | 3 [2–4] | 4 [3–5] | 2 [1–3] | <.01 |
| History of cancer, n (%) | 1 (2) | 1 (3) | 0 | .49 |
| Recurrence of dermatomyositis after transplantation, n (%) | 5 (8) | 2 (3) | 3 (5) | .99 |
Abbreviations: a‐IIM, amyopathic idiopathic inflammatory myositis; c‐IIM, classical idiopathic inflammatory myositis.
3.3. Pulmonary and hemodynamic features at listing
Compared to the a‐IIM group, there was a trend toward higher mean pulmonary artery pressure in the c‐IIM group (Table S1). Nonspecific interstitial pneumonia and usual interstitial pneumonia were the main thoracic‐CT patterns. There were no other differences in pulmonary function or hemodynamics between the groups.
3.4. Survival analysis
Nineteen patients died during the median follow‐up of 33 [7–62] months after LTx. No patients were lost to follow‐up. The 1‐, 3‐, and 5‐year survival estimates were 78%, 73%, and 70%, respectively (Figure 1). Survival rates after 1, 3, and 5 years were significantly worse in the c‐IIM group (67%, 59%, and 55%) than in the a‐IIM group (90%, 90%, and 90%) (Figure 2).
FIGURE 2.
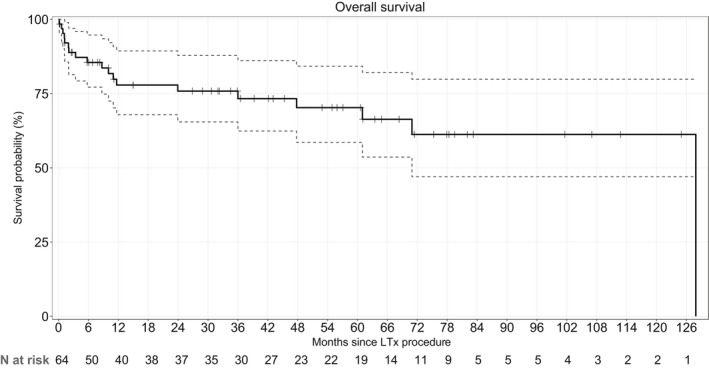
Kaplan–Meier survival estimates from the date of transplantation, with the 95% confidence intervals, in the overall population of 64 patients. Survival rates were 78%, 73%, and 70% at 1, 3, and 5 years, respectively.
A higher mortality rate and higher in‐hospital mortality were found in the group with c‐IIM compared to the group with a‐IIM (Table 1, Figure 3).
FIGURE 3.
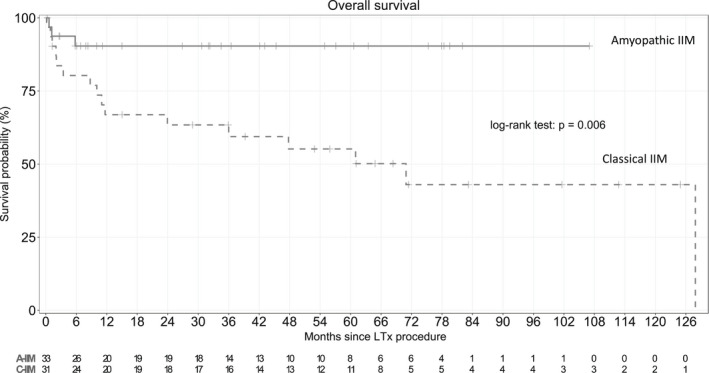
Kaplan–Meier survival estimates from the date of transplantation in the overall population according to muscle involvement phenotype (classical idiopathic inflammatory myositis [c‐IIM] versus amyopathic idiopathic inflammatory myositis [a‐IIM]). Survival rates at 1, 3, and 5 years were 67%, 59%, and 55% in the c‐IIM group versus 90%, 90%, and 90% in the a‐IIM group; respectively (p = .006). c‐IIM, classical dermatomyositis (with muscle involvement); a‐IIM, amyopathic dermatomyositis.
Table 3 reports the associations of selected variables with survival identified by univariate analysis. Variables significantly associated with poorer survival were dialysis during the ICU stay, grade 3 PGD at 72 h, c‐IIM (as opposed to a‐IIM), skin involvement, and the number of immunosuppressive lines before LTx.
TABLE 3.
Univariate analysis to identify factors associated with survival after lung transplantation
| Univariate | |||||
|---|---|---|---|---|---|
| Reference | Modality | HR | 95%CI | p‐value | |
| Sex | Male | Female | 0.74 | 0.29–1.91 | .53 |
| Age at transplantation | Continuous | 1.00 | 0.95–1.04 | .85 | |
| Body mass index at listing | Continuous | 0.95 | 0.81–1.11 | .50 | |
| Caucasian | No | Yes | 0.73 | 0.26–2.07 | .56 |
| Smoking history | No | Yes | 1.44 | 0.55–3.81 | .46 |
| CMV: donor positive / recipient negative | No | Yes | 1.17 | 0.43–3.18 | .76 |
| Blood group | O | A | 3.25 | 0.40–26.46 | .27 |
| B | 2.07 | 0.19–22.95 | .55 | ||
| AB | 2.72 | 0.17–43.59 | .48 | ||
| Lung transplant procedure | Double | Single | 0.74 | 0.17–3.24 | .69 |
| High‐priority transplant allocation program | No | Yes | 1.88 | 0.74–4.79 | .18 |
| Pulmonary hypertension | No | Yes | 1.17 | 0.25–5.53 | .84 |
| Severe pulmonary hypertension | No | Yes | 1.41 | 0.20–3.08 | .74 |
| Cardiopulmonary bypass | No | Yes | 0.60 | 0.23–1.56 | .30 |
| Induction | No | Yes | 0.63 | 0.23–1.68 | .35 |
| Dialysis during intensive unit stay | No | Yes | 3.05 | 1.14–8.21 | .03 |
| Primary graft dysfunction Grade 3 at 72 h | No | Yes | 2.47 | 0.93–6.61 | .07 |
| Ventilation time during intensive care unit stay | No | Yes | 0.99 | 0.97–1.01 | .48 |
| Hemothorax | No | Yes | 0.19 | 0.03–1.45 | .11 |
| Pneumonia | No | Yes | 0.99 | 0.97–1.01 | .48 |
| Preformed donor‐specific antibody | No | Yes | 1.41 | 0.46–4.29 | .55 |
| c‐IIM | No | Yes | 4.88 | 1.41–16.90 | .01 |
| Rapidly progressive interstitial lung disease | No | Yes | 1.94 | 0.76–4.90 | .16 |
| Skin involvement | No | Yes | 3.33 | 1.30–8.52 | .01 |
| Raynaud's phenomenon | No | Yes | 1.01 | 0.36–2.84 | .99 |
| Esophageal dysmotility | No | Yes | 3.05 | 0.68–13.69 | .14 |
| Gastroesophageal reflux disease | No | Yes | 1.60 | 0.62–4.16 | .33 |
| Swallowing impairment | No | Yes | 1.60 | 0.46–5.54 | .46 |
| Number of immunosuppressive lines before transplantation | Continuous variable | 1.36 | 0.99–1.87 | .06 | |
| 6‐min walk test | Continuous variable | 1.00 | 0.99–1.00 | .67 | |
| Forced vital capacity | Continuous variable | 0.98 | 0.94–1.02 | .31 | |
| Forced expired volume in 1 s | Continuous variable | 0.98 | 0.94–1.03 | .49 | |
| Forced expired volume in 1 s/Forced vital capacity | Continuous variable | 1.03 | 0.98–1.08 | .22 | |
| Total lung capacity | Continuous variable | 0.97 | 0.93–1.02 | .24 | |
| Diffusing capacity for carbon monoxide | Continuous variable | 1.01 | 0.96–1.06 | .78 | |
| Right atrial pressure | Continuous variable | 1.02 | 0.95–1.09 | .56 | |
| Mean pulmonary artery pressure | Continuous variable | 1.00 | 0.95–1.05 | .90 | |
| Pulmonary capillary wedge pressure | Continuous variable | 1.26 | 1.00–1.60 | .16 | |
| Cardiac index | Continuous variable | 0.56 | 0.20–1.55 | .26 | |
| Pulmonary vascular resistance | Continuous variable | 0.86 | 0.56–1.32 | .47 | |
| Predominant pattern on thoracic high‐resolution computed tomography | Nonspecific interstitial pneumonia | Usual interstitial pneumonia | 2.05 | 0.73–5.79 | .17 |
| Organizing pneumonia | 2.17 | 0.46–10.12 | .32 | ||
Abbreviations: 95%CI, 95% confidence interval; c‐IIM, classical idiopathic inflammatory myositis; CMV, cytomegalovirus; HR, hazard ratio.
3.5. Causes of death
More than half the deaths (10 of 19 patients [53%]) were caused by respiratory failure due to PGD (n = 4), pneumonia (n = 4), chronic lung allograft dysfunction (n = 1), or acute cellular rejection (n = 1). Five patients died of septic shock. The remaining causes of death were cancer (n = 1), multiple trauma (n = 1), stroke (n = 1), and catastrophic antiphospholipid syndrome (n = 1).
3.6. High‐priority transplant allocation program
Table 4 reports the main features of the groups transplanted under the standard vs. high‐priority allocation program. The group of 21 (33%) patients in the high‐priority program had a younger median age; shorter median IIM duration at LTx; higher proportions of patients with RP‐ILD, anti‐MDA5, and anti‐JO1 antibodies, preoperative invasive ventilation, and preoperative ECMO; and longer time on ventilation in the ICU. Survival was similar between the two groups (p = .18) (Figure 4). Among the 13 patients who received pre‐operative ECMO, four did not require invasive ventilation.
TABLE 4.
Comparison of the groups in the standard versus high‐priority transplant allocation programs
| Overall n = 64 | High‐priority allocation n = 21 | Standard allocation n = 43 | p‐value | |
|---|---|---|---|---|
| Male, n (%) | 35 (55) | 11 (52) | 24 (56) | .99 |
| Recipient age (years), med [IQR] | 53 [46–59] | 46 [43–53] | 56 [50–60] | <.01 |
| Connective tissue disease duration before lung transplantation (months), med [IQR] | 52 [18–140] | 14 [4–105] | 77 [40–168] | <.01 |
| Rapidly progressive interstitial lung disease, n (%) | 25 (39) | 17 (81) | 8 (19) | <.01 |
| Immunosuppressive lines, n = 53 | 3 [2–4] | 3 [1–4] | 3 [2–4] | .83 |
| c‐IIM, n (%) | 31 (48) | 10 (48) | 21 (49) | .99 |
| Antibody positivity, n (%) | ||||
| MDA5 | 13 (20) | 11 (52) | 2 (5) | <.01 |
| Jo1 | 31 (48) | 5 (24) | 26 (60) | <.01 |
| PL12 | 10 (16) | 3 (14) | 7 (16) | .99 |
| PL7 | 4 (6) | 0 | 4 (9) | .29 |
| Other | 6 (9) | 2 (10) | 4 (10) | .85 |
| Pulmonary hypertension, n (%), n = 39 | 29 (74) | 5 (83) | 24 (73) | .68 |
| Severe pulmonary hypertension, n (%), n = 39 | 13 (33) | 1 (17) | 12 (36) | .64 |
| Preoperative invasive ventilation, n (%), n = 19 | 10 (16) | 11 (53) | 0 | <.01 |
| Preoperative extracorporeal membrane oxygenation, n (%) | 13 (20) | 13 (62) | 0 | <.01 |
| Intraoperative extracorporeal membrane oxygenation, n (%) | 32 (50) | 17 (81) | 15 (35) | .13 |
| Postoperative extracorporeal membrane oxygenation, n (%) | 25 (39) | 11 (52) | 14 (33) | .21 |
| Dialysis during intensive care unit stay n (%) | 12 (19) | 6 (29) | 6 (14) | .29 |
| Primary graft dysfunction Grade 3 at 72 h, n (%) | 17 (29) | 1 (6) | 16 (39) | .02 |
| Ventilation time during intensive care unit stay (days), med [IQR] | 6 [1–22] | 14 [5–21] | 3 [1–21] | .04 |
| In‐hospital mortality, n (%) | 11 (17) | 3 (14) | 8 (19) | .74 |
| Chronic lung allograft dysfunction at last follow‐up, n (%), n = 57 | 13 (23) | 5 (25) | 8 (19) | .49 |
| Bronchiolitis obliterans syndrome | 10 (77) | 5 (100) | 5 (62) | |
| Restrictive allograft syndrome | 2 (15) | 0 | 2 (25) | |
| Mixed | 1 (8) | 0 | 1 (13) |
Abbreviation: c‐IIM, classical idiopathic inflammatory myositis.
FIGURE 4.
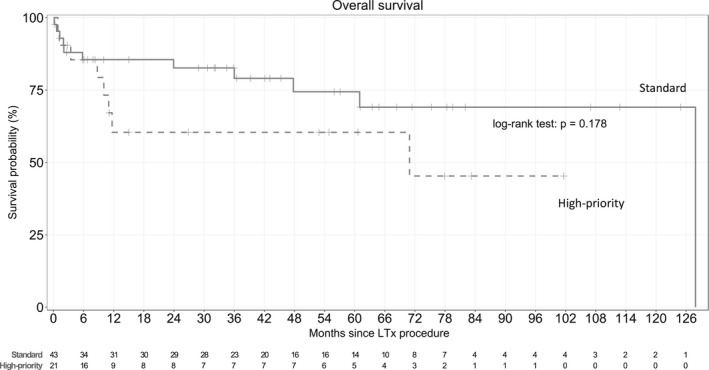
Kaplan–Meier survival estimates from the date of transplantation in the overall population according to the lung allocation program (standard versus high‐priority). Survival rates at 1, 3, and 5 years were 60%, 60%, and 60% in the high‐priority group versus 85%, 79%, and 74% in the standard group, respectively (p = .18).
3.7. Idiopathic inflammatory myositis (IIM) outcomes after lung transplantation (LTx)
After LTx, five (8%) patients experienced clinical relapse, at a median of 7.5 [1.5–70] months after surgery. Three of the five patients had mild peripheral muscle involvement and the other two had specific skin lesions. Of the 19 patients with available information about myositis‐specific antibodies after transplantation, five (26%) had persistently positive titers. Among them, two experienced clinical relapses, manifesting as mild muscle involvement in one and as specific skin lesions in the other. Cancer developed in three (5%) patients, including two with squamous‐cell skin carcinoma and one with metastatic adenocarcinoma from an unknown primary resulting in death a few weeks after the diagnosis. Of note, no patient experienced an ILD relapse in the allograft.
4. DISCUSSION
In this longitudinal study of patients with well‐characterized IIM‐ILD, outcomes after LTx were consistent with ISHLT registry data for 2010–2017. The main predictor of poorer survival was a history of muscle involvement, that is, c‐IIM as opposed to a‐IIM.
LTx in patients with end‐stage IIM‐ILD is controversial. Historically, IIM‐ILD was considered to contraindicate LTx, largely because of concerns that survival might be adversely affected by extrapulmonary manifestations, such as involvement of the respiratory and swallowing muscles. However, in our large cohort, survival rates after 1, 3, and 5 years were similar to those reported after LTx for other indications, 10 notably, for connective tissue diseases such as systemic sclerosis. 24 Furthermore, the incidence of major post‐transplantation complications such as acute rejection episodes or chronic lung allograft dysfunction did not differ from ISHLT data. 10 Finally, only five (8%) patients experienced a clinical IIM relapse. The symptoms were mild muscle weakness in three and typical skin lesions in two. The clinical impact of the relapses was thus limited, and postoperative respiratory muscle dysfunction was rare. Importantly, no patient experienced an ILD relapse in the allograft.
Our findings are supported by other studies. 13 , 25 , 26 A 2014 retrospective study showed similar 1‐, 3‐, and 5‐year mortality rates in five patients with IIM‐ILD, 48 with idiopathic pulmonary fibrosis (IPF), and 37 with ILD but neither IIM nor IPF. 13 In another retrospective study, reported in 2012, 1‐ and 5‐year survival rates were comparable in 284 patients with ILD related to connective tissue disease, including 34 with IIM‐ILD, and in 4190 patients with IPF. 26 In a recent retrospective study, no significant differences in survival, acute or chronic rejection, or extrapulmonary organ dysfunction were found in patients who underwent LTx for IPF vs. for nonscleroderma connective tissue disease, among whom 62 had IIM. 25 However, in a 2021 retrospective study, survival rates were significantly lower in the group with ILD related to DM or polymyositis than in the group of patients with IPF. 27 Importantly, only eight patients had IIM compared to 180 with IPF, and the IIM group showed trends toward a longer allograft cold‐ischemia time, higher frequency of grade 3 PGD at 72 h, and longer ICU stay. Age‐ and sex‐adjusted Cox‐proportional‐hazard analyses indicated that PGD and longer ICU stay were independently associated with poorer survival. Moreover, the inclusion of patients with several types of connective tissue disease, together with the single‐center recruitment, may have altered the findings compared to those of multicenter studies.
A strength of our study is that we were able to classify all patients according to their history of muscle involvement, that is, as having either c‐IIM or a‐IIM. This differentiation demonstrated that survival after LTx was significantly worse in the c‐IIM group, thus producing new information compared to earlier studies, most of which did not seek to identify prognostic factors. 13 , 26 , 27 Patients with c‐IIM had a longer disease duration before LTx, but pre‐LTx disease duration was not associated with the outcome by univariate analysis. However, the higher number of immunosuppressive lines may explain that the most common causes of death in this subgroup were pneumonia and septic shock.
Another strong point of our work is the comparison of patients whose transplant was allocated according to the standard vs. the high‐priority program. A third of our patients were in the high‐priority program, compared to about 15% overall in France. 28 The criterion for high‐priority listing was any event that remained immediately life‐threatening despite optimal treatment in the intensive care unit. 28 In addition to the burden of ILD, the presence of pulmonary hypertension in three‐quarters of our patients may have contributed to the frequent need for high‐priority allocation. Patients in the high‐priority sub‐group more often had RP‐ILD and positive anti‐MDA5 titers. This clinical IIM‐ILD phenotype has been described previously as an aggressive condition that results in high mortality if a respiratory failure occurs. 9 Of our 21 high‐priority patients, 13 (62%) required preoperative ECMO, and all were successfully transplanted with acceptable short‐ and long‐term outcomes, notably a lower incidence of grade 3 PGD at 72 h compared to standard‐allocation patients. ECMO as a successful bridge to LTx was recently described in patients who had IIM with RP‐ILD and respiratory failure. 29 In our cohort, four of the 13 patients who received preoperative ECMO were bridged to LTx without requiring invasive ventilation. Thus, ECMO may also obviate the need for invasive ventilation before LTx in some patients.
Among the potential limitations of our study, the general applicability of our findings deserves attention. Although our sample of 64 patients is large compared to many other studies, it remains limited. In addition, of the 68 European transplant centers invited to participate in the study, only 23 responded, potentially introducing selection bias and reducing the available information on how best to select patients with IIM‐ILD for LTx. Furthermore, we had no data on patients with IIM‐ILD who were not listed for LTx, for instance, due to dysfunction of the swallowing and/or respiratory muscles. Neither did we know how many patients died while on the waiting list. Finally, we did not know the criteria applied at each transplant center to contraindicate LTx listing of patients with IIM‐ILD.
In conclusion, our study adds valuable evidence that IIM‐ILD per se should not be considered a contraindication to LTx candidacy. The association linking a history of muscle involvement (i.e., c‐IIM) to worse survival was not sufficiently strong to make c‐IIM a contraindication to LTx, particularly given the absence of a multivariate analysis. In patients with critical respiratory failure, high‐priority allocation and ECMO as a bridge to LTx ensured acceptable posttransplantation survival. Further research is necessary to identify patient characteristics and modifiable risk factors allowing both standardized patient selection for LTx and improvements in post‐LTx outcomes.
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Supporting information
Appendix S1 Supporting information
ACKNOWLEDGMENTS
We thank Stephane Morisset (Stéphane Morisset Etudes & Consulting) for the statistical analysis and A. Wolfe for revising the draft. No funding was received for this study.
Rivière A, Picard C, Berastegui C, et al. Lung transplantation for interstitial lung disease in idiopathic inflammatory myositis: A cohort study. Am J Transplant. 2022;22:2990‐3001. doi: 10.1111/ajt.17177
Amélie Rivière, Clément Picard, Yurdagul Uzunhan, and Jérôme Le Pavec have contributed equally to this work.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med. 1975;292:344‐347. [DOI] [PubMed] [Google Scholar]
- 2. Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts). N Engl J Med. 1975;292:403‐407. [DOI] [PubMed] [Google Scholar]
- 3. 2017 European League Against Rheumatism/American College of Rheumatology Classification Criteria for Adult and Juvenile Idiopathic Inflammatory Myopathies and Their Major Subgroups. Accessed March 1, 2022. https://pubmed‐ncbi‐nlm‐nih‐gov.proxy.insermbiblio.inist.fr/29106061/.
- 4. Sontheimer RD. Would a new name hasten the acceptance of amyopathic dermatomyositis (dermatomyositis siné myositis) as a distinctive subset within the idiopathic inflammatory dermatomyopathies spectrum of clinical illness? J Am Acad Dermatol. 2002;46:626‐636. [DOI] [PubMed] [Google Scholar]
- 5. Hallowell RW, Ascherman DP, Danoff SK. Pulmonary manifestations of polymyositis/dermatomyositis. Semin Respir Crit Care Med. 2014;35:239‐248. [DOI] [PubMed] [Google Scholar]
- 6. Johnson C, Pinal‐Fernandez I, Parikh R, et al. Assessment of mortality in autoimmune myositis with and without associated interstitial lung disease. Lung. 2016;194:733‐737. [DOI] [PubMed] [Google Scholar]
- 7. Chen I‐J, Jan Wu Y‐J, Lin C‐W, et al. Interstitial lung disease in polymyositis and dermatomyositis. Clin Rheumatol. 2009;28:639‐646. [DOI] [PubMed] [Google Scholar]
- 8. Hervier B, Devilliers H, Stanciu R, et al. Hierarchical cluster and survival analyses of antisynthetase syndrome: phenotype and outcome are correlated with anti‐tRNA synthetase antibody specificity. Autoimmun Rev. 2012;12:210‐217. [DOI] [PubMed] [Google Scholar]
- 9. Allenbach Y, Uzunhan Y, Toquet S, et al. Different phenotypes in dermatomyositis associated with anti‐MDA5 antibody: study of 121 cases. Neurology. 2020;95:e70‐e78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chambers DC, Cherikh WS, Harhay MO, et al. The international thoracic organ transplant registry of the International Society for Heart and Lung Transplantation: thirty‐sixth adult lung and heart–lung transplantation report—2019; focus theme: donor and recipient size match. J Heart Lung Transplant. 2019;38:1042‐1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Delplanque M, Gatfosse M, Ait‐Oufella H, et al. Bi‐lung transplantation in anti‐synthetase syndrome with life‐threatening interstitial lung disease. Rheumatology (Oxford). 2018;57:1688‐1689. [DOI] [PubMed] [Google Scholar]
- 12. Brown Z, Holmes‐Liew C‐L, Chapman S, Glanville AR, Limaye V. Bilateral lung transplantation in antisynthetase syndrome. Intern Med J. 2019;49:1549‐1550. [DOI] [PubMed] [Google Scholar]
- 13. Ameye H, Ruttens D, Benveniste O, Verleden GM, Wuyts WA. Is lung transplantation a valuable therapeutic option for patients with pulmonary polymyositis? Experiences from the Leuven transplant cohort. Transplant Proc. 2014;46:3147‐3153. [DOI] [PubMed] [Google Scholar]
- 14. Lundberg IE, Miller FW, Tjärnlund A, Bottai M. Diagnosis and classification of idiopathic inflammatory myopathies. J Intern Med. 2016;280:39‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lundberg IE, de Visser M, Werth VP. Classification of myositis. Nat Rev Rheumatol. 2018;14:269‐278. [DOI] [PubMed] [Google Scholar]
- 16. Bottai M, Tjärnlund A, Santoni G, et al. EULAR/ACR classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups: a methodology report. RMD Open. 2017;3:e000507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mariampillai K, Granger B, Amelin D, et al. Development of a new classification system for idiopathic inflammatory myopathies based on clinical manifestations and myositis‐specific autoantibodies. JAMA Neurol. 2018;75:1528‐1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Guyatt GH, Sullivan MJ, Thompson PJ, et al. The 6‐minute walk: a new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J. 1985;132:919‐923. [PMC free article] [PubMed] [Google Scholar]
- 19. Miller MR, Hankinson J, Brusasco V, et al. ATS/ERS task force. Standardisation of spirometry. Eur Respir J. 2005;26:319‐338. [DOI] [PubMed] [Google Scholar]
- 20. Jablonski R, Bhorade S, Strek ME, Dematte J. Recognition and Management of Myositis‐Associated Rapidly Progressive Interstitial Lung Disease. Chest. 2020;158:252‐263. [DOI] [PubMed] [Google Scholar]
- 21. Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M, Williams PG, Souza R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019; 53 1801913. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6351336/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nathan SD, Barbera JA, Gaine SP, et al. Pulmonary hypertension in chronic lung disease and hypoxia. Eur Respir J. 2019;53:1801914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Johkoh T, Fukuoka J, Tanaka T. Rare idiopathic intestinal pneumonias (IIPs) and histologic patterns in new ATS/ERS multidisciplinary classification of the IIPs. Eur J Radiol. 2015;84:542‐546. [DOI] [PubMed] [Google Scholar]
- 24. Pradère P, Tudorache I, Magnusson J, et al. Working group on heart/lung transplantation in systemic sclerosis. Lung transplantation for scleroderma lung disease: an international, multicenter, observational cohort study. J Heart Lung Transplant. 2018;37:903‐911. [DOI] [PubMed] [Google Scholar]
- 25. Courtwright AM, El‐Chemaly S, Dellaripa PF, Goldberg HJ. Survival and outcomes after lung transplantation for non‐scleroderma connective tissue‐related interstitial lung disease. J Heart Lung Transplant. 2017;36:763‐769. [DOI] [PubMed] [Google Scholar]
- 26. Takagishi T, Ostrowski R, Alex C, Rychlik K, Pelletiere K, Tehrani R. Survival and extrapulmonary course of connective tissue disease after lung transplantation. J Clin Rheumatol. 2012;18:283‐289. [DOI] [PubMed] [Google Scholar]
- 27. Yang X, Wei D, Liu M, et al. Survival and outcomes after lung transplantation for connective tissue disease‐associated interstitial lung diseases. Clin Rheumatol. 2021;40:3789‐3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Agence de la biomédecine. Accessed February 17, 2022. https://rams.agence‐biomedecine.fr/greffe‐cardio‐pulmonaire‐et‐pulmonaire‐0.
- 29. Bay P, De CMP, Roux A, et al. Extracorporeal life support allows lung transplant in anti‐MDA5+ rapidly progressive‐interstitial lung disease. Eur Respir J. 2022;59:2102968. Accessed March 3, 2022. http://erj.ersjournals.com/content/early/2022/02/15/13993003.02968‐2021 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supporting information
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


