Abstract
Aims
Pre‐clinical congestion markers of worsening heart failure (HF) can be monitored by devices and may support the management of patients with HF. We aimed to assess whether congestion‐guided HF management according to device‐based remote monitoring strategies is more effective than standard therapy.
Methods and results
A comprehensive literature research for randomized controlled trials (RCTs) comparing device‐based remote monitoring strategies for congestion‐guided HF management versus standard therapy was performed on PubMed, Embase, and CENTRAL databases. Incidence rate ratios (IRRs) and associated 95% confidence intervals (CIs) were calculated using the Poisson regression model with random study effects. The primary outcome was a composite of all‐cause death and HF hospitalizations. Secondary endpoints included the individual components of the primary outcome. A total of 4347 patients from eight RCTs were included. Findings varied according to the type of parameters monitored. Compared with standard therapy, haemodynamic‐guided strategy (4 trials, 2224 patients, 12‐month follow‐up) reduced the risk of the primary composite outcome (IRR 0.79, 95% CI 0.70–0.89) and HF hospitalizations (IRR 0.76, 95% CI 0.67–0.86), without a significant impact on all‐cause death (IRR 0.93, 95% CI 0.72–1.21). In contrast, impedance‐guided strategy (4 trials, 2123 patients, 19‐month follow‐up) did not provide significant benefits.
Conclusion
Haemodynamic‐guided HF management is associated with better clinical outcomes as compared to standard clinical care.
Keywords: Heart failure, Guided management, Remote monitoring, Telemonitoring, Hospitalization, Death
Summary effect estimates for different strategies of guided management versus standard therapy related to pathogenesis of worsening heart failure. CI, confidence interval; IRR, incidence rate ratio.
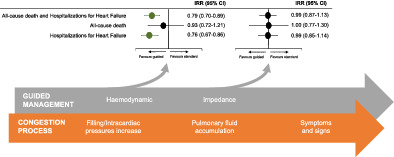
Introduction
Heart failure (HF) is a growing public health and economic problem, affecting almost 64 million people worldwide. 1 Despite the recent advances characterized by the introduction of more effective classes of drugs and device therapies, the natural history of patients with HF is still etched by poor quality of life, recurrent hospitalizations, and high rates of morbidity and mortality. 2 , 3 The hospitalization rate due to fluid overload and worsening HF remains significantly frequent, affecting patients' long‐term prognosis and burdening healthcare systems. 4 In order to improve clinical outcomes and reduce healthcare costs, a wide range of invasive and non‐invasive monitoring strategies aimed at preventing HF decompensation have been developed. 5 Several randomized controlled trials (RCTs) tested the hypothesis that careful monitoring of signs and symptoms of clinical deterioration (e.g. dyspnoea, weight gain, or peripheral oedema) through non‐invasive telemonitoring systems could lead to early medical management avoiding hospitalization. 6 However, these strategies have failed in their attempt as clinical parameters of fluid accumulation are delayed and unreliable as early signs of decompensation. 6 , 7 , 8 Later, the use of invasive devices able to automatically monitor physiological data allowed to continuously check pre‐clinical markers of worsening HF, including congestion parameters such as increased intracardiac or pulmonary artery pressures and pulmonary fluid accumulation. 9 , 10 Remote monitoring strategies of these parameters to allow a prompt and targeted therapeutic response have been tested in various RCTs, often underpowered, which have led to conflicting results. 11 , 12 , 13 , 14
Previous meta‐analyses did not provide unequivocal results as they were selectively performed on a single guided management strategy, 15 , 16 included studies testing telemonitoring systems as a substitute for in‐clinic follow‐up, or assessed heterogeneous outcomes or strategies in the same subgroup, 17 , 18 , 19 leading to questionable results. Therefore, to provide a comprehensive and updated evidence, we did a systematic review and meta‐analysis of RCTs comparing device‐based remote monitoring strategies to guide the management through congestion markers versus standard care in patients with HF.
Methods
This systematic review and meta‐analysis was carried out in accordance with the Cochrane Collaboration and Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines. 20 The protocol was registered within the international prospective register of systematic reviews (PROSPERO, CRD42022308167).
Search strategy and selection criteria
On 14 January 2022, we did a systematic and comprehensive literature research using PubMed, Embase, and Cochrane Central Register of Controlled Trials databases. In addition, we made backward snowballing research (i.e. review of references from identified articles). A combination of the following search terms was used: ‘monitoring’, ‘telemedicine’, ‘haemodynamic’, ‘impedance’, ‘implantable cardioverter defibrillator’, ‘cardiac resynchronization therapy’, ‘heart failure’. The full search strategy is available in online supplementary Table S2 . Two investigators (AZ and GP) systematically and independently screened all records retrieved from the research. Eligibility was assessed according to titles and abstracts. Articles potentially suitable were assessed for inclusion inspecting full‐text, and online appendices.
We included all RCTs comparing a strategy of guided management according to device‐based remotely monitored markers of congestion with standard therapy in patients with HF. Studies testing telemonitoring strategies only as a substitute for in‐clinic follow‐up, not reporting clinical outcomes, or with overlapping populations were excluded. We have applied no restrictions for study language, follow‐up duration, and publication date.
For the purposes of this analysis, the included trials were stratified according to the type of parameters guiding the management, resulting in two pre‐specified groups of management strategies: haemodynamic‐guided (i.e. driven by pulmonary artery and/or right ventricular pressure values) and impedance‐guided (i.e. driven by the intrathoracic impedance value, directly related to the degree of pulmonary congestion). 21
Data extraction and quality assessment
Data extraction of study design and features, patients' baseline characteristics, and outcomes was performed independently by two investigators (AZ and GP) using a standardized data worksheet. When multiple studies were reported from the same cohort of subjects, the one with the longest follow‐up was included in the analysis. Conflicts were resolved by collegial discussion.
The risk of bias assessment was independently made by two investigators (AZ and GP) according to the Cochrane Collaboration risk‐of‐bias tool (RoB2), composed of five domains: (1) randomization process; (2) deviations from intended interventions; (3) missing outcome data; (4) measurement of the outcome; and (5) selection of the reported result. 22
Outcome definition
The primary outcome was a composite of all‐cause death and hospitalizations for HF (including recurrent events). Secondary outcomes included the individual components of the primary outcome (i.e. all‐cause death and hospitalizations for HF). Endpoint definitions of each study are reported in online supplementary Table S4 .
Statistical analysis
A patient‐years approach was adopted to address different follow‐up times and recurrent events. When the number of patient‐years was not clearly reported, it was arithmetically calculated by multiplying the number of patients with the years of follow‐up (for each arm, if available). Incidence rate ratios (IRRs) and the associated 95% confidence intervals (CIs) were used as metric of choice for treatment effects and were calculated using the mixed‐effects Poisson regression model with random study effects. The heterogeneity between studies was evaluated using the Cochran's Q test, while consistency was measured by Higgins and Thompson I 2. Low heterogeneity was defined as an I 2 value <25%, moderate heterogeneity as a value of 25–50%, and high heterogeneity as a value >50%. The potential presence of publication bias was assessed by visual inspection of funnel plots and using Egger's test.
A main pre‐specified subgroup analysis was performed according to the type of monitoring strategy (haemodynamic or impedance) and findings were presented based on this analysis. Subgroup effects were compared using the Borenstein and Higgins test 23 and the credibility of subgroup differences was assessed by the ICEMAN tool, which consists of an eight‐question survey that provides a four‐level rating of the credibility of subgroup‐effect modification (very low credibility, low credibility, moderate credibility, and high credibility). 24
A pre‐specified sensitivity analysis using the leave‐one‐out approach was performed removing all studies one at a time to investigate the influence of each study on the overall effect‐size estimate. Furthermore, two post‐hoc sensitivity analyses included an analysis which added two studies testing a strategy of impedance‐guided HF management with parameters monitored during in‐clinic follow‐up without remote monitoring systems (DOT‐HF 25 and IMPEDANCE‐HF 26 ) and another analysis which excluded two studies reporting an outcome of first hospitalization instead of recurrent hospitalizations (COMPASS‐HF, 27 CONNECT‐OptiVol 14 ).
Several univariable meta‐regression analyses were performed to assess the presence of a relation between some covariates (age, left ventricular ejection fraction [LVEF], proportion of patients with atrial fibrillation, proportion of patients with HF of ischaemic cause, proportion of patients in different New York Heart Association (NYHA) functional classes, and proportion of patients treated with different drugs) and treatment effect for all outcomes. A two‐sided p < 0.05 was considered statistically significant. Statistical analysis was performed using R version 4.1.2 (The R Foundation, 2021) “meta” package. 28
Results
The comprehensive literature research retrieved 13 496 articles. The PRISMA checklist and flow diagram are shown in online supplementary Tables S1 and S3 . After screening, eight RCTs were identified, with a total of 4347 patients randomly allocated to guided management (n = 2173) or standard therapy (n = 2174). The average follow‐up duration was 15 months, providing data on 5984 patient‐years, including 3023 patient‐years in the guided‐management arm and 2961 patient‐years in the standard‐therapy arm. Four trials investigated a strategy of haemodynamic‐guided management (n = 2224 patients) during an average follow‐up of 12 months (n = 2362 patient‐years) and four trials a strategy of impedance‐guided management (n = 2123 patients) during an average follow‐up of 19 months (n = 3620 patient‐years). Key features of included trials and baseline characteristics of patients are summarized in Table 1 and online supplementary Tables S5 and S6 . 11 , 12 , 13 , 14 , 27 , 29 , 30 , 31 Trials testing a haemodynamic‐guided management were characterized by frequent data revision (daily or weekly), while trials testing an impedance‐guided management were characterized by an only‐alert‐based data revision (online supplementary Table S5 ). All patients were receiving optimal medical therapy at the date of randomization (online supplementary Table S7 ) and the clinical characteristics and therapeutic history of patients were similar in the two arms of each trial. The risk‐of‐bias assessment identified four studies at low risk of bias, three studies with some concerns, and one study at high risk of bias (online supplementary Figure S1 ).
Table 1.
Key features of randomized controlled trials included in the meta‐analysis
| Year | Device | Number of patients | Major parameters guiding management | Type of monitoring | Follow‐up | ||||
|---|---|---|---|---|---|---|---|---|---|
| Overall | Guided management | Control | Guided management | Control | |||||
| CHAMPION 11 | 2016 | CardioMEMS HF System | 550 | 270 | 280 | Pulmonary artery pressures | Non‐invasive telemonitoring | Haemodynamic | 18 months |
| COMPASS‐HF 27 | 2008 | Chronicle ICHM | 274 | 134 | 140 | Pulmonary artery and right ventricular diastolic pressures | Non‐invasive telemonitoring | Haemodynamic | 6 months |
| CONNECT‐OptiVol 14 | 2015 | ICD/CRT | 176 | 87 | 89 | Intrathoracic impedance | Usual care | Impedance | 15 months |
| GUIDE‐HF 12 | 2021 | CardioMEMS HF System | 1000 | 497 | 503 | Pulmonary artery pressures | Non‐invasive telemonitoring | Haemodynamic | 12 months |
| LIMIT‐CHF 13 | 2016 | ICD/CRT | 80 | 41 | 39 | Intrathoracic impedance | Usual care | Impedance | 12 months |
| MORE‐CARE 29 | 2017 | CRT | 865 | 437 | 428 | Intrathoracic impedance and atrial tachyarrhythmia | Usual care | Impedance | 24 months |
| OptiLink HF 30 | 2016 | ICD/CRT | 1002 | 505 | 497 | Intrathoracic impedance | Usual care with device data accessible in the clinic | Impedance | 23 months |
| REDUCEhf 31 | 2011 | Chronicle ICD | 400 | 202 | 198 | Pulmonary artery and right ventricular diastolic pressures | Non‐invasive telemonitoring | Haemodynamic | 12 months |
CRT, cardiac resynchronization therapy; ICD, implantable cardioverter defibrillator; ICHM, implantable continuous haemodynamic monitor.
Main analyses
The primary outcome of all‐cause death and hospitalizations for HF was significantly reduced with a strategy of congestion‐guided HF management compared with standard therapy (IRR 0.88, 95% CI 0.78–0.99, p = 0.034, I 2 = 47%; Figure 1 ). This result was driven by a reduction in the risk of hospitalizations for HF (IRR 0.85, 95% CI 0.75–0.97, p = 0.016, I 2 = 45%), without a significant impact on all‐cause death (IRR 0.96, 95% CI 0.80–1.16, p = 0.697, I 2 = 0%) (Figures 2 and 3).
Figure 1.
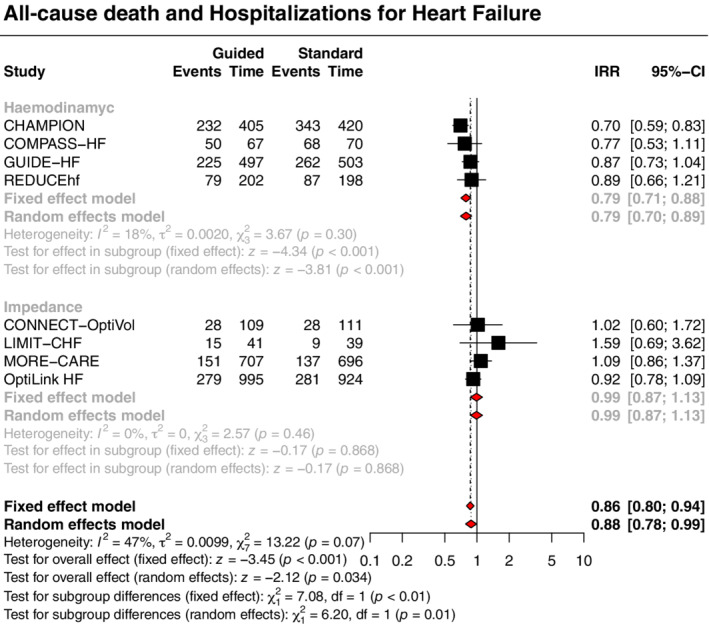
Guided management versus standard therapy for the primary outcome. CI, confidence interval; IRR, incidence rate ratio; Time, patient‐years.
Figure 2.
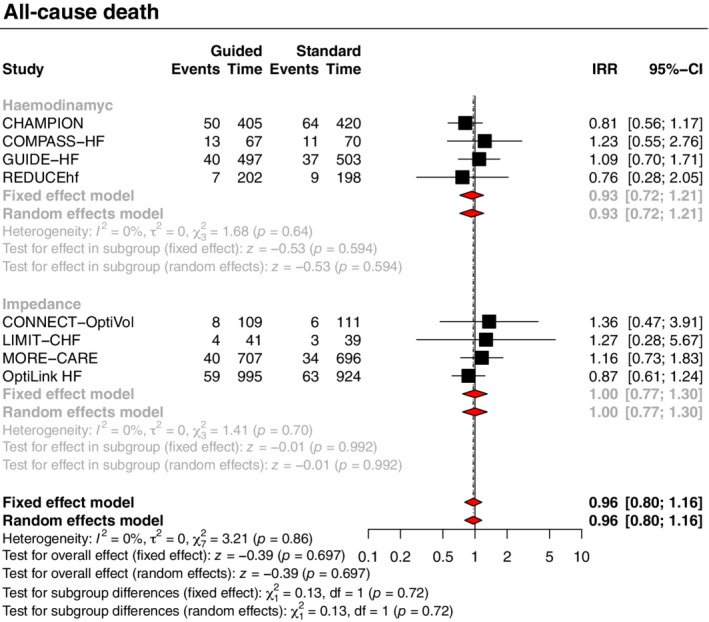
Guided management versus standard therapy for the all‐cause death outcome. CI, confidence interval; IRR, incidence rate ratio; Time, patient‐years.
Treatment effects varied according to the type of monitoring strategy (haemodynamic or impedance) with subgroup analyses showing significant interactions for the primary outcome and the outcome of HF hospitalizations, with moderate credibility due to effect modification based on between‐trial comparisons. A strategy of haemodynamic‐guided management, compared with standard therapy, was associated with a reduction in the primary outcome (IRR 0.79, 95% CI 0.70–0.89, p < 0.001, I 2 = 18%; Figure 1 ) and hospitalizations for HF (IRR 0.76, 95% CI 0.67–0.86, p < 0.001, I 2 = 20%; Figure 3 ), but no significant impact on all‐cause death (IRR 0.93, 95% CI 0.72–1.21, p = 0.594, I 2 = 0%; Figure 2 ). Conversely, a strategy of impedance‐guided management did not reduce the risks of all‐cause death (IRR 1.00, 95% CI 0.77–1.30, p = 0.992, I 2 = 0%; Figure 2 ), HF hospitalizations (IRR 0.99, 95% CI 0.85–1.14, p = 0.853, I 2 = 0%; Figure 3 ), and the composite of both (IRR 0.99, 95% CI 0.87–1.13, p = 0.868, I 2 = 0%; Figure 1 ) in comparison to standard therapy.
Figure 3.
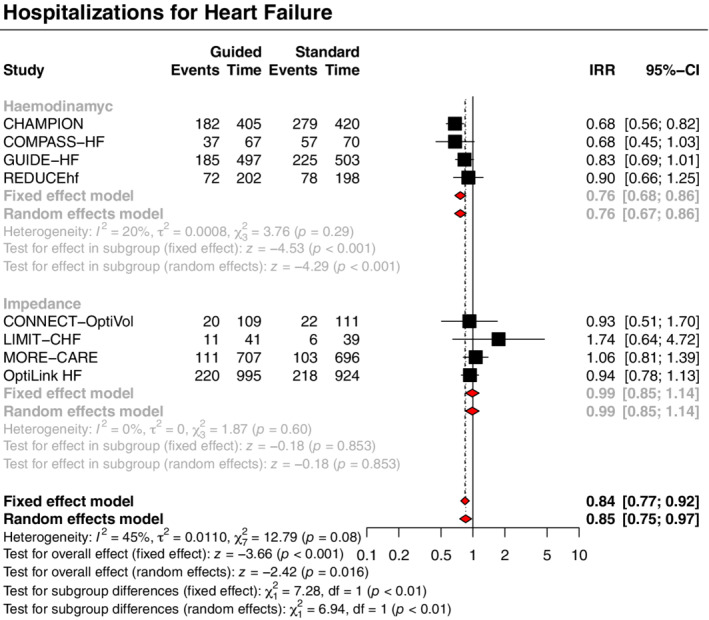
Guided management versus standard therapy for the heart failure hospitalization outcome. CI, confidence interval; IRR, incidence rate ratio; Time, patient‐years.
Sensitivity and meta‐regression analyses
At leave‐one‐out sensitivity analyses (online supplementary Figure S2 ), no trial showed significant influence on the pooled estimate for all outcomes. Both the analyses which added DOT‐HF 25 and IMPEDANCE‐HF 26 (online supplementary Table S8 and Figure S3 ) and the analyses which excluded trials reporting an outcome of first hospitalization (COMPASS‐HF 27 and CONNECT‐OptiVol 14 ; online supplementary Figure S4 ) showed findings consistent with the main analyses.
Meta‐regression analyses showed no significant relation between all covariates (age, LVEF, proportion of patients with atrial fibrillation, proportion of patients with HF of ischaemic cause, proportion of patients in different NYHA functional classes, and proportion of patients treated with angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers, beta‐blockers, mineralocorticoid receptor antagonists, and diuretic) and treatment effect for all outcomes (online supplementary Table S9 ). Funnel plots and Egger's tests suggested no evidence of publication bias or small study effect (online supplementary Figure S5 ).
Discussion
This comprehensive systematic review and meta‐analysis address the challenging task of guiding the management of HF according to device‐based remotely monitored congestion markers. The results of this meta‐analysis of eight RCTs involving 4347 patients with HF show that, compared with standard therapy, guided management according to device‐based remotely monitored pre‐clinical congestion markers is associated with a reduced risk of the composite of all‐cause death and hospitalizations for HF, mainly driven by a reduction in HF hospitalizations. Findings varied according to the type of parameters monitored (Graphical Abstract): (i) a strategy of haemodynamic‐guided management was associated with a reduction in the composite of all‐cause death and hospitalizations for HF, driven by a reduction in hospitalizations without a significant mortality reduction; (ii) a strategy of impedance‐guided management was not able to provide a significant reduction in the risks of death, HF hospitalizations, and the composite endpoint.
These results reflect the pathogenesis of HF progression from a compensated and euvolaemic state to an acutely and volume overloaded state, which occurs through various steps beginning about 30 days before the development of clinical signs. 8 A slight increase in filling and intracardiac pressures occurs early in this transition phase, 9 followed by a compensatory autonomic response characterized by sympathetic activation and vagal withdrawal, detectable by changes in several cardiac electrical activity features with varying predictive values (e.g. onset of atrial fibrillation or ventricular tachyarrhythmias, reduction of heart rate variability, and of biventricular pacing rate). 32 , 33 , 34 Then pulmonary vascular capacitance gets overwhelmed with initial pulmonary fluid accumulation, which can be detected by a reduction in intrathoracic impedance. 10 , 21 Finally, this process results in generalized fluid overload with progressive development of symptoms and signs of decompensation leading to hospitalization.
In line with the pathophysiological and clinical findings, the use of intrathoracic impedance as a unique monitoring parameter to guide the management is limited by the late onset of pulmonary congestion. 35 In contrast, a strategy of haemodynamic monitoring as a tool for continuous optimization of care appears to offer reasonable effectiveness in directing HF management because an increase in intracardiac or pulmonary artery pressures represent an early sign of worsening HF which allows a prompt and targeted therapeutic response. However, it should be emphasized that the frequency of data revision (daily or weekly for haemodynamic‐guided strategy and only‐alert‐based for impedance‐guided management) may have been a potential treatment modifier, influencing the effects provided by the impedance‐guided management strategy. Future studies are needed to explore this inference.
Previous meta‐analyses suggested a marginal reduction in the risk of HF hospitalizations with a strategy of haemodynamic‐guided management and no benefits with an heterogeneous group of strategies of impedance‐guided management. Our meta‐analysis, by adopting a patient‐years approach and strict selection criteria of RCTs, suggested a substantial difference in the effects of the different strategies of congestion‐guided management according to the monitored parameters. 17 , 18 , 19
Although haemodynamic‐guided management reduced the incidence of HF hospitalizations, it did not result in a significant mortality reduction over a 12‐month follow‐up period. However, since a reduction in HF hospitalizations or pulmonary artery pressure was previously associated with long‐term mortality benefits, 36 , 37 a longer follow‐up time and/or a larger sample size might be able to provide this finding, as already confirmed by real‐world data. 38 Furthermore, the inclusion of a proportion of patients with LVEF >40% in two trials investigating haemodynamic‐guided management (22% of patients in CHAMPION 11 and 47% of patients in GUIDE‐HF 12 ) should not be overlooked. Although a guided management according to haemodynamic parameters in this subgroup is able to reduce HF hospitalizations as well as in patients with lower LVEF, 39 its impact on mortality is still uncertain and under investigation, likely affecting overall estimates of the mortality benefit provided by this monitoring strategy.
Some trials, taken individually, did not provide sufficient evidence to support the widespread use of device‐based congestion‐guided HF management, as reported by recent guidelines and consensus statements. 2 , 40 However, the use of unreliable decompensation markers, the lack of adequate or prompt therapeutic response, and some design limitations may have skewed trial results. Major pitfalls included insufficient statistical power to detect significant differences between arms, inadequate transmission of monitored data, and/or poor treatment reaction. 13 , 14 , 29 , 31 Furthermore, frequent clinical monitoring of control arms, sometimes by non‐invasive telemonitoring, may have underestimated the favourable effect provided by the guided management. Indeed, real‐world data broadly support the routine use of monitored haemodynamic parameters in guiding HF management. 38 Finally, as predicted by clinicians' learning curves after implementation of remote monitoring systems, the large‐scale use of these tools will optimize their performance by gradually enhancing the targeted treatment response.
Our findings should be interpreted in light of public health and economic priority, with HF hospitalizations representing the main financial burden to healthcare systems and one of the strongest predictors of mortality. 4 The primary outcome of our meta‐analysis, as a surrogate of keeping patients alive and out of the hospital, addresses both sides of this issue.
The inevitable costs deriving from the implementation of these devices (e.g. CardioMEMS listed as $17 750 with Medicare), 41 the use of monitoring algorithms, and the development of telemedical evaluation units are important issues to be addressed. Some analyses suggested that haemodynamic‐guided management may be cost‐effective with a favourable clinical and economic impact. 42 , 43 However, it should be recognized that the costs of deploying telemedical evaluation units have been roughly estimated and might be a major burden for health systems. As a result, additional data on device maintenance and monitoring costs are needed to guide future global economic assessments.
The success of remote monitoring strategies will depend on the optimal data transmission, as well as on the physicians' clinical attitude to telemonitoring strategies and on patients' engagement and acceptance. Machine‐learning systems could address these limitations, implementing virtual coaching systems for both healthcare professionals and patients. 44 Future RCTs will provide further data on the effectiveness of guided management according to parameters monitored through a wide range of devices. 45
Limitations
Our study has some limitations. First, the lack of patient‐level data has foreclosed the assessment of potential treatment modifiers. Particularly, two trials testing haemodynamic‐guided management included a proportion of patients with LVEF >40%, and it cannot be ruled out that this may have influenced the impact on mortality of this monitoring strategy. 11 , 12 Second, two trials assessed an endpoint of first hospitalizations instead of recurrent hospitalizations. However, the results after removing those trials were consistent with the main analysis. Third, some studies have not reported or are heterogeneous in defining the outcome of HF hospitalizations, limiting results reliability. Fourth, one study may have enrolled a small proportion of patients without HF. 14 However, at leave‐one‐out sensitivity analysis, the exclusion of this trial provided results consistent with the main analysis. Fifth, the use of the patient‐years approach may have led to a bias due to partially reported arm‐specific follow‐up times. However, the inclusion of only RCTs and the use of the Poisson regression model reduced the risk of this bias, supporting results validity. 46
Conclusion
Compared to standard therapy, a device‐based remote monitoring strategy for haemodynamic‐guided management of patients with HF is associated with a reduction in the composite of all‐cause death and hospitalizations for HF, driven by a reduction in hospitalizations without a significant mortality benefit. Contrarily, an impedance‐guided management was not able to provide significant benefits. Further research will determine whether haemodynamic‐based remote monitoring strategy is cost‐effective in guiding the management of patients with HF.
Conflict of interest: C.T. discloses to have been involved in advisory board meetings or having received speaker fees from Abbott, Abiomed, Biotronic. F.B. discloses to have been involved in advisory board meetings or having received speaker fees from Medtronic, Abbott, Abiomed. All other authors have nothing to disclose.
Supporting information
Appendix S1. Supporting Information.
Acknowledgement
Open Access Funding provided by Universita Cattolica del Sacro Cuore within the CRUI‐CARE Agreement.
References
- 1. GBD 2017 Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990‐2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2022;24:4–131. [DOI] [PubMed] [Google Scholar]
- 3. Vaduganathan M, Claggett BL, Jhund PS, Cunningham JW, Pedro Ferreira J, Zannad F, et al. Estimating lifetime benefits of comprehensive disease‐modifying pharmacological therapies in patients with heart failure with reduced ejection fraction: a comparative analysis of three randomised controlled trials. Lancet. 2020;396:121–8. [DOI] [PubMed] [Google Scholar]
- 4. Gheorghiade M, Vaduganathan M, Fonarow GC, Bonow RO. Rehospitalization for heart failure: problems and perspectives. J Am Coll Cardiol. 2013;61:391–403. [DOI] [PubMed] [Google Scholar]
- 5. Hasan A, Paul V. Telemonitoring in chronic heart failure. Eur Heart J. 2011;32:1457–64. [DOI] [PubMed] [Google Scholar]
- 6. Drews TEI, Laukkanen J, Nieminen T. Non‐invasive home telemonitoring in patients with decompensated heart failure: a systematic review and meta‐analysis. ESC Heart Fail. 2021;8:3696–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lewin J, Ledwidge M, O'Loughlin C, McNally C, McDonald K. Clinical deterioration in established heart failure: what is the value of BNP and weight gain in aiding diagnosis? Eur J Heart Fail. 2005;7:953–7. [DOI] [PubMed] [Google Scholar]
- 8. Adamson PB. Pathophysiology of the transition from chronic compensated and acute decompensated heart failure: new insights from continuous monitoring devices. Curr Heart Fail Rep. 2009;6:287–92. [DOI] [PubMed] [Google Scholar]
- 9. Zile MR, Bennett TD, St John Sutton M, Cho YK, Adamson PB, Aaron MF, et al. Transition from chronic compensated to acute decompensated heart failure: pathophysiological insights obtained from continuous monitoring of intracardiac pressures. Circulation. 2008;118:1433–41. [DOI] [PubMed] [Google Scholar]
- 10. Lara BA, Qu F, Heist EK, Pavri BB, Van Bakel AB, Herre JM, et al. Accurate monitoring of intravascular fluid volume: a novel application of intrathoracic impedance measures for the guidance of volume reduction therapy. Int J Cardiol Heart Vasc. 2015;8:47–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Abraham WT, Stevenson LW, Bourge RC, Lindenfeld JA, Bauman JG, Adamson PB; CHAMPION Trial Study Group . Sustained efficacy of pulmonary artery pressure to guide adjustment of chronic heart failure therapy: complete follow‐up results from the CHAMPION randomised trial. Lancet. 2016;387:453–61. [DOI] [PubMed] [Google Scholar]
- 12. Lindenfeld JA, Zile MR, Desai AS, Bhatt K, Ducharme A, Horstmanshof D, et al. Haemodynamic‐guided management of heart failure (GUIDE‐HF): a randomised controlled trial. Lancet. 2021;398:991–1001. [DOI] [PubMed] [Google Scholar]
- 13. Domenichini G, Rahneva T, Diab IG, Dhillon OS, Campbell NG, Finlay MC, et al. The Lung Impedance Monitoring in Treatment of Chronic Heart Failure (the LIMIT‐CHF study). Europace. 2016;18:428–35. [DOI] [PubMed] [Google Scholar]
- 14. Lüthje L, Vollmann D, Seegers J, Sohns C, Hasenfuß G, Zabel M. A randomized study of remote monitoring and fluid monitoring for the management of patients with implanted cardiac arrhythmia devices. Europace. 2015;17:1276–81. [DOI] [PubMed] [Google Scholar]
- 15. Adamson PB, Ginn G, Anker SD, Bourge RC, Abraham WT. Remote haemodynamic‐guided care for patients with chronic heart failure: a meta‐analysis of completed trials. Eur J Heart Fail. 2017;19:426–33. [DOI] [PubMed] [Google Scholar]
- 16. Hindricks G, Varma N, Kacet S, Lewalter T, Søgaard P, Guédon‐Moreau L, et al. Daily remote monitoring of implantable cardioverter‐defibrillators: insights from the pooled patient‐level data from three randomized controlled trials (IN‐TIME, ECOST, TRUST). Eur Heart J. 2017;38:1749–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hajduczok AG, Muallem SN, Nudy MS, DeWaters AL, Boehmer JP. Remote monitoring for heart failure using implantable devices: a systematic review, meta‐analysis, and meta‐regression of randomized controlled trials. Heart Fail Rev. 2021;27:1281–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hajduczok AG, Muallem SN, Nudy MS, DeWaters AL, Boehmer JP. Letter to the editor to update the article “Remote monitoring for heart failure using implantable devices: a systematic review, meta‐analysis, and meta‐regression of randomized controlled trials”. Heart Fail Rev. 2022;27:985–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mhanna M, Beran A, Nazir S, Al‐Abdouh A, Barbarawi M, Sajdeya O, et al. Efficacy of remote physiological monitoring‐guided care for chronic heart failure: an updated meta‐analysis. Heart Fail Rev. 2021;27:1637. [DOI] [PubMed] [Google Scholar]
- 20. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yu CM, Wang L, Chau E, Chan RHW, Kong SL, Tang MO, et al. Intrathoracic impedance monitoring in patients with heart failure: correlation with fluid status and feasibility of early warning preceding hospitalization. Circulation. 2005;112:841–8. [DOI] [PubMed] [Google Scholar]
- 22. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- 23. Borenstein M, Higgins JPT. Meta‐analysis and subgroups. Prev Sci. 2013;14:134–43. [DOI] [PubMed] [Google Scholar]
- 24. Schandelmaier S, Briel M, Varadhan R, Schmid CH, Devasenapathy N, Hayward RA, et al. Development of the Instrument to Assess the Credibility of Effect Modification Analyses (ICEMAN) in randomized controlled trials and meta‐analyses. CMAJ. 2020;192:E901–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. van Veldhuisen DJ, Braunschweig F, Conraads V, Ford I, Cowie MR, Jondeau G, et al. DOT‐HF Investigators. Intrathoracic impedance monitoring, audible patient alerts, and outcome in patients with heart failure. Circulation. 2011;124:1719–26. [DOI] [PubMed] [Google Scholar]
- 26. Kleiner Shochat M, Fudim M, Shotan A, Blondheim DS, Kazatsker M, Dahan I, et al. Prediction of readmissions and mortality in patients with heart failure: lessons from the IMPEDANCE‐HF extended trial. ESC Heart Fail. 2018;5:788–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bourge RC, Abraham WT, Adamson PB, Aaron MF, Aranda JM, Magalski A, et al.; COMPASS‐HF Study Group . Randomized controlled trial of an implantable continuous hemodynamic monitor in patients with advanced heart failure: the COMPASS‐HF study. J Am Coll Cardiol. 2008;51:1073–9. [DOI] [PubMed] [Google Scholar]
- 28. Balduzzi S, Rücker G, Schwarzer G. How to perform a meta‐analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22:153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Boriani G, Da Costa A, Quesada A, Ricci RP, Favale S, Boscolo G, et al.; MORE‐CARE Study Investigators . Effects of remote monitoring on clinical outcomes and use of healthcare resources in heart failure patients with biventricular defibrillators: results of the MORE‐CARE multicentre randomized controlled trial. Eur J Heart Fail. 2017;19:416–25. [DOI] [PubMed] [Google Scholar]
- 30. Böhm M, Drexler H, Oswald H, Rybak K, Bosch R, Butter C, et al.; OptiLink HF Study Investigators . Fluid status telemedicine alerts for heart failure: a randomized controlled trial. Eur Heart J. 2016;37:3154–63. [DOI] [PubMed] [Google Scholar]
- 31. Adamson PB, Gold MR, Bennett T, Bourge RC, Stevenson LW, Trupp R, et al. Continuous hemodynamic monitoring in patients with mild to moderate heart failure: results of the Reducing Decompensation Events Utilizing Intracardiac Pressures in Patients With Chronic Heart Failure (REDUCEhf) trial. Congest Heart Fail. 2011;17:248–54. [DOI] [PubMed] [Google Scholar]
- 32. Opasich C, Rapezzi C, Lucci D, Gorini M, Pozzar F, Zanelli E, et al.; Italian Network on Congestive Heart Failure (IN‐CHF) Investigators . Precipitating factors and decision‐making processes of short‐term worsening heart failure despite “optimal”treatment (from the IN‐CHF Registry). Am J Cardiol. 2001;88:382–7. [DOI] [PubMed] [Google Scholar]
- 33. Adamson PB, Smith AL, Abraham WT, Kleckner KJ, Stadler RW, Shih A, et al.; InSync III Model 8042 and Attain OTW Lead Model 4193 Clinical Trial Investigators . Continuous autonomic assessment in patients with symptomatic heart failure: prognostic value of heart rate variability measured by an implanted cardiac resynchronization device. Circulation. 2004;110:2389–94. [DOI] [PubMed] [Google Scholar]
- 34. Singh JP, Hall WJ, McNitt S, Wang H, Daubert JP, Zareba W, et al.; MADIT‐II Investigators . Factors influencing appropriate firing of the implanted defibrillator for ventricular tachycardia/fibrillation: findings from the Multicenter Automatic Defibrillator Implantation Trial II (MADIT‐II). J Am Coll Cardiol. 2005;46:1712–20. [DOI] [PubMed] [Google Scholar]
- 35. Conraads VM, Tavazzi L, Santini M, Oliva F, Gerritse B, Yu CM, et al. Sensitivity and positive predictive value of implantable intrathoracic impedance monitoring as a predictor of heart failure hospitalizations: the SENSE‐HF trial. Eur Heart J. 2011;32:2266–73. [DOI] [PubMed] [Google Scholar]
- 36. Solomon SD, Dobson J, Pocock S, Skali H, McMurray JJV, Granger CB, et al. Influence of nonfatal hospitalization for heart failure on subsequent mortality in patients with chronic heart failure. Circulation. 2007;116:1482–7. [DOI] [PubMed] [Google Scholar]
- 37. Zile MR, Bennett TD, El Hajj S, Kueffer FJ, Baicu CF, Abraham WT, et al. Intracardiac pressures measured using an implantable hemodynamic monitor: relationship to mortality in patients with chronic heart failure. Circ Heart Fail. 2017;10:e003594. [DOI] [PubMed] [Google Scholar]
- 38. Abraham J, Bharmi R, Jonsson O, Oliveira GH, Artis A, Valika A, et al. Association of ambulatory hemodynamic monitoring of heart failure with clinical outcomes in a concurrent matched cohort analysis. JAMA Cardiol. 2019;4:556–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Adamson PB, Abraham WT, Bourge RC, Costanzo MR, Hasan A, Yadav C, et al. Wireless pulmonary artery pressure monitoring guides management to reduce decompensation in heart failure with preserved ejection fraction. Circ Heart Fail. 2014;7:935–44. [DOI] [PubMed] [Google Scholar]
- 40. Dickinson MG, Allen LA, Albert NA, DiSalvo T, Ewald GA, Vest AR, et al. Remote monitoring of patients with heart failure: a White Paper from the Heart Failure Society of America Scientific Statements Committee. J Card Fail. 2018;24:682–94. [DOI] [PubMed] [Google Scholar]
- 41. Centers for Medicare and Medicaid Services (CMS) . HHS. Medicare program; hospital inpatient prospective payment systems for acute care hospitals and the long‐term care hospital prospective payment system and fiscal year 2015 rates; quality reporting requirements for specific providers; reasonable compensation equivalents for physician services in excluded hospitals and certain teaching hospitals; provider administrative appeals and judicial review; enforcement provisions for organ transplant centers; and electronic health record (EHR) incentive program. Final rule. Fed Regist. 2014;79:49835–50536. [PubMed] [Google Scholar]
- 42. Sandhu AT, Goldhaber‐Fiebert JD, Owens DK, Turakhia MP, Kaiser DW, Heidenreich PA. Cost‐effectiveness of implantable pulmonary artery pressure monitoring in chronic heart failure. JACC Heart Fail. 2016;4:368–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cowie MR, Simon M, Klein L, Thokala P. The cost‐effectiveness of real‐time pulmonary artery pressure monitoring in heart failure patients: a European perspective. Eur J Heart Fail. 2017;19:661–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Krittanawong C, Rogers AJ, Johnson KW, Wang Z, Turakhia MP, Halperin JL, et al. Integration of novel monitoring devices with machine learning technology for scalable cardiovascular management. Nat Rev Cardiol. 2021;18:75–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kennel PJ, Rosenblum H, Axsom KM, Alishetti S, Brener M, Horn E, et al. Remote cardiac monitoring in patients with heart failure: a review. JAMA Cardiol. 2022;7:556–64. [DOI] [PubMed] [Google Scholar]
- 46. Bagos PG, Nikolopoulos GK. Mixed‐effects poisson regression models for meta‐analysis of follow‐up studies with constant or varying durations. Int J Biostat. 2009;5: Article 21. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information.


