Summary
Following knee and hip arthroplasty, transfer to a recovery area immediately following surgery and before going to ward might be unnecessary in low‐risk patients. Avoiding the recovery area in this way could allow for more targeted use of resources for higher risk patients, which may improve operating theatre flow and productivity. A prospective single‐centre cohort study on the safety of criteria for bypassing the post‐anaesthesia care unit in elective hip and knee arthroplasty was designed. Criteria were: ASA physical status < 3; peri‐operative bleeding < 500 ml; low postoperative discharge‐score (modified Aldrete‐score); and an uncomplicated surgical and neuraxial anaesthesia procedure. The primary outcome was the number of patients in need of secondary readmission to the post‐anaesthesia care unit. Events within the first 24 postoperative hours were recorded, along with readmission and complication rates. A total of 696 patients were included, with 287 (41%) undergoing total hip arthroplasty, 274 (39%) undergoing total knee arthroplasty and 135 (19%) undergoing unicompartmental knee‐arthroplasty. Of these, 207 (44%) bypassed the post‐anaesthesia care unit. Patients all received multimodal analgesia without peripheral nerve blockade. Only one patient in the ward group required secondary readmission to the post‐anaesthesia care unit. Within 24 h, 151 events were reported, with 41 (27%) in the ward group and 110 (73%) in the post‐anaesthesia care unit group. Two events in each group occurred within 2 hours of surgery. No complications were attributed to bypassing the post‐anaesthesia care unit. The use of simple pragmatic criteria for bypassing the post‐anaesthesia care unit for patients undergoing knee and hip arthroplasty with spinal anaesthesia is possible and associated with significant reduction of post‐anaesthesia care unit admission and without apparent safety issues. Confirmation is needed from other studies and external validity should be interpreted cautiously in centres with different peri‐operative regimens, organisational and staffing structures.
Keywords: enhanced recovery, hip arthroplasty, knee arthroplasty, PACU
Introduction
Evidence‐based fast track guidelines have resulted in reduced length of hospital stay in elective knee and hip arthroplasty, which has been accomplished without compromising patient safety [1, 2, 3]. The number of patients undergoing knee and hip arthroplasty is projected to increase due to changes in population demographics, challenging the existing healthcare system and requiring further optimisation of resource allocation [1, 4, 5]. Immediately following surgery, knee and hip arthroplasty patients are transferred to departments specialised in postoperative management, ensuring a stable condition before transfer to the general ward. The terminology differs, either termed recovery room or post‐anaesthesia care unit (PACU), but these areas are used for the postoperative patient to ensure sufficient analgesia and to treat early complications before discharge.
A PACU is a multidisciplinary highly specialised and staffed department, responsible for the care of postoperative patients with heterogeneous risk profiles and different needs of postoperative care and monitoring. Such units can be a potential bottleneck in surgical centres, with major influence on operating theatre logistics [6, 7]. In addition, delayed discharge from PACU may result in delaying physiotherapy and readiness to discharge from the surgical ward. A modern fast‐track setup for knee and hip arthroplasty includes: the use of evidence‐based optimised surgical procedures; reduced blood loss; multimodal analgesia, including peri‐operative glucocorticoids; local infiltration analgesia; and pre‐operative analgesics [3], all potentially reducing the need for PACU stay in the postoperative phase [8].
The aim of this study was to evaluate the efficacy and safety of predefined criteria for bypassing the PACU in knee and hip arthroplasty, with the primary outcome being the number of patients in need of readmission to PACU, and to describe all adverse events within the first 24 postoperative hours. We hypothesised that bypassing PACU using the prespecified criteria would not increase occurrence of adverse events overall, and that the majority of adverse events would occur at time‐points when patients would otherwise have been discharged from PACU [9, 10].
Methods
From 1 October 2019 to 1 November 2020, all patients undergoing knee and hip arthroplasty at Hvidovre Hospital, Denmark, were to be included. However, due to the COVID‐19 pandemic, all elective surgery was postponed from March to June 2020. Consequently, the inclusion period was extended to October 2021. Only unilateral primary procedures were included, thus excluding bilateral and revision procedures.
The surgical centre has a long experience with fast‐track arthroplasty including multimodal opioid‐sparing analgesia and early mobilisation. The analgesic protocol included acetaminophen 1 g, celecoxib 400 mg and pre‐operative intravenous methylprednisolone 125 mg, unless contraindicated (e.g. insuline dependent diabetes mellitus or ongoing systemic steroid treatment). Local infiltration with 0.2% ropivacaine 200 ml was applied in knee arthroplasty, but not in hip arthroplasty. Postoperative analgesia for patients undergoing general anaesthesia included intravenous sufentanil 0.1–0.3 μg.kg−1. Facility standard anaesthesia technique for both knee and hip arthroplasty was spinal blockade with bupivacaine 10 mg (hyperbaric for knee surgery) without the use of intrathecal opioids. Propofol sedation was given on patient request and intravenous opioids could be used peri‐operatively if deemed necessary by the anaesthetist. General anaesthesia could be used when neuraxial blockade was not possible, and in a few cases epidural or combined epidural and spinal anaesthesia were administered as preferred by the anaesthetist or patient. If spinal anaesthesia was considered insufficient during surgery, alternatives included conversion to general anaesthesia with total intravenous anaesthesia (using propofol and remifentanil) or supplemental opioids.
Total knee arthroplasty was performed using a midline incision, medial parapatellar arthrotomy, a measured resection technique with patellar resurfacing in all cases and cemented fixation. Unicompartmental knee arthroplasty was performed using a minimally invasive technique with cementless mobile bearing implants for medial procedures and fixed bearing implants with hybrid fixation (tibia cemented) for lateral procedures. Total hip arthroplasty was performed through a posterolateral approach using cementless or hybrid A fixation of components. Tourniquets were used only for unicompartmental knee arthroplasty surgery from incision until closure. In the postoperative period, patients received opioids (morphine or oxycodone) on request as rescue analgesic. No peripheral nerve blocks were used as standard care but could be used as a rescue intervention. Urinary bladder catheters were not used postoperatively. A protocol for handling postoperative urinary retention with a threshold of > 800 ml and a volume‐dependent bladder scan protocol was used in the PACU and the ward [11, 12].
Patients were discharged directly from the operating theatre to the surgical ward after fulfilling defined criteria. There had to have been successful standard neuraxial anaesthesia, < 500 ml peri‐operative blood loss and with a pre‐operative ASA physical status of 1 or 2 [13, 14]. The total postoperative discharge‐score (DASAIM‐score [9, 15, 16]) had to be < 4 and with no single component > 1. Finally, peri‐operative surgical and anaesthesia procedures had to be without complications, defined as stable cardiopulmonary conditions including an evaluation of the postoperative surgical APGAR score [17] and no requirement for postoperative oxygen, evaluated by the anaesthetist or specialised anaesthetist nurse.
These criteria could be overruled, and patients admitted to PACU at the discretion of the responsible anaesthetist if there were specific concerns on postoperative evaluation of the patient. If patients did not fulfil the criteria, they were admitted to the PACU as per usual practice and discharged to a dedicated ward when fulfilling the Danish Society of Anesthesiology and Intensive Care Medicine discharge criteria (DASAIM score [15, 16]). This score is an eight‐item report‐list (sedation; respiratory rate; oxygen saturation; blood pressure; heart rate; motor function assessment; pain; and nausea) scored from 0 to 3 (score range 0–24), and if the total score is < 4 and no single item > 1, the patient is considered for discharge to the ward. Patients were discharged from the PACU without motor‐function restrictions [9]. The surgical APGAR score [17] is a simple score (0–10) based on intra‐operative data on haemodynamic status and blood loss to predict morbidity, with lower scores associated with worse outcomes.
The dedicated knee and hip arthroplasty ward includes orthopaedic nurses trained and experienced in monitoring, detecting and treating complications, postoperative assessment of urinary retention and specialised physiotherapeutic treatment. After direct discharge from the operating theatre and discharge from the PACU to the ward, a clinical handover was given by telephone and patients were escorted without anaesthesia personnel to the ward, limiting possible delay in operating theatre usage. Collection of pre‐operative baseline characteristics included: type of surgery; sex; ASA physical status; medical history; use of anticoagulants; age; BMI; peri‐operative data from anaesthesia and surgery; and postoperative surgical APGAR and DASAIM scores at the end of surgery, on arrival at and every 30 min during PACU stay and at discharge from the PACU. Following arrival on the ward, an ‘early warning score’ was registered as per institutional guidelines [18]. Routine early warning scores onwards from this point were not registered.
All interventions within the first two postoperative hours were recorded, either in the PACU, ward or both. From the end of surgery and for the following 24 h, all events requiring an assessment from a physician were registered and evaluated similar to a previous discharge study [9]. Adverse events were grouped into: respiratory events (e.g. difficulty breathing and dyspnoea); circulatory events (e.g. chest pain, hypo‐ and hypertension); hypotension (subgroup of only hypotensive events); cerebral events (e.g. dizziness or neurological symptoms); pain events (an event leading to an increase in opioid dose); postoperative nausea and vomiting; surgical events (e.g. wound leaking or bleeding or suspicion of surgical complication); urological events (e.g. suspected or confirmed urinary retention, need for catheterisation); and other (e.g. hyperglycaemia, abdominal pain). Length of stay, complications, reasons for readmission and death within 7, 30 and 90 days after surgery were also recorded.
In the event of deterioration within the first 24 postoperative hours, patients would be assessed by the ward physician and discussed with the on‐call anaesthetist. If assessed as needing continued observation and treatment, the patient would be readmitted to PACU according to Danish practice, and only the ICU in very severe cases such as respiratory or cardiac failure. The primary outcome was the number of patients in need of readmission to the PACU or ICU after bypassing directly to the ward postoperatively. Secondary outcomes included events necessitating assessment from a physician and subsequent interventions within 24 postoperative hours. Peri‐operative data, discharge criteria, DASAIM‐score, surgical APGAR, early warning score on arrival at the ward, length of stay, readmission and mortality were also secondary outcomes.
Available data from the study centre suggested that approximately 50% of knee and hip arthroplasty patients would potentially be eligible for discharge directly to the ward following surgery. The estimated readmission rate was approximately 3%, and an actual rate not exceeding 6% in the ward group would be clinically acceptable in a non‐inferiority design. A non‐inferiority limit of 1% was acceptable, and with a power of 80% and an α of 5%, a sample size of 331 patients bypassing the PACU was needed, and a total of at least 662 patients were to be included, based on a bypass rate of 50%.
All complications and events were analysed in accordance with PACU or ward admission and place of origin, and reported with length of stay, readmissions and mortality. In case of patients dying within 90 days of surgery, the cause was analysed. Data were stored in REDCap [19, 20] and analysis was carried out using R (R Project, Vienna, Austria) and Microsoft Excel (Microsoft, Redmond, WA, USA).
Results
From 1 October 2019 to 1 November 2020, a total of 940 knee and hip arthroplasty procedures were performed at the specialised arthroplasty unit at Hvidovre Hospital and 696 (74%) were included and analysed (Fig. 1). Of the included patients, 287 (41%) were total hip arthroplasty, 274 (39%) were total knee arthroplasty and 135 (19%) were unicompartmental knee arthroplasty. No patients were excluded from the study analysis or treatment pathway due to comorbidity or other patient‐related factors.
Figure 1.
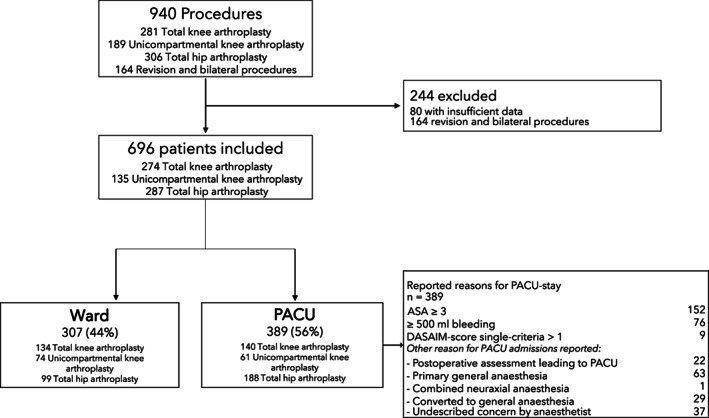
Patient flow diagram for included and excluded patients and their allocation to ward or PACU discharge following surgery. DASAIM: Danish Society of Anaesthesiology and Intensive Care Medicine score (modified Aldrete‐score).
Of those included, 307 (44%) bypassed PACU and were discharged directly to the ward postoperatively while 389 (56%) were discharged conventionally to PACU (Fig. 1). Five patients in the ward group were ASA physical status 3 but still discharged directly to the ward in violation of procedure and were included in our analysis. These violations happened at the discretion of the responsible anaesthetist. Two patients went to PACU due to logistical issues. Also, 42 (6%) receiving spinal anaesthesia had peri‐operative conversion to general anaesthesia and afterwards 39 (93%) of these patients went to PACU, while 3 (7%) bypassed the PACU in violation of protocol. These protocol violations also happened at the discretion of the responsible anaesthetist (Table 1).
Table 1.
Baseline characteristics of patients transferred directly to the general ward or admitted to PACU after elective knee‐ or hip arthroplasty. Values are number (proportion) or median (IQR [range]).
| Ward | PACU | |
|---|---|---|
| n = 307 | n = 389 | |
| Sex; female | 182 (59%) | 227 (58%) |
| BMI; kg.m‐2 | 28 (25–32 [15–47]) | 29 (25–34 [16–61]) |
| ASA physical status | ||
| 1 | 44 (14%) | 31 (8%) |
| 2 | 258 (84%) | 206 (53%) |
| 3 | 5 (2%) | 150 (39%) |
| 4 | 0 | 2 (1%) |
| Current smoker | 33 (11%) | 60 (15%) |
| Morbidity | ||
| Hypertension | 145 (47%) | 224 (58%) |
| Ischaemic heart disease/PCI/CABG | 12 (4%) | 36 (9%) |
| Arrhythmia | 13 (4%) | 59 (15%) |
| Cardiac valve‐disease | 6 (2%) | 19 (5%) |
| Other cardiac disease | 15 (5%) | 47 (12%) |
| Lung disease | 45 (15%) | 58 (23%) |
| Asthma | 30 (10%) | 32 (8%) |
| COPD | 8 (3%) | 26 (7%) |
| Other | 7 (2%) | 30 (8%) |
| Diabetes mellitus | 31 (10%) | 65 (17%) |
| NIDDM | 26 (9%) | 57 (15%) |
| IDDM | 5 (2%) | 8 (2%) |
| Use of antithrombotic | 42 (14%) | 133 (34%) |
| Potent antithrombotic | 11 (4%) | 73 (19%) |
| Non‐potent antithrombotic | 31 (10%) | 60 (15%) |
PCI, percutaneous coronary intervention; CABG, coronary arterial bypass grafting; COPD, chronic obstructive pulmonary disease; NIDDM, non‐insulin‐dependent diabetes; IDDM, insulin‐dependent diabetes.
Potent antithrombotics include: direct oral anticoagulants, vitamin‐k antagonists and low‐molecular heparin; Non‐potent antithrombotics include: aspirin, adenosine 5‐diphosphate antagonist and dipyridamole.
Procedures were completed under spinal anaesthesia in 304 (99%) of patients in the ward group vs. 309 (79%) in the PACU group. Combined epidural and spinal anaesthesia was applied only in the PACU group in four (1%) patients. Peri‐operative supplemental intravenous opioids in patients having spinal anaesthesia were applied in 28 (9%) vs. 49 (16%) in the ward and PACU groups, respectively (Table 2). Due to severe pain, two patients from the PACU group received a postoperative peripheral saphenous block during the PACU stay, and one of these ultimately had an epidural catheter inserted due to insufficiency of block and ongoing pain, before discharge from PACU.
Table 2.
Peri‐operative data of patients transferred directly to the general ward or admitted to PACU after elective knee and hip arthroplasty. Values are number (proportion) or median (IQR [range]).
| Ward | PACU | |
|---|---|---|
| n = 307 | n = 389 | |
| Type of procedure | ||
| Total knee arthroplasty | 134 (44%) | 140 (36%) |
| Unicompartmental knee arthroplasty | 74 (24%) | 61 (16%) |
| Total hip arthroplasty | 99 (32%) | 188 (48%) |
| Primary spinal anaesthesia | 307 (100%) | 309 (79%) |
| Bupivacaine; mg | 10 (10–10 [5–15]) | 10 (10–10 [5–18]) |
| General anaesthesia | 0 | 76 (20%) |
| Combined epidural and spinal | 0 | 4 (1.0%) |
| Converted to general anaesthesia | 3 (1%) | 39 (13%) |
| Sedation or supplemental analgesia during spinal anaesthesia | ||
| Opioids of all types | 28 (9%) | 48 (15.5%) |
| Sufentanil | 25 (8%) | 41 (13%) |
| Dose; μg | 8 (5–8 [3–13]) | 10 (5–10 [3–35]) |
| Alfentanil | 2 (1%) | 6 (2%) |
| Dose; mg | 0.3 (0.3–0.4 [0.3–0.5]) | 0.3 (0.3–0.4 [0.3–1.0]) |
| Pethidine | 0 | 1 (0%) |
| Dose; mg | 0 | 25 (25–25 [25–25]) |
| Propofol | 226 (74%) | 212 (69%) |
| Intra‐operative blood loss; ml | 200 (50–300 [0–500]) | 310 (100–550 [0–1930]) |
One patient in the ward group was readmitted to PACU due to chest pain occurring 15 min after arrival on the ward, while no patients from the PACU group were readmitted. This unexpected low occurrence did not allow for an assessment of non‐inferiority. The reason for PACU readmission was for an electrocardiogram and relevant laboratory tests due to chest pain, without confirmation of myocardial damage. The patient was discharged 3 h later without further events and no signs of myocardial damage within 24 h of surgery. The patient had a hospital length of stay of 1 day but was readmitted to hospital 5 days after surgery due to previously known lower back pain from a disc herniation. No other cardiac events occurred in the follow‐up period.
One or more events occurred in 34 (11%) vs. 85 (22%) discharged to the ward and PACU, respectively (Fig. 2). A total of 151 events demanding assessment by a physician occurred within the first 24 h with 41 (27%) in the ward group and 110 (73%) in the PACU group. Some patients had more than one event. Median postoperative discharge score and surgical APGAR score, along with early warning score, on arrival at the general ward were all significantly different between groups (Table 3).
Figure 2.
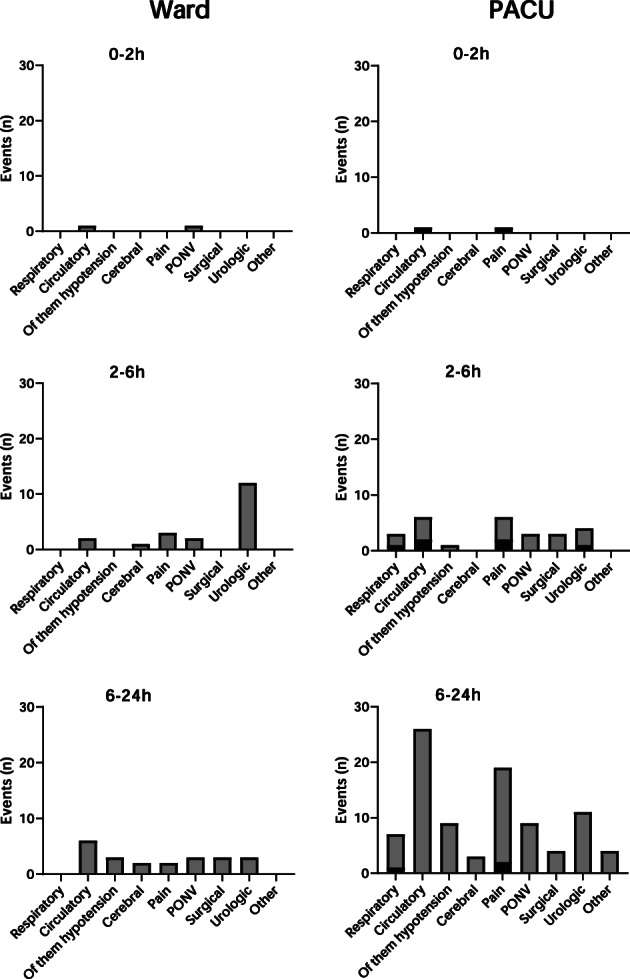
Location, number and time of occurrence of adverse events in patients transferred directly to the general ward or admitted to PACU after elective knee and hip arthroplasty. Events are grouped in time slots 0–2 h, 2–6 h and 6–24 h after surgery, and the location of occurrence of the event is marked either by grey (occurring in the general ward) or black (occurring in the PACU). Patients are discharged continuously over time from PACU onto the ward when ready to discharge, and so the number of patients in each time slot is not constant. A patient can therefore be represented with an event occurring in both the ward and the PACU within the same time slot.
Table 3.
Postoperative data of patients transferred directly to the general ward or admitted to PACU after elective knee and hip arthroplasty. Values are median (IQR [range]) or number (proportion).
| Ward | PACU | Relative risk of secondary admission to PACU | p value | |
|---|---|---|---|---|
| n = 307 | n = 389 | |||
| Surgical APGAR | 7 (7–8 [4–10]) | 7 (6–8 [2–10]) | <0.001 | |
| Score > 7 | 143 (47%) | 122 (31%) | 1.49 (95%CI 1.23–1.80) | <0.001 |
| Discharge score (DASAIM) | 3 (2–3 [0–6]) | 3 (2–4 [0–9]) | <0.001 | |
| Score < 4 | 283 (92%) | 262 (67%) | 1.37 (95%CI 1.27–1.48) | <0.001 |
| Early warning score at arrival at ward | 0 (0–1 [0–10]) | 1 (0–2 [0–5]) | <0.001 | |
| Score > 1 | 51 (17%) | 126 (32%) | 0.51 (95%CI 0.38–0.68) | <0.001 |
| Length of stay in hospital; days | 1 (1–1 [0–10]) | 1 (1–2 [0–20]) | <0.001 | |
| Length of stay > 4 days | 3 (1%) | 13 (3%) | 0.28 (95%CI 0.09–0.94) | 0.039 |
DASAIM, Danish Society of Anaesthesiology and Intensive Care Medicine score (modified Aldrete‐score).
Median (IQR [range]) time before PACU personnel reported patients ready for discharge was 110 (74–159 [2–745]) min, and actual time before leaving the PACU was 140 (109–189 [27–775]) min, with reasons for waiting > 10 min being primarily logistic, such as waiting for porters. Length of stay in hospital was significantly different between groups as was expected due to bypassing criteria. No clinically relevant difference was found between the ward and PACU groups regarding readmissions within 7, 30 and 90 days.
Three patients died during follow‐up, all within 30 days and from the PACU group. One patient died two days after surgery due to possible type 2 myocardial infarction, while another died of COVID‐19 and multi‐organ failure in the ICU after having been primarily discharged postoperatively but being readmitted on day 10. The third death occurred 28 days after total hip arthroplasty due to urinary tract infection and general decline.
Discussion
We found that 307 (44%) patients of a large unselected consecutive knee and hip arthroplasty cohort could be transferred directly to the general ward using simple criteria for bypassing PACU, with only one patient readmitted to PACU. This case was detected and treated rapidly, leading us to conclude that the suggested discharge criteria are potentially safe, while reducing PACU admission substantially. Complications on the general ward occurred in both groups but most frequently in the PACU group, as these patients were at the highest risk, most likely due to comorbidity placing them in that group. However, we were not able to assess non‐inferiority due to the very low occurrence of the primary outcome, despite the wide inclusion criteria with assumed high internal and external validity.
Knee and hip arthroplasty patients are normally transferred from the PACU to the ward when fulfilling discharge criteria such as the nationally implemented DASAIM‐score in Denmark, which is a modified Aldrete score using set criteria for discharge [15, 16]. However, these criteria were formed in the 1970s encompassing all PACU admissions and have only recently been challenged and adjusted to the advances in peri‐operative optimisation of knee and hip arthroplasty.
The potential for changing the existing dogma on PACU discharge readiness after knee and hip arthroplasty has only recently been investigated. A randomised clinical trial in 1359 patients evaluated the safety of PACU discharge without motor function assessment after total knee or hip arthroplasty and found no increased risk for complications on the general ward [9]. Another study assessed the time to meet standardised predefined PACU discharge criteria after hip and knee arthroplasty and found median time was 15 min with low‐dose spinal anaesthesia [10]. Also, the risk of postoperative urinary retention after knee and hip arthroplasty under spinal anaesthesia was assessed in a 721 patient randomised controlled trial, showing no increased risk for urinary complications [11]. When considering these three studies and advances in peri‐operative care, discharging directly to the ward and bypassing PACU to reduce resources for only patients in need seems a rational next step. Bypassing the PACU can potentially increase the logistic workflow and decrease the risk for PACU bottlenecks, and ultimately allow for further advances in early rehabilitation on the ward [6, 7, 21]. However, these effects and further safety data need to be substantiated in future studies, and our study might serve as a basis for future randomised controlled trials.
As PACUs are organised differently, the actual gain may vary from centre to centre. However, the potential of being able to safely bypass PACU in as many as 40–50% of all knee and hip arthroplasty patients is clinically important [22]. In addition, in fast track settings, final discharge to home is usually decided using functional discharge criteria [23] and having tolerable postoperative pain and nausea. Consequently, the time on the ward to complete physiotherapy and receive information on postoperative care and analgesia is a limiting factor in fulfilling these criteria in a potential day‐case setup. Nevertheless, time to first mobilisation, and the return to normal oral food‐intake [24] are easier to facilitate on the ward, supporting the importance of an early return to the ward.
Adverse events occurred in both groups, but only two events in each group happened within the first two postoperative hours, representing the time a patient would in most instances have been discharged from the PACU, similar to previous data [9, 10]. Thus, PACU bypass does not seem to push the need for observation and care onto the ward, as events are already mainly occurring on the ward as per the current standards for PACU admission. A substantially higher occurrence of events on the ward occurred in the PACU group but this is unsurprising, as the two groups had different characteristics and cannot be compared directly.
Our study has several strengths. We included 696 consecutive knee and hip arthroplasty patients at a public hospital in a socialised healthcare system, thus offering good internal and potential external validity. However, our findings may not apply to non‐enhanced recovery after surgery centres, and although we expect good external validity in centres with well‐implemented fast track criteria, this should be confirmed in similar safety trials. In addition, the use of outcomes such as all events requiring a clinical evaluation is a strength and ensures the clinical relevance of the study. Another strength is the use of prospectively collected data and the 100% follow‐up of length of stay length of stay and readmissions.
Limitations include the use of one prospective consecutive cohort without randomisation, but this was intentionally designed to assess safety and complications before subsequent randomised clinical trial development. As mentioned, the study was unexpectedly underpowered due to the almost absent primary outcome (n = 1). Another potential weakness was the possibility of selection bias in the final triage of patients as this was left to the discretion of anaesthetists who could overrule the criteria for bypassing the PACU, but the actual rate of overruling the criteria was only 9.5% of patients admitted to PACU and 5% of all patients included in the study. Bypass criteria were developed and implemented locally before the study design, ensuring organisational readiness for receiving bypass patients. However, bypass criteria could potentially be further widened to include patients admitted to the PACU with the current criteria, but this would again require new safety data. We believe the criteria are simple and can be adopted at other institutions with a similar focus on enhanced recovery.
Multivariable regression analysis was not performed, as the predefined protocol induced confounding by indication, precluding relevant conclusions based on such analyses. As the criteria for bypassing PACU are simple and pragmatic, and the fact that knee and hip arthroplasty fast track settings in general are based on internationally accepted guidelines, external validity should be high. As this is a single centre study, results must be interpreted with caution and transferability to centres with different organisational and staffing structures might be challenging. Our study includes only one patient undergoing general anaesthesia in the ward group and implementation in centres using general anaesthesia as the primary type of anaesthesia for knee and hip arthroplasty may not be valid. Further studies should be conducted in centres using this approach. Criteria for bypassing PACU were already implemented in the study centre, along with discharge from PACU without motor‐function assessment [9], precluding analysis of implementation and training issues, and no further actions were taken when starting the study.
The use of simple pragmatic criteria for bypassing PACU in patients undergoing knee and hip arthroplasty with spinal anaesthesia is possible and associated with significant reduction of PACU admission, without apparent safety issues, but needs confirmation from larger studies, and external validity should be interpreted cautiously in centres with different peri‐operative regimens and organisational and staffing structures.
Acknowledgements
The study was registered at ClinicalTrials.gov (NCT03984942). We thank M. Grentoft and K. Guldbrandsen for their assistance with data collection and all clinical staff at the Department of Anaesthesiology and the Orthopaedic Arthroplasty Unit, Hvidovre Hospital, Copenhagen University Hospital, Copenhagen, Denmark. This study was supported by an unconditional grant from Candy's foundation to HK. HK is a member of the advisory board at ‘Rapid Recovery’ by Zimmer Biomet. KG and AT received financial research support and speaker fees from Zimmer Biomet unrelated to this study. NF received speaker fees from Zimmer Biomet unrelated to this study. EA has received funding from Norpharma and Radiometer unrelated to this study. No other competing interests declared.
This article is accompanied by an editorial by W. M. Bullock and J. Gadsden, Anaesthesia 2023; 78: 14–6.
Contributor Information
N. I. Nielsen, Email: niklas.ingemann.nielsen@regionh.dk, @IngemannNiklas.
H. Kehlet, @KehletHenrik.
A. Troelsen, @ProfTroelsen.
N. B. Foss, @BangFoss.
E. K. Aasvang, @Eske1.
References
- 1. Price AJ, Alvand A, Troelsen A, et al. Knee replacement. Lancet 2018; 392: 1672–82. [DOI] [PubMed] [Google Scholar]
- 2. Hansen TB. Fast track in hip arthroplasty. European Federation of National Associations of Orthopaedics and Traumatology Open Reviews 2017; 2: 179–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Petersen PB, Kehlet H, Jørgensen CC, et al. Improvement in fast‐track hip and knee arthroplasty: a prospective multicentre study of 36,935 procedures from 2010 to 2017. Scientific Reports 2020; 10: 21233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rupp M, Lau E, Kurtz SM, Alt V. Projections of primary TKA and THA in Germany from 2016 through 2040. Clinical Orthopaedics and Related Research 2020; 478: 1622–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pabinger C, Lothaller H, Portner N, Geissler A. Projections of hip arthroplasty in OECD countries up to 2050. Hip International 2018; 28: 498–506. [DOI] [PubMed] [Google Scholar]
- 6. Sokal SM, Craft DL, Chang Y, et al. Maximizing operating room and recovery room capacity in an era of constrained resources. Archives of Surgery 2006; 141: 389–95. [DOI] [PubMed] [Google Scholar]
- 7. Watkins AC, White PF. Fast‐tracking after ambulatory surgery. Journal of Perianesthesia Nursing 2001; 16: 379–87. [DOI] [PubMed] [Google Scholar]
- 8. Aasvang EK, Luna IE, Kehlet H. Challenges in postdischarge function and recovery: The case of fast‐track hip and knee arthroplasty. British Journal of Anaesthesia 2015; 115: 861–6. [DOI] [PubMed] [Google Scholar]
- 9. Aasvang EK, Jørgensen CC, Laursen MB, et al. Safety aspects of postanesthesia care unit discharge without motor function assessment after spinal anesthesia. Anesthesiology 2017; 126: 1043–52. [DOI] [PubMed] [Google Scholar]
- 10. Lunn TH, Kristensen BB, Gaarn‐Larsen L, et al. Post‐anaesthesia care unit stay after total hip and knee arthroplasty under spinal anaesthesia. Acta Anaesthesiologica Scandinavica 2012; 56: 1139–45. [DOI] [PubMed] [Google Scholar]
- 11. Bjerregaard LS, Hornum U, Troldborg C, Bogoe S, Bagi P, Kehlet H. Postoperative urinary catheterization thresholds of 500 versus 800 ml after fast‐track total hip and knee arthroplasty: A randomized, open‐label, controlled trial. Anesthesiology 2016; 124: 1256–64. [DOI] [PubMed] [Google Scholar]
- 12. Møller T, Engedal MS, Plum LM, Aasvang EK. Reduced need for urinary bladder catheterization in the postanesthesia care unit after implementation of an evidence‐based protocol: a prospective cohort comparison study. European Urology Open Science 2021; 26: 27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Owens WD, Felts JA, Spitznagel EL. ASA physical status classifications. Anesthesiology 1978; 49: 239–43. [DOI] [PubMed] [Google Scholar]
- 14. Keats AS. The ASA classification of physical status ‐ a recapitulation (editorial views). Journal of Anesthesiology 1978; 49: 233–6. [DOI] [PubMed] [Google Scholar]
- 15. Aldrete JA. Post‐anesthetic recovery score. Journal of the American College of Surgeons 2007; 205: e3–e4. [DOI] [PubMed] [Google Scholar]
- 16. Aldrete JA, Kroulik D. A postanesthetic recovery score. Anesthesia and Analgesia 1970; 49: 924–34. [PubMed] [Google Scholar]
- 17. Wuerz TH, Regenbogen SE, Ehrenfeld JM, Malchau H, Rubash HE, Gawande AA, Kent DM. The Surgical Apgar Score in hip and knee arthroplasty. Clinical Orthopaedics and Related Research 2011; 469: 1119–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Goulden R, Hoyle MC, Monis J, et al. QSOFA, SIRS and NEWS for predicting inhospital mortality and ICU admission in emergency admissions treated as sepsis. Emergency Medicine Journal 2018; 35: 345–9. [DOI] [PubMed] [Google Scholar]
- 19. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)‐a metadata‐driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics 2009; 42: 377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. Journal of Biomedical Informatics 2019; 95: 103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Robertson A, Kla K, Yaghmour E. Efficiency in the operating room: optimizing patient throughput. International Anesthesiology Clinics 2021; 59: 47–52. [DOI] [PubMed] [Google Scholar]
- 22. Raft J, Millet F, Meistelman C. Example of cost calculations for an operating room and a post‐anaesthesia care unit. Anaesthesia Critical Care and Pain Medicine 2015; 34: 211–5. [DOI] [PubMed] [Google Scholar]
- 23. Husted H, Lunn TH, Troelsen A, Gaarn‐Larsen L, Kristensen BB, Kehlet H. Why still in hospital after fast‐track hip and knee arthroplasty? Acta Orthopaedica 2011; 82: 679–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wainwright TW, Gill M, McDonald DA, et al. Consensus statement for perioperative care in total hip replacement and total knee replacement surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations. Acta Orthopaedica 2020; 91: 3–19. [DOI] [PMC free article] [PubMed] [Google Scholar]


