Abstract
Background
Scabies is an itchy, parasitic infection of the skin. Recent reports indicate there is a decreasing efficacy of the standard treatment of choice, topical 5% permethrin cream.
Objective
To evaluate the comparative efficacy, safety and tolerability of topical benzyl benzoate (BB) with oral ivermectin in the treatment of scabies.
Methods
Patients with dermoscopy‐verified scabies visiting the dermatologic outpatient clinic were assessed for enrolment in the study. In total, 224 patients were enrolled and sequentially randomized into two equally sized groups. Group A received topical 25% or 10% BB for the daily use over a period of three consecutive days, group B received oral ivermectin (200 μg/kg body weight) twice, 1 week apart. Treatment outcome was evaluated by dermoscopy at a 3‐week follow‐up visit.
Results
Treatment resulted in a cure rate of 87% in group A and 86% in group B. After initial therapy failure in group A, six out of eight patients showed treatment response upon repeated application of BB, five of five when retreated with ivermectin and two of two with BB plus ivermectin, respectively. In group B, successful retreatment was observed in three out of three patients with ivermectin, two of two patients with BB and 11 of 11 patients with the combination of BB plus ivermectin, respectively. Tolerability and safety profile of oral ivermectin was excellent, while BB produced short burning sensations in 14%.
Conclusion
Topical BB and oral ivermectin have shown comparable good therapeutic efficacy. Therefore, both agents constitute an adequate first‐line therapy in the treatment of scabies. A combination of both agents may be considered in recalcitrant and extensively infested cases, additionally to crusted scabies.
INTRODUCTION
Human scabies is usually transmitted during skin‐to‐skin contact with persons infested with the ectoparasitic mite Sarcoptes scabiei var. hominis. Scabies is a rising major public health issue worldwide and has recently been designated as a neglected tropical disease by the World Health Organization (WHO). 1 Current guidelines propose a range of different treatment regimens taking into account topical permethrin, benzyl benzoate, sulfur, malathion, crotamiton, synergized pyrethrins and topical or oral ivermectin as the only systemic therapeutic option. 2 , 3 , 4 , 5 Although their availability differs among European countries, topical 5% permethrin together with oral ivermectin is one of the most widely prescribed first‐line scabicidal therapy. 6 However, recent reports indicate a high prevalence of resistance towards 5% permethrin cream, being the most commonly used topical treatment in Europe. 7 , 8 , 9
Resistance of mites towards permethrin has become an increasing matter of debate in the last few years. 10 , 11 , 12 , 13 Mutations concerning the “knockdown resistance” in the voltage‐gated sodium channels, which confer resistance to pyrethroids, have not yet been identified in Sarcoptes scabiei var. hominis. However, median survival times of the mites in the presence of permethrin in vitro have substantially increased from 1 h in 1994, before the widespread use of permethrin, to 3 and 6 h in 2000 and 2010, respectively, indicating a possible resistance towards permethrin. 11 , 12 , 13 , 14 A recent study in 2020 showed that after 8 h 65% and after 12 h 25% of mites were still alive ex vivo. 15 Hence, while formerly one single application for 8 h was curative in scabies, increasing therapy failures led to recommendations of repeated applications with an interval of 1 week. 2
Additionally, to the investigation of efficacy, safety and tolerability, aim of this study was to evaluate whether scabies mites have developed resistance towards topical 10% or 25% BB or oral ivermectin as it has been observed with topical 5% permethrin. 7 , 9
MATERIAL AND METHODS
Patients
This single‐center, randomized, prospective, open label trial was conducted in the Department of Dermatology and Allergology at the University Hospital of the Paracelsus Medical University in Salzburg, Austria. Patients consulting our outpatient clinic who were older than 1 year of age or weighting ≥15 kg and showing symptoms of a scabies infestation, were assessed for enrolment in this study. The diagnosis was confirmed by a meticulous dermoscopic detection of mites (“kite sign” corresponding to the head and breastplate of the mite in optional conjunction with the presence of an air‐filled intracorneal burrow system), on either hands/wrists, genitals or feet, according to the 2020 International Alliance for the Control of Scabies Consensus Criteria. 16 If no mites were detectable at these sites, search was extended to the whole body, especially closed to unspecific secondary lesions including excoriated papules or eczematous plaques. Partners or relatives of the same household were urged to visit our outpatient clinic for clinical examination. Those who were also infested were offered participation in the study in case they met the inclusion criteria, otherwise an appropriate prophylactic anti‐scabietic therapy was provided.
Additional exclusion criteria were as follows: (1) pre‐treated with topical 10% or 25% BB emulsion (2) treatment with 5% permethrin cream in (2a) the last 3 weeks or (2b) the last 2 weeks in infants, (3) scabies crustosa, (4) successful use of oral ivermectin in the past 6 months, (5) known hypersensitivity or allergy to contents of BB emulsion (6) and pregnancy or breast‐feeding.
Patients were asked about their history of anti‐scabietic pre‐treatment, the number of relevant contact persons, for example, life partners and relatives, and their occupation. Age and gender were also recorded for demographic comparison.
Written informed consent was obtained from all patients before participation in the study following the Declaration of Helsinki and after approval by the local ethics committee (Salzburg, Austria, 1104/2020). Patients were sequentially randomized into two equal groups with the exception of children weighting less than 15 kg body‐weight who were included in group A.
Statistical analysis
For the statistical analysis, we applied the χ2 test, with p < 0.05 as the level of significance. SPSS version 24 was used for all calculations. Additionally, binary logistic regressions were calculated with the outcome as the dependent variable and group as the co‐variate. Primary hypothesis was the absence of significant difference in efficacy of topical BB to oral ivermectin.
Treatment
Patients in group A received 100 g of 10% or 25% BB emulsion * for a daily application over three consecutive days. Children aged 1–5 years received topical 10% BB, otherwise a 25% emulsion was prescribed. The emulsion was applied in the evening on the whole body sparing the scalp and should not be washed off before midday of the fourth day. The application on the scalp was limited to children below the second year of life with clinical signs or dermoscopic confirmation of mites.
Patients in group B received two doses of 200 μg/kg body weight [15–24 kg: 1 tablet; 25–35 kg: 2 tablets; 36–50 kg: 3 tablets; 51–65 kg: 4 tablets; 66–79 kg: 5 tablets, ≥80 kg: 6 tablets] oral ivermectin (Scabioral; InfectoPharm, Heppenheim, Germany) on Days 0 and 7. Ivermectin was given in the evening and patients were not specifically advised to swallow ivermectin on an empty stomach.
All patients were instructed about outpatient self‐treatment or treatment conducted, for example, by a glove‐dressed life‐partner or parents, as well as behavioural and implemented household measures, both orally and by using a standardized printed handout.
Patients were examined for therapeutic success at week 3 (to 4). Patients with dermoscopic detection of mites were considered as non‐responders. These patients were allowed to repeat the treatment immediately following the same protocol, change the scabicidal treatment according to the parallel group or to receive a combination of both scabicides—taking into account the physicians' recommendation and the patients' preferences.
RESULTS
In total, 224 patients were enrolled in the study. One hundred and twelve patients (59 female, mean age 26.1 ± 18.9 years, range 1.0–78.3 years) were assigned to group A, 112 patients (47 female, mean age 24.6 ± 14.5 years, range 1.6–75.0 years) to group B. No difference in sex and age was observed between both groups.
Of the examined body regions, already 92% showed mite presence on the hands. In both groups, 43 patients (38%) had already experienced therapeutic failure with at least two treatment cycles of 5% topical permethrin before study enrolment, respectively. Some cases had even administered up to 20 applications of topical permethrin over a period of three to 6 months and were thereby psychologically stressed.
In group A, 97 of 112 patients (86.6%) were cured after one treatment cycle with topical 10% or 25% BB. Among the 15 patients who were non‐responders, 13 responded successfully to a retreatment (BB [6/8], ivermectin [5/5], or a combination of BB plus ivermectin [2/2]).
According to age, 5 of 15 (33%) patients aged 1–5 years and 1 of 6 patients (17%) aged 6–11 years showed persistence of scabies after the first treatment, necessitating treatment repetition, which were all successful (BB [4/4], combination of both agents [2/2]).
In group B, two doses of oral ivermectin in a strictly weight‐dependent dosage provided a cure rate of 85.7% (96 of 112). All retreatments were successful (ivermectin [3/3], BB [2/2] and combination of both [11/11]).
Treatment resulted in a cure rate of 87% in group A (97/112) and 86% in group B (96/112). None of the both scabicides emerged as superior in eradicating scabies (p = 0.847).
A stratified analysis did not suggest any significant difference in treatment response in relation to age (p = 0.194). Nonetheless, younger patients, particularly up to 25 years, were more likely to encounter therapeutic failure (19.1% vs. 2.8%, p = 0.001; Table 1). This finding was irrespective of the administered antiscabietic medication [BB (p = 0.014) or ivermectin (p = 0.028)].
TABLE 1.
Significant correlation between age classes (<25 vs. ≥25 years) and therapeutic outcome (‘0’ is therapeutic failure, ‘1’ is therapeutic success) within the complete study cohort (group A and B; p = 0.001)
| Patients | Percentage | ||
|---|---|---|---|
| <25 years | 0 | 29 | 19.1 |
| 1 | 123 | 80.9 | |
| Total | 152 | 100.0 | |
| ≥25 years | 0 | 2 | 2.8 |
| 1 | 70 | 97.2 | |
| Total | 72 | 100.0 | |
BB demonstrated good tolerability: 16 patients (14.3%) reported only mild adverse events, apparent as occasional mild or medium strength burning or stinging in the area where BB was applied or on excoriated skin and mucous membranes (e.g. genital mucosa) due to scratching. Reported symptom duration was 2–10 min and ceased spontaneously in all cases. None of the children aged 6 to 11 years, treated with 25% BB emulsion, reported adverse events concerning tolerability. Additionally, in 5 (4.5%) patients, a post‐therapeutic dyshidrotic eczema on the fingers and palms was observed on the hands during the follow‐up visit, irrespective of the therapeutic response. Xeroderma appeared regularly, but was not reported by the patients autonomously. Only one adult patient discontinued the therapy due to cutaneous intolerability and was therefore excluded from the study (and statistical analysis).
Ivermectin demonstrated excellent tolerability consistently. Only one patient complained about extensive skin‐dryness beyond therapy following therapy failure. Five patients (4.5%) showed a palmar dyshidrotic eczema beyond the cure of scabies.
DISCUSSION
Although no official data on current and past disease incidence exists, the soaring use of scabicides and the rise of diagnoses are indicative of an increase in the prevalence of scabies in Western Europe. This study was intended as a sequel study to our findings of reduced efficacy of topical permethrin in the treatment of scabies infested patients. 7
The notable increase in scabies infestations over the last years might be explained by an increase of at‐risk groups, such as refugees with a high prevalence of scabies and treatment failure to conventional therapies, 8 which can be attributed to increased development of resistances against permethrin. 7 , 9 , 15 , 17 In our study, 38% (86 of 224 patients) had been unsuccessfully treated with topical permethrin before study enrolment.
A French study further identified following risk factors for treatment failure: (1) the use of only one type of treatment, either topical BB or oral ivermectin vs. combination of both treatments, (2) the one‐time intake vs. two times of oral ivermectin, (3) intake of ivermectin during a meal vs. on an empty stomach, (4) absence of decontamination of furnishings and (5) absence of written documents explaining treatment modalities. 18 To address these shortcomings, herein, we aimed at reducing the confounders by administration of two doses of ivermectin 1 week apart and providing oral and written information on the correct treatment procedures, including hygienic measures.
Because it is yet unclear to what extent food intake may affect absorption and opinions diverge on whether the tablets should be taken with food or not, 2 , 8 , 19 , 20 in our study, the patients were not specifically advised to swallow ivermectin on an empty stomach.
In this study, mite prevalence on the hands was 92% and hence comparable with our previous findings (96%), in contrast to data from three population‐based surveys of scabies that found infestation of the hands in only 51.2%. 1 , 7
The European Guideline for the Management of Scabies and the German S1 guideline, with both pending revisions, recommend topical 5% permethrin or oral ivermectin, applied on Day 1 and a second time between Day 7 (ivermectin or permethrin) and 14 (ivermectin) vs. a single application of topical permethrin as first‐line therapy for adults and infants. 2 , 3 Only the European guideline further suggests daily BB in the evening on 2 consecutive days with reapplication on Day 7 as an additional first‐line therapy; however, states that the grade of recommendation is weaker than for topical permethrin or oral ivermectin due to a weaker body of evidence. 2 The Austrian guideline (Österreichische Gesellschaft für STD, ÖGSTD) recently issued an acknowledgement to stem the epidemic of scabies with ivermectin 200 μg/kg body weight and topical permethrin 5% cream, both given on Day 0 and 7. 4
To date BB, applied in the evening on three consecutive days, and washed off at the fourth day, is recommended as topical treatment of second choice by the German and Austrian guidelines.
The 10% BB preparation is approved for children with healthy skin after the first year of life, the 25% emulsion from the age of 12 years and older. 3 , 4 Data supporting the use of topical BB in infants during the first 12 months of life, and the 25% emulsion below the age of 12 years, are still lacking and application therefore remains off‐label for this age group.
High‐quality controlled trials comparing topical BB and permethrin or ivermectin are missing and yield inconsistent findings, hence no firm conclusion can be drawn. 21 Major limitations were the inconsistent timing of the follow‐up visits, differing from 1 week to 30 days, as well as the application frequencies of both treatments. In 2007, a Cochrane review concluded that there is insufficient data available to compare the relative efficacies of topical permethrin and topical BB. 22 , 23 This review also concluded that oral ivermectin appeared to be more effective than topical BB (relative risk of treatment failure with ivermectin as compared to BB was 0.50 in three trials involving 192 subjects). 22 , 24 However, a consecutive study showed that there was a higher rate of treatment failure with single‐dose ivermectin than with topical BB. 25 The latest Cochrane review did not deliver statements about BB, as only studies comparing ivermectin and permethrin were evaluated. 19 In the latest controlled trial from India in 2009, at a 2‐week follow‐up visit, the cure rate for BB was 92% (25% lotion; 35 patients, applied for two consecutive days) and 100% for ivermectin (34 patients, 200 μg/kg body weight as a single dose). The authors recommended BB in afflicted patients as a first line therapy for the first 2 weeks and consecutive shifting of uncured patients to oral ivermectin for the following 2 weeks. 26
Our clinical trial is a sequel to observations made in our Dermatology department proving loss of efficacy of topical permethrin in patients infested with scabies. 7 The herein reported outcomes confirm the high efficacy of topical BB, which, with respect to increasing resistances against topical permethrin, highlights the necessity to consider BB as an additional first‐line topical treatment. Even if not as well tolerated as topical permethrin, BB has proven equal efficacy as two doses of oral ivermectin (87% vs. 86%), additionally indicating a favourable risk–benefit ratio. Consequently, our primary hypothesis of the absence of significant difference between topical BB and oral ivermectin was achieved. Limitations of our study were the monocentric recruitment and the unblinded design.
Further, regardless of the antiscabietic medication used, patients up to 25 years were more prone for treatment failure, which might be explained by the assumption that this age class is negligent in following all instructions regarding treatment and hygienic measures correctly and has frequent skin contacts with potentially afflicted peer‐groups, partners or siblings. 27 However, this observation could be biased due to the proportionally larger cohort in this age class. A similar explanation can be considered for children of families with many children, where refractory infestations were observed more frequently.
Dyshidrotic eczema (vesicular eruption) on the fingers/palms was solely a post‐therapeutic effect and equally frequent in the BB and ivermectin groups: it is most probably a kind of allergic contact dermatitis to decomposing mite‐components within the epidermis.
With regard to the current epidemiology of resistance towards permethrin, we recommend two doses of ivermectin or topical BB for three consecutive days as a first‐line therapy for uncomplicated scabies. A repetition of the same antiscabietic agent can be offered once immediately after therapeutic failure at a 3‐week follow‐up.
A considerable advantage of oral ivermectin is (1) the lower susceptibility to (and better traceability of) application errors (2) a better acceptance of an oral intake vs. the more time‐consuming and self‐conquest needing topical application over several days and (3) the present data approving similar good tolerability even in infants in the first year of age. 20 , 28
A combination of both therapeutics has synergistic effects and is practically always sufficient to eradicate the parasite. A combination of both agents is indispensable in scabies crustosa and should be considered in retreatment of recalcitrant cases most likely appearing in adolescents and families with many children. 18 (Figure 1).
FIGURE 1.
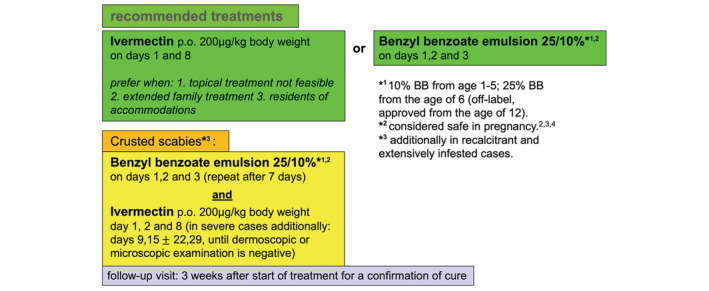
Treatment algorithm used at the Department of Dermatology and Allergology in Salzburg, Austria (adopted from 2). Instruction for outpatient self‐treatment (or treatment conducted by parents or life‐partner), behavioural and implemented household measures should be conducted both orally and by using a standardized printed handout.
CONCLUSION
Oral ivermectin or topical BB has shown good coequal therapeutic efficacy and tolerability in adults and children suffering from scabies and no reasonable evidence of resistance, contrary to permethrin. Both agents constitute therefore an adequate first‐line therapy in the treatment of scabies. Their combination should be considered for recalcitrant or extensive scabies, as in case of scabies crustosa—where it is already recommended.
AUTHOR CONTRIBUTIONS
DM was responsible for the conception, study design and interpretation of the data. DM, SS and RT contributed to recruitment of patients and AK analysed the data. DM wrote the paper with the help of TW, JWB, AH and AK. All authors read and approved the final manuscript.
FUNDING INFORMATION
None.
CONFLICT OF INTEREST
None declared.
ETHICAL APPROVAL
The authors declare that this work received all necessary ethical approvals and that patients involved all consent to participate. This study was reviewed and approved by local ethics committee.
CONSENT FOR PUBLICATION
Not applicable.
ACKNOWLEDGEMENTS
We thank Martin Wolkersdorfer and Peter Hofbauer from the clinics pharmacy for the preparation and distribution of topical benzyl benzoate.
Meyersburg D, Welponer T, Kaiser A, Selhofer S, Tatarski R, Handisurya A, et al. Comparison of topical benzyl benzoate vs. oral ivermectin in treating scabies: A randomized study. J Eur Acad Dermatol Venereol. 2023;37:160–165. 10.1111/jdv.18573
Endnote
Topical benzyl benzoate was based on an 2% cetostearyl alcohol emulsion. The emulsion was prepared by melting benzyl benzoate with 2% cetostearyl alcohol and afterwards mixing with warm, purified water for some minutes. The emulsion was prepared in the certified clinical pharmacy of the University Hospital Salzburg.
DATA AVAILABILITY STATEMENT
All data generated or analysed during this study are available from the corresponding author on reasonable request.
REFERENCES
- 1. Marks M, Engelman D, Romani L, Mason D, Sokana O, Kama M, et al. Exploration of a simplified clinical examination for scabies to support public health decision‐making. PLoS Negl Trop Dis. 2018;12:e0006996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Salavastru CM, Chosidow O, Boffa MJ, Janier M, Tiplica GS. European guideline for the management of scabies. J Eur Acad Dermatol Venereol. 2017;31:1248–53. [DOI] [PubMed] [Google Scholar]
- 3. Sunderkötter C, Feldmeier H, Fölster‐Holst R, Geisel B, Klinke‐Rehbein S, Nast A, et al. S1 guidelines on the diagnosis and treatment of scabies – short version. J Dtsch Dermatol Ges. 2016;14:1155–67. Extended version at: www.awmf.org [DOI] [PubMed] [Google Scholar]
- 4. Österreichische Gesellschaft für Sexually Transmitted Diseases und dermatologische Mikrobiologie: Skabies Therapiemanagement für Allgemeinmediziner und Fachärzte. Addendum zur Version 11/2019. Available from: http://www.oegstd.at/
- 5. Workowski KA, Bolan GA. Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64:1–137. [PMC free article] [PubMed] [Google Scholar]
- 6. Heukelbach J, Feldmeier H. Scabies. Lancet. 2006;367:1767–74. [DOI] [PubMed] [Google Scholar]
- 7. Meyersburg D, Kaiser A, Bauer JW. Loss of efficacy of topical 5% permethrin for treating scabies: an Austrian single‐center study. J Dermatolog Treat. 2020;4:1–4. [DOI] [PubMed] [Google Scholar]
- 8. Sunderkötter C, Aebischer A, Neufeld M, Löser C, Kreuter A, Bialek R, et al. Increase of scabies in Germany and development of resistant mites? Evidence and consequence. J Dtsch Dermatol Ges. 2018;17:15–23. [DOI] [PubMed] [Google Scholar]
- 9. Mazzatenta C, Piccolo V, Argenziano G, Bassi A. Is Scabies becoming less sensitive to permethrin therapy? J Eur Acad Dermatol Venereol. 2021;35:e607–9. [DOI] [PubMed] [Google Scholar]
- 10. Mounsey KE, Holt DC, McCarthy J, Currie BJ, Walton SF. Scabies: molecular perspectives and therapeutic implications in the face of emerging drug resistance. Future Microbiol. 2008;3:57–66. [DOI] [PubMed] [Google Scholar]
- 11. Thomas J, Peterson GM, Walton SF, Carson CF, Naunton M, Baby KE. Scabies: an ancient global disease with a need for new therapies. BMC Infect Dis. 2015;15:250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fraser J. Permethrin: a Top End viewpoint and experience. Med J Aust. 1994;160:806. [DOI] [PubMed] [Google Scholar]
- 13. Mounsey KE, Pasay CJ, Arlian LG, Morgan MS, Holt DC, Currie BJ, et al. Increased transcription of Glutathione S‐transferases in acaricide exposed scabies mites. Parasit Vectors. 2010;3:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Statistik Austria . Geborene nach ausgewählten demografischen und medizinischen Merkmalen seit 2009. [cited 2021 Apr]. Available from: https://www.statistik.at/web_de/statistiken/menschen_und_gesellschaft/bevoelkerung/geborene/022899.html
- 15. Pallesen K, Lassen JA, Munk NT, Hartmeyer GN, Hvid L, Bygum A. In vitro survival of scabies mites. Clin Exp Dermatol. 2020;45:712–5. [DOI] [PubMed] [Google Scholar]
- 16. Engelman D, Yoshizumi J, Hay RJ, Osti M, Micali G, Norton S, et al. The 2020 international alliance for the control of scabies consensus criteria for the diagnosis of scabies. Br J Dermatol. 2020;183:808–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pasay C, Arlian L, Morgan M, Vyszenski‐Moher D, Rose A, Holt D, et al. High‐resolution melt analysis for the detection of a mutation associated with permethrin resistance in a population of scabies mites. Med Vet Entomol. 2008;22:82–8. [DOI] [PubMed] [Google Scholar]
- 18. Aussy A, Houivet E, Hébert V, Colas‐Cailleux H, Laaengh N, Richard C, et al. Risk factors for treatment failure in scabies: a cohort study. Br J Dermatol. 2019;180:888–93. [DOI] [PubMed] [Google Scholar]
- 19. Rosumeck S, Nast A, Dressler C. Ivermectin and permethrin for treating scabies. Cochrane Database Syst Rev. 2018;4:CD012994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wilkins AL, Steer AC, Cranswick N, Gwee A. Question 1: Is it safe to use ivermectin in children less than five years of age and weighing less than 15 kg? Arch Dis Child. 2018;103:514–9. [DOI] [PubMed] [Google Scholar]
- 21. Dressler C, Rosumeck S, Sunderkötter C, Werner RN, Nast A. The Treatment of Scabies‐a systematic review of randimized controlled trials. Dtsch Arztebl Int. 2016;113:757–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Strong M, Johnstone PW. Interventions for treating scabies. Cochrane Database Syst Rev. 2007;2007:CD000320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hu S, Bigby M. Treating scabies: results from an updated Cochrane review. Arch Dermatol. 2008;144:1638–40. [DOI] [PubMed] [Google Scholar]
- 24. Currie BJ, McCarthy JS. Permethrin and ivermectin for scabies. N Engl J Med. 2010;362:717–25. [DOI] [PubMed] [Google Scholar]
- 25. Ly F, Caumes E, Ndaw CA, Ndiaye B, Mahe A. Ivermectin versus benzyl benzoate applied once or twice to treat human scabies in Dakar, Senegal: a randomized controlled trial. Bull World Health Organ. 2009;87:424–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bachewar NP, Thawani VR, Mali SN, Gharpure KJ, Shingade VP, Dakhale GN. Comparison of safety, efficacy, and cost effectiveness of benzyl benzoate, permethrin, and ivermectin in patients of scabies. Indian J Pharmacol. 2009;41:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nemecek R, Stockbauer A, Lexa M, Poeppl W, Mooseder G. Application errors associated with topical treatment of scabies: an observational study. J Dtsch Dermatol Ges. 2020;18:554–9. [DOI] [PubMed] [Google Scholar]
- 28. Levy M, Martin L, Bursztejn AC, Chiaverini C, Miquel J, Mahé E, et al. Groupe de Recherche de la Société Française de Dermatologie Pédiatrique. Ivermectin safety in infants and children under 15 kg treated for scabies: a multicentric observational study. Br J Dermatol. 2020;182:1003–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are available from the corresponding author on reasonable request.


