Abstract
This systematic review aims to assess the cost-effectiveness of cardiac telerehabilitation in comparison with centre-based cardiac rehabilitation (CR). Evidence of cost-effectiveness is an important step towards implementation and reimbursement of telerehabilitation services. Electronic databases were searched for economic evaluations of telerehabilitation programmes. Only randomized controlled trials (RCTs) published in English were eligible for inclusion. Study quality and risk of bias were assessed using the Consensus Health Economic Criteria (CHEC) list. A total of eight economic evaluations met the review inclusion criteria. The total sample size consisted of 751 patients ranging from a minimum of 46 patients to a maximum of 162 patients per study. Maximal follow-up was 5 years. A total of seven of the eight included studies demonstrated that telerehabilitation could lead to similar or lower long-term costs and are thus as cost-effective as traditional centre-based CR. There is significant heterogeneity between all included telerehabilitation interventions in duration, used technology, cost included and follow-up. Based on these small short duration trials, telerehabilitation may be as cost-effective as traditional centre-based approaches. However, more assessments of the value for money of telerehabilitation in larger and longer RCTs are needed both in high- as low-income countries.
Keywords: Heart failure, Ischaemic heart disease, Telerehabilitation, Acute coronary artery disease, Cost-effectiveness, Cost–utility
Introduction
Ischaemic heart disease (IHD) and heart failure (HF) are the most important causes of death in most European countries.1 Next to a high mortality, they are also associated with a high number of recurrent events, rehospitalizations and negative impact on quality of life.2 Ischaemic heart disease and HF also have a significant impact on healthcare budgets.1 The high rate of recurrent disease leads to the need of self-management or secondary prevention programmes. The first step of secondary prevention is often cardiac rehabilitation (CR).3 The effectiveness and cost-effectiveness of CR in reducing morbidity and mortality along with increasing quality of life and psychological well-being are well-established.4–6 A recent position paper by Ambrosetti et al.7 defined the following core components of CR: patient assessment, management and control of cardiovascular risk factors, physical activity counselling, prescription of exercise training, dietary advice, psychosocial management, and vocational support. Unfortunately, the EUROASPIRE surveys demonstrated that only few of the eligible patients participate in CR.8 Important barriers for participation are often lower health literacy or transport, familial, vocational, and schedule constraints.9,10 Telerehabilitation or home-based CR are suggested as solutions for these patients. Telerehabilitation is defined as the use of digital innovations such as smartphone applications, smartwatches, and teleconsultations to deliver CR from a distance.11 Telerehabilitation enables the remote monitoring of patients and the provision of objective feedback. This could enhance patients’ self-management skills and, subsequently support more sustainable behavioural change.11
Multiple trials have established that telerehabilitation is as effective as centre-based with similar healthcare costs.12 In recent years, the use of digital health-supported remote delivery of CR has gained attention to overcome these barriers and to improve CR access and uptake. Telerehabilitation could be used as add-on to standard CR or even replace conventional in-hospital CR.10 Some small and medium-sized trials already demonstrated that telerehabilitation could be as effective as centre-based CR.13–15 This review aims to give an overview of studies that published economic evaluations of cardiac telerehabilitation such as cost-effectiveness analysis, cost–utility analysis or cost–benefit analysis.
Methods
The review was planned and conducted in accordance with PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines.16
Eligibility criteria
Eligible studies should report findings on the cost-effectiveness of Phase II telerehabilitation of IHD or HF. Telerehabilitation is defined as remotely delivered comprehensive CR delivered with the help of technology. For this systematic review, both comprehensive telerehabilitation as exercise-only CR programmes were included. Furthermore, both telerehabilitation as add-on to standard CR and as replacement of conventional in-hospital rehabilitation were also included. Technologies must be used to offer education, to provide feedback or to monitor patients. Included technologies comprise telephone, text messaging, email, smartphone application, smartwatch or pedometer-based interventions, video consultation, and internet-based interventions. Only peer-reviewed randomized controlled trials (RCTs) comparing telerehabilitation with usual in-centre CR were eligible. The review was limited to articles published in peer-reviewed journals in English. To be eligible, studies had to assess at least one of the following main outcome measures: (i) cost-effectiveness, (ii) cost–utility. Specifically, outcomes of interest from the cost-effectiveness studies included the incremental cost-effectiveness ratio (ICER). The outcome of interest from the cost–utility analysis was cost per quality-adjusted life-year (QALY).
Search methods
For this systematic review, the following literature databases were used: Medline/Pubmed, Web of Science, Scopus, and Embase. All search terms are included in Table 1. Systematic searches were conducted by combining the search terms of category one with category two. Reference lists of key full-text articles included in the review were checked to identify any potentially eligible studies. The searches were not limited by historical time-constraints. The search results were exported to Mendeley where duplicates were excluded. Included studies were manually screened in order to select other relevant studies.
Table 1.
Complete search strategy
| Category 1 | Category 2 | Category 3 |
|---|---|---|
| Telerehabilitation | Stroke | Cost-effectiveness |
| Home exercise training | Heart diseases | Costs |
| Home-based exercise training | Myocardial ischaemia | Financial |
| Home-based cardiac rehabilitation | Coronary disease | Cost |
| Home-based CR | Heart valve disease | Cost analysis |
| Mobile health rehabilitation | Pulmonary heart disease | Budget |
| Telemedicine cardiac rehabilitation | Rheumatic heart disease | Health care cost |
| Heart failure | ||
| Cardiac arrhythmias | ||
| Peripheral arterial disease | ||
| Congenital heart defects | ||
| Brain ischaemia | ||
| Hypoxia–ischaemia brain | ||
| Aortic diseases | ||
| Cardiomyopathies | ||
| Myocarditis | ||
| Thromboembolism | ||
| Venous thromboembolism |
Study selection
As a first step, relevant articles were considered on the basis of their title and abstract. Secondly, full-text versions of selected papers were examined and assessed with regard to effect sizes and methodological quality. The first author oversaw the search strategy and removed duplicates using Mendeley. Following the above inclusion and exclusion criteria, two reviewers (M.S. and M.F.) without consideration for the results performed assessment of studies for potential inclusion independently. Any differences in opinions were resolved by a third independent reviewer. This process ensured that bias is minimized when deciding whether or not to include or exclude certain studies. The two reviewers independently conducted the data extraction from each study.
Data extraction and management
Data were extracted in duplicate by one investigator (M.S.). Domains included: author, year, country of origin, population, outcomes, intervention, comparator, details about costs (included costs, time frame, and currency) and results (both clinical as economic evaluations). Study quality and risk of bias was assessed using the Consensus Health Economic Criteria (CHEC) list, a checklist that can be used to critically appraise published economic evaluations.17 The CHEC list was completed in duplicate (M.S. and M.F.). Differences in opinions were resolved by a third independent reviewer (P.D.) (Annex 1).
Data analysis
This systematic review included several studies with very heterogeneous study designs and sources. Therefore, the results and key information obtained from each of the related articles was synthesized in a narrative summary.
Results
Selection of studies
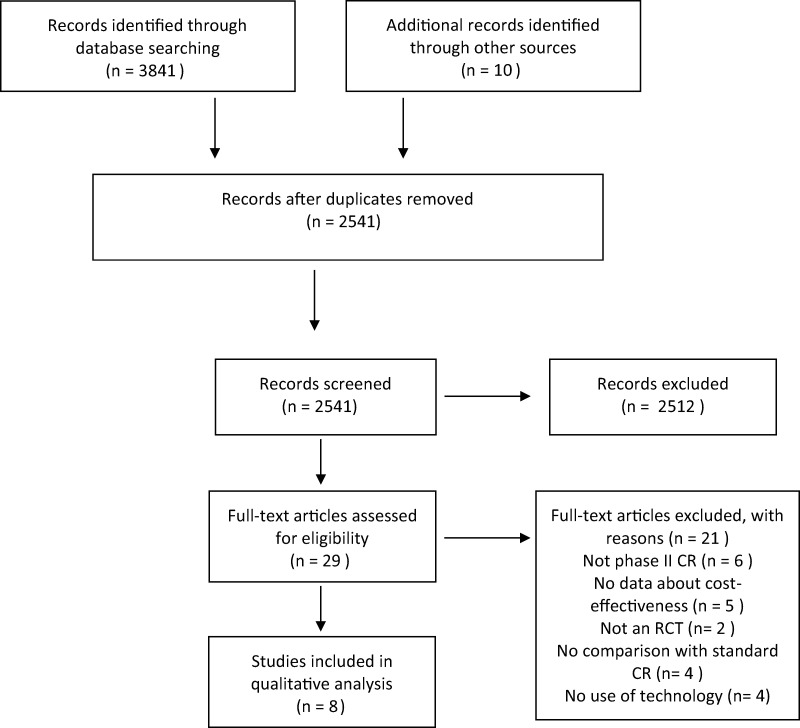
Overall, the search of MEDLINE, Web of Science, EMBASE, and Scopus yielded 3841 articles. A search through other sources identified 10 additional records. Among all, 1010 were identified as duplicates. Therefore, 2841 articles were screened through title and abstract reading. At the end of this process, 29 articles were considered eligible. After full-text assessment, another 21 articles were excluded. There were thus eight articles eligible for this systematic review. These eight studies were RCTs conducted in six countries (Australia, Belgium, Denmark, New Zealand, The Netherlands, and the UK). Details of the study selection are depicted in Figure 1.
Figure 1.
Flowchart of the results of the literature search.16
Characteristics of included studies
The total sample extracted from the eight studies consisted of 751 patients with IHD or HF ranging from a minimum of 46 patients to a maximum of 162 patients per study. The study of Frederix et al. (2017)14 is a long-term analysis of the same population as the study of Frederix et al. (2016).18 Both studies were included in this systematic review.14,18 The mean age of patients ranged from 56 to 67 years. The proportion of males ranged from 75% to 91.3%. Of the eight studies, two were undertaken in Australia,19,20 two in Belgium,14,18 and one each in the UK, Denmark,21 New Zealand,15 and the Netherlands.22 The majority of studies (n = 5) did only include patients with IHD.14,15,18,19,22 Two studies included only HF patients20,23 and one study included HF, IHD, and cardiac surgery patients.21 All but two investigated telerehabilitation as a replacement of conventional in-hospital CR. The studies of Frederix et al.14,18 studied telerehabilitation as an add-on to conventional in-hospital CR.14,18 Quality of life was assessed in six of the included studies with the EuroQol 5D (EQ-5D).14,15,18–20,23 The other two studies used the 36-Item Short Form Health Survey (SF-36).21,22 The included studies had very divergent follow-up duration ranging from 6 months to 5 years. Finally, the study interventions were heterogeneous in duration, technology used and number of contacts with a health professional during the telerehabilitation service. The included costs differed between the eight studies and are summarized in Table 2. Intervention costs such as equipment and staff costs were included in all studies. Most of the studies also included healthcare cost which could include only readmissions or a combination of primary care use, medication cost, specialist visits, and diagnostics. Only one study included non-healthcare cost such as (paid) absenteeism and presenteeism.22 Table 2 provides additional information concerning patient characteristics, interventions, and settings.
Table 2.
Characteristics of included studies
| Author (year), country | Study design and sample population | Intervention | Comparator | Costs included | Economic outcome | Conclusion |
|---|---|---|---|---|---|---|
| Cowie and Moseley23 (2013), UK |
Three group RCT HF patients Mean age 64.3 years M 91.3% 5-year follow-up 46 patients |
Eight-week home-training with a DVD and monitored by a senior CR physiotherapist by telephone (n = 15) |
Eight-week hospital-training delivered as a 1 h, interval training, aerobic circuit class, twice per week (n = 15) Control group: usual care (n = 16) |
Patients included in analysis (n = 46) Intervention (n = 15), hospital (n = 15), control (n = 16)
|
Total cost-avoidance (£) Usual care: 7206.33£ Hospital-training: 38 325.33£ Home-training: 45 531.65£ |
Home-based and centre-based training incurred similar costs. Both had lower emergency admission compared to control. |
| Whittaker and Wade19 (2014), Australia |
Two-arm RCT CAD patients Mean age 56 years M 83% 120 patients |
Six-week home-based CR. Participants in the telehealth group received a mobile phone, Wellness Diary, and a Wellness web portal, with daily text messaging (n = 60) | Six-week hospital-based outpatient CR programme, including gym sessions. Both groups received comprehensive rehabilitative care encompassing exercise, risk modification and mentoring (n = 60) |
|
Total health care cost (AU $) Intervention: $1713 Comparator: $2245 Between-group difference: $532 $2375 per patient savings in healthcare costs |
Cost of delivery by telehealth was slightly lower than centre-based CR. |
| Frederix et al.18 (2015), Belgium |
Single-centre, two-arm RCT CAD patients Mean age 61 years M 82% 1-year follow-up 139 patients |
Six weeks in-centre CR. Thereafter 6 weeks in-centre CR + TeleCR and finally 18 weeks of TELE-CR alone. Patient-specific exercise training prescriptions and self-monitoring of activity by an accelerometer. Uploading of their data on web page with a semi-automatic telecoaching system that provided weekly feedback via email and/ or SMS. Also received weekly dietary and smoking cessation advice by email and/or SMS (n = 69) | Twelve weeks of in-centre CR (n = 70) | Patients included in analysis (n = 139) Intervention (n = 69), control (n = 70)
|
Total health care cost (€) Intervention: €2156 Comparator: €2720 Between-group difference: €-564 ICER: €-21707 per QALY |
Addition of cardiac telerehabilitation to centre-based CR is more cost-effective than centre-based CR alone both after 1 year and 2-year follow-up. |
| Kidholm et al.21 (2016), Denmark |
|
Three-month telerehabilitation programme with one home-visit. Technology used: tablet computer, digital BP device, weight scale, ECG, Fitbit ultra, and follow-up software (n = 72) | The control group followed a traditional rehabilitation programme at the hospital or healthcare centre based on CR guidelines (n = 69) | Patients included in analysis (n = 134) Intervention (n = 68), control (n = 66)
|
|
Mean total cost per patient was €1700 higher in intervention group. QALY gain was higher in intervention group (not statistically significant). CU ratio was >€400 000 per QALY gained. |
| Frederix et al.14 (2017), Belgium |
|
Six weeks in-centre CR + 6 weeks in-centre CR + TeleCR. Patient-specific exercise training prescriptions and self-monitoring of activity by an accelerometer. Uploading of their data on web page with a semi-automatic telecoaching system that provided weekly feedback via email and/ or SMS. Also received weekly dietary and smoking cessation advice by email and/or SMS (n = 62) | Twelve weeks of in-centre CR (n = 64) | Patients included in analysis (n = 134) Intervention (n = 68), control (n = 66)
|
|
See Frederix et al. 2015. |
| Kraal et al.22 (2017), The Netherlands |
|
|
Twelve-week training in the outpatient clinic, supervised by two physical therapists specialized in CR. All patients received an individually tailored training programme on a cycle ergometer and treadmill n = 45) | Patients included in analysis (n = 78) Intervention (n = 37), control (n = 41)
|
|
No differences between telemonitoring and home-based training on physical fitness, physical activity level, or HRQOL. Home-based showed higher patient satisfaction and (not statistically significant) more cost-effectiveness after 1-year follow-up. |
| Hwang et al.20 (2019), Australia |
|
|
Twelve-week centre-based CR programme based on current recommended guidelines encompassing education, aerobic, and strength training exercise (n = 29) | Patients included in analysis (n = 49) Intervention (n = 23), control (n = 26)
|
|
Telerehabilitation for HF patients appeared to be less costly and as effective as centre-based CR. |
| Maddison et al.15 (2019), New Zealand |
|
Twelve weeks of individualized exercise prescription, exercise monitoring, and coaching via a bespoke telerehabilitation platform that comprised a smartphone and chest-worn wearable sensor (bespoke smartphone and web apps and custom middleware form (n = 82) | Twelve weeks of supervised exercise delivered by clinical exercise physiologists in CR clinics (n = 80) | Patients included in analysis (n = 134) Intervention (n = 65), control (n = 69)
|
|
REMOTE-CR is an effective and cost-efficient alternative model after a 2-year follow-up. Could improve overall utilization by increasing reach and satisfying participant preferences. |
CAD, coronary artery disease; CR, cardiac rehabilitation; DVD, Digital Versatile Disc; GP, general practitioner; HF, heart failure; HRQOL, health-related quality of life; ICER, incremental cost-effectiveness ration; M, male; RCT, randomized controlled trial; SMS, short-message-service; UK, United Kingdom.
Quality of included studies
Median quality score of all articles on the CHEC was 89%, the lowest quality score being 68%, whereas none of the studies had a score of 100%.
Narrative review
Cowie and Moseley23 investigated the cost-effectiveness of home-based CR for HF patients in comparison with centre-based CR and a control group in 2013 in the UK. In total, 46 patients finished the complete study period. The telerehabilitation intervention consisted of 8 weeks of home-based training with a Digital Versatile Disc and monitoring by a senior CR physiotherapist by telephone. Cowie and Moseley23 demonstrated that both the home-based and centre-based training programmes incurred similar costs, which were offset by a reduction in emergency admission costs, compared with controls. Although in-hospital training offered greater potential for reducing admission costs, with larger patient numbers the costs of home-training per patient would decrease, increasing its likelihood of being the more cost-effective option.
Whittaker and Wade19 investigated a 6-week home-based CR programme in 2014 in comparison with a standard hospital-based programme in Australia. A total of 120 patients were randomized. Participants in the telehealth group received a mobile phone, a Wellness Diary, and a Wellness web portal, with daily text messaging. Whittaker and Wade19 concluded that cost of delivery by telehealth was slightly lower than for patients attending a rehabilitation service in person both from the provider’s perspective as the patient perspective.
Frederix et al.18 performed a multi-centre RCT investigating telerehabilitation in 2015 and did a new long-term analysis based on the same population in 2017 in Belgium.14 A total of 140 patients were included in the study. Intervention group patients received a 24-week internet-based, comprehensive telerehabilitation programme in addition to the conventional centre-based CR. The programme focused on multiple CR core components and used both physical activity telemonitoring and dietary/smoking cessation/physical activity telecoaching strategies. Intervention group patients were instructed to continuously wear the hip-worn accelerometer and to weekly transmit their registered activity data to the telerehabilitation centre’s local server. These data enabled a semi-automatic telecoaching system to provide the patients with feedback, encouraging them to gradually achieve predefined exercise training goals. Frederix et al.14,18 demonstrated that the addition of cardiac telerehabilitation to conventional centre-based CR to be more cost-effective than centre-based CR alone both after 1-year and 2-year follow-up.
Kidholm et al.21 conducted an RCT in 2016 in Denmark investigating cardiac telerehabilitation in comparison with a traditional rehabilitation programme at the hospital or healthcare centre. A total of 151 patients with a clinical diagnosis of atherosclerosis (myocardial infarction, angina pectoris), coronary artery bypass surgery, valve surgery, and HF were included. The telerehabilitation intervention consisted of a 3-month programme in which the patient received a tablet computer, digital blood pressure device, weight scale, electrocardiogram, a Fitbit Ultra, and follow-up software. Kidholm et al.21 concluded that the mean total cost per patient was €1700 higher in the intervention group compared to the control group. This was mainly caused by the high number of contacts with physiotherapists in the intervention group. The QALYs gain was higher in the intervention group, but this difference was not statistically significant. Therefore, the incremental CU ratio was more than €400 000 per QALY gained.21
In 2017, Kraal et al.22 conducted an RCT in the Netherlands where they investigated home-based training with telemonitoring in comparison with hospital-based CR in 90 patients. Patients in the home-based group received three supervised training sessions in the outpatient clinic, before they continued their training programme in their home environment. In addition, they received a heart rate monitor with a chest strap (Garmin FR70) and were instructed on how to upload recorded heart rate data to a web application. Once a week the patient received feedback on training frequency, duration, and intensity via telephone by the physical therapist. They reported no differences between home-based training with telemonitoring guidance and centre-based training on physical fitness, physical activity level or health-related quality of life. However, home-based training was associated with a higher patient satisfaction and, while not statistically significant, appears to be more cost-effective after 1-year follow-up than centre-based training.22
Hwang et al.20 conducted an RCT in 2019 in Australia where they investigated the cost-effectiveness of telerehabilitation in comparison with centre-based CR in 53 HF patients. The telerehabilitation intervention consisted of 12 weeks of group-based exercise and education delivered into the home via online videoconferencing. Hwang et al.20 revealed that this telerehabilitation approach for HF patients appeared to be less costly and as effective as traditional centre-based rehabilitation.
Maddison et al.15 performed the REMOTE-CR trial in 2019 in New Zealand. They randomized 162 patients to either centre-based CR or telerehabilitation. The telerehabilitation intervention consisted of 12 weeks of individualized exercise prescription, exercise monitoring, and coaching plus theory-based behavioural strategies to promote exercise and habitual physical activity. Participants received a smartphone and chest-worn wearable sensor and received feedback through a smartphone application and direct messaging. They concluded that REMOTE-CR is an effective and cost-efficient alternative model after a 2-year follow-up that could potentially improve overall utilization rates by increasing reach and satisfying unique participant preferences.15
Discussion
This systematic review evaluates the cost-effectiveness of cardiac telerehabilitation interventions in comparison with centre-based CR. The majority of studies were conducted in a population of IHD patients. Two papers investigated telerehabilitation in HF patients and one paper included patients with a wide array of cardiovascular disease. First thing to notice is the heterogeneity between all telerehabilitation interventions. The trials strongly differed in duration, used technology, cost included, and follow-up. This makes generalization of the results difficult. There is also high heterogeneity in the delivery of CR components. Some studies only focus on exercise where other studies also focus on all core components of CR.7 Important to mention is that telerehabilitation is more than the remote delivery of exercise training. Telerehabilitation is defined as the remote provision of the core components of CR. The use of digital technology enables more personalization of the CR content to the preferences of the patient. This could possibly make CR more feasible and attractive for subgroups who are currently underrepresented in CR (women, elderly, low educated patients). In the future, more research is needed of comprehensive and personalized remote CR services. A total of seven of the eight included studies demonstrated that telerehabilitation could lead to similar or lower long-term costs as centre-based CR programmes with equal or superior clinical effects. The trial of Kidholm et al.21 was the only one that concluded that telerehabilitation was not cost-effective in comparison with centre-based CR. They revealed an incremental cost ratio of more than 500 000 Euro per QALY. This result could be explained by the high intervention cost control and no significant impact on the quality of life in comparison with the control group. Another potential explanation is the fact that only hospital visits related to heart disease were included in the estimation. However, it is possible that the added costs of telerehabilitation may result in other desirable outcomes that were not considered in the current study. Furthermore, the potential savings for the patient and for the municipalities related to the possible improvement in the patients’ quality of life were not taken into account. There is also the possibility that the volume and intensity of the CR components was too low. Both volume and intensity have a significant impact on quality of life and exercise capacity.24
An important note is that the economics of telerehabilitation will probably be very different in a real-world setting because upscaling can lead to reduced costs.
There are several limitations to this systematic review. Most studies were limited by small sample sizes. The heterogeneity of the studies in terms of duration of telerehabilitation services, the technology used, telerehabilitation as add-on or as a replacement of in-hospital CR, use of home-visits, the costs collected and the follow-up time for collection of outcomes and costs is another limitation. Finally, most trials are performed in Northwest Europe or Oceania which could reduce the generalizability to low/middle-income countries, despite 80% of cardiovascular-related deaths occurring in these countries.25 In conclusion, based on these small short duration trials, telerehabilitation may be as cost-effective as traditional centre-based approaches. However, more assessments of the value for money of telerehabilitation in larger and longer RCTs are needed both in high- as low-income countries.
Data availability
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Conflict of interest: none declared.
Annex 1 CHEC list
| CHEC list | Cowie23 | Whittaker19 | Frederix18 | Kidholm21 | Frederix14 | Kraal22 | Hwang20 | Maddison15 | |
|---|---|---|---|---|---|---|---|---|---|
| 1. | Is the study population clearly described? | X | X | X | X | X | X | X | X |
| 2. | Are competing alternatives clearly described? | X | X | X | X | X | X | X | |
| 3. | Is a well-defined research question posed in answerable form? | X | X | X | X | X | X | X | X |
| 4. | Is the economic study design appropriate to the stated objective? | X | X | X | X | X | X | X | X |
| 5. | Is the chosen time horizon appropriate in order to include relevant costs and consequences? | X | X | X | X | X | X | X | X |
| 6. | Is the actual perspective chosen appropriate? | X | X | X | X | X | X | X | X |
| 7. | Are all important and relevant costs for each alternative identified? | X | X | X | X | X | X | X | X |
| 8. | Are all costs measured appropriately in physical units? | X | X | X | X | X | X | X | X |
| 9. | Are costs valued appropriately? | X | X | X | X | X | X | X | |
| 10. | Are all important and relevant outcomes for each alternative identified? | X | X | X | X | X | X | X | X |
| 11. | Are all outcomes measured appropriately? | X | X | X | X | X | X | X | X |
| 12. | Are outcomes valued appropriately? | X | X | X | X | X | X | X | X |
| 13. | Is an incremental analysis of costs and outcomes of alternatives performed? | X | X | X | X | X | X | ||
| 14. | Are all future costs and outcomes discounted appropriately? | X | |||||||
| 15. | Are all important variables, whose values are uncertain, appropriately subjected to sensitivity analysis? | X | X | X | X | X | |||
| 16. | Do the conclusions follow from the data reported? | X | X | X | X | X | X | X | X |
| 17. | Does the study discuss the generalizability of the results to other settings and patient/client groups? | X | X | X | X | X | X | X | |
| 18. | Does the article indicate that there is no potential conflict of interest of study researcher(s) and funder(s)? | X | X | X | X | X | X | X | |
| 19. | Are ethical and distributional issues discussed appropriately? | X | X | X | |||||
| Percentage | 95% | 68% | 89% | 84% | 89% | 89% | 84% | 95% |
References
- 1.Wilkins E, Wilson L, Wickramasinghe K, Bhatnagar P, Leal J, Luengo-Fernandez R, Burns R, Rayner M, Townsend N. European Cardiovascular Disease Statistics 2017. Brussels: European Heart Network; 2017. http://www.ehnheart.org/images/CVD-statistics-report-August-2017.pdf. [Google Scholar]
- 2.Busse R, Blümel M, Scheller-Kreinsen D, Zentner A. Tackling Chronic Disease in Europe Strategies, Interventions and Challenges. Berlin: WHO, 2010. [Google Scholar]
- 3.Piepoli MF, Hoes AW, Agewall SAlbus C, Brotons C, Catapano AL, Cooney MT, Corrà U, Cosyns B, Deaton C, Graham I, Hall MS, Hobbs FDR, Løchen ML, Löllgen H, Marques-Vidal P, Perk J, Prescott E, Redon J, Richter DJ, Sattar N, Smulders Y, Tiberi M, van der Worp HB, van Dis I, Verschuren WMM, Binno S. ESC Scientific Document Group, 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J 2016;37:2315–2381. [Google Scholar]
- 4.Salzwedel A, Jensen K, Rauch B, Doherty P, Metzendorf MI, Hackbusch M, Völler H, Schmid JP, Davos CH. Effectiveness of comprehensive cardiac rehabilitation in coronary artery disease patients treated according to contemporary evidence based medicine: Update of the Cardiac Rehabilitation Outcome Study (CROS-II). Eur J Prev Cardiol 2020;27:1756–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shields GE, Wells A, Doherty P, Heagerty A, Buck D, Davies LM. Cost-effectiveness of cardiac rehabilitation: a systematic review. Heart 2018;104:1403–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Volterrani M, Iellamo F.. Cardiac rehabilitation in patients with heart failure: new perspectives in exercise training. Card Fail Rev 2016;2:63–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ambrosetti M, Abreu A, Corrà U, Davos CH, Hansen D, Frederix I, Iliou MC, Pedretti RF, Schmid JP, Vigorito C, Voller H, Wilhelm M, Piepoli MF, Bjarnason-Wehrens B, Berger T, Cohen-Solal A, Cornelissen V, Dendale P, Doehner W, Gaita D, Gevaert AB, Kemps H, Kraenkel N, Laukkanen J, Mendes M, Niebauer J, Simonenko M, Zwisler AOet al. Secondary prevention through comprehensive cardiovascular rehabilitation: from knowledge to implementation. 2020 update. A position paper from the Secondary Prevention and Rehabilitation Section of the European Association of Preventive Cardiology. Eur J Prev Cardiol 2020: doi: 10.1177/2047487320913379. Epub ahead of print. PMID: 32223332. [DOI] [PubMed] [Google Scholar]
- 8.Kotseva K, De Backer G, De Bacquer D, Rydén L, Hoes A, Grobbee D, Maggioni A, Marques-Vidal P, Jennings C, Abreu A, Aguiar C, Badariene J, Bruthans J, Castro Conde A, Cifkova R, Crowley J, Davletov K, Deckers J, De Smedt D, De Sutter J, Dilic M, Dolzhenko M, Dzerve V, Erglis A, Fras Z, Gaita D, Gotcheva N, Heuschmann P, Hasan-Ali H, Jankowski P, Lalic N, Lehto S, Lovic D, Mancas S, Mellbin L, Milicic D, Mirrakhimov E, Oganov R, Pogosova N, Reiner Z, Stöerk S, Tokgözoğlu L, Tsioufis C, Vulic D, Wood D; EUROASPIRE Investigators. Lifestyle and impact on cardiovascular risk factor control in coronary patients across 27 countries: results from the European Society of cardiology ESC-EORP EUROASPIRE V registry. Eur J Prev Cardiol 2019;26:824–835. [DOI] [PubMed] [Google Scholar]
- 9.Frederix I, Vanhees L, Dendale P, Goetschalckx K. A review of telerehabilitation for cardiac patients. J Telemed Telecare 2015;21:45–53. [DOI] [PubMed] [Google Scholar]
- 10.Neubeck L, Freedman SB, Clark AM, Bauman A, Redfern J. Participating in cardiac rehabilitation: a systematic review and meta-synthesis of qualitative data. Eur J Prev Cardiol 2012;19:494–503. [DOI] [PubMed] [Google Scholar]
- 11.Scherrenberg M, Wilhelm M, Hansen D, et al. The future is now: a call for action for cardiac telerehabilitation in the COVID-19 pandemic from the secondary prevention and rehabilitation section of the European Association of Preventive Cardiology. Eur J Prev Cardiol 2020; doi: 10.1177/2047487320939671. Epub ahead of print. PMID: 32615796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taylor RS, Dalal H, Jolly K, Moxham T, Zawada A.. Home-based versus centre-based cardiac rehabilitation. Cochrane Database Syst Rev 2010;1:CD007130. Update in: Cochrane Database Syst Rev 2015;8:CD007130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frederix I, Hansen D, Coninx K, Vandervoort P, Vandijck D, Hens N, Van Craenenbroeck E, Van Driessche N, Dendale P. Medium-term effectiveness of a comprehensive internet-based and patient-specific telerehabilitation program with text messaging support for cardiac patients: randomized controlled trial. J Med Internet Res 2015;17:e185. doi: 10.2196/jmir.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frederix I, Solmi F, Piepoli MF, Dendale P.. Cardiac telerehabilitation: a novel cost-efficient care delivery strategy that can induce long-term health benefits. Eur J Prev Cardiol 2017;24:1708–1717. [DOI] [PubMed] [Google Scholar]
- 15.Maddison R, Rawstorn JC, Stewart RAH, Benatar J, Whittaker R, Rolleston A, Jiang Y, Gao L, Moodie M, Warren I, Meads A, Gant N.. Effects and costs of real-time cardiac telerehabilitation: randomised controlled non-inferiority trial. Heart 2019;105:122–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. doi:10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evers S, Goossens M, de Vet H, van Tulder M, Ament A. Criteria list for assessment of methodological quality of economic evaluations: Consensus on Health Economic Criteria. Int J Technol Assess Health Care 2005;21:240–245. [PubMed] [Google Scholar]
- 18.Frederix I, Hansen D, Coninx K, Vandervoort P, Vandijck D, Hens N, Van Craenenbroeck E, Van Driessche N, Dendale P. Effect of comprehensive cardiac telerehabilitation on one-year cardiovascular rehospitalization rate, medical costs and quality of life: a cost-effectiveness analysis. Eur J Prev Cardiol 2016;23:674–682. [DOI] [PubMed] [Google Scholar]
- 19.Whittaker F, Wade V.. The costs and benefits of technology-enabled, home-based cardiac rehabilitation measured in a randomised controlled trial. J Telemed Telecare 2014;20:419–422. [DOI] [PubMed] [Google Scholar]
- 20.Hwang R, Morris NR, Mandrusiak A, Bruning J, Peters R, Korczyk D, Russell T. Cost-utility analysis of home-based telerehabilitation compared with centre-based rehabilitation in patients with heart failure. Heart Lung Circ 2019;28:1795–1803. [DOI] [PubMed] [Google Scholar]
- 21.Kidholm K, Rasmussen MK, Andreasen JJ, Hansen J, Nielsen G, Spindler H, Dinesen B. Cost-utility analysis of a cardiac telerehabilitation program: the teledialog project. Telemed J E Health 2016;22:553–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kraal JJ, Van den Akker-Van Marle ME, Abu-Hanna A, Stut W, Peek N, Kemps HM. Clinical and cost-effectiveness of home-based cardiac rehabilitation compared to conventional, centre-based cardiac rehabilitation: Results of the FIT@Home study. Eur J Prev Cardiol 2017;24:1260–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cowie A, Moseley O.. Home- versus hospital-based exercise training in heart failure: an economic analysis. Br J Cardiol 2014;21:76. [Google Scholar]
- 24.Keech A, Holgate K, Fildes J, Indraratna P, Cummins L, Lewis C, Yu J. High-intensity interval training for patients with coronary artery disease: Finding the optimal balance. Int J Cardiol 2020;298:8–14. [DOI] [PubMed] [Google Scholar]
- 25.Fuster V. Global burden of cardiovascular disease: time to implement feasible strategies and to monitor results. J Am Coll Cardiol 2014;64:520–522. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Conflict of interest: none declared.