Abstract
Background and purpose
A clinical risk score for sudden unexpected death in epilepsy (SUDEP) in patients with drug‐resistant focal epilepsy could help improve prevention.
Methods
A case–control study was conducted including (i) definite or probable SUDEP cases collected by the French National Sentinel Mortality Epilepsy Network and (ii) control patients from the French national research database of epilepsy monitoring units. Patients with drug‐resistant focal epilepsy were eligible. Multiple logistic regressions were performed. After sensitivity analysis and internal validation, a simplified risk score was developed from the selected variables.
Results
Sixty‐two SUDEP cases and 620 controls were included. Of 21 potential predictors explored, seven were ultimately selected, including generalized seizure frequency (>1/month vs. <1/year: adjusted odds ratio [AOR] 2.6, 95% confidence interval [CI] 1.25–5.41), nocturnal or sleep‐related seizures (AOR 4.49, 95% CI 2.68–7.53), current or past depression (AOR 2.0, 95% CI 1.19–3.34) or the ability to alert someone of an oncoming seizure (AOR 0.57, 95% CI 0.33–0.98). After internal validation, a clinically usable score ranging from −1 to 8 was developed, with high discrimination capabilities (area under the receiver operating curve 0.85, 95% CI 0.80–0.90). The threshold of 3 has good sensitivity (82.3%, 95% CI 72.7–91.8), whilst keeping a good specificity (82.7%, 95% CI 79.8–85.7).
Conclusions
These results outline the importance of generalized and nocturnal seizures on the occurrence of SUDEP, and show a protective role in the ability to alert someone of an oncoming seizure. The SUDEP‐CARE score is promising and will need external validation. Further work, including paraclinical explorations, could improve this risk score.
Keywords: case–control, epilepsy, risk score, sudden death, SUDEP
The SUDEP‐CARE score was developed to target the most at‐risk patients who suffer from drug‐resistant focal epilepsy. The higher the score is, the more is the risk of a sudden unexpected death in epilepsy event.
INTRODUCTION
Sudden unexpected death in epilepsy (SUDEP) is a non‐traumatic and non‐drowning death in patients with epilepsy. It is unrelated to the documented status epilepticus [1]. In adults, the incidence of SUDEP is estimated at 1.2/1000 patients per year [2], increasing to 9.7/1000 in candidates for epilepsy surgery [3, 4]. Generalized tonic–clonic seizures (GTCSs) represent a major risk factor [5, 6], as does intellectual disability (ID), with four to five times more SUDEP in patients with ID in addition to their epilepsy [7, 8, 9]. Similarly, nocturnal or sleep‐related seizures have recently been shown to have a three to 15 times higher risk of SUDEP [6, 10]. Comorbid depression [11, 12] might also be associated with an increased risk of SUDEP [13]. Other factors, such as the duration of epilepsy or the number of anti‐seizure medications prescribed, have been proposed as risk factors [14, 15] but with a low level of evidence [7, 13, 16].
Some recommendations advocate an honest and balanced discussion with patients and their families about the risk of SUDEP. It is suggested that this discussion should take place at the time of diagnosis [2], that it should be based on a scientific rationale and that every effort should be made to minimize the occurrence of GTCSs. However, physicians are sometimes reluctant to discuss this risk, and effective tools helping to focus on prevention efforts could improve daily care. Recent work aimed at stratifying the risk of SUDEP have shown interesting results [17, 18].
Here, the aim was to identify the clinical risk factors of SUDEP from two French national databases, the register of epilepsy‐related deaths (RSME) and the database of the epilepsy monitoring units (EMUs), to develop a risk score focused on adult patients with drug‐resistant focal epilepsy, a high‐risk population for SUDEP.
METHODS
Design
In this case–control study, the SUDEP cases were ascertained between 2010 and 2019 from the national registry of SUDEP (Réseau Sentinelle Mortalité Epilepsie, RSME [19, 20]). This programme, set up under the aegis of the French League Against Epilepsy and with the support of the French Foundation for Epilepsy Research, relies on a network of medical correspondents including all centres specialized in the exploration and treatment of epilepsy in France (N = 16 EMUs). Controls were recruited between 2010 and 2014 from a French national research database of patients being explored for drug‐resistant focal epilepsy in the same 16 EMUs. To maximize power, 10 controls were randomly selected for each SUDEP case.
Patients aged 15 years and above with focal and drug‐resistant epilepsy, according to the International League Against Epilepsy definition, were included [21]. SUDEP cases were determined according to the Nashef definition [1], and only definite or probable SUDEP cases were included.
Sudden unexpected death in epilepsy ascertainment
Sudden unexpected death in epilepsy cases were reported by neurologists, bereaved families or general practitioners. After family consent, a standardized form including detailed circumstances of death, obvious or secondary causes, medical history and comorbidities, type of seizures, frequency of seizures, anti‐seizure medication and adherence to anti‐seizure medication was completed and sent to the network coordinator. Bereaved families were interviewed by a psychologist following the recommendations made by the Institut National de la Santé et de la Recherche Médicale (INSERM) Collective Expertise for verbal autopsy [22]. The interviews made it possible to acquire detailed data regarding the context of death, the deceased's lifestyle and any particular events before the death. Relatives were also questioned about the personal family history and comorbidities of the deceased subject, the potential aetiology of the epilepsy, the description and frequency of the different types of seizures, the peri‐ictal symptoms, the current and past treatments, neurological medical monitoring, and factors of potential risk of SUDEP already identified in the literature. Autopsy reports were collected if performed. Each patient file, medical history, death circumstance and autopsy report when available were evaluated by an expert committee (composed of one paediatric neurologist, two neurologists and one epidemiologist) to validate the cause of death and the level of certainty.
Medical data collection
For each case and control, medical data were collected from the patients' medical records from the EMUs in which they underwent epilepsy diagnosis and follow‐up. For SUDEP cases, observations available were updated during an interview with the relatives of the deceased patient. The information available was previously published [19] and is summarized in Table S1. Of note are the following.
Patients were considered as suffering from depression if there was a prescription of an antidepressant or if the diagnosis was made by the major depressive disorder section of the MINI questionnaire [23]. A medical history of depression was considered if patients had previously been treated or diagnosed for depression as specified in their medical record, or had been diagnosed with the MINI (lifetime disorder).
For sleep apnoea syndrome (SAS), a medical history of SAS or a history/or current treatment in their medical record was considered.
The occurrence of peri‐ or post‐ictal events during seizures was systematically investigated by questionnaire. A peri‐ictal respiratory symptom was considered present when dyspnoea or cyanosis were reported by the patient or by witnesses. Peri‐ictal dysphoric symptoms [24] (irritability, mood change) during or close to the seizure were also recorded, as well as seizure‐related falls and the ability to alert someone of an oncoming seizure.
Statistical analysis
A comparison of cases and controls on complete data was carried out. Quantitative variables are presented with their mean and standard deviation or median with interquartile range, and qualitative variables with their frequency and associated proportions. The appropriate comparison tests were used depending on the type of variable and whether the validity conditions were met or not.
In the multivariate analyses, missing data were addressed by multiple imputation [25]. As recommended in the literature, 10 imputed datasets were created, and all analysed variables were used for the imputations [26]. Selection of variables was carried out through backward step‐by‐step multivariate logistic regression: all variables were included, and the step that presented the minimum Akaïke criterion in each imputation was kept as reference for variable selection. A final model was created with the most robust variables—those retained in at least eight of the 10 selection processes.
To limit overfitting, internal validation of the model was performed by the bootstrap method [27]. Random draw with replacement was carried out with a 1:1 rate, and 200 samples were drawn. Logistic regression was then performed on the 200 samples to build 200 bootstrap models. The optimal was calculated, corresponding to the average difference between the area under the curve of the bootstrap models applied to the bootstrapped samples and the area under the curve of the bootstrap models applied to the original dataset. A shrinkage coefficient was also calculated as the slope of observed versus predicted probabilities for each bootstrap model applied to the initial dataset [28]. The process was repeated on each of the imputed datasets; then a global analysis of imputations was carried out according to Rubin's rules [29, 30].
Sensitivity analyses
A model adjusted on other potential predictors that were not initially selected was also established for comparison. To explore potential bias from missing data on ID, a model in which missing information on ID was imputed as no ID was also performed.
SUDEP‐CARE score
A simplified model was then developed for clinical use. To simplify its use, the model was based on the rounded values of corrected estimates before exponential transformation. The discriminative abilities of this score after internal validation are presented through the area under the receiver operating curve (AUROC) as well as the sensitivity and specificity of different risk thresholds. Calibration of the score was explored through observed probabilities of SUDEP at each cut‐off and Brier score. Percentages of SUDEP in each score group are also presented with 95% confidence intervals (CIs) obtained through bootstrap.
All statistical analyses were performed using SAS 9.4 software.
Standard protocol approvals, registrations and patient consents
This study was approved by the institutional review board of Lyon University Hospital and by the national committee authorizing data collection (CNIL no. DR‐2010‐3030). Non‐opposition from the deceased's families was obtained as well as written informed consent from the controls (CPP Sud Est II no. 2010‐006‐AM6).
RESULTS
Through the RSME, 245 SUDEP were reported, of which 120 were definite or probable SUDEP cases. Fifty‐eight did not meet the eligibility criteria because they had drug‐sensitive epilepsy (n = 11) or did not suffer from focal epilepsy (n = 47). For each of the 62 SUDEP cases included, 10 controls, that is, a total of 620 controls with drug‐resistant focal epilepsy, were included. The flow chart is shown in Figure 1.
FIGURE 1.
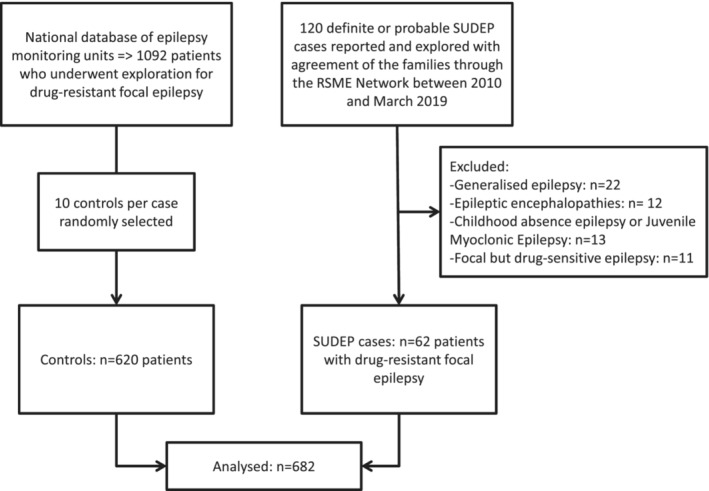
Flow chart
Initial comparison on complete data
Comparisons between cases and controls on complete data are shown in Table 1. The most important differences were found for sleep‐related or nocturnal seizures, and for the frequency of GTCSs. Patients who died of SUDEP had a higher rate of ID than the controls. Current or past depression and SAS were more common in the SUDEP cases. Per‐ or post‐critical events were significantly different between groups, with more peri‐ictal respiratory symptoms in the SUDEP cases (dyspnoea, cyanosis or desaturation) as well as more peri‐ictal dysphoric symptoms and seizure‐related falls. Being able to alert others of an oncoming seizure was less common in SUDEP cases.
TABLE 1.
Cases and control comparisons on complete data
| N | SUDEP cases | Controls | p value | |
|---|---|---|---|---|
| Sociodemographic characteristics | ||||
| Sex (men) | 682 | 32 (51.6%) | 322 (51.9%) | 0.97 |
| Age (years), mean (±SD) | 682 | 33.0 ± 11.6 | 35.2 ± 12.2 | 0.17 |
| Body mass index, median (Q1; Q3) | 644 | 22.2 (20.7; 25.9) | 23.4 (20.8; 26.2) | 0.41 |
| Comorbidities | ||||
| Intellectual disability | 441 | 16 (27.6%) | 48 (13.6%) | 0.006 |
| Cardiovascular comorbidities | 671 | 6 (9.7%) | 35 (5.7%) | 0.26 |
| Current depression | 671 | 15 (25.9%) | 97 (15.8%) | 0.05 |
| Current or past depression | 664 | 30 (51.7%) | 169 (27.9%) | <0.001 |
| History of sleep apnoea syndrome | 664 | 7 (11.5%) | 22 (3.6%) | 0.01 |
| Treatments | ||||
| Current number of anti‐seizure medications | 675 | 0.48 | ||
| 0–1 a | 14 (22.6%) | 104 (17.0%) | ||
| 2 | 32 (51.6%) | 320 (52.2%) | ||
| 3 or more | 16 (25.8%) | 189 (30.8%) | ||
| Other psychotropic drugs | 663 | 7 (12.3%) | 89 (14.7%) | 0.62 |
| Epilepsy characteristics | ||||
| Epilepsy duration (years), median (Q1; Q3) | 661 | 19.1 (10; 28.9) | 16.1 (9.5; 26.7) | 0.20 |
| Epilepsy duration (20 years or more) | 661 | 29 (49.1%) | 230 (38.4%) | 0.20 |
| Focal seizure frequency | 628 | |||
| Less than 1 per month | 12 (23.5%) | 91 (15.8%) | 0.21 | |
| From 1 per month to 1 per week | 12 (23.5%) | 192 (33.3%) | ||
| More than 1 per week | 27 (52.9%) | 294 (51.0%) | ||
| Generalized tonic–clonic seizure (GTCS) frequency | 653 | |||
| Less than 1 per year | 14 (24.1%) | 386 (64.9%) | <0.001 | |
| From 1 per year to 1 per month | 25 (43.1%) | 162 (27.2%) | ||
| More than 1 per month | 19 (32.8%) | 47 (7.9%) | ||
| Nocturnal seizures | 657 | 43 (69.4%) | 128 (21.5%) | <0.001 |
| Able to alert someone of an oncoming seizure | 636 | 17 (27.9%) | 260 (45.2%) | 0.0094 |
| Per‐ or post‐seizure respiratory symptoms | 647 | 25 (45.5%) | 81 (13.7%) | <0.001 |
| Per‐ or post‐seizure dysphoric symptoms | 656 | 13 (24.1%) | 64 (10.6%) | 0.003 |
| Seizure‐related falls | 644 | 35 (57.4%) | 154 (26.4%) | <0.001 |
| Going into the ‘prone position’ during seizure | 605 | 3 (7.1%) | 17 (3.0%) | 0.16 |
| Repeated seizure | 666 | 24 (38.7%) | 209 (34.6%) | 0.52 |
| Epilepsy surgery | 682 | 8 (12.9%) | 67 (10.8%) | 0.61 |
Only one patient did not have any anti‐epileptic drug treatment (refusal by the patient).
The rate of previous epilepsy surgery was comparable between the two groups, as was the treatment profile, the number of anti‐seizure medications used and the presence of other psychotropic drugs.
SUDEP‐CARE creation process
In the multivariate analysis (Table 2), seven variables were ultimately selected according to the 10 imputation models (Table S2): frequency of GTCSs, nocturnal or sleep‐related convulsive seizures, ability to alert others of an oncoming seizure, current or past depression, seizure‐related falls, per‐ or post‐critical respiratory symptoms, and ID.
TABLE 2.
Univariate and multivariate logistic regressions before and after bootstrap validation
| Univariate | Multivariate | After bootstrap shrinkage a | |
|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Generalized seizure frequency | |||
| Less than 1 per year | 1 (ref) | 1 (ref) | 1 (ref) |
| Between 1 per year and 1 per month | 3.87 (2–7.47) | 2.16 (1.01–4.6) | 1.86 (1.01–3.44) |
| More than 1 per month | 9.24 (4.4–19.39) | 3.26 (1.32–8.06) | 2.6 (1.25–5.41) |
| Intellectual disability | 2.1 (1.12–3.94) | 1.91 (0.84–4.33) | 1.69 (0.87–3.27) |
| Current or past depression | 2.65 (1.52–4.64) | 2.35 (1.24–4.44) | 2 (1.19–3.34) |
| Respiratory symptoms during or after seizure | 4.48 (2.55–7.85) | 1.87 (0.94–3.73) | 1.66 (0.95–2.9) |
| Nocturnal seizure | 8.18 (4.61–14.52) | 6.41 (3.39–12.13) | 4.49 (2.68–7.53) |
| Able to alert someone of an oncoming seizure | 0.5 (0.28–0.89) | 0.5 (0.26–0.97) | 0.57 (0.33–0.98) |
| Seizure‐related falls | 3.59 (2.09–6.18) | 2.08 (1.07–4.03) | 1.81 (1.06–3.09) |
Abbreviations: CI, confidence interval; OR, odds ratio.
Model 1 was used and the shrinkage coefficient given after bootstrap validation was applied.
To avoid overfitting and to allow for a better generalizability of the score, internal validation by the bootstrap method was performed. Corrected estimates are presented in Table 2, and the AUROC after bootstrap was 0.81 (95% CI 0.75; 0.86).
Sensitivity analysis
A second multivariate model, adjusted for age at inclusion or age at death and for duration of epilepsy, showed very similar results (Table 3).
TABLE 3.
Sensitivity analysis
| Multivariate analysis adjusted for age and epilepsy duration (N = 682) | Intellectual disability imputed at ‘no’ when missing a (n = 682) | Complete data and intellectual disability imputed at ‘no’ when missing a (n = 590, 48 cases and 542 controls) | |
|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Generalized seizure frequency | |||
| Less than 1 per year | 1 (ref) | 1 (ref) | 1 (ref) |
| Between 1 per year and 1 per month | 2.22 (1.02–4.79) | 2.15 (0.95–4.86) | 2.21 (0.92–5.34) |
| More than 1 per month | 3.38 (1.35–8.48) | 3.25 (1.32–8) | 3.77 (1.4–10.17) |
| Intellectual disability | 1.9 (0.81–4.46) | 3.81 (1.67–8.69) | 5.57 (2.18–14.22) |
| Current or past depression | 2.29 (1.18–4.46) | 2.37 (1.27–4.41) | 3.04 (1.46–6.31) |
| Respiratory symptoms during or after seizure | 1.93 (0.98–3.82) | 1.91 (0.96–3.81) | 1.91 (0.88–4.17) |
| Nocturnal seizure | 6.41 (3.33–12.35) | 6.85 (3.57–13.16) | 9.67 (4.34–21.54) |
| Able to alert someone of an oncoming seizure | 0.47 (0.24–0.91) | 0.5 (0.26–0.98) | 0.55 (0.26–1.19) |
| Seizure‐related falls | 2 (1.03–3.91) | 2.04 (1.06–3.94) | 1.96 (0.91–4.21) |
Abbreviations: CI, confidence interval; OR, odds ratio.
Adjusted for age and epilepsy duration.
Due to the disparity in the level of missing data for ID (6% of cases vs. 43% in the control population), missing data were considered to correspond to the absence of ID. This probably better reflects reality than models on complete or imputed data. These high levels most probably come from an underreporting of people without ID in the medical report. The results of this model, executed with multiple imputations and on complete data, are shown in Table 3. The selection process led to the same results (Table S2). As expected, the effect of ID increased (from 1.91 [0.84; 4.33] to 3.81 [1.67; 8.69]), whilst keeping other risk factor estimates close to those of the initial model.
Simplified risk stratification score: SUDEP‐CARE (CARE for ClinicAl Risk scorE)
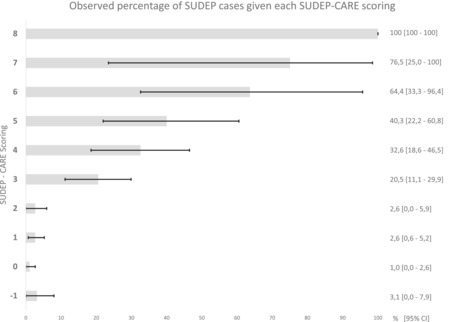
In order to be used in clinical practice, the proposed score must be simple and quick to perform. Rounded values of the parameter estimates were therefore used to construct the SUDEP‐CARE score, as shown in Table 4. The total score can be easily calculated by adding the corresponding point(s) of each risk factor present for a given patient. As expected, discrimination capabilities of the created risk score remained very good, with an AUROC of 0.81 (95% CI 0.75; 0.86) after correction (Figure S1). Comparison with a baseline model including only the frequency of GTCSs (AUROC 0.73 [95% CI 0.66–0.79]) showed significant improvement (p value 0.0002). Calibration was good as shown by Figure 2, which displays observed percentages of SUDEP cases in each score group, or with the Brier score of 0.06. The sensitivity and specificity of the different thresholds are presented in Table 5.
TABLE 4.
SUDEP‐CARE, for SUDEP ClinicAl Risk scorE
| Risk factors | Point(s) if yes | Score |
|---|---|---|
| Generalized tonic–clonic seizure frequency in the last year | ||
| <1 per year | 0 | |
| Between 1 per month and 1 per year | 1 | |
| >1 per month | 2 | |
| Presence of intellectual disability | 1 | |
| Current or past depressive disorder | 1 | |
| Respiratory symptoms during or after seizure | 1 | |
| Sleep‐related or nocturnal seizures | 2 | |
| Able to alert someone of an oncoming seizure | –1 | |
| Seizure‐related falls | 1 | |
| Total score (sum) | ||
FIGURE 2.
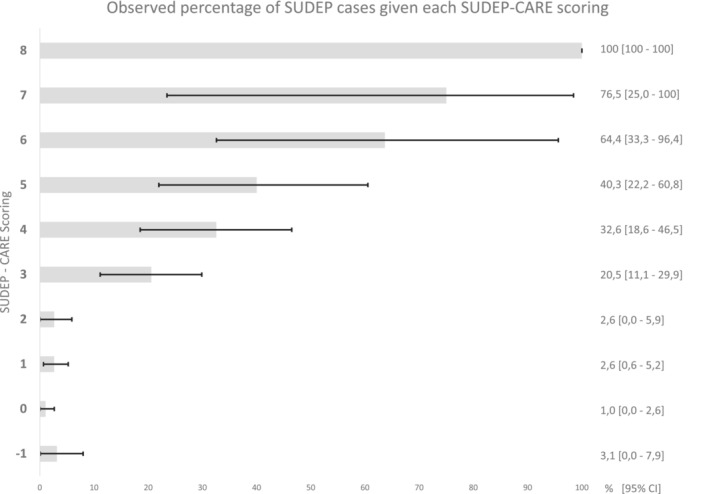
Probabilities of SUDEP observed in each score group
TABLE 5.
SUDEP‐CARE score threshold characteristics
| Thresholds | Sensitivity (95% CI) | Specificity (95% CI) | Estimated probability of SUDEP a |
|---|---|---|---|
| 8 | 3.2 (0–7.6) | 100 (100–100) | 100 (100–100) |
| 7 | 8.1 (1.3–14.8) | 99.8 (99.5–100) | 33.6 (7.5–48.2) |
| 6 | 19.4 (9.5–29.2) | 99.2 (98.5–99.9) | 19.5 (10.7–26.8) |
| 5 | 35.5 (23.6–47.4) | 96.8 (95.4–98.2) | 10.0 (6.9–12.9) |
| 4 | 58.1 (45.8–70.3) | 92.1 (90–94.2) | 6.9 (5.5–8.2) |
| 3 | 82.3 (72.7–91.8) | 82.7 (79.8–85.7) | 4.6 (4.1–5.1) |
| 2 | 87.1 (78.8–95.4) | 64.8 (61.1–68.6) | 2.4 (2.2–2.7) |
| 1 | 93.5 (87.4–99.7) | 41.0 (37.1–44.8) | 1.6 (1.5–1.7) |
| 0 | 96.8 (92.4–100) | 10.0 (7.6–12.4) | 1.1 (1.0–1.1) |
| −1 | 100 (100–100) | 0 (0–0) | 1.0 (1.0–1.0) |
Abbreviations: CI, confidence interval; SUDEP, sudden unexpected death in epilepsy.
Positive predictive value was estimated per year for an incidence of SUDEP of 10 per 1000 person years.
Prioritizing the sensitivity of SUDEP‐CARE using a threshold of 1 enabled the detection of 93.5% of SUDEP cases (sensitivity with 95% CI 87.4%; 99.7%). However, this would only allow 41.0% (95% CI 37.1%; 44.8%) of patients (specificity) to be classified as having a low SUDEP risk. To maximize sensitivity and specificity, a threshold of 3 is preferable with a sensitivity of 82.3% (95% CI 72.7%; 91.8%) and a specificity of 82.7% (95% CI 79.8%; 85.7%). Probabilities of SUDEP in each score group are presented in Figure 2.
DISCUSSION
The SUDEP‐CARE score
Our objective was to develop a clinically applicable score that stratifies patients according to their risk of SUDEP in a high‐risk population. The recently developed SUDEP‐CARE identified risk factors such as depression or nocturnal seizure, and was developed keeping only the most clinically relevant and statistically robust variables. It underwent thorough internal validation and showed excellent discrimination capabilities with a sensitivity of 82.3% (95% CI 72.7%; 91.8%) and a specificity of 82.7% (95% CI 79.8%; 85.7%) when applying the most discriminating threshold of 3. Stratifying the risk in the most at‐risk population (drug‐resistant focal epilepsy) will make it easier for practitioners to inform patients or their families. It could also be used to help refer patients with drug‐resistant focal epilepsy more quickly for epilepsy surgery.
Other risk scores have been proposed, such as the SUDEP‐7 inventory, which was developed in 2010 and revised in 2015 but whose ability to predict SUDEP was not satisfactory [31]. More recently, the SUDEP‐3 inventory was put forward. It showed better discrimination capabilities, measured by the AUROC, than the SUDEP‐7 inventory (SUDEP‐7, 0.66 [0.54–0.87]; SUDEP‐3, 0.75 [0.64–0.86]) [18].
Considering the components of the SUDEP‐3 inventory, only three risk factors were included: GTCS frequency, seizure of any type during the past year and ID. It does not include certain major risk factors like nocturnal seizures [32]. Very recently, Jha et al. [17] also proposed a tool for the individualized prediction of SUDEP, re‐analysing the data of three case–control studies and one cohort. Their final prediction model includes a large number of predictors (22 factors) with an AUROC of 0.71 (0.68–0.74). However, this could be difficult to use in clinical practice. Our score, targeted at a more homogeneous population, seems to present promising overall discrimination capabilities and should be easily usable by practitioners.
Another interesting tool has been developed in the UK by Shankar et al. [33]: the Safety Checklist. This 19‐item checklist aims to optimize the care of patients with epilepsy. It lists potential or known risk factors and evaluates global risk through discussion with the patient to mitigate risk when possible; it also attempts to score risk in patients [34]. The SUDEP‐CARE score could be complementary by allowing neurologists to quickly and objectively evaluate the level of SUDEP risk to target the most at‐risk patients and adapt information and care.
In light of the literature
In this study, the analysis of risk factors for SUDEP confirmed the major role of GTCSs, identified as a risk factor in all previous SUDEP studies. Our results also confirm that a history of nocturnal or sleep‐related convulsive seizures is an independent major risk factor of SUDEP, as shown recently [6, 10]. This could be partially linked to a lack of supervision at night, given that nocturnal supervision has a protective effect [35]. However, a specific effect of sleep‐related generalized seizures might also play a role [6].
A more original result is the association between psychiatric comorbidities, present or past, and SUDEP which is consistent with recent findings in a large Swedish cohort [6, 12].
Despite being retained in the final model, ID seems a less important risk factor in our study than in previous studies [36]. As explored in our sensitivity analysis, this is probably due to the extensive missing data on ID in the control population. When negatively imputing these data instead, estimates then matched those found in the literature.
No significant effect was found for the number of epileptic drugs taken, confirming the results of Hesdorffer et al. [16]. However, the focus here was on patients with known drug‐resistant epilepsy, which limited our conclusion on this particular factor.
The epilepsy surgery rates, as well as the sociodemographic and treatment profiles, were similar between cases and controls in this study, limiting any potential selection bias.
Strengths and limitations
As in all case–control studies, confusion bias can limit the results. Whilst predictive capabilities cannot be estimated, a score can be created that assesses the immediate risk of SUDEP and discriminates high‐risk patients from others. Our study targeted only patients with focal drug‐resistant epilepsy, which is the population available through EMUs, although this limits the generalizability of the results. Furthermore, some variables, such as respiratory symptoms or seizure‐related falls, were declarative and more at risk of information bias. Also anti‐seizure medications or other psychotropic medications could not be explored in detail due to the limited number of observations. Some factors, such as lifestyle habits, alcohol and other substance consumption, epilepsy aetiologies, level of ID, treatment compliance or paraclinical explorations with localization of the epileptogenic zone, were sadly not available in this study, but could lead to even better discrimination in the future.
Whilst missing data prevented multivariate analyses on the complete data without selection bias, multiple imputations allowed us to use the data of all the included patients, thus limiting selection bias. To ensure the validity of our findings, several sensitivity analyses were performed showing consistent results, although the importance of the risk related to ID was probably underestimated. This was taken into account, however, and did not interfere in the construction of the SUDEP‐CARE score (discrimination capabilities did not vary). The selection process also retained only the most robust factors to compose the final model and an internal validation through bootstrap was performed. This supports the overall robustness of our results, which must now undergo external validation. It is planned to use the SUDEP‐CARE score on future SUDEP cases detected in the RSME and expand the targeted population.
CONCLUSION
In practice, the SUDEP‐CARE score could be used as a simple and fast clinical decision support tool for patients with focal and drug‐resistant epilepsy. A score of 1 or higher could alert the neurologist to have a more in‐depth discussion, with the Safety Checklist for example, on the means of limiting the risk of SUDEP. These could include acting on modifiable factors or on individual decision‐making, such as the use of an alert system for the detection of seizures.
External validation is still needed, and further work including paraclinical data could help refine this score for use in research on SUDEP biomarkers or in prevention trials.
AUTHOR CONTRIBUTIONS
MCP proposed the objective, conception and design of the study. MCP, CS contributed to conception, data management and analysis of data. PR and SR ensured the coordination of the epilepsy monitoring units, including the validation of the overall database, and the completion and accuracy of the clinical data in their centre. MCP, MF ensured the coordination of the RSME network centres, including the completion and accuracy of the clinical data. CS, MCP, SR, PR, VM drafted the manuscript. CS was responsible for the statistical analysis. MCP and CS were responsible for the overall content as guarantors. All authors contributed to the critical revision of the manuscript for important intellectual content and gave their final approval of the version to be published. The corresponding author certifies that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
FUNDING INFORMATION
Financial support was provided by the FFRE (French Research Foundation for Epilepsy, Grant 2009) and the LFCE (French League Against Epilepsy, Grant UIC 8601).
CONFLICT OF INTEREST
None of the authors have any conflicts of interest to disclose.
Supporting information
Appendix S1
Appendix S2
Figures S1 and S2
Tables S1 and S2
ACKNOWLEDGEMENTS
The authors would like to thank all of the investigators and neurologists who participated in the ascertainment of cases and controls. They also thank Bernadette Larquier, head of the Bereaved Families Network of the RSME, Myriam Boulhais, psychologist of the RSME, Anna Bedbrook and Sarah Kabani for proofreading of the English.
Serrand C, Rheims S, Faucanié M, et al. Stratifying sudden death risk in adults with drug‐resistant focal epilepsy: The SUDEP‐CARE score. Eur J Neurol. 2023;30:22‐31. doi: 10.1111/ene.15566
DATA AVAILABILITY STATEMENT
The raw data after de‐identification can be shared upon individual request to Marie‐Christine Picot: mc-picot@chu-montpellier.fr.
REFERENCES
- 1. Nashef L, So EL, Ryvlin P, Tomson T. Unifying the definitions of sudden unexpected death in epilepsy: unifying the definitions of SUDEP. Epilepsia. 2012;53(2):227‐233. [DOI] [PubMed] [Google Scholar]
- 2. Harden C, Tomson T, Gloss D, et al. Practice guideline summary: sudden unexpected death in epilepsy incidence rates and risk factors: report of the guideline development, dissemination, and implementation, Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology. 2017;88(17):1674‐1680. [DOI] [PubMed] [Google Scholar]
- 3. Téllez‐Zenteno JF, Ronquillo LH, Wiebe S. Sudden unexpected death in epilepsy: evidence‐based analysis of incidence and risk factors. Epilepsy Res. 2005;65(1–2):101‐115. [DOI] [PubMed] [Google Scholar]
- 4. Ryvlin P, Nashef L, Lhatoo SD, et al. Incidence and mechanisms of cardiorespiratory arrests in epilepsy monitoring units (MORTEMUS): a retrospective study. Lancet Neurol. 2013;12(10):966‐977. [DOI] [PubMed] [Google Scholar]
- 5. Tomson T, Walczak T, Sillanpaa M, Sander JWAS. Sudden unexpected death in epilepsy: a review of incidence and risk factors. Epilepsia. 2005;46(s11):54‐61. [DOI] [PubMed] [Google Scholar]
- 6. Sveinsson O, Andersson T, Mattsson P, Carlsson S, Tomson T. Clinical risk factors in SUDEP: a nationwide population‐based case–control study. Neurology. 2020;94(4):e419‐e429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Walczak TS, Leppik IE, D'Amelio M, et al. Incidence and risk factors in sudden unexpected death in epilepsy: a prospective cohort study. Neurology. 2001;56(4):519‐525. [DOI] [PubMed] [Google Scholar]
- 8. Nashef L, Walker F, Allen P, Sander JW, Shorvon SD, Fish DR. Apnoea and bradycardia during epileptic seizures: relation to sudden death in epilepsy. J Neurol Neurosurg Psychiatry. 1996;60(3):297‐300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Young C, Shankar R, Palmer J, et al. Does intellectual disability increase sudden unexpected death in epilepsy (SUDEP) risk? Seizure. 2015;25:112‐116. [DOI] [PubMed] [Google Scholar]
- 10. Lamberts RJ, Thijs RD, Laffan A, Langan Y, Sander JW. Sudden unexpected death in epilepsy: people with nocturnal seizures may be at highest risk: nocturnal seizures as a risk for SUDEP. Epilepsia. 2012;53(2):253‐257. [DOI] [PubMed] [Google Scholar]
- 11. Ridsdale L, Charlton J, Ashworth M, Richardson MP, Gulliford MC. Epilepsy mortality and risk factors for death in epilepsy: a population‐based study. Br J Gen Pract. 2011;61(586):e271‐e278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sveinsson O, Andersson T, Carlsson S, Tomson T. The incidence of SUDEP: a nationwide population‐based cohort study. Neurology. 2017;89(2):170‐177. [DOI] [PubMed] [Google Scholar]
- 13. Nilsson L, Farahmand B, Persson P‐G, Thiblin I, Tomson T. Risk factors for sudden unexpected death in epilepsy: a case control study. Lancet. 1999;353(9156):888‐893. [DOI] [PubMed] [Google Scholar]
- 14. Hesdorffer DC, Tomson T, Benn E, et al. Combined analysis of risk factors for SUDEP: combined SUDEP analysis. Epilepsia. 2011;52(6):1150‐1159. [DOI] [PubMed] [Google Scholar]
- 15. Tomson T, Surges R, Delamont R, Haywood S, Hesdorffer DC. Who to target in sudden unexpected death in epilepsy prevention and how? Risk factors, biomarkers, and intervention study designs. Epilepsia. 2016;57:4‐16. [DOI] [PubMed] [Google Scholar]
- 16. Hesdorffer DC, Tomson T, Benn E, et al. Do antiepileptic drugs or generalized tonic–clonic seizure frequency increase SUDEP risk? A combined analysis: combined SUDEP analysis. Epilepsia. 2012;53(2):249‐252. [DOI] [PubMed] [Google Scholar]
- 17. Jha A, Oh C, Hesdorffer D, et al. Sudden unexpected death in epilepsy: a personalized prediction tool. Neurology. 2021;96(21):e2627‐e2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tarighati Rasekhi R, Devlin KN, Mass JA, et al. Improving prediction of sudden unexpected death in epilepsy: from SUDEP‐7 to SUDEP‐3. Epilepsia. 2021;62(7):1536‐1545. [DOI] [PubMed] [Google Scholar]
- 19. Donner EJ, Waddell B, Osland K, et al. After sudden unexpected death in epilepsy: lessons learned and the road forward. Epilepsia. 2016;57:46‐53. [DOI] [PubMed] [Google Scholar]
- 20. Picot M‐C, Ryvlin P, Jallon P, et al. Réseau sentinelle national de surveillance de la mortalité liée à l'épilepsie. Epilepsia. 2009;21(2):198‐199. [Google Scholar]
- 21. Kwan P, Arzimanoglou A, Berg AT, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc task force of the ILAE commission on therapeutic strategies: definition of drug resistant epilepsy. Epilepsia. 2009;51(6):1069‐1077. [DOI] [PubMed] [Google Scholar]
- 22. INSERM (dir.) . Autopsie psychologique: mise en oeuvre et démarches associées. Rapport. Les Éditions Inserm, 2008; XII‐131p.‐ (Expertise opérationnelle). http://hdl.handle.net/10608/100 [Google Scholar]
- 23. Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini‐international neuropsychiatric interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM‐IV and ICD‐10. J Clin Psychiatry. 1998;59(Suppl 20):22‐33. quiz 34–57. [PubMed] [Google Scholar]
- 24. Mula M, Jauch R, Cavanna A, et al. Interictal dysphoric disorder and periictal dysphoric symptoms in patients with epilepsy: IDD and PDS in epilepsy. Epilepsia. 2010;51(7):1139‐1145. [DOI] [PubMed] [Google Scholar]
- 25. Liu Y, De A. Multiple imputation by fully conditional specification for dealing with missing data in a large epidemiologic study. Int J Stat Med Res. 2015;4(3):287‐295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rubin DB, ed. Multiple Imputation for Nonresponse in Surveys [Internet]. John Wiley & Sons, Inc.; 1987. [cited 2019 Jul 31]. (Wiley Series in Probability and Statistics). doi: 10.1002/9780470316696 [DOI] [Google Scholar]
- 27. Steyerberg EW, Harrell FE, Borsboom GJJM, Eijkemans MJC, Vergouwe Y, Habbema JDF. Internal validation of predictive models. J Clin Epidemiol. 2001;54(8):774‐781. [DOI] [PubMed] [Google Scholar]
- 28. Steyerberg EW. Clinical Prediction Models: a Practical Approach to Development, Validation, and Updating. Springer; 2009:497 (Statistics for biology and health). [Google Scholar]
- 29. Schomaker M, Heumann C. Bootstrap inference when using multiple imputation. Stat Med. 2018;37(14):2252‐2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Marshall A, Altman DG, Holder RL, Royston P. Combining estimates of interest in prognostic modelling studies after multiple imputation: current practice and guidelines. BMC Med Res Methodol. 2009;9(1):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Odom N, Bateman LM. Sudden unexpected death in epilepsy, periictal physiology, and the SUDEP‐7 inventory. Epilepsia. 2018;59(10):e157‐e160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. DeGiorgio CM, Miller P, Meymandi S, et al. RMSSD, a measure of vagus‐mediated heart rate variability, is associated with risk factors for SUDEP: the SUDEP‐7 inventory. Epilepsy Behav. 2010;19(1):78‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shankar R, Ashby S, McLean B, Newman C. Bridging the gap of risk communication and management using the SUDEP and seizure safety checklist. Epilepsy Behav. 2020;103:106419. [DOI] [PubMed] [Google Scholar]
- 34. Shankar R, Newman C, Gales A, et al. Has the time come to stratify and score SUDEP risk to inform people with epilepsy of their changes in safety? Front Neurol. 2018;9:281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Langan Y. REVIEW sudden unexpected death in epilepsy (SUDEP): risk factors and case control studies. Seizure. 2000;9(3):179‐183. [DOI] [PubMed] [Google Scholar]
- 36. Ryvlin P, Rheims S, Lhatoo SD. Risks and predictive biomarkers of sudden unexpected death in epilepsy patient. Curr Opin Neurol. 2019;32(2):205‐212. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Appendix S2
Figures S1 and S2
Tables S1 and S2
Data Availability Statement
The raw data after de‐identification can be shared upon individual request to Marie‐Christine Picot: mc-picot@chu-montpellier.fr.


