Abstract
Objective
To test the equivalence of two doses of intravenous iron (ferric carboxymaltose) in pregnancy.
Design
Parallel, two‐arm equivalence randomised controlled trial with an equivalence margin of 5%.
Setting
Single centre in Australia.
Population
278 pregnant women with iron deficiency.
Methods
Participants received either 500 mg (n = 152) or 1000 mg (n = 126) of intravenous ferric carboxymaltose in the second or third trimester.
Main outcome measures
The proportion of participants requiring additional intravenous iron (500 mg) to achieve and maintain ferritin >30 microg/L (diagnostic threshold for iron deficiency) at 4 weeks post‐infusion, and at 6 weeks, and 3‐, 6‐ and 12‐months postpartum. Secondary endpoints included repeat infusion rate, iron status, birth and safety outcomes.
Results
The two doses were not equivalent within a 5% margin at any time point. At 4 weeks post infusion, 26/73 (36%) participants required a repeat infusion in the 500‐mg group compared with 5/67 (8%) in the 1000‐mg group: difference in proportions, 0.283 (95% confidence interval [CI] 0.177–0.389). Overall, participants in the 500‐mg arm received twice the repeat infusion rate (0.81 [SD = 0.824] versus 0.40 [SD = 0.69], rate ratio 2.05, 95% CI 1.45–2.91).
Conclusions
Administration of 1000 mg ferric carboxymaltose in pregnancy maintains iron stores and reduces the need for repeat infusions. A 500‐ mg dose requires ongoing monitoring to ensure adequate iron stores are reached and sustained.
Keywords: antenatal care, haematology: anaemia, medical disorders in pregnancy, obstetric haemorrhage, randomised controlled trials, risk management
1. INTRODUCTION
Iron deficiency (ID) is the most common nutritional disorder worldwide, listed on the World Health Organization (WHO) top five mental and physical disabilities. 1 ID is the leading underlying cause of anaemia, affecting approximately 45% of women of childbearing age in developed countries and up to 80% in lower resource settings. 2 As iron is necessary for many biological functions3, 4 pregnant women with ID or iron deficiency anaemia (IDA) frequently suffer from cardiovascular problems, reduced physical activity, impaired cognitive performance, reduced immune function, fatigue and depressive episodes. 3 , 5 , 6 These women are at a higher risk of pregnancy complications, stillbirth, postpartum haemorrhage (PPH), peri‐partum allogeneic red blood cell transfusion and death. 7 , 8 , 9 , 10 , 11 Infants of mothers with ID are at increased risk of preterm birth, growth restriction, low birthweight, perinatal death, low Apgar scores, neonatal infection, postnatal ID and impaired cognitive development. 4 , 7 , 9 , 12 In recognition of these adverse outcomes, WHO has targetted a 50% reduction of IDA in women of reproductive age by 2025. 13
Women with inadequate iron stores are ill‐prepared for the increased iron demand of pregnancy, 14 rendering up to 47% of pregnant women iron‐deficient. 15 ID is detectable, preventable and treatable, 3 with oral iron considered the first‐line treatment. Intravenous iron (IVI) is recommended when women are non‐responsive, non‐tolerant or non‐compliant with oral iron, when ID/IDA is diagnosed late in pregnancy, or in women with severe anaemia or at risk of haemorrhage. 16 , 17 , 18 , 19 , 20
Randomised controlled trials (RCTs) in pregnant women with IDA have demonstrated superior haematological outcomes after IVI compared with oral iron. 16 , 21 , 22 , 23 Doses of IVI in RCTs and observational studies have ranged from 400 to 1000 mg, with all showing improvements in iron status without serious safety concerns. 16 , 21 , 22 , 23 Higher doses come at a larger cost, and no data exist comparing adverse effects or the potential for iron excess with different doses. 24 In addition, accessibility to IVI and approved dosing schemes differ between countries, creating geographical, cultural and social barriers. 4 , 25 Clinicians lack high quality data on the optimal dose to improve and sustain iron status adequately, and to provide sufficient protection against adverse obstetric, neonatal or mental health sequelae. We therefore conducted an RCT comparing two doses of IVI (500 and 1000 mg) using an equivalence design.
2. METHODS
This was a single centre, randomised, parallel, two‐arm equivalence study with an equivalence margin of 5%. The equivalence design was chosen due to uncertainty about optimal dosing strategies in clinical management of IDA in pregnancy. The margin was determined via clinical consensus of the organisation's anaesthetic team. The trial was approved by the Northern Adelaide Local Health Network ethics committee (HREC/14/TQEH/lMH/122). All patients provided written informed consent.
2.1. Patients
Pregnant women aged over 18 years in the second or third trimester with ID, defined as serum ferritin <15 microg/ml, or serum ferritin <50 microg/ml and TSAT <20% with elevated CRP, were eligible. Participants were excluded if they had untreated B12 or folate deficiencies, known hypersensitivity to FCM, haemoglobin >130 g/l; a serious medical condition, uncontrolled systemic disease or the inability fully to comprehend or perform study procedures. Women were screened for ID during routine antenatal assessment at the Women's Assessment Unit at the Lyell McEwin Hospital, Elisabeth Vale, South Australia.
2.2. Randomisation
Randomisation was performed by a trial pharmacist using an online randomisation sequence generator and opaque envelopes. Participants were stratified into two arms based on their haemoglobin at time of screening, <105 g/l or ≥ 105 g/l. Study participants, clinicians, researchers and statistician were masked to treatment allocation until after all analyses were complete.
After data review at trial completion, 14 ineligible participants were discovered to have been mistakenly enrolled and randomised. These participants were excluded from the analysis (Figure 1); this is not expected to cause bias. 26
FIGURE 1.
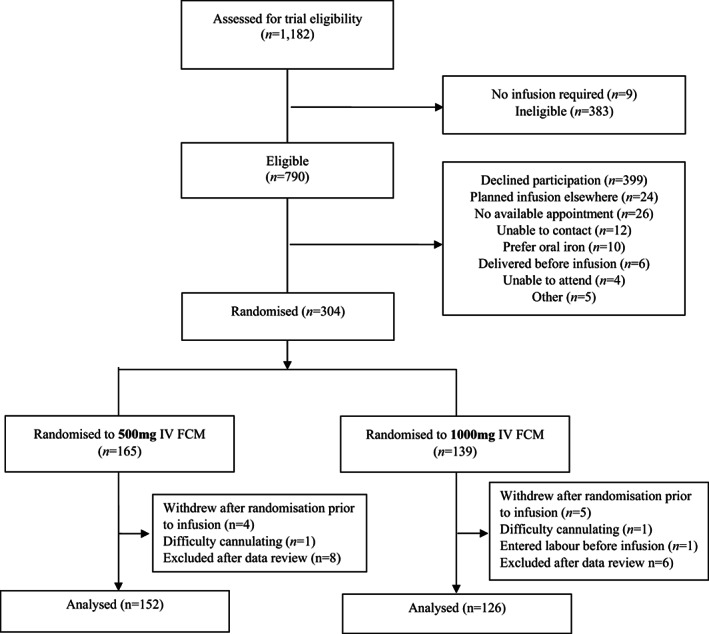
Trial flow.
2.3. Interventions
The intervention was intravenous ferric carboxymaltose (FCM), 500 mg or 1000 mg, in 250 ml of normal saline, infused over 30 min. At the first appointment after screening, FCM at the randomised dose was administered.
Iron status was monitored at five study time points: 4 weeks after the initial infusion, then at 6 weeks, 3, 6 and 12 months postpartum, or at the time of next pregnancy, whichever came first. The diagnostic criteria indicating persistent ID and requiring additional IVI were ferritin <30 microgl plus transferrin saturation <25% or, if inflammation was present (CRP >7.9 ng/l), ferritin between 30 and 50 microg/l plus a transferrin saturation <20%. If confirmed, ID was treated with a single 500‐mg FCM infusion in both groups.
2.4. Endpoints
The primary endpoint was the proportion of participants requiring additional intravenous FCM to maintain successful correction of ID, based on the diagnostic criteria. Patients were assessed at the five time points. Where participants conceived at any time during their follow‐up, they were assessed for their final follow‐up appointment and exited the study.
Secondary endpoints were repeat infusion rate prior to delivery, after delivery and overall during the study period, iron and haematological outcomes (serum iron, ferritin, transferrin and transferrin saturation), maternal pregnancy outcomes and complications including gestational diabetes, pre‐eclampsia, gestational hypertension, preterm labour, pre‐labour rupture of membranes, preterm pre‐labour rupture of membranes (PPROM), preterm birth, gestational age at birth, antenatal haemorrhage, postpartum haemorrhage, mode of delivery, neonatal outcomes (birthweight, customised birthweight centile, birth length and head circumference) and child neurodevelopment at 12 months of age (Ages and Stages Questionnaire – Third Edition; ASQ). 27 Additional infusions, iron and haematological outcomes were evaluated at the five time points.
2.5. Sample size
The study was designed assuming 12% of participants in each treatment arm would require a repeat iron infusion. 28 Using an equivalence margin of 5% and a power of 80%, 131 participants were required in each arm at baseline. After accounting for an expected 15% drop out, the required sample size was 151 in each arm of the study.
2.6. Statistical analysis
Patient characteristics were described using means (standard deviation), medians (interquartile range), or counts and frequencies, as appropriate. Primary and secondary endpoints were analysed using regression models with treatment group and haemoglobin at baseline (Hb < 105 or Hb ≥105) as covariates. Endpoints measured at multiple time points were analysed using repeated measures models with a time point by treatment group interaction. All analyses were performed in SAS v 9.4 (SAS Institute) or R v 4.2.0 (R Foundation for Statistical Computing).
2.6.1. Primary endpoint
The difference in proportions of participants who required a repeat iron infusion at each time point was analysed using a repeated measures model with generalised estimating equations (GEEs) and an independent working correlation.
Two one‐sided tests at the 0.05 level were used to assess equivalence at each time point. As the equivalence margin was 5%, 90% confidence intervals (CI) for absolute differences in proportions between groups at each time point were examined as to whether they lay within the interval (−0.05 to 0.05). To establish equivalence, the proportion of participants in both groups must lie completely within this interval.
2.6.2. Secondary endpoints
Repeat infusion rates were analysed using Poisson regression models. Serum iron, transferrin and transferrin saturation were analysed using mixed effects linear regression models adjusted for baseline. The measurement of ferritin at baseline was subject to threshold effects from limits to detection (<4 microg/l) and was singly imputed as half the limit of detection (2 microg/L).
Maternal pregnancy and birth complications, mode of delivery and neonatal outcomes were analysed using linear or logistic regression models, as appropriate. GEEs with an independent working correlation structure were used, where relevant, to account for multiple births.
For secondary clinical outcomes, we report mean differences (MDs) in the change from baseline between treatment groups, rate ratios (RRs) or odds ratios (ORs), as appropriate, with 95% CI.
2.7. Sensitivity and post‐hoc analyses
A number of sensitivity analyses were conducted on the primary outcome, described in Appendix S1. As the intervention was only administered in a clinical setting, a further post‐hoc sensitivity analysis was conducted. The outcome was whether a repeat infusion was administered or not; any participants who did not present for a follow‐up appointment were coded as not having received a repeat infusion.
3. RESULTS
Between 26 May 2015 and 14 August 2017, 1182 women were screened for trial eligibility at our antenatal assessment unit. Of a total of 304 women who were randomised, nine participants withdrew prior to the infusion, two did not receive the infusion and one entered labour before the infusion. The remaining 292 participants received an infusion and 278 were included in the analysis. The trial flow is shown in Figure 1.
The demographic and clinical characteristics of study participants at baseline are presented in Table 1. The median age of participants in both groups was 27 (interquartile range [IQR] 23–31 in the 500‐mg group and IQR 22–30 in the 1000‐mg group). Median body mass index was 26 (IQR 22–32) and 27 (IQR 23–33) in the 500‐mg and 1000‐mg groups, respectively. Participants were enrolled in the trial on average at 32 weeks' gestational age. Approximately half of all participants were anaemic at study entry. The first infusion was received on average 11–12 days after screening.
TABLE 1.
Characteristics of study participants of the two groups at baseline
| 500 mg (n = 152) | 1000 mg (n = 126) | |
|---|---|---|
| Age, median (IQR) | 27 (23–31) | 27 (22–30) |
| Body mass index, median (IQR) | 26 (22–32) | 27 (23–33) |
| Pregnant with twins | 2 (1%) | 1 (1%) |
| Anaemia, n (%) | 70 (46%) | 66 (52%) |
| Education, years, median (IQR) (n) | 13 (12–15) (n = 143) | 13 (12–15) (n = 117) |
| Income, n (%) a | ||
| <AUD $40,00 | 39 (26%) | 33 (26%) |
| AUD $40,000–60,000 | 37 (24%) | 31 (25%) |
| AUD $60,000–80,000 | 18 (12%) | 25 (20%) |
| AUD $80,000–120,000 | 32 (21%) | 20 (16%) |
| >AUD $120,000 | 13 (9%) | 5 (4%) |
| Unknown | 13 (9%) | 12 (10%) |
| Gestational age at screening, median (IQR) | ||
| Days | 231 (210–246) | 227 (210–244) |
| Weeks | 32.9 (30.0–35.1) | 32.4 (30.0–34.8) |
| Gestational age at first infusion, median (IQR) | ||
| Days | 241 (222–255) | 235 (222–253) |
| Weeks | 34.4 (31.7–36.5) | 33.6 (31.6–36.1) |
| Blood parameters, mean (SD) (n) or median (IQR) (n) | ||
| Haemoglobin b g/L | 106 (9) (n = 152) | 106 (10) (n = 126) |
| Ferritin microg/L | 6.0 (4.0–8.0) (n = 152) | 6.0 (4.0–9.0) (n = 126) |
| Iron micromol/L | 7 (5–10) (n = 143) | 7 (5–10) (n = 119) |
| Transferrin g/L | 4.3 (4.0–5.0) (n = 143) | 4.5 (4.1–5.0) (n = 119) |
| Transferrin saturation, (%) | 6 (5–10) (n = 143) | 6 (4–9) (n = 119) |
| MCH | 28 (26–30) (n = 116) | 28 (26–29) (n = 90) |
| Folate (serum), nmol/L | 28 (21–45) (n = 116) | 27 (18–41) (n = 106) |
| B12 (serum), pmol/L | 198 (162–247) (n = 149) | 202 (170–244) (n = 123) |
| B12 (active), pmol/L | 51 (37–65) (n = 115) | 49 (32–66) (n = 91) |
| CRP, ng/L | 5.7 (2.8–11.0) (n = 131) | 5.0 (2.3–10.0) (n = 113) |
| Supplements taken in pregnancy, n (%) a | ||
| None | 27 (18%) | 18 (14%) |
| Iron only | 15 (10%) | 10 (8%) |
| Iron + pregnancy multivitamin | 16 (11%) | 15 (12%) |
| Pregnancy multivitamin (no iron) | 55 (36%) | 44 (35%) |
| Other supplements (no iron) | 18 (12%) | 19 (15%) |
| Unknown | 21 (14%) | 20 (16%) |
May not add up to 100% due to rounding.
Stratification variable.
Equivalence between the two doses at a margin of 5% was not demonstrated at any time point (Table 2, Figure S1). More participants assigned to 500 mg IVI required repeat infusions at 4 weeks post‐infusion compared with those assigned to 1000 mg IVI (26/73 [36%] versus 5/67 [8%]; estimated difference in proportions, 0.28, 90% CI 0.18–0.39). Between 4 weeks post‐infusion and 6 months postpartum, the difference between the groups decreased but the 90% CI did not lie within the −0.05 to 0.05 interval, therefore equivalence was not achieved within a 5% margin (15/88 [17%] versus 9/67 [13%] at 6 months postpartum; estimated difference in proportions 0.037, 90% CI −0.059 to 0.133). The proportion of participants requiring a repeat infusion increased over time in the 1000‐mg IVI group from 5/67 (8%) at 4 weeks post‐infusion to 14/70 (20%) at 12 months postpartum.
TABLE 2.
Primary outcome: The proportion of women requiring a repeat infusion at each time point, n/N (%), and estimated difference in proportions between groups (90% CI)
| Time period | 500 mg (n = 152) | 1000 mg (n = 126) | Estimated difference in proportions, 500–1000 mg (90% CI) |
|---|---|---|---|
| 4 weeks post‐randomisation | 26/73 (36%) | 5/67 (8%) | 0.283 (0.177–0.389) |
| 6 weeks postpartum | 39/118 (33%) | 9/83 (11%) | 0.234 (0.147–0.321) |
| 3 months postpartum | 15/98 (15%) | 5/65 (8%) | 0.086 (0.012–0.160) |
| 6 months postpartum | 15/88 (17%) | 9/67 (13%) | 0.037 (−0.059 to 0.133) |
| 12 months postpartum | 27/90 (30%) | 14/70 (20%) | 0.111 (−0.001 to 0.222) |
Equivalence was not achieved in any of the sensitivity analyses of the primary outcome (Tables S1 and S2). The proportion of participants who required a repeat infusion at any time during follow‐up was also not equivalent (83/152 [55%] versus 34/126 [27%], estimated difference in proportions 0.288, 90% CI 0.191–0.384) (Table S1). In the ≥105 g/L Hb stratification group, 52/131 (40%) participants required a repeat infusion at any time point, compared with 65/147 (44%) participants in the <105 g/L Hb stratification group (estimated difference in proportions, −0.016, 90% CI −0.112 to 0.081).
Compared with participants assigned to 1000 mg IVI, participants in the 500‐mg IVI group received more than twice the repeat infusion rate prior to delivery, after delivery and overall (0.81 [0.84] versus 0.40 [SD = 0.69], rate ratio [RR] 2.05, 95% CI 1.45–2.91; P < 0.001) (Table 3).
TABLE 3.
Secondary outcomes
| Outcome | Time point | 500 mg | 1000 mg | Estimate (95% CI) | P‐value |
|---|---|---|---|---|---|
|
Rate of repeat infusions, a mean (SD) (n) |
n = 152 | n = 126 | |||
| Prior to delivery | 0.37 (0.51) (n = 76) | 0.14 (0.35) (n = 77) | 2.58 (1.38–4.82) | 0.003 | |
| After delivery | 0.64 (0.72) (n = 140) | 0.31 (0.57) (n = 111) | 2.10 (1.42–3.10) | <0.001 | |
| Overall | 0.81 (0.84) (n = 140) | 0.40 (0.69) (n = 111) | 2.05 (1.45–2.91) | <0.001 | |
|
Iron status, mean (SD) (n) | |||||
| Ferritin b microg/L (half threshold method) | 4 weeks | 57 (49) (n = 69) | 147 (100) (n = 65) | −90.1 (−115.1 to −65.1) | <0.001 |
| 6 weeks PP | 68 (61) (n = 115) | 115 (76) (n = 82) | −53.0 (−71.8 to −34.3) | 0.004 | |
| 3 months PP | 75 (50) (n = 97) | 96 (66) (n = 64) | −25.7 (−43.0 to −8.5) | <0.001 | |
| 6 months PP | 58 (30) (n = 85) | 85 (64) (n = 61) | −28.6 (−42.1 to −15.2) | <0.001 | |
| 12 months PP | 58 (52) (n = 88) | 69 (59) (n = 66) | −8.5 (−24.8 to 7.8) | 0.31 | |
| Transferrin saturation, b % | 4 weeks | 13 (6) (n = 67) | 20 (8) (n = 62) | −6.4 (−8.7 to −4.1) | <0.001 |
| 6 weeks PP | 20 (10) (n = 114) | 22 (9) (n = 81) | −1.2 (−4.0 to 1.6) | 0.40 | |
| 3 months PP | 22 (10) (n = 96) | 23 (9) (n = 64) | −1.3 (−4.0 to 1.4) | 0.34 | |
| 6 months PP | 20 (9) (n = 84) | 21 (9) (n = 58) | 0.1 (−3.1 to 3.2) | 0.96 | |
| 12 months PP | 20.98 (9.06) (n = 86) | 19.72 (9.64) (n = 64) | 1.78 (−1.36 to 4.93) | 0.27 | |
|
Antenatal and delivery, n/N (%) | |||||
| Gestational diabetes c | 23/150 (15%) | 27/121 (22%) | 0.61 (0.32–1.13) | 0.12 | |
| Pre‐eclampsia c | 4/150 (3%) | 9/122 (7%) | 0.33 (0.10–1.10) | 0.07 | |
| Preterm delivery c | 5/151 (3%) | 7/126 (6%) | 0.56 (0.17–1.82) | 0.34 | |
| Antepartum haemorrhage d | 5/150 (3%) | 9/121 (7%) | 0.43 (0.14–1.32) | 0.14 | |
| Postpartum haemorrhage (EBL >500 ml) c | 41/150 (27%) | 37/124 (30%) | 0.87 (0.51–1.48) | 0.61 | |
| PPROM | 0/150 (0%) | 6/121 (5%) | — | 0.007 e | |
| Mode of delivery e | |||||
| Normal vaginal, reference | 85/151 (56%) | 76/126 (60%) | 1.00 | ||
| Instrumental vaginal | 16/151 (11%) | 9/126 (7%) | 1.62 (0.67–3.88) | 0.28 | |
| Emergency caesarean section | 18/151 (12%) | 13/126 (10%) | 1.23 (0.57–2.69) | 0.60 | |
| Elective caesarean Section | 32/151 (21%) | 28/126 (22%) | 0.98 (0.54–1.79) | 0.95 | |
|
Neonatal outcomes, f mean (SD) | |||||
| Gestational age at delivery, days | 275 (10) (n = 151) | 275 (9) (n = 126) | 0.28 (−1.93 to 2.50) | 0.80 | |
| Birthweight, g | 3513 (532) (n = 151) | 3522 (468) (n = 126) | −28.6 (−187.2 to 130.0) | 0.72 | |
| Birthweight centile | 51 (30) (n = 151) | 52 (29) (n = 126) | −1.78 (−8.91 to 5.35) | 0.63 | |
| Birth length, cm | 50 (2) (n = 151) | 50 (2) (n = 124) | −0.17 (−1.52 to 1.17) | 0.80 | |
| Head circumference, cm | 35 (2) (n = 151) | 35 (2) (n = 126) | −0.30 (−1.25 to 0.64) | 0.53 | |
|
Child neurodevelopment, f mean (SD) (n) |
12 months | ||||
| Communication | 49 (11) (n = 77) | 51 (10) (n = 53) | −1.36 (−5.03 to 2.32) | 0.47 | |
| Gross motor | 45 (16) (n = 75) | 46 (17.3) (n = 53) | −1.67 (−7.56 to 4.21) | 0.58 | |
| Fine motor | 50 (10) (n = 77) | 51 (11) (n = 53) | −0.44 (−4.18 to 3.30) | 0.82 | |
| Problem solving | 43 (14) (n = 76) | 47 (13) (n = 53) | −3.67 (−8.31 to 0.97) | 0.12 | |
Abbreviations: MD, mean difference; OR, odds ratio; RM, repeated measures; RR, rate ratio.
RM Poisson regression: estimate is RR for 500/1000 mg adjusted for stratification.
RM linear mixed models: estimate is MD for 500–1000 mg adjusted for baseline and stratification.
Logistic regression: estimate is OR for outcome for 500/1000 mg adjusted for stratification.
Fisher's exact test.
Multinomial logistic regression: estimate is OR for category versus reference for 500/1000 mg adjusted for stratification.
Linear regression: estimate is MD for 500–1000 mg adjusted for stratification.
Participants who received 1000 mg IVI had significantly higher ferritin levels up to 6 months postpartum, compared with those receiving 500 mg IVI (Table 3). Similarly, serum iron and transferrin saturation were higher in participants who received 1000 mg IVI than those who received 500 mg IVI at 4 weeks post‐infusion, whereas transferrin was lower (Table 3). Importantly, transferrin saturations remained within normal limits in both groups, safeguarding the fetus from unnecessary transplacental iron transfer. Between‐group differences had disappeared for all markers of iron status by 12 months postpartum.
No serious adverse events were observed. Minor adverse events were observed in 3% (n = 8/276, 2 with missing data) of all participants during the first infusion, including 5/126 participants (4%) who received 1000 mg iron (dizziness n = 2, hypotension n = 1, nausea n = 1, chest tightness n = 1) and 3/150 participants (2%) who received 500 mg iron (nausea n = 2, hypotension n = 1). There was no difference between the groups (Fisher's exact test, P = 0.48). No adverse events were observed on subsequent infusions.
Preterm premature rupture of membranes (PPROM) occurred in 6/121 participants in the 1000‐mg arm only. The likelihood of other maternal complications did not differ between participants who received 500 mg and those who received 1000 mg IVI (Table 3). Similarly, no differences were found in the length of gestation, neonatal outcomes or child neurodevelopment at 12 months between the two doses (Table 3).
A post‐hoc analysis of haemoglobin levels indicated these were significantly higher in participants in the 1000‐mg IVI group than the 500‐mg IVI group at 4 weeks post‐infusion (Table S3). At each time point, the mean Hb level was in a range indicating sufficiency (>115) in both the 500‐ and 1000‐mg IVI iron groups. No haemoconcentration occurred in the participants during pregnancy, with minimum and maximum Hb levels during follow‐up of 81–167 g/L in the low dose and 99–170 g/L in the high dose arms. There were 6/140 (4%) (minimum–maximum ferritin, 4–434) and 12/116 (10%) (minimum–maximum ferritin, 6–581) participants who experienced hyperferritinaemia during follow‐up in the low and high dose arms, respectively. The ferritin value of 581 was measured 2 weeks after infusion and lay within commonly observed peak ferritin concentrations after iron infusion.
3.1. Main findings
This randomised controlled trial, comparing two pragmatic doses of intravenous iron for treating ID in pregnancy, demonstrated that 500 mg of intravenous FCM was not equivalent to the 1000‐mg dose. To achieve initial and sustained correction of ID, participants in the lower dose arm received more than twice the rate of repeat infusions compared with the higher dose arm. Participants in the higher dose arm had significantly higher ferritin levels up to 6 months postpartum, coupled with a significantly greater increase in haemoglobin, reflecting favourable iron availability and utilisation. Due to the close monitoring provided in this study, women with declining iron stores were able to receive subsequent IVI treatment. Given the higher rate of persistent ID observed in the low dose arm, we would suggest that continued monitoring after infusion of 500 g IVI is essential to ensure adequate iron stores are accomplished in pregnancy.
The ability of IVI to improve antenatal and postpartum haematological outcomes in pregnant women has been demonstrated in several previous randomised controlled trials. 16 , 21 , 22 , 23 These studies have used a range of IVI dosing strategies to achieve vital short‐term iron repletion and haemoglobin restoration in anaemic women, using doses ranging from 400 to 1000 mg. 16 The current study is, to our knowledge, the first prospective RCT to compare intravenous iron doses for successful and persistent correction of ID in pregnancy and over the first postpartum year. Recent concerns have been raised about whether IVI prescribing practice for women of reproductive age is appropriate and cost‐effective. 24 Our data suggest administration of 1000 mg can significantly reduce the need for repeat IVI, thereby improving patient health while reducing clinical load and cost. However, it is imperative that clinicians and patient do not become complacent after IVI administration, given over 20% of participants in both arms required additional infusions at 12 months postpartum to sustain satisfactory iron stores.
Iron deficiency and IDA are significant medical conditions with serious consequences for maternal and fetal outcomes. 3 , 7 , 8 , 13 Our study found no differences in pregnancy, birth‐ or infant‐related outcomes between the 500‐ and 1000‐mg IVI arms, with the exception of PPROM, which occurred only in participants receiving 1000 mg. Although the pathways leading to PPROM are complex and multifaceted, increased oxidative stress appears to play a role. It is possible that first or second trimester IDA itself, or exposure to increased iron, or differences in ferritin levels after IVI, may have contributed to oxidative stress leading to PPROM in the current study. 29 However, this must be interpreted with caution given the present study was not powered to examine this outcome.
Pregnancy is a time of increased iron demand. 4 The prevalence of ID is high, and progression from ID to IDA common. 20 Ensuring iron stores are optimal throughout pregnancy can avoid any adverse physiological and psychological outcomes associated with ID and IDA. 20 , 30 Further, should women experience a PPH, which remains the leading cause of maternal morbidity and mortality, optimal iron stores will boost Hb, which can provide a buffer to help protect against serious anaemia. 31 Although routine in our obstetrics unit, screening for ID is rarely incorporated into routine antenatal care. 32 Recent obstetrics guidelines on the management of ID and obstetric patient blood management guidelines have emphasised the need for more attentiveness and potential actions to detect and treat ID in pregnancy. 19 , 20 Despite this, IVI therapy has continued to be met with fear, scepticism and criticism, resulting in large uptake variations. 16 , 22 , 23 , 33 , 34 , 35 , 36
3.2. Strengths and limitations
The strengths of this study include the blinding of clinician, healthcare team, patient and statistician, the long follow‐up period, including longer term assessment of child health. The findings can reassure obstetric teams of the safety, efficacy and comprehensive benefits of IVI iron to treat ID and IDA in pregnancy.
The limitations include the variable number of participants who returned for each post‐infusion appointment. This is particularly relevant for the 4‐week post‐infusion time point, where only around 50% of participants in each arm attended. To address this, we conducted a post‐hoc sensitivity analysis assessing the number of participants receiving an infusion as a function of the total number of participants in each arm, assuming that patients who did not attend an appointment did not require an infusion. The results supported our non‐equivalence findings. Although our trial was conducted at a single site, we observed changes in all blood markers of iron status over the study period biologically consistent with a difference in the ability of both doses to adequately restore iron status. This is important, given that even in this smaller sample, the two‐fold difference in the need for a repeat infusion between the doses represents a significant addition to standard care, with associated costs and no differences in neonatal or child outcome.
3.3. Interpretation
The outcomes of this trial suggest that successful treatment of ID can occur with a single 1000‐ mg dose, with no adverse outcomes for antenatal progression, neonatal or child outcomes. This represents an effective treatment modality to shield from the detrimental short‐ and potential long‐term impacts of ID and IDA. Further, adequate iron stores have the potential to have a positive impact on maternal mental health during pregnancy and postpartum; maternal mental health outcomes collected during this trial will be the subject of a subsequent article. Further studies will help to optimise IVI dose, which may be particularly relevant to those countries where 1000 mg is not currently routinely used.
4. CONCLUSIONS
Low dose (500 mg) intravenous FCM treatment was not equivalent to 1000 mg within a 5% margin for successful correction of ID in pregnancy. A single 1000 mg does represents an efficient and effective method to manage ID and IDA clinically in pregnancy. A lower dose approach requires ongoing monitoring to ensure adequate iron stores are reached and sustained. Neither doses had any adverse impact on neonatal or child outcomes.
AUTHOR CONTRIBUTIONS
BF, NAH, GD and KOS planned the study design. BF, PP, NA and RC contributed to the collection of data. TLK and NAH performed the data analyses. BF wrote the first draft of the article, which was revised and critically reviewed by all authors. All authors approved the final version.
FUNDING INFORMATION
This study was funded by the National Blood Authority Australia (peer‐reviewed, grant ID 117) and University of Adelaide Priority Partner Grant. Neither funder had a role in the conduct of the research or in writing the paper.
CONFLICT OF INTERESTS
BF has received financial support to give lectures, and attend scientific advisory boards for Vifor Pharma Ltd., Switzerland, Compai Pharma, Malaysia, and Pfizer Australia. KOS, PP, RC, NA, TLK, GD and NAH have no conflict of interests to declare. Completed disclosure of interest forms are available to view online as supporting information.
Supporting information
Figure S1
Table S1
Table S2
Table S3
Appendix S1
Appendix S2
Appendix S3
Appendix S4
Appendix S5
Appendix S6
Appendix S7
Appendix S8
Appendix S9
ACKNOWLEDGEMENTS
Our sincere thanks go to the clinical trial pharmacists Jacinta Nagle, Joanne Mercorella and Susan Loeser. We are grateful for the support provided by the midwives, especially Tanya Warne, Michael Maier and Gemma Holling, from the Women's Assessment Unit of the Lyell McEwin Hospital, who worked relentlessly to recruit, care for and follow up participants. We thank Chnar Khoshnaw and Thomas M. Butler for their contributions in collecting data, and Bernhard T. Baune for his advice and support. In addition, we would like to thank Sophie Ballinger, research assistant, and Katrin Müller, Psychology Student, University of Freiburg, Germany, who joined our team on a Priority Partner Grant. Open access publishing facilitated by The University of Adelaide, as part of the Wiley ‐ The University of Adelaide agreement via the Council of Australian University Librarians.
Froessler B, Schubert KO, Palm P, Church R, Aboustate N, Kelly T‐L, et al. Testing equivalence of two doses of intravenous iron to treat iron deficiency in pregnancy: A randomised controlled trial. BJOG. 2023;130(1):15–23. 10.1111/1471-0528.17288
Trials Registry, ACTRN12615000950561 https://www.anzctr.org.au
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Kassebaum NJ. The global burden of anemia. Hematol Oncol Clin North Am. 2016;30(2):247–308. [DOI] [PubMed] [Google Scholar]
- 2. GBD 2016 Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1211–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Camaschella C. Iron deficiency. Blood. 2019;133(1):30–9. [DOI] [PubMed] [Google Scholar]
- 4. Georgieff MK. Iron deficiency in pregnancy. Am J Obstet Gynecol. 2020;223:516–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Musallam KM, Taher AT. Iron deficiency beyond erythropoiesis: should we be concerned? Curr Med Res Opin. 2018;34(1):81–93. [DOI] [PubMed] [Google Scholar]
- 6. Dama M, Van Lieshout RJ, Mattina G, Steiner M. Iron deficiency and risk of maternal depression in pregnancy: an observational study. J Obstet Gynaecol Can. 2018;40(6):698–703. [DOI] [PubMed] [Google Scholar]
- 7. Drukker L, Hants Y, Farkash R, Ruchlemer R, Samueloff A, Grisaru‐Granovsky S. Iron deficiency anemia at admission for labor and delivery is associated with an increased risk for cesarean section and adverse maternal and neonatal outcomes. Transfusion. 2015;55(12):2799–806. [DOI] [PubMed] [Google Scholar]
- 8. Daru J, Zamora J, Fernández‐Félix BM, Vogel J, Oladapo OT, Morisaki N, et al. Risk of maternal mortality in women with severe anaemia during pregnancy and post partum: a multilevel analysis. Lancet Global Health. 2018;6(5):e548–54. [DOI] [PubMed] [Google Scholar]
- 9. Nair M, Churchill D, Robinson S, Nelson‐Piercy C, Stanworth SJ, Knight M. Association between maternal haemoglobin and stillbirth: a cohort study among a multi‐ethnic population in England. Br J Haematol. 2017;179(5):829–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kassebaum NJ, Bertozzi‐Villa A, Coggeshall MS, Shackelford KA, Steiner C, Heuton KR, et al. Global, regional, and national levels and causes of maternal mortality during 1990‐2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9947):980–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Randall DA, Patterson JA, Gallimore F, Morris JM, McGee TM, Ford JB. The association between haemoglobin levels in the first 20 weeks of pregnancy and pregnancy outcomes. PLoS One. 2019;14(11):e0225123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Congdon EL, Westerlund A, Algarin CR, Peirano PD, Gregas M, Lozoff B, et al. Iron deficiency in infancy is associated with altered neural correlates of recognition memory at 10 years. J Pediatr. 2012;160(6):1027–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. WHO . World Health Organization. Global targets 2025. To improve maternal, infant and young child nutrition; 2014.
- 14. Milman N. Iron in pregnancy – how do we secure an appropriate iron status in the mother and child? Ann Nutr Metab. 2011;59(1):50–4. [DOI] [PubMed] [Google Scholar]
- 15. Kirschner W, Dudenhausen JW, Henrich W. Are there anamnestic risk factors for iron deficiency in pregnancy? Results from a feasibility study. J Perinat Med. 2016;44(3):309–14. [DOI] [PubMed] [Google Scholar]
- 16. Froessler B, Cocchiaro C, Saadat‐Gilani K, Hodyl N, Dekker G. Intravenous iron sucrose versus oral iron ferrous sulfate for antenatal and postpartum iron deficiency anemia: a randomized trial. J Matern Fetal Neonatal Med. 2013;26(7):654–9. [DOI] [PubMed] [Google Scholar]
- 17. Froessler B, Gajic T, Dekker G, Hodyl NA. Treatment of iron deficiency and iron deficiency anemia with intravenous ferric carboxymaltose in pregnancy. Arch Gynecol Obstet. 2018;298:75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Khalafallah A, Hyppa A, Chuang A, Hanna F, Wilson E, Kwok C, et al. A prospective randomised controlled trial of a single intravenous infusion of ferric carboxymaltose, versus single intravenous iron polymaltose or daily oral ferrous sulphate in the treatment of iron deficiency anaemia in pregnancy. Semin Hematol. 2018;55:223–34. [DOI] [PubMed] [Google Scholar]
- 19. Surbek D, Vial Y, Girard T, Breymann C, Bencaiova GA, Baud D, et al. Patient blood management (PBM) in pregnancy and childbirth: literature review and expert opinion. Arch Gynecol Obstet. 2020;301(2):627–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pavord S, Daru J, Prasannan N, Robinson S, Stanworth S, Girling J, et al. UK guidelines on the management of iron deficiency in pregnancy. Br J Haematol. 2019;188:819–30. [DOI] [PubMed] [Google Scholar]
- 21. The cost of blood: multidisciplinary consensus conference for a standard methodology. Transfus Med Rev. 2005;19(1):66–78. [DOI] [PubMed] [Google Scholar]
- 22. Bayoumeu F, Subiran‐Buisset C, Baka NE, Legagneur H, Monnier‐Barbarino P, Laxenaire MC. Iron therapy in iron deficiency anemia in pregnancy: intravenous route versus oral route. Am J Obstet Gynecol. 2002;186(3):518–22. [DOI] [PubMed] [Google Scholar]
- 23. Khalafallah A, Dennis A, Bates J, Bates G, Robertson IK, Smith L, et al. A prospective randomized, controlled trial of intravenous versus oral iron for moderate iron deficiency anaemia of pregnancy. J Intern Med. 2010;268(3):286–95. [DOI] [PubMed] [Google Scholar]
- 24. Shand AW, Bell J, Henry A, Grzeskowiak LE, Kidson‐Gerber G, Pearson S, et al. Rapid increase in intravenous iron therapy for women of reproductive age in Australia. Med J Aust. 2020;213:85–6. [DOI] [PubMed] [Google Scholar]
- 25. VanderMeulen H, Strauss R, Lin Y, McLeod A, Barrett J, Sholzberg M, et al. The contribution of iron deficiency to the risk of peripartum transfusion: a retrospective case control study. BMC Pregnancy Childbirth. 2020;20(1):196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fergusson D, Aaron SD, Guyatt G, Hébert P. Post‐randomisation exclusions: the intention to treat principle and excluding patients from analysis. BMJ. 2002;325(7365):652–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Agarwal PK, Shi L, Daniel LM, Yang PH, Khoo PC, Quek BH, et al. Prospective evaluation of the Ages and Stages Questionnaire 3rd edition in very‐low‐birthweight infants. Dev Med Child Neurol. 2017;59(5):484–9. [DOI] [PubMed] [Google Scholar]
- 28. Christoph P, Schuller C, Studer H, Irion O, De Tejada BM, Surbek D. Intravenous iron treatment in pregnancy: comparison of high‐dose ferric carboxymaltose versus iron sucrose. J Perinat Med. 2012;40(5):469–74. [DOI] [PubMed] [Google Scholar]
- 29. Haider BA, Olofin I, Wang M, Spiegelman D, Ezzati M, Fawzi WW, et al. Anaemia, prenatal iron use, and risk of adverse pregnancy outcomes: systematic review and meta‐analysis. BMJ. 2013;346:f3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rukuni R, Bhattacharya S, Murphy MF, Roberts D, Stanworth SJ, Knight M. Maternal and neonatal outcomes of antenatal anemia in a Scottish population: a retrospective cohort study. Acta Obstet Gynecol Scand. 2016;95(5):555–64. [DOI] [PubMed] [Google Scholar]
- 31. Drew T, Carvalho JCA. Major obstetric haemorrhage. BJA Educ. 2022;22(6):238–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Taylor CL, Brannon PM. Introduction to workshop on iron screening and supplementation in iron‐replete pregnant women and young children. Am J Clin Nutr. 2017;106(Suppl 6):1547S–54S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Auerbach M. Commentary: Iron deficiency of pregnancy – a new approach involving intravenous iron. Reprod Health. 2018;15(Suppl 1):96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Seeho SKM, Morris JM. Intravenous iron use in pregnancy: Ironing out the issues and evidence. Aust N Z J Obstet Gynaecol. 2018;58(2):145–7. [DOI] [PubMed] [Google Scholar]
- 35. Froessler B, Mol B, Dekker G, Hodyl N. Anaemic parturient and the anaesthesiologist: are we asleep at the wheel? Eur J Anaesthesiol. 2017;34(7):405–7. [DOI] [PubMed] [Google Scholar]
- 36. Ferguson MT, Dennis AT. Defining peri‐operative anaemia in pregnant women ‐ challenging the status quo. Anaesthesia. 2019;74(2):237–45. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Table S1
Table S2
Table S3
Appendix S1
Appendix S2
Appendix S3
Appendix S4
Appendix S5
Appendix S6
Appendix S7
Appendix S8
Appendix S9
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


