Abstract
Background
Pain is the most common and bothersome symptom experienced by people with hidradenitis suppurativa (HS) and has been prioritized as an outcome domain by the HIdradenitis SuppuraTiva cORe outcomes set International Collaboration (HISTORIC).
Objectives
To perform a scoping review of pain measurement in randomized control trials (RCTs) of painful skin conditions (PSCs) and use of the pain numerical rating scale (NRS) and visual analogue scale (VAS) in rheumatoid arthritis RCTs, to inform the efforts of HISTORIC to reach consensus on how to measure pain intensity in HS trials.
Methods
A search was conducted on several publication databases. Inclusion criteria were RCTs with a minimum of 10 participants that measured pain intensity.
Results
Pain NRS and VAS were used in 68% of PSC trials. Respectively, 77% and 87% of PSC and rheumatoid arthritis RCTs did not specify the recall window. The commonest recall window in PSCs when specified was 24 h. In total, 33% of PSC trials assessed maximum pain intensity and 3% average pain intensity, while 87% of rheumatoid arthritis trials did not provide details. Pain data were reported as mean difference by 76% of PSC trials and 75% of rheumatoid arthritis trials. Respectively, 10% and 11% of PSC and rheumatoid arthritis studies reported pain as the percentage of patients reaching a desirable state and only 1% and 2% reported number needed to treat.
Conclusions
While pain NRS and VAS are standard methods to measure pain intensity in PSCs, key details such as the recall window are often omitted and there is no consensus on how to report pain NRS data.
What is already known about this topic?
Pain is the most burdensome symptom experienced by patients with hidradenitis suppurativa and has been prioritized as an outcome domain by the HIdradenitis SuppuraTiva cORe outcomes set International Collaboration (HISTORIC).
What does this study add?
Our review shows substantial variation in how pain numerical rating scale (NRS) and visual analogue scale are utilized in clinical trials. This variation restricts meta‐analysis of pain intensity results.
There is a need for consensus regarding the recall window for pain NRS and maximum vs. average pain, and whether current pain should be measured.
The review looked at the tools used to measure pain in painful skin conditions and how Pain NRS and VAS were used in randomized clinical trials (RCTs) of painful skin conditions and rheumatoid arthritis. The results showed a discrepancy in how pain NRS/VAS were measured in the trials. In addition, the mean difference was the most common method of reporting pain.
Plain language summary available online
Hidradenitis suppurativa (HS) is a chronic and painful inflammatory skin condition that causes inflamed nodules, skin tunnels and abscesses affecting the intertriginous areas, and can result in substantial scarring. It is a relatively common condition, affecting approximately 1% of the European population. 1 , 2 , 3 , 4 Several studies have demonstrated the large impact that HS has on quality of life and mental health. 5 , 6 , 7 , 8 Pain was found to be the most frequently reported symptom in HS, with 85% of patients reporting pain as the most bothersome symptom. 9 A global survey showed that two‐thirds of people with HS had experienced moderate‐to‐severe pain over the previous week, with a mean pain numerical rating scale (NRS) score of 5·0. 8 Pain was the highest‐ranked outcome item by the HIdradenitis SuppuraTiva cORe outcomes set International Collaboration (HISTORIC) group and was recommended to form its own domain in the core outcome set. 10 Despite the importance of treating pain, there is little guidance on the management of HS‐related pain in the literature, 11 , 12 , 13 with substantial variation. 14
The current scoping review is part of the efforts of the HISTORIC pain workgroup to identify and validate the most preferred instrument with which to measure pain intensity. Functional effects of pain are measured in other domains, particularly the HS‐specific quality‐of‐life domain, which can now be measured using the Hidradenitis Suppurativa Quality of Life (HiSQOL) instrument. 10 , 15
There are several validated instruments for the measurement of pain. The instruments can be unidimensional, measuring only the intensity of pain, such as pain NRS, visual analogue scale (VAS) and verbal rating scale. Multidimensional instruments measure the intensity, character and impact of pain, for example, the McGill Pain Questionnaire (MPQ) and Brief Pain Inventory (BPI). The pain VAS comprises a horizontal line with a description of two extremes at either end of the line, usually no pain and worst imaginable pain. For the NRS, respondents select an integer from 0 to 10 (or 0 to 100). It should be noted that a VAS with subdivisions is in essence an NRS. The MPQ contains 78 words that cover the neurophysiological and psychological descriptions of pain from which the patient can choose the most suitable terms. 16 The BPI is comprised of 15 questions on the intensity of pain and the functional impact of pain, which are captured on an NRS. 17
Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) recommends that pain intensity be measured using a unidimensional scale, 18 , 19 and HS guidelines frequently suggest that pain is measured using a pain VAS or NRS. 20 , 21 , 22 A scoping review of unidimensional pain measurement instruments found close agreement between NRS and VAS scores, including minimal important difference. 18 NRS showed superiority with regards to compliance, responsiveness, ease of use and applicability compared with both a VAS and verbal rating scale. 18
Lack of detail regarding the frequency of pain measurement, recall window and average vs. maximum pain make it difficult to include results in meta‐analyses, even if the same pain intensity instrument was used. Other interpretation data such as minimal important difference, validated in the population of patients with HS, is also required to determine treatment success or failure in HS clinical trials.
The aim of our review was to identify which instruments have been used to measure pain intensity in clinical trials involving painful skin conditions (PSCs). We then analysed how the pain NRS or VAS, being the most commonly used instruments to measure pain intensity, was used in clinical trials involving PSCs. To widen our scope and learn from other pain measurement research groups we also reviewed how pain NRS or VAS has been used in rheumatoid arthritis (RA) clinical trials, guided by the Outcome Measures in Rheumatology (OMERACT) group recommendations for the measurement of pain in rheumatoid arthritis trials. 23 This will assist us in making recommendations for the optimal instrument and interpretation of results when measuring pain intensity in future HS clinical trials.
Methods
This scoping review is reported according to the PRISMA extension for scoping reviews. The protocol for our scoping review was prospectively registered with PROSPERO, ID CRD42019147311. Changes from our protocol will be addressed in the eligibility section.
Studies included in the review involved RA and the following PSCs: herpes zoster, HS, pyoderma gangrenosum, acne vulgaris, pemphigus vulgaris, epidermolysis bullosa, Stevens–Johnson syndrome, toxic epidermal necrolysis, and calciphylaxis. To be eligible, the study had to be a randomized controlled trial (RCT), have 10 or more participants and measure pain as an outcome. For RA studies, use of pain NRS or VAS was required. Within the PSC section, we included acne split‐face trials and excluded trials for acneiform eruptions. During the screening process, we excluded post‐herpetic neuralgia because the pain is primarily neuropathic and is beyond the scope of this review. However, we did include herpes zoster studies.
Our search terms are outlined in Appendix S1 (see Supporting Information). There were no initial language restrictions and we included RCTs from inception of the database until 10 August 2021. We searched the following databases: MEDLINE, Embase, PsycINFO, Scopus, Web of Science and Cochrane Central Register of Controlled Trials (CENTRAL). The search was conducted with the assistance of a medical information specialist (D.M.). To manage citations obtained through the database search we used EndNote software.
Screening for eligibility using titles and abstracts was done by two authors (S.B.H. and J.J.) with duplicate screening of 20% of the articles for quality assurance. There was a high level of agreement, with arbitration from J.R.I. required for very few papers. The papers remaining after the initial screening were assessed in full and assessed for eligibility. After piloting the data extraction form, two authors (S.B.H. and R.G.) independently assessed the articles and did the data extraction, again with an overlap of 20% for quality assurance. Data collection included the type of trial intervention and comparator, number of participants, and pain measurement instrument (in RCTs of PSCs only). We also collected data on the recall window, average vs. maximum pain, frequency of measurement and the methods of reporting and interpreting pain scores.
Missing data were recorded as absence of the validation information in question. Contacting study authors was not feasible due to the large number of RCTs included in our review. We excluded RCTs published only in abstract form from the analysis of instrument details including recall window, pain intensity and frequency of measurement data to account for word count issues preventing details of pain measurement being included in the report. Abstracts were included in our analysis of which pain measurement instruments had been used and the method of reporting pain data. The data analysis was performed using Microsoft Excel. The results are reported using frequencies with percentages.
Results
Figures 1 and 2 show the PRISMA flowcharts of the articles that were screened, excluded and included in the final data analysis. The search yielded 7154 and 1940 RCTs involving our selected PSCs and RA, respectively. After exclusion of studies not meeting our inclusion criteria, data were extracted from 105 RCTs of PSCs and 245 RA RCTs. The majority of eligible RCTs for PSCs were herpes zoster studies (78%), followed by HS (11%), epidermolysis bullosa (5%), pemphigus vulgaris (3%), acne vulgaris (2%) and pyoderma gangrenosum (1%) (Figure 3). None of the SJS, TEN or calciphylaxis studies met our eligibility criteria.
Figure 1.
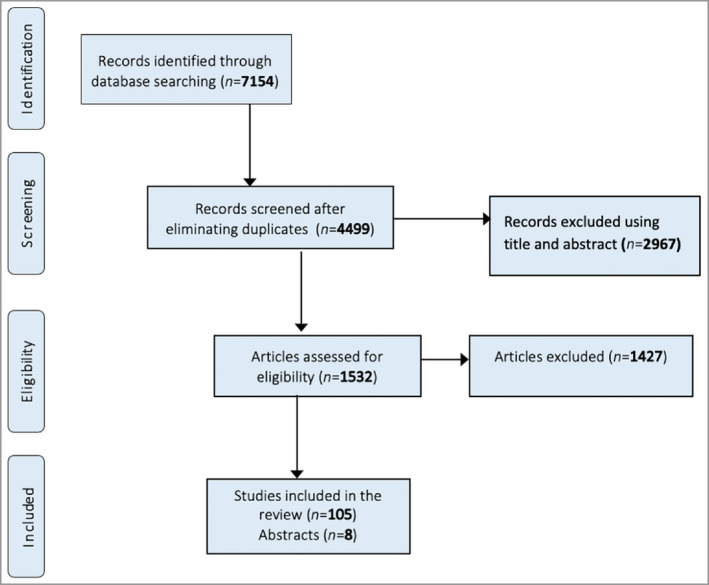
PRISMA flowchart of the search strategy used in the present study for painful skin conditions.
Figure 2.
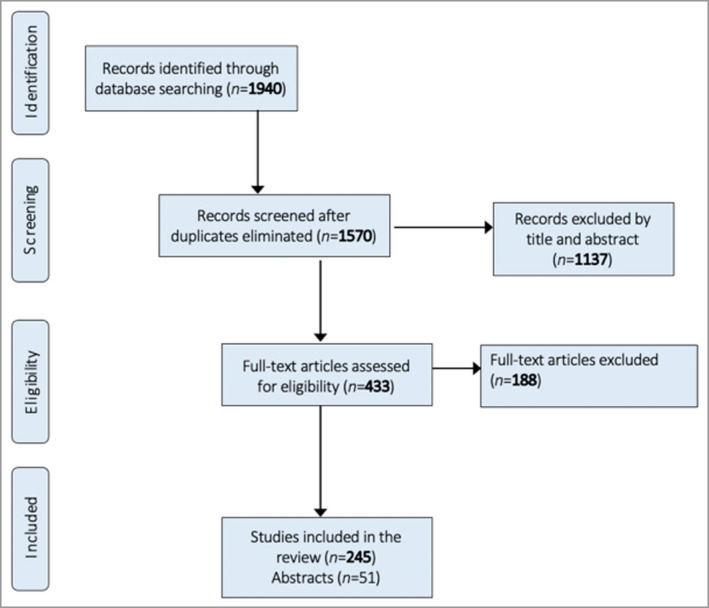
PRISMA flowchart of the search strategy used in the present study for rheumatoid arthritis.
Figure 3.
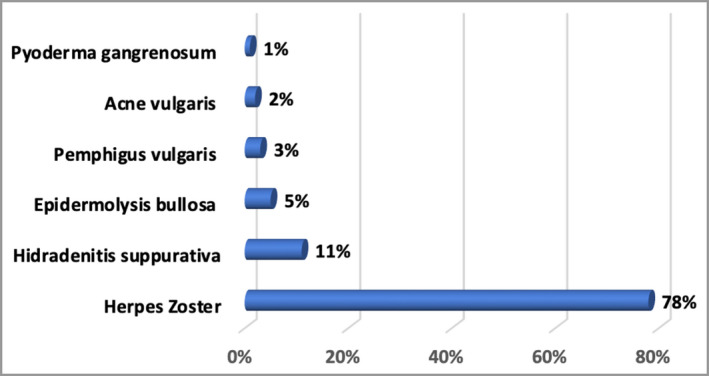
The relative number of studies included in the painful skin conditions element of the review.
Pain NRS and VAS were the most commonly used instruments, included in 68% of the PSC studies. Figure 4 provides a breakdown of the subtypes of NRS or VAS instruments, including 10% using a VAS with no subdivisions and 33% using a VAS with no mention of presence or absence of subdivisions. The remaining third used a diverse range of instruments that are detailed in Table S1 (see Supporting Information).
Figure 4.
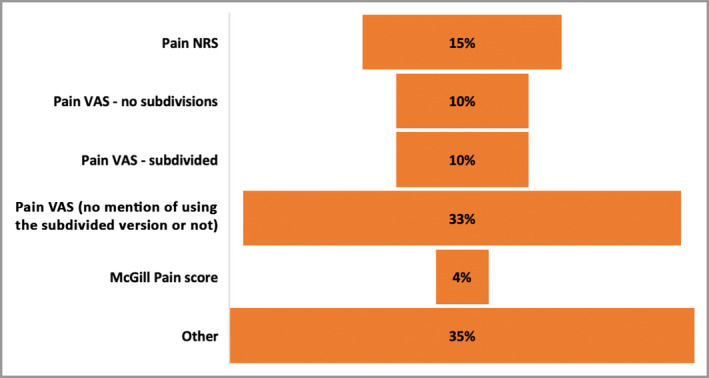
Tools used to measure pain in trials of painful skin conditions. NRS, numerical rating scale; VAS, visual analogue scale.
Overall, 70 PSC studies used pain NRS or VAS instruments, of which eight were published as abstracts (Figure 1). In the RA RCTs, out of a total of 245 articles, 51 were published as abstracts only, without a full report (Figure 2). The included RA studies used pain VAS in 98% and pain NRS in 3% (Figure 5). Overall, 77% of the PSC RCTs did not mention the recall window. Where details were provided, the recall window was 12 h, 24 h or 1 week in 2%, 14% and 2% of the total, respectively (Figure 6). For the RCTs involving RA, 87% did not mention the recall window. The rest used a recall window of 12 h, 24 h, 1 week or other in 3%, 2%, 4% and 5% of the total studies, respectively (Figure 6).
Figure 5.
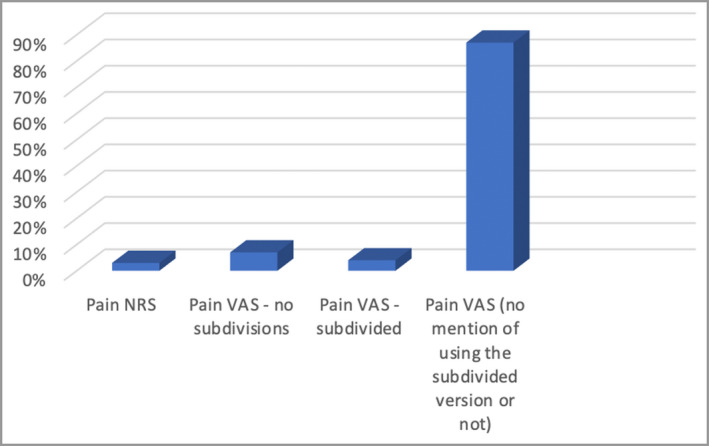
Relative usage of the numerical rating scale (NRS) and visual analogue scale (VAS) to measure pain in rheumatoid arthritis trials.
Figure 6.
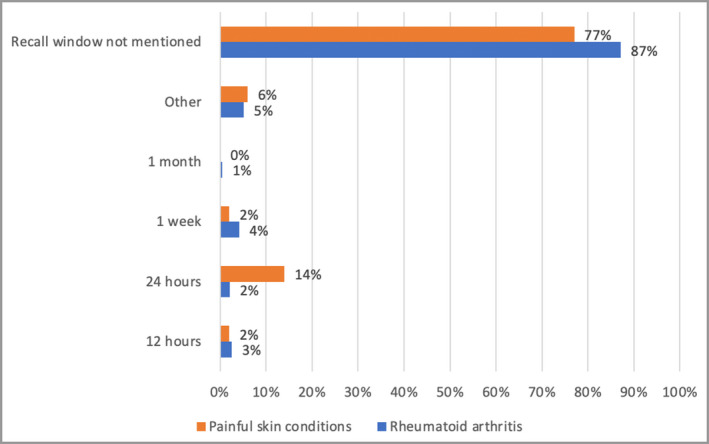
Recall window for pain numerical rating scale and visual analogue scale measurement.
Maximum pain intensity was measured in 33% of PSC RCTs, while 64% did not mention how the pain intensity was measured. In RA RCTs, 87% did not mention whether current, maximum or average pain intensity was measured (Figure 7). The frequency of measuring pain was variable in 45% of PSC studies (Table S2; see Supporting Information) and in 40% of RA RCTs (Figure 8). The most common regular frequencies of pain measurement in PSC RCTs were daily (19%) and at baseline and the end of the trial (19%).
Figure 7.
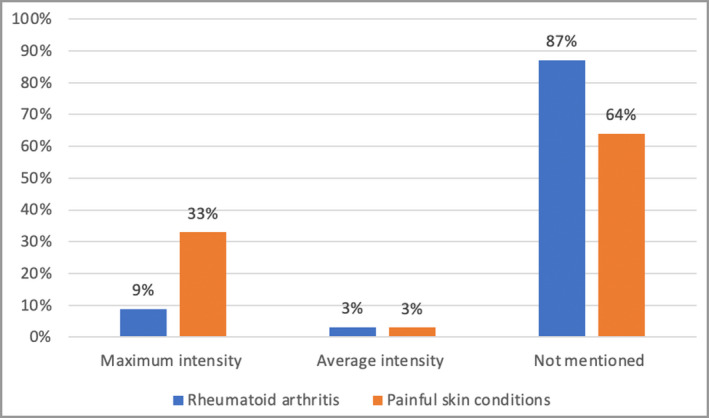
Percentage of randomized controlled trials measuring the maximum vs. the average intensity of pain experienced.
Figure 8.
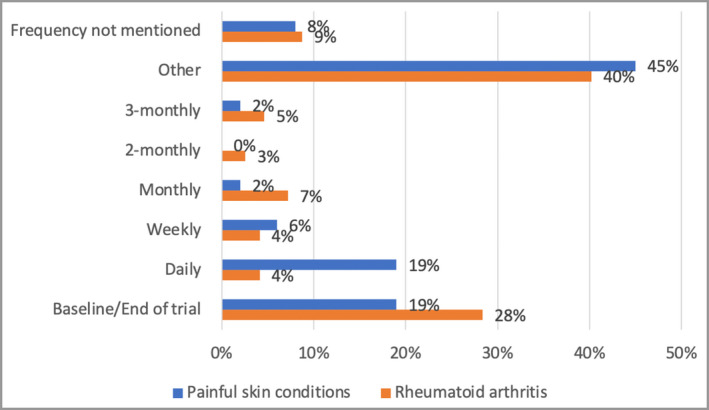
The frequency of measurement of pain.
Table 1 shows the method of interpreting pain scores in PSC and RA RCTs. The most frequent method of reporting pain scores in all RCTs was mean absolute difference compared with baseline or placebo (76% in PSCs and 75% in RA RCTs), followed by the percentage change from baseline, in RA studies (13%). The number needed to treat was reported in 1% and 2% of all PSC and RA trials, respectively.
Table 1.
Methods used to report change in pain score in the randomized controlled trials
| Reporting of pain outcomes | Painful skin conditions (n = 70) | Rheumatoid arthritis (n = 245) |
|---|---|---|
| Percentage of pain change from baseline | 10% (7) | 13% (31) |
| Mean difference (vs. baseline and/or placebo) | 76% (53) | 75% (184) |
| Percentage of patients reaching a desirable state | 10% (7) | 11% (26) |
| Relative risk reduction | 1% (1) | 0 |
| Absolute risk reduction | 0 | 0 |
| Number needed to treat | 1% (1) | 2% (4) |
| Other | 7% (5) | 10% (24) |
Discussion
The majority of the PSC data in our review were from herpes zoster trials (78%). There was a paucity of pain outcome data in RCTs for most of the PSCs included in our review, including HS, confirming a finding from other systematic reviews. 24 , 25 , 26 , 27 None of the calciphylaxis, Stevens–Johnson syndrome or toxic epidermal necrolysis studies met our eligibility criteria, despite pain having a large impact on patient wellbeing.
One of the key findings of our review is that most of the included studies using pain NRS or VAS did not mention the recall window or the type of pain intensity (average or maximum), which prevents full interpretation of the data. The validity of the data collected may be compromised without specific instructions that ensure standardization. 28 Evidence shows that patients’ pain scores are lower when they are asked to assess current pain compared with recalled pain, and almost one‐third of patients find it challenging to rate the average pain VAS over the previous week. 28 Measuring the maximum intensity of pain several times over a specific period will most likely produce more reliable data than a single average assessment. 29 It has been shown that worst pain intensity correlates better with functional interference due to pain, compared with average and least pain scores. 30 The absence of details regarding frequency of pain measurement and recall window and, where provided, the wide variation in these parameters limit meta‐analysis of trial results. Our review highlights a need to find consensus to allow pain intensity results from future studies to be compared.
The majority of studies reported the results as a mean difference or percentage change in pain intensity compared with baseline or placebo. It is generally accepted that a 2‐point reduction in absolute pain NRS score or a 30% improvement represents a clinically important change to the patient, although there is still a lack of complete consensus. 31 , 32 The OMERACT group has reported the minimal important difference for pain scores measured on a VAS from 0 to 100 mm to be a 10‐mm reduction. 23 Pain reported as the percentage of patients reaching a desirable state can provide more interpretable data. 23 , 31 , 33 , 34 , 35 Reporting pain as a dichotomous value allows for analysis of responders vs. nonresponders within the treatment group. In addition, treatment effects in subgroups can be assessed, which may be missed when reporting results as a mean or median for a continuous variable. Overall, number needed to treat is usually regarded as the most useful way to present results. 36
Some limitations of our study need to be considered. Due to the large number of articles generated by our search, it was not feasible to contact the authors to enquire about missing data. To mitigate this issue, RCTs published as abstracts were excluded from the analysis of the frequency of measurement, recall window and type of pain, acknowledging that the restricted word count permitted for an abstract may have impeded complete reporting of pain measurement.
It should be remembered that pain intensity is only one dimension of pain measurement, which also includes functional effects and the character of pain. The six core domains set out by IMMPACT for chronic pain trials are (i) pain NRS, (ii) physical functioning, (iii) emotional functioning, (iv) participant ratings of improvement and satisfaction with treatment, (v) symptoms and adverse events and (vi) participant disposition (treatment compliance and reasons for withdrawal). 37 In HS the physical and emotional impact of pain is already measured elsewhere in the HS core outcome set, via a validated HS‐specific quality‐of‐life instrument, HiSQOL. 15
Pain in HS is a mixture of acute (nociceptive) pain caused by tissue inflammation and chronic (neuropathic) pain, 38 , 39 which can be caused by direct damage to nerves or as a result of central sensitization to pain. 40 A cross‐sectional study of patients with HS has shown that up to one‐third experience neuropathic pain. 38 Pain is interconnected with several other factors including depression, anxiety, quality of life and disability, and treatments should be modified based on individual assessment. 41 , 42 The goal of HISTORIC is to generate the minimum set of outcomes that should be measured in HS clinical trials. Pain NRS or VAS does not distinguish between nociceptive and neuropathic pain, and therefore HS studies focusing on pain management may require further tools to assess pain, such as painDETECT (PD‐Q), which is a validated instrument for screening for neuropathic pain, 38 , 42 and MPQ, which evaluates the quality and intensity of pain. 16 , 43 Moreover, anxiety and depression appear to aggravate chronic pain. 39
Recently the Pain Index was proposed, building on Pain NRS‐11 by multiplying daily pain scores by pain duration over a 30‐day period, to produce a score range from 0 to 300. 44 The Pain Index showed a moderate degree of correlation with International Hidradenitis Suppurativa Severity Score System, hidradenitis suppurativa Physician Global Assessment, and Dermatology Life Quality Index. The Pain Index measures both the intensity and duration of pain; however, missing data could pose an issue when calculating scores.
Further data to help find the optimum method to measure and report pain intensity in patients with HS will be provided by the Treatment of Hidradenitis Suppurativa Evaluation Study (THESEUS), a UK‐wide prospective observational HS study. 45 THESEUS is assessing the feasibility of daily pain measurement using pain NRS. As pain is the most frequent symptom reported during flares there may be a correlation between the two parameters.
In conclusion, our scoping review showed that there is substantial heterogeneity in pain measurement in skin conditions and RA, and sufficient detail to assess whether pain NRS or VAS was used in the same, reproducible way is often lacking. The majority of RCTs reported pain as the mean change, whereas more value could be gained by reporting the proportion reaching a desirable state or number needed to treat. Our review will inform efforts to reach a consensus on how to measure pain in future HS trials.
Funding sources
None.
Conflicts of interest
L.A.V.O. is an investigator for ChemoCentryx; is a consultant to ChemoCentryx, Huron Consulting Group and MedEd Solutions; and has received speaking honoraria from Frontline Medical Communications. J.R.I. receives a stipend as Editor‐in‐Chief of the British Journal of Dermatology and an authorship honorarium from UpToDate. He is a consultant for Boehringer Ingelheim, ChemoCentryx, Novartis and UCB Pharma and has served on advisory boards for Insmed, Kymera Therapeutics and Viela Bio, all in the field of hidradenitis suppurativa (HS). He is co‐copyright holder of HiSQOL, and Investigator Global Assessment and Patient Global Assessment instruments for HS. His department receives income from copyright of the Dermatology Life Quality Index and related instruments.
Author contributions
Samar B Hasan: Conceptualization (equal); data curation (lead); formal analysis (lead); methodology (equal); resources (equal); software (lead); validation (lead); writing – original draft (lead); writing – review and editing (equal). Riham Gendra: Data curation (supporting). JaBreia J James: Data curation (supporting). Delyth Morris: Conceptualization (equal); data curation (equal); methodology (supporting); resources (lead); software (equal); visualization (equal); writing – review and editing (supporting). Lauren Orenstein: Conceptualization (lead); methodology (lead); writing – review and editing (equal). John R Ingram: Conceptualization (lead); methodology (lead); supervision (lead); writing – review and editing (lead).
Ethics statement
Not applicable.
Supporting information
Appendix S1 Search strategy.
Table S1 Other tools used to measure pain in painful skin conditions.
Table S2 Frequency of measuring pain in the ‘others’ group for painful skin conditions.
Plain language summary available online
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Jemec GBE, Heidenheim M, Nielsen NH. The prevalence of hidradenitis suppurativa and its potential precursor lesions. J Am Acad Dermatol 1996; 35:191–4. [DOI] [PubMed] [Google Scholar]
- 2. Ingram JR, Jenkins‐Jones S, Knipe D et al. Hidradenitis suppurativa prevalence and disease associations using the Clinical Practice Research Datalink. J Invest Dermatol 2016; 136 (Suppl. 2):S165. [Google Scholar]
- 3. Vinding GR, Miller IM, Zarchi K et al. The prevalence of inverse recurrent suppuration: a population‐based study of possible hidradenitis suppurativa. Br J Dermatol 2014; 170:884–9. [DOI] [PubMed] [Google Scholar]
- 4. Revuz JE, Canoui‐Poitrine F, Wolkenstein P et al. Prevalence and factors associated with hidradenitis suppurativa: results from two case–control studies. J Am Acad Dermatol 2008; 59:596–601. [DOI] [PubMed] [Google Scholar]
- 5. Von Der Werth JM, Jemec GBE. Morbidity in patients with hidradenitis suppurativa. Br J Dermatol 2001; 144:809–13. [DOI] [PubMed] [Google Scholar]
- 6. Vinding GR, Knudsen KM, Ellervik C et al. Self‐reported skin morbidities and health‐related quality of life: a population‐based nested case–control study. Dermatology 2014; 228:261–8. [DOI] [PubMed] [Google Scholar]
- 7. Thorlacius L, Cohen AD, Gislason GH et al. Increased suicide risk in patients with hidradenitis suppurativa. J Invest Dermatol 2018; 138:52–7. [DOI] [PubMed] [Google Scholar]
- 8. Garg A, Neuren E, Cha D et al. Evaluating patients’ unmet needs in hidradenitis suppurativa: results from the Global Survey Of Impact and Healthcare Needs (VOICE) Project. J Am Acad Dermatol 2020; 82:366–76. [DOI] [PubMed] [Google Scholar]
- 9. Kimball A, Okun M, Sundaram M, Bal V. Patients’ experiences with hidradenitis suppurativa: a qualitative study of symptoms and impacts. J Am Acad Dermatol 2013; 68 (Suppl. 1):AB57. [Google Scholar]
- 10. Thorlacius L, Ingram JR, Villumsen B et al. A core domain set for hidradenitis suppurativa trial outcomes: an international Delphi process. Br J Dermatol 2018; 179:642–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Horváth B, Janse IC, Sibbald GR. Pain management in patients with hidradenitis suppurativa. J Am Acad Dermatol 2015; 73 (Suppl. 1):S47–51. [DOI] [PubMed] [Google Scholar]
- 12. Savage KT, Singh V, Patel ZS et al. Pain management in hidradenitis suppurativa and a proposed treatment algorithm. J Am Acad Dermatol 2021; 85:187–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Scheinfeld N. Treatment of hidradenitis suppurativa associated pain with nonsteroidal anti‐inflammatory drugs, acetaminophen, celecoxib, gabapentin, pegabalin, duloxetine, and venlafaxine. Dermatol Online J 2013; 19:20616. [PubMed] [Google Scholar]
- 14. Jedrzejczak MJ, Ingram JR, Lowes MA et al. Expert knowledge, attitudes, and practices in management of hidradenitis suppurativa pain. JAMA Dermatol 2021; 157:464–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kirby JS, Thorlacius L, Villumsen B et al. The Hidradenitis Suppurativa Quality of Life (HiSQOL) score: development and validation of a measure for clinical trials. Br J Dermatol 2020; 183:340–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ngamkham S, Vincent C, Finnegan L et al. The McGill Pain Questionnaire as a multidimensional measure in people with cancer: an integrative review. Pain Manage Nurs 2012; 13:27–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kumar SP. Utilization of brief pain inventory as an assessment tool for pain in patients with cancer: a focused review. Indian J Palliat Care 2011; 17:108–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hjermstad MJ, Fayers PM, Haugen DF et al. Studies comparing numerical rating scales, verbal rating scales, and visual analogue scales for assessment of pain intensity in adults: a systematic literature review. J Pain Symptom Manage 2011; 41:1073–93. [DOI] [PubMed] [Google Scholar]
- 19. Dworkin RH, Turk DC, Farrar JT et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain 2005; 113:9–19. [DOI] [PubMed] [Google Scholar]
- 20. Ingram JR, Collier F, Brown D et al. British Association of Dermatologists guidelines for the management of hidradenitis suppurativa (acne inversa) 2018. Br J Dermatol 2019; 180:1009–17. [DOI] [PubMed] [Google Scholar]
- 21. Alavi A, Lynde C, Alhusayen R et al. Approach to the management of patients with hidradenitis suppurativa: a consensus document. J Cutan Med Surg 2017; 21:513–24. [DOI] [PubMed] [Google Scholar]
- 22. Zouboulis CC, Desai N, Emtestam L et al. European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa. J Eur Acad Dermatol Venereol 2015; 29:619–44. [DOI] [PubMed] [Google Scholar]
- 23. Busse JW, Bartlett SJ, Dougados M et al. Optimal strategies for reporting pain in clinical trials and systematic reviews: recommendations from an OMERACT 12 workshop. J Rheumatol 2015; 42:1962–70. [DOI] [PubMed] [Google Scholar]
- 24. Ingram JR, Woo PN, Chua SL et al. Interventions for hidradenitis suppurativa: a Cochrane systematic review incorporating GRADE assessment of evidence quality. Br J Dermatol 2016; 174:970–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Langan SM, Williams HC. A systematic review of randomized controlled trials of treatments for inherited forms of epidermolysis bullosa. Clin Exp Dermatol 2009; 34:20–5. [DOI] [PubMed] [Google Scholar]
- 26. Partridge ACR, Bai JW, Rosen CF et al. Effectiveness of systemic treatments for pyoderma gangrenosum: a systematic review of observational studies and clinical trials. Br J Dermatol 2018; 179:290–5. [DOI] [PubMed] [Google Scholar]
- 27. Martin LK, Werth VP, Villaneuva EV, Murrell DF. A systematic review of randomized controlled trials for pemphigus vulgaris and pemphigus foliaceus. J Am Acad Dermatol 2011; 64:903–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Broderick JE, Stone AA, Calvanese P et al. Recalled pain ratings: a complex and poorly defined task. J Pain 2006; 7:142–9. [DOI] [PubMed] [Google Scholar]
- 29. Jensen MP, McFarland CA. Increasing the reliability and validity of pain intensity measurement in chronic pain patients. Pain 1993; 55:195–203. [DOI] [PubMed] [Google Scholar]
- 30. Harris K, Li K, Flynn C, Chow E. Worst, average or current pain in the Brief Pain Inventory: which should be used to calculate the response to palliative radiotherapy in patients with bone metastases? Clin Oncol 2007; 19:523–7. [DOI] [PubMed] [Google Scholar]
- 31. Farrar JT, Young JP, LaMoreaux L et al. Clinical importance of changes in chronic pain intensity measured on an 11‐point numerical pain rating scale. Pain 2001; 94:149–58. [DOI] [PubMed] [Google Scholar]
- 32. Farrar JT, Portenoy RK, Berlin JA et al. Defining the clinically important difference in pain outcome measures. Pain 2000; 88:287–94. [DOI] [PubMed] [Google Scholar]
- 33. Dworkin RH, Turk DC, McDermott MP et al. Interpreting the clinical importance of group differences in chronic pain clinical trials: IMMPACT recommendations. Pain 2009; 146:238–44. [DOI] [PubMed] [Google Scholar]
- 34. Farrar JT. What is clinically meaningful: outcome measures in pain clinical trials. Clin J Pain 2000; 16 (2 Suppl.):S106–12. [DOI] [PubMed] [Google Scholar]
- 35. Moore RA, Moore OA, Derry S et al. Responder analysis for pain relief and numbers needed to treat in a meta‐analysis of etoricoxib osteoarthritis trials: bridging a gap between clinical trials and clinical practice. Ann Rheum Dis 2010; 69:374–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cook RJ, Sackett DL. The number needed to treat: a clinically useful measure of treatment effect. BMJ 1995; 310:452–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Turk DC, Dworkin RH, Allen RR et al. Core outcome domains for chronic pain clinical trials: IMMPACT recommendations. Pain 2003; 106:337–45. [DOI] [PubMed] [Google Scholar]
- 38. Garcovich S, Muratori S, Moltrasio C et al. Prevalence of neuropathic pain and related characteristics in hidradenitis suppurativa: a cross‐sectional study. J Clin Med 2020; 9:4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nielsen RM, Lindsø Andersen P, Sigsgaard V et al. Pain perception in patients with hidradenitis suppurativa. Br J Dermatol 2020; 182:166–74. [DOI] [PubMed] [Google Scholar]
- 40. van Straalen KR. Chronic pain in hidradenitis suppurativa explained through the process of central sensitization. JAMA Dermatol 2020; 156:615–16. [DOI] [PubMed] [Google Scholar]
- 41. Patel ZS, Hoffman LK, Buse DC et al. Pain, psychological comorbidities, disability, and impaired qualify of life in hidradenitis suppurativa [corrected]. Curr Pain Headache Rep 2017; 21:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Freynhagen R, Baron R, Gockel U, Tölle TR. painDETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin 2006; 22:1911–20. [DOI] [PubMed] [Google Scholar]
- 43. Melzack R. The McGill Pain Questionnaire: major properties and scoring methods. Pain 1975; 1:277–99. [DOI] [PubMed] [Google Scholar]
- 44. Zouboulis CC. Pain Index: a new prospective hidradenitis suppurativa patient‐reported outcome measure instrument. Br J Dermatol 2021; 184:1203–4. [DOI] [PubMed] [Google Scholar]
- 45. Cardiff University . THESEUS. Available at: https://www.cardiff.ac.uk/centre‐for‐trials‐research/research/studies‐and‐trials/view/theseus (last accessed 15 August 2022).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Search strategy.
Table S1 Other tools used to measure pain in painful skin conditions.
Table S2 Frequency of measuring pain in the ‘others’ group for painful skin conditions.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


