Abstract
A pharmacokinetic/pharmacodynamic (PK/PD) model was developed to describe the time course of writhings after intraperitoneal injection of acetic acid in mice. The model was applied to investigate the antinociceptive effect of trazodone and gabapentin alone and in combination. Writhings time course was described by a transit compartment model with the delay due to the transit of the acetic acid being represented by a chain of intermediate compartments. In the drug‐treated animals, the number of writhings decreases according to a k 2 factor linking drug concentration and antinociceptive effect. Compounds' potency parameters were 10.9 and 0.0459 L/μmoles/min for trazodone and gabapentin, respectively, indicating a much higher in vivo potency of trazodone in the PD writhing test. The PK/PD parameters were used to simulate the expected writhing counts in mice at combined doses without efficacy alone, assuming pharmacological additivity. Simulation results indicated that, at low dose combinations, experimental data were mostly below the simulated writhings median, suggesting possible synergic effect. Such hypothesis was tested by adding the γ parameter in the PK/PD model to represent the deviation from the assumption of no‐interaction, leading to a reduction of the objective function compared to the additive model. On this basis, several simulations were performed to identify possible starting dose combinations of trazodone and gabapentin in humans, by selecting doses yielding systemic exposures close to those being synergic in the mouse. Simulations indicated that doses of 50–100 mg trazodone could enhance gabapentin antinociceptive effect in humans, supporting the development of a low dose combination for optimal analgesia treatment.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Diabetic peripheral polyneuropathy is a common symptomatic complication in patients with types 1 and 2 diabetes to which neither single nor combined drug therapies can provide adequate pain relief. Low doses of trazodone (50–100 mg) have shown good efficacy in relieving pain in patients with diabetes affected by peripheral neuropathy or painful distal symmetrical polyneuropathy.
WHAT QUESTION DID THIS STUDY ADDRESS?
The present study allowed to characterize the synergistic interaction between trazodone and gabapentin in reducing nociception in an acute pain model in mice and provided a guide for selecting equally effective doses to be administered to humans in early discovery phases.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
The currently developed pharmacokinetic/pharmacodynamic (PK/PD) model led to the application of a model‐based translational approach to provide indications for the clinical doses of trazodone and gabapentin expected to be clinically active when given in combination in humans.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
The presented PK/PD modeling approach represents a method to allow for ranking the order of the in vivo potency of new compounds in the writhing test in mice to characterize and to quantify the additivity/synergistic effect of new combinations using time‐dependent data.
INTRODUCTION
Neuropathic pain is a chronic and debilitating condition of heterogeneous etiologies, symptoms, and underlying mechanisms. 1 Due to the small number of approved drugs and their limited clinical efficacy, the current therapeutic approach is to combine two or more drugs with different pharmacological mechanisms to simultaneously act on the intensity of pain. 2 , 3
Gabapentin, approved for some neuropathic pain forms, is a selective inhibitor of the α2‐δ subunits of voltage‐sensitive calcium channels 4 that are implicated in reducing neuronal excitability and modulation of neurotransmitter release. However, its use is accompanied by dose‐limiting side effects, requiring prolonged dose titration. Antidepressant drugs are commonly used in the clinical setting for the treatment of chronic pain 5 and combinations of antidepressants with gabapentin have shown superiority to gabapentin monotherapy both in preclinical 6 , 7 and clinical settings. 8 , 9 , 10
Trazodone is a widely used drug for the treatment of major depression, with a mild adverse effect profile. Its pharmacological action is mainly carried out through the block of the post‐synaptic serotonin (5‐HT) receptors 5‐HT2A and 5‐HT2C and the inhibition of the presynaptic 5‐HT reuptake transporter. 11 Trazodone, tested in the chronic constriction injury (CCI) rat model of chronic pain, 12 showed a dose‐dependent analgesic effect on thermal hyperalgesia. 13
The hypothesis that trazodone and gabapentin have a synergistic antinociceptive effect when concomitantly administered, reducing neurological pain, was demonstrated in a recent paper using the writhing test and an isobolographic analysis 14 and also reported in the fixed‐dose combination patent application. 15
The objective of the present study was to develop a pharmacokinetic/pharmacodynamic (PK/PD) model to be used for providing an insight into the analgesic effect of the combination of low doses of trazodone and gabapentin in humans.
In the present paper, the inhibition of writhing counts elicited by the intraperitoneal injection of acetic acid in mice was evaluated after treatment with trazodone and gabapentin in order to assess whether the administration of trazodone was able to enhance the antinociceptive effect of gabapentin.
METHODS
Animals
Experiments were performed on male CD‐1 mice weighing 20–25 g (Charles River, Italy). Room temperature and relative humidity were set at 22 ± 2°C and 55 ± 15%, respectively, and the lighting was controlled on a cycle of 12‐h light and 12‐h darkness. Food and water were freely available, except during the experimental procedure. All experimental sessions were performed between 9:00 a.m. and 1:00 p.m. to avoid diurnal variation in the behavioral tests. Each pain test was performed by an experimenter blinded to the treatments.
The experiments were carried out in accordance with the guidelines established by the European Communities Council Directive (Directive 2010/63/EU of 22 September 2010) and approved by the National Council on Animal Care of the Italian Ministry of Health (Authorization no. 59/2013‐B). All efforts were made to minimize animal suffering and to use the minimal number of animals required to produce reliable results. After the behavioral test session, animals were euthanized by CO2 inhalation according to the Italian legislation.
Drugs
Trazodone (Angelini S.p.A.) and gabapentin (Sigma‐Aldrich) were used. These drugs and their combination were dissolved in 0.9% NaCl and injected by gavage (trazodone) or intraperitoneally (gabapentin). Acetic acid was purchased from Sigma‐Aldrich, dissolved in 0.9% NaCl, and injected intraperitoneally (i.p.).
Acetic acid‐induced writhing test in mice
Fasted with water ad libitum male CD‐1 mice were i.p. injected with 16 μl/g body weight of 0.7% acetic acid and the number of writhings was counted over a 5 min period for 30 min starting immediately after administration of the acetic acid solution. The writhe was defined as a contraction of the abdominal muscles accompanied by an elongation of the body and extension of the hindlimbs. Drugs (or the vehicle in the control group) were administered 1 h before the acetic acid injection. Trazodone was administered orally (o.s.) as a single agent at 0.075, 0.15, 0.5, 1.5, 2.5, and 4.5 mg/kg; gabapentin was given i.p. as a single agent at 1.5, 5, and 15 mg/kg. The two compounds were co‐administered at the following doses of trazodone and gabapentin, respectively: 0.075–1.5, 0.15–1.5, 0.5–5, and 1.5–15 mg/kg. Doses were administered in 10 ml/kg body weight.
The doses to be tested for the present work were selected based on the results obtained through an isobolographic analysis in mice writhing test, which demonstrated that the administration of trazodone in combination with gabapentin exerted a relevant antinociceptive activity when suboptimal doses of trazodone (0.1–3 mg/kg, o.s.) were associated with suboptimal doses of gabapentin (1–100 mg/kg, i.p.). 14
Pharmacokinetic data
For nonclinical evaluations, the PKs of trazodone (1, 3, and 10 mg/kg) and gabapentin (10, 30, and 100 mg/kg) were investigated as single agents in two satellite groups of male mice (n = 12 animals per group) under fasting conditions. Twelve timepoints were selected (up to 24 h postdosing) for taking blood samples by means of a sparse data scheme to properly characterize the PK profiles of both trazodone and gabapentin. In a separate set of nonclinical analyses, the two compounds were administered alone and in combination at low (1 mg/kg trazodone + 3 mg/kg gabapentin) and high doses (10 mg/kg trazodone + 30 mg/kg gabapentin) to verify the absence of PK interactions.
For clinical dose evaluations, the PKs of trazodone was characterized using data from a phase I clinical study in which the compound was orally administered at 50 mg immediate release (IR) tablets and 30, 60, and 90 mg 6% drops to a total of 23 volunteers aged 22–54 years under fasting conditions, according to a randomized four‐way crossover design, with washout intervals of 7 days between consecutive administrations. 16 The evaluation of gabapentin PKs in humans was based on literature data, 17 where the product was orally administered at a mean daily dose of 1968 mg (min–max: 600–3600 mg) to 16 adult patients with neuropathic pain (average age: 48.7 years), with median creatinine clearance of 90.5 ml/min (min–max: 54.3–117 ml/min).
PK/PD data analysis
The PK and PD parameters of trazodone and gabapentin given alone or co‐administered were estimated using nonlinear mixed‐effect modeling as implemented in NONMEM software (version VI). 18 The population analysis approach, which takes into account both interindividual (η) and intra‐individual (ε) variability, was undertaken using the first‐order conditional estimation method with η − ε interaction.
Data manipulation and graphical data display were performed using XPOSE4 (version 4.0.1), Microsoft Office Excel 2003 and Sigma Plot (Systat Software version 10 or later). Standard non‐compartmental analysis (NCA) was performed using Phoenix WinNonlin (Certara L.P. version 6.3). Selection criteria during the model development process were based on the goodness‐of‐fit plots, changes in objective function value (OFV), parameter estimates, and their respective percent standard error (%SE). Using the likelihood ratio test, the significance was set at α = 0.01, which corresponds to a reduction of 6.64 units in the OFV (χ2 distribution) to discriminate between two nested structural models after the inclusion of one additional parameter.
Pharmacokinetic model
Population PK models were used to describe the time courses of both trazodone and gabapentin in mice and only trazodone in humans. The PK parameters were assumed to be log‐normally distributed, therefore an exponential distribution model was used to account for interindividual variability. Proportional error models were used to quantify residual error. The effect of the covariate formulation (50 mg IR tablets, 30–60–90 mg 6% drops) was investigated for trazodone PKs.
For gabapentin, the paper from Carlsson et al. 17 was used as a reference, where a population PK model for gabapentin was developed to appropriately monitor patients with neuropathic pain and for individualizing their dose regimens. Bioavailability was included in a one‐compartment disposition model as a function of dose using a hyperbolic function.
During model development, special attention was paid to investigate the PK interaction between trazodone and gabapentin in mice, in order to exclude that the pharmacological effect of the combination on writhe counts could result from a modification of the plasma concentrations of the compounds. The plasma levels of the two compounds administered alone and co‐administered were simultaneously fitted introducing for each PK parameter, one at a time, an interaction term for the concomitant treatment. The interaction term was retained in the PK model if the resulting reduction of the objective function was statistically significant using a stepwise forward additive (p value < 0.05, χ2 distribution) and a stepwise backward elimination (p value < 0.01) procedure.
Pharmacodynamic model
Unperturbed model (control group)
The time course of writhings onset following acid acetic administration was first described in the non‐treated (control) animals, showing a delay between the acid acetic injection (i.e., stimulus) and the maximum increase of writhings. This behavior was described by a transit compartment model, a commonly used model‐building strategy for time‐delayed phenomena, in which the delay in an observable event (such as writhings) arises as a consequence of the signal transit through a chain of intermediate compartments before reaching the observation compartment. The structure of the PK‐PD model is shown in Figure S1A.
In the model, W denotes the number of writhings, S represents the acid acetic stimulus at the starting time (t = 0), n is the number of transit compartments (a 0, a 1, …a n−1, a n ), while k tr and k are the transfer rate and the elimination rate constant, respectively. The optimal number of transit compartments best describing the writhing profiles in the control animals was identified as nine (a 1, …a 9). Overall, the total numbers of model compartments were equal to 11, including the first compartment where the stimulus was applied (a 0) and the observation compartment (W).
Perturbed model (treated groups)
In the animals individually treated with trazodone or gabapentin, the model assumes that the analgesic treatment elicits its effect by decreasing the number of writhings by a factor proportional to the drug concentration C(t) of each compound acting on the compartment a0 where the stimulus is applied through the constant parameter k 2, which is, therefore, an index of drug efficacy describing the relationship between the drug concentrations and antinociceptive effect (Figure S1B).
When trazodone and gabapentin are administered concomitantly, the total number of writhings is reduced by the sum of the two drugs' effects in agreement with the null‐interaction hypothesis for the additivity of the effects through the equation: k 2A C A(t) + k 2B C B(t) (Figure S1C).
The synergic effect is considered by the inclusion of the additional model parameter γ, acting in a multiplicative manner with the plasma concentration of the two compounds, as described in the following equation: k 2ACA(t) + k 2BCB(t) + γ*CA(t) CB(t) (Figure S1D).
The population PK/PD analysis was performed using a sequential approach. Population PK parameters of trazodone or gabapentin, PD control parameters (S, k tr, k, and n) and corresponding variances were previously estimated using the control group and then fixed in the PK/PD model. Finally, the PK/PD model estimated the value of k 2 of both compounds (k 2A and k 2B), the related interindividual variance and the synergic γ parameter. A more detailed description of the PK and PK/PD models is reported in Table S1.
Prediction of human pharmacological effect
The prediction of human pharmacological effect has been performed using the synergic PK/PD model on human doses producing low systemic exposures close to those found to be synergic in the mouse writhing test.
The exposures of trazodone (Table S2) were corrected to take into account the different plasma protein binding values between human and mouse (i.e., 97% and 89%, respectively [internal unpublished data]). Normalization aimed at considering the same free fraction of trazodone in both species. No correction for plasma protein binding was introduced for gabapentin, because the drug is not protein‐bound in all animal species, including mice and humans. 19 Prediction of the pharmacological effect of the drug combination in humans was based on simulated plasma concentration‐time curves of trazodone and gabapentin given alone and in combination (Table S3). One thousand virtual patients were generated for each dose group or combination of doses and the simulated percent of inhibition of the writhing counts within each individual was evaluated considering a first phase where the stimulus was applied without compound/s, followed by a second phase where the compound/s were administered (or co‐administered) 1 h before the stimulus. Between the two phases, the model compartments were reset and re‐initialized from the effect of the previous stimulus. The percent of inhibition was calculated as the ratio of the two writhing count areas (in terms of area under the curve) with and without drug administration.
Statistical analysis
An analysis of variance for repeated measures followed by Dunnett's test for multiple comparisons versus vehicle‐treated group was performed for each writhing experiment.
RESULTS
A model with zero‐order absorption (mice) or first‐order absorption rate with lag‐time (humans) and two compartments in disposition was selected for trazodone and gabapentin PKs in mice and trazodone in humans.
Trazodone and gabapentin rapid achievement of the maximum plasma concentrations and the characteristics of their distribution and elimination phases were well‐described by the PK model developed in mice for trazodone and gabapentin. Individual and population predicted values versus observed concentrations were indicative of an adequate model performance (Table 1A and B, Figure S2).
TABLE 1.
Trazodone and gabapentin population PK parameter estimates in mice
| Parameter | Units | Estimate | %CV or RSV% | %SE | Parameter | Units | Estimate | %CV or RSV% | %SE | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (a) Trazodone | (b) Gabapentin | ||||||||||
| k | h−1 | 1.25 | – | 4.48 | k | h−1 | 0.112 | – | 58.8 | ||
| V 1/F | L/kg | 5.95 | – | 6.30 | V 1/F | L/kg | 0.846 | – | 4.65 | ||
| k 12 | h−1 | 0.982 | – | 19.8 | k 12 | h−1 | 0.935 | – | 17.8 | ||
| k 21 | h−1 | 1.51 | – | 19.9 | k 21 | h−1 | 0.110 | – | 15.2 | ||
| D 1 | h | 0.0833 | – | – | D 1 | h | 0.167 | – | – | ||
| Inter‐individual variability | |||||||||||
|
|
0.0526 | 22.9 | 59.9 |
|
0.00287 | 5.36 | >100 | ||||
|
|
0.00899 | 9.48 | 192 |
|
0.337 | 58.1 | >100 | ||||
|
|
0.577 | 76.0 | 29.6 |
|
1.43 | 120 | 35 | ||||
| Intra‐individual variability | |||||||||||
|
|
0.0752 | 27.4 | 37.8 |
|
0.0598 | 24.5 | >100 | ||||
Note: Population PK parameters were estimated in mice using data of trazodone administered at 1, 3, and 10 mg/kg (panel a) and gabapentin administered at 10, 30, and 100 mg/kg (panel b).
Abbreviation: %CV, percent coefficient of variation; %SE, percent standard error; D 1, duration of the zero‐order absorption; k: elimination rate constant; k 12, k 21, intercompartment rate constants; V 1/F, volume of distribution of the central compartment; ω, interindividual variability; σ, intra‐individual variability.
There was a lack of PK interaction between the two compounds in mice, as the interaction term did not statistically decrease the objective function when applied in a stepwise sequential manner on each of the PK parameters of the two models (Tables S4 and S5). In addition, for both trazodone and gabapentin, the PK parameters calculated using the NCA on the model‐predicted concentrations were in good agreement with those calculated using the experimental values (Table S6).
The unperturbed model fitted well the individual writhing profiles of control animals observed in trazodone and gabapentin experiments. The control PD model parameters, reported in Table 2 with a sample of the fitting of the controls shown in Figure S3, indicated a moderate interindividual variability for the S and k parameters and a very low variability for the number of compartments n.
TABLE 2.
Unperturbed writhing PD model parameters
| Parameter | Units | Estimate | %CV | %SE | |
|---|---|---|---|---|---|
| k tr | 1/min | 1.36 | – | 2.12 | |
| S | – | 26.10 | – | 4.29 | |
| k | 1/min | 0.104 | – | 5.83 | |
| n | – | 9 | – | – | |
| Interindividual variability | |||||
|
|
– | 0.0231 | 15.2 | 36.1 | |
|
|
– | 0.0349 | 18.7 | 49.3 | |
|
|
– | 0.00848 | 9.2 | 52.7 | |
| Intra‐individual variability | |||||
|
|
– | 2.79 | 14.4 | ||
Abbreviations: %CV, percent coefficient of variation; %SE, percent standard error; k, elimination rate constant; k tr, transfer rate; n, number of transit compartments; PD, pharmacodynamic; S, acid acetic stimulus; ω, interindividual variability; σ, intra‐individual variability.
The PK/PD model was applied to the individual writhing count for estimating the potency parameter k 2 of trazodone and gabapentin as single agents (Table 3). The rate of the transitions between compartments and the rate of exit from the observation PD compartment were fixed to the same value as the unperturbed group. This way, the PK/PD model is highly simplified with a lesser number of parameters to be estimated.
TABLE 3.
Perturbed writhing PK/PD model parameters obtained from single‐agent administration
| Single model | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Trazodone | Gabapentin | ||||||||||
| Parameter | Units | Estimate | %CV | %SE | Parameter | Units | Estimate | %CV | %SE | ||
| k 2 | L/μmoles/min | 10.9 | 22.9 | k 2 | L/μmoles/min | 0.0459 | 32.2 | ||||
| Interindividual variability | Interindividual variability | ||||||||||
|
|
1.42 | 119.2 | 27.8 |
|
1.97 | 140.4 | 27.8 | ||||
| Intra‐individual variability | Intra‐individual variability | ||||||||||
|
|
3.20 | 19.8 |
|
2.17 | 17.1 | ||||||
Note: Model parameters results of trazodone using PD data from 0.075, 0.15, 0.5, 1.5, 2.5, and 4.5 mg/kg and gabapentin using PD data from 1.5, 5 and 15 mg/kg.
Abbreviations: %CV, percent coefficient of variation; %SE, percent standard error; k 2, potency parameter; PK/PD, pharmacokinetic/pharmacodynamic; ω, interindividual variability; σ, intra‐individual variability.
The potency parameter was widely different between the two compounds, being 10.9 and 0.0459 L/μmoles/min for trazodone and gabapentin, respectively, and indicating a much higher in vivo potency of trazodone in the PD writhing model compared to gabapentin. Variance terms indicated a large interindividual variability in the k 2 for trazodone and gabapentin (percent coefficient of variation = 119 and 140, respectively). All model parameters were accurately estimated (as shown by the values of %SE reported in Table 3).
The previously reported isobolographic analysis in the mice writhing test demonstrated that trazodone and gabapentin combination has a synergic antinociceptive effect. 14
In the present work, as reported in Figure S4, when two ineffective doses of trazodone and gabapentin were co‐administered (Figure S4A, trazodone 0.075 mg/kg + gabapentin 1.5 mg/kg; Figure S4B, trazodone 0.15 mg/kg + gabapentin 1.5 mg/kg), a marked and significant antinociception was observed (p < 0.01, p < 0.001 vs. vehicle treated group), confirming a synergistic interaction. On the contrary, when animals were treated with trazodone and gabapentin at fully active doses (Figure S4C, trazodone 0.5 mg/kg + gabapentin 5 mg/kg; Figure S4D, trazodone 1.5 mg/kg + gabapentin 15 mg/kg), a significant reduction of the writhes number was observed both with the drugs given alone and when co‐administered (p < 0.001 vs. vehicle treated group).
The same experimental dose groups were used to simulate the expected writhing numbers of 100 mice assuming additivity.
The results of the simulations indicated that most of the experimental writhing counts were below the simulated median values at the doses 0.075 mg/kg (trazodone) + 1.5 mg/kg (gabapentin) and 0.15 mg/kg (trazodone) + 1.5 mg/kg (gabapentin; Figure 1A,B), whereas a more even distribution of counts above and below the simulated median was shown for the two higher dose combinations (Figure 1C,D). This behavior further suggested a deviation from additivity at the two lower dose combinations.
FIGURE 1.
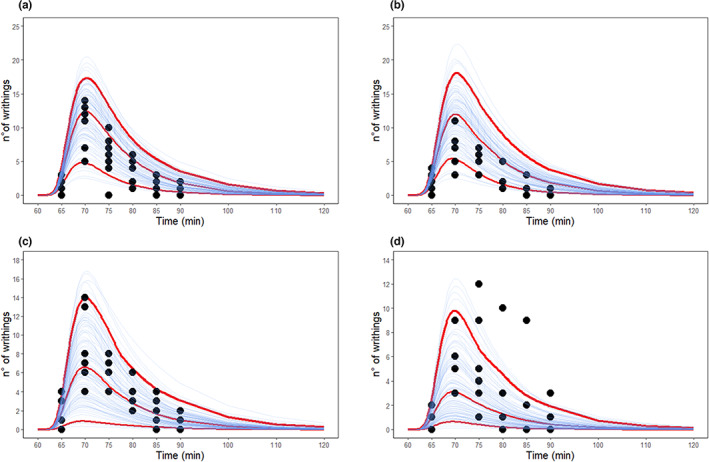
Simulation of number of writhings using the perturbed PK/PD model and assuming additivity. (a) The 0.075 mg/kg trazodone + 1.5 mg/kg gabapentin; (b) 0.15 mg/kg trazodone + 1.5 mg/kg gabapentin; (c) 0.5 mg/kg trazodone + 5 mg/kg gabapentin; (d) 1.5 mg/kg trazodone + 15 mg/kg gabapentin. Red lines: 5‐50‐95th percentiles from 100 simulations. PK/PD, pharmacokinetic/pharmacodynamic
To quantify the degree of deviation from additivity, the two combinations 0.075 mg/kg (trazodone) + 1.5 mg/kg (gabapentin) and 0.15 mg/kg (trazodone) + 1.5 mg/kg (gabapentin) were tested using the “additive” and “synergic model.” For the additive model, the objective function was equal to 1455.14 (Table 4A). For the synergic model the parameter γ, representing the deviation from no‐interaction, was equal to 16 and its introduction lowered the objective function down to 1438.13 (Table 4B) compared to the additive model. The objective function difference was significant at p < 0.001 (χ2 distribution, 1 df) with a sample of the fitting of the controls shown in Figure S5.
TABLE 4.
Perturbed writhing PK/PD model parameters
| (a) Additivity | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Combination model—pure additivity | |||||||||||
| Trazodone | Gabapentin | ||||||||||
| Parameter | Units | Estimate | %CV | %SE | Parameter | Units | Estimate | %CV | %SE | ||
| k 2 | L/μmoles/min | 12.7 | 28.4 | k 2 | L/μmoles/min | 0.0808 | 26.7 | ||||
| Interindividual variability | Interindividual variability | ||||||||||
|
|
1.43 | 119.6 | 28.4 |
|
1.67 | 129.2 | 28.2 | ||||
| Intra‐individual variability | OBJF = 1455.14 | ||||||||||
|
|
2.62 | 13.1 | |||||||||
| (b) Synergism | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Combination model—interaction | |||||||||||
| Trazodone | Gabapentin | ||||||||||
| Parameter | Units | Estimate | %CV | %SE | Parameter | Units | Estimate | %CV | %SE | ||
| k 2 | L/μmoles/min | 10.9 | 23.4 | k 2 | L/μmoles/min | 0.0490 | 32.4 | ||||
| Interindividual variance | Interindividual variance | ||||||||||
|
|
1.42 | 119.2 | 27.7 |
|
1.78 | 133.4 | 26.4 | ||||
| Combination Index | |||||||||||
| Γ | 16.0 | 24.4 | |||||||||
| Intra‐individual variability | OBJF = 1438.13 | ||||||||||
|
|
2.64 | 13.0 | |||||||||
Note: Analyses testing additive (panel A) and synergic (panel B) effects at the doses of trazodone‐gabapentin of 0.075–1.5, 0.15–1.5 mg/kg, respectively.
Abbreviations: %CV, percent coefficient of variation; %SE, percent standard error; k 2, potency parameter; PK/PD, pharmacokinetic/pharmacodynamic; ω, interindividual variability; γ, combination index; σ, intra‐individual variability.
On the contrary, the “synergic model” tested on 0.5 mg/kg (trazodone) + 5 mg/kg (gabapentin) and on 1.5 mg/kg (trazodone) + 15 mg/kg (gabapentin) did not highlight any deviations from additivity, as γ was close to zero and the two models (synergic and additive) converged to the same objective function (1429.21; Table 5A and B).
TABLE 5.
Perturbed writhing PK/PD model parameters
| (a) Additivity | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Combination model—pure additivity | |||||||||||
| Trazodone | Gabapentin | ||||||||||
| Parameter | Units | Estimate | %CV | %SE | Parameter | Units | Estimate | %CV | %SE | ||
| k 2 | L/μmoles/min | 10.2 | 20.1 | k 2 | L/μmoles/min | 0.040 | 27.5 | ||||
| Interindividual variability | Interindividual variability | ||||||||||
|
|
1.40 | 118.3 | 26.4 |
|
1.71 | 130.8 | 27.6 | ||||
| Intra‐individual variability | OBJF = 1429.211 | ||||||||||
|
|
2.71 | 13.3 | |||||||||
| (b) Synergism | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Combination model—interaction | |||||||||||
| Trazodone | Gabapentin | ||||||||||
| Parameter | Units | Estimate | %CV | %SE | Parameter | Units | Estimate | %CV | %SE | ||
| k 2 | L/μmoles/min | 10.2 | 20.1 | k 2 | L/μmoles/min | 0.0401 | 27.4 | ||||
| Interindividual variability | Interindividual variability | ||||||||||
|
|
1.40 | 118.3 | 26.4 |
|
1.71 | 130.8 | 27.6 | ||||
| Combination Index | |||||||||||
| Γ | 0.0001 | 0.0084 | |||||||||
| Intra‐individual variability | OBJF = 1429.212 | ||||||||||
|
|
2.71 | 13.3 | |||||||||
Note: Analyses testing additive (panel A) and synergic (panel B) effects at the doses of trazodone‐gabapentin of 0.5–5, 1.5–15 mg/kg, respectively.
Abbreviations: %CV, percent coefficient of variation; %SE, percent standard error; k 2, potency parameter; PK/PD, pharmacokinetic/pharmacodynamic; ω, interindividual variability; γ, combination index; σ, intra‐individual variability.
To further understand the pharmacological characteristics of the co‐administration of trazodone and gabapentin, the efficacy outcomes of different dosing regimens of the two drugs given together were predicted in mice by a model simulation according to the synergic model described above.
The combination of doses found out to be synergic from the mouse writhing test and devoid of efficacy as single agents were translated to humans by correcting the related exposures for the plasma protein binding values, with the scope of defining the possible starting dose to be further assessed and tested for efficacy in the course of appropriately designed clinical development plans.
To support translation, besides ppb, trazodone metabolism could also be made. This has been studied in the rat, rabbit, dog, baboon, and human. The data obtained suggest that trazodone, like other basic substances, is metabolized mainly by oxidation and hydrolysis reactions; moreover, the metabolic pathway of trazodone is similar in all the animal species studied and humans. 20
The results of simulations are summarized in Table 6 (PD results), indicating that low doses of both compounds (0.5, 1, 5, and 10 mg trazodone; 50 and 100 mg gabapentin), devoid of efficacy in the PD model as single agents and far below the dosages commonly used in clinical practice, 21 , 22 showed an enhanced effect when co‐administered.
TABLE 6.
Translation of PD results to humans
| Gabapentin | |||||||
|---|---|---|---|---|---|---|---|
| 0 mg | 50 mg | 100 mg | 200 mg | 400 mg | 800 mg | ||
| Trazodone (mg) | |||||||
| 0 | Mean | – | 5.4 | 10.6 | 16.4 | 22.2 | 28.2 |
| Median | – | 2.51 | 5.75 | 10.13 | 15.5 | 22.6 | |
| 1st Q | – | 1.05 | 2.28 | 4.13 | 6.5 | 9.9 | |
| 3rd Q | – | 6.43 | 14.0 | 23.2 | 32.7 | 42.4 | |
| 0.5 | Mean | 4.93 | 21.7 | 30.0 | |||
| Median | 2.76 | 17.9 | 26.2 | ||||
| 1st Q | 1.18 | 13.2 | 20.8 | ||||
| 3rd Q | 6.03 | 25.7 | 36.2 | ||||
| 1.0 | Mean | 9.20 | 29.1 | 39.2 | |||
| Median | 5.67 | 26.0 | 37.0 | ||||
| 1st Q | 2.50 | 20.6 | 30.4 | ||||
| 3rd Q | 11.75 | 33.9 | 45.7 | ||||
| 5 | Mean | 27.6 | 58.2 | 69.4 | |||
| Median | 21.7 | 57.76 | 68.8 | ||||
| 1st Q | 10.5 | 51.18 | 64.7 | ||||
| 3rd Q | 39.6 | 64.20 | 73.8 | ||||
| 10 | Mean | 37.5 | 72.09 | 81.5 | |||
| Median | 34.1 | 71.67 | 81.7 | ||||
| 1st Q | 18.6 | 66.83 | 78.2 | ||||
| 3rd Q | 53.3 | 71.67 | 81.7 | ||||
| 25 | Mean | 56.2 | |||||
| Median | 58.7 | ||||||
| 1st Q | 38.4 | ||||||
| 3rd Q | 75.0 | ||||||
| 50 | Mean | 69.41 | |||||
| Median | 73.96 | ||||||
| 1st Q | 55.09 | ||||||
| 3rd Q | 85.97 | ||||||
Note: Simulation of percentage of inhibition of pain stimulus in humans after single oral ascending doses of trazodone and gabapentin administered alone or in combination. Bold values indicate the low dose combinations of the two compounds indicating enhanced effects when co‐administered.
Abbreviation: PD, pharmacodynamic.
DISCUSSION
Trazodone is an original compound synthetized by the Angelini Research Group in the early 1970s and it is the first serotonin‐2 antagonist/reuptake inhibitor developed for the treatment of depression. 23 Trazodone is approved and marketed in several countries for the treatment of depressive disorders, with a daily dosage ranging from 150 to 600 mg. Trazodone is safe and generally well‐tolerated and its effect in patients with depression, particularly in controlling anxiety and sleep disturbances, may be seen within the first week of treatment. 24 , 25 Low doses of trazodone have also been used in neuropathic pain settings, with a good effect in pain relief and numbness observed in six patients with diabetes affected by peripheral neuropathy administered with 100 mg/day. 26 Furthermore, when 31 patients with diabetes with painful distal symmetrical polyneuropathy were treated with low doses of oral trazodone 50 or 100 mg/day for 2 weeks, 61.3% of the patients experienced symptomatic relief, whereas 22.6% experienced complete relief. 27
Based on the above evidence, trazodone was deemed an optimal candidate for the development of a fixed‐dose combination with gabapentin, aimed at the treatment of neuropathic pain, such as diabetic neuropathy.
Diabetic peripheral polyneuropathy is one of the most common symptomatic, long‐term complications in patients with both type 1 and type 2 diabetes mellitus, 28 with a median prevalence of 28%–29% in community‐based diabetic population. 29 Diabetic neuropathic pain can be constant and accompanied by cutaneous allodynia, which can substantially affect the quality of life of patients, affecting the ability to perform daily activities and having a negative influence on mood. The pain may also be a reason for withdrawal from recreational and social activities and may be associated with depression. 9 , 30 , 31 Current treatments for the management of diabetic peripheral neuropathic pain include antidepressants like tricyclic agents and serotonin‐norepinephrine reuptake inhibitors, γ‐aminobutyric acid analogues as pregabalin and gabapentin, opioids, and topical treatment. Unfortunately, there is no consensus about a single most effective drug and monotherapy rarely provides adequate pain relief. 32 A wide variety of drugs, used both alone and in combination, showed to significantly reduce neuropathic pain compared with placebo in randomized controlled trials, but pain relief remains inadequate for most of the patients. 33
Based on such premises, Angelini S.p.A. aimed at developing a fixed‐dose combination for the treatment of neuropathic pain containing trazodone and gabapentin, the latter with a full product license for the treatment of peripheral neuropathic pain in Europe.
In the mice writhing test, through an isobolographic analysis, it was recently demonstrated that the administration of trazodone in combination with gabapentin exerts a relevant antinociceptive activity when suboptimal doses of trazodone (0.1–3 mg/kg) were associated with suboptimal doses of gabapentin (1–100 mg/kg). In these experimental conditions, trazodone and gabapentin co‐administration exerts a synergistic action which was confirmed also by the calculation of the interaction index, 14 which equaled to 0.17.
Based in the above evidence, a PK/PD model was developed, leading to the application of a model‐based translational approach to provide indications of the clinical doses of trazodone and gabapentin expected to be clinically active when given in combination in humans.
The PK/PD model is established by considering that drug treatment reduced the acetic acid stimulus on the model compartment where the stimulus is applied, whereas vehicle treatment does not cause any changes of the amplitude of the stimulus.
In the animals treated with trazodone or gabapentin, due to the drug action, the amplitude of the acetic acid stimulus is reduced according to a proportionality factor k 2, multiplied by the plasma drug concentration. Consequently, k 2 represents an index of in vivo drug potency in the pharmacological model. The time course of writhings is a consequence of the acetic acid stimulus that transits in both treated and control mice through a chain of intermediate transit compartments with a unique rate constant, k tr, before reaching the observation compartment.
When the two compounds were given as single agents, the results of the PK/PD model indicated that in the “writhing test,” the in vivo potency of trazodone was much higher than gabapentin. To describe the pharmacological effect of the combination therapy, an interaction term was introduced reflecting the nature and degree of the interaction at different dose ratios of the two compounds enabling to identify synergistic drug combinations. In the present study, a synergic effect, in absence of PK interaction, can be demonstrated when low doses of both compounds are co‐administered. Additivity is shown at increasing doses of trazodone and gabapentin. Linking the antinociceptive effect of the trazodone and gabapentin combination could add valuable information to decision making in drug combination development selecting doses of both compounds devoid of side effects in humans. Based on the PK/PD model parameters, a set of simulations has been performed to support the identification of synergistic range of doses of the two compounds in combination and possible starting doses for clinical use.
It is important to consider that the simulations for dose selection have been performed under the assumption of a similar k 2 across species; this assumption can only be verified when human data are available. Moreover, it is uncertain where the line between synergism and additivity is drawn in humans based on animal data. For instance, the same combination of doses of trazodone and gabapentin could be already outside the range of synergism, resulting in just additive effect in animals, while still holding synergism, spanning across a wider range of combination of doses in humans.
During the development of the method, we verified that no drug–drug PK interaction occurred in mice and the lack of drug–drug PK interaction, which was consistent with published human data. 34 That was somehow expected because gabapentin is eliminated from the systemic circulation as unchanged drug by renal excretion and is not appreciably metabolized in animal species and humans.
There are theoretical pharmacological bases that could explain the synergistic interaction of trazodone and gabapentin. Recent preclinical studies suggested a potential role of 5‐HT2A and the mGLU2/3 heterodimer in the brain and spinal cord that could modulate the glutamate exocytosis and neuropathic pain. 35 In addition, the action of trazodone on 5‐HT2A and mGLU2/3 could be elicited by low doses of the compound.
Evidence from both animal and human studies supports gabapentin to be effective for the treatment of neuropathic pain and several specific chronic pain syndromes. Gabapentin may become an attractive therapeutic option because of its relative lack of interactions and serious adverse effects.
The capacity of gabapentin to modulate excitatory neurotransmitter release is considered a major mechanism to explain its efficacy on neuropathic pain both in preclinical and clinical settings. On such bases, we can conclude that indirect inhibition of glutamate release seems to be an effect common to both drugs, and it may well explain the synergistic antinociceptive efficacy of the trazodone and gabapentin combination observed in the CCI rat neuropathic pain model on different spontaneous and evoked behaviors.
AUTHOR CONTRIBUTIONS
L.O., B.G., F.F., F.D.B., M.T.R., P.F.D.G., and R.M.K. wrote the manuscript. L.O., B.G., F.F., and F.D.B. designed the research. F.F. and F.D.B. performed the research. L.O., B.G., F.F., and F.D.B. analyzed the data.
FUNDING INFORMATION
The present paper received funding from the following commercial source: Angelini Pharma S.p.A., that also provided support in the form of salaries for L.O., B.G., M.T.R., F.P.D.G., and R.M.K. The funder had a role in the study design, data collection and analysis, decision to publish, and preparation of the manuscript.
CONFLICT OF INTEREST
The authors declared no competing interests for this work.
Supporting information
Appendix S1
ACKNOWLEDGMENTS
The authors wish to thank Alessandro Comandini (part of Angelini Pharma S.p.A.) for his assistance in reviewing the clinical aspects of this work.
Oggianu L, Garrone B, Fiorentini F, et al. PK/PD analysis of trazodone and gabapentin in neuropathic pain rodent models: Translational PK‐PD modeling from nonclinical to clinical development. Clin Transl Sci. 2023;16:606‐617. doi: 10.1111/cts.13472
REFERENCES
- 1. Gilron I, Baron R, Jensen T. Neuropathic pain: principles of diagnosis and treatment. Mayo Clin Proc. 2015;90(4):532‐545. [DOI] [PubMed] [Google Scholar]
- 2. Moore RA, Derry CJ, Derry S, Straube S, McQuay HJ. A conservative method of testing whether combination analgesics produce additive or synergistic effects using evidence from acute pain and migraine. Eur J Pain. 2012;16(4):585‐591. [DOI] [PubMed] [Google Scholar]
- 3. Mao J, Gold MS, Backonja M. Combination drug therapy for chronic pain: a call for more clinical studies. J Pain. 2011;12(2):157‐166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Patel R, Dickenson AH. Mechanisms of the gabapentinoids and a2d‐1 calcium channel subunit in neuropathic pain. Pharmacol Res Perspect. 2016;4(2):e00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Muthuraman A, Singh N, Jaggi AS, Ramesh M. Drug therapy of neuropathic pain: current developments and future perspectives. Curr Drug Targets. 2014;15(2):210‐253. [PubMed] [Google Scholar]
- 6. Heughan CE, Sawynok J. The interaction between gabapentin and amitriptyline in the rat formalin test after systemic administration. Anesth Analg. 2002;94(4):975‐980. [DOI] [PubMed] [Google Scholar]
- 7. Garry EM, Delaney A, Anderson HA, et al. Varicella zoster virus induces neuropathic changes in rat dorsal root ganglia and behavioral reflex sensitisation that is attenuated by gabapentin or sodium channel blocking drugs. Pain. 2005;118(1–2):97‐111. [DOI] [PubMed] [Google Scholar]
- 8. Gilron I, Bailey JM, Tu D, Holden RR, Jackson AC, Houlden RL. Nortriptyline and gabapentin, alone and in combination for neuropathic pain: a double‐blind, randomised controlled crossover trial. Lancet. 2009;374(9697):1252‐1261. [DOI] [PubMed] [Google Scholar]
- 9. Tesfaye S, Wilhelm S, Lledo A, et al. Duloxetine and pregabalin: high‐dose monotherapy or their combination? The “COMBO‐DN study” – a multinational, randomized, double‐blind, parallel‐group study in patients with diabetic peripheral neuropathic pain. Pain. 2013;154(12):2616‐2625. [DOI] [PubMed] [Google Scholar]
- 10. Boyle Y, Fernando D, Kurz H, Miller SR, Zucchetto M, Storey J. The effect of a combination of gabapentin and donepezil in an experimental pain model in healthy volunteers: results of a randomized controlled trial. Pain. 2014;155(12):2510‐2516. [DOI] [PubMed] [Google Scholar]
- 11. Stahl SM. Mechanism of action of trazodone: a multifunctional drug. CNS Spectr. 2009;14(10):536‐546. [DOI] [PubMed] [Google Scholar]
- 12. Le Cudennec C, Castagné V. Face‐to‐face comparison of the predictive validity of two models of neuropathic pain in the rat: analgesic activity of pregabalin, tramadol and duloxetine. Eur J Pharmacol. 2014;735:17‐25. [DOI] [PubMed] [Google Scholar]
- 13. Okuda K, Takanishi T, Yoshimoto K, Ueda S. Trazodone hydrochloride attenuates thermal hyperalgesia in аchronic constriction injury rat model. Eur J Anaesthesiol. 2003;20(5):409‐415. [DOI] [PubMed] [Google Scholar]
- 14. Garrone B, di Matteo A, Amato A, et al. Synergistic interaction between trazodone and gabapentin in rodent models of neuropathic pain. PLoS One. 2021;16(1):e0244649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Garrone B, Durando L, Calisti F, Aziende Chimiche Riunite Angelini Francesco A.C.R.A.F. S.p.A., applicant . Combination of trazodone and gabapentin for the treatment of pain. International Patent Application WO2017067870 2017.
- 16. Oggianu L, Ke AB, Chetty M, et al. Estimation of an appropriate dose of trazodone for pediatric insomnia and the potential for a trazodone‐atomoxetine interaction. CPT Pharmacometrics Syst Pharmacol. 2020;9(2):77‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Carlsson KC, Schootbrugge M, Eriksen HO, Moberg ER, Karlsson MO, Hoem NO. A population pharmacokinetic model of gabapentin developed in nonparametric adaptive grid and nonlinear mixed effects modeling. Ther Drug Monit. 2009;31(1):86‐94. [DOI] [PubMed] [Google Scholar]
- 18. Beal, S.L. & Sheiner, L.B. NONMEM Users Guides I‐VIII. November 1989–May 2008.
- 19. Radulovic LL, Türck D, von Hodenberg A, et al. Disposition of gabapentin (neurontin) in mice, rats, dogs, and monkeys. Drug Metab Dispos. 1995;23(4):441‐448. [PubMed] [Google Scholar]
- 20. Koss FW, Busch U. Pharmacokinetics and metabolism of trazodone in different species. In: Morozov G, Saarma J, Silvestrini B, eds. Depression and the Role of Trazodone in Antidepressant Therapy; 1978. Luigi Pozzi Publisher: 11‐20. [Google Scholar]
- 21. Settimo L, Taylor D. Evaluating the dose‐dependent mechanism of action of trazodone by estimation of occupancies for different brain neurotransmitter targets. J Psychopharmacol. 2018;32(1):96‐104. [DOI] [PubMed] [Google Scholar]
- 22. Lipone P, Ehler E, Nastaj M, et al. Efficacy and safety of low doses of trazodone in patients affected by painful diabetic neuropathy and treated with gabapentin: a randomized controlled pilot study. CNS Drugs. 2020;34(11):1177‐1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stahl SM. Essential Psychopharmacology of Depression and Bipolar Disorder. 2nd ed. Cambridge University Press; 2000. [Google Scholar]
- 24. Fabre LF Jr, Feighner JP. Long‐term therapy for depression with trazodone. J Clin Psychiatry. 1983;44(1):17‐21. [PubMed] [Google Scholar]
- 25. Feighner JP. Trazodone, a triazolopyridine derivative, in primary depressive disorder. J Clin Psychiatry. 1980;41(7):250‐255. [PubMed] [Google Scholar]
- 26. Khurana RC. Treatment of painful diabetic neuropathy with trazodone. JAMA. 1983;250(11):1392. [DOI] [PubMed] [Google Scholar]
- 27. Wilson RC. The use of low dose trazodone in the treatment of painful diabetic neuropathy. J Am Podiatr Med Assoc. 1999;89(9):468‐471. [DOI] [PubMed] [Google Scholar]
- 28. Schreiber AK, Nones CF, Reis RC, Chichorro JG, Cunha JM. Diabetic neuropathic pain: physiopathology and treatment. World J Diabetes. 2015;6(3):432‐444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ziegler D, Papanas N, Vinik AI, Shaw JE. Epidemiology of polyneuropathy in diabetes and prediabetes. Handb Clin Neurol. 2014;126:3‐22. [DOI] [PubMed] [Google Scholar]
- 30. Quattrini C, Tesfaye S. Understanding the impact of painful diabetic neuropathy. Diabetes Metab Res Rev. 2003;19(Suppl 1):S2‐S8. [DOI] [PubMed] [Google Scholar]
- 31. Gore M, Brandenburg NA, Dukes E, Hoffman DL, Tai KS, Stacey B. Pain severity in diabetic peripheral neuropathy is associated with patient functioning, symptom levels of anxiety and depression, and sleep. J Pain Symptom Manage. 2005;30(4):374‐385. [DOI] [PubMed] [Google Scholar]
- 32. Javed S, Petropoulos IN, Alam U, Malik RA. Treatment of painful diabetic neuropathy. Ther Adv Chronic Dis. 2015;6(1):15‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Singleton JR, Smith AG. The diabetic neuropathies: practical and rational therapy. Semin Neurol. 2012;32(3):196‐203. [DOI] [PubMed] [Google Scholar]
- 34. Ruggieri A, Picollo R, Vecchio AD, et al. Investigations on dose proportionality and drug‐drug interaction for a fixed‐dose combination of trazodone and gabapentin. Int J Clin Pharmacol Ther. 2021;59(1):71‐86. [DOI] [PubMed] [Google Scholar]
- 35. Olivero G, Grilli M, Vergassola M, et al. 5‐HT2A‐mGlu2/3 receptor complex in rat spinal cord glutamatergic nerve endings: a 5‐HT2A to mGlu2/3 signalling to amplify presynaptic mechanism of auto‐control of glutamate exocytosis. Neuropharmacology. 2018;133:429‐439. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1


