Abstract
Cartilage injury affects numerous individuals, but the efficient repair of damaged cartilage is still a problem in clinic. Hydrogel is a potent scaffold candidate for tissue regeneration, but it remains a big challenge to improve its mechanical property and figure out the interaction of chondrocytes and stiffness. Herein, a novel hybrid hydrogel with tunable stiffness was fabricated based on methacrylated gelatin (GelMA) and iron oxide nanoparticles (Fe2O3) through chemical bonding. The stiffness of Fe2O3/GelMA hybrid hydrogel was controlled by adjusting the concentration of magnetic nanoparticles. The hydrogel platform with tunable stiffness modulated its cellular properties including cell morphology, microfilaments and Young's modulus of chondrocytes. Interestingly, Fe2O3/GelMA hybrid hydrogel promoted oxidative phosphorylation of mitochondria and facilitated catabolism of lipids in chondrocytes. As a result, more ATP and metabolic materials generated for cellular physiological activities and organelle component replacements in hybrid hydrogel group compared to pure GelMA hydrogel. Furthermore, implantation of Fe2O3/GelMA hybrid hydrogel in the cartilage defect rat model verified its remodeling potential. This study provides a deep understanding of the bio-mechanism of Fe2O3/GelMA hybrid hydrogel interaction with chondrocytes and indicates the hydrogel platform for further application in tissue engineering.
Keywords: Magnetic nanoparticles, Hydrogel, Chondrocyte, Cartilage defect, Cellular metabolism
Graphical abstract
Hydrogel platform with tunable stiffness based on Fe2O3/GelMA hybrid hydrogel for cartilage defect repair and its biomechanism.
Highlights
-
•
A novel hybrid hydrogel with tunable stiffness was constructed by chemical bonding of GelMA and magnetic nanoparticles.
-
•
The tunable stiffness hydrogel modulated cellular properties and behaviors of chondrocytes.
-
•
The hybrid hydrogel could stimulate regeneration of cartilage tissue.
1. Introduction
Joints are the vital key to ensure human movement functions, and articular cartilage is one of the most easily damaged tissues in the human body. Due to the improvement of economic level and the development of aging population in recent years, the incidence of cartilage defects caused by sports injuries, traffic accidents and arthritis has risen sharply [1]. Cartilage is a special tissue without blood vessels, nerves and lymph nodes [2,3], so the self-repairing ability of cartilage is very limited and cartilage injury is still one of the diseases that is difficult to repair in clinic [4,5]. The current clinical treatment methods generally include conservative treatment, joint debridement, autologous or allogeneic osteochondral transplantation and artificial joint replacement [6]. Unfortunately, these treatments have obvious shortcomings, such as high treatment costs, limited donor sources and autoimmune rejection. Therefore, effective articular cartilage repair is an arduous challenge in clinical treatment.
With the efforts of researchers in the fields of material science, chemistry, medicine, pharmacy and biology in the past few decades, the research on cartilage tissue engineering has opened up a new way for cartilage defects repair [[7], [8], [9]]. A variety of cartilage regeneration scaffolds have been used to induce cartilage regeneration, such as biodegradable hydrogels, nanofibers, microspheres and 3D printing scaffolds [[10], [11], [12], [13]]. Specially, hydrogel is a multi-component platform with a three-dimensional cross-linked network structure. It is insoluble in water, but has obvious swelling properties, and the mesh structure formed after cross-linking can carry a large number of water molecules. Cells can be embedded on the surface or in micropores of hydrogels to ensure effective adhesion, and they can use surrounding water as a medium to achieve intercellular signal transmission [14]. Therefore, hydrogel has been widely applied in hard and soft tissue regeneration [[15], [16], [17], [18]]. The bioactive materials for preparing hydrogel is very vital for the physical-chemical properties of hydrogel. Methacrylated gelatin (GelMA) as an excellent hydrogel material has good biocompatibility and characteristics of simulating extracellular matrix. However, the weak mechanical characteristics of GelMA hydrogels cannot meet the requirements of tissue engineering and injury remodeling [19,20]. Thus, considerable efforts have been made to improve the mechanical properties of hydrogels by changing the components or their proportions [21], improving the chemical combination mode [22], and doping micro/nano materials into the hydrogels [23]. Among these methods, the introduction of nanoparticles to strengthen the cross-linking of hydrogel is a feasible and efficient method. Robert Langer et al. developed a SiO2-combined hydrogel system that formed noncovalent bonds between SiO2 nanoparticles and polymers to enhance the mechanical properties of hydrogels [24]. Shi et al. fabricated a hyaluronic acid (HA) hydrogel by mixing bisphosphonate (BP)-modified HA solution and Fe3O4 nanoparticles to improve its mechanical strength, thus facilitating its biocompatibility in vivo [25]. On the basis of our previous studies on the core-shell multifunctional magnetic nanogels [26], we got the inspiration to bond GelMA with double bonds modified magnetic nanoparticles for hybrid hydrogel with enhanced mechanical property. Our idea of improving hydrogel's stiffness by introduction of inorganic nanoparticles is similar to the great scientists, but the bonding method and chemical reaction are very different. The covalent bonding between GelMA and double bonds modified magnetic nanoparticles is very strong, which are fundamentally different from the non-covalent bonding or physical mixing.
Meanwhile, previous researches have reported that hydrogels with low to high stiffness can induce the differentiation of stem cells into nerve cells, adipocytes, muscle cells, chondrocytes and osteoblasts, respectively [27,28]. It can be seen that hydrogels with mechanical gradients induce stem cells to differentiate into desired cells in different stiffnesses [29]. In our study, tunable stiffness hydrogel platform was designed based on GelMA and iron oxide (Fe2O3) nanoparticles through chemical bonding of carbon-carbon double bonds. The introduction of magnetic nanoparticles can improve the mechanical property of GelMA hydrogel and tune the stiffness with adding amount, which influences on the cross-linking ratios of hybrid hydrogel. After characterization of hybrid hydrogel, in vitro cell experiments were systematically studied, which elucidated the basal cellular physical property changes and cellular metabolic changes (biological property) in response to Fe2O3/GelMA hybrid hydrogel. Interestingly, we found that different stiffness of hybrid hydrogel had influence on aerobic phosphorylation of mitochondria in chondrocytes. As the oxygen tension in the cartilage layer is only about 5–7%, which is much lower than the oxygen tension in the blood vessels (∼13–17%) [30]. The energy supply of cartilage is mainly dependent on anaerobic glycolysis and supplemented by aerobic phosphorylation of mitochondria [31]. Mitochondria in cartilage energy metabolism is very important, considering that aerobic phosphorylation can produce eighteen times of ATP (thirty-six molecules) per one molecule of glucose than that of anaerobic glycolysis (two molecules of ATP) [32]. The relationship between chondrocyte metabolism and stiffness of hybrid hydrogel was revealed. In vivo cartilage defect model, the cartilage repair capacity by Fe2O3/GelMA hybrid hydrogel was further confirmed. This study provides new ideas for the design and construction of cartilage defect repair scaffolds and gives an in-depth understanding of bio-mechanism between cartilage biology and advanced biomaterials.
2. Materials and methods
2.1. Synthesis of vinyl-coated Fe2O3 nanoparticles
The synthesis method of citrate-coated magnetic nanoparticles and vinyl-coated coated magnetic nanoparticles referred to our previous studies [26,33]. Briefly, the preparation of vinyl-coated Fe2O3 nanoparticles is described as follows. A dispersion of the purified citrate-coated Fe2O3 nanoparticles (20 ml, 3.37%) was first mixed in methanol (80 ml) for 1 h at 40 °C. Concentrated ammonia solution (1.8 ml, 25%) was added, followed by stirring of the mixture at 40 °C for 30 min. Subsequently, tetraethyl orthosilicate (TEOS, 0.4 ml) was added to the reaction mixture and continuously stirred at 40 °C for 24 h. Finally, 3-(trimethoxysilyl)-propyl-methacrylate (MPS, 2.12 ml) was added and the mixture was allowed to react at 40 °C for 24 h to generate vinyl-coated Fe2O3 nanoparticles. The vinyl-coated Fe2O3 nanoparticles were collected by centrifugation (18,000 rpm) for 2 h, and then re-dispersed with ethanol (∼10 ml). Place into a dialysis bag and dialyzed for 3 days. The size and distribution of vinyl-coated Fe2O3 nanoparticles were detected by using laser diffraction particle size analyzer (ZEN369, Malvern, UK). The morphology of the magnetic nanoparticles was observed by scanning electron microscopy (SEM, JSM-5900LV, JEOL, Tokyo, Japan).
2.2. The preparation and characterization of Fe2O3/GelMA hydrogel
The magnetic nanoparticle hybrid hydrogel was prepared by heat-initiated free radical method. The concentrated vinyl-coated Fe2O3 nanoparticles was diluted by aqueous water at a final concentration of 1 mg/ml. And 50 μl of GelMA (20%, Cat: M103029, Aladdin industrial corporation, Shanghai) was mixed with different amount of vinyl-coated Fe2O3 (30, 40 and 50 μl) by sonication. Subsequently, the initiator ammonium persulfate (APS) was added to the mixture and injected them into mold for bathing in water bath at 50 °C for 30 min. The prepared magnetic hydrogel was removed and immersed in Mill-Q water to get rid of residual monomers and initiator. The synthesized Fe2O3/GelMA hybrid hydrogels were freeze-dried for characterizations. The cross-section morphology and elemental analysis of hydrogels were observed by a SEM machine. The data and images were obtained by its matching software. The surface roughness of Fe2O3/GelMA hydrogels with different concentration of Fe2O3 nanoparticles were scanned by atomic force microscopy (AFM) (Nanoscope IIIa, Digital Instruments, Santa Barbara, CA). The mechanical properties of the Fe2O3/GelMA hydrogels were determined by an Instron 5500 mechanical tester for measuring their compression modulus in wet states. The swelling behavior of the hydrogels were recorded as Swelling ratio = (Wt-W0)/W0. The Wt and W0 represent the weight of hydrogels after swelling in PBS and the weight of freeze-dried hydrogels.
2.3. Chondrocyte isolation and culture
The protocol for experimental animal usage was granted by our Institutional Review Board (IRB, No.WCHSIRB-D-2017-029) before we started all cell and tissue experiments. Chondrocytes were isolated from 2 to 3 days new born C57BL/6J mice as previously described [34]. The chondrocytes from hyaline cartilage of knee joint were collected by 0.25% trypsin digestion for 30 min at 37 °C and 0.2% type II collagenase (sigma, MO) digestion for about 12 h at 37 °C. After neutralization with 10% FBS-DMEM at 1:1 ratio, the chondrocytes were collected by centrifugation at 1000 rpm for 5 min. The isolated chondrocytes were re-suspended by fresh 10% FBS-DMEM (HyClone, Logan, UT) and cultured in the Petri dish. The chondrocytes could be passaged when the cells reach to 90% confluence. We used the chondrocytes at passage 0–1 (P0–P1).
2.4. Cellular Young's modulus detection
Chondrocytes were seeded and cultured on the Fe2O3/GelMA hydrogels and GelMA control gel for 72 h (2000 cells per hydrogel with the size in Fig. 1b). Then the cells were fixed by 0.25% glutaraldehyde for 8 h or overnight and kept still. The ethanol was used to achieve gradient dehydration (from 30%, 50%, 70%, 90%, to 100%, 15 min per gradient) and the acetone was used for final dehydration (100%) for 30 min. Then the Fe2O3/GelMA hydrogel samples were cut into 10 × 10 mm pieces (Do not disturb cell morphology). After air-dried, the samples were loaded onto AFM system (Shimadzu SPM-9700, Japan). The basic parameter is as following: the approach and retract velocities are set to be below 5 μm/s and the limit threshold is at about 2 nN. The AFM probe requires a circular probe and the elastic coefficient of the probe is calibrated by thermal noise method. The force-volume mode was set to measure the Young's modulus of choncrocytes. The distribution of force curve on the surface of the cell was collected and reconstructed to graphical drawing. Young's modulus of cells was calculated by Hertz model.
Fig. 1.
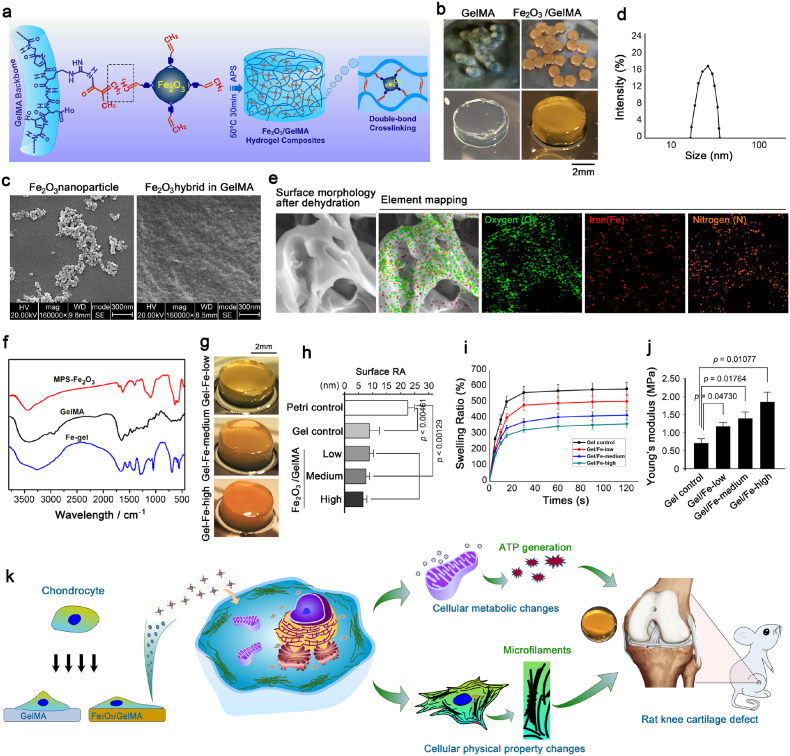
Synthesis of Fe2O3/GelMA hybrid hydrogel and its potential application in cartilage repair. a. Schematic diagram indicating the formation of the Fe2O3/GelMA hybrid hydrogel in our laboratory. b. General view of the Fe2O3/GelMA hybrid hydrogel formed in the current study. c. Representative SEM images showing the vinyl coated Fe2O3 nanoparticles (MPS-Fe2O3) and enlarged cross-section morphology of the Fe2O3/GelMA hybrid hydrogel. d. The particle size of Fe2O3 nanoparticles. e. Element mapping analysis of Fe2O3/GelMA hybrid hydrogel. The existence of oxygen, nitrogen and iron are indicated. f. FT-IR analysis of MPS-Fe2O3, GelMA and the Fe2O3/GelMA hybrid hydrogel. g. Stereomicroscope showing the morphology of the Fe2O3/GelMA hybrid hydrogel with different concentrations of Fe2O3. h. The surface roughness results of Petri dishes, pure GelMA hydrogel (gel control) and the Fe2O3/GelMA hybrid hydrogels. i. Swelling rate of Fe2O3/GelMA hybrid hydrogel. j. Young's moduli of the GelMA hydrogel and the Fe2O3/GelMA hybrid hydrogels with different concentrations of Fe2O3 nanoparticles. k. Schematic diagram indicating the changes of cellular physical and biological properties of chondrocytes co-cultured with the tunable stiffness based on Fe2O3/GelMA hybrid hydrogels, and its further application in the remodeling of cartilage defects in a rat model.
2.5. Cell viability test
To detect the cell viability in chondrocytes interacting with the Fe2O3/GelMA gels, CCK-8 assay (C0037, Beyotime Biotech) was performed following the instructions of the manufacturer. To evaluate the cell viability change under a relative long time interaction between chondrocytes and Fe2O3/GelMA gels, we kept the cells in the culture state for 9 days without changing the medium and performed sampling test every 24 h. Specifically, we put the Fe2O3/GelMA hybrid hydrogels into a 6 × 6 cm Petri dish and made them evenly fill the bottom of the Petri dish. The cells were seeded onto the Fe2O3/GelMA gels, and ensure that the seeding density of chondrocytes is more than 5000 cells per gel. Remove a gel from the Petri dish every 24 h and put the gel into a 96 well plate avoid disturbing the cells on it (The volume before solidification was designed to be 200 μl, and the diameter after curing is ∼5 mm in Fig. 1b, which just fits into the single hole of the 96-well plate). CCK-8 solution (20 μl) was added into 10% FBS-DMEM (200 μl, HyClone, Logan, UT) to form working solution (1:10). The cells were incubated for 30 min at 37 °C in cell incubator with standard humidified atmosphere of 5% CO2 and then detected by optical density (OD) at 450 nm by a microplate reader (Varioskan Flash, Thermo Fisher Scientific). The cell viability curve was generated by Boltzmann fitting.
2.6. Scanning electron microscopy (SEM) detection
To detect the cell morphology changes in chondrocytes seeded onto Fe2O3/GelMA gels (cell density at ∼2000 per hydrogel) via SEM. The chondrocytes were fixed at 8, 24 and 72 h after seeding by 2.5% glutaraldehyde for 4 h. Then the cells were undergone gradient dehydration (from 50%, 60%, 70%, 80%, 90%, to 100% in ethanol, 15 min per gradient). After coating with a thin layer of gold, the chondrocyte samples were scanned and images were obtained.
2.7. Transmission electron microscopy (TEM) detection
To detect the mitochondrion morphology, the TEM was used. Chondrocytes were collected by cell scraper after seeding on the gels for 72 h (full confluency at 1 × 106 cells per plate). The cell samples were fixed by 2.5% glutaraldehyde at 4 °C for 2 h. The ethanol was used to achieve gradient dehydration (from 30%, 50%, 70%, 90%, to 100%, 15 min per gradient) and the acetone was used for final dehydration (100%) for 30 min. Next, the 2-fold volume of embedding solution was added (ratio to acetone, v/v) to the sample for 3 h. After removal of the mixed buffer through centrifugation at 1000 rpm for 5 min, the pure embedding solution was added for an overnight. Sample section at 70 nm was achieved by ultramicrotome. After staining with 2% uranyl acetate lead citrate, the mitochondrion images were obtained by TEM.
2.8. Cellular metabolism detection
To detect the cellular metabolism changes, we used Mito-stress test Kit by Agilent Seahorse xFe24 metabolism equipment (Agilent seahorse, Santa Clara, CA). Cells were seeded onto the gels for 72 h (full confluency at 1 × 106 cells per plate) and collected by trypsin digestion. After cell counting by Automated Cell Counter (CT20™ Bio-rad, Hercules, CA), the cells were seed onto the specified plate well for seahorse equipment. Once the cells were all attached and culture for 72 h, the detection was performed. The measurement for spare respiration capacity, ATP production and glycolysis capacity were all followed the instructions of the manufacturer.
2.9. ATP-luminmeter
The intercellular ATP contents in chondrocytes were detected by using the Enhanced ATP Assay Kit (S0027, Beyotime, Shanghai, China) following the instructions of manufacturer. In brief, after chondrcoytes were seeded and cultured on Fe2O3/GelMA hybrid hydrogels for 72 h (full confluency at 1 × 106 cells per plate), the chondrocytes were lysed on ice, and 20 μl supernatant and 100 μl detection working solution were mixed and incubated for 5 min at a 96-well plate. Then the luminescence was measured by Synergy HTX Multi-Mode Microplate Reader (BioTek Instruments, Vermont). The data in the Fe2O3/GelMA hybrid hydrogels were all compared to their GelMA control groups.
2.10. Reactive oxygen species (ROS) detection
The cellular ROS was detected by Reactive Oxygen Species Assay Kit (S0033S, Beyotime) following the instruction of the manufacturer. After chondrcoytes were seeded and cultured on Fe2O3/GelMA hybrid hydrogels for 72 h (full confluency at 1 × 106 cells per plate), the chondrocytes were collected by trypsin digestion (to avid the interference of the background color of Fe2O3/GelMA gels). The suspended cells (4000 cells per sample) were mixed with the reaction buffer and incubated at 37 °C for 20 min (Shake every 5 min to make the cells fully contact with the reaction solution). The OD values were obtained at 525 nm wavelength by microplate reader. All ROS data in the Fe2O3/GelMA hybrid hydrogels were compared to their GelMA gel controls.
2.11. Western blotting
The protocol for western blotting was previously described [35]. Briefly, after being seeded and cultured on Fe2O3/GelMA hybrid hydrogels for 72 h (full confluency at 1 × 106 cells per plate), chondrocytes were lysated by RIPA (P0013B, Beyotime Biotech, Shanghai, China). One part of cell lysates was mixed with on part of sample buffer including 10% DL-Dithiothreitol (DTT, 1 M) and boiled at 100 °C for 5 min. The total proteins were separated by using electrophoretic gel (8–12%, It depends on the molecular weight) and transferred to PVDF membrane. The PVDF membrane then blocked with 5% non-fat milk for 2 h at RT. After washing, the primary antibody was added and incubated for an overnight at 4 °C. After removal of primary antibody, the secondary antibody was incubated for 2 h at RT. The blotting images were then obtained by western immobilon (P90719, Millipore). The primary antibodies used for western blotting in the current study included: AMP-activated protein kinase (total AMPKα1, 1:1,000, No. 220539, ZENBIO, China), Phospho-AMPK alpha 1 (p-AMPKα1, Ser496, 1:1,000, No. 310044, ZENBIO), IRP1(1:1,000, ab18372, Abcam, Cambridge, UK), IRP2 (ab232994), Cytochrome C (Cyt c, 1:1,000, No.250109, ZENBIO), Cyclooxygenase 2 (COX2, 1:1,000, ab15191), PPARα (1:1000, No. 518252, ZENBIO), PPARγ (1:1,000, No. 340844, ZENBIO), C/EBPα (1:1,000, No. 383901, ZENBIO), Angiopoietin-like 4 (ANGPTL4, 1:1,000, No. 383468, ZENBIO), Apolipoprotein E (APOE, 1:1,000, No. 381129, ZENBIO), Cytochrome P450 2E1 (Cyto P450 2E1, 1:1,000, ab28146) and β-actin (1:1,000, 200068-8F10, ZENBIO). The secondary antibodies used in the study included: Goat anti-mouse IgG (H&L) TRITC (1: 5,000, No. 511102, ZENBIO), Goat anti-Rabbit IgG (H&L) (1:5,000, No. 511203, ZNEBIO) and Rabbit Anti-Rat IgG (H&L) HRP (1:5,000, No. 550064, ZENBIO).
2.12. Immunofluorescence and confocal scanning microscope (CLSM)
In order to detect the protein distribution in chondrocytes, the confocal scanning microscope (CLSM) was used in the current study as previously described [36]. Briefly, after being seeded and cultured on Fe2O3/GelMA hybrid hydrogels for 72 h (Cell density was chosen according to the needs of single-cell and multicellular imaging, the former requires 2000 cells per dish, while the latter needs 5000 cells per dish, No.801002, NEST, China), chondrocytes were fixed with 4% paraformaldehyde (PFA) for 10 min. Then the cells were permeabilized with 0.5% Triton X-100 (sigma) for 10 min. After washing three times with 1 × PBS, the cells were blocked with 5% non-fat milk for 2 h at RT. The primary antibody was added and incubated for an overnight at 4 °C after wash away 5% non-fat milk by 1 × PBS three times. The antibodies used in the current study included: cyclooxygenase 2 (COX2, 1:200, ab15191), Phosphorylated PPARα (1:200, ab3484), Apolipoprotein E (APOE, 1:200, No. 381129, ZENBIO), Cytochrome P450 2E1 (Cyto P450 2E1, 1:200, ab28146). The secondary antibodies included: Goat anti-mouse IgG H&L (Alexa Fluor® 647) (ab150115), Donkey anti-rabbit IgG H&L (Alexa Fluor® 647) (ab150075). To show the cell cytoskeleton in colocalization between iron (FITC) and mitochondria (Red), we chose phalloidin-iFluor 405 (blue, ab176752) which overlaps the staining of Dapi. Images were obtained at 60× magnification.
2.13. Mitochondrial staining in living cells
The mitochondrial staining in living chondrocytes was performed by using Cell Navigator™ Mitochondrion Staining Kit (Cat: 22667, AAT Bioquest) following the instructions from the manufacturer. In brief, chondrocytes were seeded and cultured onto Fe2O3/GelMA hybrid hydrogels for 72 h (cell density: 5000 per hydrogel). After removal of culture media, the Mitolite™ Orange working solution (1000 times dilution according to the manufacturer) was added and incubated for 30 min at 37 °C in a 5% CO2 incubator (too long time incubation would weaken the cell morphology). Then the chondrocytes were fixed with 4% PFA for 15 min and permeabilized with 0.5% Triton X-100 for 10 min. The 5% non-fat milk solution was incubated for blockage for 2 h at RT. F-actin staining was achieved by using phalloidin (A12379, Alexa Fluor® 488, invitrogen) for an overnight. Images were obtained by using CLSM.
2.14. RNA sequencing
The RNA sequencing was performed and analyzed by Shanghai Lifegenes Biotechnology (Shanghai, China) as previously described [35]. In brief, chondrocytes were seeded and cultured onto Fe2O3/GelMA hybrid hydrogels for 72 h (Single well size of six-well plate, cell density: 1 × 106 per plate, full confluency), and lysated by TRI (T9424, sigma). RNA concentration was detected (RNA Nano 6000 Assay Kit) by the Bioanalyzer 2100 system (Agilent Technologies, CA). The total amount of RNA (1.5 μg per sample) was applied for the detection following the manufacturer's instruction. The clustering for the index coded samples was based on a cBot Cluster Generation System (HiSeq 4000 PE Cluster Kit, Illumia, San Diego, CA). The obtained raw data in fast q format were processed via in-house perl scripts. At this moment, clean data (clean reads) could be gained through removing reads containing adapter, reads containing ploy-N, and other low quality reads from the raw data. Paired-end clean reads were matched and aligned to the reference genome by using HISAT2 v2.1.0. We used HTSeq v0.6.1 to count the reads numbers that mapped to each gene. The fragments per kilobase of transcript per million fragments mapped (FPKM) were calculated by summing the transcripts of all genes. For comparison with differential gene expressions, the analysis was performed by using DESeq2 R package (1.26.0). Threshold (Pvalue <0.05 and FoldChange ≥2.0) was used. Gene expressions were expressed as pheatmaps generated by online R package.
2.15. Chondrogenic differentiation
Chondrogenic differentiation was carried out by using chondrogenic differentiation kit (MUXMX-90041, Cyagen, Guangzhou, China). The process of chondrogenic differentiation was followed by the manufacturer's instructions. After bone marrow mesenchymal stem cells (BMSCs) were reached to ∼90% confluence (Cell intensity: 6 × 105 per plate, single well size of twelve-well plate), the chondrogenic differentiation media were replaced and refreshed every two days. After induction for 7 days, we fixed the cells by 4% PFA and stained them with alcian blue solution provided by the kit. The images were obtained by stereomicroscope.
2.16. Quantitative real-time PCR (q-PCR)
q-PCR was performed to detect gene expressions of Col2, Sox9 and aggrecan. Total RNA was lysated by Trizol reagent (Invitrogen) and cDNA was obtained by a reverse transcription with reverse kits (Thermo scientific, Vilnius, Lithuania) based on the cell number at 1 × 106 per sample. q-PCR was performed by using SYBR Premix Ex Taq with iCycler (Bio-Rad). The primers designed were included: Col2a1 (Forward: GCCAGGATGCCCGAAAATTA, and Reverse: TCTCCCTTGTCACCACGGTC); Sox9 (Forward: TGACTACACCGACCACCAGA, and Reverse: GAGCTGTGTGTAGACGGGTT); Aggrecan (Forward: GCTTATGCCTTCCGAGCTACA, and Reverse: CTGGAACTTGGTCCACCCC); GAPDH (Forward: TCTTCTTGTGCAGTGCCAGC, and Reverse: TGAACTTGCCGTGGGTAGAG). GAPDH was used as the house keeping gene. 2−ΔΔCt method was used calculated the fold changes.
2.17. Metabonomics
The metabonomics detection was completed by Metware (Untargeted metabolomics, Metware, Wuhan, China). In brief, chondrocytes were seeded and cultured onto Fe2O3/GelMA hybrid hydrogels for 72 h. Then cells at the concentration of 1 × 106 per group were collected and subjected to metabonomics. Then cells at the concentration of 1 × 106 per group were collected and subjected to metabonomics. The Mass spectrometers (QTOF/MS-6545 and 1290 Infinity LC) were used. The raw data were converted to mzML format by Proteo Wizard and data peak values were extracted by XCMS program. The p value was set to be 0.05 based on univariate analyzed T-test. Fold change was set to be 2.0 (≥2.0) or 0.5 (≤0.5). For the final data in the current study was presented by DESeq2 online R package (1.26.0). All original data were presented in supplementary source data.
2.18. Articular cartilage defect rat model
The animal samples were obtained from HuaFuKang biotechnology company (Beijing, HFK Bioscience CO., LTD), and all protocols using in this study were approved by the Institutional Review Board (IRB) in the West China Hospital of Stomatology (No.WCHSIRB-D-2020-048), Sichuan University. To avoid any potential influence of estrogen on the cartilage remodeling [37], we choose to use male wistar rats (4-week old, ∼80 g) to establish articular cartilage defects at cartilaginous ends of femurs of knee joints in aseptic environment. The defects were created in the form of cylinder with diameter of 1.5 mm and height of 2.0 mm (The height of defect depends on whether it has penetrated into the subchondral bone). After the Fe2O3/GelMA gels were injected into the defects, the wound was sewn up and buprenorphine hydrochloride (0.2 mg/kg) (B7536, sigma) was given as an analgesic. Rats were allowed to move freely in cages in SPF-graded animal room. Rats were sacrificed at 1, 3 and 6 weeks after surgery. In each group, the rat sample number was up to 9 (n = 9). The collected samples were immediately fixed by 4% PFA for further usage.
2.19. Microtomography
As the cartilage defect penetrated into the subchondral bone area, factors from subchondral bone could infiltrate into this area to aid the remodeling of the defect. We collected the cartilaginous ends of rat femurs with defects and fixed them with 4% PFA and then scanned them by μ-CT (VIVA 40CT system, μ-CT 50, Scanco Medical, Bassersdorf, Switzerland) in our lab. The images were generated by its matched software.
2.20. Hematoxylin and Eosin (H&E) staining
H&E staining was performed to detect the tissue morphology changes of cartilage defects as previously described [38]. After frozen section, the slices of joint tissues were directly stained by Eosin. Then the slices underwent color separation by HCL-EOTH (1% acid-ethanol in 70% ethanol, 1:99 ratios). After hematoxylin staining, the slices underwent nuclear separation by ammonia-H2O (ammonium hydroxide in ddH2O, 1:500 ratios). After glycerol (10% v/v) sealing, the images were obtained by inverted light microscopy (Olympus IX71, Japan).
2.21. Von Kossa staing
Von Kossa staing was performed to observe the mineralization of joint tissues as previously described [39]. After frozen section, the slices of joint tissues were directly stained by silver nitrate (AgNO3, 5% w/v) for about 30 min in ultraviolet light. The tissues were then washed with Ca/Mg-free PBS three times and mounted by 10% glycerol (v/v). The results were obtained by inverted light microscopy (Olympus IX71, Japan).
2.22. Safranin O staining and alcian blue staining
Safranin O staining and alcian blue staining were performed to detect proteoglycan in the site of hyaline cartilage defect as previously described [39]. In the current study, we focused on the cartilage defect area and stained this area by safranin O (red) and alcian blue (blue) to detect proteoglycan formation. The difference between safranin O staining and alcian blue staining was that: before safranin O staining, the sample needs to be pretreated with iron-hematoxylin for 5 min, and the rapid oxidation of the sample in the air should be avoided after iron-hematoxylin treatment. Besides, fast green was used in safranin O staining as the counterstain. The images were obtained by inverted light microscopy (Olympus IX71, Japan).
2.23. Statistical analysis
The statistical data were based on at least three independent repeated expereimts (n ≥ 3) and presented as mean ± SD. We consider the critical significance in each analysis was present when the threshold was less than 0.05 (p < 0.05). All the data were analyzed by two-tailed Student's t-tests. The detailed statistical procedure was attached in the source data. Additionally, when comparing more than two groups, we also checked the significant differences by using SPSS 16.0 to reconfirm the results presented in manuscript.
3. Results
3.1. Synthesis and characterization of Fe2O3/GelMA hybrid hydrogels
The Fe2O3/GelMA hybrid hydrogel was designed and prepared of methacrylated gelatin (GelMA) and iron oxide nanoparticles (Fe2O3) through chemical bonding of carbon-carbon double bonds (Fig. 1a). The magnetic nanoparticles were first modified by coating with vinyl and then doped in the framework of the hydrogel in our study (detailed in method protocols). The Fe2O3/GelMA hybrid hydrogels can be prepared in different sizes according to the intended use (Fig. 1b). To further characterize the Fe2O3/GelMA hybrid hydrogel, the cross-section morphology showed that the Fe2O3 nanoparticles were embedded in the interior region of the hydrogel (Fig. 1c, Left image indicates the vinyl coated magnetic nanoparticles and right image shows internal chimeric region). The supplements of hydrogels morphology were scanned by SEM at microscale (Fig. S1). The particle size of Fe2O3 nanoparticles and PDI value were 26.2 nm and 0.117, respectively (Fig. 1d). In addition, from the elemental analysis shown in Fig. 1e, the iron element was doped evenly in the hybrid hydrogels, which proved the existence of Fe2O3 nanoparticles. From the analysis of the infrared spectrum (Fig. 1f), the Fe2O3/GelMA hydrogel retained its characteristic peak of GelMA (∼1650 cm−1 and ∼1542 cm−1), and the characteristic peak of MPS-Fe2O3 at ∼1621 cm−1 disappeared, which verified the cross-linking of the magnetic nanoparticles and GelMA. Regularly, the color of these Fe2O3/GelMA hybrid hydrogels was shown to be gradually deepened with an increasing concentration of Fe2O3 nanoparticles (Fig. 1g). We measured the surface roughness based on AFM with an improved indentation probe and found that these hydrogels showed lower surface roughness than the Petri dish control (Fig. 1h). The swelling ratio of Fe2O3/GelMA hydrogels decreased with the amount of Fe2O3 nanoparticles, which may contribute to the increased cross-linking of hybrid hydrogel (Fig. 1i). Interestingly, the internal mechanical properties of these hydrogels were significantly improved by enhancing their chemical cross-linking (Fig. 1j) (In other words, the mechanical strength of the Fe2O3/GelMA hybrid hydrogel can be tuned by controlling the concentration of Fe2O3 nanoparticles). The interaction between the tunable stiffness hydrogel platform and chondrocytes is not clear and inspires our research interest. As shown in Fig. 1k, the basal cellular physical property changes and cellular metabolic changes (biological property) of chondrocytes in response to the hybrid hydrogel were systematically elucidated. And the cartilage repair effect based on a rat knee cartilage defect model in vivo was further studied. In the following parts, we will present and discuss the results in detail.
3.2. Cellular basal physical property changes in response to Fe2O3/GelMA hybrid hydrogels
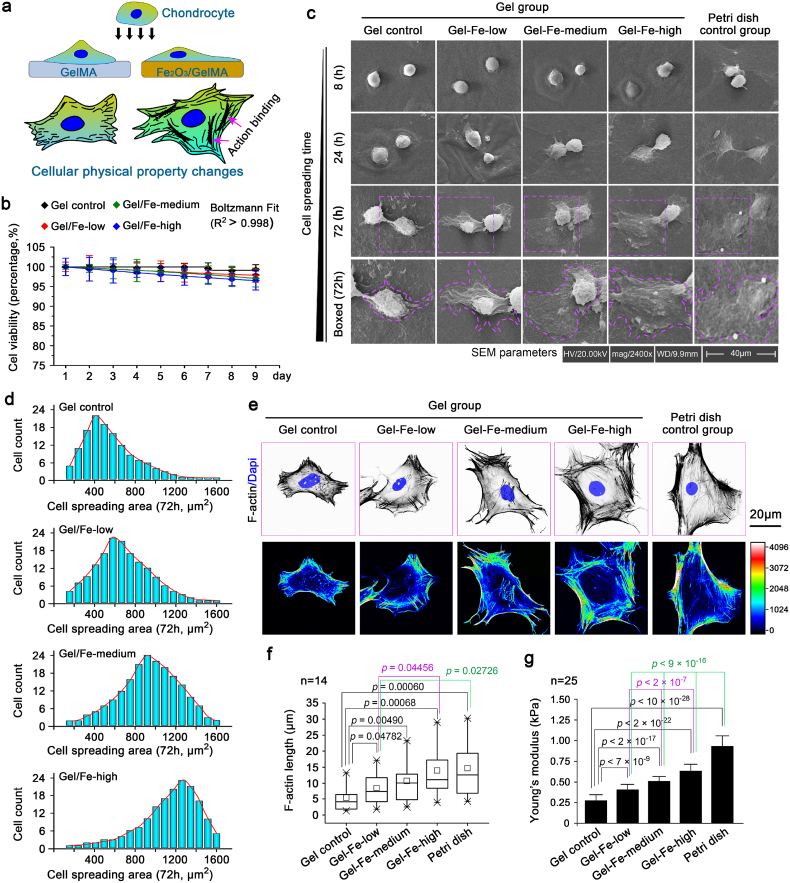
To explore the interaction between chondrocytes and Fe2O3/GelMA hybrid hydrogels, chondrocytes were first seeded onto Fe2O3/GelMA hybrid hydrogels to detect basal cellular changes (Fig. 2a). The cell proliferation was detected in a short period of interaction (Fig. S2), and the cell viability was not obviously changed after a long period of interaction for 9 days (Fig. 2b). Then, the cell morphology changes in chondrocytes were explored in depth by using SEM (Fig. 2c). We recorded the cellular morphological images at 8, 24 and 72 h after cell seeding and found that the cell spreading areas were larger on the hydrogels with higher concentrations of Fe2O3 nanoparticles (a higher stiffness). The quantification of the cell spreading area confirmed this result (Fig. 2d). We next characterized the F-actin-based microfilament organization. The F-actin was more ordered on higher stiffness hydrogels (Fig. 2e, upper image, and Fig. S3). An analysis of microfilament organization (Fig. 2e, lower image) showed a significant shift in microfilament organization from a fragmented (the softer substrates of the hydrogel control or lower Fe2O3 group) to a bundled (the stiffer substrates of higher Fe2O3 groups). The changes in F-actin bundling length were also accompanied by the changes in the stiffness of hydrogels (Fig. 2f). Most importantly, by using AFM with an improved indentation probe, the cellular Young's modulus was changed in response to hydrogel stiffness (Fig. 2g). Taking together, these results indicated physical property changes of chondrocytes interacted with Fe2O3/GelMA hybrid hydrogels.
Fig. 2.
Physical property changes of chondrocytes interacting with Fe2O3/GelMA hybrid hydrogels. a. Diagrammatic sketch indicating the cellular physical property changes of chondrocytes seeded on Fe2O3/GelMA hybrid hydrogel. b. Cell viability assay based on the CCK-8 kit showing the total activities of chondrocytes interacting with Fe2O3/GelMA hybrid hydrogels without changing the culture media. c. Morphological changes of chondrocytes on Fe2O3/GelMA gels within 72 h of cell seeding by SEM. The images were based on six independent experiments (n = 6). d. Analysis of the cell spreading areas of chondrocytes seeded on Fe2O3/GelMA hybrid hydrogels by Image J. The cell spreading calculation considered up to 132 chondrocytes in each group. e. Cytoskeleton changes were detected. The distribution changes of F-actin were determined by CLSM (upper). The changes in cellular microfilament organization (F-actin bundling) were shown by heat maps (lower). The images presented are based on four independent experiments (n = 4). f. The length of F-actin bundles in chondrocytes seeded on Fe2O3/GelMA hybrid hydrogels. There were 14 cells analyzed in each group. g. Changes in Young's moduli of chondrocytes seeded on Fe2O3/GelMA hybrid hydrogels. There were 25 cells analyzed in each group. The data in f are shown in the box (from 25, 50–75%) and whisker (minimum to maximum values) plots. All significant data presented in b, f and g are based on two-tailed Student's t-tests.
3.3. Cellular metabolic changes in response to Fe2O3/GelMA hybrid hydrogels
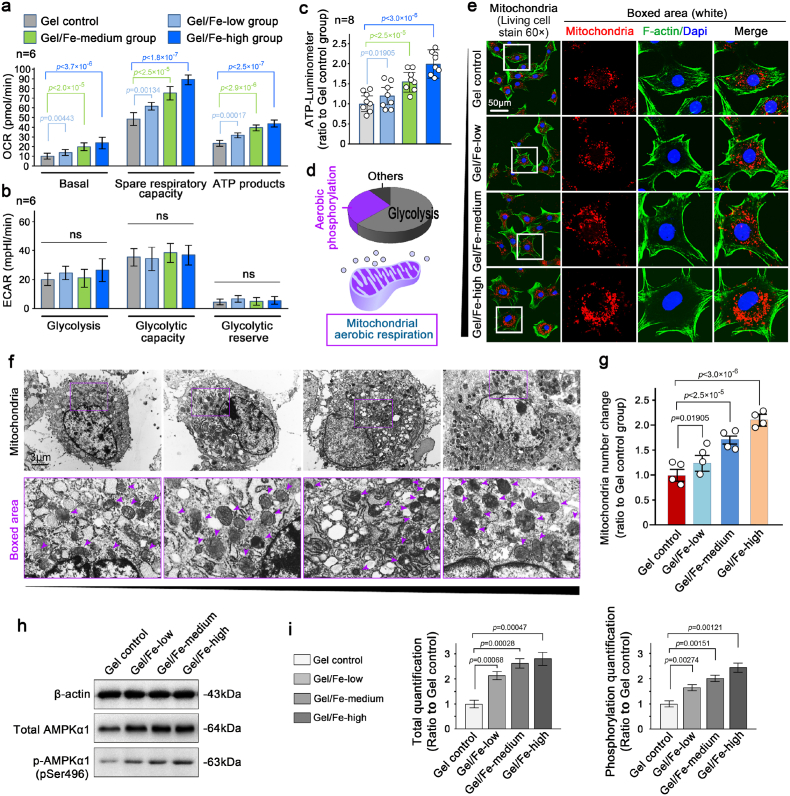
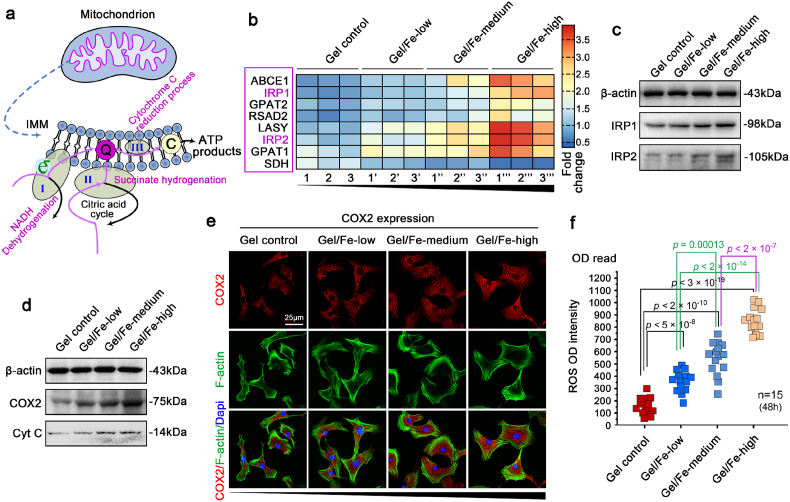
Regarding the large amount of energy consumption associated with the cellular biological property changes in response to Fe2O3/GelMA hybrid hydrogels, we first investigated the changes in energy metabolism during this cell-matrix interaction process. By using seahorse matching kits, we found that the oxygen consumption rate (OCR) was significantly increased in chondrocytes growing on hydrogels with higher Fe2O3 concentrations (Fig. 3a), while the extracellular acidification rate (ECAR), which directly reflects the glycolysis [40], was not changed (Fig. 3b). To further confirm the changes in energy metabolism, we directly detected the ATP products by using an ATP luminometer (Fig. 3c) and found that the total ATP productivity increased in chondrocytes growing on hydrogels with increased Fe2O3 concentrations. Based on the fact that aerobic respiration plays an important role in the physiological activities of chondrocytes (Fig. 3d, purple), we detected changes in the number of mitochondria after seeding chondrocytes on Fe2O3/GelMA hybrid hydrogels for 3 days (Fig. 3e). By using mitochondrial staining of living chondrocytes observed by CLSM at the microscale, the number of mitochondria increased when they were grown on the hydrogels with increased Fe2O3 concentrations. We then used TEM at the nanoscale to detect the changes in the number of mitochondria (Fig. 3f & Fig. S4) and found that more mitochondria were generated on the hydrogels with increased Fe2O3 concentrations. Quantitative analysis based on the number of mitochondria in single cell by TEM confirmed these results (Fig. 3g). As the fusion/fission dynamics of mitochondria are directly mediated by AMPK signaling, especially fission or biogenesis [41,42], we therefore detected this signaling. The results showed that the expression of total AMPK and phosphorylated AMPK was all increased in chondrocytes on hydrogels with increased Fe2O3 concentrations (Fig. 3h and i).
Fig. 3.
Mitochondrial activation in chondrocytes interacting with Fe2O3/GelMA hybrid hydrogels. a. A histogram showing mitochondrial respiration capacity in chondrocytes interacting with Fe2O3/GelMA hybrid hydrogels. OCR, oxygen consumption rate. The data presented are based on six independent experiments (n = 6). b. A histogram showing glycolysis in chondrocytes interacting with Fe2O3/GelMA hybrid hydrogels. ECAR, extracellular acidification rate. The data presented are based on six independent experiments (n = 6). c. The ATP luminometer further confirmed the total ATP generation in chondrocytes interacting with Fe2O3/GelMA hybrid hydrogels. The data presented are based on eight independent experiments (n = 8). d. A diagrammatic sketch showing the proportion of metabolic types of normal chondrocytes (upper) and the change in metabolic types interacting with Fe2O3/GelMA hybrid hydrogels appeared in mitochondrial-mediated aerobic phosphorylation (lower). e. Representative mitochondrial staining showing the changes in the number of mitochondria in living chondrocytes seeded on Fe2O3/GelMA hybrid hydrogels for 72 h. The images are representative of six independent experiments (n = 6). f. Representative TEM indicating the detailed changes of the number of mitochondria in chondrocytes seeded on Fe2O3/GelMA hybrid hydrogels for 72 h. Purple arrows indicate mitochondria. The images are representative of four independent experiments (n = 4). g. Quantification of the mitochondrial number in f. The data are based on four independent experiments (n = 4). h. Western blotting showing the protein changes in total and phosphorylated AMPKα1, which are responsible for the changes in mitochondrial number in the chondrocytes seeded on Fe2O3/GelMA hybrid hydrogels. The images are representative of three independent experiments (n = 3). i. Quantification of the total and phosphorylated AMPKα1 by western blotting shown in h. The data are based on three independent experiments (n = 3). All significant data presented in a, b, c, g and h are based on two-tailed Student's t-tests.
The Fe2O3/GelMA hybrid hydrogels are not only a scaffold platform for cell culture systems in vitro and tissue remodeling in vivo, but also a potent carrier with magnetic nanoparticles. We provided the detailed recipe in Fe2O3/GelMA hybrid hydrogels (Table S1), and it indicated, in the 200 μl system, the Fe2O3 contents were 120, 160 and 200 μg in gels with low, medium and high concentration, respectively. As ATP generation is not only affected by the number of mitochondria (Fig. 3e–g), but also by the function of mitochondria, and based on the fact that thioferritin (iron (Fe)/sulfur (S)) is a prerequisite for the first three steps in the mitochondrial respiratory chain (NADH dehydrogenation, succinate hydrogenation and cytochrome reduction process, as listed in Fig. 4a in purple), we detect all 18 known Fe/S cluster assembly proteins participating in the aerobic respiratory chain and biogenesis of mitochondria. The RNA sequencing results showed that the mRNA transcripts of 7 of these genes were increased (Fig. 4b). Importantly, iron-regulatory protein 1 (IRP1) and 2 (IRP2), which are in charge of iron uptake and sequestration, transferrin receptor expression, and ferritin levels [64], were highly expressed in chondrocytes in response to the Fe2O3/GelMA hybrid hydrogels. We used western blotting and detected the enhanced expression of IRP1 and IRP2 (Fig. 4c). The quantitative analysis further confirmed their expression (Fig. S5). We also detected an increase in cyclooxygenase-2 (COX2), the last enzyme in the mitochondrial electron transport chain that drives oxidative phosphorylation [43], and cytochrome c (Cyt c), a mobile electron carrier, an indispensable component in the electron transport chain responsible for the chief source of ATP [44] (Fig. 4d and e). Enhancement of the respiratory chain leads to more ATP generation for physiological needs in chondrocytes (Fig. S6A), which is beneficial for the repair of cartilage injury. Additionally, enhancement of the respiratory chain also induced a transient increase of reactive oxygen species (ROS) (Fig. 4f & S6B), which might has a certain impact on mitochondrial autophagy [45]. However, this transient enhancement of ROS did not impact the changes of cell viability of chondrocytes in response to hydrogels with higher Fe2O3 concentrations, as shown in Fig. 2b.
Fig. 4.
Enhancement of the mitochondrial respiratory chain in chondrocytes interacted with Fe2O3/GelMA hybrid hydrogels. a. Diagrammatic sketch indicating that interaction between chondrocytes and Fe2O3/GelMA hybrid hydrogel facilitated the first three stages of the mitochondrial respiratory chain in chondrocytes. I, II and III in blue indicates the three stages of the mitochondrial respiratory chain in energy generation. b. Pheatmap imaging showing that the expression of 8 out of 18 genes in the mitochondrial respiratory chain were enhanced, especially IRP1 and IRP2. The data were obtained from three independent experiments (n = 3). Each set of samples 1, 1′, 1″ and 1‴; 2, 2′, 2″ and 2‴; 3, 3′, 3″ and 3‴ is from the same batch of mother cells. c. Western blotting showing the protein changes of IRP1 and IRP2 in chondrocytes seeded on Fe2O3/GelMA hybrid hydrogels. The images are representative of three independent experiments (n = 3). d. Western blotting showing the protein changes of COX2 and cytochrome c (Cyt C) in chondrocytes seeded on Fe2O3/GelMA hybrid hydrogels. The images are representative of three independent experiments (n = 3). e. CLSM image showing the distribution of COX2 in chondrocytes seeded on Fe2O3/GelMA hybrid hydrogels. The images are representative of three independent experiments (n = 3). f. Optical densitometer showing the changes in reactive oxygen species released from chondrocytes seeded on Fe2O3/GelMA hybrid hydrogels. The data are based on fifteen independent experiments (n = 15). The significance was based on two-tailed Student's t-tests.
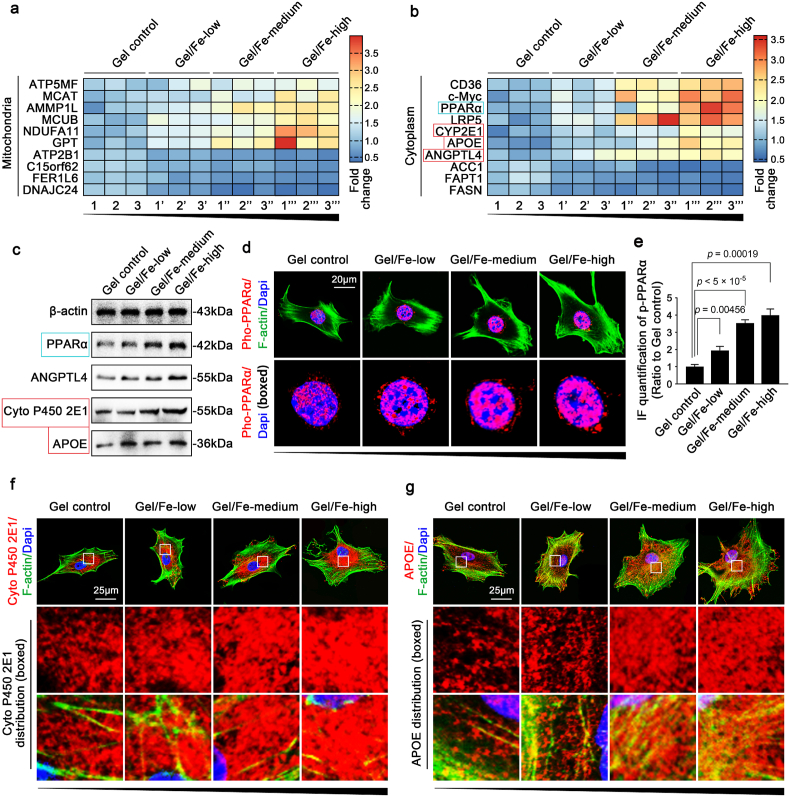
From the RNA-sequencing data, we identified other genes with altered expression involved with mitochondria (Fig. 5a). We analyzed these gene candidates and found that most of them were favorable for mitochondrial ATP generation. Cell-matrix interactions between chondrocytes and Fe2O3/GelMA hybrid hydrogels strengthen the respiratory chain of mitochondria, but they are not limited to this. In the entire cytoplasm, changes in various signaling pathways were also detected. The results indicated that peroxisome proliferator-activated receptor (PPAR) signaling was the main changed pathway (Fig. 5b). We next confirmed the changes in PPAR signaling, including PPARα, angiopoietin-like 4 (ANGPTL4), cytochrome P450 2E1 (Cyto P450 2E1), apolipoprotein E (APOE), PPARγ and CCAAT/enhancer-binding protein alpha (C/EBPα), at protein level. The expression levels of PPARα, ANGPTL4, Cyto P450 2E1 and APOE, which are all involved in mitochondrial fatty acid oxidation [46], all increased in chondrocytes seeded on Fe2O3/GelMA hybrid hydrogels (Fig. 5c), while the expression levels of PPARγ and C/EBPα did not change (Fig. S7). As PPARα, a nuclear hormone receptor, directly regulates the expression of genes involved in mitochondrial and microsomal fatty acid oxidation [47], we later observed its nuclear accumulation (Fig. 5d) in chondrocytes seeded on Fe2O3/GelMA hybrid hydrogels. The quantitative analysis further confirmed these results (Fig. 5e). Two important enzymes, cytoP450 2E1 and APOE, were shown to be enhanced throughout the cytoplasm of the chondrocytes (Fig. 5f and g).
Fig. 5.
Key signaling activation in chondrocytes interacting with Fe2O3/GelMA hybrid hydrogels. a. Pheatmap showing the total gene changes in mitochondria in addition to those in the respiratory chain. The data are based on three independent experiments (n = 3). Each set of samples 1, 1′, 1″ and 1‴; 2, 2′, 2″ and 2‴; 3, 3′, 3″ and 3‴ are from the same batch of mother cells. b. Pheatmap imaging showing that the top ten candidate genes in the cytoplasm of chondrocytes indicate enhanced lipid catabolism through enhanced mitochondrial aerobic phosphorylation. Each set of samples 1, 1′, 1″ and 1‴; 2, 2′, 2″ and 2‴; 3, 3′, 3″ and 3‴ is from the same batch of mother cells. c. Western blotting showing the protein changes of PPARα Angptl4, Cyto P450 2E1 and Apoe in chondrocytes seeded on Fe2O3/GelMA hybrid hydrogels. d. CLSM images showing the nuclear accumulation of phosphorylated PPARα signaling. The images are representative of three independent experiments (n = 3). e. Fluorescence OD quantification showing the changes in the nuclear accumulation of PPARα signaling. The data are based on three independent experiments (n = 3). The significance is based on two-tailed Student's t-tests. f. CLSM images showing the distribution changes of Cyto P450 2E1 in chondrocytes seeded on Fe2O3/GelMA hybrid hydrogels. The images are representative of three independent experiments (n = 3). g. CLSM images showing the distribution changes of Apoe in chondrocytes seeded on Fe2O3/GelMA hybrid hydrogels. The images are representative of three independent experiments (n = 3). All significant data presented in e are based on two-tailed Student's t-tests.
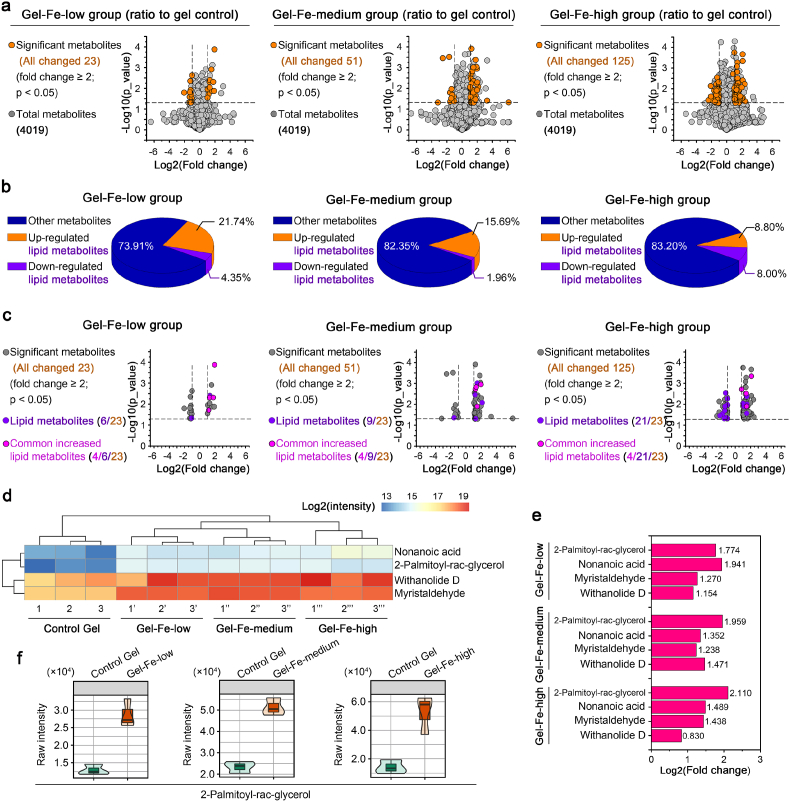
We next examined all byproducts of metabolites by the perturbations of mitochondrial aerobic respiration by metabonomics (Fig. 6). We screened total 4019 metabolites in chondrocytes interacting with Fe2O3/GelMA hybrid hydrogels compared to the GelMA control. We found that changed metabolites were more abundant in chondrocytes seeded on hydrogels with higher Fe2O3 concentrations (Fig. 6a). At the threshold of fold change ≥2 and p value < 0.05, we found 23, 51 and 125 changed metabolites in hydrogels with low, medium and high Fe2O3 concentrations, respectively. Among these changed metabolites, we then analyzed the lipid metabolites and showed their proportions among the total changed metabolites (Fig. 6b). There were 6, 9 and 21 changed lipid metabolites in hydrogels with low, medium and high Fe2O3 concentrations, respectively (Fig. 6c). Moreover, four of the same lipid metabolites were increased on these hydrogels at low, medium and high Fe2O3 concentrations. They were nonanoic acid, 2-palmitoyl-rac-glycerol, withanolide D and myristaldehyde. They could be clustered by hierarchical clustering analysis based on pheatmap analysis (Fig. 6d). Nonanoic acid is a saturated fatty acid metabolite, withanolide D is a phosphatidylcholine (PC) metabolite, and 2-palmitoyl-rac-glycerol and myristaldehyde are fatty aldehyde metabolites (the online UniProt resource pool provides all of this information). In addition, we showed change folds of these metabolites in chondrocytes interacting with hydrogels at low, medium and high Fe2O3 concentrations (Fig. 6e). Moreover, the increase in 2-palmitoyl-rac-glycerol was more reliable because it could form violin plots in each group relative to the control (Fig. 6f). Taking together, these four metabolites indicated the changes of lipid catabolism in chondrocytes in response to Fe2O3/GelMA hybrid hydrogels.
Fig. 6.
Metabonomics in chondrocytes interacting with Fe2O3/GelMA hybrid hydrogels indicates the products of lipid catabolism. a. Volcano map showing all changed metabolites in GelMA gels hybridized with Fe2O3 at low (left), medium, and high (right) concentrations compared with the GelMA control group. A p value < 0.05 and fold change ≥2 were set as the thresholds for identifying significantly changed compounds. A total 4019 metabolites were screened. b. Pie chart showing the proportion of lipid metabolites among the total metabolites. The three groups, i. e., Gel-Fe-low, Gel-Fe-medium and Gel-Fe-high, were all compared with the GelMA control group. c. Volcano map showing the four common changed lipid metabolites in GelMA gels hybridized with Fe2O3 at low (left), medium, and high (right) concentrations compared with the GelMA control group. A p value < 0.05 and fold change ≥2 were set as the thresholds for identifying significantly changed compounds. d. Pheatmap showing the four commonly changed lipid metabolites in chondrocytes growing on Fe2O3/GelMA hybrid hydrogels. The data were subjected to hierarchical clustering with the online R package. A p value < 0.05 and fold change ≥2 were set as the thresholds for identifying significantly changed compounds. The data are presented as log2(intensity). Each set of samples 1, 1′, 1″ and 1‴; 2, 2′, 2″ and 2‴; 3, 3′, 3″ and 3‴ is from the same batch of mother cells. e. A histogram showing the specific fold changes of the four lipid metabolites in chondrocytes grown on Fe2O3/GelMA hybrid hydrogels versus the GelMA control group. f. The metabolite, 2-palmitoyl-rac-glycerol-M, was confirmed by violin maps generated from the three Fe2O3/GelMA gel groups versus the GelMA control group.
3.4. Application of Fe2O3/GelMA hybrid hydrogels in cartilage defects in vivo
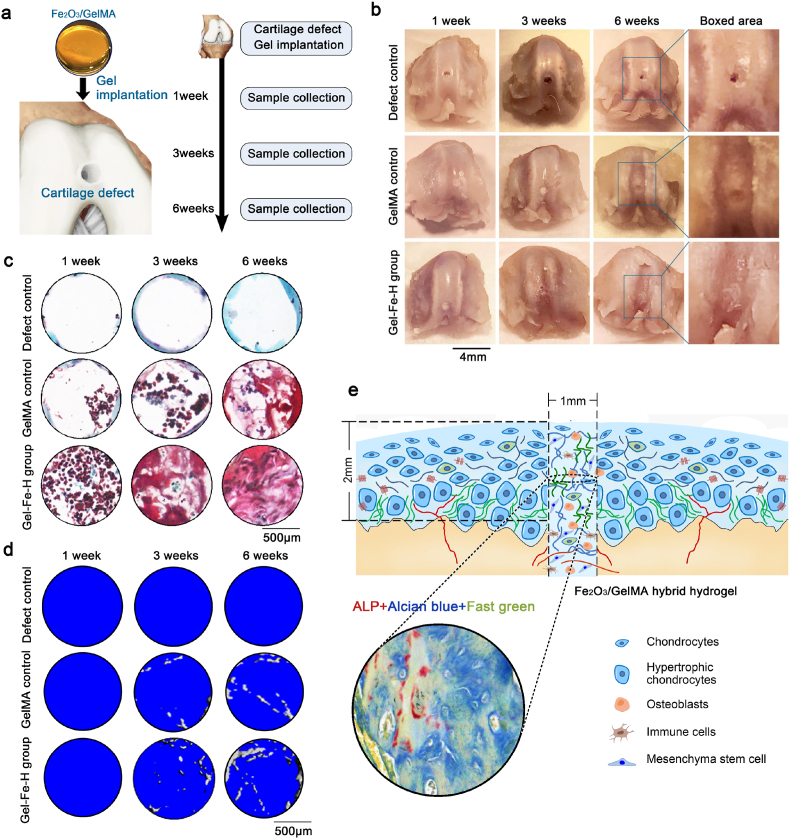
In the preliminary experiment, we examined the chondrogenic differentiation capacity of bone marrow mesenchymal stem cells (BMSCs) growing on the Fe2O3/GelMA hybrid hydrogels in vitro (Fig. S8) and found that, with chondrogenic differentiation induced media, the chondrogenic differentiation capacity of BMSCs seeded on the Fe2O3/GelMA hybrid hydrogel (Gel-Fe-high group) were shown to be stronger than these in the Petri dish group and GelMA group (Fig. S8A-Alcian blue staining, 7 days). The qPCR results confirmed higher gene expressions of Col2, Sox9 and aggrecan in BMSCs under chondrogenic differentiation induced media for 7 days (Fig. S8B). We established a rat cartilage defect model and aimed to check whether the Fe2O3/GelMA hybrid hydrogel could promote the repair of cartilage defects. We made a defect model (∼1.5 × 1.5 × 2 mm3) in the knee cartilage (the depth of defect “2” depends on whether it has penetrated into the subchondral bone under a stereomicroscope), implanted the hybrid hydrogel into the defects (hydrogels with high Fe2O3 concentrations were used), and collected knee samples at 1, 3 and 6 weeks after implantation surgery (Fig. 7a). To detect degradation of the hydrogels, the size changes of the hydrogels within 7 weeks were measured with or without cell seeding in vitro (Fig. S9). We found that the higher concentration of Fe2O3 nanoparticles hybrid in hydrogel lead to slower degradation rate (with or without chondrocyte seeding). In regard to the defect repair capacity of Fe2O3/GelMA hybrid hydrogels, we observed the morphology of the defect healing (Fig. 7b). The results showed that the Fe2O3/GelMA hybrid hydrogels had a better repair capacity for the healing of cartilage defects in a rat model. To further analyze the histological changes at the site of the repaired cartilage defects, we performed safranin O staining (Fig. 7c). The results showed that in the early stage (1 week post surgery), cartilage defects after implantation of Fe2O3/GelMA hybrid hydrogels could recruit more cells, including peripheral chondrocytes (because some cells or surroundings were shown to be red by safranin O staining). At 6 weeks post surgery, a large amount of proteoglycan matrix was accumulated in the new formed tissue at the site of the defect (red). Additionally, by μ-CT, we also found that this newly formed tissue contained osteoid components at 6 weeks post surgery (Fig. 7d). Further, we used combined staining of ALP and Alcian blue to characterize this newly formed tissue at the site of the defects and found that osteoblasts (ALP, red) and chondrocytes (Alcian blue, blue) coexisted in this newly formed tissue (Fig. 7e).
Fig. 7.
The application of Fe2O3/GelMA hydrogel in a rat cartilage defect model. a. Schematic diagram indicating the implantation of Fe2O3/GelMA hydrogel into rat cartilage defects. Cartilage samples were collected at 1, 3 and 6 weeks after implantation. b. Morphology of the remodeling of rat cartilage defects with implantation of Fe2O3/GelMA hydrogels. The results are based on 9 independent experiments (n = 9). c. Safranine O staining showing the aggregation of cartilaginous proteoglycans in the cartilage defect region. d. μ-CT indicating the generation of osteoid components in the cartilage defect region. e. Schematic diagram showing that the implantation of Fe2O3/GelMA hydrogel promotes cartilage defect remodeling with the help of multiple cell types infiltrated from the subchondral bone. Tissue staining in the circle showing that both osteoblasts (ALP staining, red) and chondrocytes (Alcian blue staining, blue) coexisted in the remodeling region (fast green was counterstained to show the background, in green).
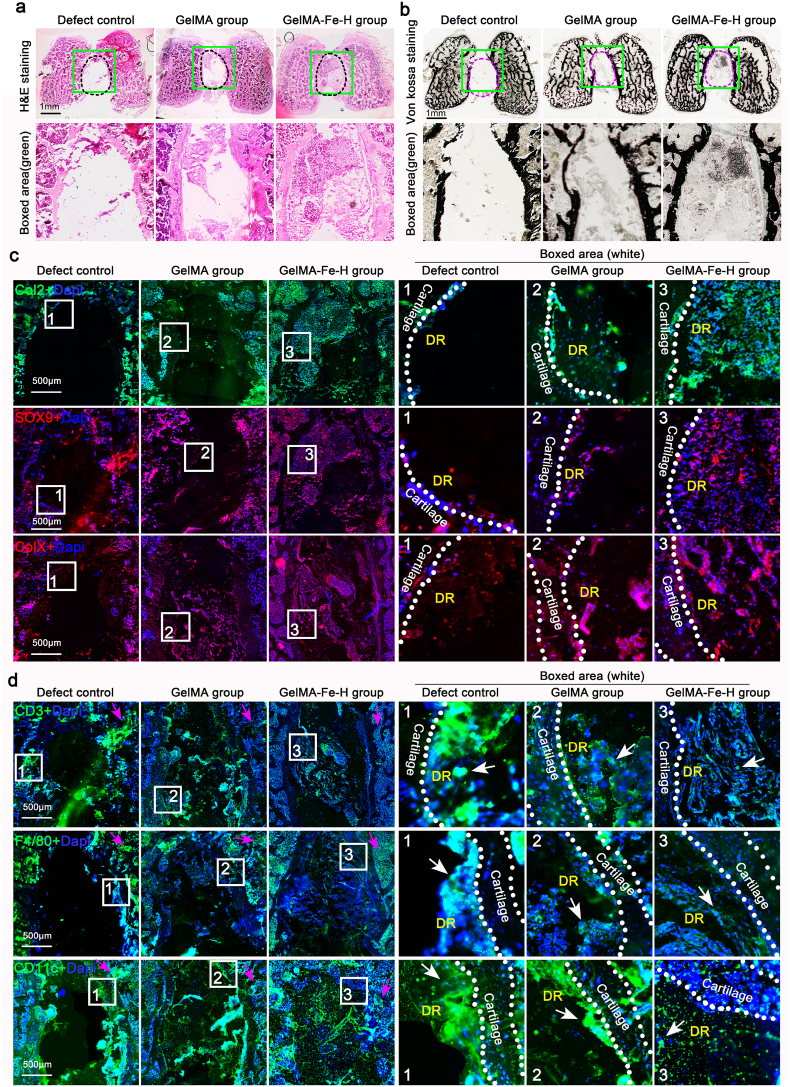
We finally performed in-depth biochemical analysis of the tissues in the cartilage defect region with the Fe2O3/GelMA hydrogel implantation for 6 weeks. Based on frozen section, we first showed the remodeling morphology of the defect region by H&E staining (Fig. 8a), and the results showed that a new tissue filler was gathered in the defect region in the Fe2O3/GelMA hydrogel group relative to the defect group (black control) and GelMA group at 6 weeks post surgery. The following mineralization analysis by von kossa staining indicated that there was weak mineralization formation in the defect region with the Fe2O3/GelMA hydrogel implantation relative to the defect group and GelMA group (Fig. 8b). This result, combined with the osteoid component analysis in Fig. 7e, indicated that these newly formed tissues contained the osteogenic components mainly originated from the subchondral bone (the ALP staining in Fig. 7e). We then detected the expressions of collagen type II (Col2) and Sox9 by immunofluorescence staining (Fig. 8c, upper). From the results, we could observe that highly expressed positive Col2 in these newly formed tissues. Moreover, the high expression of Sox9 confirmed a high content of chondrocytes in these tissues. At the same time, we detected the expressions of collagen type X (ColX), a hypertrophic chondrocyte marker [3,39], in these defect regions (Fig. 8c, lower). The occurrence of inflammation around the defect region was finally investigated (Fig. 8d). We detected the expressions of CD3, a T cell marker [48], F4/80, a macrophage marker [49] and CD11c, a dendritic cell marker [50] at/around the defect region. The results indicated that, in the region of subchondral bone marrow adjacent to defect region, the expressions of these three inflammatory markers were higher expressed in the defect group than these in the Fe2O3/GelMA hydrogel group (purple arrows in Fig. 8d, left). Meanwhile, on the side of defect region (close to the defect edge), we could also observe the expressions of these three markers and their expressions were also higher in the defect group than these in the Fe2O3/GelMA hydrogel group (white arrows in Fig. 8d, right). The result showed that lower inflammatory response in the Fe2O3/GelMA hydrogel group was beneficial to the formation of neo-tissue.
Fig. 8.
Evaluation of cartilage defect region implanted with Fe2O3/GelMA hydrogels for 6 weeks. a. H&E staining indicating the remodeling morphologies of cartilage defect region implanted with Fe2O3/GelMA hydrogels. b. Von kossa staining showing the mineralization of cartilage defect region implanted with Fe2O3/GelMA hydrogels. c. Immunofluorescence staining showing the expressions of collagen II (Col2), Sox9 and collagen X (ColX) in the defect region with the Fe2O3/GelMA hydrogel implantation. Col2, green; Sox9 and ColX, red; Dapi, blue. DR, defect region. d. Immunofluorescence staining showing the expressions of CD3, F4/80 and CD11c in the defect region with the Fe2O3/GelMA hydrogel implantation. CD3, F4/80 and CD11c, green; Dapi, blue. DR, defect region. White arrows indicate positive results of immunofluorescence staining about CD3, F4/80 and CD11c. The representative images in a-d were based on 4 independent groups. All tissue sections in a-d were stained by frozen section.
4. Discussion
The interaction between cells and biomaterials reflects the changes of biological behaviors of seeding cells, which is considered to be an intuitive embodiment of the biological effect of the material and the most objective evaluation of the material bio-application [28,51]. At the same time, the interaction also provides a reference of biological mechanism for the design, improvement and optimization of biomaterials, which is conducive to the development of biocompatible and effective new materials [7,13,25]. Based on our previous exploration of hydrogels [9,26], in the current study, we fabricated a novel hybrid hydrogel with tunable stiffness based on GelMA and iron oxide nanoparticles, underlined the cellular physical property responses and cellular metabolic changes, and applied it to cartilage remodeling based on cartilage defect.
The interaction between cells and material interface often directly affects the basic behavior of cells, such as cell proliferation, migration, differentiation, apoptosis and so on [52,53]. Hybrid hydrogels, as a good bioremediation material, play an important role in regulating cell growth and promoting tissue repair [[15], [16], [17], [18]]. In this study, a tunable stiffness hydrogel platform was designed based on the cross-linking of iron oxide nanoparticles and GelMA to achieve the advantage of magnetic nanomaterials and enhancement in the plasticity of material stiffness. We did not observe the significant changes of cell viability (Fig. 2b) and cell proliferation (Fig. S2), but the cell morphology changed accompanied with the changes of hydrogel stiffness (Fig. 2c). This result was consistent with these previously reported involving interactions between cells and the scaffold stiffnesses [54,55]. Further, we observed re-organization of F-actin-based microfilament (Fig. 2e&f) and the resultant changes in cellular Young's modulus (Fig. 2g). Based on our previously reported cellular stiffness of chondrocytes [56], this result provides further understanding of cellular physical properties in response to substrate stiffness based on this new hydrogel platform.
Cellular physical property response requires a large energy supply [31,57]. Interaction with Fe2O3/GelMA hybrid hydrogels enhanced up-regulation of oxidative phosphorylation of mitochondria. It is well-known that the energy generation of chondrocytes mainly depends on anaerobic glycolysis (approximately 70%) and is supplemented by oxidative phosphorylation of mitochondria [31,58]. Although oxidative phosphorylation of mitochondria accounts for only a small proportion of the entire energy metabolism in normal chondrocytes (Fig. 3d), it is of great importance for chondrocyte physiology because of its roles in redox homeostasis [59,60], cellular matrix synthesis and calcification [61], and the integration of chondrocyte death signals including autophagy [62,63]. In the process of mitochondrial oxidative phosphorylation, we found that the changes of mitochondrial aerobic respiration in chondrocytes in response to Fe2O3/GelMA hybrid hydrogels by characterizing the change of IRP1 and RP2 (Fig. 4c & S5), which are in charge of iron uptake and sequestration, transferrin receptor expression and ferritin levels [64], and the consequential changes of COX2, the last enzyme in the mitochondrial electron transport chain that drives oxidative phosphorylation [65], and Cyt c, a mobile electron carrier, an indispensable component in the electron transport chain responsible for the chief source of ATP [66]. The change of thioferritin (Fig. 4b&c) in mitochondrial respiratory chain reminds us of the correlation with iron nanoparticles in Fe2O3/GelMA hybrid hydrogels. The magnetic nanoparticles from the hydrogel may be engulfed by chondrocytes [67]. Because the low pH of endosomes, the iron ion could be released from the Fe2O3 nanoparticles [68,69]. Combined with the fact that thioferritin (Fe/S) is a prerequisite for the first three steps in the mitochondrial respiratory chain (NADH dehydrogenation, succinate hydrogenation and cytochrome reduction process, as listed in Fig. 4a in purple) [65,66], we inferred that the iron intake might influence the mitochondrial aerobic respiratory chain.
After the enhancement of mitochondrial respiration in chondrocytes in response to Fe2O3/GelMA hybrid hydrogels, the metabolic products of chondrocytes were changed. Cyto P450 2E1 is a monooxygenase that catalyzes many lipid reactions, including steroids, cholesterol and other lipids [70], and APOE is a major apoprotein of chylomicron, which is essential for the catabolism of triglyceride-rich lipoprotein components [71]. The changes of these two proteins indicated the activation of PPAR signaling in the interaction between chondrocytes and Fe2O3/GelMA hybrid hydrogels and the changes in lipid metabolism. From our metabonomics, we found that four lipid metabolites increased in chondrocytes in response to Fe2O3/GelMA hybrid hydrogels. They were nonanoic acid, 2-palmitoyl-rac-glycerol, withanolide D and myristaldehyde (Fig. 6). These metabolites originated from fatty acids ingested by chondrocytes not only provide energy through mitochondrial oxidation, but also importantly, serve as a material supply for generating the structural components of cellular membranes [72]. Moreover, they might be also recycled into the tricarboxylic acid cycle for energy production and cellular structural supplement [73].
In a rat cartilage defect model, we verified that the Fe2O3/GelMA hybrid hydrogels showed to be a great potent scaffold in cartilage repair. We also detected the positive expressions of ALP (Fig. 7e), a osteoblast marker, enhanced ColX expression (Fig. 8c, lower), and a hypertrophic chondrocyte marker [4,42] in these defect regions. It might be due to mineralization by stem cells recruited from the subchondral bone, and this mineralization also indicated the role of the Fe2O3/GelMA hybrid hydrogels in stem cells recruitment and osteogenesis. Meanwhile, the positive expressions of ColX and ALP point to the hypertrophic differentiation of chondrocytes [74], which might be highly undesirable for cartilage regeneration. However, as far as cartilage remodeling was concerned, it at least completely reached the generation of new cartilage tissue relative to these flaws at the early stage of defect remodeling. Taking together, we showed a remodeling role of Fe2O3/GelMA hydrogel at the early stage of cartilage defects. Although these newly formed tissues were not comparable to peripheral normal cartilage in terms of composition and function, the potentials of the Fe2O3/GelMA hybrid hydrogel to recruit different types of cells and potentially provide favorable iron also indicates that it is a promising scaffold platform in cell culture in vitro and in tissue engineering in vivo.
5. Conclusion
A novel hydrogel platform was designed based on magnetic nanoparticle cross-linked GelMA with tunable stiffness. The cellular physical properties of the chondrocytes were changed, when they interacted with Fe2O3/GelMA hybrid hydrogels. Their cellular metabolic changes included increased mitochondrial oxidative phosphorylation, which was based on breakdown of cellular lipids and the generation of cellular lipid metabolites in response to activation of PPARα signaling. The cell behavior changes could be attributed to a combined effect of hydrogel stiffness (biophysical) and a trace release of iron ions (biochemical). In a rat cartilage defect model, the Fe2O3/GelMA hybrid hydrogel was found to have a great potential for promoting the repair of defects. In summary, this study provides deep insights into the interaction between chondrocytes and the hybrid hydrogel and indicates the great potential of Fe2O3/GelMA hybrid hydrogels for further application in tissue engineering.
Data availability statement
Raw data were mainly attached in the current study. Any other data involving this study are available from the corresponding author on request.
CRediT authorship contribution statement
Chenchen Zhou: performed the cell experiments, collected and analyzed all data, Writing – original draft. Chunli Wang: performed the animal experiments. Kang Xu: performed the animal experiments. Zhixing Niu: performed the cell experiments, collected and analyzed all data. Shujuan Zou: checked all data and evaluated the statistics. Demao Zhang: performed the cell experiments, collected and analyzed all data. Zhiyong Qian: checked all data and evaluated the statistics. Jinfeng Liao: Conceptualization, this study, checked all data and evaluated the statistics, Writing – original draft, made a final approval. Jing Xie: Conceptualization, this study, performed the cell experiments, collected and analyzed all data, checked all data and evaluated the statistics, Writing – original draft, made a final approval.
Declaration of competing interest
All authors declare no conflict of interest.
Acknowledgement
This work was supported by the National Natural Science Foundation of China (81771047 and 22CXRC0216 to Jing Xie, 32171354 to Jingfeng Liao, 81901040 to Chenchen Zhou), China Postdoctoral Science Foundation (2019M653440), Sichuan Science and Technology Innovation Talent Project (2022JDRC0044), and Chengdu International Science and Technology Cooperation Project (2020-GH02-00048-HZ). We also acknowledged Dr. Yunfei Tian in the Analytical and Testing Centre of Sichuan University for his excellent assistance in AFM detection.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bioactmat.2022.07.013.
Contributor Information
Jinfeng Liao, Email: liaojinfeng.762@163.com.
Jing Xie, Email: xiejing2012@scu.edu.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Jansen M.P., Mastbergen S.C. Joint distraction for osteoarthritis: clinical evidence and molecular mechanisms. Nat. Rev. Rheumatol. 2022;18(1):35–46. doi: 10.1038/s41584-021-00695-y. [DOI] [PubMed] [Google Scholar]
- 2.Jiang Y., Tuan R.S. Origin and function of cartilage stem/progenitor cells in osteoarthritis. Nat. Rev. Rheumatol. 2015;11(4):206–212. doi: 10.1038/nrrheum.2014.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou C., Cui Y., Yang Y., Guo D., Zhang D., Fan Y., Li X., Zou J., Xie J. Runx1 protects against the pathological progression of osteoarthritis. Bone Res. 2021;9(1):50. doi: 10.1038/s41413-021-00173-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huey D.J., Hu J.C., Athanasiou K.A. Unlike bone, cartilage regeneration remains elusive. Science. 2012;338(6109):917–921. doi: 10.1126/science.1222454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Einhorn T.A., Gerstenfeld L.C. Fracture healing: mechanisms and interventions. Nat. Rev. Rheumatol. 2015;11(1):45–54. doi: 10.1038/nrrheum.2014.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ambrosi T.H., Marecic O., McArdle A., Sinha R., Gulati G.S., Tong X., Wang Y., Steininger H.M., Hoover M.Y., Koepke L.S., Murphy M.P., Sokol J., Seo E.Y., Tevlin R., Lopez M., Brewer R.E., Mascharak S., Lu L., Ajanaku O., Conley S.D., Seita J., Morri M., Neff N.F., Sahoo D., Yang F., Weissman I.L., Longaker M.T., Chan C.K.F. Aged skeletal stem cells generate an inflammatory degenerative niche. Nature. 2021;597(7875):256–262. doi: 10.1038/s41586-021-03795-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xue X., Hu Y., Deng Y., Su J. Recent advances in design of functional biocompatible hydrogels for bone tissue engineering. Adv. Funct. Mater. 2021;31 doi: 10.1002/adfm.202009432. [DOI] [Google Scholar]
- 8.Han S., Wu J. Three-dimensional (3D) scaffolds as powerful weapons for tumor immunotherapy. Bioact. Mater. 2022;17:300–319. doi: 10.1016/j.bioactmat.2022.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shan Y., Tan B., Zhang M., Xie X., Liao J. Restorative biodegradable two-layered hybrid microneedles for melanoma photothermal/chemo co-therapy and wound healing. J. Nanobiotechnol. 2022;20:238. doi: 10.1186/s12951-022-01426-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Makris E.A., Gomoll A.H., Malizos K.N., Hu J.C., Athanasiou K.A. Repair and tissue engineering techniques for articular cartilage. Nat. Rev. Rheumatol. 2015;11(1):21–34. doi: 10.1038/nrrheum.2014.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xue X., Hu Y., Wang S., Chen X., Jiang Y., Su J. Fabrication of physical and chemical crosslinked hydrogels for bone tissue engineering. Bioact. Mater. 2021;12:327–339. doi: 10.1016/j.bioactmat.2021.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu P., Liu Y., Xie J., Li J. Spatiotemporally controlled calcitonin delivery: long-term and targeted therapy of skeletal diseases. J. Contr. Release. 2021;338:486–504. doi: 10.1016/j.jconrel.2021.08.056. [DOI] [PubMed] [Google Scholar]
- 13.Sun A., He X., Ji X., Hu D., Pan M., Zhang L., Qian Z. Current research progress of photopolymerized hydrogel for tissue engineering. Chin. Chem. Lett. 2021;32:2117–2126. doi: 10.1016/j.cclet.2021.01.048. [DOI] [Google Scholar]
- 14.Truong N.F., Kurt E., Tahmizyan N., Lesher-Pérez S.C., Chen M., Darling N.J., Xi W., Segura T. Microporous annealed particle hydrogel stiffness, void space size, and adhesion properties impact cell proliferation, cell spreading, and gene transfer. Acta Biomater. 2019;94:160–172. doi: 10.1016/j.actbio.2019.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang L., Chen L., Wang J., Wang L., Gao C., Li B., Wang Y., Wu J., Quan C. Bioactive gelatin cryogels with BMP-2 biomimetic peptide and VEGF: a potential scaffold for synergistically induced osteogenesis. Chin. Chem. Lett. 2022;33:1956–1962. doi: 10.1016/j.cclet.2021.10.070. [DOI] [Google Scholar]
- 16.Jin L., Guo X., Gao D., Liu Y., Ni J., Zhang Z., Huang Y., Xu G., Yang Z., Zhang Z., Jiang X. An NIR photothermal-responsive hybrid hydrogel for enhanced wound healing. Bioact. Mater. 2022;16:162–172. doi: 10.1016/j.bioactmat.2022.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu G., Xu Y., Liu Q., Chen M., Sun H., Wang P., Li X., Wang Y., Li X., Hui X., Luo E., Liu J., Jiang Q., Liang J., Fan Y., Sun Y., Zhang X. An instantly fixable and self-adaptive scaffold for skull regeneration by autologous stem cell recruitment and angiogenesis. Nat. Commun. 2022;13:2499. doi: 10.1038/s41467-022-30243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen X., Han S., Wu W., Wu Z., Yuan Y., Wu J., Liu C. Harnessing 4D printing bioscaffolds for advanced orthopedics. Small. 2022 doi: 10.1002/smll.202106824. 10.1002/202106824. [DOI] [PubMed] [Google Scholar]
- 19.Yang J., Zhang Y.S., Yue K., Khademhosseini A. Cell-laden hydrogels for osteochondral and cartilage tissue engineering. Acta Biomater. 2017;57:1–25. doi: 10.1016/j.actbio.2017.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foyt D.A., Norman M.D.A., Yu T.T.L., Gentleman E. Exploiting advanced hydrogel technologies to address key challenges in regenerative medicine. Adv. Healthc. Mater. 2018;7(8) doi: 10.1002/adhm.201700939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Winklbauer R. Dynamic cell-cell adhesion mediated by pericellular matrix interaction - a hypothesis. J. Cell Sci. 2019;132 doi: 10.1242/jcs.231597. [DOI] [PubMed] [Google Scholar]
- 22.Benton G., Arnaoutova I., George J., Kleinman H.K., Koblinski J. Matrigel: from discovery and ECM mimicry to assays and models for cancer research. Adv. Drug Deliv. Rev. 2014;79–80:3–18. doi: 10.1016/j.addr.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 23.Zhang W., Yang G., Wang X., Jiang L., Jiang F., Li G., Zhang Z., Jiang X. Magnetically controlled growth-factor-immobilized multilayer cell sheets for complex tissue regeneration. Adv. Mater. 2017;29(43) doi: 10.1002/adma.201703795. [DOI] [PubMed] [Google Scholar]
- 24.Appel E.A., Tibbitt M.W., Webber M.J., Mattix B.A., Veiseh O., Langer R. Self-assembled hydrogels utilizing polymer-nanoparticle interactions. Nat. Commun. 2015;6:6295. doi: 10.1038/ncomms7295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi L., Zeng Y., Zhao Y., Yang B., Ossipov D., Tai C., Dai J., Xu C. Biocompatible injectable magnetic hydrogel formed by dynamic coordination network. ACS Appl. Mater. Interfaces. 2019;11:46233–46240. doi: 10.1021/acsami.9b17627. [DOI] [PubMed] [Google Scholar]
- 26.Liao J., Jia Y., Chen L., Zhou L., Li Q., Qian Z., Niu D., Li Y., Li P. Magnetic/gold core-shell hybrid particles for targeting and imaging-guided photothermal cancer therapy. J. Biomed. Nanotechnol. 2019;15:2072–2089. doi: 10.1166/jbn.2019.2839. [DOI] [PubMed] [Google Scholar]
- 27.Huebsch N., Arany P.R., Mao A.S., Shvartsman D., Ali O.A., Bencherif S.A., Rivera-Feliciano J., Mooney D.J. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat. Mater. 2010;9(6):518–526. doi: 10.1038/nmat2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xie J., Zhang D., Zhou C., Yuan Q., Ye L., Zhou X. Substrate elasticity regulates adipose-derived stromal cell differentiation towards osteogenesis and adipogenesis through β-catenin transduction. Acta Biomater. 2018;79:83–95. doi: 10.1016/j.actbio.2018.08.018. [DOI] [PubMed] [Google Scholar]
- 29.Hadden W.J., Young J.L., Holle A.W., McFetridge M.L., Kim D.Y., Wijesinghe P., Taylor-Weiner H., Wen J.H., Lee A.R., Bieback K., Vo B.N., Sampson D.D., Kennedy B.F., Spatz J.P., Engler A.J., Choi Y.S. Stem cell migration and mechanotransduction on linear stiffness gradient hydrogels. Proc. Natl. Acad. Sci. U.S.A. 2017;114(22):5647–5652. doi: 10.1073/pnas.1618239114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou S., Cui Z., Urban J.P. Factors influencing the oxygen concentration gradient from the synovial surface of articular cartilage to the cartilage-bone interface: a modeling study. Arthritis Rheum. 2004;50:3915–3924. doi: 10.1002/art.20675. [DOI] [PubMed] [Google Scholar]
- 31.Kan S., Duan M., Liu Y., Wang C., Xie J. Role of mitochondria in physiology of chondrocytes and diseases of osteoarthritis and rheumatoid arthritis. Cartilage. 2021;13(2_suppl):1102S–1121S. doi: 10.1177/19476035211063858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blanco F.J., Valdes A.M., Rego-Pérez I. Mitochondrial DNA variation and the pathogenesis of osteoarthritis phenotypes. Nat. Rev. Rheumatol. 2018;14:327–340. doi: 10.1038/s41584-018-0001-0. [DOI] [PubMed] [Google Scholar]
- 33.Chen L., Niu D., Lee C.H., Yao Y., Lui K., Ho K.M., Li P. Amphiphilic core-shell nanocomposite particles for enhanced magnetic resonance imaging. Part. Part. Syst. Char. 2016;33:756–763. doi: 10.1002/ppsc.201600095. [DOI] [Google Scholar]
- 34.Zhou C., Duan M., Guo D., Du X., Zhang D., Xie J. Microenvironmental stiffness mediates cytoskeleton re-organization in chondrocytes through laminin-FAK mechanotransduction. Int. J. Oral Sci. 2022;14(1):15. doi: 10.1038/s41368-022-00165-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wei J., Yang Y., Guo D., Xu S., Huang H., Zhang D., Xie J., Zhou X. Osteoblasts induce glucose-derived ATP perturbations in chondrocytes through noncontact communication. Acta Biochim. Biophys. Sin. 2022;54(5):625–636. doi: 10.3724/abbs.2022042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu Y., Duan M., Guo D., Kan S., Zhang L., Aili M., Zhang D., Du W., Xie J. PDGF-AA promotes cell-to-cell communication in osteocytes through PI3K/Akt signaling pathway. Acta Biochim. Biophys. Sin. 2021;53(12):1640–1649. doi: 10.1093/abbs/gmab136. [DOI] [PubMed] [Google Scholar]
- 37.Robinson J.L., Cass K., Aronson R., Choi T., Xu M., Buttenbaum R., Drissi H., Lu H.H., Chen J., Wadhwa S. Sex differences in the estrogen-dependent regulation of temporomandibular joint remodeling in altered loading. Osteoarthritis Cartilage. 2017;25:533–543. doi: 10.1016/j.joca.2016.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang D., Li X., Pi C., Cai L., Liu Y., Du W., Yang W., Xie J. Osteoporosis-decreased extracellular matrix stiffness impairs connexin 43-mediated gap junction intercellular communication in osteocytes. Acta Biochim. Biophys. Sin. 2020;52(5):517–526. doi: 10.1093/abbs/gmaa025. [DOI] [PubMed] [Google Scholar]
- 39.Xie J., Zhang D., Lin Y., Yuan Q., Zhou X. Anterior cruciate ligament transection-induced cellular and extracellular events in menisci: implications for osteoarthritis. Am. J. Sports Med. 2018;46:1185–1198. doi: 10.1177/0363546518756087. [DOI] [PubMed] [Google Scholar]
- 40.Kramer P.A., Ravi S., Chacko B., Johnson M.S., Darley-Usmar V.M. A review of the mitochondrial and glycolytic metabolism in human platelets and leukocytes: implications for their use as bioenergetic biomarkers. Redox Biol. 2014;2:206–210. doi: 10.1016/j.redox.2013.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee S.M., Jung J.H., Seo J.A., Kim Y.S. Bioinformation of volatile and nonvolatile metabolites by saccharomycopsis fibuligera KJJ81 cultivated under different conditions-carbon sources and cultivation times. Molecules. 2018;23:2762. doi: 10.3390/molecules23112762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Duarte F.V., Amorim J.A., Palmeira C.M., Rolo A.P. Regulation of mitochondrial function and its impact in metabolic stress. Curr. Med. Chem. 2015;22:2468–2479. doi: 10.2174/0929867322666150514095910. [DOI] [PubMed] [Google Scholar]
- 43.Barathova M., Grossmannova K., Belvoncikova P., Kubasova V., Simko V., Skubla R., Csaderova L., Pastorek J. Impairment of hypoxia-induced CA IX by beta-blocker propranolol-impact on progression and metastatic potential of colorectal cancer cells. Int. J. Mol. Sci. 2020;21:8760. doi: 10.3390/ijms21228760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Singh S., Singh T.G., Rehni A.K., et al. Reviving mitochondrial bioenergetics: a relevant approach in epilepsy. Mitochondrion. 2021;58:213–226. doi: 10.1016/j.mito.2021.03.009. [DOI] [PubMed] [Google Scholar]
- 45.Mohsin M., Tabassum G., Ahmad S., Ali S., Syed M.A. The role of mitophagy in pulmonary sepsis. Mitochondrion. 2021;59:63–75. doi: 10.1016/j.mito.2021.04.009. [DOI] [PubMed] [Google Scholar]
- 46.Barjaktarovic Z., Merl-Pham J., Braga-Tanaka I., Tanaka S., Hauck S.M., Saran A., Mancuso M., Atkinson M.J., Tapio S., Azimzadeh O. Hyperacetylation of cardiac mitochondrial proteins is associated with metabolic impairment and sirtuin downregulation after chronic total body irradiation of ApoE (-/-) mice. Int. J. Mol. Sci. 2019;20:5239. doi: 10.3390/ijms20205239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Busik J.V. Lipid metabolism dysregulation in diabetic retinopathy. J. Lipid Res. 2021;62 doi: 10.1194/jlr.TR120000981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chatenoud L., Bluestone J.A. CD3-specific antibodies: a portal to the treatment of autoimmunity. Nat. Rev. Immunol. 2007;7:622–632. doi: 10.1038/nri2134. [DOI] [PubMed] [Google Scholar]
- 49.Soncin I., Sheng J., Chen Q., Foo S., Duan K., Lum J., Poidinger M., Zolezzi F., Karjalainen K., Ruedl C. The tumour microenvironment creates a niche for the self-renewal of tumour-promoting macrophages in colon adenoma. Nat. Commun. 2018;9:582. doi: 10.1038/s41467-018-02834-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lindquist R.L., Shakhar G., Dudziak D., Wardemann H., Eisenreich T., Dustin M.L., Nussenzweig M.C. Visualizing dendritic cell networks in vivo. Nat. Immunol. 2004;5:1243–1250. doi: 10.1038/ni1139. [DOI] [PubMed] [Google Scholar]
- 51.Zhou C., Zhang D., Zou J., Li X., Zou S., Xie J. Substrate compliance directs the osteogenic lineages of stem cells from the human apical papilla via the processes of mechanosensing and mechanotransduction. ACS Appl. Mater. Interfaces. 2019;11(29):26448–26459. doi: 10.1021/acsami.9b07147. [DOI] [PubMed] [Google Scholar]
- 52.Zhou C., Zhang D., Du W., Zou J., Li X., Xie J. Substrate mechanics dictate cell-cell communication by gap junctions in stem cells from human apical papilla. Acta Biomater. 2020;107:178–193. doi: 10.1016/j.actbio.2020.02.032. [DOI] [PubMed] [Google Scholar]
- 53.Vining K.H., Mooney D.J. Mechanical forces direct stem cell behaviour in development and regeneration. Nat. Rev. Mol. Cell Biol. 2017;18(12):728–742. doi: 10.1038/nrm.2017.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou C., Wang Q., Zhang D., Cai L., Du W., Xie J. Compliant substratum modulates vinculin expression in focal adhesion plaques in skeletal cells. Int. J. Oral Sci. 2019;11:18. doi: 10.1038/s41368-019-0052-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cai L., Liu W., Cui Y., Liu Y., Du W., Zheng L., Pi C., Zhang D., Xie J., Zhou X. Biomaterial stiffness guides cross-talk between chondrocytes: implications for a novel cellular response in cartilage tissue engineering. ACS Biomater. Sci. Eng. 2020;6:4476–4489. doi: 10.1021/acsbiomaterials.0c00367. [DOI] [PubMed] [Google Scholar]
- 56.Chen C., Xie J., Deng L., Yang L. Substrate stiffness together with soluble factors affects chondrocyte mechanoresponses. ACS Appl. Mater. Interfaces. 2014;6(18):16106–16116. doi: 10.1021/am504135b. [DOI] [PubMed] [Google Scholar]
- 57.Hardie D.G., Ross F.A., Hawley S.A. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 2012;13(4):251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee R.B., Urban J.P. Evidence for a negative Pasteur effect in articular cartilage. Biochem. J. 1997;321(Pt1):95–102. doi: 10.1042/bj3210095. Pt1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Akbari M., Kirkwood T.B.L., Bohr V.A. Mitochondria in the signaling pathways that control longevity and health span. Ageing Res. Rev. 2019;54 doi: 10.1016/j.arr.2019.100940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.He Y., Wu Z., Xu L., Xu K., Chen Z., Ran J., Wu L. The role of SIRT3-mediated mitochondrial homeostasis in osteoarthritis. Cell. Mol. Life Sci. 2020;77:3729–3743. doi: 10.1007/s00018-020-03497-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu L., Liu H., Li L., Liu H., Cheng Q., Li H., Huang H. Mitochondrial pathology in osteoarthritic chondrocytes. Curr. Drug Targets. 2014;15:710–719. doi: 10.2174/1389450115666140417120305. [DOI] [PubMed] [Google Scholar]
- 62.Battistelli M., Salucci S., Olivotto E., Facchini A., Minguzzi M., Guidotti S., Pagani S., Flamigni F., Borzì R.M., Facchini A., Falcieri E. Cell death in human articular chondrocyte: a morpho-functional study in micromass model. Apoptosis. 2014;19:1471–1483. doi: 10.1007/s10495-014-1017-9. [DOI] [PubMed] [Google Scholar]
- 63.Sun K., Jing X., Guo J., Yao X., Guo F. Mitophagy in degenerative joint diseases. Autophagy. 2021;17:2082–2092. doi: 10.1080/15548627.2020.1822097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shi Y., Ghosh M., Kovtunovych G., Crooks D.R., Rouault T.A. Both human ferredoxins 1 and 2 and ferredoxin reductase are important for iron-sulfur cluster biogenesis. Biochim. Biophys. Acta. 2012;1823:484–492. doi: 10.1016/j.bbamcr.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Saneto R.P. Mitochondrial diseases: expanding the diagnosis in the era of genetic testing. J. Transl. Genet. Genom. 2020;4:384–428. doi: 10.20517/jtgg.2020.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rouault T.A., Maio N. Biogenesis and functions of mammalian iron-sulfur proteins in the regulation of iron homeostasis and pivotal metabolic pathways. J. Biol. Chem. 2017;292:12744–12753. doi: 10.1074/jbc.R117.789537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Im G.B., Jung E., Kim Y.H., Kim Y.J., Kim S.W., Jeong G.J., Lee T.J., Kim D.I., Kim J., Hyeon T., Yu T., Bhang S.H. Endosome-triggered ion-releasing nanoparticles as therapeutics to enhance the angiogenic efficacy of human mesenchymal stem cells. J. Contr. Release. 2020;324:586–597. doi: 10.1016/j.jconrel.2020.05.038. [DOI] [PubMed] [Google Scholar]
- 68.Niwa M., Hirayama T., Oomoto I., Wang D.O., Nagasawa H. Fe(II) Ion release during endocytotic uptake of iron visualized by a membrane-anchoring Fe(II) fluorescent probe. ACS Chem. Biol. 2018;13(7):1853–1861. doi: 10.1021/acschembio.7b00939. [DOI] [PubMed] [Google Scholar]
- 69.Pawlowski J.W., Kellicker N., Bobst C.E., Kaltashov I.A. Assessing the iron delivery efficacy of transferrin in clinical samples by native electrospray ionization mass spectrometry. Analyst. 2016;141(3):853–861. doi: 10.1039/c5an02159f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Song B.J., Abdelmegeed M.A., Cho Y.E., Akbar M., Rhim J.S., Song M.K., Hardwick J.P. Contributing roles of CYP2E1 and other cytochrome P450 isoforms in alcohol-related tissue injury and carcinogenesis. Adv. Exp. Med. Biol. 2019;1164:73–87. doi: 10.1007/978-3-030-22254-3_6. [DOI] [PubMed] [Google Scholar]
- 71.Cooper J.M., Lathuiliere A., Migliorini M., Arai A.L., Wani M.M., Dujardin S., Muratoglu S.C., Hyman B.T., Strickland D.K. Regulation of tau internalization, degradation, and seeding by LRP1 reveals multiple pathways for tau catabolism. J. Biol. Chem. 2021;296 doi: 10.1016/j.jbc.2021.100715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Furuhashi M., Hotamisligil G.S. Fatty acid-binding proteins: role in metabolic diseases and potential as drug targets. Nat. Rev. Drug Discov. 2008;7:489–503. doi: 10.1038/nrd2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Martínez-Reyes I., Chandel N.S. Mitochondrial TCA cycle metabolites control physiology and disease. Nat. Commun. 2020;11:102. doi: 10.1038/s41467-019-13668-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Morita K., Miyamoto T., Fujita N., Kubota Y., Ito K., Takubo K., Miyamoto K., Ninomiya K., Suzuki T., Iwasaki R., Yagi M., Takaishi H., Toyama Y., Suda T. Reactive oxygen species induce chondrocyte hypertrophy in endochondral ossification. J. Exp. Med. 2007;204:1613–1623. doi: 10.1084/jem.20062525. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw data were mainly attached in the current study. Any other data involving this study are available from the corresponding author on request.