Abstract
The necessity of disease models for bone/cartilage related disorders is well-recognized, but the barrier between ex-vivo cell culture, animal models and the real human body has been pending for decades. The organoid-on-a-chip technique showed opportunity to revolutionize basic research and drug screening for diseases like osteoporosis and arthritis. The bone/cartilage organoid on-chip (BCoC) system is a novel platform of multi-tissue which faithfully emulate the essential elements, biologic functions and pathophysiological response under real circumstances. In this review, we propose the concept of BCoC platform, summarize the basic modules and current efforts to orchestrate them on a single microfluidic system. Current disease models, unsolved problems and future challenging are also discussed, the aim should be a deeper understanding of diseases, and ultimate realization of generic ex-vivo tools for further therapeutic strategies of pathological conditions.
Keywords: Organoid, On-chip platform, Osteochondral unit, Ex-vivo disease model
Highlights
-
•
The organ-on-chip system is one of the best solutions to meet the requirement of ex-vivo model for bone/cartilage disorders.
-
•
We propose the concept of BCoC, summarize basic modules and current efforts to orchestrate them on microfluidic system.
-
•
The goal of BCoC is the ultimate realization of generic ex-vivo tools for strategies of pathological conditions.
1. Introduction
The bone is a highly mineralized tissue that provides mechanical support for soft organs and tissues, and maintains metallic element homeostasis either. With the global aging and obese population skyrocketing increasing these years, skeletal disorders including osteoporosis (OP) and osteoarthritis (OA) jeopardize motor ability of millions worldwide. The incidence of bone and joint diseases exponentially increases along with age. Osteoporosis affects at least 44 million citizens over 50 in the United States, similar statistics have been reported in Asia, Europe and Australia [1]. Coincidentally, global prevalence of osteoarthritis is dramatically increasing these years, as well as the economic burden. It is estimated that over 250 million people are, in varying degrees, affected by OA [2]. Despite the past decades of investigation, there are still much unknown about these diseases.
Lifelong resorption and formation processes, or the so-called bone remodeling, are taken place inside the bone, yet the unbalance of destruction and reconstruction leads to multiple skeletal disorders. Overspeed bone resorption and formation in both trabecular and cortical bone are observed in aged female, especially postmenopausal women. On contrast, relatively low rate of bone turnover and formation process is the key etiology for osteoporosis in men. There are two main kinds of anti-osteoporosis drugs, antiresorptive and anabolic ones. However, biphosphonates, the most popular antiresorptive medicine, have shown several side effects such as atypical osteonecrosis [3]. Joint disorders like OA are occasionally called “old but unsolved medical problems” [4], cause there are actually no effective clinical interventions except arthroplasty. Components including subchondral bone, cartilage, synovial and meniscus affect each other during OA progress, thus become serious obstacle to mechanism research.
Disease models are necessary for basic research. In vitro models like traditional 2D cell cultures are insufficient to exhibit the real physiological and pathological status. Cell shape, spatial characterization and cellular communication are absent in 2D cell cultures, which made 3D cell culture promising candidate for future research. With a dramatically high percent of water, biocompatible hydrogels are widely used in 3D cell culture system. For example, Dr. Mooney from Harvard University, a prominent scholar in biomaterials, reported an optimized hydrogel with faster relaxation, and achieve better initial elastic modulus, degradation, and cell-adhesion-ligand density [5]. Three-dimensional culture system not only lessens gaps between cells in dishes and real physiological tissues, but also increases abilities of drug screening and toxicity prediction [6]. Animal models are essential element in bone and joint research, but the differences in gene, shape, and joint loading mode between rodents and human lead to disparate response towards drugs or interventions [7].
The development and application of microfluidic device have already altered the traditional way in which we handle cells in ex-vivo systems. These microfabricated organ-on-a-chip could not only support cell differentiation like 3D system, but also recapitulate cellular crosstalk, tissue-tissue interfaces, spatiotemporal behavior and mechanical load. In consequence, organ-on-a-chip solution provides novel approach to ex-vivo disease models, toxic testing and drug screening. From another perspective, the term “organoids” means stem cells generated, self-assembled, multicellular structures, which were usually designed to emulate micro-structure and biological functions of natural organs or tissues [8]. Organoids provide promising access to advanced mechanism research and disease models, but the popularization is now limited because of the complicated inducing methods, long incubation period and unignorable heterogeneity.
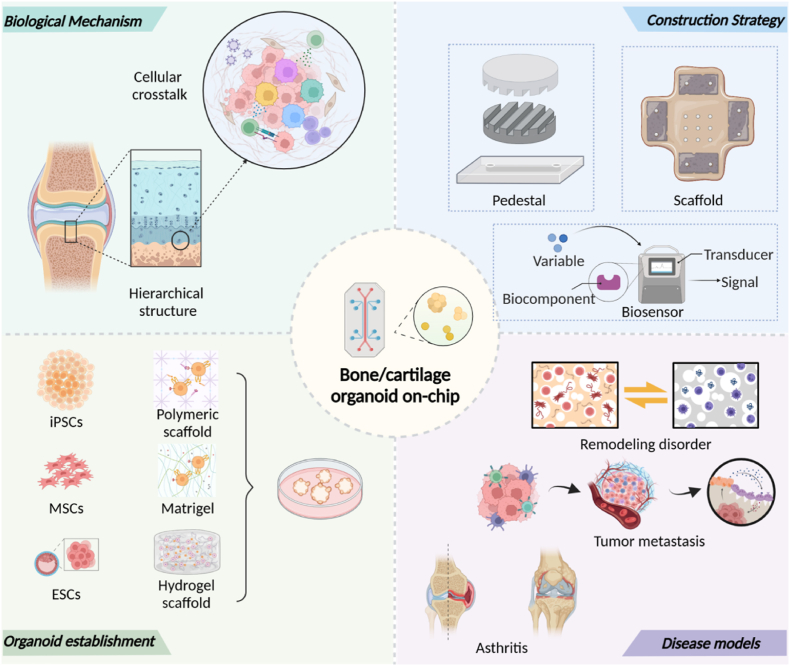
Combining both advantage of organoids and microfluidic chips, the organoid-on-a-chip platform shows advantages in three aspects: precise regulation of microenvironment, accurate emulation of multi-tissue crosstalk, and lower heterogeneity. In this review, we introduce research progress of musculoskeletal organoids and cellular crosstalk inside osteochondral unit. We also discuss concept and design philosophy of bone/cartilage-on-a-chip (BCoC) platform under biological mechanism (Fig. 1).
Fig. 1.
Schematic description of the Bone/cartilage organoid on-chip.
2. Osteochondral unit
2.1. Basic structure of osteochondral unit
The term, “osteochondral unit”, means the biological complex or funtional unit formed by articular cartilage, calcified cartilage and subchondral bone, which were anatomically adjacent in the knee joint [9]. The functional unit holds special ability of load transferring during weight bearing and body movement, and destruction of any individual part leads to disruption of the whole joint, or exactly, osteoarthritis.
The articular cartilage is an avascular, elastic multi-layer structure, which acts as buffer to absorb direct shock during movement. Comprised glycosaminoglycans (GAGs), collagen fibres and chondrocytes, the articular cartilage was thought to be non-renewable decades ago, until the spontaneous regeneration was presented in rabbit experiments at 2010 [10]. Appropriate mechanical load is necessary in cartilage development and maintenance, both overloading and underloading are detrimental to chondrocytes [11]. Cartilage overloading observed in obese patients directly led to horizontal fissuring, subchondral bone sclerosis and ultimately osteoarthritis [12]. On the other hand, underloading or non-weight bearing altered cartilage composition in clinical samples, but they could return to the baseline level in about four weeks [13]. Tissue engineering technique, which could provide proper and controllable mechanical support for cell behavior or tissue repair, has given alternative strategies for clinical therapies.
The subchondral bone beneath cartilage layer provides necessary nutritional support and proper mechanical stimulation, suggesting that alternation inside subchondral bone might directly or indirectly affect cartilage metabolism [14]. Clinical evidence showed that the non-cystic damage area in subchondral bone, or so-called bone marrow lesion (BML), could be observed in more than half of asymptomatic citizens over 50 [15]. Remarkably, nearly two-thirds of cartilage erosion took place exactly upon the BML zone, which highly suggested the intrinsic connection between subchondral bone and cartilage during OA progression [16]. It was proved in animal experiment that inhibition of transforming growth factor (TGF)-β signaling, which is closely related to bone turnover, in subchondral bone could attenuate osteoclasts activation and OA progression [17]. Another interesting work compared cartilage metabolism under different nutrition sources: synovial fluid(SF) and subchondral bone marrow (SBM)-both, SBM-only, SF-only, none and free [18]. The data showed that the dominant source sustenance for cartilage is SF, but the most severe damage was observed in the SBM-only group, indicating that subchondral bone might contribute to cartilage destruction during OA.
Synovium and synovia are also critical parts since they are in directly contact with cartilage during movement. Normally comprised by 1–3 layers, synovium and synovia help maintain the homeostasis inside the synovial joint by lubricant secretion, matrix metalloproteinases (MMP) release and macrophage filtration [19]. Synovial fibroblasts and macrophages are the major residents inside healthy synovial tissue, but excessive influx of pro-inflammatory monocytes produce pathogenic factors like tumour necrosis factor (TNF) during rheumatoid arthritis (RA) [20,21].
2.2. Crosstalk inside osteochondral unit
In a normal knee joint, the uppermost level of osteochondral unit is hyaline cartilage, calcification of matrix could be observed at the interface between cartilage and bony layers. The boundary between hyaline-calcified cartilage is normally called the tidemark, and another border between bone and carilage is named cement line. Direct cellular contacts crossing these boundaries have been observed in various disease samples. The tidemark, interface between hyaline and calcified cartilage, was first introduced in 1984, and it moves slowly towards joint space while ageing [22]. Radiological data shows that the tidemark is far more complicated than a single line or flat surface, it is more likely a complex 3D structure containing cartilage, bone, vessel, sensory nerve fibre and even inflammatory cells [23]. In some instances, uncalcified cartilage could penetrate the tidemark and reach the subchondral bone eventually, further suggesting the direct cellular contact between chondrocytes, osteoblasts, osteoclasts, mesenchymal stem cells, endothelial cells and even sensory neurons. These inter-cellular crosstalk phenomena are also observed in ex-vivo co-culture experiments. In a co-culture system containing human MSCs and bovine chondrocytes, species-specific quantitative PCR experiments showed that MSC accelerate matrix produced by chondrocytes, instead of differentiating into chondrocytes [24,25]. Exosomes have been identified as common postman for cellular or tissue-tissue communication, MSC derived exosomes could also improve cartilage repair by accelerate chondrocyte proliferation, matrix fabrication, and regulate immune phenotype [26], similar results were also observed in animal experiments while injecting exosomes into joint space [27,28].
The so-called subchondral bone marrow lesion (BML), or “bone marrow edema”, is one of the powerful clinical evidence for osteochondral unit [29]. Among the 710 patients investigated in a clinical research, BMLs were detected in 52% of these non-osteoarthritic human subjects [15]. Clinical trial presented by Hernigou et al. [30] reported a lowered yearly arthroplasty incidence after subchondral injection of MSCs, while compared with intraarticular injection. Similar research observing the effect of cell therapy inside BML showed that the direct regulation of BML sites could effectively delay or even avoid the joint replacement [31]. These clinical evidence strongly suggested the presence of functional unit consisting subchondral bone and articular cartilage.
Due to the negative potential of glycosaminoglycan, one of the most common constitutes of cartilage matrix, anionic molecules could barely pass the cartilage layer, while cationic ones are unblocked. Apart from direct contact, the synovium could alter cartilage metabolism through a ligand-receptor pattern. Through a transcription profiles analysis program, Wang et al. reported potential ligand-receptor pairs between cartilage, synovium, subchondral bone and meniscus, the results showed that ligands like tenascin-C (TNC) and fibronectin1 (FN1) were up-regulated during OA progress, as well as their receptors [32], indicating potential intervention strategies for osteoarthritis. Penetration of small molecules from subchondral bone to calcified cartilage had been described by a fluorescence loss method, and this diffusion could be increased during pathological status [33]. VEGF derived from hypertrophic chondrocytes could recruit endothelial cells and MSCs inside subchondral bone, thus promoted cartilage destruction during the endochondral ossification process [34]. These clues indicate a complete functional complex including synovium, cartilage and subchondral bone, that's why the osteochondral unit caught great attention recent years.
3. Organoid in musculoskeletal system
Current models during bone research mainly include cell culture or rodent models. However, the monolayer cell sheets in dishes, flasks or wells are far away from the in vivo multi-cell microenvironment, which might lead to misunderstanding of the real physiological status [35]. The mechanical surroundings, spatiotemporal nutrient and oxygen distribution are better displayed in novel 3D culture system. On the other hand, gene difference between rodents, rabbits or other experimental animals with human lower the efficiency and accuracy during basic research and drug development.
Organoids are ex-vivo 3D cell culture system aiming at emulating multicellular relationship, spatial structure and physiological function of real organs [36]. Landmark study from Dr. Hans Clevers reported crypt-villus structures generated from adult Lgr5 stem cells without mesenchymal niche [37]. Another pioneering work presented by Dr. Yoshiki Sasai reported a dynamic, autonomous formatted optic cup structure from ex-vivo 3D culture of mouse embryonic stem cells [38]. Various organoids, have been generated from different cell source since these two pioneering works, like liver [39], pancreas [40], pulmonary alveolus [41], thyroid [42] and kidney [43].
Bone/cartilage organoids are self-organized and self-renewing mini-tissues, which mimic structure and function of bone and cartilage under either normal or disease condition [44]. The bone is a living organ that maintains immune cells, hematopoietic cells, calcium-phosphorus metabolism and supports the body. The most popular cells source for bone/cartilage organoids are MSCs and induced pluripotent stem cells (iPSCs). There are multiple techniques and protocols for chondrocytes, adipocytes and osteoblasts induction [45,46], but endothelial cells, osteoclasts and other immune ingredients should be differentiated from iPSCs. Despite the excellent differentiation potential of iPSCs [[47], [48], [49], [50]], there are still none available protocols for synoviocytes yet.
The differentiation of stem cells is well orchestrated in the organic body, it is very difficult to recapitulate all clues ex-vivo. To guide stem cell differentiation properly, model the mechanical strength and biological 3D structure of bone, series of biocompatible materials like hydrogels are designed and applied recently. Hydrogels are powerful candidates due to the unique structure of high aqueous content and adjustable physicochemical properties [51]. Bionics is the most popular method for hydrogel design. For example, hyaluronic acid hydrogels are used for chondrocyte induction and culturing [52], hydroxyapatite (HA) supplemented hydrogels could be effective while mimicking the tidemark microenvironment [53], hydroxyapatite scaffold embedded in methacrylated gelatin hydrogel are applied for a chondro-osteo-vascular triphasic culture system [54].
4. Organ-on-a-chip model
Based on microfluidic chip technique, the organ-on-a-chip system are now developed to mimic physiological or pathological status on a mini-chip module, aiming at replacing animal model and accelerate personalized drug screening [55]. Traditional tissue engineering technique concentrates on duplicate the injured tissue or even whole organ at the human scale, and clinical application, ultimately. On contrary, these organ-on-a-chip models aim to reproduce the basic organotypic architecture, cellular constitutes, biochemical factors and biological functioning on a much smaller scale, then provide efficient platform for drug screening and basic research [56,57]. The most popular materials for chip manufacturing is polydimethylsiloxane (PDMS), a well-recognized optical material suitable for real-time image. PDMS enables a better visual observation about cell and organ response to external stimuli, or in single cell scale by some special devices.
Encouraging works based on multi-organ-on-a-chip system have shared new orientations for organ-organ communication during complex physiological reactions. Different from individual organ functions on single organ chips, the multi-organ chip models could, customized and personalized, integrate several organ modules, thus mimic cooperation behaviors in human body [58]. For example, a four organ integrated chip was designed to reproduce intestinal absorption, liver metabolism, kidney excretion and skin reaction, the multi-organ toxicity of potential drugs could be monitored during 28 days [59]. The so called absorption/distribution/metabolism/excretion/toxicity (ADMET) model is now very popular to evaluate safety and efficiency of promising medicine [60].
5. Concept and construction of bone/cartilage-on-a-chip platform
The joints are normally multiple-tissue systems, various functions of cartilage, bone, ligament, synovium and meniscus are precisely orchestrated in healthy bodies. Current therapies targeting single components like cartilage, subchondral bone, synovium and even immune system are reported to be efficient in animal models, but there are still none FDA-approved anti-OA drugs at present. One of the most critical reason is the close and veiled crosstalk between joint elements, or regulation relation with other organs like intestine and brain.
About the question of “how could microfluidic technology help organoid research”, Huh et al. [61] described it in three aspects: easier control of biomedical microenvironment, versatile construction of multi-organ system, lower viability during parallel experiments. Novel progress of joint-on-a-chip model provides new way to further explore pathological mechanism and potential interception methods, but engineering technique and biological design are major challenges yet.
5.1. Structural basis of functional osteochondral unit model
5.1.1. Pedestal materials
Fabrication materials are crucial to every microfluidic devices. Although polydimethylsiloxane (PDMS) is the most common basal material for chip manufacturing, novel ingredients are now introduced and modified to meet various needs (Fig. 2). Among the multifarious materials are elastomers like PMDS, inorganics like silicon, plastics like polystyrene (PS), and so on.
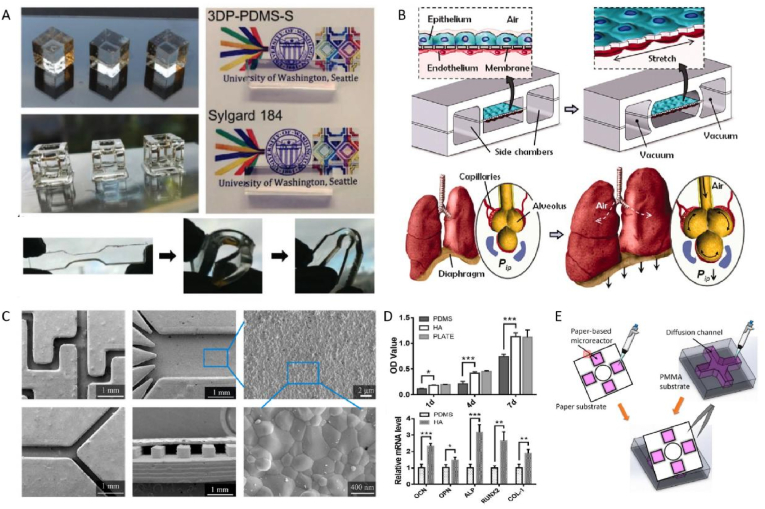
Fig. 2.
Material design of pedestal. (A) Optical transparency and elasticity of PDMS (Adapted from Ref. [63], with permission); (B) The mechanical stretching of PDMS membrane enabled a alveolar-capillary like breathing movements by applying vacuum to the side chambers (Adapted from Ref. [41], with permission); (C,D) Physicochemical and biological properties of HA-modified PDMS substrate (Adapted from Ref. [77], with permission); (E) Paper/PMMA hybrid microfluidic platform for cellular crosstalk (Adapted from Ref. [81], with permission).
Possessing wonderful economic, engineering and optical properties, PDMS occupies a major proportion of this field. The excellent optical transparency of PDMS enables the direct, continuous, precise observation during cell and micro-tissue culturing [62,63]. The relatively lower Young's modulus and better yield strain of PDMS material make it suitable to manufacture complex three dimension structure, with high replicability during mass production [64]. In a pathbreaking work presenting alveolar-vessel interface on chip, a 10 μm thick monolayer PDMS membrane was utilized as spacer between two endothelial cell layers mimicking capillaries and pulmonary alveoli, respectively [41]. In the experiment, intermittent stretch of PDMS membrane reproduced the respiratory process, altered intercellular space and permeability, so the whole-organ responses to external stimulus could be simulated. Nevertheless, other characteristics of PMDS, such as hydrophobicity and adsorption of some biomolecules, limit its further application while designing highly specific organ-on-a-chip models. The compliance of PDMS, due to it low elastic modulus of 1–3 MPa, could lead to shear force induced cell behavior during a long-term culture procedure [65]. The gas permeability of PDMS helps the maintenance of carbon dioxide level, in order to stimulate cell growth [66]. But the double-edge sword brings trouble when we want some anaerobic cells, such as chondrocytes. Additionally, uncrosslinked polymer chains might insensibly diffuse into solutions like culture mediums, the actual impact are not very aware but possibly critical in some certain circumstances [67].
Surface modification solutions are now developed and applied, like polyurethane methacrylate (PUMA) [68] and thermoset polyester (TPE) [69]. The intrinsic hydrophobic nature of PDMS could be related to biofouling, medium depletion, and biosensor deviation [70]. Pre-saturation method is a promising way to diminish undesireable adsorption, a research group from Netherlands coated PDMS membrane with fibronectin, thus achieved a vessel-on-a-chip module for cellular interactions and signaling pathways research [71]. Grafting microchannels with other polymers could be another effective solution, they are usually applied to adjust the wettability or anti-biofouling properties. For example, Sui et al. [72] introduced poly(ethylene glycol) (PEG) and amine (NH2) to PDMS for preventing unspecific protein resorption, and improved the surface dynamics and stability of modified chip. Other coatings were reported to ameliorate surface properties, such as polydopamine [73], sulfuric acid [74], surfactants like sodium dodecyl sulphate [75] and PLGA microparticles [76]. Direct coating PDMS pedestal with biocompatible lining is one of the most simple and effective way, a ceramic stereolithography printed hydroxyapatite lining was added to PDMS pedestal by Tang et al., proliferation and osteogenic differentiation of foetal derived human osteoblast cell line were increased on the modified chip [77].
In the past century, researchers relied desperately on cell dishes, flasks and well plates made by polystyrene (PS) [66]. It is seemed to be more attractive if chips were manufactured by these familiar materials, like PS. Unfortunately, the challenges on engineering these plastic materials restricted the availability. Whereas, there are many other options like poly(methyl methacrylate) (PMMA), which is widely used for adhesion agent during arthroplasty, polycarbonate (PC), and polylactic acid (PLA). Busek et al. [78] reported a PMMA-based microfluidic devices without PDMS involvement, this multilayered, pneumatic micro-pumps integrated chip module was suitable for human endothelial cells growth. Thermo-processing is promising in chip fabrication due to their high production rate and low cost. Some complex nanocomposites with appropriate biological effects could be easily bonded to PMMA base under UV or visible light [79]. Due to the excellent biocompatibility, a PMMA/PC composite interface coated with fibronectin was applied in microfluidic device, calcified aggregate formation was observe in 7 days, as well as up-regulated osteogenic differentiation, synthesis and deposition of extracellular matrix [80].
Paper based microfluidics technique is attracting recent years, due to the advantages like lightweight, easyof use and low cost. However, the lab-on-paper platform is severely restricted by the detection methods due to the poor transparency. It is normally utilized in quick diagnostic devices for blood samples, but Lei et al. [81] developed a paper/PMMA hybrid 3D microfluidic device suitable for cellular crosstalk research (Fig. 2E). The paper substrate was pre-printed with specific micro-reactors using a wax printing technique, the team printed patterns of wax to a hydrophilic paper, and then melted the wax to achieve the hydrophobic barriers between micro-reactors [82].
Glass is optically transparent and treated as ideal pedestal material for real-time observation, the reduced absorbance and biomolecule permeability [83] also make it promising in chip manufacturing, but long-term culturing is still challenging because of the air impermeability. Borosilicate glass was used in islet-on-a-chip platform recently [84], the maskless femtosecond laser ablation technique helped prototyping 3Dstructures on glass substrate [85]. Otherwise, the liquid glass, a special photocurable amorphous silica nanocomposite, was used in microfluidic device manufacturing through thermal debinding and sintering methods at a relatively low cost [86]. To make the best of biological inertness, thermal stability and other advantages of glass, neutral detergent was used to strengthen glass-glass bonding by Funano et al. [87], the productivity and the usability improvement further extended the possibility of glass based microfluidic devices in the morning.
5.1.2. Hydrogel and scaffold
Hydrogel suitable for proliferation, differentiation and other cell behaviors of bone related cells should fulfill several important requirements, like favorable biocompatibility, bioactivity, mechanical and adhesion properties [51,[88], [89], [90], [91]]. Multiple natural or synthetic materials are now utilized in bone tissue engineering and microfluidic devices, hydrogels are the most attractive among them due to the excellent tunable properties. Physicochemical characteristics of hydrogels are related to their raw materials, gelation and fabrication techniques. Different carriers for bone related cells are reported recently, depend on the various cell types and experimental design (Fig. 3).
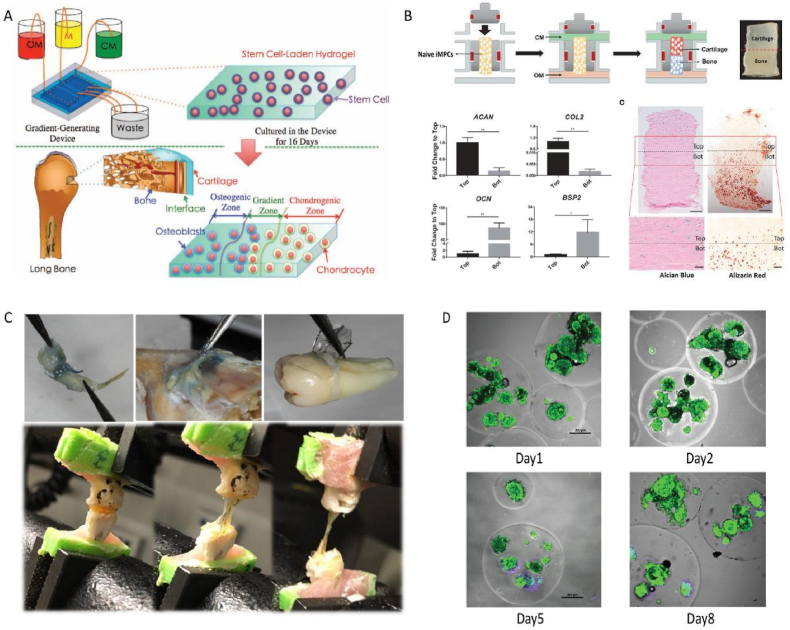
Fig. 3.
Hydrogel scaffolds applied in BCoC platforms. (A) Stem cells laden hydrogel for layered osteochondral unit construction (Adapted from Ref. [92], with permission); (B) Biphase induction of iMPCs on one single hydrogel plate (Adapted from Ref. [93], with permission); (C) Adhesiveness of chemical modified alginate hydrogel (Adapted from Ref. [94], with permission); (D) High cellular viability after encapsulation in the microgel sphere, green and purple are indicated by the Calcein-AM/PI staining (Adapted from Ref. [101], with permission).
The most popular role of hydrogels in microfluidic devices is cell scaffold, supporting cell growth as spacers. Major requirements for chondrocyte differentiation and proliferation are nutrition supply, cell adhesion, degradability and mechanical stimuli [52]. A cartilage-on-a-chip model with biomimetic interface was designed based on the permeability of hydrogel, the nutrition from different medium could diffuse anisotropically in this system [92]. MSCs were induced into chondrocytes and osteoblasts on the same hydrogel culturing device, cellular differentiation behaviors were similar to the natural conditions on the gradient-generating microfluidic device. Additionally, Lin et al. [93] developed osteochondral-on-a-chip model via diphasic induction of iPSCs on a whole piece of methacrylated gelatin. Osteogenic and chondrogenic markers are observed on the osteochondral unit, thus led to a high throughput cartilage-bone composite drug screening platform.
As cell attachment is not that easy on PDMS or glass surface, functional hydrogels are sometimes coated onto the pedestal. To enhance the cell adhesion behavior of hydrogel, the collagen mimicking, RGD peptides modified methacrylated alginate hydrogel was introduced by Mohammad et al. [94], effective chondrogenesis and endochondral ossification processes were observed in animal experiments, integrated with dopamine and MSCs. On contrast, Oliveira et al. [95] reported a quick testing system based on microfluidic devices, cell adhesion behaviors were not depended on the mechanical/viscoelastic properties of biomaterials, which is worthy of further study.
Physiological barriers, like blood-brain-barrier (BBB) and placental barrier, were normally reproduced by PDMS, PC or other polyporous membranes [96], Dr. Ingber used a PDMS membrane to counterfeit the connective tissue between vascular and alveolar epithelium [41]. Thanks to the quick progress of 3D printing technique, the pre-spatialized hydrogels are now wonderful divider which allows direct cellular contact. For instance, high-fidelity multimaterial microstructures printed by stereolithography-based platform was introduced to testified the neovascularization potential of 3D hydrogel on chip [97]. Via viscous finger patterning technique, hydrogel based BBB chip was fabricated to observe the formation of endothelial barrier layer, and validate therapeutic drugs [98].
The cell-laden hydrogel bases on novel microfabrication technologies enables not only formation of independent cellular structure, but also observation of multicellular interactions [99,100]. Alginate with excellent economic, safety and engineering properties, is now popular to fabricate hydrogel microparticles. According to Headen et al. [101], precisely controlled microgel particles with appropriate size and permeability could support cell viability and function of human islets organoid. Although multiple kinds of bone-targeting hydrogel had been introduced [102,103], studies about bone-related hydrogel droplet on chip are not yet reported.
Microstructure mimicking hierarchical nature of bone is well-recognized in tissue engineering area, thanks to the distinct advantages including cell adhesion, differentiation and mechanical support [104]. The 3D scaffold is also critical for cell growth and biological mineralization on microfluidic devices, the vessel-on-a-chip module, or “AngioChip” by the authors, enabled intercellular crosstalk and monocyte extravasation via a special porogen, poly(ethylene glycol) dimethylether (PEGDM) [105]. Based on a biodegradable PLA and gelatine scaffold, chondrocytes could be well protected on the microfluidic system, thus enables effective drug screening for OA and cartilage repair [106].
5.1.3. Biosensors
Biological activities inside musculoskeletal system, including bone modeling and remodeling, vascularization and nerve innervation, inflammation, hematopoiesis and so on, are well orchestrated in healthy body, the real-time observation of the physiological status and pathological abnormality is an eternal topic and engineering challenge. For example, during the long bone fracture healing process, MSCs and immune cells are recruited to the injury site at first time, chondrogenesis and subsequent vascularization fulfill the blank, accompanied by inflammation resolution, endochondral ossification and lengthy bone remodeling recreate the cortical-trabecular bone structure [107]. In some specific circumstance, we do want to accelerate or suppress some particular signaling and retreat at appropriate time-point, and this intervention could only be executed by experience.
In the past decades, traditional detection procedures like PCR, Western Blot and immunofluorescence staining are destructive to the experiment, and provide only endpoint results [108]. The microfluidic technique is one of the novel methods for real-time observation, the high transparency of PDMS and glass enables direct surveillance of living cells on the chip, fluorescent probes are developed to monitor the various biomarkers, but the antibody-based reporter system is normally time-consuming and laborious.
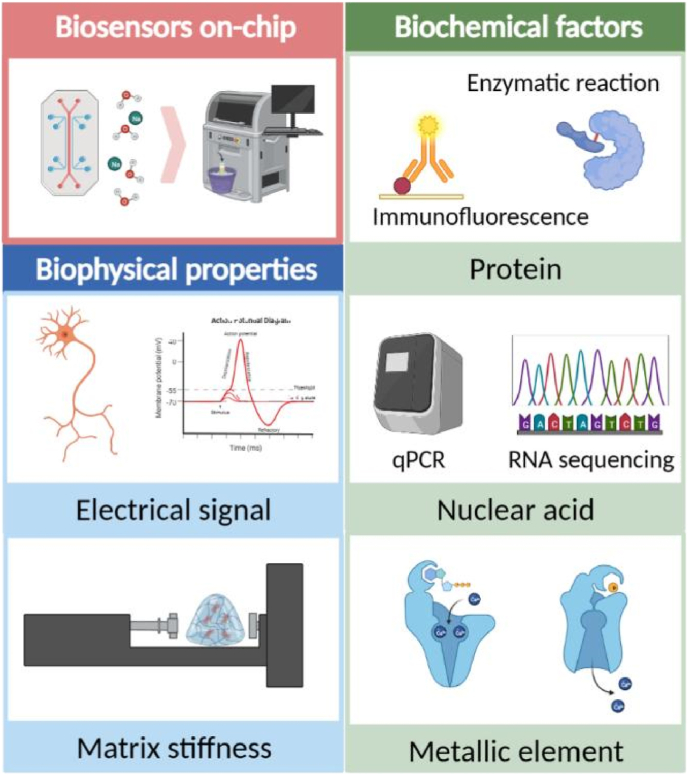
Biosensing technique is now the most promising solution to monitor the physicochemical environment in the culturing system, tremendous efforts have been made targeting markers like PH [109], oxygen pressure [110], glucose [111], COVID-19 virus [112] and others. However, there are few reports about biosensors integrated in BCoC systems, herein we introduce needs, developments and perspectives of biosensors in musculoskeletal research. Most common biochemical properties detected in BCoC device are protein, nuclear acids and metallic elements and biophysical signals during bone and cartilage metabolism are mostly electronic and mechanical ones. Due to the deep collaboration required in the fabrication of integrated biosensors, successful demonstration in microfluidic chips, especially BCoC, is relatively inadequate. Here we summarize current progression and representative works that are already, or might be, utilized in BCoC fabrication, and provide reference for future research (Fig. 4).
Fig. 4.
Need of biosensors in BCoC construction. The biophysical properties monitored in BCoC include electrical signal and matrix stiffness, while biochemical factors are mainly protein, nuclear acid and metallic element.
Electrochemical sensors detect proteins like specific enzymes and convert the chemical bonding to electric signals [108]. Theoretically, once electrodes were embedded into BCoC, those sensors could continuously report level of predefined enzyme, like alkaline phosphatase (ALP) [113]. Another remarkable protein during bone metabolism, bone morphogenetic protein (BMP), was represented on a microfluidic device by a novel, surface plasmon resonance (SPR)-based resolution [114]. Fc moiety of human lgG1 protein combined with BMP, and the BMP-receptor complex could easily captured by the sensor on the chip. A Integrated senor and chip system was reported by Ophir et al. [115], ALP activities could be demonstrated once vomited or leaked out of hepatocytes. The current or charge was exhibited by the oxidation process of the specific production which came from ALP and 1-naphtyl phosphate (1-NP) substrate. Ragones et al. [116] reported a PDMS-based integrated electrochemical sensor that is capable of for direct demonstration of biological samples on contact, or in close proximity. Two gold electrodes are connected to an Ag/AgCl quasi-reference electrode, and graphite powder was used to connect both side of the chip, these designation enabled a stable amperometric response to ALP level, which is quite promising in the BCoC chip. Calcium and vitamin D supplementation reduced bone loss and fracture incidence in the eldly [117], serum calcium level monitoring is critical to demonstrate bone metabolism status [118]. Unfortunately, there are no biosensors for microfluidic chips that detecting calcium, magnesium and other metallic element involved in bone metabolism.
There are also disadvantages of integrated biosensors, including the relatively poor sustainability and operational difficulties in calibration and replacement. Simple and effective solution is to connect the off-chip sensors with culturing system, and biomarkers could be detected within the circling culture medium. A real-time monitoring of glucose and lactate could effectively reflex mitochondrial dysfunction in liver-on-a-chip model [119]. Analogously, level and metabolism condition of interleukin 6 (IL-6) and tumor necrosis factor alpha (TNF-α) were monitored on a muscle-on-a-chip module, in order to observe biological reaction under lipopolysaccharide (LPS) or electrical stimulation [120].
While biochemical factors could be captured by enzymes, antibodies, vesicles [[121], [122], [123]] and even aptamers [124,125], electrophysical sensors could measure other properties of culturing system, like electrical and mechanical response, which might be of equal importance during BCoC construction. Not only critical in neuroscience, electrical signaling from sensory nerve could, on some levels, reflex innervation and bone-nerve regulation status in BCoC. A microfluidic device was reported by Silva et al. [126], dorsal root ganglion (DRG) neurons and MSCs were co-cultured to represent the in vivo bone sensory nerve innervation, we hypothesize that electrodes implantation would greatly help the characteristic of neurons in bone tissue. Matrix stiffness is one of the most important indicator of mechanical load. Composed by a pair of ultrasonic transmitter and receiver transducers, a novel ultrasonic platform was developed to transfer ultrasonic wave to electronic signal [127], this model provides new method to real-time monitoring the matrix calcification inside extracellular matrix.
5.2. Cell sources
Cell constitutes inside bone and cartilage are complicated and differentiated from various origins. For instance, osteoblast, chondrocytes and adipocytes come from MSCs, osteoarthritis and monocytes are derived from hematopoietic stem cells (HSC), and the endothelial progenitors are believed to be born from hemogenic endothelial cells in mesoderm along with HSC [128]. Herein we discuss cell sources to construct different modules of BCoC, separately (Table .1).
Table 1.
Cell sources for bone/cartilage organoid on a chip system.
| Tissue | Cell sources | Species | Ref. |
|---|---|---|---|
| Cartilage | Chondrocytes | Human | [129,130] |
| Chondrocytes (continuous passaged) | Human | [131] | |
| iPSCs | Human | [93] | |
| BMSCs | Human | [132] | |
| Bone | Osteoblasts | Mouse | [133] |
| Osteocytes | Mouse | [134] | |
| BMSCs | Human | [132] | |
| Foetal osteoblasts | Human | [77] | |
| iPSCs | Human | [93] | |
| Adipose tissue derived stem cells | Human | [135] | |
| Human embryonic stem cell-derived mesenchymal progenitors | Human | [136] | |
| CD14+ monocytes (osteoclasts) | Human | [137] | |
| RAW264.7 (osteoclasts) | Mouse | [138] | |
| Synovium | Fibroblast-like synoviocytes | Human | [139,140] |
| Vessel | HUVEC | Human | [141] |
| Fibroblast | Human | [142] | |
| Sensory nerve | DRG neurons | Rat | [126] |
| iPSCs | Human | [143] |
5.2.1. Cartilage
Under the principle of nonmaleficence, human chondrocytes from health individuals could only obtained from special circumstances. For instance, cartilage debris from patients suffered from amputation surgery could be ideal cell source, after informed consent and official ethic approval. In a recent work reported by our own team [144], chondrocytes and other cells from subchondral bone could be obtain from special patients which suffer from unicondylar cartilage destruction, but accept total knee arthopalsty. Due to the distinctive mechanical load, the cartilage and subchondral bone tissues from lateral side of femoral condyle and tibial plateau are visually intact. Recent report from Occhetta et al. [129] use primary chondrocytes from five volunteers without clinical situation or joint disorders, but they did not describe the specific position from which these articular cartilage samples were captured. Paggi et al. [130] from University of Twente designed a cartilage-on-a-chip model via primary chondrocytes isolated from “histologically healthy-looking cartilage” that obtained from patients underwent total knee replacement. However, a dissenting opinion has been raised by the same research team [53]. Given that all joint units are involved during osteoarthritis, the so-called healthy-looking condition is somehow suspicious.
Considering that the healthy chondrocytes from human sample are not that easy to obtain, stem cell inducing cells are the most popular alternatives. Chondrogenic induction could be processed under special medium consisting high-glucose DMEM medium, 1% penicillin-streptomycin, 0.1 μM dexamethasone, 40 μg/mL l-proline, 10 μg/mL ITS premix (insulin, transferrin, seleninic acid, bovine serum albumin, and linoleic acid), 50 μg/mL ascorbate, 10 ng/mL TGF-β3 [145]. MSCs are well-recognized chondrogenic progenitors, Lin et al. [93] designed a novel methods inducing iPSCs into MSC-like progenitor cells, and named them iMPCs. After encapsulated in gelatin scaffold, the iMPCs were induced into osteochondral unit on the chip, under exposure of chondrogenic and osteogenic medium separately in a dual-flow microfluidic chip. Despite that the term cartilage organoid has been raised 30 years ago, there are still no cartilage organoid on a chip model reported yet, methods used in cartilage organoids are also beneficial for BCoC construction. Dedifferentiated chondrocyte based cartibeads were reported by Kutaish and his college [131], the innovative method reverse the loss of chondrogenic potential during passaging, and obtain matrix rich cartilage organoid.
5.2.2. Bone
Osteoblasts are the major cells inside bone tissue that manufacture extracellular matrix and thus enable the subsequent mineralization process. In a bone-on-a-chip model reported in 2018 [133], murine calvaria derived osteoblasts were seeded in the bottom of the chamber, in a monolayer culture mode. Mature calcified osteoblastic colony with a thickness of 85 μm, as well as calcified collagen fibres, were observed in the chip within 30 days. What's more, the co-culture system of both osteoblasts and breast cancer cells demonstrated a similar phenotype with in vivo status. A simpler and more straightforward microfluidic model using a surface-etched bone wafer was reported [134]. After planted with osteocytes, the chip module could be used to test the functions of both osteoblast and osteoclasts, and the deformable design enabled the mechanotransduction, helped the researches of mechanical force related disorders. Human foetal osteoblasts (hFOBs) were also used to construct a bone-on-a chip model [77], the researchers achieve HA-PDMS composite pedestal via a stereolithography technique. Osteoblastic proliferation and calcification were significantly enhanced in the HA coating group, the osteosarcoma cell line and DOX tolerance experiments testified the comparability of this chip model for drug screening.
Stem cell inducing methods are well recognized and widely used in organoid-on-a-chip models construction. A commonly used osteogenic induction medium was prepared by high-glucose Dulbecco's modified Eagle medium, 10% FBS, 1% antibiotics-antimycotics, 0.1 μM dexamethasone, 10 mM β-glycerophosphate, and 50 μg/mL ascorbate 2-phosphate [93]. As is described earlier, MSC-like iMPCs induced from iPSCs could effectively form solid cortical-like bone tissue in a hydrogel supported culture system [93]. Pilar et al. [135] constructed a collagen hydrogel-based bone-on-a-chip model via adipose tissue derived stem cells. After 7 or 14 days of pre-differentiation, the osteogenic cells were seeded in the 3D collagen matrix and served as ex-vivo bone models. Human embryonic stem cell-derived mesenchymal progenitors are also utilized in BCoC construction [136], primary cilia were observed in this chip, which are thought to be mechanosensor in bone matrix.
Osteoclasts are critical players during the life-long bone remodeling, but they are not homologous with osteoblasts or chondrocytes [146]. A novel co-culture system containing sympathetic neurons, osteoclasts and breast cancer cells was designed to unravel the hiding mechanism of bone metastasis [137].
5.2.3. Synovium
Synovial invasion is one of the most critical pathological change during rheumatoid arthritis (RA), and is also great participant in pigmented villonodular synovitis, a rare subtype of arthritis. The major cellular constituents of synovium are fibroblast-like synoviocytes (FLS), macrophages, endothelial cells and various immune cells [147]. FLS are highly specialized mesenchymal cells that produce hyaluronan and lubricin, and treated as the key player in RA [148]. In the recent study, the synovial organoid on-chip model was proved to be highly similar with the in vivo synovium [139], involving the lining layer connection and reaction to inflammatory cytokine. In another report from the same team, a chondrocyte-FLS co-culture system demonstrated a better cartilage structure and physiology, and the alteration of chondrocytes phenotype further testified the ex-vivo chip model of RA research. A monocyte invasion chip was reported to reconstruct the abnormal accumulation of macrophages during arthritis [149].
5.2.4. Vessel and sensory nerve
The Circulation system, or the ubiquitous network of arteries, veins and capillaries, delivery nutrition and thus maintains organ viability. As promising ex-vivo organ models for drug screening and mechanism research, the organ-on-a-chip models are also strengthened by vascular network. Human umbilical vein endothelial cells (HUVEC) are the major source of vessel net on chip, Wang et al. [141] constructed a capillary network inside the microfluidic chip chamber, and enabled an effective drug screening and toxicity testing platform. Furthermore, in order to reproduce the crosstalk and synergistic effect of multi-organs, Kacey et al. [150] reported a vascular flow linked multi-organ chip, including heart, liver, bone, and skin, to further optimize drug screening platform. Unfortunately, the vessel network is not reported in bone/cartilage on-chip systems yet.
It is well explained that sensory nerve helps bone metabolism [151], but innervation on the chip platform is still technically challenging. Diana et al. [126] demonstrated a microfluidic co-culture system containing dorsal root ganglion (DRG) neurons and MSCs, the differentiation behavior of MSC was significantly up-regulated via the β-catenin-Wnt signaling pathway.
5.3. Disease-specific models
5.3.1. Bone metabolism
The life-long bone resorption and reconstruction process enable us with appropriate adaption to load and environment around, the unbalance of bone remodeling leads to various of bone related diseases, such as osteoporosis, osteopetrosis, arthritis and tumor. A tripartite co-culture device was reported to reproduce the remodeling process [152]. The load motivated osteocytes send soluble signals, achieve osteoblasts and osteoclasts downstream via the default channel. The precisely controlled microfluid could be wonderful marker of shear force, Babaliari et al. [153] demonstrated a organ-on-a-chip device for tunable control of osteoblast culturing. The proliferation rate in the fast-flow group was enhanced significantly, but the activity of ALP was relatively higher in the opposite group. These chips modeling bone metabolism in varying degrees had showed opportunity to a better ex-vivo simulation and biological research. Osteoclasts are normally regarded as initiator of bone remodeling and related disorders, but there are still not many research about osteoclast-on-a-chip device, partly because of the difficulty of induction and longtime culturing.
Tissue repair after fracture is one of the most concerned problems in clinical experience, microfluidic devices could take part in designation of bone/cartilage implantation materials. Li et al. [106] established a microfluidic drug-screening device and decided the optimum concentration of resveratrol, thus construct the filling material for articular cartilage defect. In situ repair of stem cells is now promising and well-developed for tissue defect, but the induction condition in vivo is difficult to maintain. A kind of on-chip cultured cell globule was introduced to be efficient and safe for the incoming clinical trials, the authors construct cell-imprinted substrate and chondrogenic induced MSCs separately, thus eliminated the articular defect in six months [154]. Disturbed bone remodeling status could also be found in other metabolic diseases, like diabetes and inflammatory bowel disease (IBD) [155], these complicated circumstance could be better simulated on microfluidic systems in the future.
5.3.2. Arthritis
The arthritis microenvironment is usually recapitulated on microfluidic devices in three aspects: mechanical stimulation, multicellular interaction and immune intervention (Fig. 5) [156].
Fig. 5.
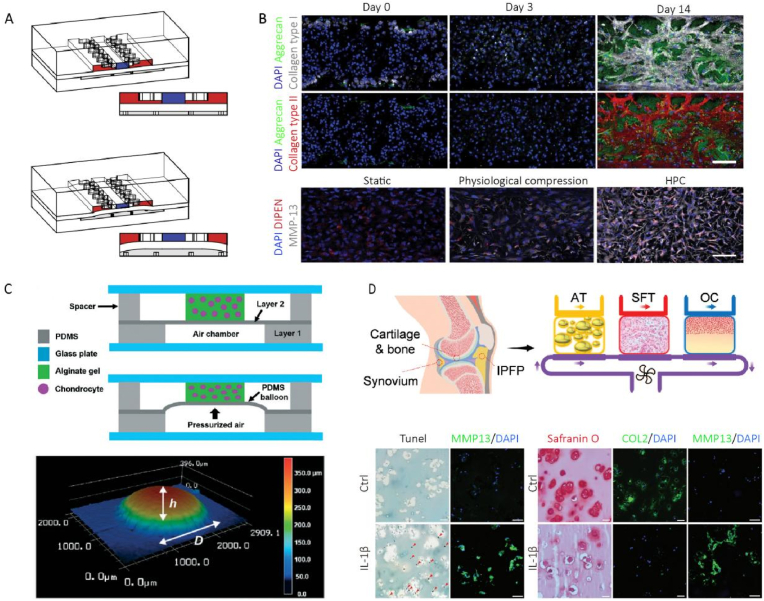
Construction of arthritis model on-chip. (A) Hyperphysiological compression induced osteoarthritic phenotype on chip, the deformation of PDMS membrane led to compression of the superior hydrogel (Adapted from Ref. [129], with permission); (B) Immunofluorescence presented the expression of aggrecan, collagen type I and collagen type II, MMP13 expression was up-regulated in the hyperphysiological compression (HPC) (Adapted from Ref. [129], with permission); (C) The balloon inflated by pressurized air supplied the compression (upper channel), 3D laser scanning microscopy measured the deformation (lower channel) (Adapted from Ref. [158], with permission); (D) Reconstruction of the joint on-chip based on cellular crosstalk (Adapted from Ref. [132], with permission).
Engineering approaches for mechanical stimulation have been well established in recent decades, like direct squeezing and substrate distension [157], the deformability of PDMS enables the culturing system with extraneous stimulation, vertically, horizontally or vortically. For instance, by pressurizing the bottom chamber, the upper chamber was vertically compressed by PDMS membrane, as well as the chondrocytes and PEG-hydrogel culturing matrix [129]. This mechanical stimulation triggered the transformation from stability to catabolism and inflammation phenotype, as well as osteoarthritic gene expression. Another research characterized the pressure activated pneumatic device performance detailedly, especially mechanical properties [158].
The most familiar kind of cellular crosstalk reproduced on chip is like synovium and cartilage. Series of research works have been presented by Prof. Lin Hang from University of Pittsburgh, they constructed osteochondral unit on the same hydrogel system via biphase induction of MSCs, the interactions of bone and cartilage could be demonstrated [159]. The limited generation of cell passage restricted the further movement, a iPSCs derived mesenchymal progenitor, so-called iMPCs, was developed to overcome this barrier [93]. During osteoarthritis and rheumatoid arthritis, the synovium and adipose tissue took part in the pathological processes, the crosstalk inside bone and cartilage is certainly insufficient to reproduce joint microenvironment. A complex bioreactor containing osteochondral unit, synovial-like fibrous tissue and adipose pad was designed and manufactured, the system demonstrated a arthritic phenotype while exposed to immune factor IL-1β, and provided a effective joint-on-a-chip model for therapeutic research [132].
Innate immune response is the most important player during RA progression, but the biological effect in OA pathology is still vague. Cohort study showed that monocytes in OA synovial fluid are closely related to patient perception, possibly via CD4+ T cell activation [160], macrophages accelerate osteogenic differentiation of adjacent MSCs on a mini-joint model [156] have also confirmed the involvement. The extravasation of monocytes is one of the pathological abnormalities during arthritis, Carlotta et al. recapitulating the articular joint space on a microfluidic system with synovium and cartilage [149], the extravasated monocytes were significantly up-regulated after pre-processing with chemokines and OA synovial fluid.
5.3.3. Tumor development and metastasis
Being euangiotic and environmentally stable, the bone is one of the most popular destinations for traveling tumor cells [161]. To mimic the process of metastasis ex-vivo, cancer cells are usually intravenously or intraperitoneally injected on immunodeficient mice models, but the success rate and absence of immune system have long limited the further development. The microfluidic devices offer new methods to a deep investigation, as a cheaper, quicker and more authentic ex-vivo model.
Cellular invasion and extravasation behaviors could be mimicked on the chip device. Metastatic breast cancer cells, HUVEC and osteocytes were co-cultured to investigate the mechanical regulation of osteocytes during bone metastasis [162], the results showed that the load induced osteocytes reduced breast cancer extravasation. Another model monitoring invasion behavior found that the co-culture of prostate cancers with MC3T3-E1 osteoblasts had up-regulated the protrusive phenotype of cancer cells [163]. Hao et al. [133] constructed a spontaneous bone-on-a-chip system, described the implantation of breast cancer cells, and thus provided a promising experimental platform for bone metastasis.
Homing of cancer cells and HSCs to bone marrow is critical pathophysiological phenomenon that closely related to bone metastasis [164], recent work of ours [165] has built bone-targeting nanocarrier based on this mechanism. Jeon et al. [166] built a vascularized bone-on-a-chip platform to study the extravasation behavior of breast cancer. Together with HUVEC, the BMSCs demonstrated a mural-cell like phenotype, α-smooth muscle actin (α-SMA) positive and wrapped around vascular networks. The seed and soil theory was further testified in this research, relative quantitative results were also provided about the anti-metastasis effect of skeletal muscle. In brief, the construction of on-chip bone metastasis niche provides new thought to break the barrier between in vitro models and physiological conditions.
6. Conclusion and perspective
Novel platform to recapitulate the physiological condition and pathological changes in bone and joint is now urgently needed, the BCoC devices cross the barrier between ex-vivo cell culture, animal models and the real pathological status in human bodies. In the long term, BCoC devices possessing multiple variables could demonstrated pathophysiology characters during bone/cartilage disorders, and share new opportunities to drug screening and therapeutic resolutions. Although there are still few well-established systems reported, the rapid pace of all supporting technologies, including engineering, biological and medical aspects, suggest the coming of a new era of ex-vivo research platform for bone and cartilage diseases.
Credit authorship contribution statement
Yan Hu: Conceptualization, Methodology, Investigation, Writing - Original Draft. Hao Zhang: Writing - Original Draft. Sicheng Wang: Methodology, Validation. Liehu Cao: Writing - Revised Draft. Fengjin Zhou: Formal analysis, Software. Yingying Jing: Project administration, Data Curation. Jiacan Su: Supervision, Funding acquisition.
Ethics approval and consent to participate
There is no need for ethics approval and consent to participate, because this is a review paper without human or animal experiments.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgement
This work was supported by grants from National Natural Science Foundation of China (No.82230071, 92249303 and 82172098 to J. Su), Shanghai Committee of Science and Technology (Laboratory Animal Research Project to J. Su), Shanghai Baoshan District Medical Health Project (No. 21-E-14 to L. Cao) and China Postdoctoral Science Foundation (No. 2022M722033 to Y. Hu).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Contributor Information
Fengjin Zhou, Email: dr.zhoufj@163.com.
Yingying Jing, Email: jingy4172@shu.edu.cn.
Jiacan Su, Email: drsujiacan@163.com.
References
- 1.Kawai M., Mödder U.I., Khosla S., et al. Emerging therapeutic opportunities for skeletal restoration. J. Nat. Rev. Drug Discov. 2011;10(2):141–156. doi: 10.1038/nrd3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hunter D.J., Bierma-Zeinstra S. Osteoarthritis. J. Lancet. 2019;393(10182):1745–1759. doi: 10.1016/S0140-6736(19)30417-9. [DOI] [PubMed] [Google Scholar]
- 3.Black D.M., Rosen C.J. Clinical practice. Postmenopausal osteoporosis.[J] N. Engl. J. Med. 2016;374(3):254–262. doi: 10.1056/NEJMcp1513724. [DOI] [PubMed] [Google Scholar]
- 4.Li Z.A., Sant S., Cho S.K., et al. Synovial joint-on-a-chip for modeling arthritis: progress, pitfalls, and potential. J. Trends Biotechnol. 2022 doi: 10.1016/j.tibtech.2022.07.011. https://www.sciencedirect.com/science/article/pii/S0167779922001937?via%3Dihub [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaudhuri O., Gu L., Klumpers D., et al. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. J. Nat. Mater. 2016;15(3):326–334. doi: 10.1038/nmat4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pampaloni F., Reynaud E.G., Stelzer E.H.K. The third dimension bridges the gap between cell culture and live tissue.[J] Nat. Rev. Mol. Cell Biol. 2007;8(10):839–845. doi: 10.1038/nrm2236. [DOI] [PubMed] [Google Scholar]
- 7.Huh D., Hamilton G.A., Ingber D.E. From 3D cell culture to organs-on-chips.[J] Trends Cell Biol. 2011;21(12):745–754. doi: 10.1016/j.tcb.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clevers H. Modeling development and disease with organoids. J. Cell. 2016;165(7):1586–1597. doi: 10.1016/j.cell.2016.05.082. [DOI] [PubMed] [Google Scholar]
- 9.Goldring S.R., Goldring M.B. Changes in the osteochondral unit during osteoarthritis: structure, function and cartilage-bone crosstalk.[J] Nat. Rev. Rheumatol. 2016;12(11):632–644. doi: 10.1038/nrrheum.2016.148. [DOI] [PubMed] [Google Scholar]
- 10.Lee C.H., Cook J.L., Mendelson A., et al. Regeneration of the articular surface of the rabbit synovial joint by cell homing: a proof of concept study. J. Lancet. 2010;376(9739):440–448. doi: 10.1016/S0140-6736(10)60668-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun H.B. Mechanical loading, cartilage degradation, and arthritis. J. Ann. New York Acad. Sci. 2010;1211:37–50. doi: 10.1111/j.1749-6632.2010.05808.x. [DOI] [PubMed] [Google Scholar]
- 12.Chen L., Yao F., Wang T., et al. Horizontal fissuring at the osteochondral interface: a novel and unique pathological feature in patients with obesity-related osteoarthritis. J. Ann. Rheumatic Dis. 2020;79(6):811–818. doi: 10.1136/annrheumdis-2020-216942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Souza R.B., Baum T., Wu S., et al. Effects of unloading on knee articular cartilage T1rho and T2 magnetic resonance imaging relaxation times: a case series.[J] J. Orthop. Sports Phys. Ther. 2012;42(6):511–520. doi: 10.2519/jospt.2012.3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu Y., Chen X., Wang S., et al. Subchondral bone microenvironment in osteoarthritis and pain. J. Bone Res. 2021;9(1):20. doi: 10.1038/s41413-021-00147-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guermazi A., Niu J., Hayashi D., et al. Prevalence of abnormalities in knees detected by MRI in adults without knee osteoarthritis: population based observational study (Framingham Osteoarthritis Study) [J]. BMJ-Br. Med. J. 2012;345:e5339. doi: 10.1136/bmj.e5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bowes M.A., McLure S.W., Wolstenholme C.B., et al. Osteoarthritic bone marrow lesions almost exclusively colocate with denuded cartilage: a 3D study using data from the Osteoarthritis Initiative.[J] Ann. Rheum. Dis. 2016;75(10):1852–1857. doi: 10.1136/annrheumdis-2015-208407. [DOI] [PubMed] [Google Scholar]
- 17.Zhen G., Wen C., Jia X., et al. Inhibition of TGF-β signaling in mesenchymal stem cells of subchondral bone attenuates osteoarthritis. J. Nat. Med. 2013;19(6):704–712. doi: 10.1038/nm.3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y., Wei L., Zeng L., et al. Nutrition and degeneration of articular cartilage. J. Knee Surg. Sports Traumatol. Arthrosc. : Off. J. ESSKA. 2013;21(8):1751–1762. doi: 10.1007/s00167-012-1977-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orr C., Vieira-Sousa E., Boyle D.L., et al. Synovial tissue research: a state-of-the-art review.[J] Nat. Rev. Rheumatol. 2017;13(8):463–475. doi: 10.1038/nrrheum.2017.115. [DOI] [PubMed] [Google Scholar]
- 20.Kurowska-Stolarska M., Alivernini S. Synovial tissue macrophages: friend or foe?[J] RMD open. 2017;3(2):e527. doi: 10.1136/rmdopen-2017-000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kurowska-Stolarska M., Alivernini S. Synovial tissue macrophages in joint homeostasis, rheumatoid arthritis and disease remission. J. Nat. Rev. Rheumatol. 2022;18(7):384–397. doi: 10.1038/s41584-022-00790-8. [DOI] [PubMed] [Google Scholar]
- 22.Havelka S., Horn V., Spohrová D., et al. The calcified-noncalcified cartilage interface: the tidemark.[J] Acta Biol. Hung. 1984;35(2–4):271–279. [PubMed] [Google Scholar]
- 23.Lyons T.J., McClure S.F., Stoddart R.W., et al. The normal human chondro-osseous junctional region: evidence for contact of uncalcified cartilage with subchondral bone and marrow spaces. J. BMC Musculoskel. Disord. 2006;7:52. doi: 10.1186/1471-2474-7-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu L., Prins H., Helder M.N., et al. Trophic effects of mesenchymal stem cells in chondrocyte co-cultures are independent of culture conditions and cell sources. J. Tissue Eng., A. 2012;18(15–16):1542–1551. doi: 10.1089/ten.TEA.2011.0715. [DOI] [PubMed] [Google Scholar]
- 25.Wu L., Leijten J.C.H., Georgi N., et al. Trophic effects of mesenchymal stem cells increase chondrocyte proliferation and matrix formation. [J]. Tissue Eng. A. 2011;17(9–10):1425–1436. doi: 10.1089/ten.TEA.2010.0517. [DOI] [PubMed] [Google Scholar]
- 26.Zhang S., Chuah S.J., Lai R.C., et al. MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. J. Biomater. 2018;156:16–27. doi: 10.1016/j.biomaterials.2017.11.028. [DOI] [PubMed] [Google Scholar]
- 27.Wong K.L., Zhang S., Wang M., et al. Intra-articular injections of mesenchymal stem cell exosomes and hyaluronic acid improve structural and mechanical properties of repaired cartilage in a rabbit model.[J] Arthroscopy : J. Arthrosc. Related Surg. Off. Publ. Arthrosc. Assoc. N. Am. Int. Arthrosc. Assoc. 2020;36(8):2215–2228. doi: 10.1016/j.arthro.2020.03.031. [DOI] [PubMed] [Google Scholar]
- 28.Wang R., Jiang W., Zhang L., et al. Intra-articular delivery of extracellular vesicles secreted by chondrogenic progenitor cells from MRL/MpJ superhealer mice enhances articular cartilage repair in a mouse injury model. [J]. Stem Cell Res. Ther. 2020;11(1):93. doi: 10.1186/s13287-020-01594-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roemer F.W., Frobell R., Hunter D.J., et al. MRI-detected subchondral bone marrow signal alterations of the knee joint: terminology, imaging appearance, relevance and radiological differential diagnosis. J. Osteoarthritis Cartilage. 2009;17(9):1115–1131. doi: 10.1016/j.joca.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 30.Hernigou P., Bouthors C., Bastard C., et al. Subchondral bone or intra-articular injection of bone marrow concentrate mesenchymal stem cells in bilateral knee osteoarthritis: what better postpone knee arthroplasty at fifteen years? A randomized study. J. Int. Orthop. 2021;45(2):391–399. doi: 10.1007/s00264-020-04687-7. [DOI] [PubMed] [Google Scholar]
- 31.Hernigou P., Delambre J., Quiennec S., et al. Human bone marrow mesenchymal stem cell injection in subchondral lesions of knee osteoarthritis: a prospective randomized study versus contralateral arthroplasty at a mean fifteen year follow-up. J. Int. Orthop. 2021;45(2):365–373. doi: 10.1007/s00264-020-04571-4. [DOI] [PubMed] [Google Scholar]
- 32.Wang M., Tan G., Jiang H., et al. Molecular crosstalk between articular cartilage, meniscus, synovium, and subchondral bone in osteoarthritis. J. Bone Joint Res. 2022;11(12):862–872. doi: 10.1302/2046-3758.1112.BJR-2022-0215.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pan J., Zhou X., Li W., et al. In situ measurement of transport between subchondral bone and articular cartilage.[J] J. Orthop. Res. : Off. Publ. Orthopaed. Res. Soc. 2009;27(10):1347–1352. doi: 10.1002/jor.20883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu K., Olsen B.R. Osteoblast-derived VEGF regulates osteoblast differentiation and bone formation during bone repair.[J] J. Clin. Invest. 2016;126(2):509–526. doi: 10.1172/JCI82585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duval K., Grover H., Han L., et al. Modeling physiological events in 2D vs. 3D cell culture. J. Physiol. (London) 2017;32(4):266–277. doi: 10.1152/physiol.00036.2016. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 36.Rossi G., Manfrin A., Lutolf M.P. Progress and potential in organoid research.[J] Nat. Rev. Genet. 2018;19(11):671–687. doi: 10.1038/s41576-018-0051-9. [DOI] [PubMed] [Google Scholar]
- 37.Sato T., Vries R.G., Snippert H.J., et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. J. Nat. 2009;459(7244):262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 38.Eiraku M., Takata N., Ishibashi H., et al. Self-organizing optic-cup morphogenesis in three-dimensional culture.[J] Nature. 2011;472(7341):51–56. doi: 10.1038/nature09941. [DOI] [PubMed] [Google Scholar]
- 39.Takebe T., Sekine K., Enomura M., et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. J. Nat. 2013;499(7459):481–484. doi: 10.1038/nature12271. [DOI] [PubMed] [Google Scholar]
- 40.Boj S.F., Hwang C., Baker L.A., et al. Organoid models of human and mouse ductal pancreatic cancer. J. Cell. 2015;160(1–2):324–338. doi: 10.1016/j.cell.2014.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huh D., Matthews B.D., Mammoto A., et al. Reconstituting organ-level lung functions on a chip. J. Sci. 2010;328(5986):1662–1668. doi: 10.1126/science.1188302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kurmann A.A., Serra M., Hawkins F., et al. Regeneration of thyroid function by transplantation of differentiated pluripotent stem cells.[J] Cell Stem Cell. 2015;17(5):527–542. doi: 10.1016/j.stem.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takasato M., Er P.X., Chiu H.S., et al. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. J. Nat. 2015;526(7574):564–568. doi: 10.1038/nature15695. [DOI] [PubMed] [Google Scholar]
- 44.Chen S., Chen X., Geng Z., et al. The horizon of bone organoid: a perspective on construction and application. [J]. Bioactive Mater. 2022;18:15–25. doi: 10.1016/j.bioactmat.2022.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pittenger M.F., Discher D.E., Péault B.M., et al. Mesenchymal stem cell perspective: cell biology to clinical progress. J. NPJ. Regen. Med. 2019;4:22. doi: 10.1038/s41536-019-0083-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Augello A., De Bari C. The regulation of differentiation in mesenchymal stem cells.[J] Hum. Gene Ther. 2010;21(10):1226–1238. doi: 10.1089/hum.2010.173. [DOI] [PubMed] [Google Scholar]
- 47.Guzzo R.M., Drissi H. Differentiation of human induced pluripotent stem cells to chondrocytes. J. Methods Mol. Biol. (Clifton, N.J.) 2015;1340:79–95. doi: 10.1007/978-1-4939-2938-2_6. [DOI] [PubMed] [Google Scholar]
- 48.Jeon O.H., Panicker L.M., Lu Q., et al. Human iPSC-derived osteoblasts and osteoclasts together promote bone regeneration in 3D biomaterials. [J]. Sci. Rep. 2016;6 doi: 10.1038/srep26761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Patsch C., Challet-Meylan L., Thoma E.C., et al. Generation of vascular endothelial and smooth muscle cells from human pluripotent stem cells.[J] Nat. Cell Biol. 2015;17(8):994–1003. doi: 10.1038/ncb3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nakajima T., Nakahata A., Yamada N., et al. Grafting of iPS cell-derived tenocytes promotes motor function recovery after Achilles tendon rupture. [J]. Nat. Commun. 2021;12(1):5012. doi: 10.1038/s41467-021-25328-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xue X., Hu Y., Deng Y., et al. Recent advances in design of functional biocompatible hydrogels for bone tissue engineering[J] Adv. Funct. Mater. 2021;31(19) [Google Scholar]
- 52.Kim I.L., Mauck R.L., Burdick J.A. Hydrogel design for cartilage tissue engineering: a case study with hyaluronic acid. J. Biomater. 2011;32(34):8771–8782. doi: 10.1016/j.biomaterials.2011.08.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Paggi C.A., Teixeira L.M., Le Gac S., et al. Joint-on-chip platforms: entering a new era of in vitro models for arthritis.[J] Nat. Rev. Rheumatol. 2022;18(4):217–231. doi: 10.1038/s41584-021-00736-6. [DOI] [PubMed] [Google Scholar]
- 54.Pirosa A., Gottardi R., Alexander P.G., et al. An in vitro chondro-osteo-vascular triphasic model of the osteochondral complex. J. Biomater. 2021;272 doi: 10.1016/j.biomaterials.2021.120773. [DOI] [PubMed] [Google Scholar]
- 55.Ma C., Peng Y., Li H., et al. Organ-on-a-Chip: a new paradigm for drug development.[J] Trends Pharmacol. Sci. 2021;42(2):119–133. doi: 10.1016/j.tips.2020.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bhatia S.N., Ingber D.E. Microfluidic organs-on-chips.[J] Nat. Biotechnol. 2014;32(8):760–772. doi: 10.1038/nbt.2989. [DOI] [PubMed] [Google Scholar]
- 57.Whitesides G.M. The origins and the future of microfluidics. J. Nat. 2006;442(7101):368–373. doi: 10.1038/nature05058. [DOI] [PubMed] [Google Scholar]
- 58.Pires de Mello C.P., Carmona-Moran C., McAleer C.W., et al. Microphysiological heart-liver body-on-a-chip system with a skin mimic for evaluating topical drug delivery. J. Lab Chip. 2020;20(4):749–759. doi: 10.1039/c9lc00861f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maschmeyer I., Lorenz A.K., Schimek K., et al. A four-organ-chip for interconnected long-term co-culture of human intestine, liver, skin and kidney equivalents. J. Lab Chip. 2015;15(12):2688–2699. doi: 10.1039/c5lc00392j. [DOI] [PubMed] [Google Scholar]
- 60.Picollet-D'hahan N., Zuchowska A., Lemeunier I., et al. Multiorgan-on-a-Chip: a systemic approach to model and decipher inter-organ communication. J. Trends Biotechnol. 2021;39(8):788–810. doi: 10.1016/j.tibtech.2020.11.014. [DOI] [PubMed] [Google Scholar]
- 61.Park S.E., Georgescu A., Huh D. Organoids-on-a-chip. J. Sci. 2019;364(6444):960–965. doi: 10.1126/science.aaw7894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Miranda I., Souza A., Sousa P., et al. Properties and applications of PDMS for biomedical engineering: a review.[J] J. Funct. Biomater. 2021;13(1) doi: 10.3390/jfb13010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bhattacharjee N., Parra-Cabrera C., Kim Y.T., et al. Desktop-stereolithography 3D-printing of a poly(dimethylsiloxane)-based material with sylgard-184 properties. J. Adv. Mater. 2018;30(22) doi: 10.1002/adma.201800001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Suzuki H., Mitsuno K., Shiroguchi K., et al. One-step micromolding of complex 3D microchambers for single-cell analysis. J. Lab Chip. 2017;17(4):647–652. doi: 10.1039/c6lc01313a. [DOI] [PubMed] [Google Scholar]
- 65.Gervais T., El-Ali J., Günther A., et al. Flow-induced deformation of shallow microfluidic channels. J. Lab Chip. 2006;6(4):500–507. doi: 10.1039/b513524a. [DOI] [PubMed] [Google Scholar]
- 66.Berthier E., Young E.W.K., Beebe D. Engineers are from PDMS-land, biologists are from polystyrenia.[J] Lab Chip. 2012;12(7):1224–1237. doi: 10.1039/c2lc20982a. [DOI] [PubMed] [Google Scholar]
- 67.Regehr K.J., Domenech M., Koepsel J.T., et al. Biological implications of polydimethylsiloxane-based microfluidic cell culture. J. Lab Chip. 2009;9(15):2132–2139. doi: 10.1039/b903043c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kuo J.S., Zhao Y., Ng L., et al. Microfabricating high-aspect-ratio structures in polyurethane-methacrylate (PUMA) disposable microfluidic devices[J] Lab Chip. 2009;9(13):1951–1956. doi: 10.1039/b902124h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fiorini G.S., Yim M., Jeffries G.D.M., et al. Fabrication improvements for thermoset polyester (TPE) microfluidic devices[J] Lab Chip. 2007;7(7):923–926. doi: 10.1039/b702548c. [DOI] [PubMed] [Google Scholar]
- 70.Shakeri A., Khan S., Didar T.F. Conventional and emerging strategies for the fabrication and functionalization of PDMS-based microfluidic devices.[J] Lab Chip. 2021;21(16):3053–3075. doi: 10.1039/d1lc00288k. [DOI] [PubMed] [Google Scholar]
- 71.van Engeland N.C.A., Pollet A.M.A.O., den Toonder J.M.J., et al. A biomimetic microfluidic model to study signalling between endothelial and vascular smooth muscle cells under hemodynamic conditions.[J] Lab Chip. 2018;18(11):1607–1620. doi: 10.1039/c8lc00286j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sui G., Wang J., Lee C., et al. Solution-phase surface modification in intact poly(dimethylsiloxane) microfluidic channels. J. Anal. Chem. 2006;78(15):5543–5551. doi: 10.1021/ac060605z. [DOI] [PubMed] [Google Scholar]
- 73.Dabaghi M., Shahriari S., Saraei N., et al. Surface modification of PDMS-based microfluidic devices with collagen using polydopamine as a spacer to enhance primary human bronchial epithelial cell adhesion. [J]. Micromachines. 2021;12(2) doi: 10.3390/mi12020132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gitlin L., Schulze P., Ohla S., et al. Surface modification of PDMS microfluidic devices by controlled sulfuric acid treatment and the application in chip electrophoresis. J. Electroph. 2015;36(3):449–456. doi: 10.1002/elps.201400269. [DOI] [PubMed] [Google Scholar]
- 75.Fatona A., Chen Y., Reid M., et al. One-step in-mould modification of PDMS surfaces and its application in the fabrication of self-driven microfluidic channels.[J] Lab Chip. 2015;15(22):4322–4330. doi: 10.1039/c5lc00741k. [DOI] [PubMed] [Google Scholar]
- 76.Montazeri L., Bonakdar S., Taghipour M., et al. Modification of PDMS to fabricate PLGA microparticles by a double emulsion method in a single microfluidic device. J. Lab Chip. 2016;16(14):2596–2600. doi: 10.1039/c6lc00437g. [DOI] [PubMed] [Google Scholar]
- 77.Tang Q., Li X., Lai C., et al. Fabrication of a hydroxyapatite-PDMS microfluidic chip for bone-related cell culture and drug screening. [J]. Bioactive Mater. 2021;6(1):169–178. doi: 10.1016/j.bioactmat.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Busek M., Nøvik S., Aizenshtadt A., et al. Thermoplastic elastomer (TPE)-Poly(Methyl methacrylate) (PMMA) hybrid devices for active pumping PDMS-free organ-on-a-chip systems. [J]. Biosensors. 2021;11(5) doi: 10.3390/bios11050162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim Y.J., Lim J.H., Lee J.M., et al. CuS/rGO-PEG nanocomposites for photothermal bonding of PMMA-based plastic Lab-on-a-Chip.[J] Nanomaterials. 2021;11(1) doi: 10.3390/nano11010176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Altmann B., Steinberg T., Giselbrecht S., et al. Promotion of osteoblast differentiation in 3D biomaterial micro-chip arrays comprising fibronectin-coated poly(methyl methacrylate) polycarbonate. J. Biomater. 2011;32(34):8947–8956. doi: 10.1016/j.biomaterials.2011.08.023. [DOI] [PubMed] [Google Scholar]
- 81.Lei K.F., Chang C., Chen M. Paper/PMMA hybrid 3D cell culture microfluidic platform for the study of cellular crosstalk.[J] ACS Appl. Mater. Interfaces. 2017;9(15):13092–13101. doi: 10.1021/acsami.7b03021. [DOI] [PubMed] [Google Scholar]
- 82.Carrilho E., Martinez A.W., Whitesides G.M. Understanding wax printing: a simple micropatterning process for paper-based microfluidics[J] Anal. Chem. 2009;81(16):7091–7095. doi: 10.1021/ac901071p. [DOI] [PubMed] [Google Scholar]
- 83.Hirama H., Satoh T., Sugiura S., et al. Glass-based organ-on-a-chip device for restricting small molecular absorption.[J] J. Biosci. Bioeng. 2019;127(5):641–646. doi: 10.1016/j.jbiosc.2018.10.019. [DOI] [PubMed] [Google Scholar]
- 84.Yi L., Wang X., Dhumpa R., et al. Integrated perfusion and separation systems for entrainment of insulin secretion from islets of Langerhans. J. Lab Chip. 2015;15(3):823–832. doi: 10.1039/c4lc01360c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schulze T., Mattern K., Früh E., et al. A 3D microfluidic perfusion system made from glass for multiparametric analysis of stimulus-secretioncoupling in pancreatic islets.[J] Biomed. Microdevices. 2017;19(3):47. doi: 10.1007/s10544-017-0186-z. [DOI] [PubMed] [Google Scholar]
- 86.Kotz F., Plewa K., Bauer W., et al. Liquid glass: a facile soft replication method for structuring glass. [J]. Adv. Mater. (Deerfield Beach, Fla.) 2016;28(23):4646–4650. doi: 10.1002/adma.201506089. [DOI] [PubMed] [Google Scholar]
- 87.Funano S., Ota N., Tanaka Y. A simple and reversible glass-glass bonding method to construct a microfluidic device and its application for cell recovery. J. Lab Chip. 2021;21(11):2244–2254. doi: 10.1039/d1lc00058f. [DOI] [PubMed] [Google Scholar]
- 88.Xue X., Hu Y., Wang S., et al. Fabrication of physical and chemical crosslinked hydrogels for bone tissue engineering. [J]. Bioactive Mater. 2022;12:327–339. doi: 10.1016/j.bioactmat.2021.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang H., Wu S., Chen W., et al. Bone/cartilage targeted hydrogel: strategies and applications.[J] Bioact. Mater. 2023;23:156–169. doi: 10.1016/j.bioactmat.2022.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wu S., Wu X., Wang X., et al. Hydrogels for bone organoid construction: from a materiobiological perspective[J] J. Mater. Sci. Technol. 2023;136:21–31. [Google Scholar]
- 91.Wang Y., Zhang H., Hu Y., et al. Bone repair biomaterials: a perspective from immunomodulatory[J] Adv. Funct. Mater. 2022;32(51) [Google Scholar]
- 92.Shi X., Zhou J., Zhao Y., et al. Gradient-regulated hydrogel for interface tissue engineering: steering simultaneous osteo/chondrogenesis of stem cells on a chip. J. Adv. Healthc. Mater. 2013;2(6):846–853. doi: 10.1002/adhm.201200333. [DOI] [PubMed] [Google Scholar]
- 93.Lin Z., Li Z., Li E.N., et al. Osteochondral tissue chip derived from iPSCs: modeling OA pathologies and testing drugs.[J] Front. Bioeng. Biotechnol. 2019;7:411. doi: 10.3389/fbioe.2019.00411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hasani-Sadrabadi M.M., Sarrion P., Pouraghaei S., et al. An engineered cell-laden adhesive hydrogel promotes craniofacial bone tissue regeneration in rats. [J]. Sci. Transl. Med. 2020;12(534) doi: 10.1126/scitranslmed.aay6853. [DOI] [PubMed] [Google Scholar]
- 95.Oliveira M.B., Luz G.M., Mano J.F. A combinatorial study of nanocomposite hydrogels: on-chip mechanical/viscoelastic and pre-osteoblast interaction characterization. J. mater. Chem. B. 2014;2(34):5627–5638. doi: 10.1039/c4tb00437j. [DOI] [PubMed] [Google Scholar]
- 96.Kim J., Lee K., Lee J.S., et al. Fungal brain infection modelled in a human-neurovascular-unit-on-a-chip with a functional blood-brain barrier. [J]. Nat. Biomed. Eng. 2021;5(8):830–846. doi: 10.1038/s41551-021-00743-8. [DOI] [PubMed] [Google Scholar]
- 97.Miri A.K., Nieto D., Iglesias L., et al. Microfluidics-enabled multimaterial maskless stereolithographic bioprinting. J. Adv. Mater. 2018;30(27) doi: 10.1002/adma.201800242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yu F., Kumar N.D.O.S., Foo L.C., et al. A pump-free tricellular blood-brain barrier on-a-chip model to understand barrier property and evaluate drug response. J. Biotechnol. Bioeng. 2020;117(4):1127–1136. doi: 10.1002/bit.27260. [DOI] [PubMed] [Google Scholar]
- 99.Namgung B., Ravi K., Vikraman P.P., et al. Engineered cell-laden alginate microparticles for 3D culture. J. Biochem. Soc. Trans. 2021;49(2):761–773. doi: 10.1042/BST20200673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang C., Grossier R., Candoni N., et al. Preparation of alginate hydrogel microparticles by gelation introducing cross-linkers using droplet-based microfluidics: a review of methods. J. Biomater. Res. 2021;25(1):41. doi: 10.1186/s40824-021-00243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Headen D.M., Aubry G., Lu H., et al. Microfluidic-based generation of size-controlled, biofunctionalized synthetic polymer microgels for cell encapsulation. J. Adv. Mater. 2014;26(19):3003–3008. doi: 10.1002/adma.201304880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tao Y., Chen Y., Wang S., et al. COMPOSITES PART B-ENGINEERING; 2022. Optimizing the Modification Density of Acid Oligopeptides to Enhance the Bone-Targeting Activity of liposomes[J] p. 247. [Google Scholar]
- 103.Ren X., Chen X., Geng Z., et al. Bone-targeted biomaterials: strategies and applications[J] Chem. Eng. J. 2022:446. [Google Scholar]
- 104.Pina S., Oliveira J.M., Reis R.L. Natural-based nanocomposites for bone tissue engineering and regenerative medicine: a review. [J]. Adv. Mater. (Deerfield Beach, Fla.) 2015;27(7):1143–1169. doi: 10.1002/adma.201403354. [DOI] [PubMed] [Google Scholar]
- 105.Zhang B., Montgomery M., Chamberlain M.D., et al. Biodegradable scaffold with built-in vasculature for organ-on-a-chip engineering and direct surgical anastomosis. J. Nat. Mater. 2016;15(6):669–678. doi: 10.1038/nmat4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ming L., Zhipeng Y., Fei Y., et al. Microfluidic-based screening of resveratrol and drug-loading PLA/Gelatine nano-scaffold for the repair of cartilage defect. J. Artif. Cell Nanomed. Biotechnol. 2018;46(sup1):336–346. doi: 10.1080/21691401.2017.1423498. [DOI] [PubMed] [Google Scholar]
- 107.Einhorn T.A., Gerstenfeld L.C. Fracture healing: mechanisms and interventions. J. Nat. Rev. Rheumatol. 2015;11(1):45–54. doi: 10.1038/nrrheum.2014.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang Y., Yu T., Ding J., et al. Bone-on-a-chip platforms and integrated biosensors: towards advanced in vitro bone models with real-time biosensing. J. Biosens. Bioelectron. 2022;219 doi: 10.1016/j.bios.2022.114798. [DOI] [PubMed] [Google Scholar]
- 109.Rupprecht C., Wingen M., Potzkei J., et al. A novel FbFP-based biosensor toolbox for sensitive in vivo determination of intracellular pH.[J] J. Biotechnol. 2017;258:25–32. doi: 10.1016/j.jbiotec.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 110.Rivera K.R., Pozdin V.A., Young A.T., et al. Integrated phosphorescence-based photonic biosensor (iPOB) for monitoring oxygen levels in 3D cell culture systems. J. Biosens. Bioelectron. 2019;123:131–140. doi: 10.1016/j.bios.2018.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liang Z., Zhang J., Wu C., et al. Flexible and self-healing electrochemical hydrogel sensor with high efficiency toward glucose monitoring. J. Biosens. Bioelectron. 2020;155 doi: 10.1016/j.bios.2020.112105. [DOI] [PubMed] [Google Scholar]
- 112.Safiabadi Tali S.H., LeBlanc J.J., Sadiq Z., et al. Tools and techniques for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)/COVID-19 detection.[J] Clin. Microbiol. Rev. 2021;34(3) doi: 10.1128/CMR.00228-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Balbaied T., Moore E. Overview of optical and electrochemical alkaline phosphatase (ALP) biosensors: recent approaches in cells culture techniques. [J]. Biosensors. 2019;9(3) doi: 10.3390/bios9030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Aykul S., Martinez-Hackert E. High-throughput, biosensor-based approach to examine bone morphogenetic protein (BMP)-Receptor interactions. J. Methods Mol. Biol. 2019;1891:37–49. doi: 10.1007/978-1-4939-8904-1_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Porat-Ophir C., Dergachev V., Belkin A., et al. Chip level agitation effects on the electrochemical sensing of alkaline-phosphatase expressed from integrated liver tissue[J] Sensor. Actuator. B Chem. 2015;213:465–473. [Google Scholar]
- 116.Ragones H., Schreiber D., Inberg A., et al. Disposable electrochemical sensor prepared using 3D printing for cell and tissue diagnostics[J] Sensor. Actuator. B Chem. 2015;216:434–442. [Google Scholar]
- 117.Dawson-Hughes B., Harris S.S., Krall E.A., et al. Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older.[J] N. Engl. J. Med. 1997;337(10):670–676. doi: 10.1056/NEJM199709043371003. [DOI] [PubMed] [Google Scholar]
- 118.Jackson R.D., LaCroix A.Z., Gass M., et al. Calcium plus vitamin D supplementation and the risk of fractures. N. Engl. J. Med. 2006;354(7):669–683. doi: 10.1056/NEJMoa055218. [DOI] [PubMed] [Google Scholar]
- 119.Bavli D., Prill S., Ezra E., et al. Real-time monitoring of metabolic function in liver-on-chip microdevices tracks the dynamics of mitochondrial dysfunction.[J] Proc. Natl. Acad. Sci. U.S.A. 2016;113(16):E2231–E2240. doi: 10.1073/pnas.1522556113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ortega M.A., Fernández-Garibay X., Castaño A.G., et al. Muscle-on-a-chip with an on-site multiplexed biosensing system for in situ monitoring of secreted IL-6 and TNF-α.[J] Lab Chip. 2019;19(15):2568–2580. doi: 10.1039/c9lc00285e. [DOI] [PubMed] [Google Scholar]
- 121.Jiang Y., Li J., Xue X., et al. Engineered extracellular vesicles for bone therapy[J] Nano Today. 2022:44. [Google Scholar]
- 122.Liu H., Zhang Q., Wang S., et al. Bioactive Materials; 2021. Bacterial Extracellular Vesicles as Bioactive Nanocarriers for Drug Delivery: Advances and perspectives[J] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Liu H., Li M., Zhang T., et al. Engineered bacterial extracellular vesicles for osteoporosis therapy[J] Chem. Eng. J. 2022:450. [Google Scholar]
- 124.Lee J., Mehrotra S., Zare-Eelanjegh E., et al. A heart-breast cancer-on-a-chip platform for disease modeling and monitoring of cardiotoxicity induced by cancer chemotherapy. [J]. Small. 2021;17(15) doi: 10.1002/smll.202004258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sun S., Liu H., Hu Y., et al. Selection and identification of a novel ssDNA aptamer targeting human skeletal muscle. [J]. Bioactive Mater. 2023;20:166–178. doi: 10.1016/j.bioactmat.2022.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Silva D.I., Santos B.P.D., Leng J., et al. Dorsal root ganglion neurons regulate the transcriptional and translational programs of osteoblast differentiation in a microfluidic platform. J. Cell death Dis. 2017;8(12):3209. doi: 10.1038/s41419-017-0034-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zareei A., Jiang H., Chittiboyina S., et al. A lab-on-chip ultrasonic platform for real-time and nondestructive assessment of extracellular matrix stiffness. J. Lab Chip. 2020;20(4):778–788. doi: 10.1039/c9lc00926d. [DOI] [PubMed] [Google Scholar]
- 128.Hou S., Li Z., Zheng X., et al. Embryonic endothelial evolution towards first hematopoietic stem cells revealed by single-cell transcriptomic and functional analyses. J. Cell Res. 2020;30(5):376–392. doi: 10.1038/s41422-020-0300-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Occhetta P., Mainardi A., Votta E., et al. Hyperphysiological compression of articular cartilage induces an osteoarthritic phenotype in a cartilage-on-a-chip model. [J]. Nat. Biomed. Eng. 2019;3(7):545–557. doi: 10.1038/s41551-019-0406-3. [DOI] [PubMed] [Google Scholar]
- 130.Paggi C.A., Hendriks J., Karperien M., et al. Emulating the chondrocyte microenvironment using multi-directional mechanical stimulation in a cartilage-on-chip. J. Lab Chip. 2022;22(9):1815–1828. doi: 10.1039/d1lc01069g. [DOI] [PubMed] [Google Scholar]
- 131.Kutaish H., Bengtsson L., Matthias Tscholl P., et al. Hyaline cartilage microtissues engineered from adult dedifferentiated chondrocytes: safety and role of WNT signaling.[J] Stem cells Transl. Med. 2022;11(12):1219–1231. doi: 10.1093/stcltm/szac074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Li Z., Lin Z., Liu S., et al. Human mesenchymal stem cell-derived miniature joint system for disease modeling and drug testing. J. Adv. Sci. 2022;9(21) doi: 10.1002/advs.202105909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hao S., Ha L., Cheng G., et al. A spontaneous 3D bone-on-a-chip for bone metastasis study of breast cancer cells. [J]. Small. 2018;14(12) doi: 10.1002/smll.201702787. [DOI] [PubMed] [Google Scholar]
- 134.Truesdell S.L., George E.L., Van Vranken C.C., et al. A lab-on-A-chip platform for stimulating osteocyte mechanotransduction and analyzing functional outcomes of bone remodeling. J. Vis. Exp. : JoVE. 2020;(159) doi: 10.3791/61076. [DOI] [PubMed] [Google Scholar]
- 135.Alamán-Díez P., García-Gareta E., Arruebo M., et al. A bone-on-a-chip collagen hydrogel-based model using pre-differentiated adipose-derived stem cells for personalized bone tissue engineering.[J] J. Biomed. Mater. Res., Part A. 2023;111(1):88–105. doi: 10.1002/jbm.a.37448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bahmaee H., Owen R., Boyle L., et al. Design and evaluation of an osteogenesis-on-a-chip microfluidic device incorporating 3D cell culture. J. Front. Bioeng. Biotechnol. 2020;8 doi: 10.3389/fbioe.2020.557111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Conceição F., Sousa D.M., Loessberg-Zahl J., et al. A metastasis-on-a-chip approach to explore the sympathetic modulation of breast cancer bone metastasis. J. Mater. Today. Bio. 2022;13 doi: 10.1016/j.mtbio.2022.100219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Middleton K., Al-Dujaili S., Mei X., et al. Microfluidic co-culture platform for investigating osteocyte-osteoclast signalling during fluid shear stress mechanostimulation. J. Biomech. 2017;59:35–42. doi: 10.1016/j.jbiomech.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 139.Rothbauer M., Höll G., Eilenberger C., et al. Monitoring tissue-level remodelling during inflammatory arthritis using a three-dimensional synovium-on-a-chip with non-invasive light scattering biosensing. J. Lab Chip. 2020;20(8):1461–1471. doi: 10.1039/c9lc01097a. [DOI] [PubMed] [Google Scholar]
- 140.Rothbauer M., Byrne R.A., Schobesberger S., et al. Establishment of a human three-dimensional chip-based chondro-synovial coculture joint model for reciprocal cross talk studies in arthritis research. J. Lab Chip. 2021;21(21):4128–4143. doi: 10.1039/d1lc00130b. [DOI] [PubMed] [Google Scholar]
- 141.Wang X., Phan D.T.T., Sobrino A., et al. Engineering anastomosis between living capillary networks and endothelial cell-lined microfluidic channels. J. Lab Chip. 2016;16(2):282–290. doi: 10.1039/c5lc01050k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Phan D.T.T., Wang X., Craver B.M., et al. A vascularized and perfused organ-on-a-chip platform for large-scale drug screening applications.[J] Lab Chip. 2017;17(3):511–520. doi: 10.1039/c6lc01422d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Vatine G.D., Barrile R., Workman M.J., et al. Human iPSC-derived blood-brain barrier chips enable disease modeling and personalized medicine applications. [J]. Cell Stem Cell. 2019;24(6):995–1005. doi: 10.1016/j.stem.2019.05.011. [DOI] [PubMed] [Google Scholar]
- 144.Hu Y., Cui J., Liu H., et al. Single-cell RNA-sequencing analysis reveals the molecular mechanism of subchondral bone cell heterogeneity in the development of osteoarthritis[J] RMD Open. 2022;8(2) doi: 10.1136/rmdopen-2022-002617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tuli R., Tuli S., Nandi S., et al. Characterization of multipotential mesenchymal progenitor cells derived from human trabecular bone. [J]. Stem cells. 2003;21(6):681–693. doi: 10.1634/stemcells.21-6-681. [DOI] [PubMed] [Google Scholar]
- 146.Boyle W.J., Simonet W.S., Lacey D.L. Osteoclast differentiation and activation. J. Nat. 2003;423(6937):337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 147.Sanchez-Lopez E., Coras R., Torres A., et al. Synovial inflammation in osteoarthritis progression.[J] Nat. Rev. Rheumatol. 2022;18(5):258–275. doi: 10.1038/s41584-022-00749-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Nygaard G., Firestein G.S. Restoring synovial homeostasis in rheumatoid arthritis by targeting fibroblast-like synoviocytes.[J] Nat. Rev. Rheumatol. 2020;16(6):316–333. doi: 10.1038/s41584-020-0413-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mondadori C., Palombella S., Salehi S., et al. Recapitulating monocyte extravasation to the synovium in an organotypic microfluidic model of the articular joint. [J]. Biofabrication. 2021;13(4) doi: 10.1088/1758-5090/ac0c5e. [DOI] [PubMed] [Google Scholar]
- 150.Ronaldson-Bouchard K., Teles D., Yeager K., et al. A multi-organ chip with matured tissue niches linked by vascular flow.[J] Nat. Biomed. Eng. 2022;6(4):351–371. doi: 10.1038/s41551-022-00882-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chen H., Hu B., Lv X., et al. Prostaglandin E2 mediates sensory nerve regulation of bone homeostasis.[J] Nat. Commun. 2019;10(1):181. doi: 10.1038/s41467-018-08097-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.George E.L., Truesdell S.L., York S.L., et al. Lab-on-a-chip platforms for quantification of multicellular interactions in bone remodeling.[J] Exp. Cell Res. 2018;365(1):106–118. doi: 10.1016/j.yexcr.2018.02.027. [DOI] [PubMed] [Google Scholar]
- 153.Babaliari E., Petekidis G., Chatzinikolaidou M. A precisely flow-controlled microfluidic system for enhanced pre-osteoblastic cell response for bone tissue engineering. J. Bioeng. 2018;5(3) doi: 10.3390/bioengineering5030066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yazdian Kashani S., Keshavarz Moraveji M., Taghipoor M., et al. An integrated microfluidic device for stem cell differentiation based on cell-imprinted substrate designed for cartilage regeneration in a rabbit model. J. Mater. Sci. Eng. C, Mater. Biol. Appl. 2021;121 doi: 10.1016/j.msec.2020.111794. [DOI] [PubMed] [Google Scholar]
- 155.Guo J., Wang F., Hu Y., et al. Exosome-based bone-targeting drug delivery alleviates impaired osteoblastic bone formation and bone loss in inflammatory bowel diseases. [J]. Cell Rep. Med. 2022 doi: 10.1016/j.xcrm.2022.100881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Banh L., Cheung K.K., Chan M.W.Y., et al. Advances in organ-on-a-chip systems for modelling joint tissue and osteoarthritic diseases. J. Osteoarthritis Cartilage. 2022;30(8):1050–1061. doi: 10.1016/j.joca.2022.03.012. [DOI] [PubMed] [Google Scholar]
- 157.Brown T.D. Techniques for mechanical stimulation of cells in vitro: a review.[J] J. Biomech. 2000;33(1):3–14. doi: 10.1016/s0021-9290(99)00177-3. [DOI] [PubMed] [Google Scholar]
- 158.Lee D., Erickson A., You T., et al. Pneumatic microfluidic cell compression device for high-throughput study of chondrocyte mechanobiology[J] Lab Chip. 2018;18(14):2077–2086. doi: 10.1039/c8lc00320c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lin H., Lozito T.P., Alexander P.G., et al. Stem cell-based microphysiological osteochondral system to model tissue response to interleukin-1β. J. Mol. Pharm. 2014;11(7):2203–2212. doi: 10.1021/mp500136b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Gómez-Aristizábal A., Gandhi R., Mahomed N.N., et al. Synovial fluid monocyte/macrophage subsets and their correlation to patient-reported outcomes in osteoarthritic patients: a cohort study. J. Arthritis Res. Ther. 2019;21(1):26. doi: 10.1186/s13075-018-1798-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Clézardin P., Coleman R., Puppo M., et al. Bone metastasis: mechanisms, therapies, and biomarkers. J. Physiol. Rev. 2021;101(3):797–855. doi: 10.1152/physrev.00012.2019. [DOI] [PubMed] [Google Scholar]
- 162.Mei X., Middleton K., Shim D., et al. Microfluidic platform for studying osteocyte mechanoregulation of breast cancer bone metastasis. J. Integr. Biol. : Quant. Biosci. Nano To Macro. 2019;11(4):119–129. doi: 10.1093/intbio/zyz008. [DOI] [PubMed] [Google Scholar]
- 163.Bischel L.L., Casavant B.P., Young P.A., et al. A microfluidic coculture and multiphoton FAD analysis assay provides insight into the influence of the bone microenvironment on prostate cancer cells. J. Integr. Biol. : Quant. Biosci. Nano To Macro. 2014;6(6):627–635. doi: 10.1039/c3ib40240a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Müller A., Homey B., Soto H., et al. Involvement of chemokine receptors in breast cancer metastasis. J. Nat. 2001;410(6824):50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 165.Hu Y., Li X., Zhang Q., et al. Exosome-guided bone targeted delivery of Antagomir-188 as an anabolic therapy for bone loss. [J]. Bioactive Mater. 2021;6(9):2905–2913. doi: 10.1016/j.bioactmat.2021.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Jeon J.S., Bersini S., Gilardi M., et al. Human 3D vascularized organotypic microfluidic assays to study breast cancer cell extravasation.[J] Proc. Natl. Acad. Sci. U.S.A. 2015;112(1):214–219. doi: 10.1073/pnas.1417115112. [DOI] [PMC free article] [PubMed] [Google Scholar]